Introduction
Cervical cancer is a common malignancy that poses a
severe threat to the health of women. Each year, ~500,000 new cases
of cervical cancer are diagnosed worldwide. In developed countries,
the morbidity of cervical cancer has decreased as advances have
been made in the diagnostic and screening methods for cervical
cancer (1); however, due to the
socioeconomic limitations in developing countries, cervical cancer
remains one of the major causes of mortality for women in those
countries (2). Therefore,
identifying novel treatment options to combat cervical cancer is of
the utmost importance.
Chemotherapy has a crucial role in reducing
pre-operative tumor size, creating more favorable conditions for
surgery and preventing post-operative recurrence and metastasis
(3). It is also the primary
treatment option for late-stage metastatic cervical cancer
(4); however, chemotherapy also
causes severe toxic effects and side effects without good
therapeutic efficacy (5–7). Several active ingredients extracted
from plants have been reported to be good alternatives to
chemotherapeutic drugs or suitable for combined use with
chemotherapeutic drugs (8).
Astragaloside IV is the main ingredient that is
extracted from Radix astragali. As a small-molecule compound,
astragaloside IV has antitumor efficacy and potency that are
several times higher than that of astragalus extract (9–11).
In vivo and in vitro data have demonstrated that
astragaloside IV has anti-inflammatory and anti-viral activities
that modulate the immune system. Treatment with astragaloside IV
has been reported to protect and enhance cardiopulmonary function
and improve the tumor microenvironment (12–15).
This herb-derived active component is widely available and produces
few toxic effects or side effects (16). Previous studies have demonstrated
that astragaloside IV exhibits significant antitumor activity and
tumor cell cytotoxic ability in vivo and in vitro
(17–20).
Uncontrolled proliferation, abnormal differentiation
and invasion, and metastatic capacities are among the most
distinctive biological features of tumor cells (21). Epithelial-mesenchymal transition
(EMT) is considered to be an important molecular mechanism
associated with the metastasis of tumors, and has a major role in
the infiltration and metastasis of malignant epithelial tumor cells
(22). EMT is a process in which,
under specific physiological and pathological conditions,
epithelial cells are transformed into mesenchymal cells. The EMT
concept was first proposed in 1982 by Greenberg and Hay (23); the study reported that lens
epithelial cells formed pseudopods and had a long and narrow
appearance in collagen gels. The epithelial cells differentiated
into mesenchymal-like cells and exhibited increased migration and
mobility (23). During EMT,
microenvironmental factors are produced that act on cell receptors,
inducing changes in cell signal transduction pathways. EMT is
considered to be an important step in the malignant progression,
invasion and migration of tumor cells. In the current study, human
SiHa cervical cancer cells were treated with astragaloside IV, and
the inhibitory effect on the invasion and migration of cervical
cancer cells was evaluated. It was aimed to investigate whether the
inhibitory effect of astragaloside was associated with EMT.
Materials and methods
Chemicals and reagents
Astragaloside IV (purity of >98% as determined by
high-performance liquid chromatography) was obtained from Nanjing
Zelang Medical Technology Co., Ltd. (Nanjing, China; cat. no.
ZL20160315021). Mitomycin C was purchased from MedChemExpress
(Monmouth Junction, NJ, USA; cat. no. HY-13316-31756). A
First-Strand cDNA Synthesis kit and Taq DNA Polymerase were
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Matrigel was purchased from Becton, Dickinson and Company (Franklin
Lakes, NJ, USA). A Transwell chambers were purchased from Corning
Inc. (Corning, NY, USA). A diaminobenzidine (DAB) kit (cat. no.
DAB-1031) and an EliVision plus kit (cat. no. kit-9902) were
purchased from Maixin Biotech Co., Ltd (Fuzhou, China). MTT cell
proliferation assay kit (cat. no. 20147849) and DMSO (cat. no.
20120322) were purchased from Jiuyi Chemical Reagent Co., Ltd.
(Shanghai, China). A penicillin/streptomycin solution (cat. no.
KGY002), 0.25% Trypsin-EDTA (cat. no. KGY001), western blot primary
antibody diluent (cat. no. KGP106), western blot secondary antibody
diluent (cat. no. KGP107), a total protein extraction kit (cat. no.
KGP250), an enhance chemiluminescence detection kit (cat. no.
KGP1127) and a BCA protein assay kit (cat. no. KGA902) were all
purchased KeyGen Biotech Co., Ltd. (Nanjing, China).
Cell culture
SiHa cervical cancer cells were obtained from
Nanjing University of Chinese Medicine (Nanjing, China). The cells
were cultured in 90% Dulbecco's modified Eagle's medium + 10% calf
serum complete medium (KeyGen Biotech Co., Ltd.) at 37°C in 5%
CO2 (volume fraction). Cells in the logarithmic growth
phase were used for subsequent experiments.
MTT assay
For the MTT assay, cells were prepared at a density
of 5×104 cells/ml, and 100 µl cell suspension was seeded
into each well of a 96-well plate. The cells were cultured for 24 h
at 37°C in a 5% CO2 incubator. Astragaloside IV was
prepared at different concentrations (800, 400, 200, 100, 50, 25,
12.5, 6.25, 3.125, 1.5625 and 0.78125 µg/ml) using complete culture
medium. To each well, 100 µl culture medium containing different
concentrations of astragaloside IV was added, and the plate was
incubated at 37°C for 24 h. Untreated cells were the negative
control group. An MTT solution was added to each well of the
96-well plate. DMSO was used to dissolve the purple formazan and
optical density (OD) values were measured at λ=490 nm (BioTek
ELx800; BioTek Instruments, Inc., Winooski, VT, USA). For each
group, the inhibitory rate and 50% inhibitory concentration
(IC50) values were calculated.
Wound healing assay
Cells in the log phase were trypsinized and cultured
together with mitomycin C (1 µg/ml) for 1 h. Then, the cells were
seeded in a 6-well plate at a density of 5×105
cells/ml/well. Different concentrations of astragaloside IV (800,
200 and 50 µg/ml) prepared in serum-free medium were added. A
negative control group was included. The following day, when the
cells were ~60% confluent, the monolayer was scratched using a
sterile pipette tip. Nonadherent cells were removed by a PBS wash,
the culture medium was replace, and the plate was incubated at 37°C
for 24 h. Subsequently, the plate was removed from the incubated,
images were acquired using a light microscope (Olympus IX51;
Olympus Corporation, Tokyo, Japan; magnification, ×100), and cell
migration was measured (Olympus IX51; Cellsens, cmactlicense 1.19;
Olympus Corporation).
Transwell invasion assay
Cells in the logarithmic phase were harvested and
seeded in a 6-well plate at a density of 5×105
cells/ml/well. The following day, when the cells were adherent,
culture medium containing different concentrations of astragaloside
IV was added (800, 200 and 50 µg/ml). A negative control group was
included. Matrigel was thawed at 4°C overnight and diluted 1:2
using serum-free medium. In the upper chamber of the Transwell
insert, 30 µl diluted Matrigel was added and at 37°C for 120 min so
that the Matrigel matrix would form a gel. The cell density was
adjusted to 5×105 cells/ml using serum-free medium. A
total of 100 µl of the cell suspension was added to the Transwell
chamber, and into the lower chamber, 500 µl culture medium
containing 20% fetal bovine serum (KeyGen Biotech Co., Ltd.) was
added. The 24-well plate was cultured at 37°C in a 5%
CO2 incubator for 24 h. The Matrigel and the nonmigrated
cells in the upper chamber were removed using a cotton swab. The
Transwell inserts were removed and inverted for air-drying for 2 h.
In a 24-well plate, 500 µl culture medium containing 0.1% crystal
violet was added. The Transwell inserts were placed in the culture
medium, with the membrane immersed in the stain, at 37°C for 2 h.
After 30 min, the membrane was removed and washed with PBS. Along
the diameter of the insert, three fields of view (FOVs) were
randomly selected, and images were acquired (Olympus IX51;
magnification, ×200). The number of cells in each FOV was
counted.
Immunocytochemical detection of
proteins
Cells (5×104 cells/ml) were air dried,
fixed in 4% paraformaldehyde for 30 min and washed with PBS three
times for 3 min each time. Culture medium containing different
concentrations of astragaloside IV was added (800, 200 and 50
µg/ml). A negative control group was included. Two drops of a 3%
H2O2-methanol solution were added to each
slide, and the slide was blocked at 20°C for 10 min and then washed
three times with PBS. Next, 75 µl goat serum (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) was added dropwise, and
the cells were incubated at room temperature for 20 min.
Subsequently, the cells were incubated with 75 µl primary antibody.
