Introduction
O-GlcNAcylation is a reversible
post-translational modification whereby a single
N-acetylglucosamine (GlcNAc) molecule is added to the
hydroxyl group of Ser/Thr protein residues. This reaction is
catalyzed by the enzyme, O-linked-N-acetylglucosamine
transferase (OGT) and the protein sugar moiety is removed using
O-GlcNAcase enzymes (OGA, NCOAT or MGEA5) (1,2).
Endometrial receptivity refers to a limited period
of time during which the endometrium is conducive to blastocyst
growth, attachment and the subsequent events of implantation
(3,4).
The sensitivity of the endometrium to the embryo is classified as
the pre-receptive, receptive and non-receptive phases (5). During the pre-receptive phase, the
uterus cannot initiate embryo implantation. However the uterine
environment does not pose a specific threat to the survival of the
embryo. During the non-receptive phase, the uterine environment is
not conducive to the survival of the embryo.
The female menstrual cycle lasts for an average of
28–30 days and is divided into the proliferative (follicular) and
secretory (luteal) phases. The uterus then becomes receptive to
blastocyst attachment in the mid-luteal phase for 4 days (7–10 days
after ovulation) (6). Normal cycle
duration is a key determinant of whether an embryo can be implanted
smoothly. Embryo implantation is the main rate-limiting step in
pregnancy and endometrial receptivity is closely associated with
embryo implantation. Endometrial receptivity can also affect
implantation and clinical pregnancy rates. The establishment of
endometrial receptivity is therefore important to ensure the
successful development of the fetus and placenta.
Previous studies have focused on the role of protein
glycosylation modification in implantation-associated events,
including embryonic development and maternal-fetal interface
recognition. The results revealed that LeY glycan,
fucosyltransferase and β1,4-galactosyltransferase regulate embryo
implantation, indicating that glycosylation modification exerts an
important effect on implantation (7,8).
At present, our understanding of O-linked
β-N- acetylglucosamine (O-GlcNAc) modifications is
mainly in the context of tumor occurrence and metastasis (9), yet the behavior of invasive cancer cells
is similar to that of invasive placental cells (10). Protein glycosylation serves an
important role in embryonic development, but the role of
O-GlcNAcylation in the regulation of implantation is yet to
be fully elucidated. The present study utilized RL95-2 and HEC-1A
endometrial carcinoma cell lines to simulate high-receptive and
low-receptive endometrial states, respectively. A chorionic tumor
cell line, JAR, was used to simulate invasive embryos (11). Using this model, the present study
aimed to determine the role of O-GlcNAcylation during the
process of embryo implantation. The results of the present study
may therefore have important significance for the improvement and
treatment of female fertility. The results may also provide a
theoretical basis for the development of clinical reproductive
regulation, pregnancy aid and anti-implantation technology.
Materials and methods
Patients and samples
Endometrial tissue (n=36; median age, 35 years; age
range, 25–45 years; 18 cases in each of the proliferative and
secretory stages) were obtained from The First Affiliated Hospital
of Dalian Medical University under the Human Research Agreement
approved by the Ethics Committee of Dalian Medical University.
Written informed consent was acquired from each patient prior to
tissue collection. Endometrial specimens were collected, embedded
in paraffin wax and screened via histopathology.
The patients included in the present study were
diagnosed with gynecological benign uterine leiomyoma and were
undergoing hysterectomy. They exhibited normal fertility and a
regular menstrual cycle. All patients had not received any hormonal
drugs for a minimum of 3 months. Patients with diabetes,
hyperthyroidism, hypothyroidism, ovarian neoplasms and other
complications were excluded.
Cell lines and cell culture
RL95-2 and HEC-1A endometrial carcinoma cell lines
and the chorionic tumor cell line, JAR, were purchased from the
American Type Culture Collection (ATCC). The JAR cell line was
established from a trophoblastic tumor of the placenta, but the
cell line was derived from a male fetus and as such should not
affect the results of the present study. RL95-2 cells were cultured
in DMEM/F12 media (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 0.1% insulin, 10% fetal bovine serum (FBS)
(ScienCell Research Laboratories, Inc.) and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). JAR cells were cultured in DMEM/F12 media (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). HEC-1A cells were maintained in McCoy's 5A media (Gibco;
Thermo Fisher Scientific, Inc.), supplemented with 10% FBS and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). All cell lines were maintained at 37°C in a humidified
atmosphere containing 5% CO2. Growth media were changed
every 2–3 days.
RNA interference
Small interfering RNA (siRNA) duplexes targeting the
OGT and OGA genes, as well as an siRNA negative control, were
synthesized by GenePharma Co., Ltd. The following siRNA sequences
were utilized: OGT sense, 5′-GCAGUAACACAGCUCUUAATT-3′ and
antisense, 5′-UUAAGAGCUGUGUUACUGCTT-3′; OGA sense,
5′-GGGAUAUCAAGAGUAUAAUTT-3′ and antisense,
5′-AUUAUACUCUUGAUAUCCCTT-3′; negative control sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. siRNA transfection was performed in
HEC-1A and RL95-2 cells. Cells were transfected with 100 pmol siRNA
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) in accordance with the manufacturer's protocol.
