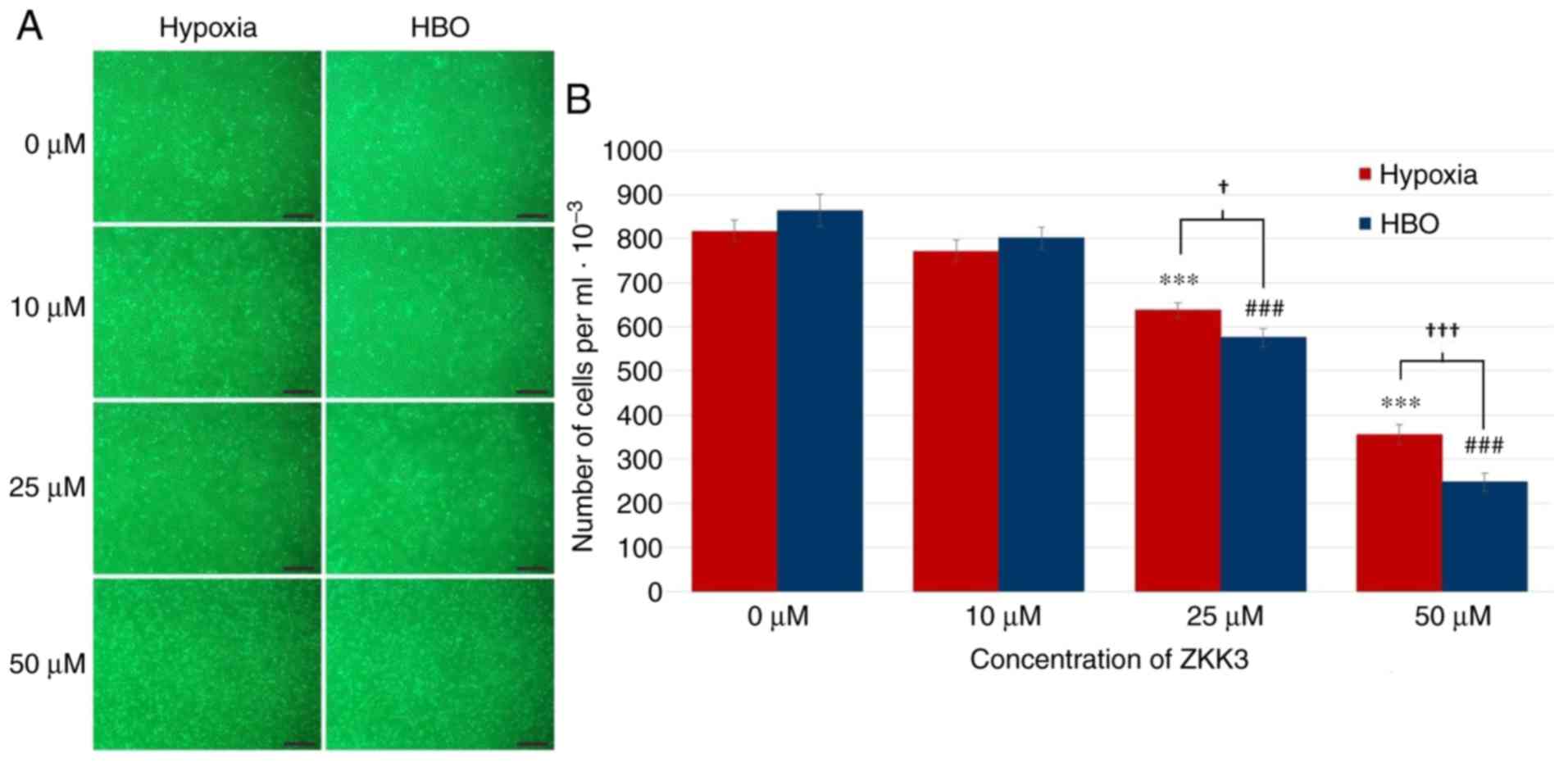
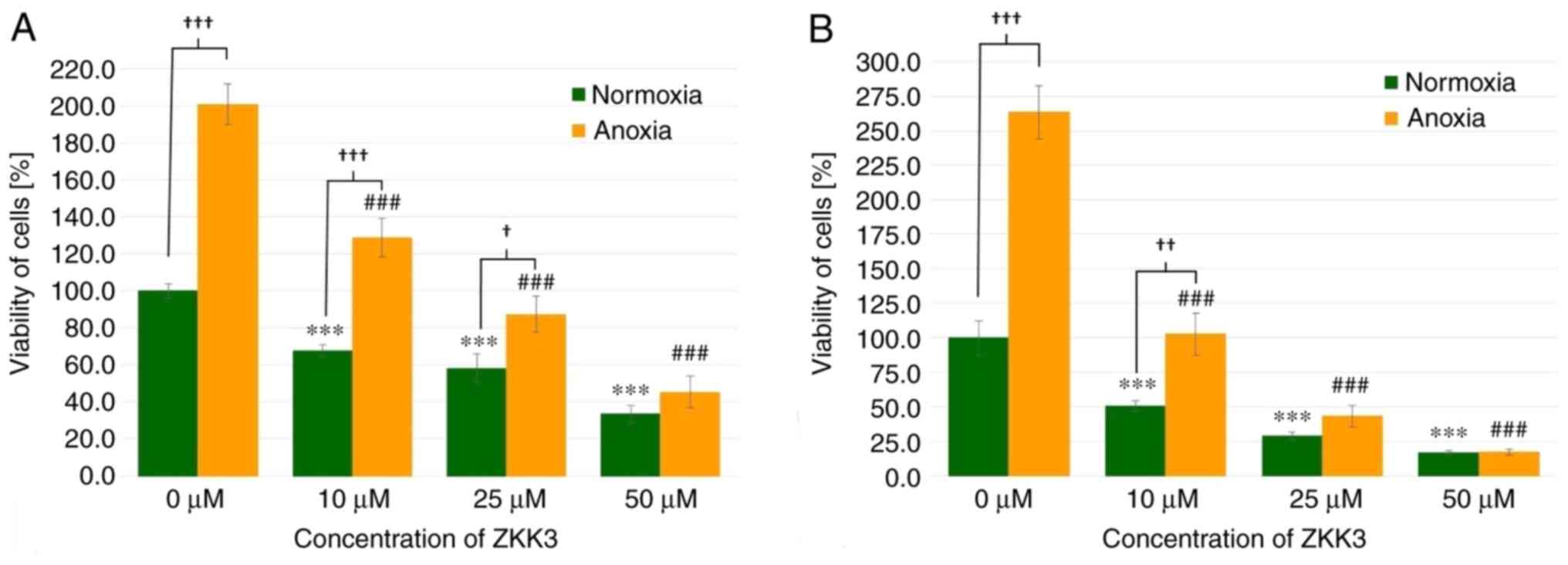
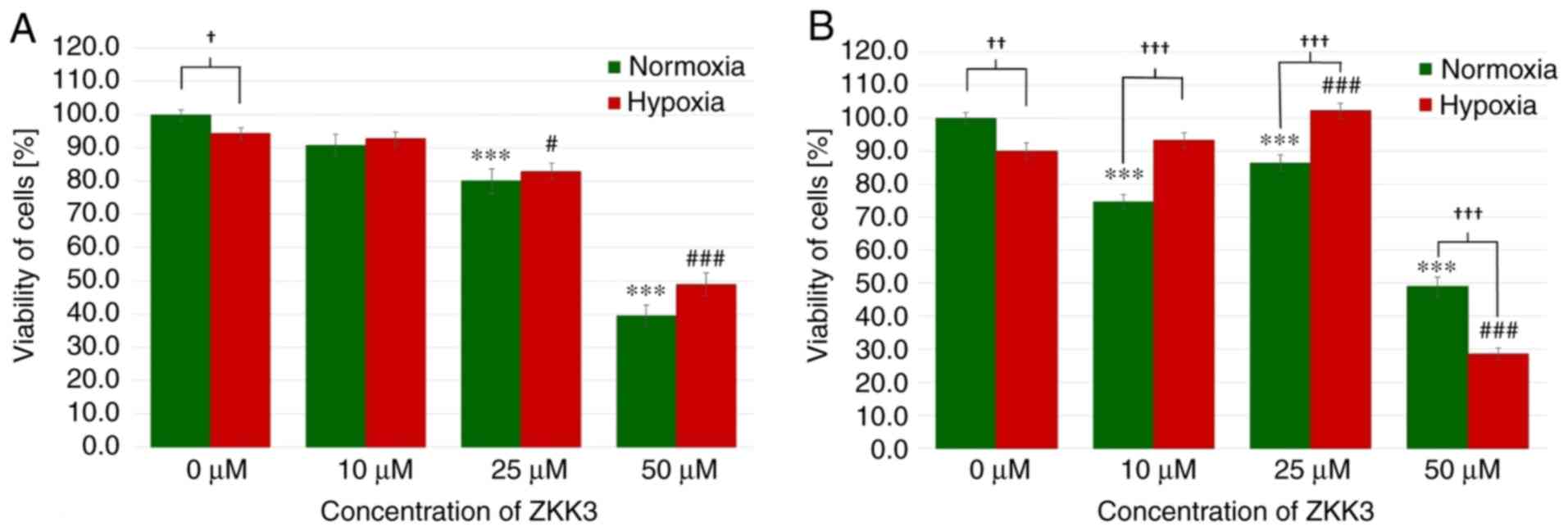
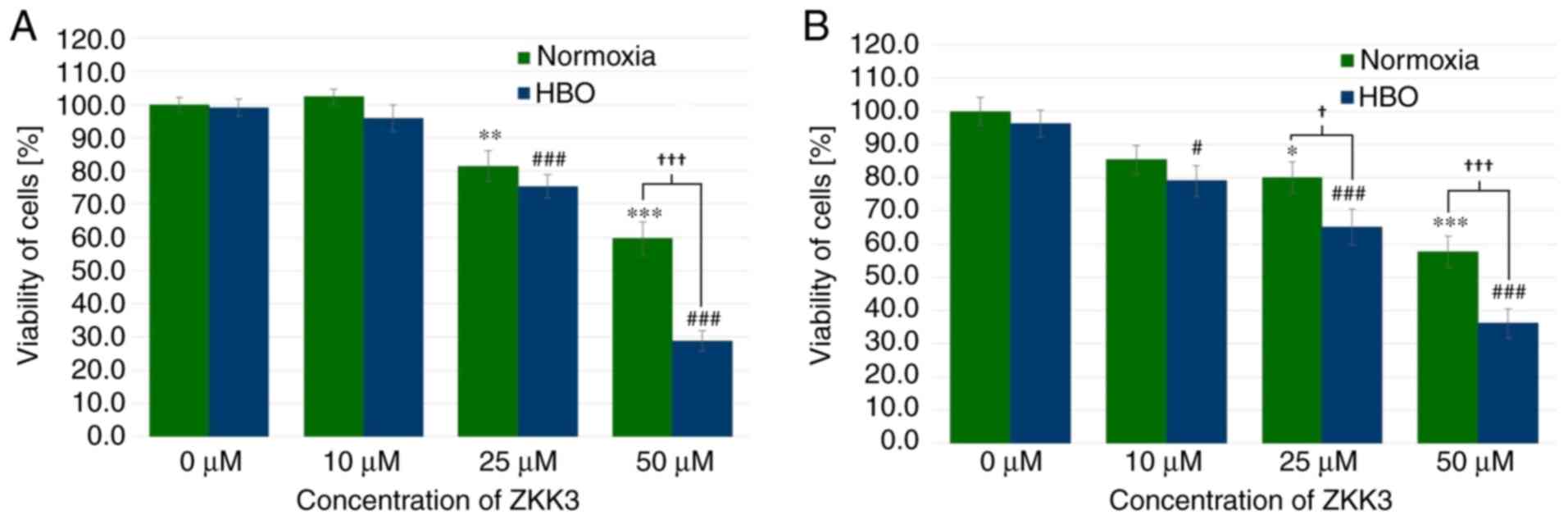
|
1
|
Batash R, Asna N, Schaffer P, Francis N
and Schaffer M: Glioblastoma multiforme, diagnosis and treatment;
recent literature review. Curr Med Chem. 24:3002–3009. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davis ME: Glioblastoma: Overview of
disease and treatment. Clin J Oncol Nurs. 20 (Suppl 5):S2–S8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanif F, Muzaffar K, Perveen K, Malhi SM
and Simjee ShU: Glioblastoma multiforme: A review of its
epidemiology and pathogenesis through clinical presentation and
treatment. Asian Pac J Cancer Prev. 18:3–9. 2017.PubMed/NCBI
|
|
4
|
Liao W, Fan S, Zheng Y, Liao S, Xiong Y,
Li Y and Liu J: Recent advances on glioblastoma multiforme and
nano-drug carriers: A review. Curr Med Chem. 2018.
|
|
5
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SY: Temozolomide resistance in
glioblastoma multiforme. Genes Dis. 3:198–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jensen RL: Brain tumor hypoxia:
Tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a
therapeutic target. J Neurooncol. 92:317–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rockwell S, Dobrucki IT, Kim EY, Marrison
ST and Vu VT: Hypoxia and radiation therapy: Past history, ongoing
research, and future promise. Curr Mol Med. 9:442–458. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Daruwalla J and Christophi C: Hyperbaric
oxygen therapy for malignancy: A review. World J Surg.
