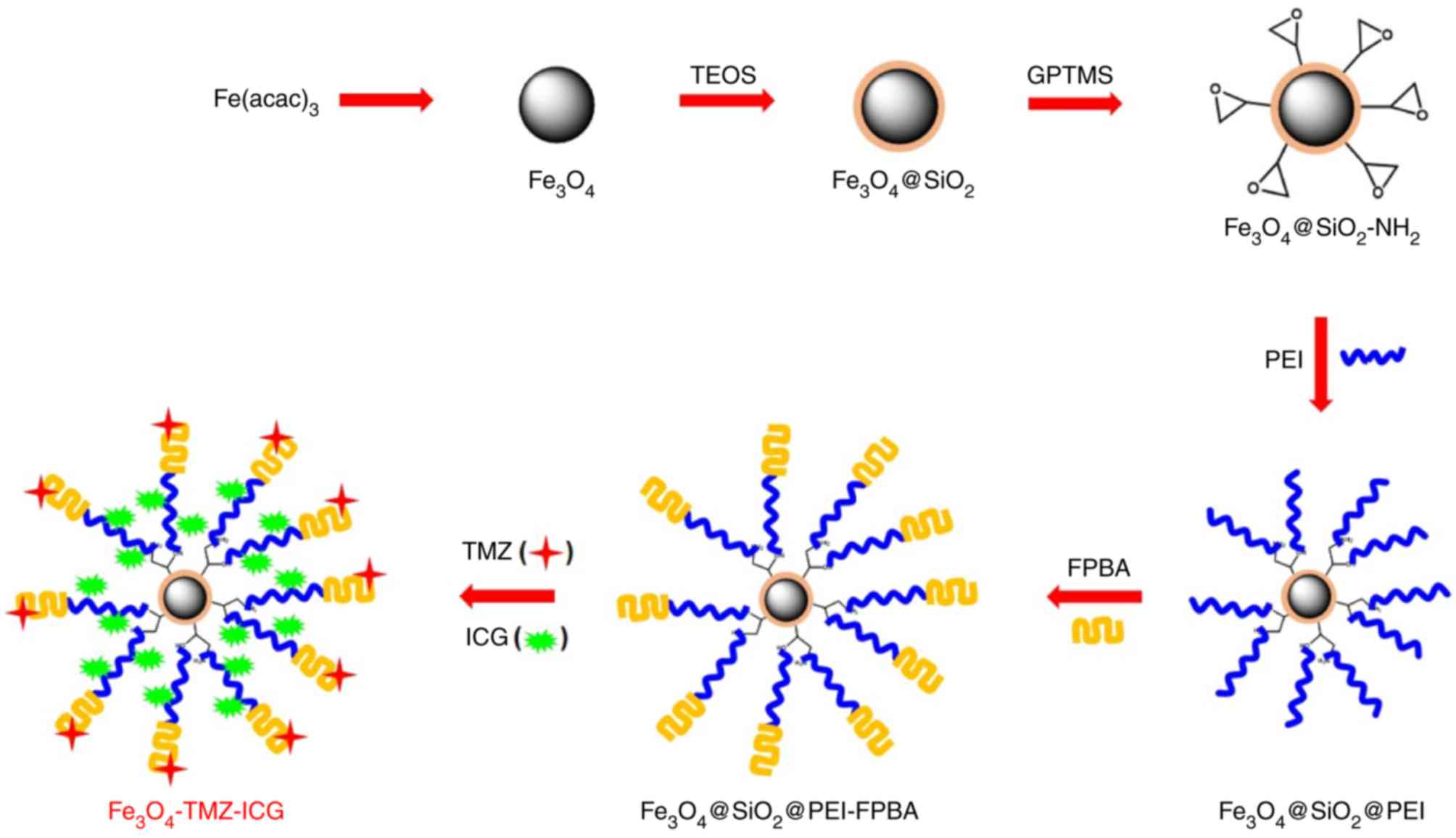
Introduction
Glioblastoma (GBM) is the most common primary brain
tumor of the central nervous system and has a high mortality rate,
with a 4.5% 5-year relative survival rate in the US following
diagnosis (1–3). A variety of cell types with fast growth
potential in GBM and the blood-brain barrier (BBB), which controls
the transportation of drugs, including antibodies, are major
obstacles of GBM treatment (4,5). Thus, the
development of a drug delivery system for GBM treatment is urgent
to overcome issues associated with the BBB (6). Chemotherapy along with surgery and
radiation therapy is the conventional methods for the treatment of
patients with GBM (7). Temozolomide
(TMZ), a commonly used oral anticancer drug inhibits the viability
of malignant glioma cells with increased permeability through the
BBB (8). Although TMZ can penetrate
BBB, it shows low cytotoxicity against human glioma cells due to
several cellular mechanisms of drug resistance (9). Therefore, a strategy for overcome this
problem is required to increase the therapeutic efficacy of
TMZ.
Chemotherapy in combination with phototherapy is
considered an effective approach for treating brain cancer as of
its minimal invasiveness (10).
Photothermal therapy uses long-wavelength near-infrared (NIR) light
with an absorption range, which allows a minimally invasive target
treatment for tumor sites without affecting normal cells (11). It was reported that chemotherapeutic
efficacy is generally increased in combination with photothermal
therapy since the cytotoxicity of chemotherapeutic agents and drug
delivery into cancer cells are enhanced at elevated temperatures
(12–14). Currently, a number of inorganic and
organic NIR photothermal agents, including metal-based particles
such as Au (15,16), Ag (17),
Pt (18,19), and Cu (20), graphene, and carbon-based
nanomaterials (21), have been
developed. However, these agents have drawbacks such as high
toxicity and low photothermal conversion efficacy in the NIR
range.
Magnetic nanoparticles (MNPs) as an effective cancer
therapeutic agent and diagnostic contrast nanocomposite are widely
applied the in biomedical field (22). Surface modifications of MNPs by
introducing functional groups increase the efficacy of cancer
therapy and bio-imaging through high drug-loading efficacy of MNPs
and diagnostic contrast agent (23).
In addition, as Fe3O4 MNPs possess broad
absorption in the NIR range, they can be applied for photothermal
therapy (24). Recently, studies have
reported the photothermal conversion process of
Fe3O4 MNPs based on the photoluminescence
emission in the NIR region (25,26);
however, few studies have sought to elucidate photothermal
properties and conversion mechanism. Fe3O4
MNPs with a good photothermal conversion efficacy in the NIR region
and low toxicity have been developed, and exhibited higher
photothermal conversion efficiency than other photoabsorbers, such
as noble metal-, carbon- and organic compound-based nanomaterials
(27). Furthermore, efficient
photothermal-induced hyperthermia using functionalized MNPs can
induce synergistic anticancer effects in combination with
chemotherapy (28).
Cancer cells are more vulnerable than normal cells
due to their immature and disorganized vasculature (29–31). Thus,
it is a feasible strategy for irreversible cellular damage through
increasing therapeutic temperature (42–47°C) in the tumor region by
photothermal therapy, leading to cellular apoptosis (32). Nanoparticle-mediated NIR thermal
therapy depends on photothermal conversion efficacy and laser
dosage (33). Additionally, the
perfusion of blood and cellular membrane permeability can be
improved by increasing the temperature in the tumor region by
photothermal-induced local hyperthermia; thereby, the efficacy of
drug delivery into the specific intracellular region is enhanced
(34,35).
On the contrary, the photothermal effects of
Fe3O4 MNPs alone is usually insufficient to
deliver appropriate thermal energy because of inevitable laser
light scattering and relatively poor specificity to target tumor
area (36). To overcome these
problems, the present study proposed ICG- and TMZ-loaded
Fe3O4 MNPs as a multimodal cancer therapeutic
agent. ICG, an FDA-proved bifunctional NIR fluorescence dye, acts
as a photothermal agent and photodynamic photosensitizer under NIR
laser irradiation and can convert absorbed NIR light to thermal
energy and reactive oxygen species (ROS) that are essential for
photothermal and photodynamic therapy (37). However, its poor photo-stability is a
major limiting factor for clinical application. In this study, we
subsequently constructed TMZ- and ICG-loaded
Fe3O4 (Fe3O4-TMZ-ICG)
MNPs for NIR laser-induced chemo-photothermal-photodynamic therapy
(chemo-phototherapy) and evaluated its anticancer effects on U-87
MG glioblastoma cells through ROS-mediated apoptosis under NIR
light irradiation. Considering these factors, the combination of
chemo-phototherapy using Fe3O4-TMZ-ICG MNPs
could be an effective approach to develop an efficient technique
for improved and effective brain cancer treatment.
Materials and methods
Materials
Iron(III) acetylacetonate [Fe(acac)3],
1,2-hexadecanediol (90%), oleic acid (90%), oleylamine (70%),
1-octadecene (90%), cyclohexane, 1-hexanol, Triton X-100,
tetraethyl orthoslicate (TEOS), ammonium hydroxide,
3-glycidyloxypropyl trimethoxysilane (GPTMS), 4-formylphenylboronic
acid (FPBA), polyethylenimine (PEI, 50 wt% solution in water),
trimethylamine, tetrahydrofuran and TMZ were obtained from
Sigma-Aldrich (Merck KGaA). Indocyanine green (ICG) was purchased
from Thermo Fisher Scientific, Inc. Toluene, absolute ethanol
(≥99.5%), n-hexane (≥99%) and dimethyl sulfoxide (DMSO) were
obtained from Duksan Company. All commercial chemicals were used
without further purification.
Preparation of
Fe3O4-TMZ-ICG MNPs
The Fe3O4 magnetic core was
synthesized by well-established thermal decomposition method
(38). In brief, Fe(acac)3
(3 mmol), 1,2-hexadecanediol (10 mmol), 1-octadecene (20 ml), oleic
acid (6 mmol) and oleylamine were stirred under nitrogen atmosphere
and the resulting solution was heated to 100°C for 30 min. Then,
the temperature was slowly increased to 200°C and stirred for 2 h.
Subsequently, the mixture was refluxed at 320°C for 1 h with
vigorous stirring and cooled down to room temperature. The products
were precipitated by the addition of excess ethanol (99.5%) and
purified by washing with n-hexane (99%) and ethanol repeatedly. The
obtained materials were separated by centrifugation at 7,000 × g
for 20 min at room temperature and re-dispersed in n-hexane.
The water soluble silica coated
Fe3O4@SiO2-NH2 MNPs
were prepared by the hydrolysis of TEOS (39) and the condensation reaction of GPTMS
(40). First, 250 mg
Fe3O4 MNPs was ultrasonically dispersed in a
mixture of 20 ml cyclohexane, 4 ml 1-hexanol, 5 ml of Triton X-100
and 0.85 ml of water for 15 min at room temperature. To this, a 2.5
ml TEOS was dropped into the solution, and was vigorously stirred
for 6 h at room temperature. Subsequently, a 0.5 ml ammonia
solution (28% in water) was added and the solution was further
stirred for 24 h. The precipitate was separated by an external
magnet and washed with ethanol. The obtained product was dried in a
vacuum oven at 60°C. Afterwards, ~200 mg
Fe3O4@SiO2 MNPs was dispersed in
80 ml of anhydrous toluene and sonicated for 30 min at room
temperature, and then 2 ml GPTMS was slowly dropped to this
suspension under nitrogen atmosphere. Next, the mixture was stirred
at 80°C for 24 h. After 24 h, the precipitate was separated by an
external magnet and washed with toluene and ethanol, and then dried
in vacuum oven overnight at 60°C.
Subsequently,
Fe3O4@SiO2-NH2 MNPs for
the drug and photosensitizer loading were modified with PEI and
FPBA for ICG and TMZ conjugations, according to previous reports
(41). First, PEI conjugation onto
Fe3O4@SiO2-NH2 MNPs was
performed via an epoxy ring opening reaction. 200 mg
Fe3O4@SiO2-NH2 MNPs was
added in 1.5 ml ethanol containing 0.0025 mg/ml PEI and the
dispersion was refluxed at 70°C for 24 h. After centrifugation at
7,000 × g for 20 min at room temperature, the precipitate was
washed with ethanol and water for several times and dried at 70°C
for 10 h. Then, 4-FPBA groups were grafted onto the surface of
Fe3O4@PEI MNPs. 200 mg
Fe3O4@PEI MNPs was dispersed in 300 ml
methanol/acetic acid (124:1), after which 200 mg FPBA was added.
The mixed solution was stirred at 40°C for 10 h. To eliminate the
unstable Schiff base, 200 mg triethylamine was added to the
resulting solution and stirred at 40°C for 10 h. The resultant
Fe3O4@PEI-FPBA MNPs were collected and washed
several times with water and ethanol.
For drug loading,
Fe3O4@PEI-FPBA MNPs were modified with TMZ in
the presence of succinic anhydride coupling agent (42). 20 mg
Fe3O4@PEI-FPBA MNPs was ultrasonically
dispersed in 5 ml methanol containing with 1 mg of TMZ for 10 min.
