Introduction
Tumor-associated macrophages (TAMs), one main immune
cell population found in most solid tumors, contribute to tumor
development, progression, drug resistance and suppression of
antitumor immune cells (1–4). There are two main TAM subtypes: M1
macrophages, which encourage inflammation, and M2 macrophage, which
encourage tissue repair (5). TAMs are
mainly of the M2 phenotype (6). TAMs
are recruited to the tumor as a response to cancer-associated
inflammation (7,8) and acquire M2 properties in response to
cytokines such as tumor growth factor (TGF)-β, interleukin (IL)-10
and macrophage colony-stimulating factor (M-CSF). An increased
amount of M2 type TAMs is associated with a worse prognosis in
several cancer types including breast cancer (9,10).
However, recent single-cell sequencing results have shown that both
M1 and M2-associated genes are frequently expressed in the same
cells and are positively correlated with one another along the same
activation trajectory. These results have challenged the model of
macrophage polarization wherein M1 and M2 activation states are two
discrete states (11). As macrophages
affect all therapeutic modalities, further investigation of the
interplay between macrophages and tumor cells is urgently
needed.
The interplay between tumor cells and macrophage
have been revealed. For example, macrophages can release
inflammatory compounds such as tumor necrosis factor (TNF)-α which
activates nuclear factor (NF)-κB and NF-κB regulates tumor cell
apoptosis, proliferation and inflammation. Moreover, macrophages
serve as a source for many pro-angiogenic factors including
vascular endothelial growth factor (VEGF), TNF-α, M-CSF/CSF1 and
IL-1 and IL-6 further contributing to tumor growth and metastasis
(12). In turn, tumors produce
factors, such as M-CSF/CSF1, monocyte chemoattractant protein-1
(MCP-1)/CCL2 and angiotensin II, triggering the amplification and
mobilization of macrophages in tumors (13–15).
Alternative splicing (AS) is a post-transcriptional
regulation process during gene expression that results in a single
gene coding for multiple proteins. AS has been proved to play
important roles in macrophage differentiation and regulation of
most tumor hallmarks (16). For
example, Liu et al found that splicing factor MBNL1
is the major regulator during the differentiation from monocytes to
macrophages (17). Human M-CSF
heterogeneity is partially derived from alternative mRNA splicing
(18). However, how tumor cells and
macrophages interplay at the alternative splicing level and its
potential application in cancer therapy remain unknown.
Understanding the tumor-macrophage crosstalk at the AS level would
reveal the molecular mechanisms underlying tumor progression, drug
resistance and suppression of immune cells and further shade light
on the identification of new cancer therapeutic strategies.
Generally, TAMs appear to have an unfavorable role
in breast cancer (19,20). Moreover, breast cancer is considered
to be a highly heterogeneous disease, and many secreted factors may
differentially contribute to the macrophage phenotype. Therefore,
the aim of the present study was to understand macrophage
‘education’ under the influence of two breast cancer types,
estrogen receptor-positive (ER+) breast cancer and
triple-negative breast cancer (TNBC) at the AS level in a
simplified setting. Through calculation of the number of common and
specific altered AS events, the present study aimed to ascertain:
i) Whether ER+ breast cancer and TNBC cells exert
similar influences on co-cultured macrophage cells; ii) whether
ER+ breast cancer and TNBC cells are affected to a
similar degree at the AS level in the presence of macrophages; iii)
which specific biological pathways are disturbed at the AS level in
macrophage cells when cocultured with ER+ breast cancer
and TNBC cells; iv) which specific biological pathways are
disturbed at the AS level in ER+ breast cancer and TNBC
cells at the presence of macrophage cells.
In the present study, we reanalyzed a previously
published dataset (GSE75130) and our results showed that differing
from transcriptional analysis, DNA repair and DNA damage processes
were enriched in both ER+ breast cancer and TNBC after
co-culturing with macrophages, which indicated the conserved
functional regulation of macrophages on tumor cells in the tumor
microenvironment. Meanwhile, macrophages were differentially
regulated from the pathway views by co-culturing with
ER+ breast cancer and TNBC cells. Sequence features of
skipped exons from different conditions were also characterized in
our analysis.
Materials and methods
GEO dataset GSE75130 was reanalyzed (21) which was originally used to
characterize the differences in macrophage activation under the
influence of either ER+ breast cancer or TNBC cells. For
detailed treatment procedures please refer to a previous study
(21).
Profiling of gene expression
Paired-end reads were mapped to the human genome
primary assembly (GRCh37) (22), and
the Ensembl human gene annotation for GRCh37 genebuild was used to
improve the accuracy of the mapping with STAR software
(STAR_2.4.2a) (23). FeatureCounts
(version 1.4.6-p5) (24) was used to
assign sequence reads to genes. Mitochondrial genes, ribosomal
genes, and genes possessing less than five raw reads in half the
samples were removed. Normalized gene expression profiles were
obtained with the edgeR package 1.6 (25).
Alternative splicing analysis
For AS analysis, MISO software (version 0.5.4)
(26) was used to analyze RNA-Seq
data and estimate the percentage of splicing isoforms. Firstly, we
utilized MISO to achieve the AS profiles for each treated sample
and 5 types of AS profiles including alternative 3′/5′ splice site
(A3SS, A5SS), skipped exons (SE), mutually exclusive exons (MXE)
and retained introns (RI) were obtained. AS profiles were
represented by percentage of splice-in (PSI/Ψ) values. Secondly, we
identified the delta PSI (ΔPSI) by comparing the cocultured sample
with individually cultured sample. Finally, we determine the
significantly different AS events by Bayes factor >6 and
|ΔPSI|>0.2.
Principal component analysis
(PCA)
PCA was performed as previously described (27). A total of 20,169 genes were accounted
for in PCA of the whole transcriptome. Expression was normalized
with the reads per million mapped reads (RPM) method. The prcomp
package from R was used to perform PCA and the default parameters
were used (28). The ggplot2 package
from R was used to draw the scatter plot (29). A total of 36,377 AS events were
utilized in the PCA of the ER+ breast cancer and TNBC
transcriptome analysis. A total of 35,476 AS events were utilized
in the PCA of the macrophage transcriptome analysis.
Functional enrichment analysis
Gene Ontology (GO) term enrichment analysis was
conducted with webserver DAVID 6.8 (30). P<0.05 was considered as
statistically significant. Background gene list was set to 16,133
esemble genes which possess alternative splicing isoforms.
Protein-protein interaction
analysis
Web server STRING (31) was utilized to explore the
protein-protein interactions. Default parameters were applied.
NCBI protein domain analysis
Protein domains of full-length CHEK2 (accession no.
CAG30304.1) was analyzed with NCBI webserver (https://www.ncbi.nlm.nih.gov) (32). Two significant protein domains were
identified. The first domain is STKc_Chk2 (accession: cd14084) and
the second is FHA (accession: cd00060).
Evolutionary conservation
Placental Mammal PhastCons scores were used to
represent evolutionary conservation. For average conservation of
exons, bigWigAverageOverBed (33) was
used to calculate the mean conservation across each exon. The
ggplot2 package from R was used to draw the box plot (29).
3′ and 5′splicing strength
To evaluate splice site strength, 3′ of the
exon-intron boundary (20 nt into intron and +3 nt into exon) and 5′
of the exon-intron boundary (3 nt into exon and +6 nt into intron),
together with the transcript sequences for these regions were
obtained by bedtools (34).
MaxEntScan (35) was used to
calculate the strength of the splice sites for the AS exons.
Repetitive element enrichment
To identify repetitive elements in AS exons, Repeat
Masker track was downloaded from UCSC Genome Browser and
intersected with AS exons by bedtools intersect (34). Repeats were grouped into families
defined by the Pfam database of repetitive DNA elements.
Phylostratum scores were used to describe gene age, as previously
reported (36).
Association between 222 splicing
factors and altered AS events
Overlap of gene-associated events and conditional
specific events (macrophages vs. macrophages co-cultured with T47D
cells; macrophages vs. macrophages co-cultured with MDA-MB-231
cells; T47D cells vs. T47D cells co-cultured with macrophages; and
MDA-MB-231 cells vs. MDA-MB-231 cells co-cultured with macrophages)
were used to assess how much of the conditional specific events
could be explained by each splicing factor. First, conditional
specific ES events were isolated by comparing splicing events from
treatment samples against those from the control samples. The
significant splicing events were determined based on Bayes factor
>6 and |ΔPSI| >0.2. Second, gene-associated ES events were
determined by correlation analyses using gene expression levels
(RPKM) and PSI values across all samples. Correlation coefficients
(R-value) and corresponding P-values were calculated with the
Pearson method, and gene-associated events were determined by
R>0.2 and P<0.0001. Common events were considered as events
that occurred (overlapped) in both gene-associated and conditional
specific events. Finally, top 5 splicing factors which explain the
most conditional specific events were isolated.
Statistical analyses
Significantly different AS events were determined by
Bayes factor >6 and |ΔPSI|>0.2 during comparison of AS events
between cocultured state and single cultured state. Fisher exact
test was applied to test significance of GO term enrichment and
P<0.05 was considered as statistically significant. R>0.2 and
P<0.0001 were applied to identify significantly associated AS
events to a specific splicing factor across 14 samples in this
study. Wilcoxon signed-rank test was applied to test significance
in Fig. 6A-D and P<0.05 was set to
determine significance. Fisher's exact test was used to test
significant enrichment of each elements and P-values were-log10
transformed to plot the heatmap in Fig.
6E.
Results
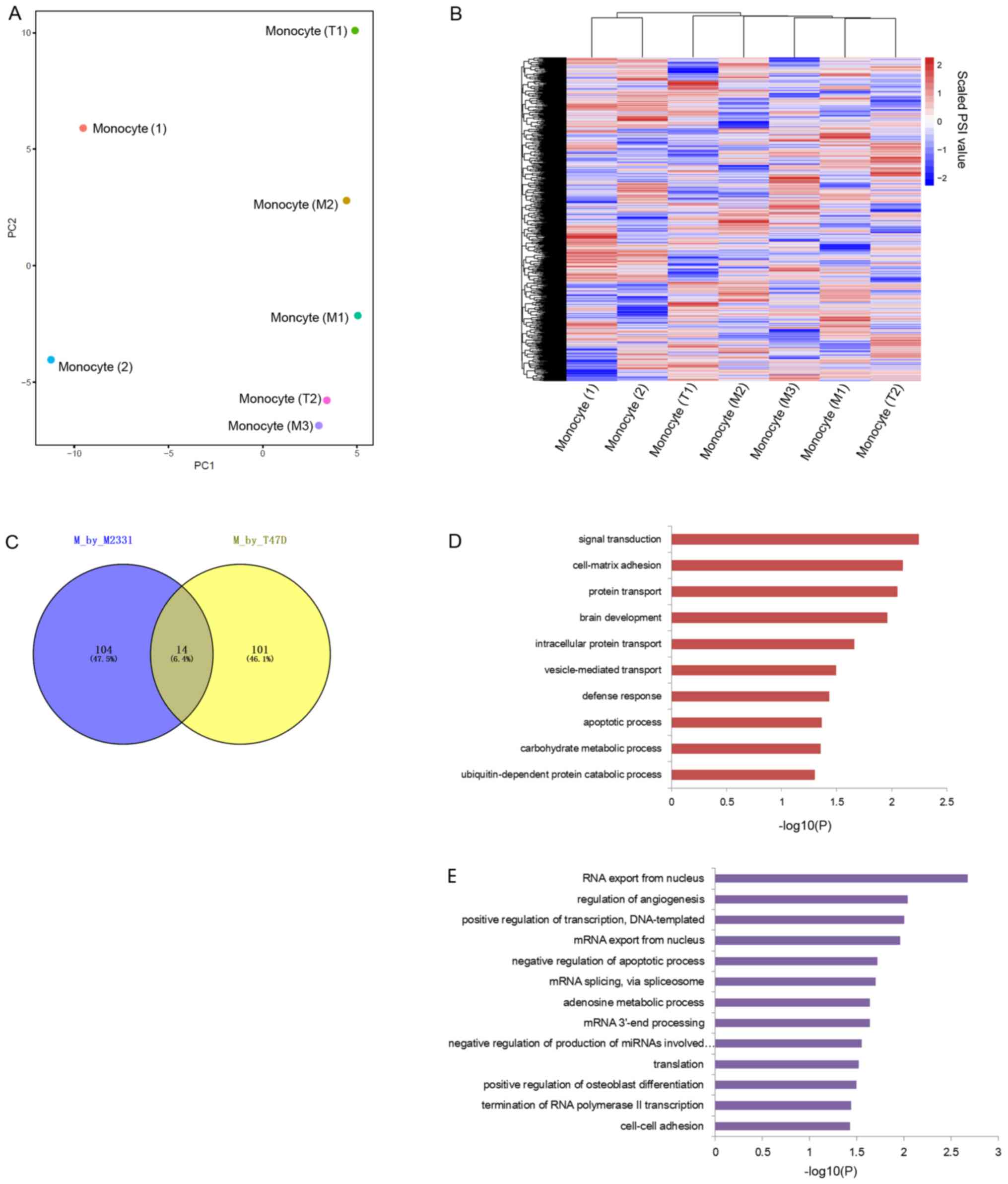
Confirmation of data reliability
To investigate the interplay between breast cancer
cells and macrophages at the alternative splicing level, we
downloaded one dataset from a previous study (21). In this previous study, freshly
isolated human peripheral monocytes were cultured with two breast
cancer cell lines (T47D, ER+ and MDA-MB-231, TNBC) in an
in vitro Transwell co-culture assay. Then, at day five, the
whole transcriptome of macrophage cells and breast tumor cells were
sequenced. Detailed sample information was previously described
(22). We firstly evaluated the data
reliability by investigating the genetic variance among samples
using gene expression profiles based on the assumption that samples
from the same cell type and treatment should have smaller genetic
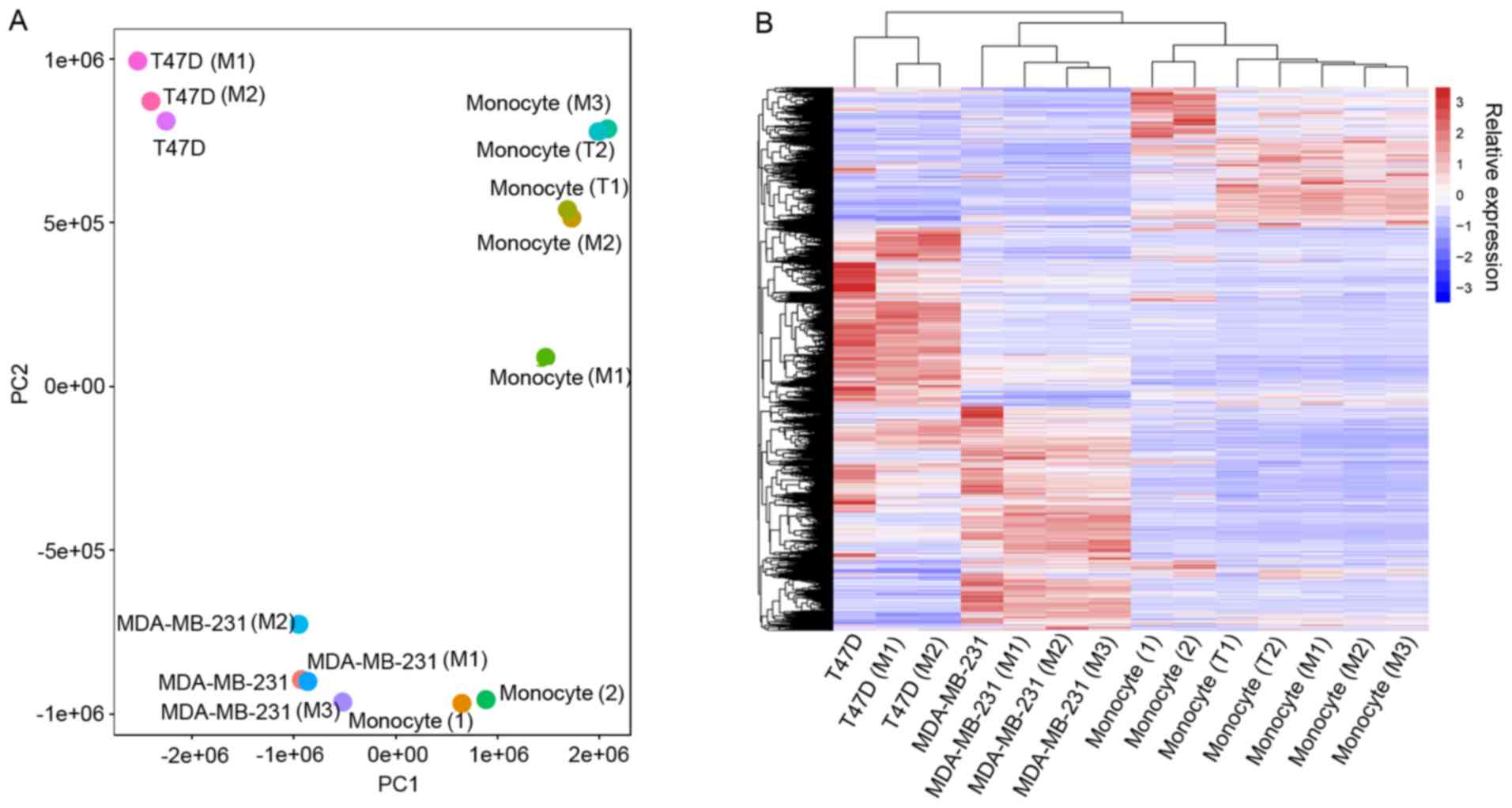
distances. PCA was performed with 20,169 genes and the results
showed that samples were grouped together according to the types
and treatments (Fig. 1A). Then
unsupervised clustering based on the same transcriptomes confirmed
the dataset reliability (Fig.
