Introduction
Betulinic acid (BTA) is a carboxylic derivative of
betulin, a naturally occurring triterpene predominantly found in
the birch bark and other plants (1). It exhibits broad-spectrum biological
activity even at low concentrations, such as anti-bacterial,
anti-inflammatory, anti-herpes simplex virus-1 or anti-malarial
(2–4). Previous studies have indicated the
potential use of BTA as a new anticancer drug (5,6). It
has been reported to induce apoptosis in various human cancer cell
lines (7–10). This process occurs independently of
the cascades that mediate programed cell death and without the
activation of p53 protein, which is responsible for the promotion
of apoptosis in cancer cells (11–13). A
very important BTA feature is its lack of cytotoxicity against
normal cells (14); therefore, it
is hypothesized that the use of BTA in cancer treatment may protect
patients from the adverse effects of many standard cytostatic drugs
(such as cisplatin). However, one of the drawbacks of BTA
therapeutic use is its low solubility. One method to overcome this
limitation could be structural modification as indicated by several
studies (3,4,15).
Another solution may be a physical method, such as electroporation,
which allows the flow of molecules into the cell.
Electroporation (EP) is a technique that enables the
formation of unstable and hydrophilic pores in cell membranes
following exposure to high-intensity short electrical pulses that
induce the formation of breaks in membranes, through which
macromolecules can enter from the intercellular space. In addition,
drugs can also penetrate the cell through the pores created by EP.
EP has not been fully explored yet. The ‘pores’ created in EP are
unstable, form quickly and disappear within a few seconds to
several minutes after the exposure of the cell to the electric
field (16,17).
The combination of EP and chemotherapy (CT) is
termed electrochemotherapy (ECT). It allows for the delivery of
drugs directly into the cell (18).
When cytotoxic agents are poorly transported into the cell, the use
of ECT enables the passage of cytostatic drugs, enhancing the local
treatment of cancer and potentially reducing the side effects of
systemic CT by reducing the required doses of drugs. ECT is much
faster and more efficient than CT alone, which is crucial for the
treatment of patients with cancer. In particular, it may be
beneficial for the treatment of drug-resistant tumors in cases when
the efficiency of the working dose has been substantially reduced,
for example by multidrug resistance mechanisms (16,18).
Furthermore, electrical pulses cause decreased blood perfusion in
vessels surrounding a tumor and can prolong contact of drugs with
cancer cells (16,19). ECT is a very promising method of
treatment for superficially located tumors. In some cases, ECT
limits the necessity for surgical intervention and saves the organ
(19). Currently, in many European
countries, bleomycin and cisplatin (CP) are the only cytostatic
drugs that are clinically approved for use in ECT protocols
(16,18). Therefore, exploration of a less
toxic, natural-origin drugs (such as BTA) for use in ECT is highly
desirable.
Recent studies have demonstrated that elevated
levels of heat shock protein (HSPs), which are ubiquitous
intracellular ‘stress proteins’ or molecular chaperones (20–22),
can increase the aggressiveness of cancer, or alter the response to
chemo- or radiotherapy (23). HSPs
are large and heterogeneous molecules involved in a multitude of
housekeeping functions within a cell (24–26).
Under physiological conditions, HSPs have an important role in
stabilizing and maintaining the conformational structure of a
protein (20,25,27).
Transcription of genes encoding HSPs may be activated by various
stimuli, including physical (temperature and radiation), chemical
(toxic compounds), and biological factors (cytokines, oxygen-free
radicals, and infections) (28).
Under cellular stress, HSPs bind to proteins with abnormal
structure, thereby preventing the formation of aggregates and
allowing the refolding of denatured proteins (25). Additionally, HSPs are indirectly
involved in silencing or decreasing the effects of stress factors
(20). Among the HSP family, HSP27
and HSP70 are reported to be involved in neoplastic processes, with
expression of HSP27 and HSP70 increased in various cancer cell
lines (29). These two chaperone
proteins inhibit programed cell death, thus supporting tumor
development and promoting CT resistance. HSP70 and HSP27 have dual
effects on cancer cells; they suppress anticancer mechanisms and
also promote the expression of genes responsible for metastases. On
the contrary, HSP70 and HSP27 can activate immune pathways that
target cancer cells (30,31). HSP70 has an important role in the
maintenance of cellular homeostasis. Overexpression of HSP70, and
HSP72 in particular, may occur in different types of cancers,
Alzheimer disease and various kidney diseases (28,32).
HSP27 is member of the small HSP subfamily, associated with a
variety of signaling pathways that are critical for cellular
functions (33). Among the other
roles, small HSPs are involved in the antioxidant defense system
within cells (34). HSPs accomplish
this via two mechanisms: Indirectly, in which HSPs increase the
cellular glutathione level; and directly way, in which HSPs
neutralize protein oxidation. Increased expression of HSP27
contributes to resistance to CT and is associated with poor
prognosis (35,36). Therefore, HSPs have a potential role
in the treatment efficacy among different types of cancers
(23,37,38).
ECT is effective in various cutaneous cancer types,
including in melanoma treatment, and in cancer of internal organs,
such as colorectal metastases (39). Therefore, in the present study, cell
lines from melanoma and ovarian metastases of colonic carcinoma
were used as a model to investigate the ECT approach in
vitro. The aim of this study was to examine the efficacy of BTA
as a novel natural-origin compound that can be used for ECT.
Cisplatin was also used with EP as the ‘gold standard’ cytostatic
drug. Whether HSPs can be used as biomarkers of the therapeutic
effects in cancer cells in the response to stress induced by ECT
was also investigated.
Materials and methods
Cell culture
Two metastatic human cancer cell lines were used,
SW626 and Me45. SW626 cells (American Type Culture Collection,
Manassas, VA, USA) are derived from an ovarian metastasis of colon
adenocarcinoma. Me45 cells are a metastatic human pigmented
malignant melanoma cell line was a kind gift from Professor Z.
Krawczyk, established in the Department of Experimental and
Clinical Radiobiology, Center of Oncology (Gliwice, Poland)
(40). Both cell lines were
cultured in polystyrene flasks as a monolayer in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 50 µg/ml
streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C in a humidified
atmosphere with 5% CO2. Cells were harvested by
trypsinization (0.25% trypsin and 0.02% EDTA).
MTT cell viability assay
MTT assay (Sigma-Aldrich; Merck KGaA) was performed
to determine the cell viability. Briefly, 1×104
cells/well were seeded into 96-well plates and cultured overnight.
The cells were incubated with selected concentrations of drugs
(0–50 µM) and with or without EP. MTT assay was performed at
selected time points after the incubation (24, 48, and 72 h) and
according to the manufacturer's protocol. Results were determined
using a multiwell scanning spectrophotometer at 570 nm (EnSpire
Multimode Plate Reader; PerkinElmer, Inc., Waltham, MA, USA). Cell
viability is expressed as normalized percentage of treated cells
compared to untreated control cells.
Chemotherapeutic compounds
In this study, two different cytostatic agents were
selected. BTA was purchased from Sigma-Aldrich (Merck KGaA), as a
naturally derived compound with a potential use in CT, and CP
(Sigma-Aldrich; Merck KGaA), as a standard cytostatic drug.
Briefly, the cells were incubated with drugs for 24, 48 and 72 h at
concentrations ranging from 1–50 µM. For further studies,
concentrations were selected according to the results obtained from
the MTT assay.
