Introduction
Lung cancer is the main cause of cancer-associated
mortality worldwide (1), and
squamous cell lung carcinoma is one of the most common types of
non-small-cell lung cancer (NSCLC). Despite treatment with surgical
resection combined with chemotherapy and radiotherapy, patients
with squamous cell lung carcinoma exhibit an overall 5-year
survival of <15% (2).
Gemcitabine (GEM) is routinely used as the standard first-line
treatment for advanced squamous cell lung carcinoma; however, the
majority of the patients inevitably develop resistance to GEM,
followed by tumor progression (3–5).
Although molecular targeting drugs and immunotherapy have been
beneficial in the treatment of lung adenocarcinoma, there is yet no
clear targeted drug treatment for squamous cell lung carcinoma
(6). Therefore, targeted prevention
and treatment of peritoneal carcinomatosis from lung cancer is
crucial for improving the quality of life and prognosis of
patients.
Hyperthermia using a number of energy sources, such
as ultrasound, laser, radiofrequency and microwaves, has long been
investigated for cancer treatment (7). Microwave hyperthermia (MWHT), due to
its low incidence of adverse reactions, easy clinical
implementation and effectiveness in improving the results of
traditional radiochemotherapy, has been widely used as an adjuvant
cancer treatment. A large number of clinical studies have confirmed
that MWHT can improve the prognosis of patients with cancer,
including hepatic, breast, bladder and lung cancer (7–13).
Indeed, the ability of MWHT to enhance the anticancer effects of
chemotherapy is well known. It has previous been demonstrated that
hyperthermia (conventional water bath) combined with GEM
significantly inhibited the growth and proliferation of cancer
cells, and promoted apoptotic cell death (14). However, traditional water bath
hyperthermia only involves thermal effects, and its preclinical
research is limited. To resolve this issue, our recent study
(15) improved the feasibility and
safety of this treatment by independently developing a novel,
non-invasive hyperthermia instrument (patent no. CN-204824903-U)
based on the use of microwaves with 433-MHz frequency for the
treatment of NSCLC in vitro and in vivo. This study
reported that MWHT induced caspase-3-dependent apoptosis and G2/M
cell cycle arrest in NSCLC cells (15). Additionally, it was demonstrated
that MWHT combined with GEM markedly inhibited the proliferation
and induced the apoptosis of human squamous cell lung carcinoma
cells in vitro (16).
However, whether autophagy is activated by MWHT in lung cancer
cells remains unknown.
Autophagy is the process by which cells encapsulate
their own cytoplasmic proteins, intracellular pathogens and damaged
organelles to form vesicles in lysosomes under starvation and
energy stress conditions (17,18).
Autophagy serves a dual role by inhibiting or promoting
tumorigenesis and cancer development, and is continually activated
in tumor cells following anticancer therapies, such as
chemotherapy, radiotherapy and hyperthermia (19,20).
Under physiological conditions, reactive oxygen species (ROS) serve
an important role in immune response, gene regulation and signaling
pathways. Studies have reported that hyperthermia induces autophagy
and changes in the intracellular ROS content (21,22).
Furthermore, ROS produced by hyperthermia not only participate in
the physiological process of cell proliferation, differentiation
and apoptosis (23,24), but can also be used as signaling
molecules participating in the activation of cell autophagy. The
autophagic process is often accompanied by changes in ROS content.
However, to the best of our knowledge, the association between ROS
and autophagy in MWHT combined with GEM treatment has not been
reported in squamous cell lung carcinoma.
In the present study, the effectiveness of MWHT in
combination with GEM treatment in human squamous cell lung
carcinoma cells was investigated, and the underlying mechanism was
examined.
Materials and methods
Cells and cell cultures
The human squamous cell lung carcinoma cell lines
NCI-H1703 and NCI-H2170 were purchased from the American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin/streptomycin at 37°C in an atmosphere containing 5%
CO2.
Treatment of cells with MWHT and
GEM
According to the experimental method applied in a
previous study (16), NCI-H1703 and
NCI-H2170 cell suspensions (1×104 cells/well) were
seeded into 96-well plates overnight. Cells were then divided into
four experimental groups and treated as follows: Control (CON)
group, which was not treated; MW group, treated with the novel
microwave applicator alone with a frequency of 433 MHz [±5 KHz;
patent no. CN-204824903-U (15)] at
42°C for 60 min; GEM group, treated with GEM (a duration of 24 h;
Lilly France, Neuilly-sur-Seine, France) alone at a dose of 5
µmol/l; and MW + GEM group, in which cells were exposed to GEM (a
duration of 24 h and a dose of 5 µmol/l), and then treated with
MWHT (performed as mentioned in the MW group) at 42°C for 60 min.
Following the various treatments, the cells were immediately
returned to the cell incubator and incubated at 37°C for 24 h prior
to further experiments.
Cell viability assay
The effects of the different treatments on cell
viability were determined by the Cell Counting Kit-8 (CCK-8) assay
(MedChem Express, Monmouth Junction, NJ, USA) according to the
manufacturer's protocol. Briefly, 10 µl CCK-8 solution was added to
cells (1×104 cells/well) in 96-well plates, and then the
cells were incubated at 37°C for 1–3 h. Subsequently, the cell
viability in each group was measured at 450 nm using a Multiskan
Spectrum spectrophotometer (Thermo Fisher Scientific, Inc.).
Cell cycle analysis by flow
cytometry
Cells were seeded in 6-well plates at a density of
1×106 cells/well, and then divided into the four
experimental groups (CON, MW, GEM and MW + GEM). After 24 h of
treatment, the cells were harvested, washed with phosphate-buffered
saline (PBS) and fixed with 70% ice-cold ethanol at −20°C
overnight. The cells were then washed further with PBS and stained
using the CycletestPlus DNA Reagent kit (BD Biosciences, San Jose,
CA, USA), according to the manufacturer's protocol. The cell cycle
distribution was analyzed by a flow cytometer (BD Biosciences).
LysoTracker Red staining
Cells were cultured in 6-well plates at a density of
5×105 cells/well and separated into the four
experimental groups (CON, MW, GEM and MW + GEM). After 24 h of
treatment, cells from the different groups were collected and
incubated with 50 nM LysoTracker Red (Beyotime Institute of
Biotechnology, Haimen, China) for 30 min at 37°C in the dark.
Samples were then analyzed using a fluorescence microscope (BX61;
Olympus Corp., Tokyo, Japan) in order to examine autophagy.
Measurement of intracellular ROS
generation
Detection of intracellular ROS production was
performed by fluorescent probe 2′,7′-dichlorodihydrofluorescein
diacetate (DCFH-DA) staining (Beyotime Institute of Biotechnology).
Briefly, cells were seeded into 6-well plates at a density of
5×105 cells/well in a volume of 2 ml and divided into
the four experimental groups (CON, MW, GEM and MW + GEM). After 24
h of treatment, the cells were preloaded with 10 µM DCFH-DA in
FBS-free RPMI-1640 medium for 30 min. Subsequent to washing three
times with PBS, the cells were mounted under a fluorescence
microscope (BX61) at an excitation wavelength of 488 nm and
emission wavelength of 525 nm. Using Image-Pro Plus software (Media
Cybernetics, Inc., Rockville, MD, USA), the mean fluorescence
intensity of the images was assessed and normalized to obtain
relative ratios, which were compared among the experimental
groups.
