Introduction
Ovarian cancer is the most lethal gynecological
malignancy and is the fifth leading cause of cancer-related
mortality among women in developing countries (1). Patients with ovarian cancer are treated
with surgical debunking, followed by chemotherapy with a
platinum-based drug or paclitaxel (2). In addition, cisplatin (also known as
DDP) is recommended as a common chemotherapeutic agent. As patients
eventually become resistant to cytotoxic agents, the overall
five-year survival rate is only 44% (3–5). Thus,
chemoresistance is one of the main causes limiting the
effectiveness of chemotherapy (6).
There is an increasing evidence regarding the
association of autophagy and chemoresistance (7). Autophagy is often known as type II
programmed cell death, and is an evolutionarily conserved catabolic
process that is responsible for the degradation and recycling of
long-lived or aggregated proteins, and excess or defective
organelles. Autophagy is induced by a group of autophagy-related
(ATG) genes and activates the conversion of cytosolic
microtubule-associated protein light chain 3A (LC3A) into LC3B
(8,9).
Autophagy is generally low under basal conditions, while altered
autophagic activity has been observed under stress conditions, such
as starvation (10), diverse diseases
(11), irradiation (12,13) and
chemotherapy (14). Although several
studies have demonstrated that various anti-tumor agents induce
autophagic cell death in cancer cells (15–17), there
is compelling evidence to indicate that autophagy contributes to
chemoresistance in various types of cancer (18,19). It
has recently been reported that miR-181a suppresses autophagy and
sensitizes gastric cancer cells to cisplatin (20) and also that the suppression of
autophagy enhances the efficacy of sunitinib in clear cell ovarian
carcinoma (21). Autophagy has also
been shown to contribute to taxol resistance in human colorectal
cancer cells (22).
Thioredoxin-related protein of 14 kDa (TRP14) is a
novel disulfide reductase that belongs to the thioredoxin (TXN)
family and was discovered in 2004 (23). It is also known as thioredoxin domain
containing 17 (TXNDC17) and thioredoxin-like 5 (TXNL5) (24). TRP14 is a TrxR1-dependent reductase
that can effectively reduce both S-nitrosothiol and L-cystine
levels. The biological function of TRP14 remains unclear however.
Previous studies have demonstrated the role of TRP14 in autophagy
in mediating taxol resistance by stimulating autophagy in
colorectal and ovarian cancer cells (22,25). Thus,
we hypothesized that there may be an association among TRP14,
autophagy and cisplatin resistance in ovarian cancer.
Although there is increasing interest in the
modulation of autophagy for cancer therapy, the role of TRP14 in
autophagy and cisplatin resistance in ovarian cancer cell lines
remains unknown, at least to the best of our knowledge. Hence, in
this study, we evaluated the level and effect of autophagy and the
function of TRP14 in cisplatin-resistant ovarian cancer cell lines.
The findings of this study demonstrate that TRP14 plays a key role
in cisplatin resistance through the induction of autophagy. Thus,
TRP14 may prove to be a potential predictor or target in ovarian
cancer therapy.
Materials and methods
Cell lines, reagents and
antibodies
The human ovarian cancer cell lines, A2780 and
SKOV3, were obtained from the European Collection of Authenticated
Cell Cultures (93112519, ECACC) and the American Type Culture
Collection (HTB-77, ATCC), respectively and maintained in RPMI-1640
(10–040-CVR, Corning, Inc.) and McCoy 5A medium (16600-108, Gibco;
Thermo Fisher Scientific) supplemented with 10% fetal bovine serum
(FBS) at 37°C in 5% CO2. The cisplatin-resistant cells,
SKOV3/DDP and A2780/DDP, were established by exposing the cells to
gradually increasing concentrations (from 0 to 25 µg/ml) of
cisplatin for 6 months (data not shown). Cisplatin was purchased
from Hansoh Pharmaceutical Co., Ltd. 3-Methyladenine (3-MA),
rapamycin (Rapa), compound C and monodansylcadaverine (MDC) were
purchased from Sigma-Aldrich. The primary antibody to TRP14
(ab121725/dilution, 1:500) was purchased from Abcam. The primary
antibodies for ATG5 (#12994/dilution, 1:1,000), Beclin1
(#3495/dilution, 1:1,000), LC3B (#3868/dilution, 1:1,000), p-5′
AMP-activated protein kinase (AMPK; #2537/dilution, 1:1,000),
p-mammalian target of rapamycin (mTOR; #5536/dilution, 1:1,000),
p-p70S6K (#9204/dilution, 1:1,000), AMPK (#5832/dilution, 1:1,000),
mTOR (#2983/dilution, 1:1,000) and p70S6K (#2708 /dilution,
1:1,000) were obtained from Cell Signaling Technology Inc. The
antibody for GAPDH (sc-47724) was purchased from Santa Cruz
Biotechnology.
Cell viability assay
Cells were plated in triplicate in 96-well plates
overnight and then were treated with or without 200 nM Rapa for 24
h or treated with 4 µM compound C for 12 h. After 12 h, the cells
were treated with various concentrations of cisplatin (from 0 to 16
µg/ml) for 24 h. Cell viability was measured using a Cell Counting
kit-8 (CCK-8, Dojindo Molecular Technologies, Inc.) assay according
to the manufacturer's instructions (25). Cell viability was calculated according
to the following equation: Cell viability=OD value of experimental
group/OD value of control group ×100%.
Western blot analysis
Cells were harvested and washed with
phosphate-buffered saline (PBS). The proteins were extracted, and
western blot analysis was performed as previously described
(26). Briefly, the cells were
dissolved (for 30 min) in lysis buffer containing 1% PMSF in RIPA
buffer (Thermo Fisher Scientific) and quantified using the BCA
Protein Assay (Thermo Fisher Scientific). Proteins (30 µg) were
resolved on 12% SDS-PAGE, transferred onto polyvinylidenedifluoride
transfer membranes (PVDF) and blocked with 5% non-fat dry milk in
TBST for 1 h at room temperature. The membranes were incubated with
the indicated antibodies overnight at 4°C, followed by the
incubation with the corresponding secondary antibodies (anti-rabbit
#7074 and anti-mouse #7076/dilution, 1:5,000, Cell Signaling
Technology Inc.) conjugated with horseradish peroxidase-conjugated
(HRP) for 1 h at room temperature. The bands were detected with an
EZ-ECL kit (BI Biological Industries, 20-500-120) in the MicroChemi
bio-imaging system (DNR). ImageJ was used for densitometry (Image J
1.46r, National Institutes of Health).
Plasmids and shRNA transfection
The pcDNA3.1(C)-TRP14 plasmid was constructed based
on the following description. The fragment (182 to 553) of TRP14
mRNA was synthesized and inserted into the pcDNA3.1(C) vector
(Invitrogen; Thermo Fisher Scientific, #V790-20). The pcDNA3.1(C)
vector was considered as the mock control. DNA oligonucleotides
carrying shRNA (Invitrogen-Life Technology; Thermo Fisher
Scientific) were constructed into the pLKO.1 plasmid (Addgene
Plasmid #8453). The packaging plasmid psPAX2 (Addgene Plasmid
#12260) and envelop plasmid pMD2.G (Addgene Plasmid #12259) were
transfected into 293T cells (from Cell Bank of the Chinese Academy
of Sciences)with recombinant plasmids using LipofectamineTM2000
(Invitrogen; Thermo Fisher Scientific). The supernatant containing
lentiviruses was collected after 36 h. The sequences of the shRNAs
were as follows, TRP14 shRNA#1, GTGCCTACACTACTTAAGT; TRP14 shRNA#2,
ACCTAACCTCACCACTGAA; ATG5 shRNA#1, GCTACTCTGGATGGGATTG; ATG5
shRNA#2, ATTGGCTCAATTCCATGAA; and control shRNA,
CACACCGTTTCGTGGCTTT. Cells were grown till 80% confluence prior to
transfection. Transfected cells were grown for 2 weeks in the
presence of puromycin (0.2 µg/ml) before they were used for
subsequent experimentation.