EP protocol
EP of cells was performed using an ECM 830 device
(BTX; Harvard Apparatus, Holliston, MA, USA). To test the viability
of cells following EP, cells were suspended in EP buffer (pH 7.4;
10 mM phosphate buffer, 1 mM MgCl2 and 250 mM sucrose)
with a low electrical conductivity and placed in cuvettes with
parallel electrodes (gap of 4 mm). The experiment was performed
according to the following selected parameters: A series of eight
electric pulses of 800–2,000 V/cm, 100 µsec long with 1 sec
intervals. Conditions were selected based on previous studies
(41,42). After pulsing, the cells were
incubated for 10 min at 37°C to enable the resealing of the cell
membrane. Subsequently, the cells were tested for viability using
the MTT test or by performing immunocytochemical (ICC) analysis.
For morphological studies, the cells were seeded on slides 1 day
before or were suspended in EP buffer immediately before EP. The
adhered cells were electroporated using the Petri Pulser™ (BTX;
Harvard Apparatus) consisting of 13 gold-plated electrodes spaced 2
mm apart. Microscopic images were collected after a specified time:
Immediately (15 sec) or 10 min after EP, using a camera connected
to an inverted microscope (Olympus CX41; Olympus Corporation,
Tokyo, Japan).
ECT in vitro
The effect of ECT with CP and BTA in comparison to
untreated control cells was analyzed in SW626 and Me45 cancer cell
lines. Safe EP parameters and non-cytotoxic concentrations of drugs
were selected (50 µM CP; 20 µM BTA for Me45 and 1 µM BTA for
SW626). The cells were prepared for the experiment as described in
the EP protocol and were subjected to electric pulses, following
suspension in cuvettes containing CP or BTA in EP buffer. After 10
min recovery time at 37°C, cells were resuspended in culture medium
and subjected to the same procedures described in EP protocol.
ICC avidin-biotin complex (ABC)
analysis
After CT, EP or ECT cells were plated
(2×103) into 10-well microscopic slides (Thermo Fisher
Scientific, Inc.). After 24, 48 or 72 h, the cells were fixed with
4% formalin for 10 min at room temperature. Blocking was performed
using PBS with 5% fetal bovine serum for 1 h at room temperature
and incubated overnight at 4°C with rabbit monoclonal antibodies
against HSP27 (G3.1; cat. no. sc-59562) or HSP70 (3A3; cat. no.
sc-32239; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) using
1:200 dilution in antibody diluent (EMD Millipore, Billerica, MA,
USA). After 24 h, the slides were incubated with biotinylated
secondary anti-rabbit antibody (DAKO LSAB 2 kit; cat. no. K0675;
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) for 30 min
at room temperature. The ICC assay was completed using the
peroxidase ABC method according to the manufacturer's protocol
(DAKO LSAB 2 kit). Briefly, slides were incubated with horseradish
peroxidase-conjugated streptavidin for 10 min, followed by 5 min
incubation with diaminobenzidine at room temperature. All slides
were counterstained with Mayer's hematoxylin for 1 min at room
temperature (Sigma-Aldrich; Merck KGaA). Blinded samples were
analyzed using an upright microscope equipped with a 40 × objective
(Olympus BX51; Olympus Corporation). The expression was determined
semi-quantitatively by counting the percentage of positively
stained cells in randomly selected fields (from a total of 100
cells per sample, with a minimum four fields analyzed). The
intensity of staining was evaluated as follows: (−) negative, (+)
weak, (++) moderate, and (+++) strong.
Statistical analysis
For the ECT experiments, as there were two factors
affecting the proliferation of cells (drug and electric pulses),
two-way analysis of variance and Tukey's multiple comparison test
in Prism software (v.7.0; GraphPad Software, Inc., La Jolla, CA,
USA) was applied to verify the statistical difference between
experimental groups. Data are expressed as the mean ± standard
deviation (n=3). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cytotoxicity analysis
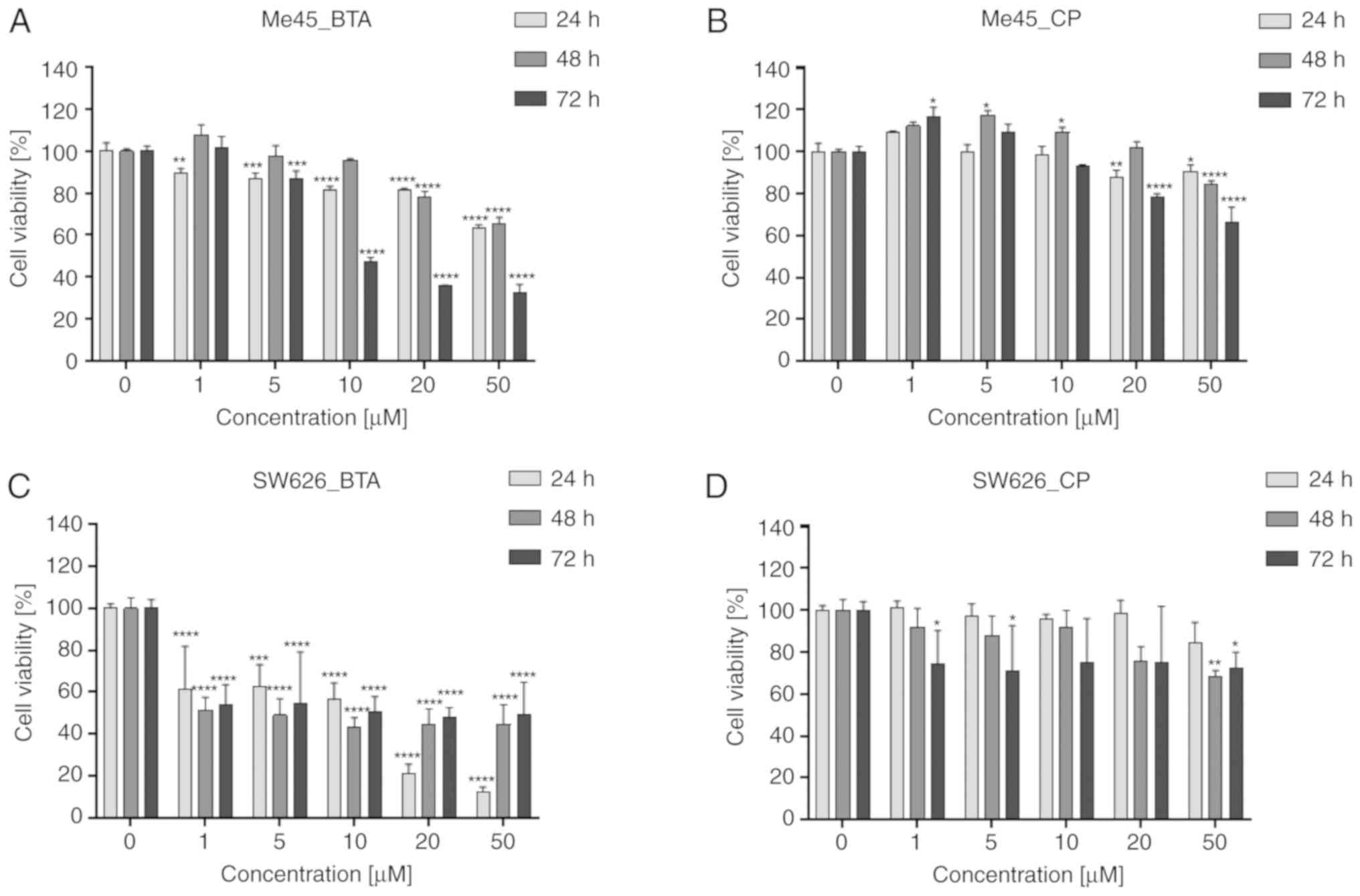
In Me45 cells, the reduction in cell survival
following treatment with CP and BTA for 24 and 48 h was ≤30% less
cells compared with the control group (Fig. 1A and B). Some values were comparable
or higher than the control cells. BTA had the most potent negative
impact on Me45 cells at concentrations >10 µM for 72 h (Fig. 1A); at this concentration, BTA caused
~70% decrease in the cell viability. The cytotoxic activity of CP
(Fig. 1B) was minimal at lower
concentrations (≤10 µM), and a higher decrease (~35%) was detected
after the longest incubation duration (72 h).
