Introduction
According to the latest 2018 cancer statistics for
USA, breast cancer (BC) is the most common malignant tumor type in
females, accounting for 30% of estimated new diagnoses (1). Simultaneously, the mortality rate of
females with malignant breast tumors is second only to those with
lung cancer in USA in 2011–2015 (1,2).
Additionally, in economically developed countries and regions, BC
is prevalent. According to the latest reports in USA, there are
~250,000 new cases annually, and the mortality toll is estimated at
411,000 annually in 2017 (3,4).
However, as economies develop further and living standards improve,
the incidence of BC increases year after year (5). Globally, ~1.7 million females are
diagnosed with BC annually, resulting in >500,000 mortalities
annually, which makes BC the most common malignant tumor type
affecting the lives and health of females, not only in USA, but
also globally in 2012 (5). At
present, BC treatment primarily includes surgery, chemotherapy,
endocrine therapy, radiotherapeutics and biotherapy (6,7).
Nevertheless, the incidence and mortality of BC remain high, and BC
still affects the quality of life of females (8). At present, scholars have reached a
consensus that BC, similar to the majority of cancer types, is
considered a specific gene-associated disease, which means that the
pathogenic process of BC involves a multi-factor, multi-stage and
multi-step complex effect (9). With
research deepening on the level of gene molecules and screening for
differentially-expressed genes (DEGs) associated with BC, research
on the functional annotation and participating signaling pathways
of DEGs helps to improve understanding regarding BC pathogenesis.
These in-depth studies notably assist in predicting the recurrence,
metastasis and prognosis of BC, which also provides a theoretical
basis for individualized precision treatment. The urgent
requirement for the early diagnosis and treatment of BC, as well as
for improving the prognosis and reducing the mortality rate, has
led researchers to shift focus to the molecular levels of BC,
aiming to determine its pathogenic mechanisms and to provide an
evidence-based foundation for targeted therapy.
The present study attempts to screen and analyze
BC-relevant gene expression profiles in the Gene Expression Omnibus
(GEO) and The Cancer Genome Atlas (TCGA) databases by computational
biology. Following consulting a large body of literature on the
subject and using computational biology statistical calculations,
COL5A1 was determined to be a crucial gene in the oncogenesis of BC
(10,11). However, coverage of the clinical
value of COL5A1 remains insufficient (12). In the present study, computational
biology-associated methods are used to mine the expression levels
of associated genes in the GEO database and analyze their
prognostic value with numerous methods. To support the calculation
of the biological data results, immunohistochemical methods are
used to verify whether the expression level of COL5A1 in BC tissue
is increased, compared with normal breast tissue. Furthermore, the
association between the COL5A1 gene and the clinicopathological
features and prognosis of BC were determined, as well as the
clinical significance of COL5A1 alterations (Fig. 1).
Methods and materials
mRNA and protein expression levels of
COL5A1
Clinical data collection of COL5A1
mRNA expression
The GEO database (https://www.ncbi.nlm.nih.gov/geo/) is the largest and
most comprehensive public gene expression database available today
(13). The BC gene probe expression
matrix and probe annotation file were mined from the GEO dataset,
and probes corresponding to the COL5A1 gene were located based on
the probe annotation file. According to the data requirements for
BC, the present study screened the chips in the GEO database. The
key word was set as ‘breast cancer’, and the chip filter conditions
were as follows: i) Human breast tissue; ii) comparative RNA
expression data chip with BC tissue and paracancer or normal breast
tissue; iii) invasive BC sample; and iv) patients without adjuvant
therapies, including radiotherapy and chemotherapy. The exclusion
conditions were as follows: i) Cell line; ii) methylation; iii)
samples that have been treated with adjuvant therapies; and iv)
cancer samples without comparison of paracancer or normal tissue.
Considering that the specific classification of BC is associated
with its prognosis, all selected GEO chips underwent secondary
screening. Invasive BC is divided into the invasive non-specific
and invasive specific types, in which non-specific BC is subdivided
into invasive ductal carcinoma (IDC) and invasive lobular carcinoma
(ILC) (8). The criteria
aforementioned currently applies to the classification of each BC
case. The lack of subdivisions for invasive specific BC may be due
to fewer clinical cases, and, if necessary, the present study
uniformly classified it as invasive specific type. If there was no
specific classification in the GEO chip, the sample was included as
an invasive BC type. A corresponding histogram was then created
using GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA,
USA) to depict the difference in COL5A1 expression levels in
invasive BC and normal tissues from each piece of GEO chip
data.
The Kaplan-Meier plotter (http://kmplot.com/analysis/index.php?p=service)
(14,15) is an online drawing tool used to
evaluate the influence of 54,685 genes on survival by mining
information from 10,471 samples collected from 5,243 patients with
BC, along with detailed survival data originating in the European
Genome-phenome Archive, TCGA and the GEO (Affymetrix microarray
only) databases. Additionally, the clinical values of specific
genes were analyzed by the Kaplan-Meier plotter with survival
curves. Gene Expression Profiling Interactive Analysis (GEPIA)
(http://gepia.cancer-pku.cn/) (16–18)
collected 9,736 tumor samples from the TCGA and Genotype-Tissue
Expression databases and constituted a web tool for analyzing the
RNA sequencing expression data. In the research, the expression
level of COL5A1 and the prognostic value material were obtained via
GEPIA and Kaplan-Meier plotter.
Prediction of clinical significance of
COL5A1 genetic alteration
The cBioPortal (http:/cbioportal.org/) (19,20),
which provides data from 20 types of cancer studies including
>5,000 tumor samples, was utilized to investigate the clinical
significance of COL5A1 genetic alteration in BC, including detailed
information regarding the mutation of COL5A1 in multiple samples,
including genetic sites, mutation types and amino acid changes. The
Kaplan-Meier survival estimate of COL5A1 in patients with BC was
also analyzed using cBioPortal.
Potential mechanism of COL5A1 in
BC
The co-expressed genes associated with COL5A1 were
obtained to investigate the possible mechanism of COL5A1 in BC. The
relative genes were processed with KEGG pathway enrichment and GO
functional annotation through WebGestalt (http://www.webgestalt.org/option.php), which is a tool
to perform functional enrichment analysis (21,22).
The KEGG enrichment analysis was performed with COL5A1-associated
genes using the ggplot2 package in R (23). For further investigation, the
biological process of cellular component organization, which had
the most accumulation and alteration rate of co-expressed genes
associated with COL5A1, was selected to analyze and discuss the
potential mechanism of BC.
Meta-analysis of COL5A1 mRNA
expression
To conduct a holistic evaluation of COL5A1
expression levels, Stata 12.0 (StataCorp LP, College Station, TX,
USA) was used for the meta-analysis of continuous variables.
Additionally, if the meta-analysis was revealed to be
heterogeneous, sources of heterogeneity were further identified
through sensitivity analysis. After removing the chips that may
cause heterogeneity in the meta-analysis, the remaining chips were
re-analyzed to confirm that heterogeneity had been excluded.
Heterogeneity indicated that the results of GEO data were
inconsistent, which may result in bias in the results of
meta-analysis. Provided that the heterogeneity value of the results
was <50%, the results of the meta-analysis were considered
credible. Finally, if the standard mean difference (SMD) was <0,
and 95% confidence interval (CI) that did not cross the 0-point
coordinate line (0.60-1.07), then the target gene was
differentially expressed in BC tissues relative to the normal
control group. Furthermore, Deeks' test was conducted to identify
any sources of publication bias.
Additionally, the expression of COL5A1 was imported
into IBM SPSS 22.0 (IBM Corp., Armonk, NY, USA) to calculate the
number of true positive (TP), true negative (TN), false positive
(FP) and false negative (FN) cases. In the calculation process,
cancer tissue was defined as the control group for the gene highly
expressed in BC tissue. GraphPad Prism 7.0 was used to plot the
Receiver Operating Characteristic (ROC) curves to show the
potential diagnostic value of the target gene in each chip.
Finally, a summary ROC curve (sROC) was plotted using Stata 12.0 to
further evaluate the potential diagnostic value of the gene of
interest as a whole. The aforementioned steps were repeated for all
sub-types of BC to further study the association of COL5A1 these
subtypes. Rversion 3.4.1 was used to statistically analyze the
relationship between CLO5A1 expression levels and clinical
parameters, molecular typing in BC cases. Ggplot2 presenting as a
drawing supplement package that can be applied to R software and
was used to graphically display differential genes in potential
pathway analysis.
Immunohistochemical verification
Source of experimental material
The study protocol was approved by The Ethical
Committee of First Affiliated Hospital of Guangxi Medical
University (Nanning, China). A total of 136 cases were included in
the present study, all of which were female, aged 29–79 years old,
with a median age of 47 years old. The tissue collection location
was the breast cancer and adjacent tissue. All tissue samples were
collected from the Department of Pathology of the First Affiliated
Hospital of Guangxi Medical University between January and December
2012.
Archived sections of patients with invasive ductal
breast carcinoma who underwent surgical resection and pathological
confirmation from January 2012 to December 2012 were selected as
experimental material. The selection criteria for the experimental
samples were as follows: i) Female patients; ii) patients diagnosed
with invasive breast carcinoma by the World Health Organization's
2012 fourth edition of the Breast Tumor Histological Classification
(24); iii) patients with
well-preserved clinical pathology data via estrogen (ER),
progesterone (PR) and oncogenes [human epidermal growth factor
receptor-2 (HER-2), P53 and Ki-67] immunohistochemical staining;
iv) patients who had not received adjuvant therapy, including
radiotherapy, chemotherapy and endocrine therapy prior to the
operation; v) patients for whom the recorded breast carcinoma was
their first-discovered primary tumor; and vi) patients who received
standardized treatments following operation. Exclusion criteria
were as follows: i) Male patients; ii) patients without complete
clinical records or complete ER, PR, HER-2, P53 and Ki-67
immunohistochemical staining or no clinical staging; and iii)
patients whose BC tissues in the archived paraffin specimens were
too small to be re-cut (25–27).
Determination principles of
immunohistochemistry in experimental samples
Human bladder collagen tissue was used as a positive
control for COL5A1, and PBS was used as a negative control
replacing the primary antibody. Each of the specimens was strictly
prepared according to the manufacturer's protocols of a mouse and
rabbit specific HRP/DAB (ABC) Detection immunohistochemistry kit
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and each stain was
provided with a positive control and a blank control.
The fixative solution of tissue specimens was 10%
neutral formalin solution, the amount of fixative solution was 5–10
times of the volume of the specimen, and the fixed time of the
specimens was 12–24 h at room temperature 15–28 °C. The specimens
were embedded in Spurr resin to form a paraffin block.
Immunohistochemistry was performed on 4 µm sections of the tissue
paraffin block. They were deparaffinized in xylene at 37°C and
rinsed in medicinal-graded ethanol (100, 95, 85, 75 and 50%).
Subsequently, 3% H2O2 prepared fresh from
distilled water was used to inactivate endogenous peroxidase, which
lasted for 15 min at room temperature and was repeatedly soaked in
PBS solution. Antibody reaction used rabbit anti-human concentrated
polyclonal COL5A1 antibody (dilution, 1:1,000; cat. no. HPA030769;
Sigma-Aldrich; Merck KGaA) in 37°C for 1 h, and then thoroughly
flushed by PBS solution. After washed, slides were incubated with
fast enzyme-labeled goat anti-mouse/rabbit IgG polymer (cat. no.
KIT-5030; Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) as
secondary antibody in 37°C for 30 min. Finally, a freshly prepared
DAB colorant (cat. no. DAB-1031; Fuzhou Maixin Biotech Co., Ltd.)
was used for dyeing. The color developing effect was observed with
a light microscope with an OLYMPUS VANOX micrographic system
(magnification, ×100 and ×200).
There were two experienced pathologists who read
each film by a double-blind method. If the results of the two
pathologists' interpretations had been inconsistent, a third
pathologist would have been asked to review the film. Positive
controls were required to exhibit positive results for each test,
and negative controls were required to exhibit negative
results.
The results of the immunohistochemical staining were
mostly consistent with the relevant literature (28). Immunohistochemical results of
sections were evaluated according to the following established
criteria. The evaluation standards were as follows: i) Results were
scored according to color intensity (I) (light yellow was one
point; yellow was two points; and yellow-brown was three points);
ii) results were digitized (1–100%) in accordance with the
percentages (P) that positive cells occupied; and iii) the two
numbers were multiplied to obtain Q, which meant that Q=PxI. When
the score was Q=0, it was considered to be negative; when the score
was 0<Q≤120, it was considered to be weakly positive; when the
score was 120<Q≤210, it was considered to be moderately
positive; and when the score was 210<Q≤300, it was considered to
be strongly positive. This experiment defined negative and weakly
positive as low-level expression levels, and moderately positive
and strongly positive as high-level expression levels.
Prognosis and patient follow-up
The return visits of the patients were assessed with
the hospital management information system. For those who did not
return in the course of the follow-up period, the patients or their
family members were provided relevant follow-up information by
telephone. The survival time of all patients was counted from the
day of surgery to March 15, 2018. In the event that the patient
succumbed, or was lost to follow-up, the date of mortality or loss
to follow-up was considered the end date.
Data analysis
The experimental data were statistically analyzed
and plotted using SPSS 22.0, R version 3.4.1 (https://www.r-project.org/), GraphPad Prism 7.0 and
Stata 12.0 software. All results are presented as mean ± standard
deviation. SPSS 22.0 was applied to calculate the χ2 of
the COL5A1 protein expression levels, as well as the association
between the clinicopathological features and the expression of
antibodies in BC. The correlation analysis of antibodies and
clinicopathological parameters was conducted using Spearman's rank
correlation test. The Kaplan-Meier plotter was also used to perform
survival analysis to demonstrate the association between COL5A1 and
BC prognosis. P<0.05 was considered to indicate a statistically
significant difference. The independent sample t-test was used to
compare the expression levels of COL5A1 between two continuous
variables. Student's t-test was used to compare the expression
levels between the two paired variables. One-way ANOVA analysis of
variance was used to compare more than two different groups.
Multiple comparisons between the groups was performed using the
Student-Newman-Keuls method. All expression data were
log2 (TPM+1) transformed for differential analysis.
Protein validation of COL5A1
expression
The immunohistochemical results were verified via
the Human Protein Atlas (HPA; http://www.proteinatlas.org/) (29,30).
The website aims at mapping the distribution of human proteins in
cells, tissues and organs using integration technologies. To ensure
the staining results were sufficiently representative, all tissues
examined were derived from 144 different individuals and 216 tumor
tissues, and immunohistochemical techniques were used to detect the
distribution and expression of COL5A1 in 48 normal human tissues,
20 tumor tissues, 47 cell lines and 12 blood cells (24–26).