30:2112–2131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vaupel P: The role of hypoxia-induced
factors in tumor progression. Oncologist. 9 (Suppl 5):S10–S17.
2004. View Article : Google Scholar
|
|
12
|
Yang L, Lin C, Wang L, Guo H and Wang X:
Hypoxia and hypoxia-inducible factors in glioblastoma multiforme
progression and therapeutic implications. Exp Cell Res.
318:2417–2426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ostrowski RP and Zhang JH: The insights
into molecular pathways of hypoxia-inducible factor in the brain. J
Neurosci Res. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Al-Waili NS, Butler GJ, Beale J, Hamilton
RW, Lee BY and Lucas P: Hyperbaric oxygen and malignancies: A
potential role in radiotherapy, chemotherapy, tumor surgery and
phototherapy. Med Sci Monit. 11:RA279–289. 2005.PubMed/NCBI
|
|
15
|
Moen I and Stuhr LE: Hyperbaric oxygen
therapy and cancer-a review. Target Oncol. 7:233–242. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stepien K, Ostrowski RP and Matyja E:
Hyperbaric oxygen as an adjunctive therapy in treatment of
malignancies, including brain tumours. Med Oncol. 33:1012016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gill AL and Bell CN: Hyperbaric oxygen:
Its uses, mechanisms of action and outcomes. QJM. 97:385–395. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayer R, Hamilton-Farrell MR, van der
Kleij AJ, Schmutz J, Granström G, Sicko Z, Melamed Y, Carl UM,
Hartmann KA, Jansen EC, et al: Hyperbaric oxygen and radiotherapy.
Strahlenther Onkol. 181:113–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaminska B, Ellert-Miklaszewska A, Oberbek
A, Wisniewski P, Kaza B, Makowska M, Bretner M and Kazimierczuk Z:
Efficacy and mechanism of antitumor action of new potential CK2
inhibitors toward glioblastoma cells. Int J Oncol. 35:1091–1100.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koronkiewicz M, Chilmonczyk Z and
Kazimierczuk Z: Proapoptotic effects of novel
pentabromobenzylisothioureas in human leukemia cell lines. Med Chem
Res. 21:3111–3118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koronkiewicz M, Chilmonczyk Z and
Kazimierczuk Z: Synergistic anti-leukemic effects of CK2 inhibitors
and pentabromobenzylisothioureas in vitro. Anticancer Res.
33:4891–4899. 2013.PubMed/NCBI
|
|
22
|
Koronkiewicz M, Kazimierczuk Z, Szarpak K
and Chilmonczyk Z: Proapoptotic effects of new
pentabromobenzylisothiouronium salts in a human prostate
adenocarcinoma cell line. Acta Pol Pharm. 69:1325–1333.
2012.PubMed/NCBI
|
|
23
|
Sundram V, Chauhan SC and Jaggi M:
Emerging roles of protein kinase D1 in cancer. Mol Cancer Res.
9:985–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eiseler T, Doppler H, Yan IK, Goodison S
and Storz P: Protein kinase D1 regulates matrix metalloproteinase
expression and inhibits breast cancer cell invasion. Breast Cancer
Res. 11:R132009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wille C, Seufferlein T and Eiseler T:
Protein Kinase D family kinases: Roads start to segregate.
Bioarchitecture. 4:111–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pucko E, Matyja E, Koronkiewicz M,
Ostrowski RP and Kazimierczuk Z: Potent antitumour effects of novel
pentabromobenzylisothioureas studied on human glial-derived tumour
cell lines. Anticancer Res. 38:2691–2705. 2018.PubMed/NCBI
|
|
27
|
Muz B, de la Puente P, Azab F and Azab AK:
The role of hypoxia in cancer progression, angiogenesis,
metastasis, and resistance to therapy. Hypoxia (Auckl). 3:83–92.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang BC: Effects of hypoxia on drug
resistance phenotype and genotype in human glioma cell lines. J
Neurooncol. 29:149–155. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Papandreou I, Krishna C, Kaper F, Cai D,
Giaccia AJ and Denko NC: Anoxia is necessary for tumor cell
toxicity caused by a low-oxygen environment. Cancer Res.