Then, the mixture was stirred at 35–40°C for 12 h. The resultant
Fe3O4-TMZ MNPs were isolated by the removal
of methanol and washed several times with methanol and water.
Finally, for the photosensitizer loading, 1 mM ICG solution in DMSO
was added in the Fe3O4-TMZ MNPs aqueous
dispersion solution with a final DMSO concentration of 10% by
volume and heated at 4°C for 12 h. The obtained products were
collected and washed several times with water and ethanol, and then
stored in distilled water for further use.
Characterization of
Fe3O4-TMZ-ICG MNPs
The crystal structures of the synthesized inorganic
Fe3O4 and SiO2-coated with
Fe3O4 MNPs were analyzed by X-ray diffraction
(XRD; X'Pert-MPD System; Philips Healthcare). The optical
properties of modified Fe3O4 MNPs were
observed under UV-vis spectroscopy (V-670; JASCO International Co.,
Ltd.). The quantities of organic functional groups and residual
Fe3O4 content in the
Fe3O4-TMZ-ICG MNPs were measured by thermal
gravimetric analysis (TGA; TGA 7, Pyris 1; PerkinElmer, Inc.).
Thermal curves of MNPs were measured with a heating rate of
10°C/min from room temperature to 600°C under a nitrogen
atmosphere.
TMZ and ICG loading contents onto the
modified Fe3O4 MNPs
The TMZ and ICG loading contents onto the modified
Fe3O4 MNPs were determined by UV-vis
spectroscopy (43). First, to
determine TMZ loading efficiency, ~1.5 mg of
Fe3O4-TMZ MNPs was transferred to 10 ml
volumetric flask, and dissolved in 0.1 N hydrochloric acid (25
µg/ml) using an ultrasonic bath. The dispersion was centrifuged at
10,700 × g for 20 min at room temperature. The amount of loaded TMZ
was determined by the difference in optical absorbance at 328 nm
between the total amount of TMZ initially added to formation and
the amount of free TMZ in the supernatant using UV-vis
spectroscophotometer (multiskan Go; Thermo Fisher Scientific,
Inc.).
Next, to assess the ICG loading content, the optical
absorption of conjugated ICG was measured by comparing absorption
data at 780 nm before and after ICG conjugation onto the
Fe3O4@PEI-FPBA MNPs under a UV-vis
spectrometer. The ICG loading content was calculated based on the
calibration curve of ICG concentration at 780 nm (44). The loading content and efficiency of
TMZ and ICG molecules onto the Fe3O4 MNPs
were calculated as follows (45):
Drug loading content=Amount of loaded
TMZ(or ICG)Amount of TMZ(or ICG)loaded MNPs×100%
Drug loading efficiency=Amount of loaded
TMZ(or ICG)Total amount of TMZ(or ICG)initially added×100%
Particle size distribution and
morphology of MNPs
The particle size and morphology were determined
with a high-resolution transmission electron microscope (HR-TEM;
JEM 2010; JEOL Ltd.). The mean size of Fe3O4
MNPs was measured by TEM operating at 200 kV FE (Field Emmision)
with 1.43 Å resolution and their particle distribution were
calculated by measuring the diameter of ~80 nanoparticles using
image analysis software (ImageJ 1.52a; National Institutes of
Health). The average hydrodynamic size of TMZ and
ICG-functionalized Fe3O4@PEI-FPBA
(Fe3O4-TMZ-ICG) MNPs were determined by a
dynamic light scattering (DLS) analyzer (LS 13320; Beckman Coulter,
Inc.) in cell media. The average size of nanoparticles was
calculated by Gaussian histogram curve fitting.
Analysis of NIR photothermal heating
effects
To investigate the photothermal effect of free ICG,
Fe3O4 only, Fe3O4-ICG
and Fe3O4-TMZ-ICG aqueous suspension with two
different Fe3O4 concentrations (0, 125 and
250 µg Fe/ml) in water (1 ml), was irradiated with 808-nm NIR laser
(Changchun New Industries Optoelectronics Technology), at a power
density of 1 W/cm2 for 10 min. The output power was
adjusted by the measurement of a handy optical power meter
(PMKIT-22-01; Newport Corporation). The temperature of the sample
was detected using a thermal camera (FLIR i5; FLIR Systems, Inc.)
above a sample holder as a function of irradiated NIR-light for the
heat conversion effect.
Cell culture
U-87 MG, the human glioblastoma cell line was
obtained from Korea cell line bank (cat. no. 30014). The U-87 MG
cell line distributed by most cell collections (including the
American Type Culture Collection, CLS and ECACC) is not the
original glioblastoma cell line established in 1968 at the
University of Uppsala, but the gene expression profile generated by
the ATCC authentication indicates that this cell line is most
probably also a glioblastoma cell line but whose origin is unknown
(46). To ensure a human cell line
for research, the STR profile of U-87 MG KCLB cell line was
authenticated by comparing an STR profile of U-87 MG ATCC using the
ATCC database. STR markers of U-87 MG KCLB cell line were identical
to those of the accession no. CVCL-0022 of the U-87 MG ATCC cell
line. Thus, the U-87 MG KCLB cell line was used as a cell model of
a glioblastoma human cell line in this study.
The cells were cultured in a monolayer in Dulbecco's
modified Eagles medium (Thermo Fisher Scientific, Inc.), which was
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.) and 1% (v/v) penicillin-streptomycin (Thermo Fisher
Scientific, Inc.). The cultured cells were incubated at 37°C in 5%
CO2, and the cell growth medium was replaced every 2
days. When the cells reached 80% confluence, the cells were
harvested with 0.025% trypsin-EDTA solution.
Sample treatment and cancer
photothermal therapy
For the photothermal therapy, U-87 MG cells were
seeded at a density of 2×105 cells/well in a 24 well
plate with 1 ml of cell medium and incubated for 24 h at 37°C. The
cells were treated with different samples for 2 h prior to exposure
of NIR laser irradiation followed by incubation for 2 or 22 h. The
cells without any treatment were used as a control. Both
Fe3O4 MNPs and ICG were utilized as NIR light
absorbers to efficiently convert optical energy into thermal energy
(47). To deliver the optimal thermal
energy to cancer cells, the concentration of ICG-entrapped
Fe3O4 MNPs
(Fe3O4-ICG-TMZ) was set to temperature in the
range of 42–45°C under 808 nm of laser at a power density 1
W/cm2 for 5 min. The amount of
Fe3O4 was equivalent in the
Fe3O4, Fe3O4-ICG, and
Fe3O4-ICG-TMZ MNPs samples (125 µg Fe/ml),
and the amount of ICG was equivalent in the free ICG and
Fe3O4-ICG-TMZ MNPs samples (3.1 µg/ml). To
investigate synergistic chemo-photothermal therapy, the
concentration of free TMZ was set to the amount of TMZ
corresponding concentration in Fe3O4-ICG-TMZ
MNPs (6.6 µg/ml). The temperatures of cell mediums were monitored
with a thermal camera during photothermal therapy.
Morphological changes and cell
viability
To investigate photothermal effect to U-87 MG cells
in vitro, the cells were seeded at a density of
2×105 cells/well in a 24-well plate with 1 ml cell
medium and incubated for 24 h at 37°C. The cells were incubated
with 125 µg Fe/ml of Fe3O4,
Fe3O4-ICG and
Fe3O4-TMZ-ICG MNPs, and equivalent free ICG
and TMZ concentration, corresponding to
Fe3O4-TMZ-ICG MNPs for 2 h. The cells were
treated with 808-nm NIR laser irradiation at 1 W/cm2 for
5 min followed by incubation for 2 h. Morphological changes of
cells were observed under a bright field of optical microscope at
×400 magnification (DMI300B; Leica Microsystems GmbH) and digital
images were captured at least three different sites for each
sample.
Next, to evaluate photothermal effect on cell
viability, cell viability was detected using WST-1 cell viability
assay kit (EZ-CyTox; Daeil Lab Inc.) according to the
manufacturer's protocols. The cells were seeded at a density of
1×104 cells/well in 96-well plates and incubated for 2 h
at 37°C. The cells were treated with various samples with or
without laser irradiation. After 2 h incubation, the absorbance was
measured at 450 nm using a microplate reader (Multiskan Go; Thermo
Fisher Scientific, Inc.).
ROS generation and cell apoptosis
To confirm apoptosis induced by ROS generation, the
cells were treated with samples and laser irradiation for 2 h.
After 2 h further incubation at 37°C, the cell medium was removed
and the cells incubated in a final concentration of 10 µM
dichloro-dihydro-fluorescein diacetate (DCFH-DA; Sigma-Aldrich;
Merck KGaA) for 30 min at 37°C. Cells were washed with PBS once,
and ROS production was monitored under a fluorescence microscope at
×200 magnification (Leica DMI300B; Leica Microsystems GmbH).
Digital images were captured at least three different sites for
each sample. After DCFH-DA was converted to DCF by esterase, DCF
exhibited green fluorescence when combined with ROS. As a result,
the DCF green intensity signifies the levels of ROS generation
(48), indicative of the apoptotic
cell pathway (49).
Live/dead cell assay
After 22 h incubation at 37°C with sample and laser
therapy, to evaluate cytotoxic effect in vitro during
photothermal therapy, a live/dead cell assay was conducted using a
calcein-AM/propidium iodide (PI) double staining kit
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
instructions. U-87 MG cells stained with calcein-AM/PI were
simultaneous imaged soon after staining under a fluorescence
microscope at ×200 magnification. Digital images were captured at
least three different sites for each sample. A calcein-AM/PI double
kit is used for a two-color fluorescence cell viability assay that
is based on the simultaneous fluorescence staining of live and dead
cells. Calcein-AM a non-fluorescent cell-permeable ester that can
passively penetrate viable cells with intact plasma membranes. The
calcein generated from calcein-AM by esterase in a viable cell
emits a green fluorescence (50).
Conversely, PI cannot pass through an intact cell membrane. PI
enters cells with compromised cell membranes and disordered areas
of dead cell membrane, and intercalates with the DNA double helix
of the cell to emit red fluorescence (51,52).
Therefore, green and red fluorescent cells indicate live and dead
cells, respectively (53). Calcein-AM
and PI fluorescent intensities were quantified using standard
imaging software (ImageJ software), and the results were expressed
as integrated density based on green and red fluorescence in each
image.
Confocal microscopy analysis
To investigate the photothermal-induced cellular
damage, cell membrane disruption was observed under a confocal
microscope (LSM 700; Zeiss GmbH). After cell treatment, the cells
were washed twice with cold PBS and fixed with cold 3.7%
formaldehyde for 20 min at room temperature. Furthermore, to
investigate internalized Fe3O4-TMZ-ICG MNPs
into the nucleus in the U-87 MG cells upon NIR laser irradiation,
cellular uptake of Fe3O4-TMZ-ICG MNPs was
monitored using a confocal microscope at ×40 magnification.
Flow cytometric analysis
To quantify in vitro cell cytotoxicity, flow
cytometry was conducted. The cell population of live/apoptotic
cells were analyzed using an Annexin V-FITC Apoptosis Detection Kit
(BD Pharmingen™; BD Biosciences) according to the manufacturer's
protocol by flow cytometry (BD FACSVerse; BD Biosciences). After 22
h incubation at 37°C, floating and attached cells in the medium
were collected by trypsinization. After collecting 5×105
cells by centrifugation at 600 × g for 1 min at 37°C, cells were
re-suspended in 100 µl of 1X binding buffer. The cell suspension
was incubated with 10 µg/ml of Annexin V-FITC/PI double staining
solution for 15 min at room temperature in the dark. An additional
400 µl 1X binding buffer was added to cell suspension, and then the
fluorescence of cells was immediately analyzed with a flow
cytometer.
