Introduction
Lung cancer, which possesses the highest incidence
of malignant cancer in males, is the most common type of cancer and
cause of cancer-associated mortalities in China and globally
according to cancer statistics data in 2012 (1). Non-small cell lung cancer (NSCLC)
accounts for almost 80% of lung cancer cases in globally in 2012
(2). Although surveillance and
clinical treatment strategies have been improved, the 5-year
survival of patients with NSCLC following curative resection is
reported to be 30–60 % in globally in 2012 (3). Therefore, elucidating the potential
mechanism that mediates the initiation and progression of NSCLC is
necessary and of great interest.
MicroRNAs (miRNAs) are a class of small non-coding
RNAs with a length of 19–25 bp, which serve an important role in
post-transcriptional regulation via target 3′untranslated region
(3′-UTR) (4,5). A single miRNA can target hundreds of
mRNAs, and a single mRNA can be coordinately regulated by multiple
miRNAs (4,5). Recently, accumulating evidences
indicated that miRNAs serve diverse roles in tumorigenesis and
cancer progression (4,5). Additionally, miRNAs have received
great attention in NSCLC research. A number of deregulated miRNAs
in NSCLC, including miR-221, miR-222, miR-449a, miR-21, miR-205,
miR-10b, miR-143 and miR-181a, have been demonstrated to regulate
cell growth, apoptosis, migration, invasion, angiogenesis, and
radiotherapy or chemotherapy resistance (6–8). These
observations indicate that deregulation of miRNA expression may be
associated with tumorigenesis of NSCLC.
miR-497, a highly conserved miRNA encoded by the
first intron of the MIR497HG (Gene ID: 100506755) gene on human
chromosome 17p13.1 (9), is a member
of the miR-15/16/195/424/497 gene cluster and has been demonstrated
as a tumor suppressor in multiple cancer types, including breast
cancer, gastric cancer, colorectal cancer, hepatocellular
carcinoma, pancreatic cancer, adrenocortical carcinoma, bladder
cancer, melanoma, ovarian cancer, and cervical cancer and other
solid tumors (10–13). Zhao et al (14) demonstrated that downregulated
miR-497 in NSCLCs could result in increased tumor growth and
angiogenesis by targeting hepatoma-derived growth factor. However,
its role and associated mechanism in NSCLC has not been fully
investigated yet. The vascular endothelial growth factor (VEGF)
family, an important regulator of angiogenesis, have been
demonstrated to serve a critical role in NSCLCs (15,16).
However, whether there is an association between miR-497 and
molecules in the VEGF family has not been determined.
In the present study, the aim was to evaluate the
expression of miR-497 in NSCLC tissues and cell lines, and
investigate possible mechanisms associated with its regulatory role
on cell behavior.
Materials and methods
Clinical samples
A total of 15 cases of paraffin-embedded NSCLC
tissue (including 8 cases of squamous carcinoma and 7 cases
adenocarcinoma; mean age, 58.7 years; age range, 33–75 years; 10
cases ≥60 years old and 5 cases <60 years old; 9 males and 6
females) and 8 cases non-cancerous normal tissue confirmed by a
lung needle biopsy were collected from patients without previous
radiotherapy or chemotherapy between January 2016 and December 2016
who underwent treatment in the Department of Oncology of the First
People's Hospital of Lianyungang (Lianyungang, China). The
exclusion criteria included: Patients who had received chest
radiotherapy or systemic chemotherapy prior to sampling; previous
history of oncology; participation in other drug clinical trials
and anti-tumor treatments; pregnant and lactating women; and known
history drug abuse (except alcohol abuse). All the samples were
from human lung biopsy tissue and confirmed by experienced
pathologists who were blinded to the study. The present study was
approved by the ethics committee of the First People's Hospital of
Lianyungang. Written informed consent was obtained from the
participating individuals.
Cell culture
The human NSCLC cancer cell line Calu-1 (human
squamous cell carcinoma) (17),
H358 (human lung adenocarcinoma, #SCSP-583) (18), H460 (human lung large-cell
carcinoma) (19), H292 (human lung
mucoepidermoid carcinoma-lymph node metastatic strain) (20), H1650 (human lung adenocarcinoma)
(21), A549 (human lung
adenocarcinoma) (22), H1975 (human
lung adenocarcinoma) (21), and
H1299 (human lung adenocarcinoma lymph node metastatic strain)
(23) were obtained from the Cell
Bank of Shanghai Institute of Biological Science, Chinese Academy
of Science (Shanghai, China). Among these cell lines, Calu-1 was
cultured with McCoy's 5A medium containing 10% (v/v) fetal bovine
serum and 1% antibiotics, while other cell lines were maintained as
monolayers when cultured in cell culture flasks with RPMI-1640
medium containing 10% (v/v) fetal bovine serum and 1% antibiotics.
Cells were cultured at 37°C in a humidified atmosphere containing
5% CO2. All the cell culture medium and additives were
purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). All 8 cell lines were used for expression analysis
miR-497 by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR), whereas Calu-1 and H1975 cells were further
employed for the cell proliferation, cell migration, cell
apoptosis, cell radiosensitivity, miR-497 and KDR interaction
analysis, and the in vivo tumor formation experiment.
Cell transfection
miR-497 mimic and miR-NC were obtained from the
Sangon Biotech Co., Ltd. (Shanghai, China) and the detailed
information regarding the sequence were from previous description
(24). Cells were grown to a
confluence of ~40% and transfected with 50 nM miR-mimic and miR-NC
encapsulated with Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) in Gibco Opti-MEM™ I Reduced Serum
medium (cat. no. 31985-062; Gibco; Thermo Fisher Scientific, Inc.)
for 48 h. All the experiments were repeated for at least 3
times.
RT-qPCR
Total NSCLC tissue RNA or cellular RNA of human
NSCLC cancer cell lines were extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR for
kinase insert domain receptor (KDR) was conducted using an One Step
SYBR® PrimeScript™ RT-PCR kit (Takara Biotechnology Co.,
Ltd., Dalian, China) and an iQ5 Real-time PCR Detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The PCR conditions
were 1 cycle for 30 sec at 95°C, 40 cycles of 5 sec at 95°C and 34
sec at 60°C, followed by 1 cycle for 15 sec at 95°C and 60 sec at
60°C. Expression of the GAPDH gene was assessed simultaneously in
all samples as an internal control. For miRNA RT-qPCR,
Taqman® MicroRNA Reverse Transcription and Taqman
MicroRNA Assay (Applied Biosystems; Thermo Fisher Scientific, Inc.)
kits were employed for experiments. The expression of U6 was used
as the internal control. Relative gene expression was determined
with the 2−ΔΔCq method (25). Oligonucleotide primers specific for
KDR and GAPDH were obtained from Sangon Biotech Co., Ltd. The
sequences of the PCR primers used are as follows: KDR,
5′-CCGTCAAGGGAAAGACTACG-3′ (forward) and 5′-AGATGCTCCAAGGTCAGGAA-3′
(reverse); miR-497, 5′-GTGCAGGGTCCGAGGT-3′ (forward), and
5′-TAGCCTGCAGCACACTGTGGT-3′ (reverse); and U6,
5′-GCTTCGGCACATATACTAAAA-3′ (forward), and
5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse).
