Introduction
Cervical cancer is one of the most common
gynecological malignancies. Its morbidity is ranked in the third
place among global female malignancies (1). Furthermore, it is ranked in the second
place in developing countries in terms of both morbidity and
mortality, only second to breast cancer. In recent years, the
morbidity of cervical cancer is constantly increasing and exhibits
a younger trend along with shortened course of disease (2). Cervical cancer screening has been
extensively popularized (2).
However, a large number of patients are diagnosed with invasive
cervical cancer. It has currently been ascertained that the genesis
of cervical cancer is related to the persistent infection of high
risk human papilloma virus (HPV) (3). According to statistics, HPV infection
has resulted in 99.7% of cervical cancer cases (3).
Currently, persistent HPV infection has been
revealed to be the initial step of cervical cancer genesis. The
genesis and development of cervical cancer is the result of a joint
action of multiple factors. Existing studies indicate that,
epigenetic modification plays a key role in tumor genesis and
development (4). MicroRNAs (miRNAs)
are important factors involved in that process. The role of miRNAs
in cervical cancer has been determined with increasingly profound
basic research on miRNAs (5).
Research has revealed that numerous miRNAs are involved in
regulating multiple biological processes of cervical cancer, such
as proliferation, apoptosis, invasion and metastasis (5). In addition, it is closely correlated
with the susceptibility and clinical prognosis of cervical cancer.
miRNAs can regulate the expression of both oncogenes and
tumor-suppressor genes (5), thus
promoting or inhibiting cancer genesis (6).
Th17 cells are a subgroup of helper T cells that has
attracted wide attention in recent years (7). Its roles in inflammatory response and
autoimmune disease have been determined (7). In recent years, the role of Th17 cells
in tumors has also been studied. In gastric cancer, Th17 cells were
determined to be markedly increased in peripheral blood (8), which was more obvious in stage III–IV
gastric cancer patients than in early-stage patients. This suggests
that Th17 cells are related to tumor development and are negatively
correlated with patient survival (9). I1-17a is the primary effector secreted
by Th17 cells that has a potent pro-inflammatory effect. Research
has revealed that its role in tumors is closely related to the
enhanced formation of tumor microvessels (9).
Tumor necrosis factor receptor-associated factors
(TRAFs) are important adaptor molecules. They play a key role in
multiple signaling pathways. There are 7 members (TRAF1-7) in the
TRAF family. TRAF1 and TRAF2 were the two first discovered members.
They serve as the signal transduction proteins of tumor necrosis
factor receptor II (TNFRII). Subsequently, the other 5 family
members (TRAF3-7) were successively discovered. TRAF6-activated
downstream signals mainly include NF-κB and AP-1. These two are
involved in the transcription and expression of multiple genes
in vivo (including inflammation- and anti-apoptosis-related
genes). Therefore, TRAF6 also possesses extensive biological
functions. It not only participates in regulating natural immune
response, but it is also closely related to the maintenance of
T-cell peripheral immune tolerance. In addition, it can take part
in the maturation and differentiation of an osteoclast in
vitro. Recent research revealed that TRAF6 also plays a
critical role in tumor genesis, development, invasion and
metastasis. The aim of the present study was to investigate whether
miRNA-146a regulates the function of Th17 cell differentiation to
modulate cervical cancer cell growth and apoptosis.
Materials and methods
Patients with cervical cancer
The present hospital-based case-control study
consisted of 68 female cervical cancer patients (range, 57–72
years) and 24 female cancer-free controls (range, 55–66 years). All
patients and cancer-free controls were recruited from The Third
Affiliated Hospital of Sun Yat-sen University (Guangzhou,
Guangdong), between January 2011 and January 2016. The cancer-free
control subjects showed no evidence of a genetic relationship with
the cases. All cervical cancer patients were undergoing surgery
treatment. The cancer tissue samples were saved at −80°C to measure
the expression of miRNA-146a. This study was approved by the Ethics
Review Board of The Third Affiliated Hospital of Sun Yat-sen
University and all patients provided written informed consent.
RNA isolation and quantitative RT-PCR
(qPCR)
Total RNA was isolated with TRIzol reagent (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) from tumor tissue
samples or cell samples. RNA in exosomes was extracted using the
TOPscript Reverse Transcriptase (Enzynomics, Inc., Daejeon, Korea).
qPCR was performed using the 7500 Fast Real-Time PCR machine
(Applied Biosystems; Thermo Fisher Scientific, Inc.) by HiFast
Probe Lo-ROX and HiFast SYBR Lo-ROX Master Mix (PCR Biosystems,
London, UK). The qPCR cycle was set to an initial 95°C for 10 min,
40 cycles at 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec.
Relative quantification analysis was performed using the
comparative quantification cycle (Cq) method (2−ΔΔCq)
(10).
Isolation of tumor-infiltrated T
cells
Cancer and peri-tumor tissues samples were cut into
small pieces and digested in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.), 10% fetal bovine serum (FBS) (Invitrogen; Thermo
Fisher Scientific, Inc.), 100 U/ml Collagenase type IV and 100
µg/ml DNase. Dissociated cells were filtered using a 75-µm cell
strainer and mononuclear cells were washed and incubated with
RPMI-1640 with 10% FBS. T cells were purified with anti-CD3
magnetic Dynabeads (Thermo Fisher Scientific, Inc.).
Exosome extraction from human
serum
Blood samples were centrifuged at 1,000 × g for 10
min at 4°C and serum samples were collected. Exosome samples were
isolated using a Total Exosome Isolation kit (Thermo Fisher
Scientific, Inc.) and incubated with exosome isolation reagent for
30 min at 4°C. Then, the pellets were centrifuged at 1,000 × g for
10 min at 4°C and resuspended with phosphate-buffered saline
(PBS).
In vitro culture of CD4+ T
cells
Lentivirus with miRNA-146a, anti-miRNA-146a and
control negative mimics were purchased from Shanghai GeneChem Co.,
Ltd., (Shanghai, China). Splenic cells were harvested from mice and
then subjected to magnetic-activated cell sorting (MACS) using a
Mouse Naive CD4+ T Cell Isolation kit (Miltenyi Biotec,
Inc., Cambridge, MA, USA) according to the manufacturer's
instructions. The STAT3 decoy ODN sequences were:
5-CATTTCCCGTAAATC-3 and 5-GATTTACGGGAAATG-3.
Cell lines and miRNA mimic
transfection
Human cervical cancer cell line HeLa was purchased
from Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) and maintained at 37°C in an atmosphere of 5%
CO2 in Dulbecco's modified Eagle's medium (DMEM)
(Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China) with 10% FBS (American Amereseo, Solon, OH, USA).
HeLa cells were transduced with lentivirus with miRNA-146a,
anti-miRNA-146a, STAT3 decoy ODN, si-TRAF6 and control negative
mimics. Transfected HeLa cells and CD4+ T cells were
co-cultured with anti-CD3 (5 mg/ml) and anti-CD28 (2 mg/ml).
Cell viability and Transwell assays
and the apoptosis rate by flow cytometer
Following transduction, cells (105
cell/well) were seeded into a 96-well plate and 20 µl of MTT was
added to the cells. The cells were incubated for 4 h at 37°C and
then, the medium was removed. Dimethyl sulphoxide (DMSO) solution
was added to the cells for 20 min at 37°C. The absorbance was
assessed using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 492 nm.
For the Transwell assay, following transduction,
cells (1×106 cells/ml) in serum-free medium containing
5% FBS were plated in the top chamber of each Transwell insert
(Corning Costar, Corning, NY, USA) and incubated at 37°C for 48 h.
Non-migrating cells on the upper surface of the filter were removed
by wiping with a cotton swab. Then, the cells that remained in the
upper chambers were removed and the cells that had migrated into to
the bottom chambers and attached to the lower surface of the
membrane were fixed and stained with dye solution containing 20%
methanol violet and 0.1% crystal violet. The cells were then
counted using an optical inverted microscope (magnification, ×200;
Nikon Corp., Tokyo, Japan).
For the apoptosis rate, cells were washed with PBS
and fixed with 4% paraformaldehyde for 15 min. The cells were then
stained with 5 µl of-FITC and 5 µl of propidium iodide (PI) for 15
min in the dark. Flow cytometry was performed on a BD AccuriC6 (BD
Biosciences, Franklin Lakes, NJ, USA) and data was analyzed using
FlowJo software (FlowJo LLC, Ashland, OR, USA).
