Introduction
Vulvar cancer is a malignant tumor that poses a
threat to women's health (1). The
most common subtype is vulvar squamous cell carcinoma (VSCC),
accounting for 80–90% of all vulvar malignant tumors. VSCC is very
common among women aged >60 years (2). In recent years, its incidence has
increased. The cause for vulvar cancer development has not been
fully elucidated. Human papillomavirus has been reported to be
associated with a subset of vulvar cancers (3). It has been demonstrated that surgically
treated vulvar intraepithelial neoplasia (VIN) has a high rate of
recurrence (3.8%) and untreated VIN in women aged >30 years has
an appreciable invasive potential (4). Non-neoplastic lesions in the vulvar
epithelium include squamous epithelial hyperplasia and lichen
sclerosus (5). At present,
epidemiological studies have reported that the malignant rate is
1–5% (6). It is not clear in
molecular biology whether there is a malignant tendency in vulvar
intraepithelial tumor-like lesions and vulvar intraepithelial
non-tumor-like lesions. Further investigating the mechanism of
tumorigenesis and progression of VSCC may uncover novel targets and
help design novel therapeutic methods for VSCC treatment.
An increasing number of studies have proven that
microRNAs play important roles in carcinogenesis (7–9). MicroRNAs
are regulatory RNAs, 18–23 nucleotides in size (10). Although they have a low molecular
weight, they play key roles at the transcriptional level of human
cells, regulating several important biological functions, such as
tumor cell proliferation, invasion and migration (11). A number of studies have demonstrated
that microRNAs are abnormally expressed in different tumors, and
they act as tumor suppressors or tumor-promoting factors (12,13). Yang
and Guo reported that miR-3147 can participate in the
epithelial-to-mesenchymal transition of VSCC cells by inhibiting
Smad4 (14). Mir-30c and let-7a were
found to be reciprocally correlated with the expression of HMGA2
(15), which promotes diverse
tumorigenic processes in VSCC (16).
The upregulation of miR-590-5p was found to promote cellular
malignant behavior in VSCC via the target gene TGFβRII (17). However, to the best of our knowledge,
the role of miR-4712-5p in VSCC has not been reported to date.
The aim of the present study was to determine
whether miR-4712-5p is dysregulated in VSCC, and whether it plays a
role in the occurrence and development of VSCC, as well as to
investigate the underlying mechanism.
Materials and methods
Tissue collection
Three pairs of freshly frozen VSCC samples and
adjacent non-cancerous tissues were used for microarray assay
(Table I). A total of 30 freshly
frozen VSCC samples and adjacent non-cancerous tissues were
examined to confirm miRNA expression and validate the targeting.
All the tissues were obtained from the Department of Gynecology at
the First Affiliated Hospital of China Medical University between
January 2011 and January 2018. All the lesions were staged using
the new vulvar cancer classification system (18). The frozen specimens were examined
pathologically. Clinical records were retrospectively reviewed.
Patients who had not undergone chemotherapy or radiation treatment
prior to surgery were enrolled. The protocol of the present study
was approved by the Ethics Committee of the First Affiliated
Hospital of China Medical University and informed consent was also
obtained from the patients.
 | Table I.Characteristics of the patients in
the microarray study. |
Table I.
Characteristics of the patients in
the microarray study.
| Sample name | Age (years) | FIGO stage | Tumor
differentiation | Lymph node
metastasis |
|---|
| A exp | 48 | IIIA | Moderate | Yes |
| a ctrl | 48 | – | – | – |
| B exp | 85 | IB | High | No |
| b ctrl | 85 | – | – | – |
| F exp | 64 | IA | High | No |
| f ctrl | 64 | – | – | – |
Cell culture
Human VSCC A431 cells and the human normal vaginal
epithelial cell line HNVEC/HL-016 (VECs) were purchased from
Shanghai Cell Bank. Cells were cultured with DMEM (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% fetal bovine serum in a 5%
CO2 incubator at 37°C and saturated humidity.
Vector construction and cell
transfection
The vector with miR-4712-5p overexpression and
corresponding negative control (NC) were synthesized at GenePharma.
The protocol of cell transfection was as follows: A431 cells were
seeded at a density of 1×105 cells/well in 6-well
culture plates. As the cells were in a good condition and the
confluence was >70%, transfection was performed. After removal
of the culture medium, the cells were washed with PBS, followed by
addition of 1 ml medium to each well. siRNA and Lipofectamine 2000
(siRNA final concentration, 50 nM; Lipofectamine 2000, 1:50) was
dissolved with Opti-MEM. The diluted siRNA and Lipofectamine 2000
were mixed and allowed to form a complex for 20 min. The complex
was added to the cell culture plate (200 µl per well) and
incubated in a 5% CO2 incubator at 37°C overnight. The
medium was replaced every 4–6 h. After 48 h, the transfection
efficiency was determined by polymerase chain reaction (PCR)
analysis.
miR-4712-5p mimics and inhibitor sequences were
cloned into the green fluorescent lentivirus (GeneChem), which was
designed upon request. A431 cells were transfected with lentivirus
and screened with puromycin to increase transfection efficiency.
The transfection efficiency was examined using PCR.
Reverse transcription-quantitative
(RT-q)PCR analysis
VSCC tissues and A431 cells were treated with TRIzol
to extract RNA using the SYBR Prime Script miRNA RT-PCR Kit
(TAKARA) reaction system as follows: SYBR Premix Ex Taq II (2X), 10
µl; PCR forward primer (10 µM), 0.8 µl;
Uni-miR qPCR primer (10 µM), 0.8 µl; ROX Reference
Dye II (50X), 0.4 µl; cDNA template, 2 µl;
ddH2O, 6 µl. In the fluorescence qPCR device, the
reaction conditions were as follows: Pre-denaturation at 95°C for 1
min, denaturation at 95°C for 15 sec, annealing at 60°C for 40 sec,
and extension at 72°C for 15 sec for a total of 40 cycles. The
melting curve was analyzed. The expression of miRNA-124-3p was
calculated by the 2−ΔΔCq method (19), and U6 snRNA was used as a relative
quantitative internal reference. We defined its negative value as
relative low expression of miR-4712-5p and its positive value as
relative high expression of miR-4712-5p (VSCC compared with
adjacent normal tissues). The primers used for qPCR are listed in
Table II.
 | Table II.Gene primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table II.