Cell proliferation
Human NSCLC cancer cell lines Calu-1 and H1975
(3×103 cells) were seeded in 96-well plates in complete
medium [Calu-1 cells were cultured with McCoy's 5A medium
containing 10% (v/v) fetal bovine serum and 1% antibiotics, H1975
cells were maintained as monolayers when cultured in cell culture
flasks with RPMI-1640 medium containing 10% (v/v) fetal bovine
serum and 1% antibiotics] and transfected with miR-497 mimic and
miR-NC, as aforementioned. After 2 days at 37°C, 5%
CO2., cell proliferation during a time period of
consecutive 4 days was evaluated by Cell Counting Kit-8 method
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according
to the manufacturer's protocols, using a microplate reader
(Molecular Devices LLC, Sunnyvale, CA, USA) to measure the
absorbance.
Cell apoptosis
Human NSCLC cancer cell lines Calu-1 and H1975 (800
cells) were seeded in 6-well plates in complete medium [Calu-1
cells were cultured with McCoy's 5A medium containing 10% (v/v)
fetal bovine serum and 1% antibiotics, H1975 cells were maintained
as monolayers when cultured in cell culture flasks with RPMI-1640
medium containing 10% (v/v) fetal bovine serum and 1% antibiotics]
and transfected with miR-497 mimic and miR-NC, as aforementioned.
After medium replacement [Calu-1 cells were cultured with McCoy's
5A medium containing 10% (v/v) fetal bovine serum and 1%
antibiotics, H1975 cells were maintained as monolayers when
cultured in cell culture flasks with RPMI-1640 medium containing
10% (v/v) fetal bovine serum and 1% antibiotics] at 48 h
post-transfection, the cells were irradiated with 2 Gy X ray to
efficiently induce apoptosis within 48 h, and then harvested and
suspended for the assay. Subsequently, ~1×107 cells were
washed in PBS, resuspended in Annexin V and propidium iodide (PI)
staining solution containing 5 µl Annexin V, 10 µl PI and 195 µl
binding buffer (Annexin V-FITC/PI Double Dyeing Cell Apoptosis Test
kit; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) and
incubated at room temperature for 20 min under the dark condition,
and then analyzed immediately with a flow cytometer (BD FACS Aria)
by BD FACSDiva software version 4.0 (BD Biosciences; Becton,
Dickinson and Company, Franklin Lakes, NJ, USA).
Clone formation
Human NSCLC cancer cell lines Calu-1 and H1975 (800
cells) were seeded in 6-well plates in complete medium [Calu-1
cells were cultured with McCoy's 5A medium containing 10% (v/v)
fetal bovine serum and 1% antibiotics, while other cell lines were
maintained as monolayers when cultured in cell culture flasks with
RPMI-1640 medium containing 10% (v/v) fetal bovine serum and 1%
antibiotics] and transfected with miR-497 mimic and miR-NC, as
aforementioned. After medium replacement [Calu-1 cells were
cultured with McCoy's 5A medium containing 10% (v/v) fetal bovine
serum and 1% antibiotics, H1975 cells were maintained as monolayers
when cultured in cell culture flasks with RPMI-1640 medium
containing 10% (v/v) fetal bovine serum and 1% antibiotics] at 48 h
post-transfection, the cells were irradiated with a series dose of
0–8 Gy X-rays and maintained at 37°C in a humidified atmosphere
containing 5% CO2 for 12 days, when they were stained
with crystal violet at room temperature for 1 h. The colony
survival (a colony was defined as ≥50 cells) was counted under a
light microscope (×40; ECLIPSETs2; Nikon Corporation, Tokyo,
Japan). The whole process was performed three times to obtain the
mean number of colony formations.
Cell invasion
A Transwell system was employed to perform the cell
invasion assay. Briefly, resuspended Calu-1 and H1975 cells [Calu-1
cells were cultured with McCoy's 5A medium containing 10% (v/v)
fetal bovine serum and 1% antibiotics, H1975 cells were maintained
as monolayers when cultured in cell culture flasks with RPMI-1640
medium containing 10% (v/v) fetal bovine serum and 1% antibiotics]
(2×105 cells) transfected with miR-497 mimic and miR-NC,
as aforementioned, were seeded into the upper chamber prefilled
with Matrigel, and the lower chamber was added with RPMI-1640
medium supplemented with 20% FBS. After the transwell plate was
maintained in a routine cell culture incubator for a specific
period of time of 48 h, the upper chamber was retained and the
membranes were obtained for hematoxylin staining at room
temperature for 1 min. The cell number of each membrane was
determined in 3 randomly picked fields (×200 magnification) under a
light microscope. All the experiments were performed in
triplicate.
Western blotting
Cells obtained from the aforementioned treatment
(routine cell culture or after transfection with miR-497 mimic and
miR-NC) were lysed in radioimmunoprecipitation buffer (Beyotime
Institute of Biotechnology, Beijing, China), followed by high-speed
centrifugation (15,000 × g for 5 min at 4°C) and protein
quantification using a bicinchoninic acid assay. Cellular proteins
(20 µg) were separated by 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes. Following blocking in 5%
non-fat milk containing PBS at room temperature for 2 h, the
membranes were incubated with anti-VEGF receptor 2 (VEGFR2)
monoclonal primary antibodies (1:1,000 dilution; Cell Signaling
Technology, Inc. Danvers, MA, USA; #9698) at room temperature for 1
h. GAPDH (1:1,000 dilution; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA; #sc-32233) was used at room temperature for 1 h as the
loading control. Appropriate horseradish peroxidase-conjugated
secondary antibodies (anti-rabbit IgG, HRP-conjugated antibody;
1:2,000 dilution; incubated at room temperature for 1 h; Cell
Signaling Technology, Inc.; cat. no. 7074) were applied to detect
labeled proteins. The protein bands were developed with a
SuperSignal Ultra Chemiluminescent Substrate (Pierce; Thermo Fisher
Scientific, Inc.) on X-ray films (Kodak, Rochester, NY, USA).
Dual luciferase reporter assay
The possible miR-497 binding sites in KDR gene
3′-UTR were predicted using bioinformatics software (Targetscan
version 7.1; http://www.targetscan.org/vert_71/). The predicted and
mutated sequences targeting on KDR 3′-UTR were amplified and cloned
into a pMIR-REPORT Dual-Luciferase miRNA Target Expression Vector
(Promega Corporation, Madison, WI, USA; named pMIR-REPORT-KDR-WT
and pMIR-REPORT-KDR-MUT, respectively). Subsequently, Calu-1 and
H1975 cells were co-transfected with 0.5 µg pMIR-REPORT-KDR-WT or
pMIR-REPORT-KDR-MUT vectors, and 100 nM negative control (NC) miRNA
or miRNA-mimics (Shanghai GenePharma Co., Ltd., Shanghai, China)
using Lipofectamine 2000. The luciferase activities were measured
using a Dual-Luciferase Reporter Assay kit (Promega Corporation) 48
h after transfection. A control reporter was co-transfected to
provide an internal control for normalizing the activity of the
experimental reporter.