Immunohistochemistry
Human endometrial tissues were sectioned (4 µm) and
dried at 56°C for 60 min. After the de-paraffinization and
re-hydration of tissue sections, antigen retrieval was performed at
100°C for 20 min in citrate buffer. Samples were then treated with
3% peroxide for 15 min at room temperature in the dark to quench
endogenous peroxidase activity. Sections were washed in PBS and
blocked for 15 min with 5% goat serum at 37°C to prevent
non-specific binding. Following incubation with an O-GlcNAc
primary antibody (Abcam; cat. no. ab2739; dilution 1:50) overnight
at 4°C, the sections were washed several times. Biotinylated
secondary antibodies (OriGene Technologies, Inc.; cat. no. sp9000;
dilution 1:1) were then added to the tissue sections and incubated
at 37°C for 30 min. After washing, the sections were incubated with
streptavidin-horseradish peroxidase (OriGene Technologies, Inc.) at
37°C for 30 min. Sections were then allowed to react with
diaminobenzidine (DAB)-peroxidase substrate (OriGene Technologies,
Inc.), counterstained with hematoxylin for 30 sec, dehydrated and
mounted in distrene dibutylypthalate xylene. Photomicrographs were
captured using an Olympus TH4-200 microscope (magnifications, ×10
and ×40). The collected images were analyzed and processed using an
Image-Pro Plus 6.0 (Media Cybernetics) image analysis system. The
mean optical density of positively stained areas of endometrial
stromal cells, glandular epithelial cells and luminal epithelial
cells was measured. PBS replaced the primary antibody for negative
controls.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cells using TRIzol
reagent (Takara Biotechnology Co., Ltd.), according to the
manufacturer's protocol. RNA samples were quantified via
spectrophotometry at 260 and 280 nm, and exhibited a 260/280 nm
ratio ranging from 1.8–2.0, which indicated a high quality of
extracted RNA. RNA (1.0 µg) was subsequently reverse transcribed
into cDNA using the All-in-One First-Strand cDNA Synthesis SuperMix
for qPCR (One-Step gDNA Removal; Beijing TransGen Biotech Co.,
Ltd.) following the manufacturer's protocol. qPCR analysis was
performed in a 10 µl reaction system containing: 5 µl 2X SYBR
Premix Ex Taq (Beijing TransGen Biotech Co., Ltd.; AT341), 0.2 µl
of each forward and reverse primer (10 µM), 0.2 µl ROX Reference
Dye I (50X) (Beijing TransGen Biotech Co., Ltd.), 3.4 µl
ddH2O and 1.0 µl cDNA. The Applied Biosystems ABI Step
One Plus real-time PCR system (Thermo Fisher Scientific, Inc.) was
utilized. Relative gene expression was determined using the
2−ΔΔCq method (12,13) with
GAPDH as the reference gene. All RT-qPCR reactions were performed
in triplicate using the following primer sequences designed with
oligo7: GAPDH (XM_001256799.2) forward, 5′-GTGAAGGTCGGAGTCAACG-3′
and reverse 5′-TGAGGTCAATGAAGGGGTC-3′; OGT (XM_017029908.1)
forward, 5′-CGGGAATCACCCTACTTCACACC-3′ and reverse,
5′-CCGCCATCACCTTCACTCGAAA-3′; OGA (XM_017015586.1) forward,
5′-TCCCCAGAGATGTCCATGCAAG-3′ and reverse,
5′-TCCTTTGGGTCCATGCTCGTA-3′.
Western blot analysis
Proteins from endometrial tissues or cells were
extracted using lysis buffer (Nanjing KeyGen Biotech Co., Ltd.) in
accordance with the manufacturer's protocol. The concentration of
protein extracts was determined using a BCA Protein Quantitative
kit (TransGen Biotech Co., Ltd.). Protein (40 µg) was denatured in
6X Protein Loading Buffer for 7 min at 100°C. Proteins were then
separated via 8% SDS-PAGE and transferred to nitrocellulose
membranes (EMD Millipore) for 90 min at 4°C. Membranes were blocked
with 5% non-fat dried milk in Tris-buffered saline (10 mmol/l Tris,
pH 7.5 and 0.14 mol/l NaCl) with 0.1% Tween-20 (TBST) for 2 h at
room temperature, followed by incubation with the following primary
antibodies overnight at 4°C: Anti-O-GlcNAc (Abcam; cat. no.
ab2739; dilution 1:1,000), anti-OGT (ProteinTech Group, Inc.; cat.
no. 11576-2-AP; dilution 1:1,000), anti-OGA (ProteinTech Group,
Inc.; cat. no. 14711-1-AP; dilution 1:1,000), anti-cyclin D1
(ProteinTech Group, Inc.; cat. no. 60186-1-Ig; dilution 1:2,000),
anti-B-cell lymphoma-xL (Bcl-xl; CLOUD-CLONE Corp.; cat. no.
PAE582Hu01; dilution 1:700), anti-matrix metalloproteinase-9 (MMP9;
Abcam; cat. no. ab38898, dilution 1:1,000), MMP2 (Bioworld
Technology, Inc.; cat. no. bs1236; dilution 1:700), anti-Snail
(ProteinTech Group, Inc.; cat. no. 26183-1-AP; dilution 1:900),
anti-E-cadherin (ProteinTech Group, Inc.; cat. no. 20874-1-AP,
dilution 1:700) and anti-β-actin (ProteinTech Group, Inc.; cat. no.
20536-1-AP; dilution 1:2,000). After being washed three times with
TBST, the membranes were probed with
horseradish-peroxidase-conjugated anti-mouse or anti-rabbit IgG
secondary antibodies (ProteinTech Group, Inc.; cat. nos. SA00001-1
and SA00001-2; dilution 1:3,000) at room temperature for 1 h and
subsequently washed further with TBST. Immunoreactive bands were
visualized using an enhanced chemiluminescence detection system,
according to the manufacturer's protocol. The entire experiment was
repeated three times. Image Lab software (Bio-Rad Laboratories,
Inc. Ver: 4.0 49710) was used for the detection and analysis of
protein bands. Protein expression was quantified via densitometric
analysis and expression was normalized to that of β-actin.
Cell adhesion assay
RL95-2 and HEC-1A cells (5×106) were
transfected in 6-well plates for 12 h. Together with the cells of
the untreated group, the transfected cells were digested with
trypsin and cultured in a 24-well plate for 12 h to ensure that
cells covered the entire surface area. CellTraceCFSE (Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. C34554; 5 µg) was
dissolved in 10 µl of DMSO and diluted with media at a ratio of
1:2,000. JAR cells were pretreated with diluted carboxyfluorescein
succinimidyl ester for 30 min. The JAR cell suspension was
subsequently added to 24-well plates at a 1:1 ratio and incubated
for 1 h at 37°C in 5% CO2 and a 90% humidity.
Fluorescent tags were added and a fluorescence microscope was used
for imaging (×10) and cell counting. The number of fluorescently
labeled cells was counted from five randomly selected fields under
a fluorescence microscope and the results of at least three
independent experiments.
Cell counting kit-8 (CCK-8) assay
HEC-1A or RL95-2 cells from control and siRNA groups
were trypsinized and seeded into 96-well culture plates at a
density of 5,000 cells/well using the prescribed media supplemented
with 10% FBS. Each group was provided with three multiple holes.
The proliferation assay was performed using a CCK-8 kit (Dojindo
Molecular Technologies, Inc.) in accordance with the manufacturer's
protocol. Following culture for 24, 48 and 72 h, 10 µl of CCK-8
solution was added to each well and incubated for 2 h at 37°C.
