Introduction
Prostate cancer (PCa) is the most common cancer in
men aged ≥65 years with an average age of ~66 at the time of
diagnosis (1). PCa ranks the third
leading cause of male cancer-associated mortality with an estimated
31,620 deaths in 2019 in the USA. Metastasis is the main cause of
PCa-related mortality, in which cancer cells spread to bladder,
bone, retroperitoneal lymph nodes, spinal cord and other areas
(2,3).
About 40% of localized PCa patients relapsed after initial therapy
(4) and they usually succumb due to
cancer metastasis or drug resistance. Thus, it is a great challenge
to determine the underlying mechanism of PCa progression,
recurrence and metastasis to improve the outcomes of treating
PCa.
Hypoxia is a tumorigenesis-related microenvironment
change which usually occurs in the earliest stage of PCa
development (5). In response to
decreasing oxygen availability, the activity of hypoxia-inducible
factors (HIFs) in cells increase and also mediate various
transcriptional changes (6). HIF
heterodimers comprise HIFα and HIFβ, the former is sensitive to
oxygen and the latter is a constitutively expressed subunit. Under
hypoxic conditions, HIF-1α dimerizes with HIF-1β and translocates
into nucleus to bind to the hypoxia-responsive element, which is
the specific sequence present in the promoter of several
hypoxia-dependent target genes (7).
Mechanistically, hypoxia was demonstrated to affect the invasive
and migratory behavior of PCa cells via epithelial-to-mesenchymal
transition (EMT), a trans-differentiation of cells for the
acquirement of plasticity and increased mobility, a process which
alters the metastatic potential of cancer cells (8). It is widely reported that hypoxia is the
inducer of the EMT process in various epithelial cancers, such as
PCa, ovarian carcinoma, lung cancer and hepatocellular carcinoma
(9–11), thus facilitating tumor cell survival
and resistance to chemo or radio-therapies (12,13).
Hypoxia-induced EMT is characterized by a decrease in epithelial
gene expression, such as E-cadherin and β-catenin and an increase
in mesenchymal associated gene expression, such as N-cadherin and
vimentin (14). Hypoxia can also
activate downstream transcription factors such as Smads, SNAIL,
SLUG, and TWIST, and inhibits the expression of E-cadherin
(15). Recently, it was reported that
chronic hypoxia-induced SLUG promotes the EMT of PCa cells by
activating expression of Ephrin-B1 (16); however, the detailed mechanisms
leading to the induction of EMT by hypoxia remains largely
unknown.
Forkhead box M1 (FoxM1) is a classic proliferation-
associated transcription factor belonging to the family of Forkhead
box (Fox) protein, which consists of a conserved forkhead
DNA-binding domain, an N-terminal repressor domain, and a
C-terminal transactivation domain (17). In addition to cell proliferation,
FoxM1 is also involved in cell cycle regulation, angiogenesis,
invasion, metastasis and EMT (18,19). FoxM1
has been reported to be upregulated and of prognostic significance
in several malignancies including breast cancer, colorectal cancer,
lung cancer, melanoma and PCa (20–25). Over
the past few decades, understanding of the function and regulation
of FoxM1 has notably improved, providing novel insights into the
roles of this transcription factor in cancer development and
progression. Additionally, certain small molecule inhibitors that
target FoxM1 have promising potential as therapeutic drugs against
PCa and have been receiving great attention by urologists and
patients. There is thus increasing interest in elucidating the
regulatory mechanisms of FoxM1 in PCa.
In our study, we reported that FoxM1 was
transcriptionally regulated by HIF-1α in PCa. FoxM1 was upregulated
in PC3 and DU145 PCa cells under hypoxic conditions, which were
associated with EMT induction and enhanced invasive ability. This
process could be inhibited by the suppression of HIF-1α activity.
Furthermore, HIF-1α binding sites were illustrated and mapped to
the promoter of FoxM1. Thus, we concluded that EMT of hypoxic PCa
cells is mediated by the transcriptional regulation of FoxM1 by
HIF-1α.
Materials and methods
Reagents and antibodies
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), penicillin and streptomycin cocktail were
purchased from HyClone (GE Healthcare). Primary antibodies against
β-actin (cat. no. 3700), E-cadherin (cat. no. 3195), vimentin (cat.
no. 3932), FoxM1 (cat. no. 5436), and HIF-1α (cat. no. 3716) were
purchased from Cell Signaling Technology; the dilutions for primary
antibody es were 1:1,000. YC-1, proteinase and phosphatase
inhibitors cocktail were from Sigma-Aldrich (Merck KGaA). Transwell
mini-cells were obtained from EMD Millipore and Matrigel was
purchased from BD Medical Technology (BD Biosciences).
Nitrocellulose membranes and Enhanced Chemiluminescence reagents
were purchased from Bio-Rad Laboratories, Inc.
Cell culture
PC3 and DU145 cell lines were purchased from the
American Type Culture Collection. Both cell lines were cultured in
DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin. Cells were incubated in an atmosphere of 95% humidity
at 37°C, 5% CO2. Culture medium was replaced with fresh
medium every other day or according to experimental designs. For
hypoxia, cells were plated and adhered overnight, then cells were
grown in a hypoxia incubator at 37°C in 1% O2, 5%
CO2 and 94% N2 for different time periods
(24, 48 and 72 h). For YC-1 treatment, cells were pretreated with
50 µM YC-1 for 24 h at 37°C, 5% CO2 and then exposed to
normoxic or hypoxic conditions. To detect the changes of HIF-1α and
FoxM1 in both mRNA and protein levels under hypoxic condition,
cells were plated and adhered overnight under normoxic condition
and then exposed to hypoxic condition for different time periods
(0, 6, 12 and 24 h).
Lentivirus preparation, siRNA, and
transfection
PLKO.1 lentiviral vector was used to package
encoding short hairpin RNAs (shRNAs) of FoxM1 with the sequence,
5′-GGAAATGCTTGTGATTCAACA-3′. To generate the lentivirus, 8 µg PAX2
packaging vector, 2 µg VSV-G and 8 µg the aforementioned plasmids
or empty vectors as the negative control were co-transfected into
90% confluent 293T cells (purchased from the American Type Culture
Collection) in 10 cm plates. Lentivirus expressing FoxM1 was
produced using pcDNA3-FoxM1 plasmid, which was sub-cloned into LV5
vectors according to the manufacturer's protocols (Shanghai
GenePharma Co., Ltd.). The pcDNA3-FoxM1 vector was generated by the
human FoxM1 coding region cDNA subcloning into the pcDNA3.1
(Invitrogen; Thermo Fisher Scientific, Inc.) plasmid. The empty
vectors were used as the negative control. The transfection reagent
used for the above protocols was Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. Cells were infected by the virus with
multiplicity of infection of 40 then selected and maintained in 2–3
µg/ml puromycin for subsequent experiments. Small interfering RNA
targeting HIF-1α was designed and synthesized by Shanghai
GenePharma Co., Ltd. The sequence of HIF-1α siRNA was as follows:
siHIF1α: 5′-AGCACUACUUCGAAGUGGCTT-3′. Cells were transfected with
X-tremeGene siRNA transfection reagents (Roche Diagnostics GmbH)
for 2–3 days, and harvested for subsequent experiments.
Matrigel migration and invasion
assay
Cells were seeded onto 6-well plates and cultured to
60% confluence. Then, cells were treated with or without hypoxia
for 24 h. Cells were digested with Trypsin-EDTA Solution (0.25%
trypsin, 0.02% EDTA) and centrifuged at 160 × g, room temperature
for 5 min. 50,000 cells were seeded onto a Transwell chamber
(Corning, Inc.) without Matrigel (migration assay) and
8×104 cells were seeded onto a Transwell chamber with
Matrigel (invasion assay) in 200 µl serum-free medium. For invasion
assay, Matrigel was diluted with serum-free medium (1:4) and coated
by 40 µl onto the Transwell chamber then put in 37°C for 1 h; 1 ml
medium containing 10% FBS was added to the lower chamber (24-well
plate) as a chemoattractant. After incubation at 37°C for 20 h,
non-migrated or non-invaded cells on the upper surface of the
filter were removed with a cotton swab. Cells invaded to the
underside of the filter membrane were stained by 0.1% crystal
violet at room temperature for 10 min and the cell numbers were
calculated statistically in five random fields using an inverted
light microscope (magnification, ×200).
Western blot analysis
Cells were prepared with radioimmunoprecipitation
assay buffer (50 mM Tris pH 8.0, 150 mM NaCl, 0.1% SDS, 1% NP-40,
and 0.5% sodium deoxycholate). Protein was separated by 12%
SDS-PAGE and transferred onto nitrocellulose membranes. The
membranes were blocked in 5% skim milk at room temperature for 1 h
and then incubated with primary antibodies at 4°C overnight.
