Introduction
Cancer is the second most common cause of mortality
in the United States, and the incidence rate is still increasing
(1). Chemotherapy is still the most
common treatment among the four major cancer therapy types:
Surgical treatment, radiotherapy, chemotherapy and biological
therapy (2). The majority of the
approaches currently used involve interference in nucleic acid
metabolism and DNA repair (3).
Currently, there are >70 types of anti-tumor drugs commonly used
in the clinic, and >400 new anti-tumor drugs have entered
clinical trials (4). However, the
majority of drugs fail due to their lack of specificity for tumor
cells.
Targeted therapy is a way of targeting tumor cells
with drugs and other active substances that kill tumor cells
without affecting the function of normal tissues and cells
(5). Subsequently, the curative
effect is improved and side effects are relatively reduced based
upon the application of target therapy. The targeted drug delivery
uses a novel nano drug delivery system, which increases the
selectivity of the drug to the target focus, thus increasing the
drug concentration in the tumor tissue (6,7).
Current, the drug delivery systems mainlyact by targeting an
antigen or antibody on the surface of tumor cells (8,9).
Doxorubicin is one of the common chemotherapeutic agents used to
treat liver cancer (10). However,
it has dose limitations due to its poor bioavailability,
hydrophobicity and cytotoxicity. The cyclin-(RGDyk)[c(RGDyK)]
peptide functionalized nanomicellar system can overcome the
drawbacks of low transport of chemotherapeutics across the
blood-tumor barrier (BTB) in multidrug-resistant cancer cells
(11). The anti-tumor efficacy of
chemotherapeutic agents was significantly facilitated with the
assistance of the c(RGDyK) drug delivery system (12,13).
However, the therapeutic effects of doxorubicin assisted by
c(RGDyK)-modified liposomes on melanoma growth and the underlying
mechanisms are largely unknown.
MicroRNAs (miRs) can act as oncogenes or tumor
suppressor genes during cancer development (14,15).
miRs regulate multiple signaling pathways and change the expression
of mRNAs (16,17). miR-21 was reported to be upregulated
in the tissues and cells of various solid tumors, including glioma,
colon cancer, gastric cancer and melanoma tissue (18). Overexpression of miR-21 in glioma
cells can inhibit apoptosis and miR-21 may have an oncogenic
function (19). miR-21 is a
potential therapeutic target for various types of cancer,
particularly melanoma (18).
Therefore, a miR-21 inhibitor could potentially facilitate the
antitumor effect of a targeted therapy. In this study, cellular and
animal models were used to explore the effect of doxorubicin
capsuled in 4c[RGDyk]-L-[CD] nanoparticle and miR-21 inhibitor on
melanoma cells, and to assess its associated mechanisms. This study
may provide an experimental basis for the treatment of
melanoma.
Materials and methods
Preparation of doxorubicin-loaded
nanometer
Doxorubicin was obtained from Beijing Huafeng United
Technology Co., Ltd. (Beijing, China). c(RGDyK) (molecular weight,
619.51) was synthesized by GL Biochem Ltd. (Shanghai, China).
Liposomes loaded with doxorubicin and c(RGDyK)-L-[CD] were prepared
in our lab using the method described previously (20). Doxorubicin (1 ml) diluted in PBS was
added into a dialysis bag. After 48 h, the dialysis bag was cut and
2 ml 10% Triton X-100 was added to destroy all the liposomes. The
final concentration of doxorubicin was 20 mg/ml.
Cell culture
Melanoma cell line B16F10 was purchased from
Shanghai Cell Bank of Chinese Academy of Sciences and cultured in
Dulbecco's modified Eagles medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT,
USA) and 100 U/ml penicillin-streptomycin (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) in 5% CO2 at 37°C. When the
cell confluence reached 90%, the culture medium was discarded and
cells were washed twice with PBS and passaged following trypsin
digestion.
miR-21 inhibitor transfection
At a density of 50–70 %, B16F10 cells
(3×103 cells) were transfected with vector (miR-21
scrambled control) or inhibitor (1 µl) using
Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions for 6 h. The miR-21
inhibitor and miR-21 negative control (NC) inhibitor were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Cells
were then cultured in DMEM containing 10% FBS for another 72 h. The
sequences of miR-21 inhibitor and vector were
5′-UCAACAUCAGUCUGAUAAGCUA-3′ and
5′-UCUACUCUUUCUAGGAGGUUGUGA-3′.
MTT assay
The effects of doxorubicin-loaded nanometer (DLN) on
melanoma cell growth were evaluated by MTT assay. The cells were
treated with different concentrations of DLN (0–20 µl) for 24–72 h.
To investigate the anti-tumor effects of association application of
DLN with miR-21 inhibitor, the experiments were divided into five
groups: Control group, vector (miR-21 inhibitor NC) group, DLN
group, miR-21 inhibitor group, and miR-21 inhibitor + DLN group.
Following the indicated treatments, an MTT assay was applied to
evaluate cell proliferation. DMSO (100 µl) was added to each well
to dissolve the formazan. The optical density was determined via
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) at
570 nm and represented cell viability.
Transwell assay
B16F10 cells in the logarithmic growth phase were
cultured in serum-free medium for 24 h. The medium was discarded
and the cells were digested using trypsin. The cells underwent
washing, centrifugation and resuspension in a low serum DMEM medium
(containing 0.2% FBS) to form a single cell suspension with a
density of 5×105/ml. The cells in different groups were
treated for 48 h. Subsequently, the cells were collected and
suspended cells (100 µl; 1×106/ml) were added into each
Transwell chamber. DMEM/F12 (600 µl) containing 10% FBS was added
into each well in a 24-well plate. The prepared Transwell chambers
were placed in the 24-well plate for 20 h at 37°C and 5%
CO2. Cells in the lower chamber were fixed in 4%
paraformaldehyde for 30 min at room temperature and stained with 3%
crystal violet for 5 min at room temperature. Four fields in the
upper and lower, left and right positions were selected for
counting in each well. The experiment was repeated for three times
and mean value was calculated.
Cell migration
B16F10 cells (3×105) were seeded in
6-well plates and treated with the indicated drugs for 72 h. After
that, the cells were collected and suspended cells (100 µl;
1×106/ml) were incubated in the plates for 24 h. A line
was drawn across the center of the wells. After incubation in
CO2 incubator at 37°C, the images were captured under a
light microscope. The migration speed was calculated based on the
formula: Cell mobility (µm/h)=[width(1)-width(2)]/72 h. Width(1) and width(2) represented the width measured at
0 and 72 h, respectively.
Flow cytometry
B16F10 cells (3×105) were seeded in
6-well plates and treated with indicated drugs for 72 h. The cells
were collected following trypsin digestion. The cells were
incubated with Annexin V-fluorescein isothiocyanate (0.5 µl) and
propidium iodide (PI; 0.5 µl; cat. no. C1062; Beyotime Institute of
Biotechnology, Haimen, China) for 30 min in the dark. Subsequently,
apoptosis was detected by flow cytometry (BD Biosciences, San Jose,
CA, USA) and data were analyzed using FlowJo 10 (FlowJo LLC,
Ashland, OR, USA). Following transfection for 72 h, the cells were
collected for PI staining (0.5 µl/ml) and the cell cycle
distribution was assessed by flow cytometry (BD Biosciences) within
1 h of staining.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
B16F10 cells (3×105) were seeded in
6-well plates and treated with indicated drugs for 72 h. RNA in
different groups was extracted using a TRIzol (Thermo Fisher
Scientific, Inc.). Subsequently, RNA was transcribed into cDNA
according to the instructions of a Reverse Transcription Kit
(Takara Biotechnology Co., Ltd., Dalian, China). Fluorescence qPCR
was utilized to detect the expression level of the target RNAs
using cDNA as the template following the instruction of
Platinum™ II Taq Hot-Start DNA Polymerase (cat. no.
14966005; Thermo Fisher Scientific, Inc.). SYBR-Green (cat. no.