Optical density (OD) values of absorbance for each well were
measured at 450 nm using Fluoroskan Ascent FL (Thermo Fisher
Scientific, Inc.). Representative results from three independent
experiments were expressed as the mean ± SEM for each
condition.
Wound healing assay
After pretreatment (O-GlcNAc knockdown or
overexpression), HEC-1A or RL95-2 cells (5×106) were
seeded in 6-well plates for 24 h. The resulting cell monolayer was
then wounded by removing a 500- to 700-µm wide strip with a
standard 200-µl pipette tip, after which samples were washed twice
with PBS to remove floating cells. An optical Olympus IX71
microscope (Olympus Corp.; magnification, ×10) was used to image
specific sites on the first day post-wound. Cells were cultured in
media supplemented with 10% FBS in 5% CO2 at 37°C for a
further 24 or 48 h and imaged at 2 or 3 days, respectively. Wound
healing was quantified by measuring the migratory distance of
cells.
Transwell invasion assay
The invasion of cells was assessed using a 24-well
Transwell Permeable Support with 8-µm pores (Corning, Inc.)
according to the manufacturer's protocol. The inner compartments of
the Transwell inserts were pre-coated with 50 µl of 1 mg/ml
Matrigel matrix (BD Biosciences) at 37°C for 1 h to solidify
samples. RL95-2 and HEC-1A cells (1×105 cells/ml) in
serum-free media were seeded into the upper chamber of the
Matrigel-coated filter. Media containing 10% FBS (500 µl) was
subsequently added to the lower chamber as a chemoattractant. After
incubation for 24 h at 37°C in 5% CO2, non-invading
cells were removed from the upper surface of the filter membrane.
Invading cells that remained on the bottom surface of the filter
membrane were fixed in methanol and stained with crystal violet for
15 min at room temperature. The number of invaded cells on each
membrane was counted from five randomly selected fields under a
light microscope (Olympus Corp., magnification, ×20).
Statistical analyses
All data of the present study are the results of at
least three independent experiments. Data are normally presented as
the mean ± SEM. A Student's t-test was used to compare the
statistical significance between two groups. SPSS version 16.0 for
Windows (SPSS, Inc., Chicago, IL, USA) was used for statistical
analyses. P<0.05 was considered to indicate a statistically
significant difference. P-values were indicated in the figures and
legends as follows: ***P<0.001, **P<0.01 and *P<0.05.
Results
Expression of O-GlcNAc in the
implantation-phase endometrium is higher than that of the
non-implanted endometrium
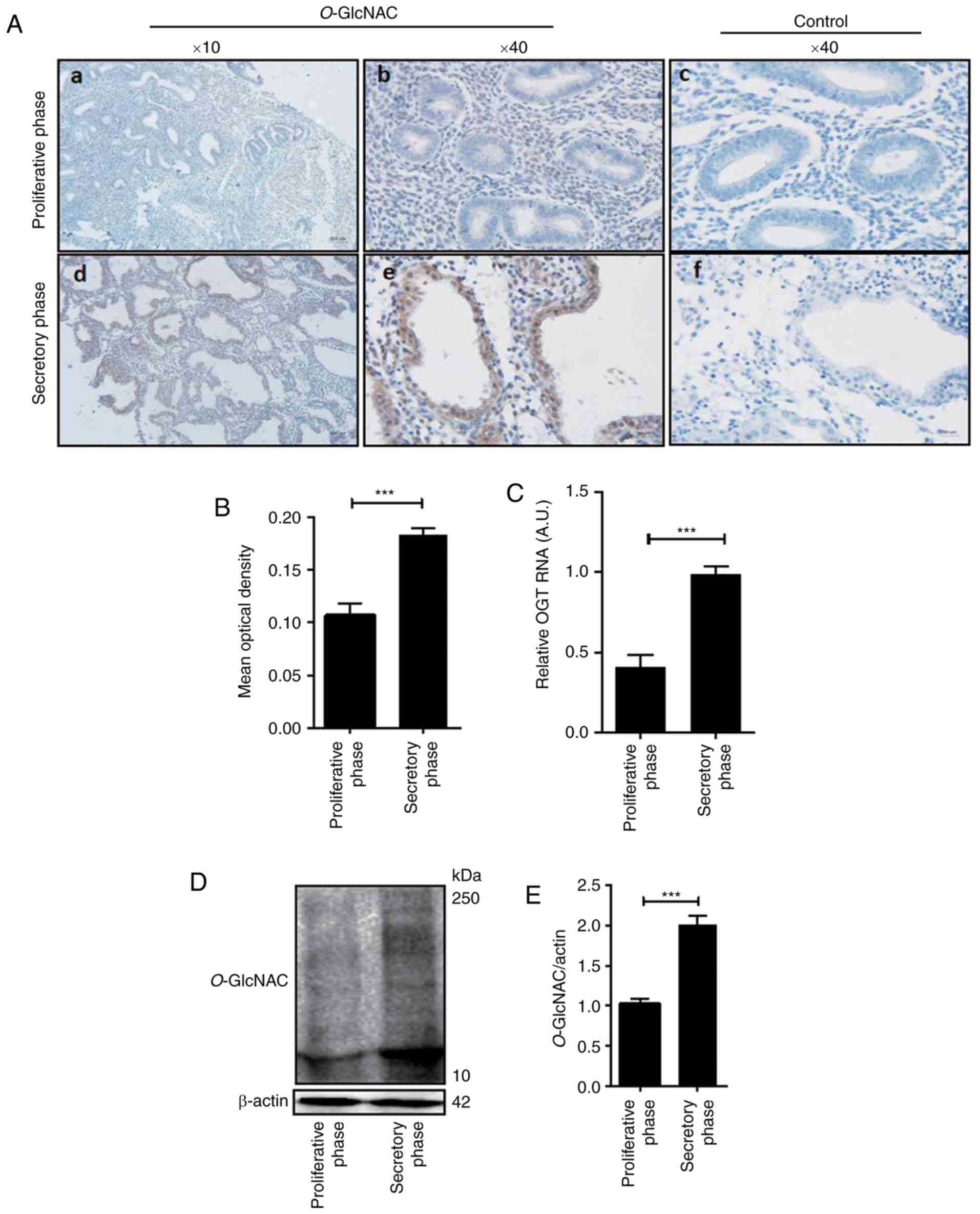
The present study detected the expression of
O-GlcNAcylation via immunohistochemistry in female
endometrial tissue of the proliferative and secretory phase. As
presented in Fig. 1, the results
revealed that compared with the control (Fig. 1A-c and -f), levels of O-GlcNAc
protein modification in the human secretory endometrium was higher
than that of the endometrium in the proliferative phase, which was
particularly low (Fig. 1A-a, -b, -d and
-e). Additionally, O-GlcNAc-modified proteins were
minimally expressed in the endometrium during the proliferative
phase (Fig. 1A-a and -b). However,
during the secretory phase, the expression of O-GlcNAc was
significantly increased (Fig. 1A-d and
-e, D and E). In humans, O-GlcNAc-modified proteins are
mainly expressed in glandular epithelia and luminal epithelia
(Fig. 1A-b and -e). Image analysis
revealed that the mean optical density (OD) of O-GlcNAc in
the secretory phase was significantly different compared with that
of the proliferative phase (P<0.05; Fig. 1B; Table
I). Furthermore, the level of OGT mRNA in the secretory
endometrium was significantly higher than that in the proliferative
phase endometrium (Fig. 1C).
