Introduction
Breast cancer is one of the most common malignancies
affecting women worldwide, accounting for 25% of newly diagnosed
cancer cases and 15% of cancer-associated mortalities in women. As
such, breast cancer represents a major threat to life expectancy
and quality of life, as well as being a socioeconomic burden
(1). Data from the Global Cancer
Report (2014) revealed that 1.677 million new cases of breast
cancer were diagnosed in 2014; of these, 187,000 cases were
reported in China, accounting for 11.2% of novel breast cancer
cases worldwide. According to the China Cancer Registration Annual
Report in 2014, the morbidity of breast cancer in Chinese women in
2012 reached 42.55/100,000 (2). At
present, China has a relatively low incidence of breast cancer;
however, China has a large population base and the morbidity of
breast cancer in China has been increasing since the 1990s. Breast
cancer now has the highest morbidity of cancers in Chinese women
and is the 6th leading cause of cancer-associated mortality
(3,4).
The Wnt protein family is a group of highly
conserved secreted signaling molecules that regulate intercellular
signal transduction during embryogenesis. In recent years, the
various Wnt protein-triggered signaling pathways have been
investigated (5). Mutations of the
Wnt gene and Wnt signaling pathway molecules can induce
developmental defects, while aberrant Wnt signal transduction can
lead to the development of human diseases, including tumors
(6). It has been demonstrated that
imbalances in the Wnt signaling pathway are associated with the
genesis and development of breast cancer (7).
As the key transfer factor of the Wnt signaling
pathway, β-catenin is expressed in a number of human tumors and its
carcinogenic potential has been extensively studied in vitro
as well as in animal experiments in vivo (8). Nuclear aggregation of β-catenin is
frequently considered to be a marker of Wnt/β-catenin signaling
pathway activation, while its stability and accumulation within the
cells is regarded as one of the most critical events in the pathway
(9). In the presence of Wnt
signaling, GSK-3β activity is inhibited and a large amount of
β-catenin accumulates within the cells, entering the nucleus and
initiating target gene expression (7).
As the downstream IE-targeted gene of the
Wnt/β-catenin signaling pathway, cyclin D1 is believed to promote
G1 to S stage transition, initiate DNA synthesis, and participate
in cell proliferation, differentiation and apoptosis in normal
cells. Excessive cyclin D1 expression leads to abnormal cell cycle
control and is associated with the genesis and development of a
number of human tumors (10). It
has been demonstrated that the cyclin D1 gene is an important
downstream target gene of the Wnt/β-catenin signaling pathway and
that cyclin D1 overexpression in numerous human tumors is
associated with the aberrant expression of β-catenin and mutations
in the Wnt/β-catenin signaling pathway (11,12).
Aberrant expression of β-catenin has been revealed to be associated
with cyclin D1 and c-myc overexpression in breast cancer (11).
MicroRNAs (miRNAs) are involved in almost all cell
biological processes (13). It has
recently been reported that a number of miRNAs are aberrantly
expressed in tumor tissues, including breast cancer (14). In addition, they serve different
roles in the various stages of tumor metastasis, including tumor
cell adhesion, migration, invasion and angiogenesis (14). The carcinogenic and antitumor
effects of miRNA in breast cancer have been established (15). However, their roles in breast cancer
metastasis have only been proposed in the past few years. Zhang
et al suggested that microRNA-216a suppresses the
proliferation, migration and invasion of glioma cells via the
Wnt/β-catenin signaling pathway (16). MicroRNA-216a may act as a regulatory
factor in human breast cancer cells. Therefore, the aim of the
present study was to assess the potential effects of microRNA-216a
on the growth of human breast cancer cells and the possible
underlying mechanism.
Materials and methods
Human samples
Patients with breast cancer (females, 55–67 years
old) and normal volunteers were recruited from the School of Basic
Medical Sciences of Xinxiang Medical University (Xinxiang, China)
between December 2016 and January 2017. The characteristics of the
patients are displayed in Table I.
All clinical samples (6 breast cancer serum and 6 normal volunteer
serum) were centrifuged at 1,000 × g for 10 min at 4°C. The serum
specimens were snap-frozen immediately after collection and were
stored at −80°C until use. All experimental protocols were approved
by the Institutional Review Board of the Department of Laboratory
Animal Science of School of Basic Medical Sciences, Xinxiang
Medical university (Xinxiang, China). Written informed consent was
obtained from all participants.
 | Table I.Basic characteristics of the patients
with breast cancer. |
Table I.
Basic characteristics of the patients
with breast cancer.
| Variables | Patients (6) | Normal (6) |
|---|
| Age (yrs.) |
|
|
|
≤55 | 3 | 4 |
|
>55 | 3 | 2 |
| Edmondson
grade |
| 0 |
| I | 0 |
|
| II | 2 |
|
|
III | 4 |
|
RNA extraction and quantitative
real-time PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
used as a template to generate cDNA using a PrimeScript™ RT Reagent
kit (DRR047A; Takara, Tokyo, Japan). The RT primer was as follows:
5′-GTCGTATCCAGTGCG-3′. PCR was performed using a miRCURY LNA™
Universal RT miRNA PCR kit (Exiqon, Vedbaek, Denmark) and SYBR
Green master mix (Exiqon) on an ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The microRNA-216a
primers were as follows: forward, 5′-ATCCAGTGCGTGTCGTG-3′ and
reverse, 5′-TGCTTAATCTCAGCTGGCA-3′.
Cell lines and cell transfection
The human breast cancer MCF-7 cells were obtained
from the American Type Culture Collection (ATCC) and maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.) and 1% antibiotics (100 IU/ml penicillin and 100
µg/ml streptomycin) and maintained in a 37°C incubator with a
humidified atmosphere of 5% CO2. MicroRNA-216a,
anti-microRNA-216a and negative control plasmids were purchased
from Sangon Biotech Co., Ltd. (Shanghai, China). The sequences were
as follows: miR-216a, 5′-TCACAGTTCCCAGCTGAGATTA-3′; anti-miR-216a,
5′-GCAGCGCCTGTGAGAGGGATGAAAA-3′; and negative control,
5′-CCCCCCCCCCCCCC-3′. Cell transfection was performed with 100 nM
of microRNA-216a, anti-microRNA-216a and negative control plasmids
using Lipofectamine™ 2000 (Invitrogen) according to the
manufacturer's instructions. Next, after transfection for 6 h, 20
µM of PNU-74654 was added to the cells for 48 h.
Determination of cytotoxicity
After transfection for 24, 48 and 72 h, MCF-7 cells
were seeded in a 96-well-plate (1×104 cells). MTT
solution (20 µl) was added to every well and incubation followed
for 4 h at 37°C. Then 150 µl of DMSO was added into every well
after removal of the medium to dissolve the crystals and incubation
followed for 20 min at 37°C. The absorbance was assessed at 492 nm
using a microplate reader (model 550; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Cell apoptosis analysis
After transfection for 48 h, MCF-7 cells were seeded
in a 6-well-plate (1–2×106 cells) and were stained with
5 µl Annexin V-APC and 5 µl of propidium iodide (1 mg/ml) (KeyGen
Biotech Co., Ltd., Nanjing, China) for 20 min in the dark. Cell
apoptosis was assessed using a flow cytometer (FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA).
Cell migration assay
After transfection for 48 h, MCF-7 cells
(1×105) were seeded in a 6-well-plate (1×105
cells) and the surface area of the cells was scratched with a
2-mm-wide tip. The plate was incubated with culture media in the
presence for 24 h. The cells in the upper Transwell chamber were
removed carefully with cotton swabs, and the cells that had
migrated to the lower chamber were fixed in 75% ethanol, stained
with 0.1% crystal violet and washed with PBS (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA). The rate of the cells
migrating into the scratched area was assessed and images were
captured using a fluorescence microscope (E1000M Eclipse; Nikon
Corporation, Tokyo, Japan).
Caspase-3/9 kits
MCF-7 cells (1×106) were seeded in a
6-well-plate (1–2×106 cells) and were lysed in Cell
Lysis Buffer (Cell Signaling Technology, Inc., Danvers, MA, USA).
