Introduction
The Wnt/β-catenin signaling pathway plays critical
roles in embryonic development and tissue homeostasis, and aberrant
Wnt signaling has been implicated in the pathogenesis of many
different human cancers, including breast cancers (1–4).
Therefore, this signaling pathway represents a target for the
development of anticancer therapeutics. Wnt/β-catenin signaling
involves multiple proteins and binding events. It is initiated by
binding of Wnt ligands to frizzled receptor (Fzd) as well as the
co-receptor low-density lipoprotein receptor-related protein 5/6
(LRP5/6). This binding, results in activation of Wnt signaling,
permitting additional binding of dishevelled (DVL), which triggers
phosphorylation of LRP5/6 at one or more cytoplasmic motifs.
Subsequently, phosphorylated LRP5/6 enhances the interaction
between DVL and Axin, which destabilizes the β-catenin destruction
complex composed of Axin, adenomatous polyposis coli (APC), casein
kinase 1 (CK1), and glycogen synthase kinase 3β (GSK-3β) (5). The destruction complex can phosphorylate
β-catenin, which is the central mediator of canonical Wnt signaling
through GSK3β, and thereby induce the degradation of β-catenin by
the ubiquitin-proteasome pathway (6).
Disaggregation of the destruction complex results in inhibition of
β-catenin phosphorylation, which leads to cytoplasmic β-catenin
accumulation. Upon reaching a sufficiently high cytoplasmic
concentration, β-catenin translocates to the nucleus where it
promotes expression of Wnt target genes, such as CD44, cyclin D1,
c-Myc, survivin and fibronectin (7–9).
Emetine, a natural alkaloid isolated from
Psychotria ipecacuanha, has been revealed to inhibit the
synthesis of various biomolecules (10,11) and
used to treat amoebiasis since the early 1900s (12). Phase I and II clinical trials
evaluating the anticancer efficacy of emetine were conducted by the
National Cancer Institute in the mid-1970s (13–16).
However, these clinical studies did not lead to the clinical
application of emetine as they revealed only marginal efficacy and
some adverse side effects, such as cardiac damage. In recent years,
derivatives of emetine have been synthesized and reported to offer
better efficacy against cancer cells along with less toxicity to
normal cells (17,18). Moreover, various studies have
demonstrated a potent cytotoxic activity of emetine and its
biological targets in a variety of human carcinoma cell lines
(19–24). Emetine has been investigated in
combination with other agents for evaluation of their synergistic
antitumor effects toward the goal of achieving effective treatment
with a reduced dose and fewer side effects (25,26).
Specifically, Visnyei et al identified emetine as an
inhibitor of glioblastoma stem cells using a molecular screening
system (27). Another study
demonstrated that emetine inhibits the hedgehog signaling pathway
by binding to hedgehog, smoothened and Gli protein, which have been
implicated in the biology of cancer stem cells (CSCs) (28). However, the molecular mechanism by
which emetine targets CSCs remains unclear, and such knowledge
could facilitate the potential application of emetine and its
structural modifications in cancer chemotherapy. Therefore, in the
present study, the effects of emetine on Wnt signaling were
investigated in multiple breast cancer cell lines. The present
results revealed emetine as a novel Wnt/β-catenin signaling
antagonist that suppresses the phosphorylation of LRP6 and
dishevelled-2 (DVL2).
Materials and methods
Reagents and plasmids
Emetine was purchased from Sigma-Aldrich; Merck
KGaA. Emetine was dissolved in dimethyl sulfoxide (DMSO) for
preparation of a stock solution at a concentration of 10 mM. For
use with cells, the stock solution was diluted with the
cell-specific media, and the final DMSO concentration was <0.1%.
The SuperTOPFlash reporter plasmid was a kind gift from Dr Karl
Willert (University of California at San Diego, San Diego, CA,
USA). The expression plasmids for Wnt1, LRP6, CK1, DVL2, β-catenin,
and β-galactosidase (β-gal) have been previously described
(29,30).
Cell culture
293T, MDA-MB-231, MDA-MB-468, Hs578T, and MCF10A
cells were obtained from the American Type Culture Collection
(ATCC). 293T and Hs578T cells were cultured in Dulbecco's Modified
Eagle's Medium (DMEM) (Gibco; Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin in 5% CO2 at 37°C. MDA-MB-231
and MDA-MB-468 cells were grown in Leibovitz's L-15 medium (Gibco;
Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS and 1% penicillin-streptomycin at 37°C in a humidified
incubator without CO2. MCF10A cells were cultured in
DMEM/Ham's F-12 (Gibco; Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 100 ng/ml cholera toxin, 20 ng/ml epidermal
growth factor (EGF), 0.01 mg/ml insulin, 500 ng/ml hydrocortisone,
and 5% chelex-treated horse serum. All of the growth factors were
purchased from Sigma-Aldrich (Merck KGaA).
Luciferase reporter gene assay
293T cells were transfected with reporter plasmid
(0.25 µg), control plasmid pCMX bgal (50 ng), and the indicated
expression plasmids (50–200 ng) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. After transfection for 24 h, the cells
were treated with emetine at concentrations of 0 (control), 6.25,
12.5, 25, 50 or 100 nM for the indicated culture time-points.
Luciferase assays were performed using a luciferase assay kit
(Promega Corp.) and the luciferase activity was normalized to β-gal
activity.
Immunoblot analyses
Cells were harvested and sonicated in lysis buffer
(20 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1%
Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol
phosphate, 1 mM sodium orthovanadate, 2 µg/ml leupeptin and 1 mM
PMSF) containing the protease inhibitor phenylmethylsulfonyl
fluoride, and protein concentrations were determined using a BCA
protein assay kit (Cell Signaling Technology, Inc.). Equal amount
of proteins (40 µg) were loaded in a 8% sodium dodecyl sulfate
(SDS)-polyacrylamide gel, and transferred to polyvinylidene
difluoride (PVDF) membranes for immunoblotting with anti-phospho
LRP6 (Ser1490) (dilution 1:1,000; cat. no. 2560s), anti-LRP6
(dilution 1:1,000; cat. no. 2568L), anti-DVL2 (dilution 1:1,000;
cat. no. 3216s), anti-non-phospho (active) β-catenin (dilution
1:2,000; cat. no. 8814s; all from Cell Signaling Technology, Inc.),
and anti-β-catenin (dilution 1:2,000; cat. no. sc-7963; Santa Cruz
Biotechnology, Inc.), anti-β-actin (dilution 1:5,000; cat. no.
HC-201; TransGen Biotech). After transferring, the PVDF membranes
were blocking using 5% non-fat powdered milk (cat. no.
A600669-0250; Sangon Biotech Co., Ltd.) at room temperature for 1
h. Then the PVDF membranes were incubated with HRP conjugated goat
anti-mouse (dilution 1:10,000; cat. no. A16066; Thermo Fisher
Scientific, Inc.) or anti-rabbit (dilution 1:10,000; cat. no.
A16096; Thermo Fisher Scientific, Inc.) IgG for 1 h at room
temperature. After incubated with ECL Plus Western Blotting
Substrate (cat. no. 32132; Thermo Fisher Scientific, Inc.), the
immunoblots were developed by either X-ray film (Kodak) or
Chemiluminescent Imaging System (cat. no. 5200; Tanon).
Densitometric analysis was carried out using the ImageJ 1.8.0
Analysis Software (National Institutes of Health), and the
quantification results were normalized to the loading control.
