Introduction
Breast cancer is one of the most frequently
diagnosed malignancy in females with an estimated 1.5 million new
cases worldwide each year (1). Due to
the fast development of diagnosis and novel treatment techniques,
the incidence has been decreasing in the developed and developing
world (2). However, it remains the
second leading cause of mortality from cancer in women (3). Thus, investigating a novel diagnosis
method and determining a novel treatment target remains urgent. A
previous study was conducted to identify novel markers that control
and regulate cell growth and differentiation, which are associated
with tumor growth, formation and progression of breast cancer
(3).
MicroRNAs (miRNAs/miRs) are small non-coding
single-stranded RNAs, which contain 20–25 nucleotides, that serve
important roles in RNA silencing and post-transcriptional
regulation of gene expression in multicellular organisms (4). Animal miRNAs are generally complementary
to a site in the 3′untranslated region (UTR) of the target mRNA by
perfect or near perfect base paring to promote the cleavage of the
target RNA, thus inducing degradation of the target gene (5). miRNAs are considered to serve roles in
cancer development, progression and metastasis, and during the
cancer development, numerous miRNAs, including miR-15, miR-16,
miR-21 and miR-29a have been discovered. Additionally, miRNAs can
serve oncogenic or tumor suppressive roles in carcinogenesis
through negative regulation of target gene expression (6–8).
In breast cancer, various miRNAs have been
discovered and they have been demonstrated to be deleted,
downregulated or upregulated, such as the miR-17-92 cluster, which
is upregulated, and miR-143 and miR-145, which are deleted
(9). They also have been determined
to have oncogenic or tumor suppressive effects, and serve important
roles in tumor initiation, antitumor drug resistance and advanced
tumor metastasis (10–13). Among the miRNAs, miR-192 was confirmed
by Lim et al (14) in 2003.
miR-192 is considered to be positive regulator of p53, which is a
human tumor suppressor gene (15).
miR-192 is also reported to be overexpressed in gastric cancer,
hepatocellular carcinoma and neuroblastoma, while downregulated in
colorectal cancer and hematological disorders, as well as in
lymphoblastic leukemia (16–20). However, its role in breast cancer
development and formation remains unknown. In the present study,
the results indicated that the miR-192 was significantly decreased
in the tumor tissue, compared with adjacent normal tissue.
Upregulation of miR-192 inhibits tumor cell proliferation by
inducing of the tumor cell apoptosis cell cycle arrest. Notably,
using a bioinformatics method, it was demonstrated that caveolin 1
(CAV1) is a direct target of miR-192 and its protein expression is
negatively regulated by miR-192. Therefore, these results
demonstrated that miR-192 serves an important role in the
regulation of breast tumor cell proliferation and apoptosis, and
the miR-192/CAV1 axis may have a potential as a therapeutic target
for treatment of breast cancer.
Materials and methods
Patient samples
A total of 58 specimens from women with breast
cancer and adjacent normal tissues samples were collected from The
Affiliated Luoyang Central Hospital of Zhengzhou University
(Luoyang, China) from January 2015 to March 2017. The patients had
a mean age of 56±12 years, and did not receive radiotherapy,
chemotherapy or any other treatment prior to or following the
operation. Patient characteristics are listed in Table SI. Tumor surgical specimens, tumor
lumps and tumor adjacent normal tissues that were at least 2 cm
from the edge of the tumor were collected, snap-frozen in liquid
nitrogen and stored at −80°C for miR-192 and CAV1-associated
assays. Written informed consent was obtained from all the study
participants. The use of tissue samples was approved by the Ethics
Committee of the Affiliated Luoyang Central Hospital of Zhengzhou
University.
Cell culture and transfection
A total of 3 breast and breast tumor cells lines
were used in the present study, which includes the normal mammary
fibroblast cell line Hs578Bst, a more aggressive breast tumor cell
line MDA-MB-231 and a less aggressive breast tumor cell line MCF-7.
All these cell lines were obtained from American Type Culture
Collection (Manassas, VA, USA) and maintained in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal
bovine serum (FBS; Gibco; Invitrogen) and 1% antibiotics (Gibco;
Thermo Fisher Scientific, Inc.) in an atmosphere of humidified air
containing 5% CO2. MCF-7 and MDA-MB-231 cells were
transfected with miR-192 mimics (miR-192 mimics:
5′-CUGACCUAUGAAUUGACAGCC-3′) or miR-Control
(5′-UUCUCCGAACGUGUCACGUTT-3′) (Shanghai Genepharma Co., Ltd.,
Shanghai, China) at 10 pmol/1×103 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols.
Detection of cell proliferation with
an MTT assay
The effect of miR-192 on proliferation of breast
cancer cells was detected with an MTT assay. Briefly, MCF-7 and
MDA-MB-231 cells were plated in 96-well plates
(3×103/well). After incubation for 24 h in a 37°C
incubator with 5% CO2, the cells were transfected with
miR-192 mimics (30 pmol) or miR-Control (30 pmol) for 12, 24 and 48
h using Lipofectamine® 2000. Subsequently, the MTT
solution (0.5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was added to each well (20 µl/well). After an additional 4 h
incubation at 37°C, MTT solution was discarded and 200 ml dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA) was added, and the plates
were shaken gently. The absorbance was measured on an ELISA reader
at a wavelength of 570 nm. For the colony formation assay, cells
were counted and plated in 12-well plates (in triplicate) at 100
cells/well. Fresh complete RPMI-1640 medium was replaced every 3
days. The number of viable colonies was determined after 14 days
culture at 37°C, and the colonies were fixed with 100% methanol for
10 min at room temperature and stained with 0.5 g crystal violet
(0.05% w/v) for 15 min at room temperature. Images of the colonies
were captured using a digital camera (Singer Instruments) and
colonies were counted. Each experiment was performed in triplicate
and repeated for at least three times.
Flow cytometry detection of cell cycle
and apoptosis
MCF-7 and MDA-MB-231 cells transfected with miR-192
mimics and miR-Control were plated in 6-well plates in complete
RPMI-1640 medium at 1×105 cells/well (Gibco; Thermo
Fisher Scientific, Inc.) for 24 h at 37°C. Subsequently, the cells
were cultured with FBS-free RPMI-1640 medium for 48 h; the medium
was then replaced with complete RPMI-1640 medium for another 24 h
at 37°C. Cells were then collected by centrifugation at 500 × g for
10 min at room temperature, fixed in 95% ethanol for 20 min at room
temperature, incubated at −20°C overnight and then washed twice
with PBS. The cells were resuspended in 1 ml FACS solution with
propidium iodide (PI; PBS, 0.1% Triton X-100, 60 µg/ml PI, 0.1
mg/ml DNase-free RNase and 0.1% trisodium citrate) and incubated on
ice for 30 min. Cells were analyzed using a FACSCalibur flow
cytometer (BD Biosciences, San Jose, CA, USA). A total of
1×104 cells were gated and counted for each sample.
