Introduction
Chondrosarcomas are malignant bone tumors formed
from cartilage cells and are the second most common primary bone
malignancy, accounting for 25.8% of primary bone cancers (1). Chondrosarcomas are a heterogeneous
group of neoplasms; however, all grades and variants are relatively
refractory to chemotherapy and radiation therapy (2). Low-to-intermediate grade
chondrosarcomas have a good prognosis following surgical
management, whereas high-grade tumors have poor outcomes; thus,
novel approaches for the management of this disease are required
(3). Chondrosarcoma is a cancer of
mesenchymal origin, of which the mechanism underlying the
mesenchymal transformation of cartilage cells remains unclear.
Proline-rich polypeptide 1 (PRP-1), also known as
galarmin, is produced by the neurosecretory cells of the brain
(4). The cytostatic,
antiproliferative, immunomodulatory, and tumor suppressor
properties of PRP-1 suggest its potential as a therapeutic agent in
human chondrosarcoma cells resistant to radiation and chemotherapy
(4–9).
Aldehyde dehydrogenase (ALDH) is an established
marker of cancer stem cells (CSCs) in a variety of neoplasms. Cells
exhibiting upregulated expression of ALDH have been isolated from
human sarcoma cell lines, including the human chondrosarcoma
SW-1353 cell line (10). ALDH1
activity can be used to identify a subpopulation of cells
characterized by significant increases in the proliferation rate,
colony formation ability and the expression of ABC transporter
genes and stemness markers when compared with control cells
(10). ALDH1 activity has been
reported to be characteristic of cells with increased colony
formation abilities, invasiveness and the expression of ABC
transporters genes (10).
CSCs provide tumors with the capacity for
self-renewal. ALDH expression in primary bone sarcomas was found to
be associated with metastatic potential; upon culturing with
disulfiram, an ALDH inhibitor, sarcoma cells exhibited decreased
proliferation (11). Regarding the
role of ALDH in self-renewal, proliferation and metastasis, ALDH
may be considered as a potential target in the treatment of human
chondrosarcoma.
The canonical Wnt/β-catenin signaling pathway plays
a critical role in embryonic development and homeostatic stem cell
self-renewal in a variety of adult tissues (12). Aberrant activation of the
Wnt/β-catenin signaling pathway has been associated with numerous
types of cancer, including colorectal cancer and leukemias
(12), while loss of β-catenin
expression has been linked to disease progression in malignant
melanoma (13,14). The role of Wnt/β-catenin signaling
has been well reported in the process of epithelial-mesenchymal
transition in carcinomas; however, its involvement in the
proliferation of bone sarcomas, including osteosarcoma and
chondrosarcoma requires further investigation. Bone sarcomas have
been associated with the aberrant activation of Wnt/β-catenin
signaling (15), which has been
demonstrated in a stem-like population of osteosarcoma cells with
high tumorigenicity (16). On the
contrary, Wnt/β-catenin signaling was proposed as an
anti-tumorigenic pathway (17). In
addition, the formation of sarcomas from mesenchymal stem cells via
inactivation of the Wnt pathway has been reported (18). This suggests that inactivation of
Wnt/β-catenin signaling in mesenchymal tumors may initiate
sarcomagenesis.
In the present study, an Aldefluor® assay
was performed to analyze the expression of ALDH in human
chondrosarcoma JJ012 cells in the presence or absence of PRP-1.
Subsequently, PRP-1-treated and untreated cells were sorted into
ALDHlow and ALDHhigh populations to determine
the expression of genes related to the Wnt signaling pathway using
a WNT Signaling Pathway RT2 Profiler PCR Array.
Additionally, western blot analysis and immunocytochemistry were
performed to determine the differences in the cytoplasmic and
nuclear expression of β-catenin in association with the regulation
of Wnt/β-catenin signaling.
Materials and methods
Tissue culture
The human chondrosarcoma JJ012 cells were obtained
from the laboratory of Dr Joel Block, Rush University, Chicago, IL,
USA. The cells were maintained in complete growth medium containing
the following: Dulbecco's modified Eagle's medium (DMEM) +
GlutaMAX™, MEM (minimum essential medium) supplemented with F-12 +
GlutaMAX™ Nutrient mixture (Ham), 10% fetal bovine serum (FBS), 1%
penicillin/streptomycin (Thermo Fisher Scientific, Inc., Waltham,
MA, USA); 25 µg/ml ascorbic acid, 100 ng/ml insulin and 100 nM
hydrocortisone (Sigma-Aldrich; Merck KGaA). The cells were
incubated at 37°C in a humidified atmosphere of 5% CO2
and periodically checked for mycoplasma.
Mycoplasma detection assay to detect
possible contamination of the cells
With the MycoAlert® Mycoplasma Detection
kit (Lonza, Inc., Rockland, ME, USA; cat. no. LT07-118; lot. no.
0000678423) 2 ml of cell culture or culture supernatant was
transferred into a centrifuge tube and centrifuged at 1,500 rpm
(200 × g) for 5 min. Cleared supernatant (100 µl) was transferred
into a luminometer well. MycoAlert™ reagent (100 µl) was added to
each sample and incubated at room temperature (20°C) for 5 min. The
plate was placed in the luminometer and the program was initiated
for Reading A. MycoAlert™ substrate (100 µl) was added to each
sample and incubated at room temperature for 10 min. The plate was
placed in the luminometer for Reading B. The ratio was calculated
as Reading B/Reading A. If contamination was detected, cells were
seeded in full growth media and treated with Plasmocin™ (InvivoGen,
Inc., San Diego, CA, USA; cat. no. ant-mpt; lot. no. MPT-38-03A)
with a concentration of 25 µg/ml for 14 days, removing and
replacing with fresh Plasmocin™ treatment containing medium every
3–4 days.
PRP-1 treatment
A subset of cultured human JJ012 chondrosarcoma
cells were treated with 10 µg/ml of PRP-1 and incubated at 37°C in
a humidified atmosphere of 5% CO2 for a period of 24 h
prior to the Aldefluor® assay. A subset of cultured
human JJ012 chondrosarcoma cells were treated with incrementally
increasing doses of 1, 5, 10 and 20 µg/ml PRP-1 and incubated at
37°C in a humidified atmosphere of 5% CO2 for a period
of 24 h prior to cytoplasmic and nuclear fractionation. PRP-1 was
initially isolated from the brain hypothalamus (4).
Aldefluor® assay and
fluorescence-activated cell sorting (FACS)
To measure cells with ALDH activity, the
Aldefluor® assay was carried out as described according
to manufacturer's protocol (Aldefluor™ kit, cat. no. 01700;
StemCell Technologies, Vancouver, BC, Canada). Briefly, cells were
harvested and resuspended in Aldefluor™ assay buffer at a
concentration of 1×106/ml. To activate the Aldefluor™
reagent, first 25 µl of DMSO was added and incubated for 15 min
with 25 µl of 2N HCl, then 360 µl of assay buffer was added to the
vial. The cells were then incubated with the activated Aldefluor™
reagent for 45 min at 37°C. Diethylaminobenzaldehyde (DEAB), a
specific ALDH inhibitor, was added as a negative control. Following
incubation, all tubes were centrifuged for 5 min at 250 × g, and
the supernatant was removed, and resuspended in Aldefluor™ assay
buffer. The cells were then transferred and strained onto Falcon 5
ml polystyrene round bottom tube with a cell strainer cap (cat. no.
352235). After labeling, the samples were sorted on a BD
Biosciences (San Jose, CA, USA) Special Order Research Product
(SORP) FACSAria II, using BD FACSDiva software (version 6.1.3) into
ALDHlow and ALDHhigh cells with and without
PRP-1 treatment. Data analysis was performed using FlowJo software
(FlowJo LLC, Ashland, OR, USA) (version 10).
RT2 profiler PCR array
The human chondrosarcoma JJ012 cells were sorted
into cryovials and flash frozen in liquid nitrogen for shipment.
Each group was repeated in triplicate. The RT2 profiler
PCR array was carried out by Qiagen, where RNA isolation and
quality control were completed (WNT Signaling Pathway
RT2 Profiler PCR Array, cat. no. PAHS-043Z; Qiagen,
Inc., Valencia, CA, USA).
Statistical analysis for
RT2 Profiler PCR array fold-changes
Fold-change was calculated using the ΔΔCt method.
Fold-change (2−ΔΔCt) is the normalized gene expression
in the test sample divided by the normalized gene expression in the
control sample. Fold-regulation represents fold-change results in a
biologically meaningful way. Fold-change values greater than one
indicated a positive or an upregulation, and the fold-regulation is
equal to the fold-change. Fold-change values less than one
indicated a negative or downregulation, and the fold-regulation was
the negative inverse of the fold-change. The P-values were
calculated based on a Student's t-test of the replicate
2−ΔΔCt values for each gene in the control group and
treatment groups.
