Introduction
Liver cancer is one of the most common lethal
cancers worldwide. It has been reported that liver cancer ranks
sixth among the most commonly diagnosed cancers worldwide, and was
the fourth major cause of cancer-related deaths in 2018. Global
cancer statistics indicate that ~841,000 new cases and 782,000
deaths occur annually (1).
Hepatocellular carcinoma (HCC) is the major histological type of
primary liver cancer, accounting for 75–85% of all cases. Infection
with hepatitis virus [mainly hepatitis B virus (HBV) and hepatitis
C virus], aflatoxin exposure, excessive alcohol consumption and
tobacco smoking, are considered as the main risk factors for the
development of HCC (2,3). In China, the predominant cause of HCC is
chronic HBV infection, and it is estimated that ~70% of these
patients have an established HBV infection history (4). Although the available therapies for HCC
patients have greatly improved over the past decades, the clinical
prognosis remains unfavorable, with a 5-year overall survival (OS)
rate of ~30% following hepatic resection (2,5).
Therefore, it is imperative to identify more sensitive diagnostic
and prognostic biomarkers for HCC.
Wingless-type MMTV integration site (WNT)
genes are a family of 19 genes that modulate both the canonical WNT
signal transduction pathway (referred to as β-catenin-dependent)
and non-canonical WNT signal transduction pathway (referred to as
β-catenin-independent) (6). Previous
studies indicated that WNT family genes are associated with
various tumor biological processes, including cell proliferation
(7,8),
invasion (8–11), metastasis (12,13) and
drug resistance (13–15). In addition, some researchers have
reported that aberrant WNT expression levels are associated
with diagnosis and prognosis prediction for certain tumors. Fu
et al (16) proved that
WNT2 can activate the WNT/β-catenin signal transduction
pathway, which ultimately promotes esophageal cancer cell growth,
and WNT2 enhances cell motility and invasiveness by inducing
epithelial-to-mesenchymal transition. Furthermore, WNT2
expression level was found to be closely associated with the poor
clinical performance status of patients with esophageal squamous
cell carc WNT inoma.
However, the diagnostic and prognostic value of the
WNT gene family expression in HBV-related HCC remains
unclear. The primary goal of the present study was to investigate
this association by collecting data from public databases and
performing a series of bioinformatics analyses.
Materials and methods
Data sources
The clinical characteristics of patients with
HBV-related HCC and the corresponding WNT gene family
expression levels were downloaded from the GSE14520 dataset of Gene
Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14520,
accessed November 28, 2018) (17,18). The
detailed process of GSE14520 genome-wide expression profile dataset
processing has been described in our previous article (19). The Cancer Genome Atlas (TCGA) database
HCC cohort was used as a validation cohort, and the data processing
of RNA sequencing was described in our previous paper (19). The raw RNA sequencing dataset of TCGA
was normalized by DESeq (20). The
clinical data of these patients in the GSE14520 dataset included
age, gender, serum alanine aminotransferase level, serum
α-fetoprotein (AFP) level, cirrhosis, main tumor size, tumor
number, Barcelona Clinic Liver Cancer (BCLC) stage,
tumor-node-metastasis (TNM) stage, survival time, and survival
status. The data for mRNA expression level of five WNT
family genes (WNT3A, WNT8A, WNT9A, WNT9B and WNT10A)
were unavailable in the Gene Expression Omnibus (GEO) database.
Therefore, only 14 WNT genes were finally analyzed in the
present study. As the dataset included in our research was obtained
from a public database, the study did not require the approval of
an ethics committee.
Bioinformatics analysis of WNT family
genes
To explore the potential biological functions and
possible pathways of WNT family genes, gene enrichment
analyses, including Gene Ontology (GO) functional analysis and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis,
were conducted by applying the Database for Annotation,
Visualization, and Integrated Discovery (DAVID) bioinformatics
online tool, version 6.8 (https://david.ncifcrf.gov/, accessed December 2, 2018)
(21). Statistically, an enrichment
P-value of <0.05 was considered to indicate statistically
significant differences. In order to further validate the results
of enrichment analysis by DAVID, application package Biological
Networks Gene Ontology tool (BiNGO) in the Cytoscape software
(version 3.7.1) (22) was used to
explore the GO terms of WNT family genes. Pearson's
correlation coefficient was calculated to assess the relevance
among WNT genes in the co-expression analysis. These data
were visualized by the correlation plot package in the R platform
(version 3.4.0). WNT gene-gene and protein-protein
interactions were investigated by using the online resource
GeneMANIA (http://genemania.org/, accessed December
6, 2018) (23) and the Search Tool
for the Retrieval of Interacting Genes/Proteins (STRING)
(https://string-db.org/cgi/input.pl,
accessed December 6, 2018) (24,25),
respectively.
Assessment of diagnostic value
The expression level of WNT family genes in
tumor tissues and corresponding adjacent non-tumor tissues were
compared via t-test statistical analysis. The public online
resource Metabolic gEne RApid Visualizer (MERAV) (http://merav.wi.mit.edu/, accessed December 8, 2018)
(26) was used to further validate
that genes of the WNT family were differentially expressed
between primary liver cancer tissues and normal liver tissues.
Receiver operating characteristic (ROC) curve analysis was selected
to determine the diagnostic value of these differentially expressed
WNT family genes.
Survival analysis
The 212 patients with HBV-related HCC were
categorized into high- and low-expression groups based on the
median WNT gene expression level in tumor tissues. In order
to investigate whether the expression level of WNT family
genes was correlated with prognosis and outcome, Cox proportional
hazard regression analysis and Kaplan-Meier survival analysis with
log-rank tests were used to evaluate the association between
WNT family gene expression, OS and recurrence-free survival
(RFS).
Joint effects analysis of WNT family
genes
Based on the results of multivariate Cox
proportional hazard regression analysis and Kaplan-Meier survival
analysis, only WNT1 and WNT6 were found to be
significantly associated with RFS. Therefore, the combined effects
of WNT1 and WNT6 were analyzed. The combinations were
as follows: Low WNT1 expression and low WNT6
expression (group 1), low WNT1 expression and high
WNT6 expression (group 2), high WNT1 expression and
low WNT6 expression (group 3), and high WNT1
expression and high WNT6 expression (group 4).
Prognostic nomogram for survival
prediction
All 212 patients with HBV-related HCC in the
GSE14520 dataset of the GEO database were identified as the source
population for nomogram construction. The variables that were
related to prognosis outcome were selected to construct the
nomogram, including sex, serum AFP level, cirrhosis, BCLC stage,
tumor size and WNT gene expression. With each variable being
assigned a score, the total point was calculated by summing up the
scores of all the variables and located onto the scale. Therefore,
the probabilities of the survival outcome could be predicted by
drawing a vertical line to the total point.
Statistical analysis
All statistical analyses were performed with the
SPSS software package, version 17.0 (SPSS Inc.). Comparison of
WNT family gene expression levels between tumor tissues and
corresponding adjacent non-tumor tissues was performed using
t-tests. The Cox proportional hazards regression model was selected
for univariate and multivariate analyses. By applying Kaplan-Meier
survival analysis with log-rank test, the association of WNT
family gene expression levels with OS and RFS time was observed.
Vertical scatter plots, ROC curves and survival curves were plotted
by GraphPad Prism software, version 7.0 (GraphPad Software, Inc.).
P<0.05 was considered to indicate statistically significant
differences.
Results
Characteristics of patients in the GEO
database
In the GSE14520 dataset of the GEO database, there
remained 212 patients with HBV-related HCC after excluding patients
who had no reported HBV infection or any available survival
information. Detailed characteristics of these patients are shown
in Table SI. Serum AFP level,
cirrhosis, tumor size, BCLC stage and TNM stage were found to be
closely associated with OS (P<0.05), whereas sex, cirrhosis,
BCLC stage and TNM stage were significantly associated with RFS
(P<0.05). The remaining characteristics did not exhibit a
significant association with OS or RFS (all P>0.05).