In the SW626 cell line, the low cell viability was
obtained after 24 h incubation with BTA (Fig. 1C). Compared with the control cells,
the survival rate was ~15 and ~20% following treatment with BTA at
20 and 50 µM, respectively, for 24 h. After incubation for 48 and
72 h, there was an increase viability of cells compared with 24 h.
This indicates that not all cells were affected by BTA and were
still able to proliferate. For cells incubated for 72 h with BTA,
the survival rate was ~50% regardless of the concentration used.
SW626 cells exhibited limited sensitivity to CP (Fig. 1D) at all tested concentrations and
all time points (≤30% reduction in viability).
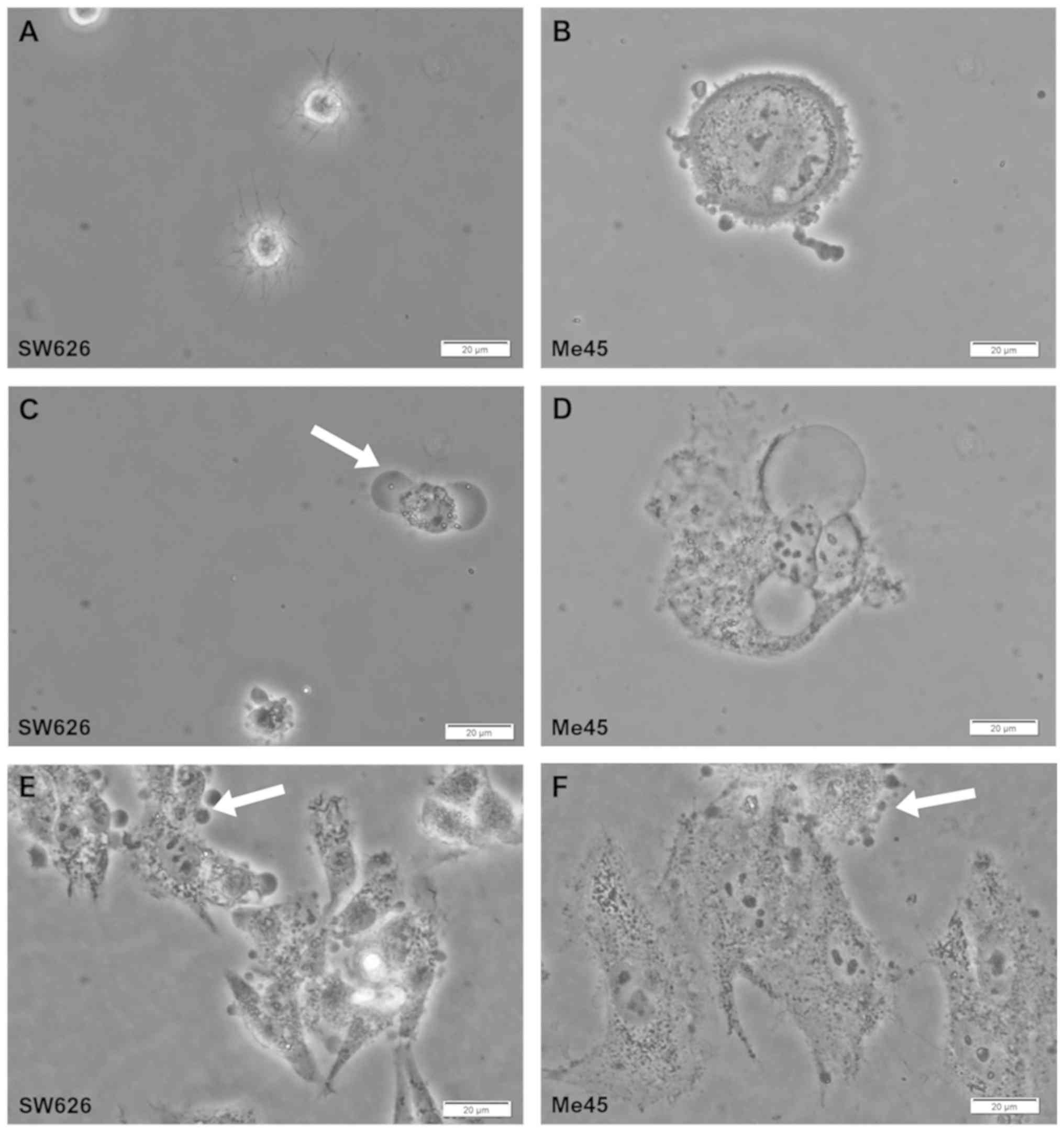
Effect of EP on cell morphology
The effect of the electrical pulse on SW626 and Me45
cancer cells was observed and recorded using a standard microscope
equipped with a camera (Fig. 2).
The pores were formed in the cancer cell membrane by stimulation
with the high electric field (1,200 V/cm). Cytoplasmic outflow was
also observed as ‘bubbles’ at 10 min after EP (Fig. 2C and D). In addition, to induce and
observe visible changes in cell morphology of the cells adhered to
the plate after EP, the intensity of the electric field was
increased to 3,000 V/cm (Fig. 2E and
F).
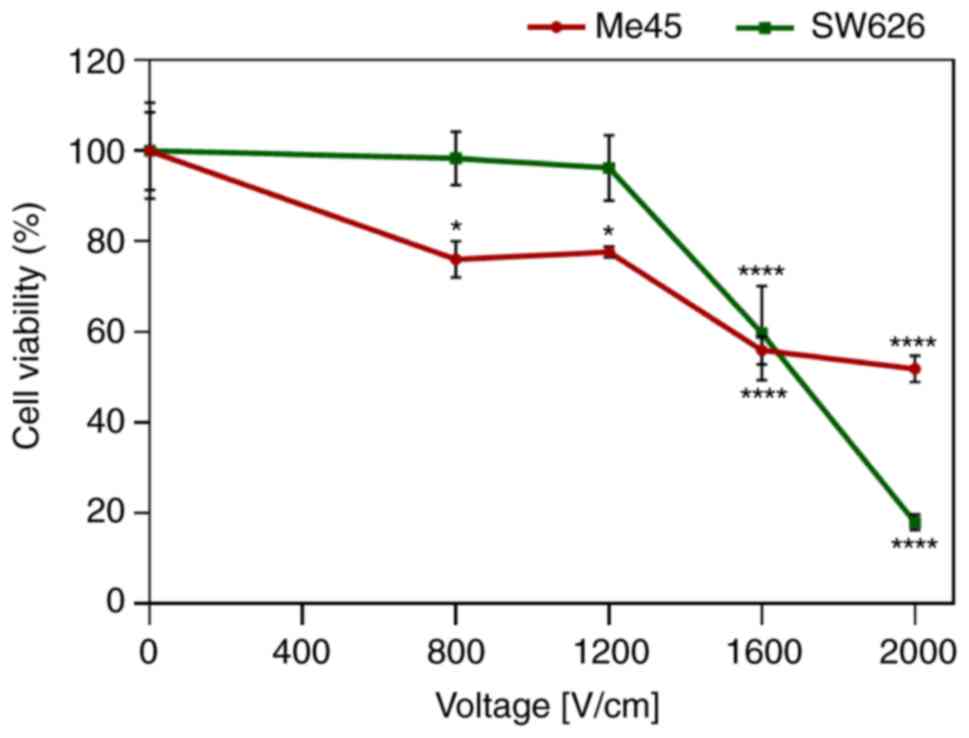
ECT
Based on the results of the cytotoxicity and the EP
analysis, the effective concentrations of drugs and parameters of
EP were selected. Both cell lines were electroporated using the
standard parameters (8 pulses, 100 µs pulse duration, 1 Hz
frequency). Two values of electric field strength (800 and 1,200
V/cm) with limited toxic effect were selected for subsequent
experiments (Fig. 3). The selection
was also based on our previous studies (43,44)
and according to standard ECT procedures where the European
Standard Operating Procedures of Electrochemotherapy protocol is
used. However, the results indicate that selected electric field
values had relatively low lethality. Only in melanoma cells was
observed a 20% decrease of cell viability observed after
electro-pulsation without any drug. This demonstrated that the
selected strengths of the electric field were sufficient for cell
permeabilization, enabling the chemotherapeutic agent to enter the
cells. Electric field strength >800 V/cm caused a significant
decrease in cell viability in both cell lines.