Brief immunocytochemistry
protocol
JJ012 chondrosarcoma cells were cultured and
incubated to confluency. A subset of cells was treated with 10
µg/ml of PRP-1 for 24 h. Cells were collected using trypsin and
then seeded directly onto coverslips (5×105
cells/coverslip) placed into 6-well clusters. Cells were cultured
overnight at 37°C in a 5% CO2 incubator. After 24 h, the
medium was removed, and samples were fixed using 1 ml of 4%
formaldehyde solution (F8775; Sigma-Aldrich; Merck KGaA) in
phosphate-buffered saline (PBS), pH 7.4 1X (Gibco; Thermo Fisher
Scientific) (10010–023) for 15 min in the incubator at 37°C.
Samples were then washed with PBS twice. Permeabilization of
samples was completed with PBS/Triton X-100 1% (T9284;
Sigma-Aldrich; Merck KGaA) for 5 min at room temperature. The
detergent was removed, and non-specific sites were blocked using
PBS containing 2% bovine serum albumin (BSA, A2153; Sigma-Aldrich;
Merck KGaA) at room temperature for 30 min. Further incubation of
samples was completed adding primary antibodies: Alexa Fluor
Conjugate 594 WGA (Thermo Fisher Scientific, Inc.; cat. no. W11262)
at a dilution of 1:200 incubated for 10 min at room temperature in
the dark; E-cadherin (Abcam; AB1416) at a dilution of 1:100
incubated for 60 min at room temperature in the dark; and β-catenin
(Abcam; cat. no. AB223075) at a dilution of 1:100 incubated for 24
h in a cold room (4°C) on a rocker. The following morning, two
consecutive washes and incubation were completed with PBS solution.
Secondary antibodies anti-rabbit DyLight 488 (Abcam; cat. no.
AB96899) at a dilution of 1:500 and anti-mouse DyLight 550 (Abcam;
cat. no. AB96872) at a dilution of 1:500 were added and samples
were incubated for 60 min at room temperature in the dark. The
second fixation step using formaldehyde for 15 min at room
temperature was performed followed by two washing steps.
4′,6-Diamino-2-phenylindole dihydrochloride (3 µM) (DAPI; D1306;
Thermo Fisher Scientific, Inc.) was used for nuclear staining for
10 min at room temperature. This was followed by two PBS washes.
Antifade mounting medium was used to mount the samples on
coverslips. ProLong Gold Antifade reagent (p10144; Life
Technologies) was applied directly to fluorescently labeled cells
on microscope slides to be used as a liquid mount and to protect
fading of fluorescent dyes during microscopy.
Imaging
Image acquisition was performed by the Analytical
Imaging Core Facility at DRI/SCCC, University of Miami (FL, USA).
Zeiss 200M, ApoTome fluorescent microscope, DAPI 49, GFP 38HE, Cy3
43, Cy5 50 filter cubes (Carl Zeiss Miscroscopy), heated stage,
Orca II ERG Hamamatsu b/w 14-bit camera and AxioVision acquisition
software were used. The coverslips were placed in regular 35-mm
Petri dishes and the cells were grown on them, covered with medium.
Once the cells were grown, the coverslips were taken out, and the
cells were fixed, stained and mounted on glass slides. For imaging
controls secondary antibodies were used without the primaries.
Gel electrophoresis and western
blotting
JJ012 chondrosarcoma cells were cultured and
incubated to confluency. Cells were collected using trypsin and
then seeded into Petri dishes at a concentration of
1×106 cells/ml. The cells were incubated for 24 h at
37°C in a 5% CO2 incubator. The next day, an ice-cold
phosphate-buffered saline wash was performed, and protease
inhibitor was added to the cell lysis buffer (C2978; Sigma-Aldrich;
Merck KGaA) in a 1:100 ratio. After the collection of cells with a
rubber scraper and lysis of cell membranes with an 18-gauge needle,
the cells were centrifuged at 15,000 × g at 4°C. The supernatant
was then collected, and protein content was measured using
NanoDrop® spectrophotometer (Thermo Fisher Scientific,
Inc.). The supernatant was frozen at −80°C until loading onto the
gels (20 µg/lane). Polyacrylamide gel electrophoresis and western
blotting reagents were supplied by Lonza, Inc. (Allendale, NJ, USA)
and related procedures were followed in accordance with the
company's protocol. The catalog numbers for the reagents and
suppliers are listed below. Pager Gold Precast Gels (59502; 10%
Tris-glycine; Lonza, Inc.); ECL reagent (RPN2109; GE Healthcare,
Little Chalfont, UK); Western Blocker solution (W0138;
Sigma-Aldrich; Merck KGaA); ProSieve Quad Color Protein marker
(4.6–300 kDa, 00193837; Lonza, Inc.); 20X reducing agent for
ProSieve ProTrack Dual Color Loading buffer (00193861; Lonza,
Inc.); ProTrack loading buffer (00193861; Lonza, Inc.); ProSieve
ProTrack Dual Color Loading buffer EX running buffer (00200307;
Lonza, Inc.); ProSieve EX Western Blot Transfer buffer (00200309;
Lonza, Inc.); Immobilon®-P polyvinylidene difluoride
membranes (P4188; Sigma-Aldrich; Merck KGaA).
Preparation of the subcellular
fraction lysates
Cytoplasmic and nuclear fractions of cells were
isolated following the manufacturer's instructions (cat. no. 40010,
lot. no. 22118072; Active Motif) using phosphatase inhibitors,
protease cocktail inhibitor and sonicator.
CGP57380 treatment
CGP57380 is a cell-permeable selective inhibitor of
β-catenin nuclear translocation which was added in incrementally
increasing doses of 1, 5, 10 and 20 µM for a period of 24 h prior
to the assay (C0993, Sigma-Aldrich; Merck KGaA).
DEAB treatment
DEAB, a specific ALDH inhibitor, from the Aldefluor™
kit was added as a negative control (5 µl/ml) for a period of 24 h
prior to lysate generation.
Antibodies for western blotting
Rabbit polyclonal antibody to β-catenin was applied
as a primary antibody (ab2365, Abcam) at a dilution of 1:1,000 and
goat anti-rabbit IgG peroxidase conjugate as a secondary antibody
(A0545; Sigma-Aldrich; Merck KGaA) at a dilution of 1:5,000. As
housekeeping proteins, mouse anti-tubulin antibody was applied for
cytoplasmic fractions (T5168; Sigma-Aldrich; Merck KGaA) at a
dilution of 1:2,000 and anti-mouse IgG (A4416; Sigma-Aldrich; Merck
KGaA) at a dilution of 1:5,000 was applied as a secondary antibody.
Mouse anti-TBP was used for nuclear fractions (T1827,
Sigma-Aldrich; Merck KGaA) at a dilution of 1:1,000 and anti-mouse
IgG (A4416; Sigma-Aldrich; Merck KGaA) at a dilution of 1:5,000 was
applied as a secondary antibody. Incubations for all primary
antibodies were carried out in a cold room while rocking for a
period of 24 h, while secondary antibodies were incubated for 2 h
under the same conditions.
Densitometric analysis for western
blot analysis
Quantitative analysis and densitometry were obtained
using integrated density analysis on ImageJ 1.52e (NIH; National
Institutes of Health, Bethesda, MD, USA) to calculate relative
optical density (OD) of β-catenin to the housekeeping protein. Bulk
JJ012 was used as the control.
Statistical analysis
Statistical analyses were performed using individual
unpaired t-tests for flow cytometry experiments, which were
repeated 10 times. Statistical analyses of relative ODs were
completed using one-way analysis of variance (ANOVA) with a post
hoc Dunnett's multiple comparisons test of all samples to control
bulk JJ012 cells expressed as 95% confidence intervals of mean
difference. All western blot experiments were repeated 2 times. All
statistical analyses were completed using GraphPad Prism 8.0.2
(GraphPad Software, Inc., San Diego, CA, USA). A P-value <0.05
was considered significant. Error bars represent standard error of
the mean (SEM) in all graphs with *P<0.05, **P<0.01,
***P<0.001 (as indicated in the figures and figure legends).
Results
PRP-1 significantly decreases
ALDHhigh cells from the bulk JJ012 cell population
Considering the demonstrated cytostatic,
antiproliferative and tumor-suppressor properties of PRP-1 and the
established ALDHhigh CSC populations in chondrosarcoma
cell lines, we sought to determine whether PRP-1 plays a role in
the expression of stem cell characteristics in bulk JJ012 human
chondrosarcoma cells. To test this, we cultured a group of bulk
JJ012 with PRP-1 and without PRP-1. These cells underwent
Aldefluor® assay and ALDH expression was detected using
FACS. ALDH inhibitor, DEAB, served as the negative control for both
treated and untreated cells to ensure the accuracy of the analysis.