Supernatants were harvested, and total protein was measured with a
BCA assay (KeyGen Biotech Co., Ltd.). Total protein (10 µg) was
incubated with Ac-DEVD-pNA and Ac-IETD-pNA at 37°C for 1 h.
The absorbance was assessed at 405 nm using a microplate reader
(model 550; Bio-Rad Laboratories, Inc.).
Western blotting
MCF-7 cells were seeded in a 6-well-plate
(1–2×106 cells) and lysed in Cell Lysis Buffer (Cell
Signaling Technology, Inc.). Supernatants were harvested, and total
protein was assessed using a BCA assay (KeyGen Biotech Co., Ltd.).
Total protein (40–80 µg) was subjected to 10% SDS-PAGE and
transferred onto nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). The membranes were then blotted with primary
antibodies against Bax (cat. no. sc-6236; 1:500), p53 (cat. no.
sc-6243; 1:500), p21 (cat. no. sc-397; 1:500), β-catenin (cat. no.
sc-7199; 1:500), cyclin D1 (cat. no. sc-717; 1:500) and GADPH (cat.
no. sc-25778; 1:500; all from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at 4°C overnight. Incubation then followed with
secondary antibody goat anti-rabbit IgG-HRP (cat. no. sc-2004;
1:5000; Santa Cruz Biotechnology, Inc.). The proteins were detected
using ECL Plus Western Blotting Detection System (GE Healthcare
Life Sciences, Chalfont, UK). A protein blank was scanned with a
Fujifilm LAS-3000 Imaging System and analyzed using MultiGauge
Software (Fujifilm, Brookvale, Australia).
Statistical analysis
Comparisons among groups were determined by one-way
ANOVA followed by Tukey's post-hoc test. The data are expressed as
the mean ± SD (n=3). P<0.05 was considered to indicate a
statistically significant difference.
Results
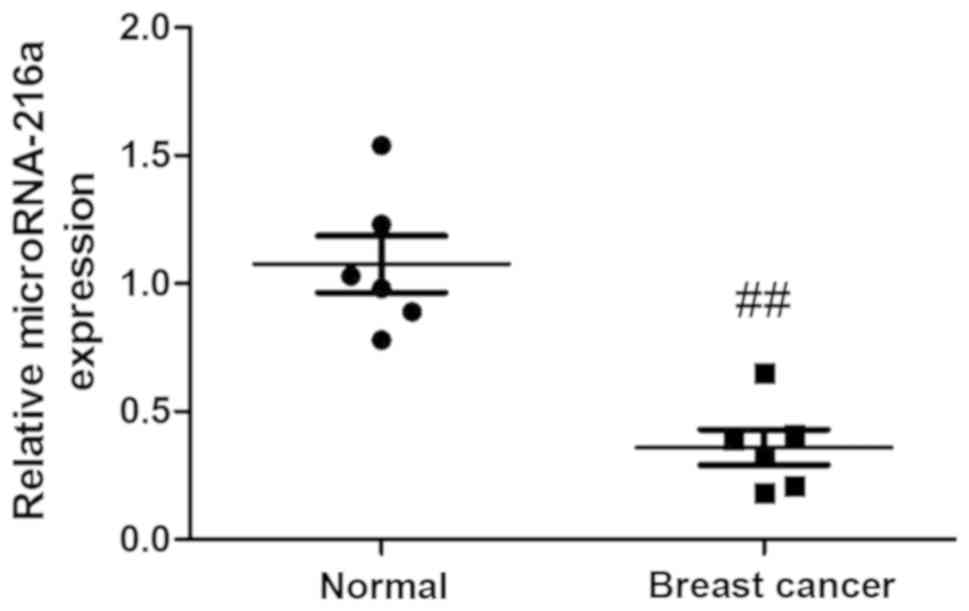
MicroRNA-216a expression in vivo
To assess the potential anticancer effects of
microRNA-216a in human breast cancer, the expression of
microRNA-216a was measured using quantitative real-time PCR. As
presented in Fig. 1, serum
microRNA-216a was significantly decreased in the breast cancer
group compared with the control group.
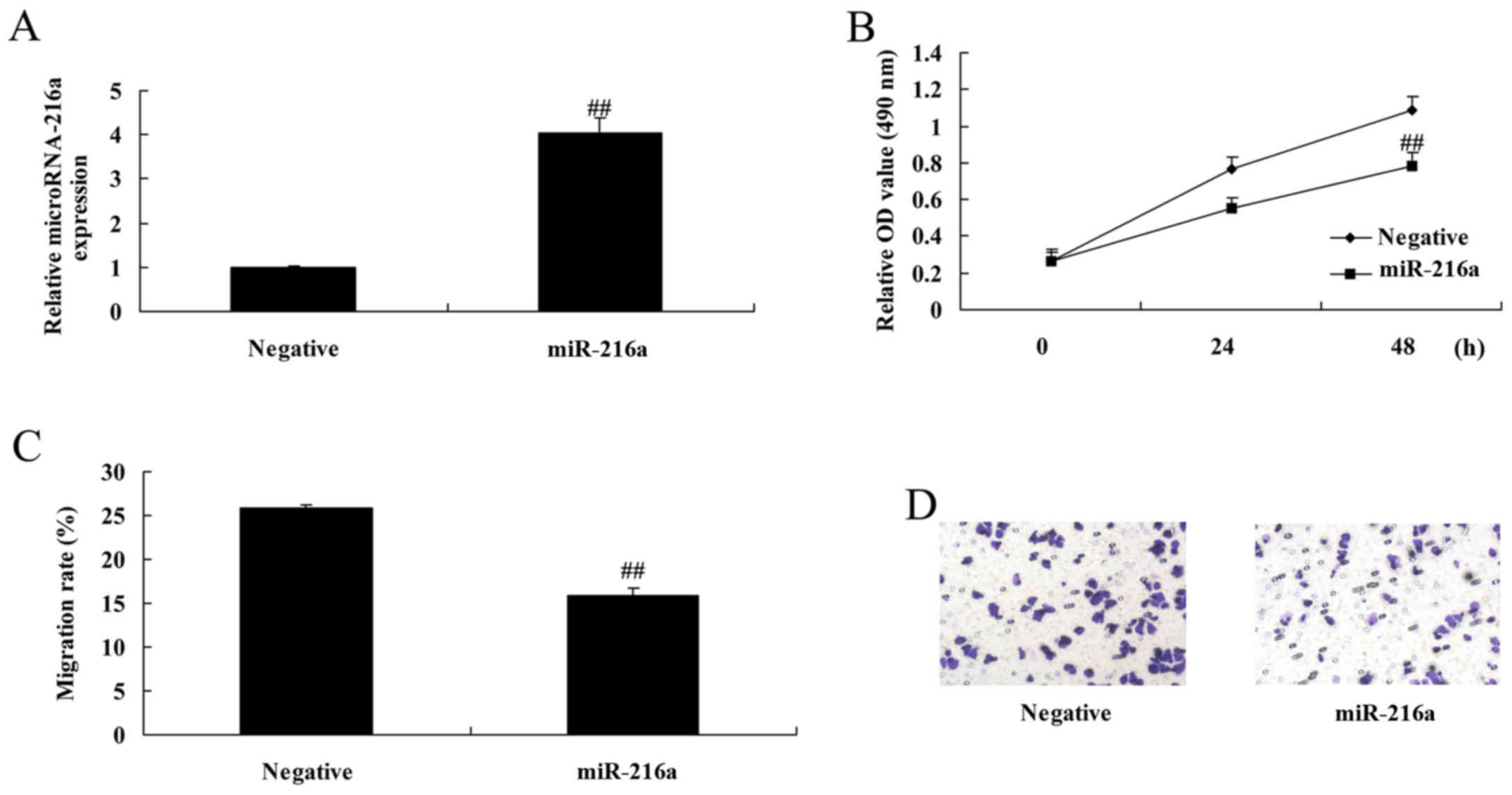
MicroRNA-216a overexpression reduces
the proliferation and migration of MCF-7 cells
The anticancer effects of microRNA-216a on MCF-7
cells were assessed. MicroRNA-216a overexpression, induced by
transfection with microRNA-216a mimics, significantly reduced the
proliferation and migration of MCF-7 cells compared with control
negative group (Fig. 2).