Western blot analysis
NCI-H1703 and NCI-H2170 cells in the logarithmic
growth phase were collected and seeded at a density of
5×105 cells/well in 6-well plates. Following treatment
according to the four experimental groups (CON, MW, GEM and MW +
GEM), the cells were then lysed in ice-cold
radioimmunoprecipitation assay lysis buffer for 30 min and then
centrifuged at 20,000 × g for 10 min at 4°C to obtain the total
protein. The protein concentration was determined by the BCA assay
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Equal amounts (40 µg) of cell lysates were separated by
8–12% SDS-PAGE and transferred to a polyvinylidene difluoride
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Subsequent to blocking with 5% non-fat milk for 1–2 h at room
temperature, the membranes were incubated with specific primary
antibodies at 4°C overnight. The following antibodies were used:
Anti-microtubule-associated protein light chain 3 (LC3; dilution
1:1,000; cat. no. sc-398822; Santa Cruz Biotechnology, Santa Cruz,
CA, USA), anti-sequestosome1/p62 (SQSTM1/p62; dilution 1:1,000;
cat. no. sc-48402; Santa Cruz Biotechnology),
anti-phosphatidylinositol 3-kinase (PI3K; dilution 1:1,000; cat.
no. 4249; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-phosphorylated-phosphatidylinositol 3-kinase (p-PI3K; dilution
1:1,000; cat. no. 4228; Cell Signaling Technology, Inc.),
anti-protein kinase B (AKT; dilution 1:1,000; cat. no. 2920; Cell
Signaling Technology, Inc.), anti-phosphorylated-protein kinase B
(p-AKT; dilution 1:1,000; cat. no. 4060; Cell Signaling Technology,
Inc.), anti-mammalian target of rapamycin (mTOR; dilution 1:1,000;
cat. no. 2983; Cell Signaling Technology, Inc.),
anti-phosphorylated-mammalian target of rapamycin (p-mTOR; dilution
1:1,000; cat. no. 5536; Cell Signaling Technology, Inc.),
anti-phosphorylated-S6 (pS6; dilution 1:1,000; cat. no. 4858; Cell
Signaling Technology, Inc.), anti-ribosomal protein S6 kinase
(p70S6k; dilution 1:1,000; cat. no. 2708; Cell Signaling
Technology, Inc.) and anti-β-actin (dilution 1:100; cat. no.
sc-47778; Santa Cruz Biotechnology). Next, the membranes were
washed three times with Tris-buffered saline and Tween 20, and then
incubated with horseradish peroxidase-conjugated goat
anti-rabbit/mouse IgG secondary antibodies (anti-rabbit IgG;
dilution 1:5,000; cat. no. sc-2357; and anti-mouse IgG; dilution
1:5,000; cat. no. sc-2005; from Santa Cruz Biotechnology) at room
temperature for a further 1–2 h. An enhanced chemiluminescence
detection reagent (Beyotime Institute of Biotechnology) was used
for signal detection, and images were captured with an Odyssey
infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA). The
relative expression level of the total protein is represented by
the ratio of the gray value of the total protein band to the gray
value of the β-actin band of the internal reference. The ratio of
the gray value of the phosphorylated protein band to the
corresponding gray value of the total protein band indicates the
relative expression of the phosphorylated protein (ImageJ Software;
v.1.8.0; National Institutes of Health, Bethesda, MD, USA). All
experiments were repeated at least three times.
Treatment of cells with N-acetyl
cysteine (NAC)
Cells were pretreated for 1 h with 5 mmol/l NAC
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), a ROS scavenger,
and then treated with MWHT and GEM as described earlier. Then, the
CCK-8 experiment and the detection of autophagy-related proteins
and PI3K/AKT/mTOR pathway proteins were performed.
Treatment of cells with
3-methyladenine (3-MA)
Cells were pretreated for 1 h with 5 mmol/l 3-MA
(Selleck Chemicals, Houston, TX, USA), a selective PI3K inhibitor,
which was dissolved in PBS, followed by treatment with MWHT and GEM
as described earlier. Subsequently, DCFH-DA staining was performed
to examine generation of cellular ROS after treatment with
autophagy inhibitors, while the expression levels of
autophagy-associated and PI3K/AKT/mTOR proteins were detected by
western blot analysis.
Statistical analysis
The statistical analysis was performed using SPSS
(version 17.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism
software (version 7.00 for Macintosh; GraphPad Software, Inc., La
Jolla, CA, USA). The one-way ANOVA followed by Tukey's post hoc
test was used for multiple comparisons. The results were considered
statistically significant when P<0.05. All analyses represented
at least three independent in vitro experiments, and the
results are expressed as the mean ± standard deviation.
Results
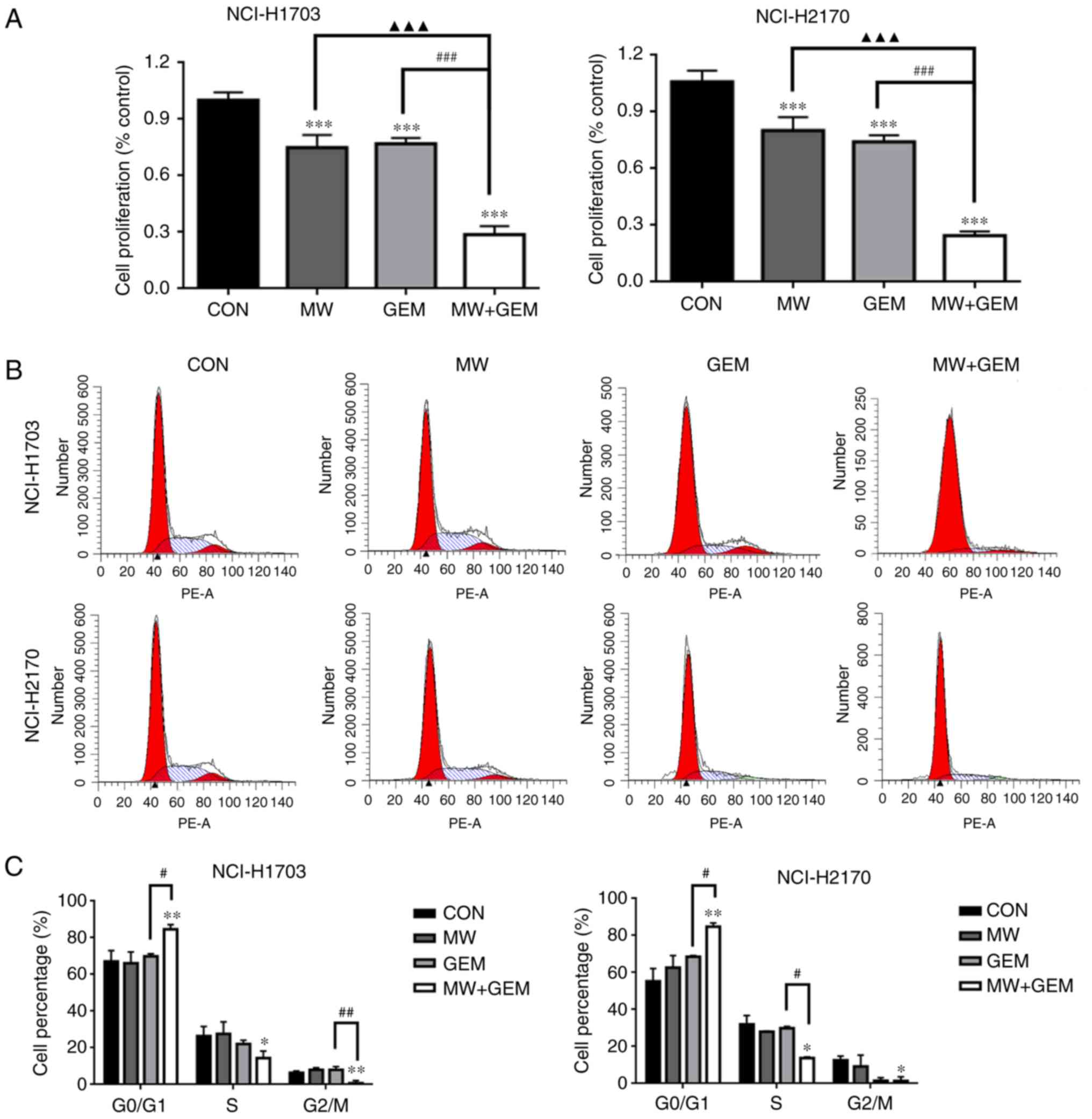
MWHT combined with GEM inhibits cell
proliferation and induces G0/G1 arrest in squamous cell lung
cancer
Our previous study investigated whether MWHT
subsequent to treatment with GEM can synergistically inhibit the
proliferation of human squamous lung carcinoma cells in
vitro (16). To further
elucidate the underlying mechanism, the cells were divided into
four groups (CON, MW, GEM and MW + GEM), and a CCK-8 assay was
conducted to evaluate cell viability. The results revealed that,
compared with the CON group, cell viability significantly decreased
in the MW, GEM and MW + GEM groups (P<0.001) in the two cell
lines (Fig. 1A). Compared with the
GEM or MW alone groups, the cell viability in the combination group
was also significantly decreased (P<0.001). To verify the causal
association of cell proliferation inhibition with cell cycle
arrest, the cell cycle distribution was analyzed by flow cytometry.