1B).
DNA damage and DNA repair pathways are
altered in both MDA-MB-231 and T47D cell lines by co-culturing with
macrophages
Next, we aimed to ascertain how macrophages educate
different breast cancer cells at the AS level. To obtain the AS
profile for each sample, we performed the Mixture of Isoforms
(MISO) pipeline (26) according to
its recommended procedure (https://miso.readthedocs.io/en/fastmiso/). Five types
of AS events including skip exon (SE), alternative 3′/5′ splice
sites (A3SS, A5SS), mutually exclusive exons (MXE), and retained
introns (RI) were analyzed. A total of 36,377 splicing events with
values at least in 7 samples were identified. Then, PCA and
unsupervised clustering were used to characterize the genetic
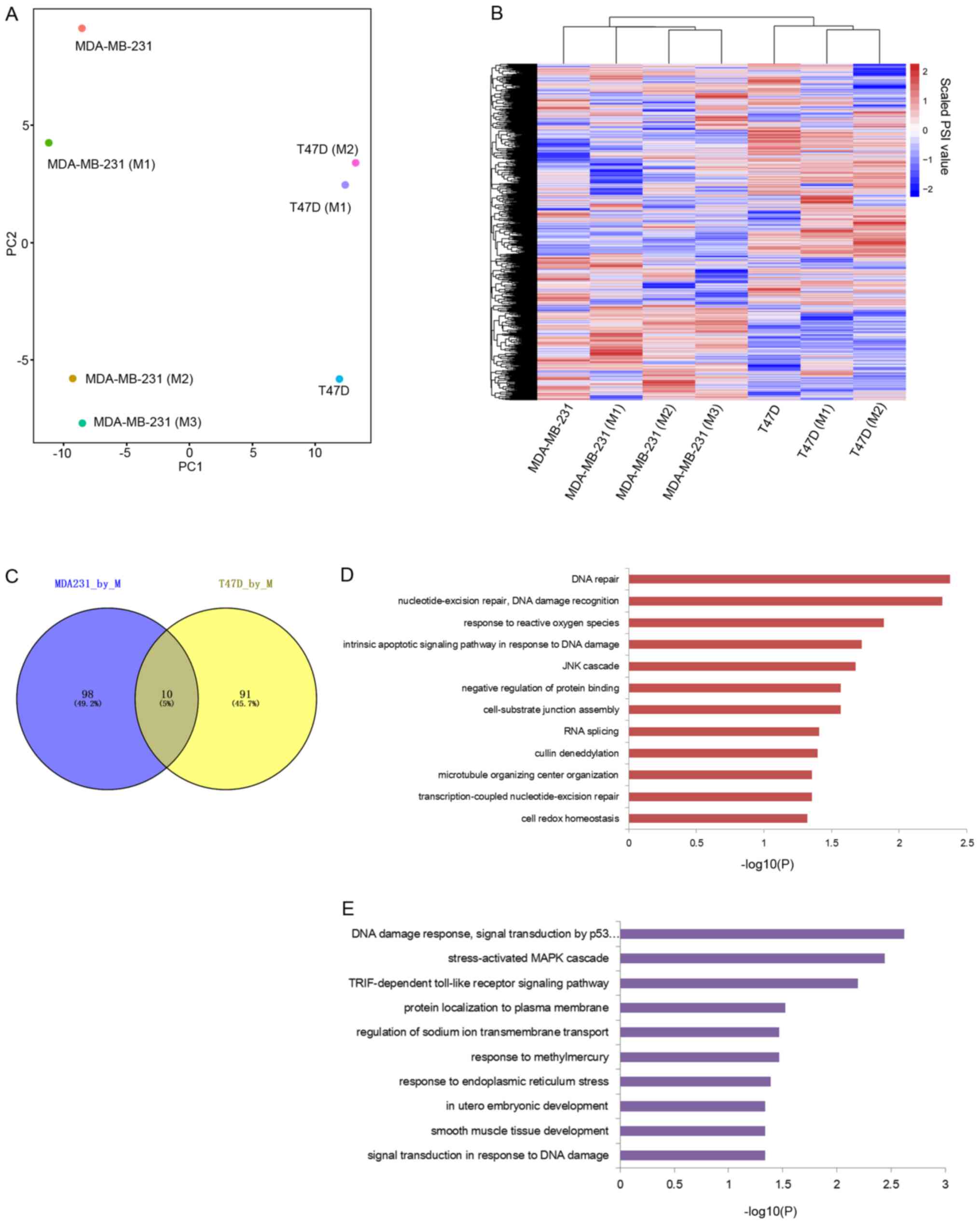
distances among samples. The PCA result showed that both T47D and
MDA-MB-231 individually cultured samples were obviously separated
from their macrophage co-cultured states (Fig. 2A). Unsupervised clustering result also
supported that co-cultured breast cancer cells were different from
the individually cultured states (Fig.
2B). These results indicated that AS profiles of both TNBC and
ER+ breast cancer were affected by co-culturing with
macrophages.
Then, it was further determined which AS events were
affected in each breast caner type and whether the altered AS
events from two breast cancer types were largely similar or
different. We isolated those altered splicing events by comparing
the co-cultured states with individually cultured states from T47D
and MDA-MB-231 cell lines, respectively. Statistically different
splicing events with delta percentage of splice-in (ΔPSI) >0.2
and bayes factor >6 were isolated. A total of 101 and 108
different splicing events were identified from T47D and MDA-MB-231
cell lines, respectively (Tables I
and II) and there were 10 common
splicing events in both T47D and MDA-MB-231 altered splicing
events. All of these results indicated that ER+ breast
cancer cells and TNBC cells were differently ‘educated’ at the AS
level by co-culturing with the macrophages (Fig. 2C).
 | Table I.Altered AS events in MDA-MB-231 cells
co-cultured with macrophages. |
Table I.
Altered AS events in MDA-MB-231 cells
co-cultured with macrophages.
| Gene | Events | ΔPSI
(SRR2922607_vs_SRR2922615) | ΔPSI
(SRR2922607_vs_SRR2922617) |
|---|
| GPS1 |
chr17:80009763:80009840:+@chr17:80010135:80010335:+@chr17:80011150:80011242:+ | 0.68 | 0.57 |
| NA |
chr17:79520100:79520153:-@chr17:79518954:79519078:-@chr17:79517274:79518229:- | 0.62 | 0.61 |
| INO80B,
WBP1 |
chr2:74685527:74685798:+@chr2:74685959:74686046:+@chr2:74686565:74686872:+ | 0.60 | 0.48 |
| TSC1 |
chr9:135786955-135786840:-@chr9:135786500-135786389:- | 0.50 | 0.44 |
| MTRF1 |
chr13:41837621:41837713:-@chr13:41836547:41836617:-@chr13:41836184:41836467:-@chr13:41834629:41835051:- | 0.47 | 0.49 |
| CTDP1 |
chr18:77477810:77478016:+@chr18:77488907:77489069:+@chr18:77496355:77496521:+ | 0.46 | 0.36 |
| HAUS2 |
chr15:42851537:42851606:+@chr15:42852980:42853068:+@chr15:42853468:42853600:+ | 0.45 | 0.44 |
| VWA9 |
chr15:65903469:65903134|65903436:-@chr15:65899497:65899763:- | 0.44 | 0.45 |
| PFDN5 |
chr12:53689235:53689423:+@chr12:53690214|53691634:53691708:+ | 0.44 | 0.66 |
| SPAG9 |
chr17:49054469:49054582:-@chr17:49053224:49053262:-@chr17:49052132:49052308:- | 0.43 | 0.25 |
| PPCDC |
chr15:75315927:75315967:+@chr15:75320588:75320794:+@chr15:75335782:75335877:
+@chr15:75340894:75341062:+ | 0.42 | 0.41 |
| OGG1 |
chr3:9796388:9796569:+@chr3:9798451:9798500:+@chr3:9807493:9808353:+ | 0.42 | 0.43 |
| ATG12 |
chr5:115177087:115177548:-@chr5:115176515:115176631:-@chr5:115176194:115176309:-@chr5:115173325:115173461:- | 0.41 | 0.34 |
| RHOC,
PPM1J |
chr1:113249700:113250025:-@chr1:113247790|113248874:113247722:- | 0.41 | 0.41 |
| RBM25 |
chr14:73566375-73566458:+@chr14:73569900-73570186:+ | 0.40 | 0.39 |
|
KIAA1551 |
chr12:32123123:32123290:+@chr12:32133812:32138891:+@chr12:32140173:32140256:+ | 0.39 | 0.37 |
| WDR26 |
chr1:224619283:224619179|224619227:-@chr1:224612220:224612356:- | 0.37 | 0.33 |
| PRPF4B |
chr6:4060647-4060920:+@chr6:4061249-4061381:+ | 0.37 | 0.34 |
| PRDX5 |
chr11:64085560:64085858:+@chr11:64087206:64087340:+@chr11:64088337:64088375:+ | 0.37 | 0.37 |
| PFKM |
chr12:48528726:48528821|48529166:+@chr12:48531504:48531629:+ | 0.36 | 0.35 |
| MAP2K7 |
chr19:7968765:7968953:+@chr19:7970693:7970740:+@chr19:7974640:7974781:+ | 0.35 | 0.31 |
| NFATC4 |
chr14:24845500:24845760|24846084:+@chr14:24846844:24848810:+ | 0.34 | 0.33 |
| NAV3 |
chr12:78515720:78516208:+@chr12:78520947:78520988:+@chr12:78522486:78522646:+ | 0.34 | 0.34 |
| APEX1 |
chr14:20923737-20923932:+@chr14:20924073-20924260:+ | 0.33 | 0.21 |
| VWA9 |
chr15:65903486:65903024|65903134:-@chr15:65899497:65899780:- | 0.33 | 0.32 |
| TAGLN |
chr11:117070040:117070148|117070545:+@chr11:117073718:117073909:+ | 0.33 | 0.33 |
| SOD2 |
chr6:160103670-160103506:-@chr6:160100330-160100096:- | 0.33 | 0.40 |
| UBXN1 |
chr11:62444990:62445106:-@chr11:62444111|62444477:62443972:- | 0.32 | 0.28 |
| SDR39U1 |
chr14:24911552:24911658:-@chr14:24911384:24911466:-@chr14:24910880:24911001:- | 0.32 | 0.29 |
| ITGA6 |
chr2:173362703:173362828:+@chr2:173366500:173366629:+@chr2:173368819:173371181:+ | 0.32 | 0.29 |
| TSPAN4 |
chr11:842824:842915:+@chr11:847201:847300:+@chr11:850288:850367:+@chr11:862550:862741:+ | 0.32 | 0.37 |
|
C16orf13 |
chr16:686094:686347:-@chr16:685612:685774:-@chr16:685281:685340:- | 0.31 | 0.32 |
| VWA9 |
chr15:65903469:65903134|65903436:-@chr15:65899497:65899780:- | 0.3 | 0.33 |
| RPAIN |
chr17:5329291-5329619:+@chr17:5331391-5331531:+ | 0.29 | 0.27 |
|
SUV420H1 |
chr11:67980944:67981068:-@chr11:67957384:67957619:-@chr11:67953248:67953395:- | 0.29 | 0.38 |
| COX7A2 |
chr6:75950892:75950981:-@chr6:75949993:75950101:-@chr6:75947391:75947704:- | 0.28 | 0.32 |
| ZNRD1 |
chr6:30029036-30029175:+@chr6:30029292-30029446:+ | 0.28 | 0.38 |
| GNB2L1 |
chr5:180669170:180669345:-@chr5:180668563|180668639:180668492:- | 0.27 | 0.29 |
| ERRFI1 |
chr1:8075555:8075752:-@chr1:8075368:8075460:-@chr1:8071779:8074456:- | 0.27 | 0.30 |
|
SLC25A35 |
chr17:8192961-8192892:-@chr17:8191750-8191082:- | 0.27 | 0.33 |
| GOLGA2 |
chr9:131036129:131036251:-@chr9:131035064:131035144:-@chr9:131030699:131030803:- | 0.27 | 0.39 |
| NA |
chr14:105180540:105181193:+@chr14:105181621:105181677:+@chr14:105185132:105185947:+ | 0.26 | 0.20 |
| RFWD3 |
chr16:74700684:74700779:-@chr16:74698522:74698643:-@chr16:74694830:74695349:-@chr16:
74685818:74686020:- | 0.26 | 0.20 |
| SEC23A |
chr14:39572797:39573033:-@chr14:39572236:39572432:-@chr14:39565102:39565343:-@chr14:39562391:39562448:- | 0.26 | 0.26 |
| RPS21 |
chr20:60962899-60962970:+@chr20:60963365-60963576:+ | 0.26 | 0.30 |
| TXNRD1 |
chr12:104680460:104680887:+@chr12:104682709:104682818:+@chr12:104705068:104705190:
+@chr12:104707023:104707095:+ | 0.26 | 0.39 |
| ALG13 |
chrX:110928193:110928331:+@chrX:110929365:110929402:+@chrX:110931115:110933623:+ | 0.25 | 0.20 |
| MTRF1 |
chr13:41837621:41837713:-@chr13:41836184:41836467:-@chr13:41834629:41835051:- | 0.25 | 0.21 |
|
C16orf13 |
chr16:686094:686347:-@chr16:685518:685774:-@chr16:685281:685340:- | 0.24 | 0.24 |
| PHC2 |
chr1:33799893:33799691|33799786:-@chr1:33797876:33798005:- | 0.24 | 0.26 |
|
C16orf13 |
chr16:686094:686347:-@chr16:685518:685774:-@chr16:685281:685340:-@chr16:684719:684797:- | 0.24 | 0.30 |
| PTPN3 |
chr9:112189230:112189402:-@chr9:112184998:112185132:-@chr9:112182704:112182880:- | 0.23 | 0.22 |
| BAX |
chr19:49464067-49464171:+@chr19:49464789-49465055:+ | 0.23 | 0.22 |
| PFKM |
chr12:48528726:48528821:+@chr12:48529074:48529166:+@chr12:48531504:48531629:+ | 0.23 | 0.23 |
| TMEM41B |
chr11:9305140-9304918:-@chr11:9302588-9302201:- | 0.23 | 0.23 |
| NCAPG2 |
chr7:158444985:158445172:-@chr7:158443524:158443664:-@chr7:158439152:158439255:- | 0.23 | 0.25 |
| MTRF1L |
chr6:153316454:153316271|153316379:-@chr6:153315648:153315811:- | 0.23 | 0.30 |
| ORMDL1 |
chr2:190648995:190649097:-@chr2:190647740:190647849:-@chr2:190647148:190647328:- | 0.22 | 0.21 |
| RHOC,
PPM1J |
chr1:113249700:113250025:-@chr1:113247722:113248874:-@chr1:113246266:113246428:- | 0.22 | 0.21 |
| BCAS3 |
chr17:59093112:59093262:+@chr17:59104227:59104271:+@chr17:59112027:59112151:+ | 0.22 | 0.23 |
| TSPAN4 |
chr11:844081:844186:+@chr11:847201:847300:+@chr11:850288:850367:+@chr11:862550:862741:+ | 0.22 | 0.27 |
| NFATC4 |
chr14:24845500-24845760:+@chr14:24846844-24848810:+ | 0.21 | 0.25 |
| DRAM2 |
chr1:111663315:111663138|111663154:-@chr1:111662499:111662581:- | 0.20 | 0.23 |
| ANKRD49 |
chr11:94229770-94230117:+@chr11:94231237-94232744:+ | 0.20 | 0.28 |
| MEF2A |
chr15:100185766:100185969:+@chr15:100211528:100211659:+@chr15:100211725:100211862:
+@chr15:100214598:100214817:+ | −0.2 | −0.33 |
| NCOR2 |
chr12:124812179:124811955|124812093:-@chr12:124810737:124810916:- | −0.20 | −0.24 |
| TSPAN4 |
chr11:847201:847300:+@chr11:850288:850367:+@chr11:862550:862741:+ | −0.20 | −0.22 |
| CYB5B |
chr16:69458498:69458760:+@chr16:69481053:69481181:+@chr16:69492996:69493024:+@chr16:
69496333:69500167:+ | −0.20 | −0.21 |
| MIR3654,
EEF1G |
chr11:62342380-62342149:-@chr11:62340214-62340056:- | −0.21 | −0.22 |
| MKNK2 |
chr19:2040133:2040176:-@chr19:2037828|2039855:2037470:- | −0.22 | −0.29 |
| NA |
chr19:3638882:3639014:-@chr19:3633435:3633518:-@chr19:3630179:3633167:- | −0.22 | −0.24 |