HY-K0501; MedChemExpress, Monmouth Junction, NJ, USA) was used as
fluorophore and the thermocycling conditions were as follows:
Initial denaturation at 95°C for 10 min, followed by 40 cycles of a
two-step PCR at 95°C for 15 sec and 60°C for 1 min. The expression
of miR-21 was normalized to U6. The expression of BCL-2, Bax and
P53 was normalized to GAPDH. The primers are listed in Table I. The 2−ΔΔCq method was
used to quantify the results as previously described (21).
 | Table I.Primer sequence for use in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequence for use in reverse
transcription-quantitative polymerase chain reaction.
| Primer name | Sequence
(5′-3′) | Primer length
(bp) | Product length
(bp) | Annealing (°C) |
|---|
| miR-21 F |
CGCCGTAGCTTATCAGACTG | 20 | 65 | 57.7 |
| miR-21 R |
CAGCCACAAAAGAGCACAAT | 20 |
|
|
| U6 F |
CTCGCTTCGGCAGCACA | 17 | 94 | 60 |
| U6 R |
AACGCTTCACGAATTTGCGT | 20 |
|
|
| BCL-2 F |
GTGCCTGCTTTTAGGAGACCGA | 22 | 128 | 62.9 |
| BCL-2 R |
GAGACCACACTGCCCTGTTGATC | 23 |
|
|
| P53 F |
AGTGCTCGCTTAGTGCTCCCT | 21 | 110 | 62.6 |
| P53 R |
GTGCATGTTTGTGCCTGTCCT | 21 |
|
|
| Bax F |
AGACACTCGCTCAGCTTCTTG | 21 | 116 | 58 |
| Bax R |
CTTTTGCTTCAGGGTTTCATC | 21 |
|
|
| GAPDH F |
GAAGGTCGGAGTCAACGGAT | 20 | 224 | 58.3 |
| GAPDH R |
CCTGGAAGATGGTGATGGG | 19 |
|
|
Western blot
B16F10 cells (3×105) were seeded in
6-well plates and treated with indicated drugs for 72 h. Protein
was extracted from the treated cells using a protein isolation kit
(cat. no. 28-9425-44, GE Healthcare Life Sciences). Protein levels
were quantified with a bicinchoninic acid protein assay kit.
Protein (25 µg/lane) was separated via SDS-PAGE on 12% gels and
transferred onto nitrocellulose membranes. The membranes were
blocked in 5% skim milk for 2 h at room temperature. The antibodies
against BCL-2 (cat. no. ab32124; Abcam, Cambridge, MA, USA;
1:1,000), Bax (cat. no. ab32503; Abcam; 1:2,000), P53 (cat. no.
A11212; ABclonal Biotech Co., Ltd., Woburn, MA, USA; 1:1,000) and
GAPDH (cat. no. AC033; ABclonal Biotech Co., Ltd.; 1:1,000) were
incubated overnight at 4°C. After washing, the membranes were
incubated with the secondary antibody (horseradish
peroxidase-labeled goat anti-rabbit IgG; 1:100; cat. no. ab131368;
Abcam) for 2 h at room temperature. Enhanced chemiluminescence kit
(cat. no. RPN2133; GE Healthcare Life Sciences) was added to the
membrane and visualized using a gel imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Establishment of in vivo tumor
model
All animal experiments were approved by the Ethics
Committee of Jiangxi Tumor Hospital (approval no. 20170312). Female
Balb/c nude mice (6-week old; 20 g) were purchased from Hunan SLAC
Experimental Animal Co. Ltd. (SCXK2016-0002) and housed in a
specific pathogen-free condition that was automatically maintained
at a temperature of 23±2°C, a relative humidity of 45–65 %, and
with a controlled 12 h light /dark cycle. The mice implanted with
the tumor cell lines were randomly divided into six groups (n=5 in
each group): Control group, doxorubicin group, DLN group, miR-21
inhibitor group, miR-21 inhibitor + doxorubicin group and miR-21
inhibitor + DLN group. B16F10 cells in the logarithmic growth phase
(1×107) were diluted in 0.2 ml PBS and injected
(subcutaneous injection) into the Balb/c nude mice. Doxorubicin,
DLN and miR-21 inhibitor group were administered for 10 consecutive
days, following attainment of a tumor size of 50 mm3. In
the miR-21 group, each mouse received miR-21 inhibitor
[intraperitoneal injection (i.p.), 20 µl]. In the miR-21
inhibitor + doxorubicin group, each mouse received 20 µl (1 µg/µl)
miR-21 inhibitor (i.p.) and 20 µl doxorubicin (5 mg/kg). In
the miR-21 inhibitor + DLN group, each mouse received 20 µl miR-21
inhibitor (i.p.) and 20 µl DLN (5 mg/kg). General conditions
of the mice were monitored daily and the tumor size was measured
every 2 days. Following 10 days of drug administration, the mice
were anesthetized using isoflurane and decapitated, and the whole
tumor was removed. The tumor specimens were fixed in 4%
paraformaldehyde in PBS (pH 7.4) at 4°C overnight and then embedded
in paraffin for tissue sectioning. The tissues were sectioned into
5 µm-thick sections. Subsequently, the slides were stained with
hematoxylin (3%) and eosin (3%) for 5 min at room temperature. The
images were captured under light microscopy.
Statistical analysis
The data were presented as the mean + standard
deviation and analyzed using SPSS 17.0 (SPSS, Inc., Chicago, IL,
USA). Statistical significance was determined by one-way analysis
of variance with Newman-Keuls as the post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
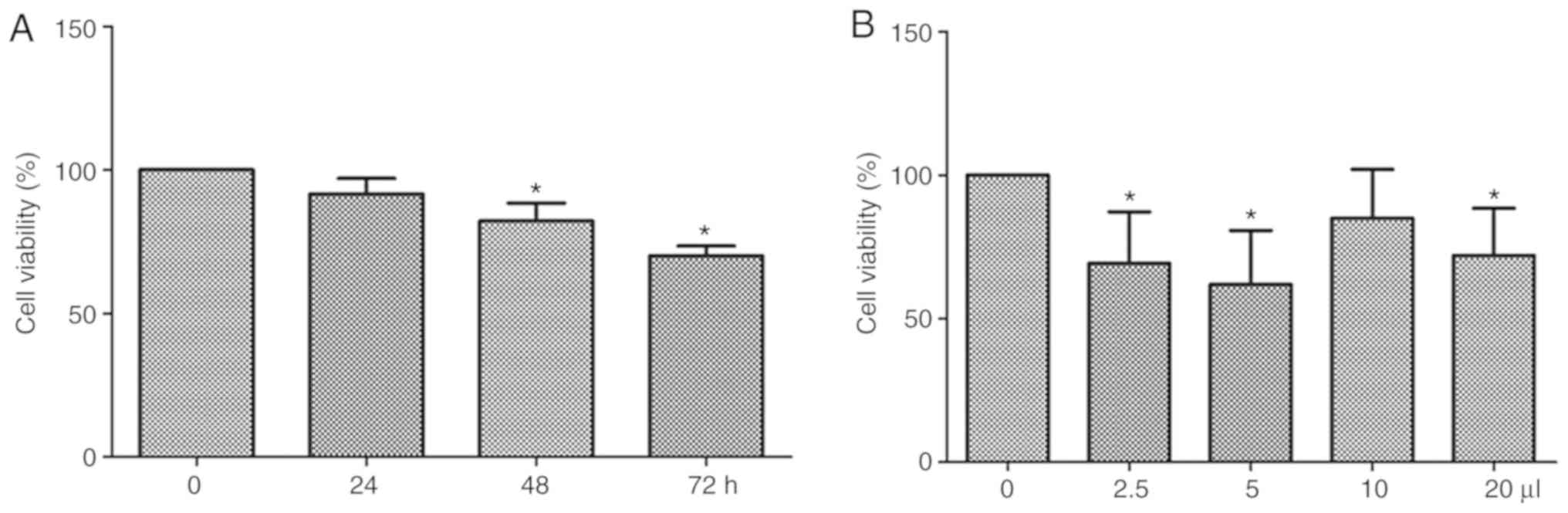
DLN inhibits cell growth of B16F10
cells
Initially, we detected the effects of 5 µl DLN on
cell growth of B16F10 cells. As shown in Fig. 1A, DLN inhibits the cell growth in a
time-dependent manner (0–72 h). Significant difference was observed
at the 48 and 72 h time points compared with the 0 h control
(P<0.05). As shown in Fig. 1B, 5
µl DLN (5 mg/ml) the optimal effects on cell growth were observed
after 72 h treatment. Therefore, 5 µl DLN was selected to treat the
cells for 72 h in the subsequent experiments.