Western blot analysis
Total cellular protein was extracted using RIPA
assay Pierce; Thermo Fisher Scientific, Inc.) and protein
concentration was determined using a BCA protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Equal amounts of protein were
separated by 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE), and electrophoretically transferred to
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% non-fat milk in Tris-buffered saline containing
Tween-20 (TBST) for 1 h at 37°C and probed with TRAF6 (1:1,000;
cat. no. sc-7221), NF-κB (1:1,000; cat. no. sc-71675) and Bax
(1:1,000; cat. no. sc-6236), p-Stat3 (1:500; cat. no. sc-8001-R)
and GAPDH (1:5,000; cat. no. sc-51631; all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. Then, the
membranes were washed with TBST for 15 min and probed with
horseradish peroxidase-labeled goat-anti-rabbit IgG (1:5,000; cat.
no. sc-2004; Santa Cruz Biotechnology, Inc.) for 1 h at 37°C and
visualization was performed by Immobilon Western Chemiluminescent
HRP Substrate (EMD Millipore, Billerica, MA, USA).
Statistical analysis
Data are presented as the mean ± SEM. Student's
t-test or one-way analysis of variance (ANOVA) followed by Tukey's
post hoc test were used to perform statistical comparisons. A value
of P<0.05 was considered to indicate a statistically significant
result.
Results
miR-146a expression in human cervical
cancer specimens
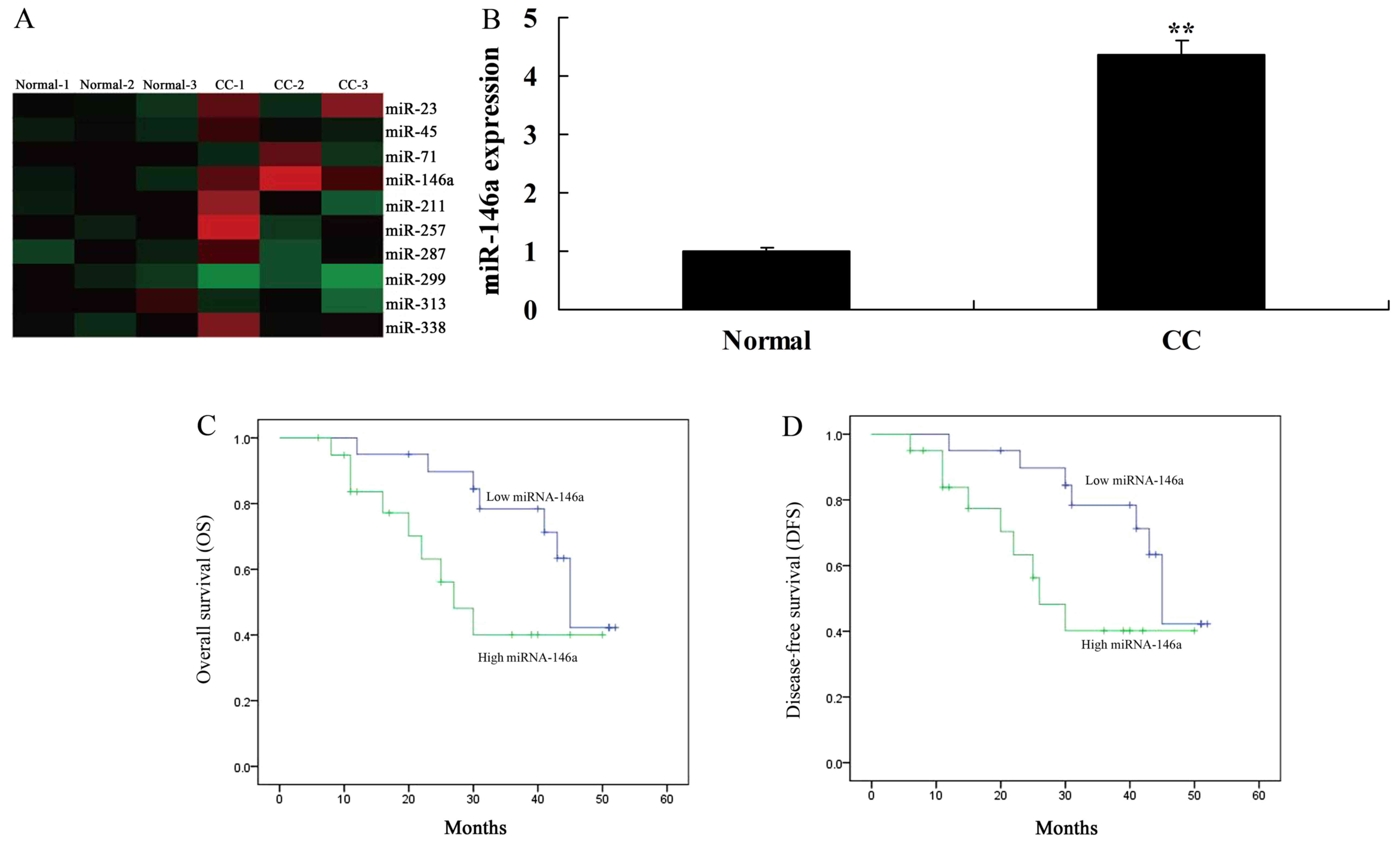
We first assessed the changes of miR-146a expression
in human cervical cancer tissue samples and normal control samples.
The results revealed that miR-146a expression was increased in
human cervical cancer tissues (Fig. 1A
and B). In addition, we found that overall survival (OS) and
disease-free survival (DFS) of patients with low miR-146a
expression were higher than those of patients with high miR-146a
expression (Fig. 1C and D).
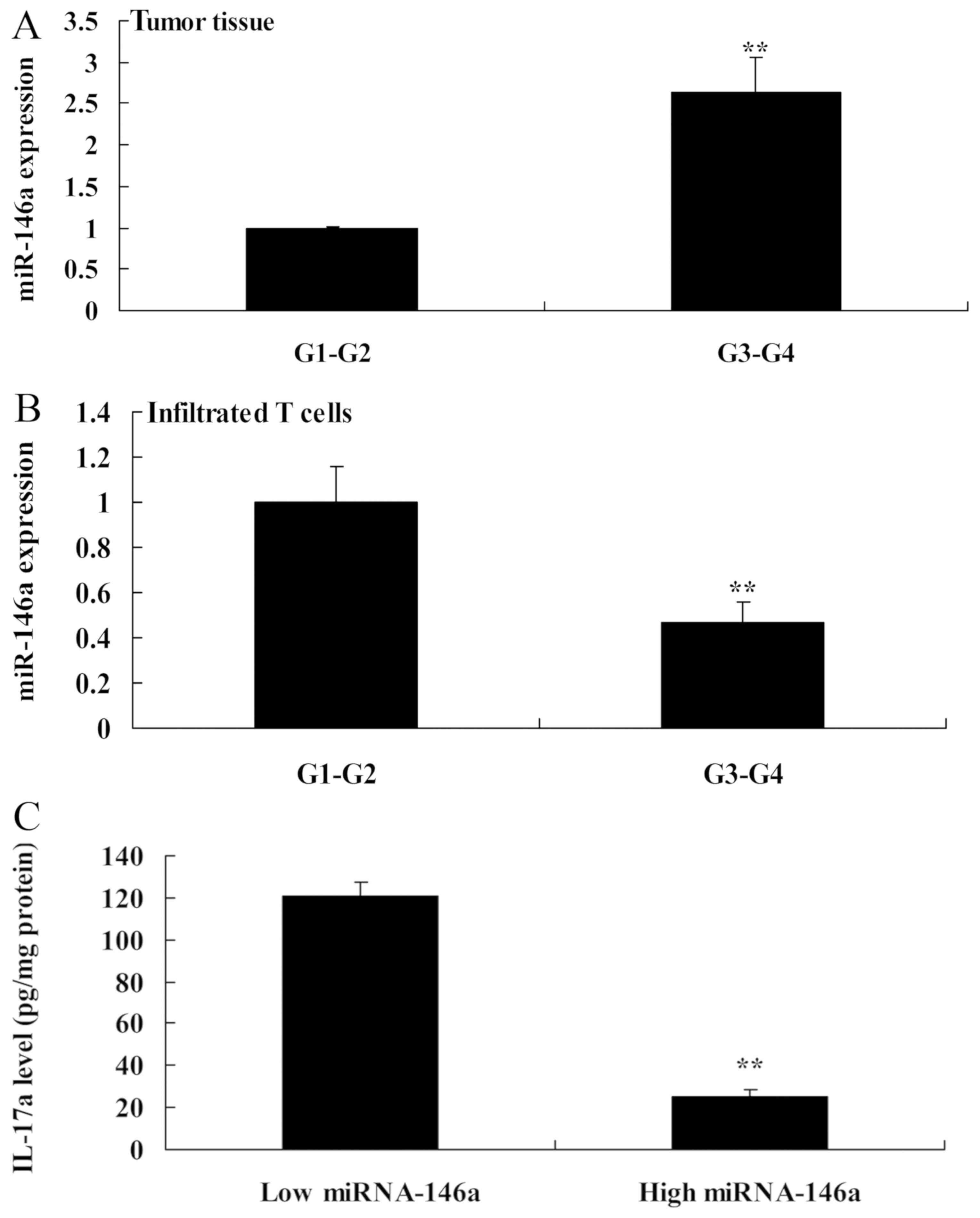
miR-146a expression in tumor tissues
and infiltrated T cells
Next, the expression of miR-146a in tumor tissues of
patients with G1-G2 grade cervical cancer was significantly lower
than that in those with G3-G4 grade (Fig. 2A). However, the expression of
miR-146a in infiltrated T cells in patients with G1-G2 grade was
extremely higher than that in those with G3-G4 grade (Fig. 2B). The IL-17a levels were lower in
patients with high miR-146a expression than those in patients with
low miR-146a expression (Fig. 2C).