Results
Association of COL5A1 mRNA expression
level and clinical significance
Overall, 20 GEO chip data were included after a
preliminary screening in the present study. The chip GSE61723,
without COL5A1 expression data, was excluded. According to the gene
chip probe annotation file, the target gene, COL5A1, corresponded
with the probes 203325_s_at, 212488_at and 212489_at, and the
investigation of COL5A1 the expression value was based on the
arithmetic mean value of the three probes above. According to the
established principles, seven microarrays containing IDC were
screened, including GSE5764, GSE10780, GSE15852, GSE21442,
GSE22544, GSE36295 and GSE61304. The microarrays containing ILC
were GSE5764 and GSE15852. Due to the lack of adequate data for
invasive specific BC, the expression analysis could not be
performed. Following integrating the data, GraphPad Prism 7.0 was
used to pool the box charts, which directly demonstrated the
difference in the expression levels of COL5A1 between BC and normal
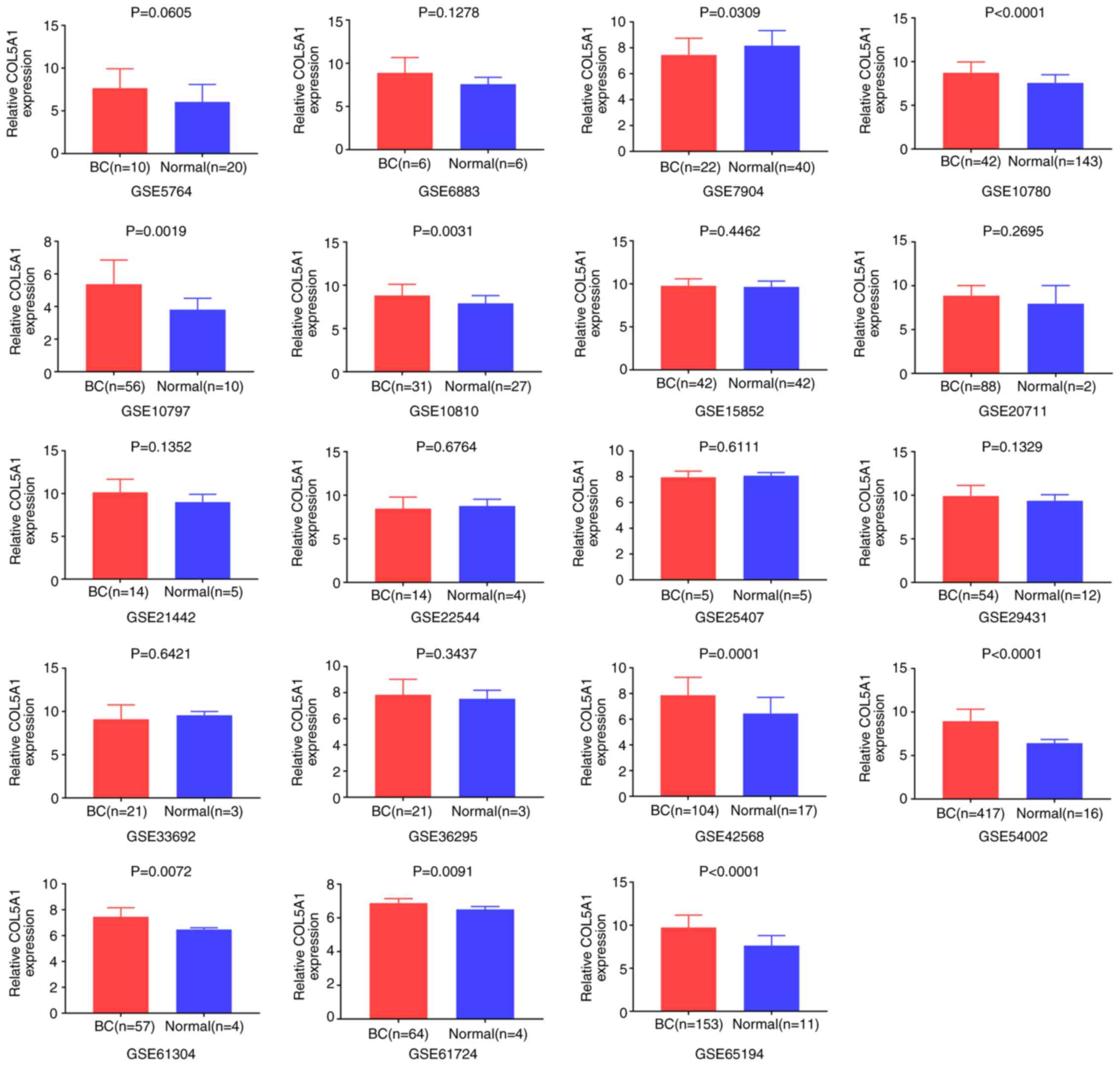
tissues. In the overall data analysis of 19 GEO chips, the results
revealed that eight selected COL5A1 chips exhibited significantly
increased expression in carcinoma tissues, compared with normal
tissues (P<0.05; Fig. 2).
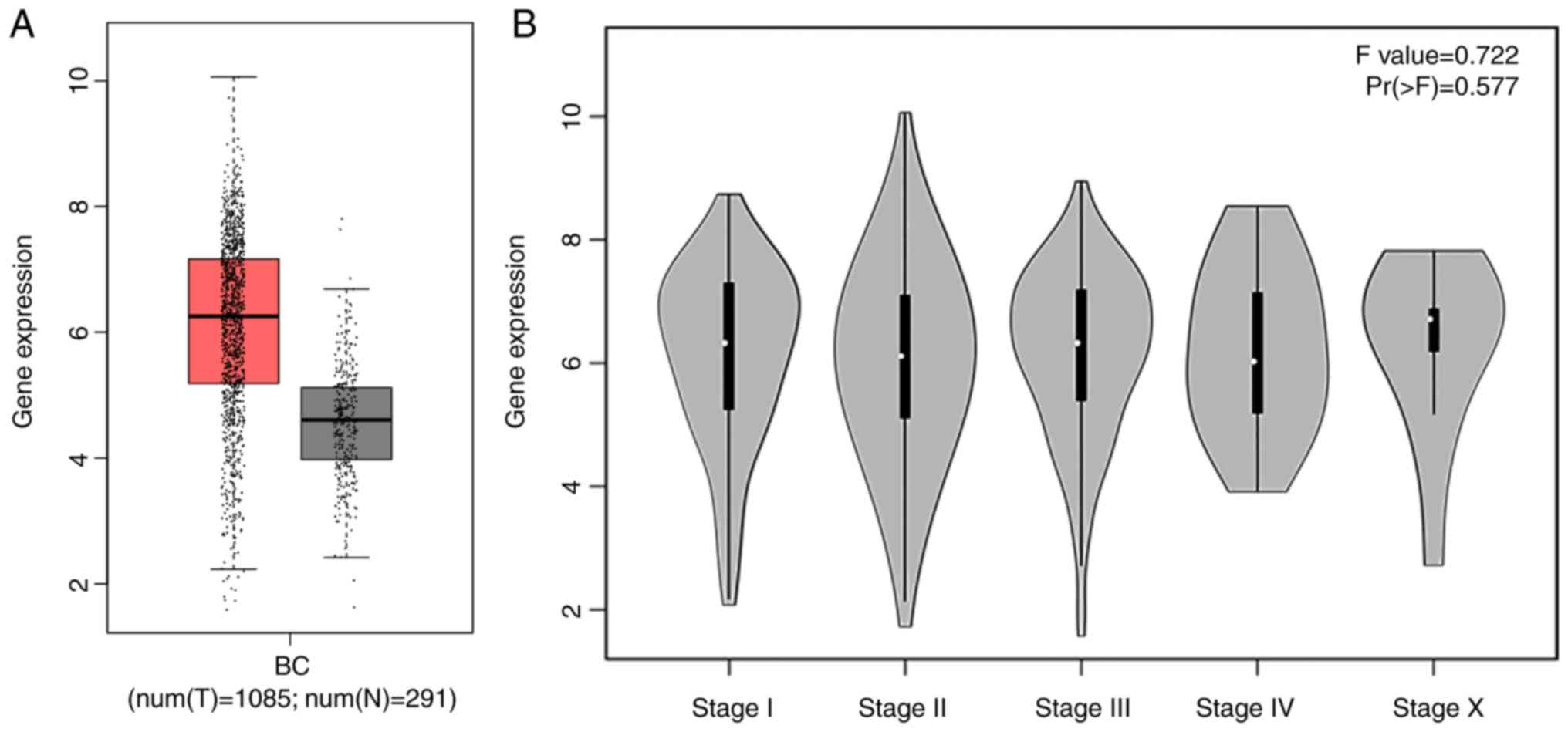
Additionally, the box chart of the overall COL5A1
expression level indicated that the gene expression was
significantly increased in cancerous BC tissues, compared with
non-cancerous BC tissues (Fig. 3A).
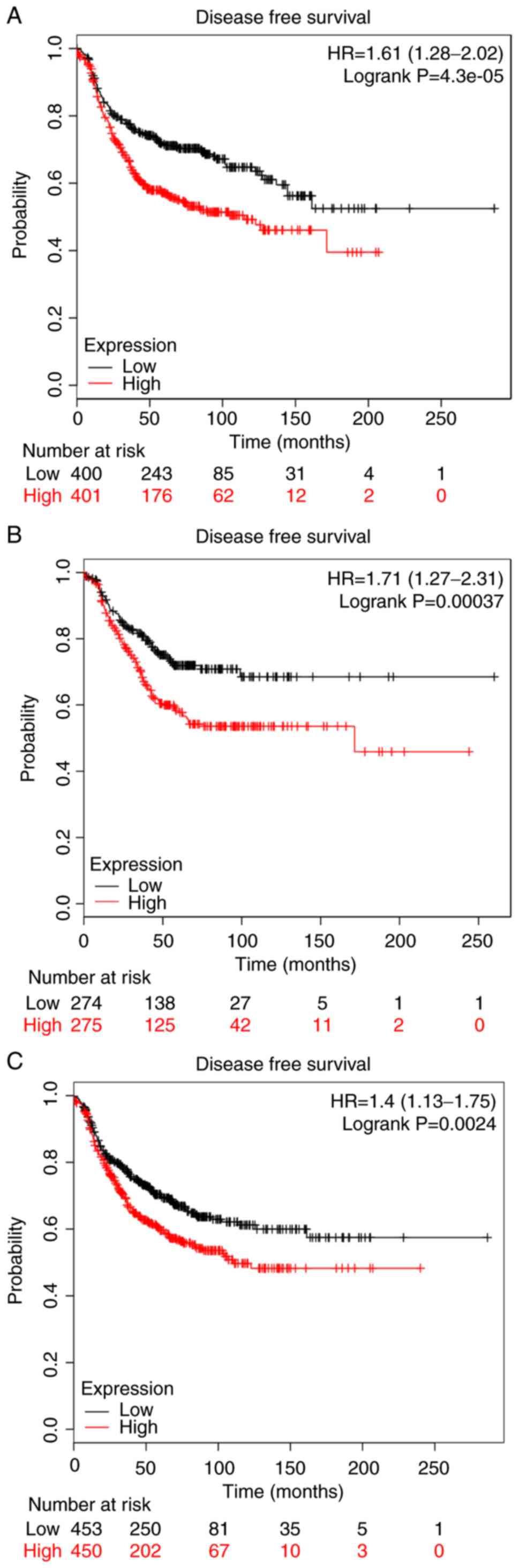
The clinical BC stage made no significant difference on COL5A1
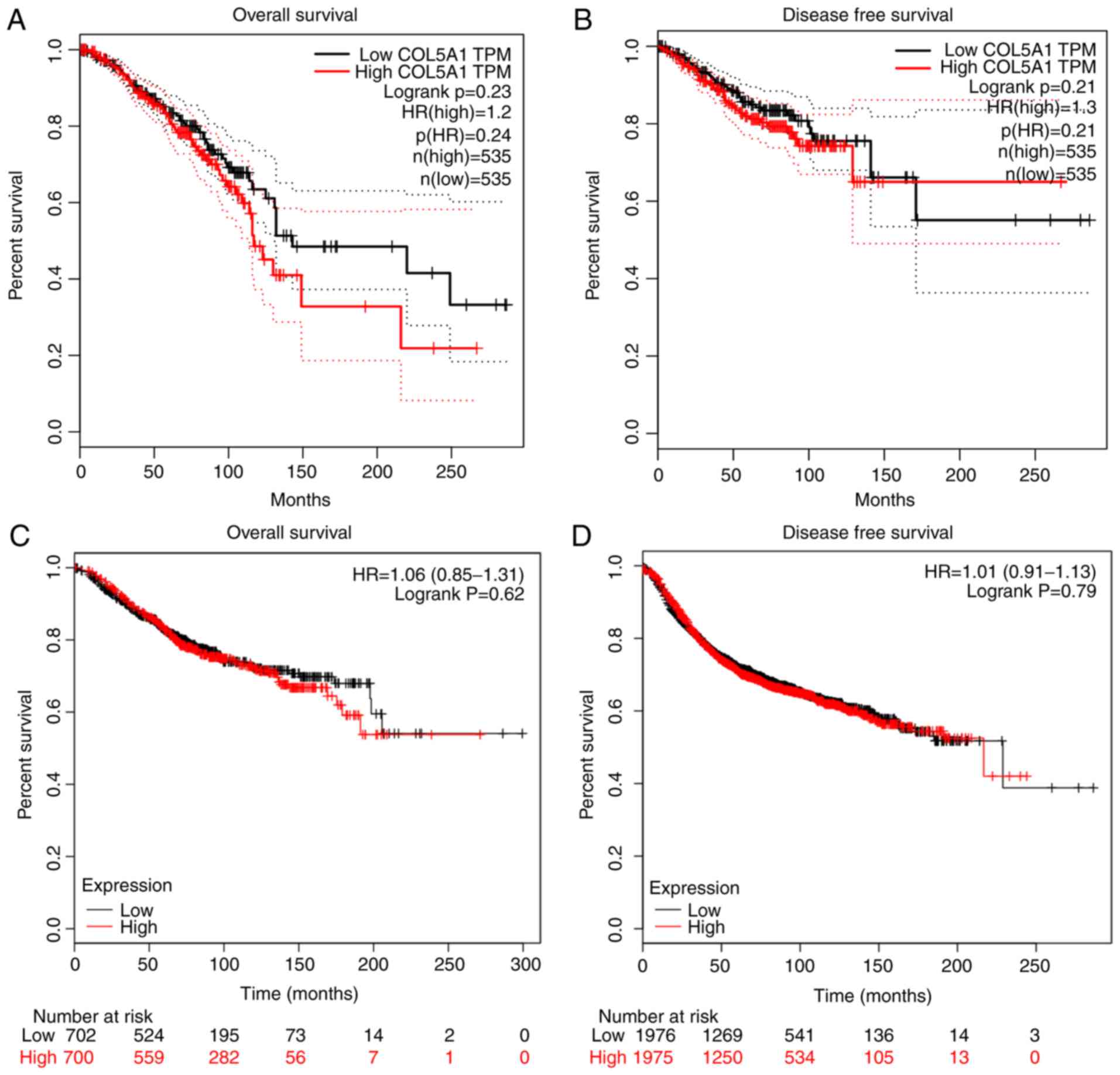
expression (Fig. 3B). The survival
prognoses of COL5A1 in BC, plotted by GEPIA and the Kaplan-Meier
plotter, demonstrated that there was no significant difference in
the overall survival (OS) and disease-free survival (DFS) time
(Fig. 4). Nevertheless, a
difference was observed in the curves between low and high
expression levels. To further investigate the association between
clinical parameters and BC, ER negative (P=4.3×10−5) and
PR negative (P=0.00037) were determined to be significant with DFS
in BC, as well as histological grade III (P=0.0024) (Fig. 5), while there was no direct
statistical evidence to reveal the association between ER, PR and
HER-2, and OS.