To identify apoptotic cells ratio, Annexin V and PI
double staining was performed using an Annexin V-Fluorescein
Isothiocyanate (FITC) Apoptosis Detection kit (Becton-Dickinson and
Company, Franklin Lakes, NJ, USA), according to the manufacturer's
protocols. After the MCF-7 and MDA-MB-231 cells were transfected
with miR-192 mimics/miR-Control for 48 h, 5×105 cells
were collected by centrifugation at 1,000 × g at room temperature
for 5 min. Cells were re-suspended in 200 µl binding buffer (BD
Biosciences), and stained with 5 µl FITC Annexin V and 1 µl PI
solution for 30 min at room temperature. Cell apoptosis was
detected by using a FACSCalibur flow cytometer (BD Biosciences).
Apoptotic cells were defined as Annexin V-positive/PI-negative.
Western blotting
Western blot analysis was performed to determine
protein expression of CAV1. Cell lysates were prepared by using
NP-40 cell lysis buffer (Beyotime Institute of Biotechnology,
Haimen, China) and the protein concentration in the supernatants
was determined using Bradford protein dye reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). A total of 30 mg proteins
were resolved by 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membrane. The membranes were blocked with 5% fat-free
dry milk in PBS for 30 min at room temperature and then incubated
with primary anti-CAV1 (1:1,000, cat. no. ab2910; Abcam, Cambridge,
MA, USA) and anti-GAPDH (1:1,000, cat. no. ab9485; Abcam)
antibodies at 4°C overnight. Subsequently, they were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibodies [goat
anti-rabbit IgG H&L (HRP); 1:3,000, cat. no. ab6721; Abcam] for
2 h at room temperature. CAV1 and GAPDH proteins were visualized
with enhanced chemiluminescence detection reagent (Pierce
Biotechnology; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNAs were extracted from the frozen tissue
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and then total RNA (0.5 µg) from each sample was
used for cDNA synthesis using the M-MLV Reverse Transcriptase kit
(Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol. The specific products of human miR-192 and
CAV1 were amplified by qPCR using the following primers: miR-192,
5′-GCTGTTATCTGGGGCGAGGG-3′ (forward) and 5′-GGTGGGACCATGAGTGCTGC-3′
(reverse); and CAV1, 5′-TGGTTTTACCGCTTGCTGTCTG-3′ (forward) and
5′-GCAAGTTGATGCGGACATTGCT′ (reverse). Verification of gene
expression levels was performed by RT-qPCR using EvaGreen (Biotium,
Inc., Freemont, CA, USA). The following thermocycling conditions
were used: Initial denaturation at 95°C for 5 min; followed by 40
cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 10
sec and extension at 72°C for 20 sec. U6 and GAPDH were used as the
internal control; the primer sequences were as follows: U6,
5′-CGAGCACAGAATCGCTTCA-3′ (forward) and 5′-CTCGCTTCGGCAGCACATAT-3′
(reverse); and GAPDH, 5′-GAAGGTGAAGGTCGGAGTC-3′ (forward) and
5′-GAAGATGGTGATGGGATTTC-3′ (reverse). The relative expression
levels of miR-192 and CAV1 were calculated with the
2−ΔΔCq method (21).
Construction of 3′UTR reporter plasmid
and luciferase assay
The putative target genes of miR-192 were predicted
by TargetScan (www.targetscan.org), PicTar (pictar.mdc-berlin.de) and
miRanda (www.microrna.org/microrna/home.do). The human CAV1
3′UTR harboring miR-192 target sequence and the
seed-sequence-mutation (miR-192-3′UTR-mut) were synthesized by
Shanghai Genepharma Co., Ltd. (Shanghai, China). The CAV1 3′UTR
reporter was generated by inserting the entire 3′UTR or 3′UTR-mut
of human CAV1 mRNA into XhoI/NotI sites of psiCHECK-2
vector (Promega Corporation) downstream of the Renilla
luciferase gene. For the luciferase assay, 1×105 cells
were transfected with the CAV1 3′UTR reporter and the miR-192
mimics in a 24-well plate using Lipofectamine® 2000,
according to the manufacturer's protocols. After 24 h, the firefly
and Renilla luciferase activities were measured and analyzed
with a Dual Luciferase Reporter Assay kit (Promega Corporation).
Relative luciferase activity was estimated by normalizing firefly
luciferase activity to that of Renilla for each assay. At 24
h post-transfection, relative luciferase activity was calculated by
normalizing firefly luminescence to Renilla luminescence
using the Dual Luciferase Reporter Assay (Promega Corporation)
according to the manufacturer's protocol.
Knockdown of CAV1 by small interfering
RNA (siRNA)
The transient transfection of CAV1 siRNA was
performed by using Lipofectamine® 2000, according to the
manufacturer's protocols. The sequences of siRNAs were as follows:
siRNA-CAV1, 5′-AGACGAGCUGAGCGAGAAGCA-3′; siRNA-control,
5′-ACTACCGTTGTTATAGGTG-3′, and they were used at a final
concentration of 50 nM. After transfection for 48 h, the cells were
collected, cell lysates were prepared and western blot analysis was
performed to analyze the effects of the knockdown. The CAV1 siRNA
was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Tumorigenicity in vivo
The lentiviral vector that overexpresses pre-miR-192
and the control lentiviral packaging plasmid was obtained from
Shanghai GeneChem Co., Ltd. (Shanghai, China). A total of 16 nude
mice (male BALB/c nude mice; age, 4 weeks; Beijing Vital River
Laboratory Animal Technology Co., Ltd., Beijing, Chin) were
randomly divided into two groups: The lentiviral control group and
lentiviral pre-miR-192 group. Mice were housed in isolated cages
under a 12-h light/dark cycle with free access to food and water at
24±2°C and 55±10% humidity. MCF-7 cells stably transfected with
pre-miR-192 mimics and pre-miR-control were inoculated bilaterally
and subcutaneously into the right flanks of nude mice. Tumor growth
was monitored and tumor size was measured using vernier calipers
every seven days, and the mice were euthanized after four weeks.
The volume of the implanted tumor was calculated using the formula:
Volume=(width2 × length)/2. All animal experiments in
the present study were approved by The Ethics Committee of The
Affiliated Luoyang Central Hospital of Zhengzhou University.
