Introduction
Melanoma is a malignant tumor that originates from
cells that produce melanin. It is characterized by the sudden
appearance, or rapid growth, of moles with deepening color and
comet-like structure. The symptoms of this type of cancer include
local pain, infection, ulcer or bleeding and enlarged lymph nodes
(1). Melanoma tumors occur most
often in the lower limbs, followed by the head, neck, upper limbs
and eyes. Early metastasis occurs via lymphatic and blood vessels
to the liver, brain, bone and mucosa, among other tissues (2). Biotherapy is a new choice for tumor
treatment (3) and is characterized
by fewer complications and less toxic and side effects than
traditional therapeutic approaches (2). Biotherapy can be used alone or as
complementary treatment following surgery, radiotherapy and
chemotherapy (4). Hence, biotherapy
is considered an important development in the treatment of
malignant tumors, particularly melanoma (5).
Dendritic cells (DCs), the most powerful antigen
presenting cells (APCs) in vivo and the only APCs that can
activate T lymphocytes, have an important role in connecting innate
and adaptive immunity (6). Their
function is to present pathogen-derived antigen peptides, via major
histocompatibility molecules (MHC), to immature T lymphocytes in
lymphoid organs. Through this process, DCs form the key link
between innate and adaptive immunity, which is essential for
antigen-specific immune responses (7).
IL-12 is an immunoregulatory cytokine that functions
as an important link in the process of immune regulation (8). IL-12 can inhibit tumor growth through
the following pathways: Stimulation of T cell activation and
promotion of the transformation of naïve T cells into T helper type
1 cells; activation of cytotoxic T lymphocytes (CTLs) and natural
killer cells and promotion of the secretion of tumor necrosis
factor (TNF)-α and other cytokines (9). Moreover, IL-12 can upregulate the
levels of vascular adhesion molecule-1 and cadherin, induce
apoptosis and promote neovascularization (10–12).
Hence, IL-12 is a promising immunotherapeutic agent that links
innate and adaptive immunity (13).
IL-12 has been observed to have antitumor effects in several
preclinical and clinical studies (14–16).
In the present study, monocytes were isolated and differentiated
into mature DCs, which were then transfected with lentiviruses
constructed to overexpress IL-12. Thereafter, these genetically
modified DCs were directly injected into melanoma tumors in model
mice, and their antitumor effects were evaluated.
Materials and methods
Preparation of DCs
Twenty specific pathogen-free male C57BL/6 mice
(weighing ~22 g; 4 weeks old) were purchased from Changzhou Cavans
Laboratory Animal Co. Ltd. [license no. SCXK (Su) 2016-0010] and
housed in a specific pathogen-free condition that was automatically
maintained at a temperature of 23±2°C, a relative humidity of 45–65
%, and with a controlled 12 h light/dark cycle and access to food
and water ad libitum. Mice were sacrificed by cervical
dislocation following anaesthesia with isoflurane, immersed in 75%
ethanol for 5–10 min, and then their spleens were removed by
aseptic surgery, and the tissues were soaked in Hank's solution
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Spleens
were rinsed repeatedly until they turned white. Then, cell
suspensions were collected, and mononuclear cells were obtained by
density gradient centrifugation (987 × g, for 20 min), using
lymphocyte separation solution (C-44010; Bio-Connect B.V., Huissen,
The Netherlands), and then washed twice with phosphate-buffered
saline (PBS) by centrifugation. Spleen mononuclear cell density was
adjusted to 1×107/ml, using lymphocyte separation
solution, and cells were plated into 6-well tissue culture plates
(2 ml/well), and incubated at 37°C in a 5% CO2
incubator. After 2 h, medium was aspirated from the suspended
cells, and replaced with Dulbecco's modified Eagle's medium,
containing 10% fetal bovine serum (FBS), and supplemented with
granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4
(0.1 g/l). On the 7th day, lipopolysaccharides (LPS) were added to
the medium at a final concentration of 1 µg/l, and after 24 h of
further incubation, cells were collected. Antibodies against CD40
(cat. no. 553723), CD80 (cat. no. 561955) and CD86 (cat. no.
561962) (all from BD Biosciences, Franklin Lakes, NJ, USA) were
used to identify mature DCs by flow cytometer method as previously
described (6). The experimental
protocols were approved by the Ethics Committee of Jiangxi
Provincial People's Hospital (Nanchang, China).
Construction of the IL-12
overexpression vector
The IL-12 gene sequence was identified in the
NCBI database (https://www.ncbi.nlm.nih.gov/gene/16159) and cloned
into the EcoRI and BstBI restriction enzyme sites of
the pCDH-CMV-MCS-EF1-CopGFP-T2A-Puro lentivirus vector
(CD513B-1-SBI; System Biosciences LLC, Palo Alto, CA, USA).
Tumor model and experimental
groups
Mouse melanoma cells were purchased from the Cell
Bank of the Shanghai Academy of Sciences (Shanghai, China) and
cultured in RPMI-1640 medium (HyClone; GE Healthcare, Chicago, IL,
USA). Cultures were supplemented with 10% fetal bovine serum (FBS)
(SKU: 04-007-1A; Biological Industries, Kibbutz Beit-Haemek,
Israel) and 100 U/ml penicillin-streptomycin (P1400; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China), in 5%
CO2 at 37°C. Cells used in the experiments exhibited
~70% confluence. B16 cells (1×107) were subcutaneously
administered into the right forelimb armpit of each mouse (C57BL/6,
male, 4-weeks old). Twelve days after injection, tumors were
formed, and mice were randomly divided into four groups: i) Tumors
injected with PBS (controls); ii) tumors injected with untreated
DCs (DC group); iii) tumors injected with DCs transfected with
empty vector (DC + vector group); and iv) tumors injected with DCs
transfected with vector overexpressing IL-12 (DC + IL-12 group).
Different types of DCs (untreated, transfected with lentiviral
vector alone, and transfected with DCs overexpressing IL-12) were
injected into the tumors on the first, third, and seventh days
after tumor formation; each mouse received 1×106 DCs
diluted in 0.2 ml PBS. One week after treatment, mice were
sacrificed by cervical dislocation, fixed on a sterile towel sheet,
and the hair was removed around the tumor to fully expose it.