Following washing with TBST, membranes were incubated with Goat
anti-rabbit (cat. no. 926-32211) or goat anti-mouse secondary
antibodies (cat. no. 926-68070) (LI-COR Biosciences) diluted at
1:3,000 in 5% skimmed milk at room temperature in the dark for 1 h.
Following washing with TBST, membranes were visualized by an
Odyssey infrared imaging system (Li-Cor Biosciences). The relative
intensity of each band was determined by using Glyko BandScan
software version 4.0 (Glyko Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells were harvested with TRIzol reagent (Thermo
Fisher Scientific, Inc.) to extract total RNA. Then, the lysates
ware reverse-transcribed into cDNA using a PrimeScript™ RT reagent
kit (Takara Bio, Inc.) according to the manufacturer's protocols.
cDNA was employed for qPCR using a CFX96 real-time PCR system
(Bio-Rad Laboratories, Inc.) with SYBR®-Green PCR Master
Mix (Takara, Dalian, China) to determine the expression of target
genes. The thermocycling conditions were defined as follows: 95°C
10 min, 1 cycle; 95°C 10 sec, 60°C 30 sec, 72°C 30 sec, 40 cycles;
72°C 10 min, 1 cycle. Relative gene expression was calculated by
the 2−ΔΔCq method (26).
The primers used were: β-actin, forward, 5′-AAGGATTCCTATGTCGGC-3′
and reverse, 5′-CTTCATGATGGAGTTGAAGGT-3′; HIF-1α,
5′-AGCTTGCTCATCAGTTGCCA-3′ and reverse, 5′-CCAGAAGTTTCCTCACACGC-3′;
FoxM1, 5′-GGAGCAGCGACAGGTTAAGG-3′ and reverse,
5′-GTTGATGGCGAATTGTATCATGG-3′.
Dual-luciferase activity assay
FoxM1 promoter report plasmid pGL3-FoxM1 was
generated by inserting different lengths promoter fragments of
FoxM1 (−1,000/+40, −878/+40, −300/+40, −220/+40, −50+40) into the
pGL3-basic plasmid (Shanghai GenePharma Co., Ltd.). The vector
construct was validated by sequencing. PC3 and DU145 cells under
normoxic or hypoxic conditions were cotransfected with ERE-TK-Luc,
pGL3-basic, and pGL3-FoxM1 using X-tremeGENE HP DNA transfection
reagent (Roche Diagnostics). Then, a dual-luciferase activity assay
was carried out using the Dual Luciferase Assay kit (Promega
Corporation) following the manufacturer's instructions. Each data
point used three wells of cells and the results were calculated
statistically.
Bioinformatics analysis
The generate a heatmap for analysis, the
RNA-sequencing data of PCa in count format was downloaded from The
Cancer Genome Atlas (TCGA: http://cancergenome.nih.gov/). Then, we sorted the
data according to the level of HIF1A expression. The top 50 samples
with high expression of HIF1A and the bottom 50 samples with low
expression of HIF1A were extracted. Furthermore, differentially
expressed genes (DEGs) were identified by edgeR package (v3.26.5,
http://www.bioconductor.org/packages/release/bioc/html/edgeR.html)
(27,28) using R software; P<0.05 and fold
change >2 were considered as statistically significant. The
expression of 50 DEGs were presented in the heatmap.
In addition, the expression of FoxM1 in TCGA
prostate normal and cancer samples with the Gleason score was
presented as a boxplot, which was analyzed using the UALCAN online
tool (http://ualcan.path.uab.edu/analysis.html) with
P<0.01 among groups. Gene Ontology (GO) and pathway enrichment
analyses were performed on the top 200 upregulated DEGs using
Metascape (http://metascape.org/gp/) (29). Moreover, the RNA-sequencing data of
PCa in FPKM format was downloaded from TCGA, after log2(x+1)
normalization, the HIF1A and FOXM1 expression were extracted;
Pearson's correlation was performed using R software.
Statistical analysis
GraphPad Prism 7 software (GraphPad Software, Inc.)
was used for the statistical analysis. The difference between two
groups was analyzed by a Student's t-test. Comparisons between
multiple groups were performed by one-way analysis of variance,
followed by Dunnett's post-hoc test in which one group was compared
against all the other groups or a Tukey's multiple comparison test
in which pairwise comparisons between all groups were performed.
Data were presented as mean ± standard deviation of three
replicates. P<0.05 was considered to indicate a statistically
significant difference.
Results
Hypoxia results in the induction of
EMT with upregulation of HIF-1α and FoxM1 in PCa cells
We firstly investigated the effects of hypoxia on
the EMT of PCa cells. Transwell migration and invasion assays were
performed to assess the migration and invasive abilities of PC3 and
DU145 cells. Compared with the normoxic control groups, the number
of migrated and invasive cells was significantly higher under
hypoxic conditions compared with normoxic conditions. This
indicated that hypoxia could increase the migration and invasion
abilities of PC3 and DU145 cells (Fig.
1A). Furthermore, the expression of epithelial and mesenchymal
phenotypic markers was determined by western blotting analysis. As
presented in Fig. 1B, compared with
the normoxic conditions, the expression of E-cadherin was
significantly decreased, while that of vimentin was significantly
increased in PC3 and DU145 cells under hypoxic conditions; the
highest levels of vimentin were observed at 48 h. Increased
expression of HIF-1α and FoxM1 protein levels were also found under
hypoxic conditions.
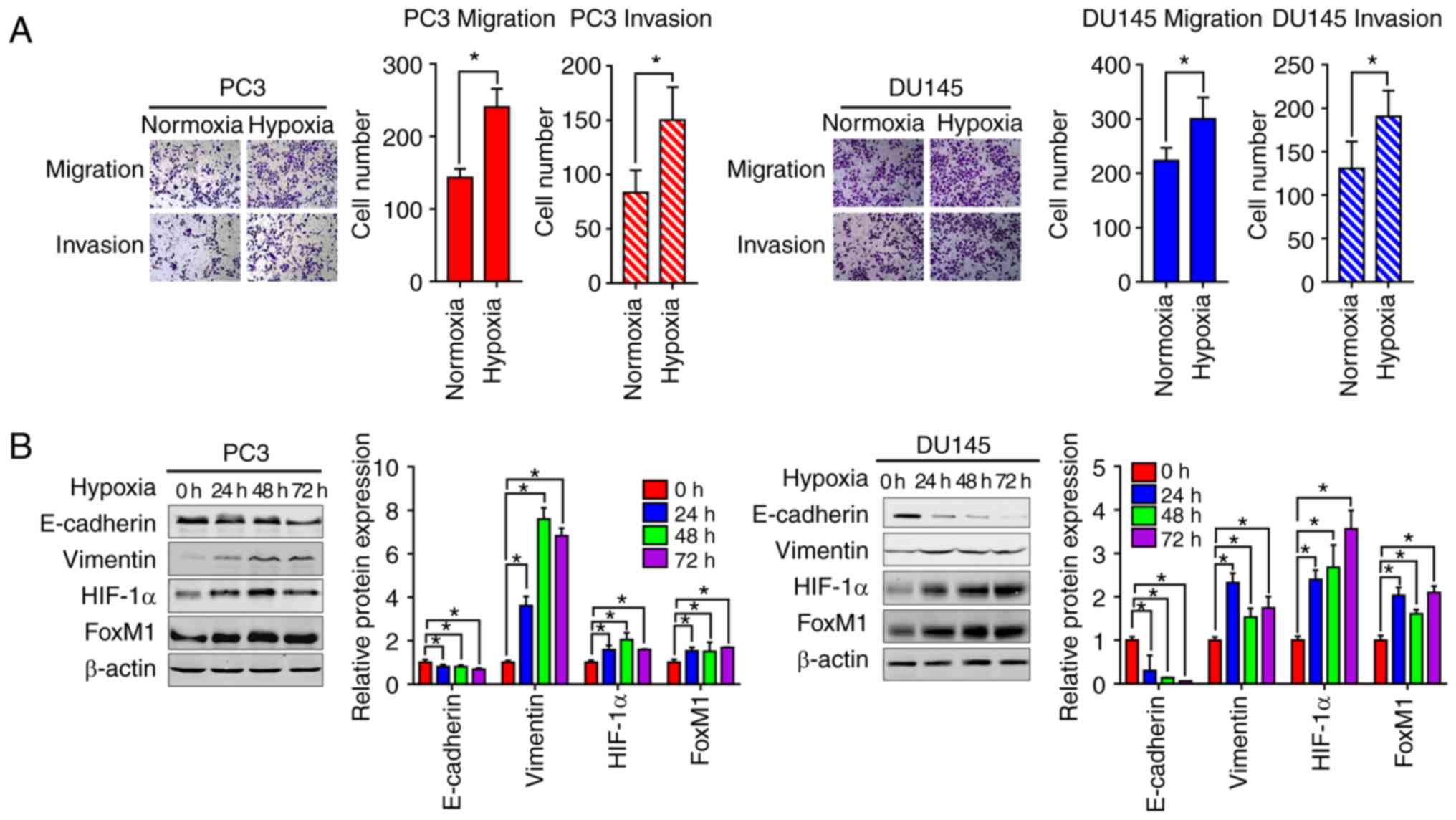 | Figure 1.Hypoxia induces EMT in PC3 and DU145
prostate cancer cells with the upregulation of HIF-1α and FoxM1.
