Introduction
Ovarian cancer is the fourth most frequent
gynecologic malignancy and the leading cause of tumor-associated
mortality in the USA (1).
Epithelial ovarian cancer, which accounts for ~90% of ovarian
cancers, is generally diagnosed at an advanced stage (2). Furthermore, the prognosis and
five-year survival have not improved significantly owing to the
high recurrence rate (3).
Therefore, it is urgent to identify the underlying mechanisms and
to develop alternative therapeutic strategies for this type of
cancer.
The ovarian surface epithelium (OSE) is the
recognized source of epithelial ovarian cancer (2,4).
Several characteristics of epithelial ovarian carcinomas imply it
is a cancer stem cell-driven disease. First, the stem properties of
OSE have been recognized previously (5), and the cancer-prone stem cell niche
was also identified at the helium area of ovary (6). Second, epithelial ovarian cancer can
differentiate into several subtypes that recapitulate the histology
of other normal gynecologic tissues (2). Third, the high recurrence rate
following the initial successful treatment may derive from a small
number of cells within the cancer population, which are: i) Capable
of repopulating the entire tumor and ii) exhibit cytoprotective
mechanisms on somatic stem cells (6–10).
Furthermore, the presence of cancer stem cell-like cells (CSCs) in
patients is reported to be associated with poor survival and
chemoresistance (11,12). Therefore, targeting CSCs may be a
potential therapeutic approach to epithelial ovarian cancer.
Emerging evidence suggests that vitamin D deficiency
is strongly associated with the risk of various human cancers,
including colorectal, breast, prostate and ovarian cancer (13). 1α,25-dihydroxyvitamin D3
[1α,25(OH)2D3], the active metabolite of
vitamin D3, functions by binding to vitamin D receptor
(VDR). 1α,25(OH)2D3, or analogues of
1α,25(OH)2D3, may serve as anticancer agents
owing to their ability to suppress proliferation, invasion,
metastasis and angiogenesis, and to induce apoptosis (14–17).
Furthermore, 1α,25(OH)2D3 is reported to
inhibit the proliferation of prostate cancer stem cells by inducing
cell cycle arrest and senescence (18). Furthermore, results from in
vitro and xenograft studies indicated that the vitamin D
analogue (BXL0124) may decrease mammosphere growth by reducing
cluster of differentiation CD44 expression levels (14,19,20).
There is preliminary evidence that
1α,25(OH)2D3 could be used to cure and
inhibit prostate and breast CSCs (21). Our previous study demonstrated that
1α,25(OH)2D3 inhibited the migration of human
ovarian cancer cells by increasing the expression of VDR and
suppressing epithelial-mesenchymal transition (17). However, the effects of vitamin
D3 on ovarian CSCs remain largely unknown. Side
population (SP) cells have been presented as functional markers of
CSCs (22–26). The present study investigated
whether 1α,25(OH)2D3 was able to inhibit the
stem cell-like phenotype of SP cells isolated from mouse OSE (MOSE)
cells, and determined its possible underlying mechanisms. The
present findings indicated that 1α,25(OH)2D3
suppressed the properties of ovarian CSCs by enhancing VDR
expression and reducing CD44 level, which may provide the novel
strategy for treating epithelial ovarian cancer.
Materials and methods
Cell culture and reagents
MOSE cells were isolated as described by Roby et
al (27). Briefly, the 4 mice
are 6–8 week old and ~20 g. All mice were housed with full of food
and water under controlled conditions of temperature at 21±2°C,
relative humidity at 55±5% and a 12:12 h light-dark cycle. Ovaries
from female breeder mice (BALB/c) were resected and, following
removal of the residual remnants of the oviducts, were incubated in
1.5 ml tubes for 30 min with trypsin in 5% CO2 at 37°C.
Following incubation, tubes were removed from the incubator and
inverted 10 times. The ovaries were transferred to a clean 1.5 ml
tube for the second digestion with trypsin, as above. Cells were
collected, pelleted by 1,000 × g/min for 5 min at 4°C, resuspended
in DMEM/F12 medium containing 20 ng/ml mEGF, 20 ng/ml mouse basic
fibroblast growth factor, 2 µg/ml insulin and 4 µg/ml heparin
sodium and seeded onto 35 mm dishes. On the 2nd day, the culture
dishes were not removed, because moving them is conducive to cell
growth and therefore the stability of the microenvironment around
the cells can be maintained. On the 3rd day, growth medium was
changed according to the cell growth. MOSE cells were continuously
passaged in vitro. MOSE cells maintained proliferation and
underwent spontaneous neoplastic transformation when subcultured
for >80 passages in vitro (see Supplementary data). The
spontaneous malignant transformation of MOSE cells in late stage
(M-L cells) were maintained in Dulbecco's modified Eagle's
medium/F-12 medium (DMEM/F12) containing 10% fetal bovine serum
(FBS), 1% penicillin, 1% streptomycin, 10 ng/ml mouse epithelial
growth factor (mEGF) and 1% insulin-transferrin-selenium in an
atmosphere of 95% humidity and 5% CO2 at 37°C. The MOSE
cells have been tested for mycoplasma and human cell lines
contamination by STR profiling and were demonstrated to be clean
(see Figs. S1 and S2; Table
SI). The reagents for cell culture were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Vitamin D3
(40,000 IU/ml) and 1α,25(OH)2D3 were
purchased from Shanghai General Pharmaceutical Company, Ltd.
(www.cpshgp.com/english/; Shanghai,
China) and Sigma-Aldrich (Merck KGaA, Darmstadt, Germany),
respectively. In the present study, cells were treated by 10 nM
1α,25(OH)2D3 in vitro, and mice were
was administrated by vitamin D3. In vivo, vitamin
D3 is successively hydroxylated by the 25-hydroxylase
and 1α-hydroxylase, and forms 1α,25(OH)2D3,
the active form of vitamin D3.
Isolation of SP cells
M-L cells (1×106 cells/ml) were
re-suspended in DMEM/F12 medium containing 2% FBS and 4%
penicillin-streptomycin, and stained with 10 µg/ml Hoechst 33342
(Sigma-Aldrich; Merck KGaA) in the presence or absence of 50 µg/ml
verapamil (Sigma-Aldrich; Merck KGaA) at 37°C for 90 min with
intermittent shaking every 10 min. Following incubation, the cells
were centrifuged by 1,000 × g/min for 5 min at 4°C and washed with
cold phosphate-buffered saline (PBS). The SP and non-side
population (NSP) cells were isolated and collected by MoFloAstrios
EQ fluorescence activated cell sorting (Summit 6.2; FACS,
www.beckmancoulter.cn/ls-discovery/flow/researchflow/moflo-astrios.html;
Beckman Coulter, Inc., Brea, CA, USA). The Hoechst dye was excited
with a UV laser at 346 nm and its fluorescence emissions were
measured with 630/22 (Hoechst 33342 Red) and 424/44 filters
(Hoechst Blue). For purification, isolated SP cells were cultured
in serum-free DMEM/F12 medium containing 20 ng/ml mEGF, 20 ng/ml
mouse basic fibroblast growth factor, 1:50 B27, 2 µg/ml insulin, 4
µg/ml heparin sodium and 6 mg/ml glucose for 10 days and were
subsequently re-suspended in StemPro Accutase Cell Dissociation
Reagent at 37°C for 10 min. The NSP cells were cultured in DMEM/F12
medium containing 20 ng/ml mEGF, 20 ng/ml mouse basic fibroblast
growth factor, 2 µg/ml insulin and 4 µg/ml heparin sodium. The
reagents in serum-free medium were purchased from Thermo Fisher
Scientific, Inc. Subsequently, the cells were centrifuged by 1,000
× g/min for 5 min at 4°C and maintained in the fresh serum-free
medium until further use.
Detection of CD44 and CD117
The SP and NSP cells were washed, re-suspended and
separately incubated with fluorescence-conjugated antibodies
against CD44 (1:1,000; cat. no. 555478), CD117 (1:1,000; cat. no.
555714) and IgG (1:1,000; cat. nos. 555749 and 555742; BD
Biosciences, San Jose, CA, USA) in 4°C for 10 min. The appropriate
concentrations of each antibody were recommended by the
manufacturer. The cells were washed and analyzed by Cytomics™ FC
500 from Beckman Coulter, Inc. In brief, unstained cells,
CD44+, CD117+ and double-stained control
cells were used to mark the four quadrants in a dot-plot for
unstained, CD44+, CD117+ and double-positive
populations. The software used for the analysis is CXP Analysis,
which is the part of FC500 flow cytometer (Beckman Coulter,
Inc.).