65:3171–3178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Osawa T, Tsuchida R, Muramatsu M, Yuasa Y
and Shibuya M: Human glioblastoma cells exposed to long-term
hypoxia and nutrient starvation stimulated induction of secondary
T-cell leukemia in mice. Blood Cancer J. 1:e62011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Riffle S, Pandey RN, Albert M and Hegde
RS: Linking hypoxia, DNA damage and proliferation in multicellular
tumor spheroids. BMC Cancer. 17:3382017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mamede AC, Abrantes AM, Pedrosa L,
Casalta-Lopes JE, Pires AS, Teixo RJ, Gonçalves AC,
Sarmento-Ribeiro AB, Maia CJ and Botelho MF: Beyond the limits of
oxygen: Effects of hypoxia in a hormone-independent prostate cancer
cell line. ISRN Oncol. 2013:9182072013.PubMed/NCBI
|
|
33
|
Yao K, Gietema JA, Shida S, Selvakumaran
M, Fonrose X, Haas NB, Testa J and O'Dwyer PJ: In vitro
hypoxia-conditioned colon cancer cell lines derived from HCT116 and
HT29 exhibit altered apoptosis susceptibility and a more angiogenic
profile in vivo. Br J Cancer. 93:1356–1363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qian J, Shen S, Chen W and Chen N:
Propofol reversed Hypoxia-induced docetaxel resistance in prostate
cancer cells by preventing epithelial-mesenchymal transition by
inhibiting Hypoxia-lnducible factor lα. Biomed Res Int.
2018:41742322018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Strese S, Fryknäs M, Larsson R and Gullbo
J: Effects of hypoxia on human cancer cell line chemosensitivity.
BMC Cancer. 13:3312013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsieh CH, Lee CH, Liang JA, Yu CY and Shyu
WC: Cycling hypoxia increases U87 glioma cell radioresistance via
ROS induced higher and long-term HIF-1 signal transduction
activity. Oncol Rep. 24:1629–1636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding JB, Chen JR, Xu HZ and Qin ZY: Effect
of hyperbaric oxygen on the growth of intracranial glioma in rats.
Chin Med J (Engl). 128:3197–3203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang YG, Zhan YP, Pan SY, Wang HD, Zhang
DX, Gao K, Qi XL and Yu CJ: Hyperbaric oxygen promotes malignant
glioma cell growth and inhibits cell apoptosis. Oncol Lett.
10:189–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kohshi K, Kinoshita Y, Terashima H, Konda
N, Yokota A and Soejima T: Radiotherapy after hyperbaric
oxygenation for malignant gliomas: A pilot study. J Cancer Res Clin
Oncol. 122:676–678. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kohshi K, Kinoshita Y, Imada H, Kunugita
N, Abe H, Terashima H, Tokui N and Uemura S: Effects of
radiotherapy after hyperbaric oxygenation on malignant gliomas. Br
J Cancer. 80:236–241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kohshi K, Beppu T, Tanaka K, Ogawa K,
Inoue O, Kukita I and Clarke RE: Potential roles of hyperbaric
oxygenation in the treatments of brain tumors. Undersea Hyperb Med.
40:351–362. 2013.PubMed/NCBI
|
|
42
|
Stuhr LE, Iversen VV, Straume O, Maehle BO
and Reed RK: Hyperbaric oxygen alone or combined with 5-FU
attenuates growth of DMBA-induced rat mammary tumors. Cancer Lett.
210:35–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Granowitz EV, Tonomura N, Benson RM, Katz
DM, Band V, Makari-Judson GP and Osborne BA: Hyperbaric oxygen
inhibits benign and malignant human mammary epithelial cell
proliferation. Anticancer Res. 25:3833–3842. 2005.PubMed/NCBI
|
|
44
|
Kawasoe Y, Yokouchi M, Ueno Y, Iwaya H,
Yoshida H and Komiya S: Hyperbaric oxygen as a chemotherapy
adjuvant in the treatment of osteosarcoma. Oncol Rep. 22:1045–1050.
