Introduction
Cancer is a major public health problem worldwide
and is the second leading cause of cancer-related deaths in the US
(1). There has been a rise in the
incidence and mortality rates of colorectal cancers (CRC) in Asia
(2). To date, complex therapies
including surgery, chemotherapy and molecular targeted therapy have
been used to treat CRC widely. However, the prognosis remains
relatively poor, mainly due to metastasis to distant organs
(3). Therefore, newer therapeutic
targets are urgently required to improve the survival of the
patients.
Rab1A is a member of the RAB family, a small GTPase,
that mediates vesicular trafficking from the endoplasmic reticulum
(ER) to the Golgi apparatus (4).
Rab1A protein is also involved in mediating signal transduction
(5), cell migration (6) and regulation of autophagy (7). Aberrant expression of Rab1A has been
linked to a range of human diseases, including Parkinson's disease
and cardiomyopathy (8,9). Recently, varying levels of Rab1A
overexpression have been reported in many malignant tumors such as
breast cancer (10), and
hepatocellular carcinomas (HCC) (11). In addition, Rab1A overexpression was
revealed to play a significant role in the development of different
tumors. Rab1A has been revealed to function as an activator of
mTORC1 in HCC (11). Thomas et
al reported overexpression of Rab1A in CRC, which was revealed
to be correlated with elevated mTORC1 signaling, tumor invasion,
progression, and poor prognosis (12).
Mammalian target of rapamycin (mTOR), as a
downstream effector in the PI3K/AKT signaling pathway, consists of
two distinct complexes designated mTORC1 and mTORC2, which are
composed of the mTOR kinase subunit and accessory proteins
(13). As part of a complicated
pathway, mTORC1 plays various roles in cell survival, growth,
metabolism, and cell cycle progression and is sensitive to
rapamycin (14). Due to the central
role of mTORC1 in growth, aberrant mTORC1 signaling has been linked
to human diseases, particularly cancer (15). mTORC1 activation promotes cell
survival through the activation of anti-apoptotic proteins, thus
contributing to tumor progression (16,17).
Despite the increasing number of mTORC1-targeted clinical trials,
development to date has been limited (18).
Rab1A functions as an oncogene and an activator of
mTORC1 in HCC and CRC (11,12). The link between Rab and p-S6K1,
which is the main signaling molecule downstream of mTORC1, was
first reported by Li et al (19) in 2010; however, the relationship
between p-S6K1 and Rab1A in CRC remains to be fully elucidated.
Therefore, in the present study, we examined the association
between Rab1A and p-S6K1 levels in CRC specimens to determine their
prognostic significance. Moreover, we validated the anticancer
effects of Rab1A knockdown in CRC cells to evaluate the importance
of Rab1A as a novel therapeutic target in CRC, particularly for
mTORC1-targeted therapy-resistant cancers.
Patients and methods
Human colorectal cancer tissues and
colorectal cancer cell lines
CRC tissues were obtained from 142 patients
undergoing radical surgery for CRC at the Department of General
Surgery, the Affiliated Suzhou Hospital of Nanjing Medical
University (Suzhou, China) from 2008–2013. The diagnosis of
colorectal adenocarcinoma was confirmed by pathological
examination. None of the patients received radiotherapy or
chemotherapy prior to surgery. All patients had complete clinical
data and were available for follow-up. This study was approved by
the Independent Ethics Committee of the Affiliated Suzhou Hospital
of Nanjing Medical University and all participants provided written
informed consent. After surgery, each patient was followed-up
regularly. Human CRC cell lines SW480 (from a primary
adenocarcinoma of the colon; adherent cells; tumorigenic: yes),
LOVO (derived from metastatic site: left supraclavicular region;
adherent cells; tumorigenic: yes), and RKO (a poorly differentiated
colon carcinoma cell line; adherent cells; tumorigenic: yes) were
purchased from the Chinese Academy of Sciences (Shanghai, China),
and the HCT116 (derived from the parental HCT116 colorectal
carcinoma cell line; adherent cells) and COLO201 (derived from
metastatic site: ascites; suspension, with some loosely adherent
cells; tumorigenic: yes) were obtained from the College of Life
Sciences, Soochow University (Suzhou, China) mainly selected due to
the fact that they are relatively representative and have different
characteristics. All five cell lines were maintained in Dulbecco's
modified Eagle's medium supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
cultured at 37°C in a humidified atmosphere containing 5%
CO2.
Immunohistochemistry (IHC)
Rab1A protein expression was analyzed in 142 CRC
tissue samples and 40 adjacent normal colorectal tissue samples by
immunohistochemical staining (IHC). The surgical specimens were
fixed in 10% formalin and embedded in paraffin and serial sections
(thickness, 5 µm) were prepared. Sections were then dewaxed,
rehydrated and endogenous peroxidase activity was blocked by
incubation with peroxide in methanol. Non-specific immunoglobulin
binding was blocked by incubation with 10% normal goat serum for 15
min. After washing with phosphate-buffered saline (PBS), sections
were incubated with the polyclonal antibodies for the detection of
Rab1A (dilution 1:100; cat. no. 11671-1-AP; ProteinTech; Wuhan
Sanying Biotechnology, Wuhan, China), p-AKT (dilution 1:100; cat.
no. 4060; Cell Signaling Technology, Inc., Danvers, MA, USA), HER2
(dilution 1:100; cat. no. ab131490) and p-S6K1 (dilution 1:100;
cat. no. ab60948) (both from Abcam, Cambridge, UK) at room
temperature for 2–3 h and stained using a tissue staining kit
(Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China)
according to the manufacturer's protocol. Immunostaining was
examined independently by two clinical pathologists who were
blinded to the patient outcome. Five high-power fields (×200,
magnification) in each section were randomly selected for analysis
according to the percentage of cells stained and the intensity of
the staining. The percentage of positively staining cells per
section was scored as follows: Absent, 0–5%; 1, 6–25%; 2, 26–50%;
3, 51–75%; 4, >75%. The staining intensity was scored as
follows: 0 (negative); 1 (weak); 2 (moderate); 3 (strong). The
percentage and intensity scores were then multiplied to achieve a
total score (staining score = percentage score × intensity score).
The total staining score was classified as follows: 0, (−); 1–4,
(+); 5–8, (++), 9–12, (+++). In the present study, we classified
all of the samples into the high expression group (++ or +++) and
the low expression group (− or +) according to the protein
expression. The IHC method and staining score were performed as
described by Yang et al and Shao et al (20,21).
Short hairpin RNA transfection of
human colorectal cancer cell lines
SW480/HCT116 cell lines stably expressing
Rab1A-specifc shRNA or scrambled control shRNA were generated using
a lentiviral shRNA transfection technique. The sequences specific
for human Rab1A (5′-CAGCAUGAAUCCCGAAUAUTT-3′) selected to inhibit
the Rab1A gene expression were synthesized by (Shanghai GenePharma
Co., Ltd., Shanghai, China). SW480 and HCT116 cells were
transfected with shRab1A or control shRNA using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. At 72 h post-transfection, cells were
subjected to puromycin selection to obtain a stable cell line. The
efficiency of Rab1A-shRNA was detected by fluorescence microscopy
and western blot analysis.
Protein extraction and western blot
analysis
Whole protein extracts from SW480/HCT116 were lysed
for 30 min in ice-cold RIPA lysis buffer (Beyotime Biotechnology,
Inc., Nantong, China) according to the manufacturer's protocol.
