Introduction
Glioblastoma multiforme (GBM) is recognized as the
most aggressive type of diffuse glioma of astrocytic lineage and is
equivalent to grade IV based on the World Health Organization (WHO)
Classification from 2007; it is divided into isocitrate
dehydrogenase (IDH) wild-type and IDH mutant-type according to
molecular typing in the most recent WHO Classification (1). GBM is the most common malignancy of
the central nervous system with an average annual age-adjusted
incidence rate of 19/100,000 individuals, accounting for 54% of all
glioma cases (2). GBM is a
refractory malignant tumor type with a median survival time of only
15 months (3). Treatment is
complex, and initially consists of maximal safe surgical resection
and subsequent radiation therapy with concurrent temozolomide
chemotherapy, followed by 6–10 or even more cycles of maintenance
temozolomide chemotherapy (4–6).
Radiation therapy has a crucial role in GBM treatment, particularly
for the tumor that is not totally surgically removed (7,8).
However, for a long time, the problem of radiotherapy resistance of
GBM, which is one of the reasons why the survival of affected
patients cannot be prolonged remains an obstacle for clinicians.
Therefore, it is important to elucidate the mechanisms underlying
radiotherapy resistance of GBM.
Legumain (LGMN), also known as asparagine
endopeptidase, is a lysosomal cysteine protease originally
identified in the seeds of legumes, which is also present in the
human body. The gene is located on chromosome 14 and it is
associated with a variety of tumor types at the stages of
development, metastasis and invasion (9). A previous study by our group indicated
that once tumor-associated macrophages, which highly expressed LGMN
on their surface, were selectively ablated by using a
doxorubicin-based prodrug activated by LGMN, tumor growth and
metastasis were markedly inhibited in a murine tumor model, this
implying an important role of LGMN in cancers (10). LGMN pseudogene 1 (LGMNP1) is a
pseudogene of LGMN located on chromosome 13 and its expression in
GBM is much higher than that in normal tissues (11,12),
implying that LGMNP1 has a certain association with GBM. To study
the function of LGMNP1 on the radioresistance of GBM, the present
study assessed whether LGMNP1 was altered after radiotherapy and
whether overexpression of this gene promoted the radiotherapy
resistance of GBM using in vitro experiments. In addition,
the underlying mechanisms were explored.
Materials and methods
Cell culture
The human GBM cell lines U87-MG (glioblastoma of
unknown origin; cell line was authenticated by STR profiling) and
T98G (purchased in 2014 from the Cell Bank of the Chinese Academy
of Sciences) were cultured in Dulbecco's modified Eagle's medium
(HyClone; GE Healthcare Life Sciences, Logan UT, USA) supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and maintained in a humidified atmosphere at 37°C
with 5% CO2.
Radiation treatment
Cells in culture were treated with an irradiator (GE
3000; GE Healthcare Life Sciences) using a 137Cs source
at an exact dose of 0 or 6.0 Gy. During irradiation, the cultures
were stored in the cell culture incubator (5% CO2 at
37°C). The cells were harvested exactly at the end of the
irradiation.
Reverse transcription-quantitative PCR. Total RNA
was extracted from cellular samples using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. UV spectrophotometry was used to determine the RNA
concentration and quality. Reverse transcription of total RNA was
performed using an iScript™ cDNA Synthesis kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) following the manufacturer's
instructions. A 7500 Fast PCR instrument (Applied Biosystems,
Thermo Fisher Scientific, Inc.) was used for quantitative PCR
amplification. The primer and probe sequences were as follows:
LGMNP1 forward primer, 5′-GGACGTGGAAGATCTGACTAACC-3′, reverse
primer, 5′-ATGATGTGGCTGGTATTGGTGTAT-3′ and probe,
5′-VIC-CAAGCAGTGCCGCC-MGB-3′. The probe was modified with MGB at
the 3′-end. GAPDH forward primer, 5′-GAAGGACTCATGACCACAGTCCA-3′,
reverse primer, 5′-GCAGGGATGATGTTCTGGAGAG-3′ and probe,
5′-ROX-CGGCCATCACGCCACAGTTTCC-3′-BHQ2. Gene alignments and primer
specificity analysis were used to choose specific primers and
probes. The composition of the reaction mixture contained 10 µl of
2X TaqMan Universal Master Mix II with UNG (Applied Biosystems,
Thermo Fisher Scientific, Inc.), 1 µl (100 ng) of cDNA, 1 µl of
probe-primer mix and 8 µl of nuclease-free water, constituting to a
final volume of 20 µl. The thermocycling conditions for qPCR were
as follows: 50°C for 2 min, 95°C for 10 min and 45 cycles of 95°C
for 15 sec and 60°C for 1 min. Data were acquired at the end of the
annealing/extension phase. Melting curve analysis was performed at
the end of each run from 50 to 95°C.
Lentiviral vector-mediated gene
overexpression
The LGMNP1 overexpression sequence was constructed
by Shanghai Hanyin Co., Ltd. (Shanghai, China). The recombinant
lentivirus and negative control (NC) lentivirus were prepared and
titered to 109 transfection U/ml. After 48 h, the
efficiency of overexpression was confirmed via RT-qPCR. To obtain
stably transfected cells (LGMNP1-OE), GBM cells were seeded in
6-well dishes at a density of 1×105 cells/well. The
cells were then infected with the same virus titer on the following
day with 8 µg/ml Polybrene. At 72 h post-viral infection, the
culture medium was replaced with selection medium containing 4
µg/ml puromycin. The puromycin-resistant cells were amplified in
medium containing 2 µg/ml puromycin for 7 days and then transferred
to medium without puromycin.
Colony formation assay
The isolated cells were seeded at 300 cells/well in
a 6-well tissue culture plate and grown for 14 days until
macroscopic cell clones were visible. The cells were then fixed
with 95% cold methanol for 15 min at 4°C and stained with 0.5%
methylene blue for 2 min in order to determine the number of
colonies by microscopy. The colony forming efficiency was
calculated as the percentage of single cells that generated
colonies on the 14th day. The colony formation rate was calculated
as follows: Colony formation rate = (number of clones/300) ×
100%.