The primary antibodies used were rabbit anti-human transforming
growth factor-β1 (TGF-β1; cat. no. KG22744-2; dilution, 1:200) and
rabbit anti-human E-cadherin (cat. no. KG22195-2; dilution, 1:200).
Both primary antibodies were obtained from KeyGen Biotech Co., Ltd.
The slides were incubated at 37°C in a wet box for 2 min. After
washing with PBS three times, and the cells were incubated at room
temperature in a wet box for 30 min. Then, the cells were washed
three times with PBS and incubated with 50 µl peroxidase-conjugated
AffiniPure goat anti-rabbit IgG (cat. no. KGAA35; KeyGen Biotech
Co., Ltd.) for 30 min at 37°C, followed by three PBS washes. Then,
two drops of freshly prepared DAB substrate were added to each
slide. After counterstaining with hematoxylin staining solution
(cat. no. D005; Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) for 10 min at 25°C, the slides were sealed, and
four areas with high expression were selected during the microscopy
evaluation (Olympus IX51) to determine protein expression. Three
slides were evaluated for each condition. Image Pro Plus 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) was used to calculate the
integral OD (IOD) over the area of the FOV. The mean density was
calculated as the IOD divided by the area of the FOV.
Western blot analysis
Cells in the logarithmic growth phase were
trypsinized and seeded in a 6-well plate at a density of
5×105 cells/ml/well. The following day, when the cells
were adherent, cell culture medium containing different
concentrations of astragaloside IV was added (800, 200 and 50
µg/ml). A negative control group was included. For each group, 200
µl precooled lysis buffer [20 nM Tris (pH 7.5), 150 mM NaCl,
Triton-X-100; cat. no. KGP701; KeyGen Biotech, Co., Ltd] was added
to the cells and mixed, and the cell mixture was placed in an ice
bath for 30 min. After routine vortex rotation, the cells were
centrifuged for 15 min at 21,912.8 × g and 4°C. The supernatants
were collected, and the protein concentrations were determined
using the bicinchoninic acid method. Proteins were separated by
SDS-PAGE on 10% gels (30 µg per lane) and transferred to
polyvinylidene difluoride membranes, which were incubated overnight
at 4°C with 5% nonfat milk. Next, the membranes were incubated
overnight with primary antibodies at 4°C. The following primary
antibodies were used: Rabbit anti-human E-cadherin (cat. no.
KG22195-2; dilution, 1:200); rabbit anti-human N-cadherin (cat. no.
KG22194; dilution, 1:200); rabbit anti-human Vimentin (cat. no.
KG22794; dilution, 1:200); rabbit anti-human phosphatidylinositol 3
kinase (PI3K; cat. no. KG22639; dilution, 1:500); rabbit anti-human
phosphorylated (p)-PI3K (cat. no. KG22638-2; dilution, 1:500);
rabbit anti-human protein kinase B (Akt; cat. no. KG21502;
dilution, 1:200); rabbit anti-human p-Akt (cat. no. KG11054-2;
dilution, 1:200); rabbit anti-human mammalian target of rapamycin
(mTOR; Ser 2448; cat. no. KGYT2914-7; dilution, 1:200); rabbit
anti-human p-mTOR (cat. no. KGYP0176-6; dilution, 1:200); rabbit
anti-human extracellular signaling-regulated kinase (ERK) 1/2 (cat.
no. KG30107-2; dilution, 1:200); rabbit anti-human c-Jun N-terminal
kinase (JNK) 1/2 (cat. no. KG22481-2; dilution, 1:200); rabbit
anti-human P38 (cat. no. KG30244-2; dilution, 1:200); rabbit
anti-human p-ERK1/2 (cat. no. KG30246-2; dilution, 1:200); rabbit
anti-human p-JNK1/2 (cat. no. KG11504-2; dilution, 1:200); rabbit
anti-human p-P38 (cat. no. KG11253-2; dilution, 1:200); and
anti-GAPDH (cat. no. KGAA002-2; dilution, 1:200). All primary
antibodies were obtained from KeyGen Biotech Co., Ltd. The
membranes were washed three times with Tris-buffered saline-Tween
(TBST) for 10 min each time and incubated with a horseradish
peroxidase-conjugated goat anti-rabbit antibody (1:4,000; cat. no.
KGAA35; KeyGen Biotech Co., Ltd.) for 1 h at 37°C. In each of the
above steps, the membrane was washed with TBST three times for 10
min each time. Images were obtained using a gel imaging system. The
protein bands were visualized using ECL detection reagents (cat.
no. KGP1201; KeyGen Biotech Co., Ltd.) Gray-scale analysis was
performed using Gel-Pro32 software (version V4.4.0.36; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Fluorescence reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA extraction was performed with SiHa cells
in the logarithmic phase. A sample of 5 µl RNA was diluted in 495
µl 1X TE buffer, and the concentration and purity of the RNA were
determined according to its absorption values at 260 and 280 nm.
The extracted RNA was reverse transcribed into cDNA using a First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) using
the following incubation conditions: 25°C for 5 min, 42°C for 60
min, and on ice for 5 min. Next, qPCR was performed using a
fluorescence qPCR instrument (ABI StepOne Plus; Thermo Fisher
Scientific, Inc.). All primers were synthesized by GenScript
(Nanjing, China) and had the following sequences: GAPDH, sense
5′-AGATCATCAGCAATGCCTCCT-3′, antisense 5′-TGAGTCCTTCCACGATACCAA-3′
(90 bp); E-cadherin, sense 5′-CAAGCAGCAGTACATTCTACA-3′ and
antisense 5′-CATTCACATCCAGCACATCCA-3′ (108 bp). GAPDH was used as
an internal reference gene. The PCR conditions were as follows:
Pre-denaturation at 95°C for 5 min, followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing/extension at 60°C for
20 sec, and denaturation at 72°C for 40 sec. At the end of each
run, a melting curve was plotted to verify the specificity of the
amplification product. Both the amplification and dissolution
curves were analyzed using Rotor-Gene Real-Time Analysis Software
6.1 (Qiagen GmbH, Hilden, Germany), and the ∆∆Cq method was used
for analysis (24). The expression
of target genes was normalized to the expression of GAPDH (ABI
StepOne version 2.3; Applied Biosystems; Thermo Fisher Scientific,
Inc.).
In vivo imaging of tumor formation in
nude mice using fluorescent probes
BABLc/nude mice were purchased from Shanghai
Lingchang Biotechnology Co., Ltd. (Shanghai China). All animals
were housed in a pathogen-free environment at 23°C with 40–70%
humidity and fed ad libitum. As 12 h light/dark cycle was
used. The procedures for care and use of animals were approved by
the Ethics Committee of the Jiangsu Health Vocational College
(approval no. IACUC-201702) and all applicable institutional and
governmental regulations concerning the ethical use of animals were
followed. SiHa cells in the logarithmic phase were harvested, and
single cell suspensions were prepared at a density of
1×107 cells/ml. The cells were injected subcutaneously
into the right armpit at a volume of 0.1 ml per mouse. The location
of the tumors did not interfere with the normal activities of the
nude mice or impair their wellbeing. The nude mice were randomly
divided into four groups (n=4 mice per group): Control group,
cisplatin group, astragaloside IV group and astragaloside IV plus
cisplatin group. For the astragaloside IV group, astragaloside IV
was administered by gastric lavage at a daily dose of 120 mg/kg.
The control group was given an equal volume of 5% sodium
carboxymethyl cellulose, whereas the cisplatin group received an
intraperitoneal injection of 2 mg/kg cisplatin. Moreover, the
combined treatment group received drug administration for 21
consecutive days. Biological probes, synthesized by fluorescence
probe Cy 5.5 labeled epidermal growth factor, was dissolved in
DMSO, prepared at a concentration of 1 mg/ml and stored at −20°C.
At 2, 4 and 6 weeks after the tumor was formed, 5 µl Cy5.5 stock
solution was diluted with DMSO to 0.2 ml and injected into each
mouse. At 10 min after injection of the fluorescent probe, the nude
mice were anesthetized, and in vivo imaging was performed at
an excitation wavelength of 680 nm (IVIS® Lumina LT
Series III; PerkinElmer, Inc., Waltham, MA, USA). The experiment
was terminated ENT when the volume of the tumor reached 2,000
mm3. The longest diameter of a single subcutaneous tumor
was 1,954 mm, radial width was 1,425 mm and the tumor volume was
1,984 mm3. Tumor volume =1/2×diameterxradial
width2.