Sphere-formation assay
The SP cells were re-suspended in StemPro Accutase
Cell Dissociation Reagent at 37°C for 10 min, terminated by the
serum-free DMEM/F12 medium. Subsequently, 1,000 cells/well were
plated in the 96-well plates and cultured in serum-free medium for
10 days. The spheres with a minimum size of 50 µm were counted
under a brightfield microscope (CKX41F; Olympus Corporation, Tokyo,
Japan) equipped with a digital camera. The sphere-formation rate
was expressed as the following formula: (Number of spheres
formed/number of plated cells) × 100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extractions from 1×106 cells SP
or NSP cells were performed using TRIzol® reagent (Life
Technologies; Thermo Fisher Scientific, Inc.). For the synthesis of
first-strand cDNA, total RNA was reverse-transcribed by the
Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics,
Basel, Switzerland). Primer sequences are available in Table I. qPCR reactions were conducted with
the SYBR-Green I Nucleic AcideGel stain (Roche Diagnostics)
according to manufacturer's protocol. Thermocycling parameters
were: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec,
60°C for 20 sec and 72°C for 30 sec. Relative expression levels
were calculated using the 2−ΔΔCq method by Livak and
Scmittgen (28). GAPDH was used as
normalization control.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| GAPDH | F:
TTGATGGCAACAATCTCCAC |
|
| R:
CGTCCCGTAGACAAAATGGT |
| CD44 | F:
AGCGGCAGGTTACATTCAAA |
|
| R:
CAAGTTTTGGTGGCACACAG |
| NANOG | F:
AGGGTCTGCTACTGAGATGCTCTG |
|
| R:
CAACCACTGGTTTTTCTGCCACCG |
| OCT4 | F:
AGAAGGAGCTAGAACAGTTTGC |
|
| R:
CGGTTACAGAACCATACTCG |
| CD133 | F:
TAGAGGGAAGTCATTCGGCT |
|
| R:
CCCAAGATACCTTCAATGCTG |
| SOX2 | F:
AAAGCGTTAATTTGGATGGG |
|
| R:
ACAAGAGAATTGGGAGGGGT |
| KLF4 | F:
CAGTGGTAAGGTTTCTCGCC |
|
| R:
GCCACCCACACTTGTGACTA |
| NOTCH1 | F:
CTGAGGCAAGGATTGGAGTC |
|
| R:
GAATGGAGGTAGGTGCGAAG |
| NOTCH2 | F:
TGTGCCGTTGTGGTAGGTAA |
|
| R:
TGCTGTGGCTCTGGCTGT |
| ABCG2 | F:
TCGCAGAAGGAGATGTGTTGAG |
|
| R:
CCAGAATAGCATTAAGGCCAGG |
| CD117 | F:
CGGTCGACTCCAAGTTCTACAAG |
|
| R:
GTTGCAGTTTGCCAAGTTGGAGT |
| β-catenin | F:
ATGGCTTGGAATGAGACTGC |
|
| R:
CTCCATCATAGGGTCCATCC |
| c-Myc | F:
CAACGTCTTGGAACGTCAGA |
|
| R:
TCGTCTGCTTGAATGGACAG |
| Cyclin D1 | F:
TGTTCGTGGCCTCTAAGATG |
|
| R:
ACTCCAGAAGGGCTTCAATC |
| VDR | F:
TGACCCCACCTACGCTGACT |
|
| R:
CCTTGGAGAATAGCTCCCTGTACT |
Western blotting
Approximately 106 cells were scraped off
with radioimmunoprecipitation buffer (cat. no. P0013; Beyotime
Institute of Biotechnology, Haimen, China). Subsequently, the
lysates were centrifuged at 7,500 × g for 30 min at 4°C and the
supernatants were collected. Protein concentrations were quantified
by the Bicinchoninic Acid assay (Beyotime Institute of
Biotechnology). Proteins (30 µg/sample) were separated by 10%
SDS-PAGE and were transferred to polyvinylidene fluoride membranes.
Membranes were blocked with 5% non-fat milk in PBS + 1% Tween-20
and incubated with primary antibodies against VDR (1:500; cat. no.
12550), Cyclin D1 (1:1,000; cat. no. 2978), β-catenin (1:1,000;
cat. no. 8480), Sex-determining region Y (SOX2; 1:1,000; cat. no.
23064), NANOG (1:1,000; cat. no. 8822), octamer-binding protein
(OCT4; 1:1,000; cat. no. 2840) and GAPDH (1:1,000; cat. no. 5174)
at 4°C overnight. Subsequently, the membranes were incubated for 1
h at room temperature with 1:3,000 anti-mouse IgG, HRP-linked
antibody (cat. no. 7076) and anti-rabbit IgG, HRP-linked antibody
(cat. no. 7074). All antibodies were purchased from Cell Signaling
Technology, Inc. (CST; Danvers, MA, USA). Protein bands were
visualized using Enhanced Chemiluminescence Detection (Merck KGaA);
to quantify the level of protein expression, densitometric analysis
was performed according to the manufacture's protocol (Gbox
Chemi-XR 5; Syngene, Frederick, MD, USA). The specific/individual
protein expressions (GeneSnap image acquisition software, GeneTools
image analysis software; Syngene) should be normalized with their
respective GAPDH loading control first. Subsequently, when drafting
the histograms, the NSP or control groups should be set to ‘1’ and
all other expression compared with that.
Immunofluorescence staining
Approximately 1×105 cells were plated
onto coverslips until they reached an optimal density of 70–80%.
The cells were fixed with 4% paraformaldehyde at 4°C for 20 min,
permeabilized with 0.1% Triton X-100 for 15 min at 4°C and blocked
with 1% FBS for 1 h at room temperature. Subsequently, the cells
were incubated with primary antibody of β-catenin (CST; 1:100; cat.
no. 8480) overnight at 4°C. Following this, the cells were labeled
with anti-rabbit IgG (H+L), F(ab')2 Fragment (Alexa
Fluor® 488-conjugated; CST; 1:1,000, cat. no. 4412) in
the dark room for 1.5 h at room temperature and washed with PBS
three times. Nuclei were stained with DAPI for 20 min at room
temperature. The signals were detected using a TCS SP2 Confocal
Laser Scanning Microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
X-ray and heavy particle resistance of
SP cells
Approximately 1×105 cells were irradiated
with 2, 4, 6, 8 or 10 Gy of X-rays using a Pantac HF-320S X-ray
generator (Shimadzu Co., Kyoto, Japan) or carbon-ion beams
accelerated by the Heavy ion medical accelerator in Chiba in the
National Institute of Radiological Sciences (Chiba, Japan).
Following irradiation, NSP cells were plated in triplicate in 60-mm
dishes for assays of clonogenicity. After culturing for 14 days at
37°C, the colonies were fixed with 75% alcohol and stained with
0.3% methyl violet for 20 min at room temperature. Colonies
containing >50 cells were counted as the survivors.
Additionally, SP cells in serum-free medium were plated (1,000
cells/well) in 96-well plates for sphere-formation assay. The
spheroid formation rates were calculated as the percentage of the
spheres >50 µm. At least three parallel samples were scored in
six replicates conducted for each aforementioned irradiation
condition. Cell survival fractions were obtained from fitting the
surviving fraction to a linear-quadratic model expressed by the
following formula: SF = exp - (αD + βD2), where SF is
the survival fraction, exp means ‘exp-function’, α and β are the
constant of the fitted curve by the results, and D is the
irradiation dose.
