Introduction
According to reports, ~1–2% of all of cancer cases
occur in the thyroid (1). Papillary
thyroid carcinoma (PTC) is the most common malignancy of the
thyroid gland. The incidence of PTC in 30–50-year-old women is
twice that of age-matched men (2).
The prevalence of PTC has been increasing at an alarming rate of
~4% per year in the United States (3). Although patients with PTC have a good
prognosis, half of patients are <40 years of age and more likely
to develop lymph node metastasis; patients may experience
recurrence or even succumb to disease. Therefore, examining the
molecular mechanism underlying the occurrence and development of TC
is of high significance.
Ku is a DNA-binding protein that is important in
double-stranded DNA break repair via the non-homologous end-joining
pathway (4,5). It is also essential for V(D)J
recombination (5). The abnormal
expression of Ku has been reported in a variety of tumors. Ku gene
mutation may result in altered DNA repair mechanisms and genomic
instability. The abnormal expression of Ku is considered to be
closely related to the occurrence and development of malignant
tumors. However, related reports are not concordant and some are
contradictory. For example, the expression of Ku70/Ku80 has been
shown to be decreased in various types of malignant tumor, such as
colon adenocarcinoma, malignant melanoma, lung cancer and cervical
cancer (6), suggesting that the
downregulation of Ku is correlated with the occurrence and
development of these tumors. By contrast, the levels of Ku in
ovarian cancer, bladder cancer, malignant lymphoma, esophageal
cancer, breast cancer, non-melanomatous skin cancer, oral cancer
and other tumor tissues have been shown to be elevated (6), revealing Ku as an oncogenic protein that
can lead to tumorigenesis and development through transcriptional
regulation, the inhibition of apoptosis and the induction of cell
proliferation. The role of Ku in the occurrence and development of
TC, and the underlying mechanism remain to be fully elucidated.
Previous studies have shown that the protein
expression of Ku is altered in human tumor tissues, with Ku
presumably associated with tumorigenesis; however, the specific
role of Ku in tumor development and the underlying mechanisms
remain undefined. In the present study, the expression of Ku80 and
its associated molecules were assessed in TC and paracancerous
tissues. Subsequently, Ku80 was knocked down to examine its effects
on cell proliferation, apoptosis, invasion and other associated
molecular mechanisms, which may provide a potential a therapeutic
target for the early prevention and treatment of TC.
Materials and methods
Cell culture
The K1 human PTC cell line (cat. no. 92030501) was
purchased from the European Collection of Authenticated Cell
Cultures and the B-CPAP TC cell line (cat. no. SCSP-543) was
purchased from the Library of Chinese Academy of Sciences
(Shanghai, China). The two cell lines were authenticated using
short tandem repeat analysis, as described in the 2012 ANSI
Standard (ASN-0002) by the ATCC Standards Development Organization
and by Capes-Davis et al (7).
The K1 cells were cultured in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum/Ham's F12/MCDB105 (2:1:1;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA); the
B-CPAP cells were maintained in RPMI 1640 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS,
Ausbian) in a humidified incubator with 5% CO2 at
37°C.
Clinical tissue sample collection
TC and normal adjacent paracancerous samples from 20
patients, Hashimoto's thyroiditis (HT) specimens from 20 patients,
HT with TC samples from 15 patients, and normal thyroid specimens
from 18 patients were evaluated for the protein expression levels
of Ku80, RET/TC and NF-κB. These samples, derived from the
depository of the Department of Pathology were histopathologically
and clinically diagnosed at Xi'an Central Hospital (Shanxi, China)
between January 2010 and December 2013, following the provision of
written informed consent from the patients or their guardians. The
Institutional Review Board of Xi'an Central Hospital approved the
use of specimens in this study.
Knockdown of Ku80 using clustered
regularly interspaced short palindromic repeats
(CRISPR)/CRISPR-associated protein 9 (Cas9) technology
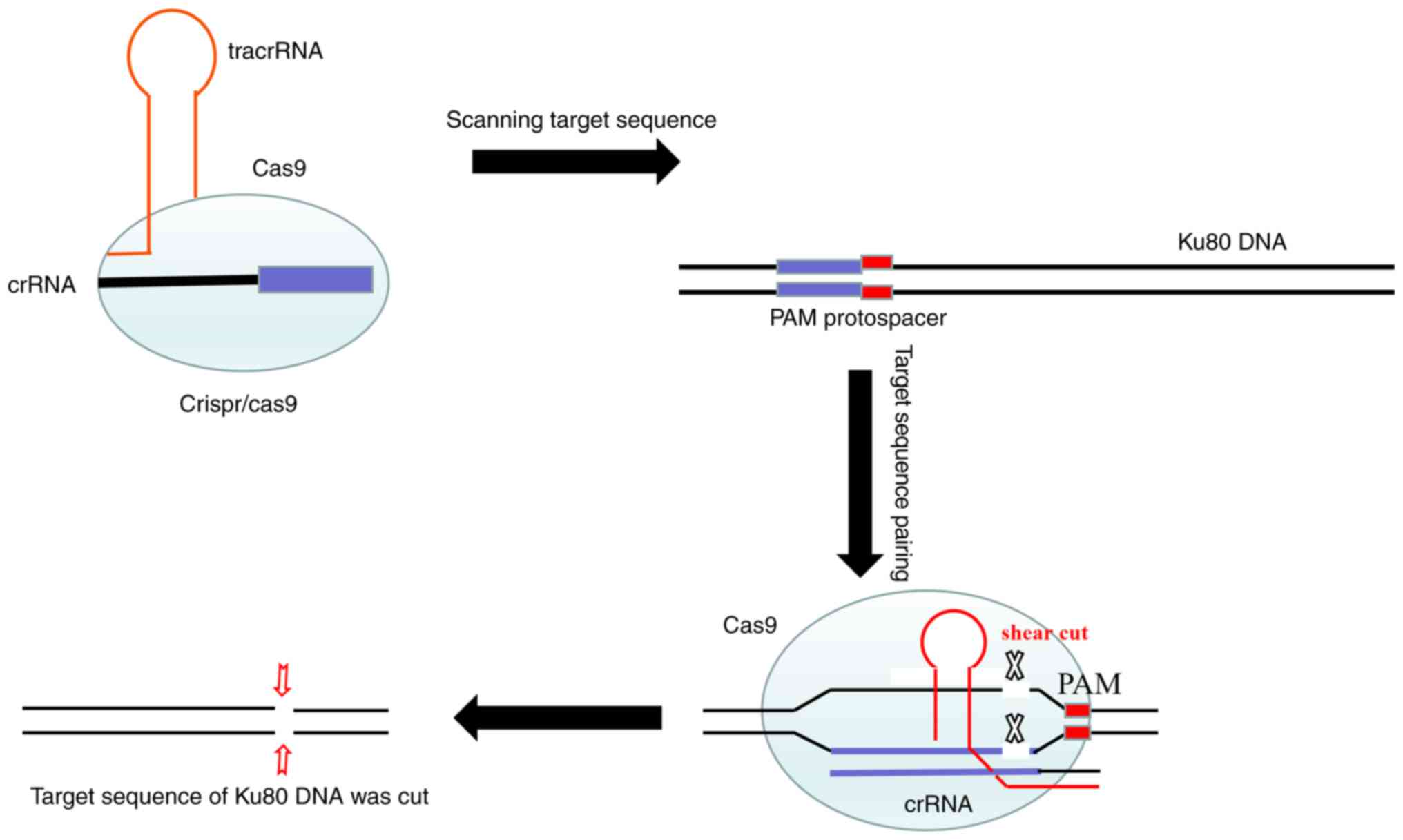
CRISPR/Cas9 technology consists of the
Cas9-puromycin (puro) lentivirus and single guide RNA
(sgRNA)-EGFPMCS lentivirus (Fig. 7). The Cas9-puro lentivirus carries the
puromycin resistance gene, and enhanced green fluorescent protein
(EGFP) is present for labeling the sgRNA-EGFPMCS
lentivirus (8). Three sgRNAs
targeting the Ku80 gene were designed. The vectors corresponding to
these lentiviruses were constructed by Shanghai GenePharma Co.,
Ltd. The sgRNA sequences were 5′-GTCATAAGCATATCGAACTA-3′,
5′-TCATATCAAGCATAACTATG-3′ and 5′-GGTTCAGAGAAGATTCTTCA-3′.