| YKT6 |
chr7:44250622:44250723:+@chr7:44251153:44251203:+@chr7:44251846:44253893:+ | −0.22 | −0.23 |
| FN1 |
chr2:216246935:216247048:-@chr2:216245534:216245803:-@chr2:216243853:216244040:- | −0.22 | −0.22 |
| MBNL2 |
chr13:98009736:98009889:+@chr13:98017390:98017425:+@chr13:98018713:98018807:
+@chr13:98043576:98046374:+ | −0.22 | −0.22 |
| ARL6IP4,
RP11-197N18.2 |
chr12:123465676:123465813|123465846:+@chr12:123466118:123466426:+ | −0.23 | −0.21 |
| UBC |
chr12:125398665:125398551|125398587:-@chr12:125398042:125398320:- | −0.24 | −0.27 |
| C9orf9 |
chr9:135763677-135763824:+@chr9:135765344-135765418:+ | −0.24 | −0.26 |
| MRRF |
chr9:125026882:125027217:+@chr9:125027741:125027824:+@chr9:125033143:125033354:+ | −0.25 | −0.32 |
| SPNS1 |
chr16:28986096:28986713:+@chr16:28986813:28986878:+@chr16:28989229:28989365:
+@chr16:28990476:28990627:+ | −0.25 | −0.26 |
|
AC124789.1 |
chr17:36607870:36608071:-@chr17:36607550:36607769:-@chr17:36606638:36606967:- | −0.25 | −0.23 |
| TBRG1 |
chr11:124495567:124495799:+@chr11:124496369:124496505:+@chr11:124496800:124496946:+ | −0.25 | −0.21 |
| H2AFY |
chr5:134696187:134696297:-@chr5:134688636:134688735:-@chr5:134686513:134686603:-
@chr5:134681658:134681747:- | −0.26 | −0.38 |
| NAV3 |
chr12:78513017:78513725:+@chr12:78515720:78516208:+@chr12:78522486:78522646:+ | −0.26 | −0.27 |
|
AC124789.1 |
chr17:36607870:36608071:-@chr17:36607550:36607637:-@chr17:36606638:36606967:- | −0.26 | −0.25 |
| LRRC28 |
chr15:99796103:99796330:+@chr15:99816781:99816821:+@chr15:99827462:99827499:+ | −0.30 | −0.42 |
| OSBPL3 |
chr7:24903115:24903218:-@chr7:24902819:24902911:-@chr7:24901232:24901388:- | −0.30 | −0.33 |
| TBRG1 |
chr11:124495567:124495799:+@chr11:124496369:124496505:+@chr11:124496589:
124496647:+@chr11:124496800:124496946:+ | −0.30 | −0.32 |
| NA |
chr17:57232800:57232317|57232492:-@chr17:57187308:57189706:- | −0.30 | −0.29 |
| EEF1D |
chr8:144679518:144679845:-@chr8:144674817:144675063:-@chr8:144671161:144672251:- | −0.31 | −0.32 |
| NBPF12 |
chr1:146395307:146395516:+@chr1:146397359:146397461:+@chr1:146398293:146398507:+ | −0.32 | −0.32 |
| NA |
chr6:30524486:30525227:+@chr6:30525927:30525989:+@chr6:30529105:30529285:+@chr6:
30529611:30529901:+ | −0.32 | −0.31 |
| UBAP2L |
chr1:154241233:154241430:+@chr1:154241838:154241888:+@chr1:154242676:154243329:+ | −0.33 | −0.20 |
| ASB8 |
chr12:48551242:48551377:-@chr12:48547308|48547312:48547151:- | −0.35 | −0.33 |
| PARP3 |
chr3:51976361:51976724:+@chr3:51977365|51977370:51977554:+ | −0.35 | −0.33 |
| EEF1D |
chr8:144679518:144679845:-@chr8:144674817:144675063:-@chr8:144672815:144672908:-
@chr8:144671161:144672251:- | −0.36 | −0.40 |
| MBNL2 |
chr13:98009736:98009889:+@chr13:98018713:98018807:+@chr13:98043576:98046374:+ | −0.36 | −0.37 |
| ATG12 |
chr5:115176515:115176631:-@chr5:115176194:115176309:-@chr5:115173325:115173461:- | −0.37 | −0.46 |
| GCOM1,
POLR2M |
chr15:57967166:57967266:+@chr15:57976600:57976872:+@chr15:57998132:57999153:
+@chr15:58001383:58001556:+ | −0.37 | −0.41 |
| COPS7B |
chr2:232646417:232646593:+@chr2:232653265:232653442:+@chr2:232658973:232659061:
+@chr2:232660816:232661018:+ | −0.37 | −0.34 |
| ZNF507 |
chr19:32836514:32836689:+@chr19:32838151:32838244:+@chr19:32843735:32845863:+ | −0.40 | −0.41 |
| EEF1D |
chr8:144679518:144679845:-@chr8:144674817:144675063:-@chr8:144672778:144672908:- | −0.41 | −0.43 |
|
C17orf70 |
chr17:79519078:79518758|79518954:-@chr17:79517274:79518229:- | −0.43 | −0.48 |
| SAFB |
chr19:5667057:5667175:+@chr19:5667358|5667364:5667461:+ | −0.47 | −0.54 |
| FGFR1 |
chr8:38314874:38315052:-@chr8:38287200:38287466:-@chr8:38285864:38285953:- | −0.47 | −0.32 |
| PIK3CD |
chr1:9711790:9711860:+@chr1:9751525:9751629:+@chr1:9770163:9770338:+@chr1:9770482:9770654:+ | −0.53 | −0.47 |
| ACADVL |
chr17:7127132-7127194:+@chr17:7127287-7127388:+ | −0.57 | −0.62 |
| STAG2 |
chrX:123095164:123095285:+@chrX:123095636:123095706:+@chrX:123155217:123155281:
+@chrX:123156381:123156521:+ | −0.57 | −0.61 |
| NME3 |
chr16:1821411-1821270:-@chr16:1821172-1821071:- | −0.67 | −0.49 |
 | Table II.Altered AS events in T47D cells
co-cultured with macrophages. |
Table II.
Altered AS events in T47D cells
co-cultured with macrophages.
| Gene | Events | ΔPSI
(SRR2922618_vs_SRR2922619) | ΔPSI
(SRR2922618_vs_SRR2922620) |
|---|
| UBQLN1 |
chr9:86284100:86284242:-@chr9:86281265:86281309:-@chr9:86279945:86280060:- | −0.49 | −0.57 |
| PFDN5 |
chr12:53689235:53689423:+@chr12:53690214|53691634:53691708:+ | −0.64 | −0.54 |
| ARMC8 |
chr3:137906148:137906441:+@chr3:137907297:137907372:+@chr3:137928659:137928735:+ | −0.61 | −0.53 |
| SENP2 |
chr3:185316200:185316333:+@chr3:185316780:185316812:+@chr3:185318553:185318643:+ | −0.59 | −0.52 |
| RRN3 |
chr16:15185172:15185228:-@chr16:15180222:15180311:-@chr16:15179986:15180115:- | −0.32 | −0.51 |
| SULF1 |
chr8:70378859:70379185:+@chr8:70408000:70408161:+@chr8:70414109:70414203:+ | −0.44 | −0.50 |
| NA |
chr5:10236590:10236727:-@chr5:10235323:10235373:-@chr5:10225620:10227759:- | −0.45 | −0.49 |
| NA |
chr6:30524486:30525227:+@chr6:30525927:30525989:+@chr6:30529611:30529901:+ | −0.47 | −0.47 |
| VAMP1 |
chr12:6574107-6574056:-@chr12:6572008-6571404:- | −0.43 | −0.45 |
| ZFAND6 |
chr15:80364903:80364989:+@chr15:80390758:80390920:+@chr15:80412670:80412840:+ | −0.38 | −0.45 |
| CNOT1 |
chr16:58663632:58663790:-@chr16:58658557:58658698:-@chr16:58657186:58657313:-
@chr16:58633140:58633415:- | −0.38 | −0.45 |
|
C16orf13 |
chr16:686094:686347:-@chr16:685612:685774:-@chr16:685281:685340:- | −0.31 | −0.45 |
| 43349 |
chrX:118759298:118759342:-@chrX:118752749|118754014:118750909:- | −0.28 | −0.45 |
| SP140L |
chr2:231253274:231253348:+@chr2:231254634:231254738:+@chr2:231256802:231256944:+ | −0.39 | −0.44 |
| TMEM91, BCKDHA,
CTC-435M10.3 |
chr19:41888677:41888826:+@chr19:41889466:41889537:+@chr19:41889768:41889987:+ | −0.29 | −0.44 |
| VAMP1 |
chr12:6574107-6574056:-@chr12:6572008-6571403:- | −0.40 | −0.43 |
| IKBKB |
chr8:42128820:42128987:+@chr8:42129601:42129723:+@chr8:42146152:42146246:
+@chr8:42147674:42147791:+ | −0.75 | −0.41 |
| FKBP14 |
chr7:30062281:30062432:-@chr7:30059829:30059920:-@chr7:30058612:30058739:- | −0.40 | −0.41 |
| NCOR2 |
chr12:124862783:124862930:-@chr12:124858959:124859009:-@chr12:124856568:124857156:- | −0.33 | −0.41 |
| ANAPC11 |
chr17:79849599:79849709|79849717:+@chr17:79852343:79852462:+ | −0.36 | −0.40 |
| MYO5A |
chr15:52638558:52638658:-@chr15:52635314:52635394:-@chr15:52632393:52632591:- | −0.37 | −0.39 |
|
C16orf13 |
chr16:686094:686347:-@chr16:685518:685774:-@chr16:685281:685340:-@chr16:684719:684797:- | −0.27 | −0.38 |
| PTPN3 |
chr9:112189230:112189402:-@chr9:112184998:112185132:-@chr9:112182704:112182880:- | −0.29 | −0.37 |
| 43349 |
chrX:118763281:118763471:-@chrX:118752749|118754014:118750909:- | −0.29 | −0.37 |
|
ATP6V0D1 |
chr16:67514860:67515089:-@chr16:67478431:67478609:-@chr16:67477002:67477081:- | −0.31 | −0.36 |
| PILRB,
CTB-161A2.4 |
chr7:99950187-99950537:+@chr7:99950620-99950746:+ | −0.37 | −0.35 |
| NA |
chr3:37396592:37396678:+@chr3:37402734:37402796:+@chr3:37407571:37408370:+ | −0.26 | −0.35 |
| MUC1 |
chr1:155160639:155160707:-@chr1:155160198:155160334:-@chr1:155159701:155159850:- | −0.37 | −0.34 |
| DALRD3 |
chr3:49058279:49058467:-@chr3:49055833:49055986:-@chr3:49055456:49055751:- | −0.33 | −0.34 |
|
RP11-66N24.4 |
chr14:24025198:24025552:+@chr14:24025952:24026243:+@chr14:24027904:24028790:+ | −0.31 | −0.34 |
| ZNRD1 |
chr6:30029036-30029175:+@chr6:30029292-30029446:+ | −0.31 | −0.33 |
| NA |
chr7:75633076:75633173:-@chr7:75630208:75630272:-@chr7:75625655:75625917:- | −0.36 | −0.32 |
| DLG1 |
chr3:196921296:196921460:-@chr3:196888511:196888609:-@chr3:196876614:196876667:- | −0.3 | −0.32 |
| NEIL2 |
chr8:11627172:11627300:+@chr8:11627683:11627844:+@chr8:11628955:11629094:+ | −0.29 | −0.32 |
|
C16orf13 |
chr16:686094:686347:-@chr16:685518:685774:-@chr16:685281:685340:- | −0.21 | −0.32 |
| TPD52L1 |
chr6:125574863:125574901:+@chr6:125578244:125578304:+@chr6:125583980:125584644:+ | −0.37 | −0.31 |
| ORMDL1 |
chr2:190648995:190649097:-@chr2:190647740:190647849:-@chr2:190647148:190647328:- | −0.36 | −0.31 |
| ORMDL1 |
chr2:190648995:190649097:-@chr2:190647328|190647849:190647148:- | −0.40 | −0.29 |
| SMPD4 |
chr2:130921947:130922018:-@chr2:130918759:130918845:-@chr2:130914824:130914969:- | −0.34 | −0.29 |
| AP001055.7,
C21orf33 |
chr21:45553494:45553720:+@chr21:45553984:45554036:+@chr21:45555942:45556055:+ | −0.26 | −0.28 |
| NA |
chr22:50964675:50964905:-@chr22:50964430:50964570:-@chr22:50961997:50962853:- | −0.25 | −0.28 |
| NA |
chr17:5326089:5326149:+@chr17:5331391:5331531:+@chr17:5335862:5336340:+ | −0.34 | −0.27 |
| NA |
chr10:14920782:14920918:+@chr10:14923499:14923644:+@chr10:14938845:14939516:+ | −0.29 | −0.27 |
| C1orf52 |
chr1:85725041:85725355:-@chr1:85724618:85724744:-@chr1:85724207:85724405:- | −0.28 | −0.27 |
|
RP11-66N24.4 |
chr14:24025198:24025552:+@chr14:24025952:24026248:+@chr14:24027904:24028790:+ | −0.27 | −0.27 |
| ISYNA1 |
chr19:18548670:18548798:-@chr19:18547289|18547915:18547140:- | −0.26 | −0.27 |
| ENTPD6 |
chr20:25176339:25176503:+@chr20:25187158:25187226:+@chr20:25187712:25188033:+ | −0.23 | −0.26 |
| NA |
chr11:72983352:72983511:+@chr11:73006775:73006860:+@chr11:73007530:73009664:+ | −0.31 | −0.25 |
| MBD1 |
chr18:47801347:47801415:-@chr18:47800556:47800723:-@chr18:47799934:47800233:- | −0.26 | −0.25 |