 | Table I.Mean optical density value of
O-GlcNAc in the endometrium of female tissue during
different phases of the menstrual cycle. |
Table I.
Mean optical density value of
O-GlcNAc in the endometrium of female tissue during
different phases of the menstrual cycle.
| Menstrual cycle
phase | Mean optical
density |
|---|
| Proliferative
phase | 0.1088±0.024 |
| Secretory
phase |
0.1837±0.017a |
Effect of O-GlcNAcylation on
endometrial cell adhesion
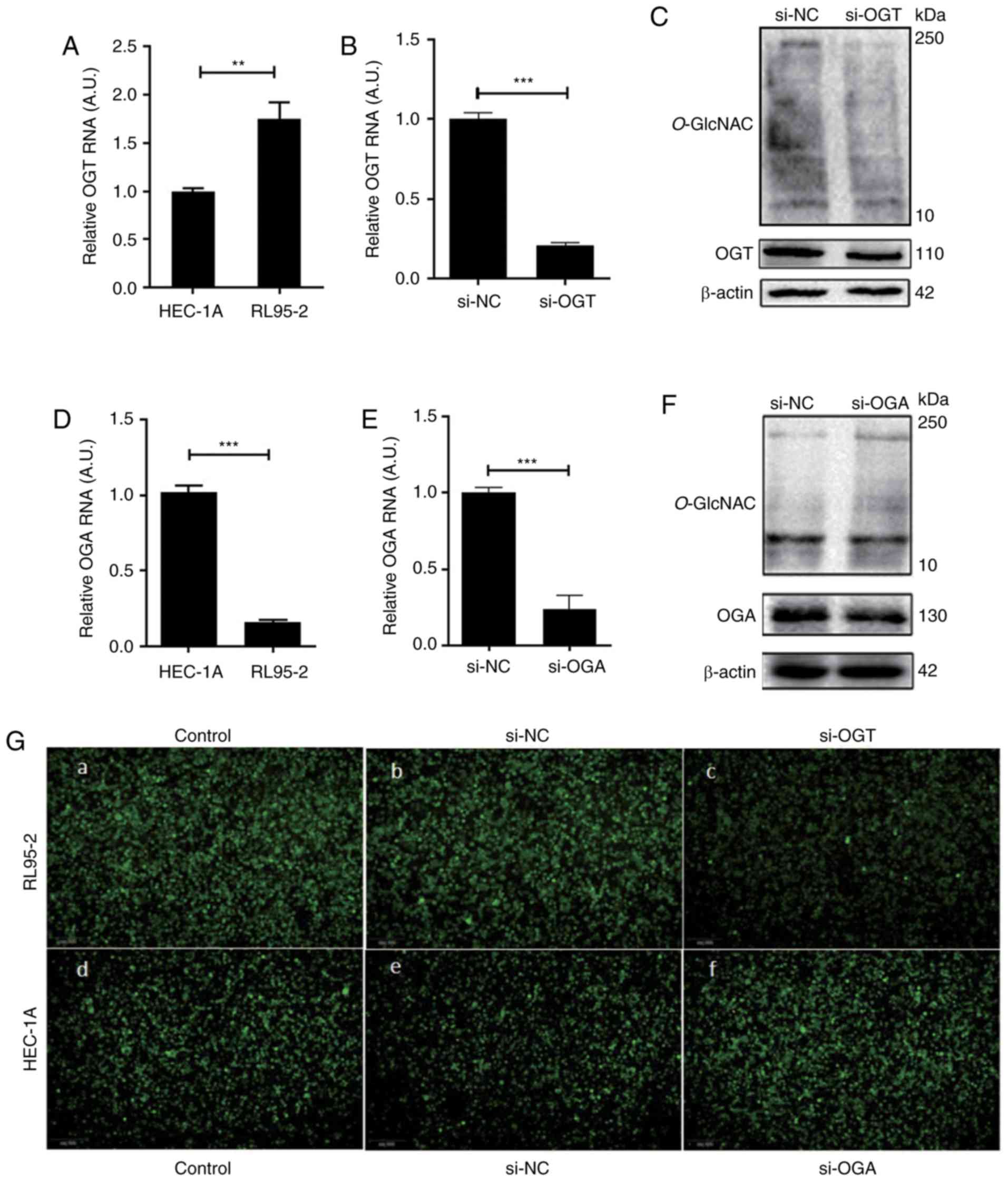
The expression of OGT and OGA genes were detected
via RT-qPCR in high receptive RL95-2 endometrial cells and low
receptive HEC-1A endometrial cells. The results revealed that the
expression of OGT in the RL95-2 cells was significantly higher than
that in the HEC-1A cells (Fig. 2A).
Conversely, the expression of OGA in the RL95-2 cells was
significantly lower than that in the HEC-1A cells (Fig. 2D). In RL95-2 cells with a high OGT
expression, an OGT siRNA was transfected to reduce the levels of
O-GlcNAcylation, which was subsequently detected via RT-qPCR
and western blotting. The results demonstrated that the level of
OGT mRNA (Fig. 2B), OGT protein
(Fig. 2C) and O-GlcNAcylation
(Fig. 2C) were significantly
decreased 48 h after transfection. However, following treatment
with OGA siRNA in HEC-1A cells, the increased expression of OGA
mRNA (Fig. 2E), OGA protein (Fig. 2F) and O-GlcNAc-modified
proteins (Fig. 2F) was
attenuated.