(A) The migration and invasion were tested by Transwell invasion
assay in PC3 (left panel) and DU145 (right panel) cells. Cells were
placed onto the upper chamber without or with Matrigel under
normoxic or hypoxic conditions (24 h) and the cell images were
obtained (magnification, ×200). Quantification analysis is shown.
(B) PC3 and DU145 cells were cultured under normoxic or hypoxic
conditions for different time periods (0, 24, 48 and 72 h), then
the protein expression levels of EMT markers, including E-cadherin,
vimentin, HIF-1α and FoxM1 were detected by western blotting.
Similar results were observed in two additional experiments. Data
are presented as the mean ± standard deviation of three replicates.
*P<0.05 vs. control. EMT, epithelial-mesenchymal transition;
FoxM1, Forkhead box M1; HIF-1α, hypoxia-inducible factor 1α. |
Bioinformatics analysis of potential
correlation between HIF-1α and FoxM1 in PCa
We next aimed to identify the possible molecular
mechanisms that were responsible for hypoxia-induced EMT. GO
enrichment analysis showed that high HIF-1α expression was
associated with ‘homophilic cell adhesion via plasma membrane
adhesion molecules’ (Fig. 2A). From
the heatmap, we found that the expression of FoxM1 was upregulated
in samples with high HIF-1α expression (Fig. 2B). Similar results were also obtained
by correlation analysis, which showed that HIF-1α expression was
positively correlated with FoxM1 expression (Fig. 2C). Furthermore, compared with normal
tissue, upregulated expression of FoxM1 was detected in primary
tumor samples (Fig. 2D); the
expression of FoxM1 also increased gradually with higher Gleason
scores (Fig. 2D). These results
indicated that HIF-1α and FoxM1 may have important interactions,
and their expression serves an important role in the invasion,
migration and progression of PCa.
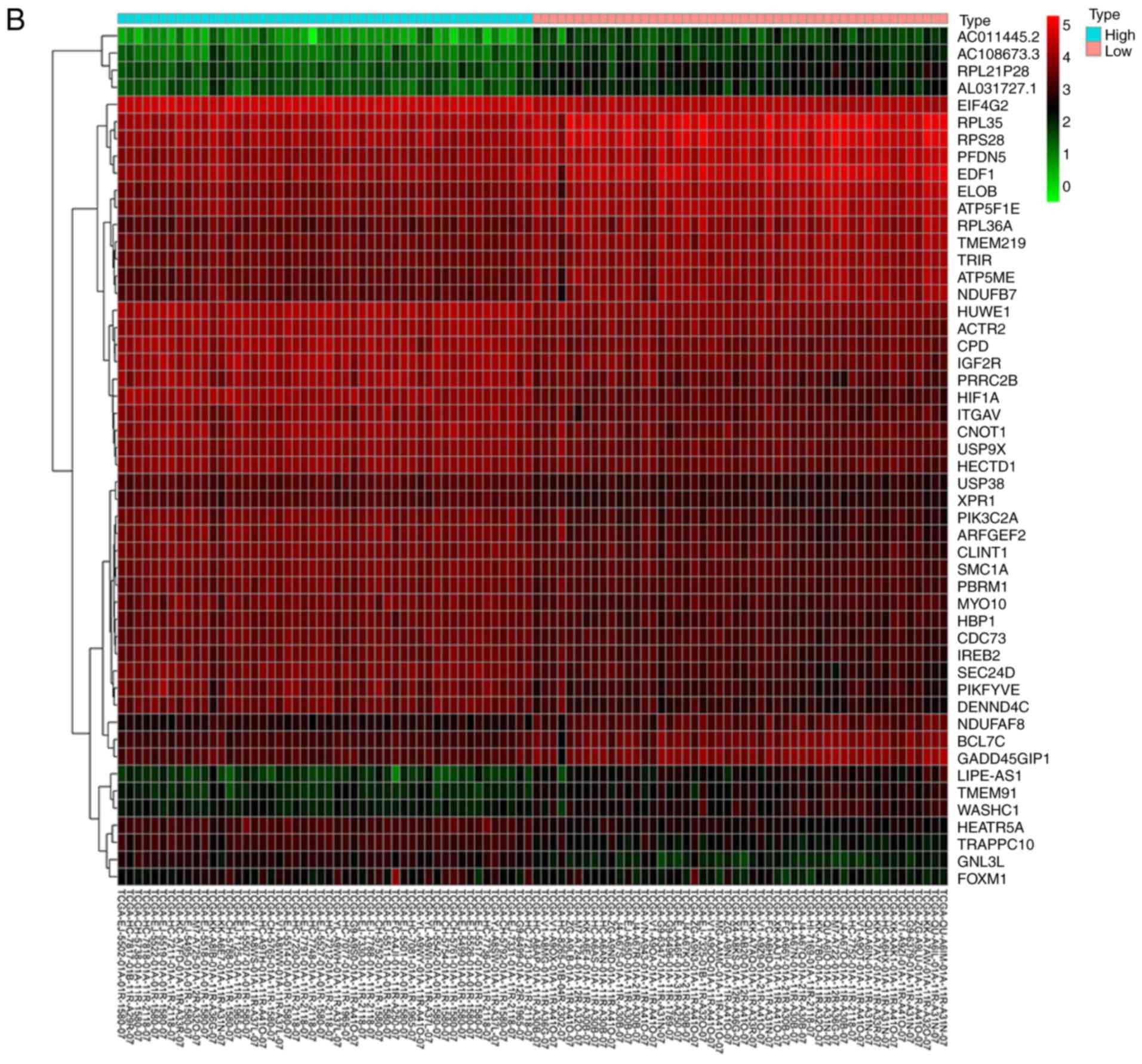 | Figure 2.Bioinformatics analysis and the
correlation between HIF-1α and FoxM1 in PCa. (A) High and low
expression of HIF1A TCGA samples were used to identify DEGs. The
results of GO and pathway enrichment applied on top 200 upregulated
DEGs are shown in the bar plot, the higher the column and the
darker the color, the smaller the P-value, and the corresponding
enrichment entry is marked on the right. (B) The relative
expression difference of DEGs was shown in the heatmap, where the
green and red represented low and high expression, respectively.
