Introduction
Breast cancer represents the leading cause of death
in women worldwide and has a poor prognosis. Estrogen and estrogen
receptors (ERs) play key roles in breast cancer progression.
Previous studies have demonstrated that ER is expressed in 75% of
breast cancer overall (1–3). Conventional cytotoxic chemotherapy is
still one of the key elements of therapeutic armamentarium, but the
effectiveness of treatment is limited.
Mucin-1 (MUC1) is a transmembrane glycoprotein
abnormally overexpressed in a wide range of human epithelial cancer
types, including colon, breast, ovarian and pancreatic cancer
(4,5).
MUC1 is localized only on the apical membrane and functions via
barrier formation and monitoring luminal events in healthy cells
(4). Overexpression and aberrant
glycosylation of MUC1 in cancer cells contribute to tumor
progression and metastasis (6). MUC1
is also a heterodimer, which consists of two subunits: A long
N-terminal fragment and short C-terminal fragment. Its cytoplasmic
tail takes part in intracellular signaling by interfering with
different proteins and affecting their function (4). The cytoplasmic tail of MUC1 interacts
with different molecules, which are also overexpressed in cancer,
such as ER, β-catenin and ErbB growth factor receptor tyrosine
kinases (7).
Our recent study synthesized and evaluated the
anticancer potential of novel diisoquinoline derivatives in breast
and gastric cancer cells (8). The
novel compounds were involved in the inhibition of AKT and
ERK1/ERK2 (8). It was also
demonstrated that the new diisoquinoline derivatives could modulate
both apoptotic pathways. Another previous study demonstrated that
all novel synthesized compounds activated the initiator and
executioner caspases, such as caspase-8, caspase-9, caspase-10 and
caspase-3, compared with untreated breast cancer cells (9).
The aim of the present study was to examine the
multi-targeted potential of a monoclonal antibody against MUC1 and
novel octahydropyrazin[2,1-a:5,4-a′]diisoquinoline derivative
(OM-86II) in ER-positive MCF-7 human breast cancer cells. The
results of the present study were compared with the findings
concerning etoposide used together with anti-MUC1 antibody as well
as with monotherapy. Etoposide is a widely used drug for
chemotherapy and its molecular mechanism of action is associated
with the inhibition of topoisomerase II (10).
Materials and methods
Materials
Stock cultures of human MCF-7 breast cancer cells
were purchased from The American Type Culture Collection. DMEM and
FBS used in cell culture were products of Gibco; Thermo Fisher
Scientific, Inc. Glutamine, penicillin and streptomycin were
obtained from Quality Biological, Inc. The JC-1 MitoScreen kit was
supplied by BD Pharmigen; BD Biosciences. ELISA kits used to detect
the concentrations of matrix metalloproteinase (MMP)-2, MMP-9,
tumor necrosis factor (TNF)-α, cyclooxygenase (COX)-2, mTOR and
soluble intercellular adhesion molecule (sICAM)1 were obtained from
Wuhan EIAab Science Co., Ltd. (cat. nos. E0100 h, E0553 h, E0133 h,
E0699 h, E14969 h and EH0161). Etoposide was obtained from
Sigma-Aldrich; Merck KGaA and the purity of the compound was
>98%. Monoclonal anti-MUC1 antibody (cat. no. MA1-06503) was a
product of Thermo Fisher Scientific, Inc.
Nuclear magnetic resonance (NMR) spectra were
recorded using a Varian VNMR500 spectrometer (Varian, Inc.).
Chemical shifts are quoted in parts per million relative to TMS for
1H and toluene-d8 for 13C NMR.
Coupling constants J are reported in Hz. Mass spectra were
recorded using an AMD-604 Intectra GmbH mass spectrometer (Waters
Corporation).
Compounds
OM-86II was synthesized using previously
standardized methods (9,11). The synthesis and physicochemical
characterization of a compound 15a was presented in our previous
study (11). Compound 15a (0.3 mmol;
169 mg) was dissolved in acetonitrile (15 ml) and cooled to 0°C.
Into intensively stirred reaction mixture, a water solution (10 ml)
of cerium ammonium nitrate (1 mmol; 326 mg) was added dropwise.
Stirring was continued at room temperature until the reaction was
over (~2 h), poured into cold sodium dithionate (60 ml; 1M),
extracted with dichloromethane (3×40 ml), dried with magnesium
sulphate, filtrated and concentrated. The crude product was
purified on silica gel using gradient DCM/MeOH (1–10% MeOH) as an
eluent (Fig. 1).
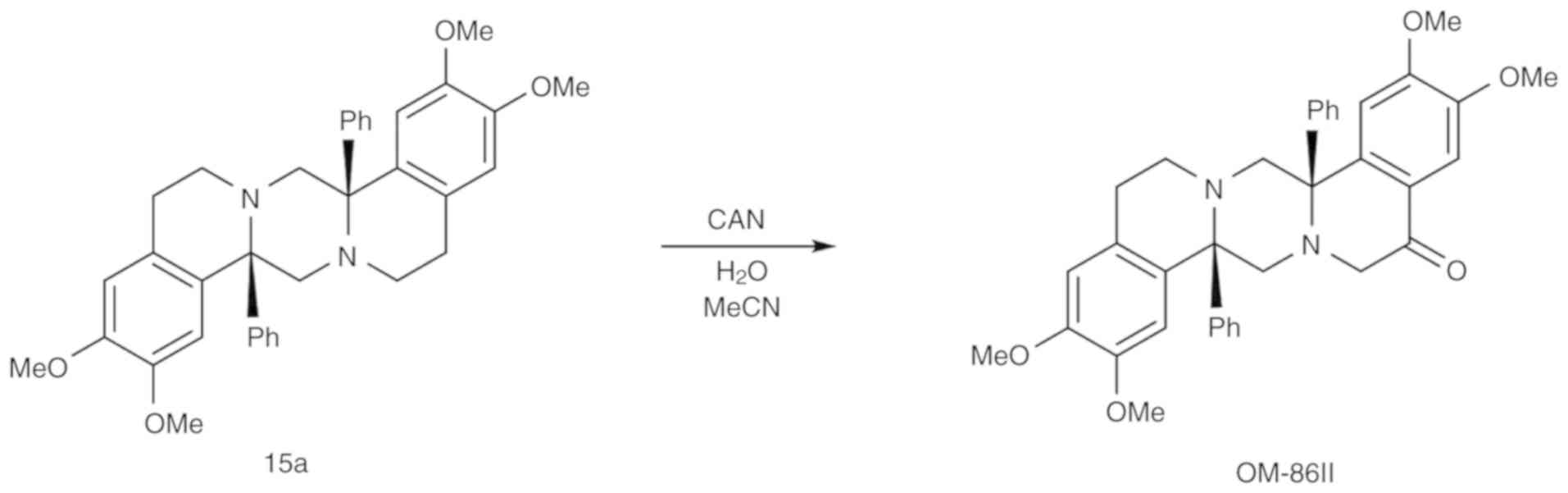 | Figure 1.Synthesis of
(8aS,16aS)-2,3,10,11-tetramethoxy-8a,16a-diphenyl-8,8a,13,14,16,16a-hexahydropyrazino[2,1-a:5,4-a′]diisoquinolin-5(6H)-one.
CAN, cerium ammonium nitrate; OM-86II,
octahydropyrazin[2,1-a:5,4-a′]diisoquinoline derivative. |
Yield: 79 mg, 45%. Semisolid. 1H NMR
(toluene-d8, 80°C, 500 MHz): 7.84 - 7.80 (m, 2H), 7.66 -
7.1 (m, 2H), 7.17 - 7.11 (m, 3H), 7.11 - 7.00 (m, 3H), 6.80 (s,
1H), 6.78 (s, 1H), 6.54 (s, 1H), 6.37 (s, 1H), 3.85 (d, J=5.6 Hz,
1H), 3.70 (d, J=9.6 Hz, 1H), 3.56 (d, J=5.6 Hz, 1H), 3.46 (s, 3H),
3.44 (s, 3H), 3.37 (s, 3H), 3.26 - 3.21 (m, 4H), 3.10 - 2.91 (m,
2H), 2.90 - 2.71 (m, 3H), 2.58 - 2.50 (m, 2H). 13C NMR
(toluene-d8, 80°C, 125 MHz): δ (ppm): 196.3, 151.5,
148.4, 148.3, 147.8, 147.0, 139.1, 134.1, 132.2, 132.0, 130.4,
130.1, 128.0, 128.0 127.7, 127.2, 126.3, 114.3, 113.7, 112.4,
111.9, 75.3, 69.0, 65.0, 57.0, 55.4, 55.1, 55.1, 55.1, 45.0, 32.9,
26.4. MS (ES, HR) m/z: (M+) calcd for
C36H36N2O5: 576.6930;
Found: 576.2626. Anal. Calcd for
C36H36N2O5: C, 74.98;
H, 6.29; N, 4.86; Found: C, 75.00; H, 6.20; N, 4.81.
Cell culture of MCF-7 cells
ER-positive breast cancer MCF-7 cells were
maintained in DMEM supplemented with 10% FBS, 2 mM glutamine, 50
U/ml penicillin, 50 mg/ml streptomycin at 37°C in a humidified
atmosphere containing 5% CO2. Sub-confluent cells were
treated with 0.05% trypsin and 0.02% EDTA in calcium-free PBS,
counted using a hemocytometer and seeded in 6-well plates (Nunc) in
2 ml growth medium (DMEM without phenol red with 10% CPSR1). The
cells that reached ~80% confluency were used for the assays.
Treatment groups and conditions
MCF-7 breast cancer cells were incubated with
anti-MUC1 (10 µg/ml), OM-86II (30 µM), OM-86II + anti-MUC1 (30 µM +
10 µg/ml), etoposide (30 µM) and etoposide + anti-MUC1 (30 µM + 10
µg/ml) for 24 and 48 h at 37°C in 5% CO2 in an
incubator.
Cell viability assay
To examine the effect of the compounds on cell
growth, MCF-7 cells were seeded in 6-well plates (2×106)
and cultured as described. Cell cultures were incubated with
varying concentrations of the compounds tested for 24 and 48 h.
Then cells were washed three times with PBS and then incubated for
4 h in 1 ml MTT solution (0.5 mg/ml PBS) at 37°C in 5%
CO2 in an incubator. The medium was removed and 1 ml 0.1
mol/l HCl in absolute isopropanol was added to the attached cells.
The absorbance of the converted dye in living cells was measured at
a wavelength of 570 nm (12).