Lentivirus mediated miR-497
knockdown
Recombinant lentiviral particles expressing miR-497
(5′-CAGCAGCACACUGUGGUUUGUA-3′) or scramble control siRNA
(5′-UUCUCCGAACGUGUCACGUTT-3′) were obtained from Shanghai
GenePharma Co., Ltd. Cells were grown to 40% confluence and
transduced with complete medium [Calu-1 cells were cultured with
McCoy's 5A medium containing 10% (v/v) fetal bovine serum and 1%
antibiotics, and H1975 cells were maintained as monolayers when
cultured in cell culture flasks with RPMI-1640 medium containing
10% (v/v) fetal bovine serum and 1% antibiotics] containing
lentiviral particles expressing miR-497 or scramble control siRNA
at concentrations of 1×108 transducing units/ml
[multiplicity of infection (MOI) of 20] (18) at 37°C for 48 h. Polybrene (cat. no.
H9268; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at a
concentration of 8 µg/ml was added simultaneously to increase
infection efficiency. No adverse effects were observed on the cell
viability by siRNA or Polybrene (data not shown). The siRNAs had no
off-target effects, and did not affect cell adherence, shape and
viability at the MOI of 20, according to manufacturer's protocol
and treatment duration. The cells were used at 48 h after
infection.
Nude mice model of ectopic tumor
Athymic nude (nu/nu) mice (male:female, 1:1 ratio;
total 8 mice) at 6 weeks old (20–22 g) were purchased from Shanghai
SLAC Laboratory Animal Co., Ltd. (Shanghai, China), housed in an
air-conditioned room at 22°C with a 12/12 h light/dark cycle and
40–60 % humidity and free access to food and water. The tumors were
generated by subcutaneous injection of 2×106 miR-497 or
scramble control siRNA lentivirus particles infected Calu-1 cell
suspended in 50 µl PBS into the dorsal region near the thigh. Mice
were then weighed and assessed for tumor size every three day by
measuring tumor length and tumor width. Furthermore, the body
weight of the mice was also determined every 5 days. At week 4
post-treatment, all the mice were sacrificed by cervical
dislocation and the tumors were excised, weighed and imaged. The
study protocol was approved by the Institutional Animal Care and
Use Committee of the First People's Hospital of Lianyungang, which
is adherent to the generally accepted international guidelines for
animal experimentation (26).
Statistical analysis
All statistical analyses were conducted using SPSS
v18 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 7.0 (GraphPad
Software Inc., San Diego, CA, USA). Data are presented as the mean
± standard deviation. The unpaired Student's t-test or one-way
analysis of variance followed by Tukey's post hoc test was used to
examine differences between groups. Dose-response cell survival
curves were fitted to a multitarget model,
S=1-(1-e-D/D0)N. S, ratio of survival tumor cells at a
given dose; D, a given dose; D0, the dose increment that reduces
the cell survival to 37% of the initial value depicted on the
exponential portion of the curve; N, back extrapolation of the
exponential portion of the survival curve to zero dose. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of miR-497 in NSCLC tissues
and cell lines
In order to investigate the role of miR-497 in
NSCLC, the expression level of miR-497 was examined in 15 NSCLC