MDC staining assay
Following treatment with 200 nM Rapa for 12 h, the
cells were washed twice with PBS and incubated with 50 mM MDC at
37°C for 1 h. The cells were washed with PBS and then fixed with 4%
paraformaldehyde (PFA) solution for 15 min. Finally, the cells were
analyzed under an Olympus BX50 fluorescence microscope
(Olympus).
RFP-LC3 analysis
The cells were then transfected with a GFP-RFP-LC3
plasmid (Addgene Plasmid #84573) using LipofectamineTM2000 and 24 h
later, the cells were transferred onto coverslips. The cells were
washed with PBS and fixed with 4% PFA in PBS for 30 min at room
temperature. The fixed cells were washed twice with PBS and then
permeabilized with 0.5% Triton X-100 in PBS for 2 min on ice.
Images were obtained under an Olympus BX50 fluorescence microscope
(Olympus).
Electron microscopy analysis
Following treatment with 200 nM Rapa for 12 h, and
the knockdown or overexpression of TRP14, the cells were harvested
and washed with PBS. The samples were processed and detected as
previously described (27). Briefly,
the cells were exposed to 10 nM paclitaxel for 24 h and fixed with
2.5% glutaraldehyde solution (Sigma-Aldrich, G5882) overnight, then
post-fixed with 1% OsO4 and dehydrated standard in graded ethanol,
embedded in 812 resin (Ted Pella, 18109). Thin sections were sliced
and stained with 2% uranyl acetate, and then examined with a
JEM-100CX transmission electron microscope (Jeol).
Drug resistance index (DRI) assay
Four groups of cells (SKOV3DDP/SKOV3 and
A2780DDP/A2780) were obtained for the preparation of cell
suspension. Cell concentration was adjusted to 5×105/ml
and 200 µl was placed in each well of a 96-well culture plate.
Following a 24 h culture, various concentrations (from 0 to 25
µg/ml) of cisplatin were added. A control group without drugs was
also set. All cells were cultured at 37°C and 5% CO2 for
24 h. Subsequenlty, 20 µl of MTT (5 mg/ml) solution were added to
each well and the cells were cultured for an additional 4 h.
Supernatants were discarded after termination of the culture and
150 µl of dimethyl sulphoxide (DMSO) were added to each well. The
plates were shaken for 10 min and a microplate reader was used to
measure the optical density (OD) value at a wavelength of 570 nm to
calculate cell survival rate. The following equation was used to
calculate the cell survival rate: Cell survival rate=(the OD value
in each experiment well/the OD value in the control well) ×100%.
The 50% of inhibition concentration (IC50) of drug was measured by
chartography. The drug resistance index (DRI)=the IC50 of
drug-resistant cells/the IC50 of parent cell line. MTT experiments
were repeated 3 times on time points(every 24 h).
Immunohistochemistry
The clinical ovarian cancer and marginal tissue
microarray (BC11115b from US Biomax) was analyzed based on the
following description. After dewaxing and hydration, the tissue
microarray slides were boiled in sodium citrate buffer for 10 min
for antigen recovery, and immersed in 3% H2O2
for 10 min to quench endogenous peroxidase. After being blocked
with 5% horse serum for 35 min, the slides were incubated with
primary antibodies against TRP14 (dilution, 1:200; ab121725,
Abcam); or Beclin1 (dilution, 1:200; ab62557, Abcam) at 4°C
overnight. Subsequently, the sections were rinsed and then coated
with HRP-conjugated secondary antibody (dilution, 1:200; ab205718,
Abcam) and incubated at 37°C for 1 h. DAB (ab64261, Abcam) was used
to visualize the immunoreactive sites.
Statistical analysis
All analyses were repeated at least 3 times. Data
are expressed as means ± SD. Statistical analysis of the data was
performed using a Student's t-test and two-tailed distribution. The
Kruskal-Wallis test was used for multiple comparisons and then Mann
Whitney U test as well as Bonferroni's correction were applied as
post hoc tests to determine the cell viability difference among the
different groups.
Results
Autophagy is induced in
cisplatin-resistant human ovarian cancer cell lines
To identify the chemoresistance of
cisplatin-resistant human ovarian cancer cell lines, the
cisplatin-resistant human ovarian cancer cell lines, SKOV3/DDP,
A2780/DDP, and the control cell lines, SKOV3/A2780, were treated
with various concentrations of cisplatin for 24 h. CCK-8 and drug
resistance index (DRI) assay were then performed to measure the
cell viability and drug sensitivity in the different cells
(Fig. 1A and B). The results revealed
that the survival of both the SKOV3 and SKOV3/DDP cells decreased
in a dose-dependent manner. However, the SKOV3/DDP cells were more
resistant to cisplatin, as compared with the SKOV3 cells
(P<0.05). Similar results were clearly observed in the A2780 and
A2780/DDP cells (Fig. 1A). Moreover,
our data suggested that the DRI was much higher in the SKOV3/DDP
compared to A2780/DDP cells, indicating that the SKOV3/DDP cells
were more resistant than the A2780/DDP cells. Recently, it has been
demonstrated that autophagy is associated with drug resistance
(25). Thus, the contribution of
autophagy to cisplatin resistance was determined in this study. As
shown in Fig 1C, the levels of LC3,
Beclin1 and ATG5, which are the characteristics of autophagy
(15,28), were elevated in the SKOV3/DDP and
A2780/DDP cells when compared with the SKOV3 and A2780 cells,
respectively (Rapa treatment was used as a positive control).
Furthermore, the acidic vesicular organelles (AVOs) were examined
by MDC staining. A greater number of the SKOV3/DDP and A2780/DDP
cells were MDC-positive cells compared with their sensitive
counterparts, the SKOV3 and A2780 cells (Fig. 1D). According to these observations,
the numbers of GFP-RFP-LC3-positive puncta were significantly
increased in the cisplatin-resistant ovarian cancer cells (Fig. 1E). More importantly, these results
were validated using transmission electron microscopy (Fig. 1F). These data clearly indicated that
cisplatin-resistant human ovarian cancer cell lines displayed an
enhanced autophagy status when compared with the control cell
lines, SKOV3 and A2780.