Gene primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer (5′→3′) |
|---|
| miR-4712-5p | Forward:
AAATGACGAGTTACTGGATGACATA |
|
| Reverse:
ATCCAGTAACTCGTCATTTTATGTC |
| RNU48 | Forward:
AGTGATGATGACCCCAGGTAA |
|
| Reverse:
CTCTTGAGTGTGTCGCTGATG |
| PTEN | Forward:
GGACGAACTGGTGTAATGATATG |
|
| Reverse:
TCTACTGTTTTTGTGAAGTACAGC |
| GAPDH | Forward:
GCATGATGCCGGCAGCTTT |
|
| Reverse:
CAGCAACTGAATGAGGCCA |
Transwell migration assay
An artificial basement membrane was formed by
Matrigel. The cells were collected, washed once with PBS, and
diluted to 1×105/ml with complete medium. A total of 0.5
ml of the cell mixture was added to the upper chamber, and 0.75 ml
of DMEM supplemented with 10% fetal bovine serum was added to the
lower chamber. After incubation at 37°C for 48 h, all samples were
stained with hematoxylin and eosin. At an original magnification of
×100 under a light microscope, three fields were randomly selected.
The number of cells which traversed the artificial basement
membrane was counted, and the mean value was calculated.
Cell Counting Kit-8 (CCK-8) assay
A431 cells in the logarithmic growth phase were
collected, and prepared into a single-cell suspension with cell
culture medium. Cells were seeded in 96-well culture plates (100
µl/well) under sterile conditions
(3×l03−5×l03/well). After 24 h of incubation,
the medium was replaced. After treatment with siRNA and blank
control, the cells were further cultured for 48 h. CCK-8 solution
(10 µl) was added to each well and further incubated for 2–4
h. Absorbance values were measured at 450 nm with a microplate
reader (MR-96A, Mindray).
Animal tumor transplantation
experiment
A total of 16 nude mice, aged 4 weeks, were
purchased from China Medical University. All animals were
maintained in individual cages under controlled ambient temperature
(24±2°C) and 40–70% humidity, with a 12-h light/dark cycle.
Standard pelleted chow and drinking water were available ad
libitum. The mice were allowed to acclimatize for at least 1
week before the experiments. The health and behavior of mice were
observed daily by animal husbandry staff at our facility. To reduce
animal suffering and distress, animal tools and nesting material
were provided to the mice. A431 and A431/miR-4712-5p cells (0.1 ml;
5×106/ml) were subcutaneously injected into the right
axilla (8 mice per group). The mice were examined every 3 days to
observe and record the growth of the tumor. After tumor
development, the weight of the mice was recorded and the length and
width of the tumor were measured to calculate the tumor volume. Two
weeks later, all animals were sacrificed by cervical dislocation,
and the tumors were resected and weighed. The experiment was
approved by the Department of Laboratory Animal of China Medical
University and the Animal Experimental Protection Agency.
Dual luciferase reporter gene
analysis
The PTEN 3′-untranslated region (UTR) sequence was
cloned into the pMIR-REPORTTM vector (Ambion). 293T cells were
grown in 24-well plates until the density reached 50% and
co-transfected with 200 ng wild-type or mutant firefly luciferase
reporter plasmid and 10 ng pMIR-REPORTTM control plasmid, 100 nM
miR-4712-5p or NC miRNA. After 48 h of culture, the cells were
harvested and examined with the luciferase activity system (Promega
Corporation).
Immunohistochemistry
The tumors from patients and nude mice were
sectioned for pathological examination. Antigen was retrieved with
citric acid at 120°C and 100 kPa. The sections were washed with
PBS, incubated with H2O2 for 30 min, blocked
with 5% bovine serum albumin for 30 min, then 10% goat serum for 30
min. Primary anti-PTEN antibody (rabbit monoclonal, 1:800 dilution,
ab32199, Abcam) was added and incubated at 4°C overnight. After
washing with PBS, secondary antibody was added and incubated at
37°C for 40 min, followed again by washing with PBS. Horseradish
peroxidase-labeled streptavidin/avidin was added and incubated at
37°C for 40 min. After washing with PBS, the sections were
developed with 3,3′-diaminobenzidine.
Western blot assay
Tissues of patients and cells collected after
transfection were lysed in RIPA lysate containing protease
inhibitor. Following centrifugation at 12,000 × g at 4°C for 20
min, the supernatant was collected. The protein concentration was
determined using the bicinchoninic acid assay. The proteins were
separated by 10% SDS-PAGE and transferred onto a polyvinylidene
fluoride membrane. The membrane was blocked with skimmed milk, and
incubated with antibodies against p-AKT (1:200 ab38449, Abcam),
cyclin D1 (1:200 ab134175, Abcam), PTEN (1:200 ab32199, Abcam),
CDK4 (1:200 ab199728, Abcam), p-GSK3β (1:200 ab68476, Abcam) and
CDK6 (1:200 ab131439, Abcam) at 4°C overnight. The membrane was
washed with TBST, incubated with secondary antibody at room
temperature for 2 h, visualized with an ECL kit (32106; Invitrogen;
Thermo Fisher Scientific, Inc.), and photographed with a gel
imaging system. The results were analyzed by absorbance using
ImageJ software (National Institutes of Health).
Statistical analysis
All results were analyzed using SPSS 19.0 (SPSS,
Inc.). Measurement data are expressed as the mean ± standard error
of the mean. The difference between groups was compared using
t-test. The difference among multiple groups was compared using
one-way analysis of variance) with Tukey's multiple comparison post
hoc test. A value of P<0.05 was considered to indicate
statistically significant differences.
Results
MicroRNA expression profile of
VSCC
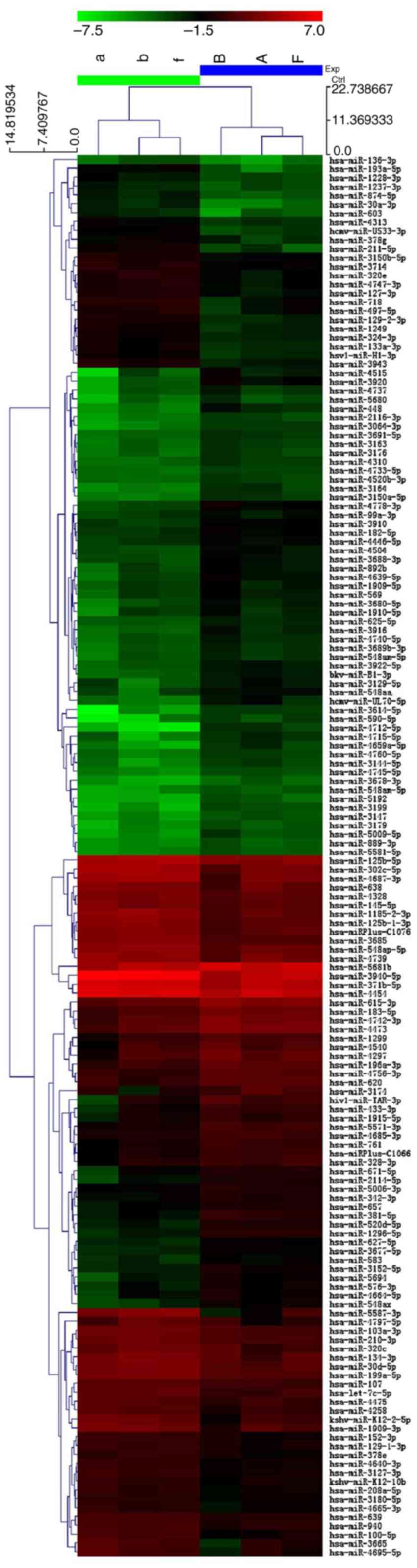
We determined the miRNA expression profile of VSCC.