As shown in Fig. 1B and C, MW + GEM
treatment induced G0/G1 phase arrest, as the cell population at
this phase was significantly increased compared with the other
three groups in NCI-H1703 cells (P<0.01 or P<0.05). By
contrast, the percentages of cells in the S and G2/M phases were
significantly decreased (P<0.05 or P<0.01) compared with the
CON group. Similar effects were also observed in NCI-H2170 cells.
These cell cycle changes may have caused the marked reduction in
NCI-H1703 and NCI-H2170 cell proliferation.
MWHT combined with GEM induces
autophagy
As autophagy contributes to cell death, the present
study then investigated whether MWHT in combination with GEM
induces autophagy in lung cancer cells. Autophagy is characterized
by the formation of numerous acidic vesicular organelles, which is
correlated with an increased number of autophagosomes and can be
detected by LysoTracker Red staining (19,24).
LysoTracker Red staining was thus conducted in the present study
and was quantified using a fluorescence microscope. The results
demonstrated that, compared with GEM or MWHT treatment alone, cell
autophagy was significantly increased (P<0.001) after combined
treatment with MWHT and GEM (Fig.
2A). To verify these findings, the expression of several marker
proteins of autophagy was further tested by western blotting. The
results revealed an increased light chain 3 (LC3)-II/LC3-I ratio
and reduced p62 expression in cells receiving combined treatment as
compared with those receiving single treatment (Fig. 2B). To further elucidate the
mechanism underlying autophagy induction by the combined treatment
with MWHT and GEM, 3-MA was used to block autophagy. Consistently,
the administration of 3-MA in MW + GEM cells decreased the ratio of
LC3-II/LC3-I protein and increased the expression of p62, as
compared with those in cells treated with 3-MA alone (Fig. 2C). Taken together, these findings
indicated the crucial role of autophagy in the treatment of
squamous cell lung carcinoma with MWHT and GEM.
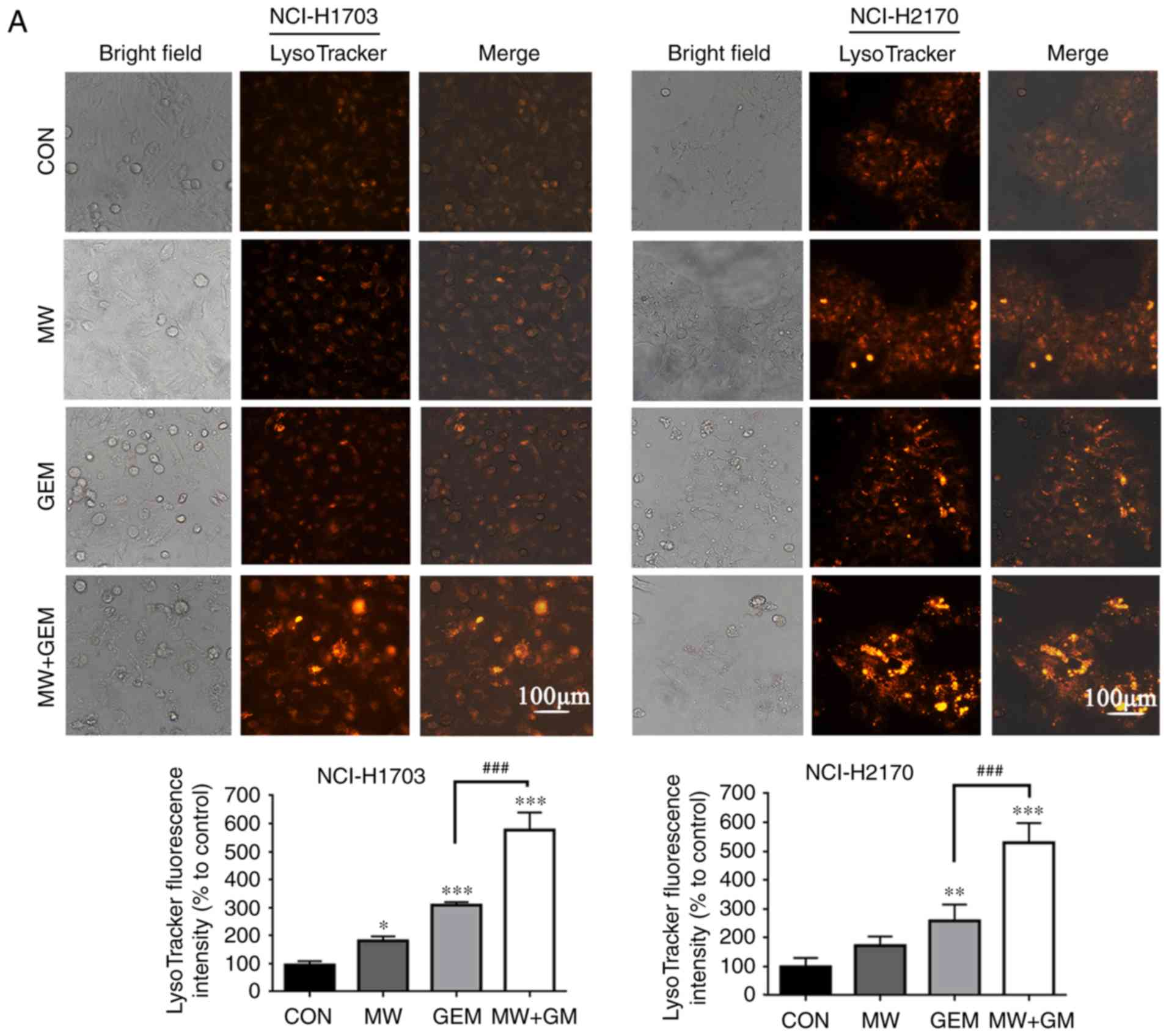 | Figure 2.MW + GEM treatment induces autophagy
in squamous cell lung carcinoma cells. (A) Representative images of
LysoTracker Red staining of cells following treatment with MW + GEM
for 24 h. Red color intensity represents acidic vesicular
organelles, indicating the autophagosomes (scale bars, 100 µm). (B)
Levels of autophagy-associated proteins LC3-II/LC3-I and p62,
analyzed by western blotting. (C) Cells were pre-incubated with
3-MA (5 mmol/l) for 1 h, and then treated with MW + GEM for 24 h,
followed by western blot analysis of autophagy-associated proteins.