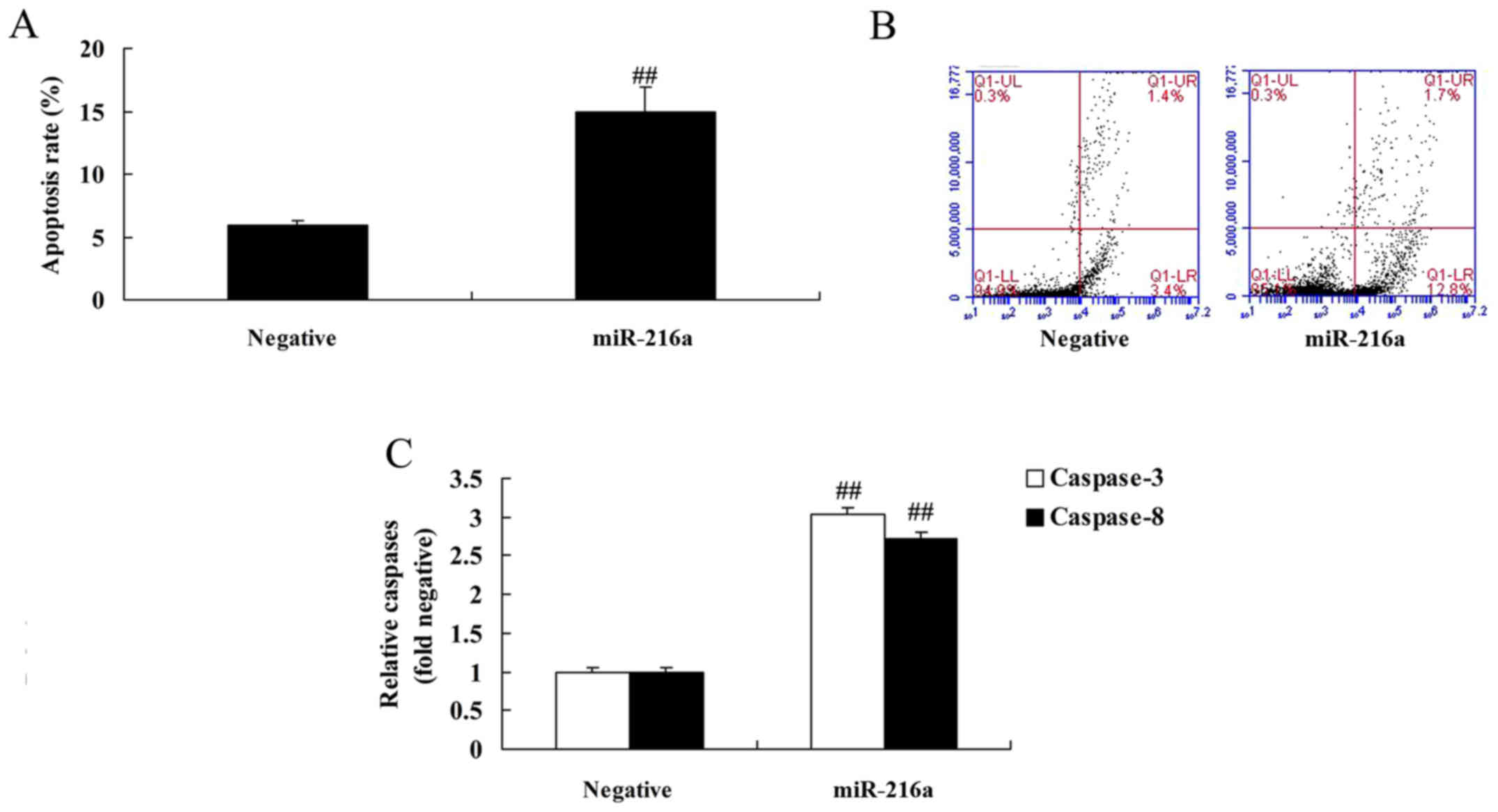
MicroRNA-216a overexpression induces
apoptosis and increases caspase-3/8 activities in MCF-7 cells
MicroRNA-216a overexpression was demonstrated to
significantly increase apoptosis and caspase-3/8 activities in
MCF-7 cells compared with the negative control group (Fig. 3).
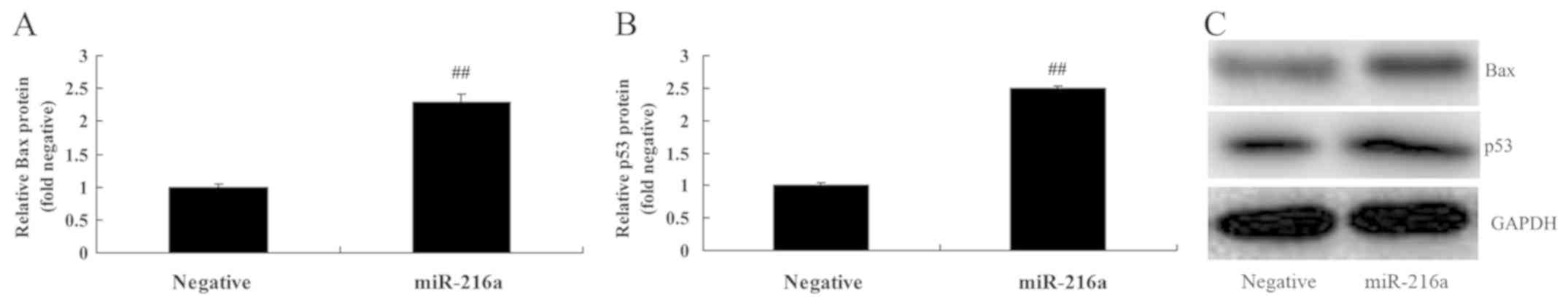
MicroRNA-216a overexpression promotes
Bax and p53 protein expression in MCF-7 cells
The results revealed that overexpression of
microRNA-216a significantly promoted Bax and p53 protein expression
in MCF-7 cells compared with the negative control group (Fig. 4).
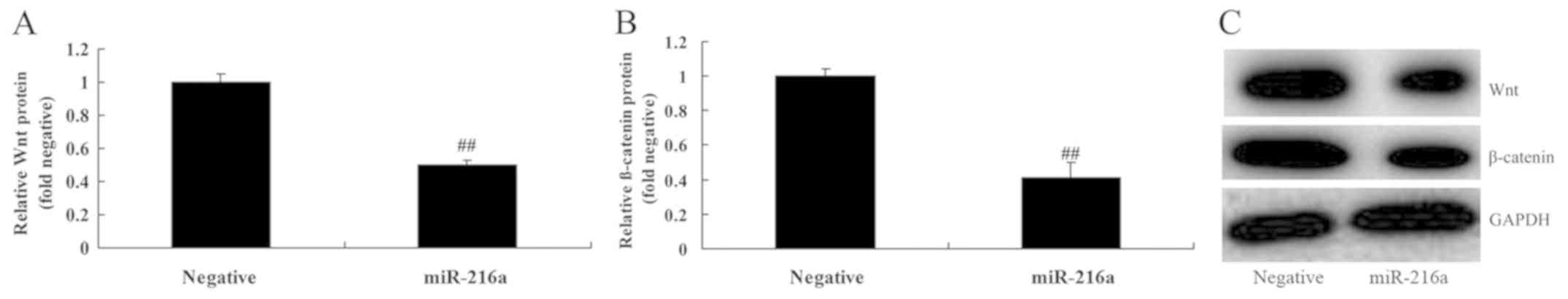
MicroRNA-216a overexpression
suppresses Wnt and β-catenin protein expression in MCF-7 cells
To investigate the anticancer mechanisms of
microRNA-216a in MCF-7 cells, Wnt and β-catenin protein expression
was assessed. The results revealed that microRNA-216a
overexpression significantly suppressed the expression of Wnt and
β-catenin proteins in MCF-7 cells compared with the negative
control group (Fig. 5).