Tumors were then collected and their volumes measured according to
formula: V=(length × width2)/2 as previously described
(17). According to IACUC
guidelines, all the tumour volumes did not exceed 4.2
cm3.
Hematoxylin and eosin (H&E)
staining
Tumors were fixed in 4% paraformaldehyde overnight
at 4°C. For staining, tissues were washed with water for several
hours, and then dehydrated in 70, 80 and 90% ethanol, xylene, and
other mixture for 15 min, and then in xylene I for 15 min, II for
15 min, until transparent. Samples were then placed in a mixture of
xylene and paraffin (1:1) for 15 min, and then paraffin was added
for 50–60 min. The tissues were then paraffin-embedded and sections
were mounted on slides, dewaxed and rehydrated. Then, the sections
were stained with hematoxylin for 3 min and eosin for 3 min at room
temperature and observed by an Olympus BX51 light microscopy
(Olympus Corp., Tokyo, Japan).
Enzyme-linked immunosorbent assay
(ELISA)
IL-12 and IL-4 in tumor tissues were detected using
ELISA kits (m1037868 and m1002149, respectively; mlbio, Shanghai,
China), following the manufacturer's instructions.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from tumors using an
UltraPure RNA Extract kit (CW0581M; CWBio, Shanghai, China). cDNA
was synthesized and used as a template for the detection of mRNA
expression. GAPDH was used as an internal reference to
calculate IL-12 and IL-4 levels in each group.
Reactions included 9.5 µl RNase-Free dH2O, 1 µl
cDNA/DNA, 2 µl primers, and 12.5 µl 2X GoldStar Taq Master Mix
(CW0960; CWBio) and were conducted using the following temperature
cycles: 95°C (denaturation) for 10 sec, 53°C (annealing) for 30
sec, and 72°C (extension) for 60 sec, for 40 cycles. The primers
were: IL-12 forward, (5′-TCCAGAGCCACCTCAAAAC-3′) and
IL-12 reverse, (5′-CGTATGCGGAAGTGAAGAAG-3′); IL-4
forward, (5′-TGTCATCCTGCTCTTTTTCTC-3′) and IL-4 reverse,
(5′-GTGGTGTTCTTCGTTGCTGT-3′); GAPDH forward,
(5′-AAGAAGGTGGTGAAGCAGG-3′) and GAPDH reverse,
(5′-GAAGGTGGAAGAGTGGGAGT-3′). The 2−ΔΔCq method was used
to quantify the results as previously described (18).
Western blotting
