Introduction
Organ transplantation is not only considered a
last-resort life-saving therapy, but also as the standard treatment
of choice for numerous patients with end-stage organ damage
(1). However, patients undergoing
transplantation may suffer from various complications, including
cancer, infection and cardiovascular disease (2). Among them, malignancy development
following organ transplantation has become a more pressing issue in
the past decade (3–5), as mortalities as a result of
cardiovascular disease and infection decrease in frequency with the
advancement of medical techniques (6). A three- to four-fold increased risk of
cancer has been observed in transplant patients in USA in 2011,
compared with the age-matched general population (3). However, the detailed mechanism of
post-transplant malignancy remains poorly understood.
Organ recipients administer immunosuppressive drugs
to prevent the body rejecting the organ. Cyclosporine A (CsA), an
inhibitor of calcineurin, is frequently used as an
immunosuppressive drug to prevent organ transplant rejection
(7). It has been well-documented
that immunosuppressant therapy increases the risk of
post-transplant cancer (4,8,9). Hojo
et al (10) demonstrated a
tumor growth-promoting effect of CsA in immunodeficient mice, which
was attributed to transforming growth factor-β upregulation.
However, Sato et al (11)
reported that CsA at high concentrations (1 µg/ml) induces
apoptosis in human lung adenocarcinoma cells in a caspase-dependent
manner. Thus, the effect of CsA on cell proliferation requires
further investigation.
Additionally, increasing evidence demonstrated that
metabolic homeostasis is crucial in maintaining human health
(12). Obesity is associated with
metabolic alterations and is considered an important risk factor
for the development of a number of cancer types, including colon,
breast, kidney and lung cancer (6,8–11).
However, in patients with cardiovascular disease, chronic renal
failure, chronic pulmonary obstructive disease, acquired immune
deficiency syndrome or rheumatoid arthritis, the presence of
obesity appears to be a paradoxical protective factor for their
survival (13,14), which is termed the ‘obesity
paradox’, and has been confirmed by clinical investigation
(15,16). In line with these observations, the
obesity paradox was also demonstrated to occur in organ recipients
(17). As increased free fatty
acids are detected in overweight and obese subjects (18), the metabolic dependence of
immunosuppressants on carcinogenesis requires further studies, as
it may account for this paradox.
Lung cancer is among the four most common cancer
types in transplant recipients in USA in 2011, particularly
following lung transplantation (4,19–21).
Therefore, in the present study, the effect of CsA on
carcinogenesis in human non-small cell lung cancer A549 cells
exposed to different metabolites (glucose or palmitic acid) was
examined, and the underlying mechanisms were determined.
Materials and methods
Cell culture
The human non-small cell lung cancer cell line A549
was obtained from Shanghai Meixuan Biological Technology Co., Ltd.
(Shanghai, China). A549 cells were cultured in a 37°C humidified
incubator with 5% CO2 in RMPI-1640 medium (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA); and 1% penicillin/streptomycin (100X;
Invitrogen; Thermo Fisher Scientific, Inc.). Medium was replaced
every 2 days.
Reagents
Reagents included: CsA (Medchem Express, Princeton,
NJ, USA); wortmannin (Wm; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany); RMPI-1640; penicillin/streptomycin (100X); FBS;
N-acetyl-cysteine (NAC; Invitrogen; Thermo Fisher Scientific,
Inc.); Palmitic acid (PA; Sigma-Aldrich; Merck KGaA);
D-Arg-2′,6′-dimethyltyrosine-Lys-Phe-NH2 (SS-31; supplied by Dr
Xing Zhang from Department of Aerospace Medicine, Fourth Military
Medical University, Xi'an, China).
Cell viability assay
Cell viability was measured using a commercial Cell
Counting Kit-8 from Medchem Express (cat. no. HY-K0301), according
to manufacturer's protocol. The cytotoxic effect of CsA against
A549 cells under glucose loading were examined as aforementioned at
the concentrations of 0, 0.1 and 1 µM at 37°C for 48 h in RMPI-1640
medium. To determine the effect of glucose in CsA-induced A549 cell
proliferation, 5, 10, 20 and 30 mM glucose was replaced with
equimolar mannitol, the isotonic control of glucose. To determine
the role of phosphoinositide 3-kinase (PI3K)/Akt signaling in
CsA-induced cell proliferation, A549 cells were treated with PI3K
inhibitor Wm (200 nM) prior to CsA treatment, and cell viability
was assessed as aforementioned. The cytotoxic effect of PA against
A549 cells were examined as above mentioned for 48 h in RMPI-1640
medium supplemented with 0, 50, 100, 200 or 500 µM PA. The
cytotoxic effect of CsA against A549 cells under normal lipid
loading were examined as aforementioned at 0.1 µM (the optimal dose
for cell proliferation) for 48 h in RMPI-1640 medium supplemented
with 200 µM PA. To determine the role of ROS in CsA-induced A549
cell proliferation, intracellular ROS scavenger (NAC; 10 µM) or
mitochondrial ROS scavenger (SS-31; 10 µM) were added to the
culture prior to CsA treatment, and cell viability was assessed as
aforementioned.
EdU incorporation assay
A549 cells were cultured in 35 mm confocal dishes
and treated with or without CsA (1 µM) at 37°C for 48 h. All cells
were treated with 50 µM EdU for 2 h at 37°C, and fixed with 4%
paraformaldehyde for at room temperature 15 min. The fixed cells
were treated with 0.3% Triton X-100 at room temperature for 10 min
and washed with PBS three times. Thereafter, the cells were exposed
to Click reaction solution (Beyotime Institute of Biotechnology,
Haimen, China) for 30 min, followed by incubation with 5 µM Hoechst
33342 at room temperature for 10 min to stain the cell nuclei.
Images were captured using an inverted confocal microscope (Zeiss
LSM 800; Carl Zeiss AG, Oberkochen, Germany) with a ×40 1.3NA
oil-immersion objective. The proliferation index was calculated by
dividing the number of EdU-labeled cells by the total number of
cells (Hoechst-positive).
PA preparation
Stock PA was dissolved in ethanol at a concentration
of 10 mM and diluted to 50, 100, 200 or 500 µM in RMPI-1640
containing 1% (w/v) bovine serum albumin (BSA; Sigma-Aldrich; Merck
KGaA). As a vehicle control, the same volume of ethanol as used in
the 50 µM PA group was diluted in RMPI-1640 containing 1% (w/v)
BSA.