Statistical analysis
GraphPad Prism software, version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA) was used to analyze data. All
the data were obtained from three independent experiments. Data are
presented as mean ± standard error. The Student's t-test was
performed to analyze the significance of differences between the
samples. One-way ANOVA was carried out for multiple comparisons
with Bonferroni's post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
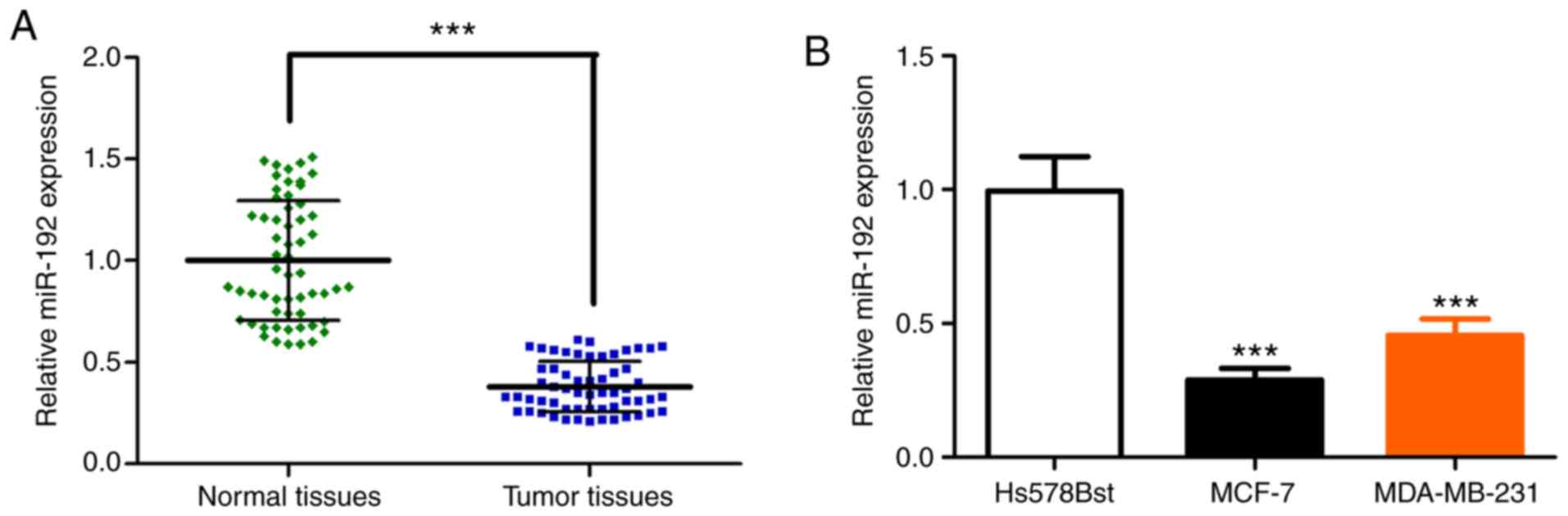
The expression of miR-192 is decreased
in breast cancer tissue and breast cancer cell lines
Total RNAs were extracted from frozen tumor tissue
of patients with breast cancer. RT-qPCR was performed to analyze
the miR-192 expression level. The relative expression of miR-192
was significantly decreased in the breast cancer tissue, compared
with adjacent normal tissues (Fig.
1A). The expression of miR-192 was also evaluated in breast
tumor cell lines MCF-7 and MDA-MB-231, as well as the normal breast
fibroblast cell line Hs578Bst. RT-qPCR analysis demonstrated that
the expression of miR-192 is significantly decreased in breast
tumor cell line MCF-7 and MDA-MB-231, compared with normal cell
line Hs578Bst (Fig. 1B). Furthermore,
the decreased expression of miR-192 was also confirmed in three
other breast cancer cell lines, including Hs578T, BCap37 and
SK-BR-3 (Fig. S1A). These results
indicated that miR-192 expression is significantly reduced in
breast tumor tissue and tumor cell lines, which is accordant with
previous studies (16,18).
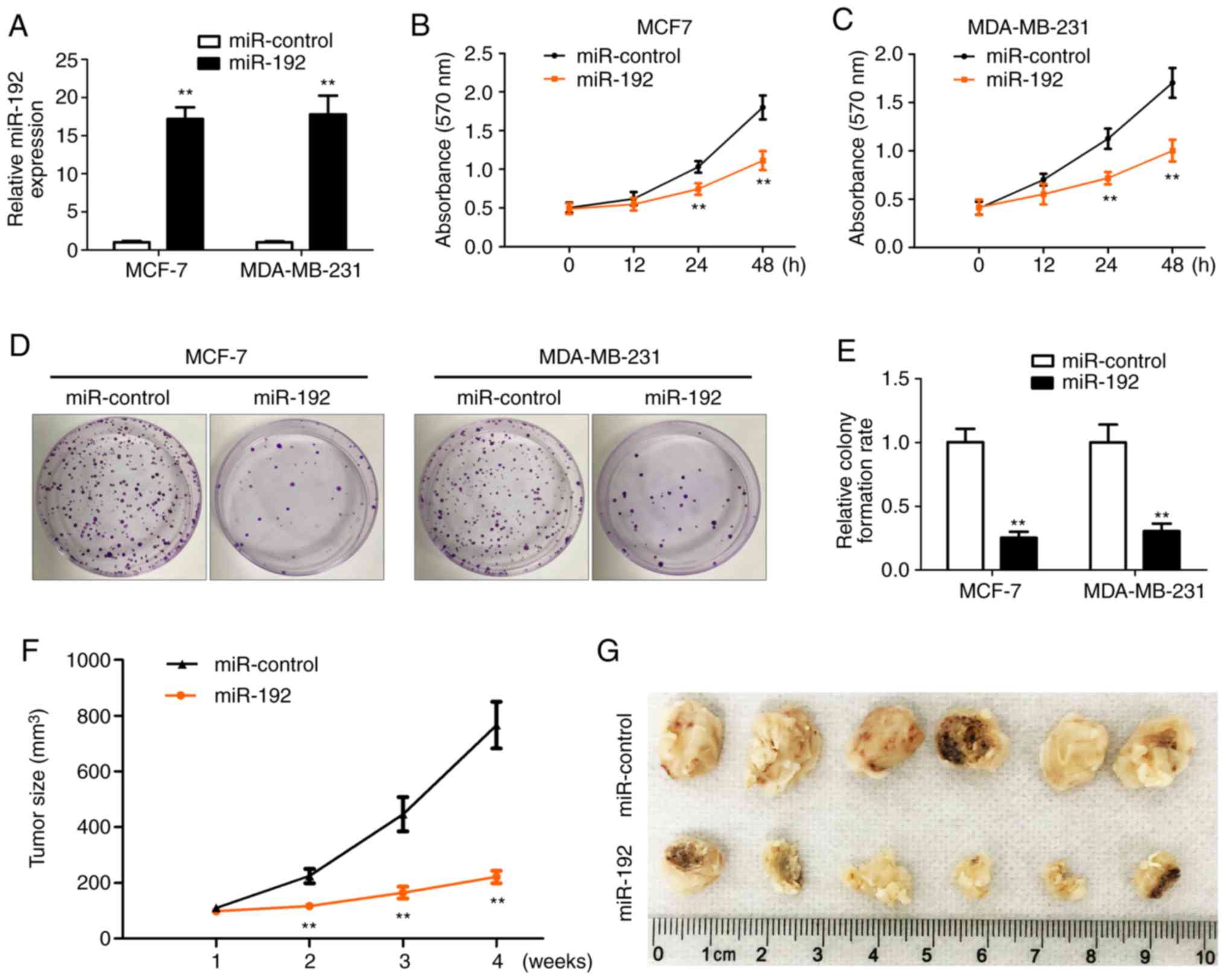
miR-192 inhibits breast tumor cell
proliferation
To determine the role of miR-192 in breast tumor
cell proliferation, breast tumor cells were transfected with
miR-192 for 12, 24 and 48 h and then an MTT assay was performed to
determine the cell proliferation. Total RNAs were extracted from
all the transfected cells and RT-qPCR was performed to check the
overexpression efficiency of miR-192. miR-192 expression was
significantly increased following transfection in MCF-7 and
MDA-MB-231 cells (Fig. 2A) compared
with cells without miR-192 transfection. The MTT assay results
indicated that cell proliferation is significantly inhibited after
24 and 48 h of transfection with miR-192 in MCF-7 and MDA-MB-231
cells (Fig. 2B and C) compared with
cells without miR-192 transfection. Colony formation results also
demonstrated that cell proliferation is significantly inhibited by
the overexpression of miR-192 in MCF-7 and MDA-MB-231 cells
(Fig. 2D and E) compared with cells
without miR-192 transfection.
