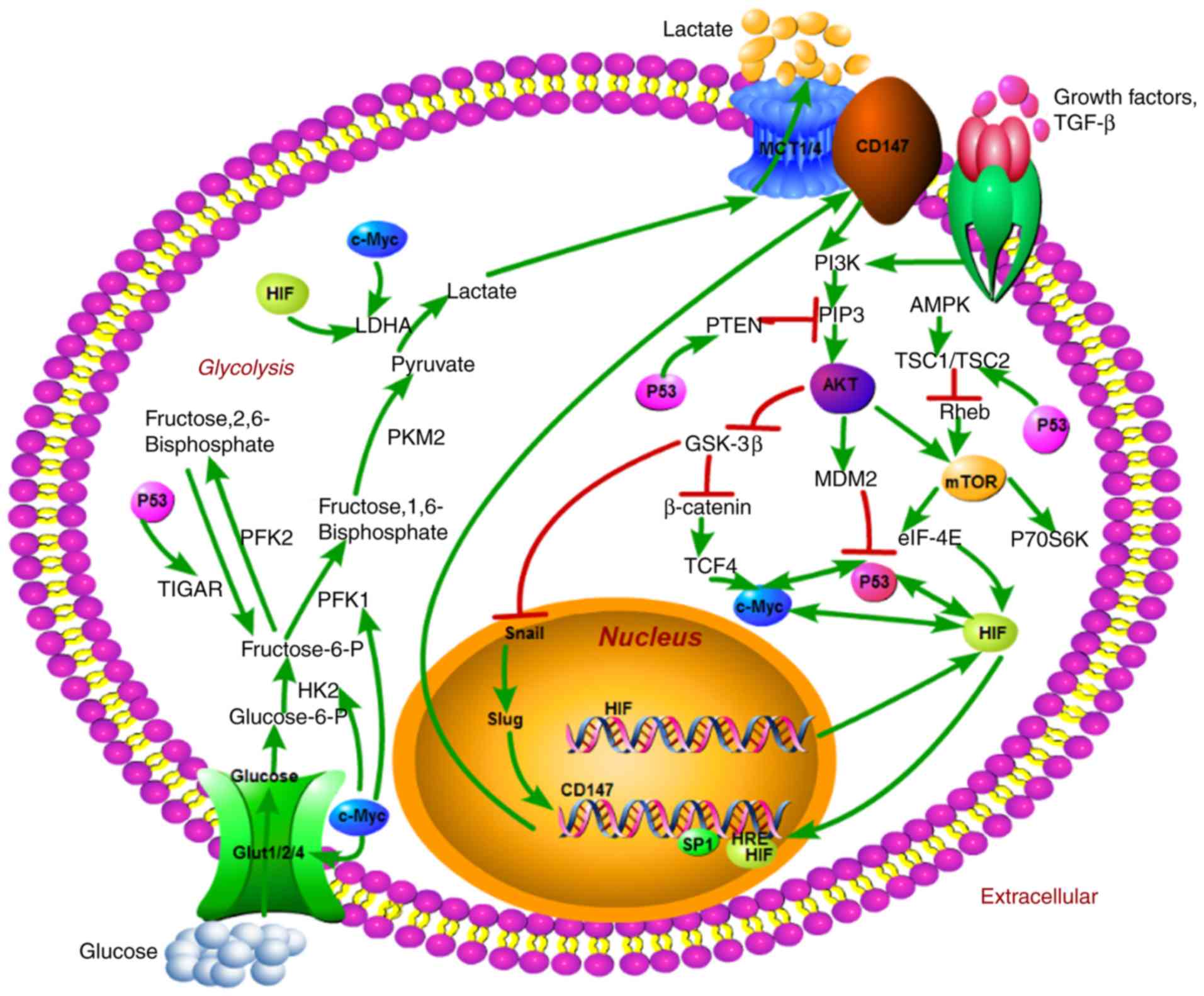
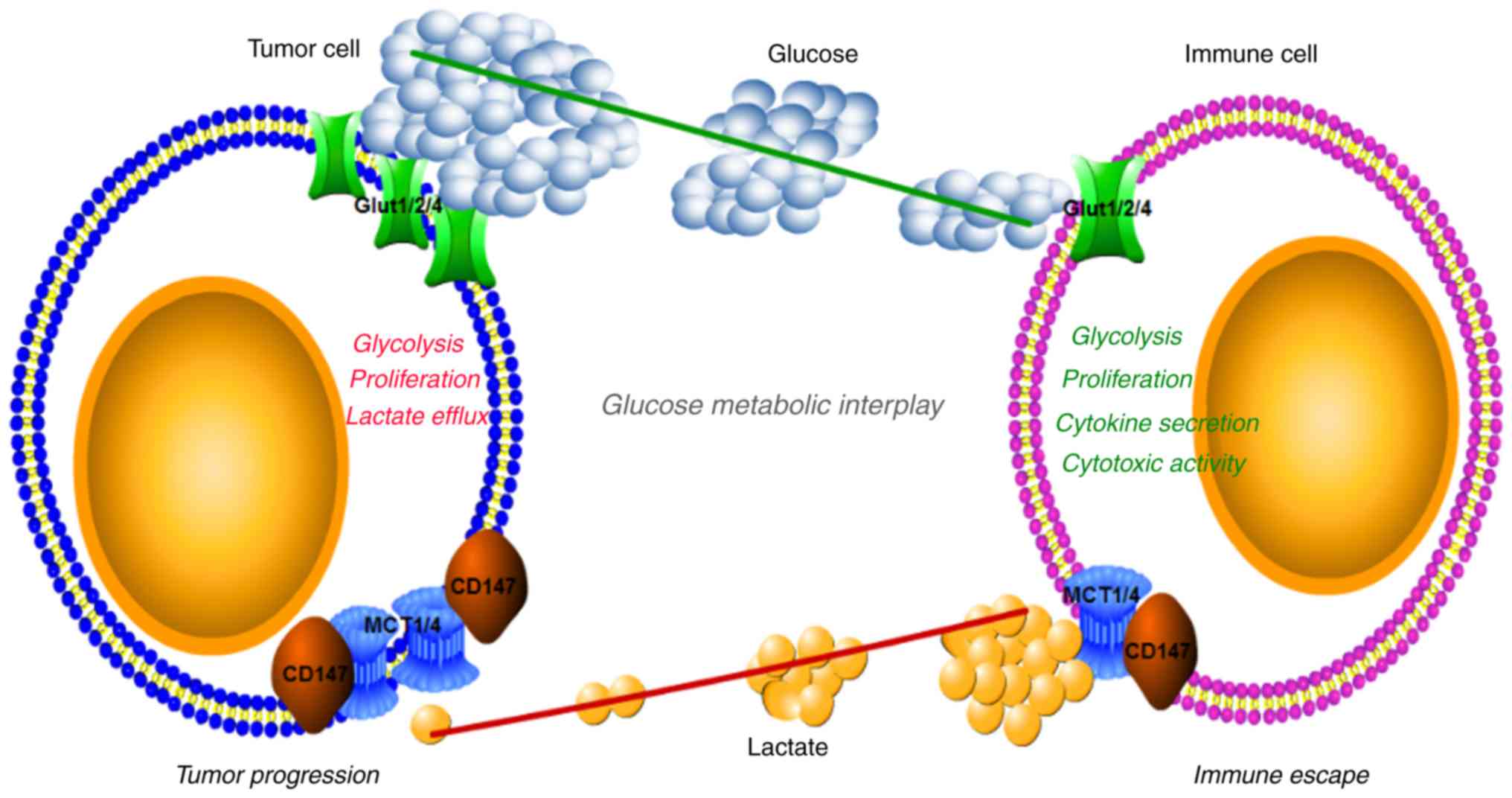
|
1
|
Kareva I and Hahnfeldt P: The emerging
‘hallmarks’ of metabolic reprogramming and immune evasion: Distinct
or linked? Cancer Res. 73:2737–2742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956.PubMed/NCBI
|
|
3
|
Li X, Peng J, Pang Y, Yu S, Yu X, Cheng
PC, Wang WZ, Han WL, Zhang J, Yin YH and Zhang Y: Identification of
a FOXP3+CD3+CD56+ population with
immunosuppressive function in cancer tissues of human
hepatocellular carcinoma. Sci Rep. 5:147572015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalathil SG and Thanavala Y: High
immunosuppressive burden in cancer patients: A major hurdle for
cancer immunotherapy. Cancer Immunol Immunother. 650:813–819. 2016.
View Article : Google Scholar
|
|
5
|
Wargo JA, Reddy SM, Reuben A and Sharma P:
Monitoring immune responses in the tumor microenvironment. Curr
Opin Immunol. 41:23–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Josefowicz SZ, Lu LF and Rudensky AY:
Regulatory T cells: Mechanisms of differentiation and function.
Annu Rev Immunol. 30:531–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hansen M and Andersen MH: The role of
dendritic cells in cancer. Semin Immunopathol. 9:307–316. 2017.
View Article : Google Scholar
|
|
8
|
Ni L and Dong C: New checkpoints in cancer
immunotherapy. Immunol Rev. 276:52–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Speiser DE, Ho PC and Verdeil G:
Regulatory circuits of T cell function in cancer. Nat Rev Immunol.
16:599–611. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang CH, Qiu J, O'Sullivan D, Buck MD,
Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ,
et al: Metabolic competition in the tumor microenvironment is a
driver of cancer progression. Cell. 162:1229–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siska PJ and Rathmell JC: T cell metabolic
fitness in antitumor immunity. Trends Immunol. 36:257–264. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gottfried E, Kunz-Schughart LA, Ebner S,
Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A and Kreutz M:
Tumor-derived lactic acid modulates dendritic cell activation and
antigen expression. Blood. 107:2013–2021. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fischer K, Hoffmann P, Voelkl S,
Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G,
Hoves S, et al: Inhibitory effect of tumor cell-derived lactic acid
on human T cells. Blood. 109:3812–3819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
MacIver NJ, Michalek RD and Rathmell JC:
Metabolic regu-lation of T lymphocytes. Annu Rev Immunol.