Clinical significance of COL5A1
genetic alteration
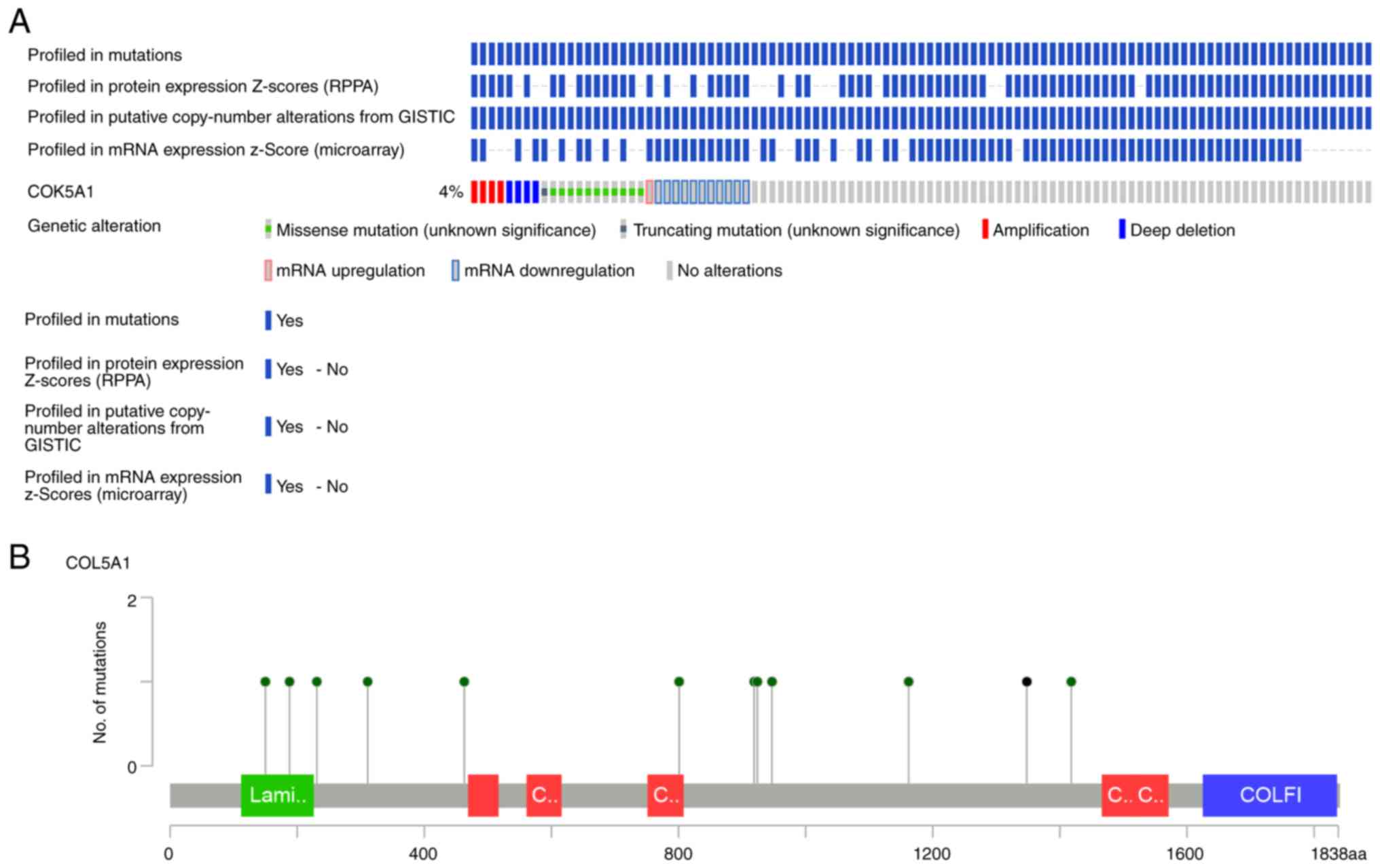
Mining the OncoPrint data demonstrated that COL5A1
altered in 32/817 (4%) sequenced samples within four cases of
amplification, 11 cases of missense mutation, four cases of deep
deletion, one case of mRNA upregulation and 11 cases of mRNA
downregulation (Fig. 6A).
Summarizing and analyzing the data revealed the somatic mutation
frequency of COL5A1 to be 1.5%, and missense mutations were most
prominent in BC (Fig. 6B). From the
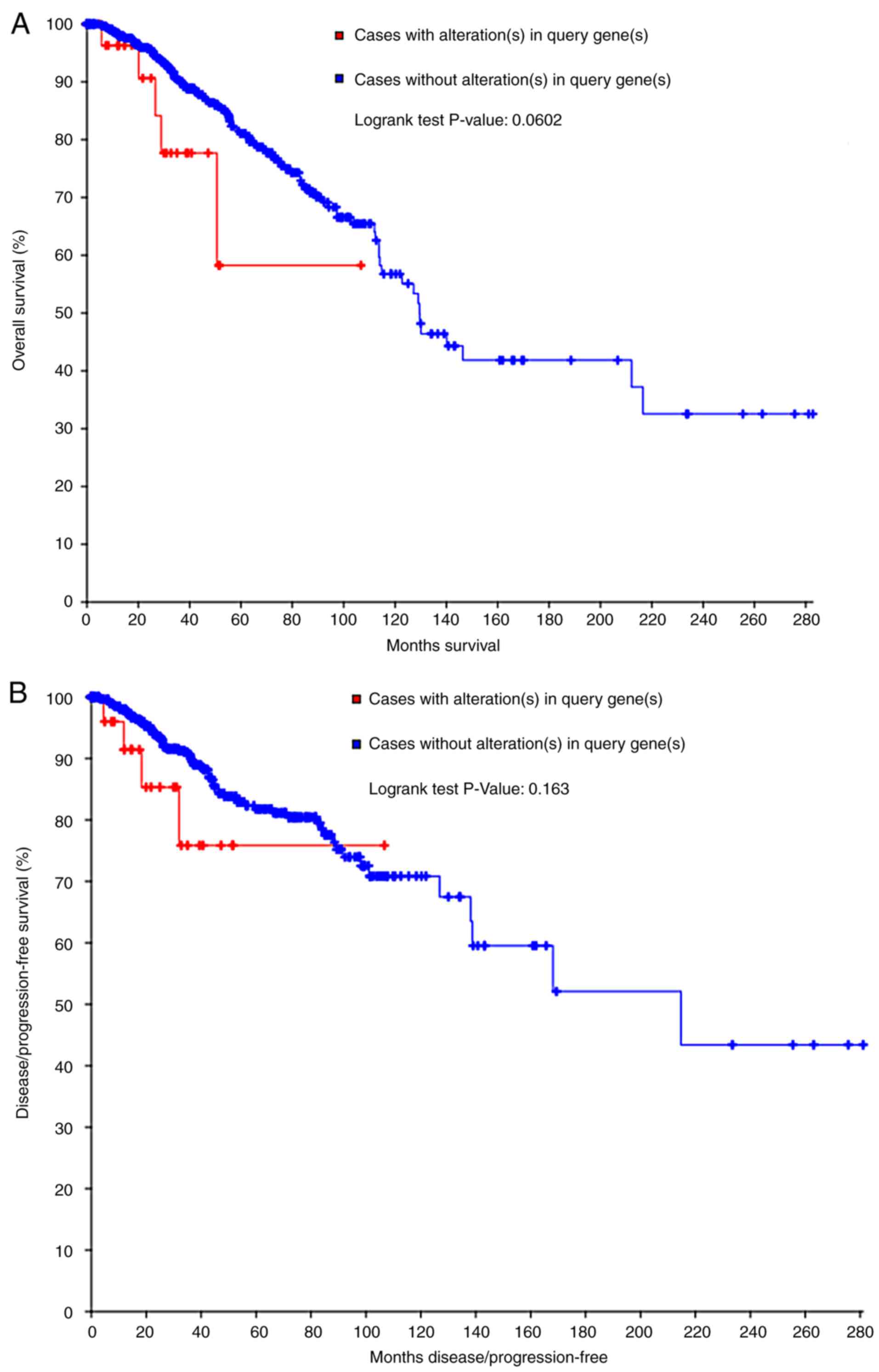
survival curves, neither the OS nor DFS estimations demonstrated
statistical differences. However, a separating tendency was
observed in Fig. 7, which indicated
that patients without COL5A1 alterations had improved prognoses,
compared with those with COL5A1 alterations.
Mechanism of COL5A1 in BC
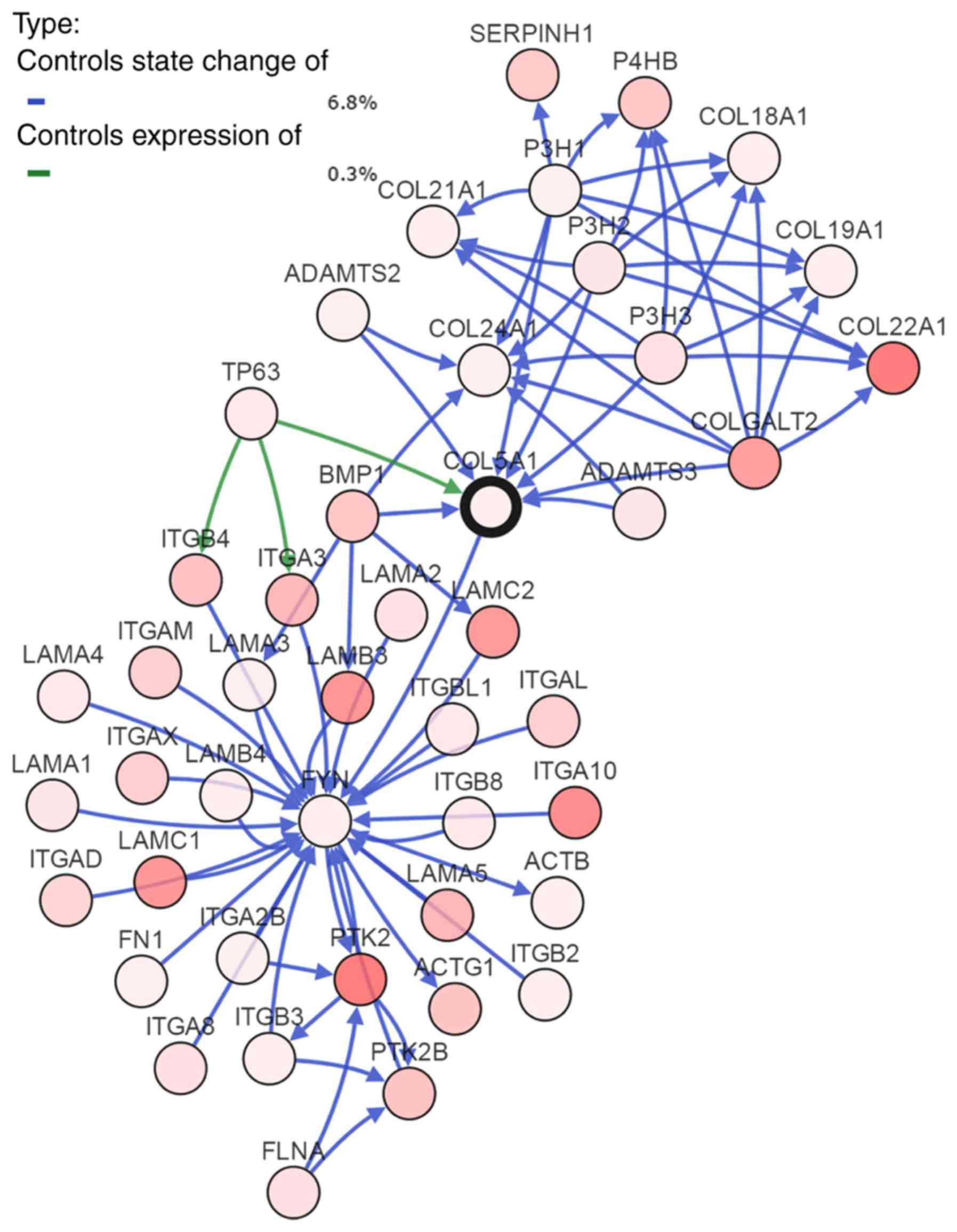
To investigate the mechanism of COL5A1 in BC, the
most frequently altered neighbor genes were obtained from
cBioPortal. A total of 50 associated genes were displayed as a gene
interaction network (Fig. 8).