Statistical analysis
All data are presented as the mean ± standard
deviation (n=3). Statistical analyses were performed with SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). The
statistical comparisons between two groups were performed by
unpaired Student's t-test and multigroup comparisons were conducted
by using one-way analysis followed by the Tukey's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
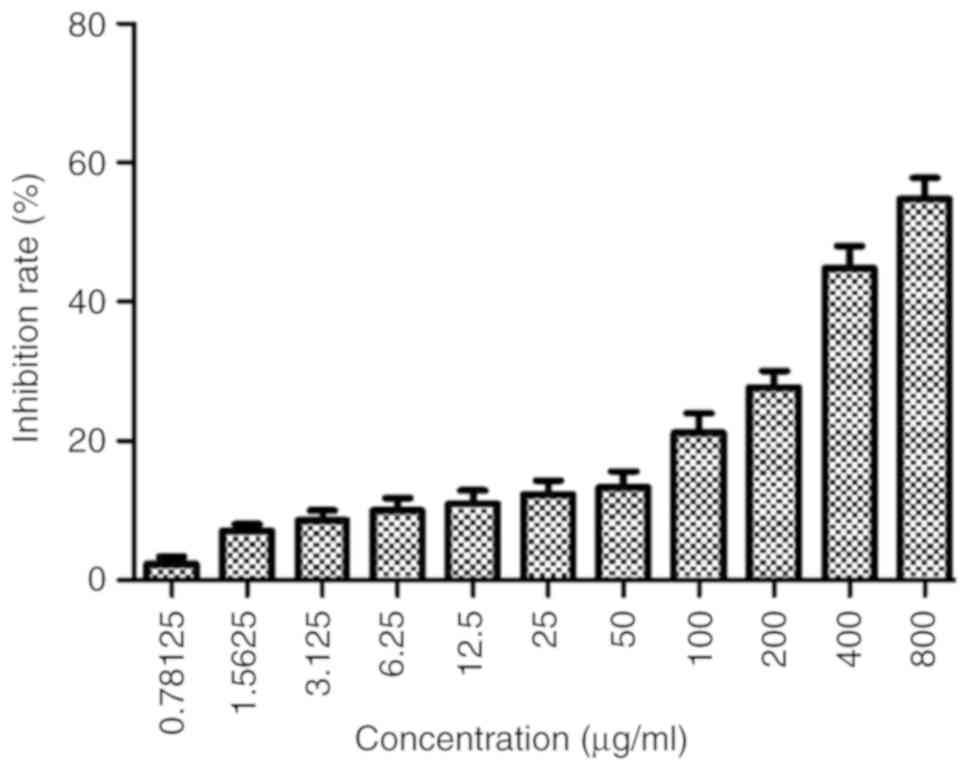
Astragaloside IV inhibits the
proliferation of SiHa cells
SiHa cells were treated with different
concentrations of astragaloside IV (800, 400, 200, 100, 50, 25,
12.5, 6.25, 3.125, 1.5625 or 0.78125 µg/ml) for 24 h (Fig. 1). The data indicated that
astragaloside IV inhibited the proliferation of the cells in a
dose-dependent manner. The IC50 value at 24 h was 628.28
µg/ml.
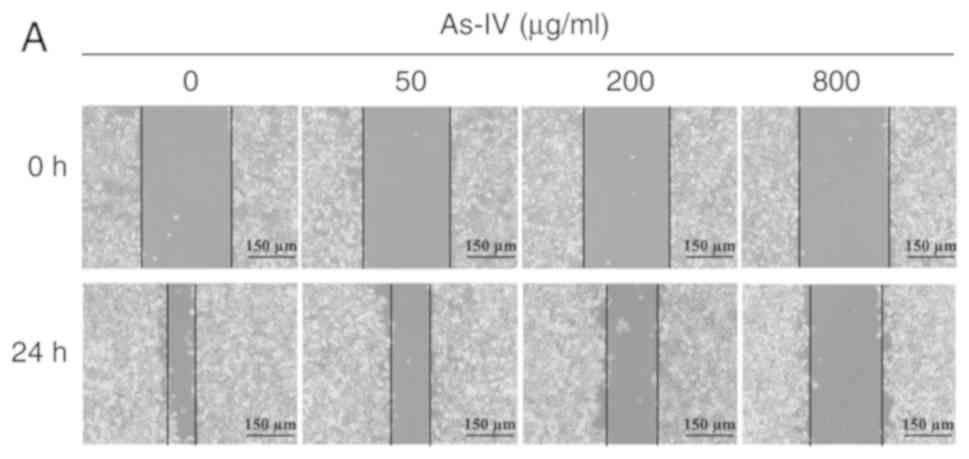
Inhibitory effects of different
concentrations of astragaloside IV on the migration of SiHa
cells
The wound healing assay is one of the most commonly
used methods to determine the migratory capacity of cells in
vitro. A monolayer of SiHa cells was scratched in vitro,
and astragaloside IV was added to determine the inhibitory effect
on the migratory capacity of SiHa tumor cells. SiHa cells in the
control group maintained the original migratory capacity, and after
treatment for 24 h, the wound distance was 96.2±4.44 µm. After
treatment with different doses of astragaloside IV (50, 200 and 800
µg/ml), the wound distance increased to 159±3.87, 238.2±5.89 and
296.8±6.18 µm, respectively, and these distances were significantly
different from the wound distance of the control (P<0.05;
Fig. 2). Thus, astragaloside IV
inhibited the migratory capacity of SiHa cells.
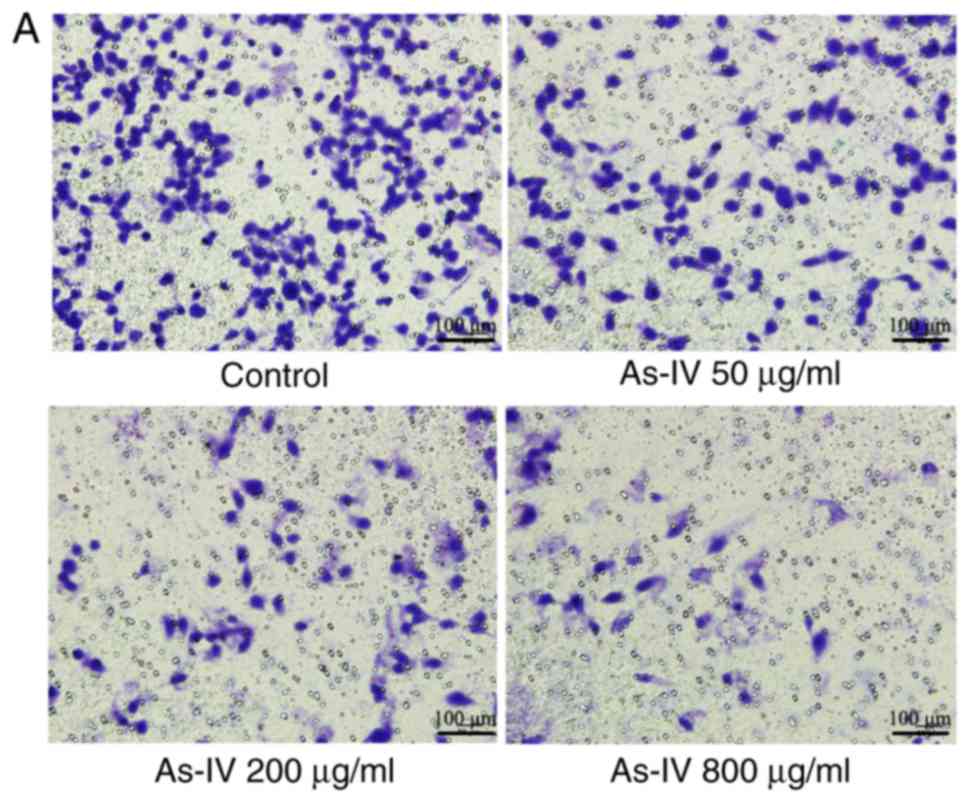
Effect of astragaloside IV on the
invasive capacity of SiHa cells
The Transwell assay, which measures the number of
cells migrating to the lower chamber of the Transwell insert, is a
classic way to determine the invasive capacity of cells. Fig. 3 demonstrated that the number of
cells migrating to the lower chamber was lower following treatment
with different concentrations of astragaloside IV than in the
control treatment group. In the control group, the number of cells
that migrated to the lower chamber was 246.6±8.56. However, after
treatment with astragaloside IV (50, 200 and 800 µg/ml) for 24 h,
the number of cells migrating to the lower chamber was 119.2±10.4,
64.2±5.07, and 24.8±8.7, respectively, which indicated that
compared with the control, astragaloside IV had a significant
inhibitory effect (P<0.05). Astragaloside IV demonstrated a
dose-dependent inhibitory effect on the invasive capacity of SiHa
cells.