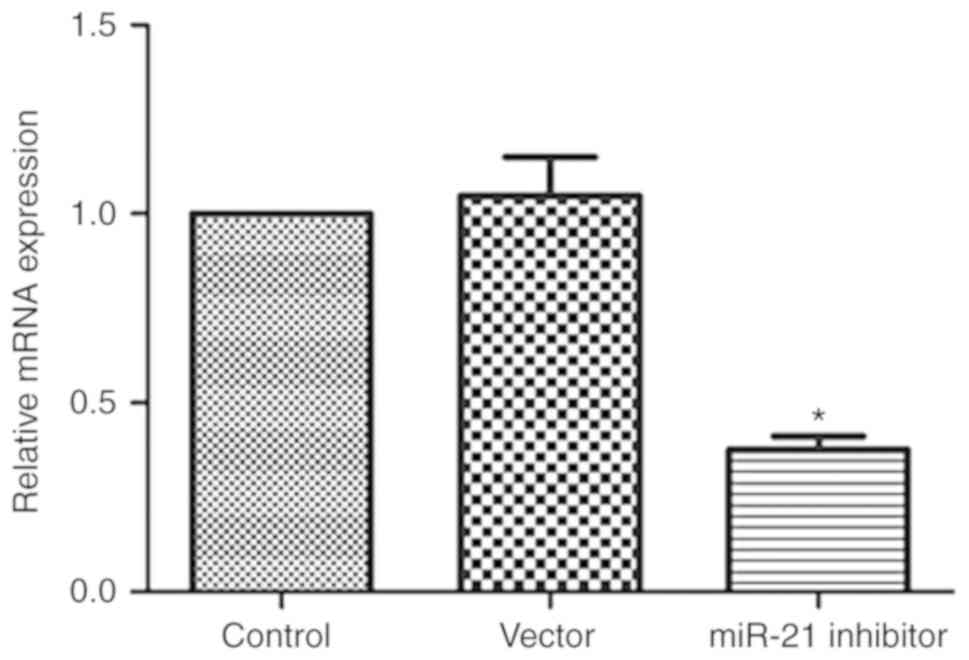
miR-21 inhibitor reduces miR-21
expression
miR-21 expression was confirmed by RT-qPCR. As shown
in Fig. 2, miR-21 inhibitor
significantly reduced miR-21 expression (P<0.05), while control
miR-21 inhibitor NC did not affect miR-21 expression.
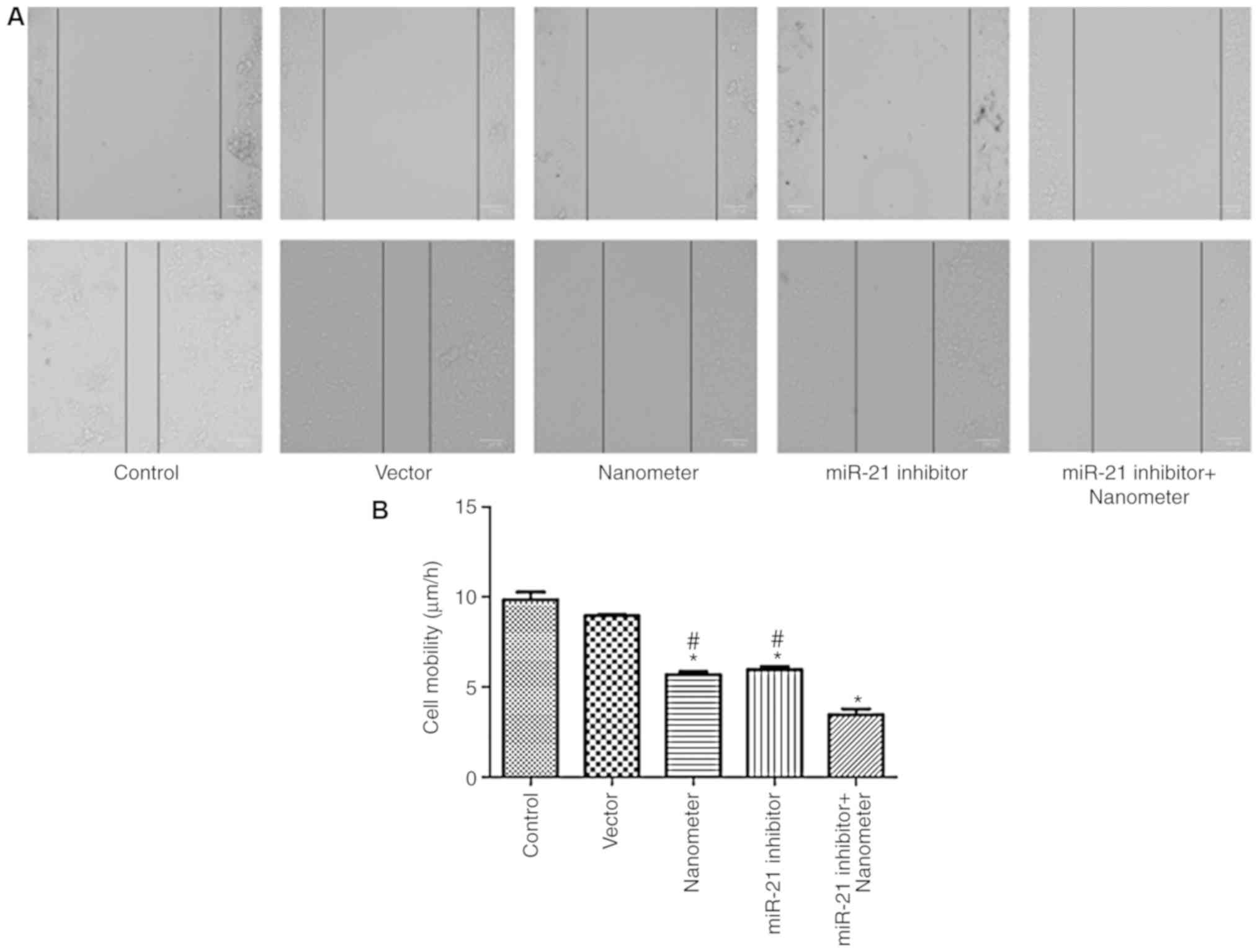
miR-21 inhibitor facilitates the
anti-migration effect of DLN
As shown in Fig. 3,
DLN significantly inhibited the migration of melanoma cells
(P<0.05). miR-21 inhibitor also significantly inhibited cell
migration compared with control (P<0.05). Combined application
of DLN and miR-21 inhibitor further inhibited cell migration
compared with single application of DLN and miR-21 inhibitor.