31:259–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerriets VA and Rathmell JC: Metabolic
pathways in T cell fate and function. Trends Immunol. 33:168–173.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang CH, Curtis JD, Maggi LB Jr, Faubert
B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih
J, Qiu J, et al: Posttranscriptional control of T cell effector
function by aerobic glycolysis. Cell. 153:1239–1251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Macintyre AN, Gerriets VA, Nichols AG,
Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen
BJ, Hale LP, et al: The glucose transporter Glut1 is selectively
essential for CD4 T cell activation and effector function. Cell
Metab. 20:61–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pearce EL, Poffenberger MC, Chang CH and
Jones RG: Fueling immunity: Insights into metabolism and lymphocyte
function. Science. 42:12424542013. View Article : Google Scholar
|
|
19
|
Chang CH and Pearce EL: Emerging concepts
in immunotherapy-T cell metabolism as a therapeutic target. Nat
Immunol. 17:364–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Sullivan D and Pearce EL: Targeting T
cell metabolism for therapy. Trends Immunol. 36:71–80. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang R, Dillon CP, Shi LZ, Milasta S,
Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger
J, et al: The transcription factor Myc controls metabolic
reprogramming upon T lymphocyte activation. Immunity. 35:871–882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi LZ, Wang R, Huang G, Vogel P, Neale G,
Green DR and Chi H: HIF1alpha-dependent glycolytic pathway
orchestrates a metabolic checkpoint for the differentiation of TH17
and Treg cells. J Exp Med. 208:1367–1376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
So L and Fruman DA: PI3K signalling in B-
and T-lymphocytes: New developments and therapeutic advances.
Biochem J. 442:465–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han JM, Patterson SJ and Levings MK: The
role of the PI3K signaling pathway in CD4+ T cell
differentiation and function. Front Immunol. 3:2452012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Powell JD, Pollizzi KN, Heikamp EB and
Horton MR: Regulation of immune responses by mTOR. Annu Rev
Immunol. 30:39–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chi HB: Regulation and function of mTOR
signaling in T cell fate decisions. Nat Rev Immunol. 12:325–338.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weidle UH, Scheuer W, Eggle D, Klostermann
S and Stockinger H: Cancer-related issues of CD147. Cancer Genomics
Proteomics. 7:157–169. 2010.PubMed/NCBI
|
|
28
|
Riethdorf S, Reimers N, Assmann V,
Kornfeld JW, Terracciano L, Sauter G and Pantel K: High incidence
of EMMPRIN expression in human tumors. Int J Cancer. 119:1800–1810.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu J, Xu HY, Zhang Q, Song F, Jiang JL,
Yang XM, Mi L, Wen N, Tian R, Wang L, et al: HAb18G/CD147 functions
in invasion and metastasis of hepatocellular carcinoma. Mol Cancer
Res. 5:605–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Q, Zhou J, Ku XM, Chen XG, Zhang L,
Xu J, Chen GS, Li Q, Qian F, Tian R, et al: Expression of CD147 as
a significantly unfavorable prognostic factor in hepatocellular
carcinoma. Eur J Cancer Prev. 16:196–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng HC, Takahashi H, Murai Y, Cui ZG,
Nomoto K, Miwa S, Tsuneyama K and Takano Y: Upregulated
EMMPRIN/CD147 might contribute to growth and angiogenesis of
gastric carcinoma: A good marker for local invasion and prognosis.
Br J Cancer. 95:1371–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang Y, Nakada MT, Kesavan P, McCabe F,
Millar H, Rafferty P, Bugelski P and Yan L: Extracellular matrix
metalloproteinase inducer stimulates tumor angiogenesis by
elevating vascular endothelial cell growth factor and matrix
metalloproteinases. Cancer Res. 65:3193–3199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang Y, Nakada MT, Rafferty P, Laraio J,
McCabe FL, Millar H, Cunningham M, Snyder LA, Bugelski P and Yan L:
Regulation of vascular endothelial growth factor expression by
EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res.
4:371–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kennedy KM and Dewhirst MW: Tumor
metabolism of lactate: The influence and therapeutic potential for
MCT and CD147 regulation. Future Oncol. 6:127–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Halestrap AP and Price NT: The
proton-linked monocarboxylate transporter (MCT) family: Structure,
function and regulation. Biochem J. 343:281–299. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Halestrap AP and Wilson MC: The
monocarboxylate transporter family-role and regulation. IUBMB Life.
64:109–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kirk P, Wilson MC, Heddle C, Brown MH,
Barclay AN and Halestrap AP: CD147 is tightly associated with
lactate transporters MCT1 and MCT4 and facilitates their cell
surface expression. EMBO J. 19:3896–3904. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li XF, Yu XZ, Song XY, Dai D and Xu WG:
The altered glucose metabolism in tumor and a tumor acidic
microenvironment associated with extracellular matrix
metalloproteinase inducer and monocarboxylate transporters.