Western blot analysis
After treatment for 24 h at 37°C, the cells were
washed twice with PBS, and lysed using a radioimmunoprecipitation
assay buffer (Sigma-Aldrich; Merck KGaA) containing protease
inhibitor (Roche Applied Science). Cytosolic and mitochondrial
fractions for cytochrome c detection were isolated using a
mitochondria isolation kit (Abcam) according to the manufacturer's
instructions. The protein concentration was determined using a
Pierce® BCA protein assay kit (Thermo Fisher Scientific,
Inc.). Total protein (25 µg) was separated using 10% SDS-PAGE and
then transferred to a nitrocellulose membrane. After blocking three
times at each 5 min interval with 5% skim milk in TBS-T
(Tris-buffered saline containing 0.1% Tween-20) at room
temperature, the membrane was reacted with Bcl-2-associated X
protein (Bax; cat. no. sc-20067, 1:200; Santa Cruz Biotechnology,
Inc.), Bcl-2 (cat. no. sc-23960, 1:200; Santa Cruz Biotechnology,
Inc.), cytochrome c (cat. no. sc-13561, 1:200; Santa Cruz
Biotechnology, Inc.), caspase-3 (cat. no. sc-271759, 1:200; Santa
Cruz Biotechnology, Inc.), cleaved caspase-3 (cat. no. 9661,
1:1,000; Cell Signaling Technology, Inc.), β-actin antibody (cat.
no. sc-47778, 1:500; Santa Cruz Biotechnology, Inc.), or cytochrome
c oxidase (COX IV; cat. no. sc-376731, 1:500; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. The membrane was washed
three times with TBS-T, 5 min each followed by incubation for 2 h
with mouse IgG κ binding protein (m-IgGκ BP) conjugated to
horseradish peroxidase (cat. no. sc-516102, 1:1,000; Santa Cruz
Biotechnology, Inc.), or anti-rabbit IgG, HRP-linked secondary
antibody (cat. no. 7074, 1:1,000; Cell Signaling Technology, Inc.)
at room temperature. β-actin and COX IV were used as loading
controls for whole cell and cytosolic proteins, and mitochondrial
proteins, respectively.
After washing three times with TBS-T for 5 min, the
bands were developed using an ECL Western Blotting Detection Kit
Reagent (Thermo Fisher Scientific, Inc.) and imaged on
Davinch-Chemi™ imaging system (CAS-400SM, Davinch-K). The relative
band intensities of each target protein expression were quantitated
using ImageJ software (version 1.52a), compared with the levels of
the β-actin or COX IV protein expression as a reference.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
After treatment for 24 h, Total RNA was isolated
using a TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Total RNA (2 µg) was
reverse-transcribed into cDNA using a cDNA synthesis kit (ET21025;
PhileKorea) under the following conditions: Incubation at 42°C for
30 min and denaturation 70°C for 10 min. For qPCR, primers were
designed using Prime-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/)
and commercially obtained from Mbiotech Inc. The reaction was
amplified with QunatiSpeed SYBR® No-Rox Kit (PhileKorea)
using primers (GAPDH, forward, 5′-AGAGGCAGGGATGTTCTG-3′ and
reverse, 5′-GACTCATGACCACAGTCCATGC-3′; Fas associated via death
domain (FADD), forward, 5′-CCGCCATCCTTCACCAGA-3′ and reverse,
5′-CAATCACTCATCAGC-3′; caspase-8, forward,
5′-CCTCATCAATCGGCTGGAC-3′ and reverse,
5′-ATGACCCTGTAGGCAGAAACC-3′). The quantification cycle (Cq) values
were obtained via qPCR with a Magnetic Induction Cycler (Bio
Molecular Systems). Transcription levels of every gene were
normalized to the levels of GADH. qPCR was performed under the
following conditions: Pre-denaturation at 95°C for 5 min,
denaturation at 90°C for 30 sec, annealing at 60°C for 40 sec and
extension at 72°C for 40 sec, for a total of 40 cycles. GAPDH was
used as a reference gene and the relative fold change values were
calculated by normalization to GAPDH expression via the
2−ΔΔCq method (54).
Relative mRNA expression, ΔΔCq=2−ΔΔCq,
where, ΔΔCq=ΔCq (a target gene)-ΔCq (a reference gene).
Statistical analysis
All statistics analyses were performed with Sigma
Plot 12.0 (Systat Software Inc., San Jose, CA, USA). The levels of
significance were calculated using a one-way ANOVA for comparisons
with the control group. The experiments for each sample were
repeated at least three times. Data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results and Discussion
Characterization of
Fe3O4-TMZ-ICG MNPs
The synthesis process of biocompatible
Fe3O4-TMZ-ICG MNPs as chemo-photothermal
therapeutic agents is presented in Fig.
1. The crystal structure of synthesized
Fe3O4 MNPs was analyzed by XRD pattern
(Fig. 2A). The distinctive
intensities of (220), (311), (400), (422), (511) and (440) peaks at
2q=30.05, 35.39, 43.01, 53.36, 65.67 and 66.72°, which corresponded
to inverse spinel structure of Fe3O4 (JCPDS
85-1436), were observed in the pristine Fe3O4
MNPs with well-defined crystallinity. After silica coating, the
obtained Fe3O4@SiO2 MNPs in the
crystalline structure showed the characteristic peaks of
Fe3O4 MNPs and the additional broad peak of
amorphous SiO2 coating shell indexed as (012), which was
observed at 20–30°. The intrinsic peaks of
Fe3O4 MNPs were well-preserved during the
process of functionalization with SiO2.
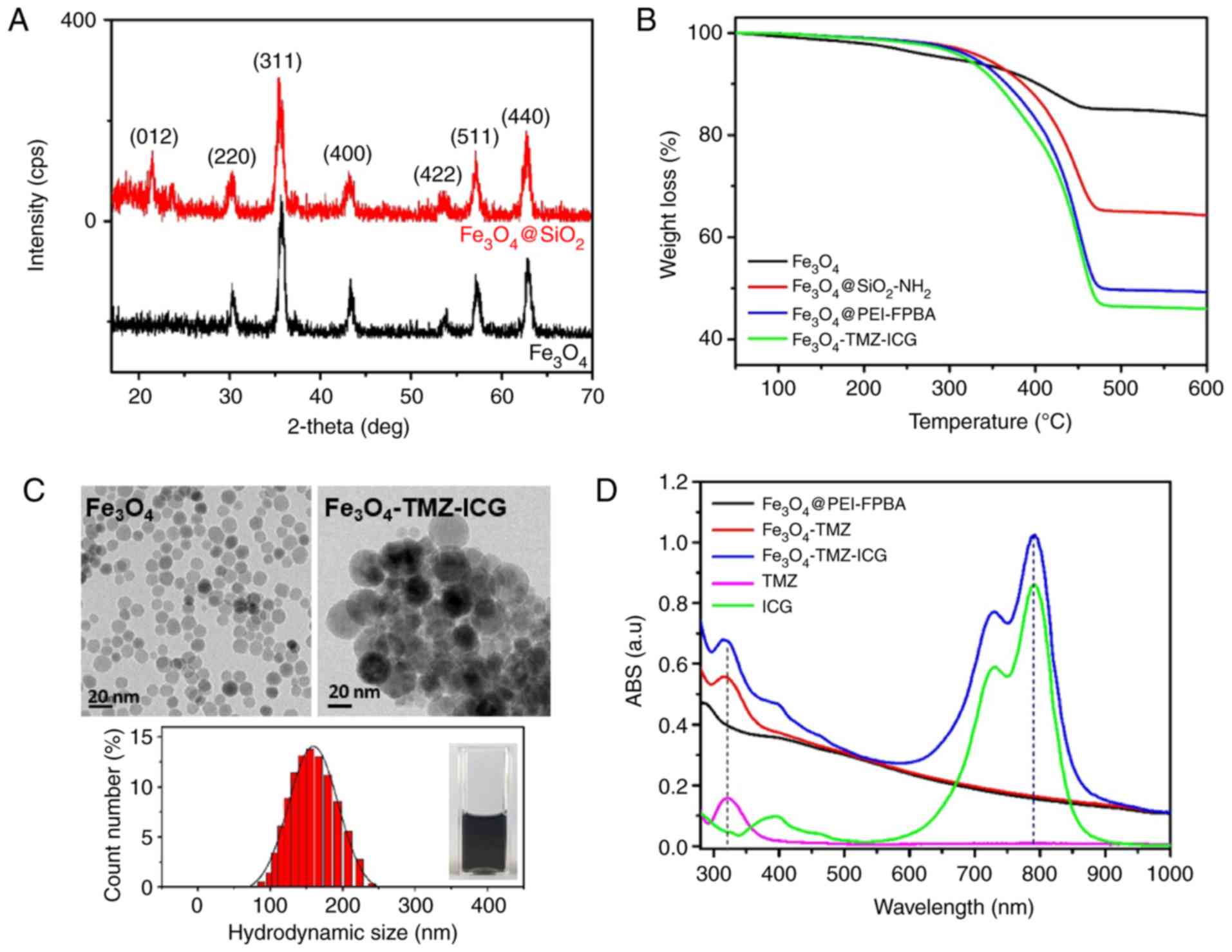 | Figure 2.Characterizations of physicochemical
properties. (A) X-ray diffraction patterns of inorganic
Fe3O4 and
Fe3O4@SiO2 MNPs. (B) Thermal
gravimetric analysis curves of Fe3O4 MNPs,
SiO2-NH2-, PEI-FPBA, and TMZ-ICG-coated
Fe3O4 MNPs. (C) High-resolution transmission
electron microscopy images of Fe3O4 and
Fe3O4-TMZ-ICG MNPs, and the average
hydrodynamic diameter of Fe3O4-TMZ-ICG MNPs
in aqueous solution by dynamic light scattering measurement (inset;
Fe3O4-TMZ-ICG MNPs aqueous dispersion). (D)
Changes in UV-vis spectra before and after TMZ and ICG loading onto
Fe3O4 MNPs. ABS, absorbance; FPBA,
formylphenylboronic acid; ICG, indocyanine green; MNPs, magnetic
nanoparticles; PEI, polyethylenimine; TMZ, temozolomide. |
The content of functional shells on the
Fe3O4 MNPs were estimated by TGA (Fig. 2B). Weight reductions indicate the
content of organic materials on the functionalized
Fe3O4 MNPs. The decrease in weight at
temperatures <200°C indicated the removal of residual adsorbed
solvent in the MNPs, and that at temperatures >250°C was
attributed to organic materials. The weight of each sample
gradually decreased at >450°C under N2 atmosphere.
The TGA thermogram of Fe3O4 MNPs showed a
constant weight reduction with increasing temperature as of the
removal of free oleic acid and decomposition of oleic acid bound to
Fe3O4 MNPs. The
Fe3O4@SiO2 MNPs showed notable
weight loss, of ~35.95% at 250–450°C, mainly due to the
decomposition of coated SiO2-NH2 on the
Fe3O4 MNPs surface. The PEI-FPBA encapsulated
Fe3O4 MNPs exhibited increased weight
reductions of ~51.31% than Fe3O4 and
Fe3O4@SiO2-NH2 MNPs due
to the decomposition of coated PEI and FPBA on the
Fe3O4 MNPs surface. At a temperature of
500°C, the functional shells on the Fe3O4
MNPs decomposed by ~100%. Using TGA thermogram analysis, final
residual naked Fe3O4 weights with high
thermal stability were determined to be 83.89, 64.32, 49.31, and
45.89% for Fe3O4,
Fe3O4@SiO2-NH2,
Fe3O4@PEI-FPBA, and
Fe3O4-TMZ-ICG MNPs, respectively.
Calculations revealed that the Fe3O4 content
of Fe3O4-TMZ-ICG MNPs could be ≤45.89
wt%.
The average size and morphology of
Fe3O4 and modified
Fe3O4-TMZ-ICG MNPs were observed by HR-TEM
images (Fig. 2C). The
Fe3O4 cores were monodispersed and spherical
in shape, and showed uniform particle size with an average size of
~13 nm. The average hydrodynamic size of
Fe3O4-TMZ-ICG MNPs measured by DLS was
159.52±34.15 nm in aqueous dispersion. The
Fe3O4-TMZ-ICG MNPs showed good colloidal
stability without any considerable aggregation (Fig. 2C inset).
Determination of TMZ- and ICG-loading
contents
UV-vis spectroscopy was employed in order to
confirm the successful TMZ- and ICG-loading onto the
Fe3O4@PEI-FPBA MNPs (Fig. 2D). The
Fe3O4-TMZ-ICG MNPs with maximum absorption at
328 and 780 nm revealed the presence of the condensation of TMZ and
electrostatic interaction of ICG with
Fe3O4@PEI-FPBA MNPs, which could be used to
quantify the TMZ and ICG concentration. TMZ- and ICG-loading
contents in Fe3O4-TMZ-ICG MNPs was determined
by the difference of optical absorbance at 328 and 780 nm,
respectively, after subtracting the optical absorbance of
Fe3O4@PEI-FPBA MNPs before TMZ- and
ICG-molecules loading. Based on the optical absorbance of TMZ- and
ICG-molecules and the linear calibration curves of TMZ- and
ICG-concentration at 328 and 780 nm, respectively, TMZ- and
ICG-loading contents were ~26.4 and 12.4 µg per 1.5 mg/ml of
Fe3O4-TMZ-ICG aqueous solution. MNPs, and the
loading efficiencies of TMZ and ICG were respectively found to be
35.2 and 21.4%. The calculated TMZ- and ICG-loading contents were
determined to be ~6.6 and 3.1 µg per 173 mg of
Fe3O4 MNPs (125 µg Fe/ml). The
Fe3O4-TMZ-ICG MNPs showed high optical
absorption in the NIR region with a high drug loading efficiency of
≤50%, suggesting that the Fe3O4-TMZ-ICG MNPs
can be applied in synergistic chemo-photothermal therapy under NIR
laser irradiation.