The results are presented as the mean ± standard deviation of three
independent experiments (n=3). *P<0.05, **P<0.01 and
***P<0.001, vs. CON group; #P<0.05 and
###P<0.001. MW, microwave hyperthermia; GEM,
gemcitabine; 3-MA, 3-methyladenine; LC3, light chain 3; CON,
control. |
MWHT combined with GEM increases ROS
levels
ROS signaling serves an important role in several
biochemical functions, including cell autophagy. Therefore, the
production of ROS was detected by DCFH-DA staining and analyzed by
fluorescence microscopy. As shown in Fig. 3A and B, the results demonstrated
that ROS production increased significantly in the MW + GEM group
when compared with the CON group (5.06-fold in NCI-H1703 cells and
5.25-fold in NCI-H2170 cells), while there was no marked increase
in ROS production in the MW (1.39-fold in NCI-H1703 cells and
1.23-fold in NCI-H2170 cells) or GEM group alone (1.40-fold in
NCI-H1703 cells and 1.39-fold in NCI-H2170 cells). Subsequently, in
order to study the effect of ROS accumulation on the
antiproliferative potential of MWHT combined with GEM, a CCK-8
assay was performed. As expected, the cell viability in the MW +
GEM group was significantly decreased (P<0.001), whereas
pretreatment with NAC markedly prevented cell death (P<0.001;
Fig. 3C). These observations
further confirmed that MWHT combined with GEM induced NCI-H1703 and
NCI-H2170 cell death via the generation of ROS.
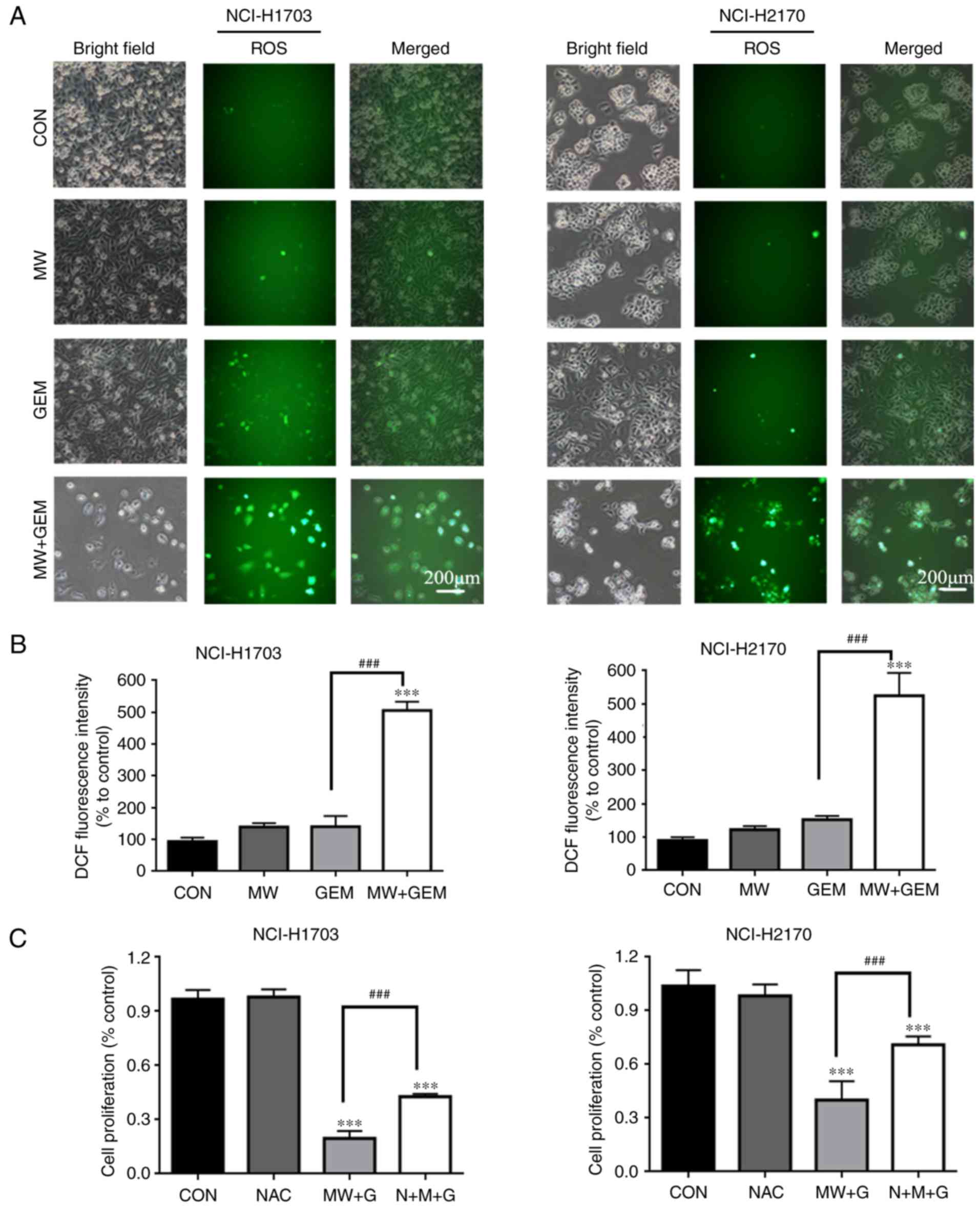 | Figure 3.MW + GEM treatment increases ROS
levels. Cells were seeded onto 6-well plates at
5×105/well, and then exposed to MW and/or GEM treatment
for 24 h. Subsequently, cells were preloaded with
2′,7′-dichlorodihydrofluorescein diacetate and then mounted under a
fluorescent microscope. (A and B) Representative images of DCF
fluorescence staining in NCI-H1703 and NCI-H2170 cells (scale bars,
200 µm). Mean fluorescence intensity in NCI-H1703 and NCI-H2170
cells. (C) Cells were preincubated with NAC (5 mM) for 1 h and then
treated with MW + GEM for 24 h. Cell viability was measured by a
Cell Counting Kit-8 assay. The results are presented as the mean ±
standard deviation of three independent experiments (n=3).
***P<0.001 vs. CON group; ###P<0.001. MW,
microwave hyperthermia; GEM, gemcitabine; ROS, reactive oxygen
species; DCF, 2′,7′-dichlorofluorescein; CON, control; NAC,
N-acetyl cysteine. |
MWHT combined with GEM activates the
PI3K/AKT/mTOR signaling pathway by inducing ROS production
Several studies have reported that PI3K/AKT/mTOR
signaling is involved in the regulation of autophagy. Therefore,
the present study investigated the effects of MWHT and GEM on
PI3K/AKT/mTOR signaling. As shown in Fig. 4A, compared with the CON and single
treatment groups, the protein expression levels of PI3K,
phosphorylated (p)-PI3K, AKT, p-AKT, mTOR, p-mTOR, PS6 and p70 S6
kinase (P70S6K) in the MW + GEM group were significantly decreased
in the two cell lines (P<0.05, P<0.01 or P<0.001). To
further examine the association between autophagy and the
PI3K/AKT/mTOR signaling pathway, the autophagy inhibitor 3-MA was
used. It was revealed that the PI3K/AKT/mTOR pathway was
upregulated (P<0.05, or P<0.001) (Fig. 4B). Taken together, these data
indicate that MWHT combined with GEM induce cell autophagy though
activation of PI3K/AKT/mTOR signaling. In addition, ROS has been
demonstrated to be an inducer for the activation of the
PI3K/AKT/mTOR signaling pathway; therefore, the present study
further assessed the effect of ROS production on the PI3K/AKT/mTOR
pathway using the ROS scavenger NAC. The results demonstrated that
pretreatment with NAC, followed by MWHT and GEM treatment,
significantly reversed the phosphorylation of PI3K, AKT and mTOR,
as compared with MW + GEM group (P<0.01 or P<0.001; Fig. 5) (35). These changes in the protein
expression levels indicated that ROS production was upstream of the
PI3K/AKT/mTOR signaling pathway.