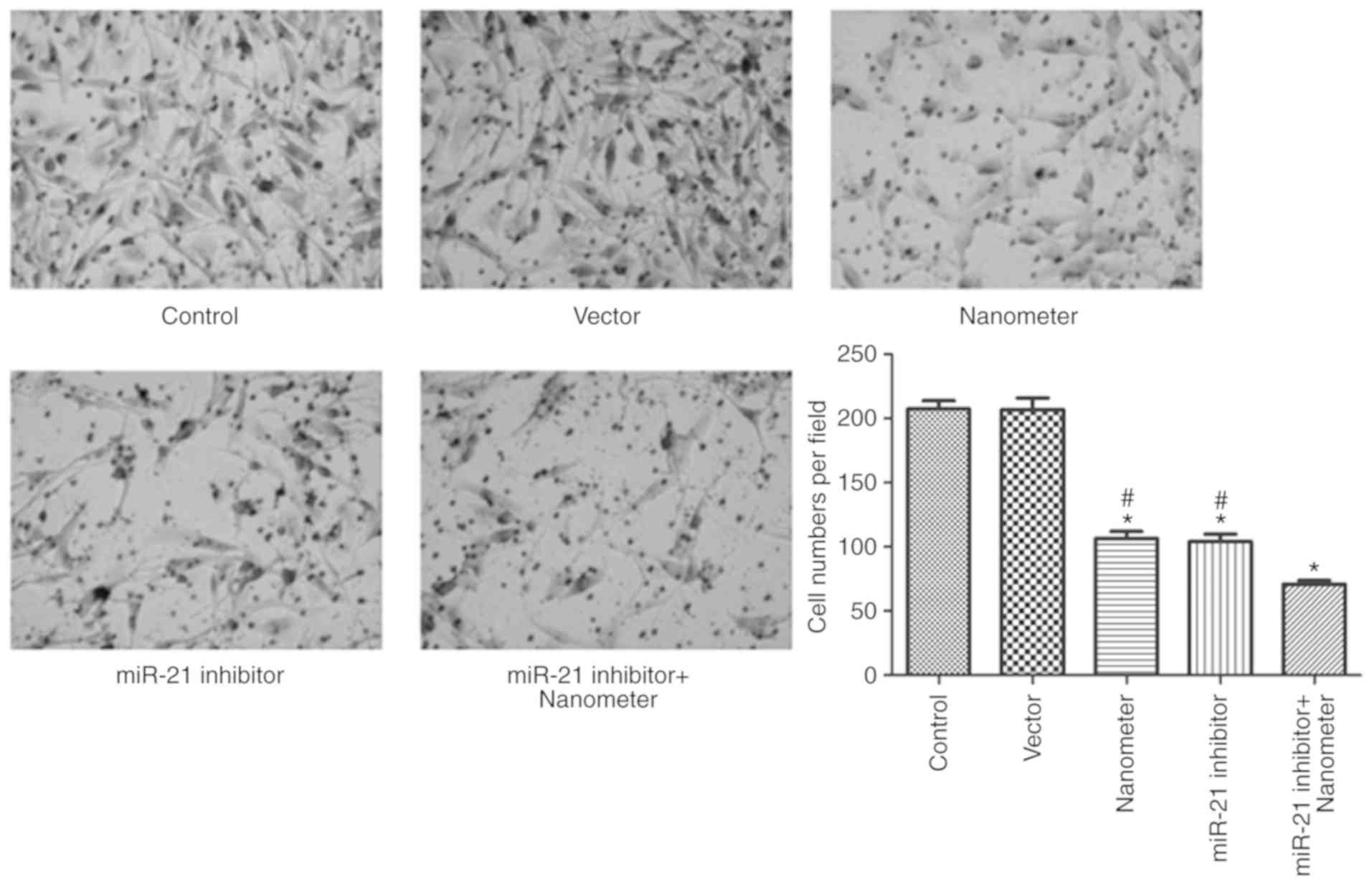
miR-21 inhibitor facilitates the
anti-invasion effect of DLN
As shown in Fig. 4,
DLN significantly inhibited invasion of melanoma cells at the
concentration of 5 µl (P<0.05). miR-21 inhibitor also
significantly inhibited cell invasion (P<0.05). Results
demonstrated that DLN and miR-21 inhibitor combination further
inhibited cell invasion compared with DLN or miR-21 inhibitor
alone.
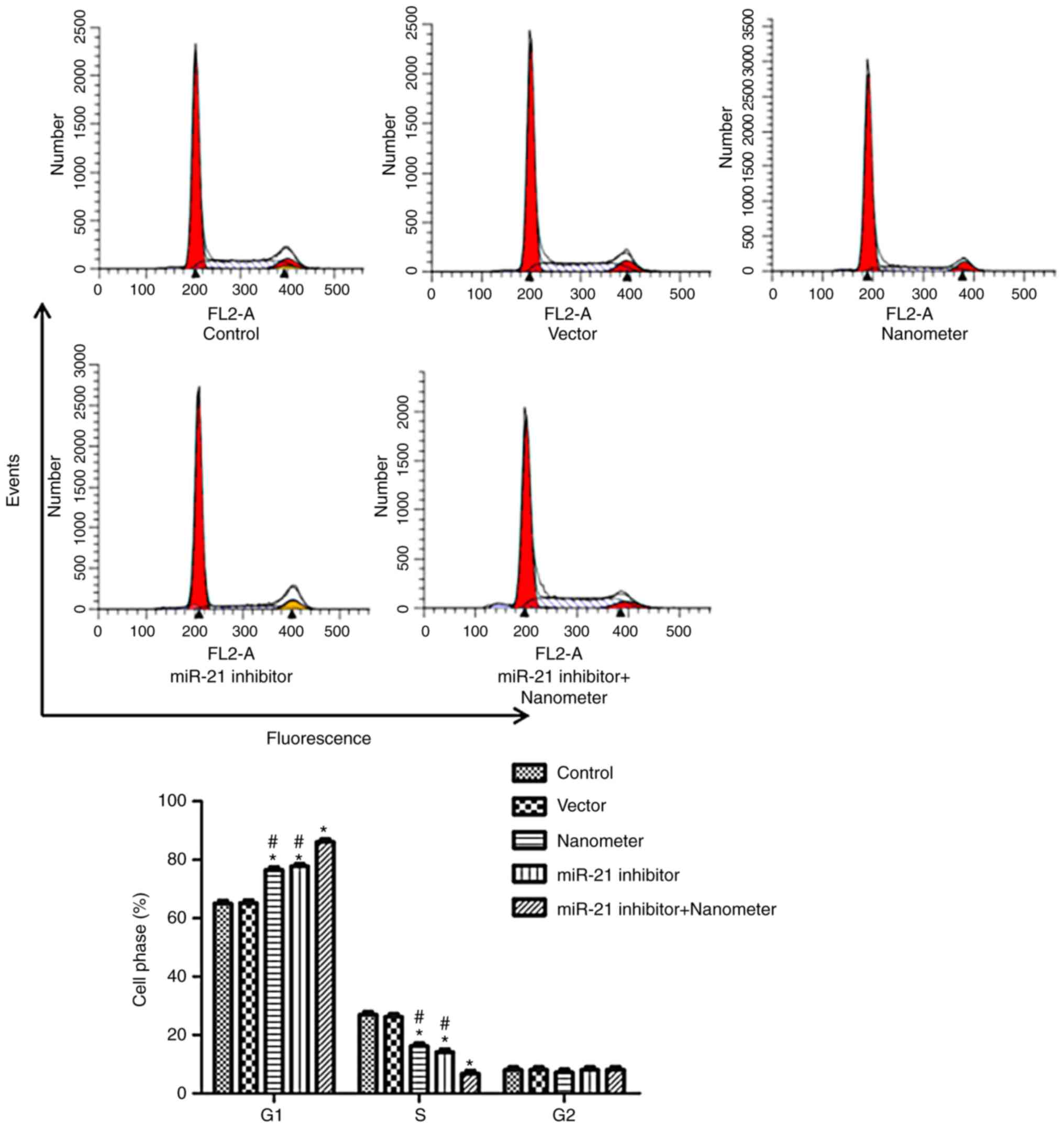
miR-21 inhibitor promotes cell cycle
arrest following DLN treatment
As shown in Fig. 5,
miR-21 inhibitor and DLN increased the number of cells at G1 phase,
and decreased the number of cells at S phase (P<0.05). miR-21
inhibitor further increased cell cycle arrest ability of DLN when
combined treatment was performed.