Bioinformatics analysis of WNT family
genes
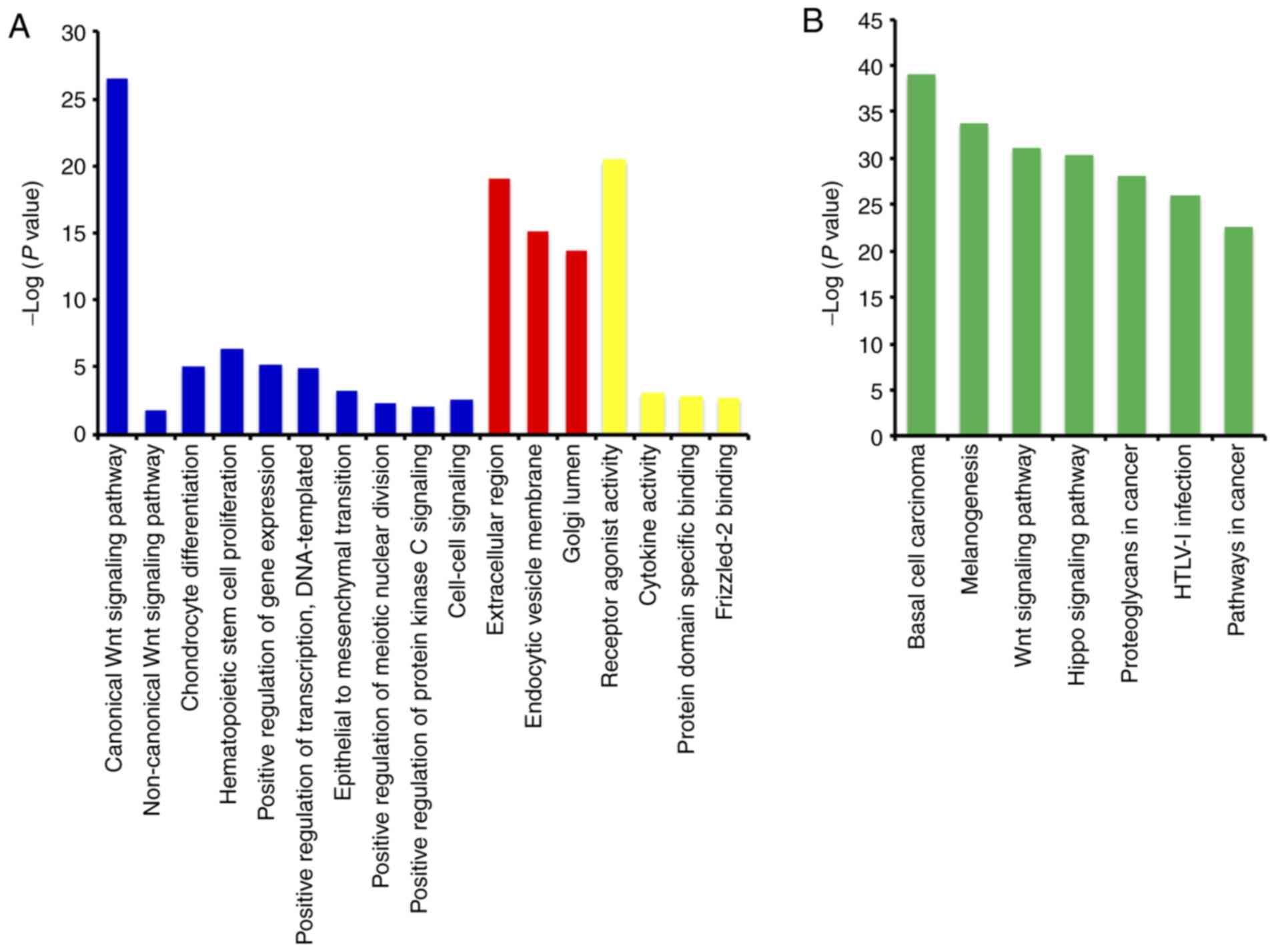
The GO function analysis indicated that WNT
family genes were mainly enriched in the regulation of cell
differentiation, cell proliferation, epithelial-to-mesenchymal
transition, and modulation of the WNT signaling pathway (Figs. 1A, S1
and S2, and Table SII). The KEGG pathway analysis
suggested that WNT family genes were associated with the WNT
signaling pathway and other pathways (Fig. 1B and Table
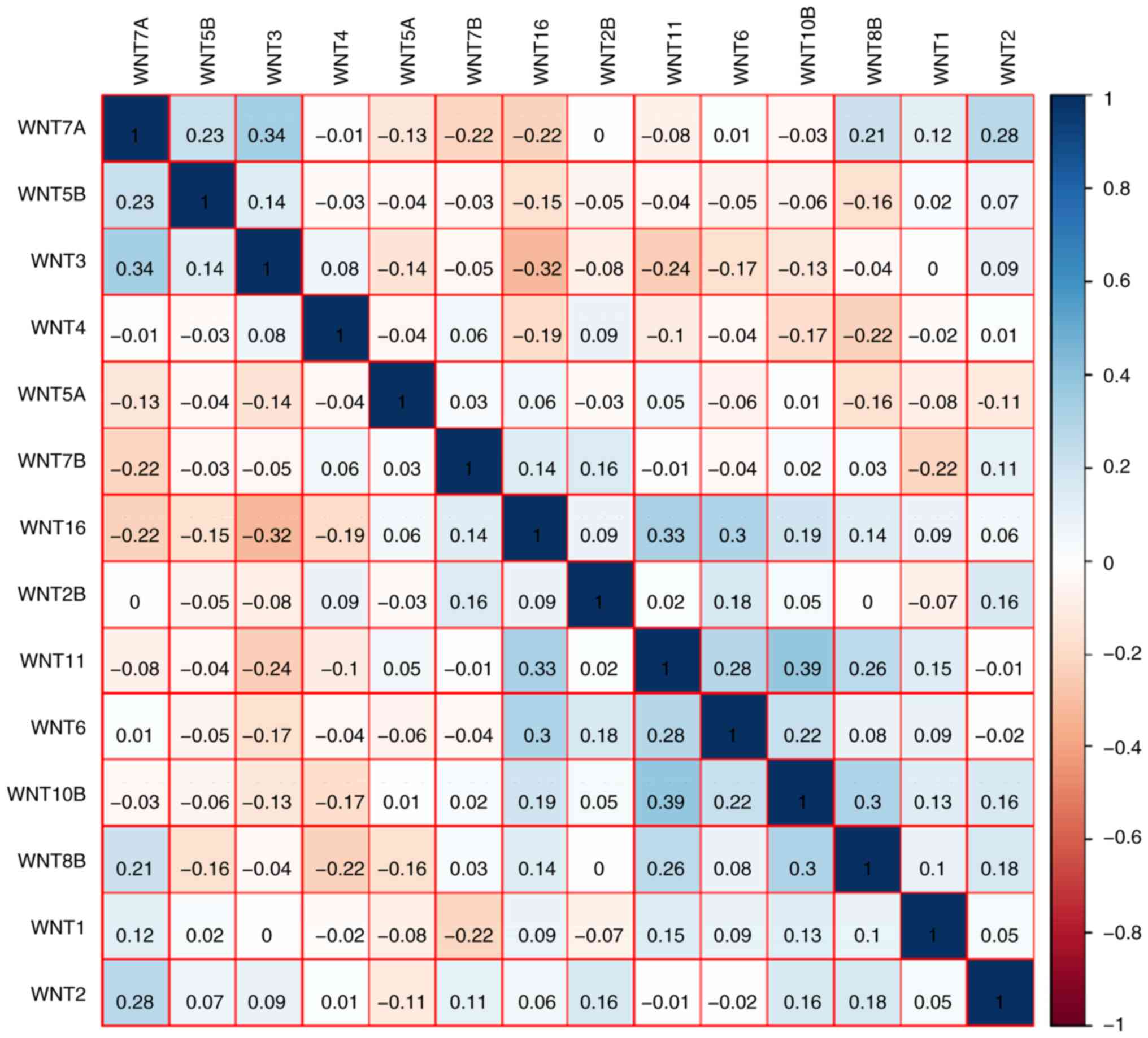
SIII). The Pearson's correlation coefficients of WNT
family genes were calculated and used to assess whether these genes
were correlated with each other. As shown in Fig. 2, the WNT family genes were
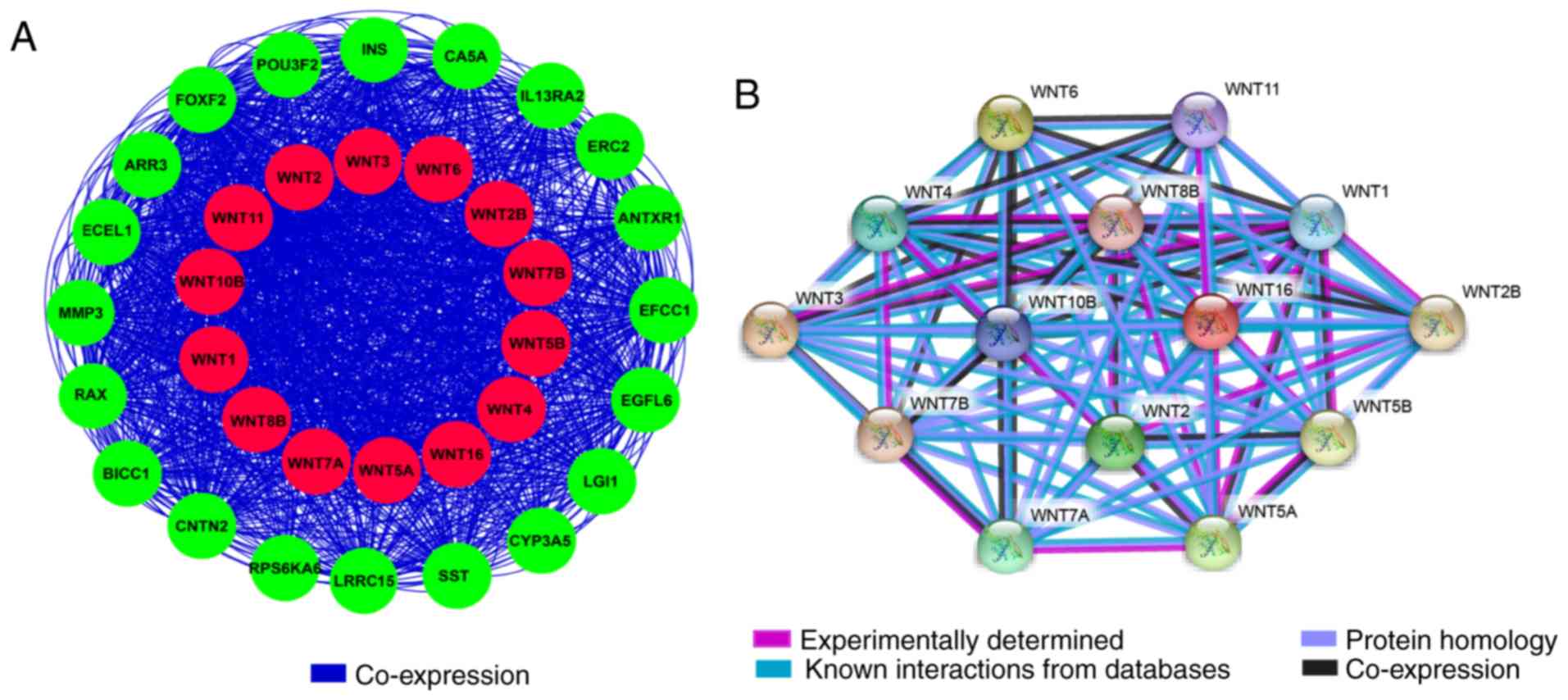
correlated to some degree. The gene-gene and protein-protein
interaction networks constructed by GeneMANIA and STRING,
respectively, indicated that the WNT family genes were
co-expressed and exhibited extensive homology at the protein level
(Fig. 3A and B).