| CRYZ |
chr1:75175782:75175931:-@chr1:75172787:75172888:-@chr1:75172583:75172678:- | −0.25 | −0.25 |
| SLMAP |
chr3:57908616:57908750:+@chr3:57911572:57911661:+@chr3:57913023:57914894:+ | −0.24 | −0.25 |
| ZMYM2 |
chr13:20567203-20567704:+@chr13:20567966-20568059:+ | −0.32 | −0.24 |
| CHEK2 |
chr22:29095826:29095925:-@chr22:29092889:29092975:-@chr22:29091698:29091861:- | −0.28 | −0.24 |
| MBD1 |
chr18:47801347:47801415:-@chr18:47800556:47800720:-@chr18:47799934:47800233:- | −0.25 | −0.24 |
| ATP5J |
chr21:27107965:27107164|27107626:-@chr21:27101942:27102112:- | −0.24 | −0.24 |
| UBE2D3 |
chr4:103749101:103749307:-@chr4:103748749:103748898:-@chr4:103747642:103747793:- | −0.22 | −0.24 |
| ATP13A3 |
chr3:194134488:194134568:-@chr3:194132928:194133017:-@chr3:194123403:194126845:- | −0.27 | −0.23 |
| MBD1 |
chr18:47801499:47801615:-@chr18:47801347:47801415:-@chr18:47800556:47800723:-
@chr18:47799934:47800233:- | −0.25 | −0.23 |
| SDR39U1 |
chr14:24911552:24911658:-@chr14:24911384:24911466:-@chr14:24910880:24911001:- | −0.23 | −0.23 |
| CTAGE5,
MIA2 |
chr14:39788413:39788495:+@chr14:39790132:39790260:+@chr14:39796068:39796226:+ | −0.27 | −0.22 |
| PAK4 |
chr19:39660172:39660397:+@chr19:39663558:39663746:+@chr19:39664216:39664650:+ | −0.22 | −0.22 |
| ZFAND5 |
chr9:74979612:74980163:-@chr9:74978386:74978522:-@chr9:74975544:74975703:- | −0.22 | −0.22 |
| CLDND1,
CPOX |
chr3:98241693:98241910:-@chr3:98240497:98240547:-@chr3:98239977:98240286:- | −0.36 | −0.21 |
| NA |
chr7:75039605:75039743:+@chr7:75039889:75040009:+@chr7:75044163:75044301:+ | −0.33 | −0.21 |
| HSPA14 |
chr10:14882074:14882156:+@chr10:14884133:14884845:+@chr10:14885370:14886747:+ | −0.25 | −0.21 |
| TPD52L1 |
chr6:125569428:125569529:+@chr6:125574863:125574901:+@chr6:125578244:125578304:
+@chr6:125583980:125584644:+ | −0.26 | −0.20 |
| LGMN |
chr14:93176017:93176217:-@chr14:93170985:93171052:-@chr14:93170152:93170706:- | −0.24 | −0.20 |
| GGCX |
chr2:85788509:85788657:-@chr2:85787938:85788108:-@chr2:85786040:85786198:- | −0.22 | −0.20 |
| OS9 |
chr12:58112776:58112965:+@chr12:58113882:58114046:+@chr12:58114189:58114301:+ | −0.22 | −0.20 |
| UBC |
chr12:125398665:125398551|125398587:-@chr12:125398042:125398320:- | 0.20 | 0.20 |
| MRPL9 |
chr1:151735622:151735431|151735466:-@chr1:151734852:151734976:- | 0.20 | 0.20 |
| EEF1D |
chr8:144679518:144679845:-@chr8:144674817:144675063:-@chr8:144672815:144672908:-
@chr8:144671161:144672251:- | 0.23 | 0.20 |
| RPL17,
RPL17-C18ORF32 |
chr18:47018628:47018834:-@chr18:47017954|47018203:47017902:- | 0.20 | 0.21 |
| ENOX2 |
chrX:129790555:129790669:-@chrX:129771378|129771384:129771203:- | 0.20 | 0.22 |
| ARL6IP4,
RP11-197N18.2 |
chr12:123465676:123465813|123465846:+@chr12:123466142:123466426:+ | 0.21 | 0.23 |
| MRPL9 |
chr1:151735622:151735426|151735466:-@chr1:151734852:151734976:- | 0.23 | 0.23 |
| NOP56 |
chr20:2636580:2636680|2636860:+@chr20:2637047:2637195:+ | 0.20 | 0.24 |
| CPSF3L |
chr1:1249681:1249745:-@chr1:1249301|1249485:1249112:- | 0.27 | 0.24 |
| NA |
chr18:674406-674161:-@chr18:673076-670324:- | 0.20 | 0.27 |
| C10orf2 |
chr10:102747293:102747370|102749210:+@chr10:102749401:102749641:+ | 0.21 | 0.28 |
| LIME1,
ZGPAT |
chr20:62367133-62368064:+@chr20:62368886-62369000:+ | 0.26 | 0.28 |
| FLOT1 |
chr6:30698877-30698696:-@chr6:30698504-30698459:- | 0.22 | 0.29 |
| GYLTL1B |
chr11:45944329-45944515:+@chr11:45944609-45944718:+ | 0.39 | 0.29 |
| MIIP |
chr1:12089280-12089951:+@chr1:12090085-12090181:+ | 0.27 | 0.33 |
| SCMH1 |
chr1:41503034:41503213:-@chr1:41499655:41499774:-@chr1:41494256:41494398:- | 0.36 | 0.33 |
| TAOK2 |
chr16:29997586-29997825:+@chr16:29999680-29999719:+ | 0.48 | 0.34 |
| SRSF5 |
chr14:70235515-70235613:+@chr14:70235899-70237257:+ | 0.29 | 0.35 |
| PQLC3 |
chr2:11304330:11304387:+@chr2:11312051:11312171:+@chr2:11315094:11315135:
+@chr2:11317863:11318998:+ | 0.34 | 0.35 |
| APEX1 |
chr14:20923385-20923487:+@chr14:20923737-20923862:+ | 0.35 | 0.35 |
| PGAP2 |
chr11:3832480:3832654:+@chr11:3838662:3838765:+@chr11:3844139:3844223:
+@chr11:3845113:3845365:+ | 0.53 | 0.39 |
| ARL6IP4,
RP11-197N18.2 |
chr12:123465676:123465813:+@chr12:123466118|123466142:123466426:+ | 0.33 | 0.42 |
| TCEA2 |
chr20:62701118-62701174:+@chr20:62701613-62701767:+ | 0.51 | 0.48 |
| PGAP2 |
chr11:3832480:3832654:+@chr11:3838583:3838765:+@chr11:3844139:3844223:
+@chr11:3845113:3845365:+ | 0.62 | 0.48 |
| WDR19 |
chr4:39274600-39274681:+@chr4:39276428-39276578:+ | 0.41 | 0.49 |
| ZNF707 |
chr8:144766622:144766712:+@chr8:144766926:144767237:+@chr8:144771374:144771471:
+@chr8:144772227:144772293:+ | 0.40 | 0.50 |
| ARSA |
chr22:51066601:51066326|51066374:-@chr22:51065984:51066220:- | 0.42 | 0.59 |
| ZBTB8OS |
chr1:33116030:33116155:-@chr1:33100369:33100393:-@chr1:33099552:33099673:- | 0.59 | 0.59 |
| RGL2 |
chr6:33261843-33261811:-@chr6:33261693-33261572:- | 0.49 | 0.60 |
| OSBPL6 |
chr2:179204399:179204491:+@chr2:179209013:179209087:+@chr2:179213951:179214116:+ | 0.56 | 0.60 |
| WDR90 |
chr16:705773-705889:+@chr16:706302-706537:+ | 0.28 | 0.64 |
| TIMM9 |
chr14:58894110:58894232:-@chr14:58893772:58893960:-@chr14:58878625:58878689:-
@chr14:58877561:58877656:- | 0.50 | 0.68 |
To further investigate the biological processes
affected by those altered AS events, Gene Ontology (GO) functional
enrichment analysis was performed. GO terms such as DNA repair,
nucleotide-excision repair, DNA damage recognition, response to
reactive oxygen species, intrinsic apoptotic signaling pathway in
response to DNA damage were significantly enriched in the
MDA-MB-231-altered AS events (Fig.
2D). Biological processes such as DNA damage response, signal
transduction by p53 class mediator resulting in cell cycle arrest,
stress-activated MAPK cascade, signal transduction in response to
DNA damage were significantly enriched in T47D-altered alternative
splicing events (Fig. 2E). Taken
together, although the altered AS events from MDA-MB-231 and T47D
cells were different, DNA repair and DNA damage-related biological
processes were significantly enriched in both breast cancer cell
lines of differing types in the presence of macrophages.
Then, we investigated how the DNA repair and DNA
damage-related AS events influence the function of corresponding
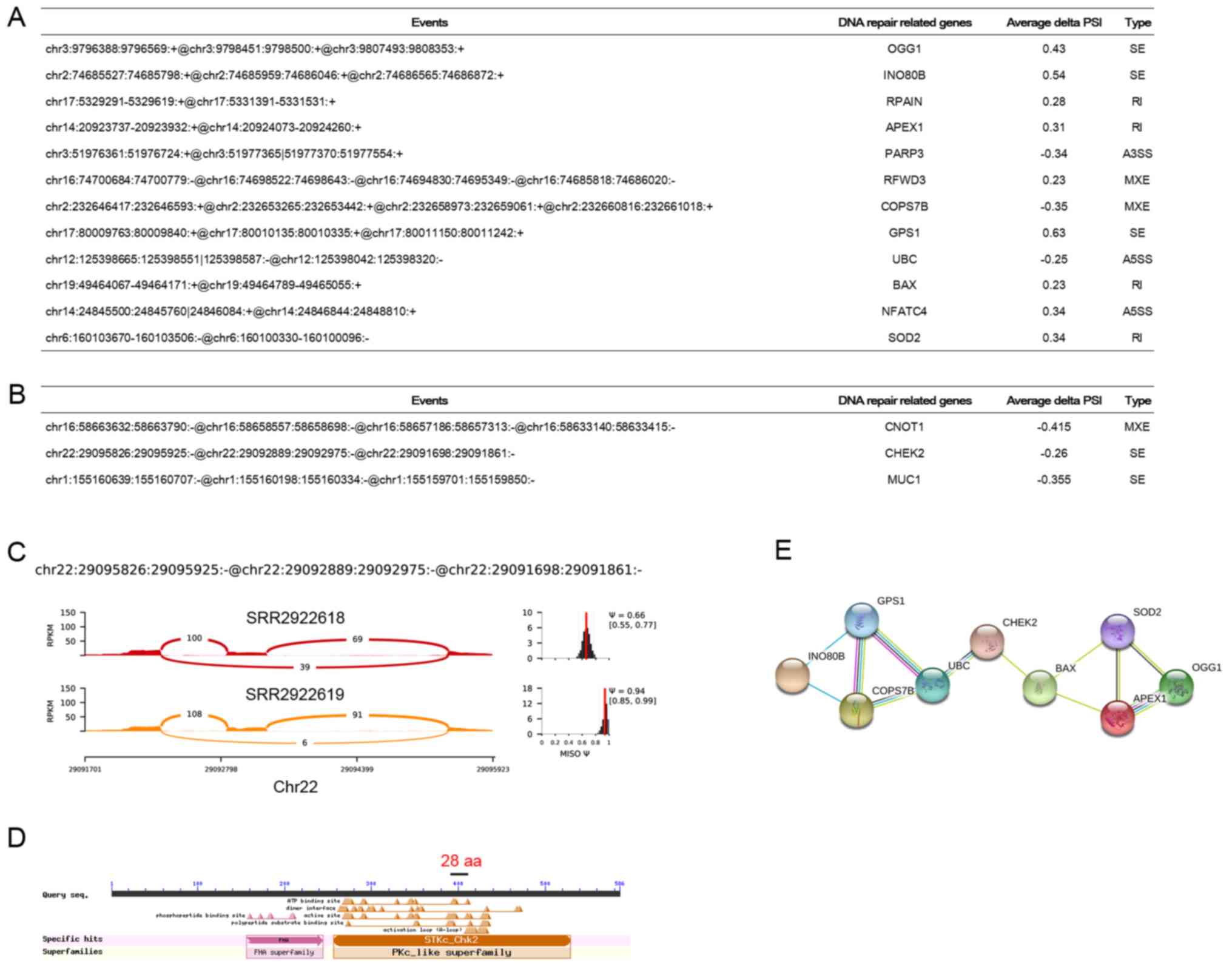
genes. Fig. 3A and B shows the DNA
repair and DNA damage-related AS events identified from MDA-MB-231
and T47D samples, respectively. Sashimi plots showed the AS events
‘chr22:29095826:29095925:-@chr22:29092889:29092975:-@chr22:29091698:29091861:-’
from gene Chek2 in T47D individual cultured sample
(SRR2922618) and co-cultured sample (SRR2922619) (Fig. 3C). CHEK2 is a cell cycle checkpoint
regulator and putative tumor suppressor (37) which contains a forkhead-associated
protein interaction domain essential for activation in response to
DNA damage and is rapidly phosphorylated in response to replication
blocks and DNA damage. CHEK2 has been shown to stabilize the
tumor-suppressor protein p53 (38,39),
leading to cell cycle arrest in G1 and it also interacts with and
phosphorylates BRCA1, allowing BRCA1 to restore survival after DNA
damage (40). According to our domain
analysis, we found that CHEK2 contains two crutial functional
domains (Fig. 3D): The first one is
STKc_Chk2, in which STKs can catalyze the transfer of a
γ-phosphoryl group from ATP to serine/threonine residues on protein
substrates, and the second one is FHA domain which is a putative
nuclear signaling domain. The skipped exon of Chek2 encodes 28 aa
which locates in the STKc_Chk2 domain (Fig. 3D). Thus the inclusion or exclusion of
the skippd exon changes the STKc_Chk2 domain of Chek2, which may
play an important role in the functional regulation of CHEK2.
Protein-protein interaction network analysis showed that these DNA
repair proteins have extensive interactions and CHEK2 may serve as
a hub gene in the network (Fig.
3E).
Altered AS events in macrophages by
co-culturing with MDA-MB-231 and T47D cells, respectively
As the differentiation of TAMs is largely regulated
by mediators secreted by tumor cells, it was ascertained how
different breast cancer cells ‘educate’ macrophages and regulate
the differentiation process at the AS level. Similarly, a total of
35,476 splicing events with values in at least 7 samples were
identified. Then, PCA and unsupervised clustering were used to
characterize the genetic distances among samples. From the PCA
result, it was revealed that macrophages individually cultured were
separated from their co-cultured states whereas the co-cultured
states of macrophage from T47D and MDA-MB-231 cells were not able
to be separated (Fig. 4A), which is
also supported by the unsupervised clustering result (Fig. 4B). Then, we isolated those altered
splicing events by comparing the co-cultured samples with
individually cultured samples. A total of 115 and 118 significant
splicing events were identified in macrophages co-cultured with
MDA-MB-231 and T47D cells, respectively (Tables III and IV). There were 14 common splicing events in
the macrophages co-cultured with T47D and MDA-MB-231 cells
(P>0.2) which indicated that ER+ breast cancer cells
and TNBC cells have different influences on macrophages at the AS
level (Fig. 4C).
 | Table III.Altered AS events in macrophages
co-cultured with MDA-MB-231 cells. |
Table III.