Oncotarget. 7:23141–23155. 2016.PubMed/NCBI
|
|
39
|
Kroemer G and Pouyssegur J: Tumor Cell
Metabolism: Cancer's Achilles' Heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2014. View Article : Google Scholar
|
|
41
|
Levine AJ and Puzio-Kuter AM: The control
of the metabolic switch in cancers by oncogenes and tumor
suppressors genes. Science. 330:1340–1344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tennant DA, Duran RV and Gottlieb E:
Targeting metabolic transformation for cancer therapy. Nat Rev
Cancer. 10:267–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Madan E, Gogna R, Bhatt M, Pati U,
Kuppusamy P and Mahdi AA: Regulation of glucose metabolism by p53:
Emerging new roles for the tumor suppressor. Oncotarget. 2:948–957.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang C, Liu J, Wu R, Liang Y, Lin M, Liu
J, Chan CS, Hu W and Feng Z: Tumor suppressor p53 negatively
regulates glycolysis stimulated by hypoxia through its target RRAD.
Oncotarget. 5:5535–5546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schwartzenberg-Bar-Yoseph F, Armoni M and
Karnieli E: The tumor suppressor p53 down-regulates glucose
transporters GLUT1 and GLUT4 gene expression. Cancer Res.
64:2627–2633. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dang CV, Le A and Gao P: MYC-induced
cancer cell energy metabolism and therapeutic opportunities. Clin
Cancer Res. 15:6479–6483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shim H, Dolde C, Lewis BC, Wu CS, Dang G,
Jungmann RA, Dalla-Favera R and Dang CV: c-Myc transactivation of
LDH-A: Implications for tumor metabolism and growth. Proc Natl Acad
Sci USA. 94:6658–6663. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Michalek RD, Gerriets VA, Jacobs SR,
Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG and
Rathmell JC: Cutting Edge: Distinct glycolytic and lipid oxidative
metabolic programs are essential for effector and regulatory
CD4+ T cell subsets. J Immunol. 186:3299–3303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Osthus RC, Shim H, Kim S, Li Q, Reddy R,
Mukherjee M, Xu Y, Wonsey D, Lee LA and Dang CV: Deregulation of
glucose transporter 1 and glycolytic gene expression by c-Myc. J
Biol Chem. 275:21797–21800. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim JW, Gao P, Liu YC, Semenza GL and Dang
CV: Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively
induce vascular endothelial growth factor and metabolic switches
hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol.
27:7381–7393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dang CV, Kim JW, Gao P and Yustein J: The
interplay between MYC and HIF in cancer. Nat Rev Cancer. 8:51–56.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Elstrom RL, Bauer DE, Buzzai M, Karnauskas
R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM and
Thompson CB: Akt stimulates aerobic glycolysis in cancer cells.
Cancer Res. 64:3892–3899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Makinoshima H, Takita M, Saruwatari K,
Umemura S, Obata Y, Ishii G, Matsumoto S, Sugiyama E, Ochiai A, Abe
R, et al: Signaling through the phosphatidylinositol 3-kinase
(PI3K)/mammalian target of rapamycin (mTOR) axis is responsible for
aerobic glycolysis mediated by glucose transporter in epidermal
growth factor receptor (EGFR)-mutated lung adenocarcinoma. J Biol
Chem. 290:17495–17504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Laplante M and Sabatini DM: mTOR signaling
at a glance. J Cell Sci. 122:3589–3594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Woo YM, Shin Y, Lee EJ, Lee S, Jeong SH,
Kong HK, Park EY, Kim HK, Han J, Chang M, et al: Inhibition of
aerobic glycolysis represses Akt/mTOR/HIF-1α axis and restores
tamoxifen sensitivity in antiestrogen-resistant breast cancer
cells. PLoS One. 10:e01322852015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cheng SC, Quintin J, Cramer RA, Shepardson
KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao
NA, Aghajanirefah A, et al: mTOR- and HIF-1α-mediated aerobic
glycolysis as metabolic basis for trained immunity. Science.
345:12506842014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lu H, Forbes RA and Verma A:
Hypoxia-inducible factor 1 activation by aerobic glycolysis
implicates the Warburg effect in carcinogenesis. J Biol Chem.
277:23111–23115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu J, Zhang C, Hu WW and Feng ZH: Tumor
suppressor p53 and its mutants in cancer metabolism. Cancer Lett.
356:197–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vousden KH and Ryan KM: p53 and
metabolism. Nat Rev Cancer. 9:691–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yeung SJ, Pan J and Lee MH: Roles of p53,
MYC and HIF-1 in regulating glycolysis-the seventh hallmark of
cancer. Cell Mol Life Sci. 65:3981–3999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ogawara Y, Kishishita S, Obata T, Isazawa
Y, Suzuki T, Tanaka K, Masuyama N and Gotoh Y: Akt enhances
Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem.