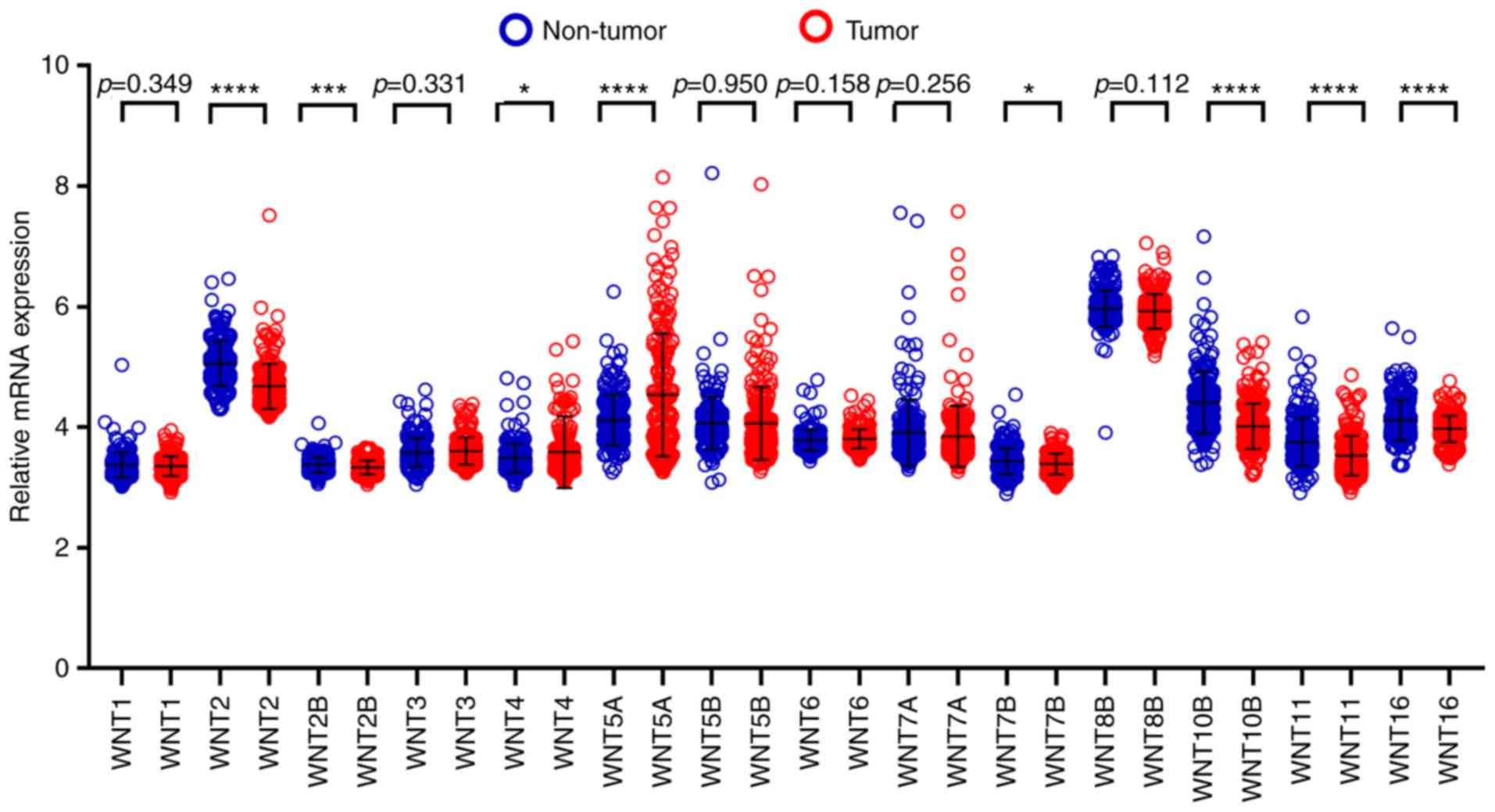
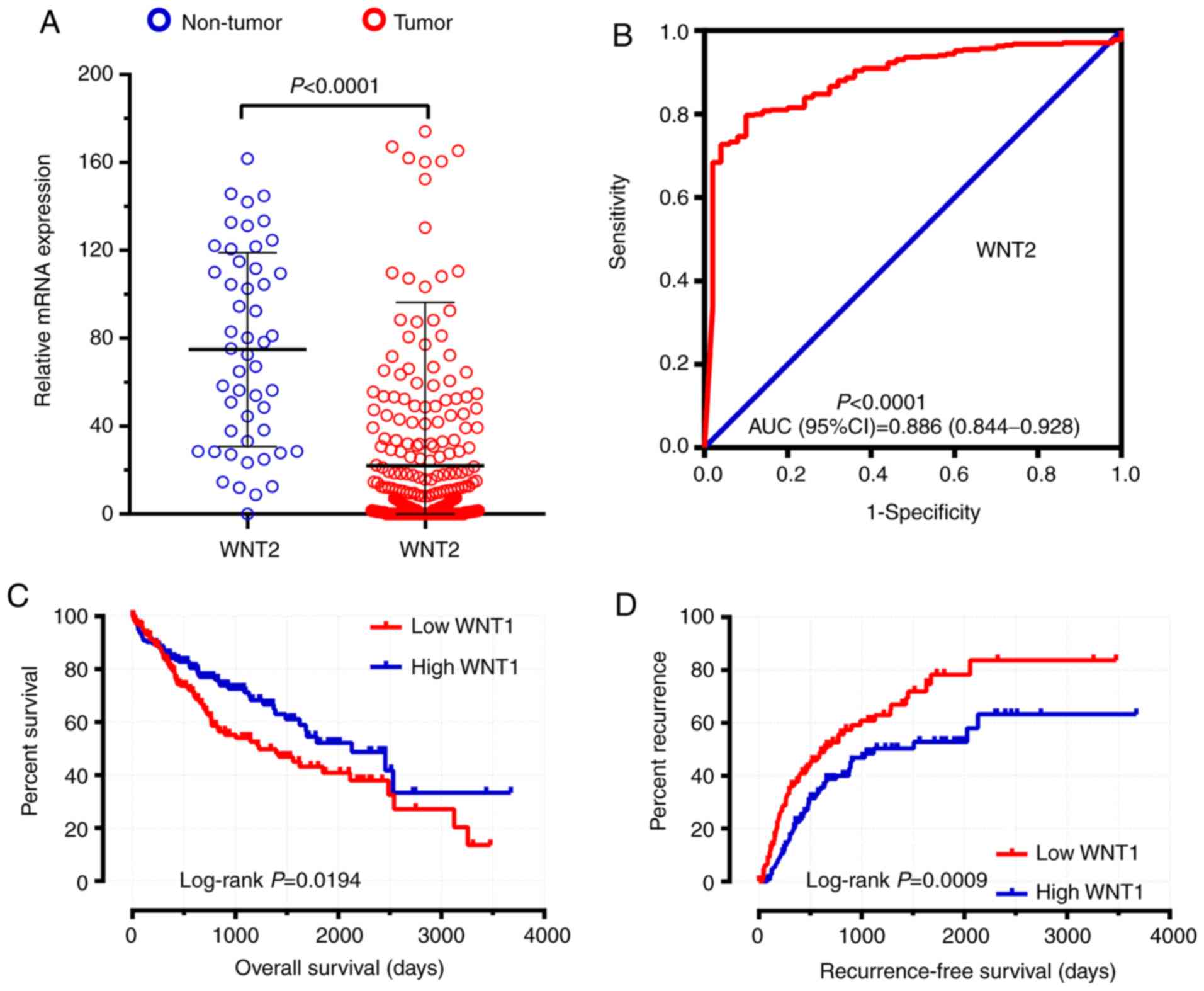
Assessment of diagnostic value
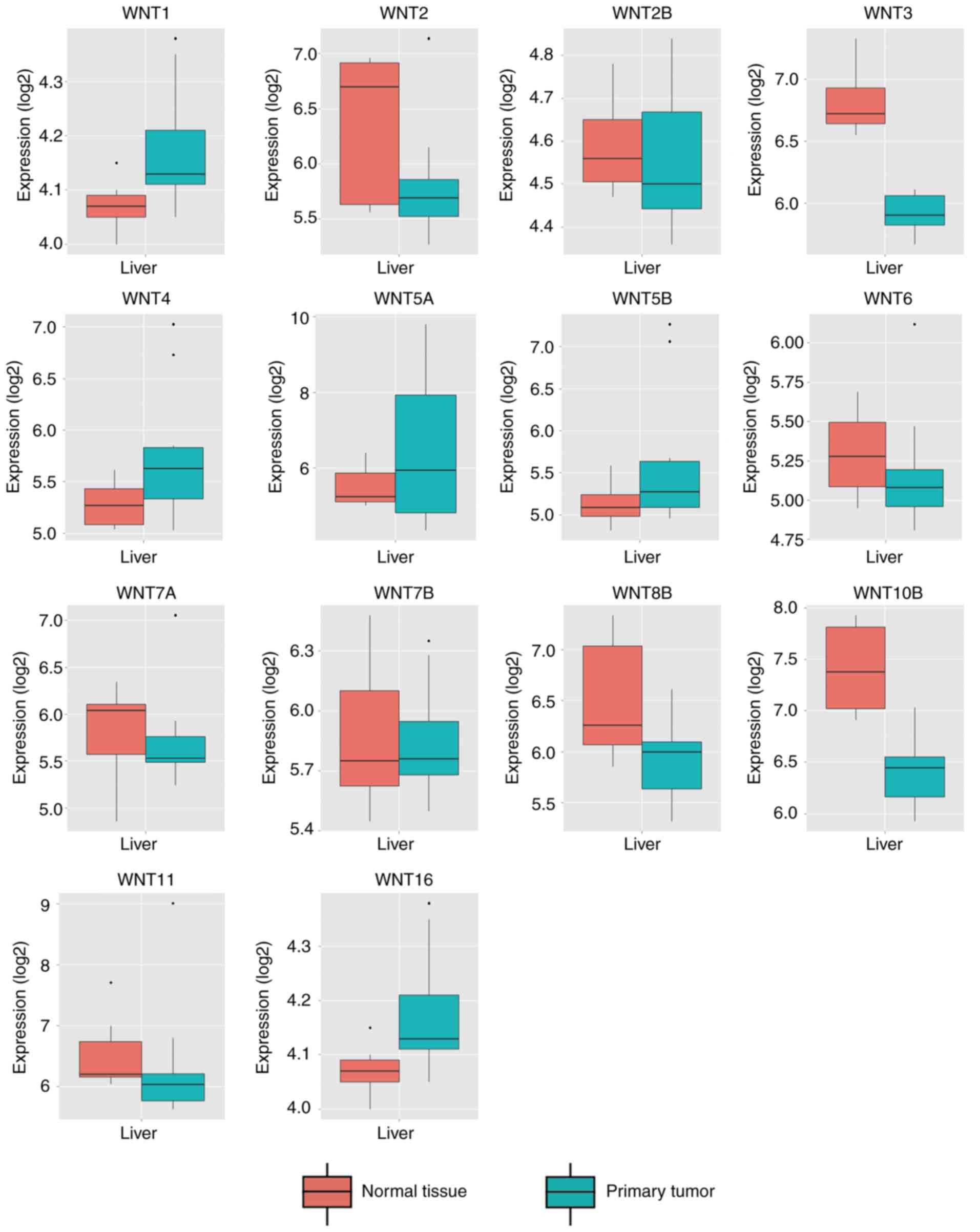
By comparing WNT family gene expression
levels between tumor tissues and corresponding adjacent non-tumor
tissues, a total of 8 WNT family genes (WNT2, WNT2B,
WNT4, WNT5A, WNT7B, WNT10B, WNT11 and WNT16) were found
to be differentially expressed in tumor and non-tumor tissues
(Fig. 4) (P<0.05). The online
resource MERAV was used to further validate genes of the WNT
family that were differentially expressed in normal liver tissues
and primary liver cancer tissues (Fig.