Altered AS events in macrophages
co-cultured with MDA-MB-231 cells.
| Gene | Events | ΔPSI
(SRR2922608_vs_SRR2922609) | ΔPSI
(SRR2922608_vs_SRR2922611) |
|---|
| WARS |
chr14:100841620:100841927:-@chr14:100840473:100840602:-@chr14:100840317:100840401:-
@chr14:100835424:100835595:- | −0.44 | −0.87 |
| WARS |
chr14:100841620:100841927:-@chr14:100840473:100840581:-@chr14:100840317:100840401:-
@chr14:100835424:100835595:- | −0.47 | −0.85 |
|
SLC25A19 |
chr17:73285170:73285453:-@chr17:73284583:73284672:-@chr17:73284469:73284542:-
@chr17:73282714:73282883:- | −0.44 | −0.54 |
| AKR1B1 |
chr7:134132813-134132732:-@chr7:134132133-134132050:- | −0.59 | −0.53 |
| NA |
chr3:148750159-148749988:-@chr3:148749150-148747904:- | −0.47 | −0.53 |
| WDR67 |
chr8:124153001:124153141:+@chr8:124154502:124154696:+@chr8:124156957:124157095:+ | −0.57 | −0.50 |
| AKR1B1 |
chr7:134132813:134132213|134132732:-@chr7:134132050:134132133:- | −0.58 | −0.49 |
| PLK3 |
chr1:45270011-45270173:+@chr1:45270322-45270451:+ | −0.35 | −0.47 |
| NA |
chr13:99852679:99853193:+@chr13:99883688:99883767:+@chr13:99890681:99890808:+ | −0.29 | −0.45 |
|
RABGAP1L |
chr1:174780970:174781098:+@chr1:174846530:174846743:+@chr1:174926594:174926686:+ | −0.44 | −0.44 |
| GBA2 |
chr9:35737936-35737745:-@chr9:35737601-35736864:- | −0.36 | −0.43 |
| AP1G2 |
chr14:24031497:24031624:-@chr14:24031171:24031275:-@chr14:24030721:24030844:- | −0.31 | −0.43 |
| IFFO1 |
chr12:6658922:6659062:-@chr12:6658642:6658650:-@chr12:6657834:6657991:- | −0.56 | −0.42 |
| HYAL2 |
chr3:50359204:50358797|50358999:-@chr3:50357000:50357966:- | −0.47 | −0.42 |
| PQLC3 |
chr2:11312051:11312171:+@chr2:11315094:11315135:+@chr2:11317863:11318998:+ | −0.31 | −0.42 |
| APEX1 |
chr14:20923385-20923487:+@chr14:20923737-20923862:+ | −0.46 | −0.39 |
| CKB |
chr14:103987600:103987771:-@chr14:103986839:103986929:-@chr14:103986459:103986648:- | −0.47 | −0.37 |
| RASSF7 |
chr11:560971:561033:+@chr11:561762:561892:+@chr11:562079:562776:+ | −0.42 | −0.37 |
| UGP2 |
chr2:64068098:64068366:+@chr2:64069193:64069338:+@chr2:64069673:64069733:
+@chr2:64083440:64083567:+ | −0.32 | −0.37 |
| UGP2 |
chr2:64068098:64068366:+@chr2:64069193:64069338:+@chr2:64082018:64082070:
+@chr2:64083440:64083567:+ | −0.38 | −0.36 |
| SPATA20 |
chr17:48624429:48624646:+@chr17:48625081:48625128:+@chr17:48625644:48625814:+ | −0.29 | −0.36 |
| UEVLD |
chr11:18557953:18558016:-@chr11:18555877:18556000:-@chr11:18552950:18554034:- | −0.41 | −0.35 |
| MX1 |
chr21:42797978:42798190:+@chr21:42799133:42799231:+@chr21:42799682:42799793:+ | −0.32 | −0.34 |
| SPHK1 |
chr17:74381532-74381735:+@chr17:74382024-74382218:+ | −0.32 | −0.34 |
| NIPBL |
chr5:37063892-37064121:+@chr5:37064629-37065921:+ | −0.38 | −0.33 |
| DYSF |
chr2:71738937:71739051:+@chr2:71740370:71740462:+@chr2:71740846:71741051:+ | −0.38 | −0.33 |
| PSTPIP1 |
chr15:77327849:77327904:+@chr15:77328143|77328152:77328276:+ | −0.31 | −0.33 |
| PXN |
chr12:120703420:120703574:-@chr12:120664642:120664920:-@chr12:120662446:120662530:-
@chr12:120661954:120662180:- | −0.30 | −0.33 |
| ZSWIM7 |
chr17:15881477-15881358:-@chr17:15881227-15881047:- | −0.25 | −0.33 |
| FAM21C |
chr10:46252460:46252587:+@chr10:46254763:46254849:+@chr10:46258836:46258937:+ | −0.24 | −0.33 |
| STXBP2 |
chr19:7707869:7707934:+@chr19:7708051:7708131:+@chr19:7709500:7709638:+ | −0.25 | −0.32 |
| NA |
chrX:30742218:30742298:+@chrX:30745583:30745669:+@chrX:30746849:30749577:+ | −0.42 | −0.31 |
| CEP350 |
chr1:180080132-180080330:+@chr1:180081572-180082515:+ | −0.35 | −0.31 |
| PCNP |
chr3:101293042:101293123:+@chr3:101298634|101298686:101298848:+ | −0.25 | −0.31 |
| ZSWIM7 |
chr17:15881477-15881358:-@chr17:15881227-15879875:- | −0.24 | −0.30 |
| DGUOK |
chr2:74173846:74174033:+@chr2:74177712:74177859:+@chr2:74185273:74185372:+ | −0.33 | −0.29 |
| NA |
chr12:9845424:9845527:+@chr12:9845630:9845711:+@chr12:9846421:9846544:+@chr12:9847356:9852151:+ | −0.50 | −0.28 |
| CKB |
chr14:103987600:103987771:-@chr14:103986806:103986929:-@chr14:103986459:103986648:- | −0.28 | −0.28 |
| C9,
DAB2 |
chr5:39388901:39388954:-@chr5:39388407:39388469:-@chr5:39382720:39383373:-@chr5:39381556:39381718:- | −0.21 | −0.28 |
| TBRG1 |
chr11:124495567:124495799:+@chr11:124496369:124496505:+@chr11:124496800:124496946:+ | −0.27 | −0.27 |
| NA |
chr12:9845424:9845527:+@chr12:9846421:9846544:+@chr12:9847356:9852151:+ | −0.45 | −0.26 |
| TNIP1 |
chr5:150460441:150460645:-@chr5:150444521:150444688:-@chr5:150443174:150443308:- | −0.31 | −0.26 |
| PARVG |
chr22:44568836:44569071:+@chr22:44577622:44577797:+@chr22:44579198:44579288:+ | −0.28 | −0.26 |
| NIN |
chr14:51226575:51227077:-@chr14:51223210:51225348:-@chr14:51221460:51221585:- | −0.22 | −0.26 |
| NA |
chr6:30181082:30181271:-@chr6:30172433:30172542:-@chr6:30168812:30168915:- | −0.25 | −0.25 |
| VPS33B |
chr15:91565384:91565833:-@chr15:91561035:91561115:-@chr15:91557614:91557663:- | −0.37 | −0.24 |
| HYAL2 |
chr3:50360084:50360281:-@chr3:50358797:50359204:-@chr3:50357000:50357966:- | −0.35 | −0.24 |
| YIPF1 |
chr1:54325729:54325826:-@chr1:54320674:54320724:-@chr1:54317392:54317943:- | −0.20 | −0.24 |
| UBE2D3 |
chr4:103748749:103749105:-@chr4:103747793|103748001:103747642:- | −0.26 | −0.23 |
|
PPP1R12A |
chr12:80201006:80201110:-@chr12:80199946:80200113:-@chr12:80199372:80199548:
-@chr12:80192274:80192364:- | −0.22 | −0.22 |
| TANK |
chr2:162016855:162016995|162016997:+@chr2:162036125:162036272:+ | −0.24 | −0.21 |
| NA |
chr12:15747871-15747991:+@chr12:15749024-15751265:+ | −0.23 | −0.21 |
|
C17orf76-AS1 |
chr17:16342641-16342728:+@chr17:16342895-16343567:+ | −0.23 | −0.21 |
| CPNE1,
NFS1 |
chr20:34252682:34252878:-@chr20:34246852:34246936:-@chr20:34220717:34220845:- | −0.30 | −0.20 |
| NA |
chr12:9845424:9845527|9846544:+@chr12:9847356:9852151:+ | −0.26 | −0.20 |
| TNIP1 |
chr5:150460441:150460645:-@chr5:150444521:150444692:-@chr5:150443174:150443308:- | −0.25 | −0.20 |
| CNN2 |
chr19:1032558:1032695:+@chr19:1036066:1036245:+@chr19:1036415:1036561:+ | −0.21 | −0.20 |
|
TNFRSF12A |
chr16:3070313:3070492:+@chr16:3071216:3071320:+@chr16:3071556:3071690:+ | −0.20 | −0.20 |
| CD44 |
chr11:35211382:35211612:+@chr11:35219668:35219793:+@chr11:35232793:35232996:
+@chr11:35236399:35236461:+ | 0.20 | 0.20 |
| GK |
chrX:30671476:30671732:+@chrX:30683628:30683701:+@chrX:30695492:30695569:+ | 0.40 | 0.20 |
| UNC45A |
chr15:91478515:91478560|91478605:+@chr15:91478774:91478935:+ | 0.20 | 0.21 |
| 43166 |
chr2:160615737:160615846|160615869:+@chr2:160619391:160619504:+ | 0.21 | 0.21 |
| TBC1D15 |
chr12:72287002:72287104:+@chr12:72288105:72288155:+@chr12:72288466:72288663:+ | 0.22 | 0.21 |
| CD44 |
chr11:35211382:35211612:+@chr11:35226059:35226187:+@chr11:35227659:35227790:+ | 0.23 | 0.21 |
| APLP2 |
chr11:129992200:129992408:+@chr11:129993507:129993674:+@chr11:129996595:129996725:+ | 0.20 | 0.22 |
| NUDT22 |
chr11:63993762-63993899:+@chr11:63994107-63994604:+ | 0.23 | 0.22 |
| DCAF6 |
chr1:167973771:167974031:+@chr1:167992226:167992285:+@chr1:168007609:168007726:+ | 0.24 | 0.22 |
| CBFB |
chr16:67116116:67116211|67116242:+@chr16:67132613:67134958:+ | 0.20 | 0.23 |
| CD44 |
chr11:35211382:35211612:+@chr11:35229652:35229756:+@chr11:35232793:35232996:+@chr11:
35236399:35236461:+ | 0.21 | 0.23 |
| DPP7 |
chr9:140009170-140009099:-@chr9:140009028-140008915:- | 0.28 | 0.23 |
| ARGLU1 |
chr13:107212005-107211780:-@chr13:107209479-107209396:- | 0.32 | 0.23 |
| BAG6 |
chr6:31620477:31620177|31620201:-@chr6:31619433:31619553:- | 0.27 | 0.24 |
| PLD1 |
chr3:171405161:171405374:-@chr3:171404475:171404588:-@chr3:171395356:171395484:- | 0.36 | 0.24 |
| RFFL,
RAD51L3-RFFL |
chr17:33353393:33353580:-@chr17:33348390:33348800:-@chr17:33344542:33344625:- | 0.37 | 0.24 |
| NCOR2 |
chr12:124812179:124811955|124812093:-@chr12:124810737:124810916:- | 0.25 | 0.25 |
| CELF2 |
chr10:11370889-11371060:+@chr10:11372419-11374591:+ | 0.32 | 0.26 |
| CELF2 |
chr10:11370889-11371060:+@chr10:11372419-11374605:+ | 0.32 | 0.26 |
| CD44 |
chr11:35211382:35211612:+@chr11:35231512:35231601:+@chr11:35232793:35232996:
+@chr11:35236399:35236461:+ | 0.26 | 0.27 |
| CELF2 |
chr10:11370889-11371060:+@chr10:11372419-11376357:+ | 0.34 | 0.27 |
|
RP11-458D21.5 |
chr1:145281369:145281704:+@chr1:145290429:145290511:+@chr1:145293114:145293272:
+@chr1:145293371:145293580:+ | 0.34 | 0.27 |
| RNASE1 |
chr14:21270956:21271036:-@chr14:21270408:21270478:-@chr14:21269515:21270252:- | 0.27 | 0.28 |
| TMX2-CTNND1,
RP11-691N7.6,CTNND1 |
chr11:57529234:57529518|57529591:+@chr11:57556509:57556627:+ | 0.24 | 0.29 |
| CD44 |
chr11:35211382:35211612:+@chr11:35219695:35219793:+@chr11:35232793:35232996:
+@chr11:35236399:35236461:+ | 0.28 | 0.29 |
| PGAP2 |
chr11:3832480:3832654:+@chr11:3838662:3838765:+@chr11:3845113:3845365:+ | 0.31 | 0.29 |
| SRRM1 |
chr1:24989151:24989295:+@chr1:24989674:24989715:+@chr1:24993306:24993416:+ | 0.34 | 0.29 |
| NSUN4 |
chr1:46810473:46810816:+@chr1:46812593:46812747:+@chr1:46818540:46818700:+ | 0.33 | 0.30 |
| CHFR |
chr12:133447310:133447369:-@chr12:133446205:133446384:-@chr12:133438053:133438220:- | 0.51 | 0.30 |
| SNX17 |
chr2:27593389:27593673:+@chr2:27594136:27594174:+@chr2:27595490:27595607:+ | 0.23 | 0.31 |
| ITGA6 |
chr2:173362703:173362828:+@chr2:173366500:173366629:+@chr2:173368819:173371181:+ | 0.26 | 0.32 |
| CHFR |
chr12:133447310:133447369:-@chr12:133446205:133446420:-@chr12:133438053:133438220:- | 0.46 | 0.32 |
| NA |
chr14:20923290:20923497|20923548:+@chr14:20923737:20923862:+ | 0.35 | 0.33 |
| PNPLA8 |
chr7:108166473:108166762:-@chr7:108161920:108161965:-@chr7:108154880:108156018:- | 0.4 | 0.34 |
| GOLIM4 |
chr3:167759180:167759262:-@chr3:167758574:167758657:-@chr3:167754624:167754782:- | 0.29 | 0.35 |
| LILRA2,
LILRB1 |
chr19:55087274:55087576:+@chr19:55088932:55089190:+@chr19:55098477:55098527:
+@chr19:55098668:55099027:+ | 0.31 | 0.35 |
| FLNB |
chr3:58124009:58124256:+@chr3:58127585:58127656:+@chr3:58128377:58128479:+ | 0.41 | 0.36 |
| CD44 |
chr11:35211382:35211612:+@chr11:35219695:35219793:+@chr11:35226059:35226187:+
@chr11:35229652:35229753:+ | 0.47 | 0.36 |
| STX2 |
chr12:131283070:131283180:-@chr12:131280540:131280665:-@chr12:131274145:131276522:- | 0.29 | 0.38 |
| POLD1 |
chr19:50910584:50910672:+@chr19:50911964|50912042:50912158:+ | 0.37 | 0.38 |
| NA |
chr13:99852679:99853193:+@chr13:99853683:99853821:+@chr13:99883688:99883767:
+@chr13:99890681:99890808:+ | 0.46 | 0.41 |
| AFTPH |
chr2:64800080:64800202:+@chr2:64806620:64806680:+@chr2:64812556:64812679:+ | 0.52 | 0.41 |
| NA |
chr1:120930039:120930293:-@chr1:120928331:120928615:-@chr1:120926128:120927417:- | 0.50 | 0.43 |
| AC069513.3,
SDHAP2 |
chr3:195389442:195389604:+@chr3:195390495:195390703:+@chr3:195390957:195391121:+ | 0.34 | 0.44 |
| ELMOD3 |
chr2:85617261-85617388:+@chr2:85617883-85618875:+ | 0.43 | 0.44 |
| ELMOD3 |
chr2:85617261-85617388:+@chr2:85617883-85618871:+ | 0.43 | 0.45 |
| FBXO38 |
chr5:147805085:147805264:+@chr5:147806776:147807285:+@chr5:147812987:147813087:+ | 0.31 | 0.46 |
| FBXO38 |
chr5:147805085:147805264:+@chr5:147806776:147807510:+@chr5:147812987:147813087:+ | 0.32 | 0.48 |
|
AC024560.3 |
chr3:197350091:197350253:-@chr3:197349058:197349201:-@chr3:197348575:197348739:- | 0.53 | 0.50 |
| NBPF14 |
chr1:148011680:148011788:-@chr1:148010884:148011056:-@chr1:148009344:148009516:
-@chr1:148008573:148008624:- | 0.20 | 0.51 |
| NQO2 |
chr6:3000067:3000319:+@chr6:3002284:3002520:+@chr6:3003903:3004023:+ | 0.61 | 0.54 |
| AFTPH |
chr2:64800080:64800202:+@chr2:64806620:64808407:+@chr2:64812556:64812679:+ | 0.57 | 0.55 |
| IFT88 |
chr13:21141208:21141395:+@chr13:21141809:21142136:+@chr13:21148519:21148614:+ | 0.58 | 0.59 |
| NQO2 |
chr6:3000067:3000319:+@chr6:3003903:3004023:+@chr6:3004729:3004885:+@chr6:3006702:3006793:+ | 0.61 | 0.61 |
| HPCAL1 |
chr2:10536961:10537046:+@chr2:10546391:10546487:+@chr2:10548688:10548860:
+@chr2:10559860:10560261:+ | 0.44 | 0.62 |
|
SLC25A19 |
chr17:73284672:73284469|73284583:-@chr17:73282714:73282883:- | 0.48 | 0.62 |
| PDE1B |
chr12:54964025-54964141:+@chr12:54966385-54966525:+ | 0.63 | 0.63 |
|
TRIM39-RPP21 |
chr6:30312906:30313005:+@chr6:30313075:30313175:+@chr6:30313268:30313350:
+@chr6:30314209:30314334:+ | 0.45 | 0.67 |
| PTGS1 |
chr9:125133465:125133551:+@chr9:125133892:125134113:+@chr9:125140178:125140294:+ | 0.77 | 0.73 |
| NA |
chr17:26646121:26646391:+@chr17:26652529:26652673:+@chr17:26653560:26655711:+ | 0.61 | 0.74 |
 | Table IV.Altered AS events in macrophages
co-cultured with T47D cells. |
Table IV.