277:21843–21850. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Warburg O, Gawehn K and Geissler AW:
Metabolism of leukocytes. Z Naturforsch B. 13B:515–516. 1958.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jacobs SR, Michalek RD and Rathmell JC:
IL-7 is essential for homeostatic control of T cell metabolism in
vivo. J Immunol. 184:3461–3469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang R and Green DR: Metabolic checkpoints
in activated T cells. Nat Immunol. 13:907–915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vander-Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Van der Windt GJ, Everts B, Chang CH,
Curtis JD, Freitas TC, Amiel E, Pearce EJ and Pearce EL:
Mitichondrial respiratory capacity is a critical regulator of
CD8+ T cell memory development. Immunity. 36:68–78.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
van der Windt GJ, O'Sullivan D, Everts B,
Huang SC, Buck MD, Curtis JD, Chang CH, Smith AM, Ai T, Faubert B,
et al: CD8 memory T cells have a bioenergetic advantage that
underlies their rapid recall ability. Proc Natl Acad Sci USA.
110:14336–14341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mannava S, Grachtchouk V, Wheeler LJ, Im
M, Zhuang D, Slavina EG, Mathews CK, Shewach DS and Nikiforov MA:
Direct role of nucleotide metabolism in C-MYC-dependent
proliferation of melanoma cells. Cell Cycle. 7:2392–2400. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shen Y, Wei Y, Wang Z, Jing Y, He H, Yuan
J, Li R, Zhao Q, Wei L, Yang T, et al: TGF-β regulates
hepatocellular carcinoma progression by inducing Treg cell
polarization. Cell Physiol Biochem. 35:1623–1632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kalathil S, Lugade AA, Miller A, Iyer R
and Thanavala Y: Higher frequencies of
GARP+CTLA-4+Foxp3+ T regulatory
cells and myeloid-derived suppressor cells in hepatocellular
carcinoma patients are associated with impaired T-cell
functionality. Cancer Res. 73:2435–2444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Galgani M, De Rosa V, La Cava A and
Matarese G: Role of metabolism in the immunobiology of regulatory T
cells. J Immunol. 197:2567–2575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Procaccini C, Carbone F, Di Silvestre D,
Brambilla F, De Rosa V, Galgani M, Faicchia D, Marone G, Tramontano
D, Corona M, et al: The protemic landscape of human ex vivo
regulatory and conventional T cells reveals specific metabolic
requirements. Immunity. 44:406–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gao P, Tchernyshyov I, Chang TC, Lee YS,
Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, et
al: c-Myc suppression of miR-23a/b enhances mitochondrial
glutaminase expression and glutamine metabolism. Nature.
458:762–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cham CM, Driessens G, O'Keefe JP and
Gajewski TF: Glucose deprivation inhibits multiple key gene
expression events and effector functions in CD8+ T
cells. J Immunol. 38:2438–2450. 2008.
|
|
77
|
Siska PJ, van der Windt GJ, Kishton RJ,
Cohen S, Eisner W, MacIver NJ, Kater AP, Weinberg JB and Rathmell
JC: Suppression of Glut1 and glucose metabolism by decreased
Akt/mTORC1 signaling drives T cell impairment in B cell leukemia. J
Immunol. 197:2532–2540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Dang EV, Barbi J, Yang HY, Jinasena D, Yu
H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al: Control of
TH17/Treg balance by hypoxia-inducible factor
1. Cell. 146:772–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen L and Flies DB: Molecular mechanisms
of T cell co-stimulation and co-inhibition. Nat Rev Immunol.
3:227–242. 2013. View Article : Google Scholar
|
|
80
|
Wieman HL, Wofford JA and Rathmell JC:
Cytokine stimulation promotes glucose uptake via
phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and
trafficking. Mol Biol Cell. 18:1437–1446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Delgoffe GM, Kole TP, Zheng Y, Zarek PE,
Matthews KL, Xiao B, Worley PF, Kozma SC and Powell JD: The mTOR
kinase differentially regulates effector and regulatory T cell
lineage commitment. Immunity. 30:832–844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu C, Chapman NM, Karmaus PW, Zeng H and
Chi H: mTOR and metabolic regulation of conventional and regulatory
T cells. J Leukoc Biol. 7:837–847. 2015. View Article : Google Scholar
|
|
83
|
Procaccini C, De Rosa V, Galgani M, Abanni
L, Calì G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava
A, et al: An oscillatory switch in mTOR kinase activity sets
regulatory T cell responsiveness. Immunity. 33:929–941. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Blagih J, Coulombe F, Vincent EE, Dupuy F,
Galicia-Vázquez G, Yurchenko E, Raissi TC, van der Windt GJ,
Viollet B, Pearce EL, et al: The energy sensor AMPK regulates T
cell metabolic adaptation and effector responses in vivo. Immunity.
42:41–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveilance to tumor
escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu Y and Cao XT: Immunosuppressive cells
in tumor immune escape and metastasis. J Mol Med. 94:509–522. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang TT, Liu GW and Wang RN: The
intercellular metabolic interplay between tumor and immune cells.
Front Immunol. 5:3582014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Slomiany MG, Grass GD, Robertson AD, Yang
XY, Maria BL, Beeson C and Toole BP: Hyaluronan, CD44, and emmprin
regulate lactate efflux and membrane localization of
monocarboxylate transporters in human breast carcinoma cells.
Cancer Res. 69:1293–1301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hirschhaeuser F, Sattler UG and
Mueller-Klieser W: Lactate: A metabolic key player in cancer.