Comet assay
Cells were lysed by placing the slides in a Coplin
jar (Thomas Scientific, Swedesboro, NJ, USA) containing 2.5 M NaCl,
0.1 M Na2EDTA, 0.1 M Tris and 1% Triton X-100 (pH 10) at
4°C for at least 1 h. Subsequently, slides were immersed in
electrophoresis solution (0.3 M NaOH and 1 mM Na2EDTA,
pH >13) for 30 min. Electrophoresis was then performed at 1.3
V/cm for 20 min in the same solution. Slides were washed twice in
cold phosphate-buffered saline (PBS) for 10 min and in water for
another 10 min. Comets were fixed by immersing the slides in 70%
ethanol for 15 min and in absolute ethanol for a further 15 min
prior to placing them on the bench to dry overnight. Comets were
stained with SYBRGold at the dilution recommended by the
manufacturer in a bath at 4°C with agitation. After 40 min,
SYBRGold solution was removed and the slides were rinsed twice with
water and left to dry at room temperature. On the day of analysis,
gels were hydrated by adding a drop of water on top of each
minigel, and a glass coverslip (24×60 mm) was used to cover all the
minigels on the slide. The semi-automated image analysis system
Comet Assay IV (Perceptive Instruments Ltd., Bury St. Edmunds, UK)
was used to evaluate 100 comets/gel in the case of the
H2O2 experiments. Percentage DNA in the tail
was the parameter selected to describe each comet. The number of
comets per gel (including so-called hedgehogs) was counted by
direct observation.
Cell apoptosis analysis
Apoptosis was analyzed by translocation of
phosphatidylserine to the cell surface using an Annexin and DAPI
apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA).
Cells were treated with 6 Gy of radiation, then collected and
washed in cold PBS. Cells were resuspended in Annexin V-FITC and
DAPI for 30 min in the dark. Cell apoptosis was analyzed on a
FACSAria flow cytometer (BD Biosciences) and quantified using
CellQuest software 5.1 (BD Biosciences). Fluorescence was captured
with an excitation wavelength of 480 nm.
Western blot analysis
The total proteins of cells were extracted with cell
lysis buffer (50 mM Tris-HCl pH 8.0, 120 mM NaCl, 0.5% NP-40 and 1
mM PMSF) and determined by BCA methods. Protein samples (30 µg)
were subjected to 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were incubated with blocking buffer [5% skimmed milk in
Tris-buffered saline containing Tween-20 (TBS-T)] at room
temperature for 1 h. The membranes were then incubated with the
following antibodies at a 1:500 dilution at 4°C overnight:
caspase-3 antibody (cat. no. 9662), caspase-7 antibody (cat. no.
12827), Bax antibody (cat. no. 14796; all from Cell Signaling
Technology, Inc., Danvers, MA, USA), active caspase-3 antibody
(cat. no. ab2302; Abcam, Cambridge, UK) and β-actin antibody (cat.
no. 4970; Cell Signaling Technology, Inc.). The membranes were
washed with TBS-T, then incubated with horseradish
peroxidase-conjugated anti-rabbit (cat. no. R2655) or anti-mouse
antibody (cat. no. M8270; both from Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at a 1:10,000 dilution at room temperature for
2 h. Detection was performed using western blot detection reagents
(Odyssey; LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. One-way analysis of variance (ANOVA) with Tukey's post
hoc test was performed for comparison of multiple groups. All
statistical analyses were performed using SPSS for Windows v. 17.0
(SPSS, Inc., Chicago, IL, USA). A two-tailed P<0.05 was
considered to indicate a statistically significant difference.
Results
LGMNP1 is significantly upregulated in
response to radiotherapy
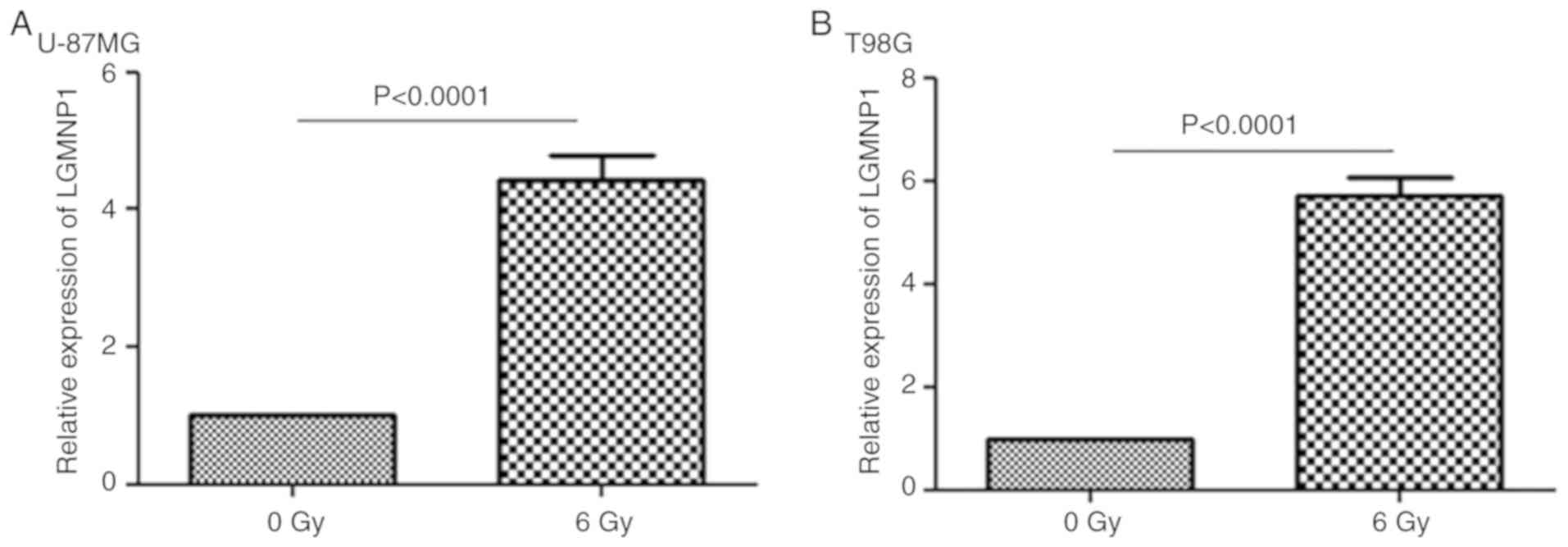
To determine the potential role of LGMNP1 in GBM
radiotherapy resistance, RT-qPCR was employed to detect the
relative expression of LGMNP1 in two glioma cell lines after
exposure to a radiation dose of 6 Gy compared with that in the
control group (0 Gy) (Fig. 1A and
B). The result indicated that in each of the two cell lines,
the relative expression of LGMNP1 in the experimental group was
significantly higher than that in the control group (P<0.0001).