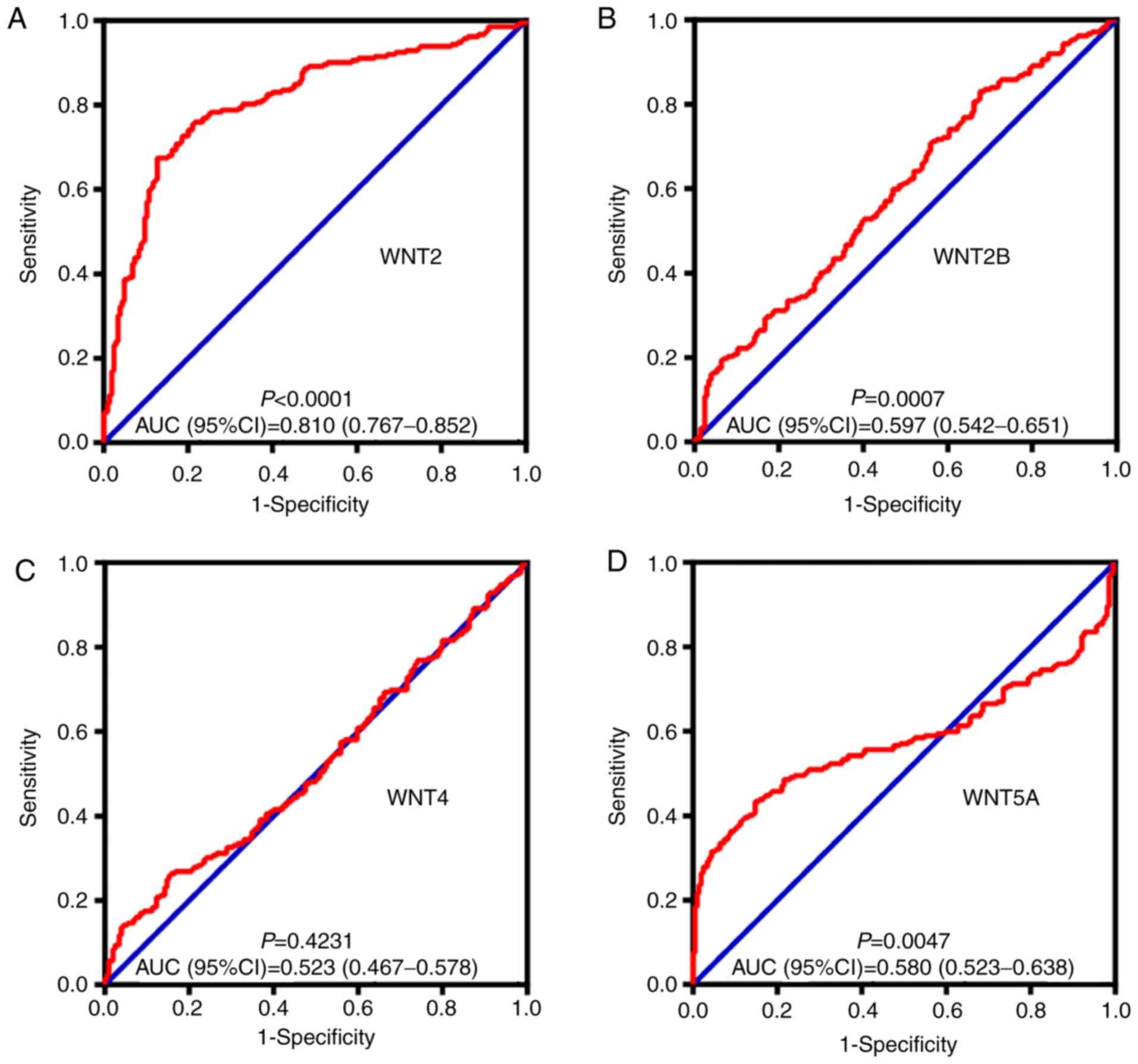
5). An ROC curve was constructed to further explore the
diagnostic value of these 8 differentially expressed genes. As
shown in Fig. 6, six WNT genes
(WNT2, WNT2B, WNT5A, WNT10B, WNT11 and WNT16) had a
potential prediction value, with all P-values <0.05 and area
under the curve (AUC) >0.500; WNT2 in particular
exhibited high accuracy in differentiating HCC tissues from
non-tumor tissue (P<0.0001, AUC=0.810, 95% CI: 0.767–0.852).
Survival analysis
The characteristics associated with clinical
prognostic outcome, including cirrhosis, tumor size and BCLC stage,
were included in the multivariate Cox regression analysis.
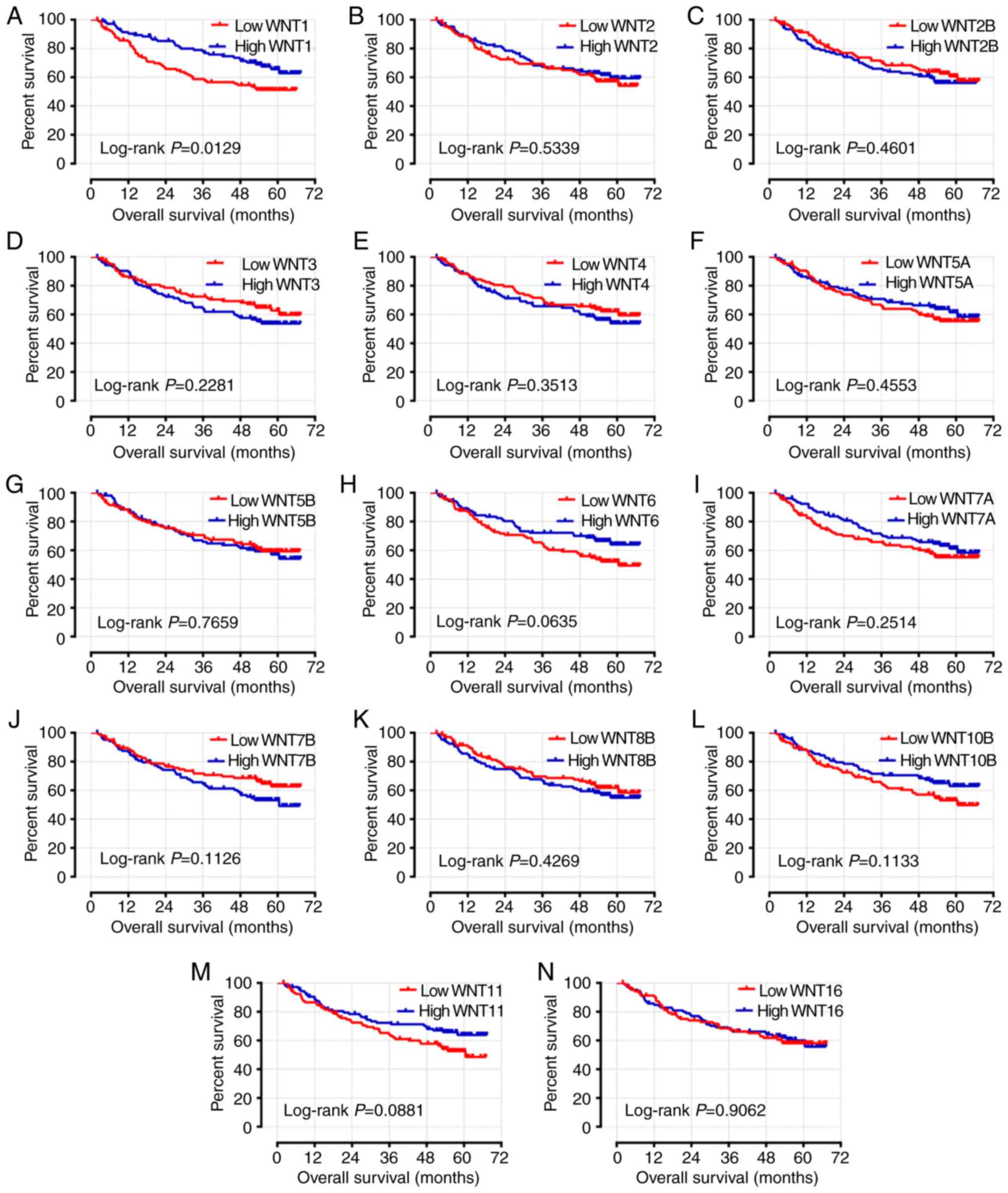
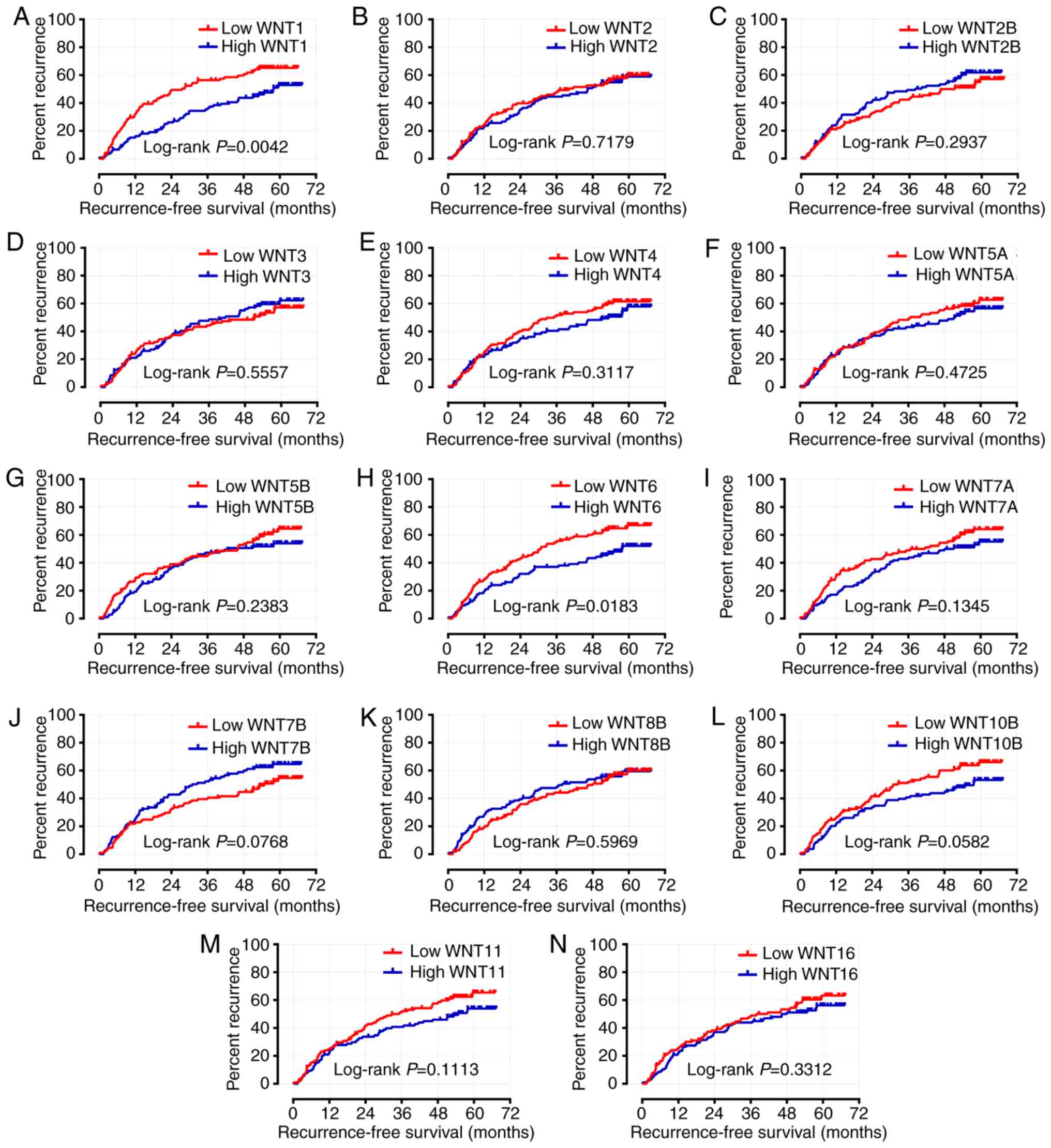
Following adjustment or these prognosis-related risk factors, the
results indicated that the expression level of WNT1 was
significantly associated with OS and RFS in the survival analysis
(adjusted P=0.033, adjusted HR=0.607, 95% CI: 0.384–0.960 and
adjusted P=0.007, adjusted HR=0.592, 95% CI: 0.404–0.868,
respectively) (Table I; Figs. 7A and 8A). The expression level of WNT6 was
closely associated with RFS (adjusted P=0.033, adjusted HR=0.665,
95% CI: 0.457–0.968), but WNT6 did not exhibit a significant
association with OS (P>0.05) (Table
I; Figs. 7H and 8H).