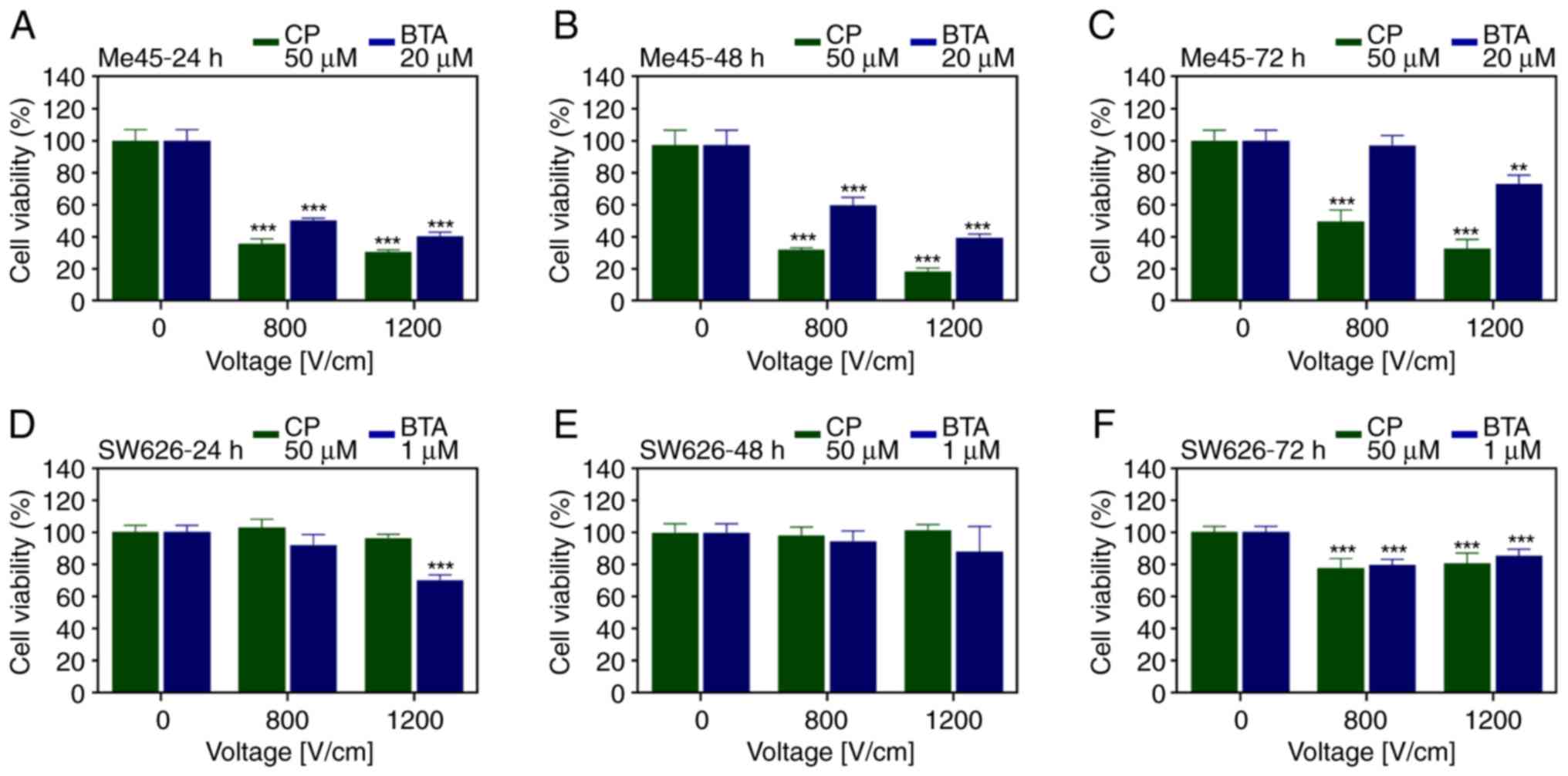
In Me45 cells, CP at 50 µM and BTA at 20 µM, and
three different incubation times (24, 48 and 72 h) were used
(Fig. 4A-C). EP at 800 and 1,200
V/cm intensity caused an increase in cell death induced by BTA at
24 and 48 h (Fig. 4A and B),
compared with BTA alone. The presence of CP in the EP buffer caused
a greater reduction in the cell viability than that of BTA in Me45
cells. As the intensity of the electrical pulses increased, there
the drug-induced cytotoxicity was increased the Me45 cells. ECT
with BTA had a most significant effect than EP or CT alone.
In SW626 cells, two sublethal concentrations of
cytostatic drugs (50 µM CP and 1 µM BTA) and three different
incubation times (24, 48 and 72 h) were used (Fig. 4D-F). According to the results, ECT
slightly improved the efficiency of the applied drug. The viability
was decreased by 30% below the level obtained for CP alone at the
same concentration. The 1,200 V/cm electric field caused a decrease
in the cell viability compared with drug treatment alone after 24 h
(Fig. 4D). No significant increase
in the cytotoxic effect of was observed after 48 h. A slight
decrease of ~20% was induced by ECT compared with CP and BTA alone
after 72 h incubation (Fig. 4E and
F).