We selected the miRNAs which were overexpressed or underexpressed
by >2-fold in bioinformatics analysis and identified 90
upregulated and 67 downregulated miRNAs in the cancer samples
(Fig. 1).
MicroRNA-4712-5p is upregulated in
VSCC tissues and cells
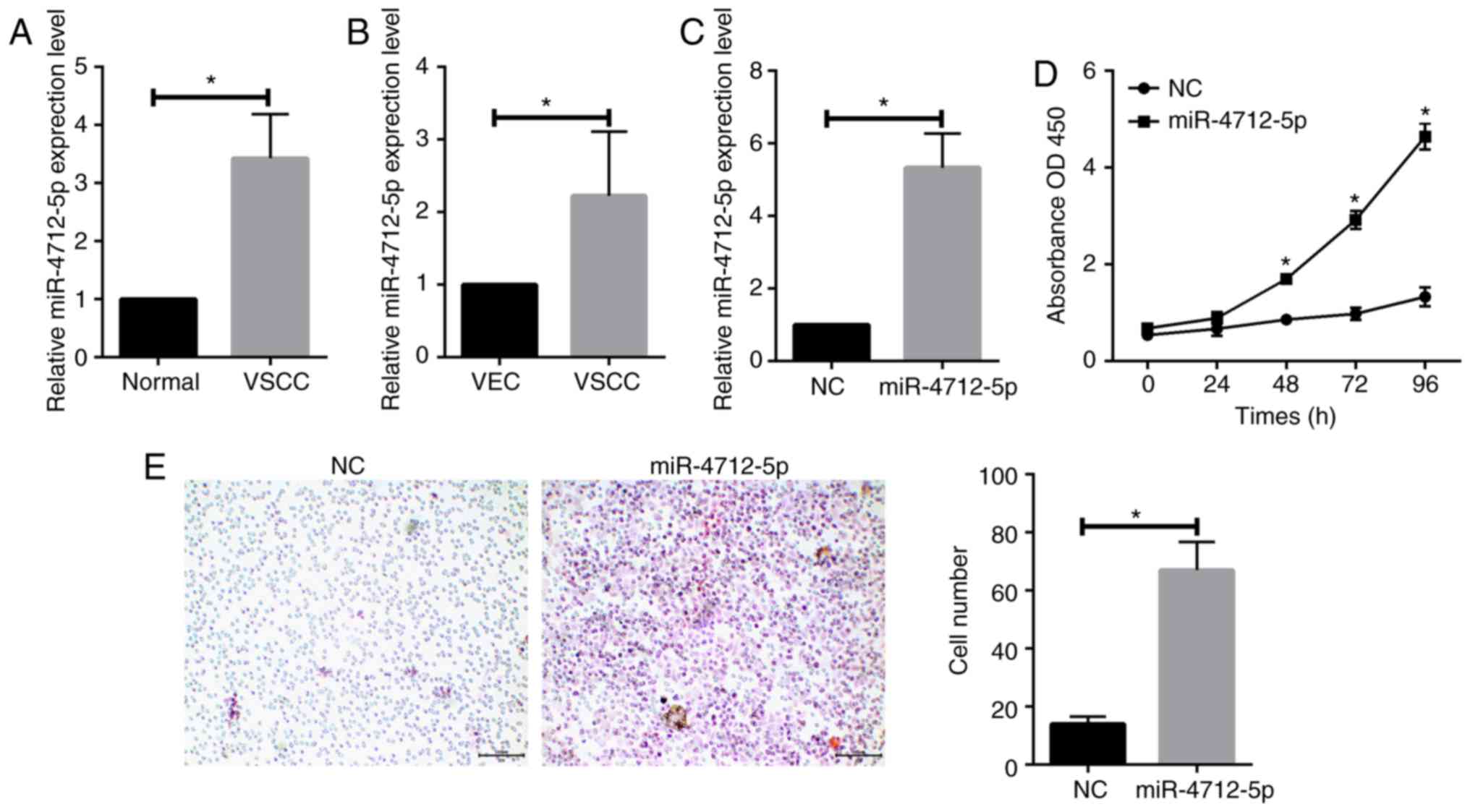
RT-qPCR was conducted to determine the expression
levels of miR-4712-5p in 56 pairs of VSCC and adjacent normal
tissues. The results revealed that the expression level of
miR-4712-5p was significantly upregulated in VSCC tissues
(P<0.05; Fig. 2A). RT-qPCR was
further used to detect the expression levels of miR-4712-5p in VSCC
cells and VECs. The results demonstrated that the expression of
miR-4712-5p in VSCC cells was significantly higher compared with
that in VECs (P<0.05; Fig.
2B).
MicroRNA-4712-5p promotes the
proliferation and invasion of VSCC
To further study the role of miR-4712-5p in VSCC,
A431 cells were separately transfected with miR-4712-5p mimics and
NC, and the transfection efficacy was verified by RT-qPCR (Fig. 2C). The CCK-8 assay was used to detect
the effect of miR-4712-5p on the proliferation of VSCC cells. On
the fifth day, the proliferation rate of cells transfected with
miR-4712-5p was significantly increased compared with day 3 of
culture (P<0.05; Fig. 2D). The
Transwell assay demonstrated that the invasiveness of cells
transfected with miR-4712-5p mimics was significantly higher
compared with that in the negative control group (P<0.05;
Fig. 2E). These results suggested
that miR-4712-5p can affect cell proliferation and invasion.
