Introduction
Lung cancer is the major cause of cancer-associated
mortality worldwide (1). Lung
cancer nanotherapeutics is a strategy to overcome the limitations
of the conventional methods available for diagnosis and treatment
(2). Nanoparticle (NP) systems have
improved the chemotherapeutic efficiency of drugs associated with
poor solubility, stability or adverse effects on their own by
providing different pathways. The lipid prodrug strategy is one of
the promising methods involving NP systems (3). The advantages of lipid-based prodrug
nanocarriers include improved drug encapsulation and stability,
balancing of the pharmacokinetics of drugs, controlled drug release
and enhanced biocompatibility (4).
D-alpha-tocopheryl polyethylene glycol 1000
succinate (TPGS), a water-soluble derivative of natural vitamin E,
has an amphiphilic structure comprised of a lipophilic alkyl tail
and a hydrophilic polar head portion (5). It has already demonstrated advantages
when applied along with various anticancer drugs, e.g. doxorubicin
(DOX), including enhanced therapeutic effects and reduced side
effects (6). Tumor cells contain a
higher concentration of glutathione (GSH, 2–8 mM) than normal cells
(7). Numerous disulfide-based
proteins and small-molecule prodrug strategies have been developed
that rely on high intracellular GSH levels (8). Furthermore, active transport of
activated disulfides into cells is increasingly recognized as an
important mechanism for cell delivery. Disulfide (S-S) bonds may be
rapidly cleaved by intracellular GSH, following which intracellular
targeting may be achieved (9). In
addition, through the disulfide bond connection, the self-assembly
and stabilization of hydrophobic prodrugs are supported (10). In the present study, TPGS was
conjugated to DOX through S-S bonds to constitute TPGS-S-S-DOX.
Hyaluronic acid (HA) is a natural line
polysaccharide. Due to its high affinity toward CD44, a cell
adhesion membrane glycoprotein overexpressed on the surface of
cancer cells, HA has high tumor-specific targeting properties to
(lung) cancer cells compared to normal cells (11). HA is not only a structural component
of the extracellular matrix of the tumor cell but also a
biologically active molecule that may promote tumor progression
through induction of cell signaling (12). In addition, HA protects tumor
tissues against immune surveillance and chemotherapeutic agents. In
the present study, HA was conjugated to TPGS to obtain HA-TPGS.
Self-assembled amphiphilic prodrug-based nanocarrier
delivery systems may overcome the limitations of common prodrugs,
which on their own may be chemically or enzymatically degraded
in vivo in an uncontrolled manner (13). Combining the advantages of prodrug
and nanomedicine strategies in cancer therapy may achieve high
therapeutic efficiency. In the present study, the advantages of
prodrug and nanomedicine strategies were combined and a
self-assembled TPGS-S-S-DOX prodrug-based and HA-TPGS
ligand-containing nanomedicine (HA-TPGS DOX-NPs) was developed for
the treatment of lung cancer. In vitro and in vivo
evaluation of the system were performed using lung cancer cell
lines and lung tumor-bearing mice, respectively.
Materials and methods
Chemicals and reagents
TPGS was obtained from Eastman Chemical Co.
(Kingsport, TN, USA). TPGS-COOH was synthesized according to
previous studies (14,15). HA (molecular weight, 7.8 kDa) was
purchased from Freda Biochem Co., Ltd. (Linyi, China). HA-TPGS was
synthesized according to a previous study (16). Polylactic acid (PLA) was purchased
from Jinan Daigang Biomaterial Co., Ltd. (Jinan, China). DOX, DMSO,
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC),
N-hydroxysuccinimide (NHS), Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS), Tween®−80 and MTT were
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell lines and culture
The A549 and HCC827 lung cancer cell lines and the
MRC-5 human embryonic lung cell line were obtained from the
American Type Culture Collection and cultured in DMEM and RPMI-1640
medium, respectively, supplemented with 10% (v/v) FBS, 1%
non-essential amino acids, 1% L-glutamine and 1%
streptomycin-penicillin (100 IU/ml) at 37°C, in a humidified
atmosphere of 95% air and 5% CO2 (17).
Animal model
HCC827 cells (1×106 cells/animal) were
injected into the lateral tail vein of 4- to 6-week-old, female
BALB/c nude mice (100 mice; Shandong University Laboratory Animal
Center, Jinan, China) to produce a lung cancer-bearing animal model
(18), mice were raised under
conventional conditions with a 12 h light/dark cycle, constant
temperature (25°C) and humidity (60%) with access to food and water
ad libitum. The animal experiments complied with the Guide
for the Care and Use of Laboratory Animals of the National
Institutes of Health (publication no. 8023; Bethesda, MD, USA) and
approval was obtained from the Institutional Animal Care and
Treatment Committee of Southeast University (Nanjing, China). A
loss of >20% of body weight; mice that could not take in food
for 24 h; who could not stand for 24 h; whose tumor weight exceeded
10% of their body weight; or whose average tumor diameter was
>20 mm were set as humane endpoints of the animal
experiments.
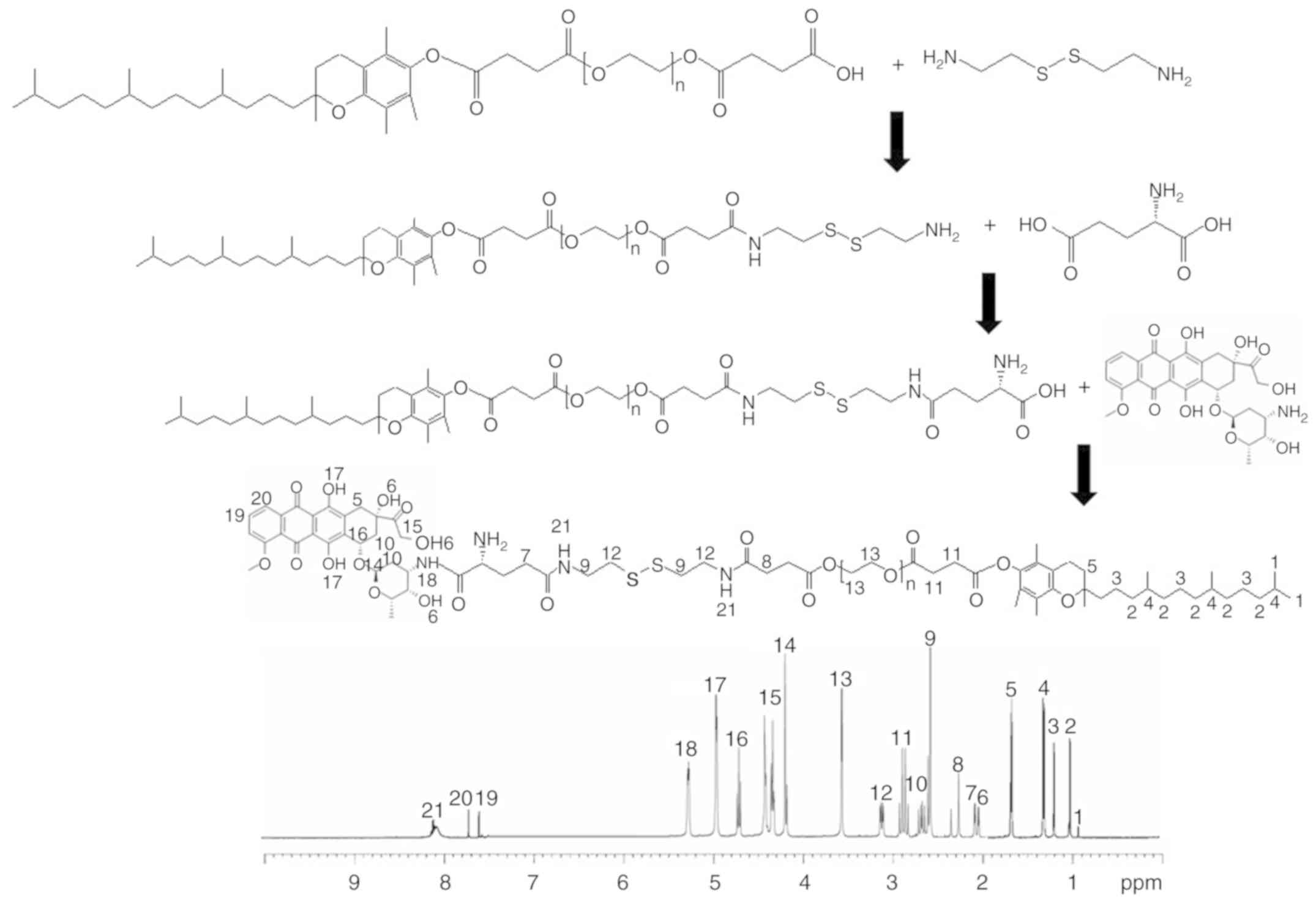
Synthesis of TPGS-S-S-DOX
The TPGS-S-S-DOX prodrug was synthesized as follows
(Fig. 1): TPGS-COOH was dissolved
in DMSO, EDC·HCl (1.2 equivalents) and NHS (1.2 equivalents) were
added, followed by stirring for 2 h at room temperature (RT).