Initially, the K1 and B-CPAP cells were seeded in 6-well plates and
infected with the Cas9-puro lentivirus. In order to obtain cells
stably expressing Cas9, at 72 h post-infection, 2.0 µg/ml of
puromycin was used for 48 h to kill uninfected cells, and K1-Cas9
and B-CPAP-Cas9 were generated (Fig.
1A). Subsequently, the log-phase K1-Cas9 and B-CPAP-Cas9 cells
were infected with the three sgRNA-EGFPMCS lentiviral
vectors, respectively. After 72 h, EGFP was observed under an
inverted fluorescence microscope (Fig.
1B). Reverse transcription-PCR was used to detect the
expression levels of LV-Cas9-puro in the K1-cas9 and B-CPAP-Cas9
cells. A cruiser enzyme digestion assay was performed for
Cas9-sgRNA activity assessment (Fig.
1C), as described below.
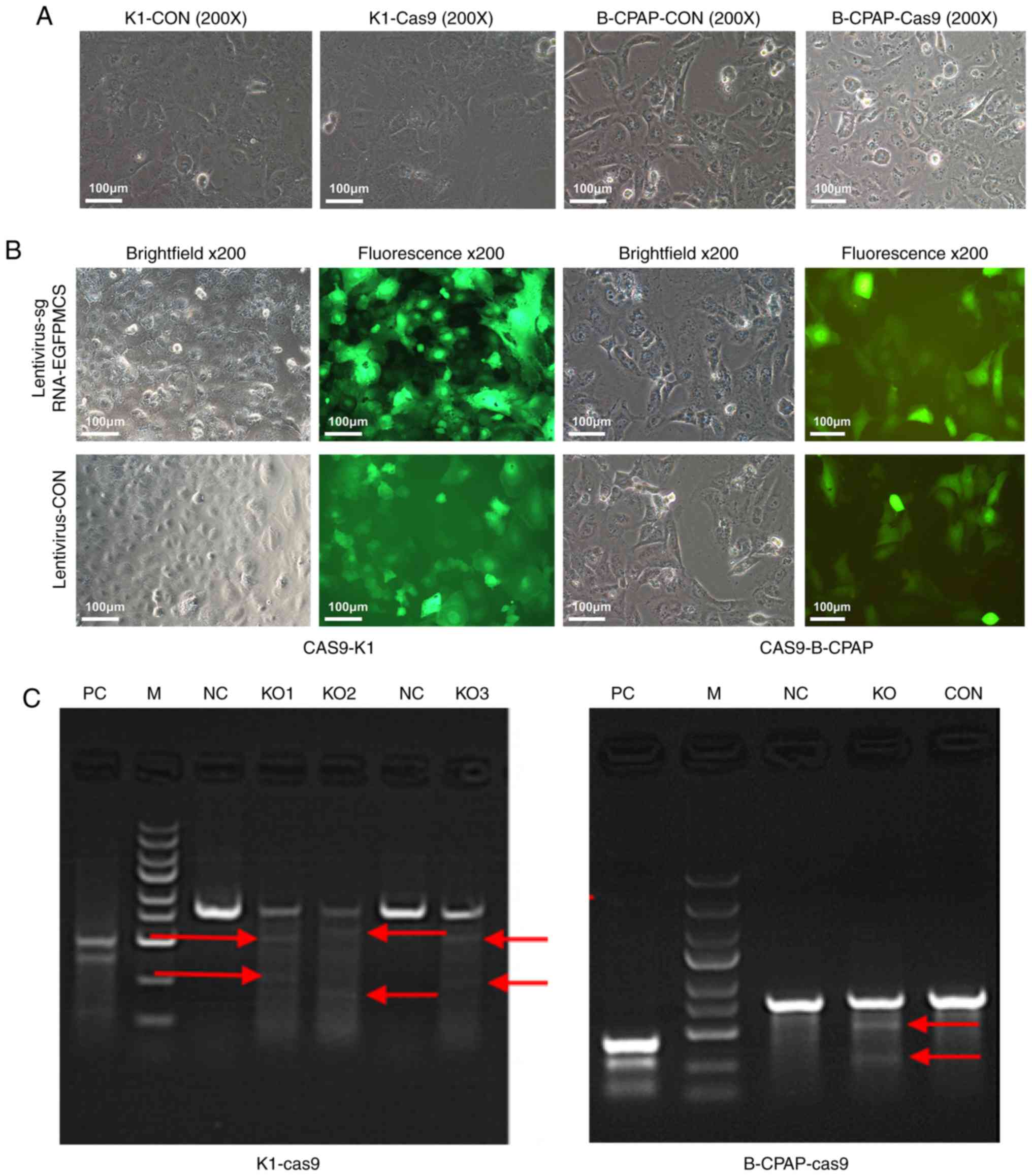 | Figure 1.Microscopy and PCR results of K1 and
B-CPAP cells infected with Cas9. (A) K1 and B-CPAP cells were grown
and infected with the lentivirus Cas9-puro or empty virus for 16 h
and subjected to bright field microscopy. (B) Cas9-K1 and
Cas9-B-CPAP cells were infected with
lentivirus-sgRNA-EGFPMCS. Samples were assessed by
bright field or fluorescence microscopy (magnification, ×200). (C)
Cruiser enzyme digestion assay for Cas9-sgRNA activity in the
K1-cas9 and B-CPAP-Cas9-stably expressing strains. K1 and B-CPAP
cells were successfully infected with the Cas9-sgRNA lentivirus.