tissues and 8 non-cancerous adjacent normal tissues, and the
results demonstrated that significantly decreased levels of miR-497
expression in NSCLC, compared with normal tissues (0.47±0.27 vs.
1.03±0.27; P<0.05; Fig. 1A).
Furthermore, the expression of miR-497 and KDR in 8 NSCLC cancer
cell lines was also examined, and the results demonstrated that KDR
and miR-497 were significantly decreased in H1299 and H1975 cells,
compared with in other cell lines, respectively (Fig. 1B; P<0.05). Additionally, H1975
and Calu-1 cell lines with reduced expression of miR-497 were
further employed for the following experiments.
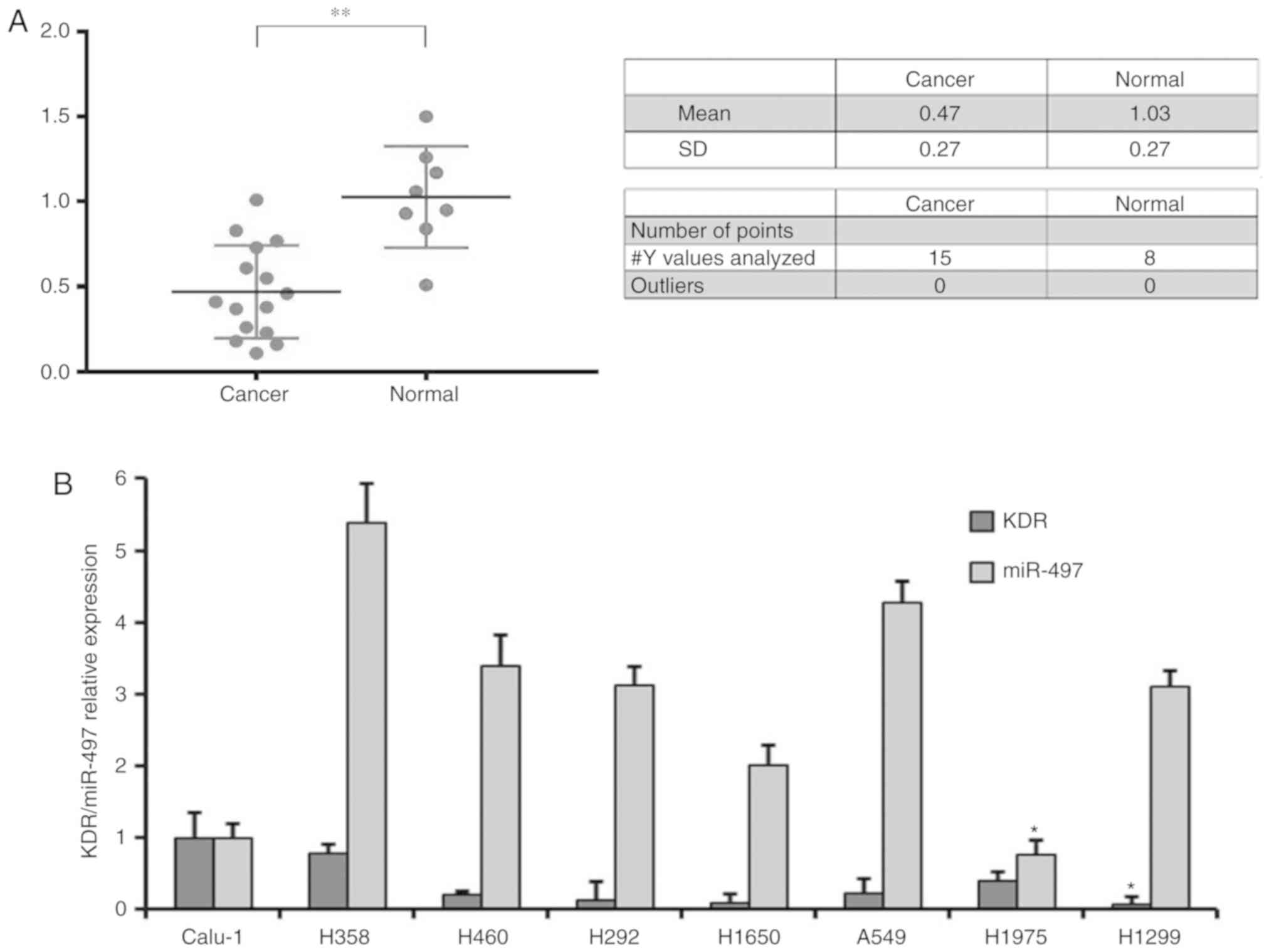 | Figure 1.Reverse transcription-quantitative
polymerase chain reaction evaluation of the expression level of
miR-497 in NSCLC tissues and cell lines. (A) A total of 15
paraffin-embedded NSCLC tissues and 8 non-cancerous adjacent normal
tissues were collected for miR-497 level evaluation. Significantly
decreased miR-497 level was determined in NSCLC tissues, compared
with the control tissues. (B) A total of 8 NSCLC cell lines,
including H1975, A549, H358, H1650, H460, Calu-1, H1299 and H292,
were collected for miR-497 level evaluation. Calu-1 and H1975 cells
were confirmed as having the lowest two expression levels of
miR-497 among the 8 cell lines. *P<0.05, compared with the other
cell lines. **P<0.01. NSCLC, non-small cell lung cancer; miR,
microRNA; KDR, kinase insert domain receptor. |
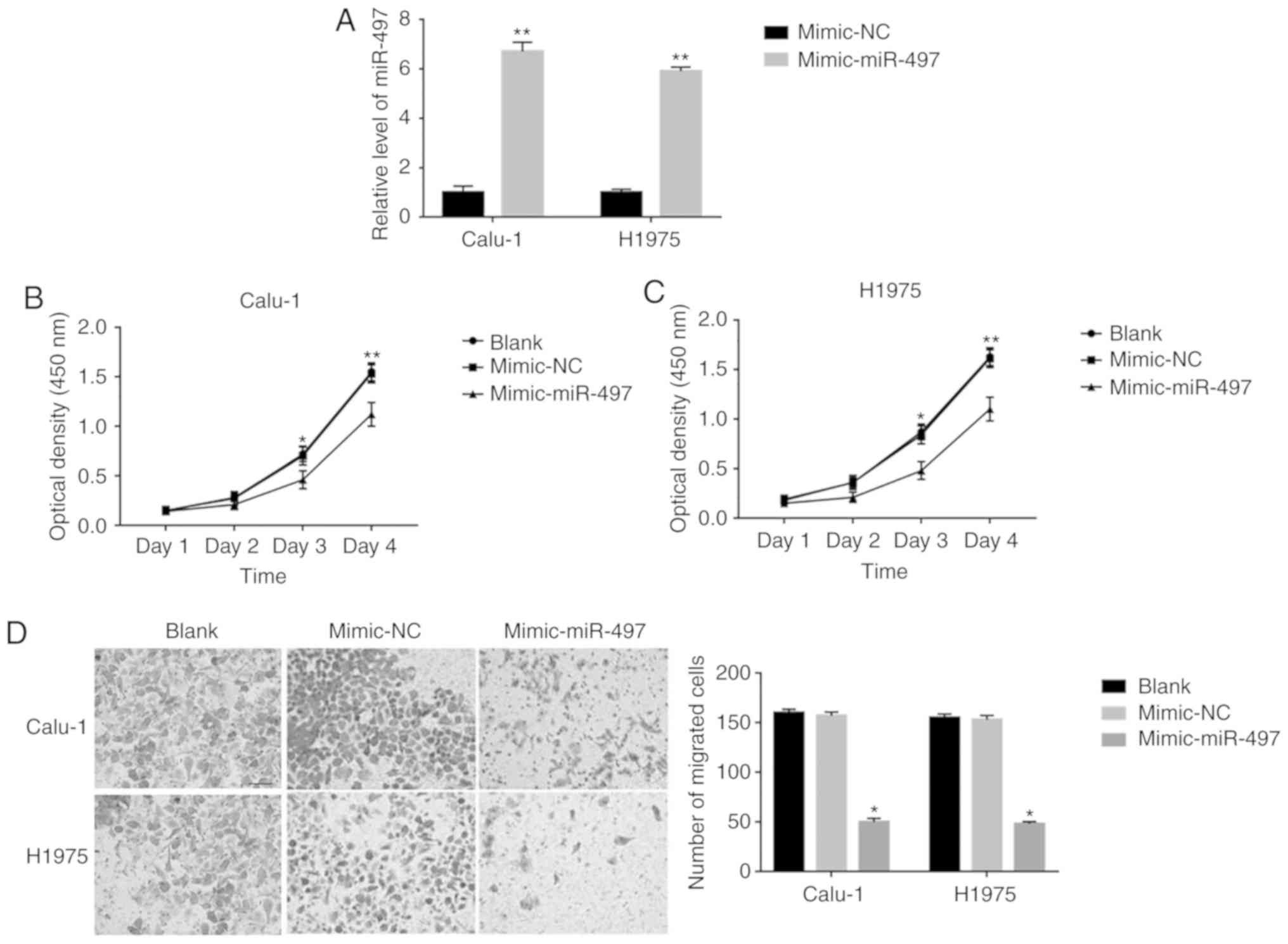
miR-497 overexpression decreases cell
proliferation and migration in Calu-1 and H1975 cells
In order to observe the effects of miR-497 on the
cell behavior of Calu-1 and H1975 cells, miR-497 overexpression was
performed on Calu-1 and H1975 cells. As depicted in Fig. 2A, a 6.7 and 5.9 times expression
level increase was exhibited in miR-497-mimic transfected Calu-1
and H1975 cells, compared with mimic-NC-transfected cells,
respectively. For cell proliferation, a significantly decreased
cell proliferation was observed on days 3 and 4 in
miR-497-mimic-transfected Calu-1 and H1975 cells, compared with
mimic-NC transfected cells (Fig. 2B and
C). Furthermore, a significantly decreased cell invasion was
also observed in miR-497-mimic-transfected Calu-1 (50±3.5 vs.
157±3.7; P<0.001) and H1975 (48±2.4 vs. 153±4.2; P<0.001)
cells, compared with mimic-NC-transfected cells (Fig. 2D). These results indicated that
miR-497 overexpression decreases cell proliferation and migration
in Calu-1 and H1975 cells.
miR-497 overexpression increases cell
apoptosis and radiosensitivity in Calu-1 and H1975 cells
The effects of miR-497 overexpression on the cell
apoptosis and radiosensitivity in Calu-1 and H1975 cells was
further evaluated. The results demonstrated that there was
significantly increased cell apoptosis following 2 Gy radiation in
miR-497-mimic-transfected Calu-1 and H1975 cells, compared with
mimic-NC-transfected cells (Fig.
3A; P<0.001). Furthermore, the clone formation was
consistently decreased after 2, 4, 6 and 8 Gy radiation, and the
sensitization enhancement ratio in Calu-1 and H1975 were 1.48 and
1.58, respectively (Fig. 3B and C),
and miR-497 significantly increased the radiosensitivity of these
cells. These results indicated the enhanced radiosensitivity
following miR-497 transfection.