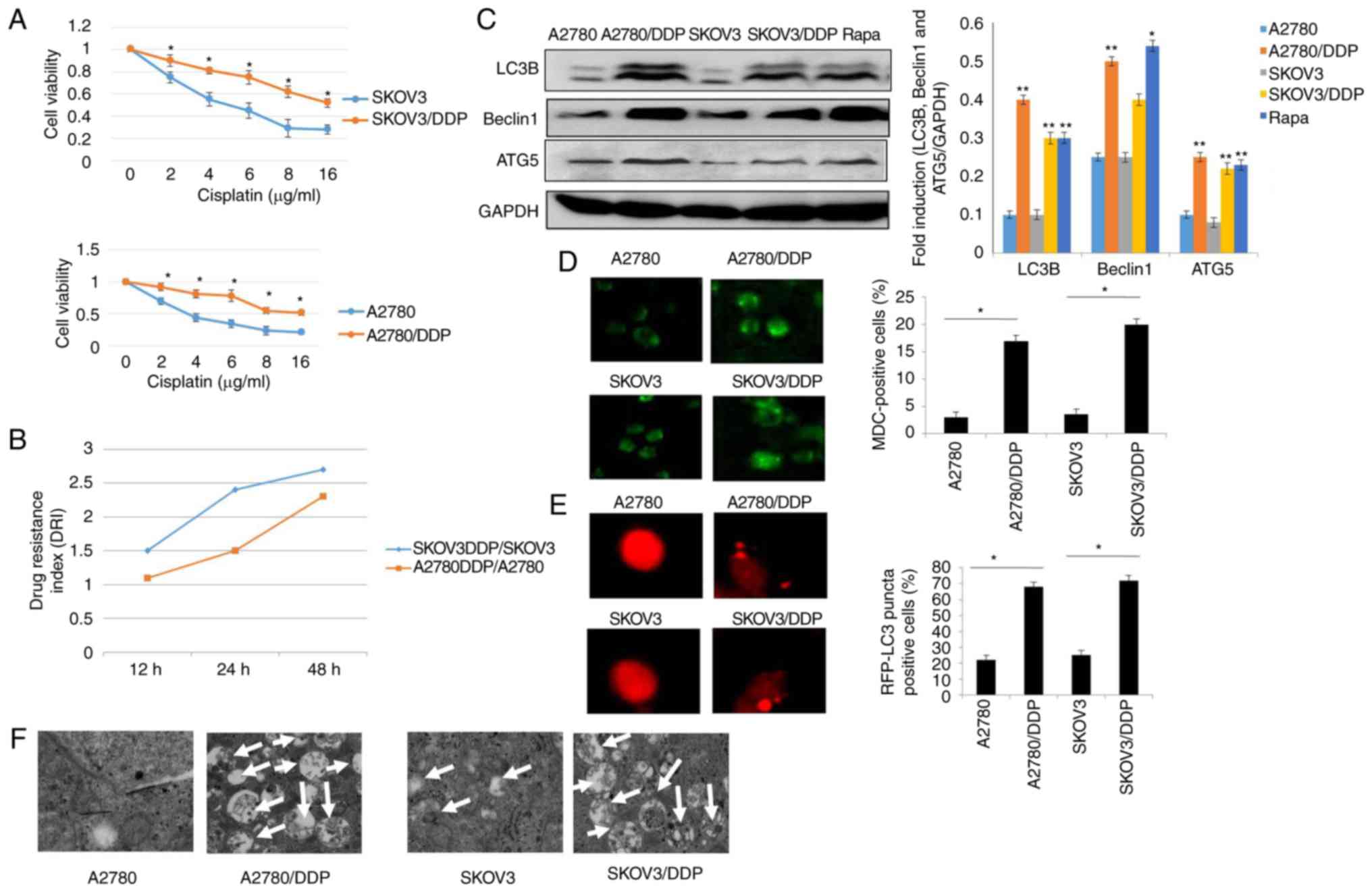 | Figure 1.Autophagy is induced in
cisplatin-resistant human ovarian cancer cell lines. (A) A2780,
A2780/DDP, SKOV3 and SKOV3/DDP cells were incubated with the
indicated concentrations of cisplatin for 24 h, and cell viability
was then measured by CCK-8 assay. Values were presented as the mean
± SEM (n=3). *P<0.05, statistical significance vs. normal A2780
and SKOV3 cells. (B) Drug resistance index (DRI) of cisplatin in
A2780, A2780/DDP, SKOV3 and SKOV3/DDP cells. (C) The levels of LC3,
Beclin1 and ATG5 were detected by western blot analysis in A2780,
A2780/DDP, SKOV3 and SKOV3/DDP cells. Rapamycin (Rapa) was used as
a positive control. GAPDH served as a loading control. Results were
repeated in independent experiments. *P<0.05 and **P<0.01,
statistical significance vs. normal A2780 and SKOV3 cells. (D) MDC
staining of A2780, A2780/DDP, SKOV3 and SKOV3/DDP cells.
*P<0.05, statistical significance vs. normal A2780 and SKOV3
cells. (E) A2780, A2780/DDP, SKOV3 and SKOV3/DDP cells were
transfected with GFP-RFP-LC3-plasmid. After 24 h, representative
images of GFP- RFP-LC3-II-positive puncta were photographed by
using a confocal fluorescence microscope. *P<0.05, statistical
significance vs. normal A2780 and SKOV3 cells. (F) The
ultrastructural changes of A2780, A2780/DDP, SKOV3 and SKOV3/DDP
cells as measured by transmission electron microscopy are shown.
The white arrows indicate the typical images of autophagosomes and
autolysosomes. |
Autophagy is required for cisplatin
resistance in human ovarian cancer cells
To elucidate the role of autophagy in the resistance
of human ovarian cancer cell lines to cisplatin, the autophagy
inhibitor, 3-MA was used (29). As
shown in Fig. 2A, the levels of LC3,
Beclin1 and ATG5 were significantly decreased in the SKOV3/DDP and
A2780/DDP cells following treatment with 5 nM 3-MA for 24 h. As
expected, treatment with 3-MA significantly decreased the viability
of the SKOV3/DDP and A2780/DDP cells (Fig. 2B). To assess the role of autophagy in
cisplatin resistance, autophagy was inhibited through genetic
methods (silencing of ATG5) in the SKOV3/DDP and A2780/DDP cells.
Following the silencing of ATG5, the viability of the SKOV3/DDP and
A2780/DDP cells exhibited a significant attenuation (Fig. 2C and D), indicating that the cytotoxic
effects of cisplatin were enhanced by ATG5 knockdown. Since the DRI
was greater in the SKOV3 cells than in the A2780 cells (Fig. 1B), Rapa (200 nM) was used to induce
autophagy in the SKOV3 cells (Fig.
2E-H). The results revealed that the expression levels of LC3,
Beclin1 and ATG were markedly upregulated by Rapa in the SKOV3
cells (Fig. 2E). Consistent with the
above-mentioned results, MDC staining, GFP-RFP-LC3 transfection and
transmission electron microscopy analysis confirmed that cisplatin
triggered autophagy in the SKOV3 cells (Fig. 2F-H), as evidence by the increased
number of GFP-RFP-LC3-positive puncta, MDC-positive cells, and
autolysosome vesicles. Moreover, cisplatin exhibited significant
cytotoxicity in the SKOV3 cells and A2780 cells in a dose-dependent
manner (Fig. 2I). These findings
suggested that cisplatin induced autophagic cell death in the SKOV3
cells. Similar results were observed in the A2780 cells (data not
shown). Taken together, these results clearly demonstrated that
autophagy modulated cisplatin resistance in human ovarian cancer
cells.