Furthermore, GO functional annotation and KEGG pathway enrichment
were performed on these genes. Subsequently, following setting the
filter to 5, 6 and 7%, the thresholds of total alteration
frequencies, genes with alteration frequencies below these
thresholds were filtered out. Results of the GO functional
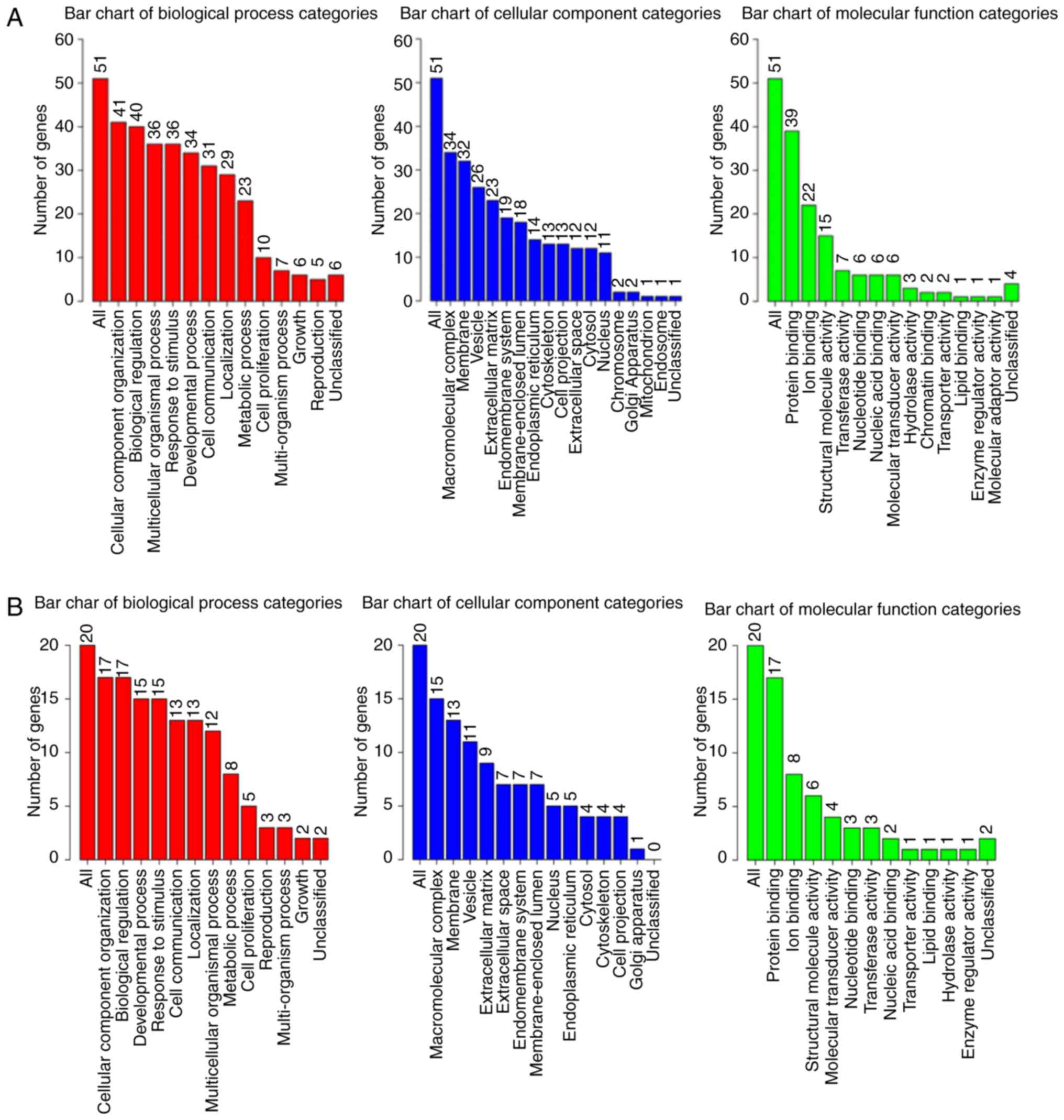
annotation were obtained, as depicted in Fig. 9.
GO analysis of the 50 associated genes indicated
that the three most significant biological processes included
cellular component organization, biological regulation and a
multicellular organismal process. Particularly in the cellular
component, all three processes involved macromolecular complex,
membrane and vesicle; and, in the molecular function, the three
most significant remained protein binding, ion binding and
structural molecular activity. In the genes of 5, 6 and 7%
alteration rates, all three GO analyses demonstrated that the most
significant biological process was cellular component organization,
along with macromolecular complex in the cellular component and
protein binding in the molecular function. The outcomes of
alteration rates were consistent with that of COL5A1-associated
genes.
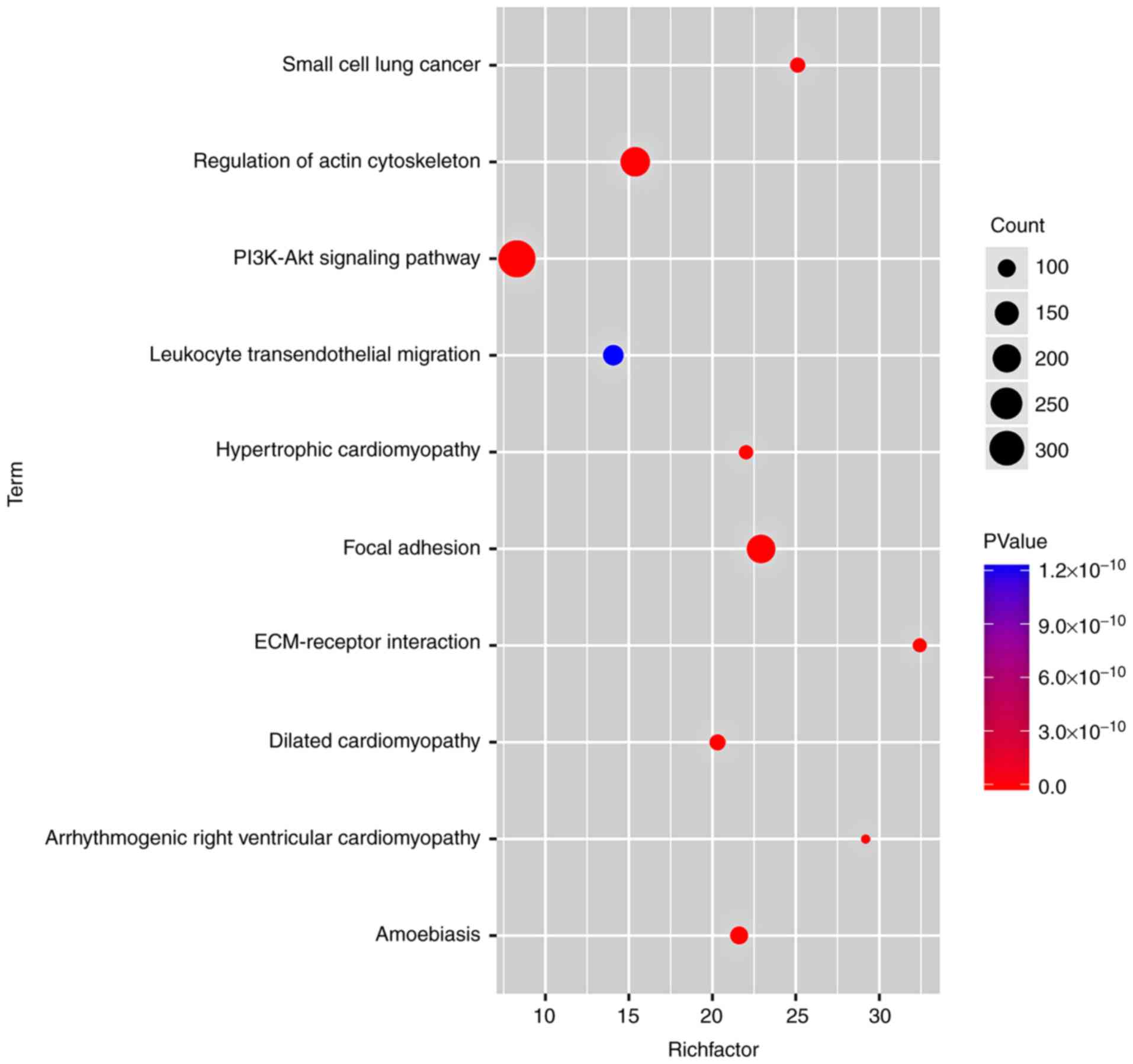
Additionally, KEGG analysis confirmed that the most
notable DEG-enriched pathways included focal adhesion,
extracellular matrix (ECM)-receptor interaction and regulation of
the actin cytoskeleton (Fig.
10).
Meta-analysis of COL5A1 mRNA
expression
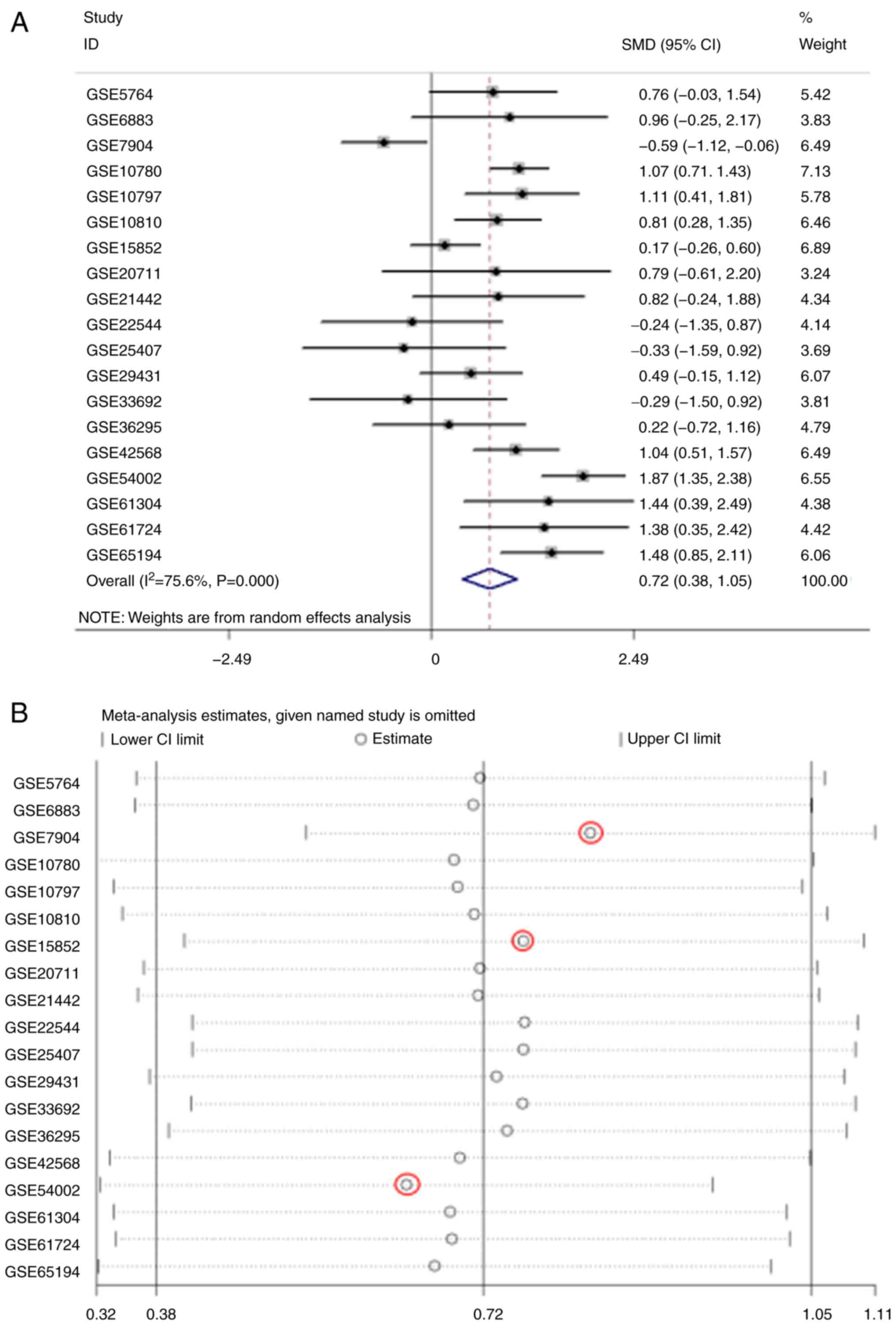
The meta-analysis of COL5A1 expression levels
demonstrated high heterogeneity from the research
(I2>50%; Fig. 11A).
To eliminate heterogeneity and reduce research error, a sensitivity
analysis was conducted to further determine the source of
heterogeneity. As a result, GSE7904, GSE15852 and GSE54002 were
considered the main sources of heterogeneity (Fig. 11B). Following removing these three
GEO chips, research heterogeneity was reduced (I2=31.8%;
P=0.108; Fig. 11C). Additionally,
Deeks' test demonstrated that the bias value of P>|t| was 0.468,
also indicating that the study of COL5A1 diagnostic significance
had no notable publication bias (Fig.
11D). In conclusion, COL5A1 mRNA expression levels were
increased in cancerous tissues, compared with non-cancerous
tissues, from the GEO database for BC (SMD, 0.84; 95% CI,
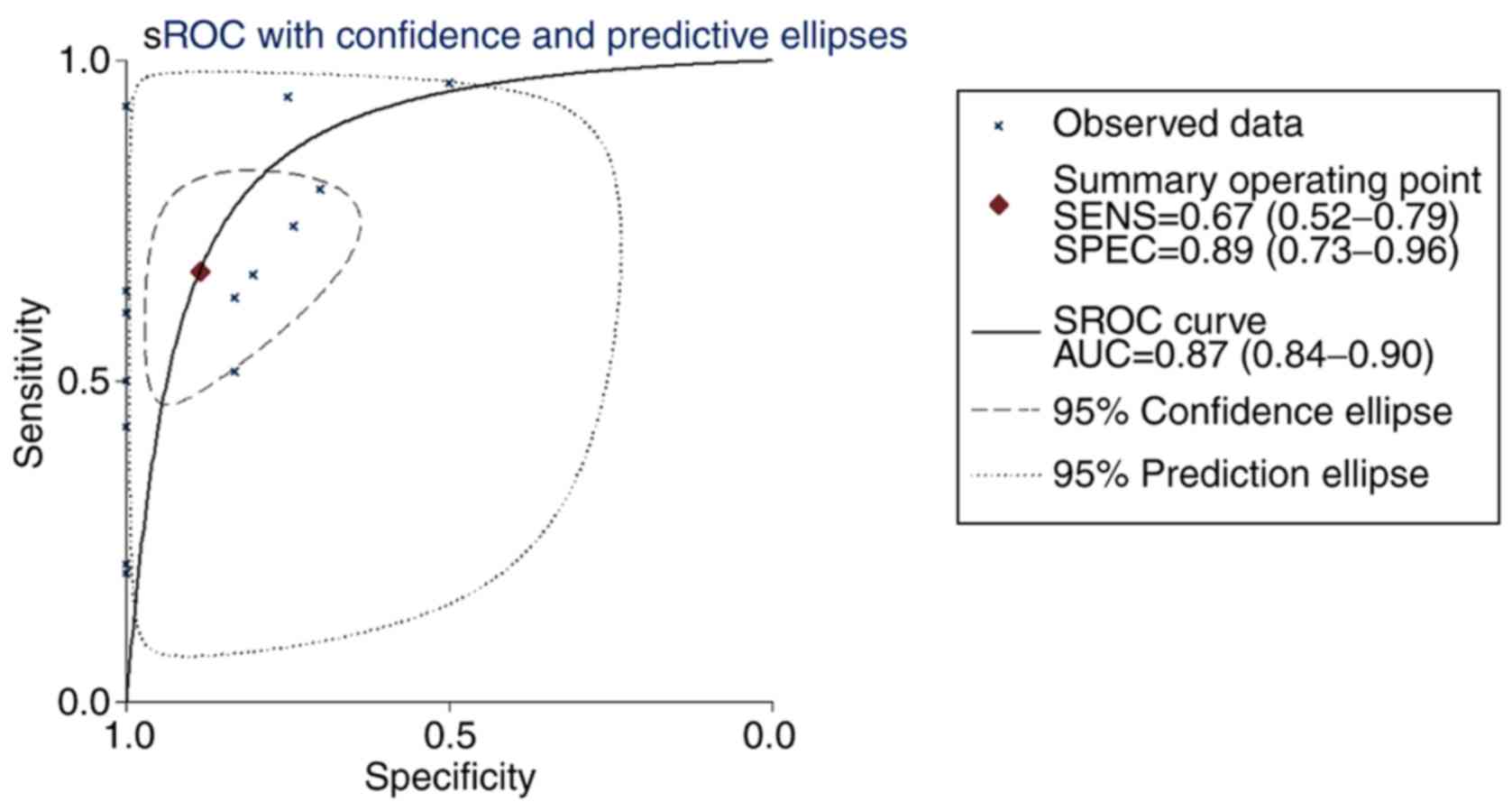
0.60-1.07). The sROC curves were plotted based on TP, FN, TN and FP
cases obtained from the GEO datasets. The area under the sROC was
0.87 (95% CI, 0.84-0.90), indicating that COL5A1 has a strong
potential diagnostic value for BC (Fig. 12).