Tumorigenesis
BALB/c nude mice (age, 4–6 weeks, 18–20 g) were
purchased from Soochow University Laboratory Animal Center (Suzhou,
China). Mice were provided with water and food ad libitum
and were housed at five animals per cage. All mice were housed
under controlled conditions of temperature at 21±2°C, relative
humidity at 55±5% and a 12:12 h light-dark cycle. All surgical
procedures and care administered to the animals were approved by
the Institutional Animal Care and Use Committee (approval number is
ECSU-201800049). In the orthotopic model, 10 µl cell suspension
(1×104 NSP or SP cells) mixed with Matrigel (1:1; BD
Biosciences; cat. no. 356234) were injected orthotopically into the
one ovary (n=2 mice/group). In the subcutaneous model, it has been
reported that SP cells has stronger tumorigenicity than NSP cells
(22,24). Therefore, different number of SP and
NSP cells were injected into mice. One hundred µl cell suspension
containing 1×104 SP cells or 1×106 NSP cells
mixed with Matrigel were injected subcutaneously into flanks of the
mice (n=4 mice/group), respectively. The subcutaneous model was
used to evaluate the effect of vitamin D3 on
tumorgenicity of SP cells. The mice in the vitamin
D3-treatment group were injected with a single dose of
vitamin D3 (1,000 IU/week) intramuscularly. The animals
were sacrificed at the indicated time intervals (4–12 weeks) when
tumor nodules were identified on their body surfaces. The
anesthesia of the mice used was 1% pentobarbital sodium (50 mg/kg),
prior to the injection of cells to their ovary. The
euthanasia/sacrifice of the mice was performed by 3% pentobarbital
sodium (150 mg/kg), followed by cervical dislocation to ensure the
death of mice. Tumor growth was monitored by measuring 2
perpendicular diameters. Tumor volumes were calculated according to
the formula: 0.5 × a × b2, where a and b are the largest
and smallest diameter, respectively.
Statistical analysis
Statistical analysis was performed using the paired
Student's t-test for the difference between two groups. One-way
analysis of variance was used where multiple comparisons were made,
followed by Bonferroni's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Categorizing MOSE cells
The capacity of anchorage-independent growth was
determined, which in an in vitro hallmark of neoplastic
transformation of cells (29).
Early (<20 passages) and intermediate (21–80 passages) MOSE
cells were unable to form colonies in soft agar. Notably,
late-passage (>81) MOSE cells were capable of forming >30 mm
colonies. Compared with MOSE cells at earlier stages, late-passage
MOSE cells exhibited an increased plating efficiency and growth
rate, another proliferative parameter that is often associated with
neoplastic change. Furthermore, the tumor formation rates were
significantly increased in vivo (27,30).
M-L cells formed tumors up to 100%, whereas M-E and M-I cells did
not form tumors. Therefore, three sequential stages of transformed
MOSE cells were defined as M-E (≤20 passages; early), M-I (21–80
passages; intermediate) and M-L (≥81 passages; late) cells,
respectively.
Identification of ovarian cancer stem
cells-like cells
Our previous study demonstrated that the acquisition
of stemness was closely associated with malignant transformation of
MOSE cells (unpublished data). SP cells are an important hallmark
for the definition of the stem cell-like characteristics (22–26);
therefore, whether SP cells may be used to enrich ovarian CSCs was
investigated. Malignant MOSE (M-L) cells were separated by FACS
into two populations. SP cells actively pump the Hoechst 33342 dye
out of the cells, while NSP cannot and therefore this is a way to
isolate these two cell populations (22–26).
The percentage of SP cells in M-L cells reached up to 24.4%
(Fig. 1A). After culturing for 3–4
days in serum-free culture medium, NSP cells gradually grew by
adherence, and acquired epithelial morphology; however, SP cells
formed larger spheres (Fig. 1B).
Results from the sphere formation assay revealed that the
sphere-forming rates of SP cells were significantly higher compared
with those of the NSP cells (Fig.
1C).
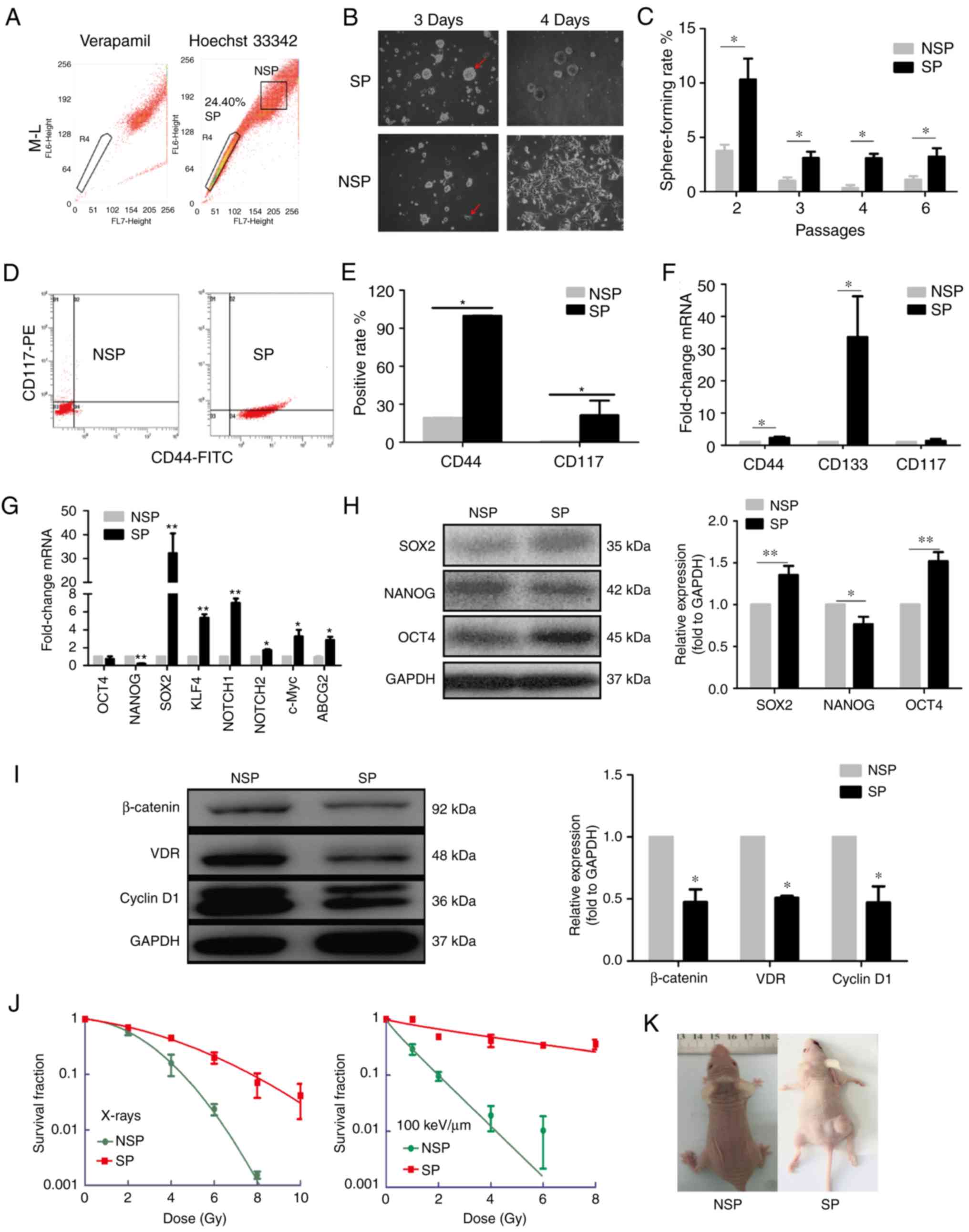 | Figure 1.Identification of SP cells with
ovarian cancer stem cell-like properties in vitro and in
vivo. (A) Results of FACS indicated that the SP cells accounted
for 24.40% of the M-L cells. (B) Morphology of SP and NSP cells
cultured in serum-free medium. (C) Sphere-forming rates of SP and
NSP cells in different passages. (D, E) Results of flow cytometry
assay indicated that CD44 and CD117 accounted for 99.8 and 21.2% of
the SP cells, respectively, which was significantly higher compared
with NSP cells. (F and G) Relative mRNA expression levels of (F)
CD44, CD117 and CD133, and (G) OCT4, NANOG, SOX2, KLF4, NOTCH1,
NOTCH2, c-Myc and ABCG2 in NSP and SP cells were examined by
reverse transcription-quantitative polymerase chain reaction. (H
and I) Relative protein expression levels of (H) SOX2, NANOG and
OCT4, and (I) β-catenin, VDR and Cyclin D1 in SP and NSP cells were
examined by western blotting. (J) Cell survival fractions with
radiation dose of X-rays or carbon ions as the abscissa and the
survival rate as the ordinate. (K) Representative images showing
the tumorigenicity of NSP and SP cells in vivo. Data are
presented as the mean ± standard deviation; n=3 in (A-J) and n=4 in
(K); *P<0.05 and **P<0.01 vs. NSP. ABCG2, ATP binding
cassette subfamily G member 2; CD, cluster of differentiation; KLF,
Krüppel-like factor; NSP, non-side population; OCT, octamer-binding
protein; SOX, Sex-determining region Y; SP, side population; VDR,
Vitamin D receptor. |
To further verify the stem cell-like properties of
SP cells, the expressions of CD44+ and
CD117+, two well-known ovarian CSCs markers, were
assessed by flow cytometry. In SP cells, the number of
CD44+ (99.8%) and CD117+ (21.2%) cells were
significantly higher compared with NSP cells (Fig. 1D and E). RT-qPCR results
demonstrated that the mRNA levels of CD44 and CD133 in SP cells
were increased, compared with the NSP cells (Fig. 1F). Notably, the mRNA expression
levels of multipotent genes, such as SOX2, Krüppel-like factor
(KLF)4, NOTCH1 and NOTCH2 were increased in SP cells, whereas NANOG
expression was decreased, compared with NSP cells (Fig. 1G). Similarly, the protein expression
levels of SOX2 and OCT4 were also significantly increased in SP
compared with NSP, and the protein expression of NANOG was
decreased. (Fig. 1H). In addition,
the mRNA expression levels of ATP binding cassette subfamily G
member (ABCG)2 and c-Myc were also significantly elevated in SP
cells (Fig. 1G). Notably, the
protein levels of VDR, β-catenin and Cyclin D1 in SP cells were
lower compared with those in NSP cells (Fig. 1I).