[3H]thymidine incorporation
assay
To examine the effect of the compounds on cell
proliferation, MCF-7 cells were seeded (2×106) in 6-well
plates and cultured as described. Cell cultures were incubated with
varying concentrations of the tested compounds and 0.5 µCi
[3H]thymidine for 24 h at 37°C. The cells were harvested
by trypsinization and washed several times in cold PBS (10
min/1.500 g) until the dpm in the washes were similar to the
reagent control. Radioactivity was determined by liquid
scintillation counting. [3H]thymidine uptake is
expressed as dpm/well (9).
Cell cycle analysis
The distribution of the cell cycle phases was
analyzed by flow cytometry. Briefly, MCF-7 breast cancer cells were
seeded into 6-well plates at a density of 2.5×105
cells/well and treated with the compounds for 24 and 48 h. After
incubation, the cells were harvested and then fixed with 1 ml 70 %
ethanol and kept overnight at −20°C. Before analysis, the cells
were resuspended in PBS, treated with 50 µg/ml DNase-free RNase A
solution (Promega Corporation), and stained for 30 min at 37°C with
100 µg/ml propidium iodide (PI; ImmunoChemistry Technologies, LLC;
cat. no. 638). The FACSCanto II flow cytometer (BD Biosciences) was
used to read the fluorescence and the results were analyzed using
FACSDiva software (version 6.1.3; BD Biosciences Systems) (13).
Determination of mitochondrial
membrane potential
Disruption of the mitochondrial membrane potential
was assessed using the lipophilic cationic probe
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine
iodide (JC-1 Mitoscreen kit; BD Biosciences) as previously
described (14). Briefly, unfixed
cells were washed and resuspended in PBS supplemented with JC-1.
The cells were then incubated for 15 min. at room temperature in
the dark, washed and resuspended in PBS for immediate flow
cytometry analysis using a FACSCanto II flow cyometer. The
percentage of cells with disrupted MMP was calculated using
FACSDiva software (version 6.1.3; BD Biosciences).
Dual acridine orange/ethidium bromide
fluorescent staining
To confirm that the compounds induce apoptosis, dual
acridine orange/ethidium bromide fluorescent staining was assessed
and visualized under a fluorescent microscope (Nikon Eclipse Ti;
Nikon Corporation). MCF-7 breast cancer cells were treated with the
compounds for 24 and 48 h. The cell suspension (250 µl) was stained
for 10 min at room temperature in the dark with 10 µl dye mixture
(10 µM acridine orange and 10 µM ethidium bromide), which was
prepared in PBS. Cells cultured in a drug-free medium were used as
controls. The morphology of two hundred cells per sample was
examined by fluorescent microscopy (magnification, ×100) within 20
min. The results were analyzed with NIS-Elements software (version
3.10; Nikon Corporation).
Flow cytometry assessment of Annexin V
binding
The effect of the compounds on the induction of
apoptosis after 24 and 48 h of incubation was assessed using a
Becton Dickinson FACSCanto II flow cytometer (BD Biosciences). The
assessment allows checking the loss of asymmetry of phospholipids
on the cell membrane. Cells were trypsinized, resuspended in DMEM
and then in binding buffer. Next, they were stained with FITC
Annexin V and PI for 15 min at room temperature in the dark,
according to the manufacturer's protocol (FITC Annexin V Apoptosis
Detection kit II; BD Biosciences). Cells cultured in a drug-free
medium were used as controls. The optimal parameter settings were
found using a positive control (cells incubated with 3%
formaldehyde in buffer during 30 min on ice). Forward scatter and
side scatter signals were detected on a logarithmic scale
histogram. FITC was detected in the FL1 channel (FL1 539;
Threshold-value 52). The results were analyzed with FACSDiva
software (version 6.1.3; BD Biosciences).
Determination of matrix
metalloproteinase (MMP)-2, MMP-9, tumor necrosis factor (TNF)-α,
cyclooxygenase (COX)-2, mTOR and soluble intercellular adhesion
molecule (sICAM)1
High sensitivity assay ELISA kits (Wuhan EIAab
Science Co., Ltd.) were used to determine the concentrations of
proteins in supernatants from cell culture or in cell lysates
(15) after 24 and 48 h of incubation
with the compounds. There was no cross-reactivity or interference
by other proteins present in biological samples. The microtiter
plate provided in this kit was pre-coated with an antigen-specific
antibody. Standards and samples were added to the appropriate
microtiter plate wells. After 2 h of incubation at 37°C, the plate
was incubated with biotin-conjugated antibody from the kits for 1 h
at 37°C. Then, the microplate wells were aspirated and washed three
times, and then incubated for 1 h at 37°C with avidin conjugated to
horseradish peroxidase. Then, a 3,3′,5,5′-tetramethylbenzidine
substrate solution was added to each well. The enzyme-substrate
reaction was terminated by the addition of a sulfuric acid solution
and the color change was measured spectrophotometrically at a
wavelength of 450±2 nm. The concentration of antigen in the samples
was determined by comparing the optical density of the samples to
the standard curve.
Statistical analysis
All numerical data are presented as the mean ± SD
from three independent experiments. The statistical analysis was
performed using GraphPad Prism Version 6.0 (GraphPad Software,
Inc.). All datasets were analyzed using ANOVA and Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Novel OM-86II combined with anti-MUC1
antibody decreases cell viability and proliferation of MCF-7 breast
cancer cells
The effects of etoposide, a novel diisoquinoline
derivative (OM-86II) and an anti-MUC1 antibody as well as etoposide
or OM-86II in combination with anti-MUC1 antibody on cell viability
and DNA biosynthesis in MCF-7 breast cancer cells were examined
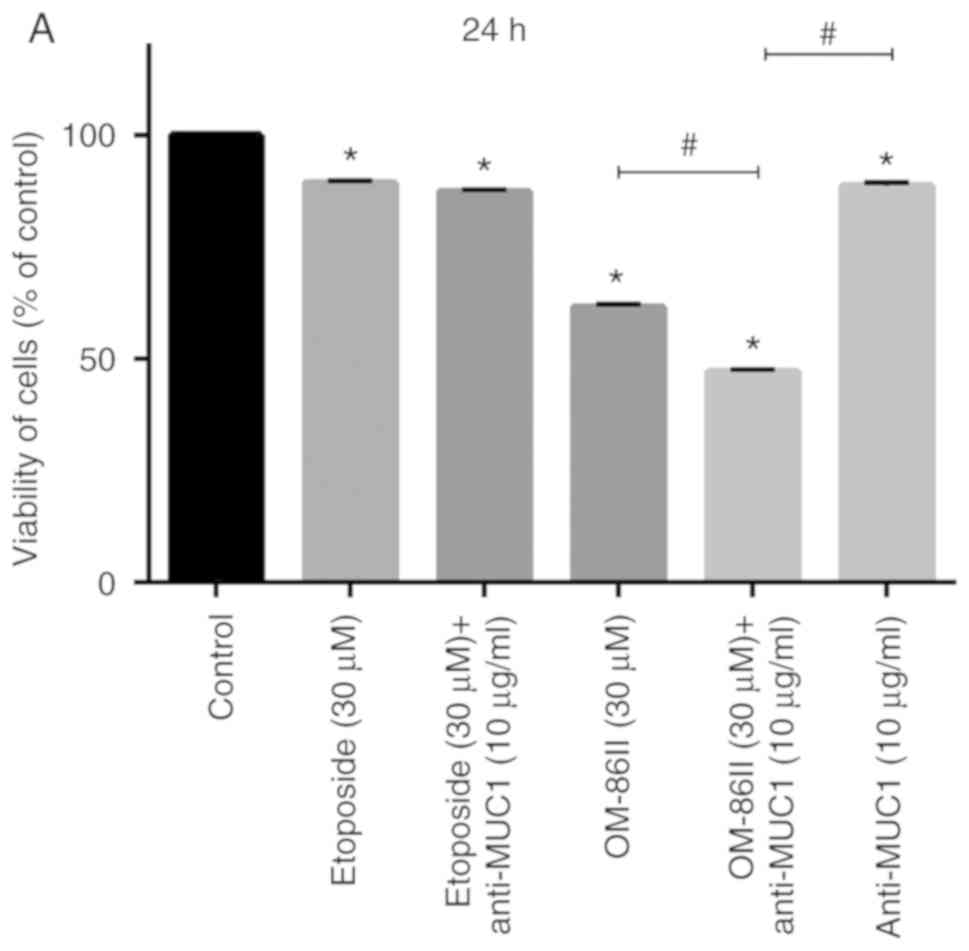
(Figs. 2 and 3). The cell viability was analyzed after 24
and 48 h of incubation with the agents tested. It was detected that
the anti-MUC1 antibody used together with OM-86II represented the
strongest cytotoxic and antiproliferative potential (Figs. 2 and 3).
Such a combination decreased the number of viable cells to 47.1 and
38.8% after 24 and 48 h of incubation, respectively (Fig. 2). The combination of etoposide and
anti-MUC1 antibody reduced the number of live cells to 87.4 and
73.2% after 24 and 48 h of incubation, respectively (Fig. 2). Monotherapy was not so efficient in
decreasing the viability of MCF-7 cells. However, the most
cytotoxic properties for monotherapy were observed after incubation
with anti-MUC1 antibody, which reduced the viability of breast
cancer cells to 53.4% after 48 h of incubation, respectively
(Fig. 2).