2,2′-Dithiodibenzoic acid (1 equivalent) was added to TPGS-COOH and
the mixture was stirred for 20 h at RT to yield TPGS-S-S. Glutamic
acid (GA) was dissolved in DMSO, and EDC·HCl (1.2 equivalents) and
NHS (1.2 equivalents) were added, followed by stirring for 2 h at
RT. TPGS-S-S was added to GA and stirred for 20 h at RT to yield
TPGS-S-S-GA. TPGS-S-S-DOX as obtained by dissolving TPGS-S-S-GA in
DMSO with added EDC·HCl (1.2 equivalents) and NHS (1.2
equivalents), and DOX (dissolved in DMSO) was then added to this
mixture, which was stirred for 20 h at RT. TPGS-S-S-DOX was
dialyzed against water for 24 h and lyophilized. The chemical
structure of TPGS-S-S-DOX was confirmed using 1H-nuclear
magnetic resonance (NMR) analysis.
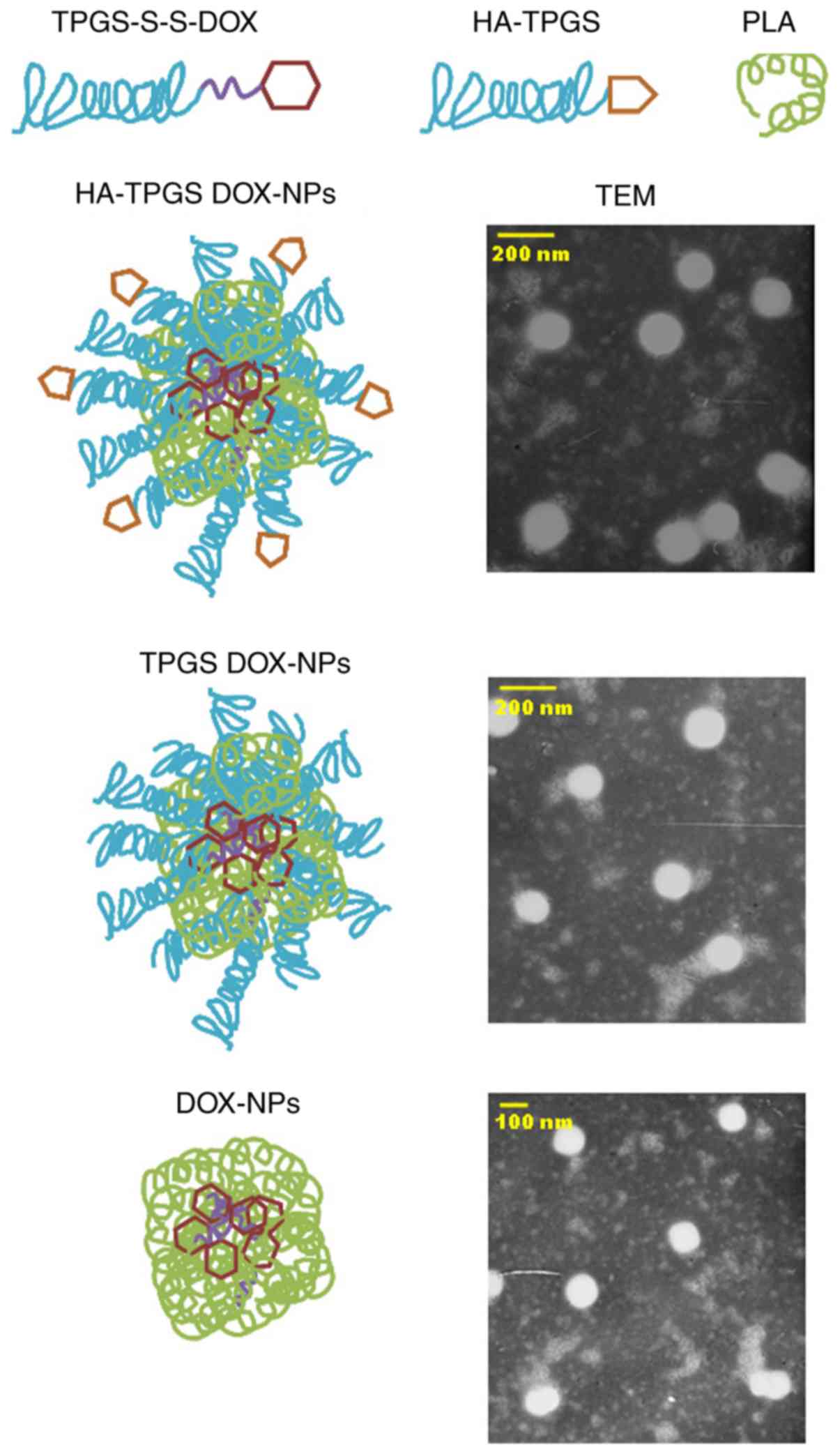
Self-assembly of HA-TPGS DOX-NPs
Self-assembled HA-TPGS DOX-NPs (Fig. 2) were prepared via a dialysis method
(19,20). In brief, 100 mg TPGS-S-S-DOX and 100
mg PLA were dissolved in 10 ml DMSO, to which 20 ml HA-TPGS (100
mg) and Tween®−80 (1%) in deionized water was added
dropwise to obtain the self-assembled HA-TPGS DOX-NPs mixture. The
mixture was subsequently transferred to a dialysis bag [molecular
weight cut-off (MWCO), 10,000 Da] and dialyzed against distilled
water for 48 h. Blank (DOX-free) HA-TPGS-containing NPs (HA-TPGS
NPs) were prepared by the same method using TPGS-S-S without the
presence of DOX. DOX-containing NPs without the HA ligand
modification (TPGS DOX-NPs) were manufactured with the same method
using TPGS without the presence of HA. DOX-loaded PLA NPs (DOX-NPs)
lacking HA-TPGS were also prepared by the same method using DOX
without TPGS and S-S. The final products were harvested after
lyophilization.
Characterization of HA-TPGS
DOX-NPs
One drop of HA-TPGS DOX-NPs, TPGS DOX-NPs or DOX-NPs
was dissolved in PBS (1 mg/ml) separately, and was carefully cast
onto a clean copper grid (21).
Subsequently, extra solution was air-dried and directly observed
under a transmission electron microscope (TEM) without staining.
The morphology of HA-TPGS DOX-NPs, TPGS DOX-NPs or DOX-NPs was
examined using a TEM (JEM-1200EX; JEOL, Ltd., Tokyo, Japan),
operated at an acceleration voltage of 100 kV.
The dynamic light scattering (DLS) method was
applied to determine the particle size, polydispersity index (PDI)
and zeta potential (ZP) of NPs using a Zetasizer®
Nano-ZS90 (Malvern Instruments, Malvern, UK) (22).