 | Table I.Prognostic values of WNT gene
expression in HBV-related HCC of the GSE14520 cohort. |
Table I.
Prognostic values of WNT gene
expression in HBV-related HCC of the GSE14520 cohort.
|
|
| OS | RFS |
|---|
|
|
|
|
|
|---|
| Gene
expression | Patient no. | No. of events | MST (months) | Crude HR (95%
CI) | Crude P-value | Adjusted HR (95%
CI) | Adjusted
P-valuea | No. of events | MRT (months) | Crude HR (95%
CI) | Crude P-value | Adjusted HR (95%
CI) | Adjusted
P-valuea |
|---|
| WNT1 |
|
|
|
|
|
|
|
|
|
|
|
| 0.007 |
|
Low | 106 | 49 | NA | 1 |
| 1 |
| 66 | 27 | 1 |
| 1 |
|
|
High | 106 | 33 | NA | 0.575 | 0.014 | 0.607 | 0.033 | 50 | 57 | 0.588 | 0.005 | 0.592 |
|
|
|
|
|
| (0.370–0.895) |
| (0.384–0.960) |
|
|
| (0.406–0.850) |
| (0.404–0.868) |
|
| WNT2 |
|
|
|
|
|
|
|
|
|
|
|
| 0.444 |
|
Low | 106 | 43 | NA | 1 |
| 1 |
| 58 | 40 | 1 |
| 1 |
|
|
High | 106 | 39 | NA | 0.872 | 0.534 | 0.784 | 0.282 | 58 | 46 | 0.935 | 0.718 | 0.865 |
|
|
|
|
|
| (0.565–1.345) |
| (0.503–1.222) |
|
|
| (0.650–1.346) |
| (0.597–1.254) |
|
| WNT2B |
|
|
|
|
|
|
|
|
|
|
|
| 0.091 |
|
Low | 106 | 38 | NA | 1 |
| 1 |
| 54 | 46 | 1 |
| 1 |
|
|
High | 106 | 44 | NA | 1.178 | 0.461 | 1.344 | 0.192 | 62 | 37 | 1.215 | 0.295 | 1.378 |
|
|
|
|
|
| (0.763–1.818) |
| (0.862–2.093) |
|
|
| (0.844–1.751) |
| (0.950–1.997) |
|
| WNT3 |
|
|
|
|
|
|
|
|
|
|
|
| 0.622 |
|
Low | 106 | 37 | NA | 1 |
| 1 |
| 56 | 51 | 1 |
| 1 |
|
|
High | 106 | 45 | NA | 1.306 | 0.230 | 1.279 | 0.285 | 60 | 41 | 1.116 | 0.556 | 1.099 |
|
|
|
|
|
| (0.845–2.018) |
| (0.814–2.008) |
|
|
| (0.775–1.606) |
| (0.754–1.601) |
|
| WNT4 |
|
|
|
|
|
|
|
|
|
|
|
| 0.113 |
|
Low | 106 | 39 | NA | 1 |
| 1 |
| 63 | 35 | 1 |
| 1 |
|
|
High | 106 | 43 | NA | 1.229 | 0.352 | 1.116 | 0.624 | 53 | 53 | 0.828 | 0.313 | 0.742 |
|
|
|
|
|
| (0.796–1.896) |
| (0.720–1.730) |
|
|
| (0.575–1.194) |
| (0.512–1.074) |
|
| WNT5A |
|
|
|
|
|
|
|
|
|
|
|
| 0.663 |
|
Low | 106 | 45 | NA | 1 |
| 1 |
| 62 | 36 | 1 |
| 1 |
|
|
High | 106 | 37 | NA | 0.847 | 0.456 | 0.915 | 0.690 | 54 | 51 | 0.875 | 0.473 | 0.922 |
|
|
|
|
|
| (0.549–1.309) |
| (0.591–1.417) |
|
|
| (0.607–1.260) |
| (0.638–1.331) |
|
| WNT5B |
|
|
|
|
|
|
|
|
|
|
|
| 0.641 |
|
Low | 106 | 40 | NA | 1 |
| 1 |
| 64 | 46 | 1 |
| 1 |
|
|
High | 106 | 42 | NA | 1.068 | 0.766 | 1.328 | 0.215 | 52 | 43 | 0.803 | 0.240 | 0.915 |
|
|
|
|
|
| (0.693–1.647) |
| (0.848–2.078) |
|
|
| (0.557–1.158) |
| (0.630–1.329) |
|
| WNT6 |
|
|
|
|
|
|
|
|
|
|
|
| 0.033 |
|
Low | 106 | 48 | 60 | 1 |
| 1 |
| 66 | 30 | 1 |
| 1 |
|
|
High | 106 | 34 | NA | 0.662 | 0.066 | 0.756 | 0.222 | 50 | 57 | 0.644 | 0.019 | 0.665 |
|
|
|
|
|
| (0.426–1.027) |
| (0.483–1.184) |
|
|
| (0.446–0.931) |
| (0.457–0.968) |
|
| WNT7A |
|
|
|
|
|
|
|
|
|
|
|
| 0.180 |
|
Low | 106 | 43 | NA | 1 |
| 1 |
| 62 | 41 | 1 |
| 1 |
|
|
High | 106 | 39 | NA | 0.776 | 0.253 | 0.764 | 0.229 | 54 | 48 | 0.757 | 0.136 | 0.778 |
|
|
|
|
|
| (0.503–1.198) |
| (0.492–1.185) |
|
|
| (0.526–1.091) |
| (0.539–1.123) |
|
| WNT7B |
|
|
|
|
|
|
|
|
|
|
|
| 0.522 |
|
Low | 106 | 36 | NA | 1 |
| 1 |
| 53 | 54 | 1 |
| 1 |
|
|
High | 106 | 46 | 60 | 1.421 | 0.115 | 1.057 | 0.812 | 63 | 30 | 1.390 | 0.078 | 1.132 |
|
|
|
|
|
| (0.918–2.200) |
| (0.670–1.667) |
|
|
| (0.963–2.004) |
| (0.775–1.655) |
|
| WNT8B |
|
|
|
|
|
|
|
|
|
|
|
| 0.721 |
|
Low | 106 | 38 | NA | 1 |
| 1 |
| 57 | 48 | 1 |
| 1 |
|
|
High | 106 | 44 | NA | 1.192 | 0.428 | 0.904 | 0.654 | 59 | 37 | 1.103 | 0.597 | 0.935 |
|
|
|
|
|
| (0.772–1.840) |
| (0.580–1.407) |
|
|
| (0.766–1.588) |
| (0.646–1.354) |
|
| WNT10B |
|
|
|
|
|
|
|
|
|
|
|
| 0.079 |
|
Low | 106 | 46 | 60 | 1 |
| 1 |
| 64 | 30 | 1 |
| 1 |
|
|
High | 106 | 36 | NA | 0.704 | 0.115 | 0.691 | 0.101 | 52 | 57 | 0.703 | 0.060 | 0.717 |
|
|
|
|
|
| (0.455–1.090) |
| (0.445–1.0750) |
|
|
| (0.487–1.014) |
| (0.495–1.039) |
|
| WNT11 |
|
|
|
|
|
|
|
|
|
|
|
| 0.371 |
|
Low | 106 | 47 | 60 | 1 |
| 1 |
| 63 | 35 | 1 |
| 1 |
|
|
High | 106 | 35 | NA | 0.685 | 0.090 | 0.740 | 0.186 | 53 | 54 | 0.744 | 0.113 | 0.843 |
|
|
|
|
|
| (0.442–1.061) |
| (0.474–1.156) |
|
|
| (0.515–1.073) |
| (0.581–1.225) |
|
| WNT16 |
|
|
|
|
|
|
|
|
|
|
|
| 0.349 |
|
Low | 106 | 40 | NA | 1 |
| 1 |
| 60 | 37 | 1 |
| 1 |
|
|
High | 106 | 42 | NA | 0.974 | 0.906 | 0.950 | 0.819 | 56 | 46 | 0.835 | 0.332 | 0.838 |
|
|
|
|
|
| (0.632–1.503) |
| (0.611–1.477) |
|
|
| (0.580–1.202) |
| (0.580–1.212) |
|
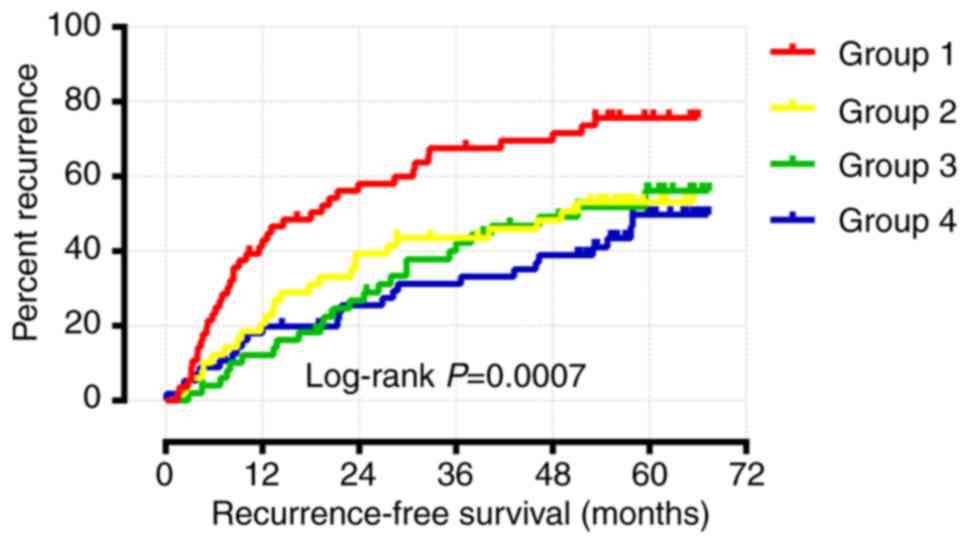
Joint effects analysis of WNT family
genes
As shown above, the multivariate Cox regression
analysis and Kaplan-Meier survival analysis demonstrated that only
WNT1 and WNT6 were significantly associated with RFS.