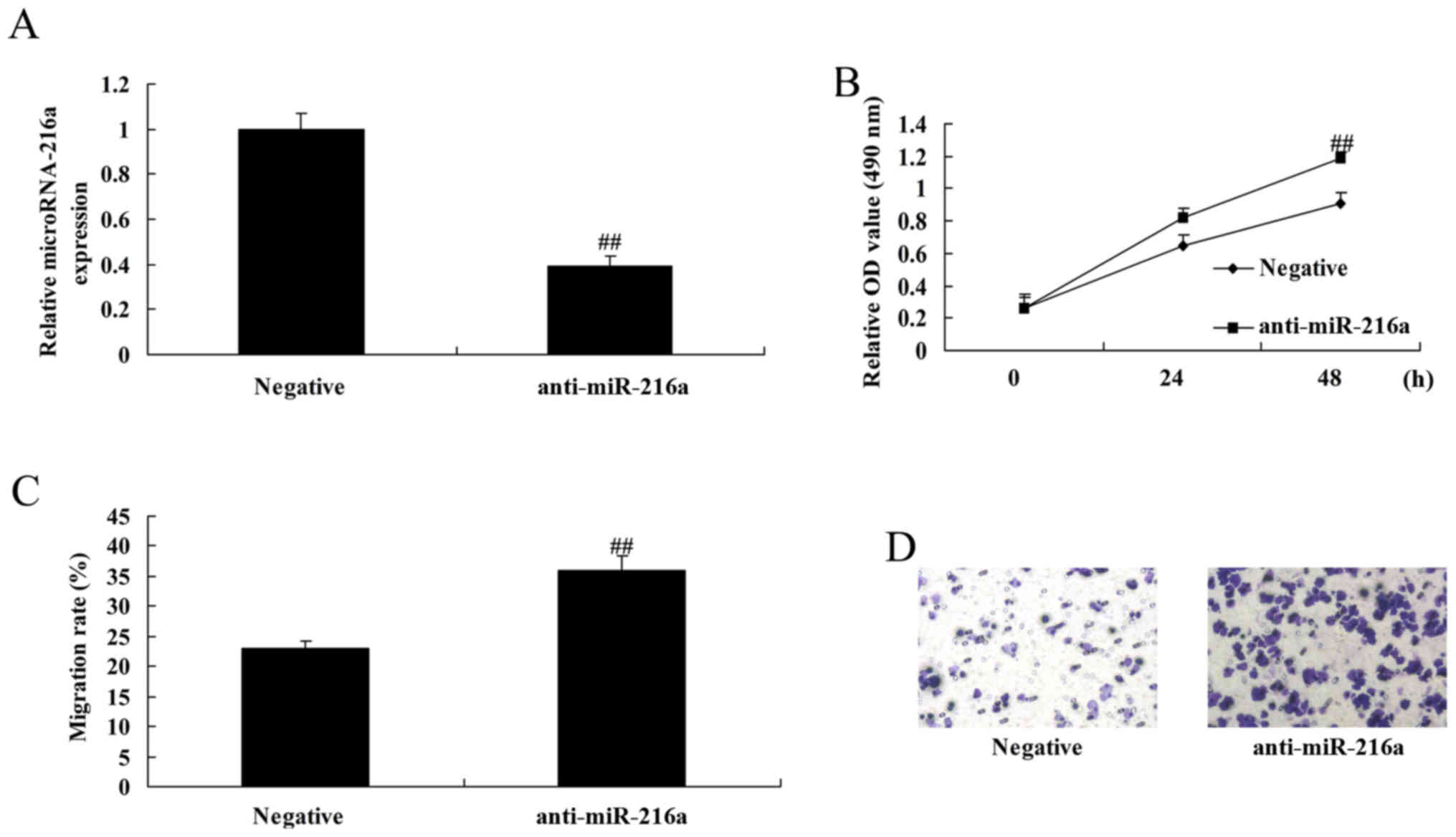
Anti-microRNA-216a increases the
proliferation and migration of MCF-7 cells
Anti-microRNA-216a mimics were used to downregulate
microRNA-216a in MCF-7 cells. Following transfection with
anti-microRNA-216a mimics, cell proliferation and migration were
significantly increased in MCF-7 cells compared with the negative
control group (Fig. 6).
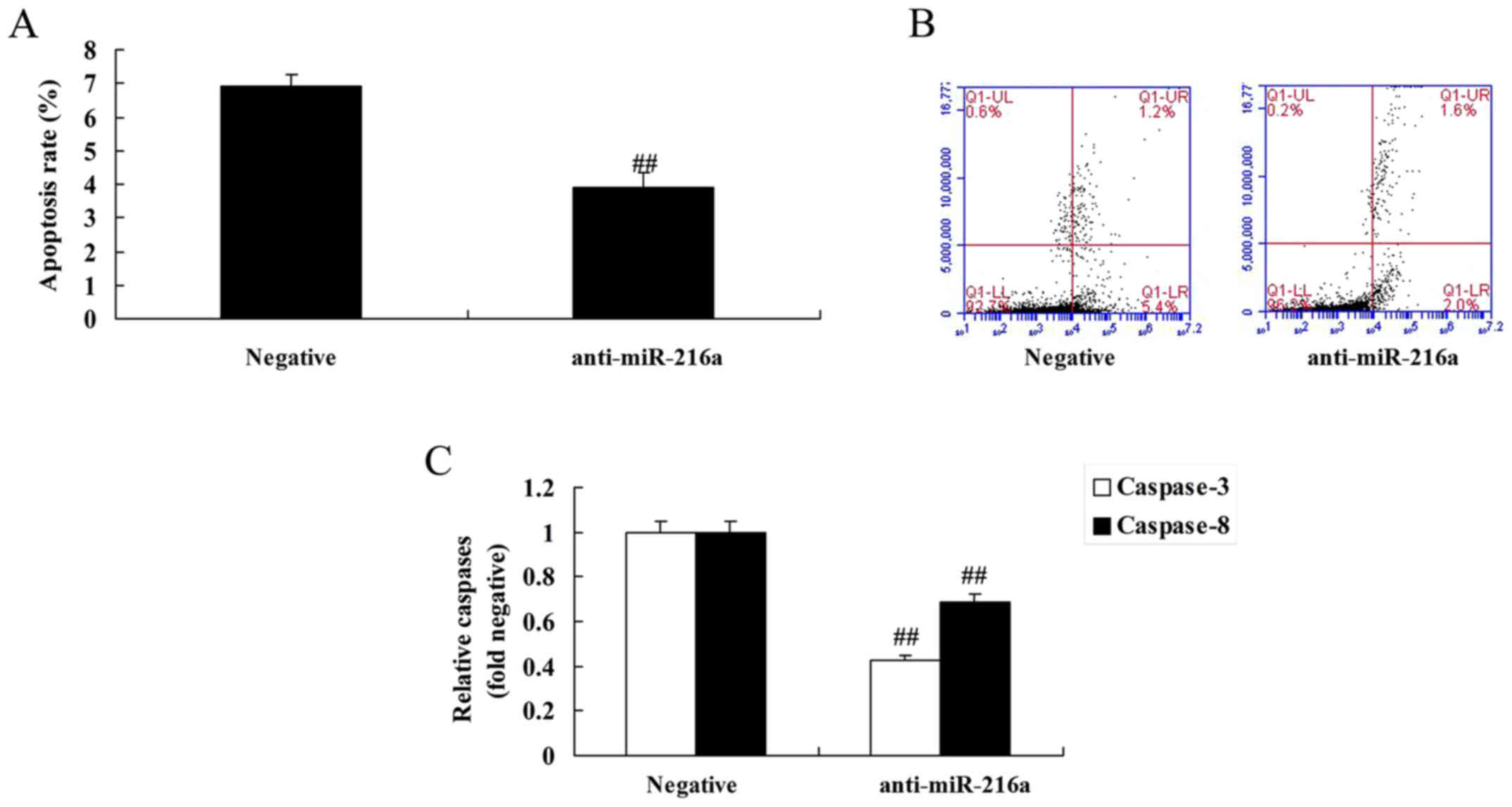
Anti-microRNA-216a inhibits apoptosis
and caspase-3/8 activities in MCF-7 cells
MicroRNA-216a inhibition resulted in a significant
decrease in apoptosis and caspase-3/8 activities in MCF-7 cells
compared with the negative control group (Fig. 7).