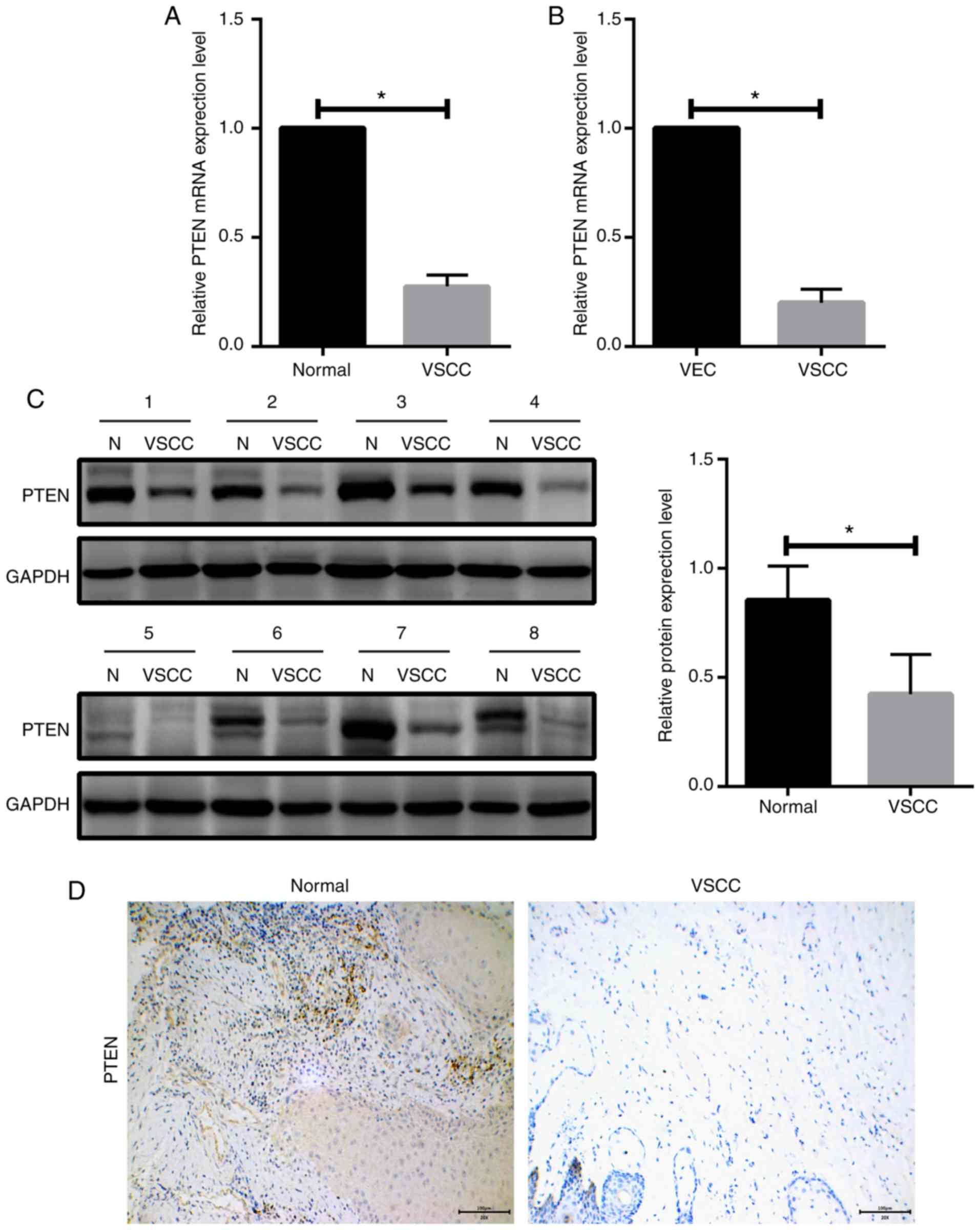
PTEN is downregulated in VSCC tissues
and cells
To examine the association between miR-4712-5p and
PTEN, we first analyzed the mRNA expression levels of PTEN in 56
paired human VSCC samples and adjacent normal tissues by RT-qPCR.
The results demonstrated that the mRNA expression level of PTEN in
VSCC tissues was significantly downregulated compared with that in
normal tissues (P<0.05; Fig. 3A).
PTEN mRNA expression levels in VSCC cells and VECs were determined
by RT-qPCR. The results demonstrated that the expression of PTEN in
VSCC cells was significantly lower compared with that in VECs
(P<0.05; Fig. 3B). We next
examined PTEN expression in VSCC tissues and adjacent normal
tissues in 8 clinicopathological samples by western blot assay. The
results revealed that the expression level of PTEN in VSCC tissues
was significantly lower compared with that in adjacent normal
tissues (P<0.05; Fig. 3C).
Immunohistochemical examination yielded the same results (Fig. 3D).
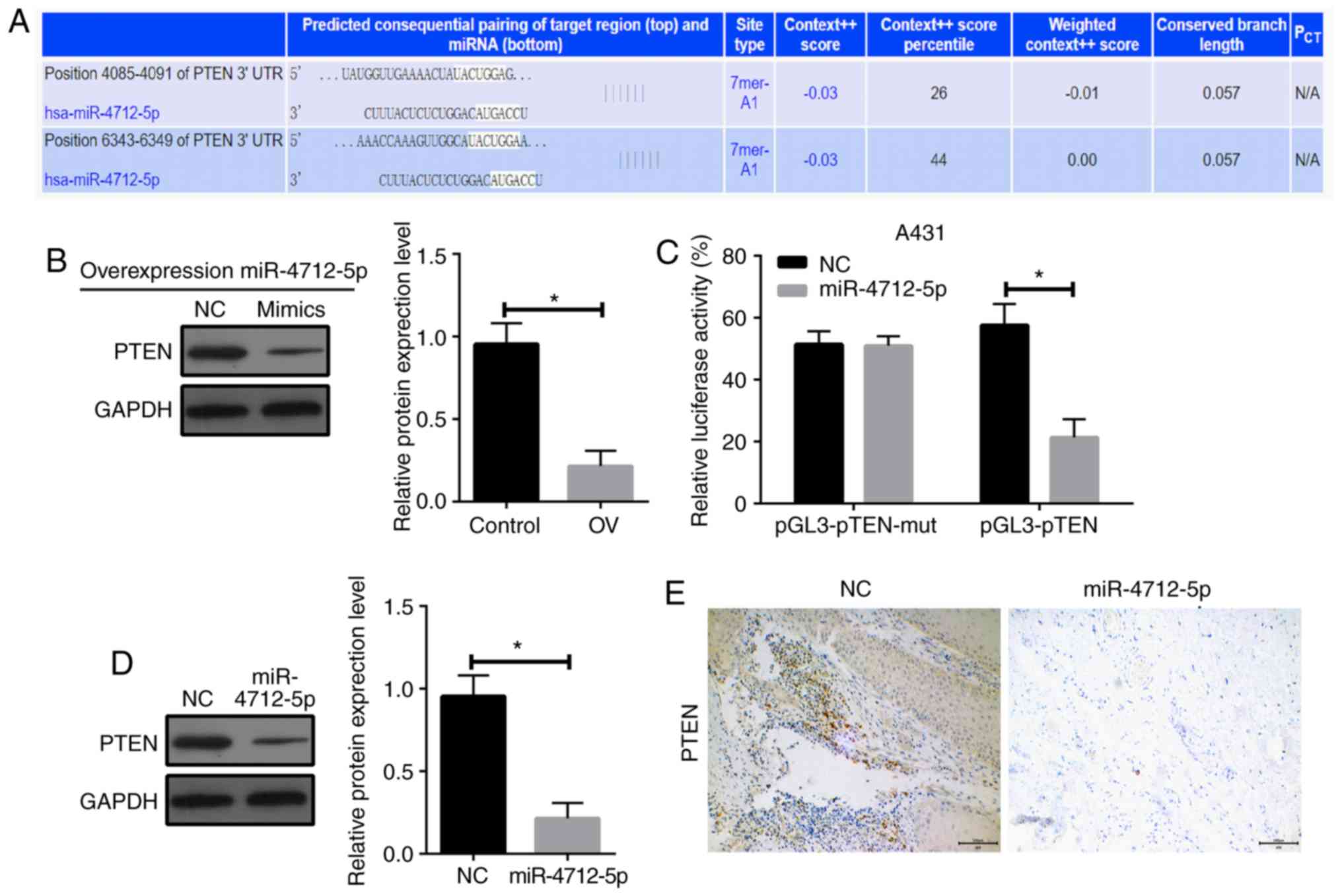
PTEN is a direct target of
microRNA-4712-5p
To investigate the potential mechanism of
miR-4712-5p affecting the proliferation of VSCC cells, we used the
public databases microRNA (http://www.microrna.org/microrna/getdownloads.do),
miRDB (http://mirdb.org) and TargetScan (http://www.targetscan.org/vert_71/) for
bioinformatics analysis (Fig. 4A).