Joint effects survival analysis for WNT1 and WNT6 was
performed following adjustment for cirrhosis, tumor size and BCLC
stage. The results suggested that group 4 (high WNT1
expression and high WNT6 expression) had the longest RFS,
whereas group 1 (low WNT1 expression and low WNT6
expression) had the shortest RFS (Table
II and Fig. 9). Therefore,
patients with a high expression of both WNT1 and WNT6
are expected to have a longer RFS.
 | Table II.Joint effects analysis for the
combination of WNT1 and WNT6. |
Table II.
Joint effects analysis for the
combination of WNT1 and WNT6.
| Group | WNT1
expression | WNT6
expression | Patients
(n=212) | MRT (months) | Crude HR (95%
CI) | Crude P-value | Adjusted HR (95%
CI) | Adjusted
P-valuea |
|---|
| 1 | Low | Low | 56 | 18 | NA | 0.001 | NA | P<0.001 |
| 2 | Low | High | 50 | 49 | 0.524
(0.318–0.862) | 0.011 | 0.457
(0.276–0.755) | 0.002 |
| 3 | High | Low | 50 | 51 | 0.478
(0.290–0.787) | 0.004 | 0.413
(0.250–0.683) | 0.001 |
| 4 | High | High | 56 | NA | 0.401
(0.243–0.662) | P<0.001 | 0.405
(0.240–0.684) | 0.001 |
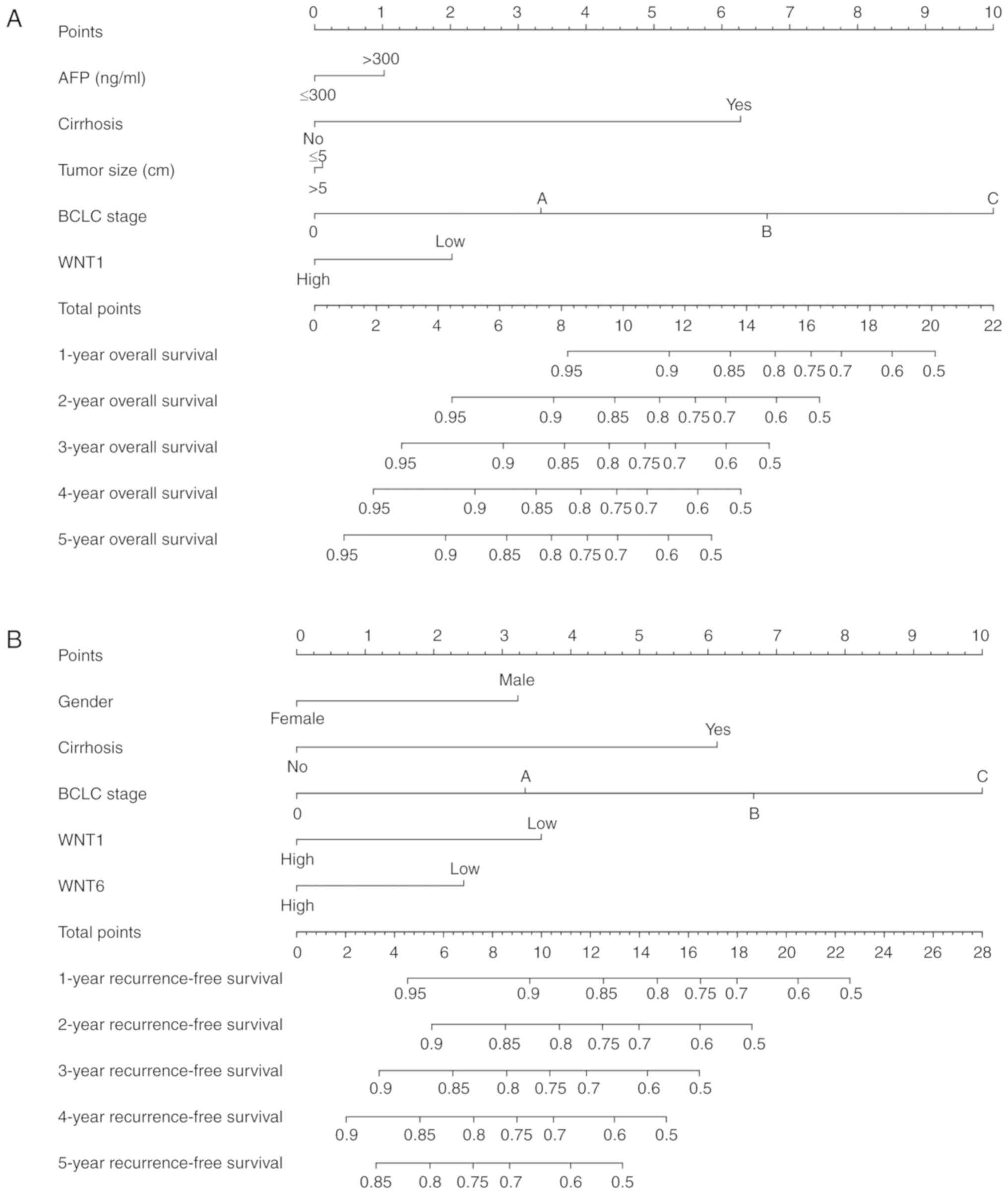
Prognostic nomogram for survival
prediction
The prognostic risk factors that may predict the
outcome of survival, including sex, serum AFP level, cirrhosis,
BCLC stage, tumor size and WNT family gene expression, were
selected to construct the nomogram, which can provide an
individualized prognosis prediction. For the 212 patients with
HBV-related HCC, nomogram analysis was performed for the
probabilities of 1-, 2-, 3-, 4- and 5-year OS (Fig. 10A) and RFS (Fig. 10B). As shown in the nomogram, the
expression level of WNT1 and WNT6 contributed to a
certain extent to the patients' clinical prognosis outcome.
TCGA validation cohort
A total of 374 tumor tissues and 50 adjacent
non-tumor tissues were included in the present study. Among those,
370 HCC patients with prognostic information were included in the
survival analysis. The expression distribution of the WNT
family genes between HCC tumor tissues and adjacent non-tumor
tissues were calculated by DESeq and are shown in Table III. WNT2 was shown to be
significantly differentially expressed between HCC and adjacent
non-tumor tissues in the TCGA cohort (Table III, Fig.
11A and B). The clinical characteristics of HCC patients in the
TCGA cohort are shown in Table SIV.
The survival analysis results of the WNT family genes are
shown in Table IV. WNT1 was
found to be associated with both the OS and RFS of HCC in the TCGA
cohort (Table IV, Fig. 11C and D).
 | Table III.Expression distribution of WNT
family genes between tumor tissue and adjacent non-tumor tissue in
the TCGA validation cohort. |
Table III.
Expression distribution of WNT
family genes between tumor tissue and adjacent non-tumor tissue in
the TCGA validation cohort.
| Gene | Log2
(fold change) | P-value | FDR |
|---|
| WNT1 | 1.84421242 | 0.946083391 | 0.999996922 |
| WNT2 | −1.769264955 | 0.002852902 | 0.022026272 |
| WNT2B | 1.360412766 | 0.373384363 | 0.65436372 |
| WNT3 | 0.066523683 | 0.810682922 | 0.928938592 |
| WNT4 | 1.309792732 | 0.026270116 | 0.120328643 |
| WNT5A | 0.650962704 | 0.073901092 | 0.249424709 |
| WNT5B | −0.101243468 | 0.644473376 | 0.842930166 |
| WNT6 | 2.792018889 | 0.42971413 | 0.703074675 |
| WNT7A | 0.13572947 | 1 | 1 |
| WNT7B | 1.071320247 | 0.324934599 | 0.610626696 |
| WNT8B | 1.918325871 | 0.070391904 | 0.241649148 |
| WNT10B | 1.121445938 | 0.298549628 | 0.582917358 |
| WNT11 | −1.50622528 | 0.000105087 | 0.001433939 |
| WNT16 | −0.077860553 | 0.822321775 | 0.934493001 |
 | Table IV.Survival analysis results of the
WNT family genes in the TCGA validation cohort. |
Table IV.