(KO1-3, three potential targets for Ku80 of K1 cells; KO, one
potential target for the Ku80 of B-CPAP cells). Red arrows
represent potential target bands of target genes. M, DL2502 ladder
marker (small to large 100, 250, 500, 750, 1,000, 1,500, 2,000,
3,000 and 5,000 bp); KO, knockout; NC, negative control; CON, blank
control; PC, positive reference; sgRNA, single guide RNA; EGFP,
enhanced green fluorescent protein. |
Cruiser enzyme digestion assay
The cruiser enzyme digestion assay was performed
using a knockout and mutation detection kit (Shanghai Jisheng
Medical Technology Co., Ltd. cat. no. MB001-100420rnx). In brief,
the steps were as follows: i) Cells were collected for detection,
and the sample genome was extracted using a genomic DNA extraction
kit; ii) primers were designed for PCR amplification (primer design
included cutting target sites and the recommended length of
products was 350–500 bp); hybridized DNA products were obtained by
PCR, according to the manufacturer's protocol; iii) enzyme
digestion and screening of positive clones was performed; and iv)
positive PCR products were detected.
Total RNA was extracted from cells using TRIzol
(Shanghai Pufei Biotech Co.; cat. no. 3101-100). The reference gene
was GAPDH, and the primers were designed and synthesized by
Shanghai GenePharma Co., Ltd. GenePharma (Shanghai, China). The
detection primer sequences are listed in Table I. The qPCR conditions were as follows:
95°C for 2 min, then 35 cycles of 95°C for 20 sec and 72°C for 30
sec, followed by a last cycle at 95°C for 5 min for denaturation of
the primers. Analysis of the products was conducted by 2% agarose
gel electrophoresis. The amplified fragment size of the positive
reference was 493 bp, and the digested fragment size was 122 BP and
371 bp, respectively. If the expected size of cleavage bands
appeared in the experimental group, this was considered a positive
clone screened by restriction enzyme digestion. The experiment was
performed in duplicate in at least two independent repeats.
 | Table I.Primers used for the Cruiser enzyme
digestion assay. |
Table I.
Primers used for the Cruiser enzyme
digestion assay.
| Target point | Upstream sequence
(5′-3′) | Downstream sequence
(5′-3′) | Amplified fragment
size (bp) | Enzyme digestion
fragment 1 | Enzyme digestion
fragment 2 |
|---|
| KO1 (K1 cell) |
AGGTGATGGGTGACTTCAGAGG |
CCGAAAGTGTGGTTCACAGACC | 801 | 528 | 273 |
| KO2 (K1 cell) |
AGGTGATGGGTGACTTCAGAGG |
CCGAAAGTGTGGTTCACAGACC | 801 | 597 | 204 |
| KO3 (K1 cell) |
CACTGTACTACTCCAAGAGCAG |
TACTTCCTTGGTCTCCATGTCC | 801 | 541 | 260 |
| KO (B-CPAP
cell) |
AGGTGATGGGTGACTTCAGAGG |
CCGAAAGTGTGGTTCACAGACC | 801 | 528 | 273 |
Immunohistochemistry (IHC)
IHC was conducted on 4-mm FFPE histologic sections
using mouse monoclonal anti-Ku80 (1:300, cat. no. 2753s; Cell
Signaling Technology, Inc., Danvers, MA, USA), mouse monoclonal
anti-RET/TC (1:250, cat. no. Sc-365943; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and mouse monoclonal anti-NF-κB P65 (1:500,
cat. no. Sc-8008; Santa Cruz Biotechnology, Inc.). The HT, HT + TC,
TC and normal paracancerous tissues were routinely dewaxed and
hydrated, and antigen retrieval was performed according to the
requirements of the manufacturers of primary antibodies.
Subsequently, 1% hydrogen peroxide was added for 20 min to quench
intrinsic peroxidase activity, and goat serum (Fuzhou Maixin
Biotech Co., Ltd., Fuzhou, China) was used for 30 min to block
nonspecific antibody binding. The slides were then incubated with
primary antibodies at 37°C for 90 min. Following washing with PBS
three times, the slides were incubated for 30 min with secondary
antibodies at room temperature (MaxVision TM HRP-Polymer anti-mouse
IHC kit; cat. no. KIT-5002; Fuzhou Maixin Biotech Co., Ltd.).
Following three additional washes, DAB solution was added to the
slides, which were then observed under a microscope. Ku80 was
observed in the nucleus. Immunofluorescence double-labeling and
laser confocal microscopy were used to localize NF-κB and RET/TC,
determining whether they were co-expressed at the cellular level.
Two independent observers assessed and scored the extent of
immunostaining. The ratio of stained cells and the degree of
staining were used as evaluation criteria. For each case, at least
1,000 tumor cells were analyzed, and the percentage of tumor cells
with positively stained nuclei was recorded. For each sample, the
ratios of cells expressing Ku80, RET/TC and NF-κB P65 varied
between 0 and 100%, and the intensity of nuclear staining varied
from weak to strong. A score was given based on the percentage of
positive cells: <5% cells, 1 point; 6–35% of cells, 2 points;
36–70% of cells, 3 points; >70% of cells, 4 points. Another
score was assigned according to the intensity of staining: Negative
staining, 1 point; weak staining (light yellow), 2 points; moderate
staining (yellow brown), 3 points; and strong staining (brown), 4
points. The final score was calculated as the product of the above
two scores. A final score ≥4 indicated high protein expression in
the tumor; otherwise, protein expression in the tumor was
considered to be low.
Cell proliferation, colony formation,
cell invasion and cell apoptosis assays
An MTT assay was used to evaluate cell
proliferation. The cells were seeded at 2×103 cells per
well in 96-well plates. At the end of each treatment, the cells
were treated with 20 µl sterile MTT (5 mg/ml, Sigma; Merck KGaA,
Darmstadt, Germany) for 4 h at 37°C. The medium was then removed
and 100 µl of dimethyl sulfoxide (Sigma; Merck KGaA) was added. The
optical density (OD) at 450 nm was measured on a microplate reader
(Tecan Infinite, Tecan Group, Ltd., Groedig, Austria) according to
the manufacturer's instructions. Data are expressed as a percentage
of the control cells. The experiments were performed in triplicate.
For the colony formation assay, 500 cells were seeded in each well
of 6-well plates and incubated for 14 days. The colonies were fixed
with 4% paraformaldehyde (Sinopharm Chemical Reagent Co. Ltd.,
Shanghai, China), stained with 500 µl Giemsa (Shanghai Dingguo
Biotechnology Co. Ltd., Shanghai, China) for 10 min and counted. An
invasion assay was performed with Boyden chambers (Corning, Inc.,
NY, USA) according to the manufacturer's protocol. To assess the
invasive ability of the cells, filters were precoated with Matrigel
prior to cell seeding into the chambers (5×104/well).
The culture system consisted of a 24-well plate with 500 and 750 µl
per well in the inner and outer chambers, respectively. The cells
were divided into three groups and assessed in triplicate.
Following incubation at 37°C for 16 h, images of the invaded cells
were captured and counted under an inverted microscope. To assess
apoptosis, logarithmic growth-phase cells were harvested and washed
twice with PBS. Sequential staining was then performed with 10 µl
of Annexin V-APC with an Annexin V-APC staining kit purchased from
eBioscience; Thermo Fisher Scientific, Inc. (cat. no. 88-8007).
Finally, apoptosis was detected by flow cytometry according to the
manufacturer's instructions (Beckman Coulter, Inc.).