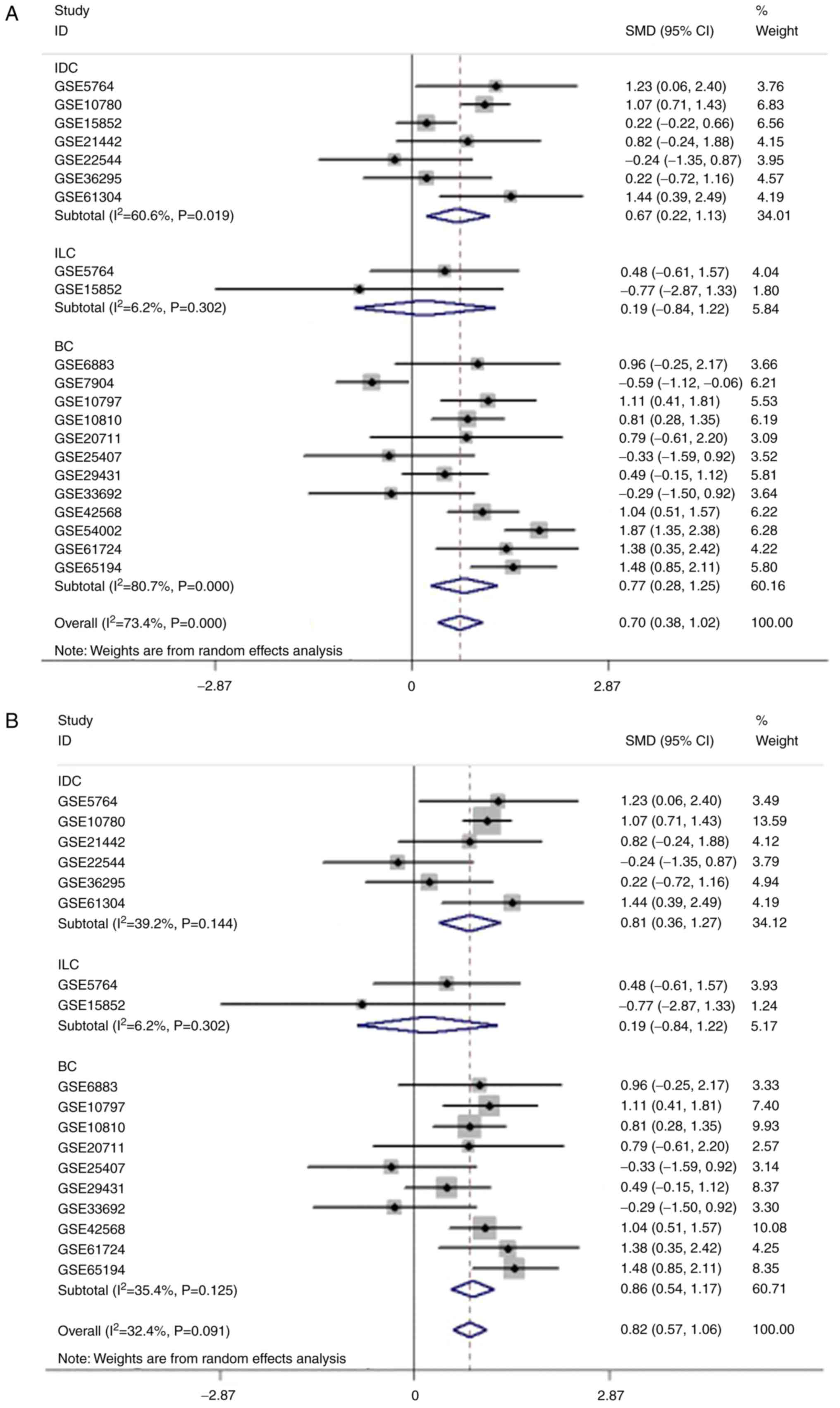
To clarify the significance of COL5A1 in BC
subtypes, the subgroup analysis was performed as aforementioned by
Stata 12.0 software. All analysis results were displayed in the
output window of the Stata 12.0 software. From the outcome of the
meta-analysis, high expression levels of COL5A1 were determined to
have clinical significance in invasive ductal BC (P=0.004), as well
as in un-subdivided invasive BC (P=0.002). The results of the
meta-analysis, including ILC, which did not indicate clinical
significance (P=0.717), indicated that the expression of COL5A1 in
BC was significantly increased, compared with non-cancer tissues
(P<0.001) (data not shown). The forest plot for subgroup
analysis demonstrated increased heterogeneity in the IDC and BC
subgroups. After excluding the three GEO chips aforementioned, the
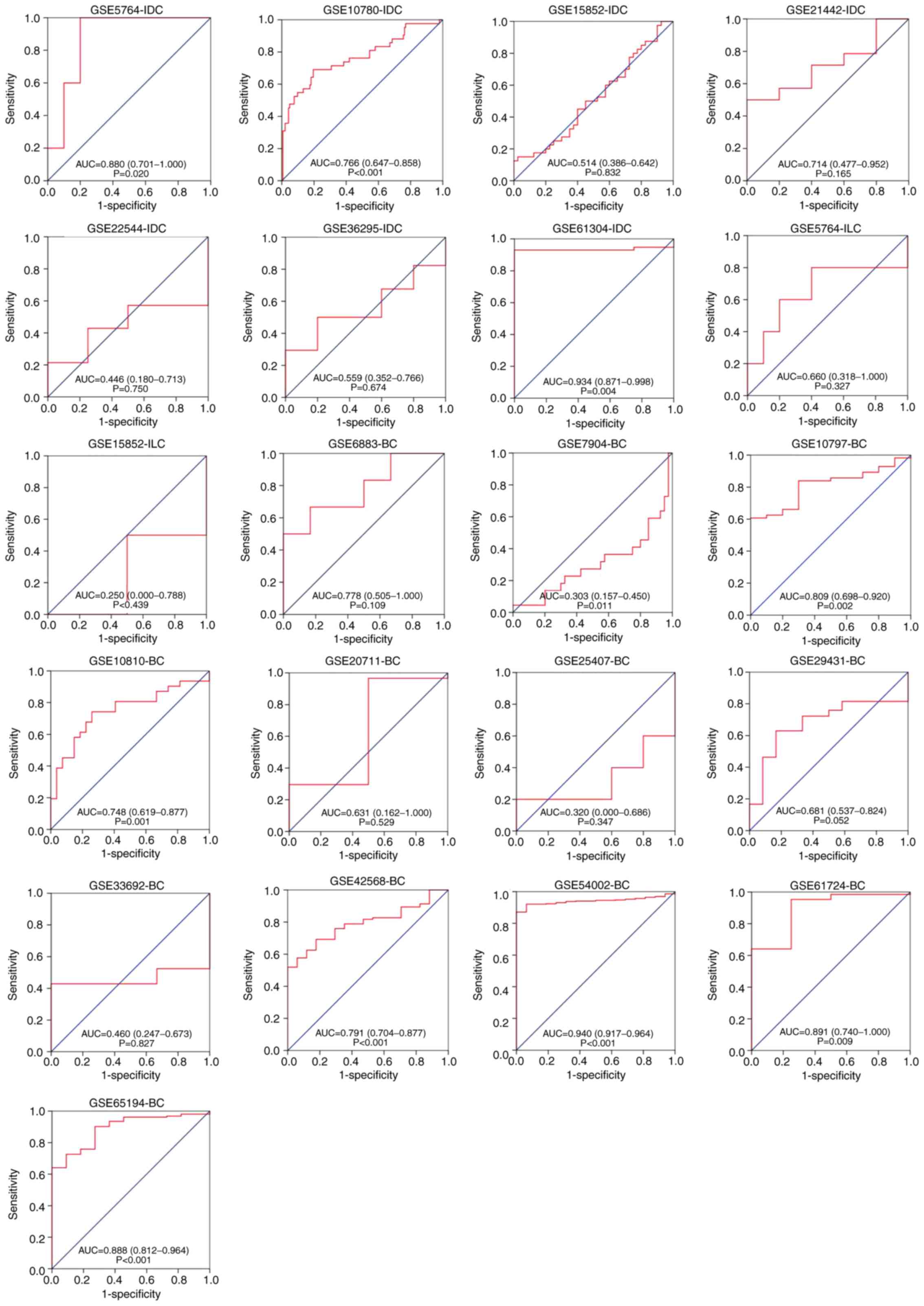
forest plot indicated decreased heterogeneity (P>0.05; Fig. 13). The ROC curves of the chip data
for each subtype are depicted in Fig.
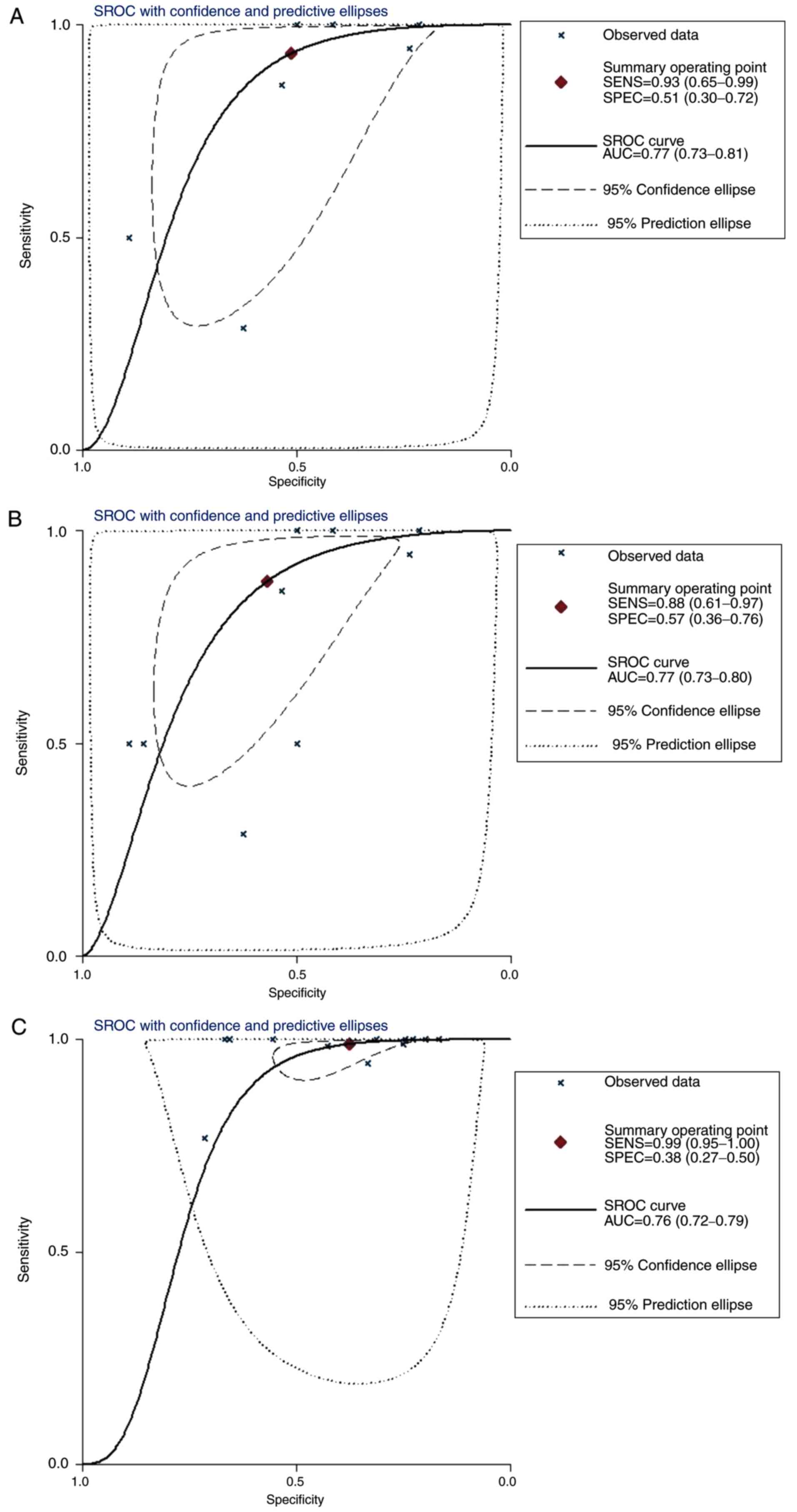
14, and the sROC curves for each subtype are depicted in
Fig. 15. The AUC was 0.77 (95% CI,
0.73-0.81) in IDC, 0.77 (95% CI, 0.73-0.80) in both IDC and ILC,
and 0.76 (95% CI, 0.72-0.79) in un-subdivided invasive BC.
Validation of COL5A1 protein expression
levels detected by immunohistochemistry
Selected BC cases and associated
clinical data
According to the selection criteria in the present
study, 136 cases of BC were considered alongside 55 pairs of normal
breast tissue adjacent to cancer. The selected patients were all
female, aged 29–79 years old, with a median age of 47 years old.
The clinical and pathological features of the 136 BC cases are
presented in Table I. During the
tracking period, the longest follow-up time was 2,242 days, and the
shortest was 162 days. There were 12 cases of death due to BC; 15
cases were lost to follow-up, and the remaining 121 cases were
entered as samples for survival analysis.
 | Table I.A total of 136 cases of clinical and
pathological features of invasive ductal carcinoma. |
Table I.