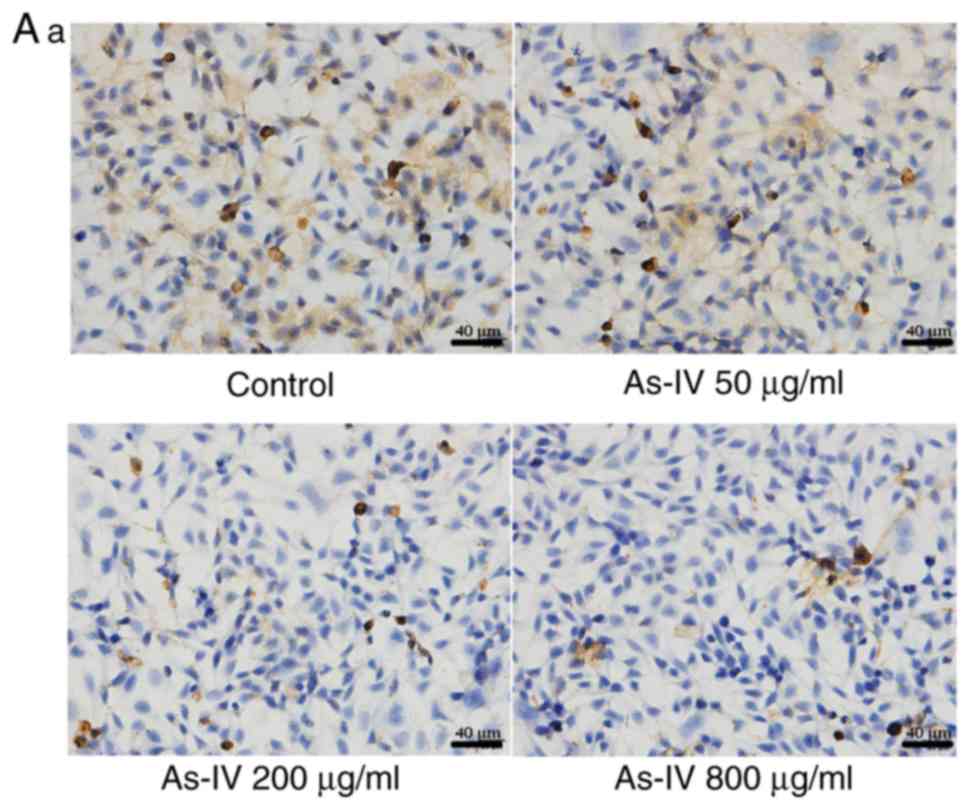
Effect of astragaloside IV on the
expression of TGF-β1 and E-cadherin
TGF-β1 and E-cadherin are key markers of EMT. As
shown in Fig. 4, the expression of
TGF-β1 was highest in the control group and decreased after
astragaloside IV treatment. Furthermore, the expression of
E-cadherin was lowest in the control group, suggesting that EMT may
have occurred in the SiHa cells. After astragaloside IV treatment,
the expression of E-cadherin increased. These results indicated
that the inhibitory effect of astragaloside IV on the migratory and
invasive capacities of SiHa cancer cells was associated with both
proteins.
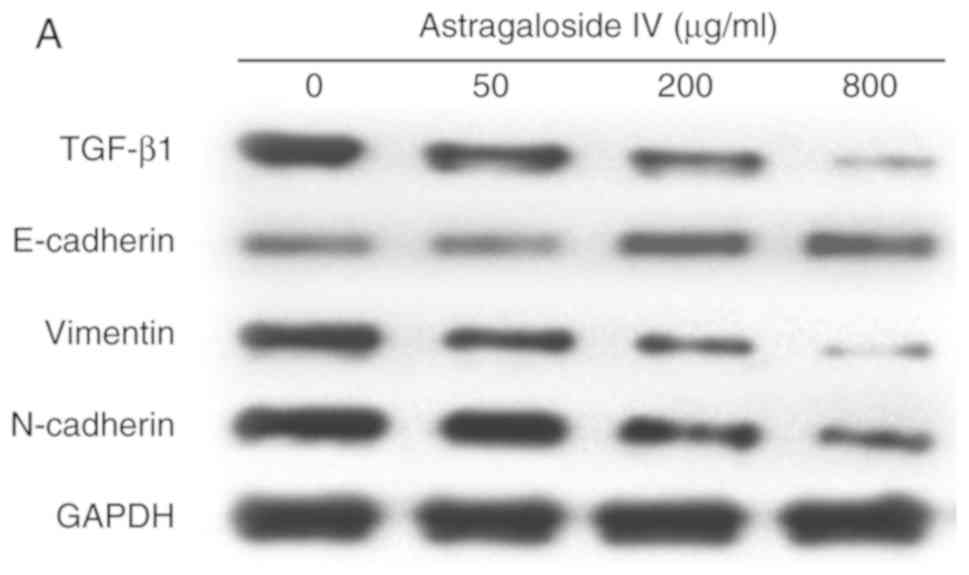
Astragaloside IV downregulates the
expression of TGF-β1, N-cadherin and Vimentin and upregulates the
expression of E-cadherin in SiHa cells
To further determine the effects of astragaloside IV
on the expression of TGF-β1, N-cadherin, Vimentin and E-cadherin,
the expression levels of the four proteins were evaluated by
western blot analysis following astragaloside IV treatment. As
shown in Fig. 5, TGF-β1, N-cadherin
and Vimentin expressions were highest in the control group and
decreased following astragaloside IV treatment. Furthermore,
E-cadherin expression was lowest in the control group and increased
in a dose-dependent manner following astragaloside IV treatment.
These data suggested that astragaloside IV inhibited the invasive
and migratory capacities of SiHa cells by downregulating TGF-β1,
N-cadherin and Vimentin expression, and upregulating E-cadherin
expression.
Astragaloside IV inhibits the
mitogen-activated protein kinase (MAPK) and PI3K signaling pathways
in SiHa cells
The MAPK and PI3K signaling pathways are associated
with the invasion and migration of SiHa cancer cells. The effect of
astragaloside IV on these two pathways was assessed using western
blot analysis. As shown in Fig. 6,
astragaloside IV inhibited the phosphorylation of P38 in the MAPK
signaling pathway, and phosphorylation of PI3K, Akt and mTOR in the
PI3K signaling pathway in a dose-dependent manner. However,
astragaloside IV had no impact on phosphorylation or expression of
other proteins. Therefore, astragaloside IV inhibited certain
mediators of the MAPK and PI3K pathways.