Cancer Res. 71:6921–6925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Le Floch R, Chiche J, Marchiq I, Naiken T,
Ilc K, Murray CM, Critchlow SE, Roux D, Simon MP and Pouysségur J:
CD147 subunit of lactate/H+ symporters MCT1 and
hypoxia-inducible MCT4 is critical for energetics and growth of
glycolytic tumors. Proc Natl Acad Sci USA. 108:16663–16668. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Martinez-Outschoorn UE, Prisco M, Ertel A,
Tsirigos A, Lin Z, Pavlides S, Wang C, Flomenberg N, Knudsen ES,
Howell A, et al: Ketones and lactate increase cancer cell
‘stemness,’ driving recurrence, metastasis and poor clinical
outcome in breast cancer: Achieving personalized medicine via
Metabolo-Genomics. Cell Cycle. 10:1271–1286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Goetze K, Walenta S, Ksiazkiewicz M,
Kunz-Schughart LA and Mueller-Klieser W: Lactate enhances motility
of tumor cells and inhibits monocyte migration and cytokine
release. Int J Oncol. 39:453–463. 2011.PubMed/NCBI
|
|
94
|
Wong TY, Phillips AO, Witowski J and
Topley N: Glucose-mediated induction of TGF-β1 and MCP-1 in
mesothelial cells in vitro is osmolality and polyol pathway
dependent. Kidney Int. 63:1404–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kottmann RM, Kulkarni AA, Smolnycki KA,
Lyda E, Dahanayake T, Salibi R, Honnons S, Jones C, Isern NG, Hu
JZ, et al: Lactic acid is elevated in idiopathic pulmonary fibrosis
and induces myofibroblast differentiation via pH-dependent
activation of transforming growth factor-β. Am J Respir Crit Care
Med. 186:740–751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Rudrabhatla SR, Mahaffey CL and Mummert
ME: Tumor microenvironment modulates hyaluronan expression: The
lactate effect. J Invest Dermatol. 126:1378–1387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gottfried E, Kreutz M and Mackensen A:
tumor metabolism as modulator of immune response and tumor
progression. Semin Cancer Biol. 22:335–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Husain Z, Huang Y, Seth P and Sukhatme VP:
Tumor-derived lactate modifies antitumor immune response: Effect on
myeloid-derived suppressor cells and NK cells. J Immunol.
191:1486–1495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Feder-Mengus C, Ghosh S, Weber WP, Wyler
S, Zajac P, Terracciano L, Oertli D, Heberer M, Martin I, Spagnoli
GC, et al: Multiple mechanisms underlie defective recognition of
melanoma cells cultured in three-dimensional architectures by
antigen-specific cytotoxic T lymphocytes. Br J Cancer.
96:1072–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Mendler AN, Hu B, Prinz PU, Kreutz M,
Gottfried E and Noessner E: Tumor lactic acidosis suppresses CTL
function by inhibition of p38 and JNK/c-Jun activation. Int J
Cancer. 131:633–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gatenby RA, Gawlinski ET, Gmitro AF,
Kaylor B and Gillies RJ: Acid-mediated tumor invasion: A
multidisciplinary study. Cancer Res. 66:5216–5223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
McCarty MF and Whitaker J: Manipulating
tumor acidification as a cancer treatment strategy. Altern Med Rev.
15:264–272. 2010.PubMed/NCBI
|
|
103
|
Hu XY and Ivashkiv LB: Cross-regulation of
signaling pathways by interferon-gamma: Implications for immune
responses and autoimmune diseases. Immunity. 31:539–550. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Nathan I, Groopman JE, Quan SG, Bersch N
and Golde DW: Immune (gamma) interferon produced by a human
T-lymphoblast cell line. Nature. 292:842–844. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Mekhail K, Gunaratnam L, Bonicalzi ME and
Lee S: HIF activation by pH-dependent nucleolar sequestration of
VHL. Nat Cell Biol. 6:642–647. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
McMahon S, Charbonneau M, Grandmont S,
Richard DE and Dubois CM: Transforming growth factor beta1 induces
hypoxia-inducible factor-1 stabilization through selective
inhibition of PHD2 expression. J Biol Chem. 281:24171–24181. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Clambey ET, McNamee EN, Westrich JA,
Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC,
Stenmark KR, Colgan SP, et al: Hypoxia-inducible factor-1
alpha-dependent induction of FoxP3 drives regulatory T-cell
abundance and function during inflammatory hypoxia of the mucosa.
Proc Natl Acad Sci USA. 109:E2784–E2793. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hung SP, Yang MH, Tseng KF and Lee OK:
Hypoxia-induced secretion of TGF-β1 in mesenchymal stem cell
promotes breast cancer cell progression. Cell Transplant.
22:1869–1882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sanjabi S, Oh SA and Li MO: Regulation of
the immune response by TGF-β: From conception to autoimmunity and
infection. Cold Spring Harb Perspect Biol. 9(pii): a0222362017.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Barsoum IB, Smallwood CA, Siemens DR and
Graham CH: A mechanism of hypoxia-mediated escape from adaptive
immunity in cancer cells. Cancer Res. 74:665–674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Park BV, Freeman ZT, Ghasemzadeh A,
Chattergoon MA, Rutebemberwa A, Steigner J, Winter ME, Huynh TV,
Sebald SM, Lee SJ, et al: TGF-β1-mediated SMAD3 enhances PD-1
expression on antigen-specific T Cells in cancer. Cancer Discov.