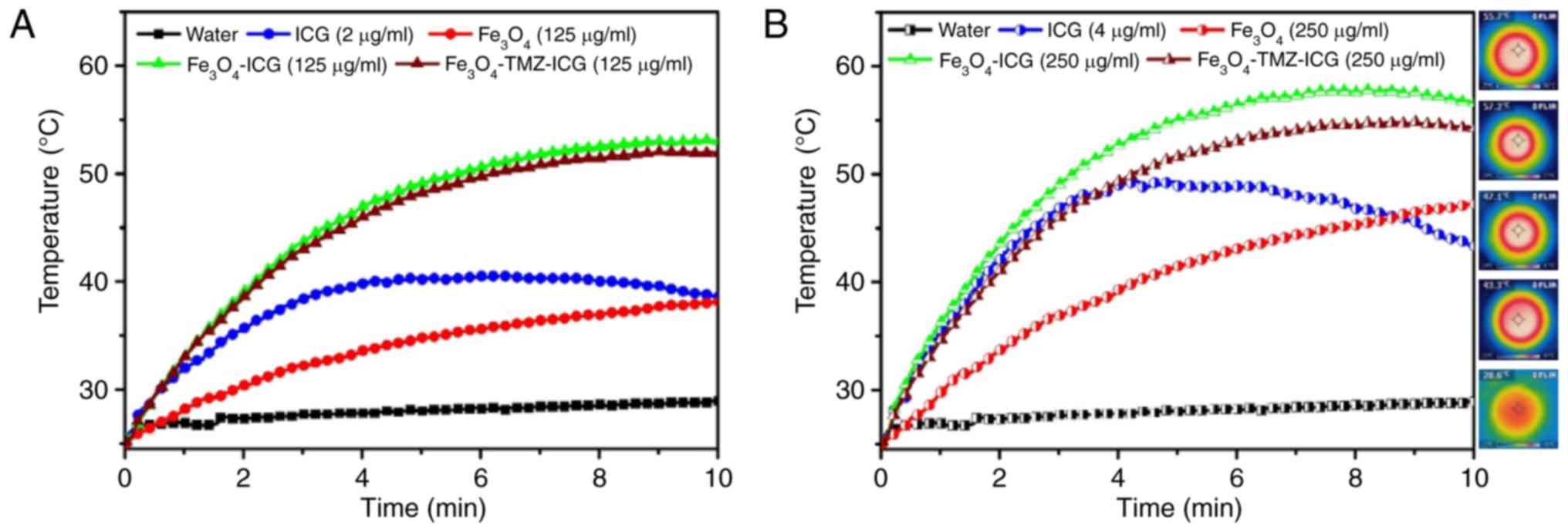
Photothermal performance of
Fe3O4-TMZ-ICG MNPs
The photothermal heating responses of free ICG,
Fe3O4 only, Fe3O4-ICG,
and Fe3O4-TMZ-ICG MNPs were examined with the
different concentrations in water (1 ml) for 10 min with 808-nm NIR
laser irradiation at 1 W/cm2. As shown Fig. 3, the temperature of
Fe3O4 solution upon 808-nm NIR laser
irradiation was increased with the increase of
Fe3O4 concentration and laser exposure time
(ΔT=9.8°C at 125 µg Fe/ml and ΔT=16.58°C at 250 µg Fe/ml for 5 min,
and ΔT=13.1°C at 125 µg Fe/ml and ΔT=22.2°C at 250 µg Fe/ml for 10
min), whereas the temperature of pure water showed no notable
change. On the contrary, the temperature of free ICG solution
quickly increased at the initial 4 min of exposure to 808-nm NIR
laser irradiation, followed by the gradual decrease due to
photo-degradation of ICG (55). ICG-
and Fe3O4-conjugated MNPs
(Fe3O4-ICG and
Fe3O4-TMZ-ICG) exhibited higher heating
efficiency with the sample concentration and laser exposure time
(ΔT=23.8°C at 125 µg Fe/ml and ΔT=29.8°C at 250 µg Fe/ml for 5 min,
and ΔT=27.7°C at 125 µg Fe/ml and ΔT=31.3°C at 250 µg Fe/ml for 10
min for Fe3O4-ICG; ΔT=23.1°C at 125 µg Fe/ml
and ΔT=26.5°C at 250 µg Fe/ml for 5 min, and ΔT=26.8°C at 125 µg
Fe/ml and ΔT=29.0°C at 250 µg Fe/ml for 10 min for
Fe3O4-TMZ-ICG) compared with the
Fe3O4 MNPs and ICG alone. Of note, the
photostability of ICG entrapped in Fe3O4 MNPs
markedly improved due to the reduced intramolecular interactions
within ICG (56).
Further, the corresponding IR thermal camera images
of ICG, Fe3O4,
Fe3O4-ICG, and
Fe3O4-TMZ-ICG solution during 10 min of NIR
laser irradiation revealed homogeneous distribution of temperature
with fast heat dissipation. These results indicate that
ICG-entrapped Fe3O4 MNPs could be an
effective photothermal agent with high heating ability and thermal
stability.
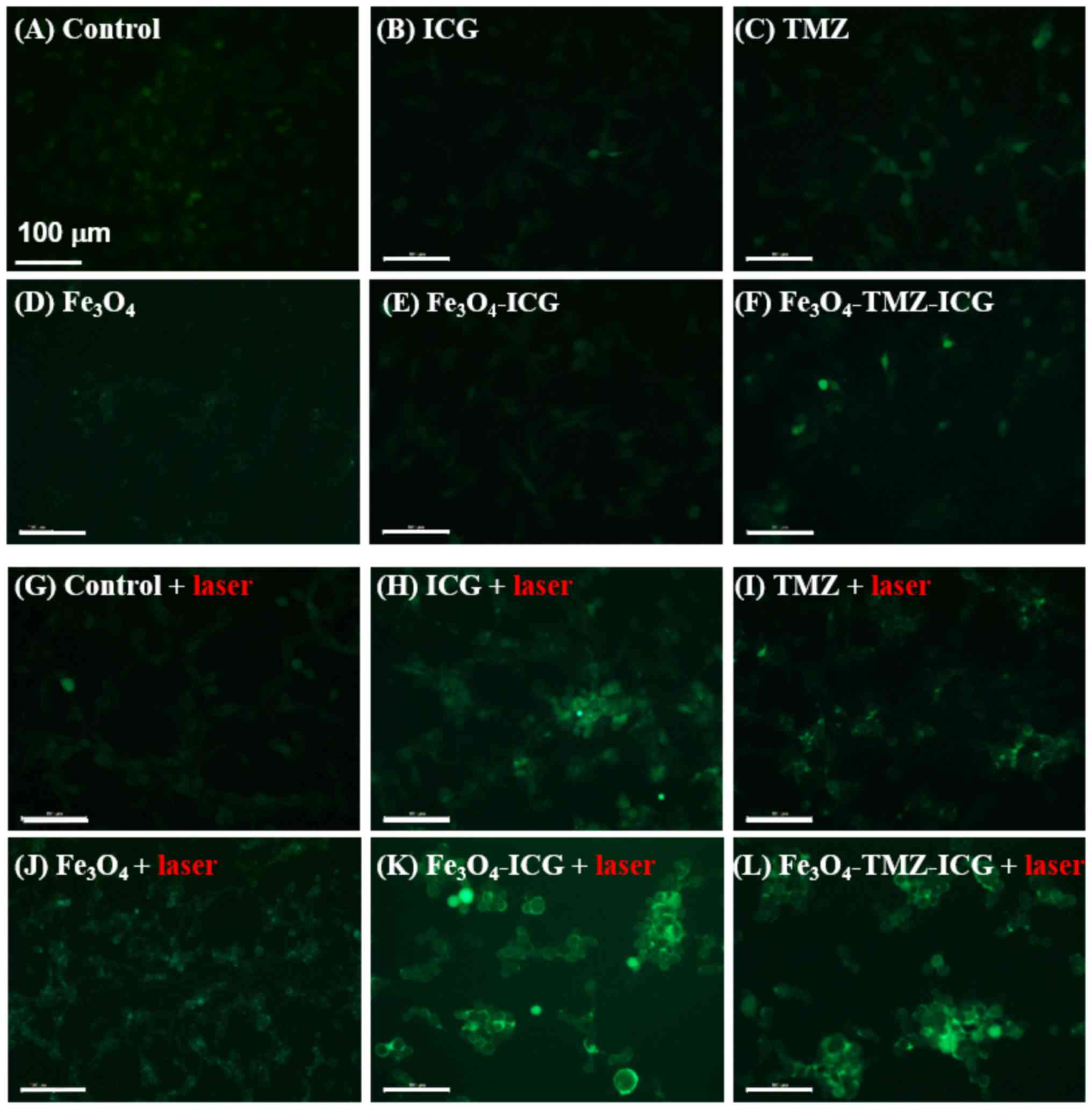
Detection of intracellular ROS
generation by chemo-photothermal therapy
ROS generation associated with cellular membrane
damage was examined using ICG- and/or TMZ-conjugated
Fe3O4 MNPs with NIR laser irradiation
(Fig. 4). Intracellular production of
ROS can cause irreversible damage to target cancer cells and
triggering of apoptosis (57). NIR
laser stimuli can induce cellular damage by not only the thermal
effect, but also via heat stress-induced ROS during photothermal
therapy to target cancer cells (58).
Furthermore, several anticancer drugs induce ROS generation via
oxidative stress (59). Importantly,
in this study, ICG was proposed to be effectively applied as a
photothermal agent and photodynamic photosensitizer to generate
heat and toxic ROS upon NIR laser irradiation for the ablation of
cancer cells (60).
The intracellular ROS generation in U-87 MG cells
treated without sample, or 3.1 µg/ml ICG, 6.6 µg/ml TMZ, and 125 µg
Fe/ml Fe3O4, Fe3O4-ICG
and Fe3O4-TMZ-ICG MNPs with or without 808-nm
NIR laser irradiation (1 W/cm2, 5 min) after 2 h was
confirmed by DCF green fluorescence stain under a fluorescence
microscope. Without 808-nm NIR laser irradiation, all cells
exhibited no considerable intracellular ROS production. In
contrast, all cells treated with samples exhibited notable ROS
production upon 808-nm NIR laser irradiation. In addition, as shown
in Fig. 4I, the cells treated with
TMZ- and ICG-loaded Fe3O4
(Fe3O4-TMZ-ICG) MNPs showed a strong green
fluorescence intensity, which indicated considerably enhanced ROS
production, along with higher cytotoxicity in cell morphology. ROS
production was more strongly detected in the
Fe3O4-TMZ-ICG MNPs-treated cells compared
with the cells treated with ICG, TMZ, Fe3O MNPs or laser
alone. Taken together, experimental results indicate that ROS
production was synergistically enhanced by the combination of
chemo-phototherapy using Fe3O4-TMZ-ICG
MNPs.
Morphological changes and cell
viability by chemo-photothermal therapy
To evaluate the cytotoxic effect in combination
with the chemotherapeutic and phototherapeutic treatments, the U-87
MG cells were incubated with the different samples (3.1 µg/ml ICG,
6.6 µg/ml TMZ, and 125 µg Fe/ml of Fe3O4,
Fe3O4-ICG and
Fe3O4-TMZ-ICG MNPs) for 2 h, followed by
808-nm NIR laser irradiation at 1 W/cm2 for 5 min. The
cells were further incubated for 22 h, and then alterations in cell
morphology were observed under an optical microscope (Fig. 5A). Both control cells and those
treated with photothermal agents (ICG, Fe3O4
and Fe3O4-ICG MNPs) without 808-nm NIR laser
treatment showed no marked morphological changes, suggesting that
ICG, Fe3O4 only, and
Fe3O4-ICG MNPs have high biocompatibility and
low cytotoxicity in vitro (Fig.
5A). Cells treated with TMZ alone and TMZ-loaded
Fe3O4-ICG MNPs as a chemo and
chemo-photothermal agent without 808-nm NIR laser treatment
(Fig. 5Ac and f), showed no
considerable damage in cell morphology compared with control cells
(Fig. 5Aa).