It was, therefore, indicated that the expression of LGMNP1 is
associated with radiotherapy.
Upregulation of LGMNP1 enhances the
colony formation ability after radiotherapy
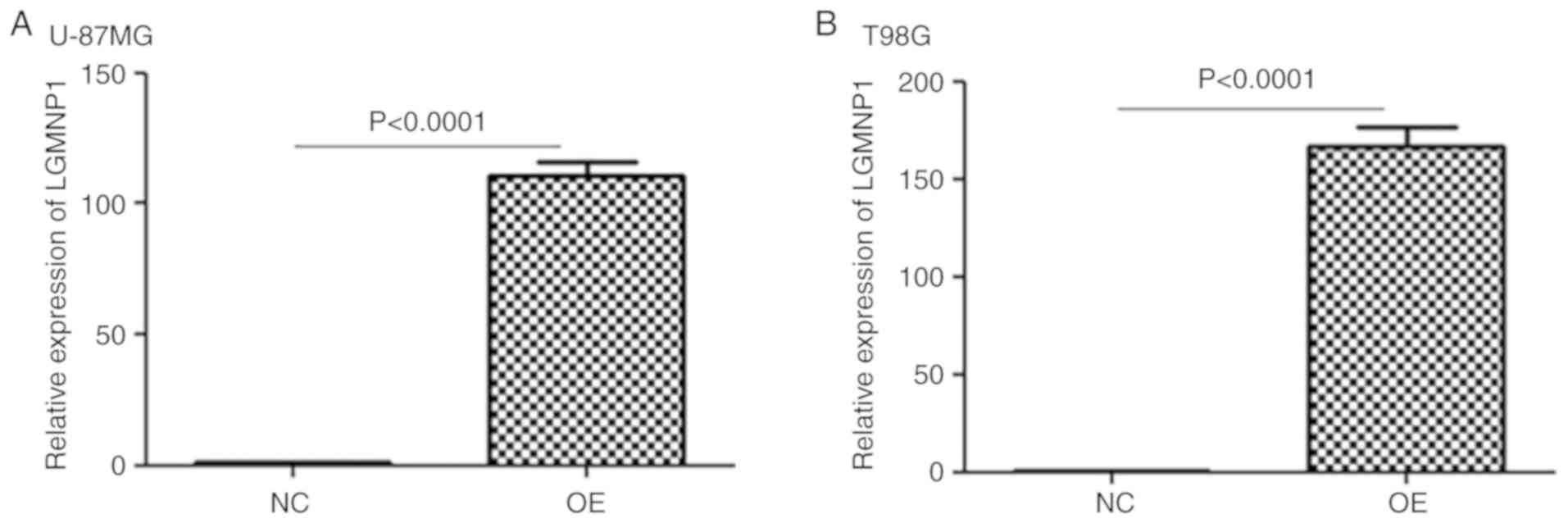
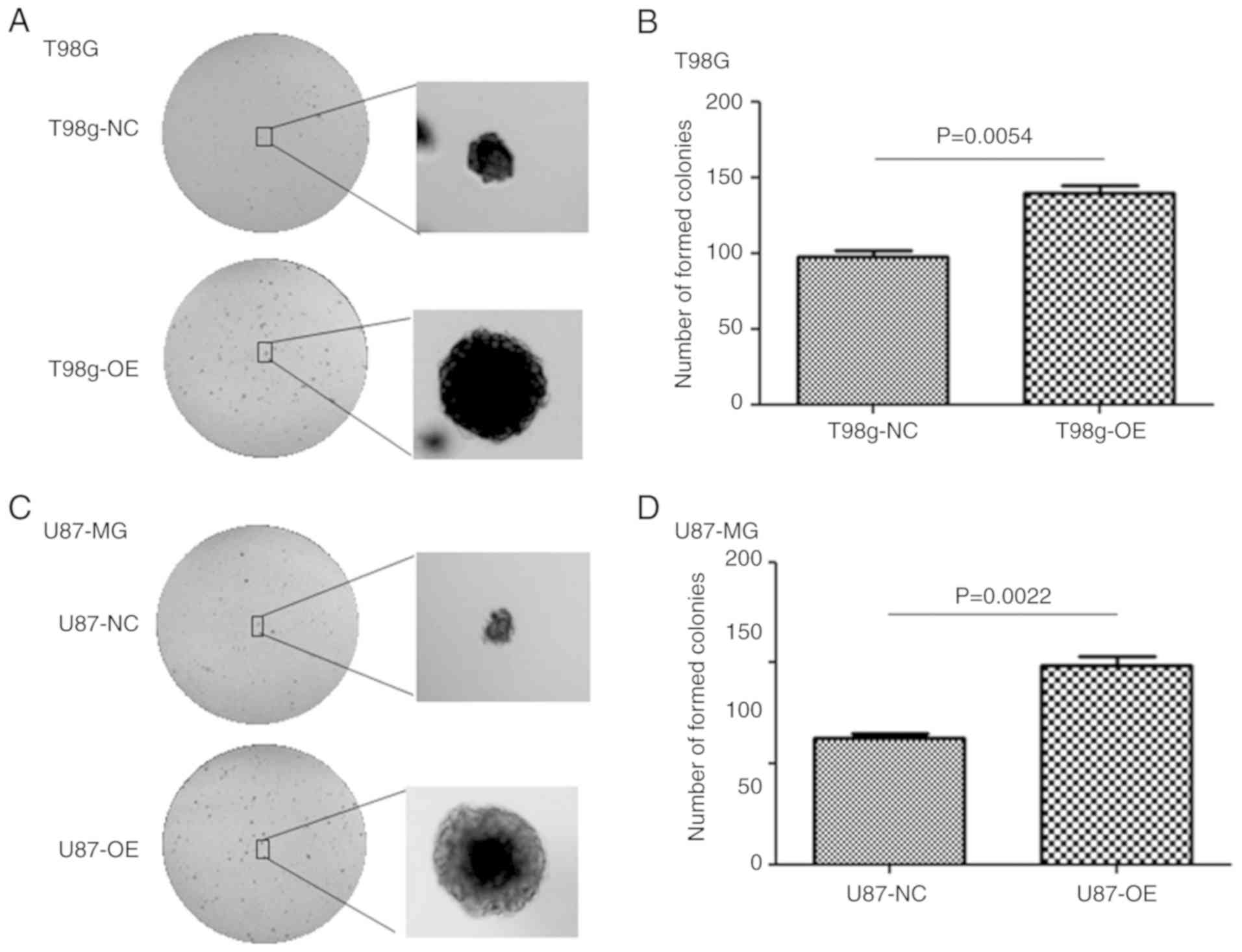
To verify the function of LGMNP1 in radiotherapy
resistance of GBM, U87-MG and T98G cells with stable overexpression
of LGMNP1 were established using the lentiviral vector-mediated
method. As is presented in Fig. 2A and
B, LGMNP1 was effectively upregulated in LGMNP1-OE cells
compared with that in the NC cells. The clonogenic assay indicated
that the colony formation ability of LGMNP1-OE cells after
radiotherapy was greater than that of the NC cells (Fig. 3). These results suggested that
overexpression of LGMNP1 contributes to radiation resistance.