Real-time polymerase chain reaction
(PCR) analyses
Total RNA was isolated using RNAiso Plus (Takara
Bio, Inc.) and reverse-transcribed into cDNA using the Primescript
RT reagent kit (Takara Bio, Inc.) according to the manufacturer's
instructions (37°C, 15 min; 85°C, 5 sec). Prepared cDNA was then
used for the quantitative PCR analysis (95°C, 5 min; 95°C, 15 sec,
60°C, 1 min) using FastStart Universal SYBR-Green Master (Roche
Applied Science). The primers used were as follows: Fibronectin
sense, 5′-ACCTACGGATGACTCGTGCTTT-3′ and antisense,
5′-TTCAGACATTCGTTCCCACTCA-3′; frizzled-7 (Fzd7) sense,
5′-CAACGGCCTGATGTACTTTAAGG-3′ and antisense,
5′-CATGTCCACCAGGTAGGTGAGA-3′; c-Myc sense, GCCACGTCTCCACACATCAG and
antisense, TCTTGGCAGCAGGATAGTCCTT; CD133 sense,
5′-AGTCGGAAACTGGCAGATAGC-3′ and antisense,
5′-GGTAGTGTTGTACTGGGCCAAT-3′; and Nanog sense,
5′-TTTGTGGGCCTGAAGAAAACT-3′ and antisense,
5′-AGGGCTGTCCTGAATAAGCAG-3′.
Lentiviral shRNA
The sequences of β-catenin shRNAs were as follows:
shβ-cat#1:
CCGGTTGTTATCAGAGGACTAAATACTCGAGTATTTAGTCCTCTGATAACAATTTTTG;
shβ-cat#2:
CCGGAGGTGCTATCTGTCTGCTCTACTCGAGTAGAGCAGACAGATAGCACCTTTTTT. For
infection with lentivirus, the cells were cultured with lentiviral
solution for 24 h in the presence of 5 µg/ml Polybrene
(Sigma-Aldrich; Merck KGaA).
Cell viability assay
MDA-MB-231 and MDA-MB-468 cells were seeded at
1×104 cells/well in 96-well plates and allowed to
incubate overnight. The cells were treated with emetine at
concentrations of 0 (control), 12.5, 25, 50, 10 and 200 nM for 48
h. MTT reagent (5 mg/ml; 20 µl/well) was added and incubated for
another 4 h. The formazan crystals were dissolved in 150 µl DMSO,
and the absorbance of the formazan solution was measured at 570
nm.
Apoptosis assay
After treatment with emetine (0–100 nM) for 24 h,
MDA-MB-231, MDA-MB-468, and MCF10A cells were collected and
incubated with Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) solutions (TransGen Biotech Co., Ltd.)
according to the manufacturer's protocol. A FACSCalibur™ (BD
Biosciences) fluorescence-activated cell-sorting (FACS) instrument
was used for quantitative fluorescence sorting, and FlowJo v10.0.8
(Tree Star, Inc.) was used for subsequent analysis.
Scratch assay
MDA-MB-231 cells were cultured in a 24-well plate,
and a 200-µl sterile pipette tip was passed through the cell
monolayer to create a wound gap. Then, the cells were incubated
with DMSO or 50 or 100 nM emetine for 24 h. Micrographs of the
scratched areas were captured using an Olympus CKX53 microscope
(Olympus Corp.).
Transwell assay
Cell migration and invasion were assessed using
Transwell assays as previously described (31). Briefly, 2×105 cells
suspended in serum-free medium were seeded in 24-well Transwell
chambers with 8-µm pore membranes. Emetine at 50 or 100 nM was
added to the top chamber, and DMEM containing 20% FBS was added to
the lower chamber as a chemoattractant. After 12 h of incubation,
the cells on the upper surface of the membrane were wiped away, and
the cells that had migrated to the lower surface of the membrane
were stained with 0.1% crystal violet and photographed under a
light microscope. For the invasion assay, the Transwell chambers
were coated with Matrigel (Corning Life Sciences). For quantitative
analysis, 33% acetic acid was used to elute the stained cells, and
the absorbance of the resultant solutions was detected at 570
nm.
Sphere formation assay
Hs578T cells were seeded at 250 cells/well in DMEM
medium [2% B-27, 10 ng/ml EGF, 10 ng/ml fibroblast growth factor
(FGF), and 10 µg/ml insulin] containing emetine (50 or 100 nM) in a
24-well Ultra-Low Attachment plate (Corning Inc.). All of the
growth factors were purchased from Sigma-Aldrich (Merck KGaA).
After 10 days in culture, spheres with a diameter greater than 50
µm were counted, and representative fields were photographed under
a light microscope. Each treatment was applied to three
replicates.
Statistical analyses
Statistical analyses were performed using GraphPad
Prism software (v5.0; GraphPad Software). The normal probability
plot was used to examine data distributions. A Student's t-test was
applied when the data exhibited normal distribution. The data were
analyzed by Student's t-test or one-way analysis of variance
(ANOVA) followed by Dunnett's t-test. A P-value <0.05 was
considered to indicate a statistically significant difference. Data
are presented as the mean ± standard deviation (SD), and are
derived from at least three independent assays, unless specified
otherwise.
Results
Emetine suppresses Wnt/β-catenin
signaling
The cell-based TOPFlash reporter system was used for
an initial screen of Food and Drug Administration (FDA)-approved
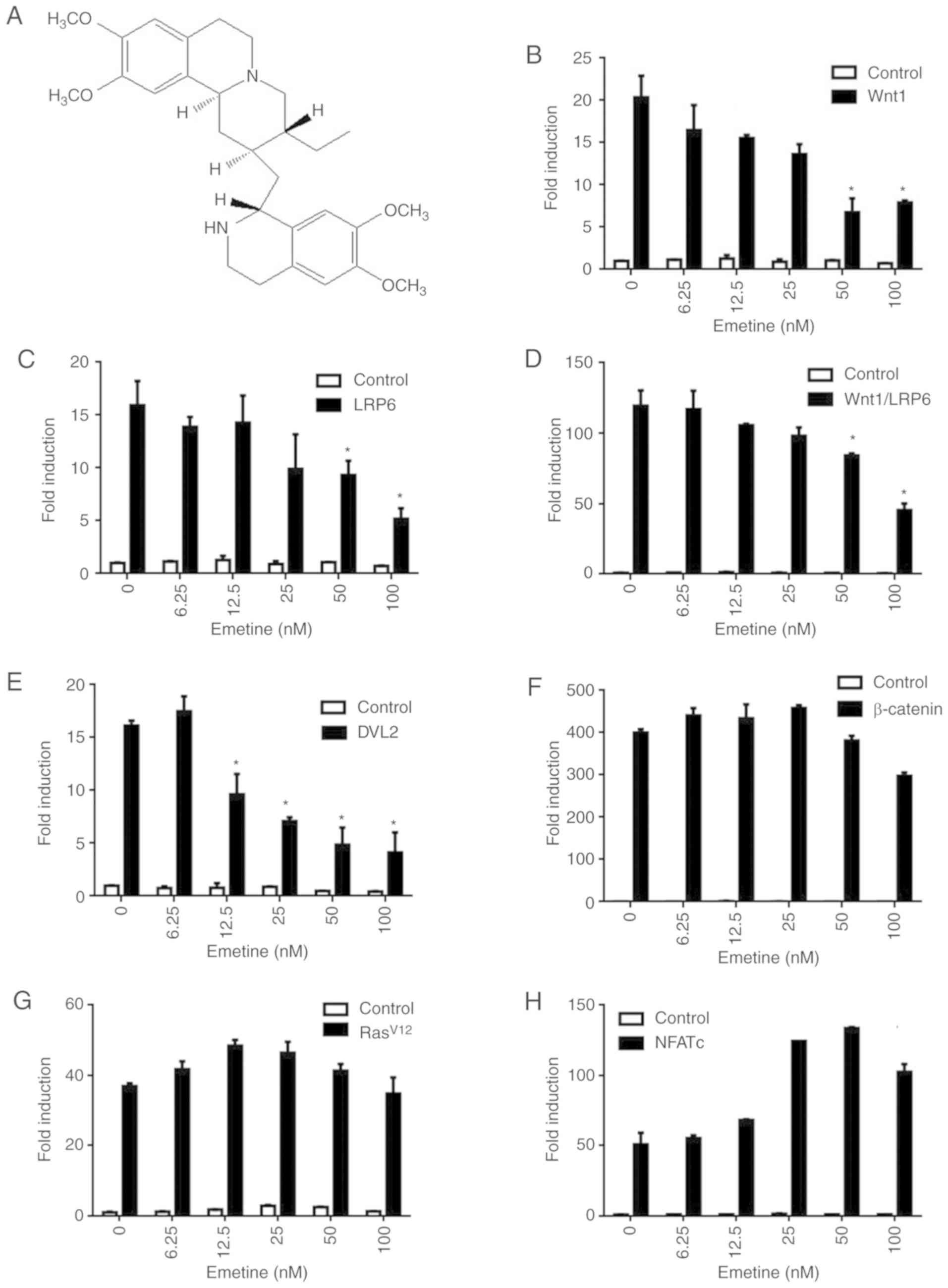
drug libraries. Emetine (Fig. 1A) was
identified as a potential inhibitor of Wnt/β-catenin signaling. To
assess the effect of emetine on Wnt signaling, 293T cells were
transfected with a SuperTOPFlash reporter plasmid together with
Wnt1, LRP6, Wnt1/LRP6, DVL2, or β-catenin expression plasmids
(Fig. 1B-F). Treatment with 6.25–100
nM emetine effectively inhibited transcription of the SuperTOPFlash
reporter activated by Wnt1 (Fig. 1B),
LRP6 (Fig. 1C), Wnt1/LRP6 (Fig. 1D) and DVL2 (Fig. 1E) compared to 0-nm treated group,
while emetine had little effect on β-catenin-induced reporter
activity (Fig. 1F). These results
indicated that emetine may target upstream components of
Wnt/β-catenin signaling. In control experiments, Wnt inhibiting
concentrations of emetine had no effect on the luciferase activity
of an activator protein 1 (AP-1) reporter gene (Fig. 1G) or a nuclear factor of activated T
cells (NFAT) reporter gene (Fig.