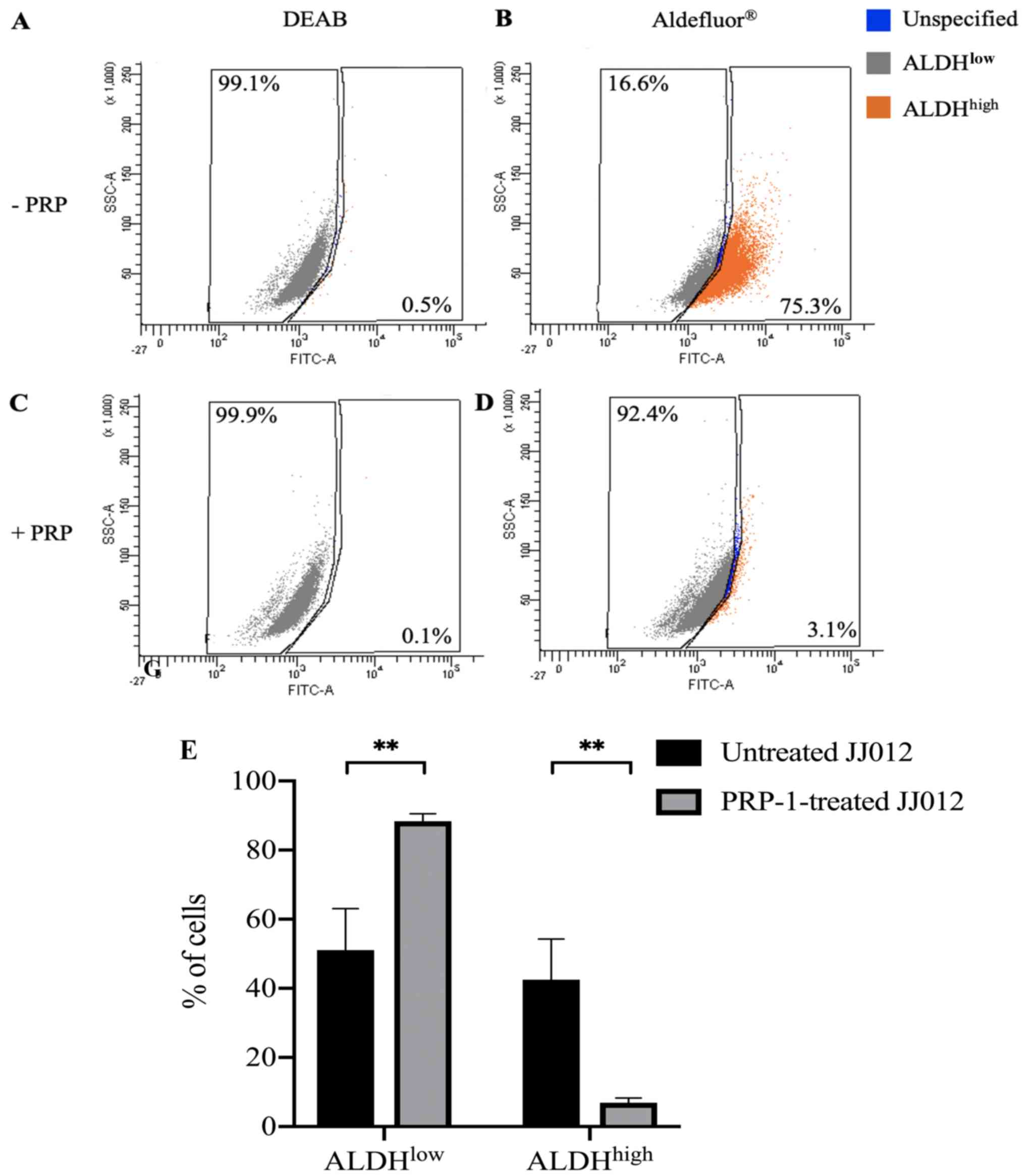
Unstained untreated and PRP-1-treated cells showed no background
fluorescence. A set of untreated cells stained with
Aldefluor® in the presence of DEAB showed 0.5%
ALDHhigh cells (Fig. 1A)
and PRP-1-treated cells stained with Aldefluor® in the
presence of DEAB showed 0.1% ALDHhigh cells (Fig. 1C).
A set of PRP-1-treated cells in the absence of DEAB
showed 3.1% ALDHhigh cells (Fig. 1D) compared to a set of untreated
cells in the absence of DEAB which showed 75.3% ALDHhigh
cells (Fig. 1B). A statistically
significant difference was found between the ALDHlow
populations in the untreated [mean (M)=51.10%, SEM=12.00%, n=10]
and PRP-1-treated JJ012 cells (M=88.39%, SEM=2.14%, n=10) stained
with Aldefluor®; t(18)=3.059, P=0.0068 (Fig. 1E). There was also a statistically
significant difference between the ALDHhigh populations
in the untreated (M=42.49%, SEM=11.82%, n=10) and PRP-1-treated
JJ012 cells (M=6.93%, SEM=1.35%, n=10) stained with
Aldefluor®; t(18)=2.990, P=0.0079 (Fig. 1E). These results demonstrated that
PRP-1 treatment significantly decreased the ALDHhigh CSC
population from a bulk JJ012 cell population. Subpopulations were
collected using flow cytometry and consisted of:
ALDHhigh cells sorted from untreated JJ012 cells
(ALDHhigh-untreated), ALDHlow cells sorted
from untreated JJ012 cells (ALDHlow-untreated) and
ALDHlow cells sorted from PRP-1-treated JJ012 cells
(ALDHlow-PRP-1). ALDHhigh cells were not able
to be collected from the PRP-1-treated JJ012 cells due to the
exceedingly low levels of these CSC populations after PRP-1
treatment.
RT2 profiler PCR array
demonstrates differential expression of Wnt signaling genes in
ALDHhigh-untreated cells compared to bulk JJ012
cells
After demonstrating the ability of PRP-1 to
eliminate CSCs in JJ012 cells, we sought to understand the pathways
involved in this population shift. Studies connecting ALDH
expression to Wnt/β-catenin signaling pathway activation in many
tumor types, including prostate cancer, liver cancer and breast
cancer, drove us to explore this pathway in chondrosarcoma cells
(19–21). We used the RT2 profiler
PCR arrays performed by Qiagen to explore the Wnt/β-catenin
signaling pathway in PRP-1-treated and untreated cells.
Our first comparison was between
ALDHhigh-untreated cells and bulk JJ012 cells. Wnt
signaling genes were profiled on three samples with technical
triplicates for each group. Notably, ALDHhigh-untreated
cells demonstrated significant downregulation of 6 Wnt signaling
genes, including TCF7L1, WNT3, FZD7, FOSL1, WNT7B and
FZD6 and upregulation of only one Wnt signaling gene,
PORCN, with putative transcription factors involved listed
(Table I). TCF7L1 had the
highest fold downregulation (6.33-fold) (P=0.000543). WNT3,
FZD7, FOSL1, WNT7B and FZD6 were downregulated in a
range from 2.48- to 2.76-fold. PORCN showed 2.43-fold
upregulation (P=0.006511). miRNAs that regulate these downregulated
Wnt signaling genes (FZD7, FZD6, FOSL1, TCF7L1 and
WNT7B) in the ALDHhigh-untreated cells were
identified (Table II).
 | Table I.Genes differentially expressed in
ALDHhigh-untreated vs. bulk JJ012 human chondrosarcoma
cells. |
Table I.
