Introduction
Gastric signet ring cell adenocarcinoma (SRCA) is
characterized by the presence of isolated or small groups of
malignant non-cohesive cells (1). The
characteristics of SRCA are its resistance to chemotherapy
(2), tissues fibrosis and peritoneal
invasion (3). Recently we reported
the upregulated expression of heparanase in SRCA, which is involved
in the acquisition of the mesenchymal phenotype and tumor cell
malignancy (4). In the present study,
it was also revealed that the epithelial-mesenchymal transition
(EMT) process could be inhibited by suramin, an heparanase
inhibitor. A recent study revealed that non-steroid
anti-inflammatory drugs such as acetyl salicylic acid inhibit
proliferation and induce cell cycle arrest as well as apoptosis in
different cancer cells (5).
Previously, we reported that an ovarian cancer cell line, OVCAR-3
NIH, expressed both cluster of differentiation (CD)-133 and CD-117
stem cell markers and secreted cytokines implicated in tumor growth
and cell differentiation (6). In
another previous study, it was indicated that poorly differentiated
ovarian cancer cells with a high proliferative index could be
transported to other tissues with no proliferation potential
(7).
The differentiation of cancer cells to
non-proliferated cells is well known. Previously, ~35 years ago,
Flynn et al (8) described the
use of 13-cis-retinoic acid for the treatment of acute
promyelocytic leukemia (APL). Today, recent clinical trials have
demonstrated that the majority of patients with APL could be
definitively cured by the combination of 2 targeted therapies:
Retinoic acid and arsenic (9). APL
cells have immature characteristics and can be differentiated to
other non-proliferative myelocyte cell lines (10). This example justifies the
investigation of differentiation-inducing factors in solid tumor
therapy. In a variety of solid tumors, a subpopulation of
tumorigenic cells was identified as cancer stem cells (CSCs)
(11). These CSCs have the ability to
initiate tumor growth in immunocompromised mice (12). CSCs, by giving rise to a large
population of differentiated progeny, make up the bulk of the tumor
but they lack tumorigenic potential (13). An association between CSC and
epithelial-mesenchymal transition (EMT) has been attributed
(14) and these CSCs could acquire
the differentiated phenotype following cytotoxic chemotherapy
(15).
On the basis of the pluripotency of CSC, the aim of
the present study was to demonstrate that the gastric cancer cell
line, KATO-III, with a high proliferative tendency could be
inhibited and differentiated into other cells in vitro using
cell-differentiating inductors.
Materials and methods
Cell lines and reagents
The human SRCA cell line used KATO-III was obtained
from the American Type Culture Collection (ATCC). The primary drug
used, acetyl salicylic acid (Aspegic), was purchased from
Sanofi-Aventis.
Cell culture
Cells were cultured in Iscove's modified Dulbecco's
medium (IMDM) containing 10% heat-inactivated fetal bovine serum
(FBS), 50 µg/ml of streptomycin, 50 IU/ml of penicillin and 2 nM of
L-glutamine (Gibco; Thermo Fisher Scientific, Inc.). Cells were
incubated at 37°C in a humidified atmosphere containing 5%
CO2.
Cytokine array
The present study examined the supernatant of
KATO-III cells grown in serum-free IMDM using a protein cytokine
array (RayBio® Human Cytokine Antibody; RayBiotech
Life); this technique is based on the principle of the sandwich
immunoassay (16). It consists of
screening, in duplicate, 174 different membrane-coupled
anti-cytokines along with the appropriate controls (experiments
were repeated 3 times).
KATO-III cells were incubated in IMDM at 37°C in a
humidified atmosphere of 5% CO2 for 24 h. Non-adherent
cells (106 cells/ml) from the culture flask were
recovered by centrifugation (130 × g), washed with PBS (1X) and
then re-suspended in serum-free IMDM. Concurrently, adherent cells
from the same flask were washed with PBS (1X) and then incubated in
the same conditions as those applied for non-adherent cells.
Following 24 h, the supernatants containing
cytokines from adherent and non-adherent cells were retrieved and
cytokines were allowed to couple with their specific antibodies
previously immobilized on the nitrocellulose membranes. The
membranes were saturated for 2 h at room temperature with bovine
serum albumin (BSA). Incubation of the array membranes with
supernatants was conducted overnight at 4°C using the corresponding
antibodies. Following several successive washes, the membranes were
incubated in the presence of a mixture of antibodies and
anti-cytokines biotinylated antibodies at 4°C overnight.
Streptavidin, coupled with horseradish peroxidase (HRP), was added
to the membranes for 2 h at room temperature. The presence of the
antibody-coupled proteins was evaluated by applying enhanced
chemiluminescence (RayBio®) to the membranes, according
to the recommendations of the manufacturer. Membranes were then
exposed to photosensitive film (Kodak X-OMAT; Kodak).
The intensity of chemiluminescence captured on the
photosensitive film was measured and recorded. Once the background
noise was removed, the results were expressed as a ratio of
chemiluminescence intensity of the experimental vs. control spots.
The positive control was considered to be 1; a ratio value <-5
indicated a reduction of the cytokine and a value >+5 indicated
an increase in cytokine expression.
RNA isolation, reverse transcription
(RT) and quantitative polymerase chain reaction (qPCR)
RNA isolation, RT and qPCR. Total RNA from the cells
was extracted using a Qiagen RNeasy Mini kit (Qiagen GmbH)
according to the manufacturer's instructions. RNA samples (70
ng/µl) were transcribed to cDNA in a 20-µl volume, using the
QuantiTect Reverse Transcription kit (Qiagen GmbH). The mRNA
expression levels of the different markers were detected by qPCR
with β-actin as the internal reference, using Mesa Blue qPCR Master
Mix Plus for SYBR® assay (Eurogentec Ltd.) on the
Mastercycler® Realplex2 (Eppendorf). The thermocycling
conditions for RT-qPCR were as follows: 95°C for 5 min, followed by
40 cycles of denaturation for 15 sec at 95°C, annealing for 20 sec
at 60°C and extension for 20 sec at 72°C. The primer sequences and
PCR product size for the target and reference genes are listed in
Table SI.
Relative quantification was performed using the
comparative quantitative cycle (Cq) method with Realplex software.
The mean Cq of triplicate measurements was used to calculate ΔCq as
the difference in Cq for the target and internal reference
(β-actin) genes. The difference between the ΔCq of the control
experiment (KATO-III) and the ΔCq of each sample were calculated to
produce ΔΔCq. The fold increase in mRNA was calculated using the
2−ΔΔCq method (17). The
PCR products of the cell lines following RT-qPCR were
electrophoresed by E-Gel Precast Agarose Electrophoresis System
(Invitrogen).