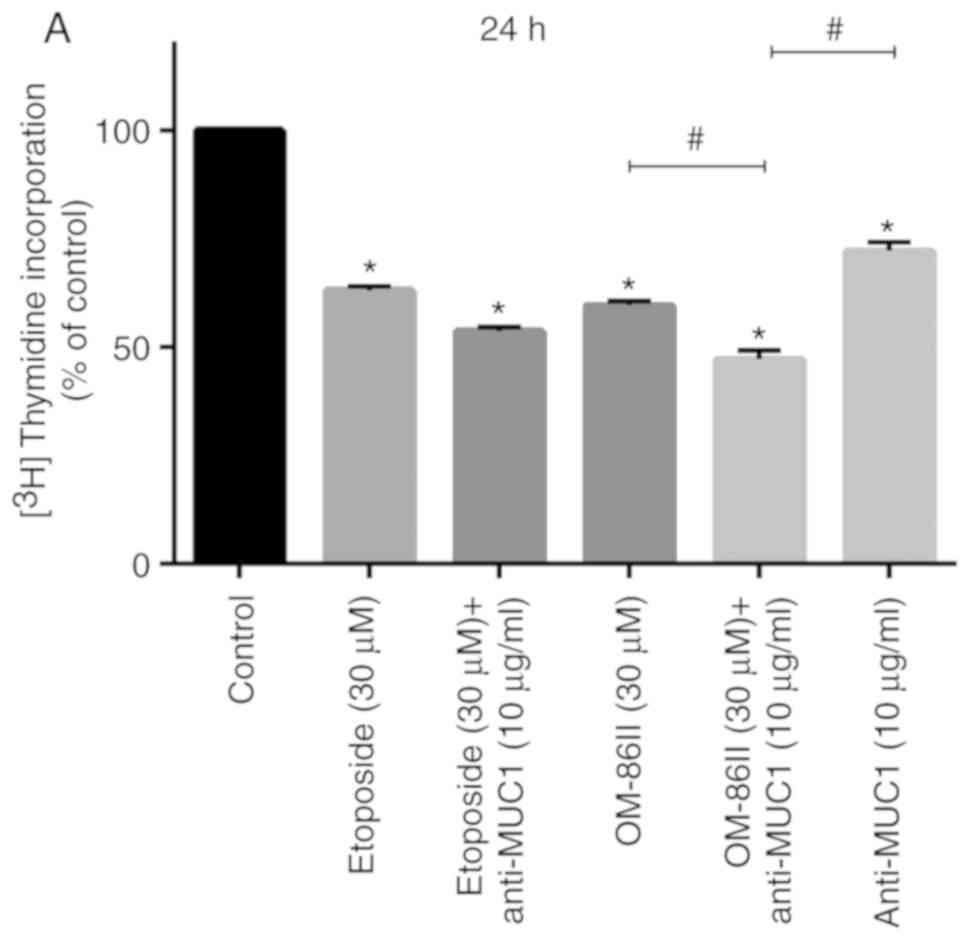
The antiproliferative potential of the compounds
tested was demonstrated by the incorporation of
[3H]-thymidine into the DNA of MCF-7 cells (Fig. 3). The combination of novel OM-86II
with anti-MUC1 represented the strongest antiproliferative activity
in MCF-7 breast cancer cells. We detected that such a combination
inhibited DNA biosynthesis to 47.15 and 26.5% after 24 and 48 h of
incubation, while etoposide with anti-MUC1 reduced
[3H]-thymidine incorporation to 53.64 and 32.12% after
24 and 48 h of incubation. Monotherapy was not so efficient, and in
this case the most antiproliferative potential was demonstrated
after incubation with OM-86II, which inhibited the
[3H]-thymidine incorporation into DNA of breast cancer
cells to 59.6 and 39.8% after 24 and 48 h of incubation (Fig. 3).
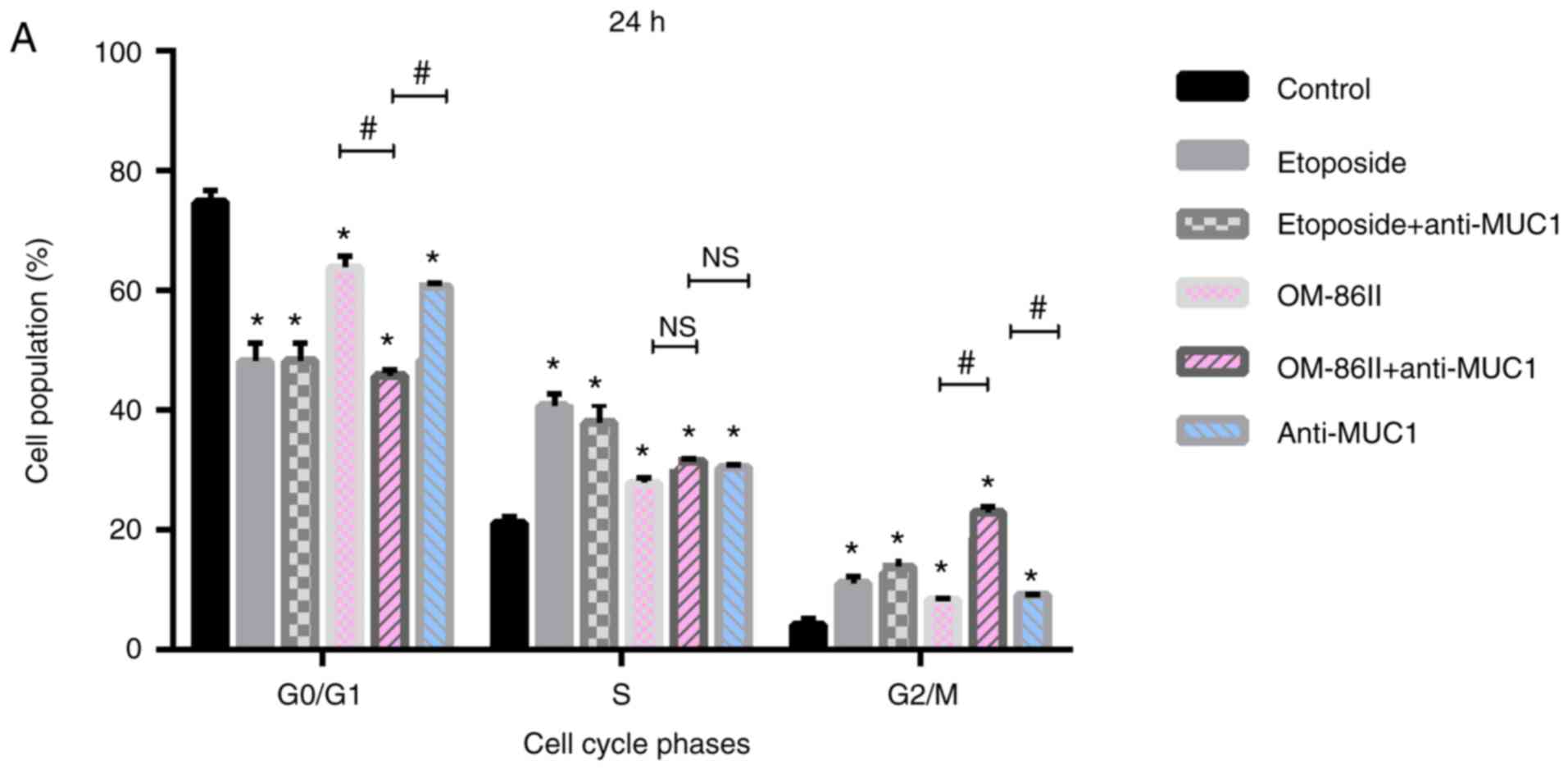
Novel OM-86II combined with anti-MUC1
antibody induces G2/M cell cycle arrest in MCF-7
cells
Anticancer agents can be designed to target specific
cell cycle checkpoints in cancer cells and are able to induce cell
death (16). Cell cycle analysis
revealed that monotherapy as well as a combination of compounds
(OM-86II, etoposide), together with anti-MUC1 antibody, induced
G2/M arrest in MCF-7 breast cancer cells. As shown in
Fig. 4A, the percentage of cells in
the G2/M phase increased from 4.1% in the untreated
control to 22.1% after treatment with OM-86II and anti-MUC1 (30 µM
+ 10 µg/ml), 13.6% after treatment with etoposide and anti-MUC1 (30
µM + 10 µg/ml), 8.3% after treatment with OM-86II (30 µM), 11%
after treatment with etoposide (30 µM) and 9% after treatment with
anti-MUC1 antibody (10 µg/ml). Upon prolongation of the exposure
time to 48 h, the highest percentages of cells were arrested in the
G2/M phase after treatment with OM-86II combined with
anti-MUC1 antibody (33.0%; Fig.
4B).
Novel OM-86II combined with anti-MUC1
antibody induces apoptosis and decreases mitochondrial membrane
potential in breast cancer cells
Dual acridine orange/ethidium bromide fluorescent
staining was performed to visualize viable, apoptotic and necrotic
cells after treatment with the compounds used alone and in
combination with anti-MUC1 antibody (Fig.
5). Control (untreated) cells were displayed as green
fluorescence. Bright green fluorescence was characteristic of early
apoptotic cells, whereas an orange color was specific to late
apoptotic cells. The present study demonstrated that the
combination of OM-86II and anti-MUC1, resulted in the highest
number of apoptotic cells. Bright green fluoresence as well as
orange fluorescence was observed. The strongest pro-apoptotic
effect was observed after 48 h of incubation (Fig. 5B).