For evaluation of drug encapsulation efficiency (EE)
and drug-loading content (DL), HA-TPGS DOX-NPs was passed through
an ultrafiltration tube (MWCO, 10,000 Da) at 15,930 × g for 30 min
(23). The amount of DOX in the
filtrate was assessed by detecting the UV absorbance at the
wavelength of 480 nm using an UV-1201 spectrophotometer (Shimadzu
Corporation, Kyoto, Japan) (24).
The stability of NPs in the presence of serum was
examined by incubation with 10% FBS (25). HA-TPGS DOX-NPs, HA-TPGS NPs, TPGS
DOX-NPs or DOX-NPs were incubated with 10% FBS (v/v) solution at
37°C with gentle stirring at 100 rpm for 1, 2, 4, 8, 24, 48 or 72
h. At each time-point, the NPs were centrifuged at 67 × g for 10
min. Their size and PDI were then measured.
In vitro drug release
The dialysis bag method was used to assess the in
vitro release of HA-TPGS DOX-NPs in comparison with TPGS
DOX-NPs and DOX-NPs (26). Of the
samples, 10 ml were placed into dialysis bags (MWCO, ~14,000),
which were then sealed and dialyzed against 100 ml PBS (pH 7.4,
37°C). A total of 200 µl release medium was withdrawn at
pre-determined intervals (0, 2, 4, 8, 16, 24, 48, 72, 96 and 120 h)
and immediately replaced with the same volume of fresh medium to
maintain sink conditions. The amount of drug released was analyzed
by the same method as in that in the aforementioned section and the
release curves were plotted.
Cellular uptake assay
Coumarin 6, which may be encapsulated into various
NPs for quantitative investigation of cellular uptake (27), was used in the present study as a
model fluorescent molecule to evaluate cellular uptake efficiency
of NPs. Coumarin 6 was encapsulated into the HA-TPGS DOX-NPs,
HA-TPGS NPs, TPGS DOX-NPs or DOX-NPs by the same synthetic method
as that stated above, and a total of 50 mg coumarin 6 was added
along with the PLA (28). A549 and
HCC827 cells were seeded in 24-well culture plates
(5×104 cells/well) and incubated for 24 h (37°C). After
the cells reached ~80% confluence, different NPs were added to
replace the medium, followed by incubation for 2, 4 or 8 h.
Subsequently, the cells were washed three times with D-Hank's
solution, collected and centrifugated (15,93 × g, 5 min), and the
fluorescence of cells was analyzed using a flow cytometer.
In vitro cytotoxicity of NPs
The in vitro cytotoxicity of HA-TPGS NPs was
evaluated by measuring the viability of cells treated with
different concentrations of NPs using an MTS assay (29). A549, HCC827 and MRC-5 cells
(5×104 cells/ml in 200 µl) were seeded separately into
96-well plates and incubated for 24 h. HA-TPGS NPs at different
concentrations were added, followed by incubation for 72 h. The
cell viability was evaluated with a CellTiter 96®
AQueous One Solution Reagent (Promega Corporation, Madison, WI,
USA). Cell culture medium (100 µl) was supplemented with fresh
medium with MTS (20 µl/well). The cell viability was calculated
using the following equation: Cell viability (%)=(absorbance of
treatment group)/(absorbance of control group) ×100. Culture medium
only served as a negative control.
In vitro anticancer efficacy
The in vitro anticancer efficacy of NPs was
assessed by determining the inhibition efficiency on A549 and
HCC827 cells using the same method as aforementioned. The only
difference was that DOX-loaded NPs and free DOX at different
concentrations were added instead of HA-TPGS NPs (30).
In vivo antitumor efficiency
A lung cancer-bearing animal model was prepared by
injecting HCC827 cells (1×106 cells/mouse) into the
lateral tail vein of BALB/c nude mice as mentioned in the
‘Animal model’ section. After 1 week, mice with tumors
(volume of 100 mm3) were randomly divided into 6 groups
(8 mice per group) that were respectively injected with 0.2 ml of
i) HA-TPGS DOX-NPs; ii) HA-TPGS NPs; iii) TPGS DOX-NPs; iv)
DOX-NPs; and v) free DOX (equivalent DOX concentration, 10 mg/kg)
or vi) 0.9% saline solution via the tail vein on days 0, 4, 8 and
12 (31). The tumors were measured
every 4 days with a Vernier caliper and the volumes were calculated
using the following equation: Tumor volume
(mm3)=(largest diameter) × (smallest diameter) × (height
of the tumor)/2. The antitumor efficacy of the drugs/NPs was
evaluated by calculating the tumor inhibition rate (TIR) according
to the following equation: TIR (%)=(tumor weight of the negative
control group-tumor weight of the treatment group)/(tumor weight of
the negative control group) ×100 (32). Images of the tumors were captured at
the end of the study. Body weight changes were monitored in order
to evaluate the systemic toxicity of the drugs/NPs.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical significances were evaluated using one-way
analysis of variance (ANOVA) followed by Tukey's test for multiple
comparisons. *P<0.05, **P<0.01 was considered to indicate a
statistically significant difference.
Results
Characterization of TPGS-S-S-DOX
To confirm the formation of TPGS-S-S-DOX,
1H-NMR analyses were performed, and the spectra are
provided in Fig. 1. The peaks,
including those for the TPGS protons, e.g. -CH2- and
-CH3 at 0.9-1.7 ppm (peaks, 1-5), the protons of PEG at
3.6 ppm (peak, 13), the-S-S-unit at 2.5-3.2 ppm (peaks, 9-12), as
well as the protons of DOX at 4.2-7.8 (peaks, 14-20), confirmed the
presence of TPGS, S-S and DOX. The peaks at 7, 8, 9, 12 and 21
illustrated the covalent conjugation of the materials through amide
linkages.
Characterization of HA-TPGS
DOX-NPs
TEM revealed the surface morphology and particle
size of HA-TPGS DOX-NPs (Fig. 2).
The image indicates that HA-TPGS DOX-NPs had a uniformly spherical
shape with a white core and grey shell. The NPs had a size of
<200 nm. These results are in agreement with the particle size
and PDI determination (Table I).
HA-TPGS DOX-NPs had a size of 172.3 nm and a PDI of 0.16. All of
the NPs exhibited a negative ZPs and EEs of >90%. The stability
of HA-TPGS DOX-NPs was evaluated in the presence of 10% FBS at 37°C
for 72 h. HA-TPGS DOX-NPs, TPGS DOX-NPs or DOX-NPs in a solution
with 10% FBS exhibited no significant changes in size or PDI
(Fig. 3).
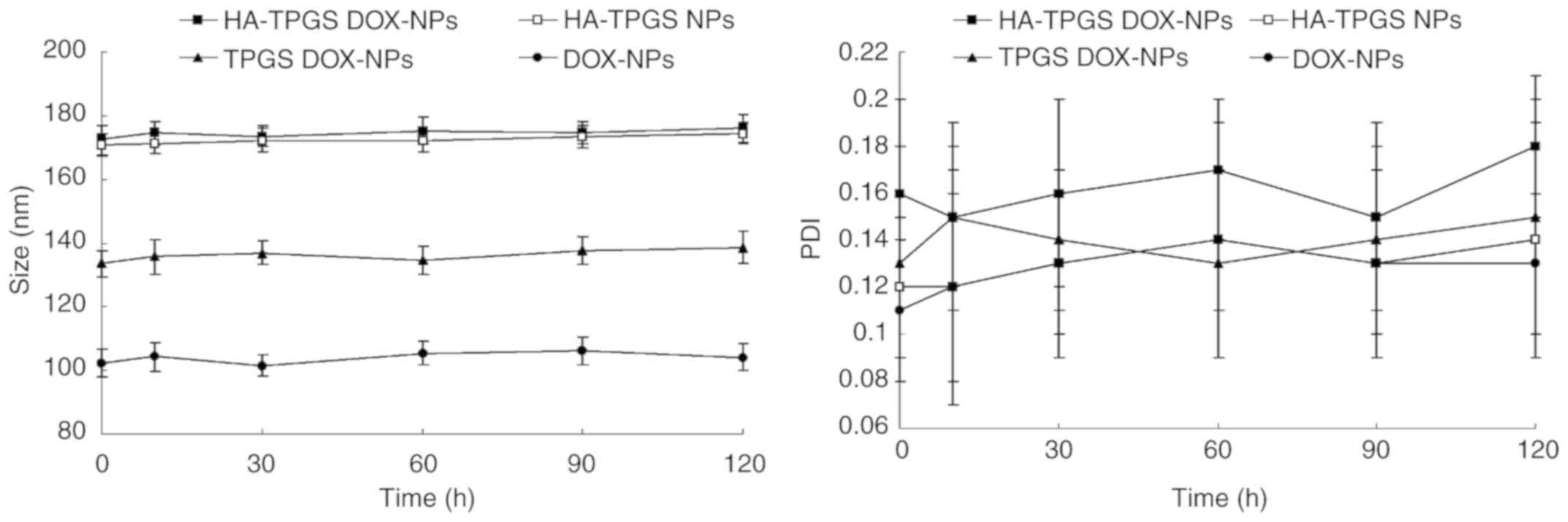 | Figure 3.Stability of HA-TPGS DOX-NPs
evaluated in the presence of serum. HA-TPGS DOX-NPs, HA-TPGS NPs,
TPGS DOX-NPs or DOX-NPs were incubated with 10% fetal bovine serum
(v/v) at 37°C with gentle stirring at 100 rpm for 1, 2, 4, 8, 24,
48 or 72 h. At each time-point, the NPs were centrifuged at 67 × g
for 10 min. Their size and polydispersity index were measured.