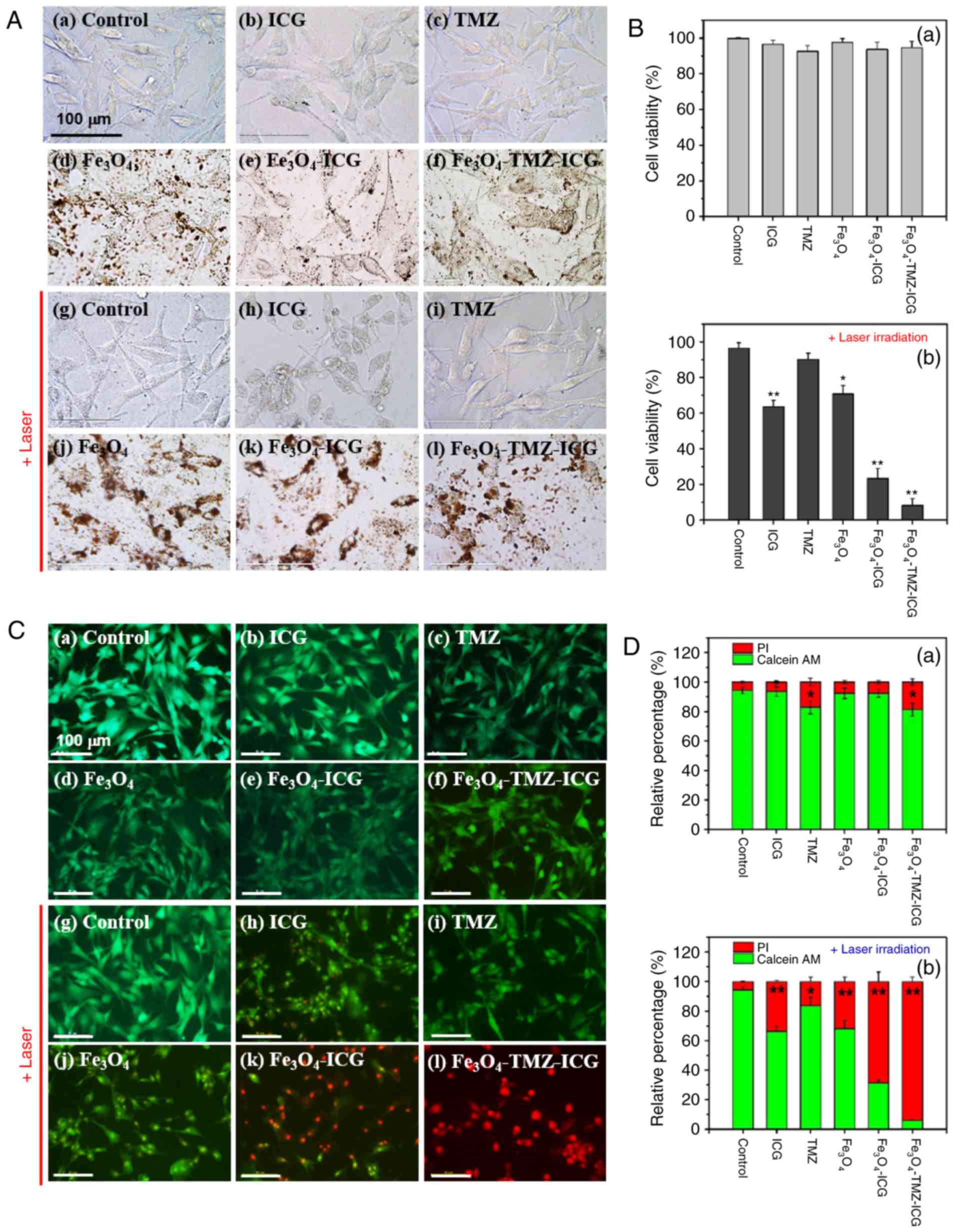 | Figure 5.In vitro cytotoxicity and
photothermal effect on U-87 MG cells. (A) In vitro
morphological changes with various treatments by optical
microscopy. (B) Relative cell viability by WST-1 assay with
different samples (a) without or (b) with laser irradiation. (C)
Fluorescence microscopy images of calcein AM/PI double stained U-87
MG cells with various treatments. (D) Quantitative analysis of
live/dead cells calculated by ImageJ software (a) without or (b)
with laser irradiation. U-87 MG cells were pre-treated with
different samples: No sample (control), 3.1 µg/ml of ICG, 6.6 µg/ml
of TMZ, and 125 µg Fe/ml of Fe3O4,
Fe3O4-ICG and
Fe3O4-TMZ-ICG magnetic nanoparticles before
NIR laser exposure. After 2 h incubation, the cells were treated
with or without NIR laser irradiation for 5 min (1
W/cm2) followed by further incubation for 22 h. All
values were presented as the mean ± standard deviation, n=3;
*P<0.05 and **P<0.01 vs. control. Scale bar, 100 µm. ICG,
indocyanine green; NIR, near-infrared; TMZ, temozolomide. |
Conversely, with 808-nm NIR laser treatment, the
cells treated with ICG, Fe3O4 and
Fe3O4-ICG MNPs as photothermal agents showed
notable cellular damage, such as cell shrinkage, nuclear
condensation, and loss of cell volume (Fig. 5Ah, j and k), whereas the control
showed no marked damage (Fig. 5Ag).
In addition, the intracellular uptake of
Fe3O4 MNPs into U-87 MG cells improved after
NIR laser irradiation (Fig. 5Aj, k and
l). Next, for the cells treated with TMZ, a chemotherapeutic
agent for brain cancer (61), with
808-nm NIR laser treatment, there was a slight reduction in cell
viability (Fig. 5Ai). Finally, the
cells treated with TMZ-loaded Fe3O4-ICG MNPs,
a chemo-photothermal agent with 808-nm NIR laser treatment, showed
notable cellular damage with a reduction in the cell
population.
Next, the viability of ICG, TMZ,
Fe3O4 alone, Fe3O4-ICG
and Fe3O4-TMZ-ICG MNPs with or without NIR
laser irradiation was measured in U-87 MG cells by WST-1 assay
(Fig. 5B). Without NIR laser
irradiation, all cells presented almost comparable cell viability
compared with that of the control. With NIR laser irradiation, the
cells treated with TMZ only showed small reductions in cell
viability (91.01%), whereas the control group with NIR laser
irradiation exhibited no marked changes in cell viability. There
was no obvious change in temperature (data not shown). ICG and
Fe3O4 MNPs with NIR laser irradiation
revealed a total of 63.55 and 70.74% viable cells, respectively.
The temperature increased up to 41.1 and 43.6°C for ICG and
Fe3O4 MNPs, respectively (data not shown).
The combination of ICG and Fe3O4
(Fe3O4-ICG) exhibited significantly decreased
cell viability (21.37%), leading to a notable temperature increase
to 51.8°C. Finally, the combination of ICG, TMZ and
Fe3O4 (Fe3O4-TMZ-ICG)
as a chemo-photothermal agent showed a significantly decreased cell
viability of 9.32%, leading to an obvious temperature increase to
51.2°C.
According to these results, photothermal therapy by
ICG, Fe3O4 and
Fe3O4-ICG MNPs with 808-nm NIR laser
irradiation induced marked morphological changes such as cell
shrinkage, membrane integrity loss, and cytoplasmic condensation,
evidencing the incidence of cell apoptosis (62). Moreover,
Fe3O4-TMZ-ICG MNPs with 808-nm NIR laser
irradiation induced severe cellular damage and showed significantly
decreased in U-87 MG glioblastoma cell viability via the
combination of chemo-photothermal therapy, resulting in subsequent
irreversible cell death.
Live/dead cell assay
To further investigate the in vitro
photothermal effect of combined chemo-photothermal therapy using
TMZ- and ICG-loaded Fe3O4 MNPs, live cells
detection after treatment with samples was carried out using
calcein AM/PI double staining. As shown in Fig. 5C, the control U-87 MG cells with and
without 808-nm NIR laser irradiation emitted green fluorescence as
the calcein-AM reagent can penetrate the live cell membrane,
indicating no apoptotic cells. Treatment with the ICG,
Fe3O4, and Fe3O4-ICG
MNPs without NIR laser irradiation showed green fluorescence,
suggesting no cytotoxicity. In addition, treatment with TMZ and
Fe3O4-TMZ-ICG MNPs showed slight
morphological changes. However, treatment with
Fe3O4 and ICG with NIR laser irradiation
showed slight yellow-green calcein-AM nuclear staining with
morphological damage, suggesting that there was the early cell
apoptosis. Moreover, treatment with
Fe3O4-TMZ-ICG MNPs with 808-nm NIR laser
irradiation exhibited intense red fluorescence due to PI staining,
suggesting that chemo-photothermal therapy using the
Fe3O4-TMZ-ICG MNPs exhibited high
cytotoxicity against U-87 MG cells.
To assess the live/dead cell ratio, calcein-AM and
PI fluorescence intensities were quantified using ImageJ software
(Fig. 5D). The cells treated with
ICG, TMZ and/or Fe3O4 showed significantly
increased cell death under NIR laser irradiation, as evidenced by
fewer green spots and enhanced red spots in the images. These
results demonstrated that chemo-photothermal therapy using
Fe3O4-TMZ-ICG MNPs induced the synergetic
effect for U-87 MG cancer cell killing capacity.
Nanoparticles intracellular uptake and
cellular damage
To assess DNA damage-induced apoptosis by the
synergistic combination of chemo-phototherapy, high-quality
morphological images in single cells were observed via confocal
microscopy. As shown in Fig. 6A-F,
cells that unexposed to 808-nm NIR laser irradiation (i.e. no
sample, 3.1 µg/ml ICG, 6.6 µg/ml TMZ, and 125 mg Fe/ml of
Fe3O4, Fe3O4-ICG and
Fe3O4-TMZ-ICG MNPs) showed no nuclear and
cellular membrane damage. The cells treated with no sample
(control) and TMZ with NIR laser irradiation also showed no notable
nuclear and cellular membrane damage. On the contrary, the cells
treated with ICG, Fe3O4, and ICG-conjugated
Fe3O4 as photothermal agents under NIR laser
irradiation exhibited considerable nuclear damage and the loss of
cellular membrane integrity (Fig.
6G-L). In particular, as shown Fig.
6J-L, Fe3O4 nanoparticles exhibited
enhanced intracellular nanoparticle uptake by 808-nm NIR laser
stimulation, thereby leading to nucleus damage. These results
suggest that Fe3O4-TMZ-ICG MNPs as a TMZ drug
carrier were more highly internalized into the nucleus of cancer
cells by NIR laser stimuli; TMZ from the surface of
Fe3O4 MNPs may bind DNA contributing to
efficient targeted drug delivery.