DEGs, differentially expressed genes; GO, Gene Ontology; FoxM1,
Forkhead box M1; HIF1A, hypoxia-inducible factor 1α; has, homo
sapiens; PCa, prostate cancer; PRAD, prostate adenocarcinoma;
TCGA, The Cancer Genome Atlas. (C) Pearson correlation analysis of
HIF1A and FoxM1 in TCGA prostate samples was performed; the red
line represented the expression trend of the two genes. (D) The
expression of FoxM1 in PRAD and normal prostate tissues (left
panel), and the relationship between FoxM1 and Gleason scores
(right panel). DEGs, differentially expressed genes; GO, Gene
Ontology; FoxM1, Forkhead box M1; HIF1A, hypoxia-inducible factor
1α; has, homo sapiens; PCa, prostate cancer; PRAD, prostate
adenocarcinoma; TCGA, The Cancer Genome Atlas. |
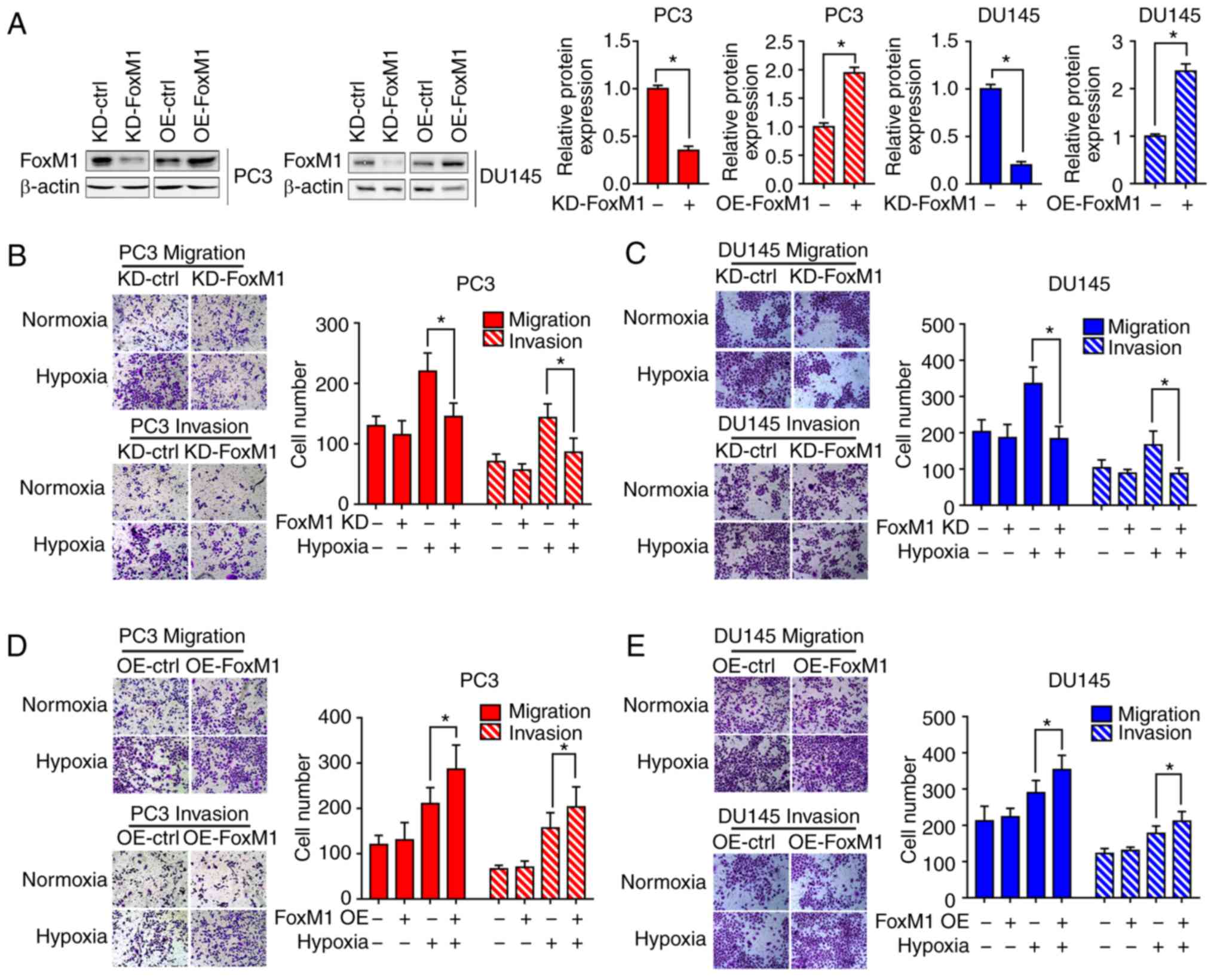
FoxM1 is required for hypoxia-induced
EMT in PC3 and DU145 PCa cells
To determine whether hypoxia-induced EMT is
dependent on FoxM1, FoxM1 knockdown (KD-FoxM1) and FoxM1
overexpression (OE-FoxM1) PC3 and DU145 (Fig. 3A) cell lines were established, and the
expression was verified by western blot analysis. Different PC3 and
DU145 cell clones were exposed to hypoxia or not for 48 h. Then,
the cell migration (Fig. 3B and D)
and invasive (Fig. 3C and E)
abilities were determined by Transwell migration and invasion
assays. Under hypoxic conditions, the number of cells migrating or
invading through the chamber was significantly decreased in
KD-FoxM1 groups compared with KD-control (ctrl) groups. In
addition, the cell migration and invasive abilities were
significantly increased in OE-FoxM1 than in OE-ctrl groups after
exposure to hypoxia.
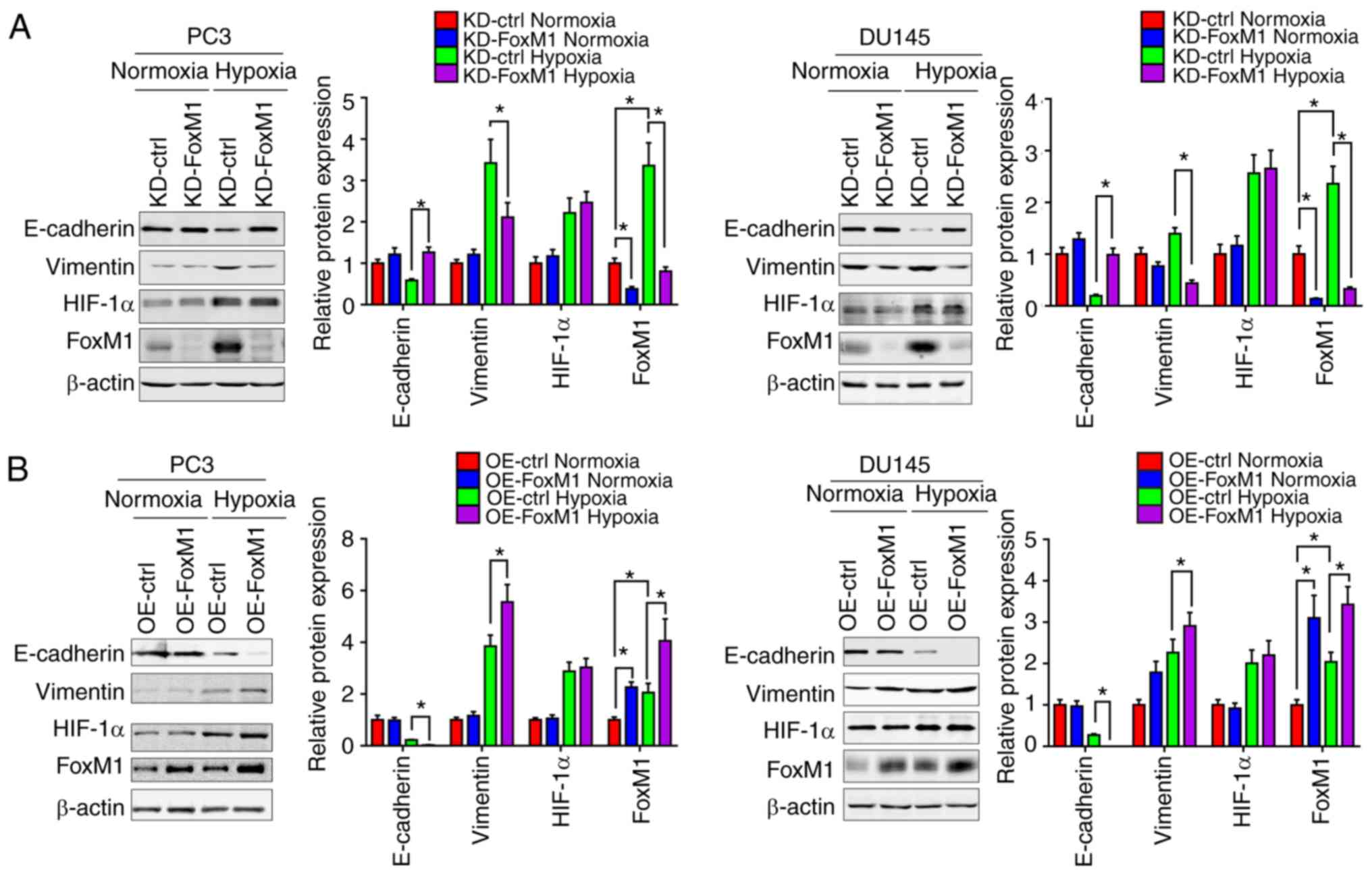
Furthermore, the protein levels of epithelial and
mesenchymal phenotypic markers in PC3 and DU145 were measured by
western blot analysis (Fig. 4A and
B). Under hypoxic conditions, compared with the control groups,
the expression of E-cadherin was increased in response to FoxM1
knockdown and decreased following FoxM1 overexpression, while the
expression of vimentin was decreased by FoxM1 knockdown and
increased by FoxM1 overexpression. Additionally, the expression of
HIF-1α exhibited no significant change when the expression of FoxM1
was manipulated, suggesting that HIF-1α may not be regulated by
FoxM1. The results indicated that FoxM1 serves an important role in
the induction of EMT by hypoxia in PC3 and DU145 cells.
Inhibition of HIF-1α blocks the
increase of FoxM1 induced by hypoxia at the mRNA level in PC3 and
DU145 PCa cells
To further study the mechanism of hypoxia on FoxM1,
the expression of HIF-1α and FoxM1 were evaluated by western
blotting (Fig. 5A) and RT-qPCR
(Fig. 5B) in PC3 and DU145 cells
exposed to hypoxia for different durations (0, 6, 12 and 24 h). As
presented in Fig. 5A and B, there
were no significant changes in the expression of HIF-1α mRNA under
different durations of hypoxia, while the protein expression levels
of HIF-1α were significantly increased in hypoxia-exposed PC3 and
DU145 cells compared with 0 h of exposure. Interestingly, increased
FoxM1 expression was detected at the protein and mRNA levels,
suggesting that FoxM1 was potentially regulated at the
transcriptional level in PC3 and DU145 under hypoxia. As we
proposed that HIF-1α was not regulated by FoxM1, we further
investigated whether the induction of FoxM1 was regulated by
HIF-1α. The RNA interference (Fig. 5C and
E) and inhibitor YC-1 (Fig. 5D and
5F) of HIF-1α were used in PC3 and DU145 cells. The inhibitory
effects of siRNA and YC-1 on HIF-1α expression was confirmed by
western blotting (Fig. 5C and D). The
results showed that knockdown of HIF-1α significantly inhibited
hypoxia-induced overexpression of FoxM1 at the protein (Fig. 5C) and mRNA level (Fig. 5E). Similarly, HIF-1α inhibitor YC-1
was also found to significantly inhibit hypoxia-induced FoxM1
expression at the protein (Fig. 5D)
and mRNA level (Fig. 5F).