Proteins were isolated from tumor tissues using a
protein isolation kit (C1053; Applygen Technologies Inc., Beijing,
China) (4°C for 30 min, 8,766 × g for 10 min). Protein
concentrations were determined using a BCA kit (Thermo Fisher
Scientific, Inc.). Proteins were subjected to 12% sodium dodecyl
sulfate polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes. Membranes were then blocked using 5%
skimmed milk, and incubated with the following primary antibodies
overnight at 4°C: Mouse monoclonal anti-GAPDH (dilution 1:2,000;
cat. no. TA-08; ZSBIO, Beijing, China); rabbit polyclonal anti-IL-4
(dilution 1:1,000; bs-2018R, BA0980); or rabbit polyclonal
anti-IL-12 (dilution 1:1,000; cat. no. ab106270; Abcam). Then
membranes were incubated with secondary antibody (HRP-labeled goat
anti-rabbit IgG; dilution 1:200; cat. no. A16104SAMPLE; Thermo
Fisher Scientific, Inc.) for 1–2 h at room temperature.
Subsequently, ECL exposure solution was used to identify
specifically bound proteins and images were generated by exposure
of the membranes to X-ray film. Grayscale values were analyzed
using Quantity One software (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analysis
All data are presented as means ± standard deviation
(SD), and were statistically analyzed using the SPSS 19.0 (IBM
Corp., Armonk NY, USA), using one-way analysis of variance (ANOVA)
followed by Bonferroni testing. P<0.05 was considered to
indicate a statistically significant difference.
Results
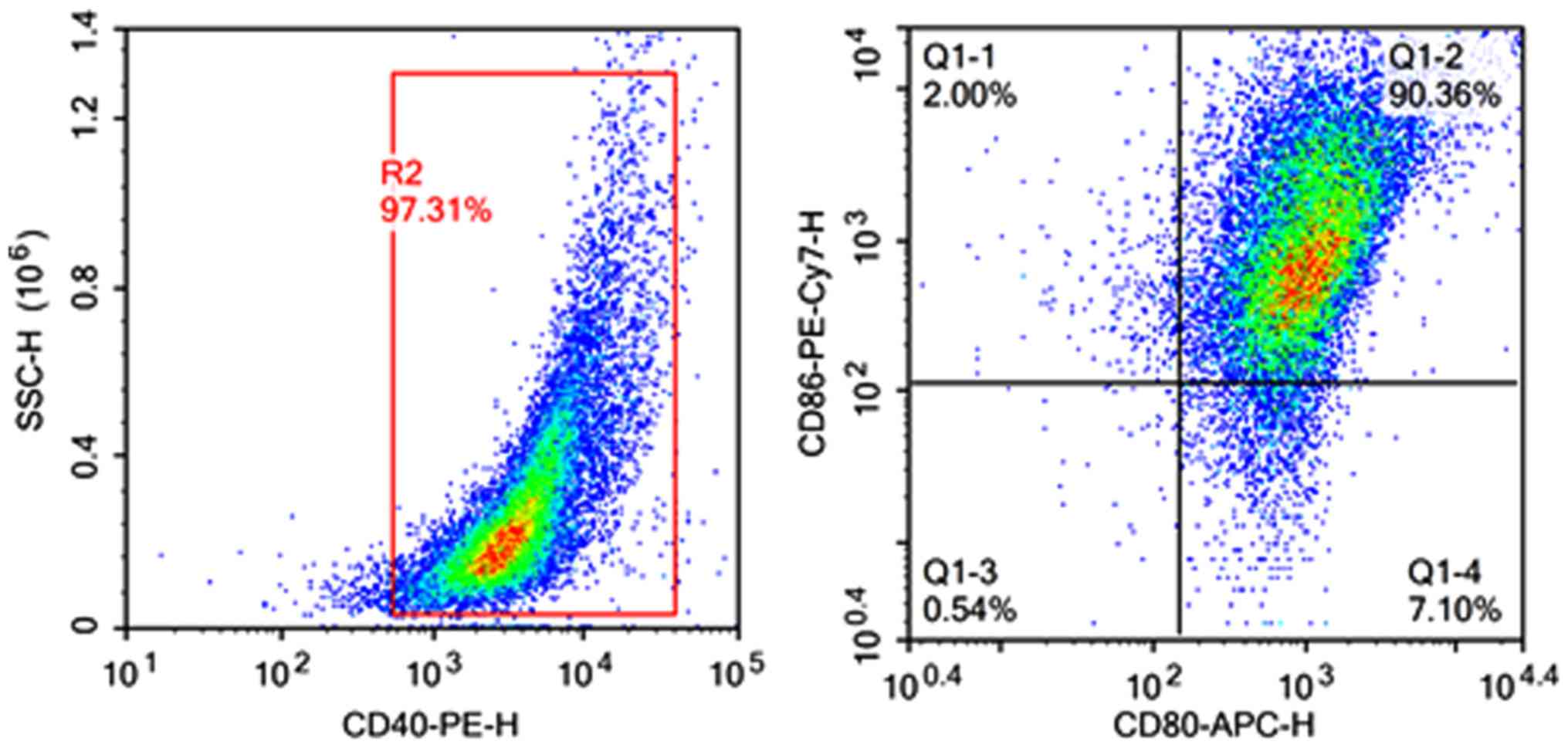
Confirmation of DC isolation
To confirm that we successfully isolated DCs from
mouse spleen, we conducted flow cytometry. Isolated DCs had
CD40-positive expression rates of 97.31%, and were >90% positive
for CD80 and CD86 (Fig. 1). These
results indicate that DCs were successfully isolated.
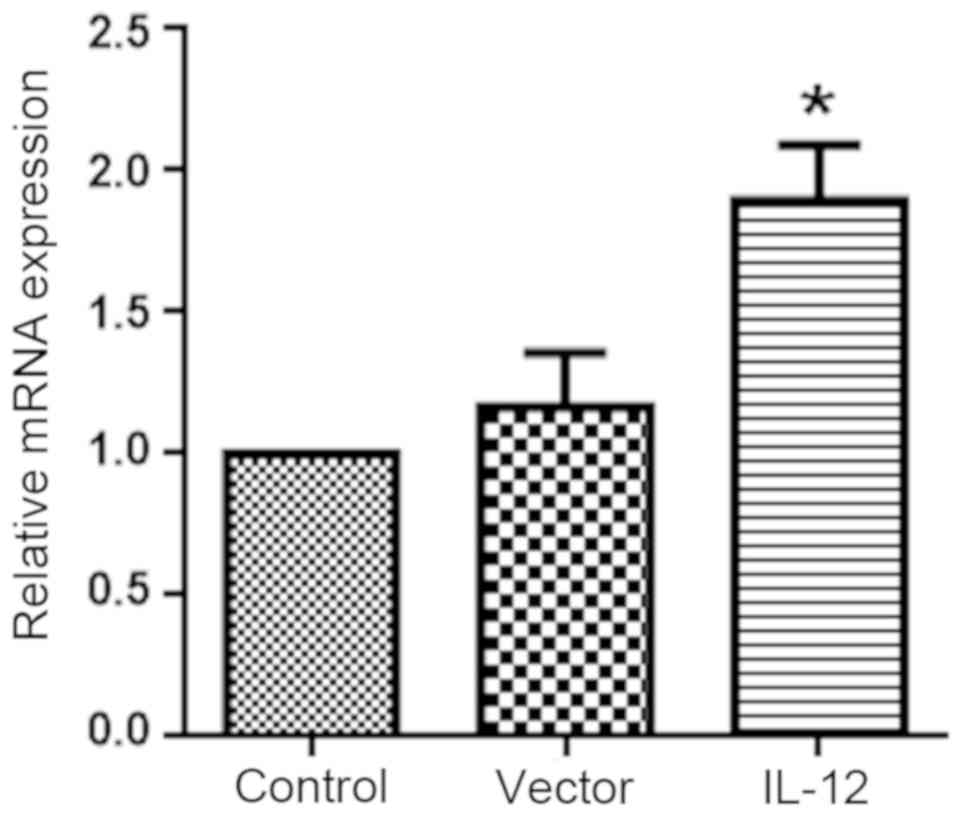
Verification of IL-12
overexpression
As shown in Fig. 2,
IL-12 expression was significantly increased in DCs transfected
with the IL-12 overexpression vector compared with the controls
(P<0.05), while transfection with the vector alone did not alter
IL-12 expression levels.