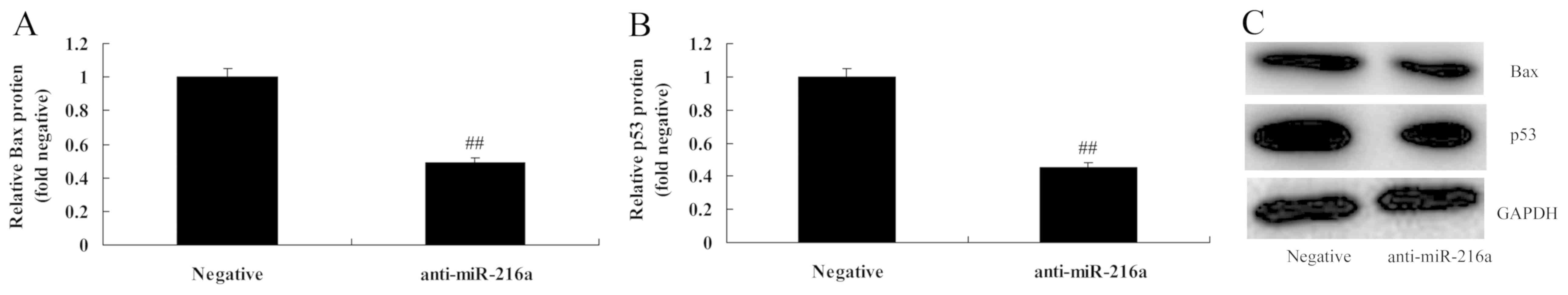
Anti-microRNA-216a suppresses Bax and
p53 protein expression in MCF-7 cells
MicroRNA-216a inhibition significantly suppressed
Bax and p53 protein expression in MCF-7 cells compared with the
negative control group (Fig.
8).
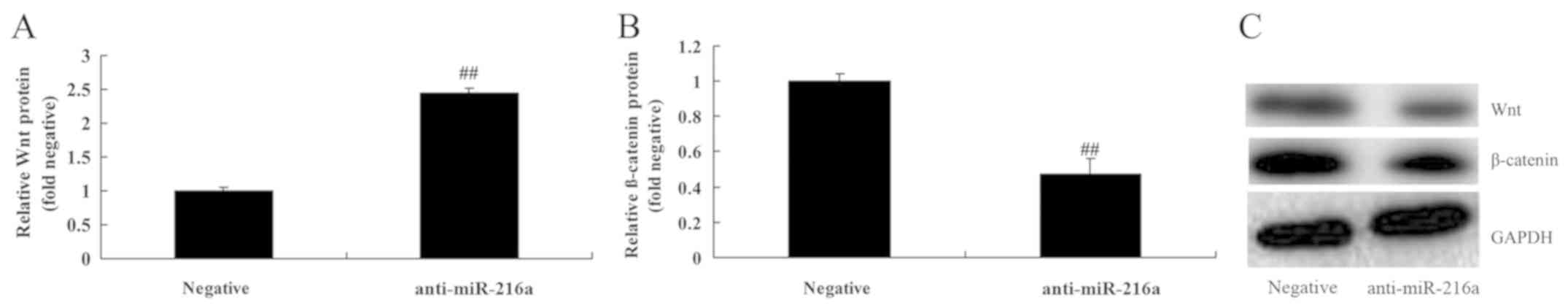
Anti-microRNA-216a promotes Wnt and
β-catenin protein expression in MCF-7 cells
To assess the anticancer mechanism of microRNA-216a
in MCF-7 cells, Wnt and β-catenin expression was assessed. The
results revealed that inhibition of microRNA-216a significantly
promoted the expression of Wnt and β-catenin in MCF-7 cells
compared with the negative control group (Fig. 9).
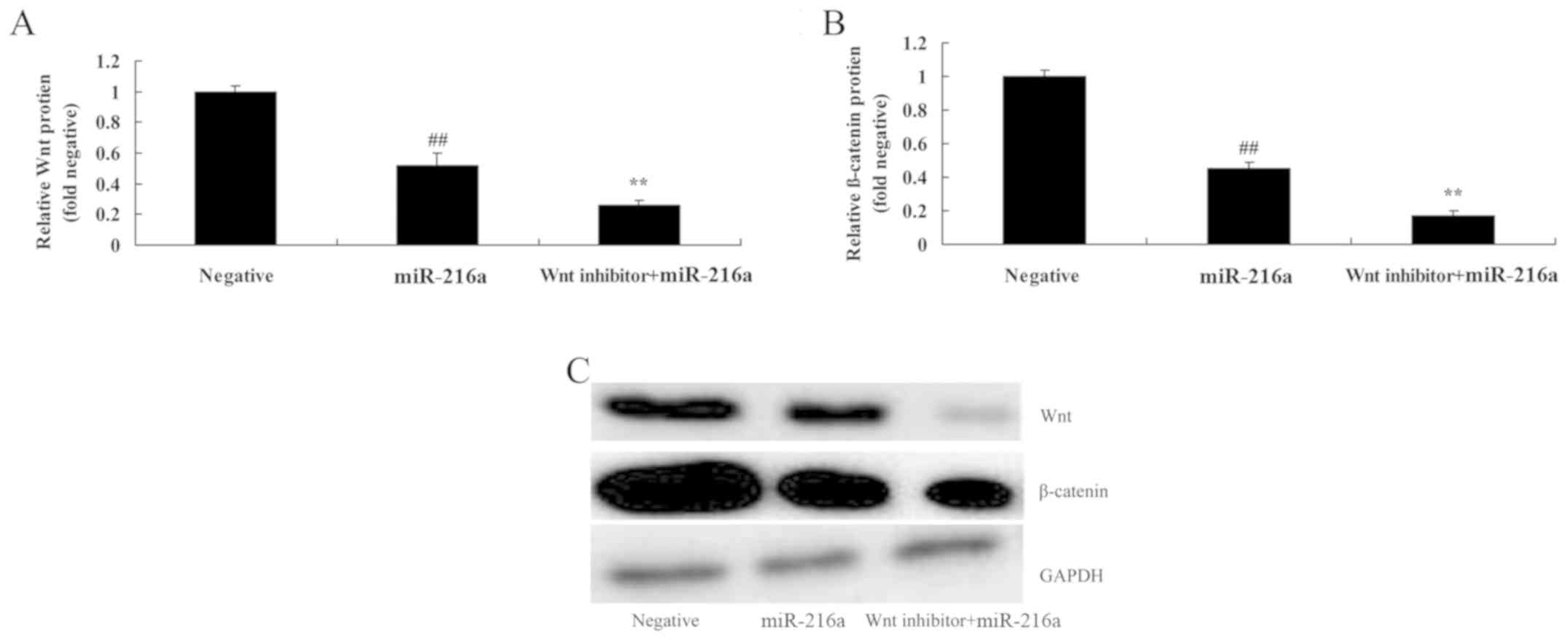
Wnt pathway inactivation suppresses
Wnt and β-catenin expression in MCF-7 cells following treatment
with microRNA-216a
To confirm whether microRNA-216 acts by affecting
Wnt in MCF-7 cells, the cells were treated with Wnt inhibitor
(PNU-74654, 20 µM) for 48 h. Wnt pathway inactivation led to a
significant increase in the anticancer effects of microRNA-216; Wnt
and β-catenin protein expression was inhibited compared with the
with microRNA-216a alone group (Fig.
10).