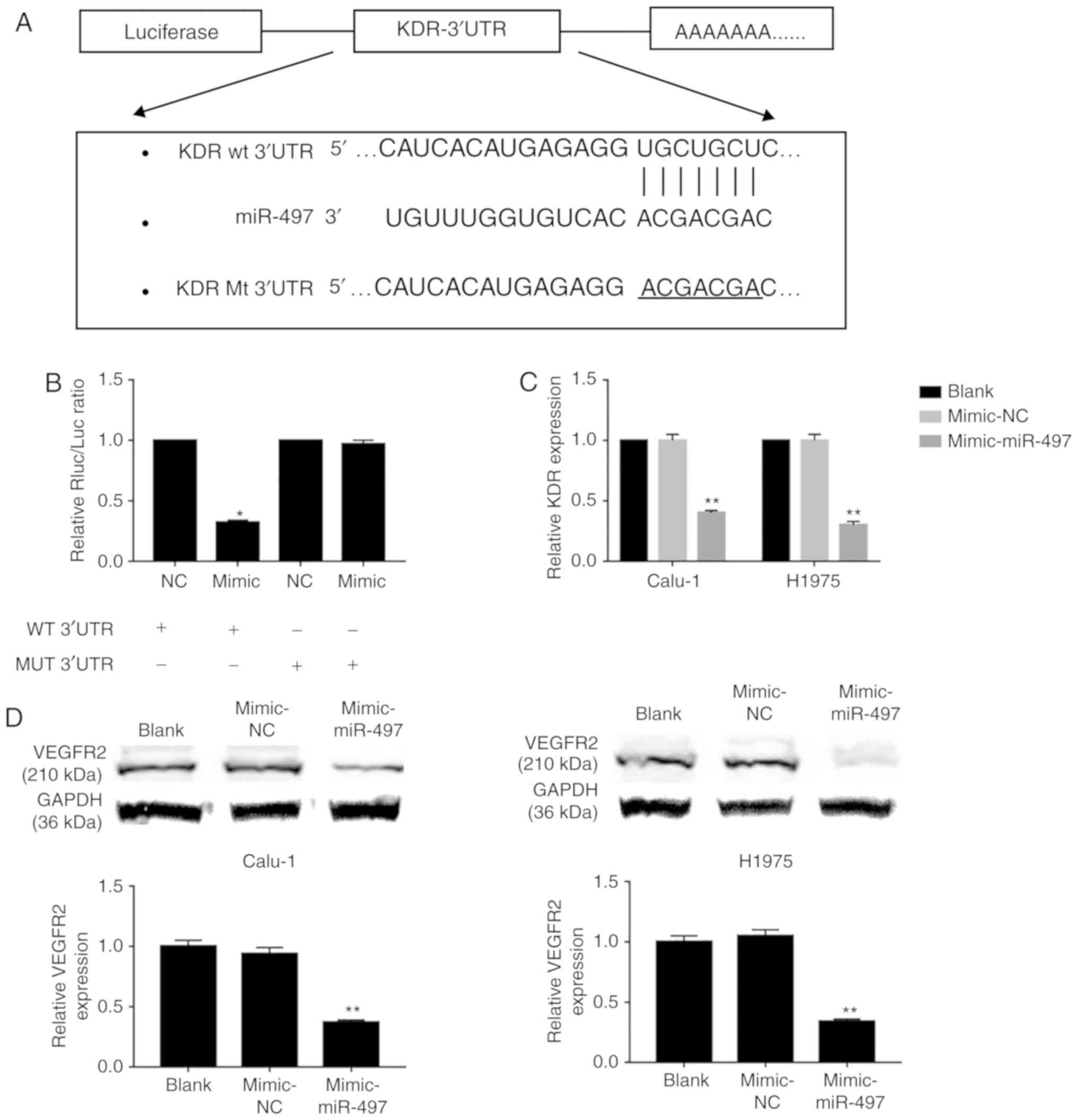
miR-497 affects the cell behavior of
Calu-1 and H1975 via targeting KDR
In order to investigate the mechanism of miR-497 in
NSCLC cell lines Calu-1 and H1975, TargetScan was searched and it
was determined that KDR may be the potential target gene of miR-497
(Fig. 4A). Subsequently the KDR
wild-type and mutated plasmids were constructed, and a luciferase
assay was performed to confirm its interaction with miR-497. The
results demonstrated that miR-497-mimic transfection significantly
decreases luciferase activity in KDR wild-type, compared with
mimic-NC transfected cells, while no difference was determined on
the cells transfected with KDR mutated plasmid following
miR-497-mimic or mimic-NC transfection (Fig. 4B). Furthermore, it was determined
that there is significantly decreased expression of KDR in
miR-497-mimic-transfected Calu-1 and H1975 cells, compared with
mimic-NC-transfected cells (Fig.
4C). Additionally, it was also observed that there is a
significantly decreased level of VEGFR2 in
miR-497-mimic-transfected Calu-1 and H1975 cells, compared with
mimic-NC-transfected cells (Fig.
4D).