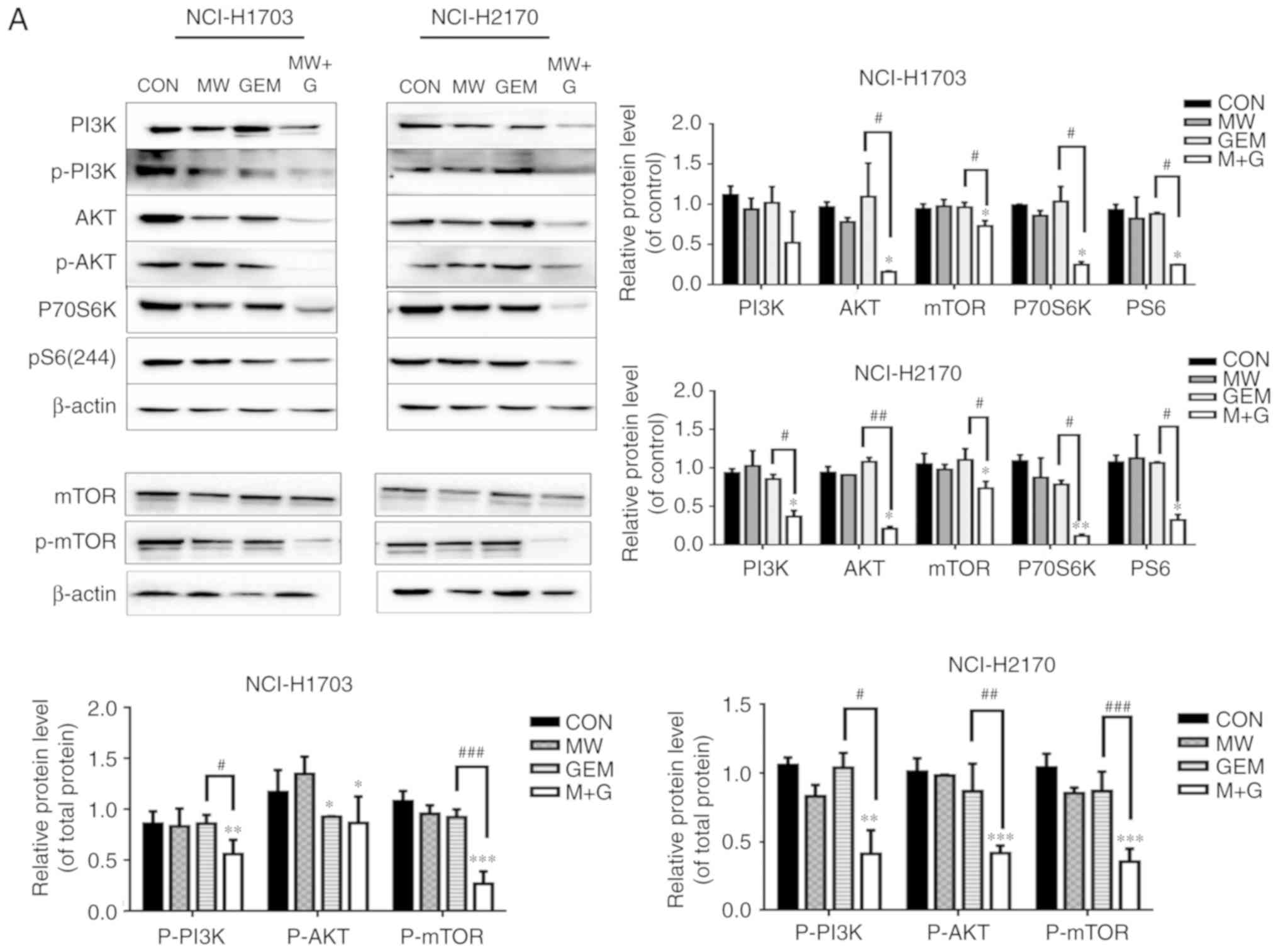 | Figure 4.MW + GEM treatment activates the
PI3K/Akt/mTOR signaling pathway. (A) Cells were exposed to various
treatments for 24 h, and the expression levels of PI3K, p-PI3K,
Akt, p-AKT, mTOR, p-mTOR, pS6 and P70S6K proteins were analyzed by
western blot assay. (B) Cells were preincubated with 3-MA (5 mM)
for 1 h and then treated with MW + GEM for 24 h, and the expression
levels of PI3K, p-PI3K, Akt, p-AKT, mTOR, p-mTOR, pS6 and P70S6K
proteins were analyzed by western blot assay. Relative expression
levels of PI3K, Akt, mTOR, pS6 and P70S6K, and the phosphorylated
proteins p-PI3K, p-Akt and p-mTOR are shown. Each band was
quantified using densitometry. The results are presented as mean ±
standard deviation of three independent experiments (n=3).
*P<0.05, **P<0.01 and ***P<0.001, vs. CON group;
#P<0.05, ##P<0.01 and
###P<0.001, vs. GEM group. MW, microwave
hyperthermia; GEM, gemcitabine; PI3K, phosphoinositide 3-kinase;
Akt, protein kinase B; mTOR, mammalian target of rapamycin; P70S6K,
p70 S6 kinase; p-, phosphorylated; CON, control. |
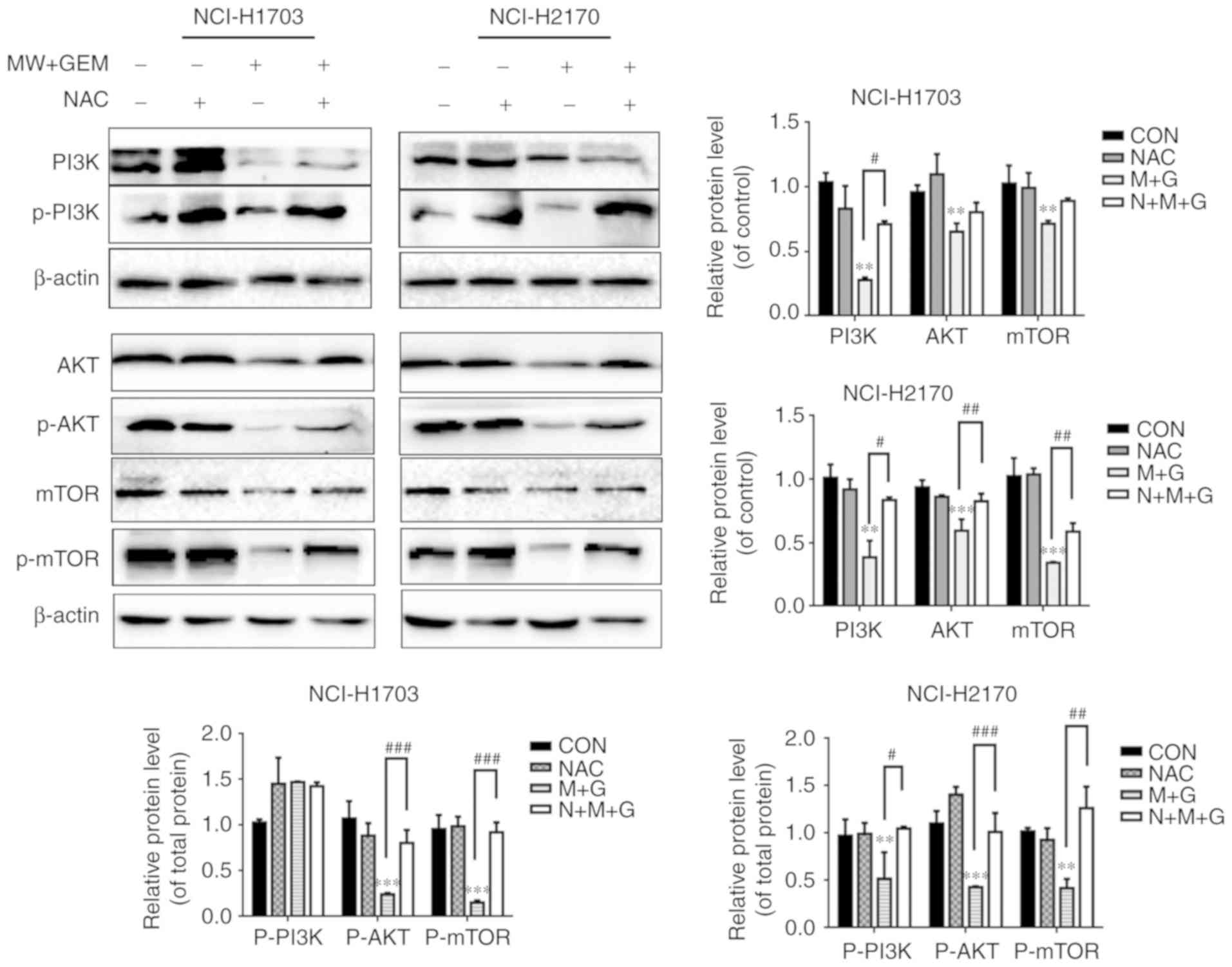 | Figure 5.Addition of ROS inhibitions could
upregulate the PI3K/Akt/mTOR signaling pathway. Cells were
preincubated with NAC (5 mM) for 1 h and then treated with MW + GEM
for 24 h. Protein expression levels of PI3K, p-PI3K, AKT, p-Akt,
mTOR and p-mTOR were analyzed by western blot analysis. The results
are presented as the mean ± standard deviation of three independent
experiments (n=3). **P<0.01 and ***P<0.001, vs. CON group;
#P<0.05, ##P<0.01 and