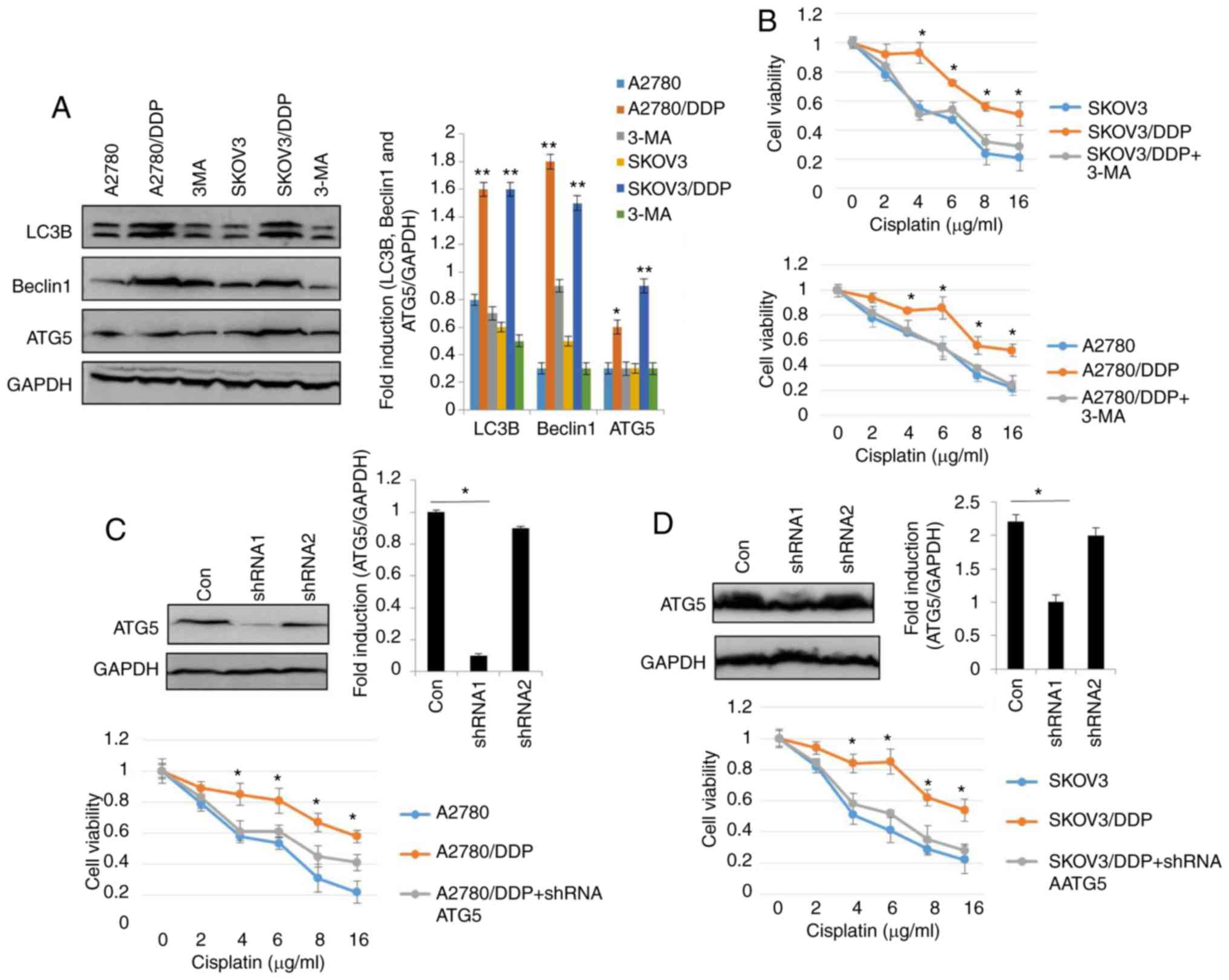 | Figure 2.Autophagy regulates cisplatin
resistance in human ovarian cancer cells. (A) The levels of LC3,
Beclin1 and ATG5 were detected by western blot analysis in
SKOV3/DDP and A2780/DDP cells treated with 5 nM 3-MA for 24 h.
GAPDH was used as an internal control. Results were repeated in
independent experiments. *P<0.05 and **P<0.01, statistical
significance vs. 3-MA-treated A2780 and SKOV3 cells. (B) The effect
of the autophagy inhibitor, 3-MA, on the viability of SKOV3/DDP and
A2780/DDP cells was tested. Cell viability was measured by CCK-8
assay. The Kruskal-Wallis was used for multiple comparisons and
then Mann Whitney U test and Bonferroni's correction were applied.
The values were presented as the means ± SEM (n=3). *P<0.05,
statistical significance vs. 3-MA-treated cells. (C) Abrogation of
ATG5 potentiates cisplatin cytotoxicity in SKOV3/DDP cells. The
Kruskal-Wallis was used for multiple comparisons and then Mann
Whitney U test and Bonferroni's correction were applied. The values
were presented as the means ± SEM (n=3). *P<0.05, statistical
significance vs. ATG5-knockdown cells. (D) Abrogation of ATG5
potentiates cisplatin cytotoxicity in A2780/DDP cells. The
Kruskal-Wallis was used for multiple comparisons and then Mann
Whitney U test and Bonferroni's correction were applied. The values
were presented as the means ± SEM (n=3). *P<0.05, statistical
significance vs. ATG5-knockdown cells. (E) The levels of LC3,
Beclin1 and ATG5 were detected by western blot analysis in SKOV3
cells treated with 200 nM Rapa for 12 h. *P<0.05 and
**P<0.01, statistical significance vs. control. (F) SKOV3 cells
were transfected with GFP-RFP-LC3-plasmid overnight. Following 12 h
of exposure to 200 nM Rapa, representative images of GFP-
RFP-LC3-II-positive puncta were obtained using a confocal
fluorescence microscope. *P<0.05, statistical significance vs.
control SKOV3 cells. Autophagy regulates cisplatin resistance in
human ovarian cancer cells. (G) MDC staining of SKOV3 cells
following treatment with 200 nM Rapa for 12 h. *P<0.05,
statistical significance vs. control SKOV3 cells. (H) Autophagosome
and autolysosome vesicles of SKOV3 cells treated with 200 nM Rapa
for 12 h were visualized by transmission electron microscopy. The
white arrows indicate the typical images of autophagosomes and
autolysosomes. (I) Effect of the autophagy activator, rapamycin
(Rapa) on the viability of SKOV3 cells and A2780 cells was
examined. Values were presented as the means ± SEM (n=3).
*P<0.05, statistical significance vs. control SKOV3 and A2780
cells. |
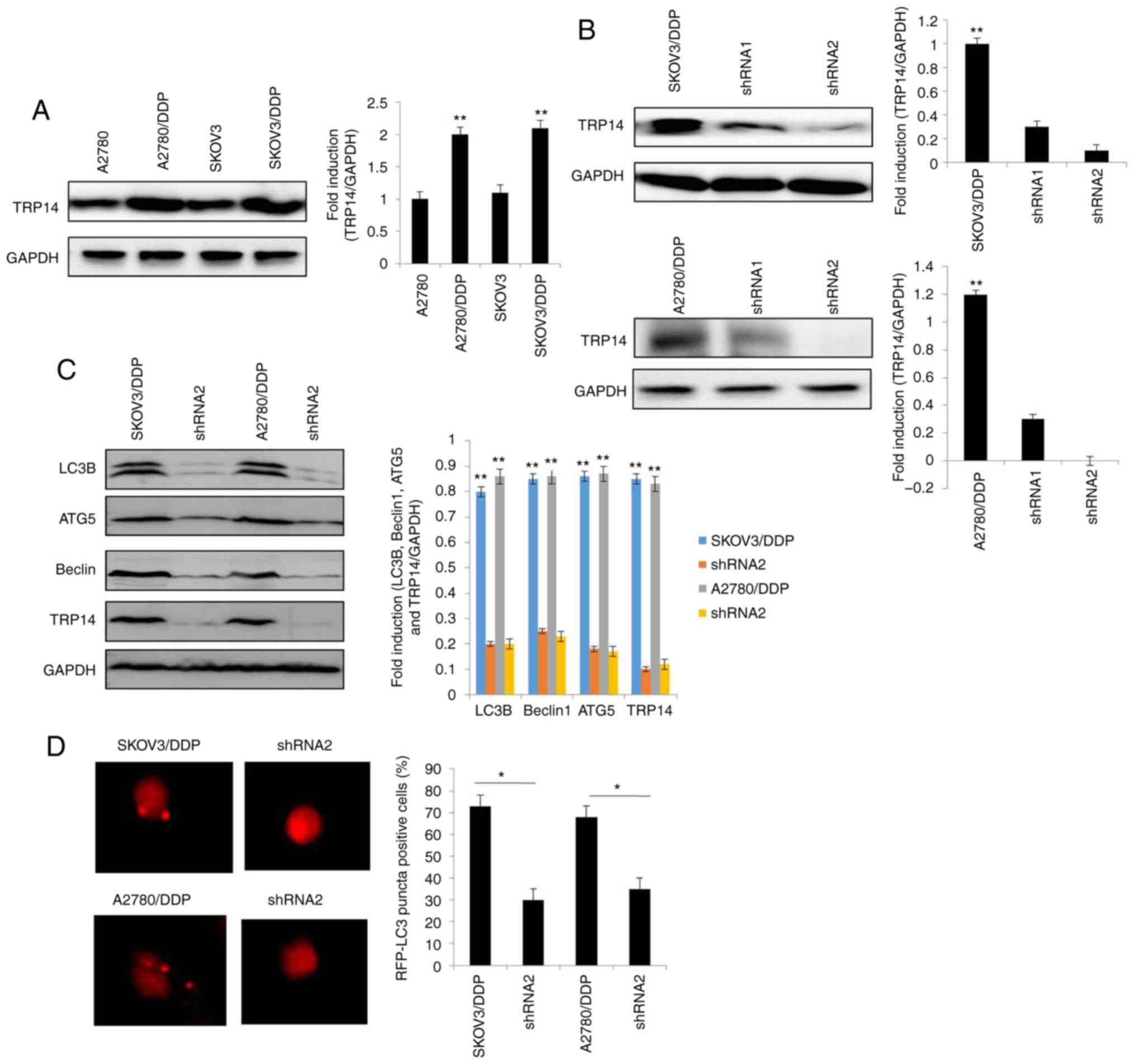
TRP14 knockdown suppresses
autophagy-induced cisplatin resistance
Previous studies have reported that TRP14 promotes
taxol resistance by inducing autophagy (22,25). Thus,
we hypothesized that TRP14 may also participate in
autophagy-induced cisplatin resistance in human ovarian cancer
cells. The results revealed that the protein expression of TRP14
was significantly increased in the SKOV3/DDP and A2780/DDP cells
when compared with the SKOV3 and A2780 cells, respectively
(Fig. 3A). To confirm the function of
TRP14 in autophagy-regulated cisplatin resistance, TRP14 was
knocked down using shRNA against different regions of TRP14 mRNA in
the SKOV3/DDP and A2780/DDP cells (Fig.