Survival analysis results of the
WNT family genes in the TCGA validation cohort.
|
| RFS | OS |
|---|
|
|
|
|
|---|
| Gene | P-value | HR | Low 95% CI | High 95% CI | P-value | HR | Low 95% CI | High 95% CI |
|---|
| WNT1 | 0.004872 | 0.5585569 | 0.37237755 | 0.83782122 | 0.038128301 | 0.67054664 | 0.459578646 | 0.978358776 |
| WNT2 | 0.165437 | 0.74242449 | 0.48738676 | 1.13091729 | 0.463552265 | 1.15543359 | 0.78518841 | 1.700262972 |
| WNT2B | 0.172666 | 0.75604408 | 0.5058112 | 1.13007116 | 0.949318195 | 0.9878679 | 0.678014074 | 1.439325557 |
| WNT3 | 0.111201 | 1.39315603 | 0.92641981 | 2.09503695 | 0.23053025 | 0.7955497 | 0.547382364 | 1.156228935 |
| WNT4 | 0.038296 | 0.65324005 | 0.43663377 | 0.97730085 | 0.308986119 | 0.82048053 | 0.560425379 | 1.201209516 |
| WNT5A | 0.25247 | 0.79158048 | 0.53045358 | 1.18125258 | 0.823749524 | 0.95871339 | 0.661532981 | 1.389396128 |
| WNT5B | 0.421213 | 0.84734859 | 0.56593122 | 1.26870474 | 0.701474468 | 0.92888443 | 0.637015977 | 1.354481386 |
| WNT6 | 0.571806 | 0.88958796 | 0.5929931 | 1.33452943 | 0.640628505 | 1.09289911 | 0.752662101 | 1.586938493 |
| WNT7A | 0.000396 | 0.47050498 | 0.31004378 | 0.71401184 | 0.668569853 | 0.92232626 | 0.636972232 | 1.335514626 |
| WNT7B | 0.156162 | 0.73979971 | 0.4877709 | 1.12205056 | 0.874887781 | 1.0304464 | 0.709391742 | 1.496803142 |
| WNT8B | 0.36875 | 0.83403019 | 0.56144901 | 1.23894839 | 0.230696394 | 0.79551932 | 0.547254355 | 1.156411068 |
| WNT10B | 0.052198 | 0.6701836 | 0.44743955 | 1.00381395 | 0.23391077 | 0.79061532 | 0.536982043 | 1.16404746 |
| WNT11 | 0.012024 | 0.59538341 | 0.397232 | 0.89237879 | 0.084433724 | 0.71556062 | 0.489264738 | 1.046523402 |
| WNT16 | 0.039333 | 0.65216604 | 0.43430059 | 0.97932296 | 0.457153569 | 1.15299749 | 0.792227421 | 1.678057559 |
Discussion
The aim of the present study was to investigate the
diagnostic and prognostic values of WNT family gene
expression in HBV-related HCC established on public databases and a
series of bioinformatics analyses. The results suggested that
WNT2 may serve as potential diagnostic biomarker for
HBV-related HCC. In addition, we demonstrated that the expression
level of WNT1 was significantly associated with clinical
prognostic outcome, with patients with a higher expression level of
WNT1 expected to have a better prognostic outcome.
Therefore, it may be concluded that WNT1 may serve as
potential prognostic biomarker for patients with HBV-related
HCC.
Previous studies confirmed that the WNT signaling
pathway plays a crucial role in numerous physiological and
pathological processes, including the regeneration of hair and skin
(27), the repair of liver and lung
after injury (28,29), hematopoiesis (30,31) and
neurogenesis (32). Furthermore,
other studies have demonstrated that the aberrant regulation of WNT
signaling may contribute to various diseases, including cancer
(6,33–35),
osteoporosis (36,37), fibrosis (38–40),
autoimmune diseases (41–43), neurological diseases (44,45), and
disorders of endocrine function (46–48). The
WNT signaling pathway has been shown to either promote or inhibit
cancer biological progression in a cancer stage- and type-specific
manner (6). WNT family genes,
as the most important component of the WNT signaling pathway,
participate in the initiation and progression of various cancers,
such as esophageal carcinoma (16),
gastric cancer (49), pancreatic
cancer (50), prostate cancer
(51), ovarian cancer (52,53), and
leukemia (54). Numerous studies have
reported that WNT family genes may regulate cell
proliferation (50,54), differentiation,
epithelial-to-mesenchymal transition (7,12) and WNT
signaling (9,16). The conclusions of those studies were
consistent with the results of gene function enrichment analysis in
DAVID.
Early discoveries confirmed that WNT family
genes may serve as potential diagnostic biomarkers for certain
types of cancer. Sin et al selected RNA sequencing as a
discovery method for specific RNA markers in bladder cancer, and
found that WNT5A detection was a valuable complementary
strategy in cystoscopy that may reduce unnecessary diagnostic
procedures for bladder cancer (55).
Jiang et al had reported that WNT6 may serve as a
diagnostic biomarker for osteosarcoma, with an AUC of 0.854, a
specificity of 88.4% and a sensitivity of 77.8% (56). Based on these previous studies, it may
be hypothesized that WNTs may also predict HCC. To test this
hypothesis and evaluate the diagnostic value of WNT genes,
an ROC curve was constructed, and the analysis suggested that
WNT2 may serve as potential diagnostic biomarker for
patients with HBV-related HCC.
In addition, we also investigated the prognostic
prediction ability of WNT family genes. The results
demonstrated that the expression level of WNT1 was
associated with OS and RFS, with patients exhibiting a higher
expression level of WNT1 having a better prognostic outcome.
Therefore, WNT1 may serve as potential prognostic biomarker
for HBV-related HCC. It has been reported that WNT family
genes may predict the prognostic outcome in several types of
cancer. As previously reported, WNT1 expression may be one
of the mechanisms underlying WNT/β-catenin signaling pathway
activation in non-small cell lung cancer, and aberrant WNT1
expression level was found to be a predictor of adverse prognosis
(57). Shi et al reported that
the WNT2B genetic variant may be a biomarker for the outcome
of patients with cutaneous melanoma (58). Jiang et al observed that high
expression of WNT6 was a predictor of poor survival of
osteosarcoma (56). Numerous studies
have demonstrated that the expression level of WNT5A is
associated with prognostic outcome and may serve as a prognostic
biomarker in hepatocellular carcinoma (59), gallbladder carcinoma (60) and medulloblastoma (61). Based on these early discoveries, a
prognostic predictive function for WNT family genes in
HBV-related HCC has been confirmed in the present study.
We herein explored the diagnostic and prognostic
value of WNT family gene expression in HBV-related HCC by
collecting data from public databases and performing a series of
bioinformatics analyses, with the aim of identifying more sensitive
biomarkers and design a novel strategy for HCC diagnosis and
treatment. There were certain limitations to the present study that
must be addressed. First, the data were obtained from a public
database and the sample size was limited; therefore, a larger
population and multi-centered clinical studies are required to
increase the credibility of our conclusions. Second, complete
clinical parameters must be included to better evaluate the
association between WNT family genes and HCC prognosis.
Third, further functional validation and clinical trials are
required to reveal the underlying molecular mechanism. Finally,
although we explored the diagnostic and prognostic value at the
mRNA level, the protein level was not investigated in the present
study. Therefore, a comprehensive research design is required to
check the consistency between mRNA and protein expression.
In conclusion, the findings of the present study
demonstrated that WNT2 may serve as diagnostic biomarker and
WNT1 may serve as prognostic biomarker for patients with
HBV-related HCC in the GSE14520 cohort. Furthermore, through
verification of the TCGA cohort, the diagnostic value of
WNT2 and the prognostic value of WNT1 may be further
validated and generalized to HCC patients. Therefore, our results
require further confirmation.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to acknowledge the
laboratory equipment and platform support sponsored by the Key
Laboratory of Early Prevention and Treatment for Regional
High-Incidence Tumors (Guangxi Medical University; Ministry of
Education, Nanning, China). The authors would also like to
acknowledge the helpful comments on this article received from our
reviewers. In addition, we would also like to thank the
contributors of GSE14520 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14520)
and The Cancer Genome Atlas (https://cancergenome.nih.gov/).
Funding
The present study was sponsored in part by the 2018
Innovation Project of Guangxi Graduate Education (grant nos.
JGY2018037 and YCBZ2018036), the Guangxi Key Laboratory for the
Prevention and Control of Viral Hepatitis (grant no.
GXCDCKL201902), the National Natural Science Foundation of China
(grant no. 81802874), the Natural Science Foundation of the Guangxi
Province of China (grant no. 2018GXNSFBA138013), the Key laboratory
of High-Incidence-Tumor Prevention & Treatment (Guangxi Medical
University), Ministry of Education (grant no. GKE2018-01), and the
Guangxi Key R & D Program (grant no. GKEAB18221019).
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
QH and XY designed this study. XW, XL, CH, TY, CY,
GL, BH, KH, GZ, ZL, XZ, HS, LS, YG, XS, TP and XY conducted this
study and analyzed the data. QH wrote the manuscript and XY revised
the manuscript. All the authors have read and approved the final
version of the manuscript for publication and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosetti C, Turati F and La Vecchia C:
Hepatocellular carcinoma epidemiology. Best Pract Res Clin
Gastroenterol. 28:753–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan JH, Wang JB, Jiang Y, Xiang W, Liang
H, Wei WQ, Qiao YL and Boffetta P: Attributable causes of liver
cancer mortality and incidence in China. Asian Pac J Cancer Prev.
14:7251–7256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Martel C, Maucort-Boulch D, Plummer M
and Franceschi S: World-wide relative contribution of hepatitis B
and C viruses in hepatocellular carcinoma. Hepatology.
62:1190–1200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balogh J, Victor D III, Asham EH,
Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM and Monsour
HP Jr: Hepatocellular carcinoma: A review. J Hepatocell Carcinoma.
3:41–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y
and Gu Q: Wnt5a suppresses colon cancer by inhibiting cell
proliferation and epithelial-mesenchymal transition. J Cell
Physiol. 229:1908–1917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiah SG, Hsiao JR, Chang WM, Chen YW, Jin
YT, Wong TY, Huang JS, Tsai ST, Hsu YM, Chou ST, et al:
Downregulated miR329 and miR410 promote the proliferation and
invasion of oral squamous cell carcinoma by targeting Wnt-7b.