Values are expressed as the mean ± standard deviation (n=3). TPGS,
D-alpha-tocopheryl polyethylene glycol 1000 succinate; DOX,
doxorubicin; HA, hyaluronic acid; NP, nanoparticle. |
 | Table I.Physicochemical characteristics of
NPs (mean ± SD, n=3). |
Table I.
Physicochemical characteristics of
NPs (mean ± SD, n=3).
| Formulation | Size (nm) | PDI | ZP (mV) | EE (%) | DL (%) |
|---|
| HA-TPGS
DOX-NPs | 172.3±6.5 | 0.16±0.04 | −39.5±3.3 | 90.2±2.8 | 7.6±0.3 |
| HA-TPGS NPs | 170.9±5.4 | 0.12±0.05 | −33.7±2.9 | N/A | N/A |
| TPGS DOX-NPs | 133.6±5.2 | 0.13±0.05 | −26.3±2.5 | 91.4±3.1 | 9.2±0.5 |
| DOX-NPs | 102.4±4.2 | 0.11±0.03 | −18.2±1.6 | 90.9±2.3 | 15.3±0.9 |
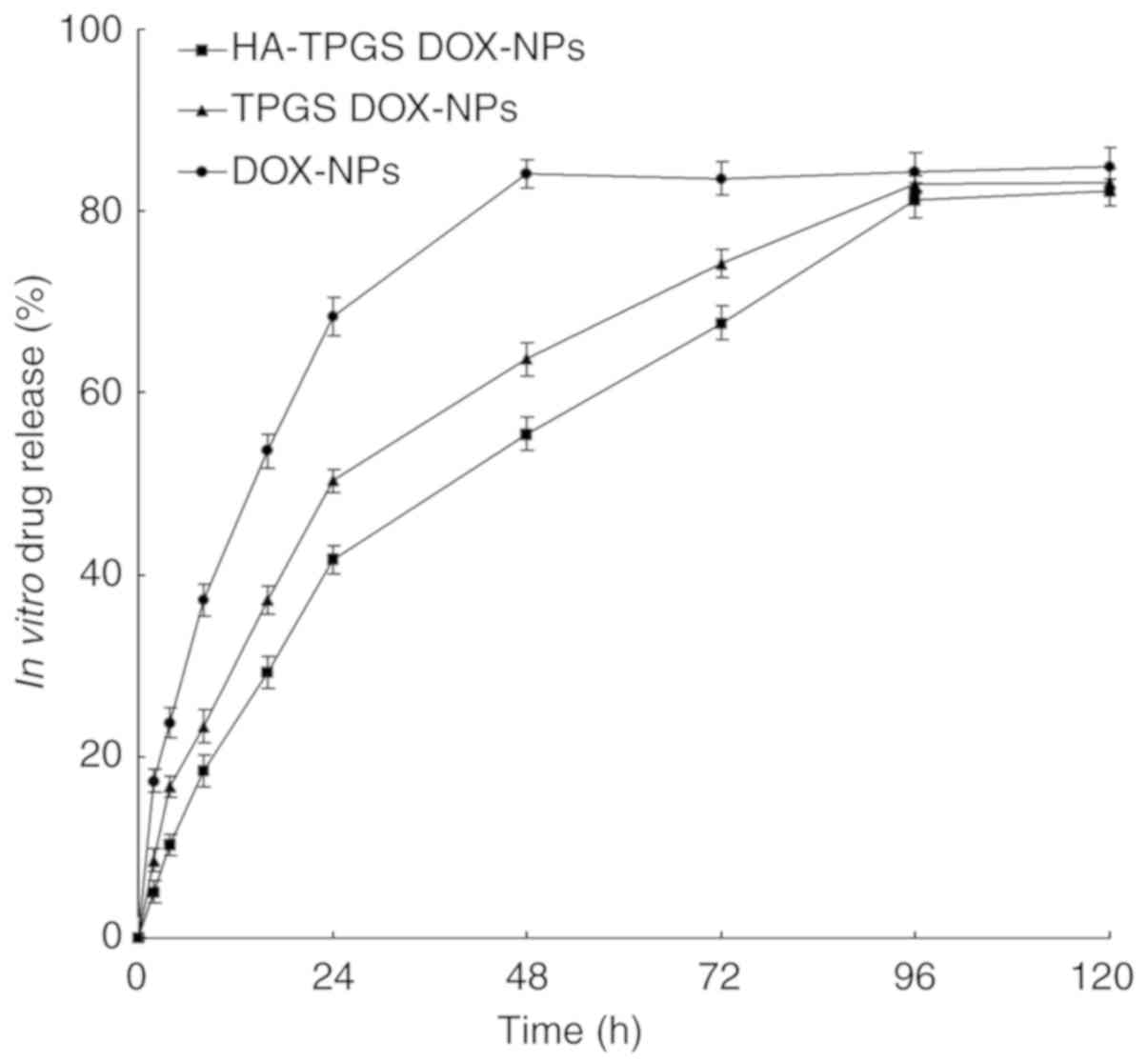
In vitro drug release
The release profiles of the NPs are presented in
Fig. 4. The release of drug from
DOX-NPs was faster than that from HA-TPGS DOX-NPs and TPGS DOX-NPs
(P<0.05). The release of DOX from DOX-NPs reached 90% within 48
h, compared with 96 h for HA-TPGS DOX-NPs and TPGS DOX-NPs,
indicating a more sustained drug release behavior of the modified
NPs. The most sustained release pattern observed for HA-TPGS
DOX-NPs may be attributed to the coating of HA and TPGS that made
the release of the drug slower.