MCF-7 stable cell line with overexpression of
miR-192 and miR-Control was inoculated into the flank of the mice
and then tumor growth was monitored and measured every 7 days.
Tumor growth was significantly inhibited by miR-192 expression and
the tumor size was smaller in the miR-192 overexpression group,
compared with the miR-control group (Fig.
2F and G). These data indicated that miR-192 serves an
important role in the regulation of tumor cell proliferation and
tumor growth. Therefore, overexpression of miR-192 inhibits tumor
cell growth in vivo and in vitro.
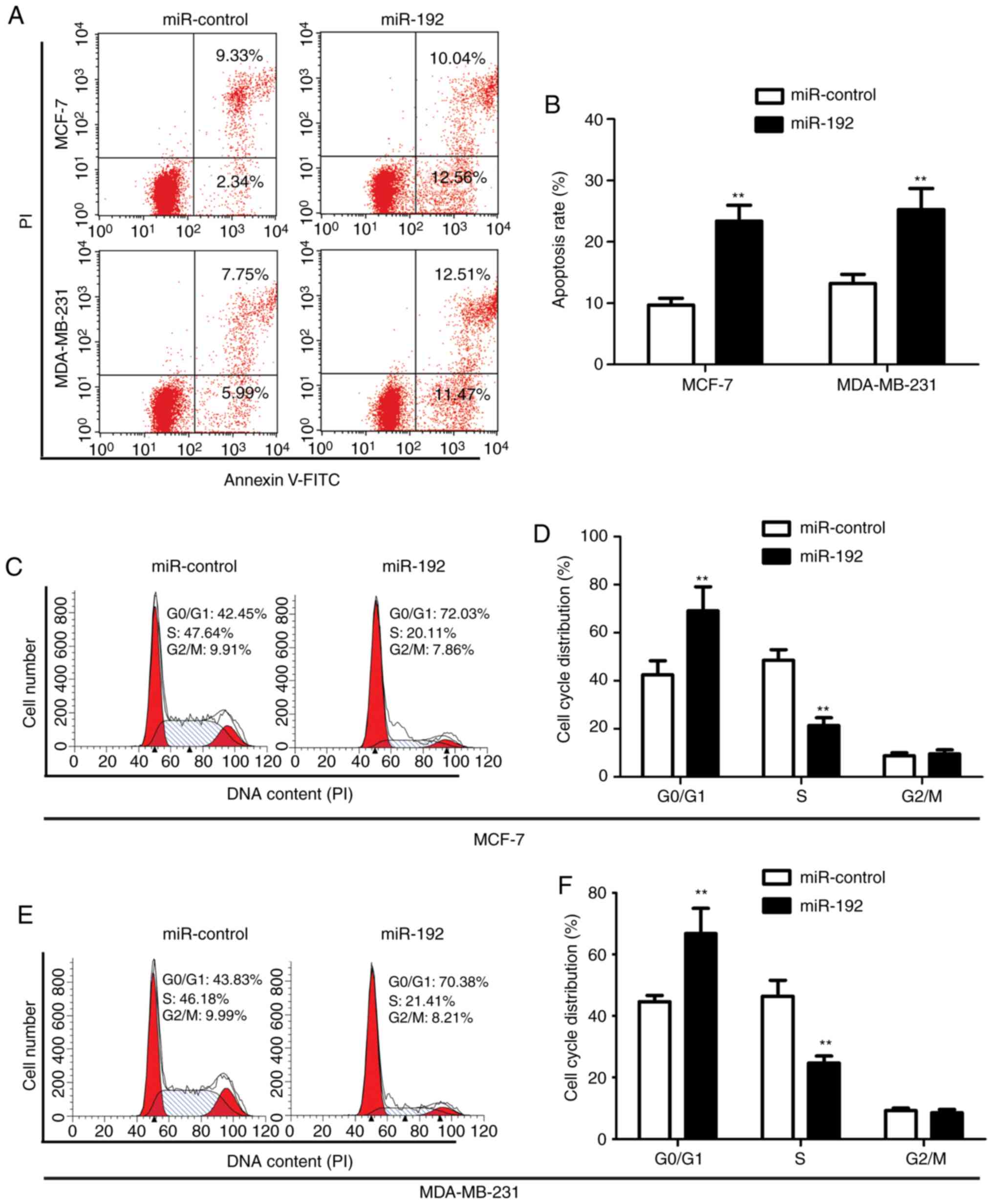
Overexpression of miR-192 induces cell
apoptosis and cell cycle arrest
To detect the effect of miR-192 on cell apoptotic
regulation, cell cycle analysis was performed by staining
transfected MCF-7 cells and MDA-MB-231 cells with Annexin-V and PI.