Cancer Res. 74:7560–7572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Jia C, Jia C, Jin X and Gu X:
MicroRNA-374a inhibits aggressive tumor biological behavior in
bladder carcinoma by suppressing Wnt/β-catenin signaling. Cell
Physiol Biochem. 48:815–826. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto H, Oue N, Sato A, Hasegawa Y,
Yamamoto H, Matsubara A, Yasui W and Kikuchi A: Wnt5a signaling is
involved in the aggressiveness of prostate cancer and expression of
metalloproteinase. Oncogene. 29:2036–2046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Sun Z, Su L, Wang F, Jiang Y, Yu
D, Zhang F, Sun Z and Liang W: miRNA-185 serves as a prognostic
factor and suppresses migration and invasion through Wnt1 in colon
cancer. Eur J Pharmacol. 825:75–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bo H, Zhang S, Gao L, Chen Y, Zhang J,
Chang X and Zhu M: Upregulation of Wnt5a promotes
epithelial-to-mesenchymal transition and metastasis of pancreatic
cancer cells. BMC Cancer. 13:4962013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Fan L, Xia X, Rao Y, Ma Q, Yang J,
Lu Y, Wang C and Huang X: Silencing Wnt2B by siRNA interference
inhibits metastasis and enhances chemotherapy sensitivity in
ovarian cancer. Int J Gynecol Cancer. 22:755–761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Webster MR, Xu M, Kinzler KA, Kaur A,
Appleton J, O'Connell MP, Marchbank K, Valiga A, Dang VM, Perego M,
et al: Wnt5A promotes an adaptive, senescent-like stress response,
while continuing to drive invasion in melanoma cells. Pigment Cell
Melanoma Res. 28:184–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen PH, Liu AJ, Ho KH, Chiu YT, Anne Lin
ZH, Lee YT, Shih CM and Chen KC: microRNA-199a/b-5p enhance
imatinib efficacy via repressing WNT2 signaling-mediated protective
autophagy in imatinib-resistant chronic myeloid leukemia cells.
Chem Biol Interact. 291:144–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu L, Zhang C, Zhang LY, Dong SS, Lu LH,
Chen J, Dai Y, Li Y, Kong KL, Kwong DL and Guan XY: Wnt2 secreted
by tumour fibroblasts promotes tumour progression in oesophageal
cancer by activation of the Wnt/β-catenin signalling pathway. Gut.
60:1635–1643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roessler S, Jia HL, Budhu A, Forgues M, Ye
QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX and Wang XW: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roessler S, Long EL, Budhu A, Chen Y, Zhao
X, Ji J, Walker R, Jia HL, Ye QH, Qin LX, et al: Integrative
genomic identification of genes on 8p associated with
hepatocellular carcinoma progression and patient survival.
Gastroenterology. 142:957–966.e12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao X, Liu X, Yang C, Wang X, Yu T, Han
C, Huang K, Zhu G, Su H, Qin W, et al: Distinct diagnostic and
prognostic values of minichromosome maintenance gene expression in
patients with hepatocellular carcinoma. J Cancer. 9:2357–2373.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anders S and Huber W: Differential
expression analysis for sequence count data. Genome Biol.
11:R1062010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maere S, Heymans K and Kuiper M: BiNGO: A
Cytoscape plugin to assess overrepresentation of gene ontology
categories in biological networks. Bioinformatics. 21:3448–3449.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mostafavi S, Ray D, Warde-Farley D,
Grouios C and Morris Q: GeneMANIA: A real-time multiple association
network integration algorithm for predicting gene function. Genome
Biol. 9 (Suppl 1):S42008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: A database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shaul YD, Yuan B, Thiru P, Nutter-Upham A,
McCallum S, Lanzkron C, Bell GW and Sabatini DM: MERAV: A tool for
comparing gene expression across human tissues and cell types.
Nucleic Acids Res. 44:D560–566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alonso L and Fuchs E: Stem cells in the
skin: Waste not, Wnt not. Genes Dev. 17:1189–1200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Monga SP: Role of Wnt/β-catenin signaling
in liver metabolism and cancer. Int J Biochem Cell Biol.
43:1021–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beers MF and Morrisey EE: The three R's of
lung health and disease: Repair, remodeling, and regeneration. J
Clin Invest. 121:2065–2073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nemeth MJ, Mak KK, Yang Y and Bodine DM:
Beta-Catenin expression in the bone marrow microenvironment is
required for long-term maintenance of primitive hematopoietic
cells. Stem Cells. 27:1109–1119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malhotra S and Kincade PW: Wnt-related
molecules and signaling pathway equilibrium in hematopoiesis. Cell
Stem Cell. 4:27–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Inestrosa NC and Arenas E: Emerging roles
of Wnts in the adult nervous system. Nat Rev Neurosci. 11:77–86.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Polakis P: Drugging Wnt signalling in
cancer. EMBO J. 31:2737–2746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Herr P, Hausmann G and Basler K: WNT
secretion and signalling in human disease. Trends Mol Med.
18:483–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kikuchi A and Yamamoto H: Tumor formation
due to abnormalities in the beta-catenin-independent pathway of Wnt
signaling. Cancer Sci. 99:202–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Day TF, Guo X, Garrett-Beal L and Yang Y:
Wnt/beta-catenin signaling in mesenchymal progenitors controls
osteoblast and chondrocyte differentiation during vertebrate
skeletogenesis. Dev Cell. 8:739–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Glass DA II, Bialek P, Ahn JD, Starbuck M,
Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA and
Karsenty G: Canonical Wnt signaling in differentiated osteoblasts
controls osteoclast differentiation. Dev Cell. 8:751–764. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Königshoff M, Balsara N, Pfaff EM, Kramer
M, Chrobak I, Seeger W and Eickelberg O: Functional Wnt signaling
is increased in idiopathic pulmonary fibrosis. PLoS One.
3:e21422008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Henderson WR Jr, Chi EY, Ye X, Nguyen C,
Tien YT, Zhou B, Borok Z, Knight DA and Kahn M: Inhibition of
Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses
pulmonary fibrosis. Proc Natl Acad Sci USA. 107:14309–14314. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Akhmetshina A, Palumbo K, Dees C, Bergmann
C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, et al:
Activation of canonical Wnt signalling is required for
TGF-β-mediated fibrosis. Nat Commun. 3:7352012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sen M, Lauterbach K, El-Gabalawy H,
Firestein GS, Corr M and Carson DA: Expression and function of
wingless and frizzled homologs in rheumatoid arthritis. Proc Natl
Acad Sci USA. 97:2791–2796. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nakamura Y, Nawata M and Wakitani S:
Expression profiles and functional analyses of Wnt-related genes in
human joint disorders. Am J Pathol. 167:97–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Keerthivasan S, Aghajani K, Dose M,
Molinero L, Khan MW, Venkateswaran V, Weber C, Emmanuel AO, Sun T,
Bentrem DJ, et al: β-catenin promotes colitis and colon cancer
through imprinting of proinflammatory properties in T cells. Sci
Transl Med. 6:225ra2282014. View Article : Google Scholar
|
|
44
|
Kalkman HO: A review of the evidence for
the canonical Wnt pathway in autism spectrum disorders. Mol Autism.
3:102012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
De Ferrari GV, Papassotiropoulos A,
Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Sáez
K, Henríquez JP, Zhao A, et al: Common genetic variation within the
low-density lipoprotein receptor-related protein 6 and late-onset
Alzheimer's disease. Proc Natl Acad Sci USA. 104:9434–9439. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
García-Jiménez C: Wnt and incretin
connections. Vitam Horm. 84:355–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Welters HJ and Kulkarni RN: Wnt signaling:
Relevance to beta-cell biology and diabetes. Trends Endocrinol
Metab. 19:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grant SF, Thorleifsson G, Reynisdottir I,
Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H,
Emilsson V, Helgadottir A, et al: Variant of transcription factor
7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet.
38:320–323. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kurayoshi M, Oue N, Yamamoto H, Kishida M,
Inoue A, Asahara T, Yasui W and Kikuchi A: Expression of Wnt-5a is
correlated with aggressiveness of gastric cancer by stimulating
cell migration and invasion. Cancer Res. 66:10439–10448. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schwartz AL, Malgor R, Dickerson E,
Weeraratna AT, Slominski A, Wortsman J, Harii N, Kohn AD, Moon RT,
Schwartz FL, et al: Phenylmethimazole decreases Toll-like receptor
3 and noncanonical Wnt5a expression in pancreatic cancer and
melanoma together with tumor cell growth and migration. Clin Cancer
Res. 15:4114–4122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li X, Placencio V, Iturregui JM, Uwamariya
C, Sharif-Afshar AR, Koyama T, Hayward SW and Bhowmick NA: Prostate
tumor progression is mediated by a paracrine TGF-beta/Wnt3a
signaling axis. Oncogene. 27:7118–7130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yoshioka S, King ML, Ran S, Okuda H,
MacLean JA II, McAsey ME, Sugino N, Brard L, Watabe K and Hayashi
K: WNT7A regulates tumor growth and progression in ovarian cancer
through the WNT/beta-catenin pathway. Mol Cancer Res. 10:469–482.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bitler BG, Nicodemus JP, Li H, Cai Q, Wu
H, Hua X, Li T, Birrer MJ, Godwin AK, Cairns P and Zhang R: Wnt5a
suppresses epithelial ovarian cancer by promoting cellular
senescence. Cancer Res. 71:6184–6194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ochoa-Hernández AB, Ramos-Solano M,
Meza-Canales ID, García-Castro B, Rosales-Reynoso MA, Rosales-Aviña
JA, Barrera-Chairez E, Ortíz-Lazareno PC, Hernández-Flores G,
Bravo-Cuellar A, et al: Peripheral T-lymphocytes express WNT7A and
its restoration in leukemia-derived lymphoblasts inhibits cell
proliferation. BMC Cancer. 12:602012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sin MLY, Mach KE, Sinha R, Wu F, Trivedi
DR, Altobelli E, Jensen KC, Sahoo D, Lu Y and Liao JC: Deep
sequencing of urinary RNAs for bladder cancer molecular
diagnostics. Clin Cancer Res. 23:3700–3710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jiang K, Li S, Li L, Wang X, Gu Y and Jin
Z: WNT6 is an effective marker for osteosarcoma diagnosis and
prognosis. Medicine (Baltimore). 97:e130112018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu X, Sun PL, Li JZ, Jheon S, Lee CT and
Chung JH: Aberrant Wnt1/β-catenin expression is an independent poor
prognostic marker of non-small cell lung cancer after surgery. J
Thorac Oncol. 6:716–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shi Q, Liu H, Han P, Li C, Wang Y, Wu W,
Zhu D, Amos CI, Fang S, Lee JE, et al: Genetic variants in WNT2B
and BTRC predict melanoma survival. J Invest Dermatol.
137:1749–1756. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ
and Liu XH: Loss of Wnt5a and Ror2 protein in hepatocellular
carcinoma associated with poor prognosis. World J Gastroenterol.
18:1328–1338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu ZC, Xiong L, Wang LX, Miao XY, Liu ZR,
Li DQ, Zou Q, Liu KJ, Zhao H and Yang ZL: Comparative study of ROR2
and WNT5a expression in squamous/adenosquamous carcinoma and
adenocarcinoma of the gallbladder. World J Gastroenterol.
23:2601–2612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee SE, Lim SD, Kang SY, Suh SB and Suh
YL: Prognostic significance of Ror2 and Wnt5a expression in
medulloblastoma. Brain Pathol. 23:445–453. 2013. View Article : Google Scholar : PubMed/NCBI
|