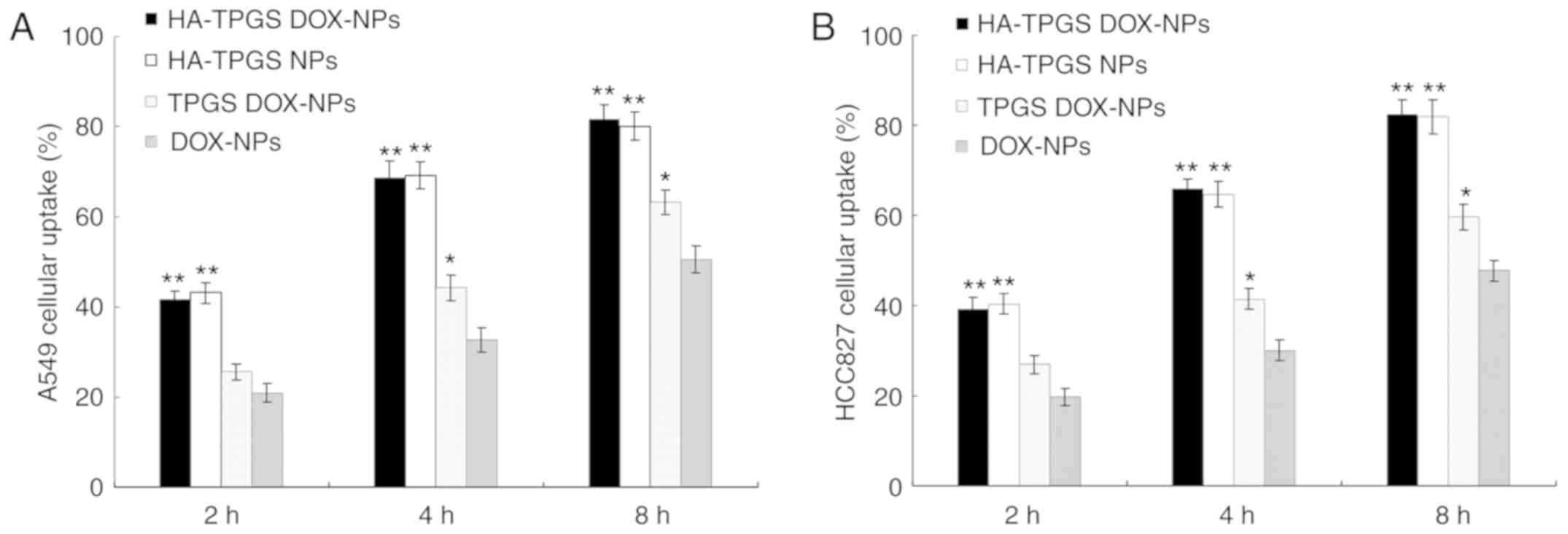
Cellular uptake assay
As indicated in Fig.
5, the uptake of NPs into A549 and HCC827 cells was increased
with time from 2 to 8 h. The cellular uptake efficiency of the
HA-modified HA-TPGS DOX-NPs and HA-TPGS NPs was markedly higher
than that of other NPs, reaching >80% at 8 h in either of the
two cell lines (P<0.01). The cellular uptake of TPGS DOX-NPs was
markedly higher than that of DOX-NPs at 4 and 8 h (P<0.05).
In vitro cytotoxicity
Table II presents
the in vitro cytotoxicity data of blank HA-TPGS NPs on A549,
HCC827 and MRC-5 cells at different concentrations. The cell
viability decreased along with the increase in the NP
concentration. Considering that the cell viability was >85% in
all groups, the NP system should be regarded as having low toxicity
and good biocompatibility.
 | Table II.Cytotoxicity evaluation of blank
HA-TPGS NPs at different concentrations (mean ± SD, n=6). |
Table II.
Cytotoxicity evaluation of blank
HA-TPGS NPs at different concentrations (mean ± SD, n=6).
| Concentration (µM)
(%) | 10 | 5 | 1 | 0.5 | 0.1 |
|---|
| A549 cell
viability | 86.9±3.7 | 88.4±2.9 | 90.1±2.6 | 93.2±2.9 | 94.1±2.2 |
| HCC827 cell
viability | 85.1±4.1 | 87.9±3.5 | 89.2±3.1 | 90.12±2.6 | 91.6±2.5 |
| MRC-5 cell
viability | 90.1±2.9 | 91.5±3.1 | 92.3±3.5 | 91.6±3.1 | 94.6±2.7 |
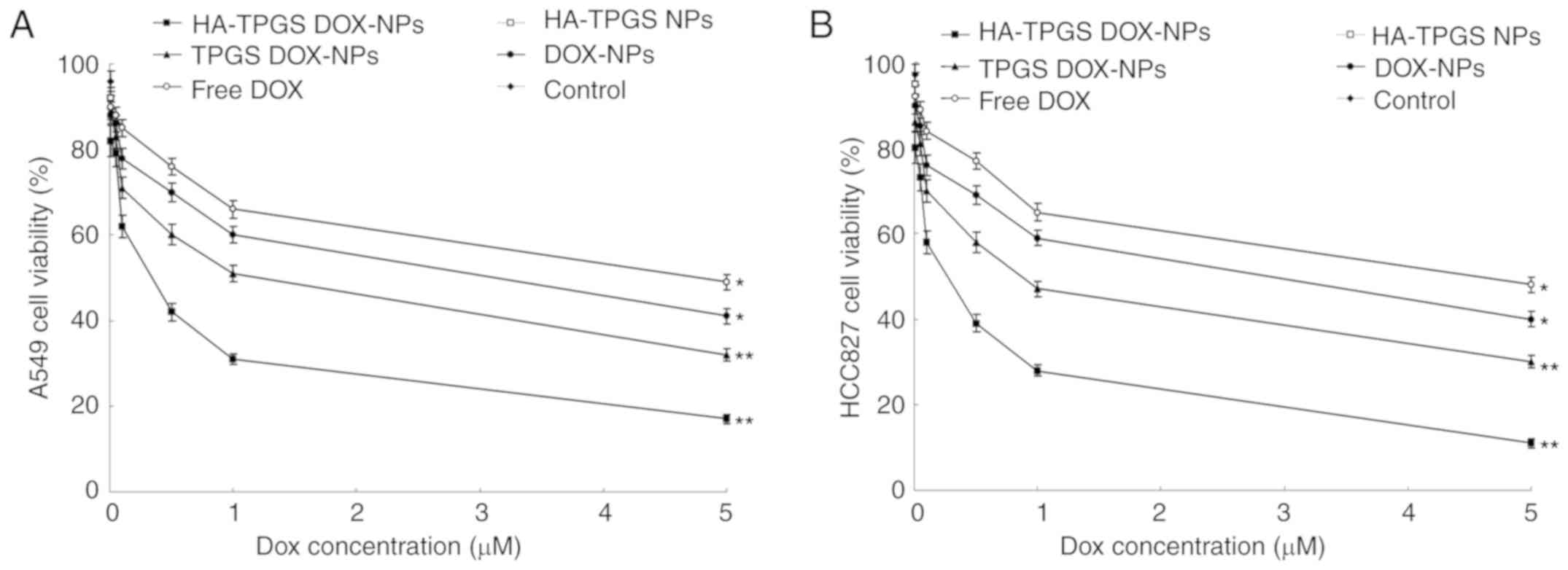
In vitro anticancer efficiency
As indicated in Fig.
6, the cell inhibitory effects of DOX-loaded NPs were
significantly higher than those of free DOX (P<0.05). HA-TPGS
DOX-NPs exhibited a significantly greater cytotoxicity than the
other NPs tested (P<0.05), and cell viabilities of 80.2, 73.4,
57.8, 39.1, 28.3 and 10.9% were measured after 72 h of incubation
with DOX-NPs with equivalent DOX concentrations of 0.01, 0.05, 0.1,
0.5, 1 and 5 µM, respectively. Notably, the inhibitory effect of
DOX-loaded NPs on HCC827 cells was greater than that on A549
cells.
In vivo antitumor efficiency
As presented in Fig.
7, the tumor volume of the mice treated with 0.9% saline
control or blank HA-TPGS NPs increased rapidly along with time and
the tumor growth curves were similar among these groups. Notably,
the tumor growth was effectively inhibited in all DOX-loaded NP
groups and slightly inhibited by free DOX (P<0.05). The TPGS
DOX-NPs exhibited a greater tumor growth inhibition efficiency
compared with that of DOX-NPs (P<0.05). The HA-TPGS DOX-NPs had
a more significant tumor suppression ability in comparison to the
other groups (P<0.01); after 28 days, the TIR of HA-TPGS
DOX-NPs, TPGS DOX-NPs, DOX-NPs and free DOX was 78.7, 55.4, 28.2
and 10.5%, respectively. The physical activity level and body
weight of the DOX-loaded NP treatment groups exhibited on obvious
change. Notably, body weight loss was identified in the free DOX
treatment, drug-free NPs and saline control groups.