Flow cytometry data demonstrated that overexpression of miR-192
significantly increases tumor cell apoptosis with an apoptotic cell
ratio of 22.6 and 24.28% in MCF-7 and MDA-MB-231 cells,
respectively, while the apoptotic ratio in the control cells was
only 11.67 and 13.74%, respectively (Fig.
3A and B).
The effect of miR-192 on cell cycle was determined
by staining cells with PI and analyzed by flow cytometry. The
results demonstrated that overexpression of miR-192 induced an
increase in the G0/G1 phase (72.03% for MCF-7 cells with miR-192
transfection vs. 42.45% for MCF-7 cells without miR-192
transfection; and 70.38% for MDA-MB-231 cells with miR-192
transfection vs. 43.83% for MDA-MB-231 cells without miR-192
transfection) and a decrease in the S phase in MCF-7 cells (20.11%
for cells without miR-192 transfection vs. 47.64% for cells with
miR-192 transfection), while there was no significant change in the
G2 phase (Fig. 3C-F). These results
indicated that overexpression of miR-192 significantly increased
the number of cells in the G0/G1 phase, while it decreased the
number of cells in the S phase in miR-192-transfected MCF-7 and
MDA-MB-231 cells.
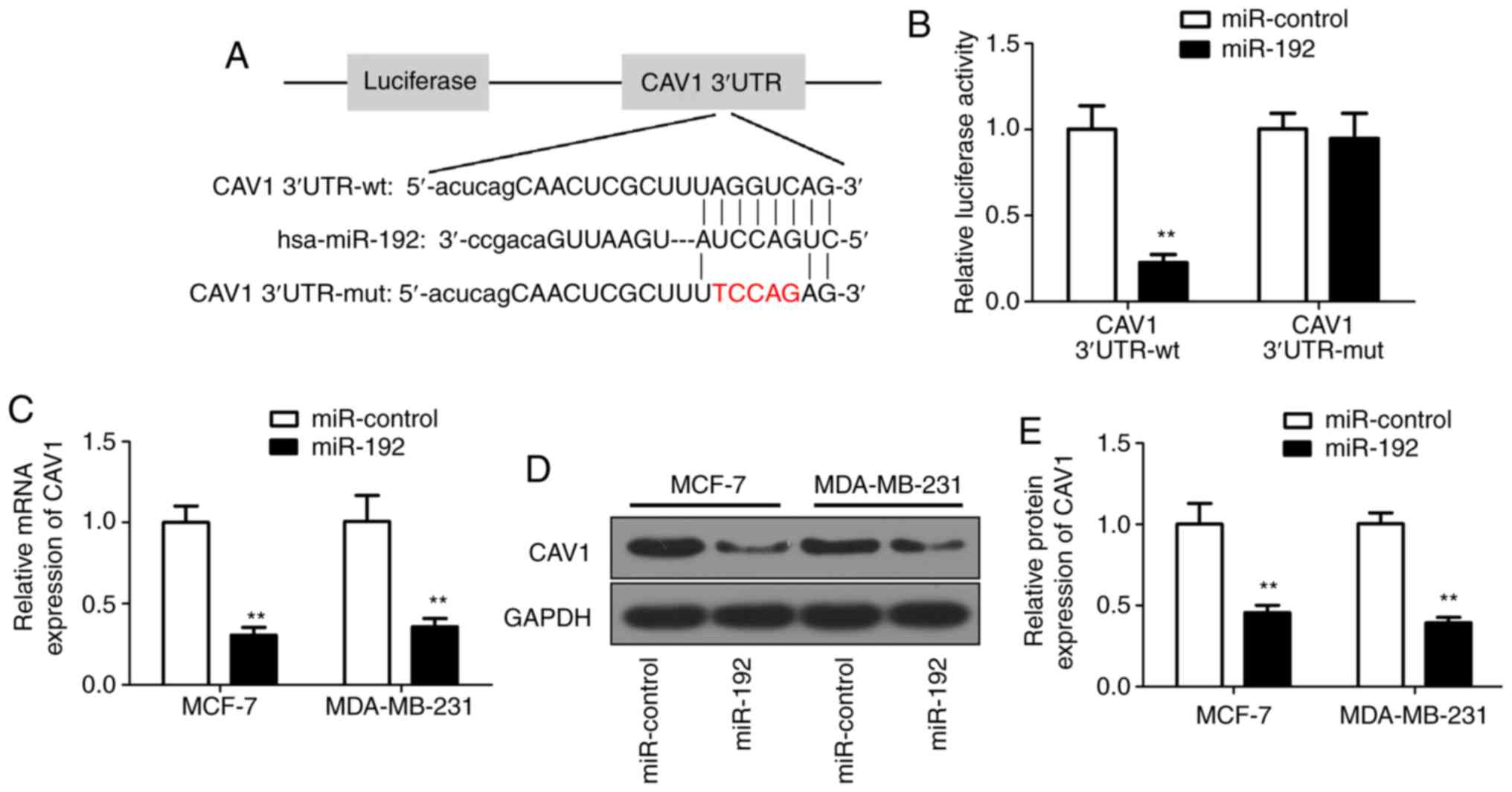
CAV1 negatively regulates the miR-192
expression
Since miRs regulate cellular processes through their
target gene (10–13), the target gene for miR-192 was
investigated. Bioinformatics analysis predicted that miR-192 may
target CAV1 (Fig. 4A). To determine
the association between miR-192 and CAV1, PGL3 luciferase reporter
vectors were constructed with wild-type or its relevant mutant
3′UTR. Subsequently, 293T cells were co-transfected with miR-192
mimics and the reporter vectors containing wild-type or mutant of
CAV1 3′UTR. A luciferase assay was performed to confirm the
luciferase intensity of PGL3/Luciferase-CAV1-3′UTR reporter. The
results demonstrated that luciferase activity was significantly
decreased in the miR-192 and wild-type reporter co-transfected
cells (Fig. 4B). Subsequently, the
CAV1 RNA expression level was evaluated in miR-192-transfected MCF7
and MDA-MB-231 cells. The RT-qPCR results demonstrated that the
CAV1 RNA expression was significantly decreased in miR-192
overexpressed cells (Fig. 4C)
compared with in untransfected cells. The correlation between the
expression levels of miR-192 and CAV1 was analyzed and the results
demonstrated that miR-192 was negatively correlated with CAV1 mRNA
expression (Fig. S1B). Furthermore,
western blot analysis results further confirmed the decreased
expression of CAV1 protein level (Fig.
4D), with a ~50% decrease in miR-192-transfected breast tumor
cells (Fig. 4E) compared with in
untransfected cells. Collectively, these results indicated that
CAV1 expression is negatively associated with miR-192, and CAV1 may
be a direct target gene of miR-192.