Determination of intracellular
reactive oxygen species (ROS) production
Intracellular ROS production was measured using a
dihydroethidium (DHE) probe. Briefly, A549 cells were treated with
or without 0.1 µM CsA under normal glucose or lipid loading at 37°C
for 48 h, and incubated with 2.5 µM DHE for 30 min at 37°C. After
cells were washed in PBS three times, fluorescence was measured
with a FluoStar Omega (BMG Labtech GmbH, Ortenberg, Germany) at
excitation and emission wavelengths of 480 and 590 nm,
respectively.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from treated A549 cells with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocols, and reverse
transcribed into cDNA with the PrimeScript RT Reagent kit with gDNA
Eraser (Takara Bio, Inc., Otsu, Japan). Subsequently, qPCR was
performed using SYBR® Premix Ex Taq II (Takara Bio,
Inc.) on a CFX96 real-time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The thermocycling conditions were as follows:
95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for
30 sec, with a final extension at 72°C for 10 min. Relative mRNA
expression levels of Cyclin D1 and p27 were quantified using the
2−ΔΔCq method (22), and
the results were normalized to β-actin as an internal control. The
sequences of primer sets used in this analysis were as follows:
Cyclin D1, forward, 5′-TGTCCTACTACCGCCTCACA-3′, and reverse,
5′-CAGGGCTTCGATCTGCTC-3′; p27, forward,
5′-TAATTGGGGCTCCGGCTAACT-3′, and reverse,
5′-TGCAGGTCGCTTCCTTATTCC-3′; and β-actin, forward,
5′-CGCCCCAGGCACCAGGGC-3′, and reverse, 5′-GCTGGGGTGTTGAAGGT-3′.
Western blotting
Following drug treatment, A549 cells were washed
with cold PBS three times and lysed in radioimmunoprecipitation
assay lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet
P-40, 1% Triton X-100 and 1 mM phenylmethylsulfonyl fluoride). Cell
lysates were incubated on ice for 15 min, and then cleared by
high-speed centrifugation (13,000 × g at 4°C for 15 min).
Subsequently, the total protein concentration was measured with a
bicinchoninic acid protein assay. Protein samples were separated by
SDS-PAGE (15%), and then transferred to a polyvinylidene difluoride
membrane. Membranes were blocked with 5% milk at room temperature
for 1 h and subsequently incubated with the appropriate primary
antibodies against Cyclin D1 (cat. no. AF1183; 1:5,000; 34 kDa;
Beyotime Institute of Biotechnology), p27 (cat. no. AP027; 1:5,000;
27 kDa; Beyotime Institute of Biotechnology), caspase-3 (cat. no.
9662; 1:1,000; 35 kDa; Cell Signaling Technology, Inc., Danvers,