A total of 136 cases of clinical and
pathological features of invasive ductal carcinoma.
| Clinicopathological
variables | Cases | % |
|---|
| Age (years) |
|
|
|
≤50 | 90 | 66.2 |
|
>50 | 46 | 33.8 |
| Histological grade
(8) |
|
|
| I | 9 | 6.6 |
| II | 88 | 64.7 |
|
III | 39 | 28.7 |
| Pathological stage
(pTNM) (8) |
|
|
| Tumor
size (cm) |
|
|
|
≤2 | 33 | 24.3 |
|
2-5 | 80 | 58.8 |
|
>5 | 23 | 16.9 |
| Lymph
node metastasis (8) |
|
|
|
0 | 49 | 36 |
|
1-3 | 43 | 31.6 |
|
4-9 | 26 | 19.2 |
|
≥10 | 18 | 13.2 |
| Distant
metastasis (8) |
|
|
|
M0 | 129 | 94.9 |
|
M1 | 7 | 5.1 |
| Clinical stage
(8) |
|
|
| I | 17 | 12.5 |
| II | 68 | 50 |
|
III | 44 | 32.4 |
| IV | 7 | 5.1 |
| Molecular types
(8) |
|
|
| Luminal
A | 16 | 12.5 |
| Luminal
B (HER-2 negative) | 63 | 49.1 |
| Luminal
B (HER-2 positive) | 15 | 11.7 |
| HER-2
(overexpression) | 16 | 12.5 |
| Triple
negative type | 18 | 14.1 |
Experimental validation of COL5A1
expression in BC and adjacent tissue
While the IHC method was applied to stain all
sections, in 136 cases of BC tissues and adjacent tissues, zero
cases were negative, 13 cases were weakly positive, 55 were
moderately positive and 68 cases had strongly positive expression
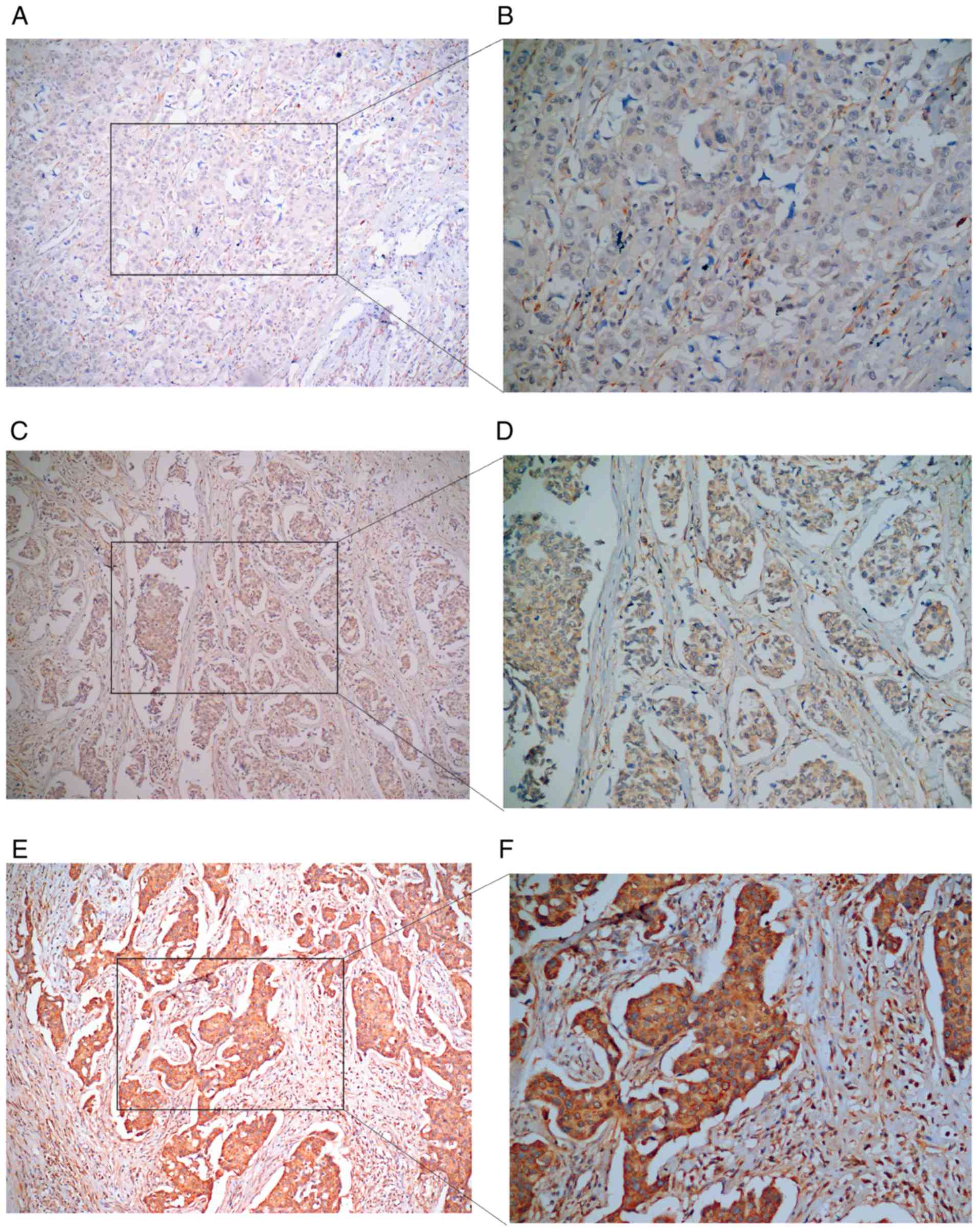
levels of COL5A1 (Table II).
Additionally, zero cases of BC tissues exhibited negative COL5A1
expression, 25 cases were weakly positive, 25 cases were moderately
positive and 21 cases were strongly positive (Figs. 16 and 17). The results are displayed in Table III.
 | Table II.Immunohistochemical staining results
of COL5A1 in cancerous and adjacent tissues. |
Table II.
Immunohistochemical staining results
of COL5A1 in cancerous and adjacent tissues.
|
| COL5A1 |
|
|---|
|
|
|
|
|---|
| Group | Negative | Weakly
positive | Moderately
positive | Strongly
positive | Sum |
|---|
| BRCA tissues | 0 | 13 | 55 | 68 | 136 |
| Adjacent
tissues | 0 | 9 | 25 | 21 | 55 |
| Sum | 0 | 22 | 80 | 89 | 191 |
 | Table III.COL5A1 expression in BC and adjacent
tissues. |
Table III.
COL5A1 expression in BC and adjacent
tissues.
|
| COL5A1 |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Group | Low expression
(%) | High expression
(%) | Sum | χ2 | P-value |
|---|
| BRCA tissues | 13 (9.6) | 123 (90.4) | 136 | 1.779 | 0.182 |
| Adjacent
tissues | 9 (16.4) | 46 (83.6) | 55 |
| Sum | 22 | 169 | 191 |
The expression of COL5A1 protein in BC tissues
demonstrated no statistically significant difference between ER,
PR, HER-2, P53 and Ki-67 (P>0.05) (Table IV), nor did it indicate a
statistically significant difference in age, histological grade,
tumor size, lymph node metastasis, distant metastasis, clinical
stage and molecular type (P>0.05; Table V).
 | Table IV.Association between COL5A1 and ER,
PR, P53, HER-2 and Ki-67 expression in breast cancer. |
Table IV.
Association between COL5A1 and ER,
PR, P53, HER-2 and Ki-67 expression in breast cancer.
|
|
| COL5A1 |
|
|
|---|
|
|
|
|
|
|
|---|
| Factors | Cases | Low expression | High
expression | χ2 | P-value |
|---|
| ER |
|
|
| 0.247 | 0.619 |
|
Negative | 45 | 3 | 42 |
|
|
|
Positive | 91 | 10 | 81 |
|
|
| PR |
|
|
| 0.247 | 0.619 |
|
Negative | 45 | 3 | 42 |
|
|
|
Positive | 91 | 10 | 81 |
|
|
| HER-2 (128
cases) |
|
|
| 0.05 | 0.823 |
|
Negative | 98 | 9 | 89 |
|
|
|
Positive | 30 | 3 | 27 |
|
|
| P53 |
|
|
| 0.003 | 0.957 |
|
Negative | 88 | 9 | 79 |
|
|
|
Positive | 48 | 4 | 44 |
|
|
| Ki-67 |
|
|
| 0.158 | 0.691 |
|
<14% | 21 | 2 | 19 |
|
|
|
≥14% | 115 | 11 | 104 |
|
|
 | Table V.Expression of COL5A1 in breast cancer
and its association with clinicopathological features. |
Table V.
Expression of COL5A1 in breast cancer
and its association with clinicopathological features.
|
|
| COL5A1 |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
variables | Cases | Low expression | High
expression | χ2 | P-value |
|---|
| Age (years) |
|
|
| 0.138 | 0.710 |
|
≤50 | 90 | 8 | 82 |
|
|
|
>50 | 46 | 5 | 41 |
|
|
| Histological grade
(8) |
|
|
| 0.248 | 0.619 |
|
I+II | 97 | 8 | 89 |
|
|
|
III | 39 | 5 | 34 |
|
|
| Tumor size
(cm) |
|
|
| 0.946 | 0.623 |
| ≤2 | 33 | 4 | 29 |
|
|
|
2-5 | 80 | 8 | 72 |
|
|
|
>5 | 23 | 2 | 21 |
|
|
| Lymph node
metastasis (8) |
|
|
| 0.013 | 0.911 |
| No
(N0) | 49 | 5 | 44 |
|
|
| Yes
(N1-N3) | 87 | 8 | 79 |
|
|
| Distant metastasis
(8) |
|
|
| 0.693 | 0.405 |
| M0 | 129 | 11 | 118 |
|
|
| M1 | 7 | 2 | 5 |
|
|
| Clinical stage
(8) |
|
|
| 0.142 | 0.707 |
|
I+II | 85 | 7 | 78 |
|
|
|
III+IV | 51 | 6 | 45 |
|
|
| Molecular type
(8) (128 cases) |
|
|
| 0.748 | 0.862 |
| Luminal
A | 16 | 1 | 15 |
|
|
| Luminal
B | 77 | 8 | 70 |
|
|
| HER-2
(overexpression) | 16 | 2 | 14 |
|
|
| Triple
negative type | 18 | 1 | 17 |
|
|
Spearman's correlation analysis of
COL5A1 expression and clinicopathological parameters
Spearman's correlation analysis between COL5A1 and
the clinicopathological parameters demonstrated that the specific
gene in BC had no statistical significance in terms of tumor size
(r=0.032; P=0.713), lymph node metastasis (r=−0.033; P=0.700),
histological grade (r=−0.093; P=0.282), clinical stage (r=−0.027;
P=0.757), molecular typing (r=0.023; P=0.791), ER (r=−0.069;
P=0.424), PR (r=−0.069; P=0.4246), Ki-67 (r=0.032; P=0.714), P53
(r=0.031; P=0.722) or HER-2 (r=0.061; P=0.493) (data not
shown).
Association between COL5A1 protein
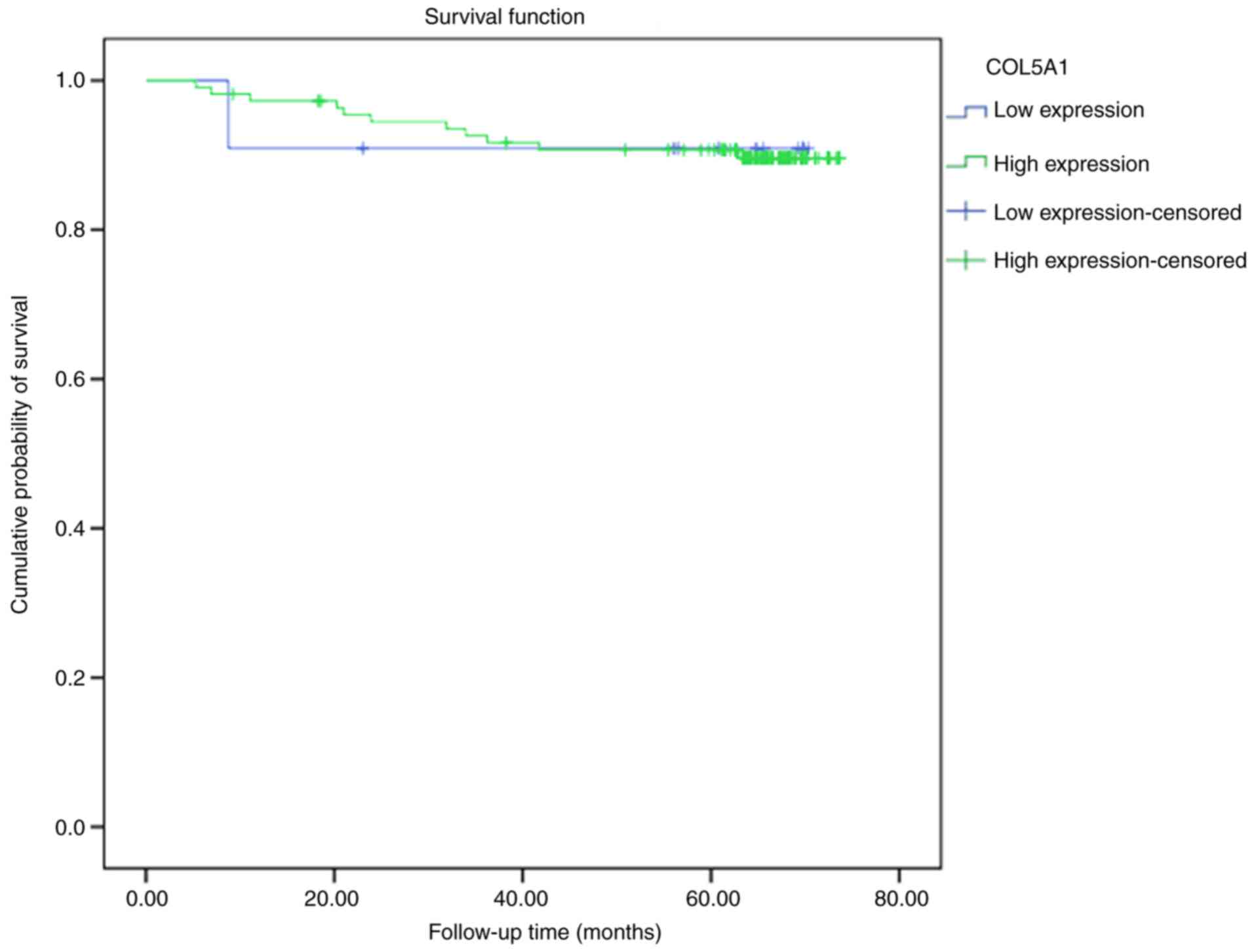
expression level and prognosis
In the 121 studied cases of BC, 11 cases had low
expression levels of COL5A1, with one case of mortality, and the
mean survival time was 64.72 months (95% CI, 54.27-75.18), and 110
cases were high-expression cases, with 11 mortalities and a mean
survival time of 68.89 months (95% CI, 65.05-71.73) (data not
shown). A log-rank test demonstrated no statistical difference
between the low and high expression levels of the COL5A1 protein in
survival curves (P=0.985; Fig.