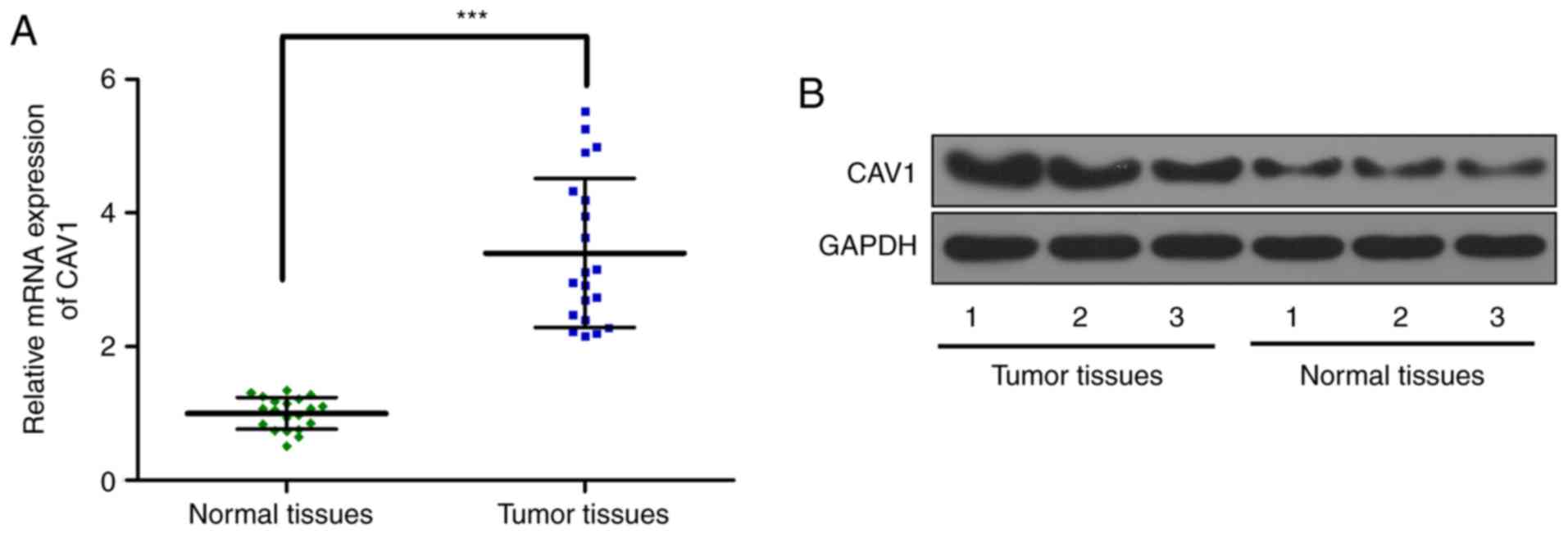
CAV1 expression increases in the
breast tumor tissue
Subsequently, the CAV1 expression in breast tumor
tissue and adjacent normal tissues was determined. The RT-qPCR
results demonstrated that CAV1 RNA expression is significantly
increased in tumor tissue (Fig. 5A)
compared with in normal adjacent tissue. Western blot analysis data
further confirmed the increased expression of CAV1 protein
expression in tumor tissue (Fig. 5B)
compared with in normal adjacent tissue. These results indicated
that the expression of CAV1 was elevated in breast tumor
tissue.
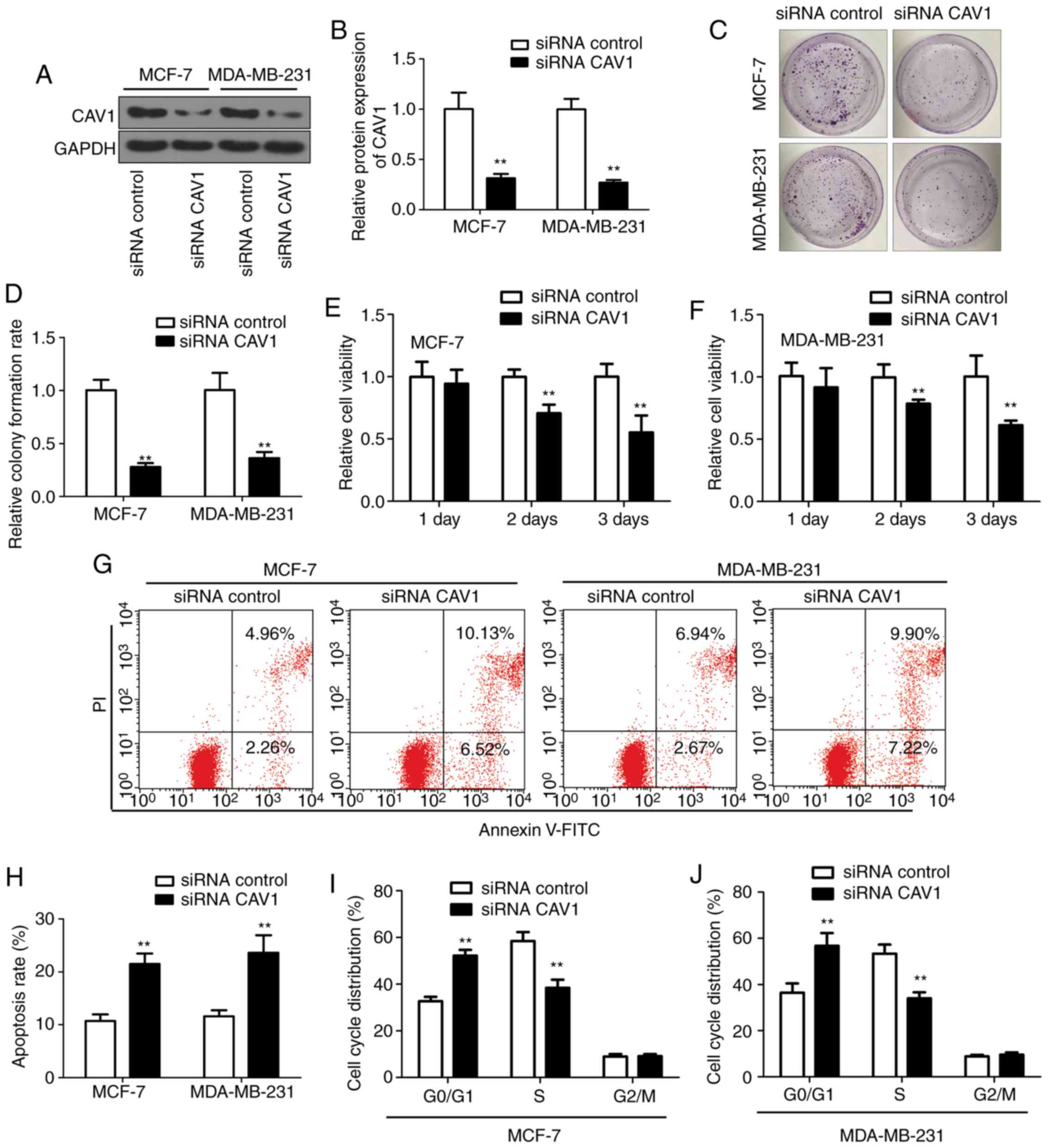
Downregulation of CAV1 inhibits breast
tumor cell proliferation and induces cell apoptosis and cell cycle
arrest
To investigate the role the CAV1 in the regulation
of tumor cell proliferation, downregulation of CAV1 was introduced
in the breast tumor cells line MCF-7 and MDA-MB-231 cells by
infecting cell with siRNA-CAV1 and siRNA-control. CAV1 expression
was significantly reduced following siRNA-CAV1 infection (Fig. 6A and B) compared with the
siRNA-control group. The colony formation experiment demonstrated
that tumor cell growth is inhibited by downregulation of CAV1
(Fig. 6C) compared with the
siRNA-control group. Additionally, the colony formation rate in the
siRNA-CAV1 group was only 30% of the siRNA-control group (Fig. 6D). Cell viability was also decreased
in the siRNA-CAV1 infected MCF-7 and MDA-MB-231 cells (Fig. 6E and F) compared with the
siRNA-control group. Since cell proliferation was inhibited by the
CAV1 expression, the effect of CAV1 on tumor cell apoptosis was
evaluated. The flow cytometry analysis results indicated that
downregulation of CAV1 with siRNA-CAV1 increased tumor cell
apoptosis, as demonstrated by staining the infected cells with
Annexin V and PI (Fig. 6G). The
apoptotic cell ratio increased ~2-folds in the CAV1 downregulation
cells, compared with the siRNA-control infected cells (Fig. 6H) in MCF-7 and MDA-MB-231 cells. Flow
cytometry analysis also demonstrated that downregulation of CAV1
significantly increased the number of cells in the G0/G1 phase and
decreased the number of cells in the S phase, while there was no
significant change in the G2/M phase (Fig. 6I and J). Collectively, these results
demonstrated that downregulation of CAV1 inhibits breast tumor cell
proliferation, and induces cell apoptosis and cell cycle
arrest.
Discussion
The abnormal expression of miRNAs serves an
important role in the cancer development and progression (22,23).
Numerous miRNAs, including miR-192, serve as tumor suppressors and
their aberrant expression serves critical roles in numerous human
cancer types, including breast cancer, prostate cancer and colon
cancer (20,24). In 2003, miR-192 was confirmed by Lim
et al (14). It is
demonstrated to serve various roles in different human cancer
types, such as gastric cancer, prostate cancer and neuroblastoma
(17,25,26). It
has been demonstrated to be overexpressed in gastric cancer,
hepatocellular carcinoma and neuroblastoma, while downregulated in
colorectal cancer and hematological disorders, as well as in
lymphoblastic leukemia (16–20). However, the expression of miR-192 and
the role of miR-192 in breast cancer remain elusive. Breast cancer,
which is the most commonly diagnosed cancer type in females, is
still considered the secondary leading cause of cancer mortalities
among females worldwide (1). Thus, it
is necessary to elucidate the role of miR-192 in the development of
breast cancer. In the present study, the results indicated that
miR-192 expression is significantly decreased in breast tumor
tissue. This observation is accordance with other studies, which