The effect of O-GlcNAcylation on the adhesion
of endometrial cells to embryonic cells was studied by performing
an adhesion assay. The results revealed that the adhesion of JAR
cells to RL95-2 cells was stronger than that of HEC-1A cells
(Fig. 2G-a and -d). Furthermore, the
adhesion of JAR cells to RL-952 cells was significantly decreased
following OGT siRNA transfection (Fig.
2G-b and -c). In addition, in HEC-1A cells, the adhesion of JAR
cells to HEC-1A cells was significantly increased following
transfection with OGA siRNA (Fig. 2G-e
and -f).
Effect of O-GlcNAcylation on the
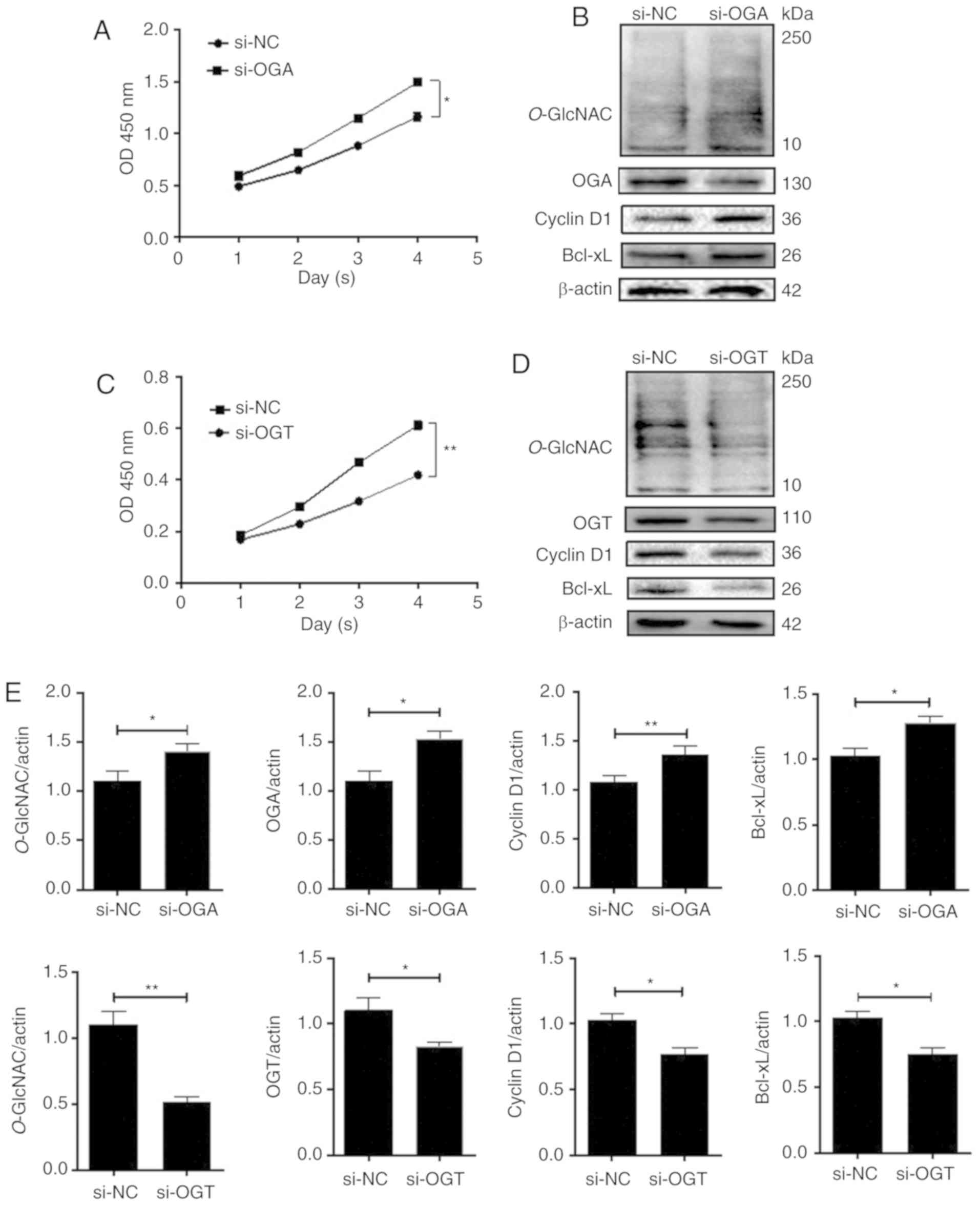
proliferation of endometrial cells
To assess the role of O-GlcNAcylation in
endometrial cells, OGA expression in HEC-1A cells and OGT
expression in RL95-2 cells were experimentally reduced. The
proliferation of cells was detected by performing a CCK-8 assay.
The results revealed that compared with the si-negative control
(NC) group, the OD value of the HEC-1A si-OGA group was
significantly increased over time. However, the OD value of the
si-OGT group was significantly decreased over time in the RL95-2
cells. These results indicated that O-GlcNAcylation may
enhance cellular proliferation (Fig. 3A
and C). Western blotting was performed to assess the expression
of the cellular proliferation-associated proteins cyclin D1 and
Bcl-xL. After treatment with si-OGA to overexpress O-GlcNAc,
levels of cyclin D1 and Bcl-xL in HEC-1A cells were significantly
increased compared with the controls (P<0.01 and P<0.05;
Fig. 3B and E). The opposite was
observed in RL-952 cells (P<0.05; Fig.
3D and E).
Effect of O-GlcNAcylation on the
migration and invasion of endometrial cells
The migration and invasion of cells was detected via
wound healing and Transwell invasion assays, respectively. Among
HEC-1A cells, the OGA-siRNA group demonstrated increased migration
(Fig. 4A-a and B) and invasion
(Fig. 4C-a) abilities when compared
with the si-NC group. Snail is the first member of the Snail family
of proteins and it serves an important role in embryo implantation
(14). During development, Snail,
together with MMP family members, regulate a variety of
extracellular matrix proteins by inhibiting the expression of
E-cadherin (15). A key protein
involved in cell cycle regulation is cyclin D1, which regulates the
transition from G1 to S phase (16).