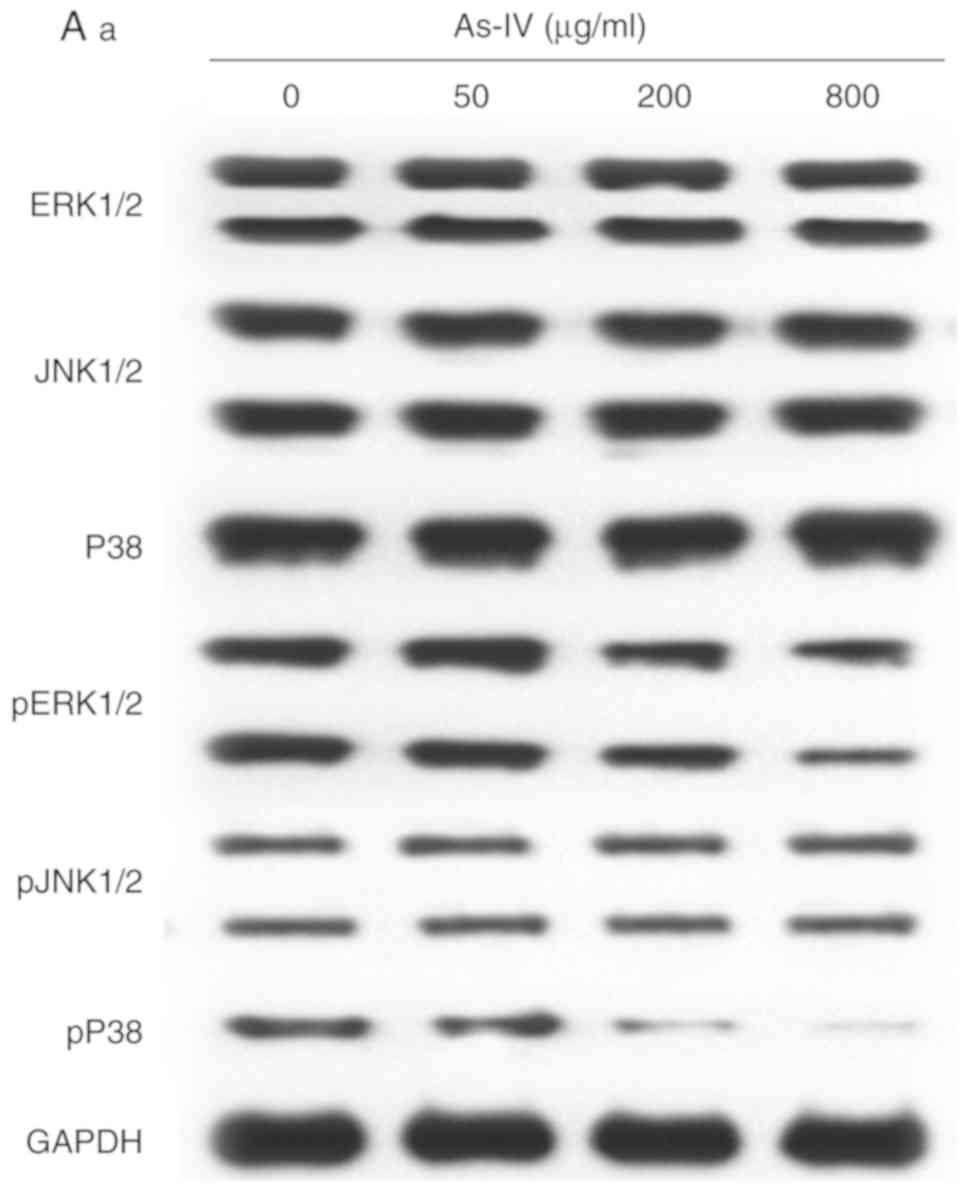 | Figure 6.Effects of astragaloside IV treatment
on the expression of ERK1/2, JNK1/2, P38, PI3K, AKT, mTOR and their
phosphorylated forms. (Aa) ERK1/2, JNK1/2 and P38 expression
determined by western blot analysis. (Ab) Densitometry analysis of
total ERK1/2, JNK1/2 and P38 relative to GAPDH. (Ac) Densitometry
analysis of phosphorylated ERK1/2, JNK1/2 and P38 relative to total
ERK1/2, JNK1/2 and P38. (Ba) PI3K, AKT and mTOR expression
determined by western blot analysis. (Bb) Densitometry analysis of
total PI3K, AKT and mTOR relative to GAPDH, and phosphorylated
PI3K, AKT and mTOR relative to total PI3K, AKT and mTOR. As-IV,
astragaloside IV; ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; p, phosphorylated; PI3K,
phosphatidylinositol 3 kinase; Akt, protein kinase B; mTOR,
mammalian target of rapamycin. *P<0.05, **P<0.01 vs. 0
µg/ml. |
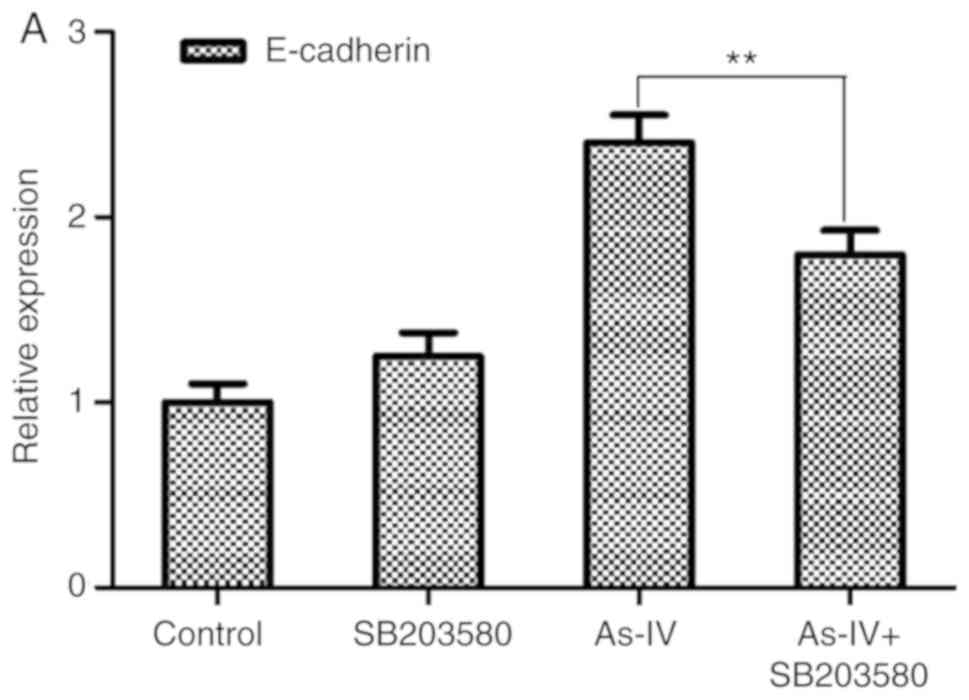
Astragaloside IV inhibits the
initiation of EMT in SiHa cells via the MAPK and PI3K signaling
pathways
The mRNA expression of E-cadherin was determined
after the combined use of astragaloside IV, the P38 MAPK inhibitor,
SB203580, and the PI3K inhibitor, LY294002. Compared with the
control treatment, the use of either inhibitor alone did not
significantly change the expression of E-cadherin (P>0.05). By
contrast, combining the pathway inhibitors significantly reduced
the effect of astragaloside IV on E-cadherin (P<0.05; Fig. 7). These data suggested that
treatment with astragaloside IV inhibited EMT in SiHa cells by
inhibiting the MAPK and PI3K signaling pathways.
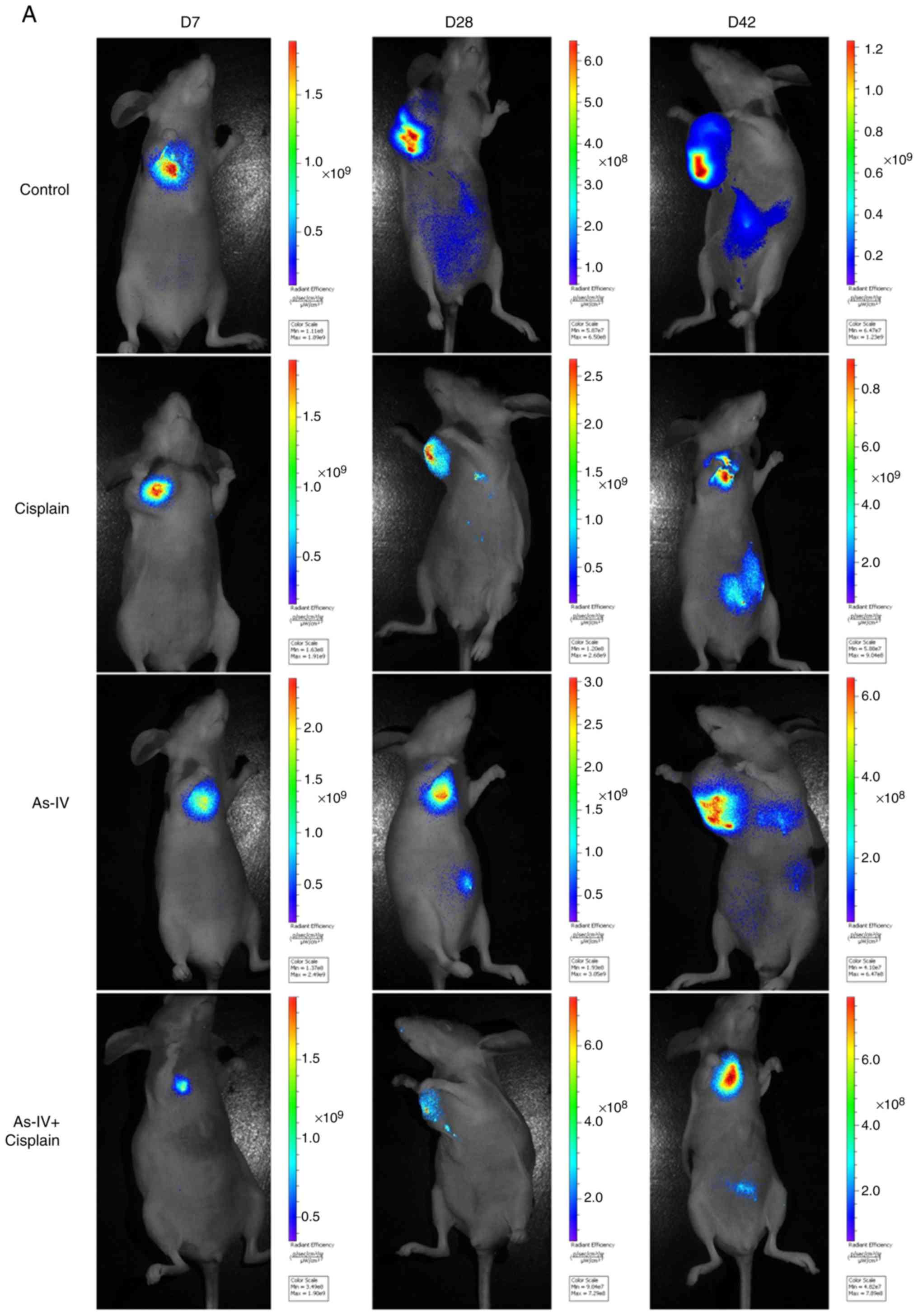
Astragaloside IV inhibits transplanted
tumor metastasis in nude mice
The findings demonstrated that compared with control
treatment, astragaloside IV treatment inhibited tumor growth and
migration. As shown in Fig. 8,
metastasis occurred within 2 weeks in the control group and was
more severe in week 6. In the cisplatin group, tumor growth was
effectively inhibited; however, extensive metastasis occurred by
week 6. Tumor metastasis was decreased in the astragaloside IV
group, and only mild metastasis occurred in week 4. Furthermore,
the tumor growth rate and degree of metastasis were lower in the
combined treatment group than the control group. These results
indicated that treatment with astragaloside IV inhibited tumor
migration and invasion in vivo and in vitro. In
addition, the treatment effect was strongest after combined
treatment with astragaloside IV and cisplatin.