Altered AS events in macrophages
co-cultured with T47D cells.
| Gene | Event | ΔPSI
(SRR2922612_vs_SRR2922610) | ΔPSI
(SRR2922612_vs_SRR2922614) |
|---|
| IL6 |
chr7:22766766:22766900:+@chr7:22767132:22767253:+@chr7:22768312:22768425:+ | −0.68 | −0.55 |
| CKB |
chr14:103987600:103987771:-@chr14:103986839:103986929:-@chr14:103986459:103986648:- | −0.5 | −0.55 |
| SUCO |
chr1:172520652:172520766:+@chr1:172522400:172522510:+@chr1:172525009:172525163:+ | −0.51 | −0.52 |
| SEP3 |
chr22:42390625-42390718:+@chr22:42392906-42394225:+ | −0.47 | −0.49 |
| ANKRD49 |
chr11:94229770:94229994|94230243:+@chr11:94231237:94232744:+ | −0.37 | −0.47 |
| NA |
chr14:70883617:70883807:-@chr14:70855187:70855323:-@chr14:70842393:70842488:- | −0.46 | −0.46 |
| IQSEC2 |
chrX:53310678:53310796:-@chrX:53308746:53308841:-@chrX:53295506:53296246:- | −0.46 | −0.46 |
| SP110 |
chr2:231084589:231084827:-@chr2:231081496:231081536:-@chr2:231079665:231079833:- | −0.51 | −0.44 |
| ANXA2 |
chr15:60688350:60688626:-@chr15:60685237:60685639:-@chr15:60678227:60678285:- | −0.46 | −0.44 |
| DCTD |
chr4:183837692:183837439|183837572:-@chr4:183836614:183836728:- | −0.35 | −0.44 |
| MGA |
chr15:42032251:42032401:+@chr15:42034744:42035370:+@chr15:42040835:42041125:+ | −0.44 | −0.43 |
|
C17orf76-AS1 |
chr17:16342641-16342728:+@chr17:16342895-16343567:+ | −0.4 | −0.43 |
| KIAA0226L,
PPP1R2P4 |
chr13:46961269:46961635:-@chr13:46952025:46952140:-@chr13:46946076:46946732:- | −0.36 | −0.43 |
| HNRNPC |
chr14:21737457:21737638:-@chr14:21731495|21731741:21731470:- | −0.36 | −0.43 |
| EXOC7 |
chr17:74087224:74087316:-@chr17:74086410:74086562:-@chr17:74085256:74085401:- | −0.36 | −0.42 |
| NA |
chr2:3605976:3606588:+@chr2:3606994|3607038:3607319:+ | −0.48 | −0.41 |
| ARIH2 |
chr3:48956254:48956431:+@chr3:48960181:48960244:+@chr3:48962151:48962272:+ | −0.31 | −0.41 |
| NA |
chr12:15747871-15747991:+@chr12:15749024-15751265:+ | −0.26 | −0.41 |
| RPS15A,
RP11-1035H13.3 |
chr16:18801656:18801566|18801632:-@chr16:18800303:18800440:- | −0.47 | −0.4 |
| RP11-691N7.6,
CTNND1 |
chr11:57529234:57529518:+@chr11:57556509:57556627:+@chr11:57558857:57559145:+ | −0.47 | −0.39 |
| UGP2 |
chr2:64068098:64068366:+@chr2:64069193:64069338:+@chr2:64069673:64069733:
+@chr2:64083440:64083567:+ | −0.24 | −0.39 |
| EXOC7 |
chr17:74087224:74087316:-@chr17:74086410:74086478:-@chr17:74085256:74085401:- | −0.35 | −0.36 |
| SLC41A2 |
chr12:105352071:105351866|105351968:-@chr12:105321875:105322472:- | −0.46 | −0.34 |
| NA |
chrX:30742218:30742298:+@chrX:30745583:30745669:+@chrX:30746849:30749577:+ | −0.4 | −0.34 |
| NHLRC3 |
chr13:39613701:39613848:+@chr13:39616242:39616442:+@chr13:39618227:39618318:+ | −0.35 | −0.34 |
| IL6 |
chr7:22766766:22766900:+@chr7:22767063:22767253:+@chr7:22768312:22768425:+ | −0.34 | −0.34 |
| ST7L |
chr1:113162266:113162405:-@chr1:113161531:113161761:-@chr1:113159435:113159517:
-@chr1:113153463:113153625:- | −0.5 | −0.33 |
| UGP2 |
chr2:64068098:64068366:+@chr2:64069193:64069338:+@chr2:64082018:64082070:
+@chr2:64083440:64083567:+ | −0.22 | −0.33 |
|
RABGAP1L |
chr1:174780970:174781098:+@chr1:174846530:174846743:+@chr1:174926594:174926686:+ | −0.46 | −0.32 |
| WASH4P |
chr16:67427-67291:-@chr16:67051-66916:- | −0.27 | −0.32 |
| ZCCHC6 |
chr9:88916191:88916516:-@chr9:88913682:88913745:-@chr9:88902648:88903659:- | −0.34 | −0.31 |
| USP8 |
chr15:50773678:50774262:+@chr15:50776472:50776558:+@chr15:50781998:50782078:+ | −0.31 | −0.31 |
| NUBP2 |
chr16:1836538:1836656|1836956:+@chr16:1837678:1837832:+ | −0.27 | −0.31 |
| TMEM19 |
chr12:72094612-72094805:+@chr12:72097757-72097836:+ | −0.36 | −0.3 |
| HNRNPH1 |
chr5:179047893:179048036:-@chr5:179046270:179046361:-@chr5:179045146:179045324:- | −0.42 | −0.29 |
| SBF1 |
chr22:50897684:50897821:-@chr22:50895463:50895540:-@chr22:50894921:50895102:- | −0.35 | −0.29 |
| BCL2L13 |
chr22:18171752:18171908:+@chr22:18178907:18178976:+@chr22:18185009:18185152:+ | −0.32 | −0.29 |
| YWHAZ |
chr8:101965078:101965221:-@chr8:101964157:101964353:-@chr8:101960824:101961128:- | −0.3 | −0.29 |
| TCOF1 |
chr5:149769450:149769586:+@chr5:149771107:149771220:+@chr5:149771520:149771739:+ | −0.25 | −0.29 |
| CHTOP |
chr1:153610771:153610924:+@chr1:153614719:153614905:+@chr1:153617540:153618782:+ | −0.34 | −0.28 |
| GIT2 |
chr12:110385061:110385309:-@chr12:110383065:110383154:-@chr12:110376970:110377052:- | −0.32 | −0.28 |
| PCM1 |
chr8:17838100:17838264:+@chr8:17840742:17840798:+@chr8:17842956:17843128:+ | −0.27 | −0.28 |
| SIGLEC11,
NUP62 |
chr19:50432583:50432988:-@chr19:50431072|50431105:50430951:- | −0.21 | −0.28 |
| ANKRD12,
RP11-21J18.1 |
chr18:9195549:9195696:+@chr18:9204474:9204542:+@chr18:9208655:9208801:+ | −0.28 | −0.27 |
| AC092755.4,
BNIP2 |
chr15:59961091:59961189:-@chr15:59960302:59960337:-@chr15:59955062:59956319:- | −0.25 | −0.27 |
| CNN2 |
chr19:1032558:1032695:+@chr19:1036066:1036245:+@chr19:1036415:1036561:+ | −0.25 | −0.27 |
| NSUN4 |
chr1:46805849:46806591:+@chr1:46806995:46807169:+@chr1:46810473:46810816:+ | −0.25 | −0.27 |
| NUBP2 |
chr16:1836538:1836656:+@chr16:1836739:1836956:+@chr16:1837678:1837832:+ | −0.25 | −0.27 |
| SRPK2 |
chr7:104767440:104767509:-@chr7:104766695:104766787:-@chr7:104766229:104766321:
-@chr7:104756823:104758469:- | −0.42 | −0.26 |
| ITGAM |
chr16:31308835:31308975:+@chr16:31309063|31309066:31309275:+ | −0.33 | −0.26 |
| OSBPL3 |
chr7:24874355:24873706|24874105:-@chr7:24870387:24870524:- | −0.32 | −0.26 |
| YWHAZ |
chr8:101965078:101965221:-@chr8:101964157:101964536:-@chr8:101960824:101961128:- | −0.26 | −0.26 |
|
C17orf76-AS1 |
chr17:16342641-16342728:+@chr17:16342895-16343017:+ | −0.2 | −0.26 |
| PXN |
chr12:120703420:120703574:-@chr12:120664642:120664920:-@chr12:120662446:120662530:
-@chr12:120661954:120662180:- | −0.28 | −0.25 |
| NA |
chr22:50964675:50964905:-@chr22:50964430:50964585:-@chr22:50961997:50962853:- | −0.53 | −0.24 |
| ANXA2 |
chr15:60690142:60690185:-@chr15:60688350:60688626:-@chr15:60685237:60685639:
-@chr15:60678227:60678285:- | −0.27 | −0.24 |
| DFFA |
chr1:10523266-10523115:-@chr1:10521759-10520603:- | −0.23 | −0.24 |
| NA |
chr1:171673674-171673527:-@chr1:171671224-171669296:- | −0.29 | −0.22 |
| CKB |
chr14:103987600:103987771:-@chr14:103986806:103986929:-@chr14:103986459:103986648:- | −0.22 | −0.22 |
| MAPK7 |
chr17:19283921-19284999:+@chr17:19285094-19285779:+ | −0.22 | −0.22 |
| SLC11A2 |
chr12:51375586-51375422:-@chr12:51373841-51373566:- | −0.39 | −0.21 |
| MPZL1 |
chr1:167742473:167742605:+@chr1:167745301:167745403:+@chr1:167757057:167761156:+ | −0.25 | −0.21 |
| ABI1 |
chr10:27054147:27054247:-@chr10:27047991:27048164:-@chr10:27040527:27040712:- | −0.33 | −0.2 |
| ABI1 |
chr10:27054147:27054247:-@chr10:27047991:27048167:-@chr10:27040527:27040712:- | −0.32 | −0.2 |
| NT5C2 |
chr10:104899163:104899236:-@chr10:104871502:104871562:-@chr10:104866346:104866463:- | 0.22 | 0.2 |
| ILK |
chr11:6624964:6625052:+@chr11:6625410|6625456:6625590:+ | 0.29 | 0.2 |
| SRRT |
chr7:100479674:100479862:+@chr7:100480386:100480711:+@chr7:100481691:100481860:+ | 0.24 | 0.21 |
| MFSD10 |
chr4:2933880-2933772:-@chr4:2933663-2933552:- | 0.2 | 0.22 |
| ACTR6 |
chr12:100594555:100594697|100594838:+@chr12:100598718:100598835:+ | 0.24 | 0.22 |
| NCOR1 |
chr17:16118676:16118874:-@chr17:16101915:16102033:-@chr17:16097776:16097953:- | 0.33 | 0.22 |
| TXNRD1 |
chr12:104680460:104680887:+@chr12:104682711:104682818:+@chr12:104705068:104705190:
+@chr12:104707023:104707095:+ | 0.39 | 0.22 |
| LILRA2,
LILRB1 |
chr19:55087274:55087576:+@chr19:55088932:55089190:+@chr19:55098477:55098527:
+@chr19:55098668:55099027:+ | 0.21 | 0.23 |
| RPL17,
RPL17-C18orf32 |
chr18:47018628:47018834:-@chr18:47017954|47018203:47017902:- | 0.29 | 0.23 |
| TXNRD1 |
chr12:104680727:104680887|104681124:+@chr12:104682709:104682818:+ | 0.32 | 0.23 |
| NA |
chr1:44679125-44679199:+@chr1:44679381-44679530:+ | 0.22 | 0.24 |
| MSRB1 |
chr16:1991406:1990779|1991258:-@chr16:1988234:1989144:- | 0.31 | 0.24 |
| WARS |
chr14:100842597:100842680:-@chr14:100841620:100841883:-@chr14:100828045:100828258:- | 0.32 | 0.24 |
| NA |
chr10:105156166:105156270:-@chr10:105155503:105155789:-@chr10:105153956:105154151:
-@chr10:105152128:105152223:- | −0.37 | 0.25 |
| IRF7 |
chr11:615647-615345:-@chr11:615259-615097:- | 0.33 | 0.25 |
| RREB1 |
chr6:7229230:7232140:+@chr6:7240671:7240835:+@chr6:7246657:7247454:+ | 0.43 | 0.25 |
| DCAF6 |
chr1:167973771:167974031:+@chr1:167992226:167992285:+@chr1:168007609:168007726:+ | 0.23 | 0.26 |
| PXN |
chr12:120663712:120663872:-@chr12:120662446:120662530:-@chr12:120661954:120662180:- | 0.39 | 0.26 |
| SRSF7 |
chr2:38976671:38976847:-@chr2:38976040:38976488:-@chr2:38975721:38975795:- | 0.37 | 0.27 |
| MLL3 |
chr7:151855948:151856157:-@chr7:151854846:151855010:-@chr7:151853290:151853431:- | 0.45 | 0.27 |
| WARS |
chr14:100842597:100842680:-@chr14:100841620:100841883:-@chr14:100835424:100835595:- | 0.24 | 0.28 |
| FBXO38 |
chr5:147805085:147805264:+@chr5:147806776:147807510:+@chr5:147812987:147813087:+ | 0.26 | 0.28 |
| NT5C3 |
chr7:33102180:33102409:-@chr7:33075546:33075600:-@chr7:33066429:33066527:- | 0.31 | 0.28 |
| NCOR2 |
chr12:124812179:124811955|124812093:-@chr12:124810737:124810916:- | 0.37 | 0.28 |
| NA |
chr6:30181082:30181271:-@chr6:30168812:30168915:-@chr6:30166443:30166930:- | 0.3 | 0.31 |
| SRSF6 |
chr20:42087001:42087149:+@chr20:42087793:42088060:+@chr20:42088411:42088535:+ | 0.33 | 0.31 |
| C10orf2 |
chr10:102750626:102750767|102750811:+@chr10:102752947:102754158:+ | 0.27 | 0.32 |
| DYRK1B |
chr19:40317925:40318065:-@chr19:40317507|40317627:40317312:- | 0.38 | 0.32 |
| HNRNPH1 |
chr5:179050670:179050596|179050637:-@chr5:179050038:179050165:- | 0.38 | 0.33 |
| ANKZF1 |
chr2:220098855-220099010:+@chr2:220099548-220100034:+ | 0.36 | 0.34 |
| ARIH2 |
chr3:48956254:48956431:+@chr3:48960181:48960244:+@chr3:48962151:48962272:
+@chr3:48982569:48982614:+ | 0.3 | 0.36 |
| CAMTA2 |
chr17:4886052:4886187:-@chr17:4885384:4885522:-@chr17:4884973:4885126:- | 0.31 | 0.36 |
| MRRF |
chr9:125027741:125027824|125028059:+@chr9:125033143:125033354:+ | 0.31 | 0.36 |
| SETMAR |
chr3:4344988:4345210:+@chr3:4354582:4354913:+@chr3:4355331:4355445:+ | 0.42 | 0.36 |
| RREB1 |
chr6:7229230:7232140:+@chr6:7240671:7240835:+@chr6:7248744:7252213:+ | 0.6 | 0.36 |
| NA |
chr1:207095163:207095378:-@chr1:207087104:207087439:-@chr1:207086274:207086387:- | 0.43 | 0.39 |
|
RP11-315D16.2 |
chr15:68497583:68498448:-@chr15:68491879:68492019:-@chr15:68489778:68489966:- | 0.48 | 0.39 |
| NA |
chr18:32820994:32821073:+@chr18:32822355:32822848:+@chr18:32823116:32823257:+ | 0.5 | 0.4 |
| FOPNL |
chr16:15982415:15982447:-@chr16:15977865:15978062:-@chr16:15973661:15973745:
-@chr16:15967348:15967484:- | 0.38 | 0.41 |
| AAMDC |
chr11:77532208:77532287:+@chr11:77552065:77552106:+@chr11:77553525:77553674:+ | 0.47 | 0.41 |
| NA |
chrX:1718085:1718325:+@chrX:1719031:1719100:+@chrX:1719552:1721411:+ | 0.48 | 0.41 |
| TCTN1 |
chr12:111078188:111078322:+@chr12:111078829:111078954:+@chr12:111082772:111082934:
+@chr12:111085016:111085141:+ | 0.42 | 0.44 |
| MON1A |
chr3:49950653:49950792:-@chr3:49948959:49949444:-@chr3:49947552:49948317:- | 0.47 | 0.44 |
| TMBIM6 |
chr12:50135340:50135394:+@chr12:50135740:50135898:+@chr12:50138196:50138325:
+@chr12:50146247:50146332:+ | 0.43 | 0.45 |
| NA |
chr5:80597402:80597489|80597517:+@chr5:80604380:80604530:+ | 0.48 | 0.45 |
| TM2D1 |
chr1:62160369:62160442:-@chr1:62152464:62152593:-@chr1:62149089:62149218:- | 0.42 | 0.46 |
| NA |
chr22:18165980:18166087:+@chr22:18185009:18185152:+@chr22:18209443:18213621:+ | 0.25 | 0.47 |
| CHD8 |
chr14:21868572:21868771:-@chr14:21868466|21868490:21868310:- | 0.35 | 0.47 |
| NA |
chr22:18171752:18171908:+@chr22:18185009:18185152:+@chr22:18209443:18213621:+ | 0.23 | 0.48 |
| SLA |
chr8:134114644:134114786:-@chr8:134087098:134087375:-@chr8:134072345:134072445:
-@chr8:134063061:134063160:- | 0.51 | 0.62 |
| ARIH2 |
chr3:48956254:48956431:+@chr3:48962151:48962272:+@chr3:48964895:48965246:+ | 0.36 | 0.64 |
Similarly, GO functional enrichment analysis was
applied to investigate the biological processes affected by those
altered AS events. GO terms such as signal transduction,
cell-matrix adhesion, protein transport, brain development were
significantly enriched in the macrophages co-cultured with
MDA-MB-231 cells (Fig. 4D). Terms
such as RNA export from the nucleus, regulation of angiogenesis,
positive regulation of transcription, DNA-templated, mRNA export
from the nucleus, negative regulation of apoptotic process were
significantly enriched in the macrophages co-cultured with the T47D
cells (Fig. 4E). Briefly, protein
transport-related processes were altered by co-culturing
macrophages with the MDA-MB-231 cells and RNA processing biological
processes were affected by co-culturing macrophages with the T47D
cells.