These results revealed that miR-146a exerted anticancer effects in
human cervical cancer and regulated cell growth and apoptosis of
Th17 cells.
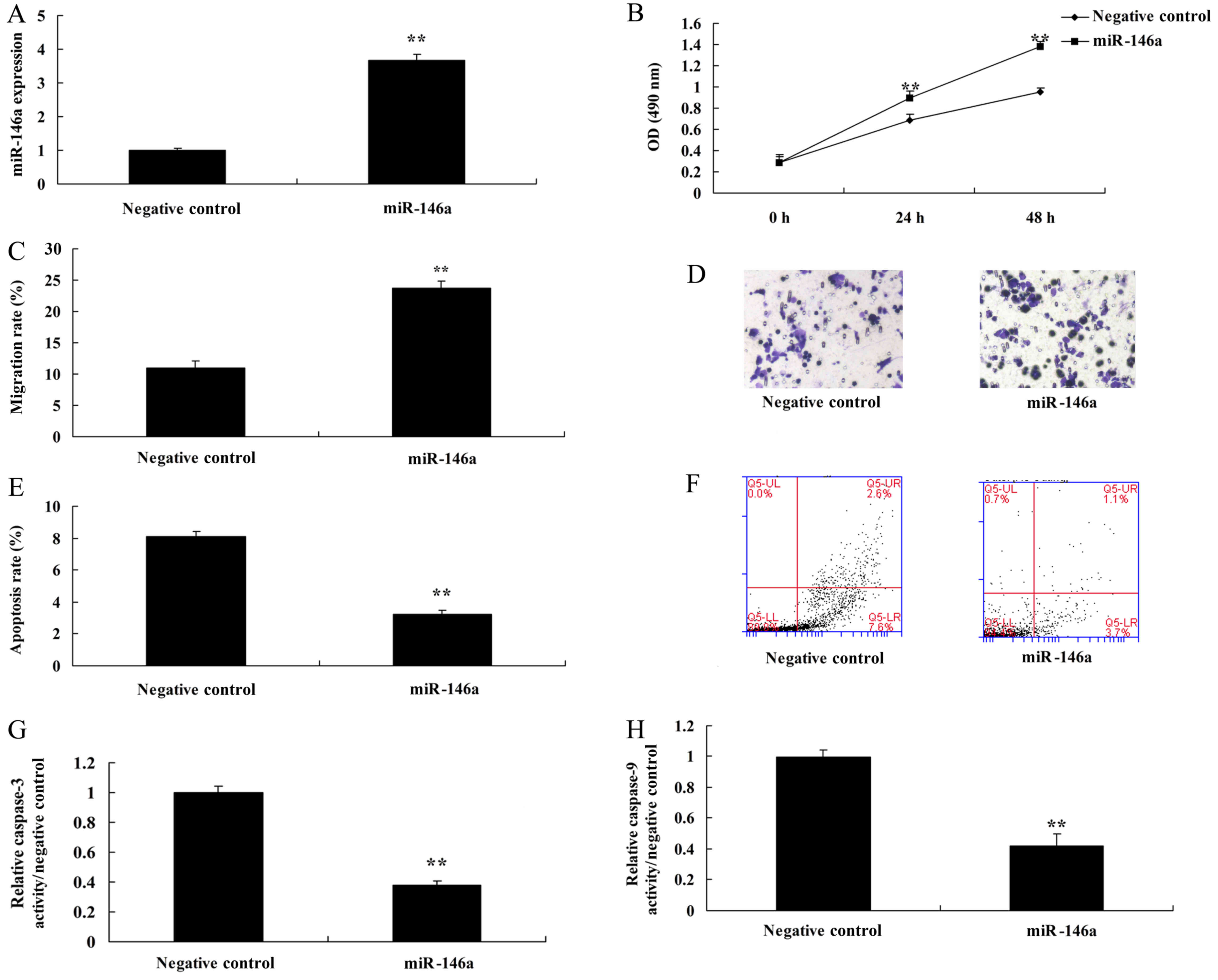
Overexpression of miR-146a promotes
cell growth and reduces apoptosis of cervical cancer cells in a
co-culture of cervical cancer and CD4+ T cells
Next, miR-146a mimics were used to increase the
expression of miR-146a in cervical cancer cells, compared with the
negative control group (Fig. 3A).
Overexpression of miR-146a promoted cell growth and the migration
rate, and reduced the apoptosis rate and caspase-3 and −9
activities in cervical cancer cells by miR-146a mimics, compared
with the negative control group (Fig.
3B-H). Additionally, we found that miR-146a regulated the
function of Th17 cell differentiation, whose mechanism required
further analysis.
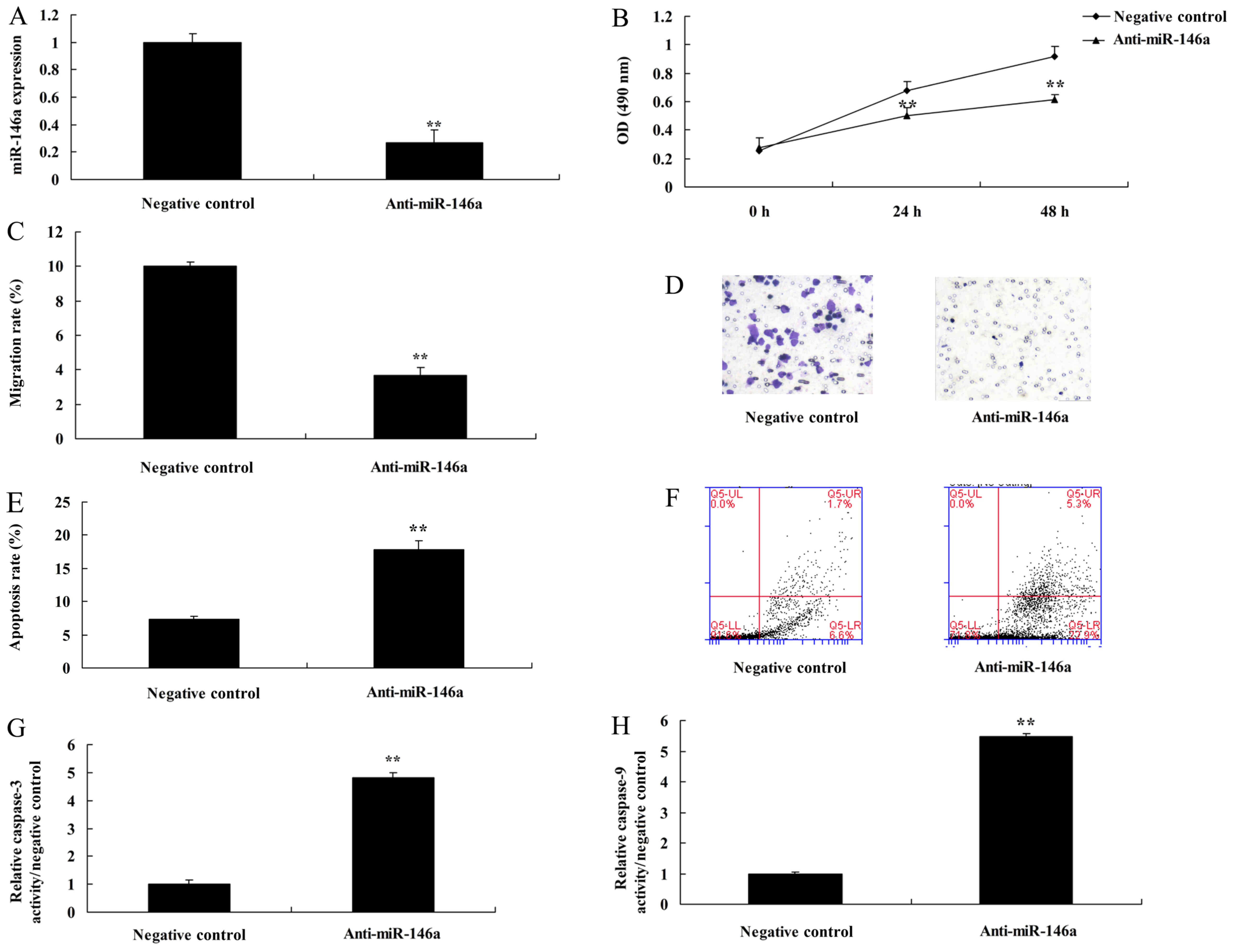
Downregulation of miR-146a inhibits
cell growth and induces apoptosis of cervical cancers in a
co-culture of cervical cancer cells and CD4+ T
cells
In determination of the function of anti-miR-146a in
a co-culture of cervical cancer and CD4+ T cells, we
found that miR-146a expression was reduced in cervical cancer cells
by anti-miR-146a mimics, compared with the negative control group
(Fig. 4A). Downregulation of
miR-146a promoted cell growth and the migration rate, and inhibited
the apoptosis rate and caspase-3 and −9 activities in cervical
cancer cells, compared with the negative control group (Fig. 4B-H).