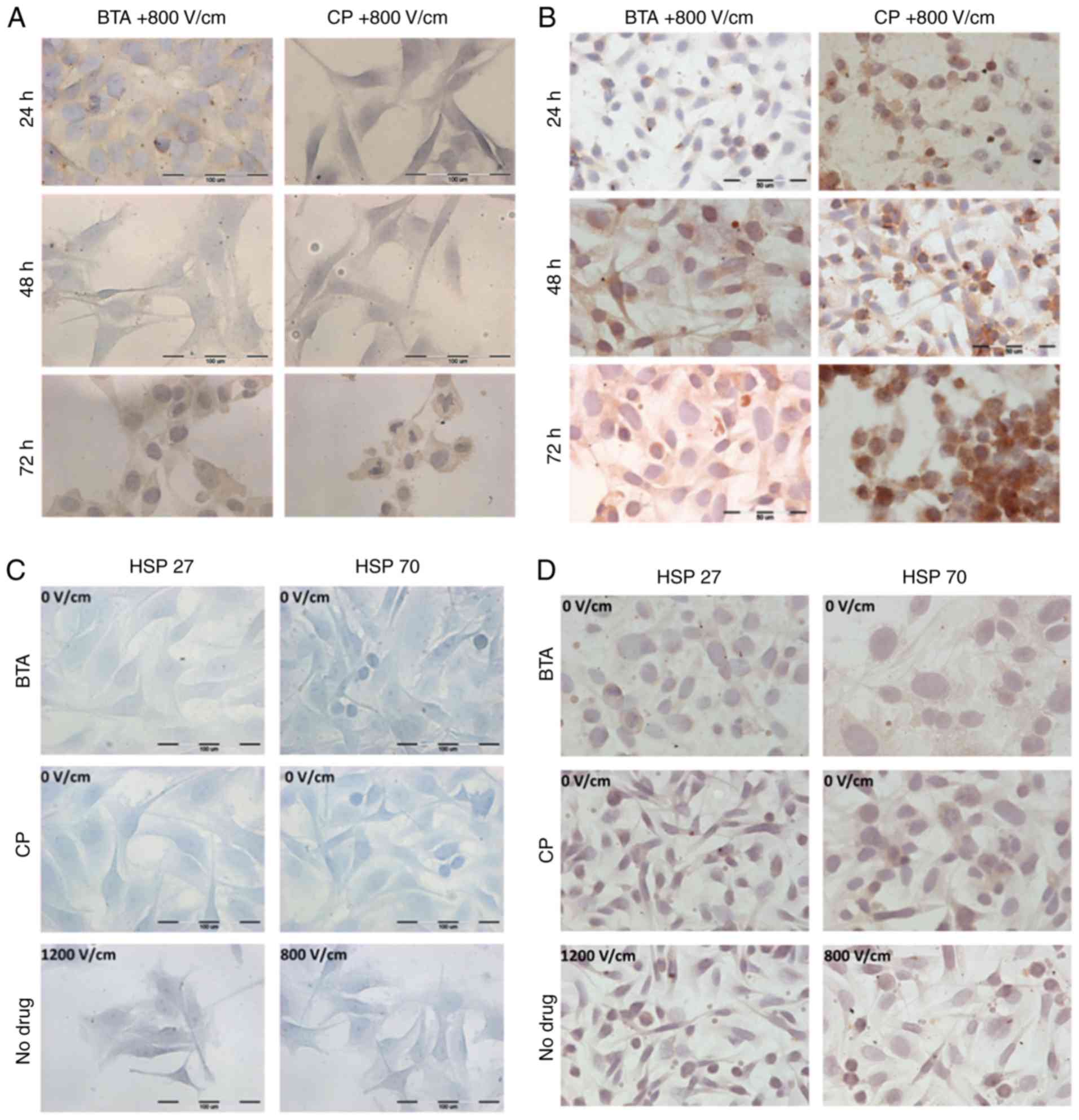
ICC for HSPs
Various studies have indicated that HSPs have an
important role in cancer progression (14). ICC analysis was used to detect
expression of the chosen HSPs in the selected cancer cell lines
after in vitro CT and ECT. The experiments were conducted
with the same parameters as in the previous experiments and were
observed using a standard upright microscope. HSPs were visualized
with various intensities depending on the cell line. Tables I and II, and Fig.
5 present semi-quantitative values related to the intensities
HSP27 and HSP70, and the number of stained cells. For SW626 cells,
the highest number of positive cells and the most intense HSP
staining (both HSP27 and HSP70) was observed after 72 h with ECT
(Fig. 5B) in particular for CP
treatment. In the case of SW626 cells whose appearances exhibited
blebbing and affected morphology, HSPs were located inside the
nucleus to a considerable extent. The intracellular localization of
HSPs was distributed among the cytoplasm and nuclear envelope. The
location of both HSPs suggests induction of apoptosis, for example
by triggering endogenic apoptosis with the mitochondrial
contribution. The relatively high intensity of HSPs in numerous
SW626 cells was also observed after the exposure of the cells to
ECT without BTA or CP (Fig. 5D). In
SW626 cells, an intense positive immunoreaction of both HSPs was
observed at 24 h post-ECT with BTA and even more so for CP, which
suggests that shock caused by the electric field combined with drug
administration had a substantial effect on the expression of HSPs.
After 48 h incubation of ECT treated cells, the amount and
intensity of both HSPs considerably decreased, whereas after 72 h
incubation, they were found to be increased again. Melanoma cells
also indicated an increased immunostaining in particular after
exposition to the strongest electroporation parameters and 72 h
(for EP-BTA and EP-CP), however the reaction was not as strong as
in case of SW626 cells. This may result from an individual HSPs
level for each cell line.
 | Table I.Evaluation of immunocytochemical
reaction with HSP27 antibody in SW626 and Me45 cancer cell lines
following ECT with CP or BTA. |
Table I.
Evaluation of immunocytochemical
reaction with HSP27 antibody in SW626 and Me45 cancer cell lines
following ECT with CP or BTA.
| A, SW626 |
|---|
|
|---|
|
|
| 24 h | 48 h | 72 h |
|---|
|
|
|
|
|
|
|---|
| Drug | EP (V/cm) | I | % | I | % | I | % |
|---|
| Control cells | 0 | ++ | 99 |
|
|
|
|
| CP (50 µM) | 0 | ++ | 94 | + | 35 | ++ | 97 |
|
| 800 | ++ | 92 | + | 50 | +++ | 100 |
|
| 1,200 | ++ | 100 | + | 75 | +++ | 100 |
| BTA (1 µM) | 0 | + | 85 | + | 40 | ++ | 98 |
|
| 800 | ++ | 90 | + | 80 | +++ | 100 |
|
| 1,200 | ++ | 100 | + | 83 | +++ | 100 |
|
| B, Me45 |
|
|
|
| 24 h | 48 h | 72 h |
|
|
|
|
|
|
| Drug | EP
(V/cm) | I | % | I | % | I | % |
|
| Control cells | 0 | − | 0 | − | 0 | − | 0 |
| CP (50 µM) | 0 | − | 0 | − | 0 | − | 0 |
|
| 800 | +++ | 100 | + | 100 | ++ | 100 |
|
| 1,200 | +++ | 100 | + | 100 | ++ | 100 |
| BTA (20 µM) | 0 | − | 0 | − | 0 | − | 0 |
|
| 800 | +++ | 100 | + | 100 | ++ | 100 |
|
| 1,200 | +++ | 100 | + | 100 | ++ | 100 |
 | Table II.Evaluation of immunocytochemical
reaction with HSP70 antibody in SW626 and Me45 cancer cell lines
following ECT with CP or BTA. |
Table II.
Evaluation of immunocytochemical
reaction with HSP70 antibody in SW626 and Me45 cancer cell lines
following ECT with CP or BTA.
| A, SW626 |
|---|
|
|---|
|
|
| 24 h | 48 h | 72 h |
|---|
|
|
|
|
|
|
|---|
| Drug | EP (V/cm) | I | % | I | % | I | % |
|---|
| Control cells | 0 | ++ | 100 | ++ | 100 | ++ | 100 |
| CP (50 µM) | 0 | ++ | 98 | + | 55 | ++ | 98 |
|
| 800 | ++ | 100 | + | 78 | +++ | 100 |
|
| 1,200 | ++ | 100 | + | 81 | ++ | 100 |
| BTA (1 µM) | 0 | +++ | 100 | +/++ | 56 | +/++ | 100 |
|
| 800 | ++ | 100 | ++ | 80 | ++/+++ | 100 |
|
| 1,200 | ++ | 100 | ++ | 87 | ++/+++ | 98 |
|
| B, Me45 |
|
|
|
| 24 h | 48 h | 72 h |
|
|
|
|
|
|
| Drug | EP
(V/cm) | I | % | I | % | I | % |
|
| Control cells | 0 | − | 0 | − | 0 | − | 0 |
| CP (50 µM) | 0 | − | 0 | − | 0 | − | 0 |
|
| 800 | − | 0 | − | 0 | − | 0 |
|
| 1,200 | ++ | 97 | ++ | 99 | ++ | 100 |
| BTA (20 µM) | 0 | − | 0 | − | 0 | − | 0 |
|
| 800 | + (pattern
distribution) | 9 | + | 98 | ++ | 100 |
|
| 1,200 | + | 92 | + | 89 | + | 100 |
In Me45 cells, the positive immunoreaction was
obtained for HSP27 only (Fig. 5C).