The results revealed that PTEN has a conserved sequence that may
bind to miR-4712-5p. It was hypothesized that miR-4712-5p promotes
tumor growth by downregulating the expression of PTEN. The
expression of PTEN protein following miR-4712-5p expression
alterations was determined by western blot assay. The results
demonstrated that the overexpression of miR-4712-5p significantly
downregulated the expression of PTEN (P<0.05; Fig. 4B). The luciferase reporter gene assay
revealed that the luciferase activity of A431 cells co-transfected
with miR-4712-5p mimics and pGL3-PTEN vector was significantly
lower compared with that of cells transfected with NC (P<0.05).
The luciferase activity of the same cells co-transfected with
miR-4712-5p mimics and pGL3-PTEN-mut vector did not differ
significantly from that of cells transfected with NC (P>0.05;
Fig. 4C). These results indicate that
the PTEN gene may be one of the direct targets of miR-4712-5p.
The expression of PTEN in the A431 cells transfected
with miR-4712-5p was compared with the blank control group. The
A431 cells transfected with miR-4712-5p exhibited significantly
decreased expression of PTEN (P<0.05) (Fig. 4D), as shown by western blot assay.
This result suggests that there is a significant correlation
between the high expression of miR-4712-5p and the low expression
of the PTEN protein. The results of the immunohistochemical
examination further proved these findings (Fig. 4E). These results indicate that PTEN
may be a target of miRNA-4712-5p.
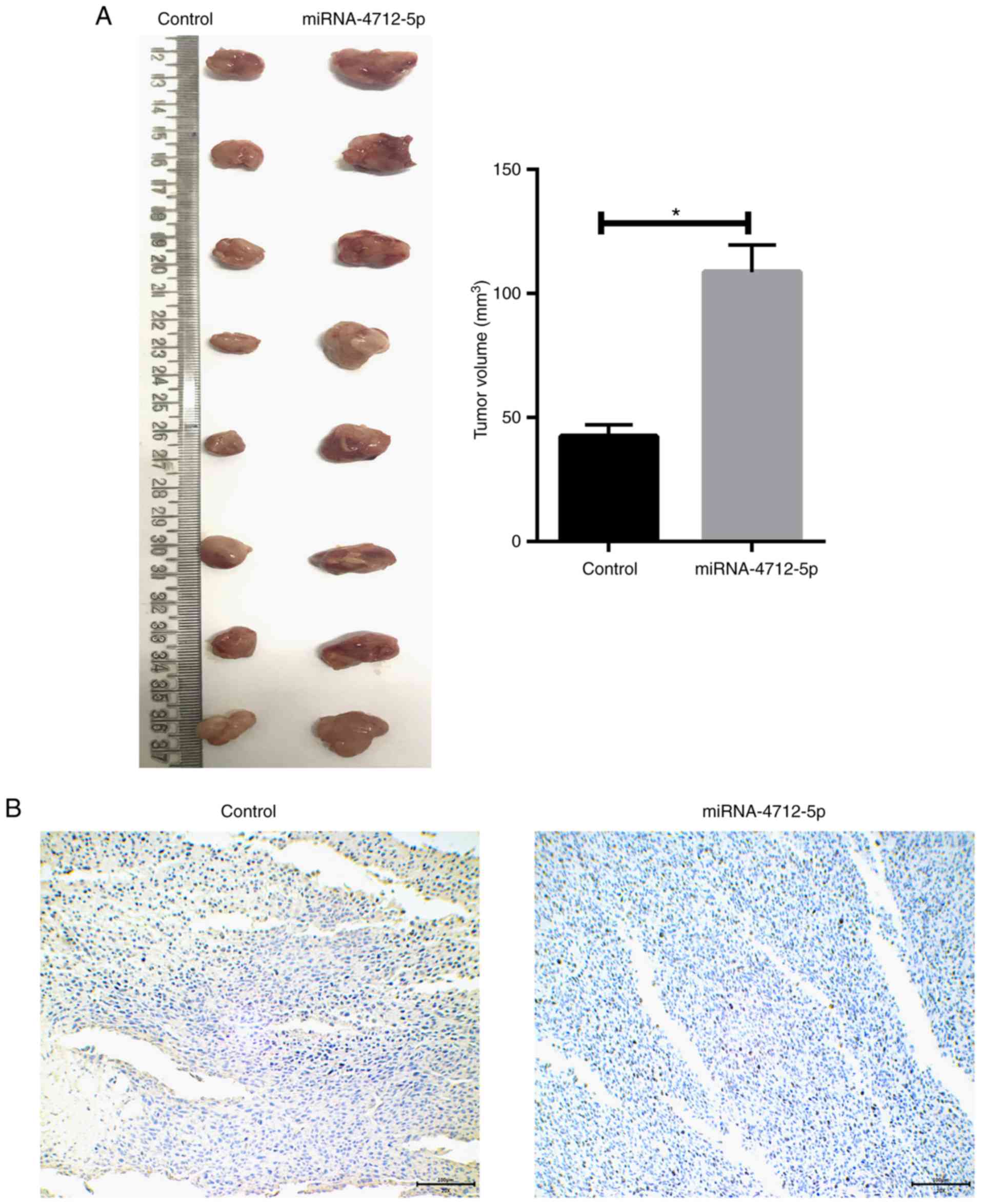
MicroRNA-4712-5p promotes tumor growth
in mouse xenograft models
The role of miRNA-4712-5p expression in a nude mouse
model was further investigated. The results demonstrated that the
tumor volume of the transfected miRNA-4712-5p cells was
significantly larger compared with that of the blank control group
(P<0.05) (Fig. 5A). We prepared
and observed the tissue sections of xenograft tumors. The
immunohistochemical results demonstrated that transfection of
miRNA-4712-5p significantly downregulated the expression of PTEN in
xenograft tumors (Fig. 5B).