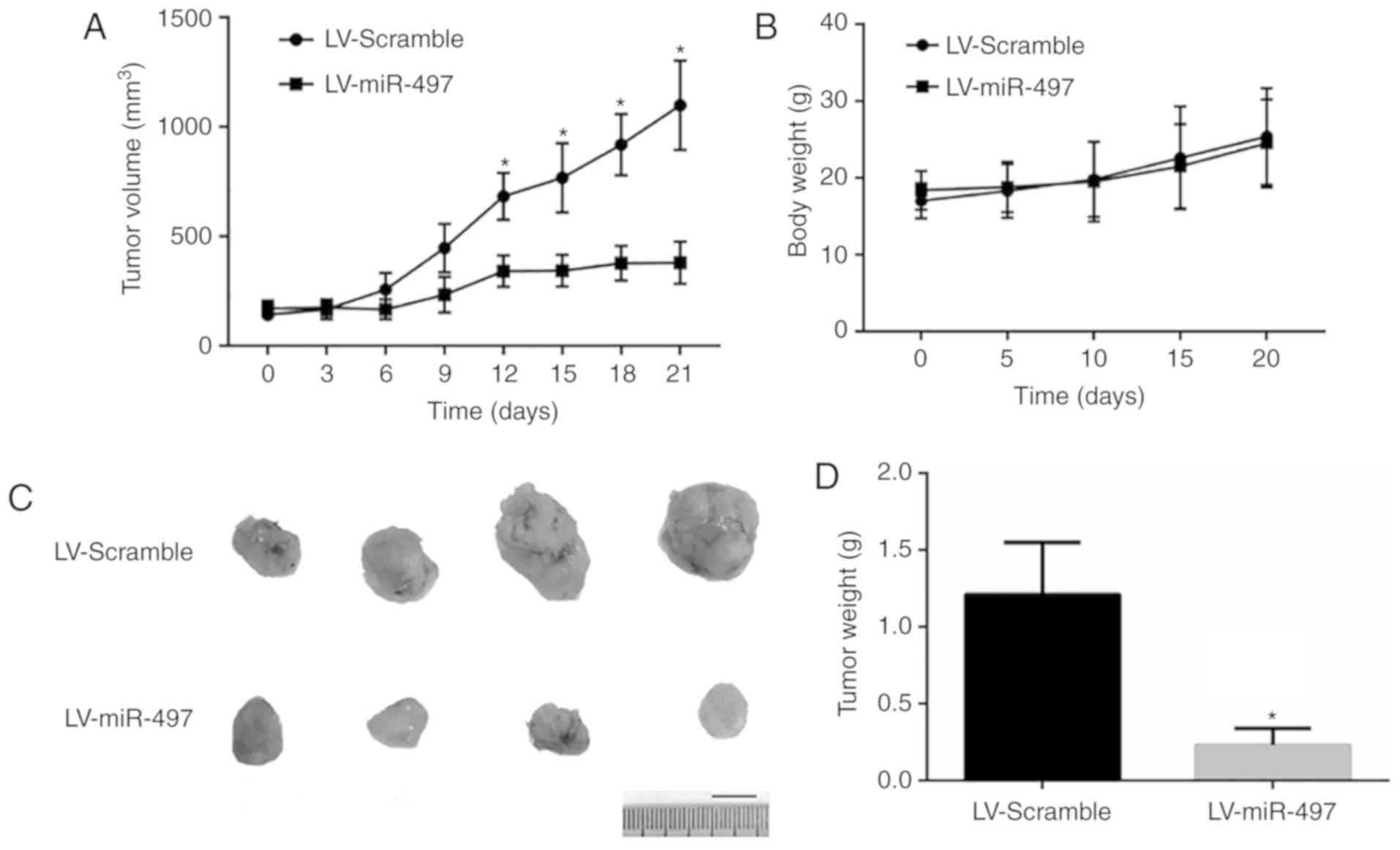
miR-497 decreases tumor growth in
vivo
Due the tumor-promoting effects of miR-497 observed
in vitro, ectopic tumor nude mice models were established to
test the effects of miR-497 in vivo. As depicted in Fig. 5, miR-497 knockdown human lung
squamous carcinoma cell line Calu-1 exhibited a decreased level of
tumor growth in vivo according to the tumor size and weight.
These results indicated that miR-497 acts as a negative regulator
on tumor growth in vivo.
Discussion
In the present study, it was demonstrated that
miR-497 was downregulated in NSCLC specimens, compared with the
adjacent normal tissues. Furthermore, an in vitro assay also
verified that miR-497 overexpression could inhibit cell
proliferation and invasion, and promote cell apoptosis and
radiosensitivity. Additionally, KDR was also confirmed as the
direct target gene of miR-497. Based on our previous study
(24,27), the data in the present study
indicated the regulatory role of miR-497 on malignant behavior of
lung cancer growth and metastasis via targeting KDR, which may
serve as a malignant phenotype prediction molecule in NSCLC.
miR-497 is a common exhibited miRNA in all human
organs and tissues, including breast, lung, liver and blood
(28,29). Dysregulated levels of miR-497 were
firstly observed in miRNA expression profile results of human
breast cancer, and significantly downregulated miR-497 level was
then confirmed in breast cancer tissues (30). Further studies also confirmed
downregulated levels of miR-497 in multiple malignant types of
cancer, including gastrointestinal cancer types, thoracic cancer,
reproduction cancer, and head and neck cancer types (31–33),
and miR-497 served as an inhibitory regulator in the development
and progression of cancer (31–33).
In the present study, by collecting NSCLC tissues, it was
determined that there is significantly downregulated miR-497
expression in NSCLC tissues, compared with normal tissues,
indicating the possible role of miR-497 in NSCLC.
According to previous studies, abnormal expression
levels of miR-497 are involved in cancer progression, including
proliferation, invasion, cell cycle regulation, apoptosis and
angiogenesis (34,35). Furthermore, multiple studies
supported the observation of miR-497 as a tumor suppressor. For
example, miR-497 overexpression in breast cancer could suppress
tumor proliferation and invasion via target gene downregulation
(36). The in vivo and in
vitro system of liver cancer also confirmed that the
upregulation of miR-497 could inhibit angiogenesis and metastasis
of liver cancer (37). In contrast,
miR-497 inhibitor transfection could result in tumor growth and
proliferation promotion effects in cancer (38). Xu et al (39) determined that the expression levels
of miR-497 were reduced in chemotherapy-resistant ovarian cancer
cells and tumor tissues due to hypermethylation of the miR-497
promoter. However, Lan et al (40) determined that miR-497 is
overexpressed in glioma and that hypoxia can induce the expression
of miR-497 at the transcriptional level by binding with the hypoxia
response element in the promoter. They also indicated that ectopic
overexpression of miR-497 promotes chemotherapy resistance in
glioma cells by targeting programmed cell death 4, a tumor
suppressor that is involved in apoptosis (40). Discrepancies regarding the role of
miR-497 in cancer may result from the heterogeneous of miR-497
expression and different tumor microenvironment. In NSCLC, miR-497
was demonstrated to exhibit a previously unappreciated role in the
suppression of VEGF-A-mediated NSCLC cancer cell growth and
invasion (31). Consistent with
previous study, in the present study, it was demonstrated that
miR-497 overexpression could inhibit cell proliferation and
invasion, promote cancer cell apoptosis and decrease cell clone
formation following radiation treatment.
A single miRNA can target hundreds of mRNAs, and a
series of target genes were determined to exert inhibitory effects
by interacting with miR-497, including cyclin E1, B-cell lymphoma,
insulin like growth factor 1 receptor and VEGFR2 (41–44).
In the present study, the dual luciferase reporter gene assay
revealed that KDR was the direct target gene for miR-497 at the
45–51 sites of 3′UTR, which is consistent with results in kidney
cancer (45). It was also
determined that miR-497 could downregulate KDR mRNA and protein
levels of VEGFR2 (45). According
to a previous study by Shi et al (46), VEGFR2knockdown could result in
decreased survival fraction of the lung cancer cells, angiogenesis
and migration during carcinogenesis. Furthermore, the direct
blockage of VEGFR2 by monoclonal antibody ramucirumab could result
in clinical benefits, compared with docetaxel only-treated
patients, in terms of progression-free survival, objective response
rate and disease control rate in Asian patients with NSCLC
(47). Additionally, ramucirumab
was well tolerated with manageable toxicity (47). Furthermore, Ding et al
(48) examined the expression of
KDR in NSCLC tissues, and they determined that the positive
immunostaining rate for VEGFR2 was 58%. Levels of VEGFR2 in lung
tumors were significantly increased compared with in the control
tissue (χ2=11.22; P=0.001). Statistically significant
correlations were observed with histological grade, clinical TNM
stage and the lymph node status (P<0.05), but not age, sex or
pathology type (P>0.05) between NSCLC tissues and control
tissues. Furthermore, significant decreased overall survival time
and progression-free survival time were observed between the groups
with increased VEGFR2 expression and those with reduced expression
(P<0.05). Reduced levels of VEGFR2 in lung cancer tissues were
consistent with the results that miR-497 directly targeted KDR and
downregulated KDR expression, thereby resulting in inhibition of
malignant behaviors of NSCLC cells. In our previous study, it was
also determined that KDR silence and pharmacological inhibition of
VEGFR2 could result in the inhibition of cell proliferation,
invasion and radiosensitivity enhancement (27), and these results were consistent
with the results in the present study regarding miR-497.