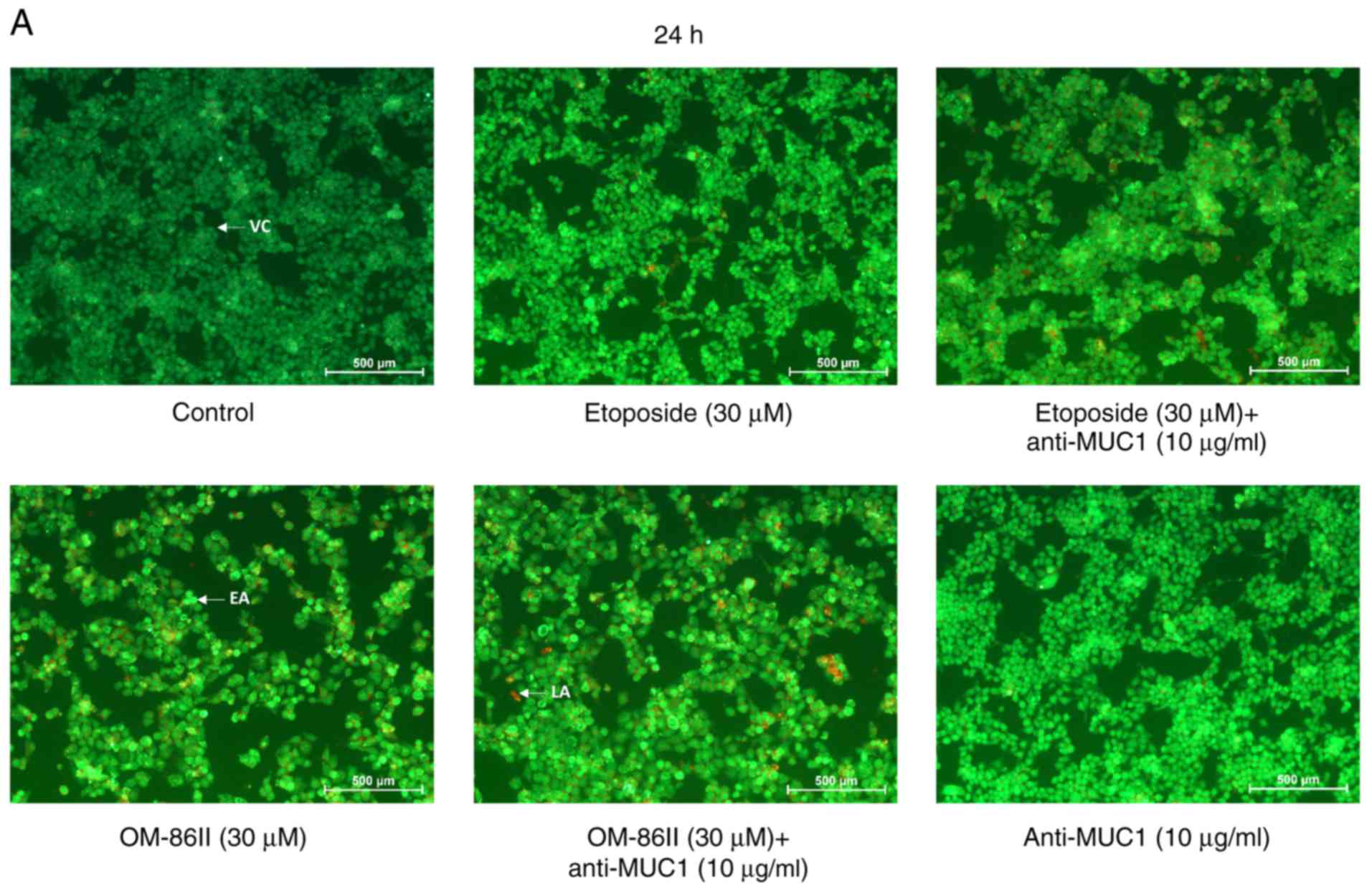 | Figure 5.Novel OM-86II combined with anti-MUC1
antibody induces apoptosis in MCF-7 breast cancer cells. Induction
of apoptosis in human MCF-7 cells treated for (A) 24 and (B) 48 h
with anti-MUC1 (10 µg/ml), OM-86II (30 µM), OM-86II + anti-MUC1 (30
µM + 10 µg/ml), etoposide (30 µM) and etoposide + anti-MUC1 (30 µM
+ 10 µg/ml) evaluated by fluorescent microscopy after acridine
orange and ethidium bromide staining. Magnification, ×100. MUC1,
mucin-1; OM-86II, octahydropyrazin[2,1-a:5,4-a′]diisoquinoline
derivative; VC, viable cells; EA, early apoptosis; LA, late
apoptosis. |
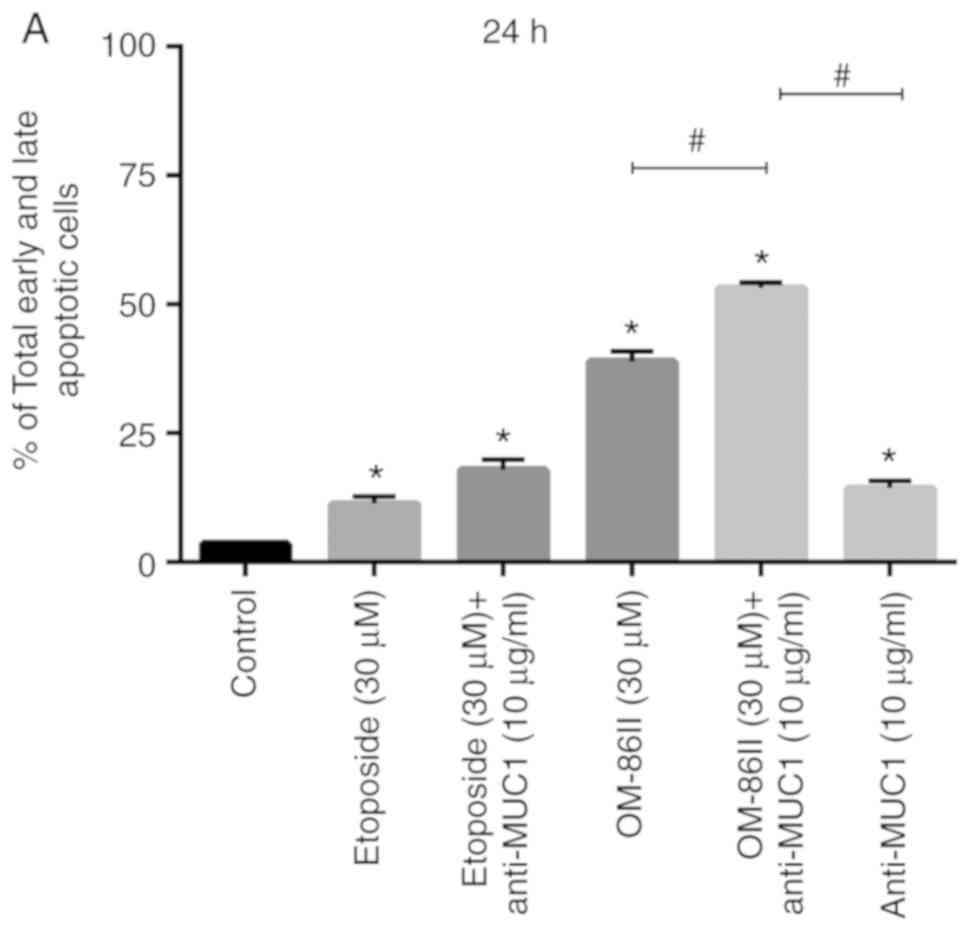
An Annexin V binding assay was performed to confirm
the results obtained by fluorescence microscopy. The results are
presented in Fig. 6. The histograms
showed viable, early and late apoptotic cells, and necrotic cells.
In total, 11.3% apoptotic cells after treatment with etoposide and
17.9% with combination of etoposide and anti-MUC1 were observed
after 24-h incubation. OM-86II demonstrated stronger pro-apoptotic
potential and 38.9% of early and late apoptotic cells were
detected. The most significant effect was observed after 24-h
incubation with anti-MUC1 and OM-86II. In that case, 53.1% of
apoptotic cells were detected (Fig.
6A). After the next 24 h of incubation, the pro-apoptotic
effect was enhanced after all treatments, but the percentage of
apoptotic cells (67.1%) was the highest after 48 h of incubation
with anti-MUC1 and OM-86II (Fig.
6B).
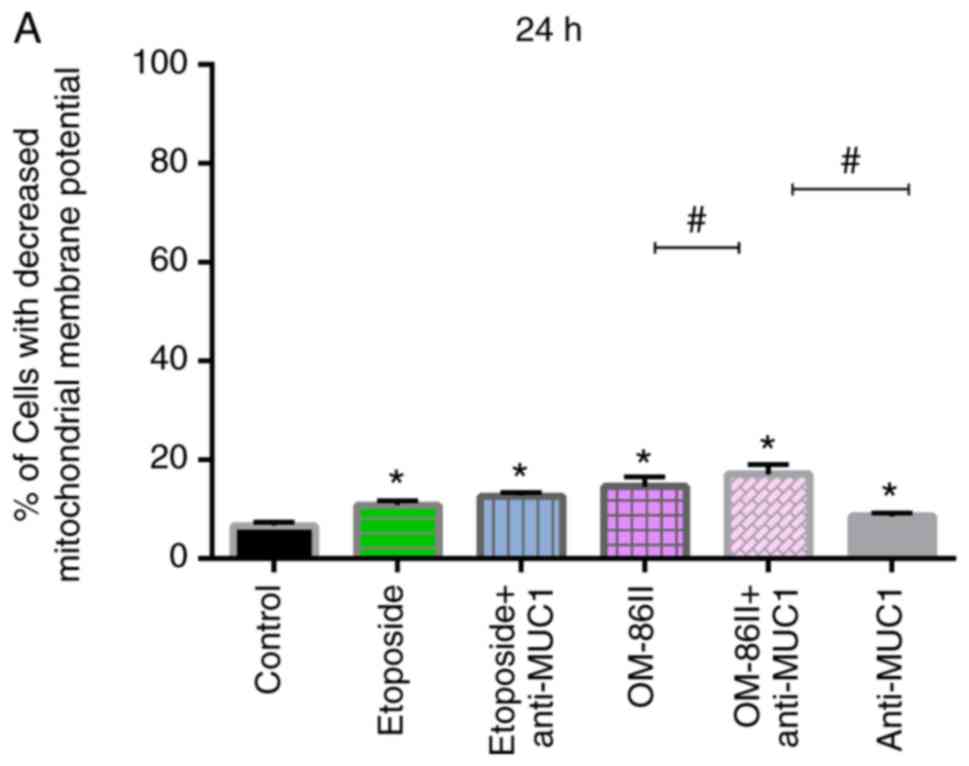
The mitochondrial membrane potential of the cells
was detected after 24 and 48 h of incubation (Fig. 7). It was identified that all the
compounds significantly decreased the mitochondrial membrane
potential compared with the control after 48 h of incubation
(Fig. 7B). In the control MCF-7
cells, 7.4% of cells with reduced mitochondrial membrane potential
were detected. After 48 h of incubation with etoposide and
etoposide with anti-MUC1, 44.9 and 46.2% of cells, respectively,
with reduced mitochondrial membrane potential were detected. The
compound OM-86II led to a higher percentage of cells with decreased
mitochondrial membrane potential (46.4%) as compared with etoposide
and anti-MUC antibody. The combination of OM-86II with anti-MUC1
antibody reduced the mitochondrial membrane potential the most; it
was observed that 55% of cells had reduced mitochondrial membrane
potential (Fig. 7B).