Overexpression of LGMNP1 confers
radioresistance by enhancing the ability of DNA damage protection
and reduction of apoptosis
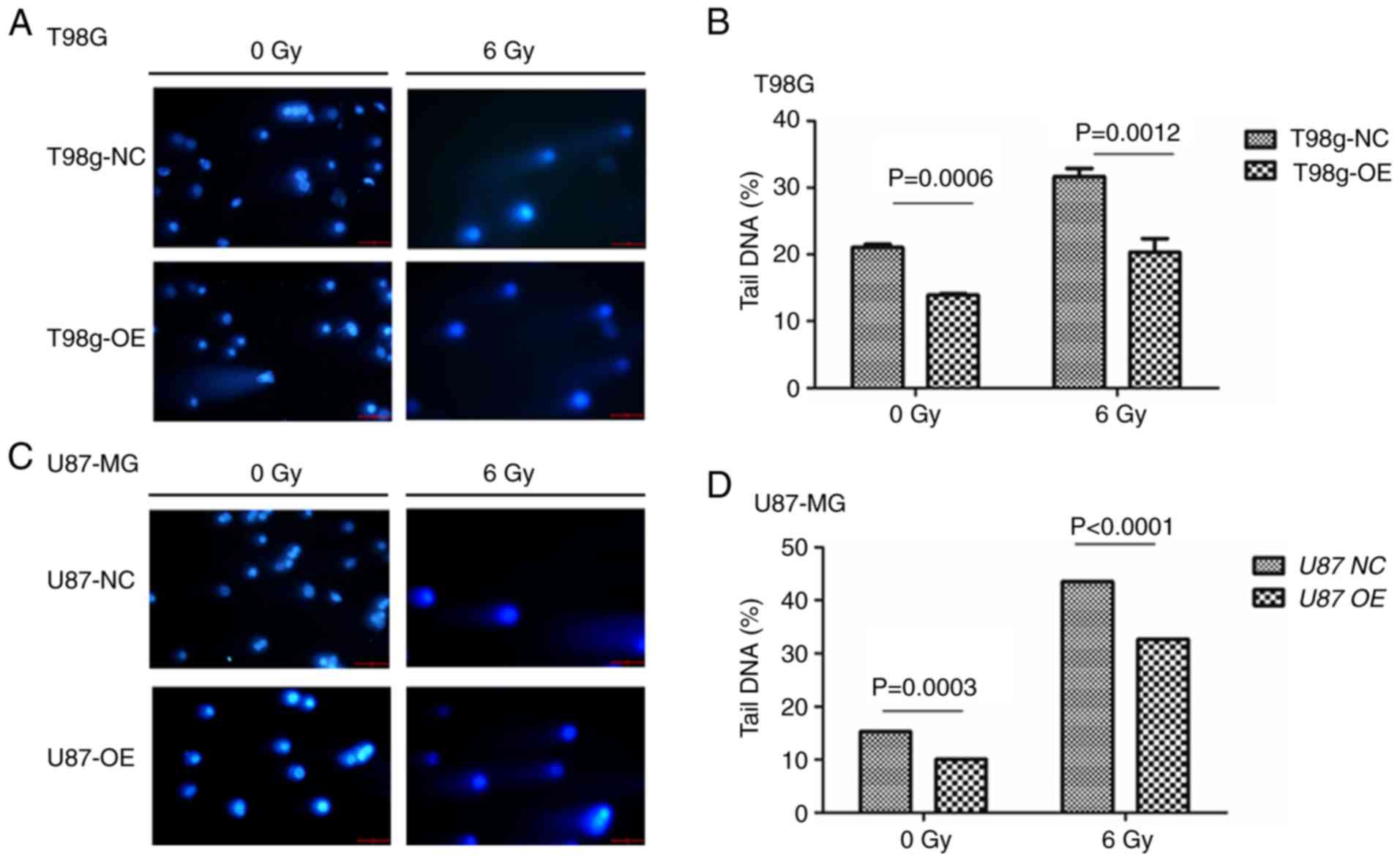
One of the mechanisms by which radiotherapy kills
tumor cells is the induction of DNA double-strand breaks. In order
to determine whether overexpression of LGMNP1 improves the capacity
for DNA damage protection after exposure to radiation damage, the
cell lines were subjected to the comet assay. The results indicated
that the percentage of tail DNA was higher in the cells exposed to
6 Gy of radiation compared with that of cells that received 0 Gy
(Fig. 4). Furthermore, the
percentage of tail DNA in the LGMNP1-OE group was less than that in
the NC group, in the T98G cell line (Fig. 4A and B) and also in the U87-MG cell
line (Fig. 4C and D). These results
suggested that overexpression of LGMNP1 improves the ability of