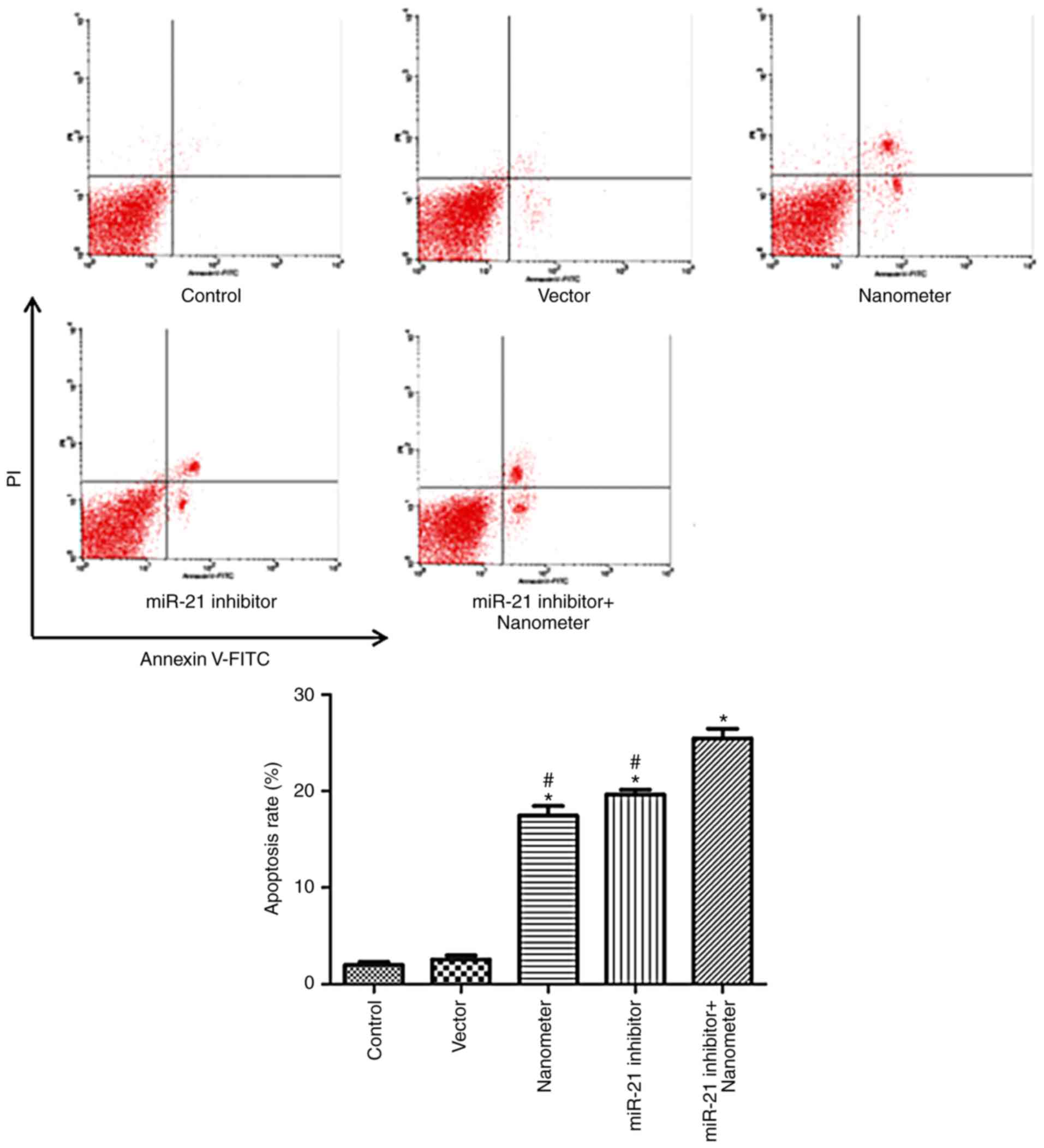
miR-21 inhibitor facilitates the
apoptosis induced by DLN
As shown in Fig. 6,
DLN induced apoptosis of melanoma cells at a concentration of 5 µl
(P<0.05). miR-21 inhibitor also induced apoptosis of melanoma
cells (P<0.05). The co-application of miR-21 inhibitor and DLN
produced a further increase in apoptosis compared with single
application of DLN and miR-21 inhibitor.
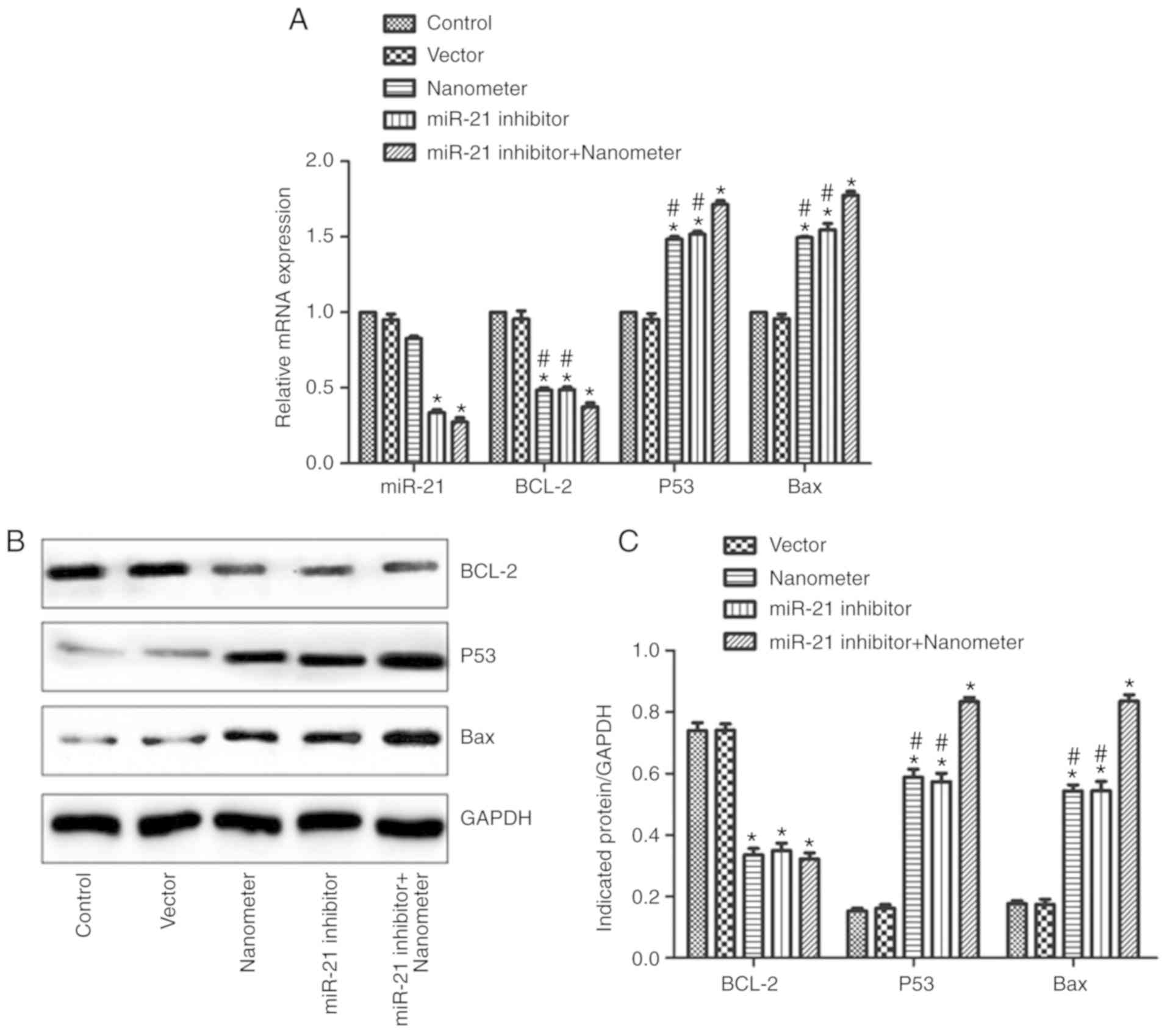
miR-21 inhibitor promotes the effects
of DLN on BCL-2, P53 and Bax
miR-21 and apoptosis-related gene expression were
also detected. As shown in Fig. 7A,
miR-21 expression in miR-21 inhibitor group and miR-21 inhibitor +
DLN group was significantly reduced compared with control
(P<0.05). By contrast, DLN treatment alone did not affect miR-21
expression. BCL-2 mRNA expression was downregulated by miR-21
inhibitor or DLN treatment (P<0.05). Additionally, the
associated application of miR-21 inhibitor with DLN further reduced
BCL-2 expression. Bax mRNA expression was upregulated by miR-21
inhibitor or DLN treatment (P<0.05). Furthermore, the associated
application of miR-21 inhibitor with DLN further promoted Bax
expression. P53 mRNA expression was upregulated by miR-21 inhibitor
or DLN treatment (P<0.05), and the combined application of
miR-21 inhibitor with DLN further promoted P53 expression. The
protein expression of BCL-2, Bax and P53 was confirmed by western
blotting, with a similar trend observed in different groups as the
mRNA expression (Fig. 7B and
C).