MA, USA), cleaved caspase-3 (cat. no. 9664; 1:1,000; 17 kDa; Cell
Signaling Technology, Inc.), phospho-protein kinase B (cat. no.
4060; 1:1,000; Akt; S473; Cell Signaling Technology, Inc.), Akt
(cat. no. 4691; 1:1,000; 60 kDa; Cell Signaling Technology, Inc.)
and β-actin (cat. no. AF0003; 1:1,000; 43 kDa; Beyotime Institute
of Biotechnology) at 4°C overnight. Subsequently, membranes were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG antibody (cat. no. A0208; 1:1,000; Beyotime Institute of
Biotechnology) or goat anti-mouse IgG antibody (cat. no. A0216;
1:1,000; Beyotime Institute of Biotechnology) for 1–2 h at room
temperature. Finally, the blots were visualized using an Enhanced
Chemiluminescence-Plus reagent (Millipore, Billerica, MA, USA), and
detected by ChemiDocXRS (Bio-Rad Laboratories, Inc.) and analyzed
with a Bio-Image Analysis system (Bio-Rad Laboratories, Inc.).
Statistical analysis
Student's unpaired t-test or one-way analysis of
variance followed by Bonferroni's post hoc test were used for
statistical analysis of cellular data. Data are shown as the mean ±
standard error of the mean. Statistical tests were performed using
GraphPad Prism software version 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cyclosporine A promotes cell
proliferation in A549 cells under glucose loading
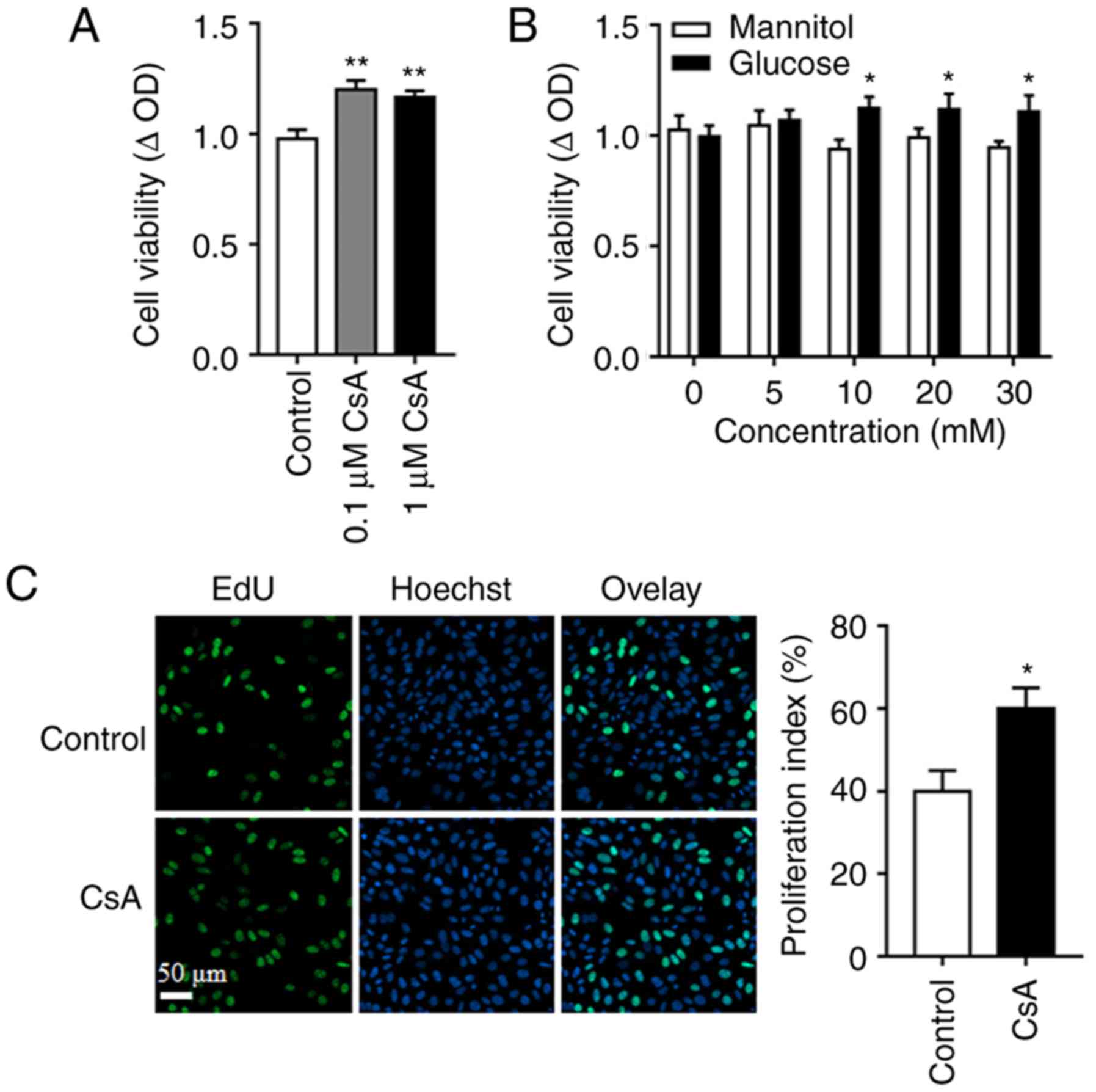
To investigate the effects of CsA on post-transplant
malignancy, A549 cell proliferation in response to different
concentrations of CsA (0, 0.1 and 1 µM) for 48 h was measured, and
the results demonstrated the pro-cancer effect of CsA (Fig. 1A). Furthermore, by replacing glucose
with mannitol, the isotonic control of glucose, it was indicated
that CsA promoted cell proliferation when glucose concentration was
high (10, 20 and 30 mM; Fig. 1B),
demonstrating that glucose is a vital factor in CsA-induced cell
proliferation. In line with these results, the number of
EdU-labeled cells following CsA treatment increased, compared with
the control group (Fig. 1C).
Involvement of PI3K/Akt signaling
pathway in CsA-induced cell proliferation
It is well-documented that aberrant Akt activation
contributes to lung carcinogenesis (23,24),
and PI3K/Akt signaling is involved in the regulation of various
cell functions, including cell survival, proliferation and cell
cycle progression (25). RT-qPCR
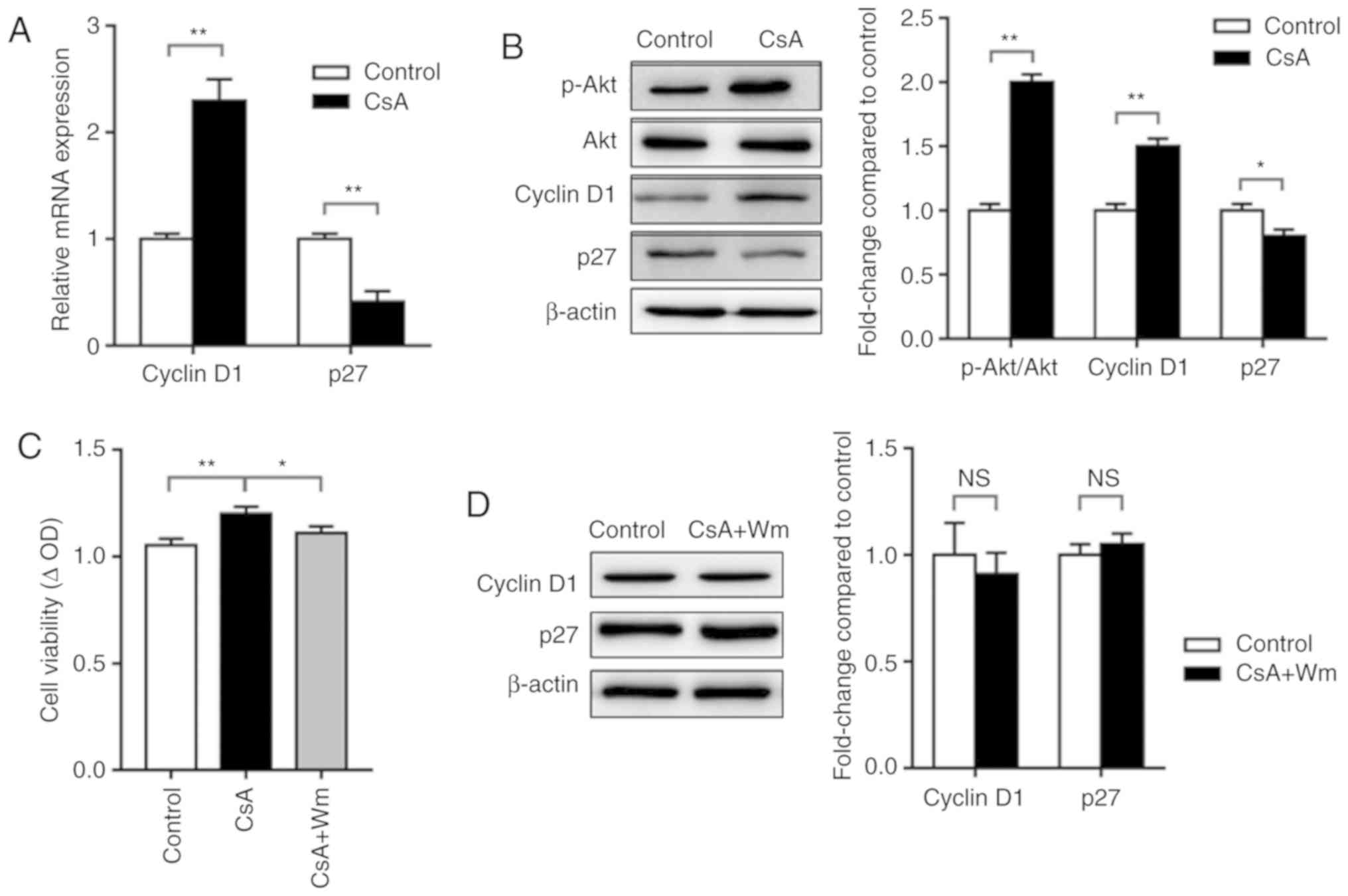
analysis of the expression of cell cycle-associated genes revealed
that 0.1 µM CsA increased Cyclin D1 mRNA expression and decreased
p27 mRNA expression (Fig. 2A).