3B). As shown in Fig. 3C-F, TRP14
knockdown significantly inhibited autophagy in the SKOV3/DDP and
A2780/DDP cells, as determined by western blot analysis, MDC
staining, transmission electron microscopy observation and
GFP-RFP-LC3 transfection. In addition, TRP14 knockdown
significantly increased the sensitivity of the SKOV3/DDP and
A2780/DDP cells to cisplatin (Fig. 3G and
H). To verify the role of autophagy in cisplatin resistance
induced by TRP14 knockdown, Rapa was used. As shown in Fig. 3G and H, Rapa promoted resistance to
cisplatin when TRP14 was knocked down. Taken together, these data
clearly indicated that TRP14 knockdown suppressed autophagy-induced
cisplatin resistance in the SKOV3/DDP and A2780/DDP cells.
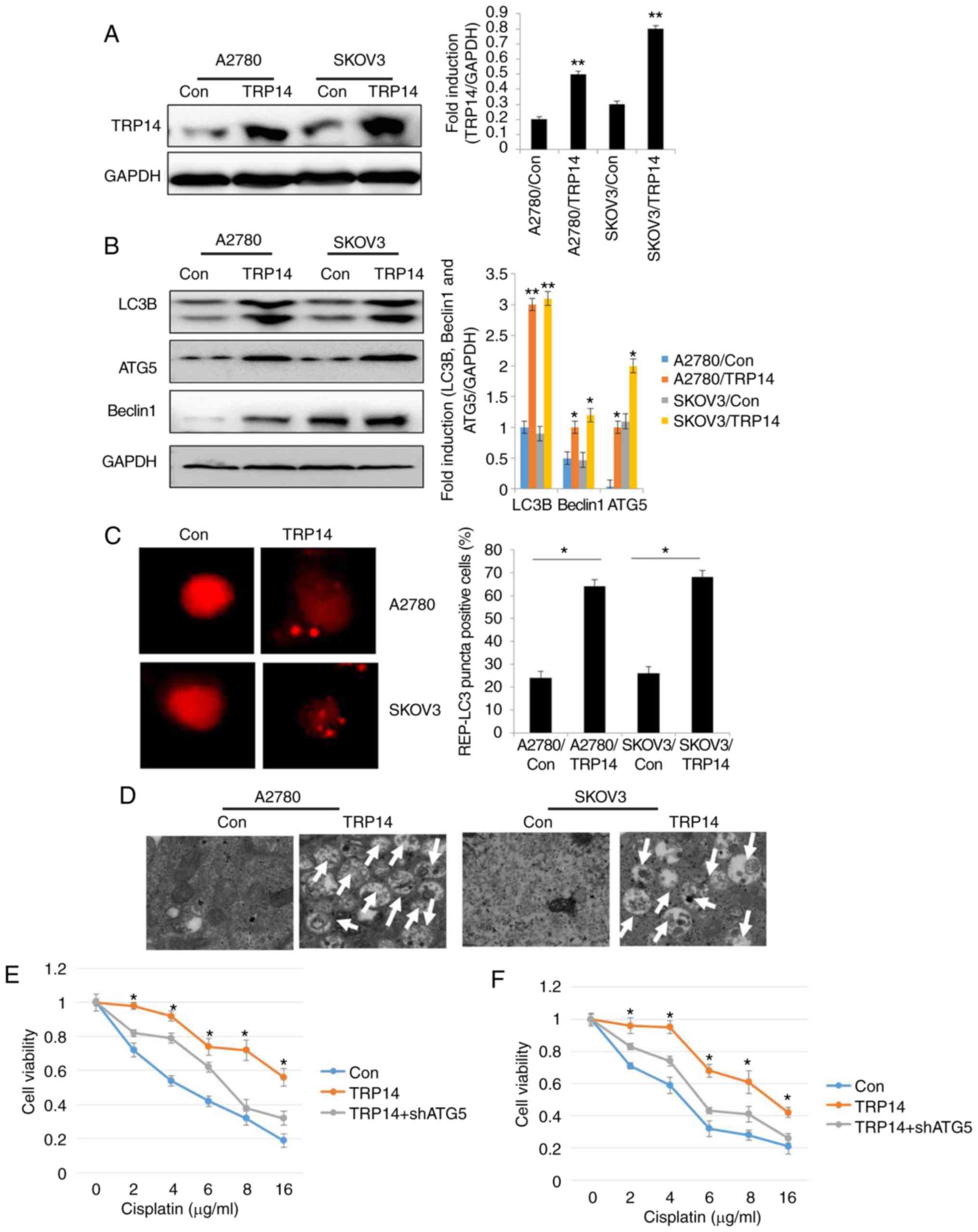
TRP14 overexpression promotes
autophagy and cisplatin resistance in human ovarian cancer
cells
To further confirm the role of TRP14, autophagy and
cisplatin resistance in human ovarian cancer cells, TRP14 was
overexpressed in the SKOV3 and A2780 cells (Fig. 4A). The results revealed that the
levels of the autophagy marker proteins, the number of RFP-positive
cells and the number of autophagosomes were significantly increased
by TRP14 overexpression (Fig. 4B-D).
These findings indicated that TRP14 induced autophagy in human
ovarian cancer cells. Moreover, TRP14 overexpression exerted
protective effects on the ovarian cancer cells against cisplatin
(Fig. 4E and F). However,
cytoprotection due to TRP14 overexpression was partly reversed by
ATG5 knockdown (Fig. 4E and F). Taken
together, these results suggested that TRP14 activated autophagy
and induced cisplatin resistance, at least partially through the
participation of ATG5.