Fluorescence-activated cell sorting
(FACS) analysis
Confluent KATO-III cells (0.1×106) were
seeded in a 25-cm2 culture flask, followed by 24 h in
either control or inductor media (StemPro®Adipogenesis,
Chondrogenesis, Osteogenesis Differentiation kit and
Neurobasal® medium) for 14 days or with 4.5 mM acetyl
salicylic acid for 6 days.
The cells and tumor spheres were dissociated as a
single cell suspension, washed with PBS and then labeled with
antibodies (10 µl/1×106 cells), including mouse
anti-human CD90 (2:100 dilution; cat. no. 559869; BD Biosciences)
and CD117 (7:100 dilution; cat. no. 550412; BD Biosciences) at 4°C
in the dark for 30 min. The samples (minimum 10,000 cells) were
analyzed by flow cytometry (FACSAria II; BD Biosciences).
Cell cycle distribution analysis
Cell cycle distribution was analyzed using the
Click-iT™ Plus EdUAlexa Fluor 647™ Flow Cytometry Assay Kit (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The KATO-III cell line was incubated with IMDM and 5% FBS for 24 h.
The following day, the cells were treated with or without 4.5 mM
acetyl salicylic acid. Following 4 days of treatment, the cells
were trypsinized, harvested and incubated in culture medium with 15
µ MEdU for 2 h. After incubation, the cells were washed with 1% BSA
in PBS and 100 µl of Click-iT fixative was added for 15 min at room
temperature. Following washing, the cells were incubated with the
Click-iT Plus reaction cocktail including fluorescent dye (Alexa
Fluor 647 picolylazide) for 30 min. The flow cytometric analysis
was performed using a BD LSR II analytical flow cytometer (BD
Biosciences). MultiCycle AV (Phoenix Flow Systems) DNA analysis
software enabled the determination of the phase of cell cycle
arrest by comparing the percentages of each cell stage (G1, S and
G2/M) between the control and treatment groups.
Cell viability assay
Cell viability was assayed by a RealTimeGlo™ kit
containing the MT Cell Viability Substrate and the
NanoLuc® Enzyme from Promega Corp. Briefly, confluent
KATO-III cells (3×103/well) were seeded on 96-well
plates, and then 24 h later in either control or conditional medium
with acetyl salicylic acid or inductor media
(StemPro®Adipogenesis, Chondrogenesis, Osteogenesis
Differentiation Kit and Neurobasal® medium).
Reagents were added directly to the cell culture and
the bioluminescence was measured at different time intervals (0,
12, 24, 48, 72 and 96 h) with a Xenius XC spectrofluorometer
(SAFAS, Monaco). Cell viability was expressed as the percentage of
the absorbance of the drug-treated cells relative to that of the
vehicle-treated cells. Each condition was tested in triplicate. The
results are representative of 3 independent experiments.
Differentiation of the KATO-III cell
line
To induce adipogenic, chondrogenic, osteogenic and
neurogenic differentiation, confluent KATO-III cells were incubated
for 14 days with the StemPro®Adipogenesis,
Chondrogenesis, Osteogenesis Differentiation kit (Gibco; Thermo
Fisher Scientific, Inc.) and Neurobasal® medium (Thermo
Fisher Scientific, Inc.). All induced cells were fixed for 30 min
in 4% paraformaldehyde at room temperature and washed with PBS. For
the assessment of calcium deposition in induced osteocytes, cells
were treated with 2% Alizarin Red S solution (pH 4.2) for 2–3 min.
The induced chondrocyte aggregates were stained with 1% Alcian Blue
solution prepared in 0.1 N HCl for 30 min and rinsed with distilled
water to neutralize the acidity. The induced adipocytes were
incubated with 60% isopropanol for 5 min and then stained with 60%
Oil Red O (0.3 g/ml isopropanol) in distilled water for 5 min. The
induced neurocytes were stained with cresyl violet solution (0.5 g
cresyl violet in 100 ml of 0.6% glacial acetic acid) for 30 min. An
inverted microscope was used to image of all stained cells.
Statistical analysis
Data were analyzed using Student's t test and one-
or two-way analysis of variance (ANOVA), followed by post-hoc
Bonferroni's multiple comparison test when appropriated.
Calculations were performed using commercial software (GraphPad
Prism version 7.01 for Windows; GraphPad Software, www.graphpad.com). A two-sided P<0.05 was retained
for statistical significance.
Results
KATO-III cell line in vitro
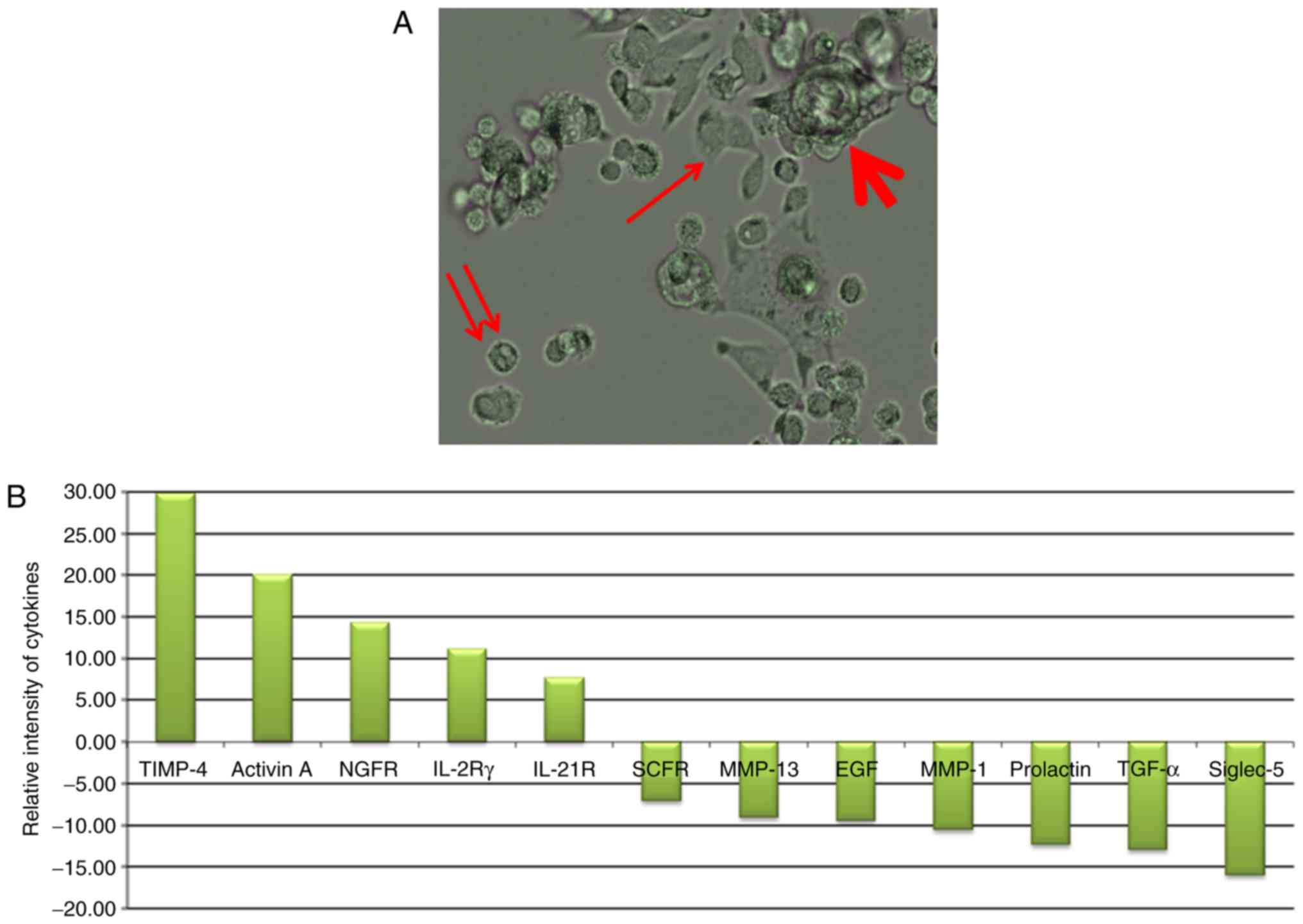
As presented in Fig.