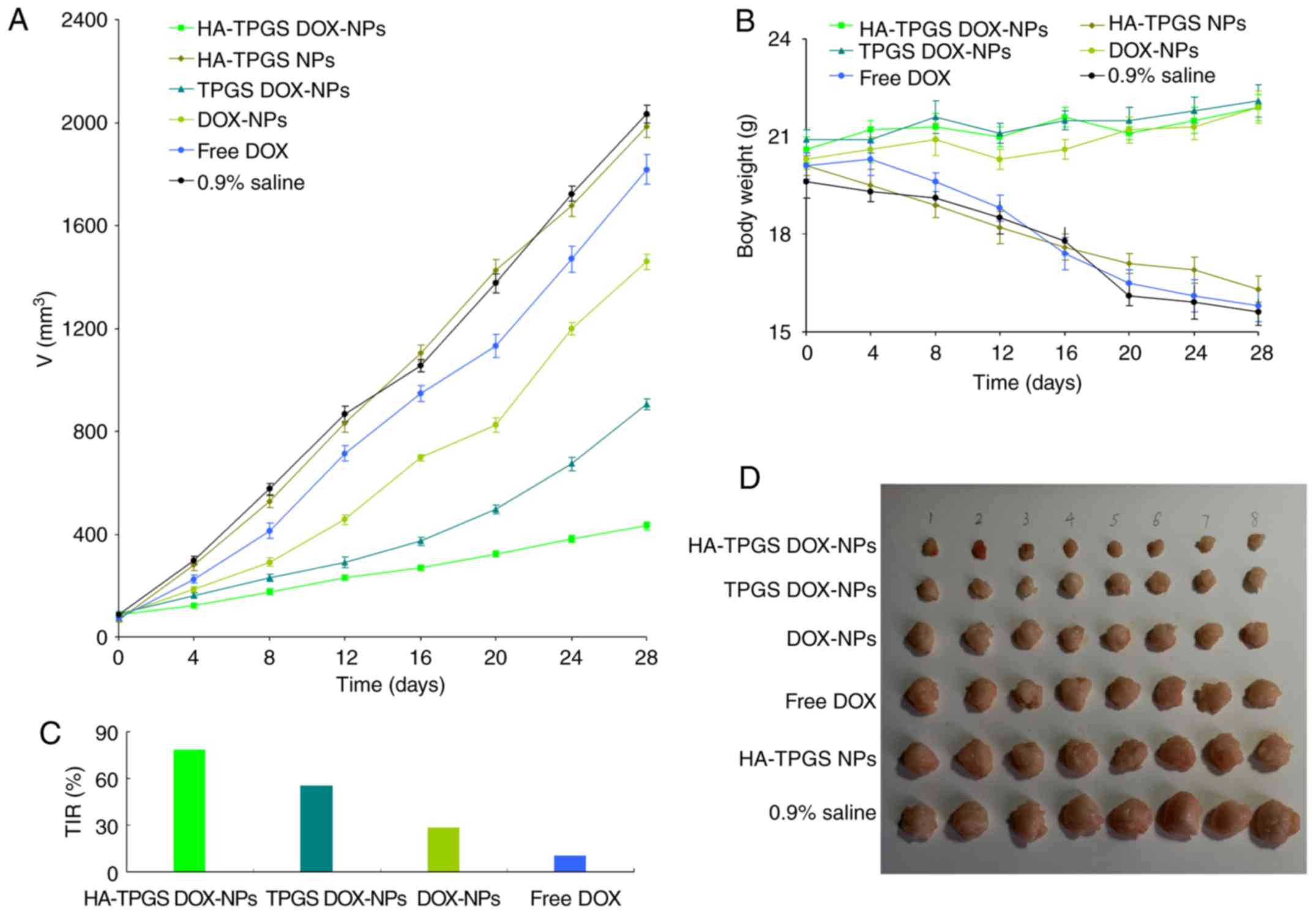 | Figure 7.In vivo antitumor efficiency
evaluated based on the (A) tumor volume, (B) body weight, (C) TIR
and (D) images of the tumors. After 1 week, mice with tumors
reaching a volume of ~100 mm3 were randomly divided into
6 groups (8 mice per group) and received 0.2 ml of HA-TPGS DOX-NPs,
HA-TPGS NPs, TPGS DOX-NPs, DOX-NPs, free DOX (equivalent DOX
concentration, 10 mg/kg) or 0.9% saline solution by intravenous
injection via the tail vein on days 0, 4, 8 and 12. The tumor was
assessed every 4 days with a Vernier caliper across its two
perpendicular diameters. The antitumor efficacy of samples was
evaluated by determining the TIR. Images of the tumors were
captured at the end of the study. Body weight changes were
monitored in order to evaluate the systemic toxicity of the
samples. Values are expressed as the mean ± standard deviation
(n=8). TIR, tumor inhibition rate; TPGS, D-alpha-tocopheryl
polyethylene glycol 1000 succinate; DOX, doxorubicin; HA,
hyaluronic acid; NP, nanoparticle. |
Discussion
Prodrugs are widely used for targeted delivery of
therapeutics to cancer cells (33).
Compared with the free active drug, prodrugs may feature a change
in the physicochemical nature of the drug and may achieve a marked
diversity for cancer therapy. In the present study, the
TPGS-S-S-DOX prodrug was constructed for the preparation of NPs
(34). Glutathione-sensitive
TPGS-S-S-DOX conjugate was designed, synthesized and characterized.
First, TPGS-S-S-GA was synthesized by EDC chemistry to conjugate
TPGS with S-S and GA. Subsequently, DOX was conjugated to
TPGS-S-S-GA to form TPGS-S-S-DOX. The chemical structure of
TPGS-S-S-DOX was confirmed using analytic methods.
PLA has been widely used for the preparation of drug
delivery systems of anticancer drugs due to its properties of
biodegradability, biocompatibility and low toxicity (35). Various methods, including
precipitation, emulsion and melting technique, have been used to
prepare PLA NP formulations. In the present study, the NPs were
self-assembled by using a dialysis method. After preparation, the
formulations were characterized. It was observed that the NPs made
of PLA displayed a negative charge of −18.2 mV that may be
attributed to the negatively charged groups of PLA. In comparison,
HA-TPGS DOX-NPs had a higher negative charge of −39.5 mV, which may
have arisen from the negatively charged HA added to PLA (36). Electron microscopy techniques,
including TEM, may easily provide the size of the NP core. However,
due to the low electron density of organic surface ligands, these
NPs are difficult to dissolve and DLS is a widely used technique
for size determination of NPs in suspension. DLS is able to measure
the hydrodynamic diameter of the NPs and determine the dimension of
the core plus shell (37). The TEM
images indicated that the size of the HA-TPGS DOX-NPs was <200
nm, while DLS suggested a size of 172 nm. PDI may be applied to
determine the size range and size distribution of the NPs (38). For polymer-based NP materials, PDI
values >0.2 are considered to have a narrow distribution. The
reason for the DL of HA-TPGS DOX-NPs being much lower than that of
DOX-NPs may be explained by the presence of HA-TPGS increasing the
weight of the carriers.
In vitro drug release of the drug-loaded NPs
may be controlled by erosion, corrosion and diffusion processes
(39). Drug depot effects may be
obtained by the carriers, which may lead to the sustained release
of hydrophobic drugs. TPGS contains a part of PEG1000
(40). PEG-modified NPs may exhibit
improved long-term circulating ability compared with the original
NPs (41). In vitro drug
release from NPs exhibits a sustained behavior; mechanistically,
this may be attributed to slow degradation of PLA and the release
of DOX from NPs depending on drug diffusion, erosion or swelling of
PLA (42). Furthermore, the HA-TPGS
shell on the outside of the polymer core has a shielding effect and
allows for slow and sustained drug release.
Cellular uptake of NPs was quantitatively
investigated by flow cytometry (42). The uptake of the NPs by A549 and
HCC827 cells increased in a time-dependent manner (43). The cellular uptake efficiency of
HA-TPGS DOX-NPs was significantly higher than that of non-modified
NPs; this may be due to the enhanced cancer cell-specific adherence
of the HA ligand. HA contained in NP systems may improve the
penetration of drugs, which may enhance the efficacy of the drugs,
reduce side effects and overcome drug resistance. In the present
study, the viability of cells treated with blank HA-TPGS NPs at
different concentrations was >85%, indicating low toxicity of
the NP system. Low cytotoxicity also indicates biocompatibility of
the system, rendering it suitable for administration (44).