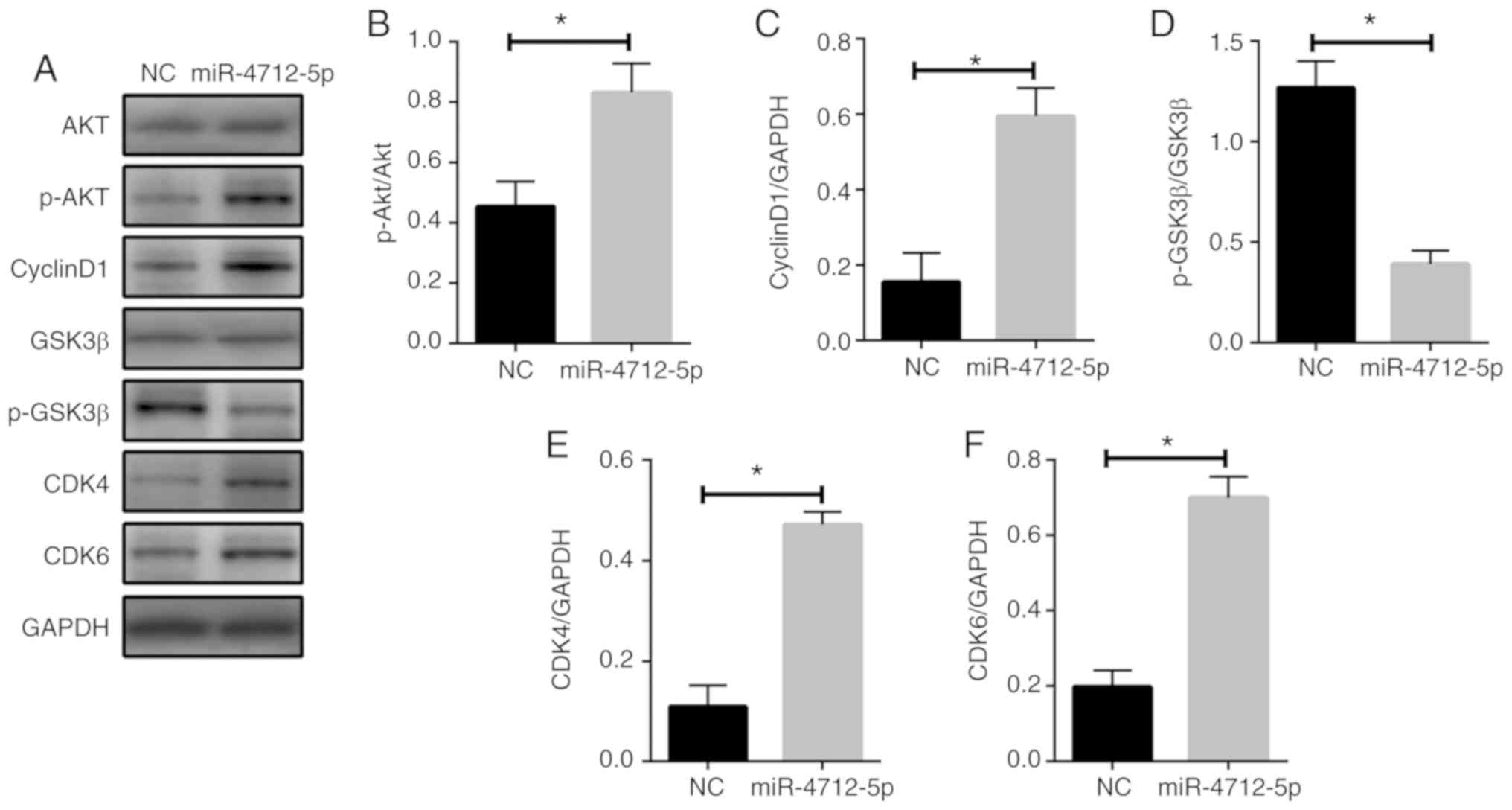
MicroRNA-4712-5p regulates cyclin D1
expression via PTEN
To further investigate the mechanism of miR-4712-5p
affecting VSCC cell proliferation in vitro and in
vivo by targeting PTEN, the expression of p-AKT, cyclin D1,
p-GSK3β, CDK4 and CDK6 in VSCC cells was detected by western blot
assay. The results demonstrated that the protein expression of
p-AKT, cyclin D1, CDK4 and CDK6 in miRNA-4712-5p cells was
significantly increased, and the expression of the p-GSK3β protein
was significantly decreased (P<0.05) (Fig. 6A-F). These results indicate that
miRNA-4712-5p affects the proliferation of VSCC cells and this
effect may be mediated by the PTEN/AKT/p-GSK3β/cyclin D1 signaling
pathway.
Discussion
Vulvar cancer belongs to the group of gynecological
malignant tumors (18). Vulvar
intraepithelial neoplasia is a type of atypical hyperplasia of the
vulva (19). Studies on VSCC are
relatively rare, even more so in China; however, its incidence has
been increasing in recent years, and it is becoming a major
gynecological concern.
miRNAs are small non-coding RNAs that regulate
post-transcriptional gene expression by interfering with the
translation of one or more target mRNAs (20). Despite their low molecular weight,
they play a key role in regulating a number of important human
biological functions at the transcriptional level, such as tumor
cell proliferation, invasion, and metastasis (21–23).
Deregulation of miRNAs is a major factor in almost all types of
cancer (24). miRNAs are also
important regulators of hematopoietic function, through controlling
the gene expression of several transcription factors necessary for
the formation, differentiation and apoptosis of hematopoietic stem
cells, so specific miRNAs may represent a potential therapeutic
target for acute lymphoblastic leukemia (25). In the present study, qPCR was used to
detect differences in RNA levels between VSCC and adjacent normal
tissues. The expression of miR-4712-5p was found to be obviously
higher in cancer tissues compared with that in adjacent normal
tissues. Histological differences suggest that the presence of
relevant microRNAs may exert some effect on the biological
properties of the tumor. To the best of our knowledge, the
mechanism of action of miR-4712-5p in VSCC has not yet been
reported. Therefore, based on the histological differences, we
propose that miR-4712-5p may act as a carcinogenic factor and
promote VSCC growth and invasion.