1H).
Emetine affects different components
of the Wnt/β-catenin signaling cascade
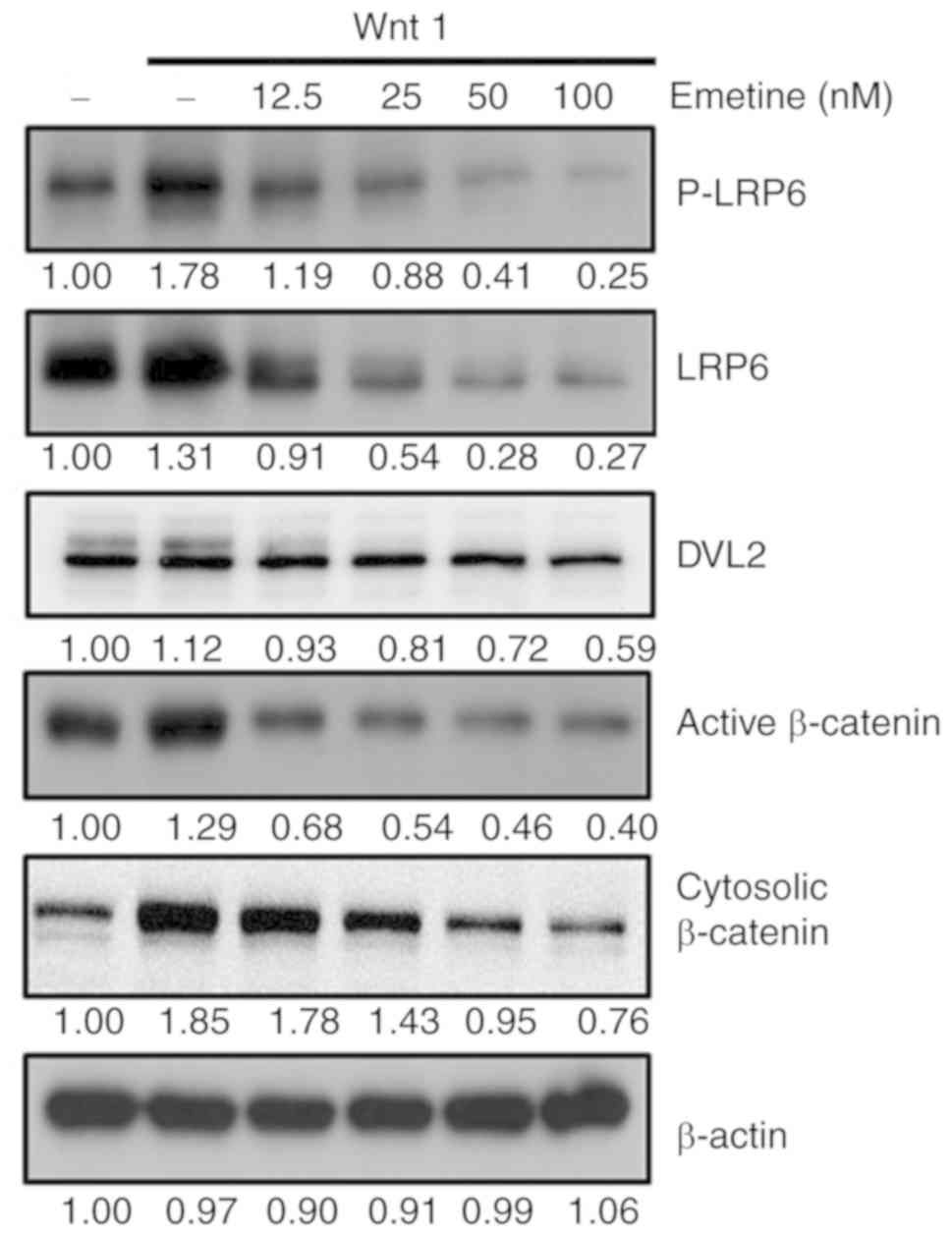
To further investigate the mechanism of the effect
of emetine on the Wnt pathway, 293T cells were transfected with a
Wnt1 expression plasmid. Overexpression of Wnt1 resulted in
enhanced levels of phosphorylated LRP6, total LRP6, DVL2, activated
β-catenin and cytosolic β-catenin (Fig.
2). Treatment with nanomolar concentrations of emetine
significantly decreased the expression levels of phosphorylated
LRP6, total LRP6, phosphorylated DVL2, active β-catenin and
cytosolic β-catenin (Fig. 2).
Notably, the extent of reduction in total and phosphorylated LRP6
was similar upon emetine treatment, suggesting that emetine may
downregulate LRP6 expression, but not its phosphorylation. These
findings indicated that emetine inhibits Wnt/β-catenin signaling by
targeting LPR6 and DVL2.