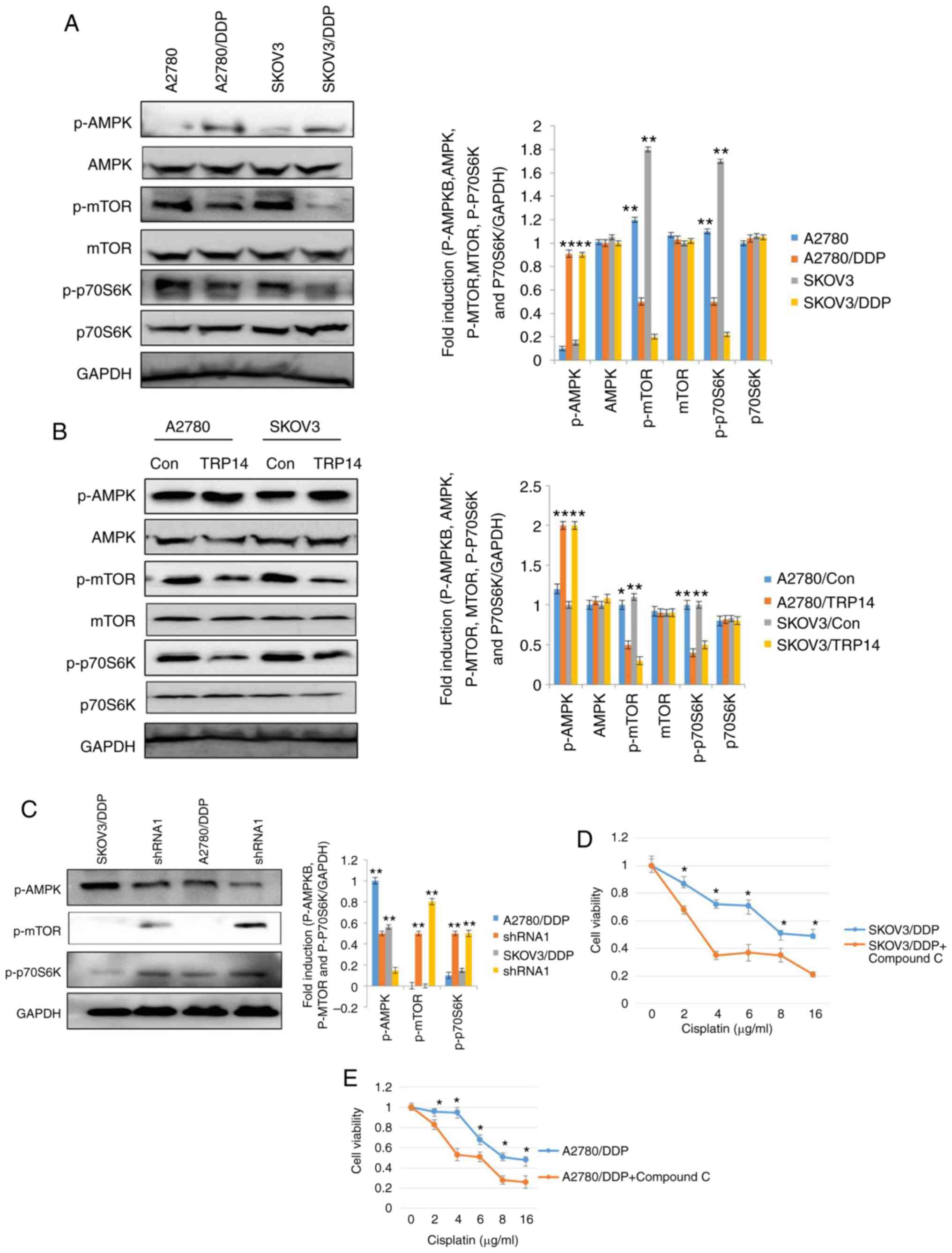
TRP14 induces autophagy and
chemoresistence via the AMPK/mTOR/p70S6K signaling pathway
Subsequenlty, the AMPK and mTOR signaling pathways
were investigated in the cisplatin-resistant human ovarian cancer
cells. According to a previous study, the AMPK and mTOR signaling
pathways regulate autophagy (30).
Western blot analysis was thus performed to elucidate the
phosphorylation levels of AMPK, mTOR and p70S6K in the SKOV3/DDP
and A2780/DDP cells. As shown in Fig.
5A, the phosphorylation levels of AMPK were increased in the
SKOV3/DDP and A2780/DDP cells, whereas the phosphorylation levels
of mTOR and p70S6K were decreased. These results indicated the
involvement of autophagy in cisplatin-resistant human ovarian
cancer cells and the role of the AMPK/mTOR/p70S6K signaling
pathway. To further confirm the involvement of AMPK, mTOR and
p70S6K signaling in cisplatin-resistant human ovarian cancer cells
and the induction of autophagy, the AMPK inhibitor, compound C, was
employed. Compound C partially, but significantly reversed
cisplatin-resistance in the SKOV3/DDP and A2780/DDP cells (Fig. 5D and E). These results clearly
indicated that cisplatin resistance was mediated by AMPK, mTOR and
p70S6K signaling in ovarian cancer cells. Our results also revealed
that TRP14 acts as an autophagy and chemoresistence promoter
(Figs. 3 and 4). Thus, we investigated the association of
the TRP14 in the AMPK/mTOR/p70S6K signaling pathway. TRP14 was
overexpressed in the SKOV3 and A2780 cells and detected the
phosphorylation levels of AMPK, mTOR and p70S6K. These results
suggested that TRP14 markedly activated the AMPK/mTOR/p70S6K
signaling pathway (Fig. 5B). To
further validate the regulation of the AMPK/mTOR/p70S6K signaling
pathway by TRP14, TRP14 shRNA2 was used in SKOV3/DDP and A2780/DDP
cells. As expected, the phosphorylation of AMPK exhibited a
decrease, whereas the phosphorylation of mTOR and p70S6K exhibited
an increase (Fig. 5C). These findings
revealed that TRP14 induced autophagy and chemoresistence via the
AMPK/mTOR/p70S6K signaling pathway.