Radioresistance is also a remarkable phenotype of
CSCs (31,32). Thus, the survival fraction of cells
irradiated by X-rays and carbon ions was determined using
clonogenic and sphere-forming assay. The dose-response curves
demonstrated that the survival fraction of SP cells was higher
compared with the NSP cells, which indicated that SP cells
exhibited resistance to both X-rays and carbon ions (Fig. 1J).
To further determine the tumorigenesis of SP and NSP
cells in vivo, subcutaneous (n=4 mice/group) and orthotopic
(n=2 mice/group) models of ovarian cancer were established in nude
mice injected with SP or NSP. Neither of the two mice in the
orthotopic NSP groups formed tumors; of the two mice that were
orthotopically implanted with SP cells, one died at
post-implantation, the other one grew a tumor with volume of 49.95
mm3 by 36 days post-implantation (data not shown). In
the subcutaneous model, different numbers of NSP and SP cells were
injected. None of the four mice injected with 1×106 NSP
cells formed any tumor by 12 weeks post-injection; however, two of
the four mice injected with 1×104 SP cells formed tumors
within 30 days post-injection, the volumes of which was 0.018 and
48 mm3 (Fig. 1K). Taken
together, these in vitro and in vivo results
demonstrated that the SP cells isolated from oncogenic
transformation-MOSE cells exhibited properties of CSCs with
self-renewal capability, multipotency and radioresistance.
1α,25(OH)2D3
inhibits stem cell-like phenotype of SP cells
To verify whether active vitamin D3 is
able to inhibit the stemness of CSCs, SP cells were treated with 10
nM 1α,25(OH)2D3. Notably, a few
1α,25(OH)2D3-treated SP cells gradually grew
by adherence following the treatment for three days, compared with
untreated SP cells. Furthermore, the number and diameter of spheres
formed were reduced in 1α,25(OH)2D3-treated
SP cells (Fig. 2A and B).
Consistent with this phenotype, the mRNA expression levels of the
multipotent genes NANOG, OCT4, SOX2, KLF4 and ABCG2 were
downregulated in 1α,25(OH)2D3-treated SP
cells, compared with the untreated SP cells (Fig. 2C and D). However, the protein levels
of NANOG, OCT4 and SOX2 were not significantly reduced in SP cells
treated with 1α,25(OH)2D3 (Fig. 2E). These results indicated that
1α,25(OH)2D3 may be able to suppress the
self-renewal capability and expression of multipotent gene
expressions including NANOG, OCT4, SOX2, KLF4 in ovarian CSCs,
although this needs to be verified.
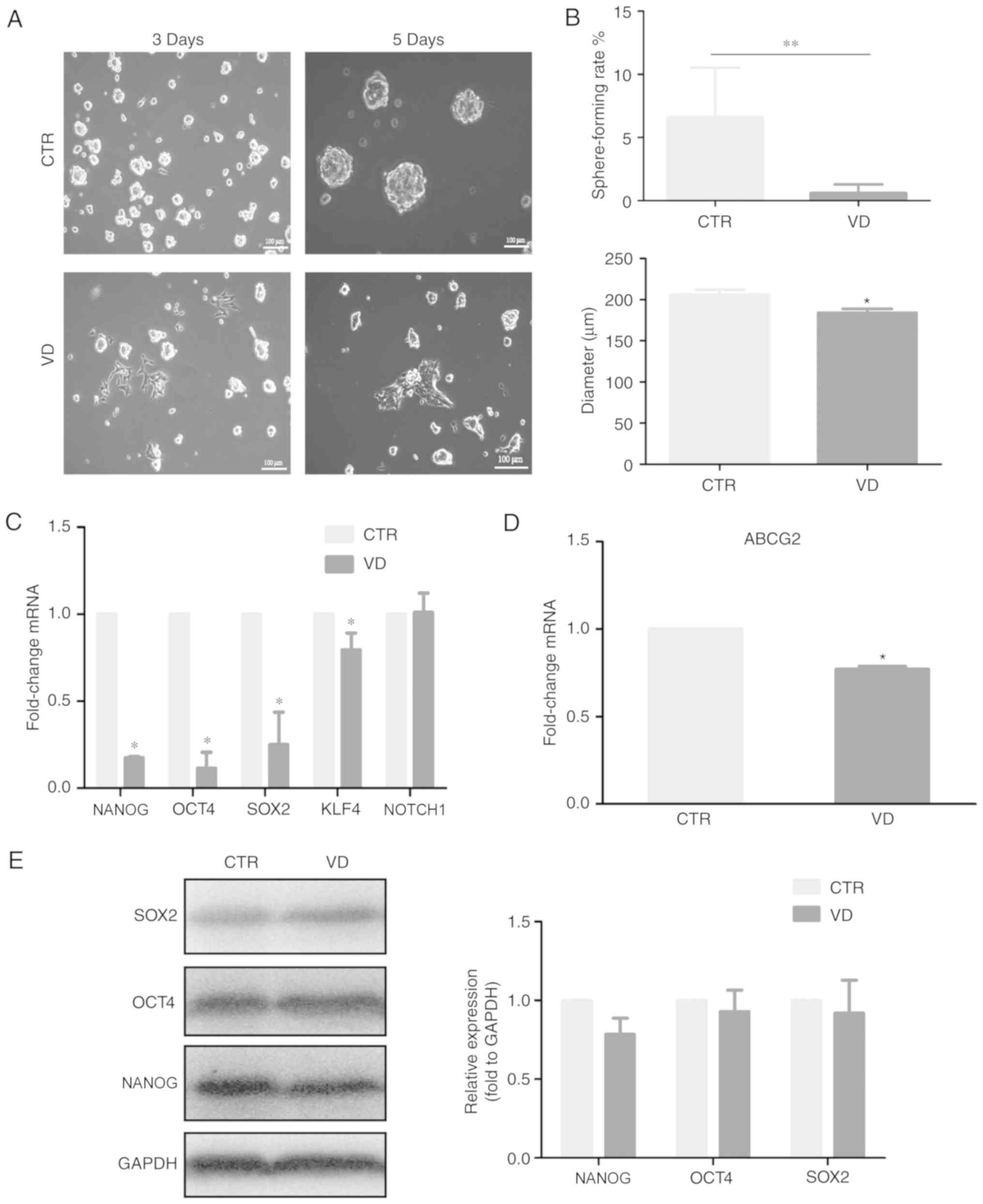 | Figure 2.1α,25(OH)2D3
inhibits cancer stem cell-like phenotype. (A) Effects of VD on the
sphere formation of SP and NSP cells cultured in suspension medium.