The in vitro anticancer efficacy of the NPs
was evaluated by assessing cell growth inhibition via an MTS assay
(45). The highest cytotoxicity
among the different NPs achieved with HA-TPGS DOX-NPs may have been
due to the HA ligand targeting its receptor on the cancer cells,
the enhanced cellular uptake of the NPs and also the enhanced drug
release through cleavage of the GSH-responsive bond with the
carriers. A DOX dose-dependent anticancer activity was observed in
all of the DOX-containing formulations. The results demonstrated
that HA-TPGS DOX-NPs had a greater cytotoxic effect on the tumor
cells compared with that of non-HA-modified DOX-NPs and free DOX.
This may also be attributed to HA having excellent dispersibility
in aqueous solution, endowing the NPs with ‘stealth’ properties
(46).
The in vivo antitumor efficacy on the NPs was
then investigated in a lung cancer-bearing BALB/c mouse model. It
was demonstrated that HA-TPGS DOX-NPs had the greatest antitumor
effect without any severe adverse effects (body weight loss)
(47–49). This result may be attributed to
multiple factors. First, the NPs may have utilized the enhanced
permeability and retention effect of solid tumors, facilitating
targeted delivery of the drugs to the tumor site (50). Furthermore, a similar structure of
the lipid shell of the NPs to that of the cell membrane leads to
high affinity of the NP systems for the cells, leading to improved
drug delivery (51). In addition,
the S-S bonds in the prodrug may be rapidly cleaved by
intracellular reducing molecules, leading to the release of DOX to
the tumor site to achieve intracellular targeting. Finally, HA
modification of NPs may provide the best antitumor effect due to
the targeting ability of the HA to the receptor on the tumor cells.
The results indicated that HA-TPGS DOX-NPs had a more prominent
antitumor efficiency than free DOX, DOX-NPs and TPGS DOX-NPs. The
safety evaluation results indicated that NPs performed well in the
animal model.
In conclusion, HA-TPGS DOX-NPs were constructed and
characterized as a lung cancer therapy system for achieving
improved efficiency of DOX. It was demonstrated that HA-TPGS
DOX-NPs have significant antitumor effects and low systemic
toxicity in vitro and in vivo. The results indicate
that HA-TPGS DOX-NPs may be a promising treatment for lung
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant
from the ‘Thirteen-Five Plan’, the Major Program of Nanjing Medical
Science and Technique Development Fund (grant no. ZDX16012).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YZ, FL, GL and LC conceived and designed the study.
GL, LC, CZ, HX, KH, NX and BW performed the experiments. GL, LC,
KH, NX and BW wrote the manuscript. YZ, FL, GL and LC reviewed and
edited the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal experiments complied with the Guide for
the Care and Use of Laboratory Animals of the National Institutes
of Health (publication no. 8023; Bethesda, MD, USA) and approval
was obtained from the Institutional Animal Care and Treatment
Committee of Southeast University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aftab S, Shah A, Nadhman A, Kurbanoglu S,
Aysıl Ozkan S, Dionysiou DD, Shukla SS and Aminabhavi TM:
Nanomedicine: An effective tool in cancer therapy. Int J Pharm.
540:132–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu B, Han L, Liu J, Han S, Chen Z and
Jiang L: Co-delivery of paclitaxel and TOS-cisplatin via
TAT-targeted solid lipid nanoparticles with synergistic antitumor
activity against cervical cancer. Int J Nanomedicine. 12:955–968.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mura S, Bui DT, Couvreur P and Nicolas J:
Lipid prodrug nanocarriers in cancer therapy. J Control Release.
208:25–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mi Y, Zhao J and Feng SS: Vitamin E TPGS
prodrug micelles for hydrophilic drug delivery with neuroprotective
effects. Int J Pharm. 438:98–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anbharasi V, Cao N and Feng SS:
Doxorubicin conjugated to D-alpha-tocopheryl polyethylene glycol
succinate and folic acid as a prodrug for targeted chemotherapy. J
Biomed Mater Res A. 94:730–743. 2010.PubMed/NCBI
|
|
7
|
Xu Z, Liu S, Kang Y and Wang M:
Glutathione- and pH-responsive nonporous silica prodrug
nanoparticles for controlled release and cancer therapy. Nanoscale.
7:5859–5868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tjin CC, Otley KD, Baguley TD, Kurup P, Xu
J, Nairn AC, Lombroso PJ and Ellman JA: Glutathione-responsive
Selenosulfide prodrugs as a platform strategy for potent and
selective mechanism-based inhibition of protein tyrosine
phosphatases. ACS Cent Sci. 3:1322–1328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Liu D, Zheng Q, Zhao Q, Zhang H,
Ma Y, Fallon JK, Fu Q, Haynes MT, Lin G, et al: Disulfide bond
bridge insertion turns hydrophobic anticancer prodrugs into
self-assembled nanomedicines. Nano Lett. 14:5577–5583. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anjukandi P, Dopieralski P, Ribas-Arino J
and Marx D: The effect of tensile stress on the conformational free
energy landscape of disulfide bonds. PLoS One. 9:e1088122014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Zhang J, Wang Y and Chen M:
Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery
of doxorubicin and miR-542-3p for triple negative breast cancer
therapy. Nanomedicine. 12:411–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu W, Qian J, Zhang Y, Suo A, Cui N, Wang
J, Yao Y and Wang H: A double-network poly(Nε-acryloyl
L-lysine)/hyaluronic acid hydrogel as a mimic of the breast tumor
microenvironment. Acta Biomater. 33:131–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duhem N, Danhier F, Pourcelle V, Schumers
JM, Bertrand O, Leduff CS, Hoeppener S, Schubert US, Gohy JF,
Marchand-Brynaert J and Préat V: Self-assembling
doxorubicin-tocopherol succinate prodrug as a new drug delivery
system: Synthesis, characterization, and in vitro and in vivo
anticancer activity. Bioconjug Chem. 25:72–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Tan S and Feng SS: Vitamin E TPGS
as a molecular biomaterial for drug delivery. Biomaterials.
33:4889–4906. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao N and Feng SS: Doxorubicin conjugated
to D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS):
Conjugation chemistry, characterization, in vitro and in vivo
evaluation. Biomaterials. 29:3856–3865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su Z, Chen M, Xiao Y, Sun M, Zong L,
Asghar S, Dong M, Li H, Ping Q and Zhang C: ROS-triggered and
regenerating anticancer nanosystem: An effective strategy to subdue
tumor's multidrug resistance. J Control Release. 196:370–383. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Almeida PV, Shahbazi MA, Mäkilä E,
Kaasalainen M, Salonen J, Hirvonen J and Santos HA: Amine-modified
hyaluronic acid-functionalized porous silicon nanoparticles for
targeting breast cancer tumors. Nanoscale. 6:10377–30387. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parvani JG, Gujrati MD, Mack MA, Schiemann
WP and Lu ZR: Silencing β3 integrin by targeted ECO/siRNA
nanoparticles inhibits EMT and metastasis of triple-negative breast
cancer. Cancer Res. 75:2316–2325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen W, Liu W, Yang H, Zhang P, Xiao C and
Chen X: A glutathione-responsive sulfur dioxide polymer prodrug as
a nanocarrier for combating drug-resistance in cancer chemotherapy.
Biomaterials. 178:706–719. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen CT, Tran TH, Amiji M, Lu X and Kasi
RM: Redox-sensitive nanoparticles from amphiphilic
cholesterol-based block copolymers for enhanced tumor intracellular
release of doxorubicin. Nanomedicine. 11:2071–2082. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu K, Chen W, Yang T, Wen B, Ding D,
Keidar M, Tang J and Zhang W: Paclitaxel and quercetin
nanoparticles co-loaded in microspheres to prolong retention time
for pulmonary drug delivery. Int J Nanomedicine. 12:8239–8255,
20147. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JY, Kim JS, Cho HJ and Kim DD:
Poly(styrene)-b-poly(DL-lactide) copolymer-based nanoparticles for
anticancer drug delivery. Int J Nanomedicine. 9:2803–2813.