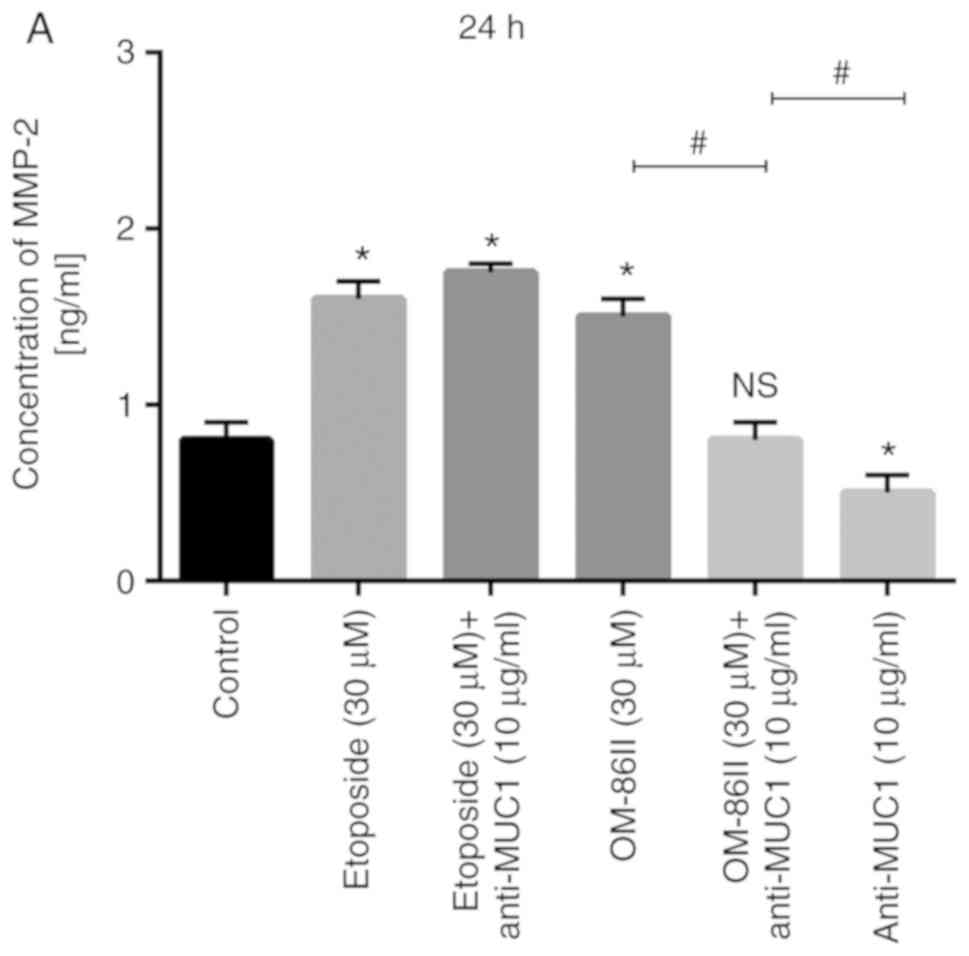
Novel OM-86II combined with anti-MUC1
antibody decreases the concentration of MMP-2 and MMP-9 in
supernatants from MCF-7 cell cultures
ELISA, a quantitative method, was chosen instead of
western blotting to measure the concentration of analyzed proteins.
The concentration of MMP-2 was detected after 24 and 48 h of
incubation with etoposide, etoposide with anti-MUC1, OM-86II,
OM-86II with anti-MUC1 and anti-MUC1 antibody (Fig. 8). It was identified that OM-86II with
anti-MUC1 antibody decreased the concentration of MMP-2 in
supernatants from the cell cultures (1.5 ng/ml) the most in
comparison with control, where the concentration of MMP-2 was 1.8
ng/ml (Fig. 8B).
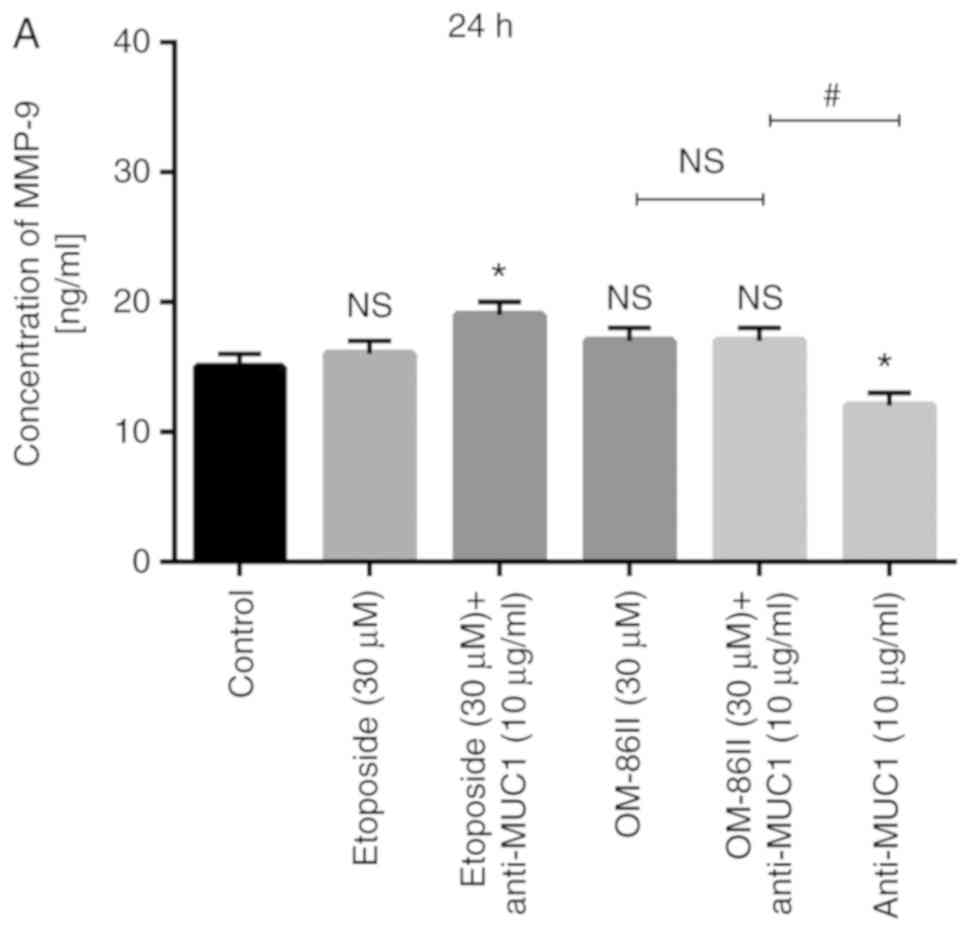
The concentration of MMP-9 was additionally detected
(Fig. 9). The most significant change
in the concentration of MMP-9 was observed after 48 h of incubation
with the compounds tested (Fig. 9B).
The concentration of MMP-9 in the control sample after 48 h was 24
ng/ml. It was demonstrated that novel OM-86II reduced the
concentration of MMP-9 to 16 ng/ml and the combination of OM-86II
with anti-MUC1 antibody reduced the concentration to 13 ng/ml.
Etoposide alone and in combination with anti-MUC1 antibody
significantly increased the concentration of MMP-9 to 48 ng/ml.
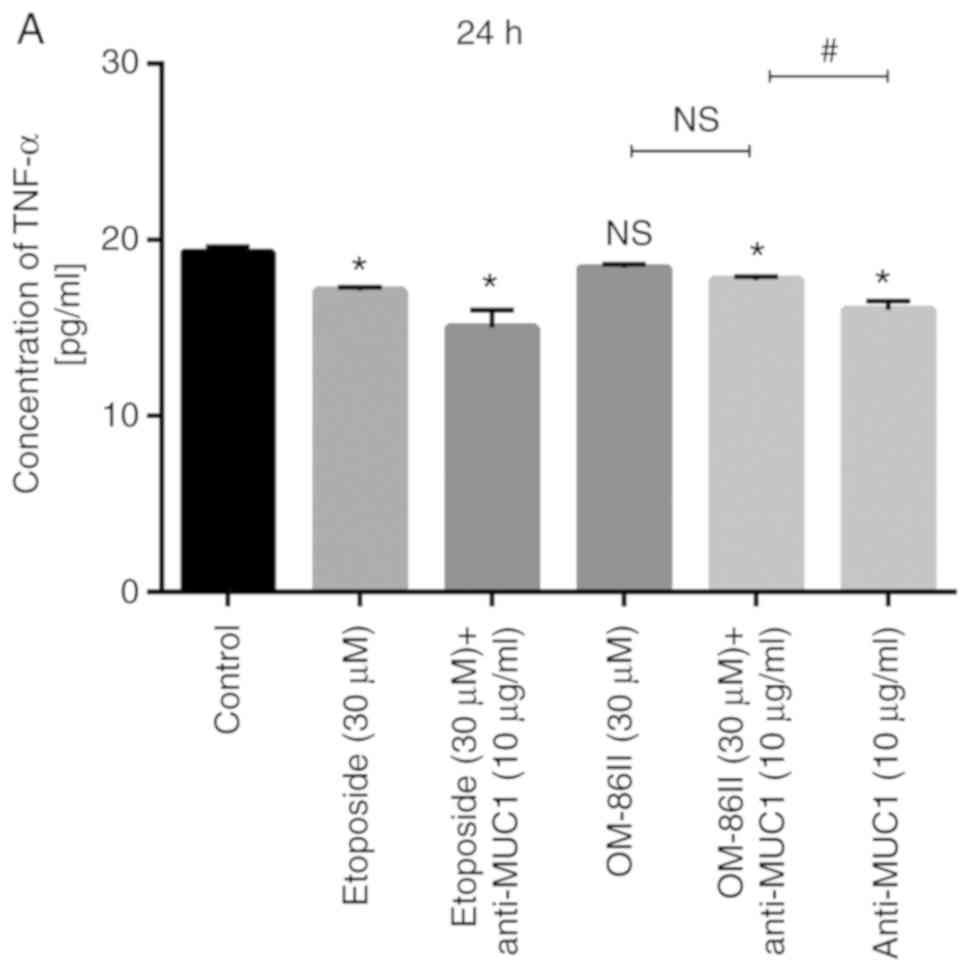
Novel OM-86II combined with anti-MUC1
antibody decreases the concentration of TNF-α and COX-2
The prolonged exposition of epithelial cells to
different factors and activation of inflammatory pathways are
associated with tumorigenesis (17).
Several previous studies have demonstrated an anticancer effect
after the inhibition of TNF-α and its receptors; such an effect was
observed in animal models of breast cancer (18–25). In
the present study, the concentration of TNF-α was detected after 24
and 48 h of incubation with the different treatments (Fig. 10). The most significant changes in
TNF-α concentration were detected after 48 h of incubation with the
compounds tested (Fig. 10B). The
strongest inhibition of TNF-α release was observed after combined
treatment with OM-86II and anti-MUC1 antibody. The concentration of
TNF-α was 15 pg/ml, as compared with 19.5 pg/ml in the control
sample.