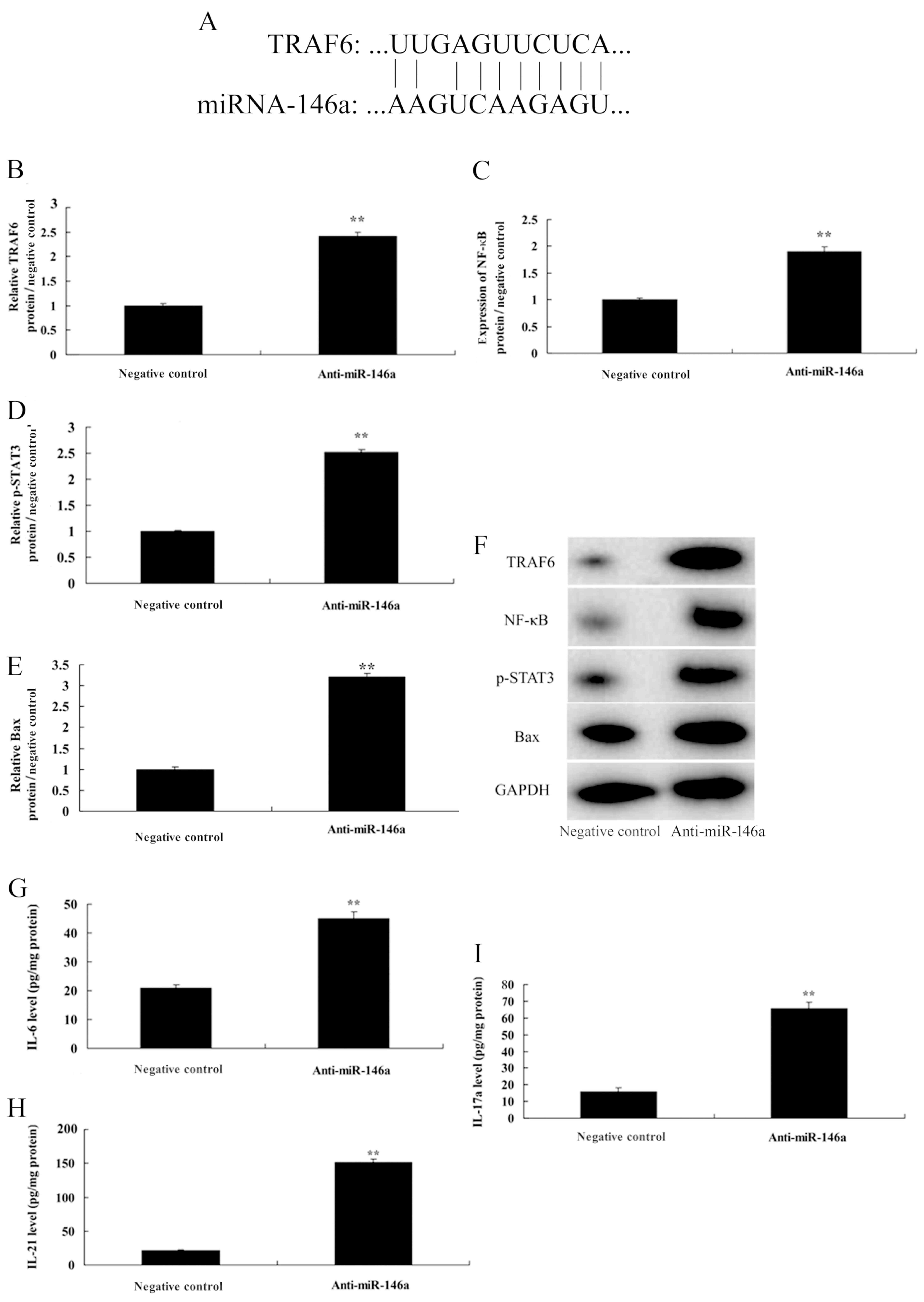
Downregulation of miR-146a induces
NF-κB signaling by targeting TRAF6
We investigated the mechanism of anti-miR-146a in
cervical cancer cells, and this study revealed miR-146a putative
binding sites and corresponding mutant sites of TRAF6 (Fig. 5A). Downregulation of miR-146a
induced TRAF6 and NF-κB protein expression, as well as Bax protein
expression in cervical cancer cells. In addition, it promoted the
protein expression of p-Stat3 in CD4+ T cells (Fig. 5B-F). Furthermore, in medium,
downregulation of miR-146a increased the levels of IL-6, IL-21 and
IL-17A, compared with the negative control group (Fig. 5H and I).
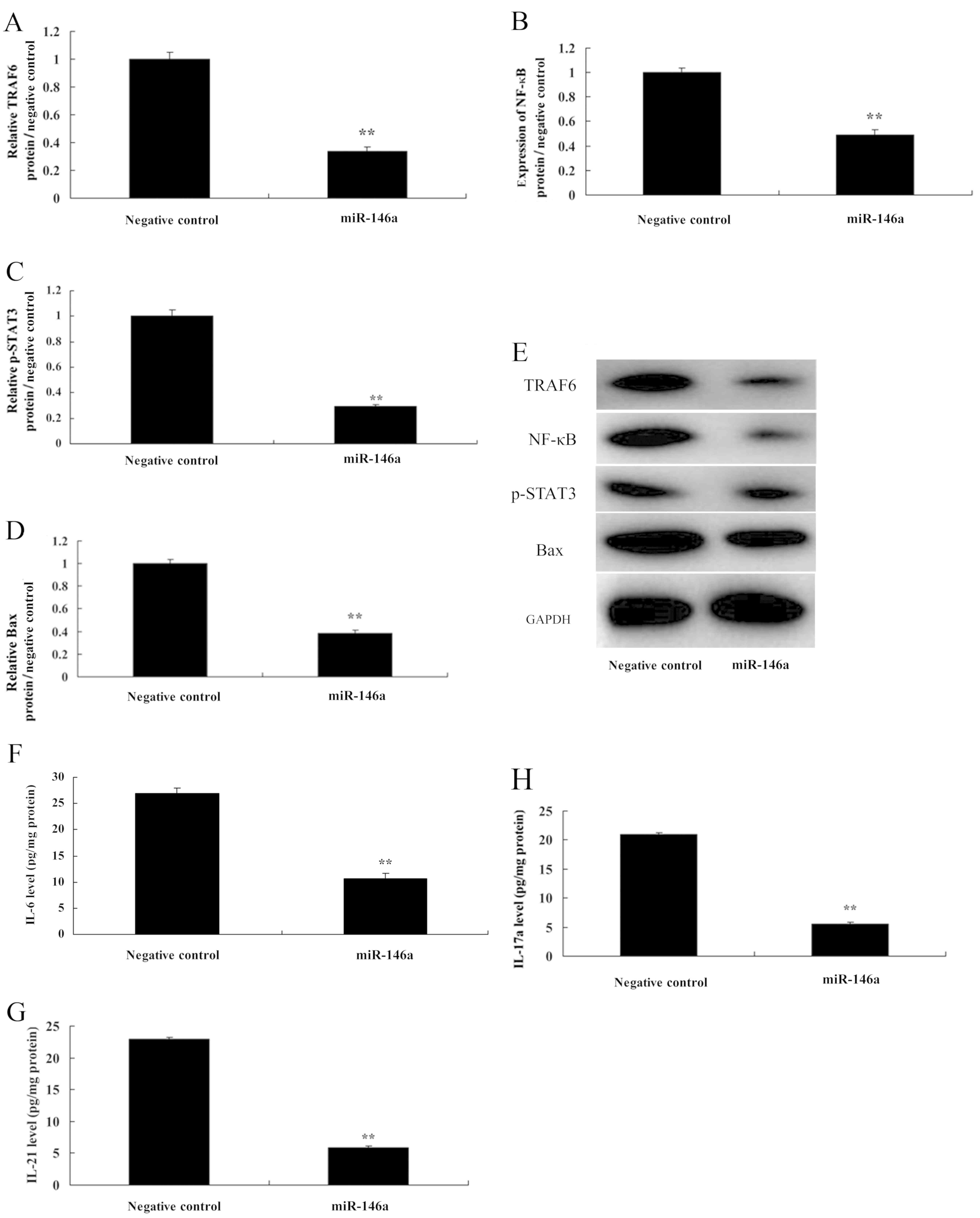
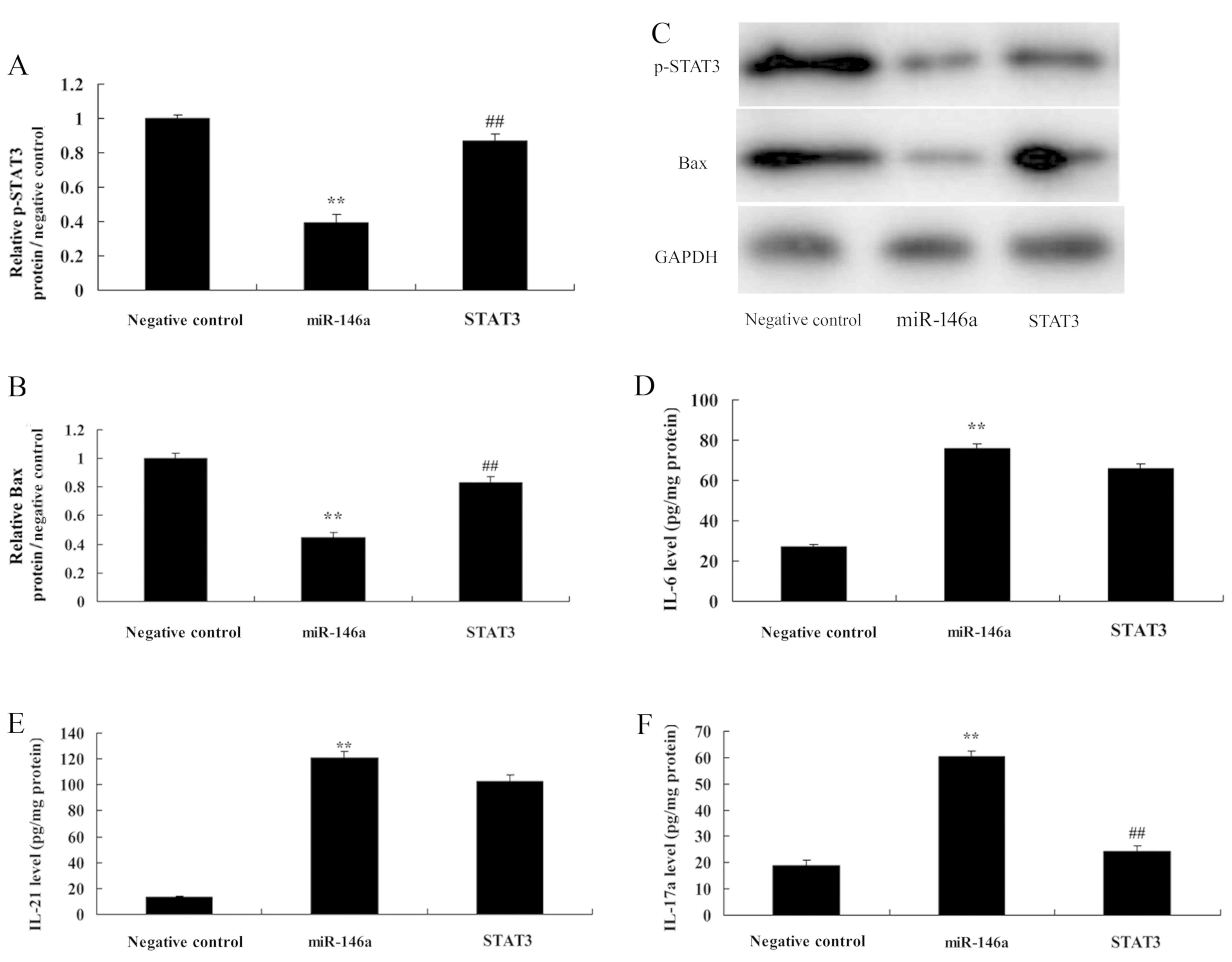
Overexpression of miR-146a induces
NF-κB signaling by targeting TRAF6
In addition, we also found that overexpression of
miR-146a suppressed TRAF6 and NF-κB protein expression, as well as
Bax protein expression in cervical cancer cells. In addition, it
reduced p-Stat3 protein expression in CD4+ T cells