2014.PubMed/NCBI
|
|
23
|
Jiang SP, He SN, Li YL, Feng DL, Lu XY, Du
YZ, Yu HY, Hu FQ and Yuan H: Preparation and characteristics of
lipid nanoemulsion formulations loaded with doxorubicin. Int J
Nanomedicine. 8:3141–3150. 2013.PubMed/NCBI
|
|
24
|
Jia Y, Yuan M, Yuan H, Huang X, Sui X, Cui
X, Tang F, Peng J, Chen J, Lu S, et al: Co-encapsulation of
magnetic Fe3O4 nanoparticles and doxorubicin
into biodegradable PLGA nanocarriers for intratumoral drug
delivery. Int J Nanomedicine. 7:1697–1708. 2012.PubMed/NCBI
|
|
25
|
Li S, Wang L, Li N, Liu Y and Su H:
Combination lung cancer chemotherapy: Design of a pH-sensitive
transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel
and baicalin. Biomed Pharmacother. 95:548–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siu FY, Ye S, Lin H and Li S:
Galactosylated PLGA nanoparticles for the oral delivery of
resveratrol: Enhanced bioavailability and in vitro
anti-inflammatory activity. Int J Nanomedicine. 13:4133–4144. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan J, Wang Y, Jia Y, Liu S, Tian C, Pan
W, Liu X and Wang H: Co-delivery of docetaxel and curcumin prodrug
via dual-targeted nanoparticles with synergistic antitumor activity
against prostate cancer. Biomed Pharmacother. 88:374–383. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ruan C, Liu L, Lu Y, Zhang Y, He X, Chen
X, Zhang Y, Chen Q, Guo Q, Sun T and Jiang C: Substance P-modified
human serum albumin nanoparticles loaded with paclitaxel for
targeted therapy of glioma. Acta Pharm Sin B. 8:85–96. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arab-Bafrani Z, Shahbazi-Gahrouei D,
Abbasian M and Fesharaki M: Multiple MTS assay as the alternative
method to determine survival fraction of the irradiated HT-29 colon
cancer cells. J Med Signals Sens. 6:112–116. 2016.PubMed/NCBI
|
|
30
|
Pedrosa P, Mendes R, Cabral R, Martins
LMDRS, Baptista PV and Fernandes AR: Combination of chemotherapy
and Au-nanoparticle photothermy in the visible light to tackle
doxorubicin resistance in cancer cells. Sci Rep. 8:114292018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alibolandi M, Ramezani M, Abnous K,
Sadeghi F, Atyabi F, Asouri M, Ahmadi AA and Hadizadeh F: In vitro
and in vivo evaluation of therapy targeting epithelial-cell
adhesion-molecule aptamers for non-small cell lung cancer. J
Control Release. 209:88–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kou CH, Han J, Han XL, Zhuang HJ and Zhao
ZM: Preparation and characterization of the Adriamycin-loaded
amphiphilic chitosan nanoparticles and their application in the
treatment of liver cancer. Oncol Lett. 14:7833–7841.
2017.PubMed/NCBI
|
|
33
|
Giang I, Boland EL and Poon GM: Prodrug
applications for targeted cancer therapy. AAPS J. 16:899–913. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan S and Wang G: Redox-responsive and
pH-sensitive nanoparticles enhanced stability and anticancer
ability of erlotinib to treat lung cancer in vivo. Drug Des Devel
Ther. 11:3519–3529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee BK, Yun Y and Park K: PLA micro- and
nano-particles. Adv Drug Deliv Rev. 107:176–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mura S, Hillaireau H, Nicolas J, Le
Droumaguet B, Gueutin C, Zanna S, Tsapis N and Fattal E: Influence
of surface charge on the potential toxicity of PLGA nanoparticles
towards Calu-3 cells. Int J Nanomedicine. 6:2591–2605.
2011.PubMed/NCBI
|
|
37
|
Shang L, Nienhaus K and Nienhaus GU:
Engineered nanoparticles interacting with cells: Size matters. J
Nanobiotechnology. 12:52014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Danaei M, Dehghankhold M, Ataei S,
Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S and
Mozafari MR: Impact of Particle size and polydispersity index on
the clinical applications of lipidic nanocarrier systems.
Pharmaceutics. 10:E572018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu J, Cheng H, Han L, Qiang Z, Zhang X,
Gao W, Zhao K and Song Y: Synergistic combination therapy of lung
cancer using paclitaxel- and triptolide-coloaded lipid-polymer
hybrid nanoparticles. Drug Des Devel Ther. 12:3199–3209. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng W, Liang C, Xu L, Liu G, Gao N, Tao
W, Luo L, Zuo Y, Wang X, Zhang X, et al: TPGS-Functionalized
polydopamine-modified mesoporous silica as drug nanocarriers for
enhanced lung cancer chemotherapy against multidrug resistance.
Small. 13:2017. View Article : Google Scholar
|
|
41
|
Rafiei P and Haddadi A: Docetaxel-loaded
PLGA and PLGA-PEG nanoparticles for intravenous application:
Pharmacokinetics and biodistribution profile. Int J Nanomedicine.
12:935–947. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nassireslami E and Ajdarzade M: Gold
coated superparamagnetic iron oxide nanoparticles as effective
nanoparticles to eradicate breast cancer cells via photothermal
therapy. Adv Pharm Bull. 8:201–209. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang L, Li X, Liu L and Zhang Q: Cellular
uptake mechanism and intracellular fate of hydrophobically modified
pullulan nanoparticles. Int J Nanomedicine. 8:1825–1834.
2013.PubMed/NCBI
|
|
44
|
Guo F, Wu J, Wu W, Huang D, Yan Q, Yang Q,
Gao Y and Yang G: PEGylated self-assembled enzyme-responsive
nanoparticles for effective targeted therapy against lung tumors. J
Nanobiotechnology. 16:572018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schrand AM, Lin JB and Hussain SM:
Assessment of cytotoxicity of carbon nanoparticles using
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) cell viability assay. Methods Mol Biol. 906:395–402.
2012.PubMed/NCBI
|
|
46
|
Garg A, Rai G, Lodhi S, Jain AP and Yadav
AK: Hyaluronic acid embedded cellulose acetate phthlate core/shell
nanoparticulate carrier of 5-fluorouracil. Int J Biol Macromol.
87:449–459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mei L, Fu L, Shi K, Zhang Q, Liu Y, Tang
J, Gao H, Zhang Z and He Q: Increased tumor targeted delivery using
a multistage liposome system functionalized with RGD, TAT and
cleavable PEG. Int J Pharm. 468:26–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dong Z, Guo J, Xing X, Zhang X, Du Y and
Lu Q: RGD modified and PEGylated lipid nanoparticles loaded with
puerarin: Formulation, characterization and protective effects on
acute myocardial ischemia model. Biomed Pharmacother. 89:297–304.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Naeem M, Bae J, Oshi MA, Kim MS, Moon HR,
Lee BL, Im E, Jung Y and Yoo JW: Colon-targeted delivery of
cyclosporine A using dual-functional Eudragit®
FS30D/PLGA nanoparticles ameliorates murine experimental colitis.
Int J Nanomedicine. 13:1225–1240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hwang H, Jeong HS, Oh PS, Kim M, Lee TK,
Kwon J, Kim HS, Lim ST, Sohn MH and Jeong HJ: PEGylated
nanoliposomes encapsulating angiogenic peptides improve perfusion
defects: Radionuclide imaging-based study. Nucl Med Biol.
43:552–558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim CE, Lim SK and Kim JS: In vivo
antitumor effect of cromolyn in PEGylated liposomes for pancreatic
cancer. J Control Release. 157:190–195. 2012. View Article : Google Scholar : PubMed/NCBI
|