1A, the gastric cancer cell line KATO-III was observed in 3
forms in the culture medium. Adherent cells (indicated by an
arrow), non-adherent cells (indicated by a double arrow) and cell
clusters (indicated by a bold arrow; some were also non-adherent)
were observed. When these cells (adherent, and combined isolated
and cluster forms of non-adherent single) were grown separately in
culture medium, they subsequently generated other forms. These
results are indicative of the internal potential of cell transition
in conditional medium. The profile of cytokines secreted under each
condition is presented in Fig. 1B.
The results revealed the high secretion of tissue inhibitor of
metalloproteinase-4 (TIMP-4), Activin A, nerve growth factor
receptor (NGFR), interleukin (IL)-2Rγ and IL-21R in adherent cells
and stem cell factor receptor (SCFR), matrix metalloproteinase
(MMP)-13, epidermal growth factor (EGF), MMP-1, Prolactin,
transforming growth factor (TGF)-α, and sialic acid binding
immunoglobulin-like lectin-5 (Siglec-5) in non-adherent
conditions.
KATO-III cells present CSC as well as
EMT markers
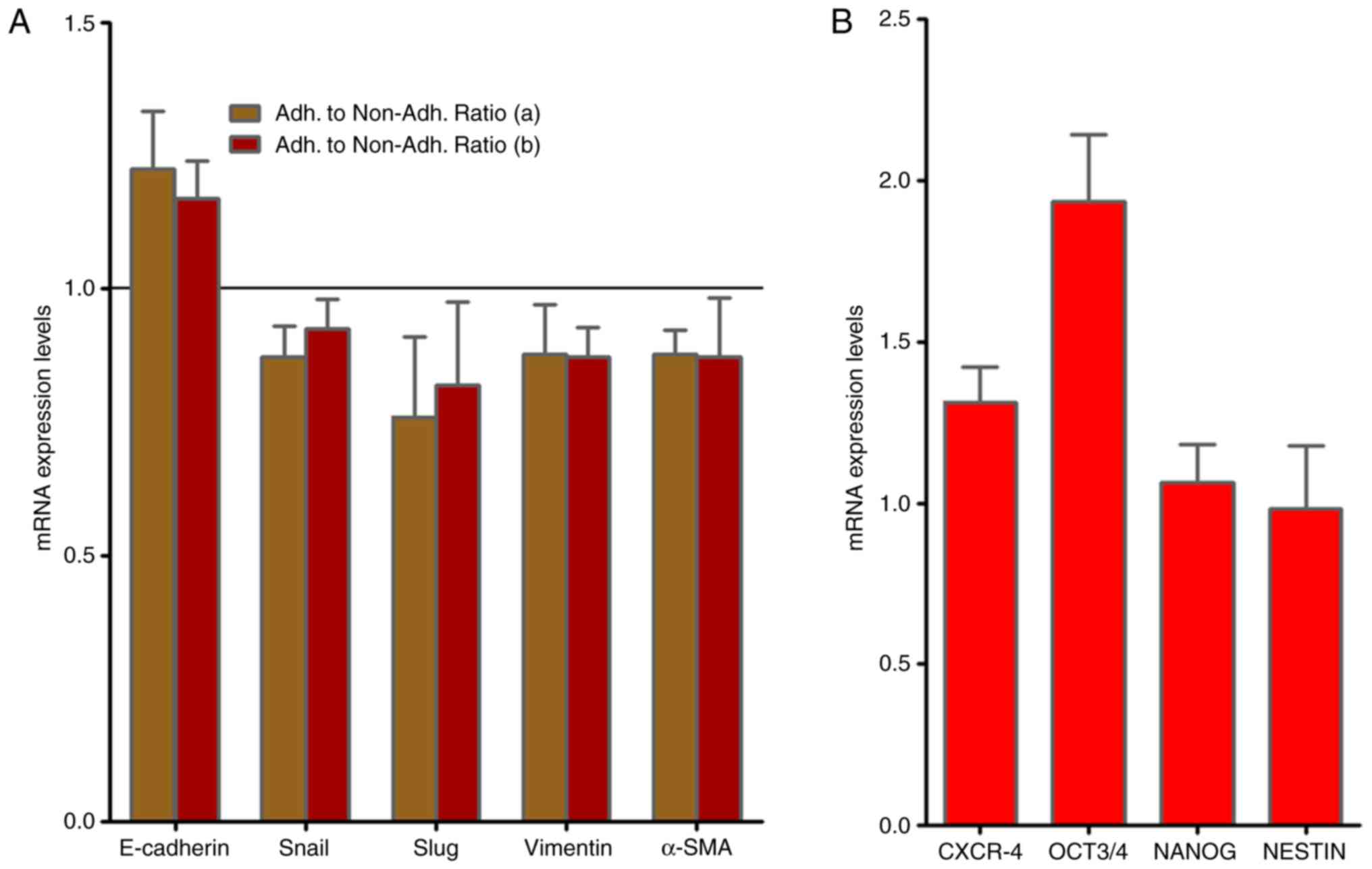
As presented in Fig.