Emetine suppresses Wnt/β-catenin
signaling in breast cancer cells
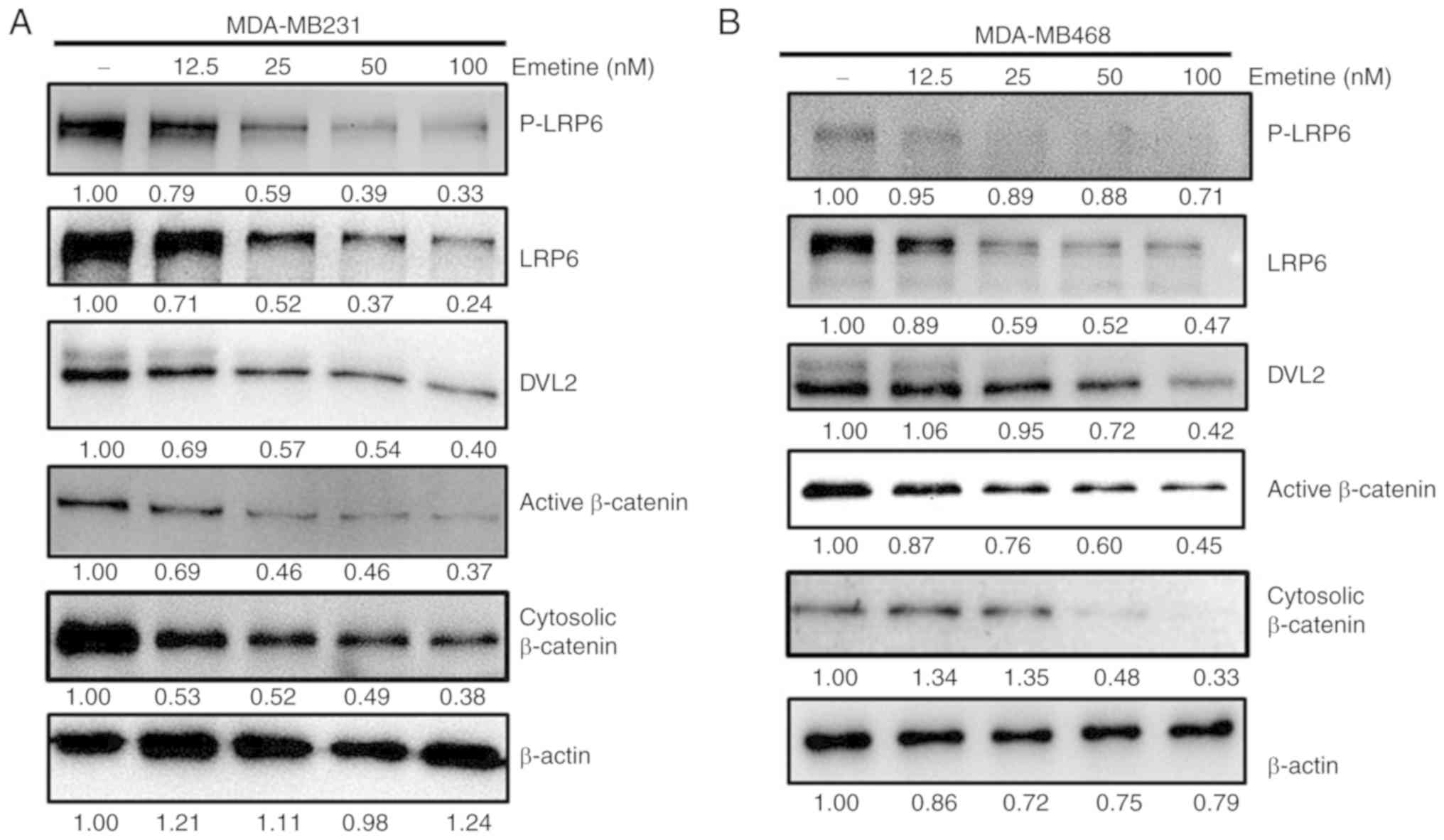
The effect of emetine on Wnt/β-catenin signaling was
next assessed in the MDA-MB-231 and MDA-MB-468 breast cancer cell
lines. Treatment with emetine decreased the levels of
phosphorylated LRP6, total LRP6, phosphorylated and
unphosphorylated DVL2, active β-catenin levels and cytosolic
β-catenin in both cell lines (Fig. 3A and
B). These results revealed that emetine suppressed
Wnt/β-catenin signaling in breast cancer cells.
Emetine downregulates the expression
of Wnt target genes in breast cancer cells
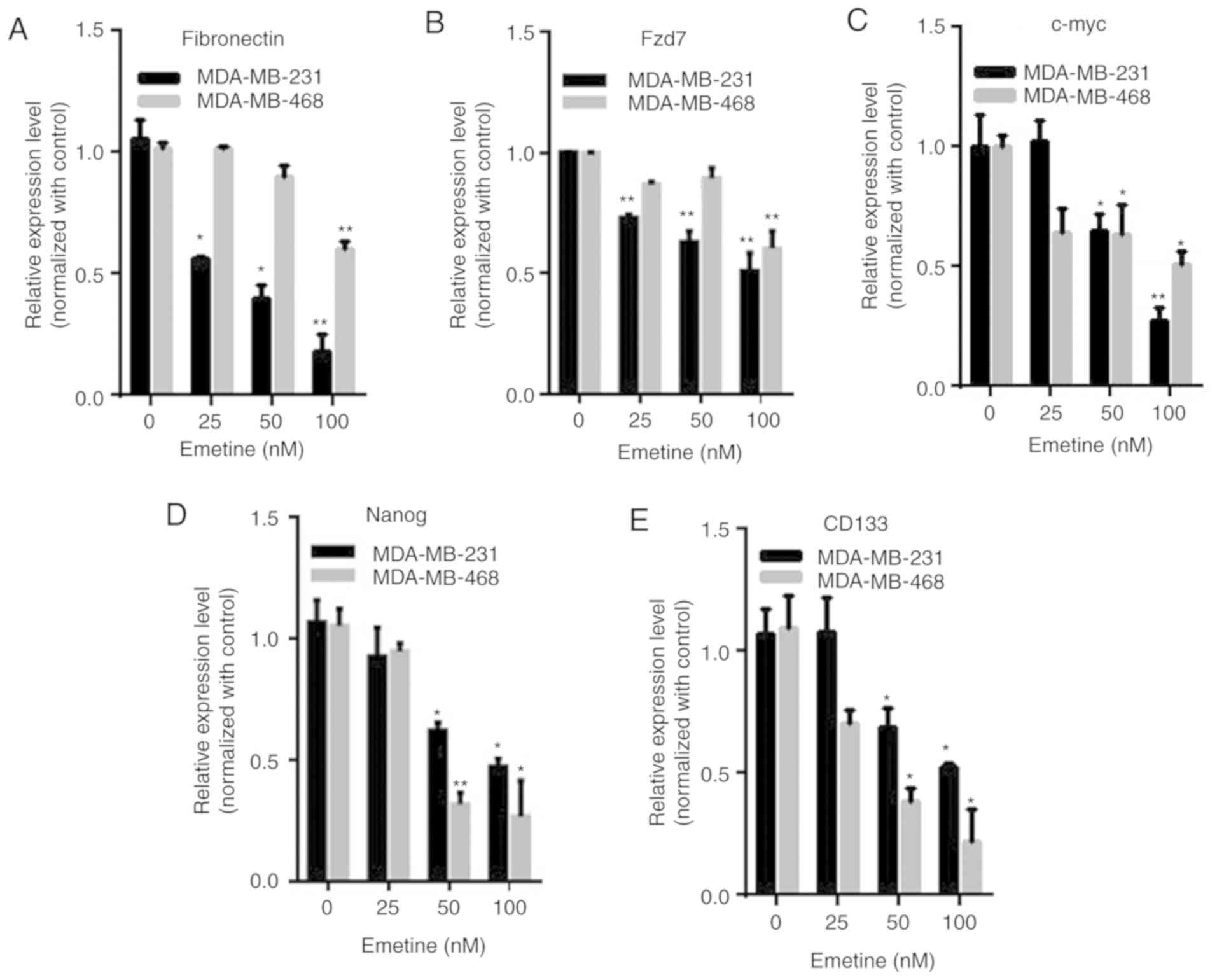
To further evaluate the inhibitory effect of emetine
on Wnt signaling in breast cancer cells, real-time PCR was
performed to detect the mRNA expression of several Wnt target
genes, including fibronectin, Fzd7, c-Myc, CD133 and Nanog. In
MDA-MB-231 and MDA-MB-468 cells, emetine dose-dependently
downregulated the transcription of fibronectin, Fzd7, c-Myc, CD133
and Nanog (Fig. 4). These results
further confirmed the suppression of Wnt signaling in breast cancer
cells treated with emetine.