reported that miR-192 serves a tumor suppressor role and is
downregulated in different tumor types (25–27).
Further results demonstrated that overexpression of miR-192 in
breast tumor cells significantly inhibited tumor cell proliferation
and colony formation. Furthermore, overexpression of miR-192
induced tumor cell apoptosis and cell cycle arrest in the G0/G1
phase. Collectively, these results indicate that miR-192 may serve
as a tumor suppressor in breast cancer.
Bioinformatic analysis predicted that miR-192 may
target CAV1. CAV1, a 21 KDa protein encoded by the CAV1
gene, is ubiquitously expressed in all cell types (28). In the past two decades, researchers
focused on investigating the role of CAV1 in the tumor development
determined that CAV1 is overexpressed in liver, colon, breast,
kidney, lung and other cancer types (29). CAV1 has been reported to serve
opposite roles as a tumor promotor or tumor suppressor, dependent
on the cancer type and stage (28,30).
Previous studies reported that high expression of CAV1 induces
tumorigenesis by inhibition of apoptosis, facilitation of
anchorage-independent growth, antitumor drug resistance and
promotion of tumor metastasis (30–32).
Regarding its tumor suppressor role, overexpression of CAV1
inhibits tumor cell progression and prolonged survival rate in
hepatocellular carcinoma (33,34). Thus,
investigation of the expression and regulation of CAV1 by miRNA in
breast tumor and breast tumor cells will further enhance the
understanding of the role of CAV1 in breast cancer.
In the present study, it was demonstrated that
miR-192 directly targets CAV1. CAV1 expression in breast tumor
tissue and breast protein expression is negatively regulated by
miR-192. Overexpression of miR-192 significantly decreased CAV1
expression in breast tumor cells. This result indicated that
miR-192 negatively regulates the expression of CAV1. Furthermore,
the present data also demonstrated that CAV1 expression is
increased in breast tumor tissues, compared with adjacent normal
tissues. Downregulation of CAV1 in tumor cell inhibited tumor cell
proliferation, and induced tumor cell apoptosis and cell cycle
arrest. Therefore, these results demonstrated that miR-192 serves
an important role in the regulation of breast tumor cell
proliferation and apoptosis. miR-192 directly targeted CAV1, which
was highly expressed in breast tumor tissues and tumor cells, to
inhibit tumor growth and progression. Thus, the miR-192/CAV1 axis
may have a potential as a therapeutic target for the treatment of
breast cancer.
miR-192 expression was decreased in breast tumor
tissues. Additionally, overexpression of miR-192 inhibited breast
tumor cell proliferation, and induced tumor cell apoptosis and cell
cycle arrest in the G0/G1 phase. Furthermore, miR-192 directly
targeted CAV1 and negatively regulated the expression of CAV1 in
breast tumor cells. CAV1 was highly expressed in breast tumor and
downregulation of CAV1 inhibited tumor cell proliferation and
induces tumor cell apoptosis and cell cycle arrest. Thus, the
miR-192-CAV1 axis should be investigated as a potential target for
treatment of breast cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PC, YF and XL were involved in the study design,
investigation, analysis and manuscript preparation. HZ, XS, BL, WJ,
XY and NZ were involved in the investigation and analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present human and animal studies were approved
by the Ethics Committee of The Affiliated Luoyang Central Hospital
of Zhengzhou University, and patients and healthy volunteers
provided written informed consent. The research was carried out in
accordance with the World Medical Association Declaration of
Helsinki.