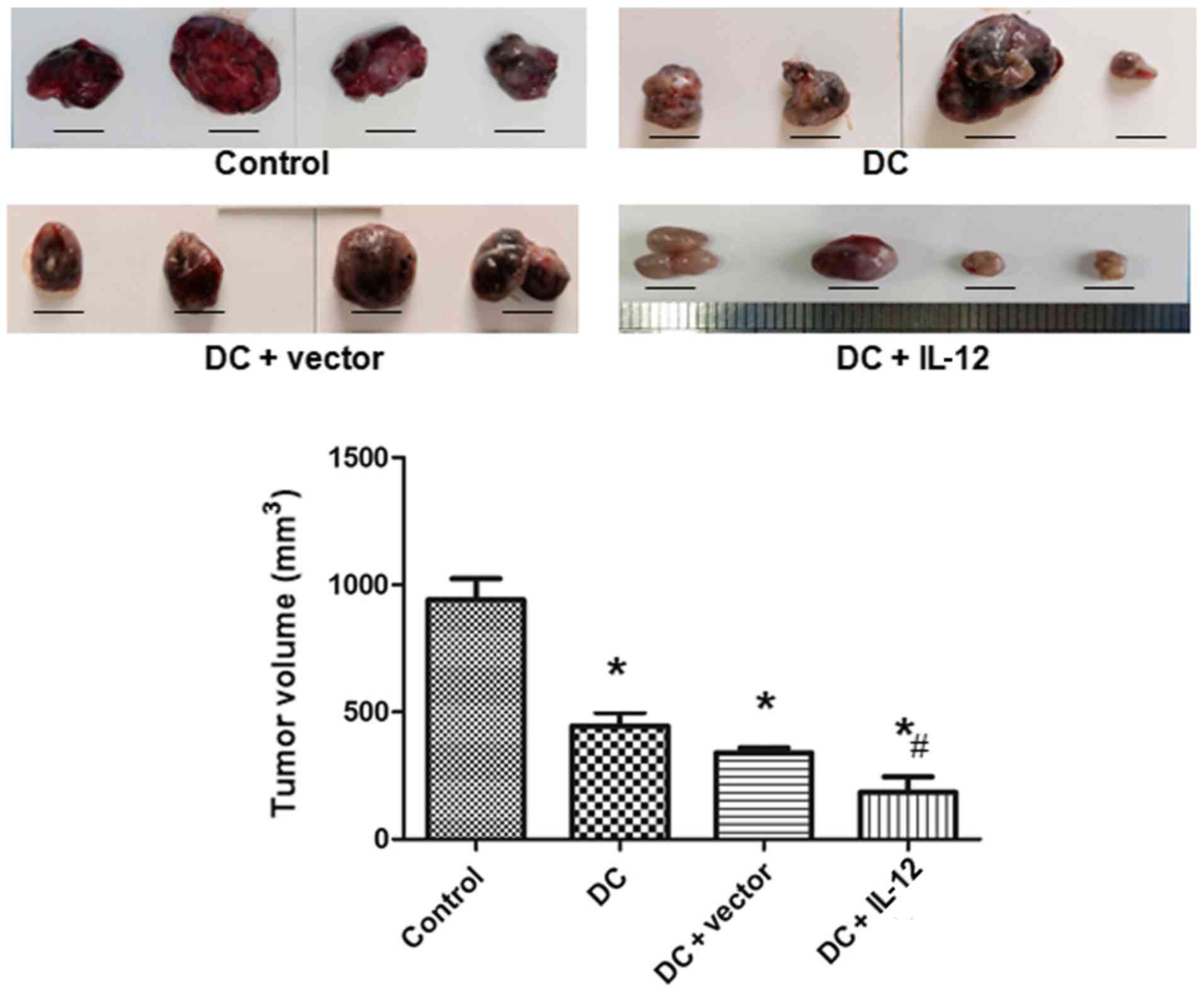
Tumor volumes in each group
To evaluate the effects of IL-12 on tumor growth,
tumor volumes were measured. The tumor volumes in each group are
presented in Fig. 3. Compared with
the control group, tumor volumes in the DC, the DC + vector, and
the DC + IL-12 groups were significantly decreased (P<0.05 vs.
control). Furthermore, the tumor volume was most markedly decreased
in the DC + IL-12 group (P<0.05 vs. DC).
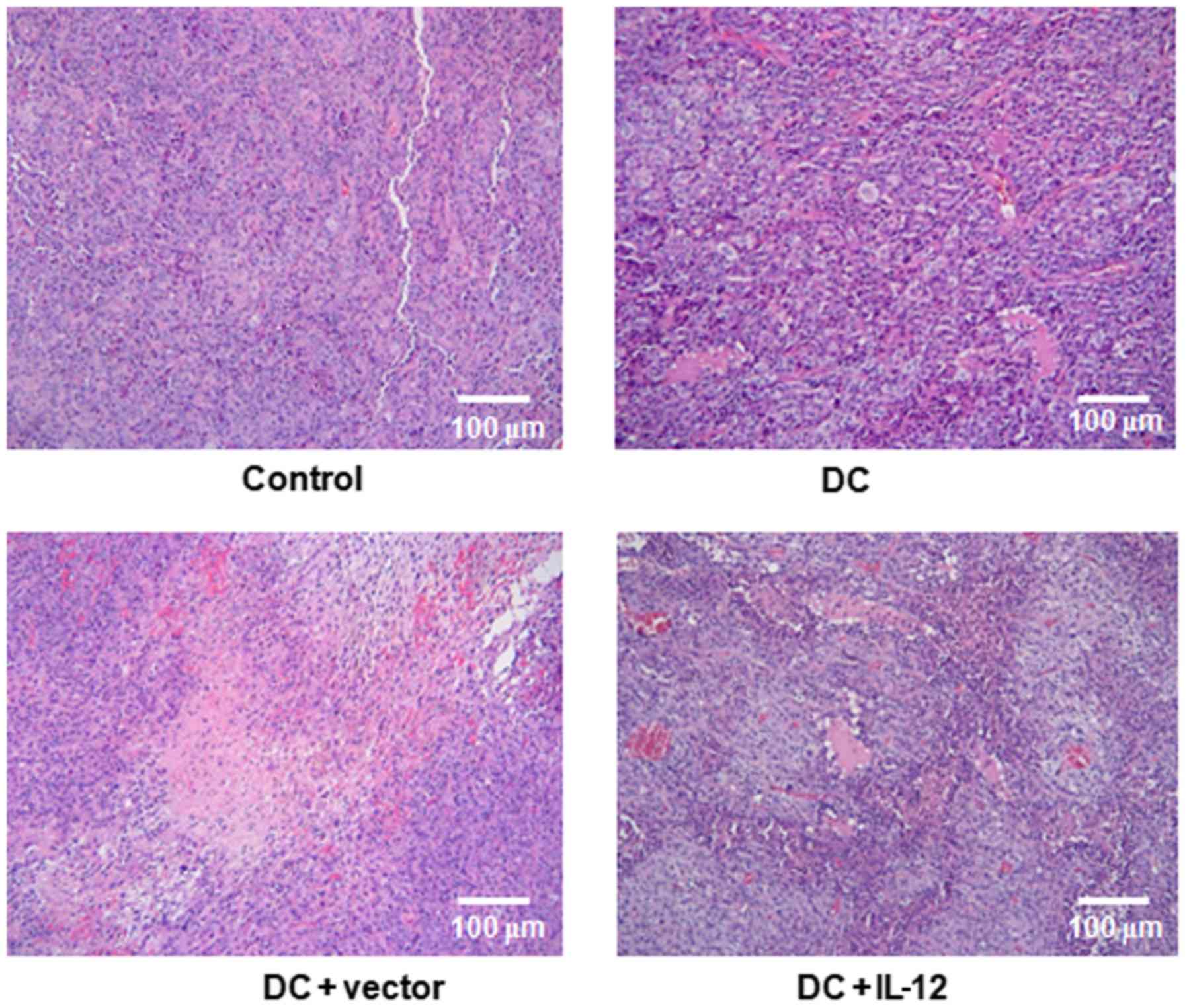
Morphological changes
Next, we examined the morphology of tumors in the
experimental groups, to determine the effects of DCs overexpressing
IL-12. As shown in Fig. 4, there
was clear proliferation of tumor cells in the control group. Small
amounts of inflammatory cell infiltration and limited areas of
tissue necrosis were observed in the DC and DC + vector groups;
however, in the DC + IL-12 group, tumors exhibited a loss of cell
structure in necrotic foci and inflammatory cell infiltration at
the edge of necrotic foci, with a large number of inflammatory
cells and a few adipocytes infiltrating the margins of the necrotic
regions.