Similarly, western blot analysis demonstrated that CsA increased
the phosphorylation of Akt and the expression of Cyclin D1, while
decreasing the expression of p27 (Fig.
2B). Pharmacological intervention of PI3K/Akt signaling with Wm
attenuated CsA-induced cell proliferation (Fig. 2C), while slightly increasing the
expression of p27 and decreasing the expression of Cyclin D1
(Fig. 2D). These results indicated
the involvement of PI3K/Akt signaling in CsA-induced cell
proliferation.
Intracellular ROS scavenger NAC
attenuates CsA-induced cell proliferation
ROS-mediated activation of Akt has been
well-documented (26), and our
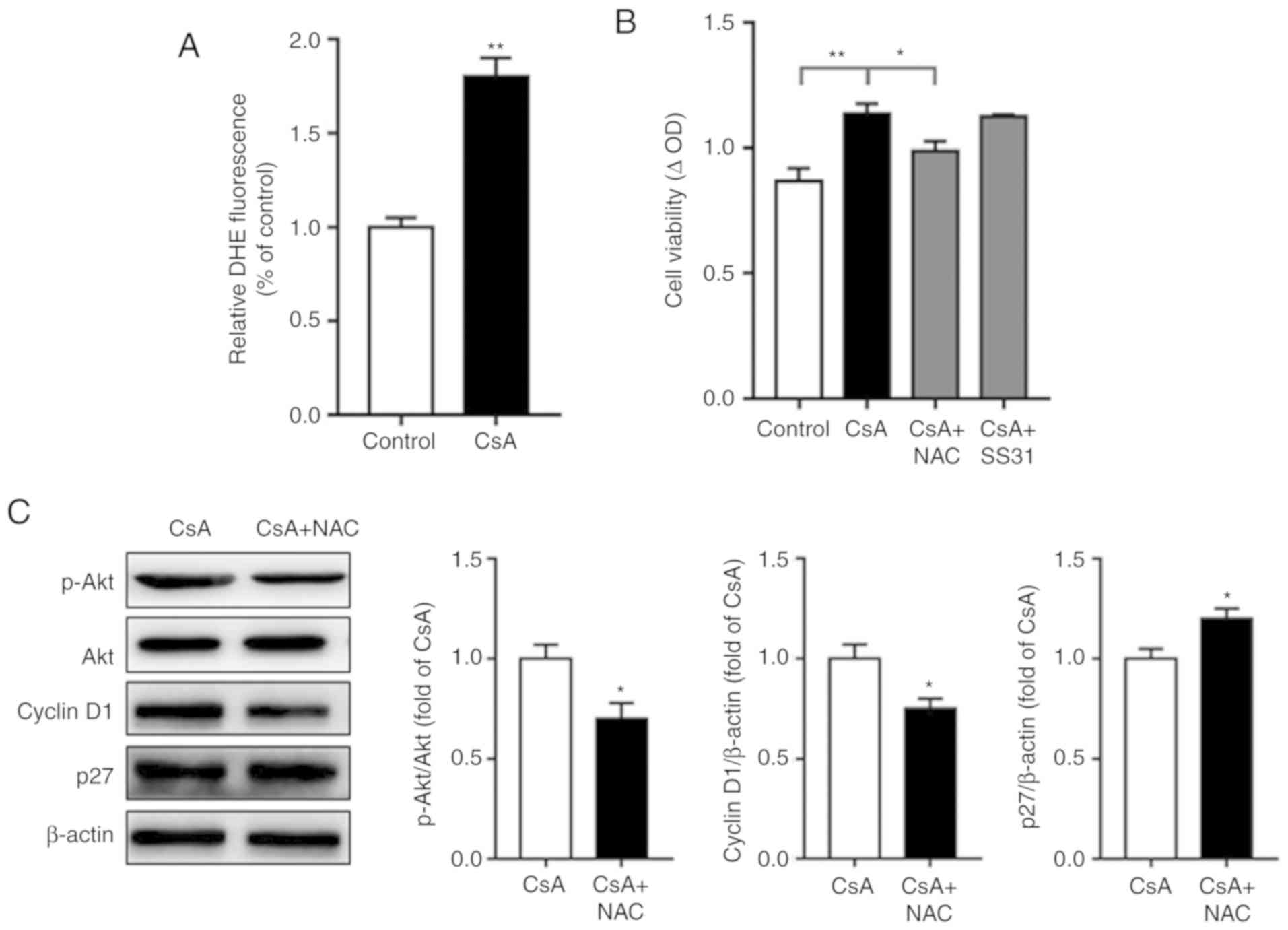
previous study demonstrated that CsA increases intracellular ROS
production in insulin-resistant C2C12 cells (27). In line with this, the present study
indicated that CsA treatment increased intracellular ROS production
in A549 cells (Fig. 3A).
Additionally, NAC attenuated CsA-induced cell proliferation,
whereas SS-31, an efficient mitochondrion-targeted antioxidant, did
not significantly affect this process (Fig. 3B), which may be due to the
predominance of glycolysis, instead of the Krebs cycle, in cancer
cells (28). Furthermore,
intracellular ROS scavenger NAC decreased CsA-mediated Akt
activation as well as Cyclin D1 expression, while increasing p27
expression (Fig. 3C). These results
indicated that ROS-mediated activation of Akt contributed to
CsA-induced cell proliferation in A549 cells under normal glucose
loading.