6:1366–1381. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wei S, Shreiner AB, Takeshita N, Chen L,
Zou W and Chang AE: Tumor-induced immune suppression of in vivo
effector T-cell priming is mediated by the B7-H1/PD-1 axis and
transforming growth factor beta. Cancer Res. 68:5432–5438. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mamori S, Nagatsuma K, Matsuura T, Ohkawa
K, Hano H, Fukunaga M, Matsushima M, Masui Y, Fushiya N, Onoda H,
et al: Useful detection of CD147 (EMMPRIN) for pathological
diagnosis of early hepatocellular carcinoma in needle biopsy
samples. World J Gastroenterol. 13:2913–2917. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Tang J, Wu YM, Zhao P, Yang XM, Jiang JL
and Chen ZN: Overexpression of HAb18G/CD147 promotes invasion an
metastasis via alpha3beta1 integrin mediated FAK-paxilli and
FAK-PI3K-Ca2+ pathways. Cell Mol Life Sci. 65:2933–2942.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dai JY, Dou KF, Wang CH, Zhao P, Lau WB,
Tao L, Wu YM, Tang J, Jiang JL and Chen ZN: The interaction of
HAb18G/CD147 with integrin α6β1 and its implications for the
invasion potential of human hepatoma cells. BMC Cancer. 9:337–346.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li
Y, Jiang JL, Zhang SH and Chen ZN: Annexin II promotes invasion and
migration of human hepatocellular carcinoma cells in vitro via its
interaction with HAb18G/CD147. Cancer Sci. 101:387–395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Baba M, Inoue M, Itoh K and Nishizawa Y:
Blocking CD147 induces cell death in cancer cells through
impairment of glycolytic energy metabolism. Biochem Biophys Res
Commun. 374:111–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Su J, Chen X and Kanekura TA:
CD147-targeting siRNA inhibits the proliferation, invasiveness, and
VEGF production of human malignant melanoma cells by
down-regulating glycolysis. Cancer Lett. 273:140–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Huang QC, Li JB, Xing JL, Li WW, Li HW, Ke
X, Zhang J, Ren TT, Shang YK, Yang HS, et al: CD147 promotes
reprogramming of glucose metabolism and cell proliferation in HCC
cells by inhibiting the p53-dependent signaling pathway. J Hepatol.
61:859–866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ke X, Fei F, Chen YK, Xu L, Zhang Z, Huang
QC, Zhang HX, Yang HS, Chen ZN and Xing JL: Hypoxia upregulates
CD147 through a combined effect of HIF-1alpha and Sp1 to promote
glycolysis and tumor progression in epithelial solid tumors.
Carcinogenesis. 33:1598–1607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Murata M, Matsuzaki K, Yoshida K, Sekimoto
G, Tahashi Y, Mori S, Uemura Y, Sakaida N, Fujisawa J, Seki T, et
al: Hepatitis B virus X protein shifts human hepatic transforming
factor (TGF)-beta signaling from tumor suppression to oncogenesis
in early chronic hetatitis B. Hepatology. 49:1203–1217. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z,
Huang XF, Chen ZN and Bian H: HAb18G/CD147 promotes
epithelial-mesenchymal transition through TGF-β signaling and is
transcriptionally regulated by Slug. Oncogene. 30:4410–4427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Christofk HR, Vander Heiden MG, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 52:230–233. 2008.
View Article : Google Scholar
|
|
127
|
Kong LM, Liao CG, Chen L, Yang HS, Zhang
SH, Zhang Z, Bian HJ, Xing JL and Chen ZN: Promoter hypomethylation
up-regulates CD147 expression through increasing Sp1 binding and
associates with poor prognosis in human hepatocellular carcinoma. J
Cell Mol Med. 15:1415–1428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Guo H, Majmudar G, Jensen TC, Biswas C,
Toole BP and Gordon MK: Characterization of the gene for human
EMMPRIN, a tumor cell surface inducer of matrix metalloproteinases.
Gene. 220:99–108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Yang H, Zou W and Chen BL: Overexpression
of CD147 in ovarian cancer is initiated by the hypoxic
microenvironment. Cell Biol Int. 37:1139–1142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Kong LM, Liao CG, Fei F, Guo X, Xing JL
and Chen ZN: Transcription factor Sp1 regulates expression of
cancer-associated molecule CD147 in human lung cancer. Cancer Sci.