RT-qPCR analysis
To assess gene expression levels, total RNA was
isolated from the cultured cells using TRIzol (Thermo Fisher
Scientific, Inc.) and reverse transcribed into cDNA with oligo (dT)
primers and the M-MLV reverse transcriptase kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
instructions. qPCR analysis was performed on a LightCycler 480 II
Real-time PCR instrument (Roche Diagnostics, Basel, Switzerland)
with SYBR Premix Ex Taq (Takara Bio, Inc., Otsu, Japan). Relative
mRNA expression was determined using the human GAPDH gene as an
endogenous reference standard. The qPCR conditions were as follows:
An initial cycle at 95°C for 5 min; 35 cycles of 95°C for 50 sec
and 55°C for 1 min; final extension at 72°C for 1 min. The
specificity of the PCR products was confirmed by melting-curve
analysis. The mRNA levels of Ku80 were assessed using the
2−ΔΔCq method (9). The
primer sequences used in this experiment are shown in Table II. To assess gene expression levels
of RET/TC, total RNA was isolated from clinical tissue samples of
the HT, HT + TC, TC and normal thyroid tissue specimens with a
total RNA isolation kit (AP-MNMS-RNA; Axygen, Union City, CA, USA)
as described by the manufacturer.
 | Table II.Primers used in the reverse
transcription-quantitative PCR analysis. |
Table II.
Primers used in the reverse
transcription-quantitative PCR analysis.
| Gene | Upstream sequence
(5′-3′) | Downstream sequence
(5′-3′) | Amplified fragment
size (bp) |
|---|
| GAPDH |
TGACTTCAACAGCGACACCCA |
CACCCTGTTGCTGTAGCCAAA | 121 |
| XRCC5 |
GCACTGACAATCCCCTTTCTG |
TGTTGAGCTTCAGCTTTAACCTG | 97 |
Automatic western blot quantitative
analysis system (WES)
A total of 168 h after infection of the K1 cells
with the Cas9-sgRNA lentivirus, total protein was extracted using a
lysis buffer (Beyotime Institute of Biotechnology) and quantitated
using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Equal quantities of protein (20 µg) were separated
by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred onto polyvinylidene difluoride membranes. The
membranes were first blocked with 5% skim milk in TBS at room
temperature for 1 h, then incubated with rabbit anti-human Ku80
antibody (1:300; cat. no. 2753S; Cell Signaling Technology, Inc.)
at 4°C overnight. The membranes were then washed with TBS/Tween 20
(TBS-T) and incubated with secondary antibodies (rabbit IgG from
the Plonox kit; 1:10,000; cat. no. ab205718; Abcam) at room
temperature for 2 h. Following incubation, the membrane was washed
with TBS-T three times (10 min each). The target band was 83 kDa,
and data were analyzed using Compass Software for Simple Western
(version 4; ProteinSimple).
Cancer phenotype, PathScan stress and
apoptosis signaling antibody array
The PathScan Antibody Array kit (Cell Signaling
Technology, Inc.) was used to assess changes in key molecules of
the signaling pathways in K1 cells co-infected with Cas9 and the
sgRNA lentivirus, according to the manufacturer's protocol. The
experiment was performed with two chips, including the cancer
phenotype and the stress and apoptotic pathways. The samples were
divided into negative control (NC) and experimental knockout (KO)
groups, and data were evaluated by chemiluminescence imaging and
gamma analysis. Each experiment was repeated three times.
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS 22.0 statistical software (IBM Corp.,
Armonk, NY, USA). Comparisons between groups for statistical
significance were made using Student's t-test. (two-tailed, paired)
P<0.05 was considered to indicate a statistically significant
difference.
Results
High expression of Ku80 in TC tissues
and cells is associated with RET/TC rearrangement and the
activation of NF-κB
Studies have found that Ku80 gene polymorphism is
associated with susceptibility to PTC (10). The fusion oncogene RET/TC, expressed
in most TC cells, activates nuclear transcription factor NF-κB,
which regulates Ku70 and Ku80 (11,12).
Therefore, the present study examined the expression of Ku80 in TC
and paracancerous tissue samples and found significantly higher
levels of Ku80 in the tumor specimens (16/20, 80%) compared with
that in the normal samples (4/20, 20%) (Fig. 2A). RT-qPCR analysis showed that the
levels of RET/TC were higher in HT (7/20, 35%) and TC (12/20, 60%)
tissues compared with those in paracancerous normal samples, in
which no expression of RET/TC was detected (P<0.01). This
indicated that RET/TC rearrangement may occur from the early lesion
of HT to TC. This finding was also confirmed by IHC in the above
tissues. The expression of RET/TC in HT (6/20, 30%) tissues was
lower than that in TC (13/20, 65%) tissues (P<0.01). This
further suggested that the expression of RET/PTC in HT may occur
from the early lesion of HT, increasing in TC (Table III, Fig.
2B). IHC was also used to detect the expression of NF-κB.
Compared with that in the normal thyroid tissue, the expression of
NF-κB in the diseased thyroid tissue was elevated, with nuclear
translocation of NF-κB present in the HT (18/20, 90%) and TC
(19/20, 95%) tissues with the expression of RET (P<0.01;
Fig. 2C), indicating that NF-κB was
in the active state. These findings suggested that the protein
expression of RET may transform HT into TC by activating NF-κB. To
confirm this result, the co-expression of NF-κB and RET/TC was
detected by immunofluorescence double labeling and laser confocal
microscopy. The results showed that NF-κB and RET/TC were
co-expressed in the HT and TC thyroid follicular epithelial cells,
confirming that the protein expression of RET may transform HT into
TC by activating NF-κB. Representative photomicrographs are shown
in Fig. 2D and the results are
summarized in Table IV.