Proteins were separated by SDS-PAGE with the quantity of 10 µg, and
then transferred to the nitrocellulose membranes (Schleicher &
Schuell BioScience, Inc., Keene, NH, USA). Standard western
blotting was performed using a polyclonal rabbit antibody against
human Rab1A (dilution 1:2,000; cat. no. ab97956), rabbit anti-human
p-S6K1 (dilution 1:1,000; cat. no. ab60948) (both from Abcam),
rabbit anti-human p-AKT (dilution 1:1,000; cat. no. 4060; Cell
Signaling Technology, Inc., Danvers, MA, USA), rabbit anti-human
HER2 (dilution 1:1,000; cat. no. ab131490; Abcam), rabbit
anti-human AKT (dilution 1:1,000; cat. no. 9272; Cell Signaling
Technology), rabbit anti-human S6K1 (dilution 1:1,000; cat. no.
ab32359; Abcam) and mouse anti-human GAPDH antibody (dilution
1:5,000; cat. no. AF7021; Affinity Biosciences, Cincinnati, OH,
USA). After blocking with 5% non-fat milk for 1 h at room
temperature, the membranes were incubated overnight with primary
detection antibodies and then with conjugated secondary detection
antibodies for 1 h at room temperature. Immunoreactive bands were
visualized by chemiluminescence and quantified using ImageJ
software (version 1.4.3.67; NIH; National Institutes of Health,
Bethesda, MD, USA).
MTT assays of cell viability
Cell viability was analyzed using MTT assay kits
(Amresco, LLC, Solon, OH, USA) according to the manufacturer's
protocol. Cells were digested, resuspended, and inoculated into
96-well culture plates. After incubation in complete medium, MTT
solution was added and cells were incubated at 37°C for 4 h. The
supernatants were then removed, and formazan crystals were
dissolved in 150 µl dimethyl sulfoxide (DMSO). After incubation for
10 min at 37°C, the absorbance at 490 nm was measured. For each
sample, five replicate wells were prepared, and the experiment was
repeated three times.
Cell migration assay
The migratory activity of the Rab1A or control
shRNA-transfected SW480 cells/HCT116 cells was evaluated in 24-well
Transwell plate assays (Corning Incorporated, Corning, NY, USA)
according to the manufacturer's protocol. Cells resuspended in
serum-free RPMI-1640 medium were plated in the upper chamber of
each well. Each lower chamber was filled with 500 µl complete
culture medium. After 24 h of incubation at 37°C, the cells on the
upper side of the membrane insert were completely removed by wiping
with a cotton swab. The cells that had invaded were fixed in
methanol, stained with 0.5% crystal violet and counted manually in
five randomly selected fields under an inverted microscope (scale
bar, 200 µm). Each experiment was repeated in triplicate.
Statistical analysis
All experiments were repeated three times.
Spearman's correlation was used to measure the correlation between
Rab1A and p-S6K1 IHC scores. Data were expressed as the means ±
standard error of the mean (SEM). Significant differences between
the groups were determined using Student's t-test and the
Chi-square test. Survival duration was calculated using the
Kaplan-Meier method and compared using the log-rank test. All
parameters that were discovered to be significant on univariate
analysis with the Cox proportional hazard model were then included
in the multivariate survival analysis. A value of P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed with SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software,
Inc., La Jolla, CA, USA). The images were combined using Microsoft
PowerPoint (PPT).
Results
Rab1A expression levels in colorectal
cancer tissues and paired adjacent tissues
We investigated the expression of Rab1A in 142 pairs
of primary human CRC and adjacent normal tissues by IHC staining.
The percentage staining and intensity scores were then multiplied
to achieve a total staining score (Fig.
1A). The results indicated that the Rab1A expression levels
were significantly higher in CRC than in paracancer tissues
(P<0.001; Fig. 1B). Furthermore,
Rab1A expression was significantly higher in patients with lymph
node invasion compared with those without (P<0.01; Fig. 1D). However, there were no
significant differences in Rab1A expression between right colon
carcinoma, left colon carcinoma and rectal carcinoma tissues
(P>0.05; Fig. 1C). Furthermore,
Rab1A expression was increased in CRC tissues from
tumor-node-metastasis (TNM) stage I–IV, although this change was
not statistically significant (P>0.05; Fig. 1E).
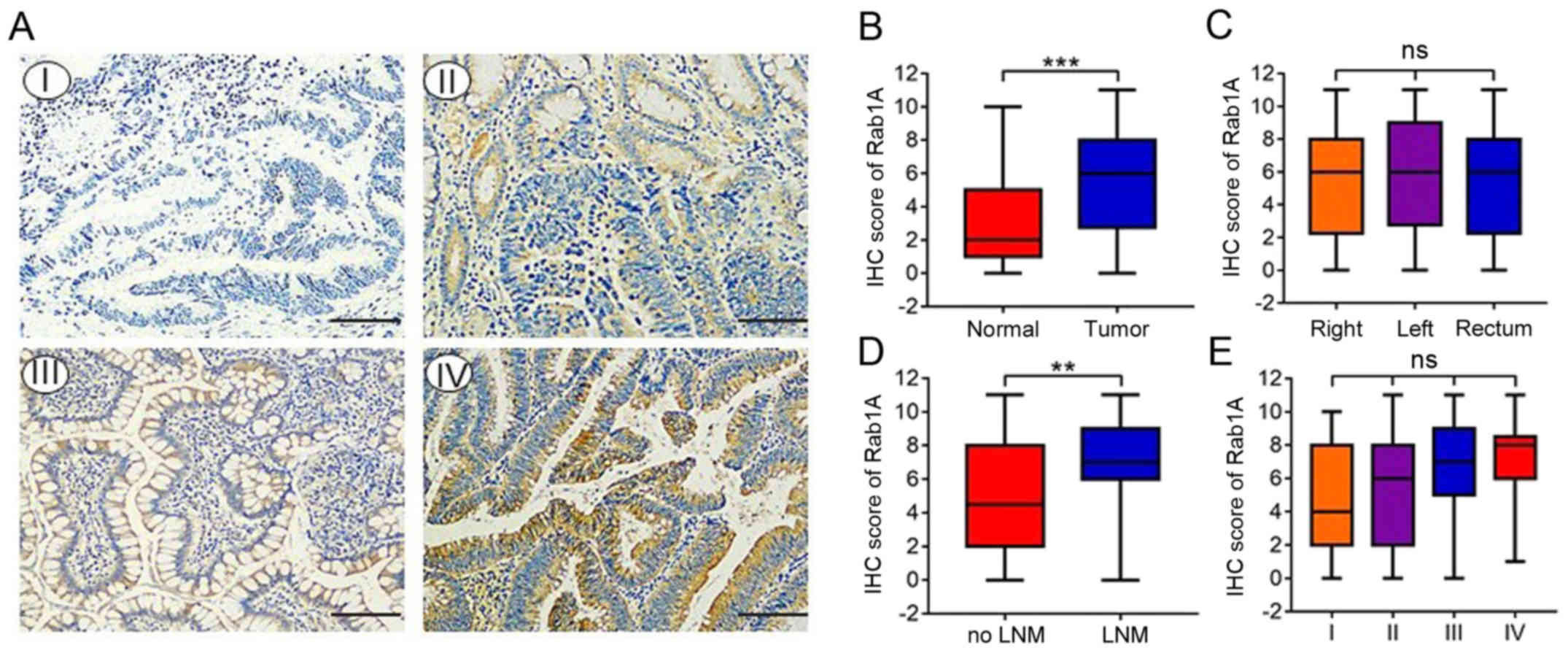 | Figure 1.Expression of Rab1A in CRC tissues.