PTEN is a potent tumor suppressor and loss of its
function is often observed in hereditary and sporadic cancers
(26). PTEN has phosphatase-dependent
and phosphatase-independent activities in cells and controls a
variety of biological processes, including maintenance of genomic
stability, cell survival, migration, proliferation and metabolism
(27–31). Even a subtle reduction in PTEN levels
and activity may increase cancer susceptibility and contribute to
tumor progression (32). Therefore,
PTEN has become a hotspot of cancer research. Liu et al have
demonstrated that miR-19b plays a carcinogenic role in the
progression of ovarian cancer and promotes metastasis and invasion
of ovarian cancer cells by inhibiting the PTEN/AKT signaling
pathway (33). Chen et al
found that the expression of miR-1297 is low in human cervical
cancer tissues, and the overexpression of miR-1297 may activate
PTEN, increasing proliferation and decreasing apoptosis of HeLa
cells (34). Cyclin D1 is an
important member of the cyclin family (35). In normal proliferating cells, the
level of cyclin D1 is extremely low, whereas its overexpression
leads to uncontrolled cell proliferation, and eventually tumor
formation; thus, it is often defined as an oncoprotein (36). When cyclin D1 is overexpressed, it
often combines with CDK4 and CDK6 to form a complex under the
stimulation of some external factors, resulting in cell cycle
abnormalities and tumorigenesis (37). Cyclin D1 protein is highly expressed
in several malignant tumors and participates in their occurrence
and development (38,39). Worsley et al considered that
the positive expression rate of cyclin D1 in ovarian borderline
tumors and well-differentiated malignant tumors was >70%, and
the positive expression in poorly differentiated tumors was ~15%,
while negative expression was found in normal ovarian and benign
tumor tissues (40). Cyclin D1
expression abnormalities are considered an early event in ovarian
cancer and are associated with tumor proliferative activity. These
findings indicate that, when cyclin D1 is overexpressed, it may
enhance the proliferative ability of the tumor.
The present study demonstrated that miR-4712-5p
targeted the PTEN protein, and A431 cells transfected with
miR-4712-5p exhibited markedly reduced expression and inhibition of
PTEN, with increased proliferative and invasive abilities of the
tumor. The luciferase activity assay was used to confirm the direct
correlation between miR-4712-5p and PTEN. As described above, the
3′-UTR of miR-4712-5p directly binds to the PTEN protein,
suggesting that miR-4712-5p acts as a carcinogen in VSCC through
the PTEN signaling pathway axis. The PTEN/AKT protein signaling
pathway has been shown to affect the proliferative capacity of
cancer cells through periodic pathways in other cancers. Our
experiments also confirmed that miR-4712-5p targeted the PTEN
protein, thereby affecting the proliferation of VSCC cells, and
this action was dependent on the PTEN/Akt/p-GSK3β/cyclin D1
signaling pathway. Following transfection with miR-4712-5p, the
expression of PTEN and p-GSK3β was markedly downregulated, while
that of p-Akt and cyclin D1 was upregulated. These results validate
our previous hypothesis that miR-4712-5p targets PTEN and promotes
the proliferation of VSCC cells through a periodic pathway. The
present study may help achieve a better understanding of the
molecular mechanisms underlying the development and progression of
VSCC and identify a new therapeutic target for vulvar cancer.
In conclusion, microRNA-4712-5p reduces the
expression of PTEN, thereby affecting the downstream p-AKT, p-GSK3β
and cyclin D1 signaling pathways and promoting the proliferation
and invasion of VSCC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of China (30973190) and Liaoning science and technology
program (2014021057).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY and XW conceived and designed the study and
drafted the manuscript. SY, YZ, YL, LW, CL, ZF, HS and WZ performed
the experiments and interpreted the results. SY, CL, ZF, HS and WZ
analyzed the data. XW contributed to acquisition of funding
support. All authors have read and approved the final version of
this manuscript.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Ethics Committee of the First Affiliated Hospital of China
Medical University and informed consent was obtained from the
patients.
Patent consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Suh DH, Kim M, Kim HJ, Lee KH and Kim JW:
Major clinical research advances in gynecologic cancer in 2015. J
Gynecol Oncol. 27:e532016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clancy AA, Spaans JN and Weberpals JI: The
forgotten woman's cancer: Vulvar squamous cell carcinoma (VSCC) and
a targeted approach to therapy. Ann Oncol. 27:1696–1705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yap ML, Allo G, Cuartero J, Pintilie M,
Kamel-Reid S, Murphy J, Mackay H, Clarke B, Fyles A and Milosevic
M: Prognostic significance of human papilloma virus and p16
expression in patients with vulvar squamous cell carcinoma who
received radiotherapy. Clin Oncol (R Coll Radiol). 30:254–261.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jones RW, Rowan DM and Stewart AW: Vulvar
intraepithelial neoplasia: Aspects of the natural history and
outcome in 405 women. Obstet Gynecol. 106:1319–1326. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hantschmann P, Sterzer S, Jeschke U and
Friese K: P53 expression in vulvar carcinoma, vulvar
intraepithelial neoplasia, squamous cell hyperplasia and lichen
sclerosus. Anticancer Res. 25:1739–1745. 2005.PubMed/NCBI
|
|
6
|
Jancárková N, Freitag P and Zivný J:
Vulvar carcinoma-retrospective study of 47 cases (epidemiology,
etiology and long-term results. Ceska Gynekol. 67:78–82. 2002.(In
Czech). PubMed/NCBI
|
|
7
|
Zhao L and Zhang Y and Zhang Y: Long
noncoding RNA CASC2 regulates hepatocellular carcinoma cell
oncogenesis through miR-362-5p/Nf-κB axis. J Cell Physiol.
233:6661–6670. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang GH, Du L, Li N, Zhang Y, Xiang Y,
Tang JH, Xia S, Zhang EE and Lv SQ: Methylation-mediated
miR-155-FAM133A axis contributes to the attenuated invasion and
migration of IDH mutant gliomas. Cancer Lett. 432:93–102. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo Y, Cao X, Chen J, Gu J, Zhao J and Sun
J: MicroRNA-224 suppresses osteoblast differentiation by inhibiting
SMAD4. J Cell Physiol. 233:6929–6937. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lekka E and Hall J: Non-coding RNAs in
disease. FEBS Lett. 592:2884–2900. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tao L, Zhang CY, Guo L, Li X, Han NN, Zhou
Q and Liu ZL: MicroRNA-497 accelerates apoptosis while inhibiting
proliferation, migration, and invasion through negative regulation
of the MAPK/ERK signaling pathway via RAF-1. J Cell Physiol.