2A, RT-qPCR analysis demonstrated no alterations in the gene
expression of the EMT-associated molecules, Slug, vimentin and
α-smooth muscle actin (SMA) mRNAs when adherent and non-adherent
KATO-III cells were grown separately for 1 week. By contrast, the
presence of the CSC markers C-X-C chemokine receptor type 4
(CXCR-4), octamer-binding transcription factor (OCT)-3/4 as well as
NANOG and NESTIN were confirmed in KATO-III cells as revealed in
Fig. 2B.
Acetyl salicylic acid modifies
heparanase, EMT and CSC marker expression in KATO-III cells in
vitro
When KATO-III cells were incubated (2, 4 and 6 days)
with acetyl salicylic acid (4.5 mM), their morphology gradually
changed (Fig. S1). Inhibition of
cell proliferation (P=0.002; Fig. 3A)
and significant downregulation of heparinase (P=0.0289) expression
in the SRCA cell line was observed in a time-dependent manner
(Fig. 3B). Mesenchymal markers such
as Slug, vimentin and α-SMA (P=0.004) were downregulated while
E-cadherin was upregulated (P=0.004; Fig.
3C). Acetyl salicylic acid also decreased the expression of the
stem cell markers, CXCR4, OCT3/4, NANOG and NESTIN (P=0.004;
Fig. 3D), and CD90 and CD117
(Fig. 3E). This phenomenon was
associated with the inhibition of cell proliferation as well as EMT
inhibition via the downregulation of heparanase and CSC
markers.
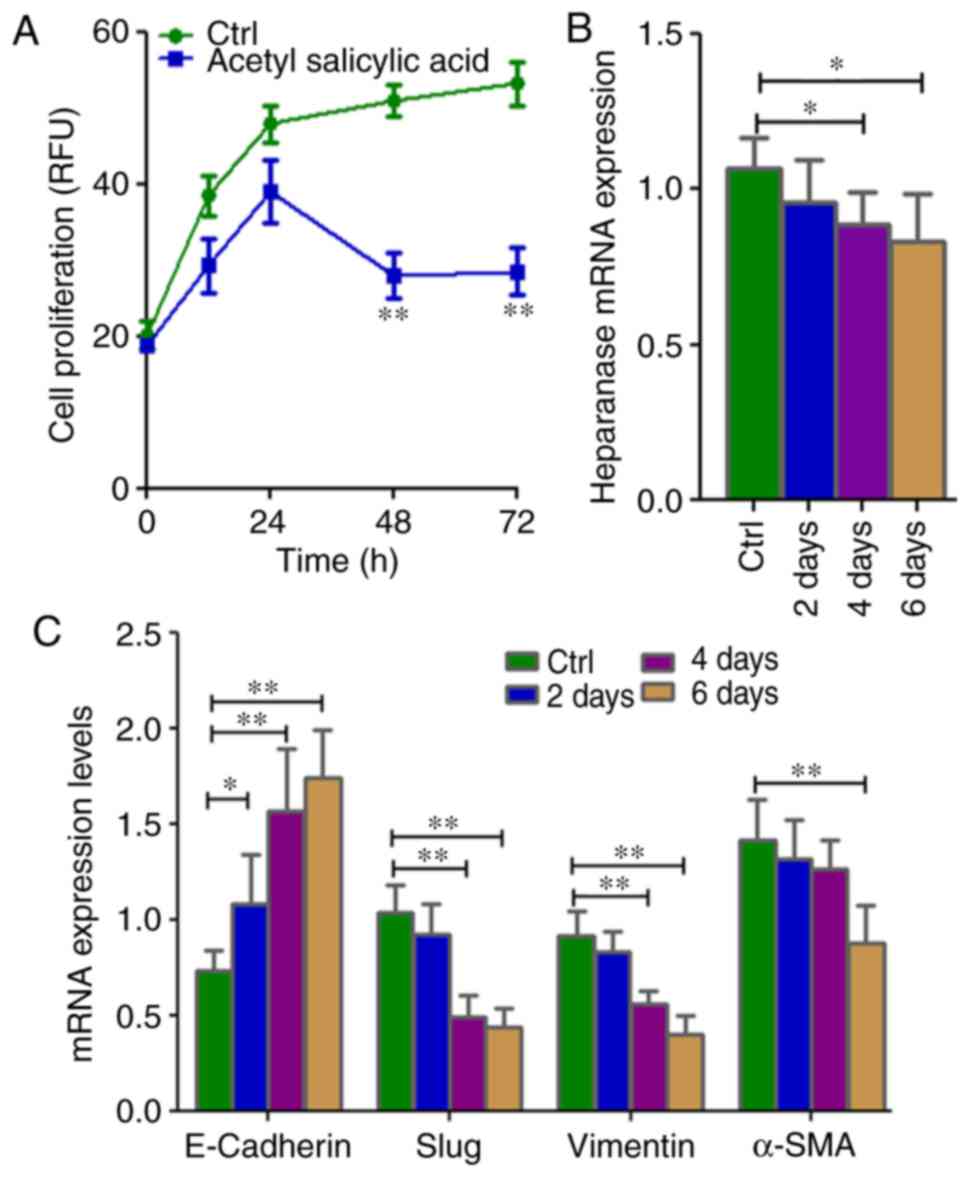 | Figure 3.Cell proliferation as well as mRNA
expression of EMT-associated genes and stem cell markers in
KATO-III following treatment with 4.5 mM acetyl salicylic acid for
6 days. (A) Cell proliferation is inhibited, and (B) the expression
of heparanase, (C) mesenchymal markers (Slug, vimentin and α-SMA)
and (D) stem cell markers CXCR-4, OCT3/4, NANOG and NESTIN as well
as (E) CD90 and CD117 were lower, while those of (C) the epithelial
marker E-cadherin was higher in KATO-III cells following treatment
with acetyl salicylic acid as determined by reverse
transcription-quantitative polymerase chain reaction and flow
cytometry. (*P<0.05 and **P<0.01). EMT,
epithelial-mesenchymal transition; α-SMA, α-smooth muscle actin;
CXCR-4, C-X-C chemokine receptor type 4; OCT3/4, octamer-binding
transcription factor 3/4; CD, cluster of differentiation. |
Acetyl salicylic acid modifies the