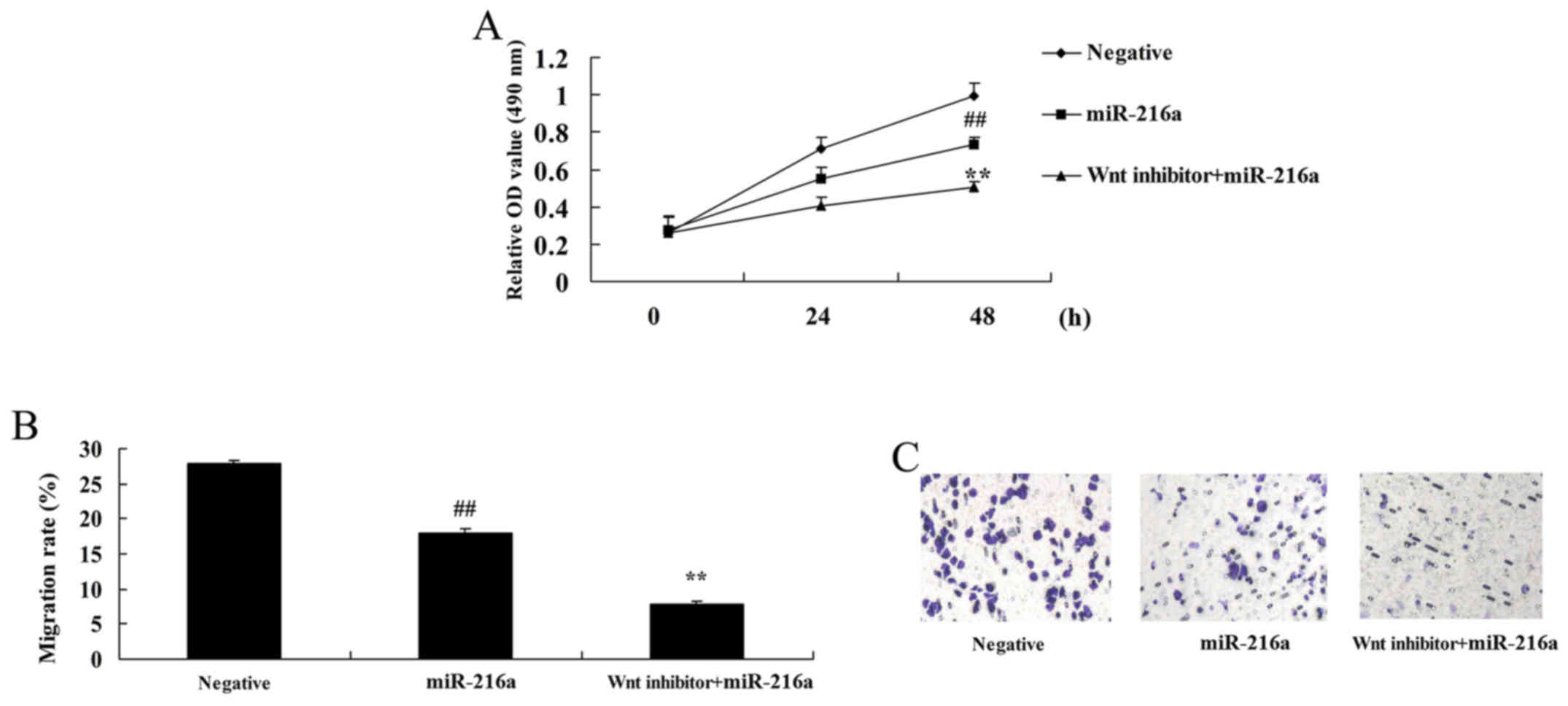
Wnt pathway inactivation reduces the
proliferation and migration of MCF-7 cells following microRNA-216a
treatment
Wnt pathway inactivation significantly reduced the
proliferation and migration of MCF-7 cells following treatment with
microRNA-216a compared with cells treated with microRNA-216a alone
(Fig. 11).
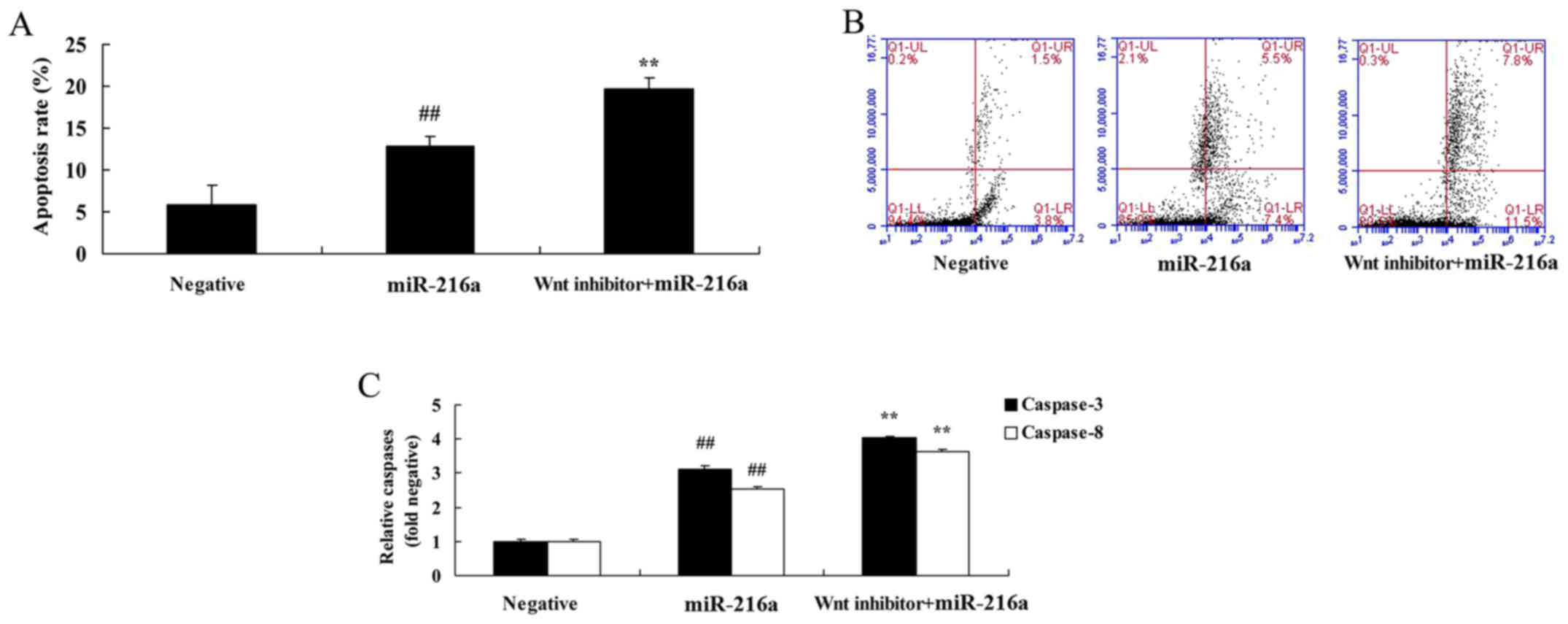
Wnt pathway inactivation induces
apoptosis and increases caspase-3/8 activities in MCF-7 cells
following microRNA-216a treatment
Compared with the microRNA-216a group, treatment
with the Wnt inhibitor significantly induced apoptosis and
increased caspase-3/8 activities in MCF-7 cells (Fig. 12).