Cancer cells with overexpressed COX-2 are resistant
to apoptosis, and COX-2 acts as a key driver in increased growth
and invasion of cancer cells via different molecular signaling
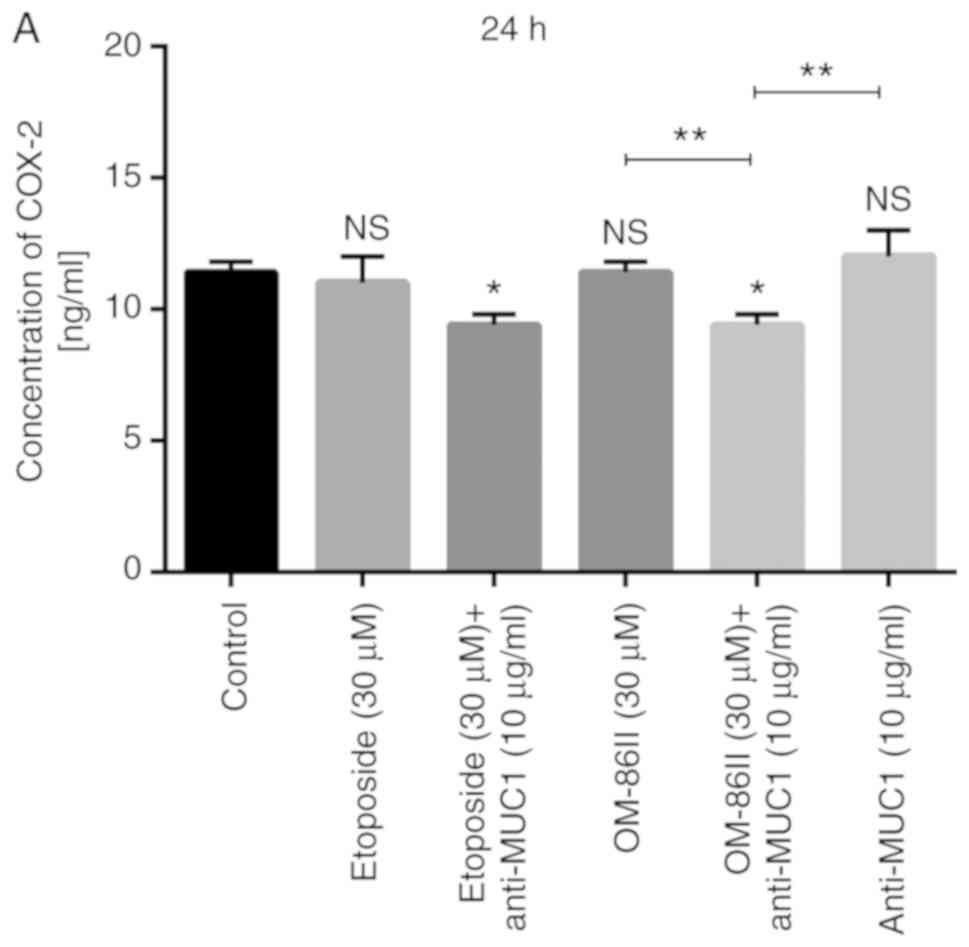
pathways (17). The present study
demonstrated that the combination therapy based on OM-86II and
anti-MUC1 antibody led to decreased concentration of COX-2 in cell
lysates in comparison with the control (Fig. 11). Etoposide and anti-MUC1 as well as
OM-86II and anti-MUC1 decreased the concentration of COX-2 to 9.4
ng/ml after 24-h incubation compared with the control, where the
concentration of COX-2 was 11.4 ng/ml (Fig. 11A). After 48 h of incubation, both
analyzed combinations of the compounds (etoposide and anti-MUC1,
and OM-86II and anti-MUC1) significantly decreased the COX-2 level.
However, the strongest effect was observed after treatment with
OM-86II and the anti-MUC1 antibody; 5 ng/ml of COX-2 was detected
in the cell lysates. The concentration of COX-2 after treatment
with etoposide and anti-MUC1 was 8 ng/ml and the difference was
also statistically significant in comparison with the control
(P<0.05; Fig. 11B).
Novel OM-86II combined with anti-MUC1
antibody decreases the concentration of mTOR
The activated PI3K/Akt/mTOR signaling pathway is
responsible for tumor growth and cancer progression (26,27).
Several agents targeted to one or more components of the
PI3K/AKT/mTOR pathway were examined for the treatment of
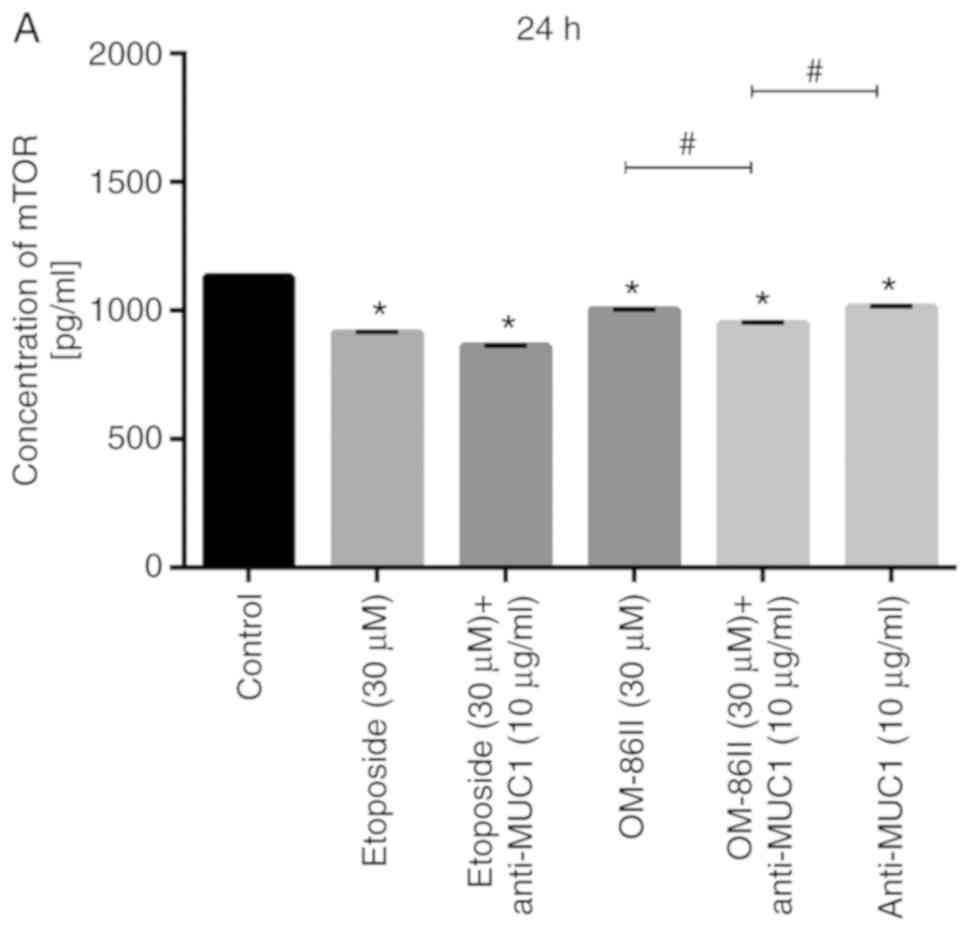
ER-positive breast cancer in clinical trials (28). The concentration of mTOR in cell
lysates was determined after 24 and 48 h of incubation with the
compounds tested (Fig. 12). Both
monotherapy and combined therapy significantly decreased the
concentration of mTOR in comparison with the untreated control
after 24 h of incubation (P<0.05; Fig. 12A). The concentration of mTOR in the
cell lysates was 949 pg/ml after treatment with OM-86II and
anti-MUC1, while in the control sample the concentration was 1,131
pg/ml. The concentration of mTOR was 861 pg/ml after treatment with
etoposide and anti-MUC1 antibody (Fig.
12A). After 48 h, it was observed that all the compounds tested
except for etoposide significantly reduced the concentration of
kinase. The lowest concentration of mTOR was detected after
incubation with OM-86II and anti-MUC1 (826 pg/ml) compared with the
control (3,729 pg/ml; Fig. 12B).
Novel OM-86II combined with anti-MUC1
antibody decreases the concentration of sICAM1
MUC1 interacts with ICAM-1 to facilitate the
migration of tumor cells (29). The
Src-CrkL-Rac1/Cdc42 signaling pathway plays the most significant
role in promoting the migratory behavior of breast cancer cells,
and upon ligation with sICAM-1 it connects with the MUC1
cytoplasmic domain and initiates cytoskeletal rearrangements
(30). The present study demonstrated
that all the tested compounds decreased sICAM1 concentration in
supernatants from cell cultures (Fig.
13). The most significant change was observed after combined
treatment. The concentration of sICAM1 after 24 and 48 h of
incubation with etoposide and anti-MUC1 was 16.8 and 5.5 ng/ml,
respectively. After 24 and 48 h of incubation OM-86II used together
with anti-MUC1 significantly decreased the sICAM1 concentration to
11.6 and 11 ng/ml, respectively.
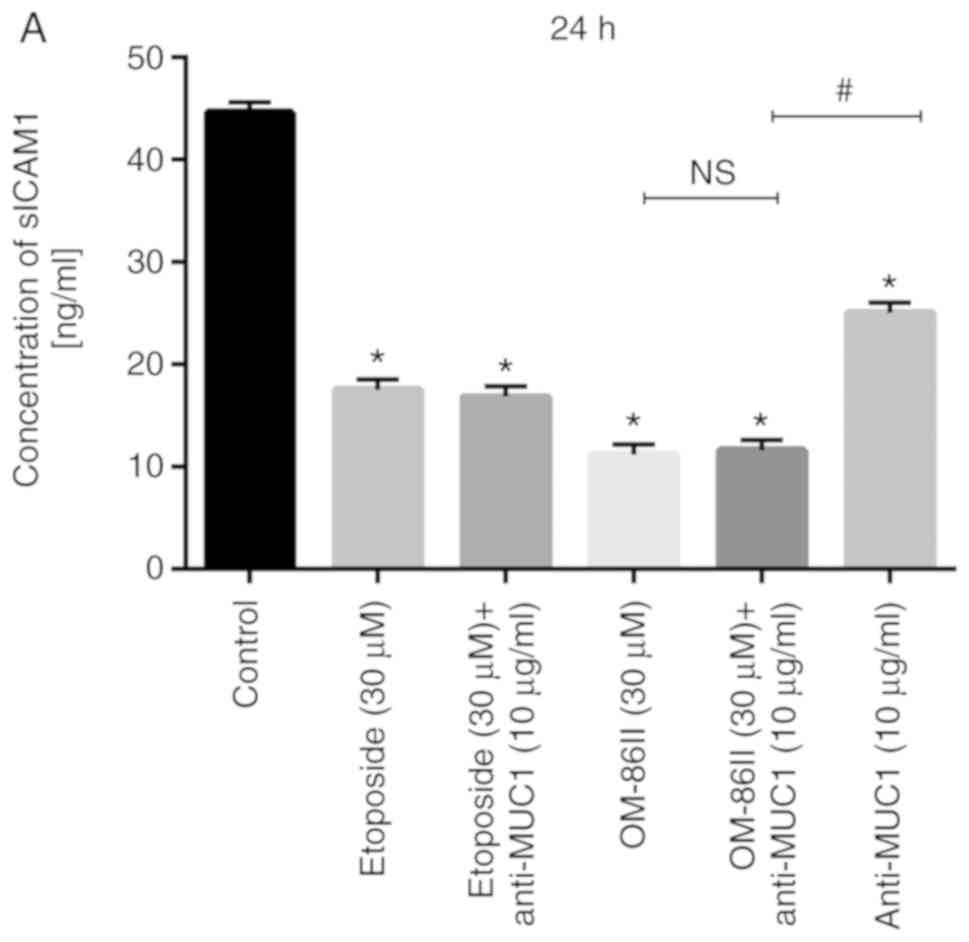 | Figure 13.Novel OM-86II combined with anti-MUC1
antibody decreases the concentration of sICAM1. Concentration of
sICAM1 in breast cancer MCF-7 cells after (A) 24 and (B) 48-h
incubation with anti-MUC1 (10 µg/ml), OM-86II (30 µM),
OM-86II+anti-MUC1 (30 µM + 10 µg/ml), etoposide (30 µM), etoposide
+ anti-MUC1 (30 µM + 10 µg/ml). Data are presented in ng/ml.