Emetine inhibits the viability,
migration and invasion of breast cancer cells while inducing
apoptosis
An MTT assay was employed to examine the effect of
emetine on the viability of breast cancer cells. The results
revealed that treatment with emetine selectively reduced the
viability of breast cancer MDA-MB-231 and MDA-MB-468 cells, but had
little effect on MCF10A human mammary epithelial cells (Fig. 5A). The effect of emetine on apoptosis
was then assessed among breast cancer cells. Evaluation of
apoptosis among MDA-MB-231 and MDA-MB-468 cells after treatment
with increasing concentrations of emetine (0, 25, 50 or 100 nM) for
24 h, revealed that emetine selectively induced apoptotic cell
death in both breast cancer cell lines compared to MCF10A cells
(Fig. 5B).
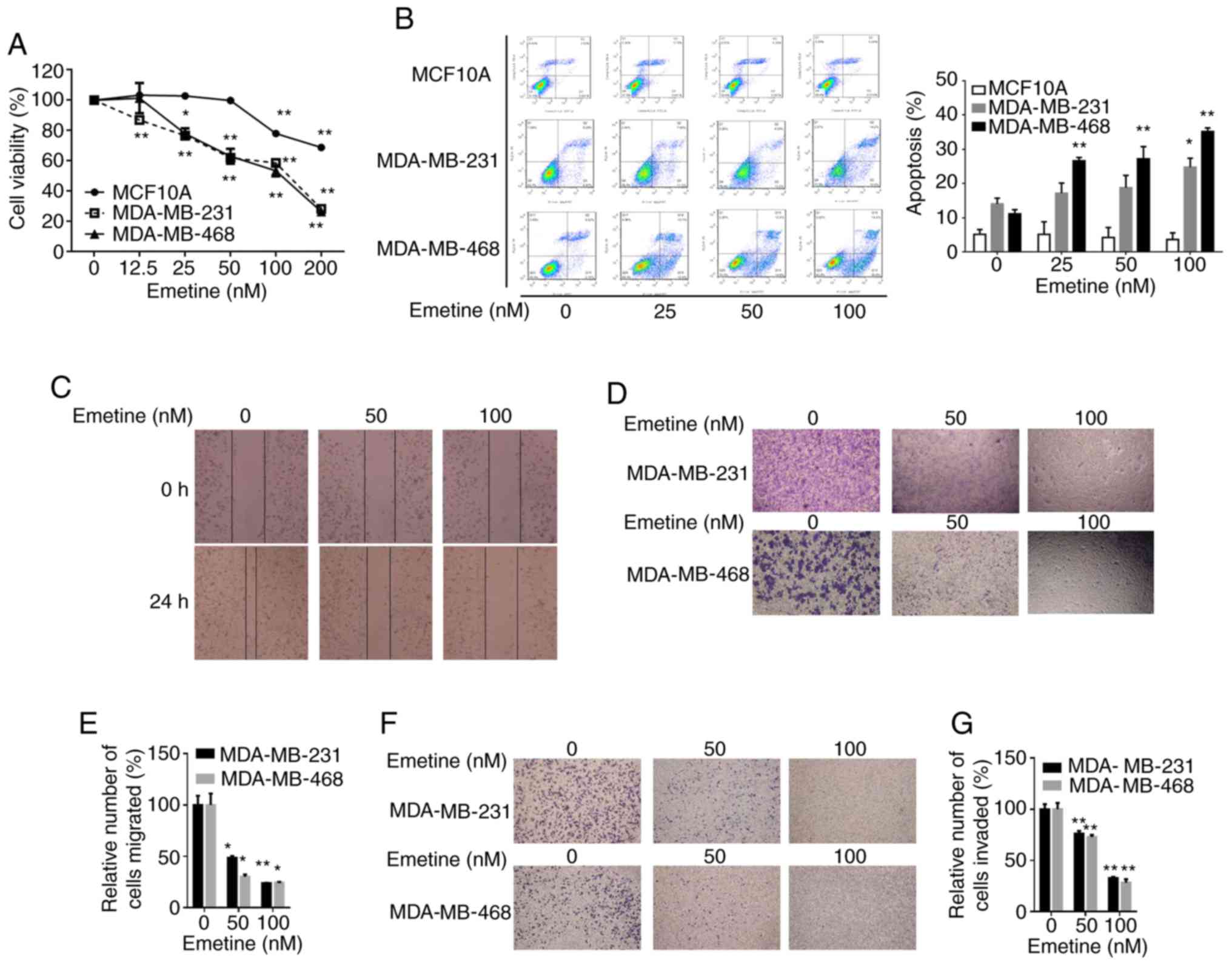 | Figure 5.Emetine promotes apoptosis and
suppresses viability, migration and invasion in breast cancer
cells, but not in MCF10A cells. MDA-MB-231, MDA-MB-468 and MCF10A
cells were treated with vehicle control (DMSO) or emetine at the
indicated concentrations for 24 h before evaluation of: (A) cell
viability using an MTT assay; (B) apoptosis by FACS; (C) migratory
ability in a scratch wound assay; (D) migratory ability in a
Transwell assay (upper image, MDA-MB-231 cells; lower image,
MDA-MB-468 cells), with quantified data presented in (E); and (F)
invasive ability in Matrigel-coated Transwells in the absence or
presence of the indicated amounts of emetine for 24 h (upper image,
MDA-MB-231 cells; lower image, MDA-MB-468 cells), with the
quantified data presented in (G). Data were collected from three
independent experiments (*P<0.05, **P<0.01). Statistical
analysis was conducted using one-way ANOVA followed by a Dunnett's
t-test (A). |
Considering the important role of the Wnt pathway in
cancer cell migration and invasion, the effects of emetine on the
migratory and invasive activities of breast cancer cells were
investigated. Results of an in vitro scratch assay revealed
that emetine treatment suppressed the migration of MDA-MB-231
breast cancer cells (Fig. 5C). The
inhibitory effects of emetine on the migration of MDA-MB-231 and
MDA-MB-468 cells were further confirmed by Transwell migration
assays (Fig. 5D and E). Using
Matrigel-coated chambers, the Transwell assays were repeated to
assess the effect of emetine on invasion by MDA-MB-231 and
MDA-MB-468 cells. Treatment with emetine significantly reduced the
numbers of cells that penetrated the membranes to reach the bottom
wells compared with the vehicle control (Fig. 5F and G). Collectively, these results
demonstrated that emetine suppressed the migratory and invasive
abilities of breast cancer cells in vitro.