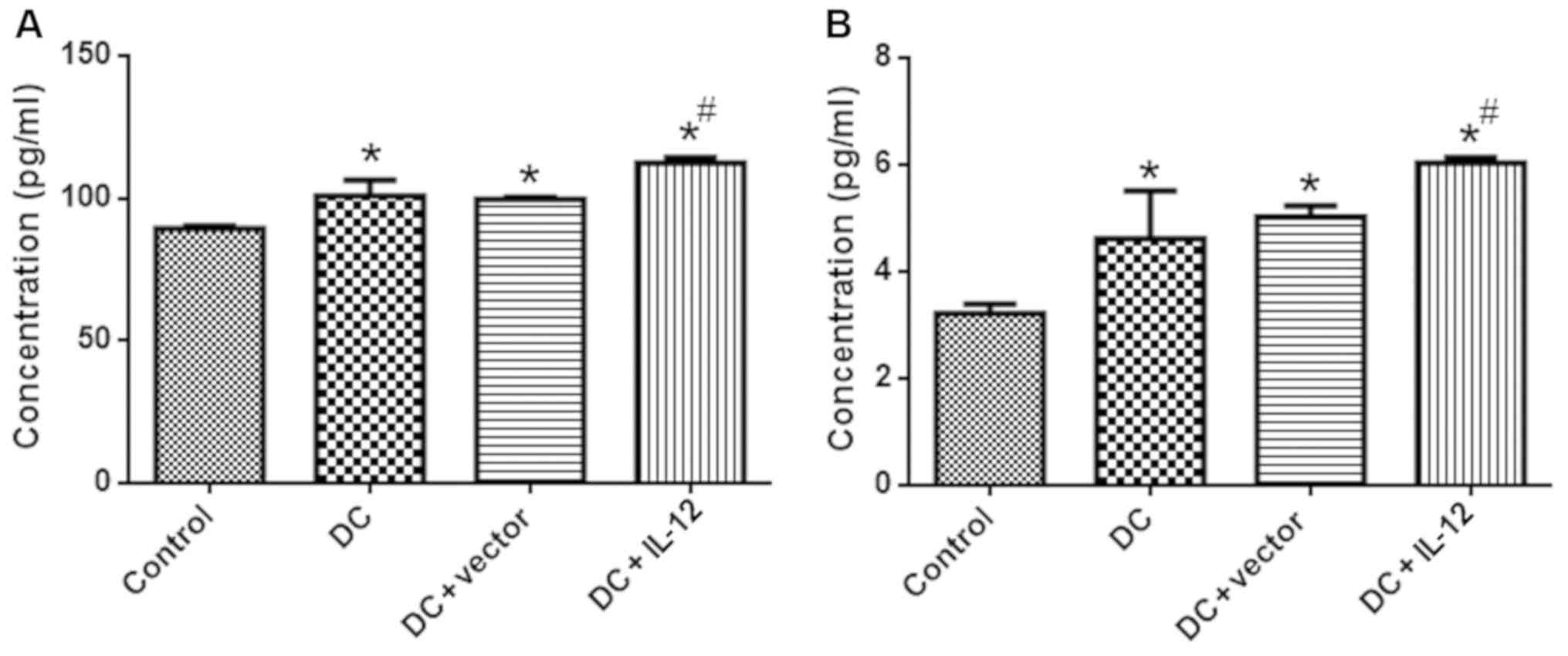
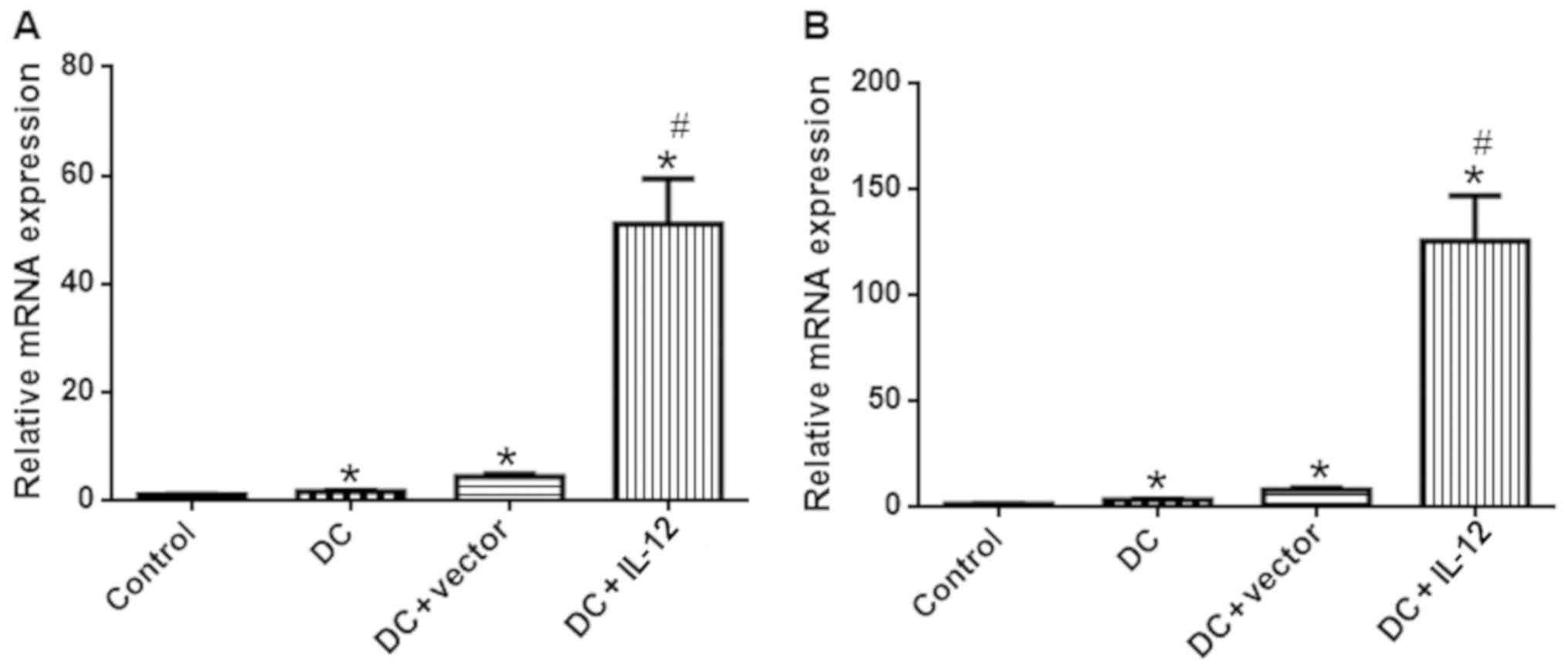
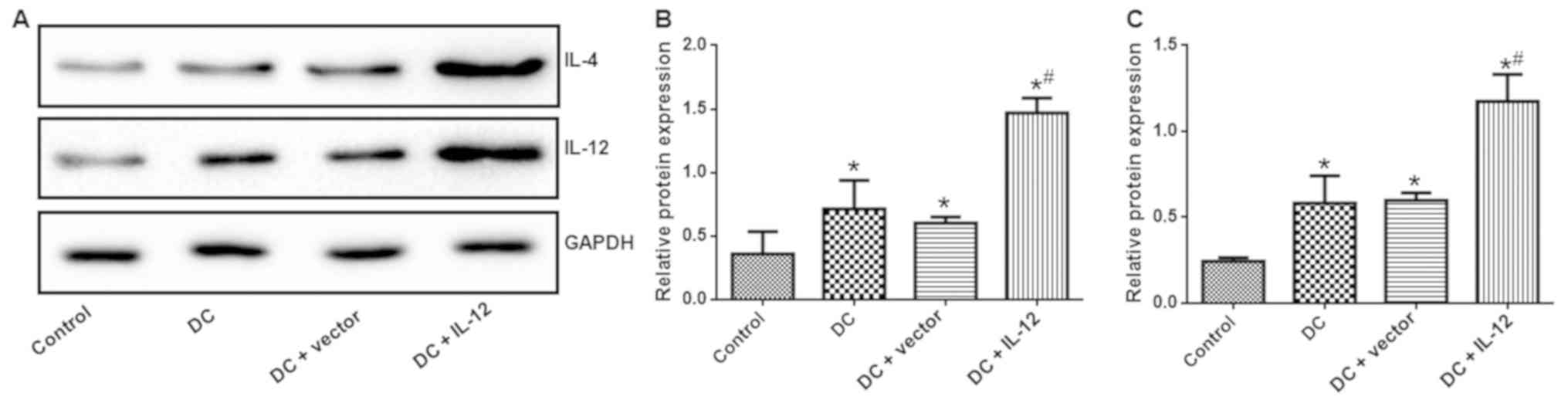
IL-12 and IL-4 expression
To evaluate the expression levels of IL-12 and IL-4
in each experimental group, we conducted ELISAs, qPCR and western
blotting. Compared with the control group, expression of IL-12 and
IL-4 was significantly higher in the DC, the DC + vector and the DC
+ IL-12 groups, as evidenced by ELISA (P<0.05 vs. control)
(Fig. 5), real-time PCR (P<0.05
vs. control) (Fig. 6) and western
blotting (P<0.05 vs. control) (Fig.
7). Expression levels of IL-12 and IL-4 were most markedly
increased in the DC + IL-12 group (P<0.05 vs. DC).
Discussion
In the present study, monocytes were isolated and
induced to develop into mature dendritic cells (DCs), which were
then transfected with a vector overexpressing interleukin-12
(IL-12). Intratumoral injection of gene-modified DCs inhibited the
growth of melanoma tumors. The possible mechanisms involved relate
to the immunotherapeutic effects of interleukin-4 (IL-4). This
study is the first to support the antitumor effects of DCs
overexpressing IL-12 in melanoma tumors.
Melanomas are skin tumors related to trauma, and
neovascularization and endothelial growth factor have key roles in
their development. Structural and functional abnormalities are
markers of neovascularization in this type of tumor, and
identification of effective treatments that can cure this disease
is the subject of intense research (19). With the continuous development of
immunological methods, biological immunotherapy has achieved
impressive results in clinical practice. Simultaneously, gene
therapy has become a promising method for cancer treatment. DCs are
the most powerful APCs and can initiate the majority of immune
responses (20). Due to lack of
understanding of the origin and differentiation of DCs, the number
of cells that can be obtained is very small, which greatly limits
study of their functional characteristics. Therefore, it is crucial
to establish technology to enable the culture of mature DCs in
vitro, to facilitate the generation of sufficient numbers of
functional DCs.