###P<0.001. MW, microwave hyperthermia; GEM,
gemcitabine; PI3K, phosphoinositide 3-kinase; Akt, protein kinase
B; mTOR, mammalian target of rapamycin; NAC, N-acetyl cysteine;
CON, control. |
MWHT combined with GEM induces
autophagy by inducing ROS production
To elucidate the interplay between ROS and
autophagy, the underlying molecular mechanism was further
investigated using western blot analysis. Notably, the protein
expression levels of p62 were found to be significantly increased
following pretreatment with NAC in the MW + GEM group for 24 h, as
compared with the group without NAC (P<0.001; Fig. 6A). By contrast, the ratio of the
LC3-II/LC3-I protein level was markedly decreased in the NAC
pretreated cells, as compared with the MW + GEM group (P<0.001;
Fig. 6A). Furthermore, when the
autophagy inhibitor 3-MA was used, no significant change was
observed in ROS production (Fig.
6B). Taken together, these results indicate that MWHT in
combination with GEM induced autophagy through ROS-mediated
PI3K/AKT/mTOR signaling in the two human squamous cell lung
carcinoma cell lines.
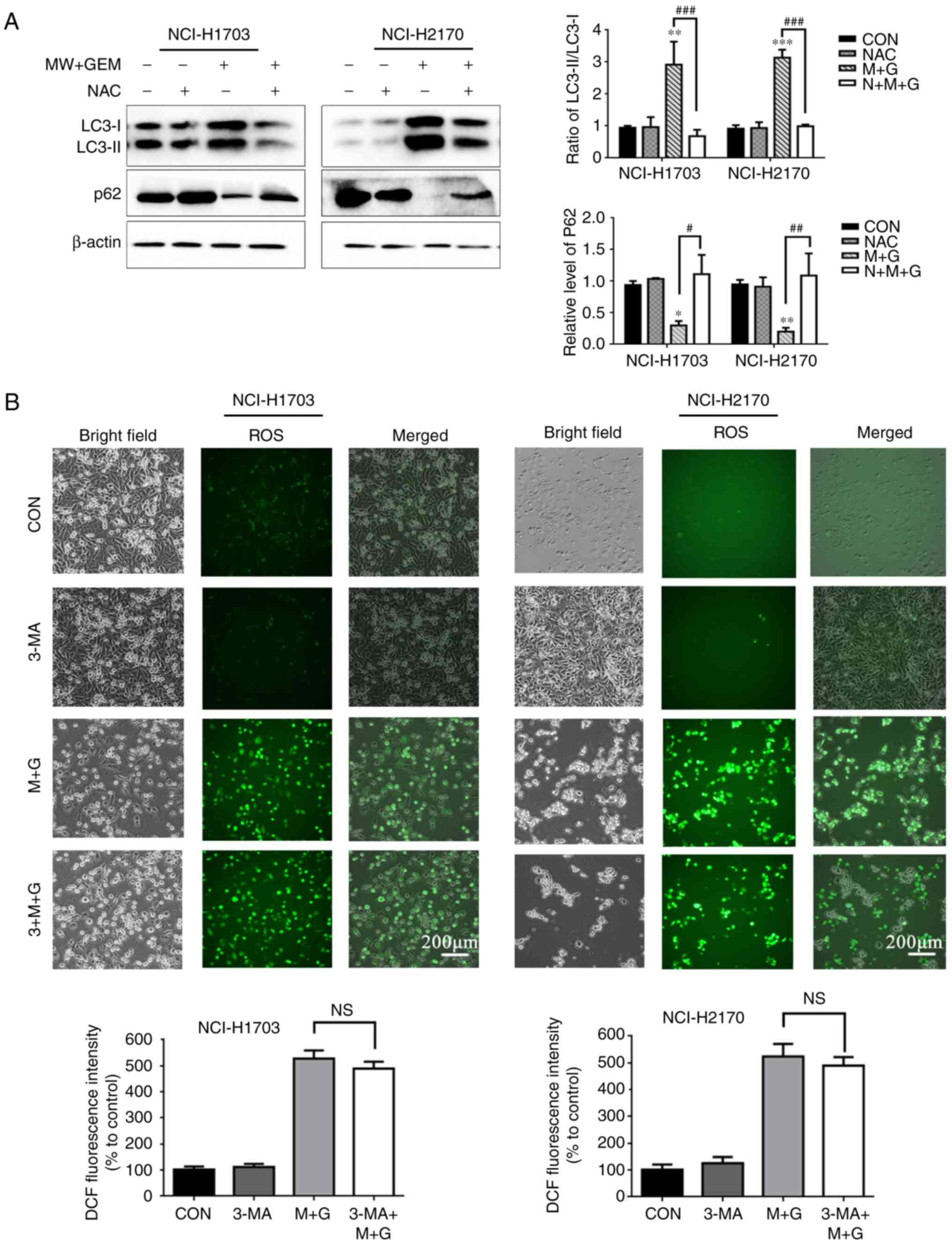 | Figure 6.Addition of autophagy inhibitions
could upregulate the PI3K/Akt/mTOR signaling pathway. MW + GEM
treatment induces autophagy by inducing ROS production. (A) Cells
were preincubated with NAC (5 mM) for 1 h and then treated with MW
+ GEM for 24 h. Protein expression levels of LC3-II/LC3-I and p62
were analyzed by western blot analysis. (B) Cells were preincubated
with 3-MA (5 mM) for 1 h and then treated with MW + GEM for 24 h.