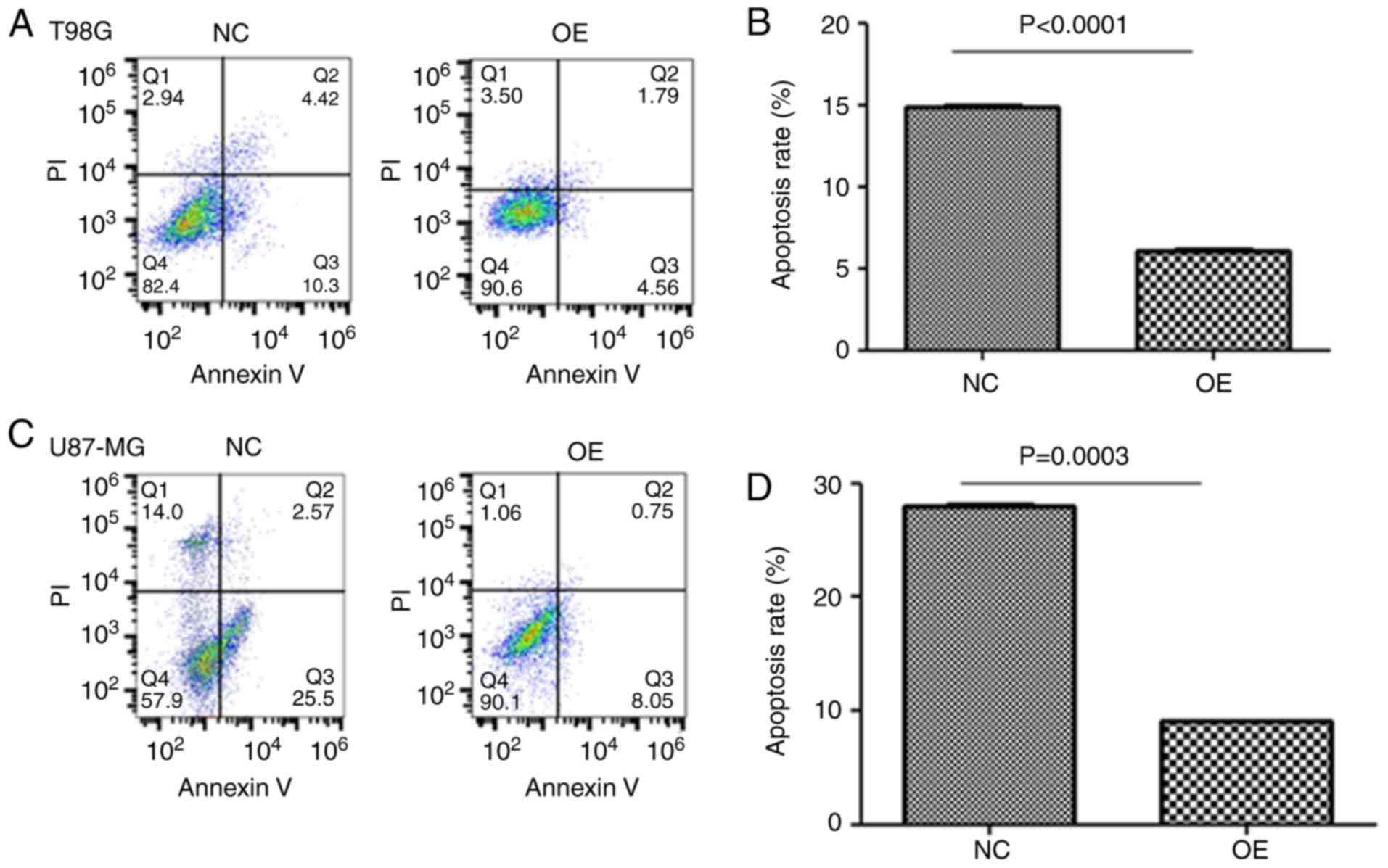
glioma cells to perform DNA damage protection. To detect apoptosis
in glioma cell lines, flow cytometric analysis was employed,
demonstrating a lower ratio of apoptosis in LGMNP1-OE vs. NC glioma
cells after radiotherapy (Fig. 5).
For further verification, the levels of apoptotic proteins were
assessed by western blot analysis. The results revealed that the
levels of apoptotic proteins, including caspase-3, active
caspase-3, caspase-7 and Bax in LGMNP1-OE glioma cells treated with
radiotherapy were decreased compared with those in the NC group,
and almost the same results were obtained with the two cell lines
(Fig. 6). Overall, these results
indicated that overexpression of LGMNP1 in GBM cell lines enhances
the capacity for DNA damage protection and reduces apoptosis
following radiation-induced damage.