cell cycle in KATO-III cells
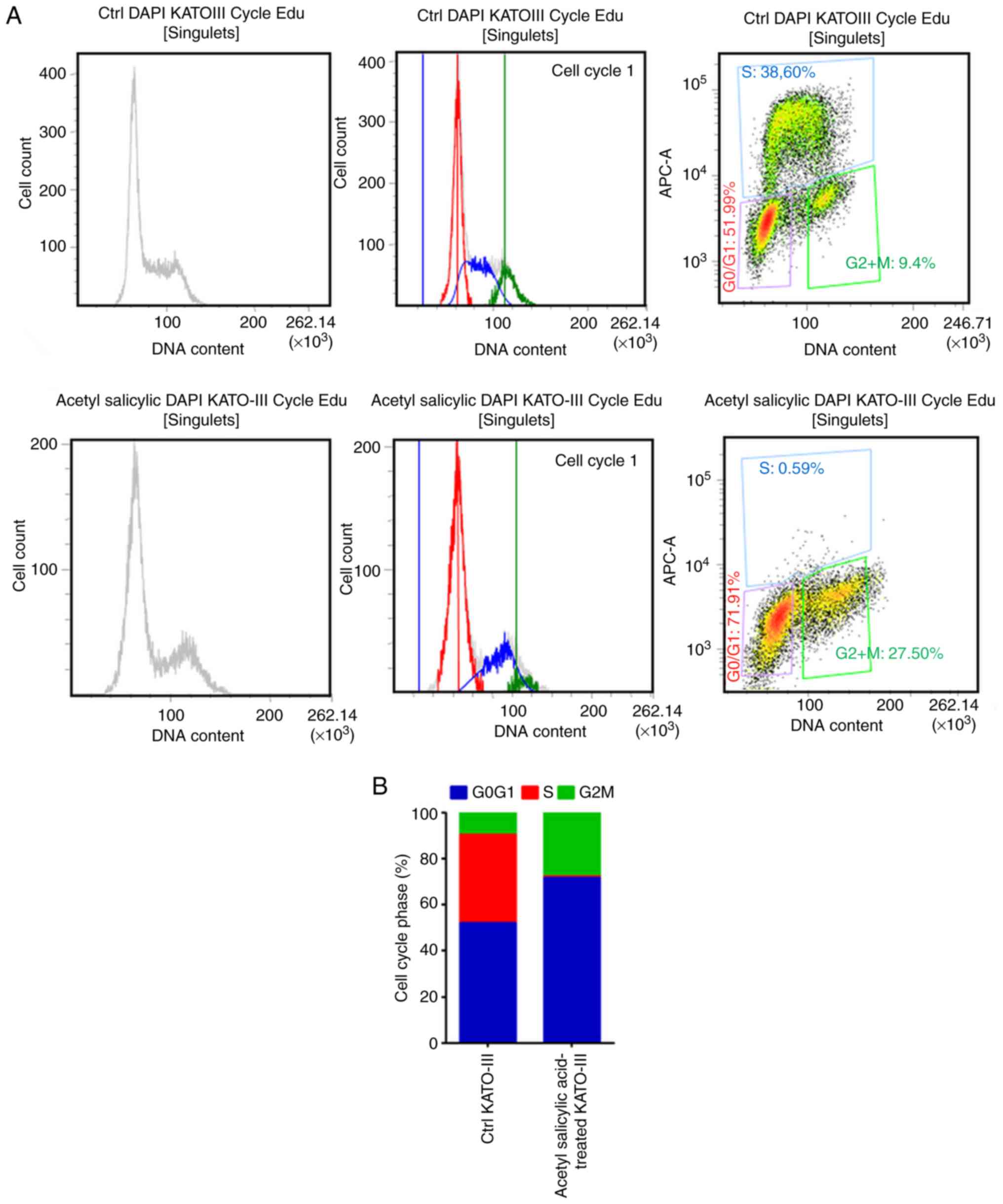
Flow cytometry was used for cell cycle analysis as
revealed in Fig. 4A. The majority of
the cells presented an increase in the number of cells in the G0-G1
phase (20%). By contrast, this phenomenon was associated with a
reduced number of cells in the S phase (reduction of 38% when
compared with the control; Fig. 4B).
These results indicated that acetyl salicylic acid via the G0-G1
phase pathway inhibits cancer cell proliferation.
Cell inducer differentiation media
downregulates heparanase and stem cell marker expression as well as
the inhibition of cell proliferation
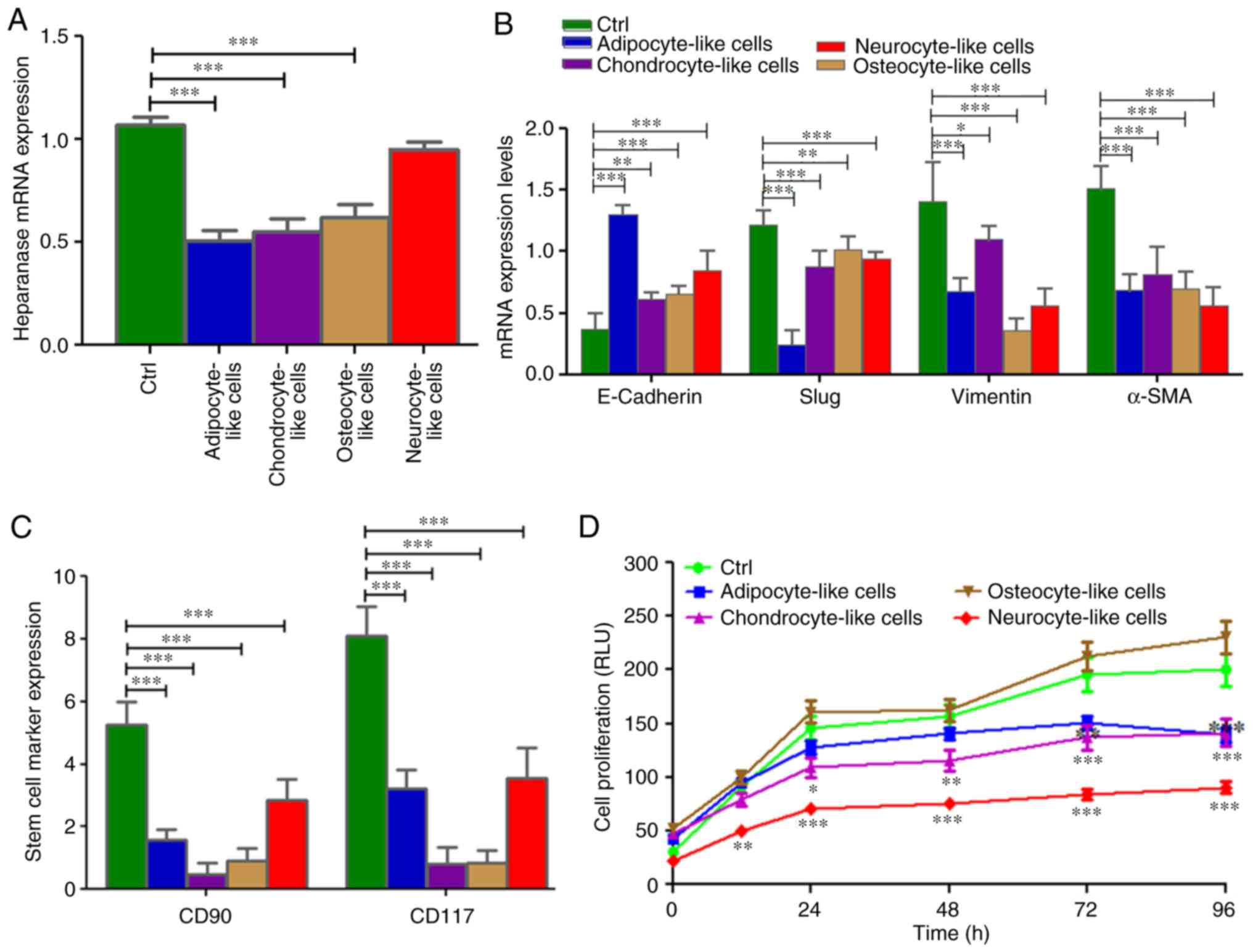
When the SRCA cell line was incubated with different
culture media targeting stem cell differentiation in adipocytes,
chondrocytes, osteocytes and neuronal cells, the expression of
heparanase significantly decreased as observed in adipocytes
(P=0.005), chondrocytes (P=0.004) and osteocytes (P=0.004; Fig. 5A). This phenomenon was associated with
a high expression of E-cadherin (P=0.004) and the downregulation
(P=0.005) of mesenchymal markers such as Slug, vimentin and α-SMA
mRNAs (Fig. 5B). By contrast, the
conditioned media, except osteocyte-inducer medium, induced the
inhibition of KATO-III cell proliferation (P=0.002; Fig. 5D). This inhibition was associated with
the downregulation (P=0.004) of the stem cell markers CD90 and
CD117 (Fig. 5C).
Cell-inducer differentiation medium
induces cancer cell differentiation
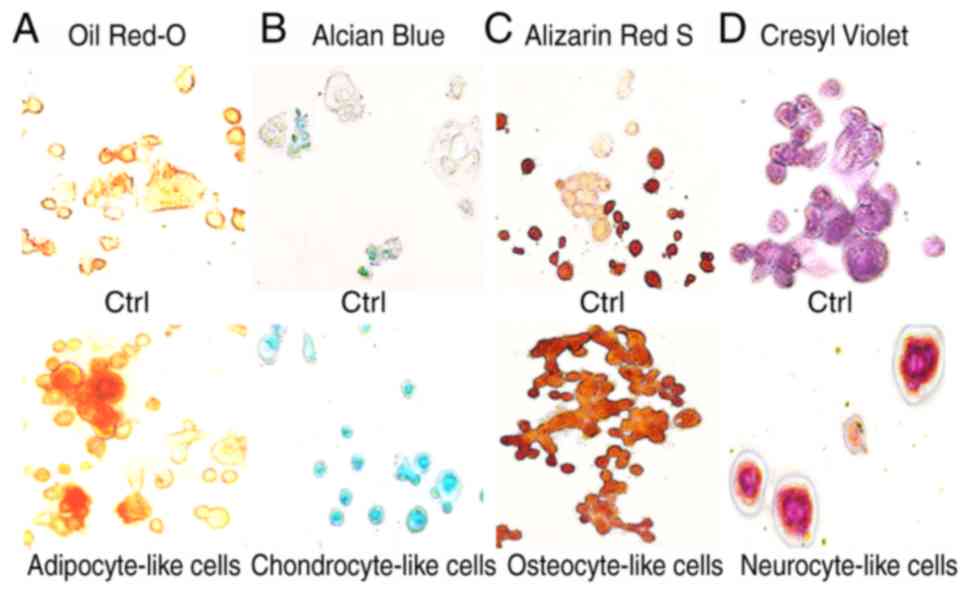
Adipogenic, chondrogenic, osteogenic and neurogenic
differentiation of the KATO-III cell line was confirmed (Fig. 6) by the visualization of
intracytoplasmic lipid drops stained red using Oil Red O (Fig. 6A); a blue coloration was observed due
to proteoglycan synthesis using Alcian blue (Fig. 6B), a red coloration was observed due
to extracellular calcium deposits using Alizarin Red S (Fig. 6C) and dark black-violet was observed
due to the extensive somata-associated accumulation of Nissl
bodies, respectively (Fig. 6D). These
results confirmed the influence of selective medium on the
mesenchymal characteristics of the KATO-III cell line.
Discussion
Apart from elimination therapies that increase the