Furthermore, since this paper did not perform a
correlation analysis between the miR-497 expression and clinical
characteristics, the role of miR-497 on prognosis of the patients
could not be evaluated. Additionally, the sample size may be
insufficient to elucidate the role of miR-497 in lung cancer
according to previous studies [17 in the study by Devery et
al (49); and 24 in the study
by Zhu et al (50)].
In conclusion, the present study demonstrated that
miR-497 was downregulated in NSCLC specimens and it served as a
tumor suppressor to inhibit cancer cell proliferation and invasion,
and increase radiosensitivity via targeting KDR. The regulatory
role between miR-497 and KDR may provide novel insight for lung
cancer progression control, radiotherapy sensitivity enhancement
and target therapy strategy.
Acknowledgements
The authors would like to thank Professor Aldo Pinto
from Department of Pharmacy, School of Pharmacy, University of
Salerno (Fisciano, Italy) for his critical reading of the present
manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472792), the
Natural Science Foundation of Jiangsu Province, China (grant no.
BK20151279), the Youth Talent Foundation of Lianyungang First
People's Hospital (grant no. QN140202), the Science and Technology
Development Program of Lianyungang City(grant no. ZD1404), the ‘521
Project’ Foundation of Lianyungang City, the ‘333 Project’
Foundation of Jiangsu Province (grant no. BRA2016301). the Science
and Technology Project Foundation of Suzhou (grant no.
SYS201504).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX, CH, LianL and XJ conceived and designed the
experiments. YX, CH, LL, KH, LW, YQ, LiangL and LijunL performed
the experiments. YX, CH, LianL, KH, LW, YQ, LiangL, LijunL and XJ
analyzed the data. YX, CH, LianL, KH, LW, YQ, LiangL, LijunL and XJ
contributed reagents, materials and analysis tools. YX, CH, LianL
and XJ contributed to the writing of the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of the First People's Hospital of Lianyungang
(Lianyungang, China). Written informed consent was obtained from
the participating individuals.
Patient consent for publication
Written informed consent was obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang S, Wang X, Zhou Q, Xu Y, Xia W, Xu W,
Ma Z, Qiu M, You R, Xu L and Yin R: Stereotactic ablative
radiotherapy versus lobectomy for stage I non-small cell lung
cancer: A systematic review. Thorac Cancer. 9:337–347. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hassan O, Ahmad A, Sethi S and Sarkar FH:
Recent updates on the role of microRNAs in prostate cancer. J
Hematol Oncol. 5:92012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du L and Pertsemlidis A: microRNA
regulation of cell viability and drug sensitivity in lung cancer.
Expert Opin Biol Ther. 12:1221–1239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Acunzo M, Visone R, Romano G, Veronese A,
Lovat F, Palmieri D, Bottoni A, Garofalo M, Gasparini P, Condorelli
G, et al: miR-130a targets MET and induces TRAIL-sensitivity in
NSCLC by downregulating miR-221 and 222. Oncogene. 31:634–642.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeon HS, Lee SY, Lee EJ, Yun SC, Cha EJ,
Choi E, Na MJ, Park JY, Kang J and Son JW: Combining
microRNA-449a/b with a HDAC inhibitor has a synergistic effect on
growth arrest in lung cancer. Lung Cancer. 76:171–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Sempere LF, Guo Y, Korc M,
Kauppinen S, Freemantle SJ and Dmitrovsky E: Involvement of
microRNAs in lung cancer biology and therapy. Transl Res.
157:200–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Itesako T, Seki N, Yoshino H, Chiyomaru T,
Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa M
and Enokida H: The microRNA expression signature of bladder cancer
by deep sequencing: The functional significance of the miR-195/497
cluster. PLoS One. 9:e843112014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Jin X, Deng X, Zhang G, Zhang B and
Ma L: The putative tumor suppressor microRNA-497 modulates gastric
cancer cell proliferation and invasion by repressing eIF4E. Biochem
Biophys Res Commun. 449:235–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie Y, Wei RR, Huang GL, Zhang MY, Yuan YF
and Wang HY: Checkpoint kinase 1 is negatively regulated by miR-497
in hepatocellular carcinoma. Med Oncol. 31:8442014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du M, Shi D, Yuan L, Li P, Chu H, Qin C,
Yin C, Zhang Z and Wang M: Circulating miR-497 and miR-663b in
plasma are potential novel biomarkers for bladder cancer. Sci Rep.
5:104372015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L, Zheng W, Song Y, Du X, Tang Y, Nie
J and Han W: miRNA-497 enhances the sensitivity of colorectal
cancer cells to neoadjuvant chemotherapeutic drug. Curr Protein
Pept Sci. 16:310–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao WY, Wang Y, An ZJ, Shi CG, Zhu GA,
Wang B, Lu MY, Pan CK and Chen P: Downregulation of miR-497
promotes tumor growth and angiogenesis by targeting HDGF in
non-small cell lung cancer. Biochem Biophys Res Commun.
435:466–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shibuya M: Vascular endothelial growth
factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A
crucial target for anti- and pro-angiogenic therapies. Genes
Cancer. 2:1097–1105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Chen J, Guo Y, Wang B and Chu H:
Strategies targeting angiogenesis in advanced non-small cell lung
cancer. Oncotarget. 8:53854–53872. 2017.PubMed/NCBI
|
|
17
|
Guo L, Zhang F, Cai Y and Liu T:
Expression profiling of integrins in lung cancer cells. Pathol Res
Pract. 205:847–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brower M, Carney DN, Oie HK, Gazdar AF and
Minna JD: Growth of cell lines and clinical specimens of human
non-small cell lung cancer in a serum-free defined medium. Cancer
Res. 46:798–806. 1986.PubMed/NCBI
|
|
19
|
Suzuki S, Takahashi T, Nakamura S, Koike
K, Ariyoshi Y, Takahashi T and Ueda R: Alterations of integrin
expression in human lung cancer. Jpn J Cancer Res. 84:168–174.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banks-Schlegel SP, Gazdar AF and Harris
CC: Intermediate filament and cross-linked envelope expression in
human lung tumor cell lines. Cancer Res. 45:1187–1197.
1985.PubMed/NCBI
|
|
21
|
Hu X, Shi S, Wang H, Yu X, Wang Q, Jiang
S, Ju D, Ye L and Feng M: Blocking autophagy improves the
anti-tumor activity of afatinib in lung adenocarcinoma with
activating EGFR mutations in vitro and in vivo. Sci Rep.