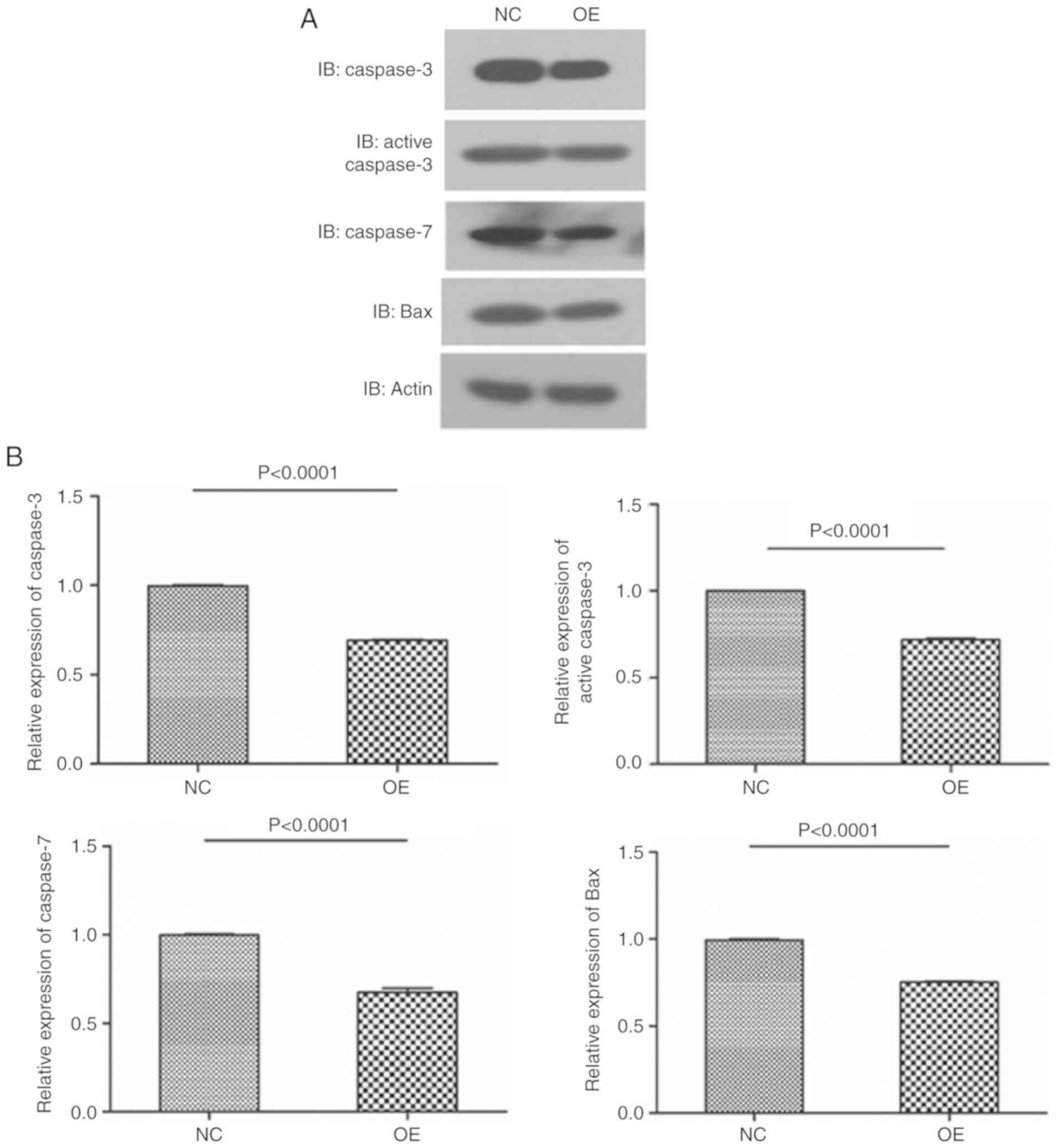 | Figure 6.Overexpression of LGMNP1 in
glioblastoma multiforme cells decreases apoptotic proteins after
radiotherapy. (A) Protein bands of caspase-3, active caspase-3,
caspase-7, Bax and β-actin from LGMNP1-OE or NC T98G cells after
radiotherapy. (B) Relative expression of caspase-3, active
caspase-3, caspase-7 and Bax in LGMNP1-OE or NC T98G cells after
radiotherapy. (C) Protein bands of caspase-3, active caspase-3,
caspase-7, Bax and β-actin from LGMNP1-OE or NC U87-MG cells after
radiotherapy. (D) Relative expression of caspase-3, active
caspase-3, caspase-7 and Bax in LGMNP1-OE or NC U87-MG cells after
radiotherapy. LGMNP1-OE, legumain pseudogene 1 overexpression; NC,
negative control. |
Discussion
With the development of surgical techniques and the
routine use of radiotherapy and chemotherapy, the two-year survival
of GBM patients has increased from 7% among cases diagnosed between
1993 and 1995 to 17% in cases diagnosed between 2005 and 2007
(3). In recent years, further
treatments have been implemented for the treatment of GBM, among
which immunotherapy, chimeric antigen receptor (CAR) T-cell
therapy, tumor vaccines, viral therapy and tumor-treating fields
(TTF) therapy have provided certain benefits. A patient with
recurrent multifocal glioblastoma receiving CAR-engineered T cells
targeting the tumor-associated antigen interleukin-13 receptor α 2
exhibited regression of all intracranial and spinal tumors
(13). Tetanus toxoid
pre-conditioning may improve the migration of the dendritic cell
vaccine and suppress tumor growth in mice and glioblastoma patients
(14). Oncolytic viruses are the
most extensively studied type of virus in glioma treatment and have
been developed for brain cancer treatment. They were demonstrated
to be safe and such therapies may also direct long-lasting immune
responses toward the tumor while reducing early antiviral reactions
(15). TTF therapy has been
evaluated in randomized phase 3 trials in GBM and was demonstrated
to prolong progression-free survival and overall survival when
administered together with standard maintenance temozolomide
chemotherapy in patients with newly diagnosed GBM (16,17).
However, this is far from enough, all of the aforementioned methods
are only effective in a limited number of patients. Therefore,
traditional radiotherapy still remains crucial for the treatment of
GBM. For newly diagnosed GBM, accurate, timely and efficient
radiotherapy is required and for recurrent GBM, radiation is also
recommended if the cancer is still susceptible (18–20).
The resistance of GBM to radiation therapy is a common problem, and
the underlying mechanisms remain to be fully elucidated. For tumor
cells, abnormal activation of the DNA damage repair pathway is not
only the root cause of tumorigenesis, but also an important
mechanism for its resistance to radiotherapy. p53, as a widely
known tumor suppressor gene interacts with Rad51 promoter and Rad51
protein to downregulate Rad51, thereby inhibiting homologous
recombination and DNA repair (21).