The color of the reaction was only visible in cells after ECT
(Fig. 5A). In the control and
samples untreated with EP, there was no positive staining of cells.
In addition, after exposure of cells to 1,200 V/cm intensity, the
cells had shrunk, the cell membrane appeared to lose its continuity
and there was an even distribution of HSP27 in the cytoplasm. The
intensity of reactions in Me45 cells varied depending on the length
of incubation time post-ECT. The most intense HSP27 ICC staining
was obtained after 24 h incubation with CP and BTA. This indicates
a strong protective response in cells caused by environmental
stress. After 48 h of incubation, the staining reaction decreased,
and then increased again after 72 h with BTA and CP.
Discussion
The results in Me45 cells confirm the utility of EP
technique. It significantly enhanced the cytotoxic effect of CP and
to some extent enhanced the effect of BTA. SW626 cells were less
susceptible to EP, thus we suppose this cell line may be
EP-resistant. ECT-CP exhibited significant and ECT-BTA exhibited a
less significant effect at the longest incubation time (72 h) in
Me45 melanoma cells. In the case of the SW626 metastatic cell line
the anticancer effect of ECT was not predominant. Thus, the data
confirm that the use of EP is dependent on the tumor type (18).
CP has been the most effective drug used in the
treatment of cancer in the past decades (41,42).
Despite this, there are many types of cancers that are resistant to
CP treatment, and this phenomena is not only dependent on drug
biodistribution in the cell, but also involves many complex
resistance mechanisms (43,44). This was also confirmed by the
results of the present study. Both selected cell lines had low
sensitivity to treatment with CP alone, and the viability was
maintained in the control level after 24 h. A significant decrease
of cell viability was exhibited after 48 and 72 h (~30%) or the
highest concentrations. The mechanism of resistance to platinum
compounds is achieved by reduced formation of cytotoxic
platinum-DNA adducts, decreased drug accumulation, and increased
inactivation of the drug by cellular proteins and non-protein thiol
groups (41). Numerous studies
reported that the use of EP in the treatment of cancer may
counterbalance drug resistance phenomena (16,43,45).
Previous studies have demonstrated that after ECT with CP, the
viability of CP-resistant cells (OvBH-1 and SKOV-3) was decreased
significantly compared with CP used alone (46). A recent study has also indicated the
advantages of EP in the treatment of neuroblastoma cells,
indicating that CP cytotoxicity was potentiated after exposure of
cells to high intensity electric pulses (47). However, certain cell lines remain
resistant to CT after EP treatment (48). In the present study, CP alone
affected the viability of SW626 cells to a certain extent, and the
use of EP significantly supported this effect. Regardless of the
use of EP, CP caused a decrease in cell viability by up to 20%,
even after 72 h incubation.
BTA has been reported to decrease the growth and
survival rate of several types of cancer (49,50).
The effect is associated with the ability of BTA to induce
programed cell death in tumor cells by triggering the mitochondrial
apoptotic pathway and inhibition of multiple pro-oncogenic factors
(13,51,52).
The present study is the first to use BTA in ECT, which may
overcome difficulty in BTA penetration through cells membranes. The
effect of BTA in ECT was evaluated in two cell lines. In Me45
cells, the application of electrical pulses significantly increased
the cytotoxic effect of BTA. Experiments on SW626 cells also
confirmed the anticancer properties of BTA at low concentrations
using EP, and significantly reduced cell survival, but with less
effect than in melanoma cells. A closer examination on the effect
of EP on the compound itself may be crucial. The data confirms the
differences in ECT sensitivities between the two cell lines
(53). One of the strategies to
increase hydrosolubility and improve the anticancer properties of
BTA is to use derivatives or analogs of BTA (e.g. with a triazole
group added) (54). Another method
to increase the toxic effect on cancer cells may be combination
therapy. In recent studies, it was proposed that combining BTA with
different active compounds, such as gemcitabine (55) or sorafenib (56), may increases the anticancer effects.
In certain of these cases, the application of EP with CP may reduce
the dose of drugs, which may minimize side effects.
HSPs expression in cells subjected to ECT does not
clearly indicate whether a tumor cells will enter the apoptotic
pathway or protect themselves. Despite this, HSP27 was detected in
both cell lines following ECT. Upregulation of HSP27 has been
reported in multiple types of malignancy, including ovarian
carcinoma and melanoma. Along HSPs have been implicated in
oncogenesis and CT resistance (36). The presence of HSP27 indicates
activation of anti-apoptotic defense mechanisms, whereas the lack
of HSP70 suggests the opposite. The current results indicate that
EP enhanced HSP27 in both cell lines at all time point, but HSP70
only in SW626 cells. Other researchers have also demonstrated that
EP induces the expression of HSP70 to a certain extent, as a result
of environmental stress (57).
However, another study demonstrated HSP70 induction may depend on
the cell line (58). The data of
the present study indicate that chemotherapeutic protocols may
modulate expression of HSP27 and HSP70 in tumor tissues.
Vargas-Roig et al (59)
observed that after chemotherapy, nuclear HSP27 and HSP70
expression was increased, and HSP70 and heat shock cognate 70
cytoplasmic expression decreased in patients with breast cancer
(59). Arts et al (60) reported that HSP27 expression was
negative before and positive after chemotherapy in only 2/30 paired
samples, whereas hsp27 expression was positive before and negative
after chemotherapy in 5/30 samples. In general, elevated levels of
HSPs are associated with drug resistance and poor prognosis
(61). Therefore, the presence of
these two proteins (HSP27 and HSP70) in untreated SW626 cells
indicates higher resistance to the applied treatment. Untreated
Me45 cells did not express HSPs; thus, they were more sensitive to
ECT. This indicates stronger intracellular defense mechanisms of
ovarian cancer cells.
Additionally, different cell lines may exhibit
variation in their tolerance to electric fields. The effect of
electric pulses depends on the size, density and shape of the cell
(62). A recent study also reported
the differences between cell lines in the kinetics of membrane
resealing; this process determines how fast the electropores in
membranes are closed following exposure to electric pulses
(63). It has been reported that
pores in the membrane of various tumor malignant cell lines reseal
much faster (up to 300%) than in normal cell lines. Furthermore, a
strong correlation between the resealing response of cancer cells
and their resistance to standard drugs, such as CP, was reported.
These properties may enhance or limit the efficiency of EP in
cancer cells. Thus, further studies are required to assess the
efficiency of this treatment modality.
In summary, the present findings indicate that ECT
protocols are highly variable depending on the type of cancer
cells. Ovarian metastatic SW626 cells were marginally more
sensitive to standard therapy with CP then Me45 melanoma cells.
Additionally, BTA, a natural compound, exhibited potent cytotoxic
effects in SW626 cells. The application of EP enhanced the effects
of BTA in Me45 melanoma cells, and may applied instead of CP. The
next stages of research should focus on further characterization of
the action of BTA on tumor cells. Furthermore, as therapies with
natural compounds appears to be safe and cause less side effects
than standard cytostatics, further research will aim to expand the
pool of test compounds with anticancer properties that can be
enhanced by EP.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Wroclaw
Medical University Statutory Funds ST.E130.16.060 (PI: M.