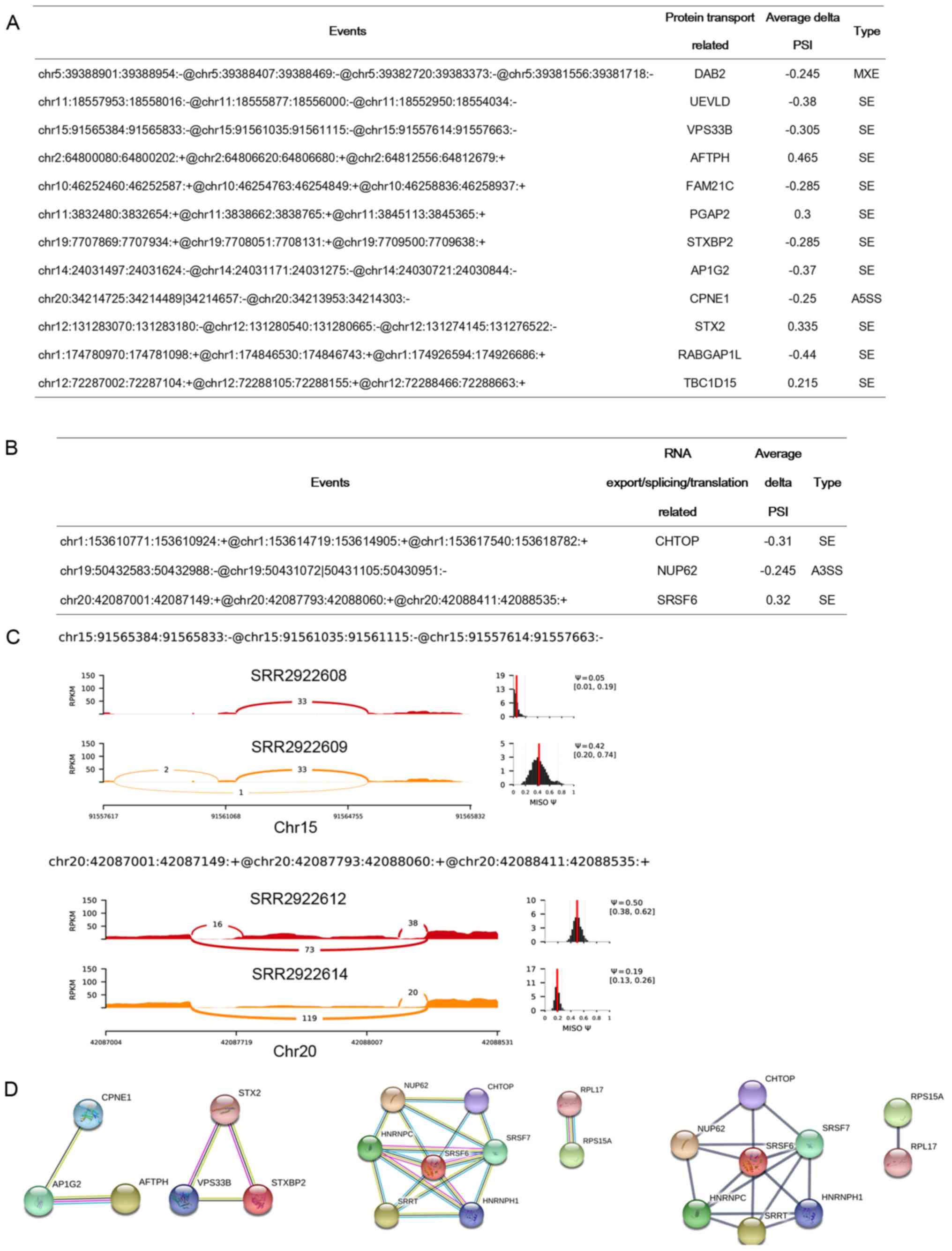
The RNA processing and protein transport-related AS
events derived from the macrophages co-cultured with the MDA-MB-231
(Fig. 5A) and T47D (Fig. 5B) cells were further analyzed.
Vacuolar protein sorting-associated protein 33B (VPS33B) is a Sec1
familiy protein involved in vesicle transport which mediates
protein sorting and plays an important role in segregation of
intracellular molecules into distinct organelles (41,42). As
known, the induced inflammatory responses by microbial ligands is
influenced by internalization of Toll-like receptors (TLRs), thus,
endosomal maturation in clearing receptors terminates inflammatory
responses. Akbar et al found that Vps33B proteins play
critical roles in the maturation of phagosomes and endosomes in
Drosophila and mammals and therefore affect the process of
maturation of macrophages (43).
Sashimi plot showed the AS event
‘chr15:91565384:91565833:-@chr15:91561035:91561115:-@chr15:91557614:91557663:-’
of gene VPS33B. The exon was largely excluded from the
macrophages co-cultured with MDA-MB-231 cells (Fig. 5C), which may influence the protein
function and further the maturation process of macrophages. The
protein encoded by SRSF6 belongs to the splicing factor SR
and may play a role in the determination of alternative splicing.
Boisson et al demonstrated that SRSF6 regulate the AS of the
IKBKG gene, which accounts for NF-κB activation and further
immune response activation (44).
Here, we showed that SRSF6 itself is regulated by AS.
Specifically, the event
‘chr20:42087001:42087149:+@chr20:42087793:42088060:+@chr20:42088411:42088535:+’
of SRSF6 was significantly included after coculturing macrophages
with T47D cells (Fig. 5C). PPI
analysis showed that protein transport-related proteins CPNE1,
AP1G2, AFTPH, STX2, VPS33B and STXBP2 were interacted intimately
(Fig. 5D). In the interaction network
of RNA processing proteins, SRSF6 has extensive interactions with
another 6 proteins and may serve as the hub protein in the network
(Fig. 5D). These results further
indicate that AS events containing genes including Vps33B
and SRSF6 may play important roles in the maturation
processes of macrophages.
Sequence feature characterization of
the altered skip exons
To investigate whether the altered AS events in
different treatments had distinct properties, we first measured the
degree of evolutionary conservation of skip exon sequences across
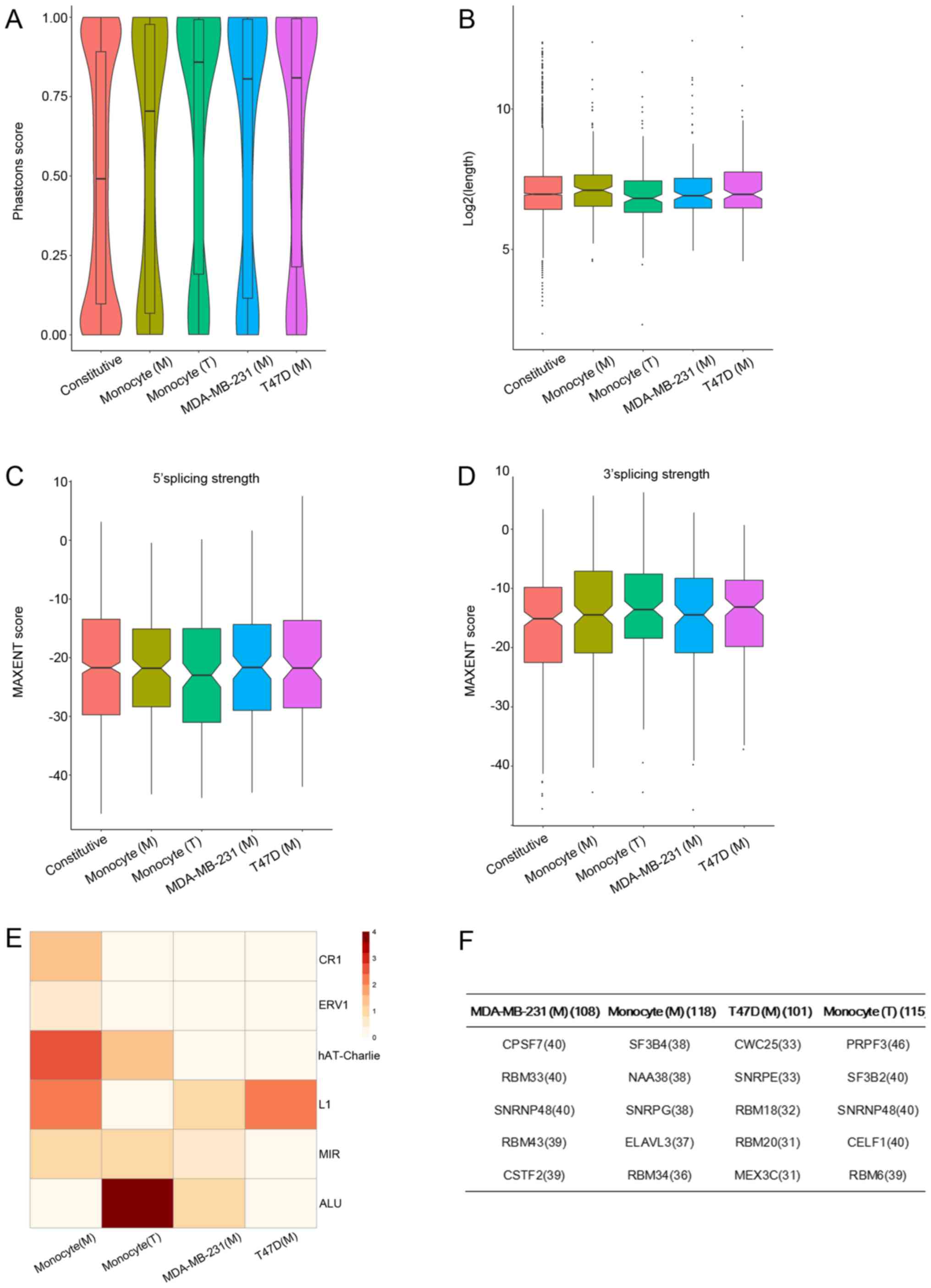
placental mammals. Unexpectedly, all the altered skip exons in
different treatments showed a higher degree of sequence
conservation equivalent compared to that of constitutive exons
(Fig. 6A). Altered skip exons in the
macrophages co-cultured with T47D cells showed highest evolutionary
conservation, whereas macrophages co-cultured with MDA-MB-231 cells
showed the lowest. There were no significant differences in skip
exon length among the different treatments (Wilcoxon signed-rank
test, P>0.05; Fig. 6B). Splicing
site strength analysis showed there were no significant differences
in splicing strength between constitutive skip and altered skip
exons and no significant differences among the different treatments
(Wilcoxon signed-rank test, P>0.05; Fig. 6C and D). Repetitive elements such as
Alu are known to be stochastically exonized (45), and we found Alu elements were more
enriched within skip exons in the macrophages cocultured with the
T47D cells, were fewer in skip exons of macrophages cocultured with
the MDA-MB-231 cells, and were almost absent from skip exons of
MDA-MB-231 co-cultured with macrophages and T47D co-cultured with
macrophages (Fisher's exact test; Fig.
6E). We conclude that altered skip exons of macrophages
cocultured with T47D cells were mostly enriched with Alu element
and had highest evolutionary conservation.