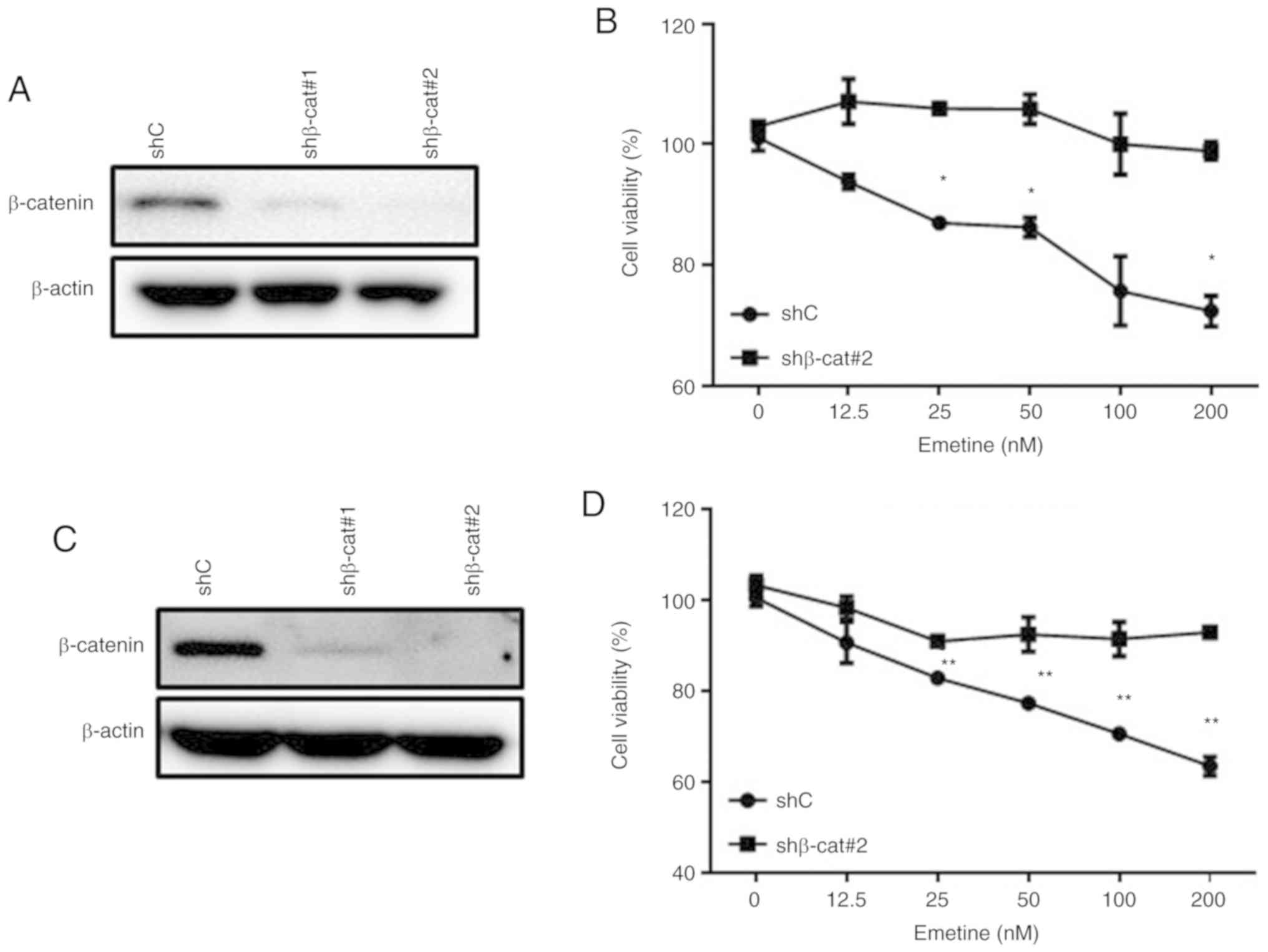
Emetine-induced reduction of cell
viability is abrogated by shRNA-mediated silencing of
β-catenin
To determine the effect of emetine on cells in which
Wnt/β-catenin signaling was already inhibited, lentivirus-mediated
shRNAs were used to suppress the expression of β-catenin, a central
mediator of the canonical Wnt/β-catenin signaling pathway. The
emetine-induced reductions in the viability of MDA-MB-231 cells
(Fig. 6A and B) and MDA-MB-468 cells
(Fig. 6C and D) were decreased after
shRNA-mediated silencing of β-catenin. These data indicated that
the cytotoxicity of emetine in breast cancer cells was mediated at
least partly through the Wnt/β-catenin signaling pathway.
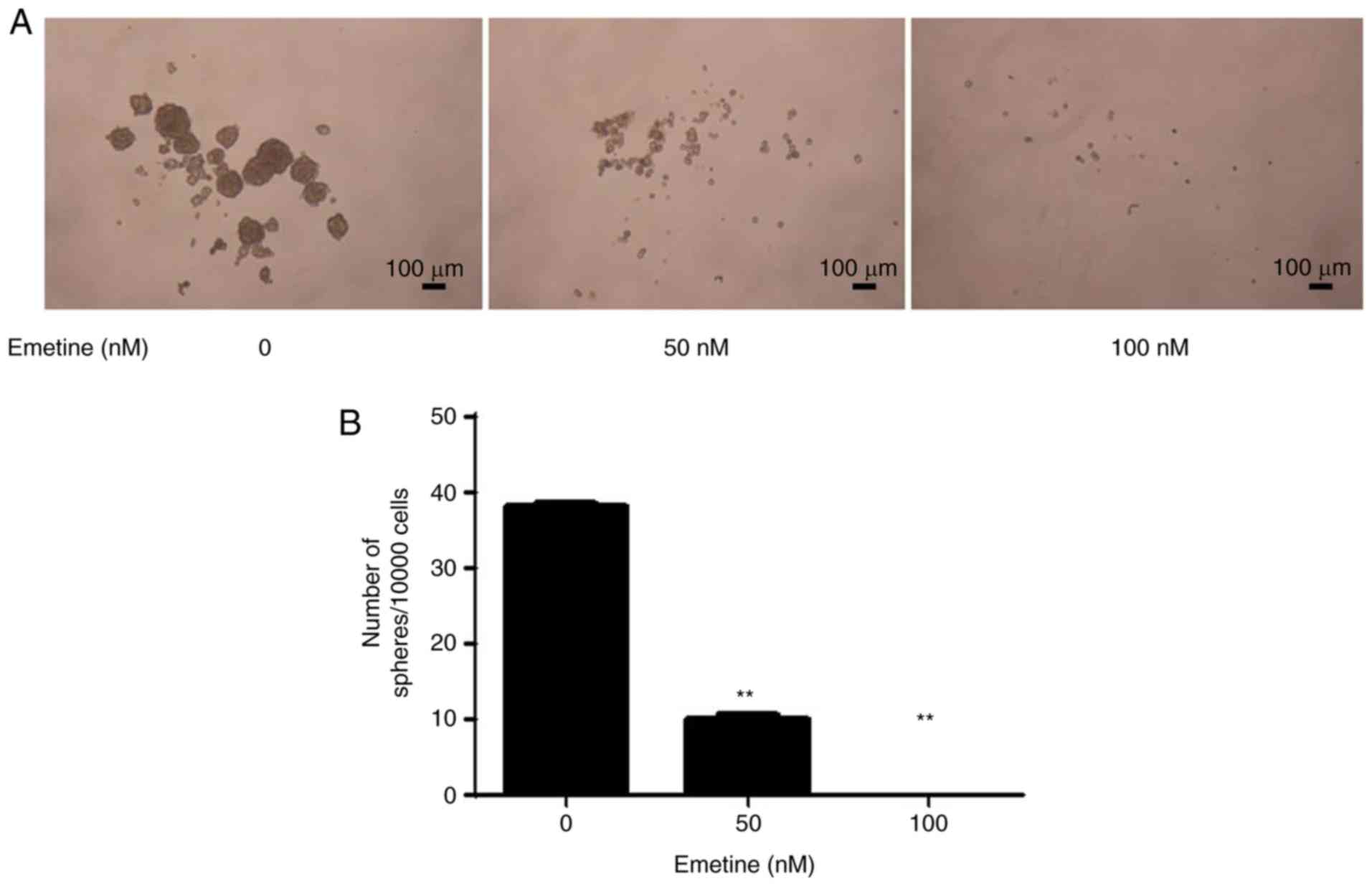
Emetine blocks the stemness of breast
cancer cells
Wnt/β-catenin signaling plays a crucial role in the
survival and maintenance of CSCs. A sphere formation assay was
performed to examine the effect of emetine on the stemness of
breast cancer cells. The breast cancer Hs578T cells were treated
with emetine at 50 and 100 nM for 10 days. As revealed in Fig. 7, treatment with emetine significantly
decreased the number and size of tumor spheres. In addition, the
mRNA expression levels of stemness marker genes (CD133 and Nanog)
in breast cancer cells were downregulated upon emetine treatment
(Fig. 4D and E).