efficacy of cancer treatments, another method to control tumor
progression is to induce the differentiation of CSCs (18). In the present study, the
differentiation of gastric SRCA cells (KATO-III) were targeted by
downregulating EMT and CSC markers. The results revealed that
KATO-III cells exhibited 3 phenotypes (adherent, non-adherent and
spheroid cluster forms) that are capable of conversion between 2
distinct forms (adherent and non-adherent), a transition we refer
to as reversible adaptive plasticity. In addition, no alteration in
the RNA expression of EMT-associated molecules was demonstrated via
qPCR when grown separately for 1 week. These results are consistent
with those of She et al (19)
who reported no difference in tumorigenicity in vivo when
the side and non-side population of the KATO-III cell line were
injected subcutaneously in nude mice.
The present study identified major cytokines
significantly secreted by non-adherent cells, including Siglec-5,
TGF-α, Prolactin, MMP-1, EGF, MMP-13 and SCFR while those of the
adherent cells were TIMP-4, Activin A, NGFR, IL-2Rγ and IL-21R.
There is evidence that Siglec-5 is involved in cell-cell
interactions and tumor dissemination (20,21), MMP-1
and MMP-13 are implicated in the regulation of extracellular matrix
degradation, prolactin was revealed to induce estrogen
responsiveness in breast cancer cells (22), TGF-α is implicated in the regulation
of cell proliferation and migration through the activation of
multiple pathways (23) and EGF and
SCFR are thought to be involved in cell differentiation (24,25)
indicating that tumor dissemination and cell proliferation may be
due to the non-adherent cells of KATO-III. Recent studies have also
demonstrated that TIMP-4, as an anti-metalloproteinase, was
implicated in cell adhesion (26),
Activin A was involved in the regulation of progesterone1 and
estradiol production in JEG-3 (27)
cells as well as the differentiation of granulosa cells via the
activation of steroid hormone receptor (28), while NGFR was a differentiating factor
for nerve cells (29) and IL-2 served
an adjunctive role in IL-21-induced B-cell differentiation
(30), suggesting that cell adhesion
and differentiation are characteristics of KATO-III adherent cells.