Discussion
The inhibitory effect of astragaloside IV on SiHa
cells was assessed using an MTT assay, and the IC50
value at 24 h was calculated to be 628.28 µg/ml. These findings
indicated that astragaloside IV treatment exerted a significant
inhibitory effect on SiHa cells in a dose-dependent manner. To
determine whether astragaloside IV had an effect on the migratory
and invasive capacities of SiHa cells, wound healing and Transwell
assays were performed. The wound healing assay, which was used to
determine the migratory capacity of the SiHa tumor cells, is based
on the fact that tumor cells possess the capacity for migration
in vitro (25). Using
Transwell assays, the invasive capacity of the tumor cells was
determined by counting the number of cells penetrating the Matrigel
(26). Both assays are commonly
used to determine the inhibitory effects of therapeutic compounds
on the migratory and invasive capacities of tumor cells (27). The migratory and invasive capacities
of SiHa cells were decreased to varying extents after 24 h of
treatment with astragaloside IV.
The major causes of cervical cancer include
activation of oncogenes, inactivation of tumor suppressor genes,
increased telomerase activity, and increased production of TGF-β
(28). TGF-β is a multifunctional
polypeptide molecule with the highest content of TGF-β1. TGF-β1 is
mainly expressed in endothelial cells, hematopoietic cells and
connective tissue cells (29,30).
TGF-β1 is a tumor suppressor in normal epithelial cells and in the
early stage of tumor development. However, in advanced tumor
stages, there is a selective loss of the tumor suppressor function
of TGF-β1, which then has a role in mediating tumor cell
proliferation, infiltration, and metastasis (31). During tumor proliferation, high
levels of TGF-β1 are associated with invasiveness (32). Furthermore, TGF-β1 is an important
cytokine in the tumor microenvironment, and several studies have
reported that TGF-β1 is involved in tumor EMT and promotes tumor
metastasis (22,33–37).
Tumor cells undergo EMT following stimulation with TGF-β1,
resulting in the transition from the paving stone-like morphology
of epithelial cells to the spindle-shaped morphology of mesenchymal
cells. E-cadherin has an important regulatory role in the adhesion
between epithelial cells (38).
Previous studies have reported that E-cadherin prevented the
invasion and metastasis of histiocytes by stabilizing intercellular
junctions (39). In fact, upon loss
of the E-cadherin gene, epithelial cells undergo EMT in several
epithelial-cell-derived cancers. This loss further enhances the
invasive and migratory capacities of tumor cells (40–43).
N-cadherin is a cell adhesion molecule. It participates in cell
adhesion and maintains the stability of tissue structures and
morphology (44). Upregulation of
N-cadherin expression in malignant epithelial tumors can reduce
cell adhesion and promote the initiation of EMT (45). Vimentin is a marker of interstitial
cells and an important component of the cytoskeleton. Increased
abnormal expression of cytoskeleton proteins alters the composition
of the cytoskeleton protein pool (46). This change makes it easier for
cancer cells to migrate and invade (47).
In the current study, the expression of TGF-β1 and
E-cadherin, proteins associated with EMT, were determined in SiHa
cells by immunocytochemistry following astragaloside IV treatment.
The staining results indicated that treatment with astragaloside IV
downregulated TGF-β1 levels and upregulated E-cadherin levels in
SiHa cells. Western blot analysis was used to determine the
specific expression of the two proteins. The denistometry analysis
results were in line with the immunocytochemistry results,
indicating that astragaloside IV treatment inhibited the invasive
and migratory capacities of SiHa cells by downregulating TGF-β1
expression and upregulating E-cadherin expression, resulting in a
reduction in EMT. Astragaloside IV can also downregulate the levels
of N-cadherin and Vimentin protein in cells. These results
indicated that astragaloside IV can inhibit the initiation of
EMT.
Molecular biology studies have revealed that the
MAPK and PI3K signaling pathways are two important pathways in the
production of TGF-β1, and these pathways include the
TGF-β1-mediated Smad-independent pathways (48). MAPKs are a serine/threonine protein
kinase family widely found in mammals. The MAPK pathway is involved
in regulating cell proliferation and differentiation, and includes
the ERK, JNK and p38 proteins. The kinases of this pathway can be
activated by various factors (49).
ERK1/2 is an important member of the MAPK family, and continuous
activation of ERK1/2 following cellular stimulation promotes cell
proliferation and malignant transformation. ERK can be activated by
numerous factors, including TGF-β1 and Ras. Activation of the Ras
signaling pathway promotes TGF-β1-induced EMT (50). JNK1/2 is expressed in nearly all
cells in the human body. JNK can be activated by several cellular
factors and oxidative injury, and ultimately acts on the caspase
family via the mitochondrial pathway, thus leading to cell
apoptosis. Furthermore, it has been reported that TGF-β1 interacts
with JNK, which may induce EMT (51). TGF-β1-activated kinase 1 (TAK1) is
an important MAPK kinase kinase in the TGF-β1-activated p38MAPK
pathway. TGF-β1 activates TAK1 through the catalytic activity of
TNF receptor-associated factor 6. Additionally, TAK1 can activate
p38, thus inducing EMT (52).
Furthermore, the PI3K/Akt/mTOR pathway is involved in TGF-β1 signal
transduction. TGF-β11 and TGF-β12 are both involved in the
activation of the PI3K pathway (53), and the induction of the TGF-β1
receptor is suppressed by treatment with a PI3K inhibitor.
Treatment with a TGF-β1 inhibitor results in weakening of the
TGF-β1-mediated activation of PI3K and its effector molecule, Akt
(54,55). Taken together, these results
indicated that TGF-β1 is associated with MAPK and PI3K signaling
pathways.
In the present study, western blot analysis
indicated that treatment with astragaloside IV had an impact on
pP38 in the MAPK signaling pathway; however, other proteins were
not affected. Regarding the PI3K pathway, astragaloside IV
treatment inhibited phosphorylation of PI3K, AKT, and mTOR to
varying degrees. Thus, astragaloside IV inhibited both the MAPK and
PI3K signaling pathways.
The MAPK and PI3K signaling mediators are widely
present in the cytoplasm and nucleus of mammalian cells, and are
involved in various physiological and pathological processes,
including proliferation, autophagy, EMT and apoptosis of tumor
cells (56–61). In the current study, treatment with
astragaloside IV inhibited cervical cancer cells through these two
pathways; however, whether this effect is associated with EMT
remains unknown. To identify the therapeutic targets involved,
inhibitors of the pathways were used, and the mRNA expression of
E-cadherin, an EMT marker, was determined by RT-qPCR. The data
revealed that E-cadherin expression was downregulated to varying
extents after combination treatment with astragaloside IV and the
pathway inhibitors. Based on these findings, it is inferred that
both pathways are targets of the inhibitory effect of astragaloside
IV on EMT in cervical cancer cells. However, a limitation of this
study was that only a single cell line was used, and that further
studies using other cell lines are required.