CsA decreases cell proliferation under
normal lipid loading
Obesity, characterized as the alteration of
metabolic balance between glucose and fatty acid oxidation, is
associated with reduced mortality, termed the ‘obesity paradox’
(29). Thus, the effect of fatty
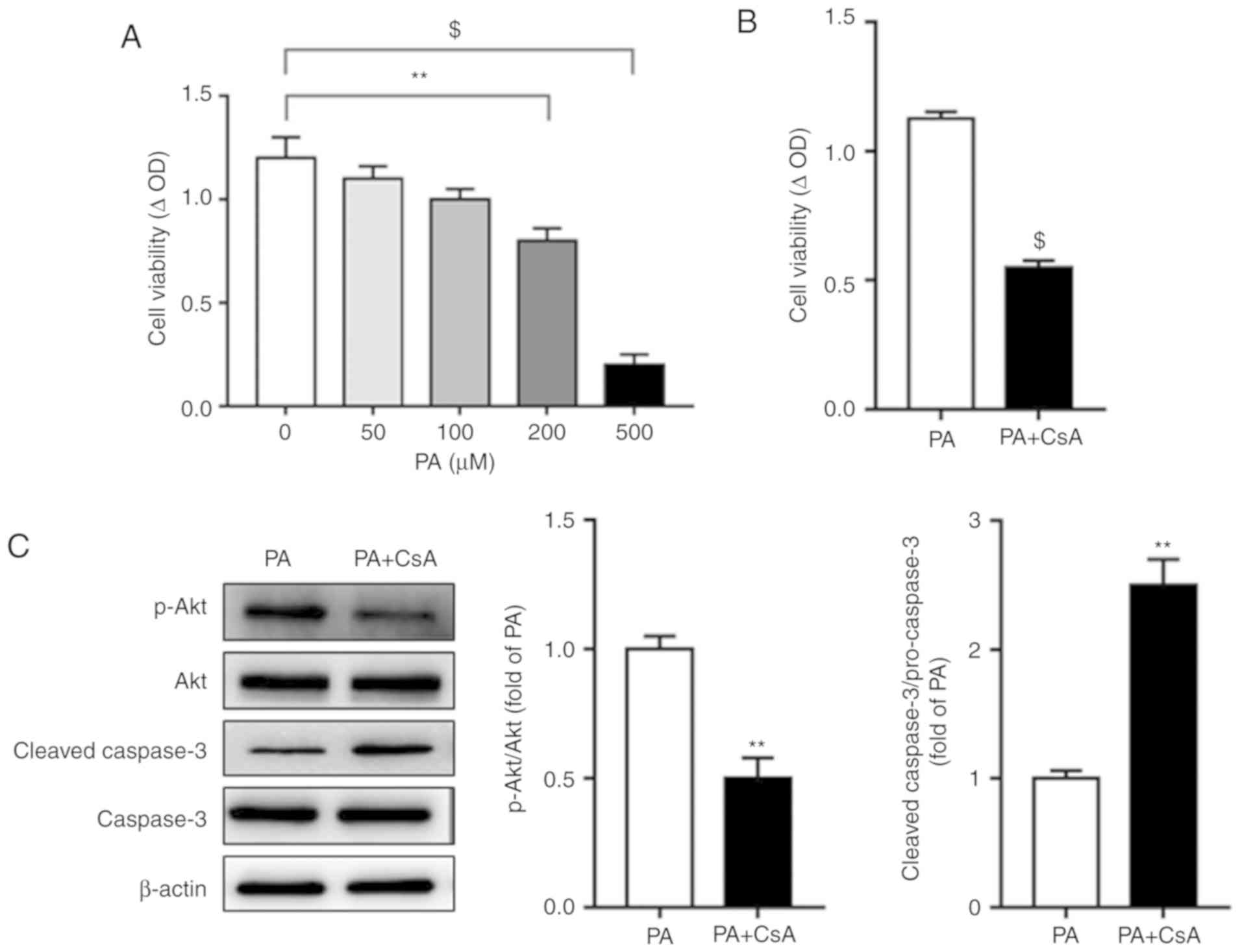
acids on CsA-mediated cell proliferation was investigated. PA is
the most prevalent saturated free fatty acid (FFA) in circulation,
accounting for ~28% of FFAs in serum (30). The effect of different
concentrations of PA on cell viability was assayed, and the results
demonstrated that 200 or 500 µM PA significantly decreased A549
cell proliferation (Fig. 4A).
Notably, under normal lipid loading (200 µM PA), CsA decreased A549
cell proliferation (Fig. 4B),
indicating a divergent role of CsA on cell proliferation in the
presence of different metabolic substrates. In line with this
observation, a decrease in cell proliferation by CsA was
accompanied by decreased Akt phosphorylation and increased cleaved
caspase-3 expression (Fig. 4C).
Excessive ROS contributes to
CsA-mediated inhibition of cell proliferation under normal lipid
loading
To investigate underlying mechanisms, intracellular
ROS levels were assessed using a DHE probe. The results
demonstrated that compared with the control group (glucose alone as
the energy source), PA significantly increased intracellular ROS
levels (Fig. 5A). Scavenging
intracellular ROS with NAC, or mitochondrial ROS with SS-31,
attenuated the CsA-mediated A549 cell proliferation inhibition
under PA load, while no significant changes in cell viability were
observed between the PA+CsA+NAC and PA+CsA+SS-31 groups (Fig. 5B). These results demonstrated the
divergent roles of CsA on cell proliferation in the presence of
different metabolic substrates.