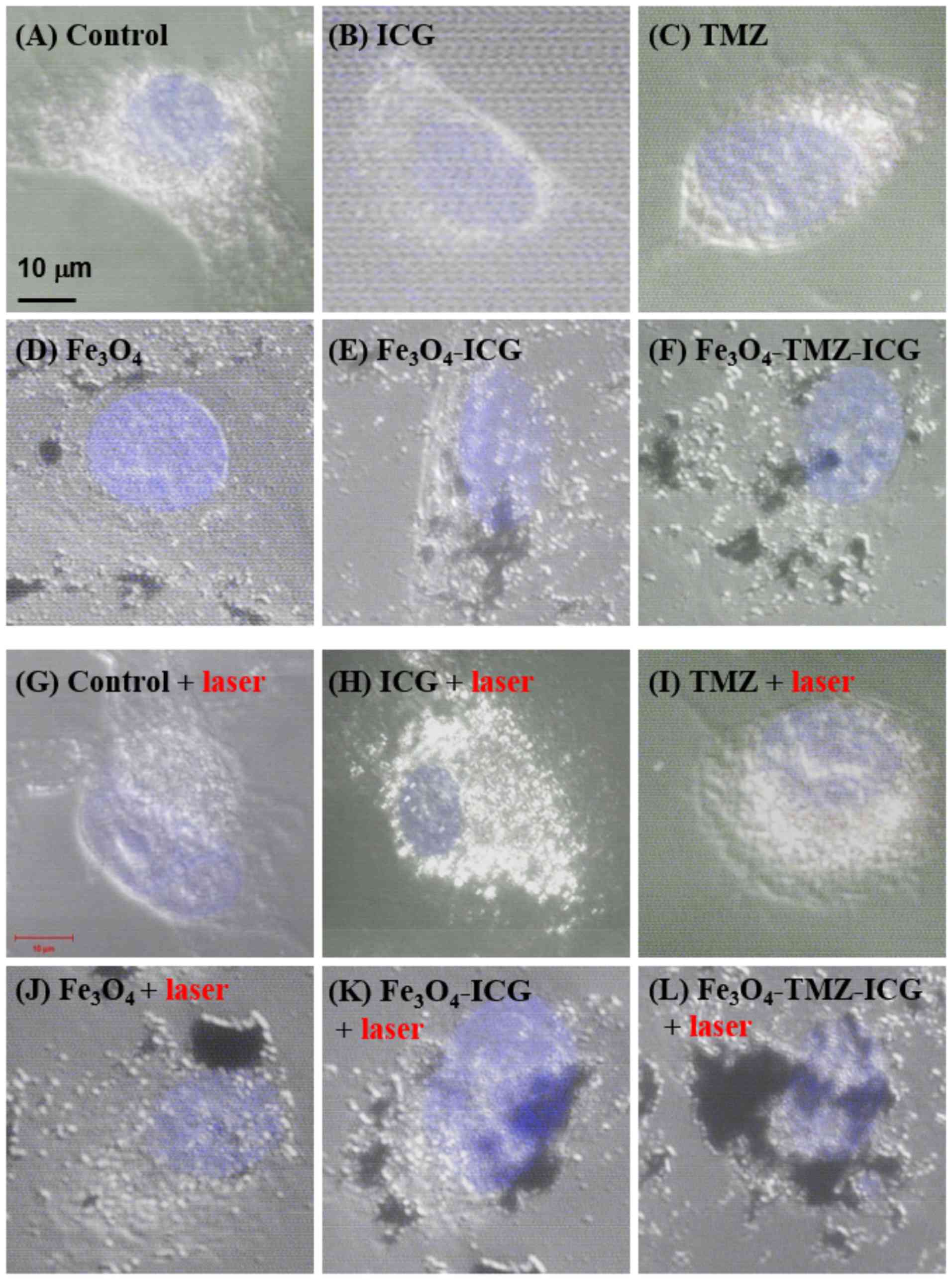 | Figure 6.Confocal laser scanning microscopy
images of photothermal effect on U-87 MG cells. The cells were
pre-treated with different samples (i.e., no sample, 3.1 µg/ml of
ICG, 6.6 µg/ml of TMZ, and 125 µg Fe/ml of
Fe3O4, Fe3O4-ICG and
Fe3O4-TMZ-ICG magnetic nanoparticles) before
NIR laser exposure. After 2 h incubation, the cells were treated
with or without NIR laser irradiation for 5 min (1
W/cm2) followed by further incubation for 22 h. Scale
bar, 10 µm. ICG, indocyanine green; NIR, near-infrared; TMZ,
temozolomide. |
Determination of cell apoptosis
To investigate the synergistic anticancer effect of
the combination of chemo-phototherapy compared to chemotherapy and
phototherapy alone, flow cytometry analysis was conducted after
treatment with samples (i.e., no sample, 3.1 µg/ml of ICG, 6.6
µg/ml of TMZ, and 125 µg Fe/ml of Fe3O4,
Fe3O4-ICG and
Fe3O4-TMZ-ICG MNPs) using Annexin V-FITC/PI
double staining (Fig. 7). For the
control with or without 808-nm NIR laser treatment, there was no
cytotoxicity, indicating the control group was not affected by NIR
laser irradiation. For the cells after treatment with samples
without 808-nm NIR laser, there were no significant cytotoxicity
compared with the control. In contrast, the cells after treatment
of samples with 808-nm NIR laser exhibited significantly higher
cytotoxicity with an increase in the proportion of cell apoptosis,
except for TMZ treatment. This suggested that the decrease in cell
viability may be due to apoptosis by phototherapy and/or
chemo-phototherapy. Here, the observed percentages of apoptotic
cells (early and late apoptotic cells) after 808-nm NIR laser
treatment were 2.06% in control, 35.3% in ICG-treated (3.1 µg/ml),
6.23% in TMZ-treated (6.6 µg/ml), 30.27% in
Fe3O4-treated (125 µg Fe/ml), 71.40% in
Fe3O4-ICG-treated (125 µg Fe/ml), and 85.58%
in Fe3O4-TMZ-ICG-treated (125 µg Fe/ml)
cells, respectively. Specifically, the
Fe3O4-TMZ-ICG MNPs without 808-nm NIR laser
irradiation showed a cytotoxicity of 6.7% against U-87 MG cells;
however, U-87 MG cells treated with the
Fe3O4-TMZ-ICG MNPs under 808-nm NIR laser
irradiation showed significantly increased cell cytotoxicity of
96.68% after 12 h treatment. In addition, treatment with
Fe3O4-TMZ-ICG and 808-nm NIR laser
irradiation at 1 W/cm2 for 5 min led to a total of ~80%
apoptotic cells. Therefore, the Fe3O4-TMZ-ICG
MNPs with 808-nm NIR laser irradiation at 1 W/cm2 may be
effectively utilized as a photothermal-induced anticancer
therapeutic agent in synergistic cancer therapy.
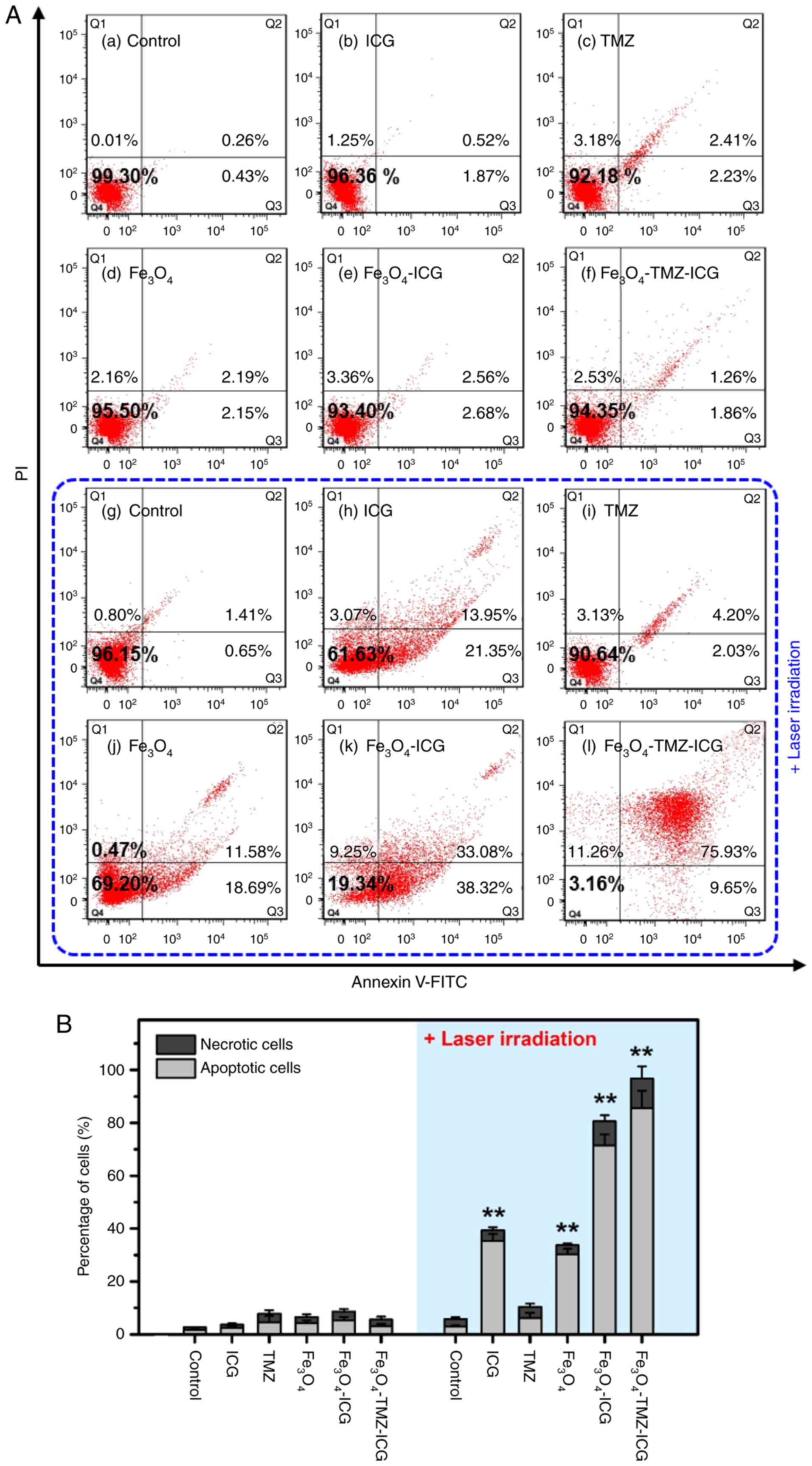 | Figure 7.Photothermally-induced apoptosis
analysis by flow cytometry in U-87 MG cells. (A) Representative of
dot plots and (B) quantification of the apoptotic and necrotic
cells in U-87 MG cells stained with Annexin V-FITC/PI double
staining kit. The cells were pre-treated with different samples: No
sample (control), 3.1 µg/ml of ICG, 6.6 µg/ml of TMZ, and 125 µg
Fe/ml of Fe3O4,
Fe3O4-ICG and
Fe3O4-TMZ-ICG magnetic nanoparticles, before
near-infrared laser exposure. After 2 h incubation, the cells were
treated with or without NIR laser irradiation for 5 min (1
W/cm2) followed by further incubation for 22 h. All
values were presented as the mean ± standard deviation, n=3;
**P<0.01 vs. control. FITC, fluorescein isothiocyanate; ICG,
indocyanine green; NIR, near-infrared; PI, propidium iodide; TMZ,
temozolomide. |
Promotion of intrinsic and extrinsic
apoptotic pathway
Apoptosis known as programmed cell death is
regulated by specific cellular signaling pathways which maintain
the balance between cell proliferation and cell death (49). Cancer can occur when the balance of
cell division and cell death is disturbed, and defects in apoptosis
can cause tumor pathogenesis (63).
Thus, a key goal of cancer therapy is to promote the apoptosis of
cancer cells without damage to normal cells (64). It has been reported that
nanoparticle-mediated photothermal therapy induces apoptosis
(65,66). Apoptosis is characterized by two main
intrinsic and extrinsic apoptotic pathways (67). Upon exposure to external and internal
stress stimuli, p53, a nuclear transcription factor, promotes
apoptotic function by the regulation of apoptotic protein
expression of both extrinsic and intrinsic pathways (68). In the intrinsic pathway, p53 modulates
the Bcl-2 family of proteins, including pro-apoptotic Bax and
anti-apoptotic Bcl-2. The Bcl-2 protein family comprise central
regulators of the intrinsic apoptotic pathway, which control
cytochrome c release from the mitochondria to the cytosol
and induce the activation of caspase-3 (69).
In the present study, ICG,
Fe3O4, and ICG-conjugated
Fe3O4 MNPs with NIR laser stimuli was
determined to induce apoptotic cell death through ROS generation
and photothermal effects. Intrinsic apoptotic pathways can be
initiated by elevated intracellular ROS generation (70). Thus, we first evaluated ROS-mediated
intrinsic apoptotic protein including Bax, Bcl-2, cytochrome
c, and caspase-3 by western blotting. As depicted in
Fig. 8A and B, ICG,
Fe3O4, and ICG-conjugated
Fe3O4 MNPs with NIR laser irradiation
significantly upregulated pro-apoptotic Bax, but downregulated
anti-apoptotic Bcl-2 protein expression compared with the control.
Next, we confirmed that cytochrome c accumulation in the
cytosolic extract was significantly increased by chemo-photothermal
therapy using ICG, Fe3O4, and their
conjugates, compared with the control. In addition, western blot
analysis showed upregulation of caspase-3 activation by
chemo-photothermal therapy using ICG, TMZ,
Fe3O4, and their conjugates, as evidenced by
increased caspase-3 cleavage fragments. Our data demonstrated that
the combination of chemo-photothermal therapy using
Fe3O4-TMZ-ICG MNPs notably promoted the
intrinsic apoptosis-related pathway compared with ICG, TMZ, and
Fe3O4 alone. The fold increase in Bax,
cytochrome c release into the cytosol, and caspase-3
activation, and the fold decrease in Bcl-2 expression due to
Fe3O4-TMZ-ICG MNPs was 5.79, 3.91, 4.92 and
0.16 compared with the control, respectively. These results suggest
that chemo-photothermal therapy exhibited notable anti-cancer
effects against U-87 MG cells through stimulation of intrinsic
apoptosis-related pathway.