(Fig. 6A-E). Then, in medium,
overexpression of miR-146a decreased the levels of IL-6, IL-21 and
IL-17A, compared with the negative control group (Fig. 6F-H). These results confirmed that
miR-146a/NF-κB signaling played a critical role in determining the
Th17 cell differentiation by TRAF6.
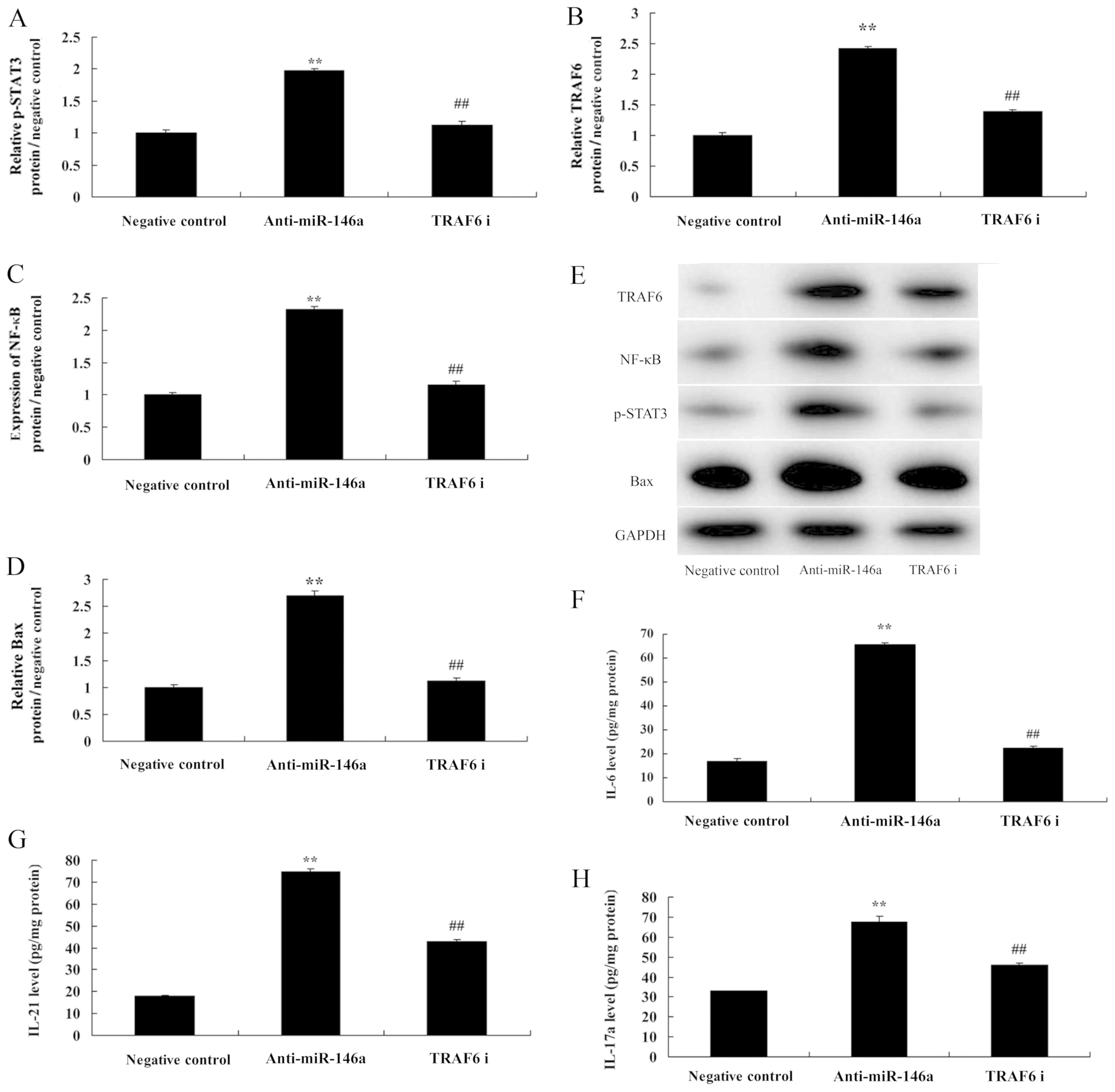
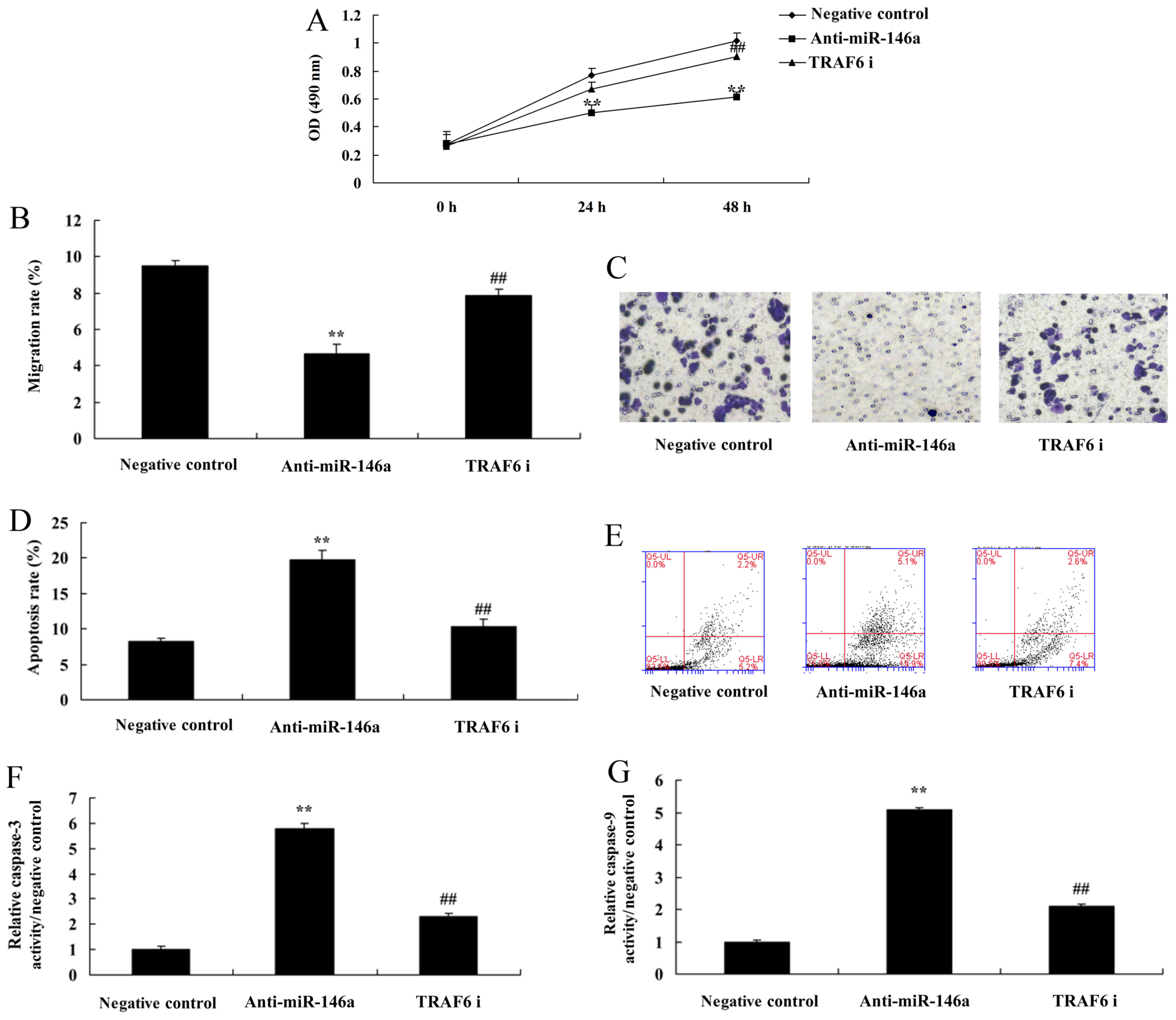
si-TRAF6 reduces the function of
miR-146a in a co-culture of cervical cancer and CD4+ T
cells
Next, we further investigated the role of TRAF6 in
the mechanism of miR-146a in cervical cancer cells. To this end,
TRAF6 protein expression was downregulated using si-TRAF6 plasmid.
The inhibition of TRAF6 suppressed TRAF6 and NF-κB protein
expression as well as Bax protein expression in cervical cancer
cells by anti-miR-146a. In addition, it also suppressed p-Stat3
protein expression in CD4+ T cells (Fig. 7A-E). The inhibition of TRAF6
decreased the levels of IL-6, IL-21 and IL-17A, compared with the
anti-miR-146a group (Fig. 7F-H).
The inhibition of TRAF6 attenuated the functions of anti-miR-146a