*P<0.05 vs. control group; #P<0.05. sICAM1,
soluble intercellular adhesion molecule 1; MUC1, mucin-1; OM-86II,
octahydropyrazin[2,1-a:5,4-a′]diisoquinoline derivative; ns, not
significant. |
Discussion
Current evidence suggested that MUC1 is involved in
growth, invasion, promotion of angiogenesis and chemoresistance to
programmed cell death, induced by DNA damage, oxidative stress and
hypoxia (31–35). Therapies targeting MUC1 include
monoclonal antibodies, vaccines or small molecules (aptamers).
However, none of them are currently used in clinical application
and there is still a need to evaluate the most promising strategy
in anticancer treatment based on MUC1 as a target (36). Combination of a monoclonal antibody
with novel chemotherapeutic agents represents a more efficient
approach in cancer treatment.
Disorders of cell cycle control and resistance to
apoptosis represent the most characteristic features of cancer
cells (37). The present study
demonstrated that combined treatment based on an anti-MUC1 antibody
with a novel diisoquinoline derivative (OM-86II) is an effective
strategy in decreasing the number of viable cells and inhibiting
the proliferation of MCF-7 breast cancer cells. The tested
compounds led to the induction of apoptosis, decreased the
mitochondrial membrane potential and induced a G2/M cell
cycle arrest in MCF-7 cells.
The extracellular matrix (ECM) plays an essential
role in the regulation of different signaling pathways, such as
PI3K/AKT, ERK and Src-FAK, and its function in tumor progression
has also been demonstrated in many previous studies (38,39). MMPs
are the enzymes responsible for degradation of ECM proteins and
promotion of breast cancer progression (40). Metalloproteinases take part in the
remodeling of the ECM in tumor invasion (41). A high serum MMP-2 level is associated
with an adverse prognosis in node-positive breast carcinoma
(42). MMP-9 plays a crucial role in
cancer growth and invasion. Its overexpression was correlated with
poor prognosis and worse patient survival (43). MMP-9 is also responsible for
destruction of collagen type IV and other ECM components (44). It is known that both MMP-2 and MMP-9
are key players of breast cancer invasion and metastasis (45). The present study demonstrated that
OM-86II with an anti-MUC1 antibody significantly decreased the
level of the MMPs analyzed. The association between the process of
chronic inflammation and tumor progression is still of interest;
anti-inflammatory agents can be beneficial in cancer therapy. The
expression of pro-inflammatory cytokine TNF-α was increased in 85%
of breast tumors in patients, whereas it was only minimally
expressed in normal breast epithelial cells (46). A previous study conducted by Hosseini
et al (47) demonstrated that
β-D mannuronic acid decreased the relative mRNA expression level of
inflammatory chemokines and other factors responsible for tumor
growth, such as vascular endothelial growth factor, MMP-2, MMP-9
and hypoxia-inducible factor-1α. In the present study, it was
demonstrated that the combination of anti-MUC1 with OM-86II
decreased the concentration of pro-inflammatory cytokine TNF-α in
the cell culture media.
In a previous study where COX-2 expression was
analyzed, it was identified that COX-2 was only expressed in
tumors, and its expression was correlated with unfavorable
prognosis (17). The effect of
treatments on the concentration of COX-2 was studied in the present
study and it was identified that anti-MUC1 used together with
OM-86II significantly decreased the concentration after 24 and 48 h
of incubation. Such a strategy was more efficient than monotherapy
and the combination of anti-MUC1 with etoposide.
In MCF-7 breast cancer cells, the activated
PI3K/AKT/mTOR signaling pathway (48)
leads to increased cellular growth and survival (26,27).
Therefore, the effect of the compounds tested on the concentration
of mTOR was determined in MCF-7 cell lysates. After 48 h of
incubation with the combination of anti-MUC1 and OM-86II, the
highest decrease in mTOR concentration was observed compared with
the other compounds. The inhibitory effect was also much stronger
than the combination of anti-MUC1 and etoposide.
Some researchers have shown that breast cancer
types, which exhibit increased expression of MUC1, are more likely
to metastasize. MUC1 is able to induce the Src-CrkL-Rac1/Cdc42
signaling pathway upon ligation to the ICAM-1 (29,30). The
activated pathway leads to increased migration of breast cancer
cells (30). Rahn et al
(49) showed that breast cancer cells
with overexpressed MUC1 were able to migrate through a layer of
sICAM-1 expressing cells in an in vitro transendothelial
migration assay. Thielemann et al (50) assessed the concentrations of the
sICAM-1 in the serum of female patients with breast cancer. They
identified increased concentrations of sICAM-1 in the serum of
women with breast cancer compared with the serum of healthy
controls (50). In the present study,
it was observed that combination of anti-MUC1 antibody with
etoposide or OM-86II significantly decreased the level of sICAM1
after 24 and 48 h of incubation in breast cancer cells.
Existing literature has suggested that the addition
of monoclonal antibody to chemotherapeutic agents represents a
promising strategy in anticancer treatment. The addition of
anti-MUC1 antibody to cisplatin or a novel platinum(II) complex
resulted in better pro-apoptotic activity and was more efficient
than monotherapy in breast cancer cells (15,51).
Another previous study showed that anti-MUC1 monoclonal antibody
(C595) with docetaxel reduced the tumor burden and ascites in an
in vivo ovarian cancer model (52). Slamon et al (53) demonstrated that the addition of
trastuzumab to chemotherapy had more benefits than monotherapy. The
final effect of such a treatment was longer survival of patients as
well as decreased risk of death (53). The role of MUC1 in resistance to
trastuzumab (Herceptin) is well documented (35,54).
Fessler et al (35) noticed
that cancer cells, which exhibit Herceptin resistance, were also
resistant to doxorubicin and cyclophosphamide. The resistance to
these chemotherapeutic agents was decreased by the combination of
the original drug and MUC1 inhibitor (35).
The present study demonstrated that combination
therapy based on anti-MUC1 antibody and a novel diisoquinoline
derivative (OM-86II) inhibited the proliferation of breast cancer
cells. Its inhibitory effects were associated with induction of
cell cycle arrest and apoptosis. Moreover, such a combination was
able to block the multiple intracellular signaling pathways
responsible for tumor growth promotion and breast cancer
progression. It was demonstrated that anti-MUC1 antibody with
OM-86II decreased the concentration of MMP-2, MMP-9, sICAM1 and
mTOR. In addition, combined therapy exhibited anti-inflammatory
activity; decreased concentrations of pro-inflammatory cytokine
TNF-α and COX-2 were observed. The present study suggested that the
combination of anti-MUC1 with novel OM-86II represents a potential
multi-targeted strategy in breast cancer treatment.
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Science
Centre (grant no. DEC-2017/01/X/NZ7/01315).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AG, AB and KB designed the study. AG wrote the
manuscript, and performed the experiments and statistical analysis
of the data. WS, RC and AS performed the in vitro
experiments. AB analyzed the results and coordinated the study. ZK
analyzed data and described the synthesis process. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
COX-2
|
cyclooxygenase-2
|
|
ECM
|
extracellular matrix
|
|
ER
|
estrogen receptor
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
MUC1
|
mucin-1
|
|
sICAM1
|
soluble intercellular adhesion
molecule 1
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Anderson WF, Chatterjee N, Ershler WB and
Brawley OW: Estrogen receptor breast cancer phenotypes in the
surveillance, epidemiology, and end results database. Breast Cancer
Res Treat. 76:27–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cleator SJ, Ahamed E, Coombes R and
Palmieri CA: A 2009 update on the treatment of patients with
hormone receptor-positive breast cancer. Clin Breast Cancer 9
Suppl. 1:S6–S17. 2009. View Article : Google Scholar
|
|
3
|
Davies E and Hiscox S: New therapeutic
approaches in breast cancer. Maturitas. 68:121–128. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nath S and Mukherjee P: MUC1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krishn SR, Kaur S, Smith LM, Johansson SL,
Jain M, Patel A, Gautam SK, Hollingsworth MA, Mandel U, Clausen H,
et al: Mucins and associated glycan signatures in colon
adenoma-carcinoma sequence: Prospective pathological implication(s)
for early diagnosis of colon cancer. Cancer Lett. 374:304–314.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanson RL and Hollingsworth MA: Functional
consequences of differential O-glycosylation of MUC1, MUC4, and
MUC16 (downstream effects on signaling). Biomolecules. 6:E342016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cascio S and Finn OJ: Intra- and
extra-cellular events related to altered glycosylation of MUC1
promote chronic inflammation, tumor progression, invasion, and
metastasis. Biomolecules. 6:E392016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pawłowska N, Gornowicz A, Bielawska A,
Surażyński A, Szymanowska A, Czarnomysy R and Bielawski K: The
molecular mechanism of anticancer action of novel
octahydropyrazino[2,1-a:5,4-a′]diisoquinoline derivatives in human
gastric cancer cells. Invest New Drugs. 36:970–984. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gornowicz A, Pawłowska N, Czajkowska A,
Czarnomysy R, Bielawska A, Bielawski K, Michalak O,
Staszewska-Krajewska O and Kałuża Z: Biological evaluation of
octahydropyrazin[2,1-a:5,4-a′]diisoquinoline derivatives as potent
anticancer agents. Tumour Biol. 39:10104283177016412017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cuya SM, Bjornsti MA and van Waardenburg
RCAM: DNA topoisomerase-targeting chemotherapeutics: What's new?
Cancer Chemother Pharmacol. 80:1–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kałuża Z, Bielawski K, Ćwiek R, Niedziejko
P and Kaliski P: C2-symmetric hemiaminal ethers and diamines: New
ligands for copper-catalyzed desymmetrization of meso-1,2-diols and
asymmetric Henry reactions. Tetrahedron Asymmetry. 24:1435–1442.