Genes differentially expressed in
ALDHhigh-untreated vs. bulk JJ012 human chondrosarcoma
cells.
| Gene symbol | Fold
regulation | P-value | Transcription
factors |
|---|
| TCF7L1 | −6.33 | 0.000543 | Pax-5, ER-α, E47,
MAZR, MZF-1, Olf-1, AP-2α, AP-2αA, AP-2β, AP-2γ |
| WNT3 | −2.76 | 0.032755 | HNF-4α1, HNF-4α2,
p53, Pax-4a, AP-2α, AP-2αA, AP-β, AP-2 γ, c-Myb, Pax-2, Pax-2a,
Pax-2b, Gfi-1, CBF(2), CBF-A, CBF-B, CBF-C, CP1A, CP1C, NF-Y,
NF-YA, NF-YB, NF-YC, AP-2γ, LCR-F1, Gfi-1, Arnt, MAZR, STAT5A,
LUN-1, Pax-5, ARP-1, E47, POU2F1, POU2F1a, STAT1, STAT1α, STAT1β,
STAT3, STAT5B, STAT5A, c-Myb |
| FZD7 | −2.66 | 0.011918 | HNF-4α1, MAZR, Sp1,
Olf-1, GATA-1, SRY, HSF2, Brachyury, CREB, delta-CREB, C/EBPα,
ATF-2, CRE-BP1, E4BP4, CUTL1, Egr-1, HEN1 |
| FOSL1 | −2.64 | 0.025993 | ATF-2, Ik-3, ATF,
CRE-BP1, CREB, delta-CREB, SRF, SRF (504 AA), Hlf |
| WNT7B | −2.64 | 0.019711 | p53, Nkx5-1, Olf-1,
c-Myc, Max, Bach2, CREB, delta-CREB, AhR, Arnt, Pax-5, CP2, RREB-1,
MAZR, MZF-1, CP2, E47, AREB6, MyoD, Zic1, ZIC2/Zic2, Zic3 |
| FZD6 | −2.48 | 0.044088 | Pax-4a, Pax-2,
Pax-2a, E2F, E2F-1 |
| PORCN | 2.43 | 0.006511 | CREB, kx2-5, Ik-3,
RFX1, LCR-F1, c-Jun, AP-1, c-Fos, GATA-1, GATA-3, XBP-1 |
 | Table II.miRNAs that regulate the
downregulated genes in the ALDHhigh-untreated vs. the
bulk JJ012 human chondrosarcoma cells. |
Table II.
miRNAs that regulate the
downregulated genes in the ALDHhigh-untreated vs. the
bulk JJ012 human chondrosarcoma cells.
| miRNA name | Target gene |
|---|
| hsa-miR-338-5p | FZD7 |
|
hsa-miR-519b-3p | FZD6 |
|
hsa-miR-519c-3p | FZD6 |
|
hsa-miR-519a-3p | FZD6 |
| hsa-miR-593-3p | FOSL1 |
| hsa-miR-101-3p | FZD6 |
|
hsa-miR-199b-5p | FZD6 |
|
hsa-miR-199a-5p | FZD6 |
| hsa-miR-568 | FOSL1,
FZD7 |
| hsa-miR-1283 | TCF7L1 |
|
hsa-miR-130b-3p | FOSL1,
FZD6 |
| hsa-miR-1294 | TCF7L1 |
|
hsa-miR-301a-3p | FOSL1,
FZD6 |
| hsa-miR-301b | FOSL1,
FZD6 |
| hsa-miR-646 | FOSL1 |
| hsa-miR-505-3p | WNT7B |
|
hsa-miR-130a-3p | FOSL1,
FZD6 |
|
hsa-miR-548d-5p | FZD7 |
|
hsa-miR-548a-5p | FZD7 |
|
hsa-miR-548b-5p | FZD7 |
RT2 profiler PCR array
shows differential expression of Wnt signaling genes in
ALDHlow-PRP-1 cells compared to bulk JJ012 cells
In the present study, we compared
ALDHlow-PRP-1 cells to bulk JJ012 cells to determine
whether addition of PRP-1 results in a differential expression of
Wnt genes compared to an untreated and unsorted JJ012 population
using a RT2 profiler PCR array. We identified
significant upregulation of 5 Wnt signaling genes in the
PRP-1-treated ALDHlow cells, including BCL9, PORCN,
RHOU, FZD2 and RPLP0, with putative transcription
factors involved listed (Table
III). MMP7 was 3.50-fold down-regulated (P=0.018004) in
the ALDHlow-PRP-1 cells compared to the bulk JJ012
cells. Notably, PORCN was 2.43-fold upregulated (P=0.006511)
in the ALDHhigh-untreated cells (Table I) while PORCN was 3.54-fold
upregulated (P=0.030693) in the ALDHlow-PRP-1 cells
(Table III) compared to the bulk
JJ012 cells. miRNAs that regulate the upregulated Wnt signaling
genes RHOU and BCL9 in ALDHlow-PRP-1 cells
were identified (Table IV).
 | Table III.Genes differentially expressed
ALDHlow-PRP-1 vs. bulk JJ012 human chondrosarcoma
cells. |
Table III.
Genes differentially expressed
ALDHlow-PRP-1 vs. bulk JJ012 human chondrosarcoma
cells.
| Gene symbol | Fold
regulation | P-value | Transcription
factors |
|---|
| BCL9 | 3.86 | 0.035605 | STAT1, STAT1α,
STAT1β, STAT2, STAT3, STAT4, STAT5A, STAT5B, STAT6, MZF-1, RFX1,
HNF-1, HNF-1A, Cdc5, Meis-1, Meis-1a, Meis-1b, FOXL1 |
| PORCN | 3.54 | 0.030693 | CREB, kx2-5, Ik-3,
RFX1, LCR-F1, c-Jun, AP-1, c-Fos, GATA-1, GATA-3, XBP-1 |
| MMP7 | −3.50 | 0.018004 | HNF-4α1, Bach1,
c-Jun, AP-1, c-Fos, FosB, Fra-1, JunB, JunD, Bach2, POU2F1,
POU2F1a, Oct-B1, oct-B2, oct-B3, POU2F2, POU2F2 (Oct-2.1), POU2F2B,
POU2F2C |
| RHOU | 2.68 | 0.019367 |
|
| FZD2 | 2.64 | 0.005308 |
|
| RPLP0 | 2.21 | 0.008985 |
|
 | Table IV.miRNAs that regulate overexpressed
genes in PRP-1-treated ALDHlow vs. the bulk JJ012 human
chondrosarcoma cells. |
Table IV.
miRNAs that regulate overexpressed
genes in PRP-1-treated ALDHlow vs. the bulk JJ012 human
chondrosarcoma cells.
| miRNA name | Target genes |
|---|
| hsa-miR-525-5p | RHOU |
|
hsa-miR-520a-5p | RHOU |
| hsa-miR-767-3p | RHOU |
| hsa-miR-101-3p | BCL9 |
| hsa-miR-218-5p | BCL9 |
| hsa-miR-124-3p | RHOU |
| hsa-miR-506-3p | RHOU |
| hsa-miR-562 | RHOU |
| hsa-miR-1284 | BCL9 |
| hsa-miR-204-5p | BCL9 |
|
hsa-miR-1301-3p | BCL9 |
| hsa-miR-211-5p | BCL9 |
| hsa-miR-559 | BCL9 |
|
hsa-miR-548a-5p | BCL9 |
| hsa-miR-548i | BCL9 |
|
hsa-miR-548d-5p | BCL9 |
|
hsa-miR-548h-5p | BCL9 |
|
hsa-miR-548c-5p | BCL9 |
|
hsa-miR-548j-5p | BCL9 |
|
hsa-miR-548b-5p | BCL9 |
RT2 profiler PCR array
shows differential expression of Wnt signaling genes in the
ALDHlow-PRP-1 cells compared to the
ALDHhigh-untreated cells
After showing PRP-1 treatment eliminates CSCs in
JJ012 cells, we aimed to determine the differences in Wnt signaling
genes between ALDHhigh-untreated cells and
ALDHlow-PRP-1 cells using a RT2 profiler PCR
array.
The arrays identified two significantly
downregulated cancer genes from the Wnt pathway in the
ALDHlow-PRP-1 cells, including the CCND2 gene,
encoding G1/S specific cyclin D2 and the MMP7 gene, encoding
a matrix metalloproteinase, with putative transcription factors
involved listed (Table V).
CCND2 was downregulated 4.51-fold (P=0.004252) and
MMP7 was downregulated 3.25-fold (P=0.000044). miRNAs that
regulate the downregulated Wnt signaling gene CCND2 in the
ALDHlow-PRP-1 cells were identified (Table VI). Numerous studies have
demonstrated the importance of miRNA regulators, their
downregulation, and their role in overexpression of CCND2 in
association with high-grade osteosarcomas and resistance to
chemotherapy (22–25). In fact, CCND2 was found to be
upregulated in metastatic osteosarcoma compared to the primary
tumor (26). Together, these
experimental results demonstrate the important role that
CCND2 plays in the development of the progression of cancer,
chemoresistance and metastasis that may also be present in
chondrosarcoma.
 | Table V.Genes differentially expressed in
ALDHlow-PRP-1 vs. ALDHhigh-untreated human
JJ012 chondrosarcoma cells. |
Table V.