In this study, granulocyte-macrophage
colony-stimulating factor (GM-CSF), and IL-4 were used to induce DC
differentiation in vitro. GM-CSF is required for maintenance
of the differentiation, development, survival and function of DCs
(21). GM-CSF mainly promotes the
development of myeloid cells and differentiation of hematopoietic
stem cells (HSCs) into DCs, monocytes and macrophages, with
primarily monocytes produced (22).
IL-4 can increase and stabilize the expression of the CD molecules
induced by GM-CSF. Treatment with IL-4 and a very low concentration
of GM-CSF can stimulate DCs, promote their maturation, and
upregulate the expression of MHC-II and costimulatory molecules.
IL-4 can also promote the secretion of IL-12, and maintain DCs in
an immature state (23). In this
study, we induced the generation of mature DCs using this method,
which were then identified by flow cytometry.
IL-12 is a newly discovered cytokine with strong
antitumor effects that can be produced by B cells, mast cells,
neutrophils, and thymic stromal cells, but is primarily secreted by
DCs (24). IL-12-transfected DCs
have exhibited clear antitumor effects on melanoma, kidney and
glioma (25–27). Furthermore, IL-12 can induce mouse
erythroleukemia cells to differentiate into DCs, and the
differentiated cells were found to have antigen presenting function
(28). The results of this study
showed that DCs overexpressing IL-12 can reduce melanoma tumor
volumes. Furthermore, our pathological findings demonstrated that
tumors treated with DCs overexpressing IL-12 had enlarged necrotic
surfaces, with large numbers of inflammatory cells at the margins
of necrotic foci. These results suggest that IL-12-transfected DCs
can inhibit the growth of tumors and have clear antitumor
effects.
IL-4 promotes antigen presentation and tumor cell
killing by macrophages, and may regulate the expression of MHC
class II antigen (29). IL-4 has
synergistic effects with GM-CSF, IL-3 and LPS, and can also induce
peripheral blood mononuclear cells to secrete G-CSF and M-CSF, and
enhance neutrophil-mediated phagocytosis, cell killing activity,
and antibody-dependent cellular cytotoxicity (30). IL-4 is also a mouse macrophage
chemokine that promotes the production of the interleukin-1
receptor antagonist (31). In this
study, we found that DCs transfected with IL-12 could increase IL-4
expression, which may also inhibit tumor cell growth. For an
example, IL-4 overexpression was found to suppress tumor
development through p21-mediated activation of Janus kinase-signal
transducer of activators of transcription (STAT) pathways in
melanoma models (32); however,
elucidation of the exact mechanisms involved in our experiment will
require further investigation.
Patient DCs can specifically eliminate tumor cells
and do not injure the majority of healthy cells (33), confirming the efficacy of
immunotherapy using DCs. IL-12 is a cytokine with immunoregulatory
activity and functions as an important link in the process of
immune regulation (8). In this
study, we overexpressed IL-12 in DCs, leading to enhanced
anticancer effects against melanoma. Our results provide
experimental evidence for future clinical application of
immunotherapy in different types of cancer.
This study had some limitations. First, although
overexpression of IL-12 also lead to increased IL-4, we did not
clarify the exact mechanisms involved. Second, if possible, in
vitro experiments should be also conducted to verify the
function of DCs overexpressing IL-12 in the treatment of cancer.
Overall, the exact mechanisms underlying the antitumor effects of
DCs overexpressing IL-12 warrant further investigation.
In conclusion, an intratumoral injection of DCs
overexpressing IL-12 was found to exert enhanced antitumor effects
in melanoma. Biotherapy using DCs overexpressing IL-12 is a
potential treatment strategy for melanoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Planning Project of Jiangxi Science and Technology
Department (nos. 20121BBG70052 and 20151BBA13053).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
WY, YL and HW conceived and designed the
experiments; WY, YL, LZ, XZ, ZZ and MZ performed the experiments
and analyzed the data; WY, YL and HW wrote the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Ethics Committee of Jiangxi Provincial People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thomson CH, Cassell O, Peach H, Holloway
S, Garioch J and Moncrieff M: Neuropathic pain and quality of life
after wide local excision and sentinel lymph node biopsy for
melanoma: A multicentre study. Melanoma Res. 27:121–125. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Vries IJ, Lesterhuis WJ, Barentsz JO,
Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ,
Boezeman JB, Adema GJ, et al: Magnetic resonance tracking of
dendritic cells in melanoma patients for monitoring of cellular
therapy. Nat Biotechnol. 23:1407–1413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng Y, Ma XL, Wei YQ and Wei XW:
Potential roles and targeted therapy of the CXCLs/CXCR2 axis in
cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer.
1871:289–312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barry RJ, Tallouzi MO, Bucknall N, Mathers
JM, Murray PI, Calvert MJ, Moore DJ and Denniston AK: Anti-tumour
necrosis factor biological therapies for the treatment of uveitic
macular oedema (UMO) for non-infectious uveitis. Cochrane Database
Syst Rev. 12:CD0125772018.PubMed/NCBI
|
|
5
|
Mandalà M and Rutkowski P: Rational
combination of cancer immunotherapy in melanoma. Virchows Arch.
474:433–447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Summerfield A and McCullough KC: Dendritic
cells in innate and adaptive immune responses against influenza
virus. Viruses. 1:1022–1034. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mellman I: Dendritic cells: Master
regulators of the immune response. Cancer Immunol Res. 1:145–149.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Cao S, Kim S, Chung EY, Homma Y,
Guan X, Jimenez V and Ma X: Interleukin-12: An update on its
immunological activities, signaling and regulation of gene
expression. Curr Immunol Rev. 1:119–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lasek W, Zagożdżon R and Jakobisiak M:
Interleukin-12: Still a promising candidate for tumor
immunotherapy? Cancer Immunol Immunother. 63:419–435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tagawa T, Albanese M, Bouvet M, Moosmann
A, Mautner J, Heissmeyer V, Zielinski C, Lutter D, Hoser J,
Hastreiter M, et al: Epstein-Barr viral miRNAs inhibit antiviral
CD4+ T cell responses targeting IL-12 and peptide
processing. J Exp Med. 213:2065–2080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cemazar M, Ambrozic Avgustin J, Pavlin D,
Sersa G, Poli A, Krhac Levacic A, Tesic N, Lampreht Tratar U, Rak M
and Tozon N: Efficacy and safety of electrochemotherapy combined
with peritumoral IL-12 gene electrotransfer of canine mast cell
tumours. Vet Comp Oncol. 15:641–654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Komai-Koma M, Wang E, Kurowska-Stolarska
M, Li D, McSharry C and Xu D: Interleukin-33 promoting Th1
lymphocyte differentiation dependents on IL-12. Immunobiol.