(B) Effects of VD on the number and diameter of spheres in SP
cells. (C and D) Examination of changes in the mRNA expression
levels of (C) multipotent genes and (D) ABCG2 in SP cells treated
with VD were determined by reverse transcription-quantitative
polymerase chain reaction. (E) Examination of changes in the
protein expression levels of SOX2, OCT4 and NANOG in SP cells
treated with VD were determined by western blot. Data are presented
as the mean ± standard deviation; n=3; *P<0.05 and **P<0.01
vs. CTR. ABCG2, ATP binding cassette subfamily G member; CTR,
Control; NSP, non-side population; OCT, octamer-binding protein;
SOX, Sex-determining region Y; SP, side population; VD,
1α,25(OH)2D3. |
1α,25(OH)2D3
increases β-catenin expression but decreases Cyclin D1 expression
in SP cells
We know that 1α,25(OH)2D3
exhibits its biological effects mainly through binding to VDR
(13). It was determined that the
protein and mRNA expression levels of VDR were increased in SP
cells treated by 1α,25(OH)2D3 (Fig. 3A, D and E). Subsequently, whether
1α,25(OH)2D3 engaged in modulating
VDR-mediated genes of ovarian CSCs was determined. The results of
RT-qPCR demonstrated that 1α,25(OH)2D3 had
the tendency to increase β-catenin but significantly decreased
Cyclin D1 mRNA expression levels in SP cells compared with Control
cells (Fig. 3B and C). Western blot
results demonstrated that 1α,25(OH)2D3
treatment enhanced β-catenin and reduced Cyclin D1 protein
expression levels in a dose-dependent manner (Fig. 3F and G). However, immunofluorescence
staining indicated that the expression of β-catenin was mainly
located in the nuclei of vehicle-treated SP cells, and β-catenin
was blocked in cytoplasm following treatment with
1α,25(OH)2D3 (Fig. 3H). Combined with the suppression of
stem cell-like properties demonstrated in Fig. 2, these results implicated that
1α,25(OH)2D3 may inhibit the stemness of SP
cells through increasing VDR and cytoplasmic β-catenin and
decreasing Cyclin D1 expression levels.
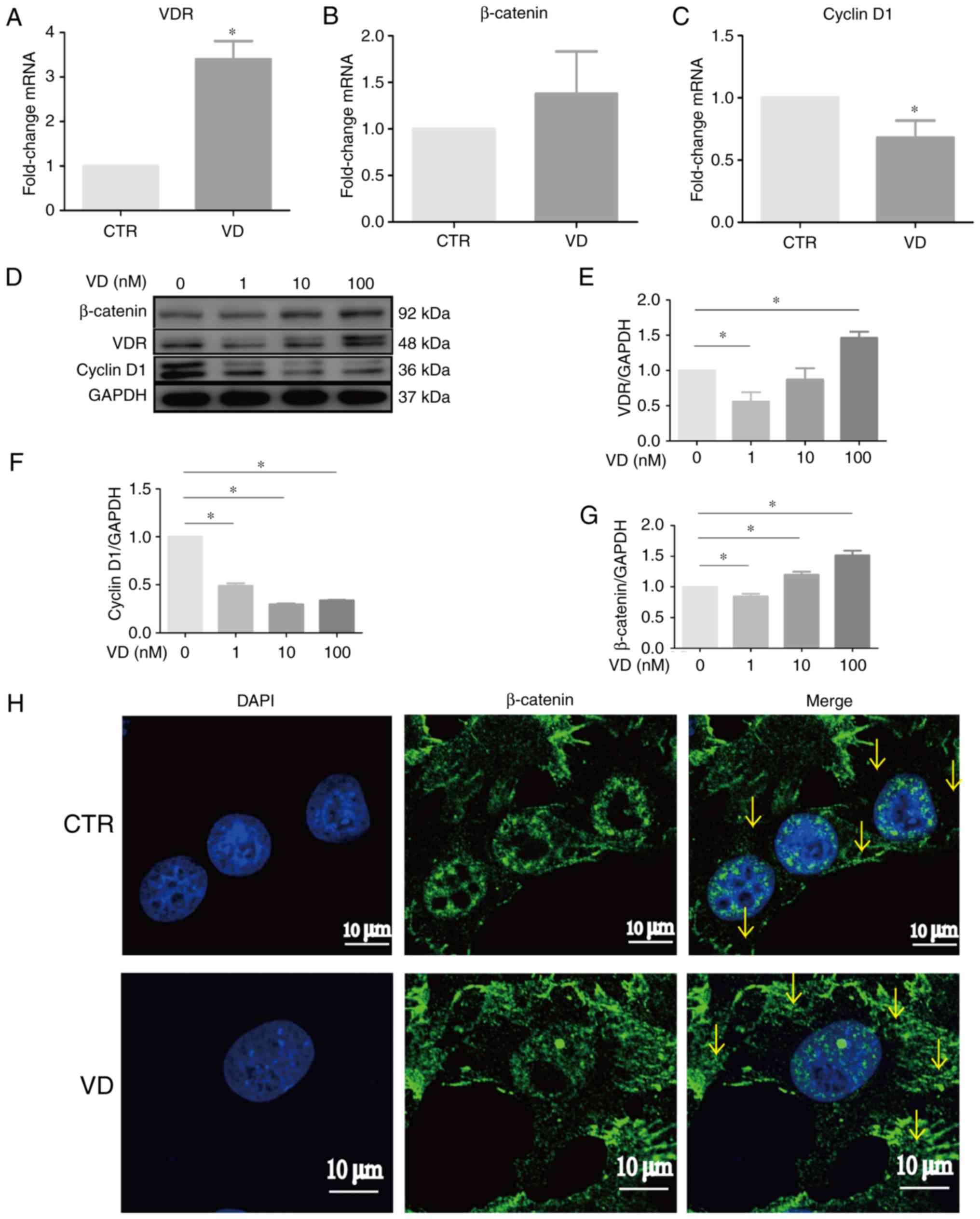 | Figure 3.1α,25(OH)2D3
regulates the VDR/β-catenin signaling pathway to inhibit the
stemness of SP cells. (A-C) Relative changes in the mRNA levels of
VDR, β-catenin and Cyclin D1 were examined in SP cells treated with
10 nM 1α,25(OH)2D3. (D-G) Relative changes in
the protein levels of VDR, β-catenin and Cyclin D1 were examined in
SP cells treated with various concentration of
1α,25(OH)2D3. (H) Representative images of SP
cells untreated and treated with 1α,25(OH)2D3
showing β-catenin expression by confocal laser scanner microscope;
nuclei were visualized by DAPI staining. Arrow pointed to the
β-catenin in cytoplasm. Data are represented as the mean ± standard
deviation; n=3; *P<0.05 vs. CTR. CTR, control; VD,
1α,25(OH)2D3NSP, non-side population; SOX,
Sex-determining region Y; SP, side population; VDR, vitamin D
receptor. |
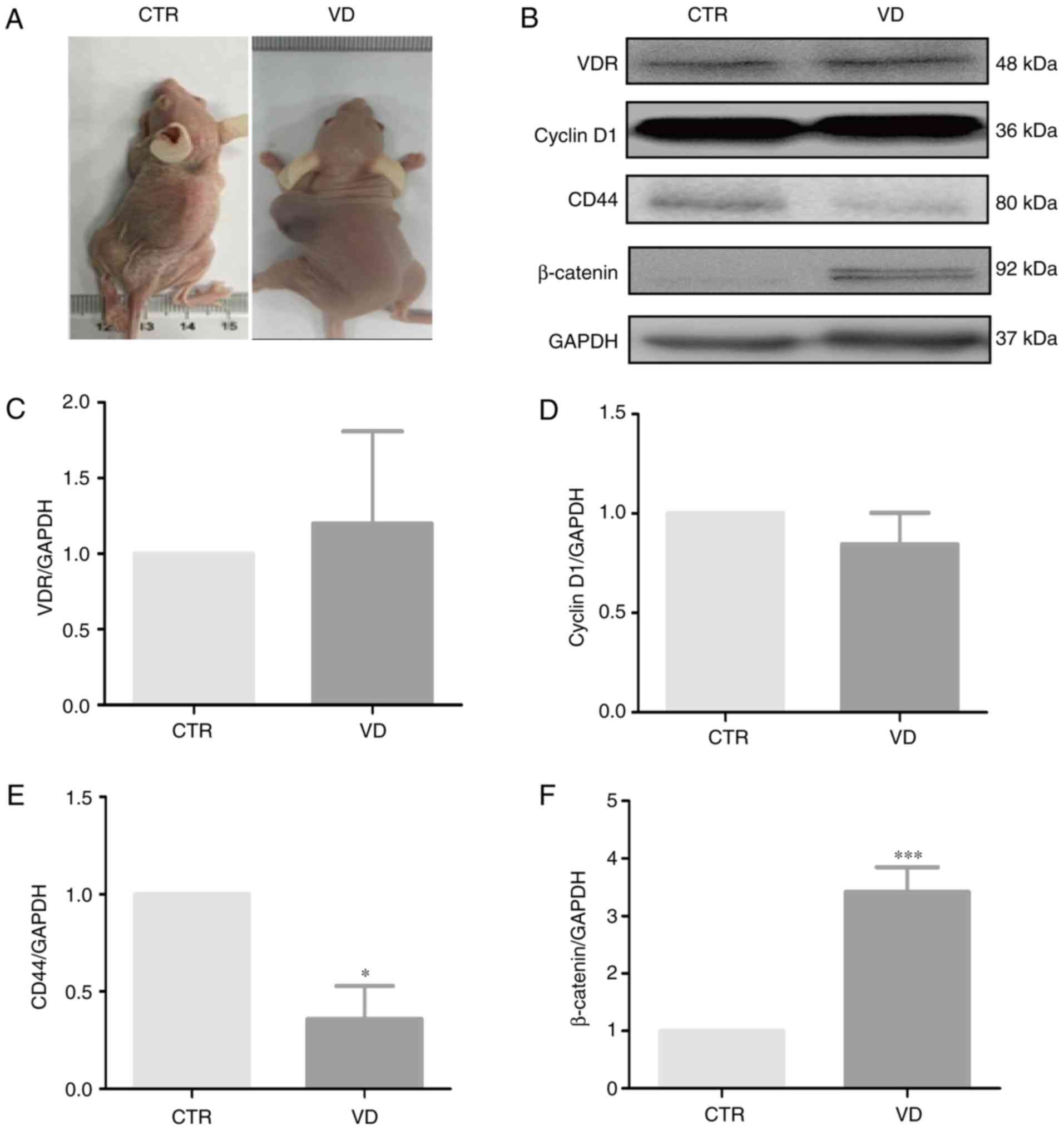
Vitamin D3 inhibits the
tumorigenic phenotype of SP cells in vivo
To further determine whether vitamin D3
had suppressive effects on tumorigenesis of SP cells,
~1×104 SP cells were subcutaneously inoculated into nude
mice. Tumor formation was monitored by palpation and direct body
dissection. In two of the four vehicle-treated Control mice tumor
nodules were palpable as early as 30 days post-inoculation. By
contrast, two of the four mice treated with vitamin D3
exhibited signs of tumor growth at 45 days post-inoculation
(Fig. 4A). To determine whether
vitamin D3 also modulates the expression of
stemness-associated proteins in vivo, total protein was
extracted from tumor tissues and analyzed by western blotting
(Fig. 4B). The results indicated
that vitamin D3 treatment led to increased VDR and
decreased Cyclin D1 protein expression levels, but the changes were
not significant (Fig. 4C and D).
Furthermore, vitamin D3 decreased the expression levels
of CD44, whereas it increased the expression levels of β-catenin
in vivo compared with Control mice (Fig. 4E and F). These results demonstrated
that vitamin D3 may have delayed the onset of tumor
formation derived from injection of ovarian CSCs by reducing CD44
and increasing β-catenin expression, but this needs to be validated
further.
Discussion
Accumulating evidence demonstrates that CSCs serve
crucial roles in the development of chemoresistance, tumor relapse
and metastasis in patients with ovarian cancer (33,34).