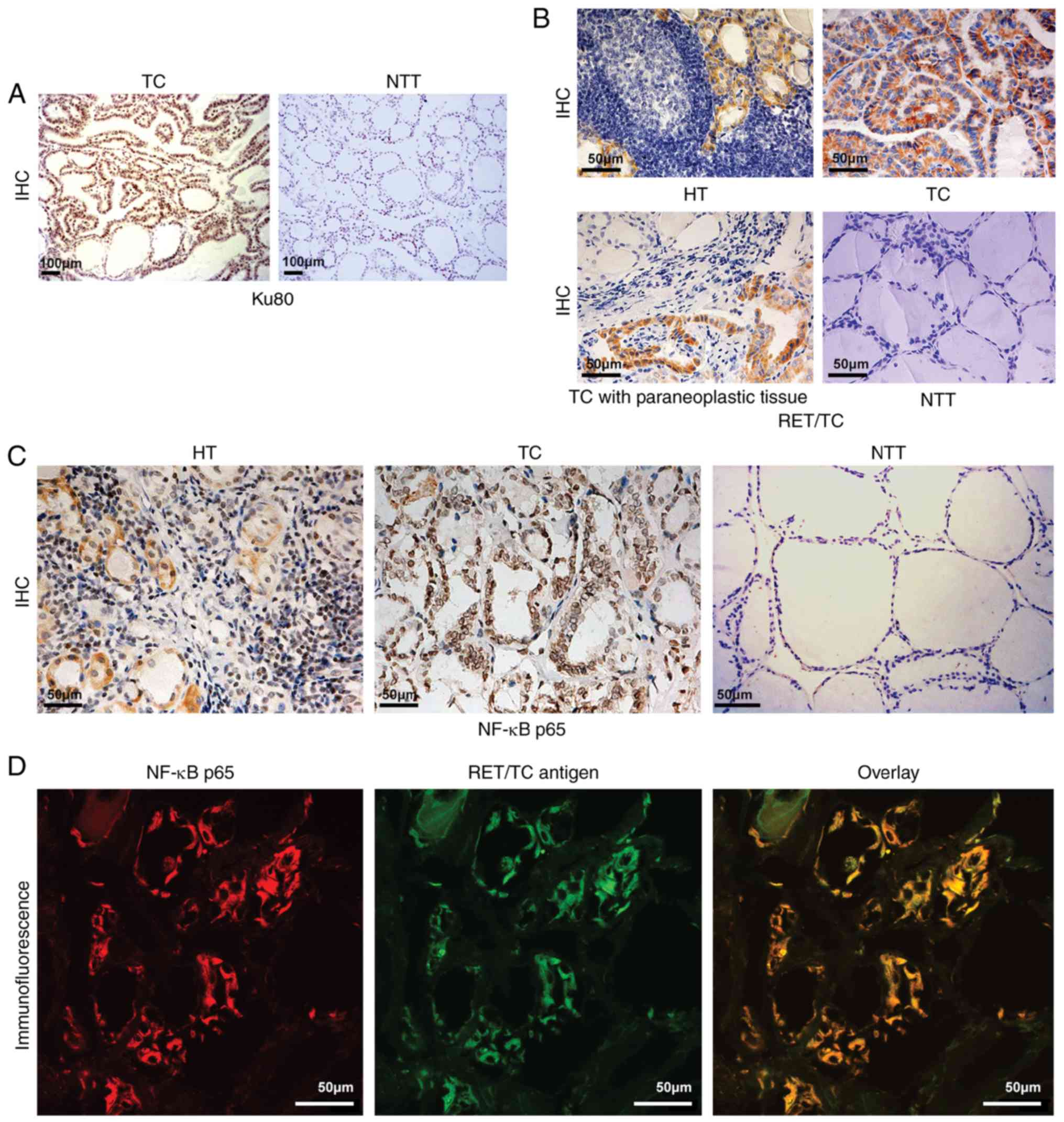 | Figure 2.Ku80, RET/TC and NF-κB p65 expression
in NTT, TC, HT and paracancerous tissues. (A) IHC staining for Ku80
assessment in TC and adjacent tissues (magnification, ×100). Ku80
was expressed in TC tissues (strong positive staining of nuclei)
but not in adjacent tissues (no positive staining of nuclei or
cytoplasm). (B) IHC detection of RET/TC protein expression in the
diseased thyroid tissue (magnification, ×40). HT exhibited weak
positive staining of the cytoplasm; TC exhibited strong positive
staining of the cytoplasm; TC with paraneoplastic (slightly weakly
positive staining of cytoplasm; NTT exhibited negative staining of
the cytoplasm and nuclei. (C) Assessment of NF-κB p65 protein
expression in the diseased thyroid tissue by IHC (magnification,
×40). HT exhibited weak positive cytoplasmic and nuclear staining;
TC exhibited strong positive cytoplasmic and nuclear staining; NTT
exhibited almost negative staining of the cytoplasm or nuclei. (D)
Immunofluorescence double-labeling and laser confocal microscopy
for RET/TC antigen and NF-κB detection (magnification, ×600). NF-κB
expression is shown as red fluorescence, RET/TC antigen expression
is shown as green fluorescence. Laser confocal microscopy results
show NF-κB and RET/TC were co-expressed in the cytoplasm and/or
nuclei of thyroid follicular epithelial cells. TC, thyroid
carcinoma; HT, Hashimoto's thyroiditis; NTT, normal thyroid tissue;
IHC, immunohistochemistry; NF-κB, nuclear factor-κB. |
 | Table III.Positive rate of RET/PTC (%) in
diseased and normal thyroid tissue. |
Table III.
Positive rate of RET/PTC (%) in
diseased and normal thyroid tissue.
|
|
|
|
|
| P-value |
|---|
|
|
|
|
|
|
|
|---|
| Factor | A | B | C | D | A vs. D | B vs. D | C vs. D | B vs. C | A vs. C vs.
(B+C) |
|---|
| Disease | HT | HT + PTC | PTC | NTT |
|
|
|
|
|
| N | 20 | 15 | 20 | 18 |
|
|
|
|
|
| PCR | 7 (35%) | ND | 12 (60%) | 0 (0) | 0.009a | ND | 0.0001a | ND | 0.113 |
| IHC | 6 (30%) | 9 (60%) | 13 (65%) | 0 (0) | 0.021a | 0.0001a | 0.0001a | 0.762 | 0.019a |
 | Table IV.Immunohistochemical data for NF-κB
and its co-expression with the RET/TC antigen. |
Table IV.
Immunohistochemical data for NF-κB
and its co-expression with the RET/TC antigen.
|
| Positive