Representative images of DCF fluorescence staining in NCI-H1703 and
NCI-H2170 cells (scale bars, 200 µm). The results are presented as
the mean ± standard deviation of three independent experiments
(n=3). *P<0.05, **P<0.01 and ***P<0.001, vs. CON group;
#P<0.05, ##P<0.01 and
###P<0.001. MW, microwave hyperthermia; GEM,
gemcitabine; NAC, N-acetyl cysteine; LC3, light chain 3; p-,
phosphorylated; 3-MA, 3-methyladenine; ROS, reactive oxygen
species; DCF, 2′,7′-dichlorofluorescein; p-, phosphorylated; CON,
control; NS, not significant. |
Discussion
The present study investigated the anticancer
effects of MWHT combined with GEM on the squamous cell lung
carcinoma NCI-H1703 and NCI-H2170 cell lines, and demonstrated that
the combined treatment effectively inhibited cancer cell
proliferation by inducing cell cycle arrest. Furthermore, the
results revealed that the combined treatment predominantly induced
autophagic cell death through the inhibition of the ROS-mediated
PI3K/AKT/mTOR signaling pathway in squamous cell lung
carcinoma.
The cell cycle, consisting of the G0/G1, S and G2/M
phases, regulates a number of cell processes, including
proliferation, differentiation and apoptosis. The loss of cell
cycle control, particularly loss of the G1/S and G2/M checkpoints,
is closely associated with tumorigenesis. It has previously been
demonstrated that GEM can cause G1/S arrest in tumor cells
(25), which is consistent with the
experimental results of the present study. By contrast,
hyperthermia has been reported to cause G2/M phase arrest (15,26);
however, the combined effect of GEM and hyperthermia is complex,
and may involve G2/M phase block (27), as well as G0/G1 phase block
(28). The experimental results
presented in the present study demonstrated that the number of
cells in the G0/G1 phase increased significantly upon co-treatment
with MWHT and GEM, which is in accordance with previous findings
(28), indicating that the combined
effect of MWHT and GEM causes G0/G1 phase arrest in squamous cell
lung carcinoma.
A growing body of evidence indicates that autophagy
closely regulates tumor response by protecting cell survival or
contributing to cell death. Autophagy is a programmed intracellular
degradation process, during which the cells are transported to the
lysosomes and digested to meet metabolic needs, organelle turnover
and maintenance of cell homeostasis (17). The process of autophagy is mainly
divided into five stages, as follows: i) Phagophore formation; ii)
Atg5-Atg12 conjugation; iii) LC3 processing; iv) autophagosome
formation; and v) autolysosome formation. The increase of
autolysosomes, the conversion of LC3-I to LC3-II and the
downregulation of the p62 protein are often used as markers of
autophagy (18). In the present
study, it was demonstrated that MWHT combined with GEM increased
the number of autolysosomes, upregulated LC3-II levels and
downregulated the expression of the p62 protein compared with the
control group. These results indicate that thermal inhibition of
the proliferation of squamous cell lung carcinoma cells may be
closely associated with the induction of autophagy.
ROS is crucial for the regulation of cell death and
survival in cancer. It has been reported that ROS can induce the
dissociation of autophagy molecules Beclin 1 and B-cell lymphoma-2,
thus activating the Beclin 1-induced autophagic pathway, inhibiting
mTOR and increasing the expression of LC3-II, thereby initiating
autophagy-associated pathways to induce cell death (29). In the present study, MWHT combined
with GEM treatment resulted in a significant increase in ROS
generation, while pretreatment with the ROS inhibitor NAC markedly
reversed the inhibition of cell proliferation and autophagy induced
by MWHT and GEM. In addition, compared with cells only treated with
MWHT and GEM, the ROS level was not significantly altered when
cells were co-treated with MWHT + GEM and 3-MA, which was
consistent with the results of the Zhang et al (30). The present study results thus
indicated that ROS acts upstream of autophagy in squamous cell lung
carcinoma.
Previous studies have reported that several key
molecules and signaling pathways may regulate autophagy (31,32).
Among them, the PI3K/AKT/mTOR signaling pathway has been
extensively investigated. The role of this pathway is pivotal in
the regulation of cell proliferation, differentiation and survival
under normal physiological conditions, as well as during
pathophysiological processes (33,34).
In the present study, it was observed that treatment of lung cancer
cells with MWHT and GEM decreased the levels of phosphorylated
PI3K, AKT and mTOR, suggesting that autophagy was induced via
suppression of PI3K/AKT/mTOR signaling in these cells. Furthermore,
intracellular ROS production reportedly serves a key role in
PI3K/AKT/mTOR inactivation and autophagy (35). In the present study, autophagy and
LC3 expression were significantly restored by a ROS scavenger, NAC,
in NCI-H1703 and NCI-H2170 cells. Furthermore, pretreatment with
NAC nearly attenuated the phosphorylation of PI3K, AKT and mTOR.
Taken together, these data indicated that MWHT combined with GEM
induced cell autophagy though the activation of ROS-mediated
PI3K/AKT/mTOR signaling.
In conclusion, to the best of our knowledge, the
present study results are the first to demonstrate that treatment
with MWHT combined with GEM induces G0/G1 cell cycle arrest and
causes cell autophagy that is regulated via ROS-mediated
PI3K/AKT/mTOR signaling. These results contribute to the
understanding of the biological effects of thermo-chemotherapy on
cancer cells, and indicate that the combination of MWHT and GEM is
a promising anticancer therapy in human squamous cell lung
carcinoma. However, it is important to investigate the interplay
between apoptosis and autophagy, and determine whether their
genetic and pharmacological manipulation may affect the efficacy of
thermal anticancer treatments. In addition, further research on
animal models is required in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation (no. U1504822). The funder had
no part in the study design, data collection and analysis, decision
to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding authors on reasonable
request.
Authors' contributions
YangY, SLM and DKY designed the experiments; SLM and
DKY were involved in project administration; YangY, SLM and DKY
wrote and edited the manuscript; YangY, CLY, ZJZ, XXZ, TSL and YaY
performed the experiments and analyzed the data. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heist RS, Mino-Kenudson M, Sequist LV,
Tammireddy S, Morrissey L, Christiani DC, Engelman JA and Iafrate
AJ: FGFR1 amplification in squamous cell carcinoma of the lung. J
Thorac Oncol. 7:1775–1780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Derman BA, Mileham KF, Bonomi PD, Batus M
and Fidler MJ: Treatment of advanced squamous cell carcinoma of the
lung: A review. Transl Lung Cancer Res. 4:524–532. 2015.PubMed/NCBI
|
|
4
|
Sun Y, Wu YL, Zhou CC, Zhang L, Zhang L,
Liu XY, Yu SY, Jiang GL, Li K, Qin SK, et al: Second-line
pemetrexed versus docetaxel in Chinese patients with locally
advanced or metastatic non-small cell lung cancer: A randomized,
open-label study. Lung Cancer. 79:143–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palumbo R, Sottotetti F, Trifirò G, Piazza
E, Ferzi A, Gambaro A, Spinapolice EG, Pozzi E, Tagliaferri B,
Teragni C, et al: Nanoparticle albumin-bound paclitaxel
(nab-paclitaxel) as second-line chemotherapy in HER2-negative,
taxane-pretreated metastatic breast cancer patients: Prospective
evaluation of activity, safety, and quality of life. Drug Des Devel
Ther. 9:2189–2199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duan J, Wang Z, Bai H, An T, Zhuo M, Wu M,
Wang Y, Yang L and Wang J: Epidermal growth factor receptor variant
III mutation in Chinese patients with squamous cell cancer of the
lung. Thorac Cancer. 6:319–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hurwitz M and Stauffer P: Hyperthermia,
radiation and chemotherapy: The role of heat in multidisciplinary
cancer care. Semin Oncol. 41:714–729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salahi S, Maccarini PF, Rodrigues DB,
Etienne W, Landon CD, Inman BA, Dewhirst MW and Stauffer PR:
Miniature microwave applicator for murine bladder hyperthermia
studies. Int J Hyperthermia. 28:456–465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asano M, Tanaka S, Sakaguchi M, Matsumura
H, Yamaguchi T, Fujita Y and Tabuse K: Normothermic microwave
irradiation induces death of HL-60 cells through Heat-independent
apoptosis. Sci Rep. 7:114062017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Li C, Ge H, Liang M, Ma G, Ling L,
Pan H, Gong H, Xie H, Ding Q, et al: Technical analysis of US
imaging for precise microwave ablation for benign breast tumours.