221:412–417. 2016. View Article : Google Scholar
|
|
13
|
Lu X: Impact of IL-12 in cancer. Curr
Cancer Drug Target. 17:682–697. 2017. View Article : Google Scholar
|
|
14
|
Li L, Jiang Y, Lao S, Yang B, Yu S, Zhang
Y and Wu C: Mycobacterium tuberculosis-Specific
IL-21+IFN-γ+CD4+ T cells are
regulated by IL-12. PLoS One. 11:e01473562016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng C, Feng M, Jiao R, Liu D, Jin Y, Zhao
X and Xiao R: Effect of Dezocine on IL-12 and IL-10 secretion and
lymphocyte activation by culturing dendritic cells from human
umbilical cord blood. Europ J Pharmacol. 796:110–114. 2017.
View Article : Google Scholar
|
|
16
|
Thada S, Ponnana M, Sivangala R, Joshi L,
Alasandagutti M, Ansari MS, Schumann RR, Valluri V and Gaddam S:
Polymorphisms of IFN-γ (+874A/T) and IL-12 (+1188A/C) in
tuberculosis patients and their household contacts in Hyderabad,
India. Hum Immunol. 77:559–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ribas A, Hamid O, Daud A, Hodi FS, Wolchok
JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, et al:
Association of pembrolizumab with tumor response and survival among
patients with advanced melanoma. JAMA. 315:1600–1609. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
See P, Dutertre CA, Chen J, Günther P,
McGovern N, Irac SE, Gunawan M, Beyer M, Händler K, Duan K, et al:
Mapping the human DC lineage through the integration of
high-dimensional techniques. Science. 356:eaag30092017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhattacharya P, Thiruppathi M, Elshabrawy
HA, Alharshawi K, Kumar P and Prabhakar BS: GM-CSF: An immune
modulatory cytokine that can suppress autoimmunity. Cytokine.
75:261–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng J, Li Y, Pennati A, Yuan S, Wu JH,
Waller EK and Galipeau J: GM-CSF and IL-4 fusion cytokine induces B
cell-dependent hematopoietic regeneration. Mol Ther. 25:416–426.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lutz MB, Suri RM, Niimi M, Ogilvie AL,
Kukutsch NA, Rössner S, Schuler G and Austyn JM: Immature dendritic
cells generated with low doses of GM-CSF in the absence of IL-4 are
maturation resistant and prolong allograft survival in vivo. Eur J
Immunol. 30:1813–1822. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fukao T, Tanabe M, Terauchi Y, Ota T,
Matsuda S, Asano T, Kadowaki T, Takeuchi T and Koyasu S:
PI3K-mediated negative feedback regulation of IL-12 production in
DCs. Nat Immunol. 3:875–881. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kamensek U, Cemazar M, Lampreht Tratar U,
Ursic K and Sersa G: Antitumor in situ vaccination effect of TNFα
and IL-12 plasmid DNA electrotransfer in a murine melanoma model.
Cancer Immunol Immunother. 67:785–795. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oliveira Brito PK, Goncalves TE, Fernandes
FF, Miguel CB, Rodrigues WF, Lazo Chica JE, Roque-Barreira MC and
da Silva TA: Systemic effects in naïve mice injected with
immunomodulatory lectin ArtinM. PLoS One. 12:e01871512017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sonabend AM, Velicu S, Ulasov IV, Han Y,
Tyler B, Brem H, Matar MM, Fewell JG, Anwer K and Lesniak MS: A
safety and efficacy study of local delivery of interleukin-12
transgene by PPC polymer in a model of experimental glioma.
Anticancer Drugs. 19:133–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kobiyama K, Temizoz B, Kanuma T, Ozasa K,
Momota M, Yamamoto T, Aoshi T, Kuroda E and Ishii KJ:
Species-dependent role of type I IFNs and IL-12 in the CTL response
induced by humanized CpG complexed with β-glucan. Eur J Immunol.
46:1142–1151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshimoto T, Yasuda K, Tanaka H, Nakahira
M, Imai Y, Fujimori Y and Nakanishi K: Basophils contribute to
T(H)2-IgE responses in vivo via IL-4 production and presentation of
peptide-MHC class II complexes to CD4+ T cells. Nat
Immunol. 10:706–712. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeap WH, Wong KL, Shimasaki N, Teo EC,
Quek JK, Yong HX, Diong CP, Bertoletti A, Linn YC and Wong SC: CD16
is indispensable for antibody-dependent cellular cytotoxicity by
human monocytes. Sci Rep. 6:343102016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Helmy A, Guilfoyle MR, Carpenter KLH,
Pickard JD, Menon DK and Hutchinson PJ: Recombinant human
interleukin-1 receptor antagonist promotes M1 microglia biased
cytokines and chemokines following human traumatic brain injury. J
Cereb Blood Flow Metab. 36:1434–1448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee HL, Park MH, Song JK, Jung YY, Kim Y,
Kim KB, Hwang DY, Yoon do Y, Song MJ, Han SB and Hong JT: Tumor
growth suppressive effect of IL-4 through p21-mediated activation
of STAT6 in IL-4Rα overexpressed melanoma models. Oncotarget.
7:23425–23438. 2016.PubMed/NCBI
|
|
33
|
Sznol M: Betting on immunotherapy for
melanoma. Curr Oncol Rep. 11:397–404. 2009. View Article : Google Scholar : PubMed/NCBI
|