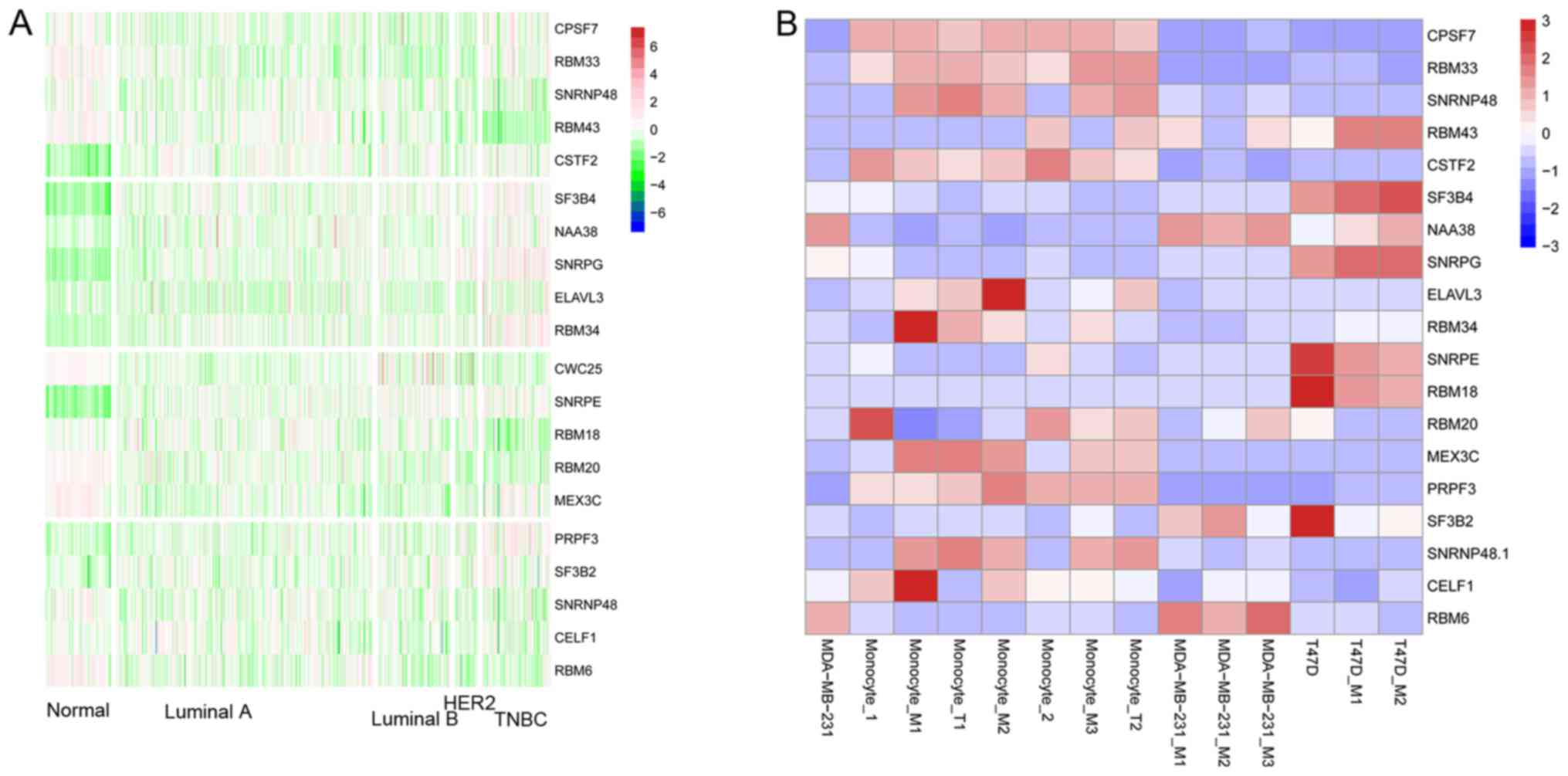
Finally, the splicing factors responsible for the AS
events in the different cells and treatments were determined. Here,
we calculated the associations between splicing factor expression
and the PSI values across the 14 samples. The top 5 splicing
factors which possessed the most associated splicing events in each
of the 4 cell conditions are shown in Fig. 6F. In the MDA-MB-231 cells co-cultured
with macrophages, CPSF7, RBM33, SNRNP48, RBM43 and
CSTF2 were the top five. Akman et al confirmed that
CSTF2 is a major regulator of 3′UTR shortening in TNBC and
contributes to the proliferation phenotype (46). In T47D cells co-cultured with
macrophages, CWC25, SNRPE, RBM18, RBM20 and MEX3C
were the top five. Meanwhile, in the macrophages co-cultured with
MDA-MB-231 cells, SF3B4, NAA38, SNRPG, ELAVL3 and
RBM34 were the top five and in the macrophages co-cultured
with T47D cells, PRPF3, SF3B2, SNRNP48, CELF1 and
RBM46 were the top five. Lin et al found that
knockdown of CELF1 in primary human macrophages led to
increased inflammatory response to M1 stimulation (47). Subsequently, the expression pattern of
candidate splicing factors among normal and 4 breast cancer
subtypes was explored. Generally, splicing factors responsible for
each condition showed similar expression pattern among the samples
(Fig. 7A). In addition, we
characterized the expression pattern of these splicing factors
among samples from the GSE75130 dataset. Splicing factors such as
CPSF7, RBM33, SNRNP48 responsible for the altered AS events
in MDA-MB-231 cells cocultured with macrophages showed a similar
expression pattern among the different treatments (Fig. 7B).
Discussion
In the present study, mutual editing of alternative
splicing (AS) between tumor cells and macrophage was investigated
by re-exploring a previous dataset. Importantly, it was found that
ER+ and triple-negative breast cancer (TNBC) were
differentially regulated from the AS events view when co-cultured
with macrophages. However, DNA repair and DNA damage processes were
enriched in both ER+ and TNBC after co-culturing with
macrophages. Meanwhile, macrophages were also differentially
regulated from the biological processes view by co-culturing with
ER+ and TNBC. These results revealed a new view of the
mutual regulation between tumor cells and macrophages, in which
biological pathways were dramatically different identified at the
expression level.
At the expression level, macrophages co-cultured
with MDA-MB-231 cells were enriched in the superpathway of
citrulline metabolism, cellular effects of sildenafil (Viagra),
GNRH signaling, citrulline biosynthesis, factors promoting
cardiogenesis in vertebrates and bladder cancer signaling. Whereas
at the AS level, RNA processing and translation-related processes
were enriched. In macrophages co-cultured with T47D cells,
biological pathways such as granulocyte adhesion and diapedesis,
inhibition of matrix metalloproteases, agranulocyte adhesion and
diapedesis, VDR/RXR activation were enriched at the transcription
level (21), whereas, biological
processes such as protein transport related pathways were enriched
at the AS level. In T47D cells co-cultured with macrophages, acute
phase response signaling, G-protein coupled receptor signaling,
LXR/RXR activation, hepatic fibrosis/hepatic stallate cell
activation were enriched at the transcriptional level (21), whereas DNA repair and DNA damage
processes were enriched at the AS level. In the MDA-MB-231 cells
co-cultured with macrophages, axonal guidance signaling, RhoGDI
signaling, relaxin signaling, CCR3 signaling in eosinophil pathways
were enriched at the transcriptional level (21), whereas also DNA repair and DNA damage
related pathways were enriched at the AS level. Here, we see that
the biological pathways affected at the transcription level and
alternative splicing level were very different which suggested that
AS may provide a new view on the interplay regulation between tumor
cells and macrophages.
Another interesting phenomenon was that although the
altered AS events from ER+ breast cancer and TNBC cells
co-cultured with macrophages were not overlapping, the biological
pathways such as DNA repair and DNA damage was enriched in both
cell line treatments. It has been established that macrophage cells
exert their DNA repair functions to normal cells. For example,
double-strand breaks (DSBs) activate the cytosolic DNA sensor
stimulator of interferon (IFN) genes, thus inducing the production
of IFNα and IFNβ, which promote innate immune responses including
macrophages (21). In addition, human
monocytes, by releasing macrophage-derived heparin-binding
epidermal growth factor (HB-EGF), enhance DNA damage response (DDR)
in neighboring cells suffering from DNA damage. Consequently,
HB-EGF-treated cells exhibit higher double-strand break (DSB)
rejoining and display lower levels of residual DSBs (21). This study also has limitations. For
example, we did not experimentally verify the functions of
candidate splicing factors in regulation of AS profiles under
different treatments. In addition, the roles of key AS events in
the cross-talk between breast cancer cells and macrophages warrant
further research. Further investigation of the functions of
splicing factors and key altered AS events would largely improve
our understanding of the molecular mechanisms involved in
cancer-macrophage crosstalk.
In short, our results here showed that macrophages
play roles in the regulation of DNA damage and DNA repair pathways,
which improve our understanding concerning the interplay between
macrophages and tumor cells. Thus, the specific roles of
macrophages in the regulation of tumor cells require further
research, which may shed light on the improvement of cancer
immunotherapy.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Yunnan Applied
Basic Research Projects (grant no. 2016FB038) and the National
Natural Science Foundation of China (grant no. 31801249).
Availability of data and materials
The datasets generated during this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LD and HZ designed the experiments, interpreted the
results, and wrote the manuscript. WD and DL performed the data
analysis. PZ, LS, HD, YL, XB and YW revised this work critically
and helped to interpret the data. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that there was no financial
support or relationship that may pose a conflict of interest and
all the authors have no conflict of interests.
References
|
1
|
Spill F, Reynolds DS, Kamm RD and Zaman
MH: Impact of the physical microenvironment on tumor progression
and metastasis. Curr Opin Biotechnol. 40:41–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Danhier F, Feron O and Preat V: To exploit
the tumor microenvironment: Passive and active tumor targeting of
nanocarriers for anti-cancer drug delivery. J Control Release.
148:135–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engblom C, Pfirschke C and Pittet MJ: The
role of myeloid cells in cancer therapies. Nat Rev Cancer.
16:447–462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mills CD: M1 and M2 macrophages: Oracles
of health and disease. Crit Rev Immunol. 32:463–488. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balkwill F, Charles KA and Mantovani A:
Smoldering and polarized inflammation in the initiation and
promotion of malignant disease. Cancer Cell. 7:211–217. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Wang LA, Zhou DB, Cui QC, Zhao DC
and Wu YJ: Expression of tumor-associated macrophages and vascular
endothelial growth factor correlates with poor prognosis of
peripheral T-cell lymphoma, not otherwise specified. Leukemia
Lymphoma. 52:46–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang BC, Gao J, Wang J, Rao ZG, Wang BC
and Gao JF: Tumor-associated macrophages infiltration is associated
with peritumoral lymphangiogenesis and poor prognosis in lung
adenocarcinoma. Med Oncol. 28:1447–1452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Azizi E, Carr AJ, Plitas G, Cornish AE,
Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M,
et al: Single-cell map of diverse immune phenotypes in the breast
tumor microenvironment. Cell. 174:1293–1308.e36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu
L, Grzesik DA, Qian H, Xue XN and Pollard JW: Macrophages regulate
the angiogenic switch in a mouse model of breast cancer. Cancer
Res. 66:11238–11246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin EY, Nguyen AV, Russell RG and Pollard
JW: Colony- stimulating factor 1 promotes progression of mammary
tumors to malignancy. J Exp Med. 193:727–740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cortez-Retamozo V, Etzrodt M, Newton A,
Ryan R, Pucci F, Sio SW, Kuswanto W, Rauch PJ, Chudnovskiy A,
Iwamoto Y, et al: Angiotensin II drives the production of
tumor-promoting macrophages. Immunity. 38:296–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oltean S and Bates DO: Hallmarks of
alternative splicing in cancer. Oncogene. 33:5311–5318. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu HF, Lorenzini PA, Zhang F, Xu S, Wong
MS, Zheng J and Roca X: Alternative splicing analysis in human
monocytes and macrophages reveals MBNL1 as major regulator. Nucleic
Acids Res. 46:6069–6086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shadle PJ, Aldwin L, Nitecki DE and Koths
K: Human macrophage colony-stimulating factor heterogeneity results
from alternative mRNA splicing, differential glycosylation, and
proteolytic processing. J Cell Biochem. 40:91–107. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leek RD, Lewis CE, Whitehouse R, Greenall
M, Clarke J and Harris AL: Association of macrophage infiltration
with angiogenesis and prognosis in invasive breast carcinoma.
Cancer Res. 56:4625–4629. 1996.PubMed/NCBI
|
|
20
|
Mahmoud SM, Lee AH, Paish EC, Macmillan
RD, Ellis IO and Green AR: Tumour-infiltrating macrophages and
clinical outcome in breast cancer. J Clin Pathol. 65:159–163. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hollmen M, Roudnicky F, Karaman S and
Detmar M: Characterization of macrophage-cancer cell crosstalk in
estrogen receptor positive and triple-negative breast cancer. Sci
Rep. 5:91882015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galperin MY, Fernandez-Suarez XM and
Rigden DJ: The 24th annual Nucleic Acids Research database issue: A
look back and upcoming changes. Nucleic Acids Res. 45:5627. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao Y, Smyth GK and Shi W: FeatureCounts:
An efficient general purpose program for assigning sequence reads
to genomic features. Bioinformatics. 30:923–930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katz Y, Wang ET, Airoldi EM and Burge CB:
Analysis and design of RNA sequencing experiments for identifying
isoform regulation. Nat Methods. 7:1009–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lever J, Krzywinski M and Atman N: Points
of Significance principal component analysis. Nat Methods.
14:641–642. 2017. View Article : Google Scholar
|
|
28
|
Hotelling H: Analysis of a complex of
statistical variables into principal components. J Educ Psychol.
24:417–441. 1933. View Article : Google Scholar
|
|
29
|
Ginestet C: ggplot2: Elegant graphics for
data analysis. J R Stat Soc A Stat. 174:245. 2011. View Article : Google Scholar
|
|
30
|
Huang DW, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marchler-Bauer A, Bo Y, Han L, He J,
Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR,
et al: CDD/SPARCLE: Functional classification of proteins via
subfamily domain architectures. Nucleic Acids Res. 45:D200–D203.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kent WJ, Zweig AS, Barber G, Hinrichs AS
and Karolchik D: BigWig and BigBed: Enabling browsing of large
distributed datasets. Bioinformatics. 26:2204–2207. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quinlan AR and Hall IM: BEDTools: A
flexible suite of utilities for comparing genomic features.
Bioinformatics. 26:841–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eng L, Coutinho G, Nahas S, Yeo G, Tanouye
R, Babaei M, Dörk T, Burge C and Gatti RA: Nonclassical splicing
mutations in the coding and noncoding regions of the ATM gene:
Maximum entropy estimates of splice junction strengths. Hum Mutat.
23:67–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Domazet-Loso T and Tautz D: An ancient
evolutionary origin of genes associated with human genetic
diseases. Mol Biol Evol. 25:2699–2707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai Z, Chehab NH and Pavletich NP:
Structure and activation mechanism of the CHK2 DNA damage
checkpoint kinase. Mol Cell. 35:818–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nevanlinna H and Bartek J: The CHEK2 gene
and inherited breast cancer susceptibility. Oncogene. 25:5912–5919.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao H, Traganos F and Darzynkiewicz Z:
Phosphorylation of p53 on Ser15 during cell cycle caused by Topo I
and Topo II inhibitors in relation to ATM and Chk2 activation. Cell
Cycle. 7:3048–3055. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meijers-Heijboer H, van den Ouweland A,
Klijn J, Wasielewski M, de Snoo A, Oldenburg R, Hollestelle A,
Houben M, Crepin E, van Veghel-Plandsoen M, et al: Low-penetrance
susceptibility to breast cancer due to CHEK2(*)1100delC in
noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 31:55–59. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pevsner J, Hsu SC, Hyde PS and Scheller
RH: Mammalian homologues of yeast vacuolar protein sorting (vps)
genes implicated in Golgi-to-lysosome trafficking. Gene. 183:7–14.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Halachmi N and Lev Z: The Sec1 family: A
novel family of proteins involved in synaptic transmission and
general secretion. J Neurochem. 66:889–897. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akbar MA, Mandraju R, Tracy C, Hu W,
Pasare C and Krämer H: ARC Syndrome-linked Vps33B protein is
required for inflammatory endosomal maturation and signal
termination. Immunity. 45:267–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boisson B, Honda Y, Ajiro M, Bustamante J,
Bendavid M, Gennery AR, Kawasaki Y, Ichishima J, Osawa M, Nihira H,
et al: Rescue of recurrent deep intronic mutation underlying cell
type-dependent quantitative NEMO deficiency. J Clin Invest.
129:583–597. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stower H: Alternative splicing: Regulating
Alu element ‘exonization’. Nature Rev Genet. 14:152–153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Akman HB, Öyken M, Tuncer T, Can T and
Erson-Bensan AE: 3′UTR shortening and EGF signaling: Implications
for breast cancer. Hum Mol Genet. 24:6910–6920. 2015.PubMed/NCBI
|
|
47
|
Lin J, Hu Y, Nunez S, Foulkes AS, Cieply
B, Xue C, Gerelus M, Li W, Zhang H, Rader DJ, et al:
Transcriptome-wide analysis reveals modulation of human macrophage
inflammatory phenotype through alternative splicing. Arterioscl
Throm Vas Biol. 36:1434–1347. 2016. View Article : Google Scholar
|