2013. View Article : Google Scholar
|
|
12
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: Assessment of radiosensitivity. Cancer Res.
47:943–946. 1987.PubMed/NCBI
|
|
13
|
Czarnomysy R, Surażyński A, Muszynska A,
Gornowicz A, Bielawska A and Bielawski K: A novel series of
pyrazole-platinum(II) complexes as potential anti-cancer agents
that induce cell cycle arrest and apoptosis in breast cancer cells.
J Enzyme Inhib Med Chem. 33:1006–1023. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh SK, Moretta D, Almaguel F, Wall NR,
De León M and De León D: Differential effect of proIGF-II and
IGF-II on resveratrol induced cell death by regulating surviving
cellular localization and mitochondrial depolarization in breast
cancer cells. Growth Factors. 25:363–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gornowicz A, Bielawska A, Czarnomysy R,
Gabryel-Porowska H, Muszyńska A and Bielawski K: The combined
treatment with novel platinum(II) complex and anti-MUC1 increases
apoptotic response in MDA-MB-231 breast cancer cells. Mol Cell
Biochem. 408:103–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gordon JL, Brown MA and Reynolds MM:
Cell-based methods for determination of efficacy for candidate
therapeutics in the clinical management of cancer. Diseases.
6:E852018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hugo HJ, Saunders C, Ramsay RG and
Thompson EW: New insights on COX-2 in chronic inflammation driving
breast cancer growth and metastasis. J Mammary Gland Biol
Neoplasia. 20:109–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Romieu-Mourez R, François M, Abate A,
Boivin MN, Birman E, Bailey D, Bramson JL, Forner K, Young YK,
Medin JA and Galipeau J: Mesenchymal stromal cells expressing
ErbB-2/neu elicit protective antibreast tumor immunity in vivo,
which is paradoxically suppressed by IFN-gamma and tumor necrosis
factor-alpha priming. Cancer Res. 70:7742–7747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Warren MA, Shoemaker SF, Shealy DJ, Bshar
W and Ip MM: Tumor necrosis factor deficiency inhibits mammary
tumorigenesis and a tumor necrosis factor neutralizing antibody
decreases mammary tumor growth in neu/erbB2 transgenic mice. Mol
Cancer Ther. 8:2655–2663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Houghton J, Li H, Fan X, Liu Y, Liu JH,
Rao VP, Poutahidis T, Taylor CL, Jackson EA, Hewes C, et al:
Mutations in bone marrow-derived stromal stem cells unmask latent
malignancy. Stem Cells Dev. 19:1153–1166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sangaletti S, Tripodo C, Ratti C, Piconese
S, Porcasi R, Salcedo R, Trinchieri G, Colombo MP and Chiodoni C:
Oncogene-driven intrinsic inflammation induces leukocyte production
of tumor necrosis factor that critically contributes to mammary
carcinogenesis. Cancer Res. 70:7764–7775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamaguchi T, Wakabayashi H, Matsumine A,
Sudo A and Uchida A: TNF inhibitor suppresses bone metastasis in a
breast cancer cell line. Biochem Biophys Res Commun. 407:525–530.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rubio MF, Werbajh S, Cafferata EG,
Quaglino A, Coló GP, Nojek IM, Kordon EC, Nahmod VE and Costas MA:
TNF-alpha enhances estrogen-induced cell proliferation of
estrogen-dependent breast tumor cells through a complex containing
nuclear factor-kappa B. Oncogene. 25:1367–1377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rivas MA, Tkach M, Beguelin W, Proietti
CJ, Rosemblit C, Charreau EH, Elizalde PV and Schillaci R:
Transactivation of ErbB-2 induced by tumor necrosis factor alpha
promotes NF-kappaB activation and breast cancer cell proliferation.
Breast Cancer Res Treat. 122:111–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rivas MA, Carnevale RP, Proietti CJ,
Rosemblit C, Beguelin W, Salatino M, Charreau EH, Frahm I, Sapia S,
Brouckaert P, et al: TNF alpha acting on TNFR1 promotes breast
cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent
pathways. Exp Cell Res. 314:509–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saal LH, Johansson P, Holm K,
Gruvberger-Saal SK, She QB, Maurer M, Koujak S, Ferrando AA,
Malmström P, Memeo L, et al: Poor prognosis in carcinoma is
associated with a gene expression signature of aberrant PTEN tumor
suppressor pathway activity. Proc Natl Acad Sci USA. 104:7564–7569.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciruelos Gil EM: Targeting the
PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer.
Cancer Treat Rev. 40:862–871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horm TM and Schroeder JA: MUC1 and
metastatic cancer: Expression, function and therapeutic targeting.
Cell Adh Migr. 7:187–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen Q, Rahn JJ, Zhang J, Gunasekera N,
Sun X, Shaw AR, Hendzel MJ, Hoffman P, Bernier A and Hugh JC: MUC1
initiates Src-CrkLRac1/Cdc42-mediated actin cytoskeletal protrusive
motility after ligating intercellular adhesion molecule-1. Mol
Cancer Res. 6:555–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sachdeva M and Mo YY: MicroRNA-145
suppresses cell invasion and metastasis by directly targeting mucin
1. Cancer Res. 70:378–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei X, Xu H and Kufe D: MUC1 oncoprotein
stabilizes and activates estrogen receptor alpha. Mol Cell.
21:295–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Woo JK, Choi Y, Oh SH, Jeong JH, Choi DH,
Seo HS and Kim CW: Mucin 1 enhances the tumor angiogenic response
by activation of the AKT signaling pathway. Oncogene. 31:2187–2198.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mikami Y, Hisatsune A, Tashiro T, Isohama
Y and Katsuki H: Hypoxia enhances MUC1 expression in a lung
adenocarcinoma cell line. Biochem Biophys Res Commun.
379:1060–1065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fessler SP, Wotkowicz MT, Mahanta SK and
Bamdad C: MUC1* is a determinant of trastuzumab (Herceptin)
resistance in breast cancer cells. Breast Cancer Res Treat.
118:113–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pillai K, Pourgholami MH, Chua TC and
Morris DL: MUC1 as a potential target in anticancer therapies. Am J
Clin Oncol. 38:108–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Etti IC, Abdullah R, Kadir A, Hashim NM,
Yeap SK, Imam MU, Ramli F, Malami I, Lam KL, Etti U, et al: The
molecular mechanism of the anticancer effect of Artonin E in MDA-MB
231 triple negative breast cancer cells. PLoS One. 12:e01823572017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim SH, Turnbull J and Guimond S:
Extracellular matrix and cell signalling: The dynamic cooperation
of integrin, proteoglycan and growth factor receptor. J Endocrinol.
209:139–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Walker C, Mojares E and Del Río Hernández
A: Role of extracellular matrix in development and cancer
progression. Int J Mol Sci. 19:E30282018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jena MK and Janjanam J: Role of
extracellular matrix in breast cancer development: A brief update.
Version 2 F1000Res. 7:2742018. View Article : Google Scholar
|
|
41
|
Fingleton B: Matrix metalloproteinases:
Roles in cancer and metastasis. Front Biosci. 11:479–491. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leppä S, Saarto T, Vehmanen L, Blomqvist C
and Elomaa I: A high serum matrix metalloproteinase-2 level is
associated with an adverse prognosis in node-positive breast
carcinoma. Clin Cancer Res. 10:1057–1063. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18:E32492018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pellikainen JM, Ropponen KM, Kataja VV,
Kellokoski JK, Eskelinen MJ and Kosma VM: Expression of matrix
metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special
reference to activator protein-2, HER2, and prognosis. Clin Cancer
Res. 10:7621–7628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Di Cara G, Marabeti MR, Musso R, Riili I,
Cancemi P and Pucci Minafra I: New insights into the occurrence of
matrix metalloproteases −2 and −9 in a cohort of breast cancer
patients and proteomic correlations. Cells. 7:E892018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Katanov C, Lerrer S, Liubomirski Y,
Leider-Trejo L, Meshel T, Bar J, Feniger-Barish R, Kamer I,
Soria-Artzi G, Kahani H, et al: Regulation of the inflammatory
profile of stromal cells in human breast cancer: Prominent roles
for TNF-α and the NF-κB pathway. Stem Cell Res Ther. 6:872015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hosseini F, Hassannia H, Mahdian-Shakib A,
Jadidi-Niaragh F, Enderami SE, Fattahi M, Anissian A, Mirshafiey A
and Kokhaei P: Targeting of crosstalk between tumor and tumor
microenvironment by β-D mannuronic acid (M2000) in murine breast
cancer model. Cancer Med. 6:640–650. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Miller TW, Rexer BN, Garrett JT and
Arteaga CL: Mutations in the phosphatidylinositol 3-kinase pathway:
Role in tumor progression and therapeutic implications in breast
cancer. Breast Cancer Res. 13:2242011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rahn JJ, Chow JW, Horne GJ, Mah BK,
Emerman JT, Hoffman P and Hugh JC: MUC1 mediates transendothelial
migration in vitro by ligating endothelial cell ICAM-1. Clin Exp
Metastasis. 22:475–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Thielemann A, Baszczuk A, Kopczyński Z,
Nowak A and Grodecka-Gazdecka S: The clinical usefulness of
assessing the concentration of cell adhesion molecules sVCAM-1 and
sICAM-1 in the serum of women with primary breast cancer. Contemp
Oncol (Pozn). 18:252–259. 2014.PubMed/NCBI
|
|
51
|
Gornowicz A, Kałuża Z, Bielawska A,
Gabryel-Porowska H, Czarnomysy R and Bielawski K: Cytotoxic
efficacy of a novel dinuclear platinum(II) complex used with
anti-MUC1 in human breast cancer cells. Mol Cell Biochem.
392:161–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang L, Chen H, Pourgholami MH, Beretov J,
Hao J, Chao H, Perkins AC, Kearsley JH and Li Y: Anti-MUC1
monoclonal antibody (C595) and docetaxel markedly reduce tumor
burden and ascites, and prolong survival in an in vivo ovarian
cancer model. PLoS One. 6:e244052011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Raina D, Uchida Y, Kharbanda A, Rajabi H,
Panchamoorthy G, Jin C, Kharbanda S, Scaltriti M, Baselga J and
Kufe D: Targeting the MUC1-C oncoprotein downregulates HER2
activation and abrogates trastuzumab resistance in breast cancer
cells. Oncogene. 33:3422–3431. 2014. View Article : Google Scholar : PubMed/NCBI
|