(A) Immunohistochemical (IHC) staining of Rab1A in 142 pairs of
primary human CRC and adjacent normal tissues (magnification, ×200)
(scale bar, 100 µm). The expression of Rab1A protein was negative
(I), weak (II), positive (III), or strong positive (IV). (B)
Staining scores of Rab1A expression in CRC tissues and adjacent
normal tissues. (C) Staining scores of Rab1A expression in right
colon carcinoma, left colon carcinoma and rectal carcinoma. (D)
Staining scores of Rab1A expression in lymph node invasion-positive
and negative patients. (E) Staining scores of Rab1A expression in
four TNM stages. **P<0.01, ***P<0.001. CRC, colorectal
carcinoma; TNM, tumor-node-metastasis; LNM, lymph node
metastasis. |
The relationship between Rab1A
expression levels and clinicopathological parameters in CRC
patients
We investigated the association between
clinicopathological parameters and Rab1A expression; the results
were summarized in Table I. Rab1A
expression was significantly associated with lymph node invasion
(P<0.001; Table I), degree of
differentiation (P<0.05; Table
I), venous invasion (P<0.05; Table I) and TNM stage (P<0.001;
Table I). However, no significant
differences were observed between Rab1A expression and other
clinicopathological variables such as age, sex, tumor size, neural
invasion, tumor location or depth of tumor invasion (P>0.05;
Table I).
 | Table I.Association between Rab1A expression
and clinicopathological factors in 142 patients with CRC. |
Table I.
Association between Rab1A expression
and clinicopathological factors in 142 patients with CRC.
|
| Rab1A |
|---|
|
|
|
|---|
| Factors | Negative | Positive | P-value |
|---|
| Sex |
|
|
|
|
Male | 31 | 58 | 0.727 |
|
Female | 20 | 33 |
|
| Age (years) |
|
|
|
|
≤60 | 14 | 28 | 0.678 |
|
>60 | 37 | 63 |
|
| Size (cm) |
|
|
|
| ≤5 | 33 | 46 | 0.103 |
|
>5 | 18 | 45 |
|
| Depth of
invasion |
|
|
|
|
T1-2 | 13 | 17 | 0.340 |
|
T3-4 | 38 | 74 |
|
| Lymph node
invasion |
|
|
|
|
Negative | 42 | 42 |
<0.001b |
|
Positive | 9 | 49 |
|
| Degree of
differentiation |
|
|
|
|
Well | 47 | 72 | 0.043a |
|
Poor | 4 | 19 |
|
| Venous
invasion |
|
|
|
|
Negative | 34 | 41 | 0.013a |
|
Positive | 17 | 50 |
|
| Neural
invasion |
|
|
|
|
Negative | 38 | 57 | 0.149 |
|
Positive | 13 | 34 |
|
| TNM staging |
|
|
|
|
I–II | 41 | 42 |
<0.001b |
|
III–IV | 10 | 49 |
|
| Tumor location |
|
|
|
|
Right | 19 | 29 | 0.777 |
|
Left | 15 | 31 |
|
|
Rectum | 17 | 31 |
|
Rab1A overexpression is associated
with poor prognosis in CRC patients
To explore the influence of high Rab1A expression on
overall survival of CRC patients, we first analyzed the survival
curves in CRC patients according to Rab1A expression level. The
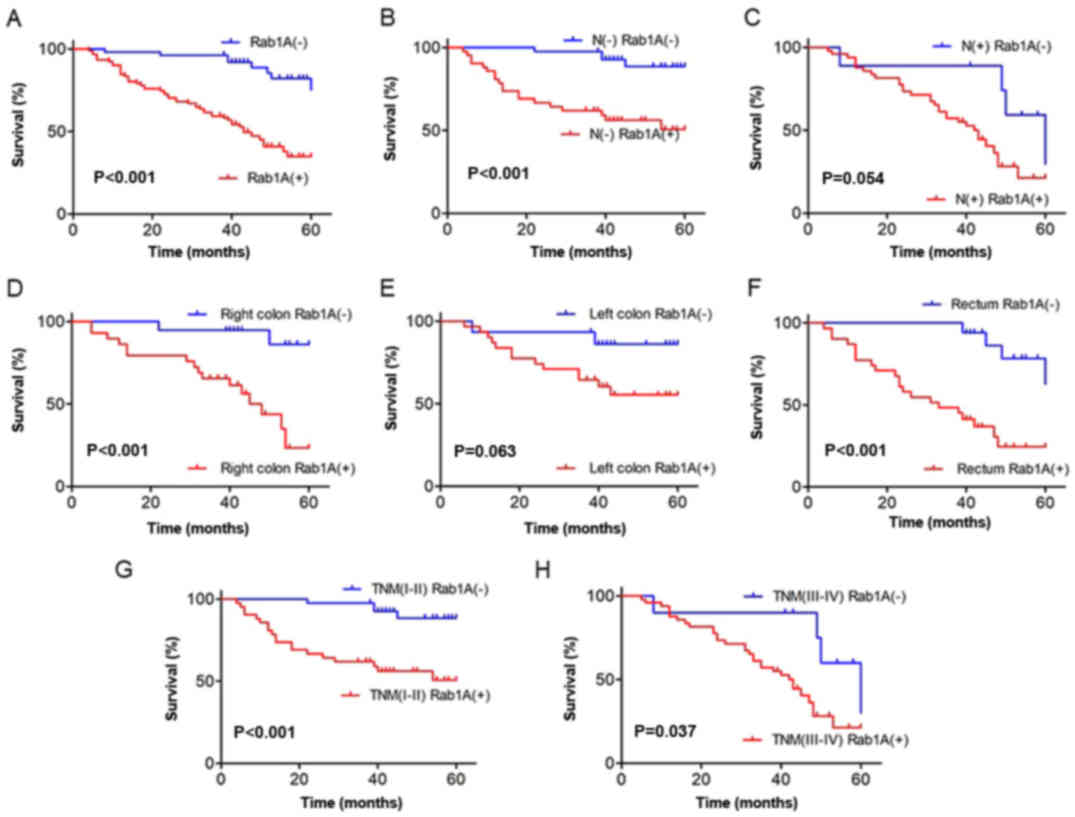
results revealed that the survival of patients with positive Rab1A
expression was significantly worse than those with negative Rab1A
expression (P<0.001; Fig. 2A).
Subsequently, we performed subgroup analysis on lymph node
invasion, tumor location and TNM staging. The results revealed that
high Rab1A expression was associated with poor prognosis in lymph
node invasion-negative patients (P<0.001; Fig. 2B). A small but statistically
insignificant difference was observed in lymph node
invasion-positive patients (P=0.054; Fig. 2C). Furthermore, our results
suggested that high expression of Rab1A was associated with poor
prognosis in patients with right colon carcinoma and rectal cancer
(P<0.001, P<0.001; Fig. 2D and
F), but not in patients with left colon carcinoma (P=0.063;
Fig. 2E). Moreover, higher Rab1A
expression was associated with a worse 5-year survival rate in
patients with both TNM stages I–II and III–IV (P<0.001, P=0.037;
Fig. 2G and H).
Next, we performed univariate and
multivariate analyses of Rab1A expression and clinicopathological
variables
The univariate analysis revealed that Rab1A
expression (P<0.001; Table II),
lymph node invasion (P<0.001; Table
II), degree of differentiation (P=0.005; Table II), and TNM staging (P=0.001;
Table II) were related with poor
prognosis. In the multivariate analysis, only Rab1A expression was
confirmed as an independent prognostic factor for the survival of
CRC patients (P<0.001; Table
II).
 | Table II.Results of univariate and
multivariate analyses of the survival of patients with CRC by Cox's
proportional hazard model. |
Table II.