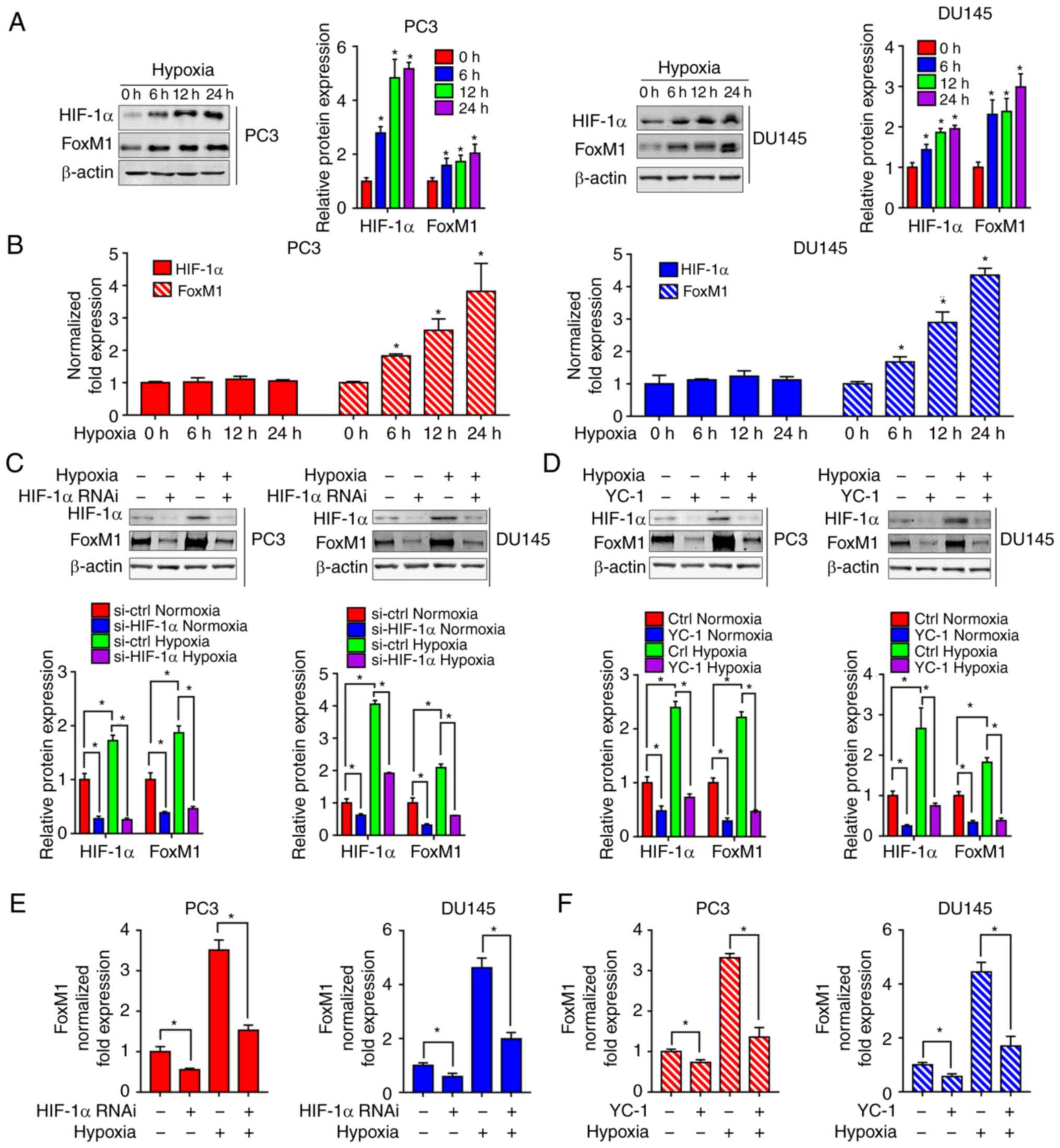 | Figure 5.Inhibition of HIF-1α blocks
hypoxia-induced upregulation of FoxM1. (A) PC3 (left panel) and
DU145 (right panel) cells were cultured under normoxic or hypoxic
conditions for different durations (0, 6, 12 and 24 h). HIF-1α and
FoxM1 protein levels were detected by western blotting. (B) PC3 and
DU145 cells were cultured under normoxic or hypoxic conditions for
different durations (0, 6 12 and 24 h). HIF-1α and FoxM1 mRNA
levels were detected by RT-qPCR. (C and D) PC3 and DU145 cells were
transfected with specific RNAi duplexes targeting for HIF-1α or
pretreated with HIF-1α inhibitor YC-1 for 24 h, and then exposed to
hypoxia for an additional 24 h. Then, the protein expression levels
of HIF-1α and FoxM1 were determined by western blotting. (E and F)
Under similar conditions, the mRNA expression levels of FoxM1 were
determined by RT-qPCR. Data are presented as the mean ± standard
deviation of three replicates. *P<0.05. ctrl, control; FoxM1,
Forkhead box M1; HIF-1α, hypoxia-inducible factor 1α; RNAi, RNA
interference; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
HIF-1α regulates the transcription of
FoxM1 during hypoxia in PC3 and DU145 PCa cells
To investigate the response of the FoxM1 promoter to
HIF-1α, RNA interference (Fig. 6A)
and the inhibitor (Fig. 6B) of HIF-1α
were applied to PC3 and DU145 cells. Then, FoxM1 promoter
activities were evaluated by a dual-luciferase assay under normoxic
or hypoxic conditions. The results showed that downregulation of
HIF-1α significantly decreased the FoxM1 promotor activity under
hypoxic conditions compared with the corresponding control. This
suggested that HIF-1α activation is important for the
transcriptional regulation of FoxM1. Furthermore, as shown in
Fig. 6C, the promotor was
investigated to identify potential HIF-1 binding sites. Six
possible HIF-1 binding sites (HBS) were identified from upstream
−1,000 bp to the translation start site: −885 bp (HBS-1, inverted),
−875 bp (HBS-2, inverted), −249 bp (HBS-3, inverted), −203 bp
(HBS-4, inverted), −25 bp (HBS-5, inverted), −23 bp (HBS-6,
inverted). Therefore, different sequences of the FoxM1 promoter
were cloned: S-1 (−1,000 bp ~ +40 bp), S-2 (−878 bp ~ +40 bp), S-3
(−300 bp ~ +40 bp), S-4 (−220 bp ~ +40 bp), S-5 (−50 bp ~ +40 bp)
(Fig. 6D). The sequences were
transfected into PC3 and DU145 cells then the transcriptional
activities were detected by dual luciferase assay under normoxic or
hypoxic conditions. Compared with the full length sequence S-1,
sequences S-2 and S-3 did not reduce the transcription activity,
while sequences S-4 and S-5 led to a significant decrease in
activity. Additionally, the activities between S-4 and S-5 had no
notable differences, while S-5 still exhibited transcriptional
activity under hypoxia compared with the normoxic control. These
results suggest HBS-3 and HBS-5/6, but not HBS-1, HBS-2 and HBS-4,
are required for the FoxM1 transcriptional activity regulated by
HIF-1α under hypoxic conditions.