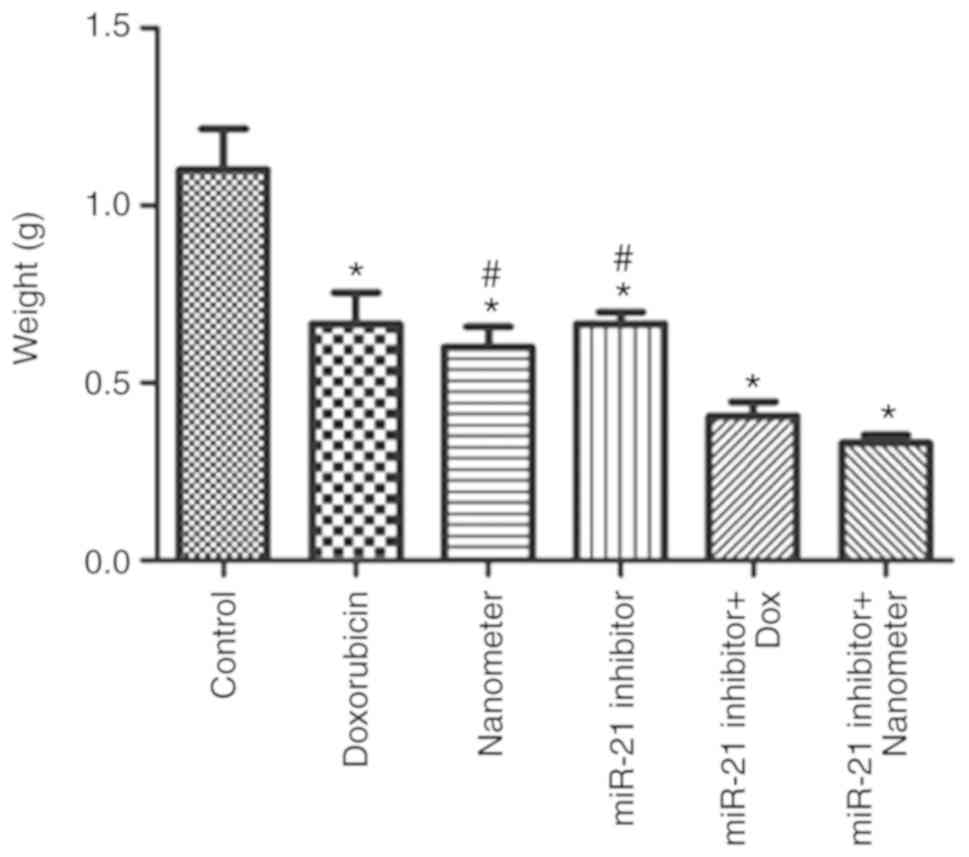
miR-21 inhibitor facilitates the
anti-tumor effects of DLN in vivo
Mouse body temperature and behaviors were monitored
each day. In the present study, the mice displayed normal wellbeing
and all animals presented with a single subcutaneous tumor in the
forelimb armpit and the longest diameter was <10 mm.
Furthermore, no ulceration or abrasion of tumor surface was
observed. The maximum tumor burden was observed in the control
group (~8% of the body weight). As shown in Fig. 8, tumor weight in the doxorubicin,
DLN, miR-21 inhibitor groups were significantly reduced compared
with control (P<0.05). The combined treatment of miR-21
inhibitor with DLN further inhibited tumor growth compared with
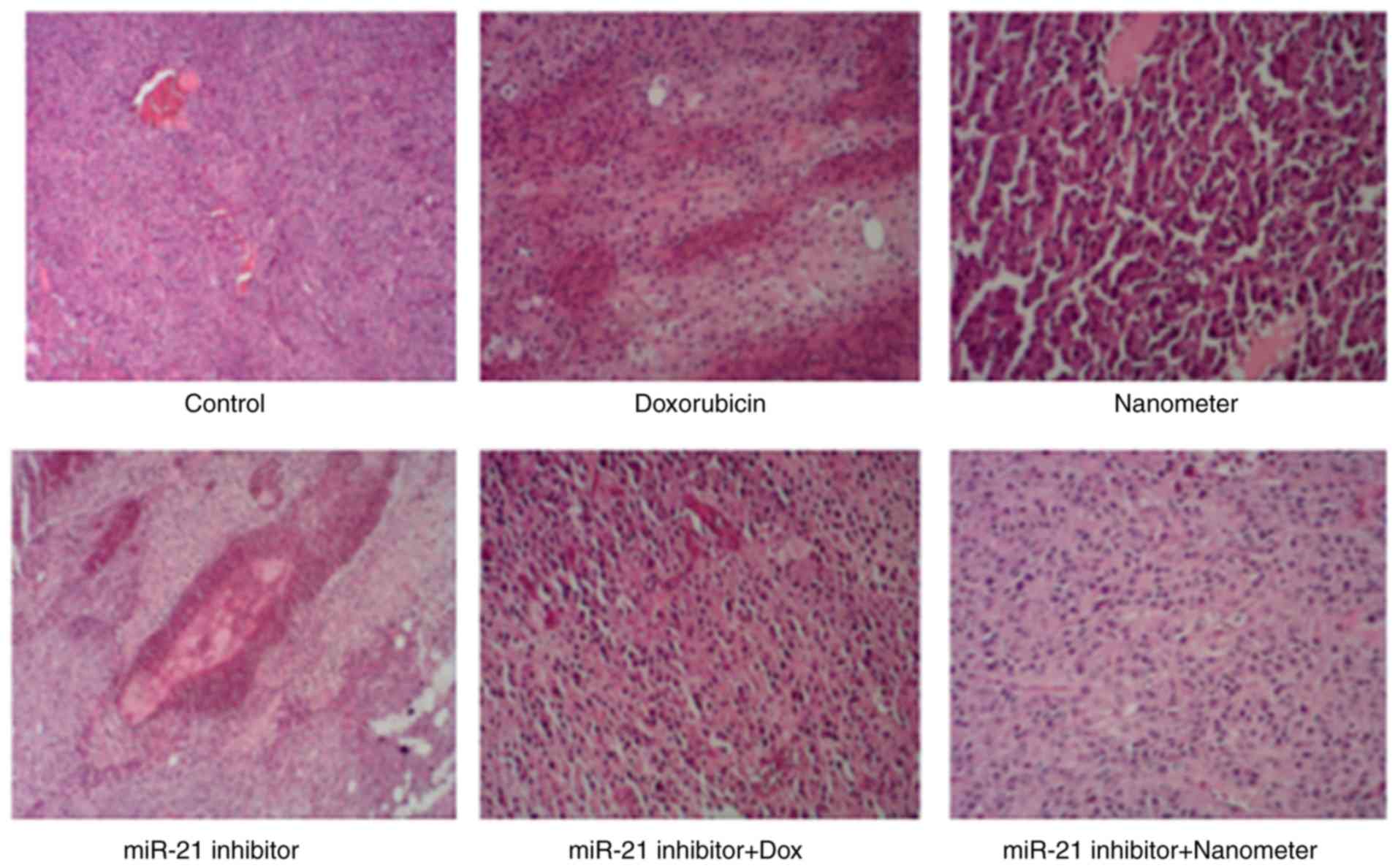
individual of miR-21 inhibitor or DLN treatment. As shown in
Fig. 9, the tumor tissue in control
group was rich in blood vessels. The tumor cells were arranged
closely and the size was relatively consistent. Tumor cells in the
doxorubicin group were loosely arranged, and tumor cells in the DLN
group were significantly enlarged. Tumor cells in miR-21 inhibitor
group exhibited vacuoles and necrosis. More tumor cells in miR-21
inhibitor + doxorubicin group exhibited necrosis. In addition,
tumor cells in miR-21 inhibitor + DLN group were loosely
arranged.