Results of univariate and
multivariate analyses of the survival of patients with CRC by Cox's
proportional hazard model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR | 95.0% CI | P-value | HR | 95.0% CI | P-value |
|---|
| Sex
(male/female) | 1.301 | 0.752–2.252 | 0.347 |
|
|
|
| Age (≤60 or >60
years) | 0.964 | 0.547–1.697 | 0.899 |
|
|
|
| Size of cancer (≤5
or >5 cm) | 0.672 | 0.402–1.125 | 0.131 |
|
|
|
| Depth of invasion
(T1-2/T3-4) | 0.459 | 0.208–1.012 | 0.054 |
|
|
|
| Lymph node
metastasis (negative/positive) | 0.382 | 0.225–0.649 |
<0.001b | 0.002 |
<0.001->10.000 | 0.914 |
| Degree of
differentiation (poor/well) | 0.432 | 0.239–0.781 | 0.005a | 0.619 | 0.331–1.160 | 0.134 |
| Venous invasion
(negative/positive) | 0.772 | 0.461–1.294 | 0.326 |
|
|
|
| Neural invasion
(negative/positive) | 0.793 | 0.468–1.344 | 0.389 |
|
|
|
| TNM stage
(I–II/III–IV) | 0.394 | 0.231–0.670 | 0.001a | >10.000 |
<0.001->10.000 | 0.920 |
| Rab1A expression
(low/high) | 0.183 | 0.086–0.390 |
<0.001b | 0.227 | 0.104–0.499 |
<0.001b |
Subgroup analysis of prognostic
factors based on Rab1A expression level
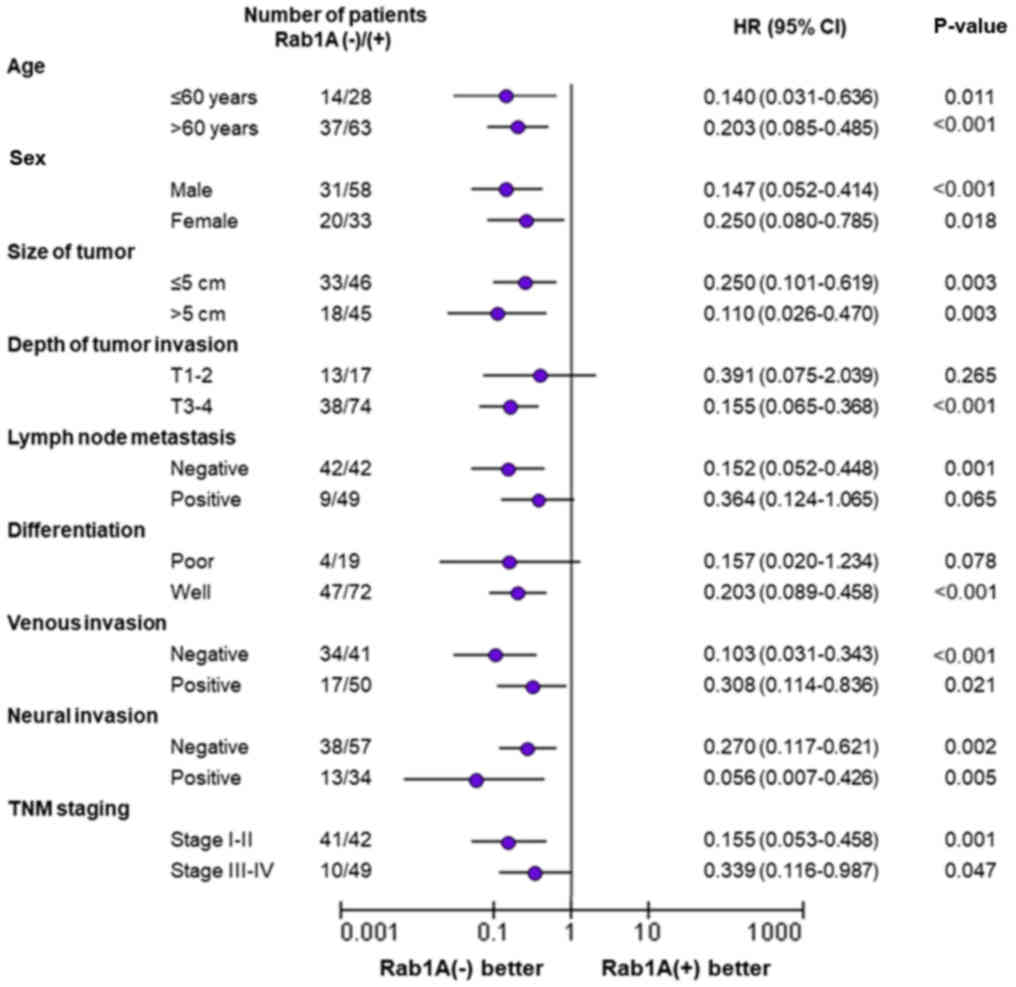
In further investigations, we performed subgroup
analysis of the relationship between Rab1A expression and survival
time. The results revealed that Rab1A overexpression was associated
with worse survival of CRC patients compared to those with low
Rab1A expression, regardless of patient age, sex, tumor size,
venous invasion, neural invasion or the TNM staging. Moreover, high
Rab1A expression was associated with markedly shorter survival time
in CRC patients with stage T3-T4 tumor depth (P<0.001; Fig. 3), negative lymph node metastasis
(P=0.001; Fig. 3) and high-level
differentiation (P<0.001; Fig.
3). In other words, patients with the depth of tumor invasion
(stage T3-T4), lymph node metastasis (negative) or stage of
differentiation (high-level), exhibited higher levels of Rab1A
expression and were associated with poorer survival time. In
contrast, there was no significant difference in survival time
between patients with stage T1-T2 disease (P=0.265; Fig. 3), positive lymph node metastasis
(P=0.065; Fig. 3) or poor
differentiation (P=0.078; Fig. 3)
regardless of the Rab1A expression level.