The present study reports that vitamin D3 not only
inhibits self-renewal capability and multipotent gene expressions
of ovarian CSCs in vitro, but it may also delay the onset of
tumor formation. Furthermore, 1α,25(OH)2D3
treatment increased both the mRNA and protein expression levels of
VDR and β-catenin, whereas it decreased the expression of Cyclin D1
and certain stemness-associated genes, including CD44, NANOG, OCT4,
SOX2, KLF4 and ABCG2 in ovarian CSCs in vitro. Notably, the
reduced expression of CD44 was demonstrated in vitro and
in vivo.
Owing to repeating disruption and repairing with
ovulation-associated remodeling, OSE with stem-like properties have
been identified (5), and the
junction area of hilum and oviduct contains cancer-prone stem cell
niche (6). Ovarian CSCs with
multipotent and self-renewal capability are usually purified using
the surface markers CD44, CD117 and CD133, or functional marker
such as isolation of SP and ALDH+ cells (22,35,36).
It is reported that SP cells isolated from SKOV-3 cells displayed
stem-like phenotype (25,37,38).
In the present study, SP cells were isolated from malignant
transformation MOSE cells, and demonstrated that both mRNA and
protein expression levels of SOX2 and Oct4 were increased in SP
cells. Additionally, they exhibited self-renewal capability and
radioresistance in vitro, and an increased tumor growth
in vivo. Notably, both mRNA and protein expression levels of
VDR were reduced in SP cells. In general, high VDR expression is
associated with reduced mortality and improved prognosis in breast
and prostate tumors (39,40). 1α,25(OH)2D3
activates and represses its target genes, such as RXRA, SMAD3,
CCND3, by combining with VDR. Previous studies showed that
1α,25(OH)2D3 inhibit proliferation and
angiogenesis, and induce apoptosis of cancer cells in human cancers
including ovarian cancer (41–43).
However, it is not clear whether 1α,25(OH)2D3
could reduce stemness of CSCs or promote differentiation through
binding to VDR.CD44, a marker for stem cells of several cancers,
serves an important role in ovarian CSCs (44,45).
Additionally, a number of studies have reported that
1α,25(OH)2D3 has potent effects on prostate
and breast cancer stem cells, and demonstrated that
1α,25(OH)2D3 and its analogue inhibited the
proliferation of prostate and breast stem cells by inducing
senescence and by decreasing CD44 expression levels (14,18–20).
The present study results also indicated that
1α,25(OH)2D3 suppressed the self-renewal
capacity of ovarian CSCs by reducing CD44 expression levels, as
well as the expressions of multipotent genes, such as Oct4, SOX2,
NANOG, KLF4 and ABCG2 in vitro. Notably,
1α,25(OH)2D3 treatment decreased the mRNA
expression levels of Oct4, SOX2 and NANOG, but not their protein
levels. Previous studies have indicated that Oct4, SOX2 and NANOG
often function in combination with each other to be involved in
self-renewal and proliferation (46,47).
CD44v3, a CD44 variant isoform, was reported to interact with
Oct4-SOX2-NANOG (48). At the
transcriptional level, Oct4, SOX2 and NANOG form a positive
autoregulatory loop which is important for the maintenance of the
undifferentiated state. At the post-translational level, non-coding
RNAs are emerging as a key player in the control of cell
proliferation and cell fate determination during differentiation
(48). For example, microRNA-302 is
controlled by a promoter containing Oct4-SOX2-NANOG-binding sites
in human head and neck squamous cell carcinoma (48). It is worth exploring whether
1α,25(OH)2D3-decreased CD44 may weaken the
interaction with Oct4-SOX2-NANOG, which may result in no changes at
the protein level, even though the mRNA expression levels of Oct4,
SOX2 and NANOG were significantly decreased. In addition,
1α,25(OH)2D3 increased the β-catenin
expression levels and decreased Cyclin D1 expression.
β-Catenin is a dual function protein, involved in
both stemness and contribution to metastasis. On the one hand,
β-catenin, as an important molecule of the Wnt signaling pathway,
sustains stem cell renewal ability by maintaining multipotency in
certain cell types (49). On the
other hand, β-catenin, as a proto-oncogene, drives metastasis
formation by translocation to the nucleus. Alterations in the
localization and expression levels of β-catenin are associated with
many cancers, including hepatocellular, colorectal, lung, breast,
ovarian and endometrial cancer (50–55).