The present study also reported estrogen and progesterone receptors
in KATO-III cells (data not shown).
The present study also identified the CSC phenotypes
of Slug, Snail, vimentin, NANOG, NESTIN, OCT3/4 and CXCR-4. Recent
studies have suggested that these CSCs are long-lived, and display
quiescent potentials in a dormant state, and are responsible for
angiogenic induction, cell proliferation, apoptotic resistance,
self-renewal and differentiation (31,32). In
the present study, to the best of our knowledge, it was revealed
for the first time in a human SRCA cell line (KATO-III) that
gastric cancer stem cells exist in the CD90- and CD117-positive
fraction that had potential to differentiate into adipocyte,
chondrocyte-, osteocyte- and neurocyte-like cells.
The present results revealed that KATO-III
differentiated into adipocyte-, chondrocyte, osteocyte and
neurocyte-like cells using inducer media. The inhibitory effect of
inducer media on KATO-III proliferation, EMT and stem cell marker
expression was observed. This inducing media also significantly
downregulated the expression of heparanase. This research is
consistent with that of Masola et al (33) on the heparanase-mediated EMT of renal
tubular cells. Epidemiological evidence has indicated the
chemopreventive effect of acetyl salicylic acid in cancer treatment
however the molecular basis for this effect is not fully known
(34,35). Consistently, it has been reported that
regular acetyl salicylic acid users have a lower risk of breast
(36,37), gallbladder (38), prostate (39), gastric (40,41) and
non-small cell lung cancer (42). A
growing body of evidence has revealed that acetyl salicylic acid is
the inhibitor of the enzyme, heparanase (43). The aim of the present study was also
to focus on the regulation of EMT by altering the expression of EMT
markers by using acetyl salicylic acid. This was investigated in
the KATO-III cell line, which revealed the significant inhibitory
effect of acetyl salicylic acid on EMT and heparanase expression as
well as cell proliferation. The present results are in agreement
with those of Masola et al (44) who reported heparanase involvement in
EMT and the cell proliferation of renal tubular cells. The results
of the present study demonstrated that acetyl salicylic acid
induced a marked degree of inhibition of EMT and proliferation in
the KATO-III cell line. Similarly, acetyl salicylic acid also
induced G0/G1 cell cycle arrest up to 20% and inhibited the S phase
by up to 38% of the cell population. The limitation of this study
was the inability to isolate single and cluster forms of
non-adherent KATO-III cells independently due to unavailability of
a technique. Incorporating new technologies in future may
potentially isolate such types of non-adherent cells for further
studies at the protein level.
In conclusion, the inducer media had the ability to
induce the differentiation of KATO-III cells. Acetyl salicylic acid
has been revealed to be effective in the prevention and treatment
of EMT-associated metastasis. Therefore, it is important to
continue efforts to identify therapies that can treat cancers no
longer susceptible to current treatments. The results of the
present study provide a basis for the development of more effective
treatment strategies for controlling gastric cancer in the
future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SS performed the PCR experiments, the KATO III cell
treatment and the flow cytometry. MP interpreted the data for the
study. MM substantially contributed to the conception of the study
and wrote the study. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu B, El Hajj N, Sittler S, Lammert N,
Barnes R and Meloni-Ehrig A: Gastric cancer: Classification,
histology and application of molecular pathology. J Gastrointest
Oncol. 3:251–261. 2012.PubMed/NCBI
|
|
2
|
Endo K, Maehara Y, Ichiyoshi Y, Kusumoto
T, Sakaguchi Y, Ohno S and Sugimachi K: Multidrug
resistance-associated protein expression in clinical gastric
carcinoma. Cancer. 77 (Suppl 8):1681–1687. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maehara Y, Sakaguchi Y, Moriguchi S, Orita
H, Korenaga D, Kohnoe S and Sugimachi K: Signet ring cell carcinoma
of the stomach. Cancer. 69:1645–1650. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah S, Fourgeaud C, Derieux S, Mirshahi
S, Contant G, Pimpie C, Lo Dico R, Soria J, Pocard M and Mirshahi
M: The close relationship between heparanase and epithelial
mesenchymal transition in gastric signet-ring cell adenocarcinoma.
Oncotarget. 9:33778–33787. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu YX, Feng JY, Sun MM, Liu BW, Yang G,
Bu YN, Zhao M, Wang TJ, Zhang WY, Yuan HF and Zhang XD: Aspirin
inhibits the proliferation of hepatoma cells through controlling
GLUT1-mediated glucose metabolism. Acta Pharmacol Sin. 40:122–132.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ducros E, Mirshahi S, Azzazene D,
Camilleri-Broët S, Mery E, Al Farsi H, Althawadi H, Besbess S,
Chidiac J, Pujade-Lauraine E, et al: Endothelial protein C receptor
expressed by ovarian cancer cells as a possible biomarker of cancer
onset. Int J Oncol. 41:433–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaci R, Shahid S, Réa L, Philipe B, Amu T,
Marc P and Massoud M: Neural signature expressed by cells from
ovarian carcinoma (A Case Report). Immunochem Immunopathol.
1:22015. View Article : Google Scholar
|
|
8
|
Flynn PJ, Miller WJ, Weisdorf DJ, Arthur
DC, Brunning R and Branda RF: Retinoic acid treatment of acute
promyelocytic leukemia: In vitro and in vivo observations. Blood.
62:1211–1217. 1983.PubMed/NCBI
|
|
9
|
de Thé H Pandolfi PP and Chen Z: Acute
promyelocytic leukemia: A paradigm for oncoprotein-targeted cure.
Cancer Cell. 32:552–560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kastner P, Lawrence HJ, Waltzinger C,
Ghyselinck NB, Chambon P and Chan S: Positive and negative
regulation of granulopoiesis by endogenous RARalpha. Blood.
97:1314–1320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Werner S, Stenzl A, Pantel K and
Todenhöfer T: Expression of epithelial mesenchymal transition and
cancer stem cell markers in circulating tumor cells. Adv Exp Med
Biol. 994:205–228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zabala M, Lobo NA, Qian D, van Weele LJ,
Heiser D and Clarke MF: Overview: Cancer stem cell self-renewal.