High levels of p-S6K1 are closely
related with Rab1A expression levels in CRC patients
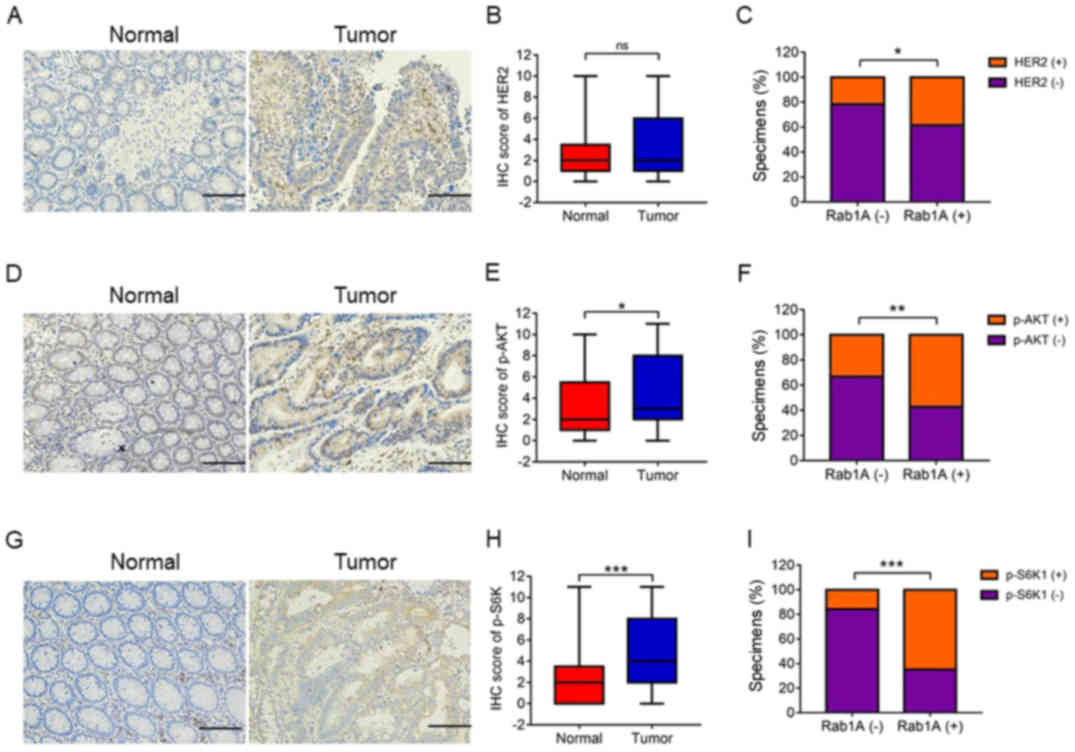
Previous studies have shown that Rab1A is an
activator of mTORC1 and HER2, which are the main upstream signaling
molecules in the AKT/mTOR/S6K1 pathway. In the present study, we
investigated the association between Rab1A expression and
HER2/AKT/mTORC/S6K1 axis. Firstly, we performed IHC staining of
p-S6K1, HER2 and p-AKT levels in 142 pairs of CRC and adjacent
normal tissues. IHC analysis revealed high expression levels of
p-S6K1 and p-AKT in CRC tissues compared with those in adjacent
normal tissues (P<0.001, P<0.05; Fig. 4D and E, and 4G and H). There was no significant
difference in HER2 expression levels between the CRC tissues and
adjacent normal tissues (P>0.05; Fig. 4A and B). Notably, the levels of
HER2, p-AKT and p-S6K1 were positively associated with Rab1A
expression in CRC tissues, particularly p-S6K1 (P<0.05,
P<0.01, P<0.001; Fig. 4C, 4F and
I). In addition, Pearson χ2 test analysis revealed
that Rab1A expression was closely related with the levels of HER2,
p-AKT and p-S6K1 (P=0.039, P=0.001, P<0.001; Table III). In conclusion, high Rab1A
expression levels were positively associated with the
HER2/AKT/mTORC/S6K1 axis. Moreover, Rab1A expression was
significantly associated with p-S6K1 levels.
 | Table III.Statistics of HER2/p-S6K1/p-AKT and
Rab1A expression in CRC patients. |
Table III.
Statistics of HER2/p-S6K1/p-AKT and
Rab1A expression in CRC patients.
| Expression | Rab1A negative | Rab1A positive | P-value |
|---|
| HER2 negative | 40 | 56 | 0.039a |
| HER2 positive | 11 | 35 |
|
| p-S6K1
negative | 43 | 32 |
<0.001c |
| p-S6K1
positive | 8 | 59 |
|
| p-AKT negative | 37 | 39 | 0.001b |
| p-AKT positive | 14 | 52 |
|
Both high Rab1A and p-S6K1 levels are
associated with a poor prognosis
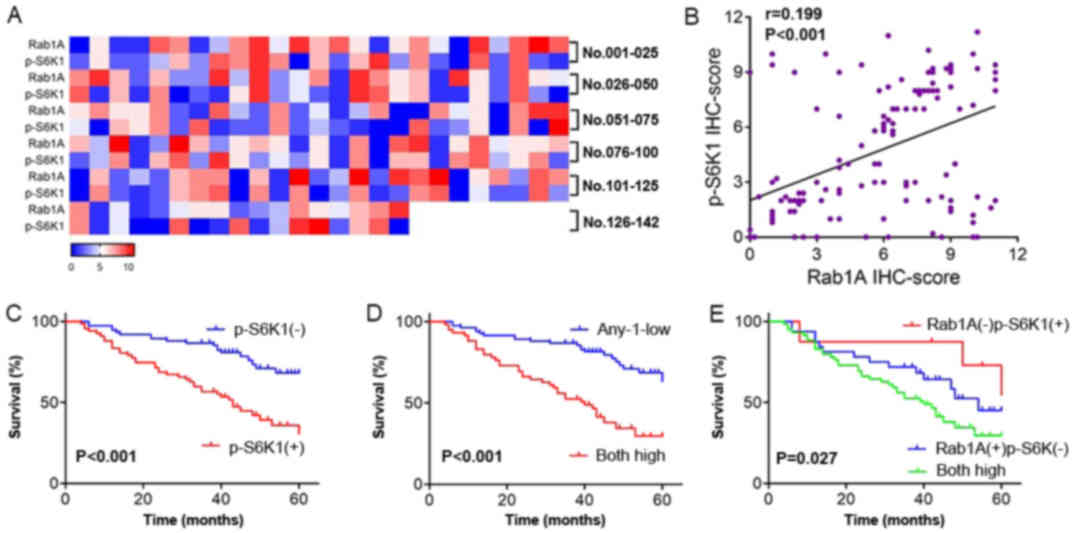
We observed that high p-S6K1 levels were closely
related with high Rab1A expression. We next generated a heat map
displaying the expression levels of Rab1A and p-S6K1 detected in
142 CRC patients through IHC analysis. Moreover, we compared the
levels of Rab1A and p-S6K1 detected in 142 CRC tissues in a scatter
plot of IHC scores. The results revealed that Rab1A expression was
positively related to p-S6K1 (P<0.001; Fig. 5A and B). The relationship between
p-S6K1/Rab1A expression levels and clinicopathological variables
was then explored; the results are summarized in Table IV. We concluded that high levels of
both Rab1A and p-S6K1 were significantly related with tumor size
(P=0.046; Table IV), lymph node
invasion (P<0.001; Table IV),
degree of differentiation (P=0.012; Table IV), venous invasion (P=0.002;
Table IV) and TNM stage
(P<0.001; Table IV) compared
with low levels of either Rab1A or p-S6K1. No positive associations
were obtained with other clinicopathological variables, such as
age, sex, neural invasion, tumor location or depth of tumor
invasion (P>0.05; Table
IV).
 | Table IV.Association between Rab1A/p-S6K1
positive or negative and clinicopathological factors in 142
patients with CRC. |
Table IV.
Association between Rab1A/p-S6K1
positive or negative and clinicopathological factors in 142
patients with CRC.
|
| Rab1A and
p-S6K1 |
|
|---|
|
|
|
|
|---|
| Factors | Any-1-low | Both high | P-value |
|---|
| Sex |
|
|
|
|
Male | 54 | 35 | 0.486 |
|
Female | 29 | 24 |
|
| Age (years) |
|
|
|
|
≤60 | 25 | 17 | 0.866 |
|
>60 | 58 | 42 |
|
| Size (cm) |
|
|
|
| ≤5 | 52 | 27 | 0.046a |
|
>5 | 31 | 32 |
|
| Depth of
invasion |
|
|
|
|
T1-2 | 19 | 11 | 0.541 |
|
T3-4 | 64 | 48 |
|
| Lymph node
invasion |
|
|
|
|
Negative | 65 | 19 |
<0.001c |
|
Positive | 18 | 40 |
|
| Degree of
differentiation |
|
|
|
|
Well | 75 | 44 | 0.012a |
|
Poor | 8 | 15 |
|
| Venous
invasion |
|
|
|
|
Negative | 53 | 22 | 0.002b |
|
Positive | 30 | 37 |
|
| Neural
invasion |
|
|
|
|
Negative | 59 | 36 | 0.209 |
|
Positive | 24 | 23 |
|
| TNM staging |
|
|
|
|
I–II | 64 | 19 |
<0.001c |
|
III–IV | 19 | 40 |
|
| Tumor location |
|
|
|
|
Right | 33 | 15 | 0.170 |
|
Left | 23 | 23 |
|
|
Rectum | 27 | 21 |
|
We also explored the influence of the high levels of
p-S6K1 on overall survival of patients with CRC by Kaplan-Meier
analysis. Compared with low p-S6K1 levels, high levels of p-S6K1
were associated with a poorer prognosis in CRC patients
(P<0.001; Fig. 5C). Moreover,
the survival of patients with high levels of both Rab1A and p-S6K1
was significantly worse than those with low levels of either Rab1A
or p-S6K1 (P<0.001; Fig. 5D).