Furthermore, the level of β-catenin can be modulated by VDR in
colon cancer cells (56). In
addition, 1α,25(OH)2D3 suppresses the
expression of Cyclin D1 in epidermal carcinoma (57). These studies indicated that
β-catenin and Cyclin D1 are both downstream target genes of
1α,25(OH)2D3. In the present study,
1α,25(OH)2D3 increased the expression of VDR
and decreased the expression of Cyclin D1 at mRNA and protein
levels. Notably, 1α,25(OH)2D3 significantly
increased the expression of β-catenin, which was inconsistent with
previous studies. However, immunofluorescence staining verified
that 1α,25(OH)2D3 only increased the
expression of β-catenin in cytoplasm, consistent with Pálmer's
study (58), which may result in
the decrease of Cyclin D1. These results demonstrated that
1α,25(OH)2D3 inhibited the stemness of CSCs
through blocking the localization of β-catenin in cytoplasm.
However, there are several limitations in the
present study. The number of mice used in the present study was not
sufficient, which may have reduced the statistical power. Since the
previous studies showed that SP cells exhibited more tumorigenicity
than NSP cells in subcutaneous model in vivo (22,24),
in the present study, 1×106 NSP cells were
subcutaneously injected into mice. Two of the four mice injected
with 1×104 SP cells formed tumors within 30 days
post-injection, but none of the four mice injected with
1×106 NSP cells formed any tumor by 12 weeks.
Additionally, whether SP cells would display more tumorgenicity
than NSP cells in the orthotopic model need to be investigated.
Unfortunately, the successful rate of the orthotopic operation was
low and could barely get enough orthotopic mice for further study.
In addition, the direct or indirect interaction between VDR and
β-catenin, which is also very interesting and may be a novel
underlying mechanism, was not investigated.
In conclusion, the present study demonstrated that
1α,25(OH)2D3 restrained stem cell-like
properties of ovarian cancer cells by enhancing VDR and had the
tendency to promote cytoplasmic β-catenin, and reducing CD44
expression levels. These results may provide a novel strategy for
vitamin D3 in diminishing the stemness of CSCs. Future
studies utilizing human ovarian cancer tissues to examine the role
of vitamin D3 in stem cell-like cell self-renewal may
extend our understanding of ovarian cancer biology, which may lead
to chemotherapeutic agents that may suppress stemness and improve
the clinical outcome for patients with epithelial ovarian
cancer.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Miss Hemei Zhang
(Wenzhou Center for Disease Control and Prevention), Dr Fei Jiang
(Soochow University), Dr Jianmei Wan (Soochow University), Miss
Ping Wang (Fuzhou General Hospital), Professor Guangming Zhou
(Soochow University) and Professor Zengli Zhang (Soochow
University) for contributing materials and writing the
manuscript.
Funding
The present study was supported by The National
Natural Scientific Funding of China (grant nos. 81673151, 81372979,
8137298 and 111335011), in part, by The State Key Laboratory of
Radiation Medicine and Protection, Medical College of Soochow
University, Collaborative Innovation Center of Radiation Medicine,
Jiangsu Higher Education Institutions.
Availability of data and materials
The data sets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
BL and LL conceived and designed the experiments.
LL and MJ performed the experiments. LL, MJ and YH analyzed the
data. YH contributed to the material and analysis tools. MJ and BL
drafted and revised the manuscript. All authors have read and
approved the final manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All surgical procedures and care administered to
the animals were approved by the Institutional Animal Care and Use
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurman RJ and Shih Ie M: The origin and
pathogenesis of epithelial ovarian cancer: A proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang HL, MacLaughlin DT and Donahoe PK:
Somatic stem cells of the ovary and their relationship to human
ovarian cancers. StemBook; Cambridge MA: Harvard Stem Cell
Institute: 2008–2009
|
|
5
|
Szotek PP, Chang HL, Brennand K, Fujino A,
Pieretti-Vanmarcke R, Lo Celso C, Dombkowski D, Preffer F, Cohen
KS, Teixeira J, et al: Normal ovarian surface epithelial
label-retaining cells exhibit stem/progenitor cell characteristics.
Proc Natl Acad Sci USA. 105:12469–12473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flesken-Nikitin A, Hwang CI, Cheng CY,
Michurina TV, Enikolopov G and Nikitin AY: Ovarian surface
epithelium at the junction area contains a cancer-prone stem cell
niche. Nature. 495:241–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aguilar-Gallardo C, Rutledge EC,
Martínez-Arroyo AM, Hidalgo JJ, Domingo S and Simón C: Overcoming
challenges of ovarian cancer stem cells: Novel therapeutic
approaches. Stem Cell Rev. 8:994–1010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao J: Cancer stem cells and
chemoresistance: The smartest survives the raid. Pharmacol Ther.
160:145–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang S, Cui B, Lai H, Liu G, Ghia EM,
Widhopf GF II, Zhang Z, Wu CC, Chen L, Wu R, et al: Ovarian cancer
stem cells express ROR1, which can be targeted for
anti-cancer-stem-cell therapy. Proc Natl Acad Sci USA.
111:17266–17271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Cardenas H, Fang F, Condello S,
Taverna P, Segar M, Liu Y, Nephew KP and Matei D: Epigenetic
targeting of ovarian cancer stem cells. Cancer Res. 74:4922–4936.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chau WK, Ip CK, Mak AS, Lai HC and Wong
AS: c-Kit mediates chemoresistance and tumor-initiating capacity of
ovarian cancer cells through activation of
Wnt/β-catenin-ATP-binding cassette G2 signaling. Oncogene.
32:2767–2781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abubaker K, Latifi A, Luwor R, Nazaretian
S, Zhu H, Quinn MA, Thompson EW, Findlay JK and Ahmed N: Short-term
single treatment of chemotherapy results in the enrichment of
ovarian cancer stem cell-like cells leading to an increased tumor
burden. Mol Cancer. 12:242013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feldman D, Krishnan AV, Swami S,
Giovannucci E and Feldman BJ: The role of vitamin D in reducing
cancer risk and progression. Nat Rev Cancer. 14:342–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pervin S, Hewison M, Braga M, Tran L, Chun
R, Karam A, Chaudhuri G, Norris K and Singh R: Down-regulation of
vitamin D receptor in mammospheres: Implications for vitamin D
resistance in breast cancer and potential for combination therapy.
PLoS One. 8:e532872013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pereira F, Larriba MJ and Muñoz A: Vitamin
D and colon cancer. Endocr Relat Cancer. 19:R51–R71. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Larriba MJ and Muñoz A: SNAIL vs. vitamin
D receptor expression in colon cancer: Therapeutics implications.
Br J Cancer. 92:985–989. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou YF, Gao SH, Wang P, Zhang HM, Liu LZ,
Ye MX, Zhou GM, Zhang ZL and Li BY:
1α,25(OH)2D3 suppresses the migration of
ovarian cancer SKOV-3 cells through the inhibition of
epithelial-mesenchymal transition. Int J Mol Sci. 17:12852016.
View Article : Google Scholar :
|
|
18
|
Maund SL, Barclay WW, Hover LD, Axanova
LS, Sui G, Hipp JD, Fleet JC, Thorburn A and Cramer SD:
Interleukin-1α mediates the antiproliferative effects of
1,25-dihydroxyvitamin D3 in prostate progenitor/stem
cells. Cancer Res. 71:5276–5286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choudhury S, Almendro V, Merino VF, Wu Z,
Maruyama R, Su Y, Martins FC, Fackler MJ, Bessarabova M, Kowalczyk
A, et al: Molecular profiling of human mammary gland links breast
cancer risk to a p27+ cell population with progenitor
characteristics. Cell Stem Cell. 13:117–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
So JY, Lee HJ, Smolarek AK, Paul S, Wang
CX, Maehr H, Uskokovic M, Zheng X, Conney AH, Cai L, et al: A novel
Gemini vitamin D analog represses the expression of a stem cell
marker CD44 in breast cancer. Mol Pharmacol. 79:360–367. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
So JY, Wahler J, Das Gupta S, Salerno DM,
Maehr H, Uskokovic M and Suh N: HES1-mediated inhibition of Notch1
signaling by a Gemini vitamin D analog leads to decreased
CD44+/CD24−/low tumor-initiating
subpopulation in basal-like breast cancer. J Steroid Biochem Mol
Biol. 148:111–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yasuda K, Torigoe T, Morita R, Kuroda T,
Takahashi A, Matsuzaki J, Kochin V, Asanuma H, Hasegawa T, Saito T,
et al: Ovarian cancer stem cells are enriched in side population
and aldehyde dehydrogenase bright overlapping population. PLoS One.