Discussion
While the exact molecular mechanisms responsible for
the effective antitumor activity of emetine remained unclear,
previous research demonstrated that emetine can induce apoptosis
through prevention of protein biosynthesis (21), DNA interaction (32) and increasing of pro-apoptotic factors
(26,33). This compound was also reported to
reduce activation of hypoxia-induced factor-1α (HIF-1α) in breast
tumor cells (34). The results of the
present study revealed that emetine is a novel inhibitor of the Wnt
signaling pathway. In the present experiments, emetine reduced
phosphorylation of LRP6 and DVL2 and inhibited the expression of
Wnt target genes in multiple lines of breast cancer cells. Together
our results illustrated a novel mechanism for the antitumor
activity of emetine.
Aberrant activation of the Wnt/β-catenin pathway has
been implicated in the development of breast cancers (4), and primary cells from breast tumors as
well as breast cancer cell lines were revealed to express several
Wnt ligands and Fzd receptors (35,36).
Previous studies also demonstrated that LRP6 expression is
upregulated in human breast cancer cells (37). Moreover, upregulation of DVL and
phosphorylated DVL proteins has been revealed in several breast
tumor cell lines (38,39). In the present study, it was revealed
that emetine decreased the phosphorylation of LRP6 and DVL2 as well
as the activation of β-catenin in breast cancer cells, which
resulted in effective inhibition of Wnt signaling. Notably, the
inhibitory effect of emetine on viability of breast cancer cells
were abolished in β-catenin-knockdown cells (Fig. 6). Additionally, the effects of emetine
on Wnt signaling occurred at concentrations comparable to those
required for inhibiting viability, migration and invasion, and
inducing apoptosis in breast cancer cells. These results indicated
that the antitumor activity of emetine was associated with its
inhibitory effects on the Wnt signaling pathway.
CSCs have been defined as tumor-initiating cells
that play critical roles in tumor development, recurrence and
treatment resistance (40). The
Wnt/β-catenin pathway is recognized to be important in the
regulation of CSC biology (41), and
thus, blocking the Wnt/β-catenin pathway could potentially
eliminate CSC populations, resulting in complete cure of a cancer.
In support of this hypothesis, one study revealed that emetine
inhibits the stemness of glioblastoma stem cells (27). Several Wnt target genes, including
Nanog and CD133, have been established as CSC markers (42–44). In
the present study, it was observed that the expression of stemness
marker genes Nanog and CD133 was reduced in breast cancer cells
following emetine treatment. Emetine also suppressed the sphere
formation of breast cancer cells. These results indicated that
emetine may have the potential of inhibiting breast cancer stem
cells. Collectively, the results of the present study indicated
that emetine may be a promising therapeutic agent against breast
CSCs. However, further research is required to fully characterize
the inhibitory action of emetine on breast CSCs. The
CD44+/CD24− breast CSCs will be isolated from
human breast cancer cell lines and primary breast cancer tissues.
Emetine effects on breast CSC activity will be examined using
sphere formation assay. The breast CSC xenograft models will be
generated by implanting these CD44+/CD24−
breast CSCs. The effect of emetine on the in vivo
tumor-seeding ability of breast CSCs will be assessed using breast
CSC xenograft mice.
In recent years, derivatives of emetine achieved via
structural modifications have also been revealed to have antitumor
effects in various cancers (45). For
example, novel emetine dithiocarbamate analogs were synthesized and
revealed to have anti-tumorigenic activity against prostate cancer
cells as well as minimal toxicity to normal prostate cells
(46). It will be interesting to
assess whether these novel emetine analogs also inhibit the
Wnt/β-catenin signaling cascade in future studies.
Acknowledgements
The authors would like to thank the Cancer Research
Center, Department of Pharmacology, and Shenzhen University Health
Science Center for providing the facilities used to carry out this
study.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 81802662), the Nature
Science Foundation of Guangdong Province (grant no.
2017A030310329), the Medical Science and Technology Research
Foundation of Guangdong Province (grant no. A2019475), the Shenzhen
Basic Research Program (grant no. JCYJ20170817094611664), the
Shenzhen Peacock Plan (grant nos. 827000183 and 827000186), the
Shenzhen Peacock Innovation Team Project (grant no.
KQTD20140630100658078), and the Shenzhen University Research
Project (grant nos. 2016085 and 2017087).
Availability of data and materials
Data and materials are available upon request to the
corresponding author.
Authors' contributions
QS performed the research, analyzed the data and
wrote the manuscript. QF, SLi, JL and SLiu performed the research.
ZW, ZS, JS and DL analyzed and interpreted the data. DL designed
the research, analyzed and interpreted the data, and wrote the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
There authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
APC
|
adenomatosis polyposis coli
|
|
CK1
|
casein kinase 1
|
|
CSCs
|
cancer stem cells
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
DVL
|
dishevelled
|
|
EGF
|
epidermal growth factor
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FBS
|
fetal bovine serum
|
|
FGF
|
fibroblast growth factor
|
|
Fzd
|
frizzled
|
|
GSK3β
|
glycogen synthase kinase-3β
|
|
LRP5/6
|
low-density lipoprotein
receptor-related protein5/6
|
|
PVDF
|
polyvinylidene difluoride
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
References
|
1
|
Boras-Granic K and Hamel PA:
Wnt-signalling in the embryonic mammary gland. J Mammary Gland Biol
Neoplasia. 18:155–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pohl SG, Brook N, Agostino M, Arfuso F,
Kumar AP and Dharmarajan A: Wnt signaling in triple-negative breast
cancer. Oncogenesis. 6:e3102017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morgan RG, Ridsdale J, Tonks A and Darley
RL: Factors affecting the nuclear localization of β-catenin in
normal and malignant tissue. J Cell Biochem. 115:1351–1361. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
MacDonald BT and He X: Frizzled and LRP5/6
receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect
Biol. 4:a0078802012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu G, Huang H, Garcia Abreu J and He X:
Inhibition of GSK3 phosphorylation of beta-catenin via
phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One.
4:e49262009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng X, Tamai K, Doble B, Li S, Huang H,
Habas R, Okamura H, Woodgett J and He X: A dual-kinase mechanism
for Wnt co-receptor phosphorylation and activation. Nature.
438:873–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akinboye ES, Rosen MD, Denmeade SR,
Kwabi-Addo B and Bakare O: Design, synthesis, and evaluation of
pH-dependent hydrolyzable emetine analogues as treatment for
prostate cancer. J Med Chem. 55:7450–7459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grollman AP: Inhibitors of protein
biosynthesis. V. Effects of emetine on protein and nucleic acid
biosynthesis in HeLa cells. J Biol Chem. 243:4089–4094.
1968.PubMed/NCBI
|
|
12
|
Lambert AC: The treatment of amoebic
dysentery with emetine and bismuth iodide. Br Med J. 1:116–118.
1918. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kane RC, Cohen MH, Broder LE, Bull MI,
Creaven PJ and Fossieck BE Jr: Phase I–II evaluation of emetine
(NSC-33669) in the treatment of epidermoid bronchogenic carcinoma.