Additionally, high levels of both Rab1A and p-S6K1 were associated
with a poorer prognosis than high levels of either Rab1A or p-S6K1
alone (P=0.027; Fig. 5E).
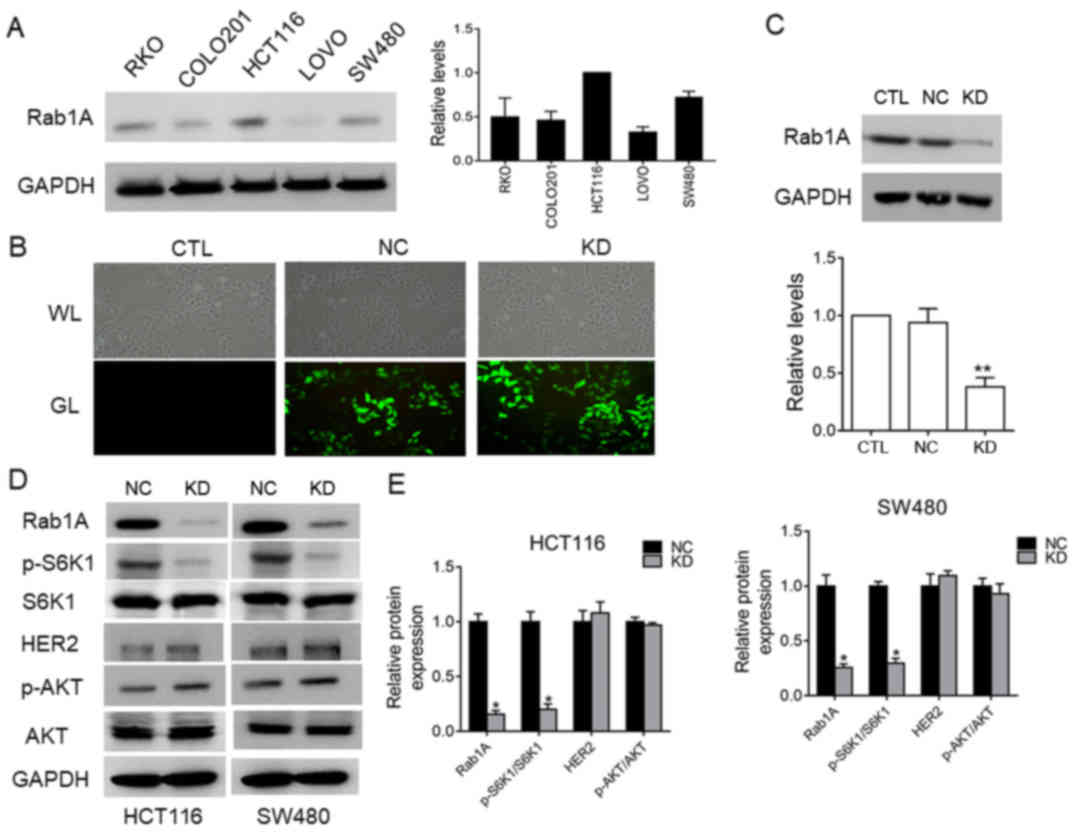
Rab1A expression in five colorectal
cancer cell lines and sh-RNA-mediated deletion of the Rab1A gene in
SW480 cells
We examined Rab1A expression levels in five of human
colorectal cancer cell lines (HCT116, SW480, LOVO, COLO201 and RKO)
by western blot analysis (Fig. 6A).
Rab1A expression was relatively high in HCT116 and SW480 cells, and
relatively low in LOVO cells. We next investigated the effects of
shRNA-mediated-knockdown of Rab1A expression SW480 cells.
Evaluation of the transfection efficiency by western blot analysis
revealed that Rab1A expression was distinctly knocked down in cells
transfected with Rab1A-shRNA compared to the expression in
control-shRNA-transfected and untreated cells (P<0.05; Fig. 6B and C). These results confirmed
effective and specific suppression of Rab1A expression in SW480
cells.
Rab1A expression is positively related
to p-S6K1 levels and regulates p-S6K1 in HCT116 cells and SW480
cells
To further clarify the relationship between Rab1A
and p-S6K1 in HCT116 cells and SW480 cells, we examined the
expression levels of p-S6K1, HER2 and p-AKT by western blotting
after Rab1A knockdown. The results revealed that p-S6K1 levels were
significantly decreased following Rab1A knockdown (P<0.05;
Fig. 6D and E), while there was no
significant change in the levels of HER2 and p-AKT (P>0.05;
Fig. 6D and E), indicating that
Rab1A expression is positively related with p-S6K1 levels but has
no effect on the HER2/AKT/mTORC1 axis. To sum up, we concluded that
Rab1A expression is not only closely related to p-S6K1 levels, but
also mediates targeted regulation of p-S6K1 in HCT116 and SW480
cells.
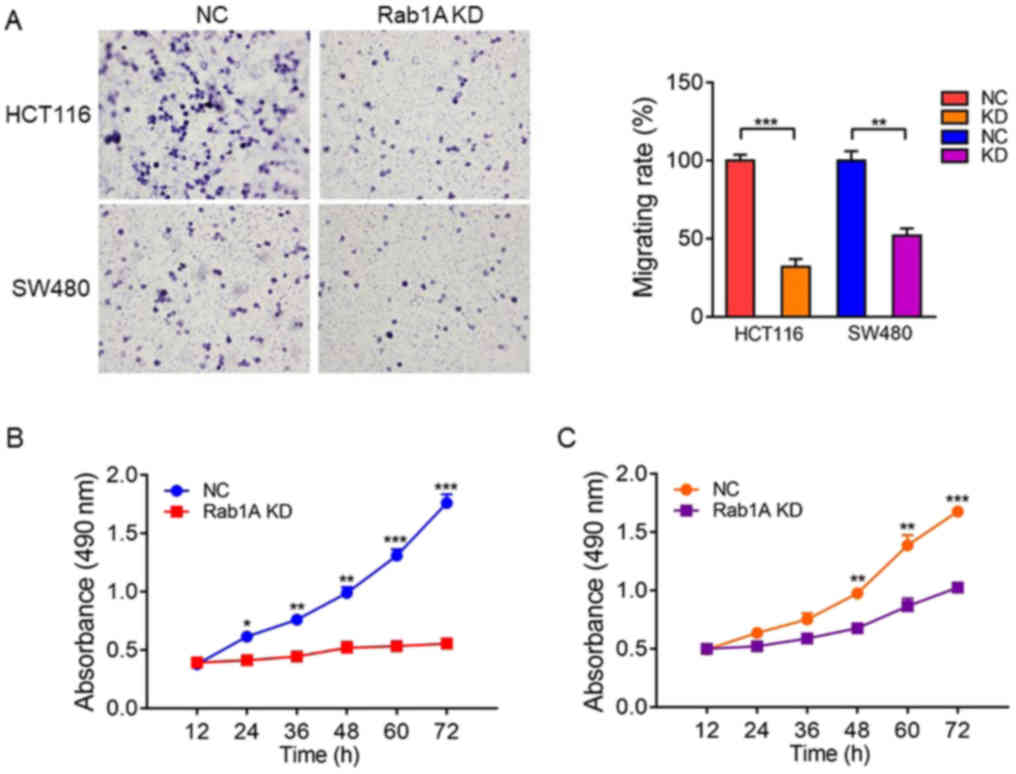
Rab1A knockdown inhibits the migration
and proliferation of HCT116 and SW480 cells
We explored the effect of Rab1A on migration and
proliferation of HCT116 and SW480 cells in vitro. Transwell
migration assays revealed that the migration ability of SW480 and
HCT116 cells was significantly inhibited following Rab1A knockdown
(P<0.05; Fig. 7A). Furthermore,
MTT assays performed at 12, 24, 36, 48, 60 and 72 h after
transfection revealed that the proliferation of HCT116 and SW480
cells transfected with Rab1A-shRNA was significantly inhibited
compared with that of the cells transfected with control-shRNA
(P<0.05; Fig. 7B and C).