on cell growth, the migration rate, and the apoptosis rate as well
as caspase-3 and −9 activities in cervical cancer cells, compared
with the anti-miR-146a group (Fig.
8).
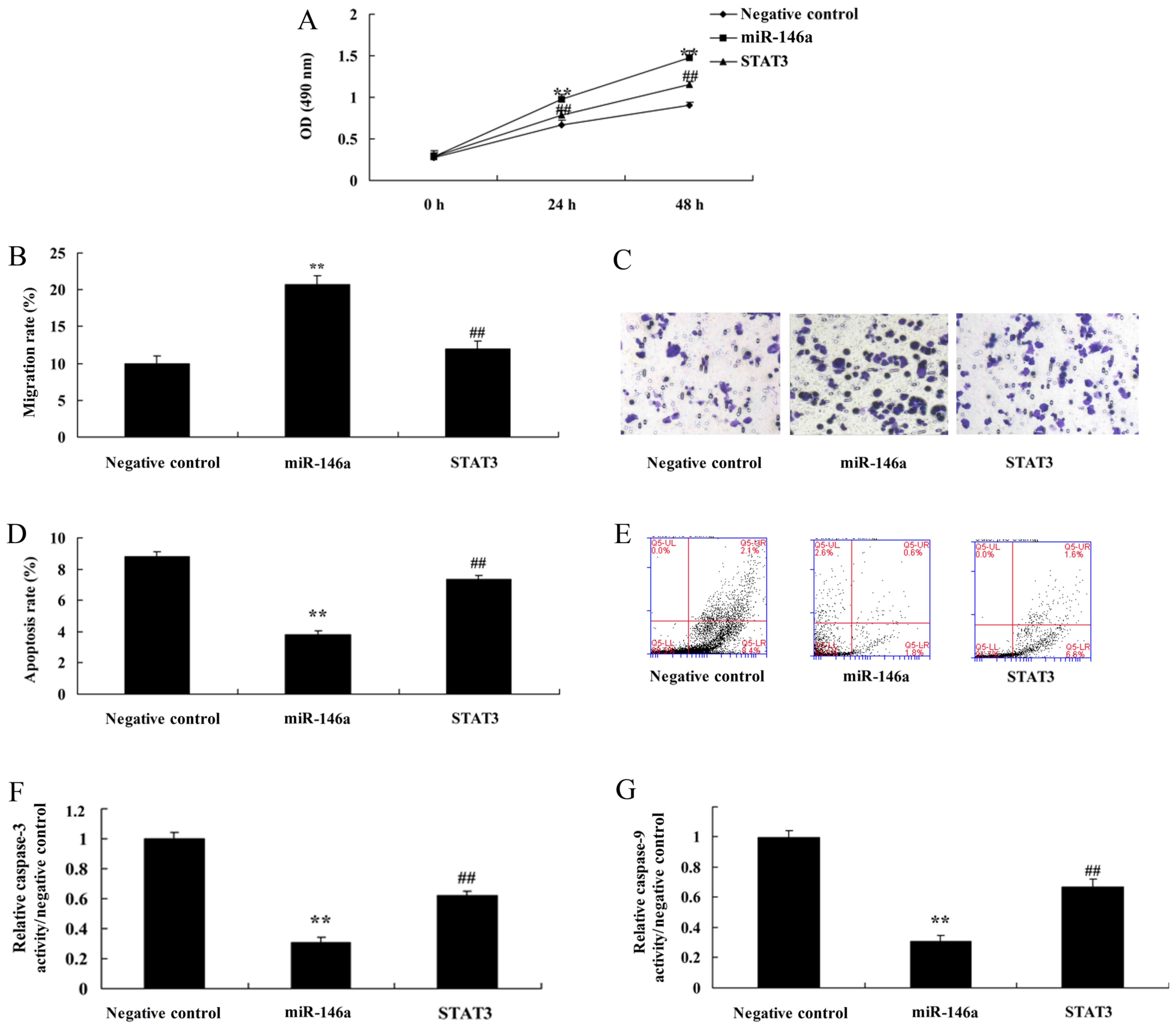
STAT3 inhibits the function of
miR-146a in a co-culture of cervical cancer and CD4+ T
cells
Finally, we examined the role of STAT3 in the
mechanism of miRNA-146a in cervical cancer by Th17 cells and used
STAT3 decoy ODN to induce the protein expression. STAT3 decoy ODN
induced the protein expression of p-STAT3 in CD4+ T
cells, and induced Bax protein expression in cervical cancer cells
by miR-146a, compared with the miR-146a group (Fig. 9A-C). However, STAT3 decoy ODN failed
to affect IL-6 and IL-21 levels, and reduced IL-17a levels in the
medium, compared with the miR-146a group (Fig. 9D-F). In addition, STAT3 decoy ODN
attenuated the functions of miR-146a on cell growth, the migration
rate, the apoptosis rate and caspase-3 and −9 activities in
cervical cancer cells by miR-146a, compared with the miR-146a group
(Fig. 10).