Bcl-xL is an important member of the Bcl-2 family of proteins, that
is an important anti-apoptotic protein (17). Western blot analysis demonstrated that
levels of Snail were increased in the si-OGA group and that
E-cadherin expression was negatively correlated with Snail
expression (P<0.05; Fig. 4D and
E). MMP2 and MMP9 expression were also significantly increased
in the si-OGA group (P<0.05; Fig. 4D
and E). Furthermore, the OGT-siRNA group demonstrated reduced
migration (Fig. 4A-b and B) and
invasion (Fig. 4C-b) abilities of
RL95-2 cells compared with the si-NC group. The expression levels
of Snail, E-cadherin, MMP2 and MMP9 were opposite to those of the
HEC-1A cells (P<0.05; Fig. 4D and
E). The results indicated that O-GlcNAcylation may
enhance cell migration and invasion by increasing the expression of
related proteins.
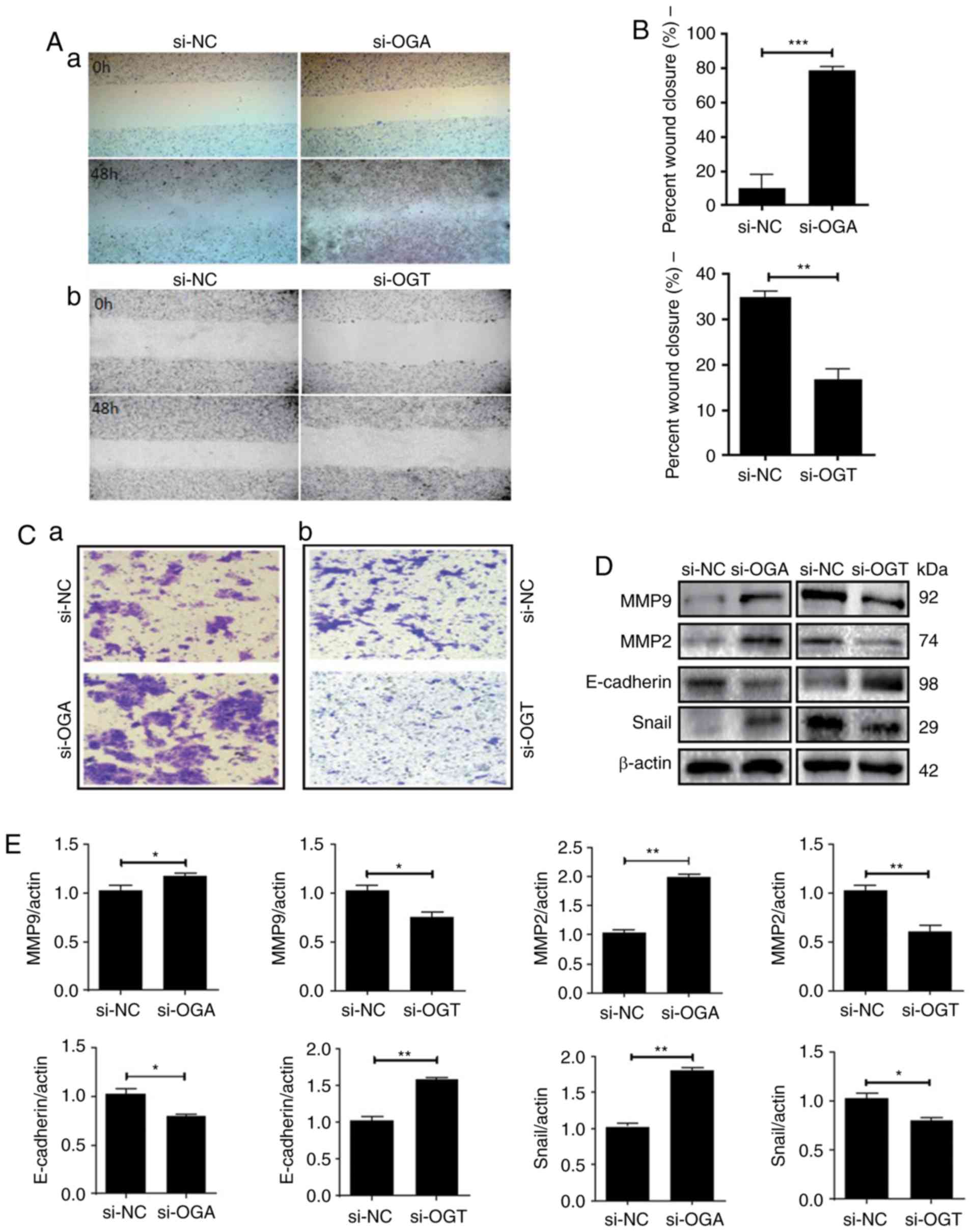 | Figure 4.O-GlcNAc expression influences
the migration and invasion of endometrial cells. (A-a, B and C-a)
Treatment of HEC-1A cells with OGA-specific siRNA promoted cell
migration and invasion. (D and E) si-OGA increased the expression
of MMP9, MMP2 and Snail, and decreased the expression of
E-cadherin. (A-b, B and C-b) Treatment of RL95-2 cells with si-OGT
abolished cell migration and invasion. (D and E) si-OGT decreased
the expression of MMP9, MMP2 and Snail, and increased the
expression of E-cadherin. Data are presented as the mean ± standard
deviations of at least three independent experiments; *P<0.05,
**P<0.01 and ***P<0.001. O-GlcNAc, O-linked
β-N-acetylglucosamine; OGA, O-GlcNAcase; siRNA, small
interfering RNA; MMP, matrix metallopeptidase; OGT, O-GlcNAc
transferase. |
Discussion
‘Classical’ protein glycosylation occurs only in
cell membrane and secretory proteins. Glycosylation primarily
consists of highly complex arrays of glycans, which can be divided
into two types: N- and O-glycans (18). The modification of proteins by
O-GlcNAc (O-GlcNAcylation) occurs in a myriad of
carbohydrate post-translational modifications. Unlike ‘traditional
glycosylation’, O-GlcNAc is generally not subject to further
modifications that produce more complex glycans and is localized
mainly within the cytoplasm or nucleoplasm (19).