Discussion
Colorectal cancer is an important health problem
worldwide (22). Despite the
development of various diagnostic and treatment strategies,
including chemotherapy, the prognosis is poor and the survival rate
is low (23). The
HER2/AKT/mTOR/S6K1 pathway plays a significant role in CRC. The
emergence of rapamycin has prolonged the survival rate in CRC
modestly, but diverse side-effects and severe drug-resistance
remain challenges. Therefore, identification of novel therapeutic
targets for CRC are urgently required (24,25),
particularly for mTORC1-targeted therapy-resistant cancers. Based
on this perspective, we investigated the expression patterns and
association between Rab1A and p-S6K1 and their clinical
significance in CRC patients. Moreover, we validated the anticancer
effects of Rab1A expression by shRNA-mediated knockdown in CRC
cells.
Rab1A is a small GTPase well known for its role in
regulating ER-to-Golgi vesicular transport (26). Rab1A has recently been reported to
function as an oncogene and is overexpressed in various types of
cancers, such as tongue squamous carcinoma (27), cervical (28), breast (10) and prostate cancer (29,30),
hepatocellular carcinomas (11),
and lung cancer (31). In our
present study, we first investigated the Rab1A expression status
and evaluated its clinical significance and relationship with
overall survival. Our results revealed that Rab1A expression levels
were significantly higher in CRC tissues than in paracancerous
tissues, and higher in patients with lymph node invasion than in
those without. Furthermore, Rab1A expression increased as TNM
staging progressed from I to IV in CRC, although the relationship
was not statistically significant. However, there were no
significant differences in Rab1A expression between right colon
carcinoma, left colon carcinoma and rectal carcinoma, indicating
that Rab1A expression is not related to tumor location. High Rab1A
expression was significantly associated with several
clinicopathological parameters such as lymph node invasion, poor
degree of differentiation, venous invasion and high TNM stage.
Previous studies have demonstrated that Rab1A
overexpression leads to poor survival in HCC (11) and gastric cancer (32). In accordance with a previous study
in HCC (11), we found that the
survival of Rab1A-positive patients was significantly worse than
that of Rab1A-negative patients and higher Rab1A expression was
associated with a worse 5-year survival rate in patients with I–II
and III–IV disease staging. Furthermore, univariate and
multivariate analysis indicated that Rab1A expression was the only
independent prognostic factor for CRC patients, which is consistent
with the results reported by Wang et al (33). Subgroup analysis of the
relationships of high/low Rab1A expression levels with other
prognostic factors revealed that Rab1A overexpression was
associated with poorer survival of CRC patients compared with that
associated with low Rab1A expression, regardless of patient age,
sex, tumor size, venous invasion, neural invasion and TNM staging.
These findings suggest that high Rab1A levels play a significant
role in the prognosis of CRC.
Previous studies have demonstrated that Rab1A
functions as an activator of mTORC1 in CRC, prostate cancer and HCC
(11,12,29).
In addition to the function of Rab1A upstream of mTORC1, it is
possible that Rab1A, which is involved in autophagy, influences
downstream molecules, such as the other autophagic small GTPases
Rab7 (34) and Rab12 (35), which are regulated by mTORC1/ULK.
HER2 is the main upstream signaling molecule in the AKT/mTOR/S6K1
pathway and p-S6K1 is downstream of mTORC1 (36). Based on this information, we first
reported the associations between Rab1A and upstream or downstream
significant targets of mTORC1 such as p-S6K1, Her-2 and p-AKT in
CRC patients. IHC analysis revealed that high Rab1A expression was
positively related with the HER2/AKT/mTORC/S6K1 axis, although no
significant relationship between Rab1A expression and the
HER2/AKT/mTORC axis was identified by western blot analysis.
Notably, Rab1A expression was found to be closely associated with
p-S6K1 levels both by IHC and western blot analysis, suggesting
that Rab1A is positively associated with p-S6K1 rather than the
HER2/AKT/mTORC axis. Furthermore, high levels of both p-S6K1 and
Rab1A were related with several clinicopathological factors such as
size, lymph node invasion, and degree of differentiation. Moreover,
we first reported that high levels of both Rab1A and p-S6K1 were
associated with a worse prognosis compared with high levels of
Rab1A or p-S6K1 alone. These findings indicated that high levels of
both p-S6K1 and Rab1A had significantly more detrimental effects on
the prognosis of patients with CRC compared with the effect of high
levels of Rab1A or p-S6K1 alone. Our in vitro studies
indicated that p-S6K1 levels sharply decreased after Rab1A
knockdown rather than p-AKT/HER-2. We searched the relevant data of
RAB1A, HER2, AKT, mTOR, S6K1 and analyzed the association in TCGA
dataset via GEPIA platform. It revealed that Rab1A was an activator
of mTORC1 and closely related with mTOR and p-S6K1. However, RAB1A
was also moderately correlated with AKT, and not related with
HER-2. It suggested that Rab1A activated the mTORC1 pathway
independent of HER2 (data not shown). In conclusion, Rab1A was not
only closely related with p-S6K1 levels, but also targeted
regulation of p-S6K1, rather than the HER2/AKT/mTORC axis.
Rab1A overexpression in HCC cell lines and Rab1A
silencing has been revealed to significantly inhibit the growth and
migration abilities of HCC cell lines (11). In our comparisons of five different
HCC cell lines, Rab1A expression was relatively high in HCT116 and
SW480 cells, and relatively low in LOVO cell lines. In accordance
with the results in HCC tissues, knockdown of Rab1A expression
inhibited the migration and proliferation of HCT116 cells and SW480
cells, suggesting that Rab1A promotes viability and migration
abilities via regulation of the HER2/AKT-independent mTOR/S6K1
pathway in colorectal cancer. However, some limitations exist in
our study, such as the specific mechanism of how Rab1A activates
mTOR independently of HER2/AKT. Thus, further research is required
to clarify the specific regulatory mechanisms between Rab1A and
HER2/AKT.
In conclusion, our results revealed that Rab1A
expression was increased in CRC tissues. Overexpression of Rab1A
was related with clinicopathological parameters and indicated a
poor prognosis. Furthermore, high levels of p-S6K1 were detected in
CRC tissues and Rab1A expression had a positive association with
p-S6K1 levels. In addition, high levels of both Rab1A and p-S6K1
were associated with a poorer prognosis compared with high levels
of either Rab1A or p-S6K1 alone. Moreover, we determined that Rab1A
knockdown significantly reduced the migration and proliferation
abilities of CRC cell lines by targeting regulation of p-S6K1.
Thus, our study revealed that Rab1A plays an important role in CRC
and may provide a new perspective on targeted therapy of CRC,
particularly for mTORC1-targeted therapy-resistant cancers.
Acknowledgements
Not applicable.