Cancer Chemother Rep. 59:1171–1172. 1975.PubMed/NCBI
|
|
14
|
Mastrangelo MJ, Grage TB, Bellet RE and
Weiss AJ: A phase I study of emetine hydrochloride (NSC 33669) in
solid tumors. Cancer. 31:1170–1175. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Panettiere F and Coltman CA Jr: Experience
with emetine hydrochloride (NSC 33669) as an antitumor agent.
Cancer. 27:835–841. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siddiqui S, Firat D and Olshin S: Phase II
study of emetine (NSC-33669) in the treatment of solid tumors.
Cancer Chemother Rep. 57:423–428. 1973.PubMed/NCBI
|
|
17
|
Akinboye ES, Bamji ZD, Kwabi-Addo B, Ejeh
D, Copeland RL, Denmeade SR and Bakare O: Design, synthesis and
cytotoxicity studies of dithiocarbamate ester derivatives of
emetine in prostate cancer cell lines. Bioorg Med Chem.
23:5839–5845. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akinboye ES, Rosen MD, Bakare O and
Denmeade SR: Anticancer activities of emetine prodrugs that are
proteolytically activated by the prostate specific antigen (PSA)
and evaluation of in vivo toxicity of emetine derivatives. Bioorg
Med Chem. 25:6707–6717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bicknell GR, Snowden RT and Cohen GM:
Formation of high molecular mass DNA fragments is a marker of
apoptosis in the human leukaemic cell line, U937. J Cell Sci.
107:2483–2489. 1994.PubMed/NCBI
|
|
20
|
Moller M, Weiss J and Wink M: Reduction of
cytotoxicity of the alkaloid emetine through P-glycoprotein
(MDR1/ABCB1) in human Caco-2 cells and leukemia cell lines. Planta
Μed. 72:1121–1126. 2006.
|
|
21
|
Moller M and Wink M: Characteristics of
apoptosis induction by the alkaloid emetine in human tumour cell
lines. Planta Μed. 73:1389–1396. 2007.
|
|
22
|
Rosenkranz V and Wink M: Alkaloids induce
programmed cell death in bloodstream forms of trypanosomes
(Trypanosoma b. brucei). Molecules. 13:2462–2473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Watanabe N, Iwamoto T, Dickinson DA, Iles
KE and Forman HJ: Activation of the mitochondrial caspase cascade
in the absence of protein synthesis does not require c-Jun
N-terminal kinase. Arch Biochem Biophys. 405:231–240. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kong HS, Lee S, Beebe K, Scroggins B,
Gupta G, Lee MJ, Jung YJ, Trepel J and Neckers L: Emetine promotes
von Hippel-Lindau-independent degradation of hypoxia-inducible
factor-2α in clear cell renal carcinoma. Mol Pharmacol.
78:1072–1078. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Foreman KE, Jesse JN III, Kuo PC and Gupta
GN: Emetine dihydrochloride: A novel therapy for bladder cancer. J
Urol. 191:502–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Q, Yogosawa S, Iizumi Y, Sakai T and
Sowa Y: The alkaloid emetine sensitizes ovarian carcinoma cells to
cisplatin through downregulation of bcl-xL. Int J Oncol.
46:389–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Visnyei K, Onodera H, Damoiseaux R,
Saigusa K, Petrosyan S, De Vries D, Ferrari D, Saxe J, Panosyan EH,
Masterman-Smith M, et al: A molecular screening approach to
identify and characterize inhibitors of glioblastoma stem cells.
Mol Cancer Ther. 10:1818–1828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mayank and Jaitak V: Molecular docking
study of natural alkaloids as multi-targeted hedgehog pathway
inhibitors in cancer stem cell therapy. Comput Biol Chem.
62:145–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl
Acad Sci USA. 108:13253–13257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M,
Leoni LM, Kipps TJ, Corr M and Carson DA: Activation of the Wnt
signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad
Sci USA. 101:3118–3123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mandal CC and Ghosh-Choudhury N, Yoneda T,
Choudhury GG and Ghosh-Choudhury N: Simvastatin prevents skeletal
metastasis of breast cancer by an antagonistic interplay between
p53 and CD44. J Biol Chem. 286:11314–11327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vlckova L, Vondrejs V and Necasek J: The
interaction of emetine with DNA and its effect on the adsorption of
certain bacteriophages. Folia Microbiol. (Praha). 15:76–81.
1970.
|
|
33
|
Aoki T, Shimada K, Sakamoto A, Sugimoto K,
Morishita T, Kojima Y, Shimada S, Kato S, Iriyama C, Kuno S, et al:
Emetine elicits apoptosis of intractable B-cell lymphoma cells with
MYC rearrangement through inhibition of glycolytic metabolism.
Oncotarget. 8:13085–13098. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou YD, Kim YP, Mohammed KA, Jones DK,
Muhammad I, Dunbar DC and Nagle DG: Terpenoid
tetrahydroisoquinoline alkaloids emetine, klugine, and
isocephaeline inhibit the activation of hypoxia-inducible factor-1
in breast tumor cells. J Nat Prod. 68:947–950. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Milovanovic T, Planutis K, Nguyen A, Marsh
JL, Lin F, Hope C and Holcombe RF: Expression of Wnt genes and
frizzled 1 and 2 receptors in normal breast epithelium and
infiltrating breast carcinoma. Int J Oncol. 25:1337–1342.
2004.PubMed/NCBI
|
|
36
|
Benhaj K, Akcali KC and Ozturk M:
Redundant expression of canonical Wnt ligands in human breast
cancer cell lines. Oncol Rep. 15:701–707. 2006.PubMed/NCBI
|
|
37
|
King TD, Suto MJ and Li Y: The
Wnt/β-catenin signaling pathway: A potential therapeutic target in
the treatment of triple negative breast cancer. J Cell Biochem.
113:13–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nagahata T, Shimada T, Harada A, Nagai H,
Onda M, Yokoyama S, Shiba T, Jin E, Kawanami O and Emi M:
Amplification, up-regulation and over-expression of DVL-1, the
human counterpart of the Drosophila disheveled gene, in primary
breast cancers. Cancer Sci. 94:515–518. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schlange T, Matsuda Y, Lienhard S, Huber A
and Hynes NE: Autocrine WNT signaling contributes to breast cancer
cell proliferation via the canonical WNT pathway and EGFR
transactivation. Breast Cancer Res. 9:R632007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dawood S, Austin L and Cristofanilli M:
Cancer stem cells: Implications for cancer therapy. Oncology
(Williston Park). 28:1101–1107, 1110. 2014.PubMed/NCBI
|
|
41
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Horst D, Kriegl L, Engel J, Jung A and
Kirchner T: CD133 and nuclear beta-catenin: The marker combination
to detect high risk cases of low stage colorectal cancer. Eur J
Cancer. 45:2034–2040. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ibrahim EE, Babaei-Jadidi R, Saadeddin A,
Spencer-Dene B, Hossaini S, Abuzinadah M, Li N, Fadhil W, Ilyas M,
Bonnet D and Nateri AS: Embryonic NANOG activity defines colorectal
cancer stem cells and modulates through AP1- and TCF-dependent
mechanisms. Stem Cells. 30:2076–2087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Iv Santaliz-Ruiz LE, Xie X, Old M, Teknos
TN and Pan Q: Emerging role of nanog in tumorigenesis and cancer
stem cells. Int J Cancer. 135:2741–2748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Uzor PF: Recent developments on potential
new applications of emetine as anti-cancer agent. EXCLI J.
15:323–328. 2016.PubMed/NCBI
|
|
46
|
Bamji ZD, Washington KN, Akinboye E,
Bakare O, Kanaan YM and Copeland RL Jr: Apoptotic effects of novel
dithiocarbamate analogs of emetine in prostate cancer cell lines.
Anticancer Res. 35:4723–4732. 2015.PubMed/NCBI
|