Although the majority of glucose is metabolized via
glycolytic pathways, ~2–5% of the total glucose entering the cell
is channeled into the nutrient-sensing hexosamine biosynthetic
pathway (HBP) (20). In this pathway,
the first and rate-limiting enzyme, glutamine: Fructose-6-phosphate
amidotransferase, irreversibly uses an amino group from glutamine
to convert fructose-6-phosphate into glucosamine-6-phosphate and
glutamate. Glucosamine-6-phosphate is further metabolized to
UDP-GlcNAc, which serves as the monosaccharide donor for
O-GlcNAcylation (20). The
level of O-GlcNAc modification in a protein is dependent on
the concentration of the nucleotide sugar donor UDP-GlcNAc, a high
energy compound second only to ATP (21). A common feature of cancer cells is the
ability to increase glucose intake via the HBP pathway and elevate
substrate UDP-GlcNAc levels, resulting in
hyper-O-GlcNAcylation.
In recent years, there has been increasing evidence
that O-GlcNAcylation and its regulators (OGT and OGA) serve
an important role in the regulation of metabolic reprogramming in
tumors by changing key transcription factors, metabolic enzymes and
major carcinogenic signaling pathways (22–24). Two
studies on breast and thyroid cancers revealed that the activity of
OGA enzymes was increased in cancer tissue, although the expression
levels of OGA protein were not determined (9,25). In this
study, western blotting results determined that the level of
O-GlcNAcylation was decreased in certain tumor tissues
compared with the control group. However, previous studies have
demonstrated increased levels of O-GlcNAcylation in various
cancer tissues. For example, compared with the corresponding
para-cancerous tissue, O-GlcNAc modification was reported to
be increased in breast, lung and colon cancer tissue sections
(26,27). The expression of OGT and OGA also
appeared to increase in lung and colon tissues. Similarly, in
patients with chronic lymphoblastic leukemia, the level of
O-GlcNAc-modified proteins increased alongside OGT and OGA
protein levels, when compared with normal lymphocytes (28). Therefore, O-GlcNAc modification
may be a regulatory mechanism associated with metabolic changes and
the pathogenesis of cancer.
Previous experimental results have revealed that
O-GlcNAc modification is crucial for embryonic development.
Knockout of the OGT mouse gene exerts fatal effects on
embryonic development (29). Jang
et al (30) reported that
octamer-binding transcription factor 4 and sex determining
region-box 2 in embryonic stem cells are also glycosylated by
O-GlcNAc and the absence of O-GlcNAcylation reduces
the self-renewal ability of stem cells and hinders the
reprogramming of somatic cells.
The human endometrium undergoes a complex series of
organized proliferative and secretory changes in each menstrual
cycle, exhibiting only a short period of receptivity, known as the
‘window’ for embryo implantation. Embryo implantation is the main
limiting step during pregnancy and endometrial receptivity is
closely associated with embryo implantation. The establishment of
endometrial receptivity is important to ensure for the development
of the fetus and placenta. The rate of embryo implantation and
clinical pregnancy can be affected by the degree of endometrium
receptivity.
The process of malignant tumor invasion and
metastasis may be considered similar to that of embryo
implantation. It has been reported that the epithelial to
mesenchymal transition (EMT) process, which is important for tumor
cell invasion (31,32), is also necessary for embryo adhesion
and invasion as it mediates the remodeling process of human
endometrial cells (33). Therefore,
the present study hypothesized that O-GlcNAcylation could
also serve an important regulatory role in the establishment of
endometrial receptivity and embryo implantation. To test this
hypothesis, different clinical and hormonal tissue samples of human
endometrium were collected. The tissue revealed a reduced
expression of O-GlcNAc-modified proteins in the endometrium
during the proliferative phase. However, O-GlcNAc levels
increased significantly during the secretory phase. In addition,
the expression of O-GlcNAcylation in glandular epithelia or
luminal epithelia was higher than that of stromal cells.
The present study then utilized an in vitro
implantation model to clarify the role and molecular mechanism of
O-GlcNAc modification in endometrial receptivity. RNA
interference was used to decrease or increase the expression of
O-GlcNAcylation. OGT-siRNA was transfected into RL95-2
cells, and OGA-siRNA was transfected into HEC-1A cells. Cellular
function assays were subsequently performed to detect the effects
of O-GlcNAcylation on the proliferation, invasion and
migration of endometrial cells. CCK-8 and wound healing assays were
also performed to analyze cell proliferation and invasion and
migration, respectively. Furthermore, the expression of
EMT-associated proteins in RL95-2 and HEC-1A cells was detected.
The results revealed that increased levels of O-GlcNAc
modification promoted cell proliferation, migration and invasion,
while also increasing the rate of cellular adhesion, thereby
promoting embryo implantation and development. This indicated that
during the embryo implantation, O-GlcNAc modification may
promote the transformation of endometrial cell EMT and increase the
migration of endometrial epithelial cells. The migration of
epithelial cells causes the destruction and remodeling of the
epithelial barrier at the implantation site, which changes the
receptivity of the endometrium to promote embryo adhesion and
implantation. However, the mechanisms involved in these processes
are yet to be fully elucidated and require further study.
Over the past decade, significant progress has been
made in our understanding of the broad role of post-translational
modifications in basic cellular processes, including
phosphorylation, acetylation, methylation and ubiquitination
(34,35). Although O-GlcNAc modification
was discovered 30 years ago, its biological functions, including
the modification of protein structures and interactions, cellular
signaling, gene regulation, and physiological and metabolic
regulation, have only begun to be understood. There is a growing
body of evidence which indicates that O-GlcNAc serves as a
nutritional sensor that links the metabolic state of a system with
the regulation of cellular signal transduction, transcription and
protein degradation (36). With
respect to endometrial receptivity and embryo implantation, the
precise role of O-GlcNAc modification in these complex
processes still requires further elucidation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Scientific Grants (grant. nos. 31570798, 31971209, and
81901511), the Program for Liaoning Excellent Talents in University
(grant no. LR2017042), by the Liaoning Key R&D Program
(2019JH2/10300017), and by the program for Liaoning Provincial
Program for Top Discipline of Basic Medical Sciences.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YK and LS designed the experiments. XH, HL and XL
performed the experiments. MD and HZ collected and analyzed the
data. BY collected and analyzed the endometrial tissues. YX an AL
interpreted the data for the study. All authors reviewed and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Endometrial tissues were obtained from The First
Affiliated Hospital of Dalian Medical University under the Human
Research Agreement approved by the Ethics Committee of Dalian
Medical University. Written informed consent was acquired from each
patient prior to tissue collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torres CR and Hart GW: Topography and
polypeptide distribution of terminal N-acetylglucosamine residues
on the surfaces of intact lymphocytes. Evidence for O-linked
GlcNAc. J Biol Chem. 259:3308–3317. 1984.PubMed/NCBI
|
|
2
|
Holt GD and Hart GW: The subcellular
distribution of terminal N-acetylglucosamine moieties. Localization
of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol
Chem. 261:8049–8057. 1986.PubMed/NCBI
|
|
3
|
Paria BC, Huet-Hudson YM and Dey SK:
Blastocyst's state of activity determines the ‘window’ of
implantation in the receptive mouse uterus. Proc Natl Acad Sci USA.