cases/total cases (%) |
|---|
|
|
|
|---|
| Group | RET antigen | NF-κB | RET + NF-κB |
|---|
| HT | 6/20
(30)a | 18/20
(90)a | 6/20 (30) |
| HT + TC | 9/15
(60)a | 14/15
(93.3)a | 9/15 (60) |
| TC | 13/20
(65)a | 19/20
(95)a | 13/20 (65) |
| NTT | 0/18 (0) | 5/18 (27.8) | 0/18 (0) |
Knockdown of Ku80 suppresses TC cell
proliferation, invasion and colony formation
To assess the role of Ku80 in TC cell proliferation,
invasion and colony formation, the CRISPR/Cas9 technique was used
to knock down Ku80 in K1 and B-CPAP cells. A total of 168 h after
infection, the knockdown efficacy of Ku80 was determined by western
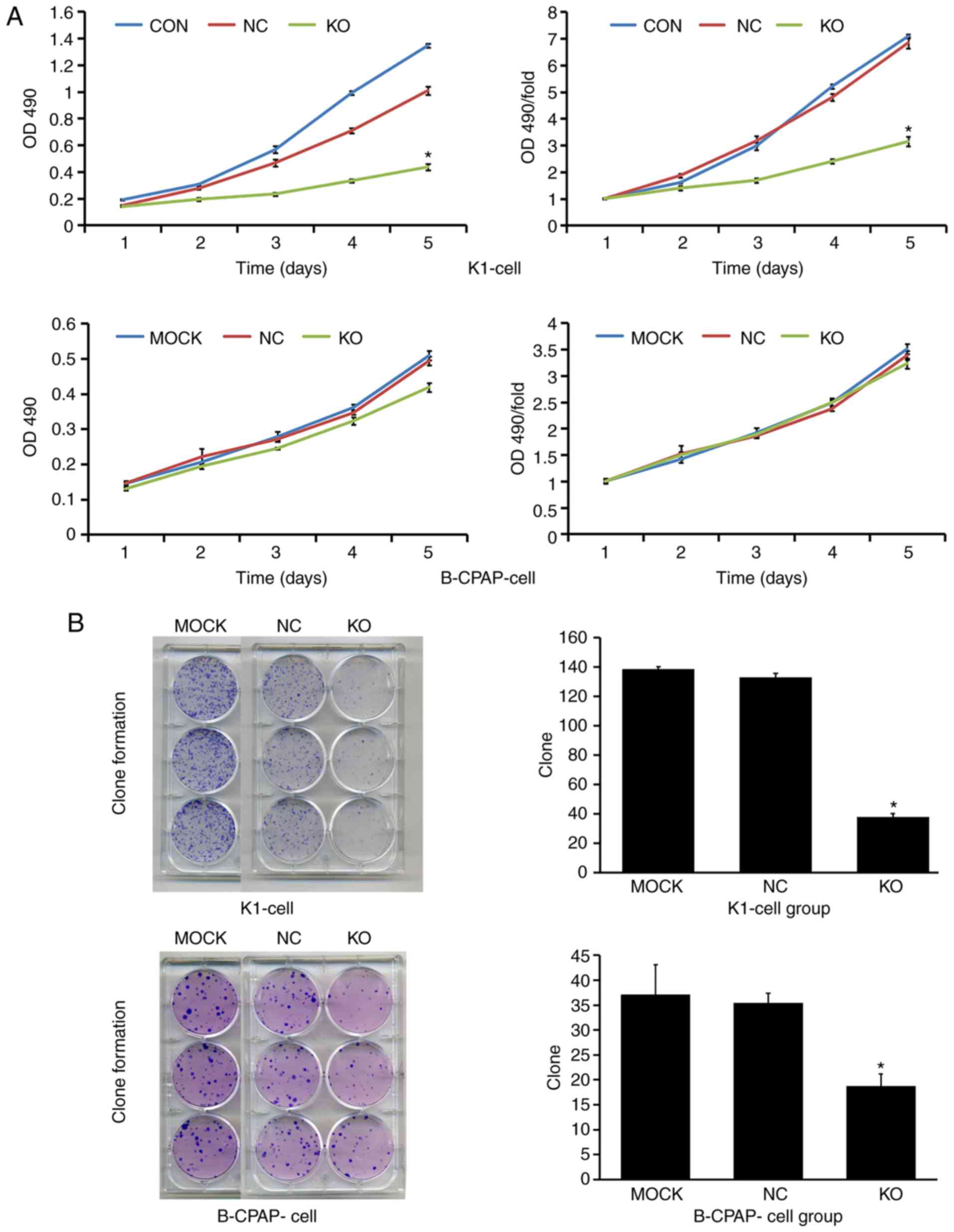
blotting and RT-qPCR analysis, respectively. As shown in Fig. 3A, compared with the NC group, the Ku80
sgRNA-infected K1 cells exhibited a 99.3% reduction in protein
levels of Ku80, indicating a high knockdown efficacy. In addition,
RT-qPCR analysis showed that the mRNA levels of Ku80 were reduced
by 56% in the Ku80 sgRNA-infected B-CPAP cells. To further evaluate
the effect of Ku80 on proliferation, the proliferative ability of
sgRNA-infected K1 and sgRNA-infected B-CPAP cells were then
assessed using an MTT assay, which revealed that the proliferation
rates of the K1 cells were significantly decreased on days 4 and 5
following Ku80 knockdown compared with those of the control cells
(P<0.05). The proliferative ability of the B-CPAP cells on the
fifth day did not differ significantly from that on the first day
(Fig. 4A). These results were
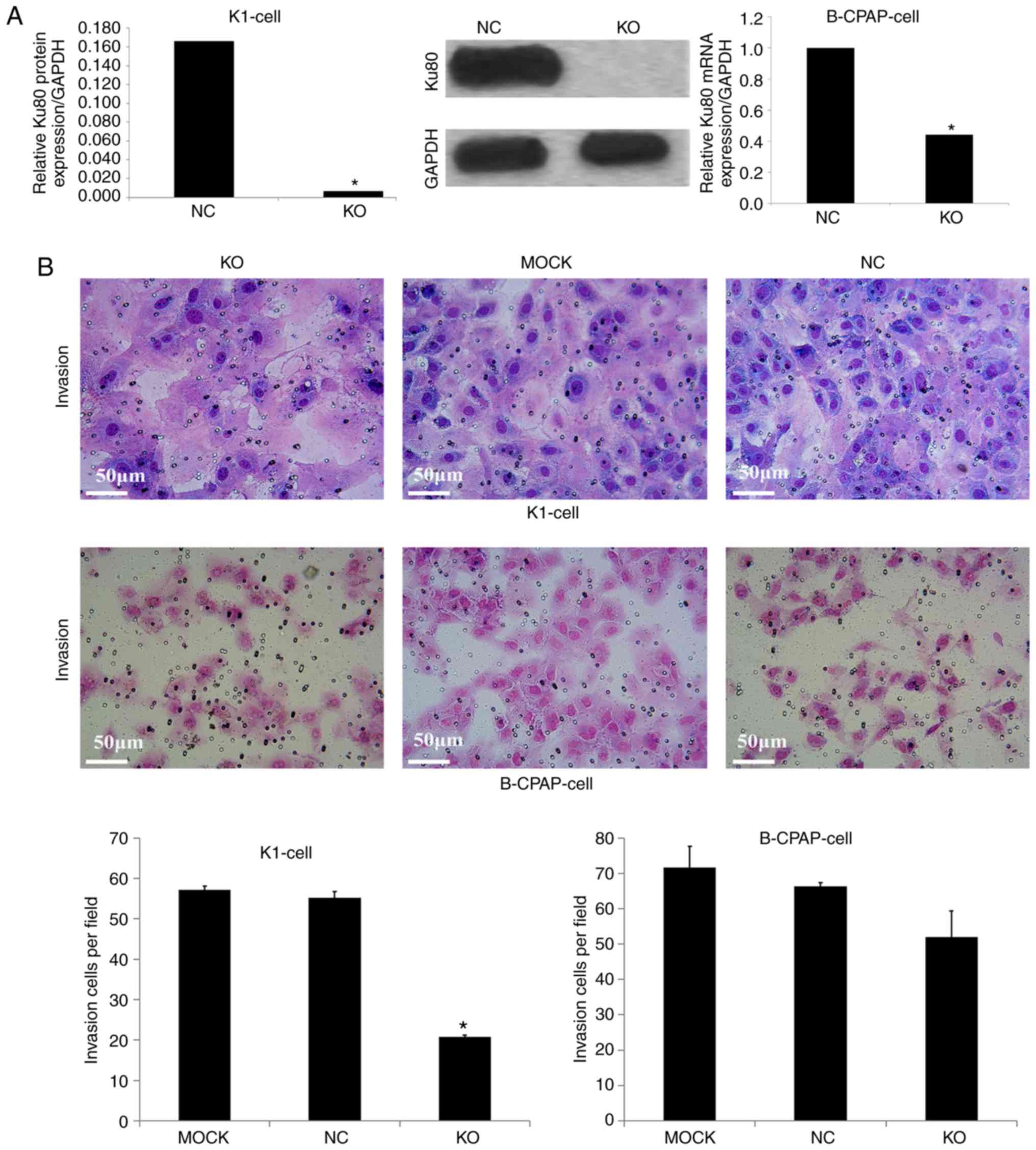
confirmed by colony formation and invasion assays; in K1 cells, the
numbers of invasive cells and cell clones formed were markedly
decreased following Ku80 knockdown compared with numbers in the
control group. The invasive ability of the sgRNA-B-CPAP cells was
not significantly decreased (P>0.05), however, the clone
formation ability was significantly decreased (P<0.05) (Figs. 3B and 4B). The proliferation and invasiveness of
the sgRNA-B-CPAP cells were not decreased compared with those in
the control group, possibly due to the rate of Ku80 knockdown being
lower than that of the K1 cells. Collectively, these findings
suggested that Ku80 was important in the proliferation, invasion
and colony formation of TC cells in vitro.