Funding
This study was supported by the Beijing Medical and
Health Foundation (B17523).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JM, JuW and CZ conceived and designed the
experiments; ZC, JiW, MX, XK, LZ and XS performed the experiments;
ZC, XS, XK and LZ collected and analyzed the data; ZC, JuW and JiW
revised the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Independent Ethics
Committee of the Affiliated Suzhou Hospital of Nanjing Medical
University (Suzhou, China) and all participants provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ng SC and Wong SH: Colorectal cancer
screening in Asia. Br Med Bull. 105:29–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hutagalung AH and Novick PJ: Role of Rab
GTPases in membrane traffic and cell physiology. Physiol Rev.
91:119–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Charng WL, Yamamoto S, Jaiswal M, Bayat V,
Xiong B, Zhang K, Sandoval H, David G, Gibbs S, Lu HC, et al:
Drosophila Tempura, a novel protein prenyltransferase α subunit,
regulates notch signaling via Rab1 and Rab11. PLoS Biol.
12:e10017772014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang C, Yoo Y, Fan H, Kim E, Guan KL and
Guan JL: Regulation of Integrin β 1 recycling to lipid rafts by
Rab1a to promote cell migration. J Biol Chem. 285:29398–29405.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka M, Mun S, Harada A, Ohkawa Y,
Inagaki A, Sano S, Takahashi K, Izumi Y, Osada-Oka M, Wanibuchi H,
et al: Hsc70 contributes to cancer cell survival by preventing
Rab1A degradation under stress conditions. PLoS One. 9:e967852014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coune PG, Bensadoun JC, Aebischer P and
Schneider BL: Rab1A over-expression prevents Golgi apparatus
fragmentation and partially corrects motor deficits in an
alpha-synuclein based rat model of Parkinson's disease. J
Parkinsons Dis. 1:373–387. 2011.PubMed/NCBI
|
|
9
|
Wu G, Yussman MG, Barrett TJ, Hahn HS,
Osinska H, Hilliard GM, Wang X, Toyokawa T, Yatani A, Lynch RA, et
al: Increased myocardial Rab GTPase expression: A consequence and
cause of cardiomyopathy. Circ Res. 89:1130–1137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu H, Qian M, Zhao B, Wu C, Maskey N, Song
H, Li D, Song J, Hua K and Fang L: Inhibition of RAB1A suppresses
epithelial-mesenchymal transition and proliferation of
triple-negative breast cancer cells. Oncol Rep. 37:1619–1626. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu BH, Li XX, Yang Y, Zhang MY, Rao HL,
Wang HY and Zheng XF: Aberrant amino acid signaling promotes growth
and metastasis of hepatocellular carcinomas through Rab1A-dependent
activation of mTORC1 by Rab1A. Oncotarget. 6:20813–20828.
2015.PubMed/NCBI
|
|
12
|
Thomas JD, Zhang YJ, Wei YH, Cho JH,
Morris LE, Wang HY and Zheng XF: Rab1A is an mTORC1 activator and a
colorectal oncogene. Cancer Cell. 26:754–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Chu Y, Wang W and Yuan W: mTORC
signaling in hematopoiesis. Int J Hematol. 103:510–518. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han G, Gong H, Wang Y, Guo S and Liu K:
AMPK/mTOR-mediated inhibition of survivin partly contributes to
metformin-induced apoptosis in human gastric cancer cell. Cancer
Biol Ther. 16:77–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Don AS and Zheng XF: Recent clinical
trials of mTOR-targeted cancer therapies. Rev Recent Clin Trials.
6:24–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Kim E, Yuan H, Inoki K,
Goraksha-Hicks P, Schiesher RL, Neufeld TP and Guan KL: Regulation
of mTORC1 by the Rab and Arf GTPases. J Biol Chem. 285:19705–19709.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang K, Jiang L, Hu Y, Yu J, Chen H, Yao Y
and Zhu X: Short hairpin RNA- mediated gene knockdown of FOXM1
inhibits the proliferation and metastasis of human colon cancer
cells through reversal of epithelial-to-mesenchymal transformation.
J Exp Clin Cancer Res. 34:402015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao X, Kuai X, Pang Z, Zhang L, Wu L, Xu
L and Zhou C: Correlation of Gli1 and HER2 expression in gastric
cancer: Identification of novel target. Sci Rep. 8:3972018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou WW, Chu YP and An GY: Significant
difference of neutrophil-lymphocyte ratio between colorectal
cancer, adenomatous polyp and healthy people. Eur Rev Med Pharmacol
Sci. 21:5386–5391. 2017.PubMed/NCBI
|
|
23
|
Zhang Z, Dong X, Yang X, Wan D, Sun L, Gu
M, Li M, Zhu Z, Wang J, Shang Z, et al: Expression and clinical
significance of absent in melanoma 2 in colorectal cancer. Biomed
Pharmacother. 94:843–849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Phyu SM, Tseng CC, Fleming IN and Smith
TA: Probing the PI3K/Akt/mTor pathway using 31P-NMR spectroscopy:
Routes to glycogen synthase kinase 3. Sci Rep. 6:365442016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Satoh A, Wang Y, Malsam J, Beard MB and
Warren G: Golgin-84 is a rab1 binding partner involved in Golgi
structure. Traffic. 4:153–161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimada K, Uzawa K, Kato M, Endo Y, Shiiba
M, Bukawa H, Yokoe H, Seki N and Tanzawa H: Aberrant expression of
RAB1A in human tongue cancer. Br J Cancer. 92:1915–1921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nikoshkov A, Broliden K, Attarha S,
Sviatoha V, Hellström AC, Mints M and Andersson S: Expression
pattern of the PRDX2, RAB1A, RAB1B, RAB5A and RAB25 genes in normal
and cancer cervical tissues. Int J Oncol. 46:107–112. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun T, Wang X, He HH, Sweeney CJ, Liu SX,
Brown M, Balk S, Lee GS and Kantoff PW: MiR-221 promotes the
development of androgen independence in prostate cancer cells via
downregulation of HECTD2 and RAB1A. Oncogene. 33:2790–2800. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abd Elmageed ZY, Yang Y, Thomas R, Ranjan
M, Mondal D, Moroz K, Fang Z, Rezk BM, Moparty K, Sikka SC, et al:
Neoplastic reprogramming of patient-derived adipose stem cells by
prostate cancer cell-associated exosomes. Stem Cells. 32:983–997.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Liu F, Qin X, Huang T, Huang B,
Zhang Y and Jiang B: Expression of Rab1A is upregulated in human
lung cancer and associated with tumor size and T stage. Aging
(Albany NY). 8:2790–2798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu B, Huang C, Yang X, Li X, Li L and Ding
Y: Significance and prognostic role of human epidermal growth
factor receptor 2 and RAB1A expression in gastric cancer. Oncol
Lett. 15:5185–5192. 2018.PubMed/NCBI
|
|
33
|
Wang ZK, Cheng ZW, Chen SJ, Zhu XG, Gu YP,
Yang XD, Sun L, Liu WT, Zhang YJ, Yuan JF, et al: Aberrant
expression of Rab1A and its prognostic significance in human
colorectal cancer. Eur Rev Med Pharmacol Sci. 22:4509–4517.
2018.PubMed/NCBI
|
|
34
|
Kim YM, Jung CH, Seo M, Kim EK, Park JM,
Bae SS and Kim DH: mTORC1 phosphorylates UVRAG to negatively
regulate autophagosome and endosome maturation. Mol Cell.
57:207–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu J, Fotouhi M and McPherson PS:
Phosphorylation of the exchange factor DENND3 by ULK in response to
starvation activates Rab12 and induces autophagy. EMBO Rep.
16:709–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vaira V, Lee CW, Goel HL, Bosari S,
Languino LR and Altieri DC: Regulation of survivin expression by
IGF-1/mTOR signaling. Oncogene. 26:2678–2684. 2007. View Article : Google Scholar : PubMed/NCBI
|