Discussion
In this study, in vitro cellular and animal
models were used to explore the effect of DLN combined with miR-21
inhibitor on melanoma tumor cells. Our data revealed that DLN had
stronger anticancer effects compared with direct application of
doxorubicin. From a mechanistic perspective, DLN did not affect
miR-21 expression to exert its antitumor effects, which was
facilitated by miR-21 inhibitor. These data provided novel idea for
the application of DLN in melanoma treatment.
Melanoma is a skin cancer caused by the
dedifferentiation of melanocytes (22). Melanoma is characterized by strong
immune escape ability (23).
Currently, the treatment of melanoma is typically early surgery,
followed by chemotherapy combined with biological therapy. As
malignant tumor is caused by unlimited proliferation and abnormal
differentiation (24), the
available treatments involve stimulating differentiation and
apoptosis. However, traditional chemotherapeutic drugs still have
various problems. The most prominent is that chemotherapy drugs not
only kill tumor cells, but also selectively damage normal cells,
particularly hematopoietic tissue and cells (25). The C[RGDyk]-modified liposome
targeted delivery system is one of the common active
tumor-targeting drug delivery systems (26). This system improves therapeutic
efficacy in bone metastasis from breast cancer (27) and glioblastoma (28). In this study, the anticancer effect
of DLN in melanoma was investigated.
MTT assay was used to determine the appropriate
concentration and appropriate time for the administration of the
drug delivery system, which provided the basis for the following
experiments. Based upon the data, 5 µl DLN (5 mg/ml) was selected
and the cells were treated for 72 h. In the current study, the
effect of 10 µl DLN on cell proliferation was not observable.
Although the reasons were not identified, high concentrations of
C[RGDyk]-modified liposome may affect the bioavailability of
doxorubicin. These data receive more attention in a future study.
miR-21 is a potential therapeutic target in melanoma (18,29,30).
In this present study, miR-21 inhibitor was applied in melanoma
cells. The results confirmed that the miR-21 inhibitor
significantly reduced the expression of miR-21 in melanoma cells.
More importantly, miR-21 inhibitor further promoted the anti-tumor
effects of DLN, including inhibiting cell proliferation, invasion
and migration, and promoting apoptosis of melanoma cells. In this
study, the reduced proliferation following treatment with DLN may
affect the cell invasion and migration results. Therefore, we
resuspended the cells after treatments and similar numbers of cells
in different groups were used in the analysis of cell migration and
invasion, which may alleviate the bias of migration and invasion
caused by reduced cell proliferation.
Invasive melanomas are characterized by the growth
of melanocyte nests, which are surrounded by interstitial matrix
and various types of stromal and immune cells. The surrounding
tissue is typically matrix rich (31). Integrins are heterodimeric,
transmembrane receptors that function as mechanosensors, adhesion
molecules and signal transduction platforms in a multitude of
biological processes, particularly in tumorigenesis (32). Self-aggregated pegylated
poly-nanoparticles were tagged with c(RGDyK) peptide for targeted
doxorubicin delivery to integrin-rich tumors (33). This may relieve the side effects of
chemotherapeutics on normal organs. Although the cytotoxicity of
DLN was not investigated in the current study, several studies have
already verified the safety of DLN (34,35).
The data of the current study demonstrated that DLN
may promote melanoma cell apoptosis and affect cell cycle, thus
arresting cells at G1 phase and affecting cell proliferation.
Although miR-21 inhibitor also demonstrated anti-cancer effect in
melanoma, the co-application of DLN may further promote the effect.
miR-21 can regulate multiple signaling pathways and change the
expression of tumor-associated protein-coding mRNAs (36). Notably, DLN treatment did not cause
a reduction of miR-21, which indicated that the effect of DLN was
independent of miR-21. However, additional miR-21 inhibition
promoted the anti-cancer effect of DLN.
BCL-2 is a cell-survival factor, while Bax is the
most important apoptosis-associated factor. Bax protein can form
heteromeric two polymers with BCL-2 (37). The ratio of Bax/BCL-2 is the key
factor determining apoptosis (38).
P53 can increase the expression of Bax and downregulate the
expression of BCL-2 to promote apoptosis (39). P53 can also modulate apoptosis
through the death receptor pathway (40). The current study demonstrated that
miR-21 inhibitor aggravated the effect of DLN on the expression of
BCL-2, Bax and P53. These results suggest that the induced
apoptosis may be associated changes in the expression of these
genes.
In order to further investigate the effect of DLN
and miR-21 inhibitor on tumor growth, a tumor formation assay in
nude mice was performed. The results revealed that DLN was more
effective in inhibiting tumor development than doxorubicin. Based
on previous publications (12,41,42),
DLN may have high bioavailability, hydrophobicity and cytotoxicity
in tumor cells. miR-21 inhibitor facilitates the anti-cancer
activity of DLN in melanoma in vivo as demonstrated by the
reduced tumor growth and the morphological changes. However,
further experiments are essential for the verification of the
mechanisms in vivo.
There were other limitations in this study. The
mechanisms associated with the anti-tumor effects of miR-21
inhibitor and DLN require further investigation, although apoptosis
may partially explain the effects. Additionally, only one dose of
DLN was used in the study. More doses of DLN combined with miR-21
inhibitor should be delivered to further clarify the efficacy. For
potential future clinical use, the toxicity of combination of DLN
with miR-21 inhibitor in normal cells should also be evaluated.
In conclusion, miR-21 inhibitor promoted the
anti-cancer effects of DLN in melanoma cells. Furthermore, the data
revealed that the anti-cancer effect of DLN is miR-21-independent,
and involves BCL-2, Bax and P53 expression. This study may provide
an alternative treatment for melanoma.
Acknowledgements
Not applicable.
Funding
This research was supported by the Key R&D
project in Jiangxi Province (grant no. 20161ACG70016).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Author's contributions
XW and CZ conceived and designed the experiments;
XW, XY and JZ performed the experiments and analyzed the data; XW
and CZ wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of Ethics Committee of Jiangxi Tumor Hospital (no.
20170312).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gopalani SV, Janitz AE and Campbell JE:
Trends in cervical cancer incidence and mortality in Oklahoma and
the United States, 1999–2013. Cancer Epidemiol. 56:140–145. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gunda D, Kido I, Kilonzo S, Nkandala I,
Igenge J and Mpondo B: Prevalence and associated factors of
incidentally diagnosed prostatic carcinoma among patients Who had
transurethral prostatectomy in Tanzania: A retrospective study.
Ethiop J Health Sci. 28:11–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lv Y, Song Y, Ni C, Wang S, Chen Z, Shi X,
Jiang Q, Cao C and Zuo Y: Overexpression of lymphocyte antigen 6
complex, locus E in gastric cancer promotes cancer cell growth and
metastasis. Cell Physiol Biochem. 45:1219–1229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hasan S, Taha R and Omri HE: Current
opinions on chemoresistance: An overview. Bioinformation. 14:80–85.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu X, Xing X, Dowlut D, Zeng Y, Liu J and
Liu X: Integrating phosphoproteomics into kinase-targeted cancer
therapies in precision medicine. J Proteomics. 191:68–79. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jindal AB, Bachhav SS and Devarajan PV: In
situ hybrid nano drug delivery system (IHN-DDS) of antiretroviral
drug for simultaneous targeting to multiple viral reservoirs: An in
vivo proof of concept. Int J Pharm. 521:196–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma X, Gong N, Zhong L, Sun J and Liang XJ:
Future of nanotherapeutics: Targeting the cellular sub-organelles.
Biomaterials. 97:10–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ying M, Chen G and Lu W: recent advances
and strategies in tumor vasculature targeted nano-drug delivery
systems. Curr Pharm Des. 21:3066–3075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garg T, Bhandari S, Rath G and Goyal AK:
Current strategies for targeted delivery of bio-active drug
molecules in the treatment of brain tumor. J Drug Target.
23:865–887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Y, Liu W, Gao F, Fang X and Chen Y:
c(RGDyK)-decorated Pluronic micelles for enhanced doxorubicin and
paclitaxel delivery to brain glioma. Int J Nanomedicine.