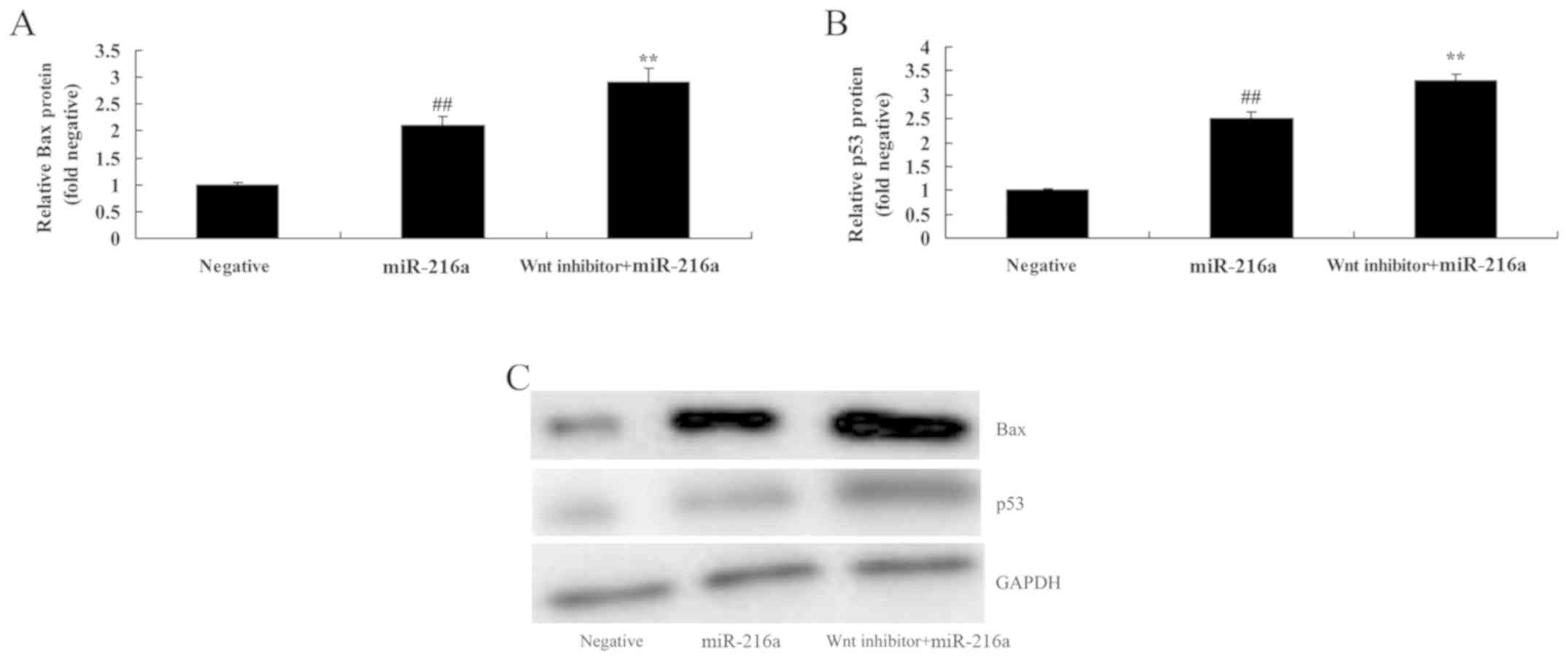
Wnt pathway inactivation promotes Bax
and p53 protein expression in MCF-7 cells following microRNA-216a
treatment
To determine whether changes in Wnt inactivation
occurred due to the effects of microRNA-216a on Bax and p53 protein
expression, the expression levels of Bax and p53 in MCF-7 cells
were assessed. Treatment with the Wnt inhibitor significantly
promoted Bax and p53 protein expression in MCF-7 cells following
microRNA-216a treatment compared with cells treated with
microRNA-216a alone (Fig. 13).
Discussion
According to statistics from the Union for
International Cancer Control, breast cancer is a malignancy with
high morbidity in women, accounting for ~25%. Each year, 1.2
million women develop breast cancer worldwide, of whom 500,000
succumb to the disease. The morbidity and mortality of breast
cancer are the highest of all malignancies affecting females, and
are increasing at an annual rate of 0.3–8% (17). In the USA, breast cancer accounts
for 15.3% of all cancer morbidity, second only to prostate cancer,
and has a mortality of 7.3%. The morbidity and mortality of breast
cancer varies between populations from different geographical
regions, races and ethnic origins (18). The genesis of breast cancer is the
accumulative result of multi-stage, multi-step and multi-gene
abnormalities under the action of environmental and genetic
factors, among which the key step is oncogene activation and
inactivation of the tumor suppressor gene (19). The results of the present study
demonstrated that serum microRNA-216a is significantly decreased in
patients with breast cancer. MicroRNA-216a overexpression reduced
the proliferation and migration, induced apoptosis, increased
caspase-3/8 activities and promoted Bax and p53 expression in MCF-7
cells. Li et al reported that microRNA-216a inhibits growth
and metastasis by targeting eukaryotic translation initiation
factor 4B in oral squamous cell carcinoma (20). In the present study, we only used
MCF-7 cells, which is a limitation. In future, more breast cancer
cell lines or in vivo models of breast cancer should be
studied. miRNA-126a may be a useful marker for monitoring responses
to chemotherapy in the future.
The Wnt signal transduction pathway is a growth and
development regulation pathway with multiple steps and multiple
sites of action and is mediated by multiple intracellular and
extracellular factors (21).
Excessive activation and imbalance of the Wnt pathway induces
dysplasia or tumor formation (22).
The Wnt pathway is comprised of three pathways; the
Wnt/Ca2+ pathway, the PCP pathway and the canonical Wnt
pathway (22). The canonical Wnt
pathway is an important signal transduction pathway that triggers a
cascade reaction via the specific binding of Wnt and the membrane
receptor to alter intra-nuclear gene expression (6). The molecules involved in the canonical
Wnt pathway include Wnt protein, Frizzled, E-cadherin, β-catenin,
Dishevledr, APC protein, GSK-3β and Axin protein, as well as
transcriptional factor/lymphoid enhancer factor and ubiquitin
(23). In the present study,
microRNA-216a suppressed Wnt and β-catenin protein expression in
MCF-7 cells. Zhang et al suggested that microRNA-216a
suppresses the proliferation, migration and invasion of glioma
cells via the Wnt/β-catenin signaling pathway (16). In the present study, only Wnt
inhibitor + miR-216a were used. In future, Wnt inhibitor +
anti-miR-216a should be utilized.
The Wnt signaling pathway regulates the
embryogenesis and morphogenesis of tissues and organs in nematodes,
fruit flies and even higher vertebrates (9). The Wnt pathway is inactive in normal
mature cells, and the majority of β-catenin in the cytoplasm binds
with E-cadherin. A small amount of β-catenin forms a protein
polymer with GSK-3β, APC and Axin; β-catenin in the polymer is
phosphorylated, binds covalently with Ub and is degraded (24). Therefore, almost no free β-catenin
is present in limitedly growing cells of the maturely and normally
growing body in the absence of Wnt signaling. However, when the Wnt
pathway is activated, Wnt binds with the cell surface receptor Fz
and GSK-3β is inactivated, while β-catenin is not degraded under
the participation of Dsh (6). When
cytoplasmic β-catenin reaches a certain level it is transferred to
the cell nucleus, accumulates gradually and enters the nucleus.
Once there, it interacts with transcription factors, initiates
transcription and regulates the expression of corresponding genes
(25). The phosphorylation status
of these components mediates the transmission of growth and
development signals. Excessive transduction and abnormal activation
may therefore give rise to malignant transformation of cells and
tumor genesis (26). In the present
study, it was determined that the anticancer effects of
microRNA-216a were reversed by anti-microRNA-216a via the
Wnt/β-catenin signaling pathway. The microRNA-216a/Wnt/β-catenin
signaling pathway may regulate other cancers, and therefore, future
studies should investigate the function of microRNA-216a in a range
of tumor types. Meanwhile, microRNA-216a may also affect additional
pathways to inhibit cell growth.
Wnt binding with Fz acts like a switch in the
pathway; β-catenin is a critical component of the pathway that
binds with E-cadherin and Tcf/Lef as well as forming complexes with
GSK-3β, APC and Axin. Cytoplasmic and nuclear β-catenin levels are
altered via binding with a variety of components, thus affecting
the expression of some genes (27,28).
In the present study, it was demonstrated that Wnt pathway
inactivation increased the anticancer effects of microRNA-216a in
MCF-7 cells, specifically proliferation and apoptosis. Zhang et
al suggested that microRNA-216a suppresses the proliferation,
migration, and invasion of glioma cells via the Wnt/β-catenin
signaling pathway (16).
Collectively, these results revealed that microRNA-216a adjusts the
Wnt/β-catenin signaling pathway to induce apoptosis of breast
cancer. The results of the present study indicated that
microRNA-216a may be a novel treatment for targeting breast cancer
cell growth via the Wnt/β-catenin signaling pathway.
Acknowledgements
We would like to thank Dr Tianyun Wang and the
International Joint Research Lab for Recombiant Pharmaceutical
Protein Expression System of Henan for technical support.