Int J Hyperthermia. 34:1179–1185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han JB, Kong FW, Ding H, Zhang YF, Liu JM,
Wei Q, Hu L, Zhao L, Xu CJ and Yi YX: Hepatectomy combined with
microwave ablation of the spleen for treatment of hepatocellular
carcinoma complicated with splenomegaly: A retrospective study. Mol
Clin Oncol. 6:204–208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kouloulias V, Triantopoulou S, Vrouvas J,
Gennatas K, Oozounoglou N, Kouvaris J, Karaiskos P, Aggelakis P,
Antypas C, Zygogianni A, et al: Combined chemoradiotherapy with
local microwave hyperthermia for treatment of T3N0 laryngeal
carcinoma: A retrospective study with long-term follow-up. Acta
Otorhinolaryngol Ital. 34:167–173. 2014.PubMed/NCBI
|
|
13
|
Othman T, Goto S, Lee JB, Taimura A,
Matsumoto T and Kosaka M: Hyperthermic enhancement of the apoptotic
and antiproliferative activities of paclitaxel. Pharmacology.
62:208–212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adachi S, Kokura S, Okayama T, Ishikawa T,
Takagi T, Handa O, Naito Y and Yoshikawa T: Effect of hyperthermia
combined with gemcitabine on apoptotic cell death in cultured human
pancreatic cancer cell lines. Int J Hyperthermia. 25:210–219. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao YY, Wu Q, Wu ZB, Zhang JJ, Zhu LC,
Yang Y, Ma SL and Zhang SR: Microwave hyperthermia promotes
caspase-3-dependent apoptosis and induces G2/M
checkpoint arrest via the ATM pathway in non-small cell lung cancer
cells. Int J Oncol. 53:539–550. 2018.PubMed/NCBI
|
|
16
|
Yang Y, Zhao Y, Ma S and Yang D: Microwave
hyperthermia combined with gemcitabine inhibits proliferation and
induces apoptosis of human lung squamous carcinoma cells. Zhongguo
Fei Ai Za Zhi. 21:805–814. 2018.(In Chinese). PubMed/NCBI
|
|
17
|
White EJ, Martin V, Liu JL, Klein SR, Piya
S, Gomez-Manzano C, Fueyo J and Jiang H: Autophagy regulation in
cancer development and therapy. Am J Cancer Res. 1:362–372.
2011.PubMed/NCBI
|
|
18
|
Rosenfeldt MT and Ryan KM: The multiple
roles of autophagy in cancer. Carcinogenesis. 32:955–963. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Zhang T, Sun W, Wang Z, Zuo D,
Zhou Z, Li S, Xu J, Yin F, Hua Y, et al: Erianin induces G2/M-phase
arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway
in human osteosarcoma cells in vitro and in vivo. Cell Death Dis.
7:e22472016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ba MC, Long H, Wang S, Wu YB, Zhang BH,
Yan ZF, Yu FH and Cui SZ: Hyperthermia enhances radiosensitivity of
colorectal cancer cells through ROS inducing autophagic cell death.
J Cell Biochem. 119:3763–3774. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen F, Wang CC, Kim E and Harrison LE:
Hyperthermia in combination with oxidative stress induces
autophagic cell death in HT-29 colon cancer cells. Cell Biol Int.
32:715–723. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Cai F, Chen X, Luo M, Hu L and Lu
Y: The role of mitochondria-derived reactive oxygen species in
hyperthermia-induced platelet apoptosis. PLoS One. 8:e750442013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou CH, Lin FL, Hou SM and Liu JF:
Hyperthermia induces apoptosis through endoplasmic reticulum and
reactive oxygen species in human osteosarcoma cells. Int J Mol Sci.
15:17380–17395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martinotti S, Ranzato E, Parodi M, Vitale
M and Burlando B: Combination of
ascorbate/epigallocatechin-3-gallate/gemcitabine synergistically
induces cell cycle deregulation and apoptosis in mesothelioma
cells. Toxicol Appl Pharmacol. 274:35–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furusawa Y, Iizumi T, Fujiwara Y, Zhao QL,
Tabuchi Y, Nomura T and Kondo T: Inhibition of checkpoint kinase 1
abrogates G2/M checkpoint activation and promotes apoptosis under
heat stress. Apoptosis. 17:102–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Michalakis J, Georgatos SD, Romanos J,
Koutala H, Georgoulias V, Tsiftsis D and Theodoropoulos PA:
Micromolar taxol, with or without hyperthermia induces mitotic
catastrophe and cell necrosis in HeLa cells. Cancer Chemother
Pharmacol. 56:615–622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haveman J, Rietbroek RC, Geerdink A, Van
Rijn J and Bakker PJ: Effect of hyperthermia on the cytotoxicity of
2′,2′-difluorodeoxycytidine (gemcitabine) in cultured SW1573 cells.
Int J Cancer. 62:627–630. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ba MC, Long H, Cui SZ, Gong YF, Yan ZF,
Wang S and Wu YB: Mild hyperthermia enhances sensitivity of gastric
cancer cells to chemotherapy through reactive oxygen
species-induced autophagic death. Tumor Biol.
39:10104283177119522017. View Article : Google Scholar
|
|
30
|
Zhang L, Wang H, Xu J, Zhu J and Ding K:
Inhibition of cathepsin S induces autophagy and apoptosis in human
glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K
and JNK signaling pathways. Toxico Lett. 228:248–259. 2014.
View Article : Google Scholar
|
|
31
|
Dewaele M, Maes H and Agostinis P:
ROS-mediated mechanisms of autophagy stimulation and their
relevance in cancer therapy. Autophagy. 6:838–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiong Y, Yepuri G, Forbiteh M, Yu Y,
Montani JP, Yang Z and Ming XF: ARG2 impairs endothelial autophagy
through regulation of MTOR and PRKAA/AMPK signaling in advanced
atherosclerosis. Autophagy. 10:2223–2238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Q, Zhu H, Xu X, Li L, Tan H and Cai
X: Inactivated Sendai virus induces apoptosis and autophagy via the
PI3K/Akt/mTOR/p70S6K pathway in human nonsmall cell lung cancer
cells. Biochem Biophys Res Commun. 465:64–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Papadimitrakopoulou V: Development of
PI3K/AKT/mTOR pathway inhibitors and their application in
personalized therapy for non-small-cell lung cancer. J Thorac
Oncol. 7:1315–1326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou M, Shen S, Zhao X and Gong X:
Luteoloside induces G0/G1 arrest and pro-death autophagy through
the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human
non-small cell lung cancer cell lines. Biochem Biophys Res Commun.
494:263–269. 2017. View Article : Google Scholar : PubMed/NCBI
|