2009.PubMed/NCBI
|
|
45
|
Ohgami Y, Elstad CA, Chung E, Shirachi DY,
Quock RM and Lai HC: Effect of hyperbaric oxygen on the anticancer
effect of artemisinin on molt-4 human leukemia cells. Anticancer
Res. 30:4467–4470. 2010.PubMed/NCBI
|
|
46
|
Sun S, Lee D, Lee NP, Pu JK, Wong ST, Lui
WM, Fung CF and Leung GK: Hyperoxia resensitizes chemoresistant
human glioblastoma cells to temozolomide. J Neurooncol.
109:467–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lu XY, Cao K, Li QY, Yuan ZC and Lu PS:
The synergistic therapeutic effect of temozolomide and hyperbaric
oxygen on glioma U251 cell lines is accompanied by alterations in
vascular endothelial growth factor and multidrug
resistance-associated protein-1 levels. J Int Med Res. 40:995–1004.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dagistan Y, Karaca I, Bozkurt ER, Ozar E,
Yagmurlu K, Toklu A and Bilir A: Combination hyperbaric oxygen and
temozolomide therapy in C6 rat glioma model. Acta Cir Bras.
27:383–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu Z, Ma J, Liu B, Dai C, Xie T, Ma X, Li
M, Dong J, Lan Q and Huang Q: Hyperbaric oxygen therapy sensitizes
nimustine treatment for glioma in mice. Cancer Med. 5:3147–3155.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Beppu T, Kamada K, Nakamura R, Oikawa H,
Takeda M, Fukuda T, Arai H, Ogasawara K and Ogawa A: A phase II
study of radiotherapy after hyperbaric oxygenation combined with
interferon-beta and nimustine hydrochloride to treat supratentorial
malignant gliomas. J Neurooncol. 61:161–170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ogawa K, Yoshii Y, Inoue O, Toita T, Saito
A, Kakinohana Y, Adachi G, Ishikawa Y, Kin S and Murayama S:
Prospective trial of radiotherapy after hyperbaric oxygenation with
chemotherapy for high-grade gliomas. Radiother Oncol. 67:63–67.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ogawa K, Yoshii Y, Inoue O, Toita T, Saito
A, Kakinohana Y, Adachi G, Iraha S, Tamaki W, Sugimoto K, et al:
Phase II trial of radiotherapy after hyperbaric oxygenation with
chemotherapy for high-grade gliomas. Br J Cancer. 95:862–868. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ogawa K, Ishiuchi S, Inoue O, Yoshii Y,
Saito A, Watanabe T, Iraha S, Toita T, Kakinohana Y, Ariga T, et
al: Phase II trial of radiotherapy after hyperbaric oxygenation
with multiagent chemotherapy (procarbazine, nimustine, and
vincristine) for high-grade gliomas: Long-term results. Int J
Radiat Oncol Biol Phys. 82:732–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yahara K, Ohguri T, Udono H, Yamamoto J,
Tomura K, Onoda T, Imada H, Nishizawa S and Korogi Y: Radiotherapy
using IMRT boosts after hyperbaric oxygen therapy with chemotherapy
for glioblastoma. J Radiat Res. 58:351–356. 2017.PubMed/NCBI
|
|
55
|
Zhou W, Dosey TL, Biechele T, Moon RT,
Horwitz MS and Ruohola-Baker H: Assessment of hypoxia inducible
factor levels in cancer cell lines upon hypoxic induction using a
novel reporter construct. PLoS One. 6:e274602011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jones RB, Dorsett KA, Hjelmeland AB and
Bellis SL: The ST6Gal-I sialyltransferase protects tumor cells
against hypoxia by enhancing HIF-1α signaling. J Biol Chem.
293:5659–5667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo
C, Han S, Liu J, Sun S and Han Z: Hypoxia-inducible factor-1 alpha
contributes to hypoxia-induced chemoresistance in gastric cancer.
Cancer Sci. 99:121–128. 2008.PubMed/NCBI
|
|
58
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein Survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
60
|
Zhang SF, Zhang CW, Song YL, Zhang J and
Xu JG: Prognostic role of survivin in patients with glioma.