After radiation, an accelerated senescence response was observed in
p53 wild-type GBM cells (22). In
addition, it is known that glioma stem cells expressing CD133 as a
biomarker contribute to radioresistance of glioma through
preferential activation of the DNA damage checkpoint response and
an increase in DNA repair capacity (23). Beyond that, vascular endothelial
growth factor (VEGF) has been thoroughly studied, since it is one
of the angiogenic growth factors that is highly expressed in GBM. A
tumor blood flow study indicated that the blood volume increased
following radiotherapy, which may be associated with the expression
of VEGF (24). Furthermore, VEGF
binds to the vascular endothelial growth factor receptor 1 and
activates a signal transduction cascade, neutralizing antibodies to
the receptor inhibited angiogenesis and enhanced the
radiation-induced response, and a greater additive effect was
achieved in GBM when VEGF blocking antibody was combined with
radiation (25,26). Advanced research on the epidermal
growth factor receptor (EGFR) using in vitro and in
vivo experiments indicated that high levels of EGFR or EGFRvIII
were able to facilitate DNA double-strand break repair, which was
mediated by the AKT pathway (26–28).
The occurrence of tumor radiotherapy resistance is thought not to
be a single signaling process. Studies have revealed that blockade
of transforming growth factor β signaling enhanced the response of
glioblastoma patients to radiation therapy and prolonged their
survival (29). Furthermore,
combined inhibition of poly(ADP-ribose) polymerase and heat shock
protein 90 enhanced the radiosensitivity of human glioma cells
(30). The mechanisms require to be
elucidated in further studies.
LGMN is known for its cysteine endopeptidase
activity in lysosomes, where it contributes to antigen processing
for class II major histocompatibility complex presentation.
However, it also occurs extracellularly and even translocates to
the cytosol and the nucleus. LGMN has also been reported to be
associated with the development of a variety of tumor types,
including breast cancer, gastric carcinoma, ovarian and colorectal
cancer, and it may even serve as a biomarker for such tumors
(31–34). Furthermore, LGMN was indicated to
promote the proliferation and invasiveness of prostate cancer cells
via the PI3K/AKT signaling pathway (35). In addition, knockdown of LGMN was
reported to suppress cervical cancer cell migration and invasion
(36). Pseudogenes are not truly
non-functional genes, although they do not encode proteins, but
non-coding RNAs formed by transcription have certain functions. It
has been indicated that abnormal expression of pseudogenes may have
vital roles in tumors (37). For
instance, phosphatase and tensin homolog (PTEN) is under the
regulatory control of PTEN pseudogene-expressed non-coding
RNA-PTENpg1, which encodes antisense RNA (asRNA) that regulates
PTEN transcription by balancing the two PTENpg1 asRNA isoforms, α
and β (38). It is possible that
LGMNP1 regulates LGMN expression in a similar manner to enhance the
DNA repair capacity and produce radiotherapy resistance. According
to the results of the present study, LGMNP1 was upregulated once
the glioma cells were exposed to radiotherapy, and when LGMNP1 was
ectopically overexpressed, the glioma cells were more resistant to
radiotherapy. The comet assay and the apoptosis detection assays
indicated less amount of DNA double-strand breaks and the amounts
of apoptotic cells and proteins were decreased. In summary,
upregulation of LGMNP1 was indicated to enhance the radioresistance
of glioma and targeting LGMNP1 may be a novel strategy to increase
sensitivity to radiotherapy, however the mechanism still requires
further exploration.
In conclusion, LGMNP1 was upregulated in GBM cell
lines after administration of ionizing radiation. LGMNP1 confered
radiotherapy resistance by increasing the capacity for DNA damage
protection and reducing apoptosis in glioma cells in vitro.
The therapy targeting LGMNP1 may be a promising method to reverse
radioresistance of GBM.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81402042 and 81772654), the
Shanghai Pudong New Area Science and Technology Development Fund
(grant no. PKJ2016-Y45), the Shanghai Pudong New Area Health and
Family Planning Commission (grant nos. PWZzk2017-16 and
PWRL2017-03) and the Shanghai Science and Technology Committee
(grant no. 16140902900).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LR and YL designed and supervised the research, YQ
was also involved in the conception of the study. HX and BC took
part in the fund raising, experimental design, data acquisition,
manuscript writing, performed the majority of the experiments and
drafted the manuscript. ZW and JX helped with the design and
performance of the experiments of western blotting and lentiviral
vector-mediated gene overexpression. CL helped with the colony
formation and comet assays. YL and YQ contributed to the revising
of the manuscript. All authors were involved in the conception of
the study, read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 15
(Suppl 2):ii1–ii56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Darefsky AS, King JT Jr and Dubrow R:
Adult glioblastoma multiforme survival in the temozolomide era: A
population-based analysis of Surveillance, Epidemiology, and End
Results registries. Cancer. 118:2163–2172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thakkar JP, Dolecek TA, Horbinski C,
Ostrom QT, Lightner DD, Barnholtz-Sloan JS and Villano JL:
Epidemiologic and molecular prognostic review of glioblastoma.
Cancer Epidemiol Biomarkers Prev. 23:1985–1996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Dietrich PY, Ostermann Kraljevic
S, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G,
Miralbell R, et al: Promising survival for patients with newly
diagnosed glioblastoma multiforme treated with concomitant
radiation plus temozolomide followed by adjuvant temozolomide. J
Clin Oncol. 20:1375–1382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wind JJ, Young R, Saini A and Sherman JH:
The role of adjuvant radiation therapy in the management of
high-grade gliomas. Neurosurg Clin N Am. 23247–258. (viii)2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caruso C, Carcaterra M and Donato V: Role
of radiotherapy for high grade gliomas management. J Neurosurg Sci.
57:163–169. 2013.PubMed/NCBI
|
|
9
|
Zhen Y, Chunlei G, Wenzhi S, Shuangtao Z,
Na L, Rongrong W, Xiaohe L, Haiying N, Dehong L, Shan J, et al:
Clinicopathologic significance of legumain overexpression in
cancer: A systematic review and meta-analysis. Sci Rep.