In this study, in vitro experiments
demonstrated that astragaloside IV treatment inhibited EMT by
inhibiting TGF-β1, thus exerting an inhibitory effect on the
invasion and migration of cervical cancer cells. However, plant
extracts are affected by several factors inside the human body,
including absorption, distribution, metabolism and secretion. To
determine the in vivo effect of astragaloside IV treatment,
tumor transplantation experiments with nude mice were performed.
Tumor growth and metastasis were visualized by in vivo
imaging, which demonstrated that treatment with astragaloside IV
alone inhibited the metastatic ability of the cervical cancer
cells. Notably, compared with the treatment with cisplatin alone,
the combined use of astragaloside IV with cisplatin effectively
prevented the metastasis of the cervical cancer cells.
In conclusion, astragaloside IV treatment reduced
the invasive and migratory capacities of cervical cancer cells
in vitro by inhibiting pP38 in the MAPK signaling pathway,
and PI3K in the PI3K signaling pathway, while downregulating TGF-β1
expression. These effects led to an increased level of the EMT
marker, E-cadherin. Antitumor effects of astragaloside IV treatment
were observed in vivo. Taken together, astragaloside IV is a
natural active ingredient with low toxicity, and its combined use
with cisplatin effectively prevented tumor growth and
metastasis.
Acknowledgements
Not applicable.
Funding
The present study was supported by Key medical
talent project in Jiangsu province (grant no. QNRC2016529) and
Scientific Research Team of Jiangsu Health Vocational College
(grant no. JKKYTD201703).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CN supervised and directed this study. LZ performed
the majority of the experiments. LZ and CN contributed to the
conception and design of the experiments. LZ and JZ contributed to
the cell culture and RNA extraction. XQ and HH helped with
interpretation of data. JZ analyzed the data and LZ wrote this
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animals were housed in a pathogen-free
environment and fed ad libitum. The procedures for care and
use of animals were approved by the Ethics Committee of the Jiangsu
Health Vocational College (approval no. IACUC-201702) and all
applicable institutional and governmental regulations concerning
the ethical use of animals were followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thaxton L and Waxman AG: Cervical cancer
prevention: Immunization and screening 2015. Med Clin North Am.
99:469–477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dappa E, Elger T, Hasenburg A, Düber C,
Battista MJ and Hötker AM: The value of advanced MRI techniques in
the assessment of cervical cancer: A review. Insights Imaging.
8:471–481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campos NG, Burger EA, Sy S, Sharma M,
Schiffman M, Rodriguez AC, Hildesheim A, Herrero R and Kim JJ: An
updated natural history model of cervical cancer: Derivation of
model parameters. Am J Epidemiol. 180:545–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Phillips P and Phillips J: Hysterectomy
with radiotherapy or chemotherapy or both for women with locally
advanced cervical cancer. Clin Nurse Spec. 31:189–190. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ly A, Cheng HH and Alwan L: Hepatitis C
infection and chemotherapy toxicity. J Oncol Pharm Pract.
25:474–480. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YS, Tian J, Han Y, Han SM and Shi SB:
Gemcitabine plus vinorelbine as second-line therapy in patients
with metastatic esophageal cancer previously treated with
platinum-based chemotherapy. Oncol Res. 24:129–135. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majithia N, Temkin SM, Ruddy KJ, Beutler
AS, Hershman DL and Loprinzi CL: National cancer
institute-supported chemotherapy-induced peripheral neuropathy
trials: Outcomes and lessons. Support Care Cancer. 24:1439–1447.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Momtazi-Borojeni AA, Ghasemi F, Hesari A,
Majeed M, Caraglia M and Sahebkar A: Anti-cancer and
radio-sensitizing effects of curcumin in nasopharyngeal carcinoma.
Curr Pharm Des. 24:2121–2128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qing LS, Peng SL, Liang J and Ding LS:
Astragalosidic acid: A new water-soluble derivative of
astragaloside IV prepared using remarkably simple TEMPO-mediated
oxidation. Molecules. 22:E12752017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu WN, Sun LF and Yang H: Inhibitory
effects of astragaloside IV on bleomycin-induced pulmonary fibrosis
in rats via attenuation of oxidative stress and inflammation.
Inflammation. 39:1835–1841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Hou X, Xu R, Liu C and Tu M:
Research review on the pharmacological effects of astragaloside IV.
Fundam Clin Pharmacol. 31:17–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou X, Sun X, Gong X, Yang Y, Chen C,
Shan G and Yao Q: Astragaloside IV from Astragalus membranaceus
ameliorates renal interstitial fibrosis by inhibiting inflammation
via TLR4/NF-кB in vivo and in vitro. Int Immunopharmacol. 42:18–24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu L, Yin G, Cheng L, Fan Y, Xiao W, Yu
G, Xing M, Jia R, Sun R, Ma X, et al: Astragaloside IV ameliorates
acute pancreatitis in rats by inhibiting the activation of nuclear
factor-κB. Int J Mol Med. 35:625–636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai H, Jia G, Lu M, Liang C, Wang Y and
Wang H: Astragaloside IV inhibits isoprenaline-induced cardiac
fibrosis by targeting the reactive oxygen species/mitogen-activated
protein kinase signaling axis. Mol Med Rep. 15:1765–1770. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang HL, Zhou QH, Xu MB, Zhou XL and Zheng
GQ: Astragaloside IV for experimental focal cerebral ischemia:
Preclinical evidence and possible mechanisms. Oxid Med Cell Longev.
2017:84243262017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Li Z, He W, Xu L, Wang J, Shi J
and Sheng M: Effects of astragaloside IV against the
TGF-β11-induced epithelial-to-mesenchymal transition in peritoneal
mesothelial cells by promoting Smad 7 expression. Cell Physiol
Biochem. 37:43–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai PC, Liu DL, Zhang L, Ye J, Wang Q,
Zhang HW, Lin XH and Lai GX: Astragaloside IV sensitizes non-small
cell lung cancer cells to gefitinib potentially via regulation of
SIRT6. Tumour Biol. 39:10104283176975552017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye Q, Su L, Chen D, Zheng W and Liu Y:
Astragaloside IV induced miR-134 expression reduces EMT and
increases chemotherapeutic sensitivity by suppressing CREB1
signaling in colorectal cancer cell line SW-480. Cell Physiol
Biochem. 43:1617–1626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B, Wang F, Liu N, Shen W and Huang T:
Astragaloside IV inhibits progression of glioma via blocking
MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 491:98–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang S, Tang D, Zang W, Yin G, Dai J, Sun
YU, Yang Z, Hoffman RM and Guo X: Synergistic inhibitory effect of
traditional chinese medicine astragaloside IV and curcumin on tumor
growth and angiogenesis in an orthotopic nude-mouse model of human
hepatocellular carcinoma. Anticancer Res. 37:465–473. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang G, Fang X, Yang Y and Song Y:
Silencing of CEMIP suppresses Wnt/β-catenin/Snail signaling
transduction and inhibits EMT program of colorectal cancer cells.
Acta Histochem. 120:56–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zong W, Yu C, Wang P and Dong L:
Overexpression of SASH1 inhibits TGF-β11 induced EMT in gastric
cancer cells. Oncol Res. 24:17–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greenburg G and Hay ED: Epithelia
suspended in collagen gels can lose polarity and express
characteristics of migrating mesenchymal cells. J Cell Biol.
95:333–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gough W, Hulkower KI, Lynch R, McGlynn P,
Uhlik M, Yan L and Lee JA: A quantitative, facile, and
high-throughput image-based cell migration method is a robust
alternative to the scratch assay. J Biomol Screen. 16:155–63. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kovaříková P, Michalova E, Knopfová L and
Bouchal P: Methods for studying tumor cell migration and
invasiveness. Klin Onkol. 27 (Suppl 1):S22–S27. 2014.(In Czech).
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He AD, Wang SP, Xie W, Song W, Miao S,
Yang RP, Zhu Y, Xiang JZ and Ming ZY: Platelet derived TGF-β1
promotes cervical carcinoma cell growth by suppressing KLF6
expression. Oncotarget. 8:87174–87181. 2017.PubMed/NCBI
|
|
29
|
Peng G, Masood K, Gantz O and Sinha U:
Neuromuscular electrical stimulation improves radiation-induced
fibrosis through TGF-β11/MyoD homeostasis in head and neck cancer.