Genes differentially expressed in
ALDHlow-PRP-1 vs. ALDHhigh-untreated human
JJ012 chondrosarcoma cells.
| Gene symbol | Fold
regulation | P-value | Transcription
factors |
|---|
| CCND2 | −4.51 | 0.004252 | GATA-1, STAT1,
STAT1α, STAT1β, STAT2, STAT3, STAT4, STAT5A, STAT5B, STAT6, Pax-3,
c-Rel, HEN1, PPAR-γ1, PPAR-γ2, HNF-4α1, HNF-4α2, E47, Lmo2 |
| MMP7 | −3.25 | 0.000044 | HNF-4α1, Bach1,
c-Jun, AP-1, c-Fos, FosB, Fra-1, JunB, JunD, Bach2, POU2F1,
POU2F1a, Oct-B1, Οct-B2, Οct-B3, POU2F2, POU2F2 (Oct-2.1), POU2F2B,
POU2F2C |
 | Table VI.miRNAs that regulate the
downregulated gene CCND2 in the ALDHlow-PRP-1
cells vs. the ALDHhigh-untreated human chondrosarcoma
cells. |
Table VI.
miRNAs that regulate the
downregulated gene CCND2 in the ALDHlow-PRP-1
cells vs. the ALDHhigh-untreated human chondrosarcoma
cells.
| miRNA name | Target gene |
|---|
|
hsa-miR-200a-3p | CCND2 |
| hsa-miR-141-3p | CCND2 |
|
hsa-miR-548d-3p | CCND2 |
| hsa-miR-506-3p | CCND2 |
| hsa-miR-124-3p | CCND2 |
| hsa-miR-548p | CCND2 |
| hsa-miR-154-5p | CCND2 |
| hsa-miR-19b-3p | CCND2 |
| hsa-miR-19a-3p | CCND2 |
| hsa-miR-656-3p | CCND2 |
| hsa-miR-1183 | CCND2 |
| hsa-miR-18b-5p | CCND2 |
| hsa-miR-18a-5p | CCND2 |
| hsa-miR-29b-3p | CCND2 |
| hsa-miR-520h | CCND2 |
|
hsa-miR-520g-3p | CCND2 |
| hsa-miR-29c-3p | CCND2 |
| hsa-miR-29a-3p | CCND2 |
| hsa-miR-1269a | CCND2 |
| hsa-miR-634 | CCND2 |
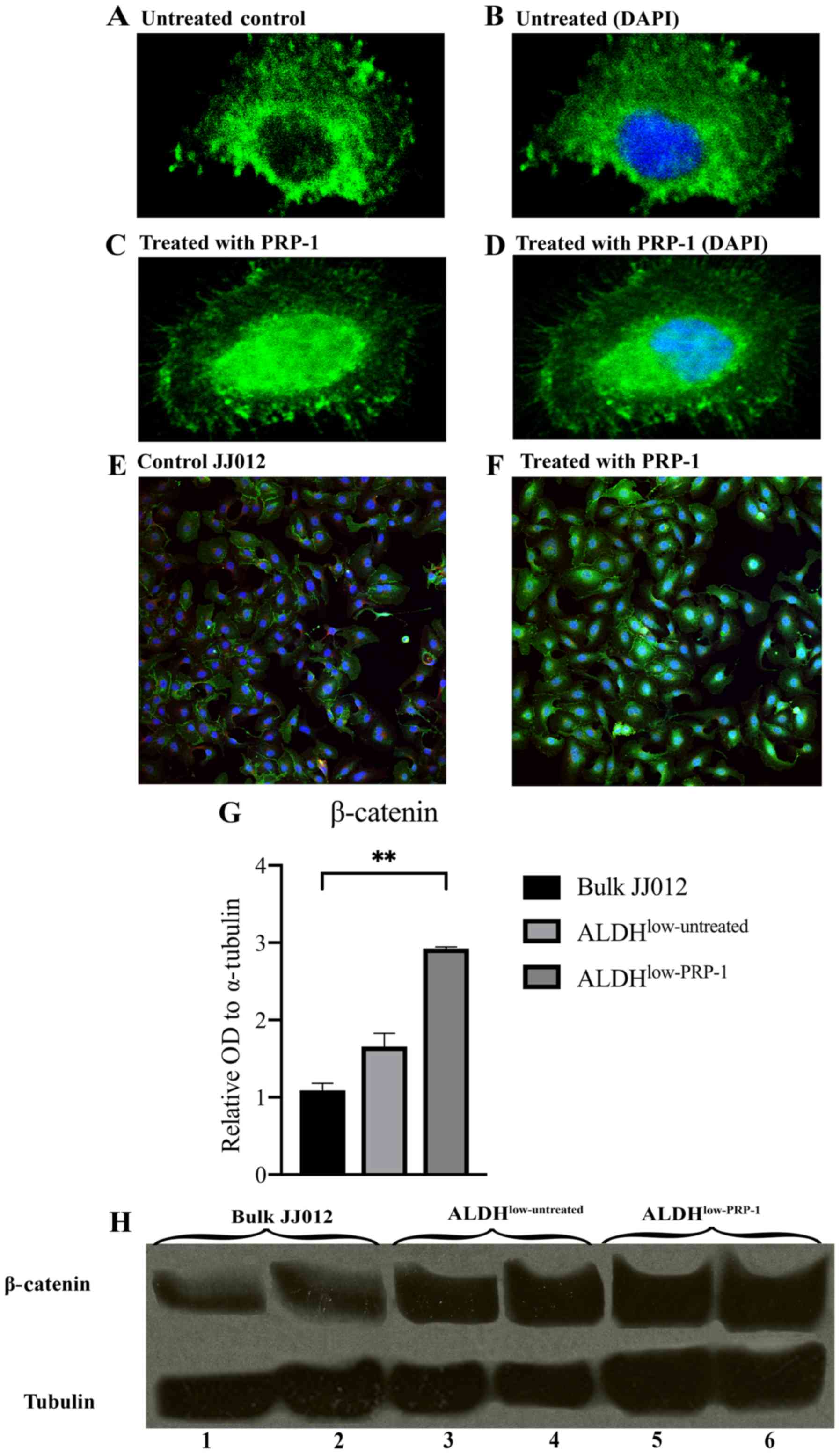
Immunocytochemistry demonstrates
increased nuclear β-catenin expression in the PRP-1-treated
JJ012
Immunofluorescent stained cell images demonstrated
that β-catenin was present in the nuclei in the PRP-1-treated JJ012
cells vs. in the cytoplasm of untreated JJ012 cells. Single-cell
images are depicted (Fig. 2A-D) and
a wider field of multiple cells is shown (Fig. 2E and F).
Western blot analysis indicates that
β-catenin protein expression is increased in the
ALDHlow-PRP-1 cells
To confirm the findings from the RT2
profiler PCR arrays and immunocytochemistry, western blot analysis
was carried out. One-way ANOVA found significant differences in the
mean relative optical density (OD) of β-catenin between bulk JJ012
cells, ALDHlow-untreated and ALDHlow-PRP-1
cells [F(2,3)=70.60, P=0.0030]. Post hoc Dunnett's tests determined
no significance difference between ALDHlow-untreated and
bulk JJ012 cells, but a significant increase in relative OD of
β-catenin was found between ALDHlow-PRP-1 and bulk JJ012
cells (1.834±0.611, P=0.0023) (Fig. 2G
and H). This supports the finding that PRP-1 regulates
β-catenin protein expression.