Discussion
Cervical cancer is one of the common malignancies in
females worldwide (1). In recent
years, the cervical cancer screening technique has been improved
and popularized in developed areas (1). Most precancerous lesions of the
uterine cervix can be timely discovered and treated. Therefore, the
morbidity of cervical cancer has been markedly reduced (11). However, in developing countries,
especially remote and less developed areas, morbidity ranks at the
top among all female malignancies (11). This can be ascribed to insufficient
screening popularization and backward medical technology (11). In addition, with the social
development cervical cancer patients exhibit a younger trend
(12). Cervical cancer is also
associated with high mortality. It is ranked in the 3rd place among
all malignancies worlwide (12).
Therefore, it has severely affected the lives and health of women
worldwide. In the present study, miRNA-146a expression was
increased in human cervical cancer. Wang et al revealed that
miRNA-146a expression was upregulated in cervical carcinogenesis
(13).
TRAF6 is an important NF-κB regulatory factor that
has important biological functions. TRAF6 not only possesses an
immune regulatory function but in addition, it is also involved in
osteoclast maturation and differentiation. TRAF6 also plays a key
role in tumor genesis, development, invasion and metastasis. NF-κB
activation by TRAF6 is necessary in lung cancer and esophageal
carcinoma. Our study revealed that downregulation of miRNA-146a
induced NF-κB signaling by targeting TRAF6. He et al
revealed that upregulation of miR-146a protects the small intestine
against small intestine ischemia and I/R injury by downregulating
the TLR4/TRAF6/NF-κB pathway (14).
Th17 cells are a subgroup of CD4+ T cells
that can mediate cytokines. Specifically, they can guarantee that
cells exert different immune effects (7). Research has revealed that there is a
dynamic balance in such cells. Imbalances play vital roles in the
genesis and development of multiple inflammations, autoimmune
diseases and tumors (15). Among
the Th17-related cytokines, IL-6, IL-17 and IL-23 have a positive
response value in the autoimmune regulation of the body (15). In addition, it displays high
detection significance in local lesion response (7). Therefore, it is vital to detect Th17
cells in the blood and tissues of such patients. Our data
demonstrated that downregulation of miRNA-146a promoted Th17
cytotoxicity to induce apoptosis of cervical cancer cells. Li et
al revealed that miR-146a blocks the autocrine IL-6- and
IL-21-induced Th17 differentiation pathways in autoreactive
CD4+ T cells (16).
The signal transduction and transcription activator
protein family is a class of important cytokine signaling proteins
(17). Multiple cytokines can
transfer signals to the cells through the classical STAT signaling
pathway (17). Thus, gene
expression of specific target cells can be altered. Activation of
the STAT signaling pathway determines the differentiation direction
of T cells. Thus, it regulates a series of physiological and
pathological processes (18). Of
them, the STAT3 protein has been indicated to play a critical role
in regulating Th17 cell differentiation (18). Abnormalities in the STAT3 signaling
pathway is related to the genesis of multiple diseases, such as
infections, tumors and autoimmune diseases (19,20).
In the present study, we demonstrated that the downregulation of
miRNA-146a induced TRAF6 and NF-κB protein expression, increased
IL-6, IL-17A and IL-21 levels, and promoted p-STAT3 protein
expression. Zhou et al revealed that microRNA-146a mediated
the IL-6/STAT3 signaling mechanism of microRNA-146a-mediated
IL-6/STAT3 signaling in lumbar intervertebral disc degeneration
(21).
Cytokines IL-6, IL-21 and IL-23 play major roles in
Th17 cell differentiation. In addition, signal transfer is mainly
achieved through the STAT3 signaling pathway (22). Typically, IL-6 is regarded as the
initial factor of Th17 cell differentiation. TGF-β plays a
synergistic part. IL-6 activates the STAT3 protein (22). This can enhance the expression of
IL-21, IL-6 and IL-23 genes, so that cells secrete IL-21 and IL-23
(23). IL-21 activates the STAT3
protein and further promotes Th17 cell differentiation and
secretion (24). The STAT3 protein
regulates the TH17 cell differentiation-related cytokines (25). Briefly, the STAT3 protein directly
binds with IL-17 and IL-17F, and regulates IL-17F secretion and
promotes Th17 cell differentiation (25). In this study, we found STAT3
inhibited the function of miRNA-146a in a co-culture of cervical
cancer and Th17 T cells. Ye et al suggests that miR-146a
suppresses the STAT3/VEGF pathways n primary human retinal
microvascular endothelial cells (26).
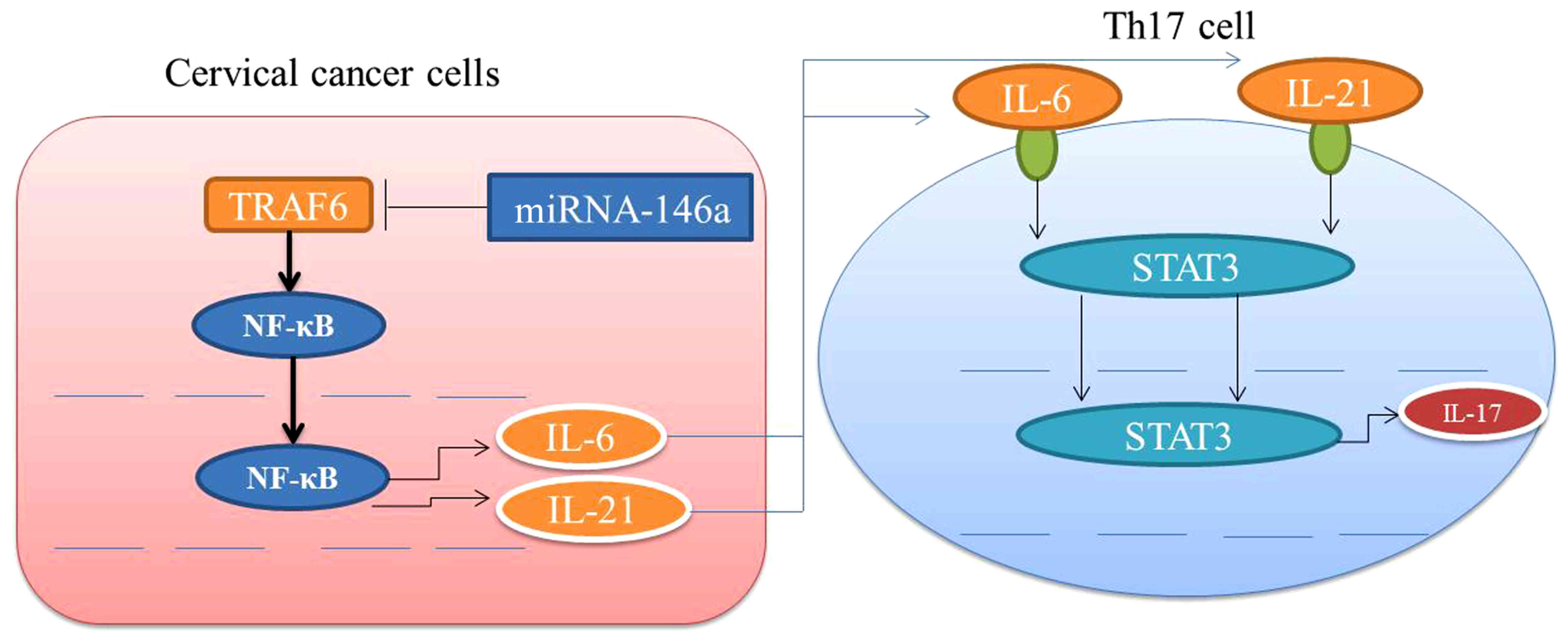
Collectively, this study demonstrated that
miRNA-146a regulates the function of Th17 cell differentiation to
modulate cervical cancer cell growth and apoptosis through NF-κB
signaling by targeting TRAF6 (Fig.