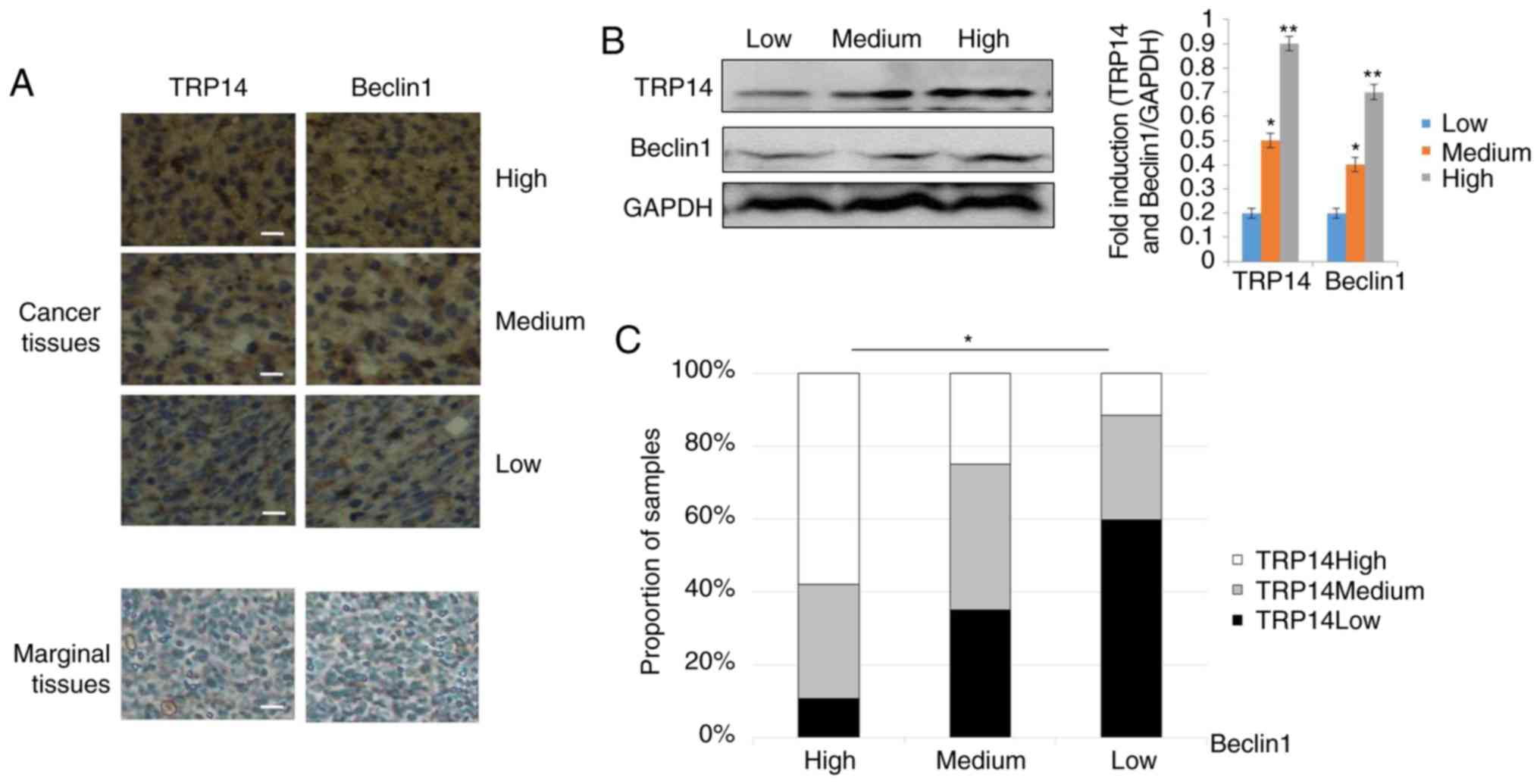
Positive association of TRP14 and
Beclin1 proteins in ovarian cancer tissues
The above-mentioned results supported the conclusion
that TRP14 promotes paclitaxel resistance by inducing autophagy via
the AMPK/mTOR/p70S6K signaling pathway in ovarian cancer. To
further determine whether there was an association between TRP14
and Beclin1, we used a tissue array of human ovarian cancer. The
tissue array was composed of 5 cases of clear cell carcinoma, 62
cases of serous adenocarcinoma, 12 cases of mucinous
adenocarcinoma, 1 case of endometrioid carcinoma, 10 cases of lymph
node metastatic adenocarcinoma and 10 cases of marginal tissue. The
representative strains from the specimens are shown in Fig. 6A and B. The results revealed that
along with the increment in the TRP14 protein levels, the
expression of Beclin1 was markedly increased (Fig. 6C). Collectively, these results
strongly suggested that the expression of TRP14 and Beclin1
proteins are positively associated in ovarian cancer tissues.
Discussion
Cisplatin is known as the ‘penicillin of cancer’,
and is widely used in the treatment of various types of cancer,
including ovarian cancer. However, cisplatin-based resistance is a
major obstacle in chemotherapy (31).
The mechanisms associated with cisplatin resistance in ovarian
cancer remain unclear. Hence, in this study, we aimed to elucidate
the possible underlying mechanisms of the effects of cisplatin on
ovarian cancer cells using sensitive SKOV3 (A2780) and resistant
SKOV3/DDP (A2780/DDP) cells as the ideal pairs of cell models.
Autophagy plays a dual role in cancer. According to
some early reports, autophagy was considered to be a tumor
suppressor (32), while others
consider that cancer cells rely on autophagy (33). More importantly, there is increasing
evidence to indicate that the activation of autophagy is involved
in the chemoresistance of cancer cells, while the inhibition of
autophagy significantly improves the effects of therapy (34–36). In
this study, the levels of LC3, Beclin1 and ATG5 were compared in
both ovarian cancer cells. Accordingly, the protein expression
levels of LC3, Beclin1 and ATG5 were high in the
cisplatin-resistant cells, while they were low in the sensitive
cells (Fig. 1C). Consistent with
these results, MDC staining, GFP-RFP-LC3 transfection and
transmission electron microscopy analysis confirmed that
cisplatin-resistant ovarian cancer cells triggered autophagy
(Fig. 1). In line with previous
findings (15), this study revealed
that the inhibition of autophagy by treatment with 3-MA or the
silencing of ATG5 augmented cisplatin cytotoxicity (Fig. 2), suggesting the importance of
autophagy in the pharmacological modulation of ovarian cancer cells
in response to cisplatin. Moreover, the induction of autophagy by
Rapa increased the survival of the SKOV3 and A2780 cells (Fig. 2E-I). These data indicated that
autophagic cell death was a possible mechanism for
cisplatin-induced cytotoxicity and changes in autophagy in the
sensitivity to chemotherapy.
There is increasing evidence to indicate that TRP14
promotes taxol resistance by inducing autophagy (25). To confirm whether TRP14 also
participates in autophagy-mediated cisplatin resistance in ovarian
cancer cells, the expression of TRP14 was initially detected. The
protein levels of TRP14 were substantially increased in SKOV3/DDP
and A2780/DDP cells (Fig. 3A). To
illustrate the role of TRP14 in the resistance of ovarian cancer,
TRP14 was knocked down using shRNA (Fig.
3B). The results revealed that cisplatin sensitivity was
increased when TRP14 was substantially suppressed by shRNA
(Fig. 3D-H). By contrast, autophagy
was induced and cisplatin sensitivity was attenuated when TRP14 was
overexpressed (Fig. 4). Taken
together, these results clearly demonstrated that TRP14 induced
cisplatin resistance, at least partly by inducing autophagy.
Based on the clinical data of the connection between
autophagy and the AMPK/mTOR signaling pathway, the signaling
pathway in resistant human ovarian cancer cells was evaluated. As
shown in Fig. 5, the overexpression
of TRP14 in the SKOV3 and A2780 cells activated the AMPK signal,
and inhibited the mTOR and p70S6K signaling pathways. Based on
these data, TRP14 may modulate these molecules, and it may be
located upstream of these molecules. Moreover, the data from a
tissue array (Fig. 6) suggested that
the protein expression of TRP14 and Beclin1 exhibited a positive
association, supporting the view that TRP14 activated autophagy in
ovarian cancer cells.
In conclusion, this study, identified that TRP14
mediated cisplatin resistance through the induction of autophagy in
human ovarian cancer cells via the AMPK/mTOR/p70S6K signaling
pathway. Moreover, TRP14 and Beclin1 were positively associated in
human ovarian cancer and marginal tissues. Although the detailed
underlying mechanisms require further investigation, TRP14 may be
regarded as a promising therapeutic target for managing cisplatin
resistance in human ovarian cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81772772 and
81302242).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
MHC, WXT and TMX conceived and designed the
experiments. WXT, XJL and ZLZ performed the experiments. JL and WJZ
performed data analysis. ZLZ, XJL, JL and WJZ contributed the
provision of the reagents/materials/analysis tools. MHC and TMX
contributed intellectually to the interpretation and discussion of
the results. WXT contributed to the writing of the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The tissue samples used in this study were from a
tissue microarray; thus, ethics approval does not apply.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Cho YJ, Woo JH, Lee JS, Jang DS, Lee KT
and Choi JH: Eclalbasaponin II induces autophagic and apoptotic
cell death in human ovarian cancer cells. J Pharmacol Sci.
132:6–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang B, Liu X, Liu Y, Kong D, Liu X,
Zhong R and Ma S: Inhibition of autophagy sensitizes MDR-phenotype
ovarian cancer SKVCR cells to chemotherapy. Biomed Pharmacother.