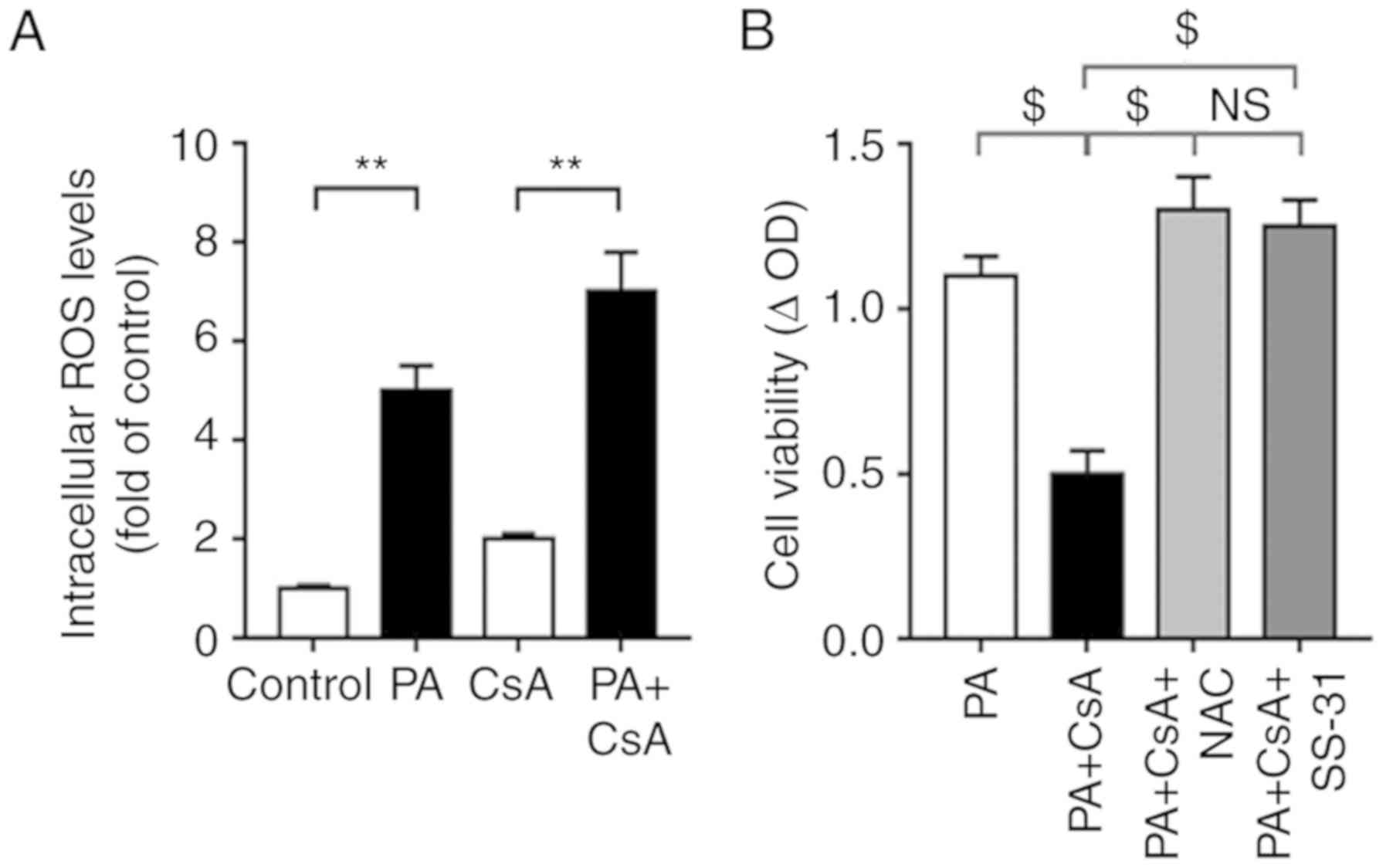 | Figure 5.Excessive ROS contributes to
CsA-mediated inhibition of cell proliferation under normal lipid
loading. (A) Following treatment with control, CsA, PA and PA+CsA,
intracellular ROS production was measured using a dihydroethidine
probe. (B) Cell proliferation was assessed in A549 cells when
incubating NAC (intracellular ROS scavenger) or SS-31
(mitochondrial ROS scavenger) prior to CsA treatment under normal
lipid loading. All values are expressed as the mean ± standard
error of the mean of three independent experiments. **P<0.01 and
$P<0.0001. NS, no significance; CsA, cyclosporine A;
ROS, reactive oxygen species; NAC, N-acetyl-cysteine; SS-31,
D-Arg-2′,6′-dimethyltyrosine-Lys-Phe-NH2; PA, palmitic acid; CsA,
cyclosporine A. |
Discussion
Increasing evidence demonstrated that organ
transplantation is associated with an increased risk of (3–5).
Recently, the immunosuppressor CsA was demonstrated to contribute
to post-transplant malignancy (8,9).
However, the effects of CsA on cell proliferation were unclear and
required further investigation. In the present study, the
pleiotropic effects of CsA on carcinogenesis in the presence of
different metabolic substrates (glucose or PA) were reported. When
cultured under glucose loading, CsA increased cell proliferation as
well as Akt/Cyclin D1 signaling; however, this pro-cancer effect
was reversed when supplemented with 200 µM PA.
Although malignancy accounts for a small percentage
of mortality in the first year after transplantation, the
International Society for Heart and Lung Transplantation Registry
reports in 2012 that malignancy accounts for ~15% of mortalities
beyond 5 years post-transplantation based on data from centers
globally (4,5). Non-melanoma skin cancer and
post-transplant lymphoproliferative disease are the most common
post-transplant malignancies (5).
However, lung cancer, including non-small cell and small cell lung
carcinoma, is increasingly becoming a frequent complication in
patients (31). Genetic, cellular,
molecular and environmental factors all serve a crucial role in
post-transplant carcinogenesis (4).
Previous studies demonstrated that tumor incidence increases with
time following organ transplantation and is associated with the
intensity of immunosuppression (32,33).
Certain studies indicated that CsA treatment inhibits
carcinogenesis (34,35), whereas others reported contradictory
results (11,36). Thus, further investigation of CsA on
carcinogenesis is of critical importance.
Increasing studies demonstrated that among patients
with cancer, elevated body mass index (BMI) is associated with
improved survival, compared with normal-weight patients, indicating
the existence of an ‘obesity paradox’ (29). Furthermore, a paradoxical U-shaped
association of BMI with outcomes is also observed in transplant
recipients (37,38). Obesity is characterized by metabolic
abnormalities, including hyperglycemia, insulin resistance and
hyperlipidemia; thus, alterations of metabolic substrate preference
may be of critical importance in this paradox (39). The efficient use of CsA as an
immunosuppressant has been limited by its toxic effects, including
nephrotoxicity, hepatotoxicity and neurotoxicity. Additionally,
obese patients have an increased risk of toxicity, compared with
lean subjects, and a smaller dose is required for obese
transplantation recipients (40),
indicating that alterations of metabolic substrates may contribute
to differential toxicities of CsA in obese and lean subjects. In
the present study, the pleiotropic effects of CsA in the regulation
of cell proliferation in human lung adenocarcinoma cells exposed to
different metabolic substrates was reported.
An association between ROS and CsA-induced toxicity
has been reported (41). The
results of the present study, as well as previous studies,
demonstrated that CsA treatment induces ROS production and lipid
peroxidation (27,42,43).