8:e681872013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szotek PP, Pieretti-Vanmarcke R, Masiakos
PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F,
Maclaughlin DT and Donahoe PK: Ovarian cancer side population
defines cells with stem cell-like characteristics and Mullerian
Inhibiting Substance responsiveness. Proc Natl Acad Sci USA.
103:11154–11159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu L, McArthur C and Jaffe RB: Ovarian
cancer stem-like side-population cells are tumourigenic and
chemoresistant. Br J Cancer. 102:1276–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rizzo S, Hersey JM, Mellor P, Dai W,
Santos-Silva A, Liber D, Luk L, Titley I, Carden CP, Box G, et al:
Ovarian cancer stem cell-like side populations are enriched
following chemotherapy and overexpress EZH2. Mol Cancer
Ther. 10:325–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sales-Pardo I, Avendaño A, Martinez-Muñoz
V, García-Escarp M, Celis R, Whittle P, Barquinero J, Domingo JC,
Marin P and Petriz J: Flow cytometry of the side population: Tips
& tricks. Cell Oncol. 28:37–53. 2006.PubMed/NCBI
|
|
27
|
Roby KF, Taylor CC, Sweetwood JP, Cheng Y,
Pace JL, Tawfik O, Persons DL, Smith PG and Terranova PF:
Development of a syngeneic mouse model for events related to
ovarian cancer. Carcinogenesis. 21:585–591. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de la Mare JA, Jurgens T and Edkins AL:
Extracellular Hsp90 and TGFβ regulate adhesion, migration and
anchorage independent growth in a paired colon cancer cell line
model. BMC cancer. 17:2022017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McCloskey CW, Goldberg RL, Carter LE,
Gamwell LF, Al-Hujaily EM, Collins O, Macdonald EA, Garson K,
Daneshmand M, Carmona E, et al: A new spontaneously transformed
syngeneic model of high-grade serous ovarian cancer with a
tumor-initiating cell population. Front Oncol. 4:532014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lobo NA, Shimono Y, Qian D and Clarke MF:
The biology of cancer stem cells. Annu Rev Cell Dev Biol.
23:675–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerweck LE and Wakimoto H: At the
crossroads of cancer stem cells, radiation biology, and radiation
oncology. Cancer Res. 76:994–998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Steg AD, Bevis KS, Katre AA, Ziebarth A,
Dobbin ZC, Alvarez RD, Zhang K, Conner M and Landen CN: Stem cell
pathways contribute to clinical chemoresistance in ovarian cancer.
Clin Cancer Res. 18:869–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nuti SV, Mor G, Li P and Yin G: TWIST and
ovarian cancer stem cells: Implications for chemoresistance and
metastasis. Oncotarget. 5:7260–7271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang R, Wu D, Yuan Y, Li X, Holm R, Trope
CG, Nesland JM and Suo Z: CD117 expression in fibroblasts-like
stromal cells indicates unfavorable clinical outcomes in ovarian
carcinoma patients. PLoS One. 9:e1122092014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Wang J, Chen D, Yang J, Yang C,
Zhang Y, Zhang H and Dou J: Evaluation of characteristics of
CD44+CD117+ ovarian cancer stem cells in
three dimensional basement membrane extract scaffold versus two
dimensional monocultures. BMC Cell Biol. 14:72013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yanamoto S, Kawasaki G, Yamada S,
Yoshitomi I, Kawano T, Yonezawa H, Rokutanda S, Naruse T and Umeda
M: Isolation and characterization of cancer stem-like side
population cells in human oral cancer cells. Oral Oncol.
47:855–860. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ruan Z, Liu J and Kuang Y: Isolation and
characterization of side population cells from the human ovarian
cancer cell line SK-OV-3. Exp Ther Med. 10:2071–2078. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hendrickson WK, Flavin R, Kasperzyk JL,
Fiorentino M, Fang F, Lis R, Fiore C, Penney KL, Ma J, Kantoff PW,
et al: Vitamin D receptor protein expression in tumor tissue and
prostate cancer progression. J Clin Oncol. 29:2378–2385. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ditsch N, Toth B, Mayr D, Lenhard M,
Gallwas J, Weissenbacher T, Dannecker C, Friese K and Jeschke U:
The association between vitamin D receptor expression and prolonged
overall survival in breast cancer. J Histochem Cytochem.
60:121–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang F, Li P, Fornace AJ Jr, Nicosia SV
and Bai W: G2/M arrest by 1,25-dihydroxyvitamin
D3 in ovarian cancer cells mediated through the
induction of GADD45 via an exonic enhancer. J Biol Chem.
278:48030–48040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang F, Bao J, Li P, Nicosia SV and Bai
W: Induction of ovarian cancer cell apoptosis by
1,25-dihydroxyvitamin D3 through the down-regulation of
telomerase. J Biol Chem. 279:53213–53221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang X, Jiang F, Li P, Li C, Ma Q,
Nicosia SV and Bai W: Growth suppression of ovarian cancer
xenografts in nude mice by vitamin D analogue EB1089. Clin Cancer
Res. 11:323–328. 2005.PubMed/NCBI
|
|
44
|
Hiraga T, Ito S and Nakamura H: Cancer
stem-like cell marker CD44 promotes bone metastases by enhancing
tumorigenicity, cell motility, and hyaluronan production. Cancer
Res. 73:4112–4122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kashyap V, Rezende NC, Scotland KB,
Shaffer SM, Persson JL, Gudas LJ and Mongan NP: Regulation of stem
cell pluripotency and differentiation involves a mutual regulatory
circuit of the NANOG, OCT4, and SOX2 pluripotency transcription
factors with polycomb repressive complexes and stem cell microRNAs.
Stem Cells Dev. 18:1093–1108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Boyer LA, Lee TI, Cole MF, Johnstone SE,
Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG,
et al: Core transcriptional regulatory circuitry in human embryonic
stem cells. Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bourguignon LY, Wong G, Earle C and Chen
L: Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes
miR-302 expression leading to self-renewal, clonal formation, and
cisplatin resistance in cancer stem cells from head and neck
squamous cell carcinoma. J Biol Chem. 287:32800–32824. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schneider S, Steinbeisser H, Warga RM and
Hausen P: Beta-catenin translocation into nuclei demarcates the
dorsalizing centers in frog and fish embryos. Mech Dev. 57:191–198.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hoffmeyer K, Raggioli A, Rudloff S, Anton
R, Hierholzer A, Del Valle I, Hein K, Vogt R and Kemler R:
Wnt/β-catenin signaling regulates telomerase in stem cells and
cancer cells. Science. 336:1549–1554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee E, Madar A, David G, Garabedian MJ,
Dasgupta R and Logan SK: Inhibition of androgen receptor and
beta-catenin activity in prostate cancer. Proc Natl Acad Sci USA.
110:15710–15715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li S, Li S, Sun Y and Li L: The expression
of beta-catenin in different subtypes of breast cancer and its
clinical significance. Tumour Biol. 35:7693–7698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang H, Wang H, Makki MS, Wen J, Dai Y,
Shi Q, Liu Q, Zhou X and Wang J: Overexpression of β-catenin and
cyclinD1 predicts a poor prognosis in ovarian serous carcinomas.
Int J Clin Exp Pathol. 7:264–271. 2014.PubMed/NCBI
|
|
54
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sebio A, Kahn M and Lenz HJ: The potential
of targeting Wnt/β-catenin in colon cancer. Expert Opin Ther
Targets. 18:611–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Larriba MJ, Ordóñez-Moran P, Chicote I,
Martín-Fernández G, Puig I, Muñoz A and Pálmer HG: Vitamin D
receptor deficiency enhances Wnt/beta-catenin signaling and tumor
burden in colon cancer. PLoS One. 6:e235242011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang YJ, Teichert AE, Fong F, Oda Y and
Bikle DD: 1α,25(OH)2-dihydroxyvitamin D3/VDR protects
the skin from UVB-induced tumor formation by interacting with the
beta-catenin pathway. J Steroid Biochem Mol Biol. 136:229–232.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pálmer HG, González-Sancho JM, Espada J,
Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros
AG, Lafarga M and Muñoz A: Vitamin D3 promotes the
differentiation of colon carcinoma cells by the induction of
E-cadherin and the inhibition of beta-catenin signaling. J Cell
Biol. 154:369–387. 2001. View Article : Google Scholar : PubMed/NCBI
|