18).
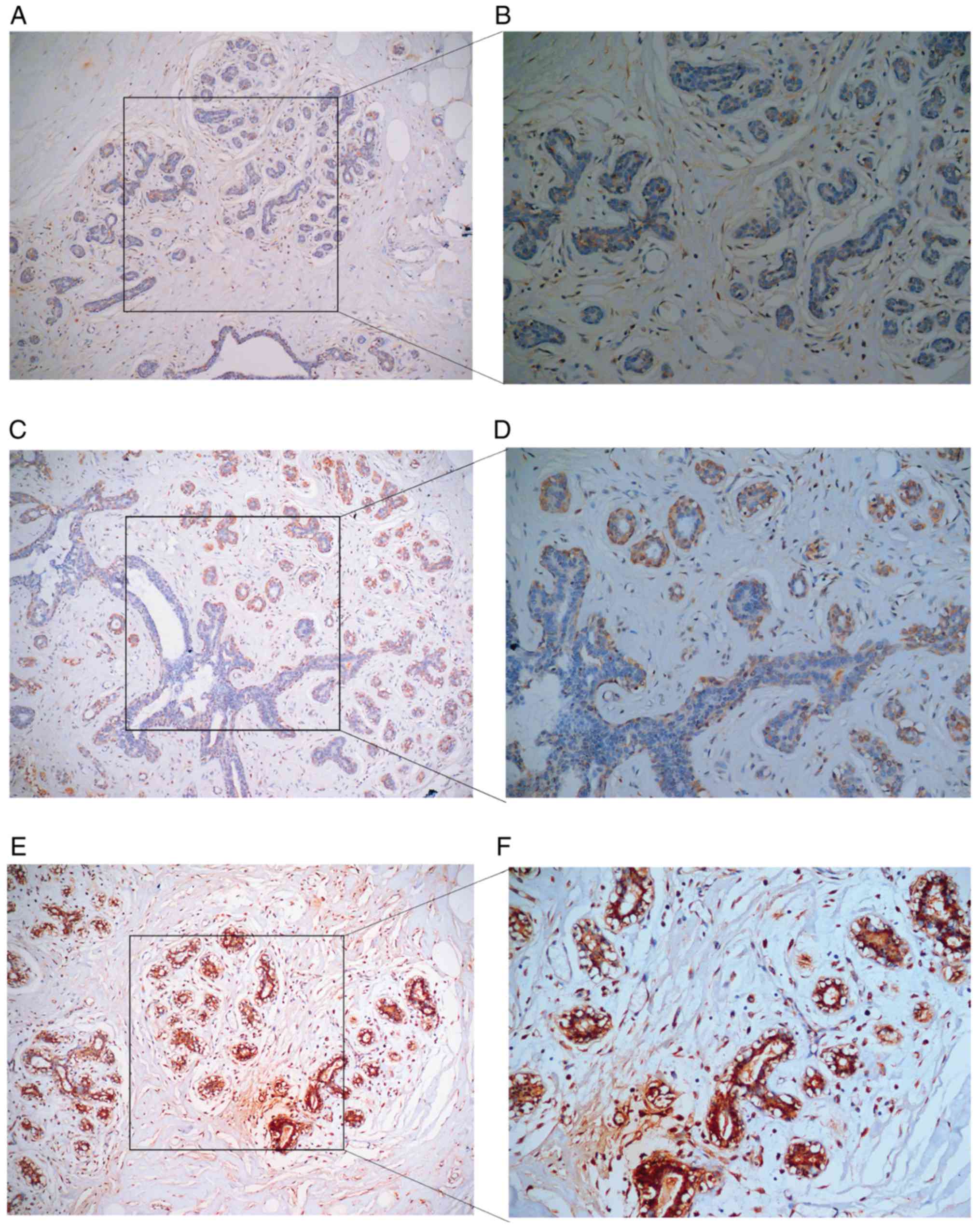
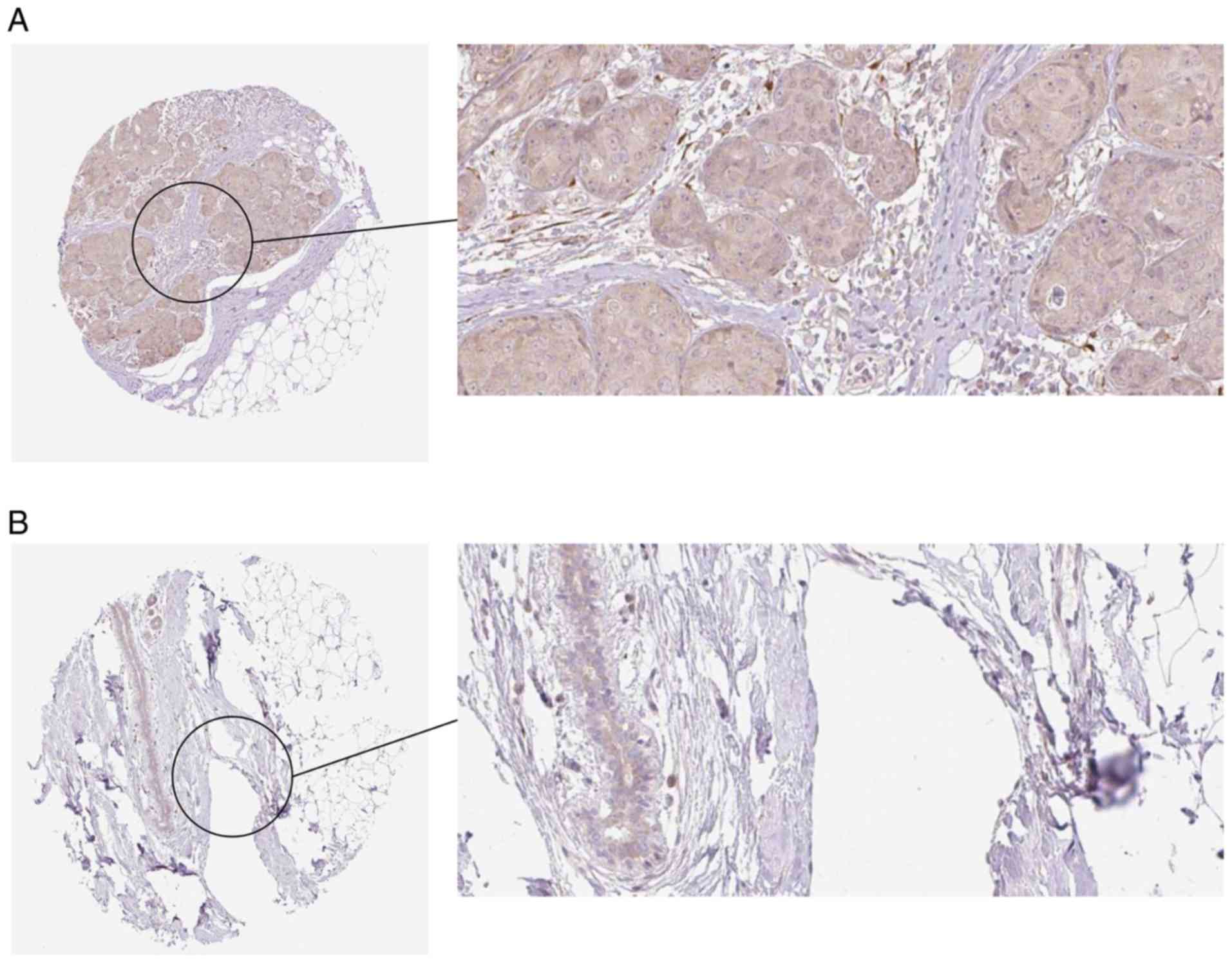
HPA Database verification
The results from the HPA Database further
demonstrated a trend toward a high expression of COL5A1 at the
protein level in BC (Fig. 19).
Discussion
At present, BC is a cancer seriously affecting
women's health globally in 2018 (1,2). Due
to its complex pathogenesis, research on its molecular pathogenic
factors has become prevalent. With the rapid development of medical
technology, increasing numbers of molecular markers associated with
BC have been determined and investigated (31,32),
continually raising the accuracy of early BC screening. However,
due to individual differences, single biomarkers cannot meet the
requirements of universal diagnosis, and the markers for multi-gene
combined screening are more conducive to diagnosis and evaluation
of BC prognosis (33,34). The discovery of markers can also
help develop individualized treatment, including molecular targeted
therapies. Elucidating the association between gene expression and
clinicopathological characteristics can support physicians in
selecting appropriate treatments for their patients in clinical
practice.
The present study obtained the differential genes
associated with BC through screening the GEO and TCGA databases.
The genes that appeared more than five times in each database were
intersected to gain the significant difference genes. Genes with
significant differences were then used to perform, GO and KEGG
pathway analyses. The present literature review revealed that
COL5A1 has been regarded as a risk biomarker for poor prognoses in
ovarian cancer and kidney carcinoma. Considering that COL5A1 may
function in BC development, the present study further investigated
this target gene.
The COL5A1 gene encodes COL5A1, which is in smaller
fibrillar collagen in mammals. Numerous studies on COL5A1 primarily
focused on single nucleotide polymorphisms, motor injuries and
connective tissue injuries (35–39).
COL5A1 is rarely reported in cancer research, which is, instead,
primarily predicted by bioinformatics (40,41).
However, previous studies indicated that COL5A1 is predicted to
have a notable role in BC (42,43).
Therefore, the accuracy of this prediction required further
experimental verification.
Through a meta-analysis of the BC gene expression
profiles from 19 GEO chips, expression levels of COL5A1 were
determined to be increased in BC tissues, compared with normal
breast tissues, in 14 chips, with nine chips having significantly
different expression (P<0.05). Additionally, the expression of
COL5A1 was significantly increased in the 1,085 BC cases from TCGA
database, compared with their adjacent tissues. A forest plot
indicated the significance of COL5A1 expression (SMD, 0.84; 95% CI,
0.60-1.07). In addition, the AUC of COL5A1 was 0.87 (95% CI,
0.84-0.90), indicating that COL5A1 has a strong potential
diagnostic value for early BC. In a subgroup analysis, COL5A1 also
had diagnostic significance in the IDC subtype (AUC, 0.77; 95% CI,
0.73-0.81). Although the OS and DFS survival curves did not
demonstrate statistical significance, a different trend which
indicated that elevated COL5A1 mRNA level predicted poor prognosis
of BC has been observed. If more cases can be included, the
association between COL5A1 and the clinical prognosis of BC may be
demonstrated further. Furthermore, research heterogeneity has been
reduced following eliminating the three GEO chips considered to be
the sources of the heterogeneity (GSE7904, GSE15852 and GSE54002).
Therefore, no publication bias existed, and the results were
credible. For molecular type, ER and PR negative were determined to
be significantly associated with DFS in BC, as well as histological
grade III. Therefore, the high expression of COL5A1 mRNA indicated
poor prognosis for patients with ER and PR negative. The increased
COL5A1 mRNA expression level also indicated that patients may have
an increased histological grade type and malignant degree of BC.
This indicated that clinicians may predict the prognosis of
patients with BC according to their COL5A1 mRNA levels, in order to
timely communicate with patients and adjust the treatments.
At the protein level, the HPA database confirmed the
high expression of COL5A1 in BC, which was consistent with the
in-house immunohistochemistry. There are a number of pathological
types of BC, with invasive types accounting for >70% (29,30).
The in-house cases were strictly selected to ensure the reliability
of experimental results. The present immunohistochemical results
confirmed that COL5A1 protein was highly expressed in invasive BC
(90.4%), but they also exhibited no statistical significance,
compared with normal tissue. The expression level of COL5A1 was not
statistically significant with the age, histological grade, tumor
size, lymph node metastasis, distant metastasis, clinical stage,
molecular type, ER, PR, HER-2, P53 and Ki-67 of patients with BC
(P>0.05). However, COL5A1 may be more appropriate as a combined,
rather than individual, marker for diagnosing and treating BC.
Therefore, it will be necessary for future studies to consider more
research samples to verify, and further clarify, these
observations. Furthermore, the protein level of COL5A1 in other
subtypes of BC will be verified in the planned, follow-up, in-house
experiment after sufficient clinical cases are collected.
The present study also determined that the COL5A1
mutation (4% mutation rate) is associated with the prognosis of
patients with BC, and patients with BC with COL5A1 mutation may
have a reduced prognosis. Investigating the underlying mechanisms,
GO analysis indicated that the most important biological process is
cellular component organization, as well as macromolecular
complexes in molecular function and protein binding in cellular
components. The GO pathway for screening genes, based on the
alteration rate in line with the aforementioned pathways, indicated
the reliability of pathway prediction. According to the KEGG
pathway analysis, the 50 most frequently-altered neighboring genes
may affect BC by regulating focal adhesion, ECM-receptor
interactions and regulation of the actin cytoskeleton. Previous BC
studies focused on tumor epithelial cells (44–46),
while other factors, including microenvironment, myoepithelial
cells and the potential role of stromal cells in tumor progression,
have not been well studied. In vivo and in vitro
studies demonstrated that cells constituting the microenvironment,
including muscle epithelium and endothelial cells, fibroblasts and
myofibroblasts, and ECM molecules may regulate the growth and
survival of normal breast tissue and the invasion of BC cells
(44,47–49).
Associated studies confirmed that the ECM-receptor interaction
signaling pathway is associated with BC bone metastasis (50), and it is involved in thyroid
papillary carcinoma (51), oral
squamous cell carcinoma (52) and
early lung adenocarcinoma (53).
Additionally, research on ovarian cancer research containing 10
genes [adipocyte enhancer-binding protein 1, COL11A1, COL5A1,
COL6A2, lysyl oxidase, periostin, snail family transcriptional
repressor 2, thrombospondin 2 (THBS2), tissue inhibitor of
metalloproteinases 3 (TIMP3) and versican] associated with ovarian
cancer predicted a poor prognosis for patients with ovarian cancer
and included the joint gene COL5A1 (31,54,55).
Subsequently, 10 genes (COL1A1, COL5A1, COL11A1, fibronectin 1,
intercellular adhesion molecule 1, integrin subunit αL, integrin
subunit αM, integrin subunit β2, THBS2 and TIMP1) were determined
to be associated with poor prognosis in renal cell carcinoma,
including COL5A1 (56). COL5A1 is
featured in all of these studies, and it is the specific gene in
the present study that involves the interaction of ECM receptors,
all of which indicates that this particular gene serves an
essential role in the cancer pathogenesis. Following a
comprehensive multi-factor analysis, the present study considered
COL5A1 as crucial to BC development.