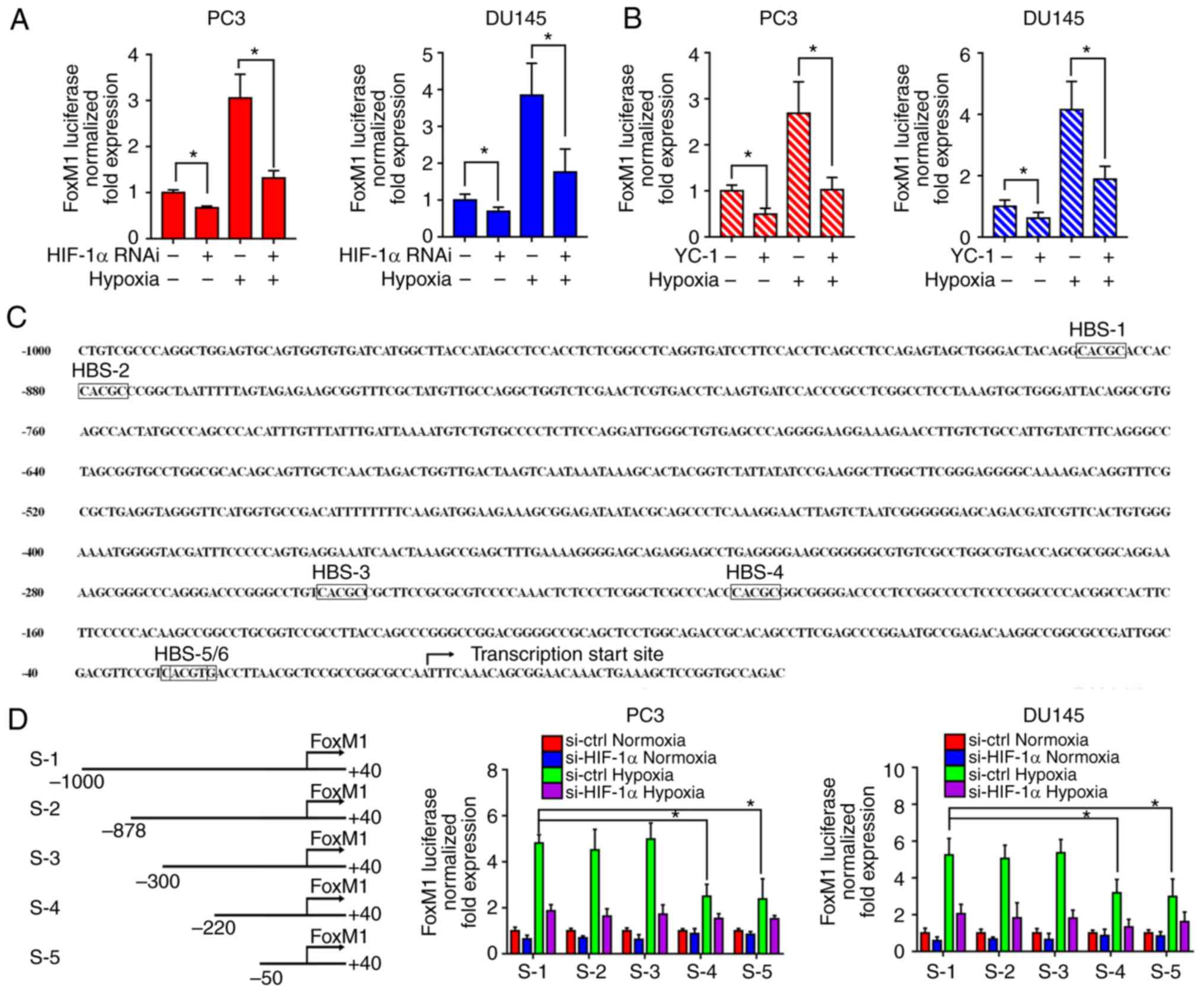 | Figure 6.HIF-1α transcriptionally regulates
FoxM1 under hypoxic conditions. (A and B) PC3 and DU145 cells were
transfected with specific RNAi duplexes for HIF-1α or pretreated
with HIF-1α inhibitor YC-1 for 24 h, and then exposed to hypoxia
for additional 24 h. Then, the transcriptional activities of FoxM1
promotor were analyzed by luciferase assay under normoxic or
hypoxic conditions. (C) Six possible HIF-1α binding sites were
identified from upstream 1,000 to the translation start site of the
FoxM1 promoter. The transcription start site was indicated. (D)
Different lengths of reporter gene constructs (HBS-1 to 5, S-1 to
5) were designed. Then, the transcription activities of different
constructs were analyzed by a dual-luciferase assay under normoxic
or hypoxic conditions with or without HIF-1α knockdown in PC3 and
DU145 cells. Data are presented as the mean ± standard deviation of
three replicates. *P<0.05. ctrl, control; FoxM1, Forkhead box
M1; HIF-1α, hypoxia-inducible factor 1α; HBS, HIF-1 binding sites;
RNAi, RNA interference. |
Discussion
EMT is a cell process through which cancer cells
acquire increased metastatic potential (30). During this process, cells obtain a
phenotype similar to fibroblasts and epithelial-specific protein
markers, such as E-cadherin are downregulated, while that of
mesenchymal protein markers such as vimentin are increased
(31). The induction and regulation
of EMT in PCa has been extensively studied by researchers.
Accumulating evidence has suggested the association between hypoxia
and EMT in PCa (32–34). We previously reported that
overexpression of HIF-1α induced EMT in PCa LNCaP and PC3 cells
both in vitro and in vivo (35). Mechanically, chronic hypoxia-induced
slug promotes EMT of PCa cells by activating the expression of
Ephrin-B1 (11). Additionally,
Annexin A1 may be a key mediator of hypoxia-related EMT processes
in PCa (36). Furthermore, monoamine
oxidase A has been identified to induce EMT and stabilize HIF-1α,
which then activates vascular endothelial growth factor
(VEGF)-A/FOXO1/TWIST1 pathway in high-grade PCa (37). In our study, using bioinformatics
analysis, we identified DEGs in PCa tissues with high or low
expression of HIF-1α. Our data showed that DEGs which were
associated with ‘homophilic cell adhesion via plasma adhesion
molecules’ were of statistical significance between two groups
exhibiting differential expression of HIF-1α. Changes in cell
adhesion are considered as key elements in determining the
development of invasive and metastatic tumors. Additionally, loss
of cell adhesion is one of the hallmarks of EMT. In our study, EMT
induction together with downregulation of E-Cadherin, which is a
key cell adhesion molecule, were observed after exposure to
hypoxia. However, the detail mechanisms involved in hypoxia
induced-EMT in PCa remains unclear.
Recently, gene analysis using the high-throughput
platforms has been developed as a promising tool with various
clinical applications, such as the molecular diagnosis and
classification of cancers, and the prediction of tumor response and
patient prognosis. Several gene expression profiles related to PCa
have been studied with microarray technology, revealing hundreds of
DEGs that are involved in the process of tumorigenesis (38–40),
serving a potential role in the identification of novel therapeutic
targets. The present study applied bioinformatics analysis to
identify DEGs in PCa with low or high expression of HIF-1α, with a
particular focus on possible hub genes that are likely to play key
roles in the progression of PCa. Under hypoxic conditions, cancer
cells initiate a signaling pathway which triggers upregulation of
the corresponding gene to adapt to the environment. These genes are
regulated by the activation of the transcription factor HIF-1α,
which serves an important role for the HIF family (41,42). In
our study, several key DEGs were identified. Of those genes, FoxM1,
which might be a potential target in other cancers was selected for
further investigation.
FoxM1 is a transcription factor that belongs to the
Forkhead superfamily and regulates the expression of target genes
through the binding sequence TAAACA (43,44). FoxM1
has been reported to serve important roles in cell proliferation,
cell cycle, cell differentiation, angiogenesis and metastasis
(17,45). Previous studies have shown that in a
variety of human malignant cancers FoxM1 is upregulated, which
indicates the poor prognosis of patients (46–50). It
was reported that FoxM1 expression in prostate epithelial cells is
critical for prostate carcinogenesis (51). Additionally, the FoxM1 pathway was
determined to act as a master regulator of PCa subtype 1 (PCS1)
tumors, and targeting FoxM1 reduces cell growth and stemness in
PCS1 tumors in vitro and in vivo (52). It was revealed that, in patients with
PCa, high FoxM1 expression was associated with advanced tumor
stages, high Gleason score and poor prognosis, suggesting the vital
role of FoxM1 in PCa development and progression (53). Consistent with published data, in our
study, the expression levels of FoxM1 were upregulated in PCa and
were associated with Gleason scores as determined by bioinformatics
analysis. Importantly, hypoxia-induced EMT was reported to be
regulated by FoxM1. Our results imply that the inhibition of FoxM1
blocked the EMT process and FoxM1 is critical for hypoxia-induced
EMT in PC3 and DU145 PCa cells.
In the present study, overexpression of exogenous
FoxM1 or knockdown of FoxM1 alone had no notable effects on EMT and
cancer cell migration/invasion. Although FoxM1 was identified to be
a direct target and downstream of HIF-1α in PCa, the data we
obtained suggested that FoxM1 might not directly mediate
hypoxia/HIF-1α-induced EMT. There could be several reasons for this
phenomenon. Firstly, under hypoxic conditions, the tumor
microenvironment sustains major EMT-inducing pathways to facilitate
tumor metastasis, such as transforming growth factor-β (TGFβ),
nuclear factor-κB and Notch signaling pathways (54–56).