Medicine (Baltimore). 97:e05712018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li JC, Han Y, Zhou D, Zhou Y, Ye M, Wang H
and Du Z: Downregulation of Survivin gene expression affects
ionizing radiation resistance of human T98 glioma cells. Cell Mol
Neurobiol. 38:861–868. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Joseph JV, Conroy S, Pavlov K, Sontakke P,
Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den
Dunnen WF and Kruyt FA: Hypoxia enhances migration and invasion in
glioblastoma by promoting a mesenchymal shift mediated by the
HIF1α-ZEB1 axis. Cancer Lett. 359:107–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mendichovszky I and Jackson A: Imaging
hypoxia in gliomas. Br J Radiol 84 Spec No. 2:S145–S158. 2011.
View Article : Google Scholar
|
|
64
|
Rapisarda A, Uranchimeg B, Scudiero DA,
Selby M, Sausville EA, Shoemaker RH and Melillo G: Identification
of small molecule inhibitors of hypoxia-inducible factor 1
transcriptional activation pathway. Cancer Res. 62:4316–4324.
2002.PubMed/NCBI
|
|
65
|
Kummar S, Raffeld M, Juwara L, Horneffer
Y, Strassberger A, Allen D, Steinberg SM, Rapisarda A, Spencer SD,
Figg WD, et al: Multihistology, target-driven pilot trial of oral
topotecan as an inhibitor of hypoxia-inducible factor-1α in
advanced solid tumors. Clin Cancer Res. 17:5123–5131. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Stuhr LE, Raa A, Oyan AM, Kalland KH,
Sakariassen PO, Petersen K, Bjerkvig R and Reed RK: Hyperoxia
retards growth and induces apoptosis, changes in vascular density
and gene expression in transplanted gliomas in nude rats. J
Neurooncol. 85:191–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Selvendiran K, Kuppusamy ML, Ahmed S,
Bratasz A, Meenakshisundaram G, Rivera BK, Khan M and Kuppusamy P:
Oxygenation inhibits ovarian tumor growth by downregulating STAT3
and cyclin-D1 expressions. Cancer Biol Ther. 10:386–390. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Terraneo L, Virgili E, Caretti A,
Bianciardi P and Samaja M: In vivo hyperoxia induces
hypoxia-inducible factor-1alpha overexpression in LNCaP tumors
without affecting the tumor growth rate. Int J Biochem Cell Biol.
51:65–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Storz P: Mitochondrial ROS-radical
detoxification, mediated by protein kinase D. Trends Cell Biol.
17:13–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
La Valle CR, George KM, Sharlow ER, Lazo
JS, Wipf P and Wang QJ: Protein kinase D as a potential new target
for cancer therapy. Biochim Biophys Acta. 1806:183–192.
2010.PubMed/NCBI
|
|
71
|
Azoitei N, Kleger A, Schoo N, Thal DR,
Brunner C, Pusapati GV, Filatova A, Genze F, Möller P, Acker T, et
al: Protein kinase D2 is a novel regulator of glioblastoma growth
and tumor formation. Neuro Oncol. 13:710–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bernhart E, Damm S, Wintersperger A,
DeVaney T, Zimmer A, Raynham T, Ireson C and Sattler W: Protein
kinase D2 regulates migration and invasion of U87MG glioblastoma
cells in vitro. Exp Cell Res. 319:2037–2048. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fleegal MA, Hom S, Borg LK and Davis TP:
Activation of PKC modulates blood-brain barrier endothelial cell
permeability changes induced by hypoxia and posthypoxic
reoxygenation. Am J Physiol Heart Circ Physiol. 289:H2012–H2019.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Son JH, Cho YC, Sung IY, Kim IR, Park BS
and Kim YD: Melatonin promotes osteoblast differentiation and
mineralization of MC3T3-E1 cells under hypoxic conditions through
activation of PKD/p38 pathways. J Pineal Res. 57:385–392. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ha WH, Seong HS, Choi NR, Park BS and Kim
YD: Recombinant human bone morphogenic protein-2 induces the
differentiation and mineralization of osteoblastic cells under
hypoxic conditions via activation of protein kinase D and p38
mitogen-activated protein kinase signaling pathways. Tissue Eng
Regen Med. 14:433–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gozal E, Roussel AL, Holt GA, Gozal L,
Gozal YM, Torres JE and Gozal D: Protein kinase C modulation of
ventilatory response to hypoxia in nucleus tractus solitarii of
conscious rats. J Appl Physiol (1985). 84:1982–1990. 1998.
View Article : Google Scholar : PubMed/NCBI
|