Zalewski).
Availability of data and materials
The datasets used in this study are available from
the corresponding author upon reasonable request.
Authors' contributions
JS, MK, JZ, MZ, JM and JK participated in the design
of the study, data interpretation, and manuscript drafting. JM,
JKut, and ACh performed the experiments. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BTA
|
betulinic acid
|
|
CP
|
cisplatin
|
|
ECT
|
electrochemotherapy
|
|
EP
|
electroporation
|
|
HSP
|
heat shock protein
|
References
|
1
|
Fulda S: Betulinic acid for cancer
treatment and prevention. Int J Mol Sci. 9:1096–1107. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osunsanmi FO, Shode FO and Opoku AR:
Anti-inflammatory activity of betulinic acid and its acetyl
derivative from melaleuca bracteata. S Afr J Bot. 103:342.
2016.http://dx.doi.org/10.4314/tjpr.v17i10.13
View Article : Google Scholar
|
|
3
|
Pavlova NI, Savinova OV, Nikolaeva SN,
Boreko EI and Flekhter OB: Antiviral activity of betulin, betulinic
and betulonic acids against some enveloped and non-enveloped
viruses. Fitoterapia. 74:489–492. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bringmann G, Saeb W, Assi LA, François G,
Sankara Narayanan AS, Peters K and Peters EM: Betulinic acid:
Isolation from Triphyophyllum peltatum and Ancistrocladus
heyneanus, antimalarial activity, and crystal structure of the
benzyl ester. Planta Med. 63:255–257. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Damle AA, Pawar YP and Narkar AA:
Anticancer activity of betulinic acid on MCF-7 tumors in nude mice.
Indian J Exp Biol. 51:485–491. 2013.PubMed/NCBI
|
|
6
|
Eiznhamer DA and Xu ZQ: Betulinic acid: A
promising anticancer candidate. IDrugs. 7:359–373. 2004.PubMed/NCBI
|
|
7
|
Rzeski W, Stepulak A, Szymański M,
Sifringer M, Kaczor J, Wejksza K, Zdzisińska B and
Kandefer-Szerszeń M: Betulinic acid decreases expression of bcl-2
and cyclin D1, inhibits proliferation, migration and induces
apoptosis in cancer cells. Naunyn Schmiedebergs Arch Pharmacol.
374:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ehrhardt H, Fulda S, Fuhrer M, Debatin KM
and Jeremias I: Betulinic acid-induced apoptosis in leukemia cells.
Leukemia. 18:1406–1412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmidt ML, Kuzmanoff KL, Ling-Indeck L
and Pezzuto JM: Betulinic acid induces apoptosis in human
neuroblastoma cell lines. Eur J Cancer. 33:2007–2010. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chintharlapalli S, Papineni S, Ramaiah SK
and Safe S: Betulinic acid inhibits prostate cancer growth through
inhibition of specificity protein transcription factors. Cancer
Res. 67:2816–2823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drag-Zalesinska M, Kulbacka J, Saczko J,
Wysocka T, Zabel M, Surowiak P and Drag M: Esters of betulin and
betulinic acid with amino acids have improved water solubility and
are selectively cytotoxic toward cancer cells. Bioorg Med Chem
Lett. 19:4814–4817. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pisha E, Chai H, Lee IS, Chagwedera TE,
Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD and
Brown DM: Discovery of betulinic acid as a selective inhibitor of
human-melanoma that functions by induction of apoptosis. Nat Med.
1:1046–1051. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fulda S and Kroemer G: Targeting
mitochondrial apoptosis by betulinic acid in human cancers. Drug
Discov Today. 14:885–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cichewicz RH and Kouzi SA: Chemistry,
biological activity, and chemotherapeutic potential of betulinic
acid for the prevention and treatment of cancer and HIV infection.
Med Res Rev. 24:90–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bache M, Bernhardt S, Passin S, Wichmann
H, Hein A, Zschornak M, Kappler M, Taubert H, Paschke R and
Vordermark D: Betulinic acid derivatives NVX-207 and B10 for
treatment of glioblastoma-an in vitro study of cytotoxicity and
radiosensitization. Int J Mol Sci. 15:19777–19790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skolucka N, Saczko J, Kotulska M, Kulbacka
J and Choromanska A: Electroporation and its application. Pol
Merkur Lekarski. 28:501–504. 2010.(In Polish). PubMed/NCBI
|
|
17
|
Kotulska M, Kubica K, Koronkiewicz S and
Kalinowski S: Modeling the induction of lipid membrane
electropermeabilization. Bioelectrochemistry. 70:64–70. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sersa G, Miklavcic D, Cemazar M, Rudolf Z,
Pucihar G and Snoj M: Electrochemotherapy in treatment of tumours.
Eur J Surg Oncol. 34:232–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kotulska M: Electrochemotherapy in cancer
treatment. Adv Clin Exp Med. 16:601–607. 2007.
|
|
20
|
Kazmierczuk A and Kilianska ZM: The
pleiotropic activity of heat-shock proteins. Postepy Hig Med Dosw
(Online). 63:502–521. 2009.(In Polish). PubMed/NCBI
|
|
21
|
Schlesinger MJ: Heat-shock proteins-the
search for functions. J Cell Biol. 103:321–325. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cymerys J and Niemialkowski M:
Heat-shock-proteins-molecular-perpetual-motion. Post Biol Kom.
31:332–339. 2004.
|
|
23
|
Kaigorodova EV and Bogatyuk MV: Heat shock
proteins as prognostic markers of cancer. Curr Cancer Drug Targets.
14:713–726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kulczynska A, Kostur A and Piszcz J: Heat
shock proteins in the pathogenesis and treatment of cancer. Acta
Haematol Pol. 41:253–259. 2010.pthit.pl/download,ahp,538.
|
|
25
|
Kazmierczuk A and Kilianska ZM: Role of
heat shock proteins in cell apoptosis. Postepy Hig Med Dosw
(Online). 64:273–283. 2010.(In Polish). PubMed/NCBI
|
|
26
|
Liberek K, Lewandowska A and Zietkiewicz
S: Chaperones in control of protein disaggregation. EMBO J.
27:328–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verbeke P, Fonager J, Clark BF and Rattan
SI: Heat shock response and ageing: Mechanisms and applications.
Cell Biol Int. 25:845–857. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Musial K and Zwolinska D: Heat shock
proteins in chronic kidney disease. Pediatr Nephrol. 26:1031–1037.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garrido C, Schmitt E, Cande C, Vahsen N,
Parcellier A and Kroemer G: HSP27 and HSP70: potentially oncogenic
apoptosis inhibitors. Cell Cycle. 2:579–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Calderwood SK: Heat shock proteins and
cancer: Intracellular chaperones or extracellular signalling
ligands? Philos Trans R Soc Lond B Biol Sci. 373:201605242018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee CT and Repasky EA: Opposing roles for
heat and heat shock proteins in macrophage functions during
inflammation: A function of cell activation state? Front Immunol.
3:1402012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Tan MS, Lu RC, Yu JT and Tan L:
Heat shock proteins at the crossroads between cancer and
Alzheimer's disease. Biomed Res Int. 2014:2391642014.PubMed/NCBI
|
|
33
|
Vidyasagar A, Wilson NA and Djamali A:
Heat shock protein 27 (HSP27): Biomarker of disease and therapeutic
target. Fibrogenesis Tissue Repair. 5:72012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian X, Zhao L, Song X, Yan Y, Liu N, Li
T, Yan B and Liu B: HSP27 inhibits homocysteine-induced endothelial
apoptosis by modulation of ROS production and mitochondrial
caspase-dependent apoptotic pathway. Biomed Res Int.