101:1463–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kono K: Current status of cancer
immunotherapy. J Stem Cells Regen Med. 10:8–13. 2014.PubMed/NCBI
|
|
132
|
Sangro B, Gomez-Martin C, de la Mata M,
Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E,
Alfaro C, Sarobe P, et al: A clinical trial of CTLA-4 blockade with
tremelimumab in patients with hepatocellular carcinoma and chronic
hepatitis C. J Hepatol. 59:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Prat A, Navarro A, Paré L, Reguart N,
Galván P, Pascual T, Martínez A, Nuciforo P, Comerma L, Alos L, et
al: Immune-related gene expression profiling after PD-1 blockade in
non-small cell lung carcinoma, head and neck squamous cell
carcinoma and melanoma. Cancer Res. 77:3540–3550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cabel L, Riva F, Servois V, Livartowski A,
Daniel C, Rampanou A, Lantz O, Romano E, Milder M, Buecher B, et
al: Circulating tumor DNA changes for early monitoring of anti-PD1
immunotherapy: A proof-of-concept study. Ann Oncol. 28:1996–2001.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Patsoukis N, Bardhan K, Chatterjee P, Sari
D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, et al:
PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis
and promoting lipolysis and fatty acid oxidation. Nat Commun.
6:66922015. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Kizaka-Kondoh S, Tanaka S, Harada H and
Hiraoka M: The HIF-1-active microenvironment: An environmental
target for cancer therapy. Adv Drug Deliv Rev. 61:623–632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Li B and Simon MC: Molecular pathways:
Targeting MYC-induced metabolic reprogramming and oncogenic stress
in cancer. Clin Cancer Res. 19:5835–5841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Okkenhaug K, Graupera M and Vanhaesebroeck
B: Targeting PI3K in cancer: Impact on tumor cells, their
protective stroma, angiogenesis, and immunotherapy. Cancer Discov.
6:1090–1105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Thorpe LM, Yuzugullu H and Zhao JJ: PI3K
in cancer: Divergent roles of isoforms, modes of activation and
therapeutic targeting. Nat Rev Cancer. 15:7–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Troncone M, Cargnelli SM, Villani LA,
Isfahanian N, Broadfield LA, Zychla L, Wright J, Pond G, Steinberg
GR and Tsakiridis T: Targeting metabolism and AMP-activated kinase
with metformin to sensitize non-small cell lung cancer (NSCLC) to
cytotoxic therapy; translational biology and rationale for current
clinical trials. Oncotarget. 8:57733–57754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Luchsinger JA, Ma Y, Christophi CA, Florez
H, Golden SH, Hazuda H, Crandall J, Venditti E, Watson K, Jeffries
S, et al: Diabetes Prevention Program Research Group: Metformin,
lifestyle intervention, and cognition in the diabetes prevention
program outcomes study. Diabetes Care. 40:958–965. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Anisimov VN, Berstein LM, Egormin PA,
Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina
TE, Semenchenko AV, Provinciali M, et al: Effect of metformin on
life span and on the development of spontaneous mammary tumors in
HER-2/neu transgenic mice. Exp Gerontol. 40:685–693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Dowling RJ, Zakikhani M, Fantus IG, Pollak
M and Sonenberg N: Metformin inhibits mammalian target of
rapamycin-dependent translation initiation in breast cancer cells.
Cancer Res. 67:10804–10812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Libby G, Donnelly LA, Donnan PT, Alessi
DR, Morris AD and Evans JM: New users of metformin are at low risk
of incident cancer: A cohort study among people with type 2
diabetes. Diabetes Care. 32:1620–1625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Jiralerspong S, Palla SL, Giordano SH,
Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi
GN and Gonzalez-Angulo AM: Metformin and pathologic complete
responses to neoadjuvant chemotherapy in diabetic patients with
breast cancer. J Clin Oncol. 27:3297–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Christofk HR, Vander Heiden MG, Wu N,
Asara JM and Cantley LC: Pyruvate kinase M2 is a
phosphotyrosine-binding protein. Nature. 452:181–186. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Bian H, Zheng JS, Nan G, Li R, Chen C, Hu
CX, Zhang Y, Sun B, Wang XL, Cui SC, et al: Randomized trial of
[131I] metuximab in treatment of hepatocellular
carcinoma after percutaneous radiofrequency ablation. J Natl Cancer
Inst. 106(pii): dju2392014.PubMed/NCBI
|
|
150
|
Calvaresi EC and Hergenrother PJ: Glucose
conjugation for the specific targeting and treatment of cancer.
Chem Sci. 4:2319–2333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Ciuleanu TE, Pavlovsky AV, Bodoky G, Garin
AM, Langmuir VK, Kroll S and Tidmarsh GT: A randomised Phase III
trial of glufosfamide compared with best supportive care in
metastatic pancreatic adenocarcinoma previously treated with
gemcitabine. Eur J Cancer. 45:1589–1596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Baeuerle PA and Reinhardt C: Bispecific
T-cell engaging antibodies for cancer therapy. Cancer Res.
69:4941–4944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Lameris R, de Bruin RC, Schneiders FL, van
Bergen en Henegouwen PM, Verheul HM, de Gruijl TD and van der Vliet
HJ: Bispecific antibody platforms for cancer immunotherapy. Crit
Rev Oncol Hematol. 92:153–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Smith DM, Simon JK and Baker JR Jr:
Applications of nanotechnology for immunology. Nat Rev Immunol.
13:592–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Metcalfe SM and Fahmy TM: Targeted
nanotherapy for induction of therapeutic immune responses. Trends
Mol Med. 18:72–80. 2012. View Article : Google Scholar : PubMed/NCBI
|