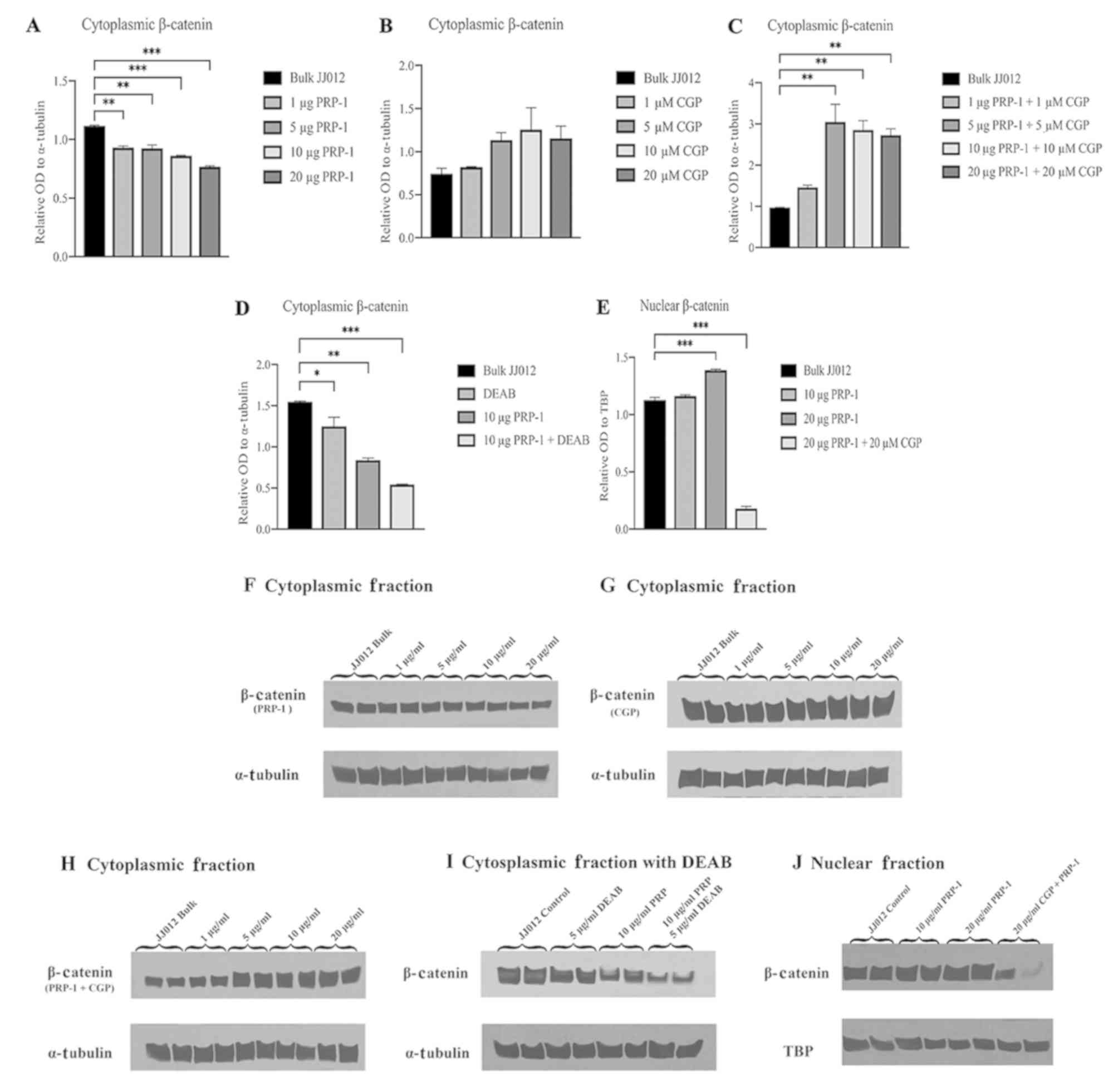
Western blot analyses of cytoplasmic
and nuclear fractions of PRP-1-treated JJ012 cells demonstrate
nuclear translocation of β-catenin
To determine whether PRP-1 has an effect on
subcellular regulation of the Wnt/β-catenin pathway, western blot
analysis was performed on cytoplasmic and nuclear fractions using
bulk JJ012 cells as a control compared to various combinations of
PRP-1, CGP57380 (CGP; a β-catenin nuclear translocation inhibitor)
and DEAB (a direct ALDH inhibitor). One-way ANOVA of the western
blot experiments with incrementally increasing PRP-1 concentrations
found a statistically significantly difference in relative OD of
β-catenin between the groups [F(4,5)=67.93, P=0.0002]. Post hoc
Dunnett's test demonstrated a significant decrease in the mean
relative OD in cells treated with 1 µg PRP-1 (−0.1867±0.0762,
P=0.0011), 5 µg PRP-1 (−0.1923±0.0763, P=0.0010), 10 µg PRP-1
(−0.2555±0.0762, P=0.0003) and 20 µg PRP-1 (−0.3485±0.0762,
P<0.0001) compared to the bulk JJ012 control cells (Fig. 3A and F). One-way ANOVA of
experiments with the addition of β-catenin nuclear translocation
inhibitor CGP57380 (27,28) in increasing concentrations
demonstrated no significant differences in mean relative OD of
cytoplasmic β-catenin protein between the groups [F(4,5)=2.649,
P=0.1570] (Fig. 3B and G). When
adding CGP57380 (CGP) with increasing PRP-1 concentrations, one-way
ANOVA demonstrated significant differences in mean relative OD of
cytoplasmic β-catenin protein between the groups [F(4,5)=16.71,
P=0.0043]. Post hoc analysis using Dunnett's test demonstrated a
significant increase in relative OD of cytoplasmic β-catenin with 5
µg PRP-1 + 5 µM CGP (2.076±1.1198, P=0.0040), 10 µg PRP-1 + 10 µM
CGP (1.881±1.0504, P=0.0062) and 20 µg PRP-1 +20 µM CGP
(1.754±1.1199, P=0.0083) compared to the bulk JJ012 control cells
(Fig. 3C and H). One-way ANOVA
analysis of JJ012 cells treated with DEAB found significant
differences in the mean relative OD of cytoplasmic β-catenin
between groups [F(3,4)=62.33, P=0.0008]. Post hoc Dunnett's test
found a significant decrease in relative OD of cytoplasmic
β-catenin following treatment with DEAB alone (−0.2969±0.2876,
P=0.0452), 10 µg PRP-1 alone (−0.7082±0.2876, P=0.0020) and 10 µg
PRP-1 + DEAB (−1.006±0.2873, P=0.0005) compared to the bulk JJ012
control cells (Fig. 3D and I). When
examining the relative OD of nuclear β-catenin in the JJ012 cells
treated with PRP-1 and CGP, one-way ANOVA found significant
differences in means between groups [F(3,4)=1182, P<0.0001].
Post hoc analysis using Dunnett's test demonstrated a significantly
increased intensity of nuclear β-catenin in the high-dose (20 µg)
PRP-1 (0.2612±0.0797, P=0.0007) and significantly decreased
intensity of nuclear β-catenin following 20 µg PRP-1 + 20 µM CGP
(−0.9474±0.0797, P<0.0001) compared to the bulk JJ012 control
cells (Fig. 3E and J).
Discussion
Chondrosarcomas represent a heterogeneous group of
cartilaginous malignancies that exhibit both radioresistance and
chemoresistance (2). In order to
investigate potential novel therapies for the treatment of this
disease, improved understanding of the cellular mechanisms driving
the proliferation and metastasis of chondrosarcoma is required.
PRP-1 has been previously reported as a potential therapeutic agent
with cytostatic, antiproliferative and tumor-suppressive properties
(5–9). In the present study, PRP-1 was
observed to notably decrease the abundance of ALDHhigh
CSCs in a population of JJ012 cells. ALDH, a marker of
chondrosarcoma stem cells, correlated with increases in thee
proliferative capacity and colony formation abilities of tumor
cells (10). The potential of PRP-1
to almost completely eliminate ALDHhigh CSCs suggests
the application of PRP-1 in inhibiting the formation of
chondrosarcoma (11,29). The present study proposed the
potential of PRP-1 in reducing the population of
ALDHhigh CSCs in monolayer culture; however, the
viability of ALDHlow cancer cells was not analyzed.
Thus, further investigation is required to establish the effets of
PRP-1 on the viability of chondrosarcoma cells and on three
dimensional models of chondrosarcoma.
Additionally, the role of the Wnt/β-catenin
signaling pathway in various populations of JJ012 chondrosarcoma
cells was investigated. ALDHhigh JJ012 cells exhibited
significantly downregulated Wnt signaling gene expression compared
to bulk JJ012 cells. When comparing ALDHlow-PRP-1 cells
with bulk JJ012 cells, significant upregulation in the expression
of Wnt signaling genes was found in the ALDHlow-PRP-1
cells, with certain exceptions. These results suggest that the
activation and inactivation of the Wnt/β-catenin signaling pathway
serve differing roles in chondrosarcomas, similar to findings in
human mesenchymal stem cells and malignant fibrous histiocytoma
(18). PRP-1 was reported to be
involved in the regulation of Wnt/β-catenin pathway; however, the
role of certain dysregulated Wnt/β-catenin signaling genes was not
identified, which poses as a limitation to our study. To confirm
the findings of the PCR array analysis, immunocytochemistry and
western blotting were conducted to determine the cellular
expression of β-catenin. Increased nuclear β-catenin expression in
the PRP-1-treated JJ012 cells as demonstrated in
immunocytochemistry and upregulated β-catenin protein expression in
ALDHlow-PRP-1 cells as determined by western blotting
suggest that PRP-1 may directly induce the nuclear translocation of
β-catenin.