Knockdown of Ku80 promotes TC cell
apoptosis
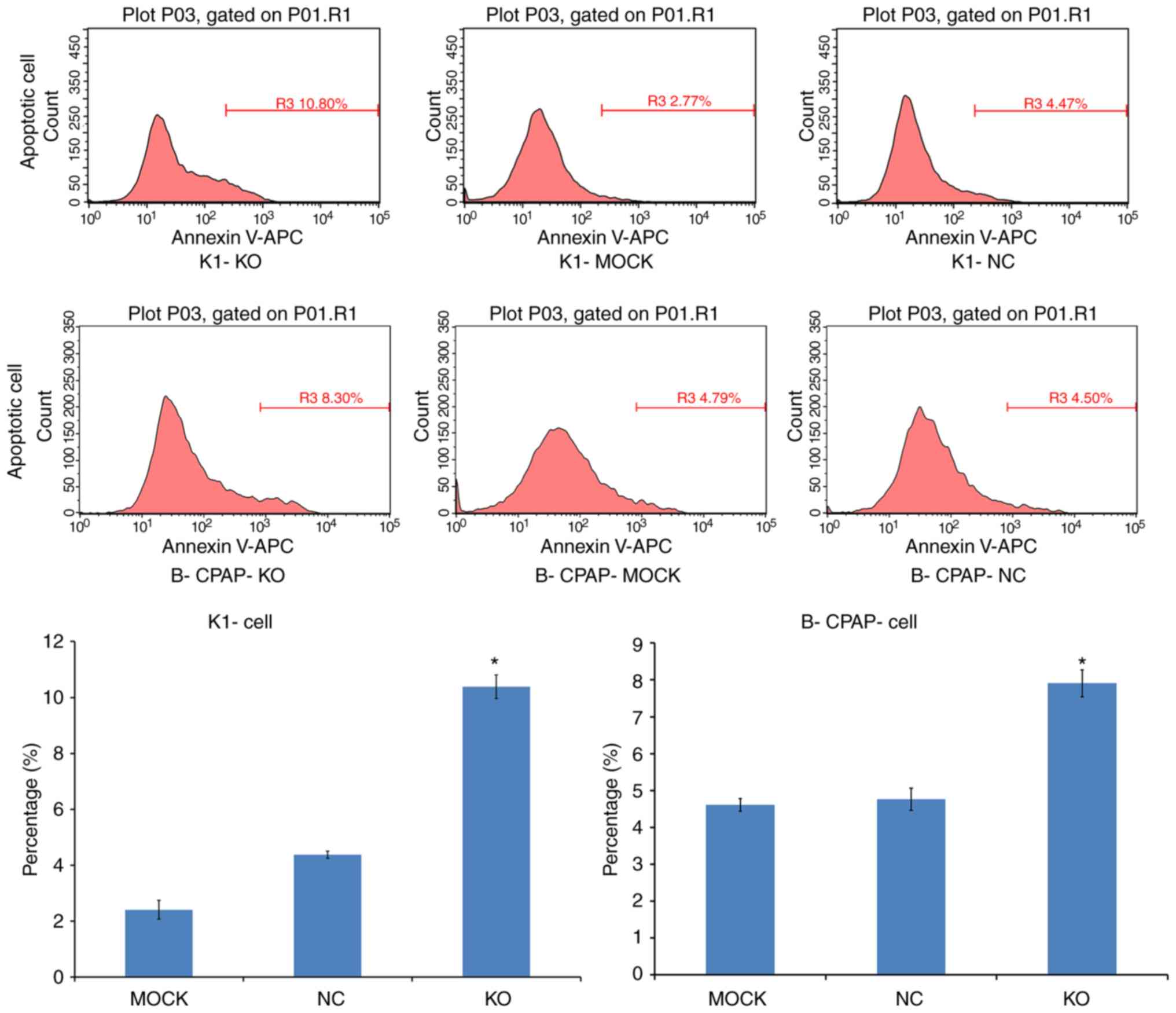
Apoptosis was assessed by Annexin V-APC single
staining. Flow cytometric analysis demonstrated that, compared with
the control, the apoptotic rates of the sgRNA-K1 cells were
4.38±0.12 and 10.38±0.42% in the NC and KO cells, respectively. The
percentages of apoptotic sgRNA-B-CPAP cells were 5.01±0.24 and
7.82±0.22% in the NC and KO groups, respectively. Both infected
cell lines exhibited significantly increased apoptotic rates
(P<0.05; Fig. 5), indicating that
Ku80 suppressed TC cell apoptosis.
Identification of genes mediating the
activity of Ku80 in TC cells
To detect and compare key signaling molecules in K1
cells, PathScan stress and apoptosis signaling antibody array
analysis was performed for K1 cells infected with CRISPR/Cas9 or
control lentiviruses. Chemiluminescence and gray scale data
indicated that, compared with the NC group, the KO group had
significantly reduced protein expression levels of vimentin, CD45,
proliferating cell nuclear antigen (PCNA), Ki-67, phosphorylated
(p-)retinoblastoma (Rb), hypoxia-inducible factor 1α (HIF-1α),
survivin and EGF receptor in cancer phenotype pathways (Fig. 6A, P<0.05). Similarly, the protein
expression levels of phosphorylated (p-)ERK1/2, p-Akt, p-HSP27,
p-Smad2, p-SAPK/JNK, p-TGF-β-activated kinase 1 (TAK1) and survivin
(involved in apoptotic signaling pathways) were also downregulated
in the KO group (Fig. 6B, P<0.05).
These data further indicated that Ku80 altered apoptosis by
regulating multiple genes in the MAPK pathway.