Inflammatory cytokines including tumor necrosis factor α, TGFβ,
interleukin (IL)-1, IL-6 and IL-8, secreted by surrounding
inflammatory cells may also play an essential role in
hypoxia-induced EMT (57–59). More importantly, there is growing
experimental evidence that HIF-1α modulates the EMT by regulating
the expression and activity of major transcription factors,
including TWIST, SNAIL, SLUG, SIP1 and zinc finger E-box binding
homeobox 1 (60). In our study, we
failed to observed the direct effects FoxM1 on EMT in PCa. We
speculated that hypoxia/HIF-1α-induced EMT in our model system may
be mediated by an unknown transcriptional factor or a signaling
pathway. Thus, we proposed the possible interplay between this
unknown transcriptional factor and FoxM1. Based on published
literature, hypoxia increases androgen receptor (AR) activity in
PCa LNCaP cells (61) and androgens
activate HIF-1, driving VEGF expression in androgen-sensitive LNCaP
cells (62). Recently, a negative
interplay/crosstalk between androgen/AR and hypoxia/HIF1 was
proposed (63). Our unpublished data
suggested that the transcription factors FoxM1, SOX9 and SOX2 are
direct targets of AR. Given the fact that hypoxia/HIF1 was
determined to associate with SOX9 (64) and SOX2 (65), we proposed that AR/SOX2 or AR/SOX9
signaling may be involved in crosstalk with FoxM1, leading to
hypoxia/HIF1-induced EMT; however, further investigation is
required. In addition, the particular genetic background of PCa may
be another possible explanation. The results of our study may be
PCa cell-type specific; further research is warranted to address
this critical issue.
In our study, we also found that hypoxia upregulated
the expression of HIF-1α at the protein level but not at mRNA
level, suggesting the possible involvement of regulation of HIF-1α
protein stability. However, the expression of FoxM1 increased
significantly in a time-dependent manner at both the protein and
mRNA levels under hypoxic condition. Previous studies have reported
that HIF-1α could transcriptionally regulate FoxM1 in several
cancer cell lines (66). To confirm
this mechanism of hypoxia-induced EMT in PC3 and DU145 PCa cells,
we used HIF-1α specific RNA interference to knockdown the
expression and HIF-1α inhibitor YC-1 to inhibit the activity of
HIF-1α. The results showed that reduction of HIF-1α significantly
prevented FoxM1 protein and mRNA expression. The dual-luciferase
assay also demonstrated that knockdown of HIF-1α expression reduced
the activity of FoxM1 transcriptional promoter induced by hypoxia.
In the process of HIF-1α regulating downstream target genes, the
binding site on the target gene promoter which could bind HIF-1α is
essential for transcriptional regulation (67,68).
Therefore, the FoxM1 promoter was reconstructed and analyzed from
upstream −1,000 to the translation start site. Our results
demonstrated that the sequence from upstream −330 to the
translation start site is critical for the transcriptional
activation of FoxM1 regulated by HIF-1α. Analysis of different
fragment length revealed that HBS-3 and HBS-5/6 on the FoxM1
promoter are likely to be binding sites for HIF-1α. These data
indicated that FoxM1 is directly activated by HIF-1α binding to the
HBS within the FoxM1 promoter during hypoxia-induced EMT.
In summary, our findings show that FoxM1 can be
stimulated by hypoxia in PC3 and DU145 PCa cells which is dependent
on the activation of the FoxM1 transcriptional promoter by HIF-1α.
This induction of FoxM1 leads to the EMT of cells, involving
decreased expression of epithelial protein markers, increased
expression of mesenchymal protein markers and promoting cell
invasive ability. This suggests that FoxM1 plays a key role in the
hypoxia-induced EMT process of PCa. Future investigations are
required to determine the suppression of FoxM1 as a potential
therapeutic strategy for treating PCa.
Acknowledgements
Not applicable.
Funding
This study was partly supported by the National
Natural Science Foundation of China (grant no. NSFC 81672538 to
JZ).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CT, KW, SX and XW performed the experiments. CT and
JZ wrote the manuscript. TL collected and interpreted the data and
performed the bioinformatics analysis. KW, SX and XW provided
technical assistance. DH and JZ designed this study and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schatten H: Brief overview of prostate
cancer statistics, grading, diagnosis and treatment strategies. Adv
Exp Med Biol. 1095:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holm HV, Dahl AA, Klepp OH and Fossa SD:
Modern treatment of metastatic prostate cancer. Tidsskr Nor
Laegeforen. 137:803–805. 2017.(In English, Norwegian). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grubb RL III and Kibel AS: Prostate
cancer: Screening, diagnosis and management in 2007. Mo Med.
104:408–414. 2007.PubMed/NCBI
|
|
5
|
Gomez CR, Kosari F, Munz JM, Schreiber CA,
Knutson GJ, Ida CM, El Khattouti A, Karnes RJ, Cheville JC,
Vasmatzis G and Vuk-Pavlović S: Prognostic value of discs large
homolog 7 transcript levels in prostate cancer. PLoS One.
8:e828332013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deep G and Panigrahi GK: Hypoxia-induced
signaling promotes prostate cancer progression: Exosomes role as
messenger of hypoxic response in tumor microenvironment. Crit Rev
Oncog. 20:419–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J,
Sun T, Wang J, Sun R and Liu Y: Hypoxia promotes vasculogenic
mimicry formation by inducing epithelial-mesenchymal transition in
ovarian carcinoma. Gynecol Oncol. 133:575–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaikh D, Zhou Q, Chen T, Ibe JC, Raj JU
and Zhou G: cAMP-dependent protein kinase is essential for hypoxia-
mediated epithelial-mesenchymal transition, migration, and invasion
in lung cancer cells. Cell Signal. 24:2396–2406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Liu Y, Yan X, Xu Y, Luo F, Ye J,
Yan H, Yang X, Huang X, Zhang J and Ji G: HIFs enhance the
migratory and neoplastic capacities of hepatocellular carcinoma
cells by promoting EMT. Tumour Biol. 35:8103–8114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hay ED: Role of cell-matrix contacts in
cell migration and epithelial-mesenchymal transformation. Cell
Differ Dev. 32:367–375. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan MI, Hamid A, Adhami VM, Lall RK and
Mukhtar H: Role of epithelial mesenchymal transition in prostate
tumorigenesis. Curr Pharm Des. 21:1240–1248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cowin P, Rowlands TM and Hatsell SJ:
Cadherins and catenins in breast cancer. Curr Opin Cell Biol.
17:499–508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iwasaki K, Ninomiya R, Shin T, Nomura T,
Kajiwara T, Hijiya N, Moriyama M, Mimata H and Hamada F: Chronic
hypoxia-induced slug promotes invasive behavior of prostate cancer
cells by activating expression of ephrin-B1. Cancer Sci.
109:3159–3170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao GB, Li XZ, Zeng S, Liu C, Yang SM,
Yang L, Hu CJ and Bai JY: Regulation of the master regulator FOXM1
in cancer. Cell Commun Signal. 16:572018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmad A, Wang Z, Kong D, Ali S, Li Y,
Banerjee S, Ali R and Sarkar FH: FoxM1 down-regulation leads to
inhibition of proliferation, migration and invasion of breast
cancer cells through the modulation of extra-cellular matrix
degrading factors. Breast Cancer Res Treat. 122:337–346. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang C, Chen H, Tan G, Gao W, Cheng L,
Jiang X, Yu L and Tan Y: FOXM1 promotes the epithelial to
mesenchymal transition by stimulating the transcription of Slug in
human breast cancer. Cancer Lett. 340:104–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abdeljaoued S, Bettaieb I, Nasri M, Adouni
O, Goucha A, El Amine O, Boussen H, Rahal K and Gamoudi A:
Overexpression of FOXM1 is a potential prognostic marker in male
breast cancer. Oncol Res Treat. 40:167–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Yu W, Zhou L, Wu S, Yang Y, Wang
J, Tian Y, He D, Xu Y, Huang J, et al: Relationship among diet
habit and lower urinary tract symptoms and sexual function in
outpatient-based males with LUTS/BPH: A multiregional and
cross-sectional study in China. BMJ Open. 6:e0108632016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong FF, Qu ZQ, Yuan HH, Wang JY, Zhao M,
Guo YH, Shi J, Gong XD, Zhu YL, Liu F, et al: Overexpression of
FOXM1 is associated with EMT and is a predictor of poor prognosis
in non-small cell lung cancer. Oncol Rep. 31:2660–2668. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito T, Kohashi K, Yamada Y, Maekawa A,
Kuda M, Furue M and Oda Y: Prognostic significance of forkhead box
M1 (FoxM1) expression and antitumour effect of FoxM1 inhibition in
melanoma. Histopathology. 69:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Liu Y, Yuan B, Yin L, Peng Y, Yu X,
Zhou W, Gong Z, Liu J, He L and Li X: FOXM1 promotes the
progression of prostate cancer by regulating PSA gene
transcription. Oncotarget. 8:17027–17037. 2017.PubMed/NCBI
|
|
25
|
Li L, Wu D, Yu Q, Li L and Wu P:
Prognostic value of FOXM1 in solid tumors: A systematic review and
meta-analysis. Oncotarget. 8:32298–32308. 2017.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCarthy DJ, Chen Y and Smyth GK:
Differential expression analysis of multifactor RNA-Seq experiments
with respect to biological variation. Nucleic Acids Res.