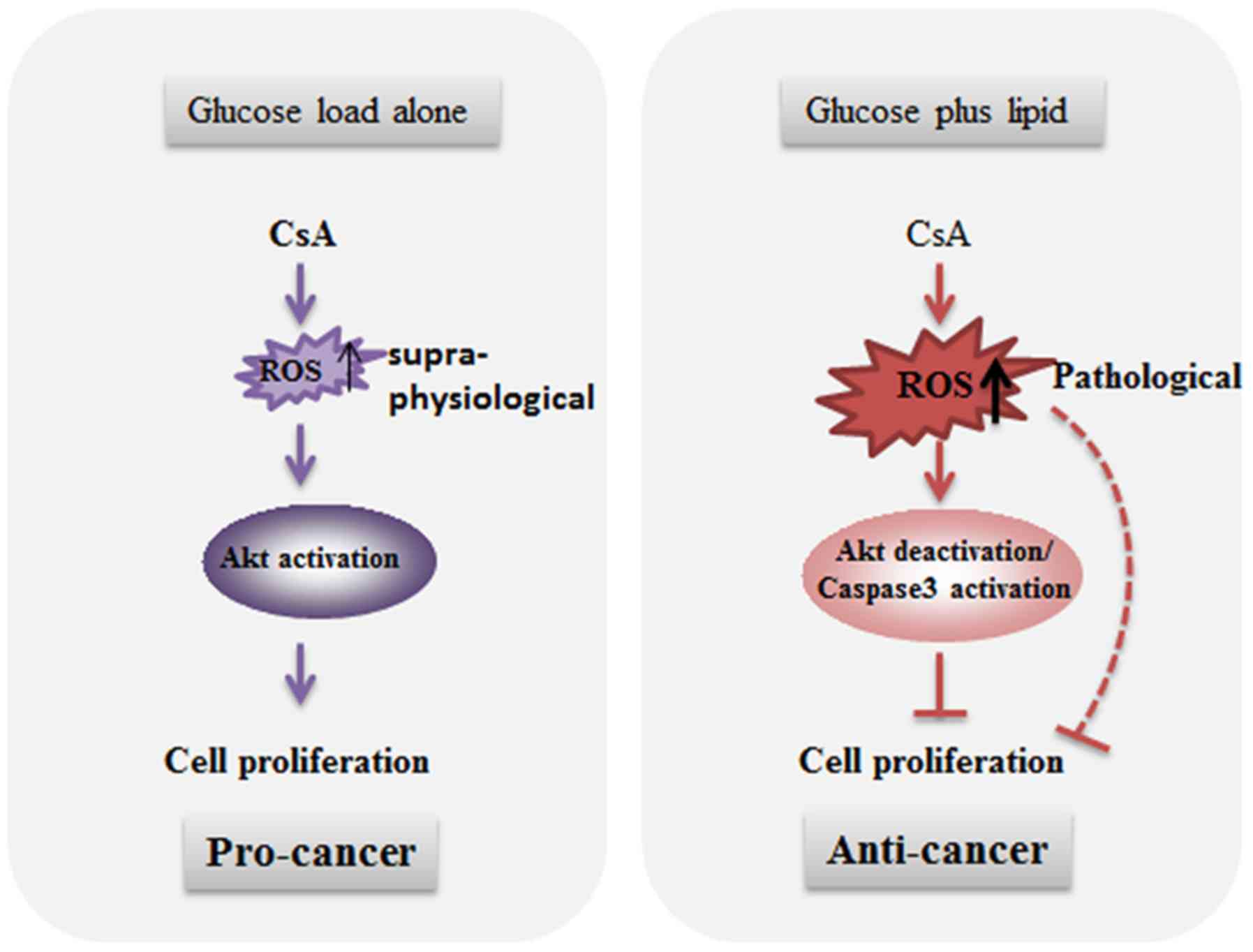
It has been well established that supra-physiological levels of ROS
may activate/deactivate certain signaling molecules, such as Akt,
and affect a number of physiological processes, including
regulation of cell cycle, cell proliferation and survival (26). Furthermore, pathological levels of
ROS have an important role in apoptosis induction. Therefore, the
results demonstrated that supra-physiological levels of ROS induced
by CsA promoted Akt signaling and cell proliferation under glucose
loading, whereas under glucose/PA loading, CsA treatment induced
pathological levels of ROS production and decreased cell
viability.
Collectively, the data indicated a divergent role of
CsA in the regulation of A549 cell proliferation with different
metabolic substrates (Fig. 6),
indicating that the CsA-mediated increase in cell proliferation
could contribute to increased post-transplant cancer risk.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Science
Foundation of China (grant no. 31500928; XQ), the Fundamental
Research Funds for the Central University (grant no. G2018KY0303;
XQ), Shaanxi Provincial Research Center for the Project of
Prevention and Treatment of Respiratory Disease (grant no.
2016HXKF04; ZC) and the Shaanxi Key Laboratory of Ischemic
Cardiovascular Disease for the Open Fund Project (grant no.
2016ZDKF03; ZC).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC designed the research, analyzed the results and
revised the manuscript. XQ performed the experiments, interpreted
the results of experiments and drafted the manuscript. Both authors
approved the final version of manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CsA
|
cyclosporine A
|
|
BMI
|
body mass index
|
|
PA
|
palmitic acid
|
|
Wm
|
Wortmannin
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Saidi RF and Hejazii Kenari SK: Clinical
transplantation and tolerance: Are we there yet? Int J Organ
Transplant Med. 5:137–145. 2014.PubMed/NCBI
|
|
2
|
Katabathina V, Menias CO, Pickhardt P,
Lubner M and Prasad SR: Complications of immunosuppressive therapy
in solid organ transplantation. Radiol Clin North Am. 54:303–319.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Engels EA, Pfeiffer RM, Fraumeni JF Jr,
Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly
AR, Clarke CA, et al: Spectrum of cancer risk among US solid organ
transplant recipients. JAMA. 306:1891–1901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perez-Callejo D, Torrente M, Parejo C,
Laporta R, Ussetti P and Provencio M: Lung cancer in lung
transplantation: Incidence and outcome. Postgrad Med J. 94:15–19.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Christie JD, Edwards LB, Kucheryavaya AY,
Benden C, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Stehlik J,
Hertz M, International Society of Heart and Lung Transplantation:
The registry of the International Society for Heart and Lung
Transplantation: 29th adult lung and heart-lung transplant
report-2012. J Heart Lung Transplant. 31:1073–1086. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chapman JR, Webster AC and Wong G: Cancer
in the transplant recipient. Cold Spring Harb Perspect Med. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
7.Cohen DJ, Loertscher R, Rubin MF, Tilney
NL, Carpenter CB and Strom TB: Cyclosporine: A new
immunosuppressive agent for organ transplantation. Ann Intern Med.
101:667–682. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Penn I and Starzl TE: Immunosuppression
and cancer. Transplant Proc. 5:943–947. 1973.PubMed/NCBI
|
|
9
|
Dantal J and Soulillou JP:
Immunosuppressive drugs and the risk of cancer after organ
transplantation. N Engl J Med. 352:1371–1373. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hojo M, Morimoto T, Maluccio M, Asano T,
Morimoto K, Lagman M, Shimbo T and Suthanthiran M: Cyclosporine
induces cancer progression by a cell-autonomous mechanism. Nature.