PRP-1 decreased the cytoplasmic expression levels of
β-catenin; however, opposing effects were observed following
treatment with the nuclear translocation inhibitor CGP57380,
indicating the nuclear translocation of β-catenin following PRP-1
treatment. Additionally, a dose-dependent increase in nuclear
β-catenin expression was reported in response to PRP-1; however,
treatment with CGP57380 significantly decreased the nuclear
expression of β-catenin protein. Of note, treatment of JJ012 cells
with DEAB, a specific ALDH inhibitor, followed by the
administration of PRP-1 revealed reductions in cytoplasmic
β-catenin protein expression. This indicated that decreased ALDH
expression, and therefore CSC activity, may induce the nuclear
translocation of β-catenin in chondrosarcoma cells. Activation of
the Wnt/β-catenin signaling pathway has been reported to serve an
important role in the normal maintenance of mesenchymal tissue
(30). Additionally, inhibition of
Wnt signaling by a known Wnt inhibitor, Dickkopf-related protein 1
(Dkk-1), has been determined to prevent osteogenesis under
conditions of bone repair (31). In
addition, it was demonstrated that upregulated levels of Dkk-1
serve a role in the pathogenesis of osteosarcoma by inhibiting the
repair of the surrounding bone (32). These studies suggest that
inactivation of the Wnt/β-catenin signaling pathway may be involved
in the development and progression of sarcomas, which opposes the
effects of activation of this pathway in a variety of carcinomas
and other types of tumors (12).
The present study identified several dysregulated
genes involved in the Wnt/β-catenin signlaing pathway, including
FOSL1, FZD1, RHOU and BCL9 (33–36).
Of note, when comparing ALDHlow-PRP-1 cells with
ALDHhigh-untreated cells, two important cancer genes,
MMP7, a matrix metalloproteinase, and CCND2, coding
for cyclin D2 involved in G1/S phase progression were reported to
be downregulated in ALDHlow-PRP-1 cells. The results of
the present study indicated that PRP-1 may downregulate certain Wnt
genes and assume the role as a regulator of the Wnt/β-catenin
pathway. In addition, microRNAs (miRNAs) that were associated with
these dysregulated genes were identified; however, their role in
the activation or suppression of these genes was not determined.
Thus, further investigation is required to elucidate the roles of
these specific miRNAs in the regulation of the Wnt/β-catenin
signaling pathway in human chondrosarcoma cells.
Considering that chondrosarcomas are of mesenchymal
origin, previously reported derivation of sarcomas from mesenchymal
stem cells as determined by inactivation of the Wnt/β-catenin
signaling pathway support the findings of the present study
(18).
CCND2 has been associated with the
progression of sarcomas, particularly osteosarcoma (37). Numerous studies have demonstrated
the importance of miRNA regulators, their downregulation and role
in overexpression of CCND2 in high-grade osteosarcomas and
resistance to chemotherapy (22–25).
Additionally, CCND2 was reported to be upregulated in
metastatic osteosarcoma compared with primary tumor samples
(26). Collectively, these results
suggest the important role served by CCND2 in cancer
progression, chemoresistance and metastasis that may also occur in
chondrosarcoma.
MMP7 has been associated with an increased
level of invasiveness of endothelial cells infected by Kaposi's
sarcoma herpesvirus (36).
Inactivation of Wnt signaling has been demonstrated to increase the
expression of MMP7 in osteosarcoma, which opposes the
aforementioned findings reported in carcinomas (37). Investigation into chondrosarcoma
cells revealed that MMP7 upregulation promoted cell motility
and invasion, leading to increased lung metastasis in vivo
(38). Considering these findings,
reductions in the expression of MMP7 in
ALDHlow-PRP-1 cells indicate the potential of PRP-1 to
inhibit the progression and metastasis of chondrosarcoma.
This present study reported the role of PRP-1 in
activating the Wnt pathway and the translocation of β-catenin to
the nucleus, in addition to downregulating the expression of
oncogenes MMP7 and CCND2. It is likely that in these
cases, PRP-1 also normalized the expression of unexpected targets
of the noncanonical pathway. For example, overexpression of
MMP7 was reported to be induced by the noncanonical WNT
signaling pathway (39).
Wnt/β-catenin signaling cascades often intersect with other
signaling pathways, resulting in synergistic or antagonistic
effects on stem cell behavior; thus, various β-catenin
co-activators may lead to different outcomes. The interaction of
β-catenin with different transcription factors and the potential
effects of these interactions for the direct crosstalk between the
Wnt/β-catenin and non-Wnt signaling pathways require further
investigation (40,41). Collectively, our results support
that inactivation of the Wnt/β-catenin signaling pathway in
mesenchymal tumors may initiate sarcomagenesis in chondrosarcoma
(18). Furthermore, Wnt signaling
was proposed to promote or inhibit tumor initiation, metastasis and
drug resistance in a cancer stage-specific and a cancer
type-specific manner (42).
The findings of the present study suggest that
activation of the Wnt/β-catenin signaling pathway may serve an
antitumor role in sarcomas as demonstrated by the upregulated
expression of genes associated with this pathway in
ALDHlow-PRP-1 cells. ALDH activity and reductions in the
abundance of CSCs were demonstrated to promote β-catenin
translocation into the nucleus. PRP-1 was determined to be involved
in regulating the Wnt/β-catenin signaling targets; however, whether
this regulation alone or in combination with other signaling events
underlies the depletion of the CSC population requires further
investigation.
Acknowledgements
We would like to acknowledge the skilled assistance
of the Flow Cytometry Shared Resource of the Sylvester
Comprehensive Cancer Center at the University of Miami Miller
School of Medicine, for the provision of sophisticated fluorescence
and cell sorting services. Our appreciation is expressed to the
Analytical Imaging Core facility staff of DRI/SCCC, University of
Miami who provided immunocytochemistry imaging service.
Funding
The present study was supported in part by a gift
from the Ratcliffe Foundation to the Miami Center of Orthopedic
Research and Education.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
AKH, AM and KAG conceived and designed the study.
AKH, AM, CG, AS, SS and JB performed the experiments. AKH, AS and
SS performed the statistical analyses and produced the figures. AKH
and AM wrote the manuscript. AKH, AM, CG and KAG reviewed and
edited the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dorfman HD and Czerniak B: Bone cancers.
Cancer 75 (1 Suppl). S203–S210. 1995. View Article : Google Scholar
|
|
2
|
Whelan JS and Davis LE: Osteosarcoma,
chondrosarcoma, and chordoma. J Clin Oncol. 36:188–193. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Angelini A, Guerra G, Mavrogenis AF, Pala
E, Picci P and Ruggieri P: Clinical outcome of central conventional
chondrosarcoma. J Surg Oncol. 106:929–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galoyan A: Neurochemistry of brain
neuroendocrine immune system: Signal molecules. Neurochem Res.
25:1343–1355. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galoian K, Temple TH and Galoyan A:
Cytostatic effect of the hypothalamic cytokine PRP-1 is mediated by
mTOR and cMyc inhibition in high grade chondrosarcoma. Neurochem
Res. 36:812–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galoian KA, Guettouche T, Issac B, Qureshi
A and Temple HT: Regulation of onco and tumor suppressor MiRNAs by
mTORC1 inhibitor PRP-1 in human chondrosarcoma. Tumour Biol.
35:2335–2341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galoian K, Qureshi A, Wideroff G and
Temple HT: Restoration of desmosomal junction protein expression
and inhibition of H3K9-specific histone demethylase activity by
cytostatic proline-rich polypeptide-1 leads to suppression of
tumorigenic potential in human chondrosarcoma cells. Mol Clin
Oncol. 3:171–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galoian K, Qureshi A, D'Ippolito G,
Schiller PC, Molinari M, Johnstone AL, Brothers SP, Paz AC and
Temple HT: Epigenetic regulation of embryonic stem cell marker
miR302C in human chondrosarcoma as determinant of antiproliferative
activity of proline-rich polypeptide 1. Int J Oncol. 47:465–472.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galoian K, Abrahamyan S, Chailyan G,
Qureshi A, Patel P, Metser G, Moran A, Sahakyan I, Tumasyan N, Lee
A, et al: Toll like receptors TLR1/2, TLR6 and MUC5B as binding
interaction partners with cytostatic proline rich polypeptide 1 in
human chondrosarcoma. Int J Oncol. 52:139–154. 2018.PubMed/NCBI
|
|
10
|
Lohberger B, Rinner B, Stuendl N, Absenger
M, Liegl-Atzwanger B, Walzer SM, Windhager R and Leithner A:
Aldehyde dehydrogenase 1, a potential marker for cancer stem cells
in human sarcoma. PLoS One. 7:e436642012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greco N, Schott T, Mu X, Rothenberg A,
Voigt C, McGough RL III, Goodman M, Huard J and Weiss KR: ALDH
activity correlates with metastatic potential in primary sarcomas
of bone. J Cancer Ther. 5:331–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kageshita T, Hamby CV, Ishihara T,
Matsumoto K, Saida T and Ono T: Loss of beta-catenin expression
associated with disease progression in malignant melanoma. Br J
Dermatol. 145:210–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maelandsmo GM, Holm R, Nesland JM, Fodstad
Ø and Flørenes VA: Reduced beta-catenin expression in the cytoplasm
of advanced-stage superficial spreading malignant melanoma. Clin
Cancer Res. 9:3383–3388. 2003.PubMed/NCBI
|
|
15
|
Chen C, Zhao M, Tian A, Zhang X, Yao Z and
Ma X: Aberrant activation of Wnt/β-catenin signaling drives
proliferation of bone sarcoma cells. Oncotarget. 6:17570–17583.