2016:48478742016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng G, Zhang Z, Liu H, Xiong Y, Luo L,
Jia X, Peng C, Zhang Q, Li N, Gu Y, et al: HSP27-mediated
extracellular and intracellular signaling pathways synergistically
confer chemo-resistance in squamous cell carcinoma of tongue. Clin
Cancer Res. 24:1163–1175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y and Shen X: Heat shock protein 27
protects L929 cells from cisplatin-induced apoptosis by enhancing
akt activation and abating suppression of thioredoxin reductase
activity. Clin Canc Res. 13:2855–2864. 2007. View Article : Google Scholar
|
|
37
|
Wang XP, Wang QX, Lin HP, Xu B, Zhao Q and
Chen K: Recombinant heat shock protein 70 functional peptide and
alpha-fetoprotein epitope peptide vaccine elicits specific
anti-tumor immunity. Oncotarget. 7:71274–71284. 2016.PubMed/NCBI
|
|
38
|
Shevtsov M and Multhoff G: Heat shock
protein-peptide and HSP-based immunotherapies for the treatment of
cancer. Front Immunol. 7:1712016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Edhemovic I, Brecelj E, Gasljevic G,
Marolt Music M, Gorjup V, Mali B, Jarm T, Kos B, Pavliha D, Grcar
Kuzmanov B, Cemazar M, et al: Intraoperative electrochemotherapy of
colorectal liver metastases. J Surg Oncol. 110:320–327. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kumala S, Niemiec P, Widel M, Hancock R
and Rzeszowska-Wolny J: Apoptosis and clonogenic cell survival in
three tumour cell lines exposed to gamma rays or chemical genotoxic
agents. Cell Mol Biol Lett. 8:655–665. 2003.PubMed/NCBI
|
|
41
|
Helm CW and States JC: Enhancing the
efficacy of cisplatin in ovarian cancer treatment-could arsenic
have a role. J Ovarian Res. 2:22009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wezgowiec J, Kulbacka J, Saczko J,
Rossowska J, Chodaczek G and Kotulska M: Biological effects in
photodynamic treatment combined with electropermeabilization in
wild and drug resistant breast cancer cells. Bioelectrochemistry.
123:9–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Michel O, Kulbacka J, Saczko J, Mączyńska
J, Błasiak P, Rossowska J and Rzechonek A: Electroporation with
cisplatin against metastatic pancreatic cancer: In vitro study on
human primary cell culture. BioMed Res Int. 2018:73645392018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gothelf A, Mir LM and Gehl J:
Electrochemotherapy: Results of cancer treatment using enhanced
delivery of bleomycin by electroporation. Cancer Treat Rev.
29:371–387. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saczko J, Kamińska I, Kotulska M, Bar J,
Choromańska A, Rembiałkowska N, Bieżuńska-Kusiak K, Rossowska J,
Nowakowska D and Kulbacka J: Combination of therapy with
5-fluorouracil and cisplatin with electroporation in human ovarian
carcinoma model in vitro. Biomed Pharmacother. 68:573–580. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Esmekaya MA, Kayhan H, Coskun A and
Canseven AG: Effects of cisplatin electrochemotherapy on human
neuroblastoma cells. J Membr Biol. 249:601–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cemazar M, Sersa G and Miklavcic D:
Electrochemotherapy with cisplatin in the treatment of tumor cells
resistant to cisplatin. Anticancer Res. 18:4463–4466.
1998.PubMed/NCBI
|
|
49
|
Zhao J, Li R, Pawlak A, Henklewska M,
Sysak A, Wen L, Yi JE and Obmińska-Mrukowicz B: Antitumor activity
of betulinic acid and betulin in canine cancer cell lines. In Vivo.
32:1081–1088. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu J, Wang X, Zhang H, Yue J, Sun Y, Zhang
X and Zhao Y: Synthesis of triterpenoid derivatives and their
anti-tumor and anti-hepatic fibrosis activities. Nat Prod Res.
16:1–7. 2018. View Article : Google Scholar
|
|
51
|
Chintharlapalli S, Papineni S, Lei P,
Pathi S and Safe S: Betulinic acid inhibits colon cancer cell and
tumor growth and induces proteasome-dependent and -independent
downregulation of specificity proteins (Sp) transcription factors.
BMC Cancer. 11:3712011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun L, Cao J, Chen K, Cheng L, Zhou C, Yan
B, Qian W, Li J, Duan W, Ma J, et al: Betulinic acid inhibits
stemness and EMT of pancreatic cancer cells via activation of AMPK
signaling. Int J Oncol. 54:98–110. 2019.PubMed/NCBI
|
|
53
|
Periasamy G, Teketelew G, Gebrelibanos M,
Sintayehu B, Gebrehiwot M, Karim A and Geremedhin G: Betulinic acid
and its derivatives as anti-cancer agent: A review. Arch Appl Sci
Res. 6:47–58. 2014.
|
|
54
|
Yang S, Liang N, Li H, Xue W, Hu D, Jin L,
Zhao Q and Yang S: Design, synthesis and biological evaluation of
novel betulinic acid derivatives. Chem Cent J. 6:1412012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pandita A, Kumar B, Manvati S, Vaishnavi
S, Singh SK and Bamezai RN: Synergistic combination of gemcitabine
and dietary molecule induces apoptosis in pancreatic cancer cells
and down regulates PKM2 expression. PLoS One. 9:e1071542014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kutkowska J, Strzadala L and Rapak A:
Sorafenib in combination with betulinic acid synergistically
induces cell cycle arrest and inhibits clonogenic activity in
pancreatic ductal adenocarcinoma cells. Int J Mol Sci.
19:E32342018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mlakar V, Todorovic V, Cemazar M, Glavac D
and Sersa G: Electric pulses used in electrochemotherapy and
electrogene therapy do not significantly change the expression
profile of genes involved in the development of cancer in malignant
melanoma cells. BMC Cancer. 9:2992009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dressel R, Baraki H, Langer F and Gunther
E: Reduced susceptibility of electroporated tumor cell lines to
killing by cytotoxic lymphocytes. Biochem Biophys Res Commun.
250:259–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vargas-Roig LM, Gago FE, Tello O, Aznar JC
and Ciocca DR: Heat shock protein expression and drug resistance in
breast cancer patients treated with induction chemotherapy. Int J
Cancer. 79:468–475. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Arts HJ, Hollema H, Lemstra W, Willemse
PH, De Vries EG, Kampinga HH and Van der Zee AG:
Heat-shock-protein-27 (hsp27) expression in ovarian carcinoma:
Relation in response to chemotherapy and prognosis. Int J Cancer.
84:234–238. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tóth ME, Gombos I and Sántha M: Heat shock
proteins and their role in human diseases. Acta Biol Szeged.
59:121–141. 2015.https://www2.sci.u-szeged.hu/ABS/2015/Acta%20HPb/59121.pdf
|
|
62
|
Talele S and Gaynor P: Non-linear time
domain model of electropermeabilization: Effect of extracellular
conductivity and applied electric field parameters. J Electrost.
66:328–334. 2008.https://doi.org/10.1016/j.elstat.2008.02.002
View Article : Google Scholar
|
|
63
|
Hui TH, Zhou ZL, Fong HW, Ngan RK, Lee TY,
Au JS, Ngan AH, Yip TT and Lin Y: Characterizing the malignancy and
drug resistance of cancer cells from their membrane resealing
response. Sci Rep. 6:266922016. View Article : Google Scholar : PubMed/NCBI
|