11). miRNA-146a may functions as an oncogene in cervical cancer
via Th17 cell differentiation by targeting TRAF6.
Acknowledgements
This study was supported by Guangdong Science and
Technology project (2016A020215074).
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TL designed the experiment. ML, CX, XX, JD, LC and
RO performed the experiment. TL and ML analyzed the data. TL wrote
the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Board of The Third Affiliated Hospital of Sun Yat-sen University
(Guangzhou, Guangdong), and all patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsunoda AT, Marnitz S, Soares Nunes J,
Mattos de Cunha Andrade CE, Scapulatempo Neto C, Blohmer JU,
Herrmann J, Kerr LM, Martus P, Schneider A, et al: Incidence of
histologically proven pelvic and Para-aortic lymph node metastases
and rate of upstaging in patients with locally advanced cervical
cancer: Results of a prospective randomized trial. Oncology.
92:213–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Azevedo C, Thuler LCS, de Mello MJG, de
Oliveira Lima JT, da Fonte ALF, Fontão DFS, Carneiro VCG, Chang TMC
and Ferreira CG: Phase II trial of neoadjuvant chemotherapy
followed by chemoradiation in locally advanced cervical cancer.
Gynecol Oncol. 146:560–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matijevic M, Hedley ML, Urban RG, Chicz
RM, Lajoie C and Luby TM: Immunization with a poly (lactide
co-glycolide) encapsulated plasmid DNA expressing antigenic regions
of HPV 16 and 18 results in an increase in the precursor frequency
of T cells that respond to epitopes from HPV 16, 18, 6 and 11. Cell
Immunol. 270:62–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Varghese VK, Shukla V, Kabekkodu SP,
Pandey D and Satyamoorthy K: DNA methylation regulated microRNAs in
human cervical cancer. Mol Carcinog. 57:370–382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shishodia G, Verma G, Das BC and Bharti
AC: miRNA as viral transcription tuners in HPV-mediated cervical
carcinogenesis. Front Biosci. 10:21–47. 2018. View Article : Google Scholar
|
|
6
|
Liang C, Ding J, Yang Y, Deng L and Li X:
MicroRNA-433 inhibits cervical cancer progression by directly
targeting metadherin to regulate the AKT and β-catenin signalling
pathways. Oncol Rep. 38:3639–3649. 2017.PubMed/NCBI
|
|
7
|
Hou F, Li Z, Ma D, Zhang W, Zhang Y, Zhang
T, Kong B and Cui B: Distribution of Th17 cells and
Foxp3-expressing T cells in tumor-infiltrating lymphocytes in
patients with uterine cervical cancer. Clin Chim Acta.
413:1848–1854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu Q, Lou XM and He Y: Preferential
recruitment of Th17 cells to cervical cancer via CCR6-CCL20
pathway. PLoS One. 10:e01208552015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Tian X, Mumtahana F, Jiao J,
Zhang T, Croce KD, Ma D, Kong B and Cui B: The existence of Th22,
pure Th17 and Th1 cells in CIN and cervical cancer along with their
frequency variation in different stages of cervical cancer. BMC
Cancer. 15:7172015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wakatsuki M, Kato S, Ohno T, Karasawa K,
Kiyohara H, Tamaki T, Ando K, Tsujii H, Nakano T, Kamada T, et al:
Clinical outcomes of carbon ion radiotherapy for locally advanced
adenocarcinoma of the uterine cervix in phase 1/2 clinical trial
(protocol 9704). Cancer. 120:1663–1669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sewali B, Okuyemi KS, Askhir A, Belinson
J, Vogel RI, Joseph A and Ghebre RG: Cervical cancer screening with
clinic-based Pap test versus home HPV test among Somali immigrant
women in Minnesota: A pilot randomized controlled trial. Cancer
Med. 4:620–631. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Tang S, Le SY, Lu R, Rader JS,
Meyers C and Zheng ZM: Aberrant expression of oncogenic and
tumor-suppressive microRNAs in cervical cancer is required for
cancer cell growth. PLoS One. 3:e25572008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He X, Zheng Y, Liu S, Shi S, Liu Y, He Y,
Zhang C and Zhou X: MiR-146a protects small intestine against
ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-kappaB
pathway. J Cell Physiol. 233:2476–2488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tristão FS, Rocha FA, Carlos D,
Ketelut-Carneiro N, Souza COS, Milanezi CM and Silva JS:
Th17-inducing cytokines IL-6 and IL-23 are crucial for granuloma
formation during experimental paracoccidioidomycosis. Front
Immunol. 8:9492017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Wang X, Choi IY, Wang YC, Liu S,
Pham AT, Moon H, Smith DJ, Rao DS, Boldin MP, et al: miR-146a
modulates autoreactive Th17 cell differentiation and regulates
organ-specific autoimmunity. J Clin Invest. 127:3702–3716. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon JH, Sudo K, Kuroda M, Kato M, Lee IK,
Han JS, Nakae S, Imamura T, Kim J, Ju JH, et al: Phosphorylation
status determines the opposing functions of Smad2/Smad3 as STAT3
cofactors in TH17 differentiation. Nat Commun.
6:76002015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SY, Lee SH, Yang EJ, Kim JK, Kim EK,
Jung K, Jung H, Lee K, Lee HH, Lee BI, et al: Coenzyme Q10 inhibits
Th17 and STAT3 signaling pathways to ameliorate colitis in mice. J
Med Food. 20:821–829. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SH, Park JS, Byun JK, Jhun J, Jung K,
Seo HB, Moon YM, Kim HY, Park SH and Cho ML: Corrigendum: PTEN
ameliorates autoimmune arthritis through down-regulating STAT3
activation with reciprocal balance of Th17 and Tregs. Sci Rep.
7:422672017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Betts BC, Sagatys EM, Veerapathran A,
Lloyd MC, Beato F, Lawrence HR, Yue B, Kim J, Sebti SM, Anasetti C,
et al: CD4+ T cell STAT3 phosphorylation precedes acute
GVHD, and subsequent Th17 tissue invasion correlates with GVHD
severity and therapeutic response. J Leukoc Biol. 97:807–819. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou T, Lin H, Cheng Z, Ji C, Zhang C and
Tian J: Mechanism of microRNA-146a-mediated IL-6/STAT3 signaling in
lumbar intervertebral disc degeneration. Exp Ther Med.
14:1131–1135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu N, Xu M, Mizoguchi I, Furusawa J,
Kaneko K, Watanabe K, Mizuguchi J, Itoh M, Kawakami Y and Yoshimoto
T: Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22,
and IL-23, in inflammatory diseases. Clin Dev Immunol.
2013:9685492013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Xu K, Wu J, Luo C, Li Y, Wu X, Gao
H, Feng G and Yuan BZ: The changes of Th17 cells and the related
cytokines in the progression of human colorectal cancers. BMC
Cancer. 12:4182012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wan CK, Andraski AB, Spolski R, Li P,
Kazemian M, Oh J, Samsel L, Swanson PA II, McGavern DB, Sampaio EP,
et al: Opposing roles of STAT1 and STAT3 in IL-21 function in
CD4+ T cells. Proc Natl Acad Sci USA. 112:9394–9399.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Halwani R, Sultana A, Vazquez-Tello A,
Jamhawi A, Al-Masri AA and Al-Muhsen S: Th-17 regulatory cytokines
IL-21, IL-23, and IL-6 enhance neutrophil production of IL-17
cytokines during asthma. J Asthma. 54:893–904. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye EA and Steinle JJ: miR-146a suppresses
STAT3/VEGF pathways and reduces apoptosis through IL-6 signaling in
primary human retinal microvascular endothelial cells in high
glucose conditions. Vision Res. 139:15–22. 2017. View Article : Google Scholar : PubMed/NCBI
|