Cancer Stem Cells. Academic Press; pp. 25–58. 2016, View Article : Google Scholar
|
|
13
|
Ginestier C, Wicinski J, Cervera N,
Monville F, Finetti P, Bertucci F, Wicha MS, Birnbaum D and
Charafe-Jauffret E: Retinoid signaling regulates breast cancer stem
cell differentiation. Cell Cycle. 8:3297–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brambilla E, Moro D, Gazzeri S, Brichon
PY, Nagy-Mignotte H, Morel F, Jacrot M and Brambilla C: Cytotoxic
chemotherapy induces cell differentiation in small-cell lung
carcinoma. J Clin Oncol. 9:50–61. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Azzazene D, Al Thawadi H, Al Farsi H,
Besbes S, Geyl C, Mirshahi S, Pardo J, Faussat AM, Jeannette S,
Therwath A, et al: Plasma endothelial protein C receptor influences
innate immune response in ovarian cancer by decreasing the
population of natural killer and TH17 helper cells. Int J Oncol.
43:1011–1018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
She JJ, Zhang PG, Wang X, Che XM and Wang
ZM: Side population cells isolated from KATO III human gastric
cancer cell line have cancer stem cell-like characteristics. World
J Gastroenterol. 18:4610–4617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crocker PR: Siglecs: Sialic-acid-binding
immunoglobulin-like lectins in cell-cell interactions and
signalling. Curr Opin Struct Biol. 12:609–615. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu FT and Rabinovich GA: Galectins as
modulators of tumour progression. Nat Rev Cancer. 5:29–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gutzman JH, Miller KK and Schuler LA:
Endogenous human prolactin and not exogenous human prolactin
induces estrogen receptor α and prolactin receptor expression and
increases estrogen responsiveness in breast cancer cells. J Steroid
Biochem Mol Biol. 88:69–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Lv X, Jiang C, Cordes CM, Fu L,
Lele SM and Davis JS: Transforming growth factor alpha (TGFα)
regulates granulosa cell tumor (GCT) cell proliferation and
migration through activation of multiple pathways. PLoS One.
7:e482992012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barberán S and Cebrià F: The role of the
EGFR signaling pathway in stem cell differentiation during
planarian regeneration and homeostasis. Semin Cell Dev Biol.
87:45–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Pierce LJ and Spangrude GJ:
Distinct roles of IL-7 and stem cell factor in the OP9-DL1 T-cell
differentiation culture system. Exp Hematol. 34:1730–1740. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Radisky ES and Radisky DC: Matrix
metalloproteinase-induced epithelial-mesenchymal transition in
breast cancer. J Mammary Gland Biol Neoplasia. 15:201–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ni X, Luo S, Minegishi T and Peng C:
Activin A in JEG-3 cells: Potential role as an autocrine regulator
of steroidogenesis in humans. Biol Reprod. 62:1224–1230. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ueno N, Nishimatsu S and Murakami K:
Activin as a cell differentiation factor. Prog Growth Factor Res.
2:113–124. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu Y, Wang J, Xu Y, Koch AE, Cai Z, Chen
X, Galson DL, Taichman RS and Zhang J: CXCL16 functions as a novel
chemotactic factor for prostate cancer cells in vitro. Mol Cancer
Res. 6:546–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berglund LJ, Avery DT, Ma CS, Moens L,
Deenick EK, Bustamante J, Boisson-Dupuis S, Wong M, Adelstein S,
Arkwright PD, et al: IL-21 signalling via STAT3 primes human naive
B cells to respond to IL-2 to enhance their differentiation into
plasmablasts. Blood. 122:3940–3950. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bao B, Ahmad A, Azmi AS, Ali S and Sarkar
FH: Overview of cancer stem cells (CSCs) and mechanisms of their
regulation: Implications for cancer therapy. Curr Protoc Pharmacol.
14:14–25. 2013.
|
|
32
|
Soltysova A, Altanerova V and Altaner C:
Cancer stem cells. Neoplasma. 52:435–440. 2005.PubMed/NCBI
|
|
33
|
Masola V, Zaza G, Granata S, Gambaro G,
Onisto M and Lupo A: Everolimus-induced epithelial to mesenchymal
transition in immortalized human renal proximal tubular epithelial
cells: Key role of heparanase. J Transl Med. 11:2922013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thun MJ, Jacobs EJ and Patrono C: The role
of aspirin in cancer prevention. Nat Rev Clin Oncol. 9:259–267.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mohammed I, Musa MO and Umar A: Effects of
acetylsalicylic acid and salicylic acid on the growth of HeLa
cervical cancer cells line. Am J Pharm Pharmacol. 1:32–44.
2014.
|
|
36
|
Mangiapane S, Blettner M and Schlattmann
P: Aspirin use and breast cancer risk: A meta-analysis and
meta-regression of observational studies from 2001 to 2005.
Pharmacoepidemiol Drug Saf. 17:115–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takkouche B, Regueira-Méndez C and Etminan
M: Breast cancer and use of nonsteroidal anti-inflammatory drugs: A
meta-analysis. J Natl Cancer Inst. 100:1439–1447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu E, Sakoda LC, Gao YT, Rashid A, Shen
MC, Wang BS, Deng J, Han TQ, Zhang BH, Fraumeni JF Jr and Hsing AW:
Aspirin use and risk of biliary tract cancer: A population-based
study in Shanghai, China. Cancer Epidemiol Biomarkers Prev.
14:1315–1318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dasgupta K, Di Cesar D, Ghosn J, Rajan R,
Mahmud S and Rahme E: Association between nonsteroidal
anti-inflammatory drugs and prostate cancer occurrence. Cancer J.
12:130–135. 2006.PubMed/NCBI
|
|
40
|
Jolly K, Cheng KK and Langman MJ: NSAIDs
and gastrointestinal cancer prevention. Drugs. 62:945–956. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Husain SS, Szabo IL and Tarnawski AS:
NSAID inhibition of GI cancer growth: Clinical implications and
molecular mechanisms of action. Am J Gastroenterol. 97:542–553.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Van Dyke AL, Cote ML, Prysak G, Claeys GB,
Wenzlaff AS and Schwartz AG: Regular adult aspirin use decreases
the risk of non-small cell lung cancer among women. Cancer
Epidemiol Biomarkers Prev. 17:148–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dai X, Yan J, Fu X, Pan Q, Sun D, Xu Y,
Wang J, Nie L, Tong L, Shen A, et al: Aspirin inhibits cancer
metastasis and angiogenesis via targeting heparanase. Clin Cancer
Res. 23:6267–6278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Masola V, Gambaro G, Tibaldi E, Brunati
AM, Gastaldello A, D'Angelo A, Onisto M and Lupo A: Heparanase and
syndecan-1 interplay orchestrates fibroblast growth
factor-2-induced epithelial-mesenchymal transition in renal tubular
cells. J Biol Chem. 287:1478–1488. 2012. View Article : Google Scholar : PubMed/NCBI
|