Notably, a study by Ren et al determined that
a high expression of COL5A1 indicated an improved prognosis in
patients with BC without lymph node metastasis. Firstly, the study
by Ren et al supports the high expression of COL5A1 in IDC
of BC (10), which is consistent
with the conclusions of the present study. Secondly, due to
different sample sources in the two studies, including race,
regional environment and lifestyle, may result in different
conclusions should be considered. Death factors may also impact
lymph node metastasis, which means mortality cases without lymph
node metastasis should probably not be considered. The present
study evaluated the clinical value of COL5A1 in BC, including OS,
DFS, clinical parameters and molecular mechanisms, and supplemented
with immunohistochemistry to verify bioinformatics results based on
high-throughput data. Additionally, the sample size was large, and
the results are highly reliable.
Although COL5A1 has been verified at the mRNA and
protein levels, this biomarker is also highly expressed at the
transcription level in BC. However, the difference in expression is
not reflected at the protein level. Additionally,
post-transcriptional modification and post-translational
modification, including glycosylation, hydrolytic processing and
phosphorylation, may induce protein degradation in vivo
during this process, thereby affecting protein expression (57,58).
However, in the course of the experiment, unavoidable experimental
errors and losses may have occurred in the detection of mRNA and
the verification of the immunohistochemistry.
In conclusion, using computational biology and
immunohistochemistry, the present study demonstrated that COL5A1 is
highly expressed at the mRNA and protein levels in BC. Based on the
survival curves, the patients with BC with high COL5A1 expression
have a reduced prognosis. Furthermore, COL5A1 may impact the
development of BC by regulating pathways, including focal adhesion,
ECM-receptor interaction and the regulation of the actin
cytoskeleton. Nonetheless, further clinical trials, including more
BC cases, are required to verify the results of the present study.
It is considered that COL5A1 may be used as an effective single, or
combined, indicator for clinical diagnosis and prediction of BC in
the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW and QS analyzed and interpreted data, and drafted
the manuscript. CHM and JYH performed the majority of the
experiments as well as statistical analysis, and supervised the
progression of research. JSP, LLP, HPL, YWD, SJF, DT, GC and ZBF
participated in sample collection and provided information from the
databases. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of First Affiliated Hospital of Guangxi Medical
University. All the patients signed the written informed consents
based on the guidelines of the First Affiliated Hospital of Guangxi
Medical University prior to participating in the present study. All
tissue samples were collected anonymous according to the ethical
and legal standards.
Patient consent for publication
All patients provided written informed consent
before participation and agreed to publication of the present
study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
COL5A1
|
collagen type V α-1 chain
|
|
BC
|
breast cancer
|
|
IHC
|
immunohistochemistry
|
|
TCGA
|
The Cancer Genome Atlas
|
|
GEO
|
Gene Expression Omnibus
|
|
DEGs
|
differentially-expressed genes
|
|
GEPIA
|
Gene Expression Profiling Interactive
Analysis
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
ROC
|
Receiver Operating Characteristic
|
|
sROC
|
summary ROC curve
|
|
ER
|
estrogen
|
|
PR
|
progesterone
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang ZJ, Yu Y, Chi JR, Guan M, Zhao Y and
Cao XC: The combined pN stage and breast cancer subtypes in breast
cancer: A better discriminator of outcome can be used to refine the
8th AJCC staging manual. Breast Cancer. 25:315–324. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
An N, Shi Y, Ye P, Pan Z and Long X:
Association between MGMT promoter methylation and breast cancer: A
Meta-analysis. Cell Physiol Biochem. 42:2430–2440. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Casey MC, Sweeney KJ, Brown JA and Kerin
MJ: Exploring circulating micro-RNA in the neoadjuvant treatment of
breast cancer. Int J Cancer. 139:12–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Savci-Heijink CD, Halfwerk H, Koster J and
Van de Vijver MJ: Association between gene expression profile of
the primary tumor and chemotherapy response of metastatic breast
cancer. BMC Cancer. 17:7552017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin YH, Hua QF, Zheng JJ, Ma XH, Chen TX,
Zhang S, Chen B, Dai Q and Zhang XH: Diagnostic value of ER, PR, FR
and HER-2-targeted molecular probes for magnetic resonance imaging
in patients with breast cancer. Cell Physiol Biochem. 49:271–281.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akram M, Iqbal M, Daniyal M and Khan AU:
Awareness and current knowledge of breast cancer. Biol Res.
50:332017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polyak K: Breast cancer: Origins and
evolution. J Clin Invest. 117:3155–3163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren W, Zhang Y, Zhang L, Lin Q, Zhang J
and Xu G: Overexpression of collagen type V α1 chain in human
breast invasive ductal carcinoma is mediated by TGF-β1. Int J
Oncol. Mar 15–2018.(Epub ahead of print). doi:
10.3892/ijo.2018.4317. View Article : Google Scholar
|
|
11
|
Lee S, Lee J, Sim SH, Lee Y, Moon KC, Lee
C, Park WY, Kim NK, Lee SH and Lee H: Comprehensive somatic genome
alterations of urachal carcinoma. J Med Genet. 54:572–578. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chai F, Liang Y, Zhang F, Wang M, Zhong L
and Jiang J: Systematically identify key genes in inflammatory and
non-inflammatory breast cancer. Gene. 575:600–614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41:D991–D995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou GX, Liu P, Yang J and Wen S: Mining
expression and prognosis of topoisomerase isoforms in
non-small-cell lung cancer by using Oncomine and Kaplan-Meier
plotter. PLoS One. 12:e01745152017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gyorffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang HL, Chang KK, Mei J, Zhou WJ, Liu LB,
Yao L, Meng Y, Wang MY, Ha SY, Lai ZZ, et al: Estrogen restricts
the apoptosis of endometrial stromal cells by promoting TSLP
secretion. Mol Med Rep. 18:4410–4416. 2018.PubMed/NCBI
|
|
18
|
Sas-Korczynska B, Reinfuss M, Mitus JW,
Pluta E, Patla A and Walasek T: Radiotherapy alone as a method of
treatment for sinonasal mucosal melanoma: A report based on six
cases and a review of current opinion. Rep Pract Oncol Radiother.
23:402–406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Vasaikar S, Shi Z, Greer M and
Zhang B: WebGestalt 2017: A more comprehensive, powerful, flexible
and interactive gene set enrichment analysis toolkit. Nucleic Acids
Res. 45:W130–W137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai Y, Sun L and Qiang W: A new strategy
to uncover the anticancer mechanism of Chinese compound formula by
integrating systems pharmacology and bioinformatics. Evid Based
Complement Alternat Med. 2018:67078502018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frank GA, Danilova NV, Andreeva I and
Nefedova NA: WHO classification of tumors of the breast, 2012. Arkh
Patol. 75:53–63. 2013.(In Russian). PubMed/NCBI
|
|
24
|
Gyorffy B, Pongor L, Bottai G, Li X,
Budczies J, Szabó A, Hatzis C, Pusztai L and Santarpia L: An
integrative bioinformatics approach reveals coding and non-coding
gene variants associated with gene expression profiles and outcome
in breast cancer molecular subtypes. Br J Cancer. 118:1107–1114.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan W, Zhao Y and He J: Anti-breast cancer
activity of selected 1,3,5-triazines via modulation of EGFR-TK. Mol
Med Rep. 18:4175–4184. 2018.PubMed/NCBI
|
|
26
|
Li X, Huang X, Zhang J, Huang H, Zhao L,
Yu M, Zhang Y and Wang H: A novel peptide targets CD105 for tumour
imaging in vivo. Oncol Rep. 40:2935–2943. 2018.PubMed/NCBI
|
|
27
|
Noorlag R, van der Groep P, Leusink FK,
Leusink FK, van Hooff SR, Frank MH, Willems SM and van Es RJ: Nodal
metastasis and survival in oral cancer: Association with protein
expression of SLPI, not with LCN2, TACSTD2, or THBS2. Head Neck.
37:1130–1136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357:eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thul PJ, Akesson L, Wiking M, Mahdessian
D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels LM,
et al: A subcellular map of the human proteome. Science.
356:eaal33212017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Zhang Y, Huang Q and Li C:
Integrated bioinformatics analysis reveals key candidate genes and
pathways in breast cancer. Mol Med Rep. 17:8091–8100.
2018.PubMed/NCBI
|
|
31
|
Kim GJ, Kim DH, Min KW, Kim YH and Oh YH:
Loss of p27kip1 expression is associated with poor
prognosis in patients with taxane-treated breast cancer. Pathol Res
Pract. 214:565–571. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jimenez-Morales S, Pérez-Amado CJ, Langley
E and Hidalgo-Miranda A: Overview of mitochondrial germline
variants and mutations in human disease: Focus on breast cancer
(Review). Int J Oncol. 53:923–936. 2018.PubMed/NCBI
|
|
33
|
Do SI, Kim HS, Kim K, Lee H, Do IG, Kim
DH, Chae SW and Sohn JH: Predictive and prognostic value of
sphingosine kinase 1 expression in patients with invasive ductal
carcinoma of the breast. Am J Transl Res. 9:5684–5695.
2017.PubMed/NCBI
|
|
34
|
Kim JH, Jung ES, Kim CH, Youn H and Kim
HR: Genetic associations of body composition, flexibility and
injury risk with ACE, ACTN3 and COL5A1 polymorphisms in Korean
ballerinas. J Exerc Nutrition Biochem. 18:205–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lim ST, Kim CS, Kim WN and Min SK: The
COL5A1 genotype is associated with range of motion. J Exerc
Nutrition Biochem. 19:49–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Makoukji J, Makhoul NJ, Khalil M, El-Sitt
S, Aldin ES, Jabbour M, Boulos F, Gadaleta E, Sangaralingam A,
Chelala C, et al: Gene expression profiling of breast cancer in
Lebanese women. Sci Rep. 6:366392016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu G, Wu K and Sheng Y: Elucidation of
the molecular mechanisms of anaplastic thyroid carcinoma by
integrated miRNA and mRNA analysis. Oncol Rep. 36:3005–3013. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
An F, Zhang Z, Xia M and Xing L: Subpath
analysis of each subtype of head and neck cancer based on the
regulatory relationship between miRNAs and biological pathways.
Oncol Rep. 34:1745–1754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumari K, Das B, Adhya AK, Rath AK and
Mishra SK: Genome-wide expression analysis reveals six contravened
targets of EZH2 associated with breast cancer patient survival. Sci
Rep. 9:19742019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Di Y, Chen D, Yu W and Yan L: Bladder
cancer stage-associated hub genes revealed by WGCNA co-expression
network analysis. Hereditas. 156:72019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abrahams Y, Laguette MJ, Prince S and
Collins M: Polymorphisms within the COL5A1 3′-UTR that alters mRNA
structure and the MIR608 gene are associated with Achilles
tendinopathy. Ann Hum Genet. 77:204–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ritelli M, Dordoni C, Venturini M,
Chiarelli N, Quinzani S, Traversa M, Zoppi N, Vascellaro A,
Wischmeijer A, Manfredini E, et al: Clinical and molecular
characterization of 40 patients with classic Ehlers-Danlos
syndrome: Identification of 18 COL5A1 and 2 COL5A2 novel mutations.
Orphanet J Rare Dis. 8:582013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Riches A, Campbell E, Borger E and Powis
S: Regulation of exosome release from mammary epithelial and breast
cancer cells-a new regulatory pathway. Eur J Cancer. 50:1025–1034.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rejon C, Al-Masri M and McCaffrey L: Cell
polarity proteins in breast cancer progression. J Cell Biochem.
117:2215–2223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McCuaig R, Wu F, Dunn J, Rao S and
Dahlstrom JE: The biological and clinical significance of
stromal-epithelial interactions in breast cancer. Pathology.
49:133–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pizon M, Schott DS, Pachmann U and
Pachmann K: B7-H3 on circulating epithelial tumor cells correlates
with the proliferation marker, Ki-67, and may be associated with
the aggressiveness of tumors in breast cancer patients. Int J
Oncol. 53:2289–2299. 2018.PubMed/NCBI
|
|
47
|
Luo M, Clouthier SG, Deol Y, Liu S,
Nagrath S, Azizi E and Wicha MS: Breast cancer stem cells: Current
advances and clinical implications. Methods Mol Biol. 1293:1–49.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Byler S, Goldgar S, Heerboth S, Leary M,
Housman G, Moulton K and Sarkar S: Genetic and epigenetic aspects
of breast cancer progression and therapy. Anticancer Res.
34:1071–1077. 2014.PubMed/NCBI
|
|
49
|
Chen X, Pei Z, Peng H and Zheng Z:
Exploring the molecular mechanism associated with breast cancer
bone metastasis using bioinformatic analysis and microarray genetic
interaction network. Medicine (Baltimore). 97:e120322018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao H and Li H: Network-based
meta-analysis in the identification of biomarkers for papillary
thyroid cancer. Gene. 661:160–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li S, Chen X, Liu X, Yu Y, Pan H, Haak R,
Schmidt J, Ziebolz D and Schmalz G: Complex integrated analysis of
lncRNAs-miRNAs-mRNAs in oral squamous cell carcinoma. Oral Oncol.
73:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen M, Liu B, Xiao J, Yang Y and Zhang Y:
A novel seven-long non-coding RNA signature predicts survival in
early stage lung adenocarcinoma. Oncotarget. 8:14876–14886.
2017.PubMed/NCBI
|
|
53
|
Epstein SG, Drucker L, Pomeranz M, Fishman
A, Pasmanik-Chor M, Tartakover-Matalon S and Lishner M: First
trimester human placenta prevents breast cancer cell attachment to
the matrix: The role of extracellular matrix. Mol Carcinog.
56:62–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Giussani M, Landoni E, Merlino G, Turdo F,
Veneroni S, Paolini B, Cappelletti V, Miceli R, Orlandi R, Triulzi
T and Tagliabue E: Extracellular matrix proteins as diagnostic
markers of breast carcinoma. J Cell Physiol. 233:6280–6290. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Boguslawska J, Kedzierska H, Poplawski P,
Rybicka B, Tanski Z and Piekielko-Witkowska A: Expression of genes
involved in cellular adhesion and extracellular matrix remodeling
correlates with poor survival of patients with renal cancer. J
Urol. 195:1892–1902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cheon DJ, Tong Y, Sim MS, Dering J, Berel
D, Cui X, Lester J, Beach JA, Tighiouart M, Walts AE, et al: A
collagen-remodeling gene signature regulated by TGF-β signaling is
associated with metastasis and poor survival in serous ovarian
cancer. Clin Cancer Res. 20:711–723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Alqinyah M and Hooks SB: Regulating the
regulators: Epigenetic, transcriptional, and post-translational
regulation of RGS proteins. Cell Signal. 42:77–87. 2018. View Article : Google Scholar : PubMed/NCBI
|