11:1629–1641. 2016.PubMed/NCBI
|
|
11
|
Chen Y, Zhang W, Huang Y, Gao F, Sha X and
Fang X: Pluronic-based functional polymeric mixed micelles for
co-delivery of doxorubicin and paclitaxel to multidrug resistant
tumor. Int J Pharm. 488:44–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Z, Luo X, Zhang X, Liu J and Jiang Q:
Targeted delivery of 10-hydroxycamptothecin to human breast cancers
by cyclic RGD-modified lipid-polymer hybrid nanoparticles. Biomed
Mater. 8:0250122013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bohme D and Beck-Sickinger AG: Drug
delivery and release systems for targeted tumor therapy. J Pept
Sci. 21:186–200. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ooi CY, Carter DR, Liu B, Mayoh C, Beckers
A, Lalwani A, Nagy Z, De Brouwer S, Decaesteker B, Hung TT, et al:
Network modeling of microRNA-mRNA interactions in neuroblastoma
tumorigenesis identifies miR-204 as a direct inhibitor of MYCN.
Cancer Res. 78:3122–3134. 2018.PubMed/NCBI
|
|
15
|
Li M, Zhang F, Su Y, Zhou J and Wang W:
Nanoparticles designed to regulate tumor microenvironment for
cancer therapy. Life Sci. 201:37–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nogales-Cadenas R, Cai Y, Lin JR, Zhang Q,
Zhang W, Montagna C and Zhang ZD: MicroRNA expression and gene
regulation drive breast cancer progression and metastasis in PyMT
mice. Breast Cancer Res. 18:752016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghosal S, Saha S, Das S, Sen R, Goswami S,
Jana SS and Chakrabarti J: miRepress: Modelling gene expression
regulation by microRNA with non-conventional binding sites. Sci
Rep. 6:223342016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wandler A, Riber-Hansen R, Hager H,
Hamilton-Dutoit SJ, Schmidt H, Nielsen BS, Stougaard M and
Steiniche T: Quantification of microRNA-21 and microRNA-125b in
melanoma tissue. Melanoma Res. 27:417–428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hermansen SK, Dahlrot RH, Nielsen BS,
Hansen S and Kristensen BW: MiR-21 expression in the tumor cell
compartment holds unfavorable prognostic value in gliomas. J
Neurooncol. 111:71–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo L, Ding W and Zheng LM: The
C(RgdyK)-conjugated Fe3O4 nanoparticles with high drug load for
dual-targeting integrin alpha(v)beta3-expressing cancer cells. J
Nanosci Nanotechnol. 14:4858–4864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamamoto A: Recent therapeutic strategies
for metastatic melanoma: Introduction to invited articles. Int J
Clin Oncol. Apr 6–2018.(Epub ahead of print). View Article : Google Scholar
|
|
23
|
Kim IK, Lane AM, Jain P, Awh C and
Gragoudas ES: Ranibizumab for the prevention of radiation
complications in patients treated with proton beam irradiation for
choroidal melanoma. Trans Am Ophthalmol Soc. 114:T22016.PubMed/NCBI
|
|
24
|
Mohamed AA, Richards CJ, Boyle K and Faust
G: Severe inflammatory ileitis resulting in ileal perforation in
association with combination immune checkpoint blockade for
metastatic malignant melanoma. BMJ Case Rep. 2018(pii): bcr
2018-224913 2018.
|
|
25
|
Neto OV, Raymundo S, Franzoi MA, do Carmo
Artmann A, Tegner M, Müller VV, Hahn RZ, Alves GV, Schwartsmann G,
Linden R and Antunes MV: DPD functional tests in plasma, fresh
saliva and dried saliva samples as predictors of 5-fluorouracil
exposure and occurrence of drug-related severe toxicity. Clin
Biochem. 56:18–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin C, Zhang X, Chen H, Bian Z, Zhang G,
Riaz MK, Tyagi D, Lin G, Zhang Y, Wang J, et al: Dual-ligand
modified liposomes provide effective local targeted delivery of
lung-cancer drug by antibody and tumor lineage-homing
cell-penetrating peptide. Drug Deliv. 25:256–266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ke X, Lin W, Li X, Wang H, Xiao X and Guo
Z: Synergistic dual-modified liposome improves targeting and
therapeutic efficacy of bone metastasis from breast cancer. Drug
Deliv. 24:1680–1689. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Belhadj Z, Zhan C, Ying M, Wei X, Xie C,
Yan Z and Lu W: Multifunctional targeted liposomal drug delivery
for efficient glioblastoma treatment. Oncotarget. 8:66889–66900.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang HL, Si LB, Zeng A, Long F, Qi Z,
Zhao R and Bai M: MicroRNA-21 antisense oligonucleotide improves
the sensitivity of A375 human melanoma cell to Cisplatin: An in
vitro study. J Cell Biochem. 119:3129–3141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin KY, Chen CM, Lu CY, Cheng CY and Wu
YH: Regulation of miR-21 expression in human melanoma via
UV-ray-induced melanin pigmentation. Environ Toxicol. 32:2064–2069.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cifdaloz M, Osterloh L, Graña O,
Riveiro-Falkenbach E, Ximénez-Embún P, Muñoz J, Tejedo C, Calvo TG,
Karras P, Olmeda D, et al: Systems analysis identifies
melanoma-enriched pro-oncogenic networks controlled by the RNA
binding protein CELF1. Nat Commun. 8:22492017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Juliano RL, Ming X, Nakagawa O, Xu R and
Yoo H: Integrin targeted delivery of gene therapeutics.
Theranostics. 1:211–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang X, Sha X, Xin H, Chen L, Gao X, Wang
X, Law K, Gu J, Chen Y, Jiang Y, et al: Self-aggregated pegylated
poly (trimethylene carbonate) nanoparticles decorated with c(RGDyK)
peptide for targeted paclitaxel delivery to integrin-rich tumors.
Biomaterials. 32:9457–9469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qiu L, Hu Q, Cheng L, Li L, Tian C, Chen
W, Chen Q, Hu W, Xu L, Yang J, et al: cRGDyK modified pH responsive
nanoparticles for specific intracellular delivery of doxorubicin.
Acta Biomater. 30:285–298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Y, Zhang W, Huang Y, Gao F and Fang
X: In vivo biodistribution and anti-tumor efficacy evaluation of
doxorubicin and paclitaxel-loaded pluronic micelles decorated with
c(RGDyK) peptide. PLoS One. 11:e01499522016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ribas J and Lupold SE: The transcriptional
regulation of miR-21, its multiple transcripts, and their
implication in prostate cancer. Cell Cycle. 9:923–929. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q and Zhang L, Yuan X, Ou Y, Zhu X,
Cheng Z, Zhang P, Wu X, Meng Y and Zhang L: The relationship
between the Bcl-2/Bax proteins and the mitochondria-mediated
apoptosis pathway in the differentiation of adipose-derived stromal
cells into neurons. PLoS One. 11:e01633272016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fan Y, Yang F, Cao X, Chen C, Zhang X,
Zhang X, Lin W, Wang X and Liang C: Gab1 regulates SDF-1-induced
progression via inhibition of apoptosis pathway induced by
PI3K/AKT/Bcl-2/BAX pathway in human chondrosarcoma. Tumour Biol.
37:1141–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ou X, Lu Y, Liao L, Li D, Liu L, Liu H and
Xu H: Nitidine chloride induces apoptosis in human hepatocellular
carcinoma cells through a pathway involving p53, p21, Bax and
Bcl-2. Oncol Rep. 33:1264–1274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dolka I, Król M and Sapierzyński R:
Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved
caspase-3 and p53) expression in canine mammary tumors: An
immunohistochemical and prognostic study. Res Vet Sci. 105:124–133.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li C, Shen J, Wei X, Xie C and Lu W:
Targeted delivery of a novel palmitylated D-peptide for
antiglioblastoma molecular therapy. J Drug Target. 20:264–271.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li G, Song YZ, Huang ZJ, Chen K, Chen DW
and Deng YH: Novel, nano-sized, liposome-encapsulated
polyamidoamine dendrimer derivatives facilitate tumour targeting by
overcoming the polyethylene glycol dilemma and integrin saturation
obstacle. J Drug Target. 25:734–746. 2017. View Article : Google Scholar : PubMed/NCBI
|