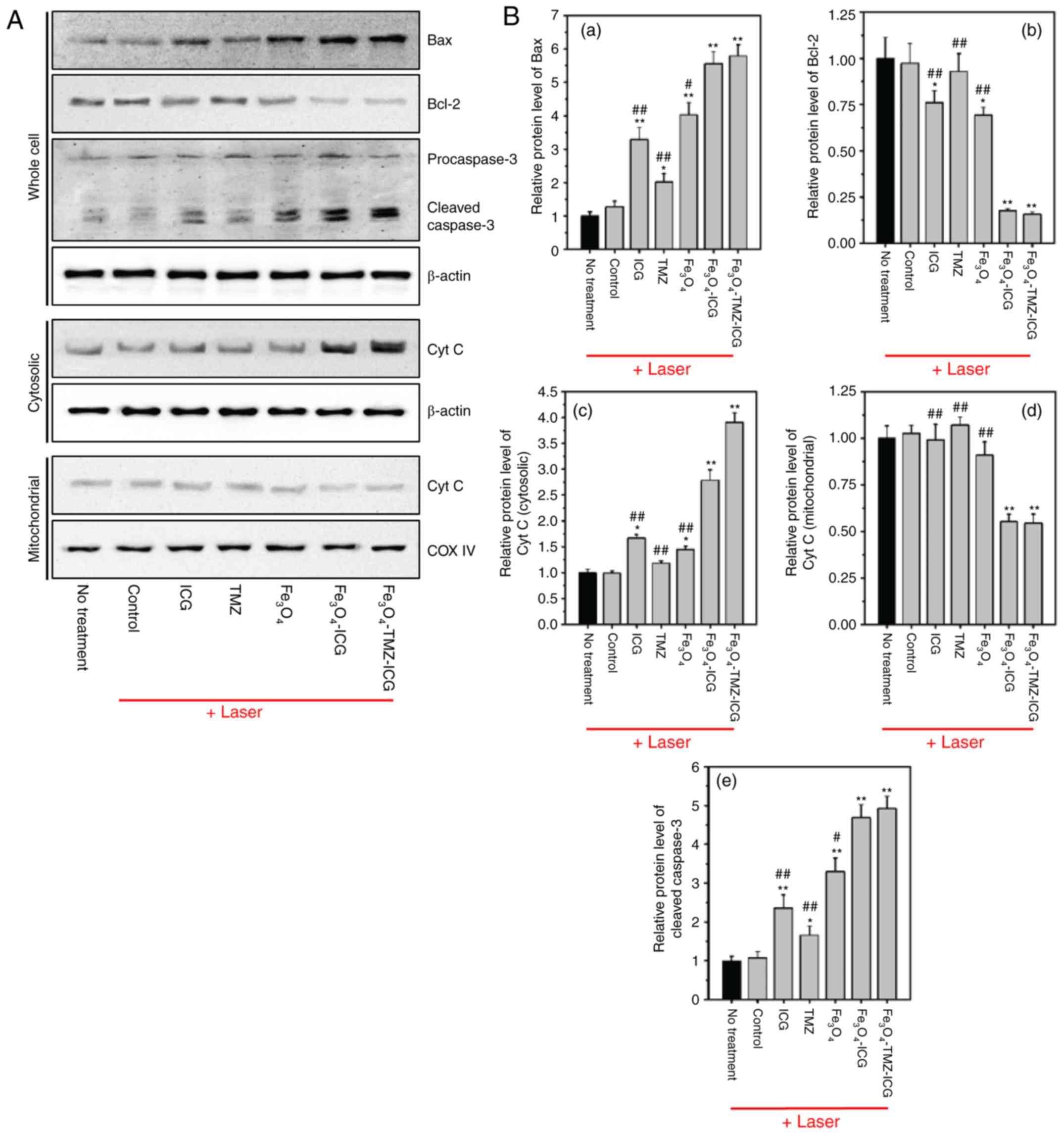 | Figure 8.Western blotting analysis of
apoptosis-related proteins regulated by photothermal therapy in
U-87 MG cells. (A) Representative western of Bax, Bcl-2,
procaspase-3, cleaved caspase-3, cytosolic cytochrome c, and
mitochondrial cyt c. β-actin and COX IV were used as loading
controls. (B) Quantitative analysis of relative protein expression
of Bax, Bcl-2, cleaved caspase-3, cytosolic cyt c, and
mitochondrial cyt c using ImageJ software. Quantification of
protein expressions of Bax, Bcl-2, and cleaved caspase-3 were
normalized to β-actin; that of cytosolic cyt c, and mitochondrial
cyt c were normalized to β-actin and COX IV, respectively. All
values were presented as the mean ± standard deviation, n=3;
*P<0.05 and **P<0.01 vs. untreated control (no treatment);
#P<0.05 and ##P<0.01 vs.
Fe3O4-TMZ-ICG with NIR laser irradiation;
Bax, Bcl-2-associated X protein; cyt c, cytochrome c; COX
IV, cytochrome c oxidase; ICG, indocyanine green; NIR,
near-infrared; TMZ, temozolomide. |
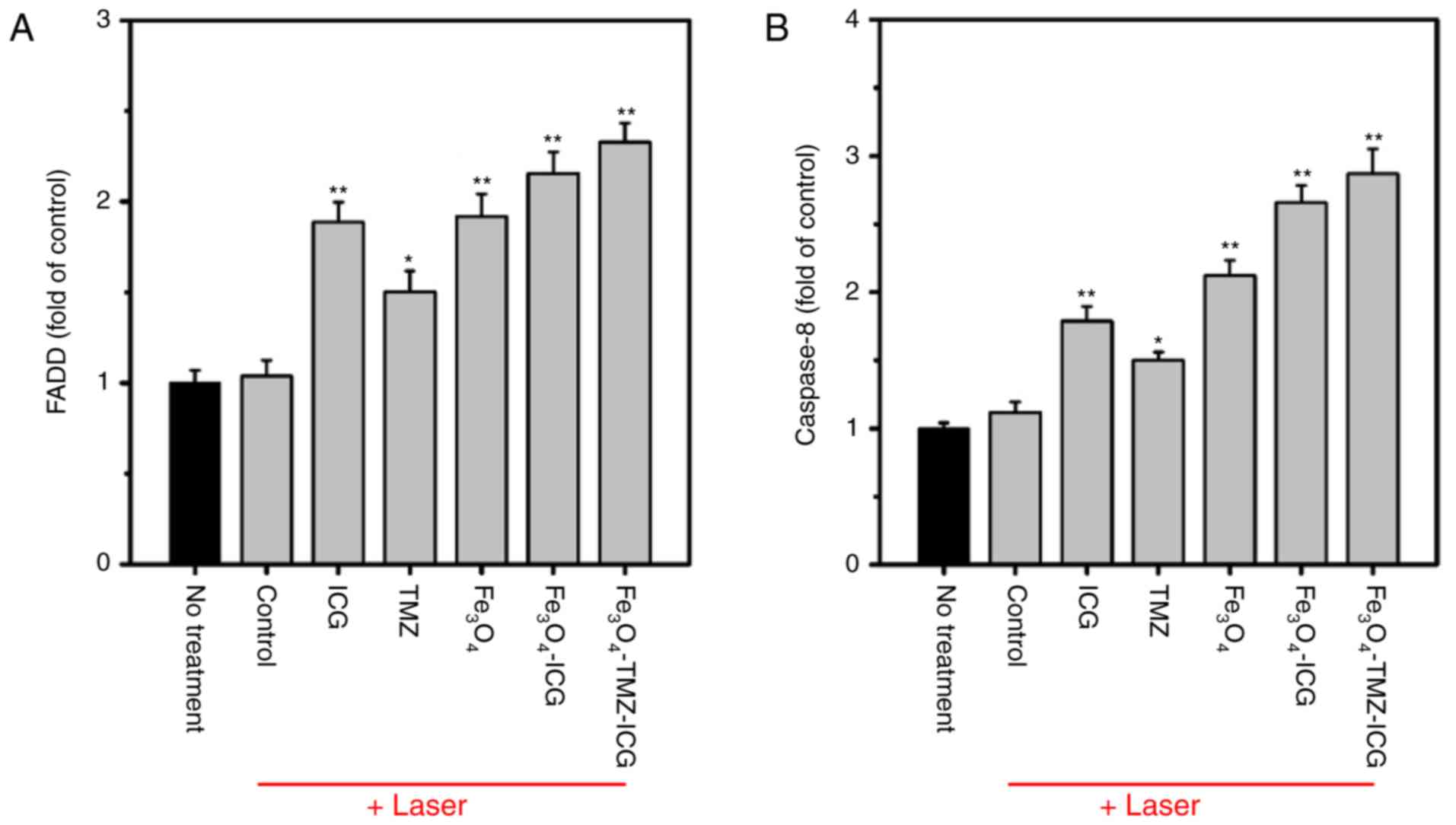
The extrinsic pathway is initiated by the binding
of an extracellular death ligand to its cell surface death
receptors, and influences the activation of the death receptor
FADD-caspase-8 pathway, which can directly activate caspase-3
(71). To investigate the molecular
mechanism of extrinsic apoptosis in chemo-photothermal therapy
using ICG, Fe3O4, and ICG-conjugated
Fe3O4 MNPs, the mRNA expression of FADD and
caspase-8 genes were analyzed by RT-qPCR. As shown in Fig. 9, ICG, Fe3O4, and
ICG-conjugated Fe3O4 MNPs with NIR laser
irradiation significantly upregulated the mRNA expression of FADD
and caspase-8 compared with the control. The combination of
chemo-photothermal therapy using
Fe3O4-TMZ-ICG MNPs resulted in markedly
higher mRNA expression of FADD and caspase-8 (2.33 and 2.87-fold,
respectively) compared with ICG, TMZ, and
Fe3O4 alone. These results support the
chemo-photothermal effects of Fe3O4-TMZ-ICG
MNPs in promoting the apoptotic of U-87 MG cells via upregulation
of extrinsic apoptosis-related genes. Taken together,
chemo-photothermal therapy using
Fe3O4-TMZ-ICG MNPs exhibited the most notable
anti-cancer effect by inducing apoptosis through activation of the
intrinsic and extrinsic pathways.
In this study, we prepared ICG- and TMZ-loaded
Fe3O4 MNPs, and demonstrated their
chemo-phototherapeutic synergistic effect against U-87 MG
glioblastoma cells. The ICG-embedded Fe3O4
MNPs exhibited excellent photothermal effect and photostability
under NIR laser irradiation. Further, TMZ- and ICG-loaded
Fe3O4 MNPs exhibited synergistic cell
cytotoxicity when applied via chemo-phototherapy. The experimental
results showed that the combination of chemo-phototherapy using the
Fe3O4-TMZ-ICG MNPs induced effective cancer
cell death mediated by enhanced ROS generation, and modulated both
the intrinsic and extrinsic apoptotic pathways. Therefore, our
results demonstrated that the synthesized NIR-light-responsive
Fe3O4-TMZ-ICG MNPs could be promising
phototherapeutic agents for the treatment of glioblastoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data used and analyzed during the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
YMK prepared the nanoparticles and drafted the
manuscript. JYJ contributed to the design of nanoparticles and
performed the cell experiments. SHC characterized the nanoparticles
and interpreted the data. YO performed the in vitro
experiments and contributed to the final version of the manuscript.
WHC made substantial contributions to the design of the present
study and supervised the experiments. All authors discussed the
results and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
Di Meco F, et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ladomersky E, Scholtens DM, Kocherginsky
M, Hibler EA, Bartom ET, Otto-Meyer S, Zhai L, Lauing KL, Choi J,
Sosman JA, et al: The coincidence between increasing age,
immunosuppression, and the incidence of patients with glioblastoma.
Front Pharmacol. 10:2002019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ostrom QT, Gittleman H, Truitt G, Boscia
A, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors diagnosed in
the United States in 2011–2015. Neuro Oncol. 20 (Suppl 4):iv1–iv86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harder BG, Blomquist MR, Wang J, Kim AJ,
Woodworth GF, Winkles JA, Loftus JC and Tran NL: Developments in
blood-brain barrier penetrance and drug repurposing for improved
treatment of glioblastoma. Front Oncol. 8:4622018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shergalis A, Bankhead A III, Luesakul U,
Muangsin N and Neamati N: Current challenges and opportunities in
treating glioblastoma. Pharmacol Rev. 70:412–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Tellingen O, Yetkin-Arik B, De Gooijer
MC, Wesseling P, Wurdinger T and de Vries HE: Overcoming the
blood-brain tumor barrier for effective glioblastoma treatment.
Drug Resist Updat. 19:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minniti G, Muni R, Lanzetta G, Marchetti P
and Enrici RM: Chemotherapy for glioblastoma: Current treatment and
future perspectives for cytotoxic and targeted agents. Anticancer
Res. 29:5171–5184. 2009.PubMed/NCBI
|
|
8
|
Friedman HS, Kerby T and Calvert H:
Temozolomide and treatment of malignant glioma. Clin Cancer Res.
6:2585–2597. 2000.PubMed/NCBI
|
|
9
|
Agarwala SS and Kirkwood JM: Temozolomide,
a novel alkylating agent with activity in the central nervous
system, may improve the treatment of advanced metastatic melanoma.
Oncologist. 5:144–151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Neal DP, Hirsch LR, Halas NJ, Payne JD
and West JL: Photo-thermal tumor ablation in mice using near
infrared-absorbing nanoparticles. Cancer Lett. 209:171–176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang J and Chen YC: Nanomaterials for
photohyperthermia: A review. Curr Pharm Des. 19:6622–6634. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Guo Z, Huang D, Liu Z, Guo X and
Zhong H: Synergistic effect of chemo-photothermal therapy using
PEGylated graphene oxide. Biomaterials. 32:8555–8561. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Sun Y, Huang R, Cang H, Cai Z and
Sun B: pH-sensitive prodrug conjugated polydopamine for
NIR-triggered synergistic chemo-photothermal therapy. Eur J Pharm
Biopharm. 128:260–271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen R, Zheng X, Qian H, Wang X, Wang J
and Jiang X: Combined near-IR photothermal therapy and chemotherapy
using gold-nanorod/chitosan hybrid nanospheres to enhance the
antitumor effect. Biomater Sci. 1:285–293. 2013. View Article : Google Scholar
|
|
15
|
El-Sayed IH, Huang X and El-Sayed MA:
Selective laser photo-thermal therapy of epithelial carcinoma using
anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett.