Funding
This study was supported by the National Natural
Science Foundation of China (no. 81502313) and the Doctoral
Scientific Research Activation Foundation of Xinxiang Medical
University (no. XYBSKYZZ201603).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
QX designed the experiment; SW, YZ, ZZ, CQ and XY
performed the experiment; QX and SW analyzed the data; QX wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institutional Review Board of the Department of Laboratory Animal
Science of School of Basic Medical Sciences, Xinxiang Medical
university (Xinxiang, China). Written informed consent was obtained
from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taylor-Phillips S, Wallis MG, Jenkinson D,
Adekanmbi V, Parsons H, Dunn J, Stallard N, Szczepura A, Gates S,
Kearins O, et al: Effect of using the same vs different order for
second readings of screening mammograms on rates of breast cancer
detection: A randomized clinical trial. JAMA. 315:1956–1965. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pu Z, Zhang X, Chen Q, Yuan X and Xie H:
Establishment of an expression platform of OATP1B1 388GG and 521CC
genetic polymorphism and the therapeutic effect of tamoxifen in
MCF-7 cells. Oncol Rep. 33:2420–2428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moss SM, Wale C, Smith R, Evans A, Cuckle
H and Duffy SW: Effect of mammographic screening from age 40 years
on breast cancer mortality in the UK Age trial at 17 years'
follow-up: A randomised controlled trial. Lancet Oncol.
16:1123–1132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pei L, Zhou Y, Tan G, Mao F, Yang D, Guan
J, Lin Y, Wang X, Zhang Y, Zhang X, et al Outcomes research
consortium, : ultrasound-assisted thoracic paravertebral block
reduces intraoperative opioid requirement and improves analgesia
after breast cancer surgery: A randomized, controlled,
single-center trial. PLoS One. 10:e01422492015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun X, Xu C, Tang SC, Wang J, Wang H, Wang
P, Du N, Qin S, Li G, Xu S, et al: Let-7c blocks estrogen-activated
Wnt signaling in induction of self-renewal of breast cancer stem
cells. Cancer Gene Ther. 23:83–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lii CK, Chang JW, Chen JJ, Chen HW, Liu
KL, Yeh SL, Wang TS, Liu SH, Tsai CH and Li CC: Docosahexaenoic
acid inhibits 12-O-tetradecanoylphorbol-13-acetate-induced
fascin-1-dependent breast cancer cell migration by suppressing the
PKCδ- and Wnt-1/β-catenin-mediated pathways. Oncotarget.
7:25162–25179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morrow KA, Das S, Meng E, Menezes ME,
Bailey SK, Metge BJ, Buchsbaum DJ, Samant RS and Shevde LA: Loss of
tumor suppressor Merlin results in aberrant activation of
Wnt/β-catenin signaling in cancer. Oncotarget. 7:17991–18005. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei H, Wang H, Ji Q, Sun J, Tao L and Zhou
X: NRBP1 is downregulated in breast cancer and NRBP1 overexpression
inhibits cancer cell proliferation through Wnt/β-catenin signaling
pathway. Onco Targets Ther. 8:3721–3730. 2015.PubMed/NCBI
|
|
9
|
Cui J, Li P, Liu X, Hu H and Wei W:
Abnormal expression of the Notch and Wnt/β-catenin signaling
pathways in stem-like ALDHhiCD44+ cells correlates
highly with Ki-67 expression in breast cancer. Oncol Lett.
9:1600–1606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farahmand L, Darvishi B, Majidzadeh AK and
Madjid Ansari A: Naturally occurring compounds acting as potent
anti-metastatic agents and their suppressing effects on Hedgehog
and WNT/beta-catenin signalling pathways. Cell Prolif.
50:e122992017. View Article : Google Scholar
|
|
11
|
Li K, Ying M, Feng D, Du J, Chen S, Dan B,
Wang C and Wang Y: Fructose-1,6-bisphosphatase is a novel regulator
of Wnt/β-Catenin pathway in breast cancer. Biomed Pharmacother.
84:1144–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shrivastava S, Jeengar MK, Thummuri D,
Koval A, Katanaev VL, Marepally S and Naidu VGM: Cardamonin, a
chalcone, inhibits human triple negative breast cancer cell
invasiveness by downregulation of Wnt/β-catenin signaling cascades
and reversal of epithelial-mesenchymal transition. Biofactors.
43:152–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Cai B, Shen L, Dong Y, Lu Q, Sun S,
Liu S, Ma S, Ma PX and Chen J: MiRNA-29b suppresses tumor growth
through simultaneously inhibiting angiogenesis and tumorigenesis by
targeting Akt3. Cancer Lett. 397:111–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Das S: Identification and targeting of
microRNAs modulating acquired chemotherapy resistance in Triple
negative breast cancer (TNBC): A better strategy to combat
chemoresistance. Med Hypotheses. 96:5–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu B, Su F, Chen M, Li Y, Qi X, Xiao J,
Li X, Liu X, Liang W, Zhang Y, et al: Serum miR-21 and miR-125b as
markers predicting neoadjuvant chemotherapy response and prognosis
in stage II/III breast cancer. Hum Pathol. 64:44–52. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Xu K, Shi L, Zhang L, Zhao Z, Xu
H, Liang F, Li H, Zhao Y, Xu X, et al: Overexpression of
microRNA-216a suppresses proliferation, migration, and invasion of
glioma cells by targeting leucine-rich repeat-containing G
protein-coupled receptor 5. Oncol Res. 25:1317–1327. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu M, Wang S, Cui S, Duan X, Fan Z and Yu
Z: The feasibility of the ACOSOG Z0011 Criteria to Chinese Breast
Cancer Patients: A multicenter study. Sci Rep. 5:152412015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li T, Wang B, Wang Z, Ragaz J, Zhang J,
Sun S, Cao J, Lv F, Wang L, Zhang S, et al: Bevacizumab in
combination with modified FOLFOX6 in heavily pretreated patients
with HER2/neu-negative metastatic breast cancer: A Phase II
Clinical Trial. PLoS One. 10:e01331332015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Láng I, Bell R, Feng FY, Lopez RI, Jassem
J, Semiglazov V, Al-Sakaff N, Heinzmann D and Chang J: Trastuzumab
retreatment after relapse on adjuvant trastuzumab therapy for human
epidermal growth factor receptor 2-positive breast cancer: Final
results of the Retreatment after HErceptin Adjuvant trial. Clin
Oncol (R Coll Radiol). 26:81–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li L and Ma HQ: MicroRNA-216a inhibits the
growth and metastasis of oral squamous cell carcinoma by targeting
eukaryotic translation initiation factor 4B. Mol Med Rep.
12:3156–3162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vadnais C, Shooshtarizadeh P, Rajadurai
CV, Lesurf R, Hulea L, Davoudi S, Cadieux C, Hallett M, Park M and
Nepveu A: Autocrine activation of the Wnt/β-catenin pathway by CUX1
and GLIS1 in breast cancers. Biol Open. 3:937–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin L, Gao Y, Zhang X, Wang J, Ding D,
Zhang Y, Zhang J and Chen H: Niclosamide sensitizes triple-negative
breast cancer cells to ionizing radiation in association with the
inhibition of Wnt/β-catenin signaling. Oncotarget. 7:42126–42138.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmed K, Shaw HV, Koval A and Katanaev VL:
A second WNT for old drugs: Drug repositioning against
WNT-dependent cancers. Cancers (Basel). 8(pii): E662016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo L, Yilamu D, Sun L, Liu S and Ma F:
Association among the expression of β-catenin, cyclin D1 and
estrogen receptor-β in human breast cancer. Exp Ther Med.
10:1423–1428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu W, Lin C and Li Y: Rottlerin induces
Wnt co-receptor LRP6 degradation and suppresses both Wnt/β-catenin
and mTORC1 signaling in prostate and breast cancer cells. Cell
Signal. 26:1303–1309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lill C, Schneider S, Ghanim B, Brunner M,
Heiduschka G, Loewe R and Thurnher D: Expression of β-catenin and
cyclin D1 in Merkel cell carcinomas of the head and neck. Wien Klin
Wochenschr. 125:501–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou XL, Qin XR, Zhang XD and Ye LH:
Downregulation of Dickkopf-1 is responsible for high proliferation
of breast cancer cells via losing control of Wnt/beta-catenin
signaling. Acta Pharmacol Sin. 31:202–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Yang Z, Li P, Bledsoe G, Chao L
and Chao J: Kallistatin antagonizes Wnt/β-catenin signaling and
cancer cell motility via binding to low-density lipoprotein
receptor-related protein 6. Mol Cell Biochem. 379:295–301. 2013.
View Article : Google Scholar : PubMed/NCBI
|