7:45592017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen QY, Wu LJ, Wu YQ, Lu GH, Jiang ZY,
Zhan JW, Jie Y and Zhou JY: Molecular mechanism of trifluoperazine
induces apoptosis in human A549 lung adenocarcinoma cell lines. Mol
Med Rep. 2:811–817. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giaccone G, Battey J, Gazdar AF, Oie H,
Draoui M and Moody TW: Neuromedin B is present in lung cancer cell
lines. Cancer Res. 52 (Suppl 9):S2732–S2736. 1992.
|
|
24
|
Liu Y, Qiao Y, Hu C, Liu L, Zhou L, Liu B,
Chen H and Jiang X: VEGFR2 inhibition by RNA interference affects
cell proliferation, migration, invasion, and response to radiation
in Calu-1 cells. Clin Transl Oncol. 18:212–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji Y, Strawn TL, Grunz EA, Stevenson MJ,
Lohman AW, Lawrence DA and Fay WP: Multifaceted role of plasminogen
activator inhibitor-1 in regulating early remodeling of vein bypass
grafts. Arterioscler Thromb Vasc Biol. 31:1781–1787. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferdowsian HR and Beck N: Ethical and
scientific considerations regarding animal testing and research.
PLoS One. 6:e240592011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Qiao Y, Hu C, Liu Y, Xia Y, Wang L,
Liu B, Chen H and Jiang X: Endostatin exerts radiosensitizing
effect in non-small cell lung cancer cells by inhibiting VEGFR2
expression. Clin Transl Oncol. 18:18–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ruan K, Fang X and Ouyang G: MicroRNAs:
Novel regulators in the hallmarks of human cancer. Cancer Lett.
285:116–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rapa I, Votta A, Felice B, Righi L,
Giorcelli J, Scarpa A, Speel EJ, Scagliotti GV, Papotti M and
Volante M: Identification of MicroRNAs differentially expressed in
lung carcinoid subtypes and progression. Neuroendocrinology.
101:246–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu A, Lu J, Wang W, Shi C, Han B and Yao
M: Role of miR-497 in VEGF-A-mediated cancer cell growth and
invasion in non-small cell lung cancer. Int J Biochem Cell Biol.
70:118–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang S, Mo Y, Midorikawa K, Zhang Z, Huang
G, Ma N, Zhao W, Hiraku Y, Oikawa S and Murata M: The potent tumor
suppressor miR-497 inhibits cancer phenotypes in nasopharyngeal
carcinoma by targeting ANLN and HSPA4L. Oncotarget. 6:35893–35907.
2015.PubMed/NCBI
|
|
33
|
Liu A, Huang C, Cai X, Xu J and Yang D:
Twist promotes angiogenesis in pancreatic cancer by targeting
miR-497/VEGFA axis. Oncotarget. 7:25801–25814. 2016.PubMed/NCBI
|
|
34
|
Yang G, Xiong G, Cao Z, Zheng S, You L,
Zhang T and Zhao Y: miR-497 expression, function and clinical
application in cancer. Oncotarget. 7:55900–55911. 2016.PubMed/NCBI
|
|
35
|
Zhao X, Zhao Z, Xu W, Hou J and Du X:
Down-regulation of miR-497 is associated with poor prognosis in
renal cancer. Int J Clin Exp Pathol. 8:758–764. 2015.PubMed/NCBI
|
|
36
|
Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang
Y, Zou C, Zhang X, Liu S, Wang X, et al: Analysis of miR-195 and
miR-497 expression, regulation and role in breast cancer. Clin
Cancer Res. 17:1722–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y,
Li PY, Wang M, Lin JS and He XX: miR-497 suppresses angiogenesis
and metastasis of hepatocellular carcinoma by inhibiting VEGFA and
AEG-1. Oncotarget. 6:29527–29542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei C, Luo Q, Sun X, Li D, Song H, Li X,
Song J, Hua K and Fang L: MicroRNA-497 induces cell apoptosis by
negatively regulating Bcl-2 protein expression at the
posttranscriptional level in human breast cancer. Int J Clin Exp
Pathol. 8:7729–7739. 2015.PubMed/NCBI
|
|
39
|
Xu S, Fu GB, Tao Z, OuYang J, Kong F,
Jiang BH, Wan X and Chen K: miR-497 decreases cisplatin resistance
in ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget.
6:26457–26471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lan J, Xue Y, Chen H, Zhao S, Wu Z, Fang
J, Han C and Lou M: Hypoxia-induced miR-497 decreases glioma cell
sensitivity to TMZ by inhibiting apoptosis. FEBS Lett.
588:3333–3339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Han Z, Zhang Y, Yang Q, Liu B, Wu J, Zhang
Y, Yang C and Jiang Y: miR-497 and miR-34a retard lung cancer
growth by co-inhibiting cyclin E1 (CCNE1). Oncotarget.
6:13149–13163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen L, Li J, Xu L, Ma J, Li H, Xiao X,
Zhao J and Fang L: miR-497 induces apoptosis of breast cancer cells
by targeting Bcl-w. Exp Ther Med. 3:475–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu JW, Wang TX, You L, Zheng LF, Shu H,
Zhang TP and Zhao YP: Insulin-like growth factor 1 receptor
(IGF-1R) as a target of miR-497 and plasma IGF-1R levels associated
with TNM stage of pancreatic cancer. PLoS One. 9:e928472014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang
B, Shu Y and Liu P: miR-497 modulates multidrug resistance of human
cancer cell lines by targeting BCL2. Med Oncol. 29:384–391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pengcheng S, Ziqi W, Luyao Y, Xiangwei Z,
Liang L, Yuwei L, Lechen L and Wanhai X: MicroRNA-497 suppresses
renal cell carcinoma by targeting VEGFR-2 in ACHN cells. Biosci
Rep. 37:BSR201702702017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi L, Zhang S, Wu H, Zhang L, Dai X, Hu
J, Xue J, Liu T, Liang Y and Wu G: miR-200c increases the
radiosensitivity of non-small-cell lung cancer cell line A549 by
targeting VEGF-VEGFR2 pathway. PLoS One. 8:e783442013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hosomi Y, Yoh K, Kasahara K, Yamada K,
Takahashi T, Hida TTK, Yoshioka H, Kato T, Takeda K, Nishio M, et
al: Docetaxel + ramucirumab (DR) versus docetaxel + placebo (D) as
second-line treatment for advanced non-small cell lung cancer
(NSCLC): A randomized, phase II, double-blind, multicenter trial in
Japan. J Clin Oncol. 33:8054. 2015. View Article : Google Scholar
|
|
48
|
Ding M, Liu L, Hu C, Liu Y, Qiao Y and
Jiang X: Expression of VEGFR2 and NRP-1 in non-small cell lung
cancer and their clinical significance. Chin J Cancer Res.
26:669–677. 2014.PubMed/NCBI
|
|
49
|
Devery AM, Wadekar R, Bokobza SM, Weber
AM, Jiang Y and Ryan AJ: Vascular endothelial growth factor
directly stimulates tumour cell proliferation in non-small cell
lung cancer. Int J Oncol. 47:849–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu J, Zeng Y, Li W, Qin H, Lei Z, Shen D,
Gu D, Huang JA and Liu Z: CD73/NT5E is a target of miR-30a-5p and
plays an important role in the pathogenesis of non-small cell lung
cancer. Mol Cancer. 16:342017. View Article : Google Scholar : PubMed/NCBI
|