239:129–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang X, Jain PK, El-Sayed IH and El-Sayed
MA: Plasmonic photothermal therapy (PPTT) using gold nanoparticles.
Lasers Med Sci. 23:217–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wiley BJ, Chen Y, McLellan JM, Xiong Y, Li
ZY, Ginger D and Xia Y: Synthesis and optical properties of silver
nanobars and nanorice. Nano Lett. 7:1032–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirsch LR, Gobin AM, Lowery AR, Tam F,
Drezek RA, Halas NJ and West JL: Metal nanoshells. Ann Biomed Eng.
34:15–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Conde J, Doria G and Baptista P: Noble
metal nanoparticles applications in cancer. J Drug Deliv.
2012:7510752012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Lu W, Huang Q, Li C and Chen W:
Copper sulfide nanoparticles for photothermal ablation of tumor
cells. Nanomedicine (Lond). 5:1161–1171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robinson JT, Tabakman SM, Liang Y, Wang H,
Casalongue HS, Vinh D and Dai H: Ultrasmall reduced graphene oxide
with high near-infrared absorbance for photothermal therapy. J Am
Chem Soc. 133:6825–6831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao J, Gu H and Xu B: Multifunctional
magnetic nanoparticles: Design, synthesis, and biomedical
applications. Acc Chem Res. 42:1097–1107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee S, George Thomas R, Ju Moon M, Ju Park
H, Park IK, Lee BI and Yeon Jeong Y: Near-infrared heptamethine
cyanine based iron oxide nanoparticles for tumor targeted
multimodal imaging and photothermal therapy. Sci Rep. 7:21082017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen S, Kong F, Guo X, Wu L, Shen H, Xie
M, Wang X, Jin Y and Ge Y: CMCTS stabilized Fe3O4 particles with
extremely low toxicity as highly efficient near-infrared
photothermal agents for in vivo tumor ablation. Nanoscale.
5:8056–8066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sadat M, Kaveh Baghbador M, Dunn AW,
Wagner HP, Ewing CR, Zhang J, Xu H, Pauletti GM, Mast DB and Shi D:
Photoluminescence and photothermal effect of
Fe3O4 nanoparticles for medical imaging and
therapy. Appl Phys Lett. 105:0919032014. View Article : Google Scholar
|
|
26
|
Chen H, Burnett J, Zhang F, Zhang J,
Paholak H and Sun D: Highly crystallized iron oxide nanoparticles
as effective and biodegradable mediators for photothermal cancer
therapy. J Mater Chem B. 2:757–765. 2014. View Article : Google Scholar
|
|
27
|
Yuan G, Yuan Y, Xu K and Luo Q:
Biocompatible PEGylated Fe3O4 nanoparticles
as photothermal agents for near-infrared light modulated cancer
therapy. Int J Mol Med Sci. 15:18776–18788. 2014. View Article : Google Scholar
|
|
28
|
Zhang Y: Photothermal effect of PS coated
Fe3O4 nanoparticles via near-infrared laser and effect of mimic
body tissue depth on hyperthermic ablation of MDA-MB-231Univ
Cincinnati; 2015
|
|
29
|
Baronzio G, Parmar G, Ballerini M, Szasz
A, Baronzio M and Cassutti V: A brief overview of hyperthermia in
cancer treatment. J Integr Oncol. 3:1152014. View Article : Google Scholar
|
|
30
|
Fernandes C, Suares D and Yergeri MC:
Tumor microenvironment targeted nanotherapy. Front Pharmacol.
9:12302018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siemann DW: The unique characteristics of
tumor vasculature and preclinical evidence for its selective
disruption by tumor-vascular disrupting agents. Cancer Treat Rev.
37:63–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahmed K and Zaidi SF: Treating cancer with
heat: Hyperthermia as promising strategy to enhance apoptosis. J
Pak Med Assoc. 63:504–508. 2013.PubMed/NCBI
|
|
33
|
Melamed JR, Edelstein RS and Day ES:
Elucidating the fundamental mechanisms of cell death triggered by
photothermal therapy. ACS Nano. 9:6–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
May JP and Li SD: Hyperthermia-induced
drug targeting. Expert Opin Drug Deliv. 10:511–527. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song C, Park H, Lee C and Griffin R:
Implications of increased tumor blood flow and oxygenation caused
by mild temperature hyperthermia in tumor treatment. Int J
Hyperthermia. 21:761–767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bao Z, Liu X, Liu Y, Liu H and Zhao K:
Near-infrared light-responsive inorganic nanomaterials for
photothermal therapy. Asian J Pharm Sci. 11:349–364. 2016.
View Article : Google Scholar
|
|
37
|
Zheng X, Zhou F, Wu B, Chen WR and Xing D:
Enhanced tumor treatment using biofunctional indocyanine
green-containing nanostructure by intratumoral or intravenous
injection. Mol Pharm. 9:514–522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie J, Peng S, Brower N, Pourmand N, Wang
SX and Sun S: One-pot synthesis of monodisperse iron oxide
nanoparticles for potential biomedical applications. Pure Appl
Chem. 78:1003–1014. 2006. View Article : Google Scholar
|
|
39
|
Jiang L, Zhou X, Wei G, Lu X, Wei W and
Qiu J: Preparation and characterization of poly(glycidyl
methacrylate)-grafted magnetic nanoparticles: Effects of the
precursor concentration on polyol synthesis of
Fe3O4 and [PMDETA]0/[CuBr2]0 ratios on
SI-AGET ATRP. Appl Surf Sci. 357:1619–1624. 2015. View Article : Google Scholar
|
|
40
|
Feng G, Jiang L, Wen P, Cui Y, Li H and Hu
D: A new ion-exchange adsorbent with paramagnetic properties for
the separation of genomic DNA. Analyst. 136:4822–4829. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li H, Shan Y, Qiao L, Dou A, Shi X and Xu
G: Facile synthesis of boronate-decorated polyethyleneimine-grafted
hybrid magnetic nanoparticles for the highly selective enrichment
of modified nucleosides and ribosylated metabolites. Anal Chem.
85:11585–11592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bertucci A, Prasetyanto EA, Septiadi D,
Manicardi A, Brognara E, Gambari R, Corradini R and De Cola L:
Combined delivery of temozolomide and anti-miR221 PNA using
mesoporous silica nanoparticles induces apoptosis in resistant
glioma cells. Small. 11:5687–5695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Razak AA, Masthanamma S, Omshanthi B,
Suresh V and Obulamma P: Development and validation of UV method of
temozolomide in bulk and capsule formulation. Int J Pharm Sci Res.
4:14192013.
|
|
44
|
Ma Y, Tong S, Bao G, Gao C and Dai Z:
Indocyanine green loaded SPIO nanoparticles with phospholipid-PEG
coating for dual-modal imaging and photothermal therapy.
Biomaterials. 34:7706–7714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim JH, Ramasamy T, Tran TH, Choi JY, Cho
HJ, Yong CS and Kim JO: Polyelectrolyte complex micelles by
self-assembly of polypeptide-based triblock copolymer for
doxorubicin delivery. Asian J Pharm Sci. 9:191–198. 2014.
View Article : Google Scholar
|
|
46
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Niu C, Xu Y, An S, Zhang M, Hu Y, Wang L
and Peng Q: Near-infrared induced phase-shifted
ICG/Fe3O4 loaded PLGA nanoparticles for
photothermal tumor ablation. Sci Rep. 7:54902017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eruslanov E and Kusmartsev S:
Identification of ROS using oxidized DCFDA and flow-cytometry.
Advanced protocols in oxidative stress II Springer. 57–72. 2010.
View Article : Google Scholar
|
|
49
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sanfilippo S, Canis M, Ouchchane L,
Botchorishvili R, Artonne C, Janny L and Brugnon F: Viability
assessment of fresh and frozen/thawed isolated human follicles:
Reliability of two methods (Trypan blue and Calcein AM/ethidium
homodimer-1). J Assist Reprod Genet. 28:1151–1156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou S, Cui Z and Urban J: Dead cell
counts during serum cultivation are underestimated by the
fluorescent live/dead assay. Biotechnol J. 6:513–518. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tawakoli PN, Al-Ahmad A, Hoth-Hannig W,
Hannig M and Hannig C: Comparison of different live/dead stainings
for detection and quantification of adherent microorganisms in the
initial oral biofilm. Clin Oral Investig. 17:841–850. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Grudzinski IP, Bystrzejewski M, Cywinska
MA, Kosmider A, Poplawska M, Cieszanowski A and Ostrowska A:
Cytotoxicity evaluation of carbon-encapsulated iron nanoparticles
in melanoma cells and dermal fibroblasts. J Nanopart Res.
15:18352013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yan F, Wu H, Liu H, Deng Z, Liu H, Duan W,
Liu X and Zheng H: Molecular imaging-guided
photothermal/photodynamic therapy against tumor by iRGD-modified
indocyanine green nanoparticles. J Control Release. 224:217–228.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dong Z, Gong H, Gao M, Zhu W, Sun X, Feng
L, Fu T, Li Y and Liu Z: Polydopamine nanoparticles as a versatile
molecular loading platform to enable imaging-guided cancer
combination therapy. Theranostics. 6:1031–1042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Matés JM and Sánchez-Jiménez FM: Role of
reactive oxygen species in apoptosis: Implications for cancer
therapy. Int J Biochem Cell Biol. 32:157–170. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Aioub M, Panikkanvalappil SR and El-Sayed
MA: Platinum-coated gold nanorods: Efficient reactive oxygen
scavengers that prevent oxidative damage toward healthy, untreated
cells during plasmonic photothermal therapy. ACS Nano. 11:579–586.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Oliva CR, Moellering DR, Gillespie GY and
Griguer CE: Acquisition of chemoresistance in gliomas is associated
with increased mitochondrial coupling and decreased ROS production.
PLoS One. 6:e246652011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu B, Li C, Xing B, Yang P and Lin J:
Multifunctional simpleUCNPs@PDA-ICG nanocomposites
for upconversion imaging and combined photothermal/photodynamic
therapy with enhanced antitumor efficacy. J Mater Chem B.
4:4884–4894. 2016. View Article : Google Scholar
|
|
61
|
Stupp R, van den Bent MJ and Hegi ME:
Optimal role of temozolomide in the treatment of malignant gliomas.
Curr Neurol Neurosci Rep. 5:198–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Samali A, Holmberg CI, Sistonen L and
Orrenius S: Thermotolerance and cell death are distinct cellular
responses to stress: Dependence on heat shock proteins. FEBS Lett.
461:306–310. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Reed JC: Apoptosis-targeted therapies for
cancer. Cancer Cell. 3:17–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mohammadinejad R, Moosavi MA, Tavakol S,
Vardar DÖ, Hosseini A, Rahmati M, Dini L, Hussain S, Mandegary A
and Klionsky DJ: Necrotic, apoptotic and autophagic cell fates
triggered by nanoparticles. Autophagy. 15:4–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mocan L, Matea C, Tabaran FA, Mosteanu O,
Pop T, Mocan T and Iancu C: Photothermal treatment of liver cancer
with albumin-conjugated gold nanoparticles initiates Golgi
Apparatus-ER dysfunction and caspase-3 apoptotic pathway activation
by selective targeting of Gp60 receptor. Int J Nanomed.
10:5435–5445. 2015.
|
|
67
|
Hongmei Z: Extrinsic and intrinsic
apoptosis signal pathway review. Apoptosis and Medicine In Tech
Open. 2012. View
Article : Google Scholar
|
|
68
|
Haupt S, Berger M, Goldberg Z and Haupt Y:
Apoptosis-the p53 network. J Cell Sci. 116:4077–4085. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Perfettini JL, Reed JC, Israël N, Martinou
JC, Dautry-Varsat A and Ojcius DM: Role of Bcl-2 family members in
caspase-independent apoptosis during Chlamydia infection. Infect
Immun. 70:55–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yee C, Yang W and Hekimi S: The intrinsic
apoptosis pathway mediates the pro-longevity response to
mitochondrial ROS in C. elegans. Cell. 157:897–909. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Andón FT and Fadeel B: Programmed cell
death: Molecular mechanisms and implications for safety assessment
of nanomaterials. Acc Chem Res. 46:733–742. 2012. View Article : Google Scholar : PubMed/NCBI
|