5:165992015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin Y, Wei C, Liu Y, Qiu Y, Liu C and Guo
F: Selective ablation of tumor-associated macrophages suppresses
metastasis and angiogenesis. Cancer Sci. 104:1217–1225. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cancer Genome Atlas Research Network, ;
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C, Stuart JM, et al: The Cancer
Genome Atlas Pan-Cancer analysis project. Nat Genet. 45:1113–1120.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng LL, Zhou KR, Liu S, Zhang DY, Wang
ZL, Chen ZR, Yang JH and Qu LH: dreamBase: DNA modification, RNA
regulation and protein binding of expressed pseudogenes in human
health and disease. Nucleic Acids Res. 46:D85–D91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown CE, Alizadeh D, Starr R, Weng L,
Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J,
Simpson J, et al: Regression of glioblastoma after chimeric antigen
receptor t-cell therapy. N Engl J Med. 375:2561–2569. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mitchell DA, Batich KA, Gunn MD, Huang MN,
Sanchez-Perez L, Nair SK, Congdon KL, Reap EA, Archer GE,
Desjardins A, et al: Tetanus toxoid and CCL3 improve dendritic cell
vaccines in mice and glioblastoma patients. Nature. 519:366–369.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaufmann JK and Chiocca EA: Glioma virus
therapies between bench and bedside. Neuro Oncol. 16:334–351. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hottinger AF, Pacheco P and Stupp R: Tumor
treating fields: a novel treatment modality and its use in brain
tumors. Neuro Oncol. 18:1338–1349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stupp R, Taillibert S, Kanner AA, Kesari
S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink
KL, et al: Maintenance therapy with tumor-treating fields plus
temozolomide vs temozolomide alone for glioblastoma: A randomized
clinical trial. JAMA. 314:2535–2543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryu S, Buatti JM, Morris A, Kalkanis SN,
Ryken TC and Olson JJ; AANS/CNS Joint Guidelines Committee, : The
role of radiotherapy in the management of progressive glioblastoma:
A systematic review and evidence-based clinical practice guideline.
J Neurooncol. 118:489–499. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lutz ST, Jones J and Chow E: Role of
Radiation Therapy in Palliative Care of the Patient With Cancer. J
Clin Oncol. 32:2913–2919. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gallego O: Nonsurgical treatment of
recurrent glioblastoma. Curr Oncol. 22:E273–E281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arias-Lopez C, Lazaro-Trueba I, Kerr P,
Lord CJ, Dexter T, Iravani M, Ashworth A and Silva A: p53 modulates
homologous recombination by transcriptional regulation of the RAD51
gene. EMBO Rep. 7:219–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quick QA and Gewirtz DA: An accelerated
senescence response to radiation in wild-type p53 glioblastoma
multiforme cells. J Neurosurg. 105:111–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gorski DH, Beckett MA, Jaskowiak NT,
Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM,
Kufe DW, et al: Blockade of the vascular endothelial growth factor
stress response increases the antitumor effects of ionizing
radiation. Cancer Res. 59:3374–3378. 1999.PubMed/NCBI
|
|
25
|
Geng L, Donnelly E, McMahon G, Lin PC,
Sierra-Rivera E, Oshinka H and Hallahan DE: Inhibition of vascular
endothelial growth factor receptor signaling leads to reversal of
tumor resistance to radiotherapy. Cancer Res. 61:2413–2419.
2001.PubMed/NCBI
|
|
26
|
Hatanpaa KJ, Burma S, Zhao D and Habib AA:
Epidermal growth factor receptor in glioma: Signal transduction,
neuropathology, imaging, and radioresistance. Neoplasia.
12:675–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mukherjee B, McEllin B, Camacho CV,
Tomimatsu N, Sirasanagandala S, Nannepaga S, Hatanpaa KJ, Mickey B,
Madden C, Maher E, et al: EGFRvIII and DNA double-strand break
repair: A molecular mechanism for radioresistance in glioblastoma.
Cancer Res. 69:4252–4259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liccardi G, Hartley JA and Hochhauser D:
Importance of EGFR/ERCC1 interaction following radiation-induced
DNA damage. Clin Cancer Res. 20:3496–3506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang M, Kleber S, Roehrich M, Timke C,
Han N, Tuettenberg J, Martin-Villalba A, Debus J, Peschke P,
Wirkner U, et al: Blockade of TGF-β signaling by the TGFβ R-I
kinase inhibitor LY2109761 enhances radiation response and prolongs
survival in glioblastoma. Cancer Res. 71:7155–7167. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dungey FA, Caldecott KW and Chalmers AJ:
Enhanced radiosensitization of human glioma cells by combining
inhibition of poly(ADP-ribose) polymerase with inhibition of heat
shock protein 90. Mol Cancer Ther. 8:2243–2254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu M, Shao GR, Zhang FX, Wu WX, Xu P and
Ruan ZM: Legumain protein as a potential predictive biomarker for
Asian patients with breast carcinoma. Asian Pac J Cancer Prev.
15:10773–10777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haugen MH, Boye K, Nesland JM, Pettersen
SJ, Egeland EV, Tamhane T, Brix K, Maelandsmo GM and Flatmark K:
High expression of the cysteine proteinase legumain in colorectal
cancer-implications for therapeutic targeting. Eur J Cancer.
51:9–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo P, Zhu Z, Sun Z, Wang Z, Zheng X and
Xu H: Expression of legumain correlates with prognosis and
metastasis in gastric carcinoma. PLoS One. 8:e730902013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu Q, Tang M and Wang X: The expression
of asparaginyl endopeptidase promotes growth potential in
epithelial ovarian cancer. Cancer Biol Ther. 18:222–228. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu W, Shao Y, Yang M, Jia M and Peng Y:
Asparaginyl endopeptidase promotes proliferation and invasiveness
of prostate cancer cells via PI3K/AKT signaling pathway. Gene.
594:176–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng F and Liu W: Knockdown of legumain
suppresses cervical cancer cell migration and invasion. Oncol Res.
23:7–12. 2016. View Article : Google Scholar
|
|
37
|
Kalyana-Sundaram S, Kumar-Sinha C, Shankar
S, Robinson DR, Wu YM, Cao X, Asangani IA, Kothari V, Prensner JR,
Lonigro RJ, et al: Expressed pseudogenes in the transcriptional
landscape of human cancers. Cell. 149:1622–1634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Johnsson P, Ackley A, Vidarsdottir L, Lui
WO, Corcoran M, Grandér D and Morris KV: A pseudogene
long-noncoding-RNA network regulates PTEN transcription and
translation in human cells. Nat Struct Mol Biol. 20:440–446. 2013.
View Article : Google Scholar : PubMed/NCBI
|