J Surg Oncol. 114:27–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rhee YH, Moon JH, Choi SH and Ahn JC:
Low-level laser therapy promoted aggressive proliferation and
angiogenesis through decreasing of transforming Growth factor-β1
and increasing of Akt/hypoxia inducible factor-1α in anaplastic
thyroid cancer. Photomed Laser Surg. 34:229–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen W, Zhou S, Mao L, Zhang H, Sun D,
Zhang J, Li J and Tang JH: Crosstalk between TGF-β1 signaling and
miRNAs in breast cancer metastasis. Tumour Biol. 37:10011–10019.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin RL and Zhao LJ: Mechanistic basis and
clinical relevance of the role of transforming growth factor-β in
cancer. Cancer Biol Med. 12:385–393. 2015.PubMed/NCBI
|
|
33
|
Ma F, Li W, Liu C, Li W, Yu H, Lei B, Ren
Y, Li Z, Pang D and Qian C: MiR-23a promotes TGF-β11-induced EMT
and tumor metastasis in breast cancer cells by directly targeting
CDH1 and activating Wnt/β-catenin signaling. Oncotarget.
8:69538–69550. 2017.PubMed/NCBI
|
|
34
|
Lim WC, Kim H, Kim YJ, Choi KC, Lee IH,
Lee KH, Kim MK and Ko H: Dioscin suppresses TGF-β11-induced
epithelial-mesenchymal transition and suppresses A549 lung cancer
migration and invasion. Bioorg Med Chem Lett. 27:3342–3348. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mitra T and Roy SS: Co-activation of TGFβ
and Wnt signalling pathways abrogates EMT in ovarian cancer cells.
Cell Physiol Biochem. 41:1336–1345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang JX, Zhai JF, Yang XT and Wang J:
MicroRNA-132 inhibits migration, invasion and epithelial
mesenchymal transition by regulating TGFβ1/Smad2 in human non-small
cell lung cancer. Eur Rev Med Pharmacol Sci. 20:3793–3801.
2016.PubMed/NCBI
|
|
37
|
Zhang S, Sun WY, Wu JJ, Gu YJ and Wei W:
Decreased expression of the type III TGF-β1 receptor enhances
metastasis and invasion in hepatocellullar carcinoma progression.
Oncol Rep. 35:2373–2381. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu L, Fu X, Chen X, Han X and Dong P: M2
macrophages induce EMT through the TGF-β1/Smad2 signaling pathway.
Cell Biol Int. 41:960–968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su S, Lin X, Ding N, Zhang H, Zhang Q,
Ding Y, Hou X and Tian Y: Effects of PARP-1 inhibitor and ERK
inhibitor on epithelial mesenchymal transitions of the ovarian
cancer SKOV3 cells. Pharmacol Rep. 68:1225–1229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matos ML, Lapyckyj L, Rosso M, Besso MJ,
Mencucci MV, Briggiler CI, Giustina S, Furlong LI and Vazquez-Levin
MH: Identification of a novel human E-cadherin splice variant and
assessment of its effects upon EMT-related events. J Cell Physiol.
232:1368–1386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Benzina S, Beauregard AP, Guerrette R,
Jean S, Faye MD, Laflamme M, Maïcas E, Crapoulet N, Ouellette RJ
and Robichaud GA: Pax-5 is a potent regulator of E-cadherin and
breast cancer malignant processes. Oncotarget. 8:12052–12066. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shin J, Song IS, Pak JH and Jang SW:
Upregulation of annexin A1 expression by butyrate in human melanoma
cells induces invasion by inhibiting E-cadherin expression. Tumour
Biol. 37:14577–14584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Z, Bu X, Chen H, Wang Q and Sha W:
Bmi-1 promotes the invasion and migration of colon cancer stem
cells through the downregulation of E-cadherin. Int J Mol Med.
38:1199–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Singh R, Mandhani A, Agrawal V and Garg M:
Positive correlation between matrix metalloproteinases and
epithelial-to-mesenchymal transition and its association with
clinical outcome in bladder cancer patients. Cancer Microenviron.
11:23–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng QM, Chen XY, Bao QF, Yu J and Chen
LH: ILK enhances migration and invasion abilities of human
endometrial stromal cells by facilitating the
epithelial-mesenchymal transition. Gynecol Endocrinol.
34:1091–1096. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang L, Dai F, Liu Y, Yu X, Huang C, Wang
Y and Yao W: RhoA/ROCK signaling regulates smooth muscle phenotypic
modulation and vascular remodeling via the JNK pathway and vimentin
cytoskeleton. Pharmacol Res. 133:201–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Song IH, Kim KR, Lim S, Kim SH and Sung
CO: Expression and prognostic significance of
epithelial-mesenchymal transition-related markers and phenotype in
serous ovarian cancer. Pathol Res Pract. 214:1564–1571. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sokolova O, Kähne T, Bryan K and Naumann
M: Interactome analysis of transforming growth factor-β-activated
kinase 1 in Helicobacter pylori-infected cells revealed novel
regulators tripartite motif 28 and CDC37. Oncotarget.
9:14366–14381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li H, He Q, Meng F, Feng X, Chen J, Li L
and Liu J: Methionine sulfoxide reductase B1 regulates
proliferation and invasion by affecting mitogen-activated protein
kinase pathway and epithelial-mesenchymal transition in u2os cells.
Biochem Biophys Res Commun. 12:806–813. 2018. View Article : Google Scholar
|
|
50
|
Liang Z, Wu R, Xie W, Zhu M, Xie C, Li X,
Zhu J, Zhu W, Wu J, Geng S, et al: Curcumin reverses tobacco
smoke-induced epithelial-mesenchymal transition by suppressing the
MAPK pathway in the lungs of mice. Mol Med Rep. 17:2019–2025.
2018.PubMed/NCBI
|
|
51
|
Zhang C, Liu T, Wang G, Wang H, Che X, Gao
X and Liu H: Rac3 regulates cell invasion, migration and EMT in
lung adenocarcinoma through p38 MAPK pathway. J Cancer.
8:2511–2522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu M, Chen WH, Wang CY, Mao CQ and Wang J:
Reciprocal regulation of miR-1254 and c-Myc in oral squamous cell
carcinoma suppresses EMT-mediated metastasis and tumor-initiating
properties through MAPK signaling. Biochem Biophys Res Commun.
484:801–807. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu Y, Kong F, Zhang C, Ma C, Xia H, Quan
B and Cui H: CD133 mediates the TGFβ1-induced activation of the
PI3K/ERK/P70S6K signaling pathway in gastric cancer cells. Oncol
Lett. 14:7211–7216. 2017.PubMed/NCBI
|
|
54
|
Saito S, Zhuang Y, Shan B, Danchuk S, Luo
F, Korfei M, Guenther A and Lasky JA: Tubastatin ameliorates
pulmonary fibrosis by targeting the TGFβ-PI3K-Akt pathway. PLoS
One. 12:e01866152017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Khan GJ, Gao Y, Gu M, Wang L, Khan S,
Naeem F, Semukunzi H, Roy D, Yuan S and Sun L: TGF-β1 causes EMT by
regulating N-Acetyl glucosaminyl transferases via downregulation of
non muscle myosin II-A through JNK/P38/PI3K pathway in lung cancer.
Curr Cancer Drug Targets. 18:209–219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nie C, Zhou J, Qin X, Shi X, Zeng Q, Liu
J, Yan S and Zhang L: Diosgenin induced autophagy and apoptosis in
a human prostate cancer cell line. Mol Med Rep. 14:4349–4359. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nie C, Zhou J, Qin X, Shi X, Zeng Q, Liu
J, Yan S and Zhang L: Reduction of apoptosis by
proanthocyanidin-induced autophagy in the human gastric cancer cell
line MGC-803. Oncol Rep. 35:649–658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang R, Deng D, Shao N, Xu Y, Xue L, Peng
Y, Liu Y and Zhi F: Evodiamine activates cellular apoptosis through
suppressing PI3K/AKT and activating MAPK in glioma. Onco Targets
Ther. 11:1183–1192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xia D, Tian S, Chen Z, Qin W and Liu Q:
miR302a inhibits the proliferation of esophageal cancer cells
through the MAPK and PI3K/Akt signaling pathways. Oncol Lett.
15:3937–3943. 2018.PubMed/NCBI
|
|
60
|
Dinsmore CJ and Soriano P: MAPK and PI3K
signaling: At the crossroads of neural crest development. Dev Biol.
(pii): S0012-1606(17)30599-7. 2018.PubMed/NCBI
|
|
61
|
Song ZY, Wang F, Cui SX and Qu XJ:
Knockdown of CXCR4 inhibits CXCL12-induced angiogenesis in HUVECs
through downregulation of the MAPK/ERK and PI3K/AKT and the
Wnt/β-catenin pathways. Cancer Invest. 36:10–18. 2018. View Article : Google Scholar : PubMed/NCBI
|