2015.PubMed/NCBI
|
|
16
|
Yi XJ, Zhao YH, Qiao LX, Jin CL, Tian J
and Li QS: Aberrant Wnt/β-catenin signaling and elevated expression
of stem cell proteins are associated with osteosarcoma side
population cells of high tumorigenicity. Mol Med Rep. 12:5042–5048.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Basu-Roy U, Seo E, Ramanathapuram L, Rapp
TB, Perry JA, Orkin SH, Mansukhani A and Basilico C: Sox2 maintains
self renewal of tumor-initiating cells in osteosarcomas. Oncogene.
31:2270–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matushansky I, Hernando E, Socci ND, Mills
JE, Matos TA, Edgar MA, Singer S, Maki RG and Cordon-Cardo C:
Derivation of sarcomas from mesenchymal stem cells via inactivation
of the Wnt pathway. J Clin Invest. 117:3248–3257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cojoc M, Peitzsch C, Kurth I, Trautmann F,
Kunz-Schughart LA, Telegeev GD, Stakhovsky EA, Walker JR, Simin K,
Lyle S, et al: Aldehyde dehydrogenase is regulated by β-catenin/TCF
and promotes radioresistance in prostate cancer progenitor cells.
Cancer Res. 75:1482–1494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JY, Lee HY, Park KK, Choi YK, Nam JS
and Hong IS: CWP232228 targets liver cancer stem cells through
Wnt/β-catenin signaling: A novel therapeutic approach for liver
cancer treatment. Oncotarget. 7:20395–20409. 2016.PubMed/NCBI
|
|
21
|
Shan S, Lv Q, Zhao Y, Liu C, Sun Y, Xi K,
Xiao J and Li C: Wnt/β-catenin pathway is required for epithelial
to mesenchymal transition in CXCL12 over expressed breast cancer
cells. Int J Clin Exp Pathol. 8:12357–12367. 2015.PubMed/NCBI
|
|
22
|
Di Fiore R, Fanale D, Drago-Ferrante R,
Chiaradonna F, Giuliano M, De Blasio A, Amodeo V, Corsini LR, Bazan
V, Tesoriere G, et al: Genetic and molecular characterization of
the human osteosarcoma 3AΒ-OS cancer stem cell line: A possible
model for studying osteosarcoma origin and stemness. J Cell
Physiol. 228:1189–1201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He C, Gao H, Fan X, Wang M, Liu W, Huang W
and Yang Y: Identification of a novel miRNA-target gene regulatory
network in osteosarcoma by integrating transcriptome analysis. Int
J Clin Exp Pathol. 8:8348–8357. 2015.PubMed/NCBI
|
|
24
|
Di Fiore R, Drago-Ferrante R, Pentimalli
F, Di Marzo D, Forte IM, D'Anneo A, Carlisi D, De Blasio A,
Giuliano M, Tesoriere G, et al: MicroRNA-29β-1 impairs in vitro
cell proliferation, self-renewal and chemoresistance of human
osteosarcoma 3AB-OS cancer stem cells. Int J Oncol. 45:2013–2023.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Di Fiore R, Drago-Ferrante R, Pentimalli
F, Di Marzo D, Forte IM, Carlisi D, De Blasio A, Tesoriere G,
Giordano A and Vento R: Let-7d miRNA shows both antioncogenic and
oncogenic functions in osteosarcoma-derived 3AB-OS cancer stem
cells. J Cell Physiol. 231:1832–1841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanamori M, Sano A, Yasuda T, Hori T and
Suzuki K: Array-based comparative genomic hybridization for
genomic-wide screening of DNA copy number alterations in aggressive
bone tumors. J Exp Clin Cancer Res. 31:1002012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang W, Wen Q, Luo J, Chu S, Chen L, Xu L,
Zang H, Alnemah MM, Li J, Zhou J and Fan S: Suppression of
β-catenin nuclear translocation by CGP57380 decelerates poor
progression and potentiates radiation-induced apoptosis in
nasopharyngeal carcinoma. Theranostics. 7:2134–2149. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bell JB, Eckerdt F, Dhruv HD, Finlay D,
Peng S, Kim S, Kroczynska B, Beauchamp EM, Alley K, Clymer J, et
al: Differential response of glioma stem cells to arsenic trioxide
therapy is regulated by MNK1 and mRNA translation. Mol Cancer Res.
16:32–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Ren Y, Yu X, Qian F, Bian BS, Xiao
HL, Wang WG, Xu SL, Yang J, Cui W, et al: ALDH1A1 defines invasive
cancer stem-like cells and predicts poor prognosis in patients with
esophageal squamous cell carcinoma. Mod Pathol. 27:775–783. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arango NA, Szotek PP, Manganaro TF, Oliva
E, Donahoe PK and Teixeira J: Conditional deletion of beta-catenin
in the mesenchyme of the developing mouse uterus results in a
switch to adipogenesis in the myometrium. Dev Biol. 288:276–283.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gregory CA, Gunn WG, Reyes E, Smolarz AJ,
Munoz J, Spees JL and Prockop DJ: How Wnt signaling affects bone
repair by mesenchymal stem cells from the bone marrow. Ann N Y Acad
Sci. 1049:97–106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee N, Smolarz AJ, Olson S, David O,
Reiser J, Kutner R, Daw NC, Prockop DJ, Horwitz EM and Gregory CA:
A potential role for Dkk-1 in the pathogenesis of osteosarcoma
predicts novel diagnostic and treatment strategies. Br J Cancer.
97:1552–1559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kirikoshi H and Katoh M: Expression of
WRCH1 in human cancer and down-regulation of WRCH1 by
beta-estradiol in MCF-7 cells. Int J Oncol. 20:777–783.
2002.PubMed/NCBI
|
|
34
|
Vallejo A, Perurena N, Guruceaga E, Mazur
PK, Martinez-Canarias S, Zandueta C, Valencia K, Arricibita A,
Gwinn D, Sayles LC, et al: An integrative approach unveils FOSL1 as
an oncogene vulnerability in KRAS-driven lung and pancreatic
cancer. Nat Commun. 8:142942017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schiavone D, Dewilde S, Vallania F,
Turkson J, Di Cunto F and Poli V: The RhoU/Wrch1 Rho GTPase gene is
a common transcriptional target of both the gp130/STAT3 and Wnt-1
pathways. Biochem J. 421:283–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mani M, Carrasco DE, Zhang Y, Takada K,
Gatt ME, Dutta-Simmons J, Ikeda H, Diaz-Griffero F, Pena-Cruz V,
Bertagnolli M, et al: BCL9 promotes tumor progression by conferring
enhanced proliferative, metastatic, and angiogenic properties to
cancer cells. Cancer Res. 69:7577–7586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ewen ME, Sluss HK, Sherr CJ, Matsushime H,
Kato J and Livingston DM: Functional interactions of the
retinoblastoma protein with mammalian D-type cyclins. Cell.
73:487–497. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guan PP, Yu X, Guo JJ, Wang Y, Wang T, Li
JY, Konstantopoulos K, Wang ZY and Wang P: By activating matrix
metalloproteinase-7, shear stress promotes chondrosarcoma cell
motility, invasion and lung colonization. Oncotarget. 6:9140–9159.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jovanovic V, Dugast AS, Heslan JM,
Ashton-Chess J, Giral M, Degauque N, Moreau A, Pallier A,
Chiffoleau E, Lair D, et al: Implication of matrix
metalloproteinase 7 and the noncanonical wingless-type signaling
pathway in a model of kidney allograft tolerance induced by the
administration of anti-donor class II antibodies. J Immunol.
180:1317–1325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lien WH and Fuchs E: Wnt some lose some:
Transcriptional governance of stem cells by Wnt/β-catenin
signaling. Genes Dev. 28:1517–1532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|