90:10159–10162. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshinaga K: Uterine receptivity for
blastocyst implantation. Ann N Y Acad Sci. 541:424–431. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Psychoyos A: Hormonal control of
ovoimplantation. Vitam Horm. 31:201–256. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim HJ and Dey SK: HB-EGF: A unique
mediator of embryo-uterine interactions during implantation. Exp
Cell Res. 315:619–626. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu J, Fan J, Xu Y, Xie Y, Gong T and Kong
Y: Regulatory function of β1, 4-galac tosyltransferase I expression
on Lewis-Y glycan and embryo implantation. Gene. 562:220–225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu J, Sui LL, Cui D, Ma YN, Zhu CY and
Kong Y: Effects of LeY glycan expression on embryo implantation.
Eur Rev Med Pharmacol Sci. 20:3327–3335. 2016.PubMed/NCBI
|
|
9
|
Slawson C, Pidala J and Potter R:
Increased N-acetyl-beta- glucosaminidase activity in primary breast
carcinomas corresponds to a decrease in N-acetylglucosamine
containing proteins. Biochim Biophys Acta. 1537:147–157. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murray MJ and Lessey BA: Embryo
implantation and tumor metastasis: Common pathways of invasion and
angiogenesis. Semin Reprod Endocrinol. 17:275–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hannan NJ, Paiva P, Dimitriadis E and
Salamonsen LA: Models for study of human embryo implantation:
Choice of cell lines? Biol Reprod. 82:235–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2018. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma XH, Hu SJ, Yu H, Xu LB and Yang ZM:
Differential expression of transcriptional repressor snail gene at
implantation site in mouse uterus. Mol Reprod Dev. 73:133–141.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dey SK, Lim H, Das SK, Reese J, Paria BC,
Daikoku T and Wang H: Molecular cues to implantation. Endocr Rev.
25:341–373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin XM: Signal transduction mediated by
Bid, a pro-death Bcl-2 family proteins, connects the death receptor
and mitochondria apoptosis pathways. Cell Res. 10:161–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dennis JW, Nabi IR and Demetriou M:
Metabolism, cell surface organization, and disease. Cell.
139:1229–1241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Queiroz RM, Carvalho E and Dias WB:
O-GlcNAcylation: The sweet side of the cancer. Front Oncol.
4:1322014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marshall S, Bacote V and Traxinger RR:
Discovery of a metabolic pathway mediating glucose-induced
desensitization of the glucose transport system. Role of hexosamine
biosynthesis in the induction of insulin resistance. J Biol Chem.
266:4706–4712. 1991.PubMed/NCBI
|
|
21
|
Wells L, Vosseller K and Hart GW: A role
for N-acetylglucosamine as a nutrient sensor and mediator of
insulin resistance. Cell Mol Life Sci. 60:222–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee TI and Young RA: Transcriptional
regulation and its misregulation in disease. Cell. 152:1237–1251.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koo CY, Muir KW and Lam EW: FOXM1: From
cancer initiation to progression and treatment. Biochim Biophys
Acta. 1819:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khan Z and Bisen PS: Oncoapoptotic
signaling and deregulated target genes in cancers: Special
reference to oral cancer. Biochim Biophys Acta. 1836:123–145.
2013.PubMed/NCBI
|
|
25
|
Krzeslak A, Pomorski L and Lipinska A:
Elevation of nucleocytoplasmic beta-N-acetylglucosaminidase
(O-GlcNAcase) activity in thyroid cancers. Int J Mol Med.
25:643–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C,
Yang J, Han F, Lu X and Yu W: GlcNAcylation plays an essential role
in breast cancer metastasis. Cancer Res. 70:6344–6351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X,
Cong Q and Yu W: O-GlcNAcylation is a novel regulator of lung and
colon cancer malignancy. Biochim Biophys Acta. 1812:514–519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi Y, Tomic J, Wen F, Shaha S, Bahlo A,
Harrison R, Dennis JW, Williams R, Gross BJ, Walker S, et al:
Aberrant O-GlcNAcylation characterizes chronic lymphocytic
leukemia. Leukemia. 24:1588–1598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shafi R, Iyer SP, Ellies LG, O'Donnell N,
Marek KW, Chui D, Hart GW and Marth JD: The O-GlcNAc transferase
gene resides on the X chromosome and is essential for embryonic
stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA.
97:5735–5739. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jang H, Kim TW, Yoon S, Choi SY, Kang TW,
Kim SY, Kwon YW, Cho EJ and Youn HD: O-GlcNAc regulates
pluripotency and reprogramming by directly acting on core
components of the pluripotency network. Cell Stem Cell. 11:62–74.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lefebvre T, Pinte S, Guérardel C, Deltour
S, Martin-Soudant N, Slomianny MC, Michalski JC and Leprince D: The
tumor suppressor HIC1 (hypermethylated in cancer 1) is O-GlcNAc
glycosylated. Eur J Biochem. 271:3843–3854. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tai HC, Khidekel N, Ficarro SB, Peters EC
and Hsieh-Wilson LC: Parallel identification of O-GlcNAc-modified
proteins from cell lysates. J Am Chem Soc. 126:10500–10501. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schlummer S, Vetter R, Kuder N, Henkel A,
Chen YX, Li YM, Kuhlmann J and Waldmann H: Influence of serine
O-glycosylation or O-phosphorylation close to the vJun nuclear
localisation sequence on nuclear import. Chembiochem. 7:88–97.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Teng CB, Diao HL, Ma XH, Xu LB and Yang
ZM: Differential expression and activation of Stat3 during mouse
embryo implantation and decidualization. Mol Reprod Dev. 69:1–10.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cameron AM, Lawless SJ and Pearce EJ:
Metabolism and acetylation in innate immune cell function and fate.
Semin Immunol. 28:408–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hart GW, Housley MP and Slawson C: Cycling
of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins.
Nature. 446:1017–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|