82:98–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gossner G, Choi M, Tan L, Fogoros S,
Griffith KA, Kuenker M and Liu JR: Genistein-induced apoptosis and
autophagocytosis in ovarian cancer cells. Gynecol Oncol. 105:23–30.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozols RF: Treatment goals in ovarian
cancer. Int J Gynecol Cancer. 15:3–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piccart MJ, Lamb H and Vermorken JB:
Current and future potential roles of the platinum drugs in the
treatment of ovarian cancer. Ann Oncol. 12:1195–1203. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strzyz P: Autophagy: Mitochondria encaged.
Nat Rev Mol Cell Biol. 19:2122018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guan Y, Li YP, Zhao G and Li YQ: HMGB1
promotes the starvation-induced autophagic degradation of
alpha-synuclein in SH-SY5Y cells Atg 5-dependently. Life Sci.
202:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fritzen AM, Frosig C, Jeppesen J, Jensen
TE, Lundsgaard AM, Serup AK, Schjerling P, Proud CG, Richter EA and
Kiens B: Role of AMPK in regulation of LC3 lipidation as a marker
of autophagy in skeletal muscle. Cell Signal. 28:663–674. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen GH, Liu HC, Zhang YD, Liang J, Zhu Y,
Zhang M, Yu D, Wang C and Hou J: Silencing PFKP inhibits
starvation-induced autophagy, glycolysis, and epithelial
mesenchymal transition in oral squamous cell carcinoma. Exp Cell
Res. 370:46–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
13
|
Wang Y, Gan G, Wang B, Wu J, Cao Y, Zhu D,
Xu Y, Wang X, Han H, Li X, et al: Cancer-associated fibroblasts
promote irradiated cancer cell recovery through autophagy.
EbioMedicine. 17:45–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leng SL, Hao YL, Du DB, Xie S, Hong L, Gu
H, Zhu X, Zhang J, Fan D and Kung HF: Ursolic acid promotes cancer
cell death by inducing Atg5-dependent autophagy. Int J Cancer.
133:2781–2790. 2013.PubMed/NCBI
|
|
16
|
Wu BW, Tan MD, Cai WL, Wang B, He PH and
Zhang XP: Arsenic trioxide induces autophagic cell death in
osteosarcoma cells via the ROS-TFEB signaling pathway. Biochem
Bioph Res Commun. 496:167–175. 2018. View Article : Google Scholar
|
|
17
|
Wang XY, Wei SH, Zhao Y, Shi C, Liu P,
Zhang C, Lei Y, Zhang B, Bai B, Huang Y and Zhang H:
Anti-proliferation of breast cancer cells with itraconazole:
Hedgehog pathway inhibition induces apoptosis and autophagic cell
death. Cancer Lett. 385:128–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Z, Teo AE and McCarty N: ROS-induced
CXCR4 signaling regulates mantle cell lymphoma (MCL) cell survival
and drug resistance in the bone marrow microenvironment via
autophagy. Clin Cancer Res. 22:187–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar A, Singh UK and Chaudhary A:
Targeting autophagy to overcome drug resistance in cancer therapy.
Future Med Chem. 7:1535–1542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao J, Nie YQ, Wang H and Lin Y: MiR-181a
suppresses autophagy and sensitizes gastric cancer cells to
cisplatin. Gene. 576:828–833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DeVorkin L, Hattersley M, Kim P, Ries J,
Spowart J, Anglesio MS, Levi SM, Huntsman DG, Amaravadi RK, Winkler
JD, et al: Autophagy inhibition enhances sunitinib efficacy in
clear cell ovarian carcinoma. Mol Cancer Res. 15:250–258. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang ZD, Wang AH, Li H, Zhi H and Lu F:
STAT3-dependent TXNDC17 expression mediates taxol resistance
through inducing autophagy in human colorectal cancer cells. Gene.
584:75–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong W, Yoon HW, Lee SR and Rhee SG:
Identification and characterization of TRP14, a thioredoxin-related
protein of 14 kDa-new insights into the specificity of thioredoxin
function. J Biol Chem. 279:3142–3150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pader I, Sengupta R, Cebula M, Xu J,
Lundberg JO, Holmgren A, Johansson K and Arnér ES:
Thioredoxin-related protein of 14 kDa is an efficient L-cystine
reductase and S-denitrosylase. Proc Natl Acad Sci USA.
111:6964–6969. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang SF, Wang XY, Fu ZQ, Peng QH, Zhang
JY, Ye F, Fu YF, Zhou CY, Lu WG, Cheng XD and Xie X: TXNDC17
promotes paclitaxel resistance via inducing autophagy in ovarian
cancer. Autophagy. 11:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song ZB, Ni JS, Wu P, Bao YL, Liu T, Li M,
Fan C, Zhang WJ, Sun LG, Huang YX and Li YX: Testes-specific
protease 50 promotes cell invasion and metastasis by increasing
NF-kappaB-dependent matrix metalloproteinase-9 expression. Cell
Death Dis. 26:e17032015. View Article : Google Scholar
|
|
27
|
Zhang WJ, Song ZB, Bao YL, Li WL, Yang XG,
Wang Q, Yu CL, Sun LG, Huang YX and Li YX: Periplogenin induces
necroptotic cell death through oxidative stress in HaCaT cells and
ameliorates skin lesions in the TPA- and IMQ-induced psoriasis-like
mouse models. Biochem Pharmacol. 105:66–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng X, Gong F, Chen Y, Jiang Y, Liu J, Yu
M, Zhang S, Wang M, Xiao G and Liao H: Autophagy promotes
paclitaxel resistance of cervical cancer cells: Involvement of
warburg effect activated hypoxia-induced factor 1-α-mediated
signaling. Cell Death Dis. 14:e13672014. View Article : Google Scholar
|
|
29
|
Shin D, Kim EH, Lee J and Roh JL: RITA
plus 3-MA overcomes chemoresistance of head and neck cancer cells
via dual inhibition of autophagy and antioxidant systems. Redox
Biol. 13:219–227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Gao JF, Zhang Y, Xu W, Hao Y, Xu Z
and Tao L: Natural pyrethrins induce autophagy of HepG2 cells
through the activation of AMPK/mTOR pathway. Environ Pollut.
241:1091–1097. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bao LJ, Jaramillo MC, Zhang ZB, Zheng YX,
Yao M, Zhang DD and Yi XF: Nrf2 induces cisplatin resistance
through activation of autophagy in ovarian carcinoma. Int J Clin
Exp Pathol. 7:1502–1513. 2014.PubMed/NCBI
|
|
32
|
Laddha SV, Ganesan S, Chan CS and White E:
Mutational landscape of the essential autophagy gene BECN1 in human
cancers. Mol Cancer Res. 12:485–490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuo YZ, Tai YH, Lo HI, Chen YL, Cheng HC,
Fang WY, Lin SH, Yang CL, Tsai ST and Wu LW: MiR-99a exerts
anti-metastasis through inhibiting myotubularin-related protein 3
expression in oral cancer. Oral Dis. 20:e65–e75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qiao SX, Tao SS, de la Vega MR, Park SL,
Vonderfecht AA, Jacobs SL, Zhang DD and Wondrak GT: The
antimalarial amodiaquine causes autophagic-lysosomal and
proliferative blockade sensitizing human melanoma cells to
starvation- and chemotherapy-induced cell death. Autophagy.
9:2087–2102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ajabnoor GMA, Crook T and Coley HM:
Paclitaxel resistance is associated with switch from apoptotic to
autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis.
26:e2602012. View Article : Google Scholar
|
|
36
|
Yin X, Zhang N and Di W: Regulation of
LC3-dependent protective autophagy in ovarian cancer cells by
protein phosphatase 2A. Int J Gynecol Cancer. 23:630–641. 2013.
View Article : Google Scholar : PubMed/NCBI
|