397:530–534. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato M, Tsujino I, Fukunaga M, Mizumura K,
Gon Y, Takahashi N and Hashimoto S: Cyclosporine a induces
apoptosis of human lung adenocarcinoma cells via caspase-dependent
pathway. Anticancer Res. 31:2129–2134. 2011.PubMed/NCBI
|
|
12
|
Brestoff JR and Artis D: Immune regulation
of metabolic homeostasis in health and disease. Cell. 161:146–160.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calle EE and Kaaks R: Overweight, obesity
and cancer: Epidemiological evidence and proposed mechanisms. Nat
Rev Cancer. 4:579–591. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calle E, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gonzalez MC, Pastore CA, Orlandi SP and
Heymsfield SB: Obesity paradox in cancer: New insights provided by
body composition. Am J Clin Nutr. 99:999–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fonarow GC, Srikanthan P, Costanzo MR,
Cintron GB and Lopatin M; ADHERE Scientific Advisory Committee
Investigators, : An obesity paradox in acute heart failure:
Analysis of body mass index and inhospital mortality for 108,927
patients in the acute decompensated heart failure national
registry. Am Heart J. 153:74–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park J, Ahmadi SF, Streja E, Molnar MZ,
Flegal KM, Gillen D, Kovesdy CP and Kalantar-Zadeh K: Obesity
paradox in end-stage kidney disease patients. Prog Cardiovasc Dis.
56:415–425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boden G: Obesity and free fatty acids.
Endocrinol Metab Clin North Am. 37:635–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roithmaier S, Haydon AM, Loi S, Esmore D,
Griffiths A, Bergin P, Williams TJ and Schwarz MA: Incidence of
malignancies in heart and/or lung transplant recipients: A
single-institution experience. J Heart Lung Transplant. 26:845–849.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Raemdonck D, Vos R, Yserbyt J,
Decaluwe H, De Leyn P and Verleden GM: Lung cancer: A rare
indication for, but frequent complication after lung
transplantation. J Thorac Dis. 8:S915–S924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katabathina VS, Menias CO, Tammisetti VS,
Lubner MG, Kielar A, Shaaban A, Mansour J, Surabhi VR and Hara AK:
Malignancy after solid organ transplantation: Comprehensive imaging
review. Radiographics. 36:1390–1407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Testa JR and Tsichlis PN: AKT signaling in
normal and malignant cells. Oncogene. 24:7391–7393. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lawlor MA and Alessi DR: PKB/Akt: A key
mediator of cell proliferation, survival and insulin responses? J
Cell Sci. 114:2903–2910. 2001.PubMed/NCBI
|
|
26
|
Clerkin JS, Naughton R, Quiney C and
Cotter TG: Mechanisms of ROS modulated cell survival during
carcinogenesis. Cancer Lett. 266:30–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin X, Li X, Liu C and Chen Z: A novel
mechanism of pre-transplant insulin resistance contributing to
post-transplant complications: Cyclosporin A-induced
O-GlcNAcylation. Biochem Biophys Res Commun. 492:172–177. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moreno-Sanchez R, Rodriguez-Enriquez S,
Marin-Hernandez A and Saavedra E: Energy metabolism in tumor cells.
FEBS J. 274:1393–1418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lenno H, Sperrin M, Badric E and Renehan
AG: The obesity paradox in cancer: A review. Curr Oncol Rep.
18:562016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klein S and Wolfe RR: Carbohydrate
restriction regulates the adaptive response to fasting. Am J
Physiol. 262:E631–E636. 1992.PubMed/NCBI
|
|
31
|
Robbins HY and Arcasoy SM: Malignancies
following lung transplantation. Clin Chest Med. 32:343–355. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dantal J, Hourmant M, Cantarovich D, Giral
M, Blanch G, Dren B and Soulillou JP: Effect of long-term
immunosuppression in kidney-graft recipients on cancer incidence:
Randomised comparison of two cyclosporin regimens. Lancet.
351:623–628. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wimmer CD, Rentsch M, Crispin A, Illner
WD, Arbogast H, Graeb C, Jauch KW and Guba M: The janus face of
immunosuppression-de novo malignancy after renal transplantation:
The experience of the Transplantation Center Munich. Kidney Int.
71:1271–1278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Masuo T, Okamura S, Zhang Y and Mori M:
Cyclosporine A inhibits colorectal cancer proliferation probably by
regulating expression levels of c-Myc, p21(WAF1/CIP1) and
proliferating cell nuclear antigen. Cancer Lett. 285:66–72. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawahara T, Kashiwagi E, Ide H, Li Y,
Zheng Y, Ishiguro H and Miyamoto H: The role of NFATc1 in prostate
cancer progression: Cyclosporine A and tacrolimus inhibit cell
proliferation, migration, and invasion. Prostate. 75:573–584. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yokoyama I, Hayashi S, Sato E, Kobayashi
T, Negita M, Uchida K and Takagi H: Enhancement of tumor
proliferation by cyclosporine A in early phase of experimental
hepatic metastasis. Jpn J Cancer Res. 85:704–709. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chaikriangkrai K, Jhun HY, Graviss EA and
Jyothula S: Overweight-mortality paradox and impact of six-minute
walk distance in lung transplantation. Ann Thorac Med. 10:169–175.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
DiCecco SR and Francisco-Ziller N: Obesity
and organ transplantation: Successes, failures, and opportunities.
Nutr Clin Pract. 29:171–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Després JP and Lemieux I: Abdominal
obesity and metabolic syndrome. Nature. 444:881–887. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Flechner SM, Kolbeinsson ME, Tam J and Lum
B: The impact of body weight on cyclosporine pharmacokinetics in
renal transplant recipients. Transplantation. 47:806–810. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee J: Use of antioxidants to prevent
cyclosporine a toxicity. Toxicol Res. 26:163–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Perez de Lema G, Arribas I, Prieto A,
Parra T, De Arriba G, Rodríguez-Puyol D and Rodríguez-Puyol M:
Cyclosporin A-induced hydrogen peroxide synthesis by cultured human
mesangial cells is blocked by exogenous antioxidants. Life Sci.
62:1745–1753. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McGrath LT, Treacy R, McClean E and Brown
JH: Oxidative stress in cyclosporin and azathioprine treated renal
transplant patients. Clin Chim Acta. 264:1–12. 1997. View Article : Google Scholar : PubMed/NCBI
|