Patient consent for publication
All patients and healthy volunteers provided written
informed consent prior to their inclusion within the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Youlden DR, Cramb SM, Dunn NA, Muller JM,
Pyke CM and Baade PD: The descriptive epidemiology of female breast
cancer: An international comparison of screening, incidence,
survival and mortality. Cancer Epidemiol. 36:237–248. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruvkun G: Clarifications on miRNA and
cancer. Science. 311:36–37. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xi Y, Shalgi R, Fodstad O, Pilpel Y and Ju
J: Differentially regulated micro-RNAs and actively translated
messenger RNA transcripts by tumor suppressor p53 in colon cancer.
Clin Cancer Res. 12:2014–2024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurozumi S, Yamaguchi Y, Kurosumi M, Ohira
M, Matsumoto H and Horiguchi J: Recent trends in microRNA research
into breast cancer with particular focus on the associations
between microRNAs and intrinsic subtypes. J Hum Genet. 62:15–24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kayani M, Kayani MA, Malik FA and Faryal
R: Role of miRNAs in breast cancer. Asian Pac J Cancer Prev.
12:3175–3180. 2011.PubMed/NCBI
|
|
11
|
Mulrane L, McGee SF, Gallagher WM and
O'Connor DP: miRNA dysregulation in breast cancer. Cancer Res.
73:6554–6562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Serpico D, Molino L and Di Cosimo S:
microRNAs in breast cancer development and treatment. Cancer Treat
Rev. 40:595–604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi Ru, Miyazaki H and Ochiya T: The
roles of microRNAs in breast cancer. Cancers (Basel). 7:598–616.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim LP, Lim LP, Glasner ME, Yekta S, Burge
CB and Bartel DP: Vertebrate microRNA genes. Science. 299:15402003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pichiorri F, Suh SS, Rocci A, De Luca L,
Taccioli C, Santhanam R, Zhou W, Benson DM Jr, Hofmainster C, Alder
H, et al: Downregulation of p53-inducible microRNAs 192, 194, and
215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma
development. Cancer Cell. 18:367–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schotte D, De Menezes RX, Akbari Moqadam
F, Khankahdani LM, Lange-Turenhout E, Chen C, Pieters R and Den
Boer ML: MicroRNA characterize genetic diversity and drug
resistance in pediatric acute lymphoblastic leukemia.
Haematologica. 96:703–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feinberg-Gorenshtein G, Guedj A, Shichrur
K, Jeison M, Luria D, Kodman Y, Ash S, Feinmesser M, Edry L,
Shomron N, et al: MiR-192 directly binds and regulates Dicer1
expression in neuroblastoma. PLoS One. 8:e787132013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song B, Wang Y, Kudo K, Gavin EJ, Xi Y and
Ju J: miR-192 Regulates dihydrofolate reductase and cellular
proliferation through the p53-microRNA circuit. Clin Cancer Res.
14:8080–8068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan Y, Ge G, Pan T, Wen D, Chen L, Yu X,
Zhou X and Gan J: A serum microRNA panel as potential biomarkers
for hepatocellular carcinoma related with hepatitis B virus. PLoS
One. 9:e1079862014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin Z, Selaru FM, Cheng Y, Kan T, Agarwal
R, Mori Y, Olaru AV, Yang J, David S, Hamilton JP, et al:
MicroRNA-192 and-215 are upregulated in human gastric cancer in
vivo and suppress ALCAM expression in vitro. Oncogene.
30:1577–1585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Augello C, Vaira V, Caruso L, Destro A,
Maggioni M, Park YN, Montorsi M, Santambrogio R, Roncalli M and
Bosari S: MicroRNA profiling of hepatocarcinogenesis identifies
C19MC cluster as a novel prognostic biomarker in hepatocellular
carcinoma. Liver Int. 32:772–782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin Y, Lu J, Wen J, Shen Y and Wen X:
Regulation of growth of human bladder cancer by miR-192. Tumor
Biol. 36:3791–3797. 2015. View Article : Google Scholar
|
|
25
|
Xu YJ and Fan Y: MiR-215/192 participates
in gastric cancer progression. Clin Transl Oncol. 17:34–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun J, Fan Z, Lu S, Yang J, Hao T and Huo
Q: MiR-192 suppresses the tumorigenicity of prostate cancer cells
by targeting and inhibiting nin one binding protein. Int J Mol Med.
37:485–492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng S, Cong S, Zhang X, Bao X, Wang W, Li
H, Wang Z, Wang G, Xu J, Du B, et al: MicroRNA-192 targeting
retinoblastoma 1 inhibits cell proliferation and induces cell
apoptosis in lung cancer cells. Nucleic Acids Res. 39:6669–6678.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta R, Toufaily C and Annabi B: Caveolin
and cavin family members: Dual roles in cancer. Biochimie.
107:188–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burgermeister E, Liscovitch M, Röcken C,
Schmid RM and Ebert MP: Caveats of caveolin-1 in cancer
progression. Cancer Lett. 268:187–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Wang N, Liu P, Peng F, Tang H,
Chen Q, Xu R, Dai Y, Lin Y, Xie X, et al: Caveolin-1, a
stress-related oncotarget, in drug resistance. Oncotarget.
6:37135–37150. 2015.PubMed/NCBI
|
|
31
|
Patani N, Martin LA, Reis-Filho JS and
Dowsett M: The role of caveolin-1 in human breast cancer. Breast
Cancer Res Treat. 131:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Savage K, Lambros MB, Robertson D, Jones
RL, Jones C, Mackay A, James M, Hornick JL, Pereira EM, Milanezi F,
et al: Caveolin 1 is overexpressed and amplified in a subset of
basal-like and metaplastic breast carcinomas: A morphologic,
ultrastructural, immunohistochemical, and in situ hybridization
analysis. Clin Cancer Res. 13:90–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang SF, Yang JY, Huang CH, Wang SN, Lu
CP, Tsai CJ, Chai CY and Yeh YT: Increased caveolin-1 expression
associated with prolonged overall survival rate in hepatocellular
carcinoma. Pathology. 42:438–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chatterjee M, Ben-Josef E, Thomas DG,
Morgan MA, Zalupski MM, Khan G, Andrew Robinson C, Griffith KA,
Chen CS, Ludwig T, et al: Caveolin-1 is associated with tumor
progression and confers a multi-modality resistance phenotype in
pancreatic cancer. Sci Rep. 5:108672015. View Article : Google Scholar : PubMed/NCBI
|