233:6578–6588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao H, Zhao H, Zhang Y and Zhou Y:
MicroRNA199b promotes cell proliferation and invasion in Wilms'
tumour by directly targeting runt-related transcription factor 3.
Mol Med Rep. 18:1812–1819. 2018.PubMed/NCBI
|
|
13
|
Liang Y, Zhang P, Li S, Li H, Song S and
Lu B: MicroRNA-873 acts as a tumor suppressor in esophageal cancer
by inhibiting differentiated embryonic chondrocyte expressed gene
2. Biomed Pharmacother. 105:582–589. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang XH and Guo F: miR3147 serves as an
oncomiR in vulvar squamous cell cancer via Smad4 suppression. Mol
Med Rep. 17:6397–6404. 2018.PubMed/NCBI
|
|
15
|
Agostini A, Brunetti M, Davidson B, Trope
CG, Heim S, Panagopoulos I and Micci F: Expressions of miR-30c and
let-7a are inversely correlated with HMGA2 expression in squamous
cell carcinoma of the vulva. Oncotarget. 7:85058–85062. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hetland TE, Holth A, Kærn J, Flørenes VA,
Tropé CG and Davidson B: HMGA2 protein expression in ovarian serous
carcinoma effusions, primary tumors, and solid metastases. Virchows
Arch. 460:505–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X and Wu X: miRNA expression profile
of vulvar squamous cell carcinoma and identification of the
oncogenic role of miR-590-5p. Oncol Rep. 35:398–408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sznurkowski JJ: Vulvar cancer: Initial
management and systematic review of literature on currently applied
treatment approaches. Eur J Cancer Care (Engl). 25:638–646. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Preti M, Scurry J, Marchitelli CE and
Micheletti L: Vulvar intraepithelial neoplasia. Best Pract Res Clin
Obstet Gynaecol. 28:1051–1062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paul P, Chakraborty A, Sarkar D, Langthasa
M, Rahman M, Bari M, Singha RS, Malakar AK and Chakraborty S:
Interplay between miRNAs and human diseases. J Cell Physiol.
233:2007–2018. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang LL, Zhang LF, Guo XH, Zhang DZ, Yang
F and Fan YY: Downregulation of miR-335-5p by long noncoding RNA
ZEB1-AS1 in gastric cancer promotes tumor proliferation and
invasion. DNA Cell Biol. 37:46–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Zhou L, Shi M, Kuang Y and Fang
L: Downregulation of miRNA-15a and miRNA-16 promote tumor
proliferation in multiple myeloma by increasing CABIN1 expression.
Oncol Lett. 15:1287–1296. 2018.PubMed/NCBI
|
|
23
|
Hu L, Wu H, Wan X, Liu L, He Y, Zhu L, Liu
S, Yao H and Zhu Z: MicroRNA-585 suppresses tumor proliferation and
migration in gastric cancer by directly targeting MAPK1. Biochem
Biophys Res Commun. 499:52–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rupaimoole R, Calin GA, Lopez-Berestein G
and Sood AK: miRNA deregulation in cancer cells and the tumor
microenvironment. Cancer Discov. 6:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ultimo S, Martelli AM, Zauli G, Vitale M,
Calin GA and Neri LM: Roles and clinical implications of microRNAs
in acute lymphoblastic leukemia. J Cell Physiol. 233:5642–5654.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pulido R: PTEN inhibition in human disease
therapy. Molecules. 23:E2852018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mukherjee A and Karmakar P: Attenuation of
PTEN perturbs genomic stability via activation of Akt and
down-regulation of Rad51 in human embryonic kidney cells. Mol
Carcinog. 52:611–618. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng D, Zhang L, Yang G, Zhao L, Peng F,
Tian Y, Xiao X, Chung RT and Gong G: Hepatitis C virus NS5A drives
a PTEN-PI3K/Akt feedback loop to support cell survival. Liver Int.
35:1682–1691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tian K, Di R and Wang L: MicroRNA-23a
enhances migration and invasion through PTEN in osteosarcoma.
Cancer Gene Ther. 22:351–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Howitt J, Low LH, Putz U, Doan A, Lackovic
J, Goh CP, Gunnersen J, Silke J and Tan SS: Ndfip1 represses cell
proliferation by controlling Pten localization and signaling
specificity. J Mol Cell Biol. 7:119–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Worby CA and Dixon JE: PTEN. Annu Rev
Biochem. 83:641–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bera A, Ghosh-Choudhury N, Dey N, Das F,
Kasinath BS, Abboud HE and Choudhury GG: NFκB-mediated cyclin D1
expression by microRNA-21 influences renal cancer cell
proliferation. Cell Signal. 25:2575–2586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu DT, Yao HR, Li YY, Song YY and Su MY:
MicroRNA-19b promotes the migration and invasion of ovarian cancer
cells by inhibiting the PTEN/AKT signaling pathway. Oncol Lett.
16:559–565. 2018.PubMed/NCBI
|
|
34
|
Chen Z, Zhang M, Qiao Y, Yang J and Yin Q:
MicroRNA-1297 contributes to the progression of human cervical
carcinoma through PTEN. Artif Cells Nanomed Biotechnol. 46 (Suppl
2):S1120–S1126. 2018. View Article : Google Scholar
|
|
35
|
John RR, Malathi N, Ravindran C and
Anandan S: Mini review: Multifaceted role played by cyclin D1 in
tumor behavior. Indian J Dent Res. 28:187–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qie S and Diehl JA: Cyclin D1, cancer
progression, and opportunities in cancer treatment. J Mol Med
(Berl). 94:1313–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kozar K and Sicinski P: Cell cycle
progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell
Cycle. 4:388–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hong Y, Huang X, An L, Ye H, Ma K, Zhang F
and Xu Q: Overexpression of COPS3 promotes clear cell renal cell
carcinoma progression via regulation of Phospho-AKT(Thr308), Cyclin
D1 and Caspase-3. Exp Cell Res. 365:163–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun W, Guo F and Liu M: Upregulated WDR5
promotes gastric cancer formation by induced cyclin D1 expression.
J Cell Biochem. 119:3304–3316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Worsley SD, Ponder BA and Davies BR:
Overexpression of cyclin D1 in epithelial ovarian cancers. Gynecol
Oncol. 64:189–195. 1997. View Article : Google Scholar : PubMed/NCBI
|