40:4288–4297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a biologist-oriented resource for the analysis of
systems-level datasets. Nat Commun. 10:15232019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalluri RJ: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grunert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang
SY, Liu CJ, Teng SC and Wu KJ: Direct regulation of TWIST by
HIF-1alpha promotes metastasis. Nat Cell Biol. 10:295–305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boddy JL, Fox SB, Han C, Campo L, Turley
H, Kanga S, Malone PR and Harris AL: The androgen receptor is
significantly associated with vascular endothelial growth factor
and hypoxia sensing via hypoxia-inducible factors HIF-1a, HIF-2a,
and the prolyl hydroxylases in human prostate cancer. Clin Cancer
Res. 11:7658–7663. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mak P, Leav I, Pursell B, Bae D, Yang X,
Taglienti CA, Gouvin LM, Sharma VM and Mercurio AM: ERbeta impedes
prostate cancer EMT by destabilizing HIF-1alpha and inhibiting
VEGF-mediated snail nuclear localization: Implications for Gleason
grading. Cancer Cell. 17:319–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo Y, He DL, Ning L, Shen SL, Li L, Li X,
Zhau HE and Chung LW: Over-expression of hypoxia-inducible
factor-1alpha increases the invasive potency of LNCaP cells in
vitro. BJU Int. 98:1315–1319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bizzarro V, Belvedere R, Migliaro V,
Romano E, Parente L and Petrella A: Hypoxia regulates ANXA1
expression to support prostate cancer cell invasion and
aggressiveness. Cell Adh Migr. 11:247–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu JB, Shao C, Li X, Li Q, Hu P, Shi C, Li
Y, Chen YT, Yin F, Liao CP, et al: Monoamine oxidase A mediates
prostate tumorigenesis and cancer metastasis. J Clin Invest.
124:2891–2908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng WS, Tao H, Hu EP, Liu S, Cai HR, Tao
XL, Zhang L, Mao JJ and Yan DL: Both genes and lncRNAs can be used
as biomarkers of prostate cancer by using high throughput
sequencing data. Eur Rev Med Pharmacol Sci. 18:3504–3510.
2014.PubMed/NCBI
|
|
39
|
Han Y, Jin X, Li H, Wang K, Gao J, Song L
and Lv Y: Microarray analysis of copy-number variations and gene
expression profiles in prostate cancer. Medicine (Baltimore).
96:e72642017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tan J, Jin X and Wang K: Integrated
bioinformatics analysis of potential biomarkers for prostate
cancer. 25:455–460. 2019.PubMed/NCBI
|
|
41
|
Soni S and Padwad YS: HIF-1 in cancer
therapy: Two decade long story of a transcription factor.
56:503–515. 2017.PubMed/NCBI
|
|
42
|
Semenza GL: Regulation of mammalian O2
homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol.
15:551–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Korver W, Roose J, Heinen K, Weghuis DO,
de Bruijn D, van Kessel AG and Clevers H: The human TRIDENT/HFH-
11/FKHL16 gene: Structure, localization, and promoter
characterization. Genomics. 46:435–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Laoukili J, Stahl M and Medema RH: FoxM1:
At the crossroads of ageing and cancer. Biochim Biophys Acta.
1775:92–102. 2007.PubMed/NCBI
|
|
45
|
Nandi D, Cheema PS, Jaiswal N and Nag A:
FoxM1: Repurposing an oncogene as a biomarker. Semin Cancer Biol.
52:74–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang DK, Son CH, Lee SK, Choi PJ, Lee KE
and Roh MS: Forkhead box M1 expression in pulmonary squamous cell
carcinoma: Correlation with clinicopathologic features and its
prognostic significance. Hum Pathol. 40:464–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun HC, Li M, Lu JL, Yan DW, Zhou CZ, Fan
JW, Qin XB, Tang HM and Peng ZH: Overexpression of Forkhead box M1
protein associates with aggressive tumor features and poor
prognosis of hepatocellular carcinoma. Oncol Rep. 25:1533–1539.
2011.PubMed/NCBI
|
|
48
|
Huynh KM, Soh JW, Dash R, Sarkar D, Fisher
PB and Kang D: FOXM1 expression mediates growth suppression during
terminal differentiation of HO-1 human metastatic melanoma cells. J
Cell Physiol. 226:194–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xia L, Mo P, Huang W, Zhang L, Wang Y, Zhu
H, Tian D, Liu J, Chen Z, Zhang Y, et al: The
TNF-α/ROS/HIF-1-induced upregulation of FoxMI expression promotes
HCC proliferation and resistance to apoptosis. Carcinogenesis.
33:2250–2259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Wen L, Zhao SH, Ai ZH, Guo JZ and
Liu WC: FoxM1 expression is significantly associated with
cisplatin-based chemotherapy resistance and poor prognosis in
advanced non-small cell lung cancer patients. Lung Cancer.
79:173–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cai Y, Balli D, Ustiyan V, Fulford L,
Hiller A, Misetic V, Zhang Y, Paluch AM, Waltz SE, Kasper S and
Kalin TV: Foxm1 expression in prostate epithelial cells is
essential for prostate carcinogenesis. J Biol Chem.
288:22527–22541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ketola K, Munuganti RSN, Davies A, Nip KM,
Bishop JL and Zoubeidi A: Targeting prostate cancer subtype 1 by
forkhead box M1 pathway inhibition. Clin Cancer Res. 23:6923–6933.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim MY, Jung AR, Kim GE, Yang J, Ha US,
Hong SH, Choi YJ, Moon MH, Kim SW, Lee JY and Park YH: High FOXM1
expression is a prognostic marker for poor clinical outcomes in
prostate cancer. J Cancer. 10:749–756. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zavadil J and Bottinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Giannoni E, Bianchini F, Calorini L and
Chiarugi P: Cancer associated fibroblasts exploit reactive oxygen
species through a proinflammatory signature leading to epithelial
mesenchymal transition and stemness. Antioxid Redox Signal.
14:2361–2371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chuang MJ, Sun KH, Tang SJ, Deng MW, Wu
YH, Sung JS, Cha TL and Sun GH: Tumor-derived tumor necrosis
factor-alpha promotes progression and epithelial-mesenchymal
transition in renal cell carcinoma cells. Cancer Sci. 99:905–913.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sullivan NJ, Sasser AK, Axel AE, Vesuna F,
Raman V, Ramirez N, Oberyszyn TM and Hall BM: Interleukin-6 induces
an epithelial-mesenchymal transition phenotype in human breast
cancer cells. Oncogene. 28:2940–2947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
St John MA, Dohadwala M, Luo J, Wang G,
Lee G, Shih H, Heinrich E, Krysan K, Walser T, Hazra S, Zhu L, et
al: Proinflammatory mediators upregulate snail in head and neck
squamous cell carcinoma. Clin Cancer Res. 15:6018–6027. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jiang J, Tang YL and Liang XH: EMT: A new
vision of hypoxia promoting cancer progression. Cancer Biol Ther.
11:714–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Park SY, Kim YJ, Gao AC, Mohler JL, Onate
SA, Hidalgo AA, Ip C, Park EM, Yoon SY and Park YM: Hypoxia
increases androgen receptor activity in prostate cancer cells.
Cancer Res. 66:5121–5129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mabjeesh NJ, Willard MT, Frederickson CE,
Zhong H and Simons JW: Androgens stimulate hypoxia-inducible factor
1 activation via autocrine loop of tyrosine kinase
receptor/phosphatidylinositol 3′-kinase/protein kinase B in
prostate cancer cells. Clin Cancer Res. 9:2416–2425.
2003.PubMed/NCBI
|
|
63
|
Geng H, Xue C, Mendonca J, Sun XX, Liu Q,
Reardon PN, Chen Y, Qian K, Hua V, Chen A, et al: Interplay between
hypoxia and androgen controls a metabolic switch conferring
resistance to androgen/AR-targeted therapy. Nat Commun. 9:49722018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Amarilio R, Viukov SV, Sharir A,
Eshkar-Oren I, Johnson RS and Zelzer E: HIF1alpha regulation of
Sox9 is necessary to maintain differentiation of hypoxic
prechondrogenic cells during early skeletogenesis. Development.
134:3917–3928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bae KM, Dai Y, Vieweg J and Siemann DW:
Hypoxia regulates SOX2 expression to promote prostate cancer cell
invasion and sphere formation. Am J Cancer Res. 6:1078–1088.
2016.PubMed/NCBI
|
|
66
|
Xia LM, Huang WJ, Wang B, Liu M, Zhang Q,
Yan W, Zhu Q, Luo M, Zhou ZZ and Tian DA: Transcriptional
up-regulation of FoxM1 in response to hypoxia is mediated by HIF-1.
J Cell Biochem. 106:247–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang GL and Semenza GL: Purification and
characterization of hypoxia-inducible factor 1. J Biol Chem.
270:1230–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|