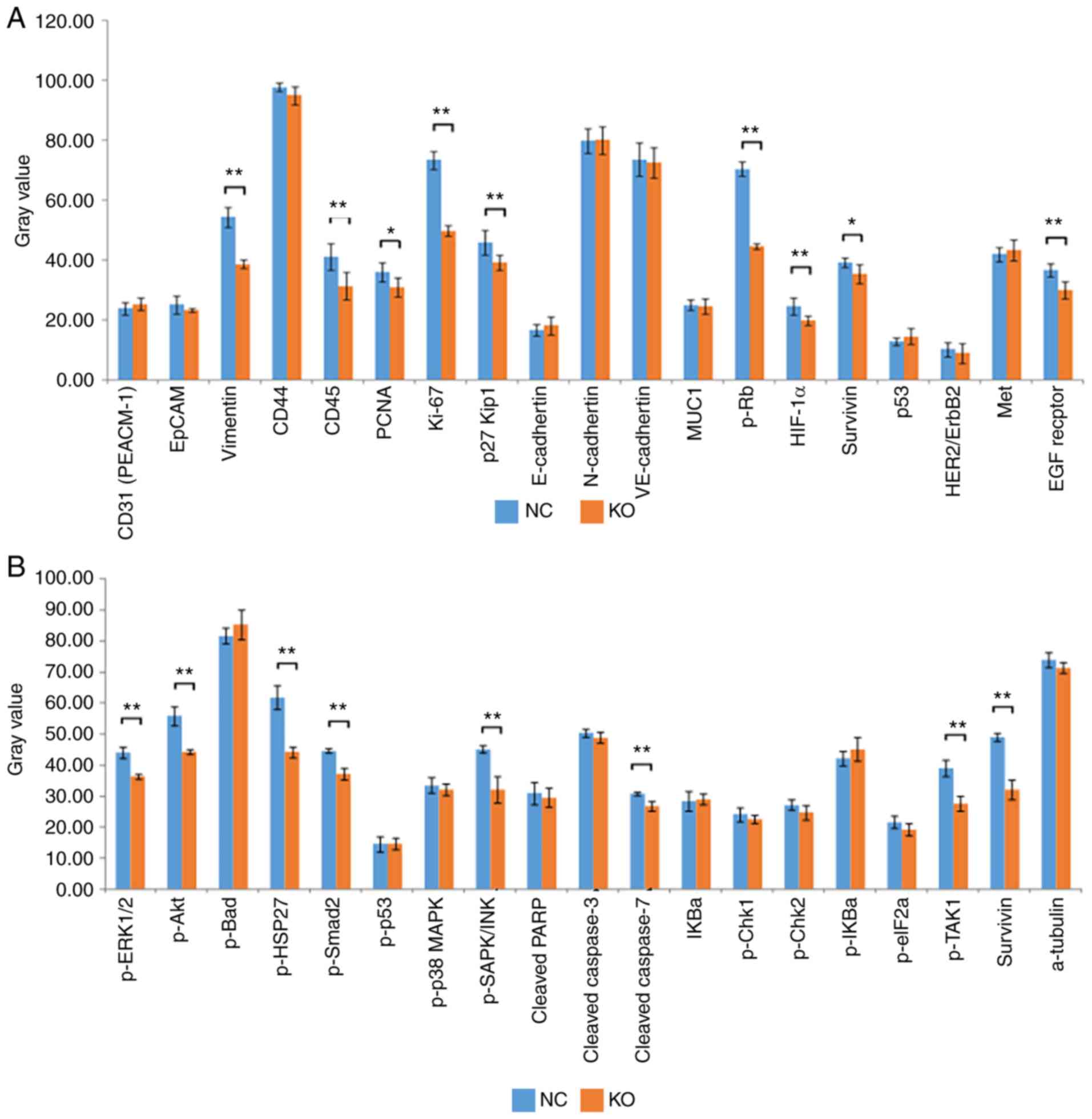 | Figure 6.Identification of Ku80
knockdown-related gene expression. (A) Statistical analysis of gray
values revealed that the expression levels of vimentin, CD45, PCNA,
Ki-67, p-Rb, HIF-1α, survivin and EGF receptor in cancer phenotype
pathways were significantly decreased in the KO group compared with
those in the control group (*P<0.05, **P<0.01). (B)
Statistical analysis of gray values revealed that the expression
levels of p-ERK1/2, p-Akt, p-HSP27, p-Smad2, p-SAPK/JNK, p-TAK1 and
survivin involved in the molecular profile of stress and apoptotic
signaling pathways were significantly decreased in the KO group
compared with those in the control group (**P<0.01). KO,
experimental group; NC, negative control group; p-,
phosphorylated. |
Discussion
TC is the most common type of endocrine malignancy
and represents the leading cause of endocrine organ cancer-related
mortality. As with other types of cancer, early diagnosis of TC is
important for improving patient survival rate and treatment
outcomes (13). Although TC has a
good prognosis, there is a risk of recurrence which represents a
threat to human life. In recent years, the incidence of PTC has
been steadily increasing worldwide (14). The etiology of PTC is most closely
associated with exposure to radiation (15). Little progress has been made in
mechanistic research. The present study demonstrated that the
occurrence of TC is closely related to the Ku80 protein. It was
found that silencing Ku80 significantly inhibited tumor cell
proliferation, invasion and colony formation, and induced
apoptosis. In addition, statistical analysis of gray scale values
showed that the protein levels of vimentin, CD45, PCNA, Ki-67,
p-Rb, HIF-1α, EGF receptor, p-ERK1/2, p-Akt, p-HSP27, p-Smad2,
p-SAPK, JNK, p-TAK1 and survivin were significantly lower following
Ku80 knockdown compared with those in the control (P<0.05).
These findings reveal potential novel targets for TC treatment.
The Ku protein is the main repair protein for DNA
double-strand breaks (DSBs). It is composed of Ku80 and Ku70
monomers and serves an important role in DNA DSB injury repair
(16). In addition to repairing DNA
DSBs, Ku has other important biological functions, including
involvement in cell cycle regulation, transcriptional regulation,
immunoglobulin-encoding gene V(D)J chain rearrangement and
maintenance of telomere structural stability (17). Studies have shown that the protein
expression of Ku70/Ku80 is increased in various tumors, including
ovarian cancer, bladder cancer, colon cancer, cervical cancer,
malignant lymphoma, esophageal cancer, lung cancer, breast cancer,
non-melanomatous skin cancer and cancer of the oral cavity
(6), revealing Ku as an oncogenic
protein. The increased activity of Ku inhibits apoptosis and
promotes cell proliferation through transcriptional regulation,
leading to tumorigenesis and cancer development. However, the
mechanisms underlying the effects of Ku in TC have rarely been
reported. In order to clarify the association between Ku and TC,
the present study analyzed the level of Ku in human TC tissue
specimens and TC cells. As shown above, compared with the control
groups, tumor samples and cells showed significantly increased
expression of Ku80, corroborating previous reports. To further
determine the specific role of Ku80, a series of experiments were
performed following Ku80 silencing. Ku80 knockdown resulted in
reduced proliferation, invasion and colony formation in tumor
cells, whereas apoptosis was induced. Although the proliferative
activity and invasion rate of B-CPAP cells in the MTT and invasion
assays, respectively, did not differ significantly to those in the
respective control groups, the overall trends of cell proliferation
and invasion were downward. This suggests that the expression of
Ku80, encoded by the XRCC5 gene, is extremely high in tumor cells,
and the Ku80-knockdown efficiency in B-CPAP cells was not high
enough, causing residual Ku80 to exert effects. In addition,
following Ku80 knockdown, the protein expression levels of
vimentin, CD45, PCNA, Ki-67, p-Rb, HIF-1α, EGF receptor, p-ERK1/2,
p-Akt, p-HSP27, p-Smad2, p-SAPK/JNK, p-TAK1, survivin and others,
which promote cell proliferation, metastasis, invasion and
angiogenesis, were significantly reduced. This further demonstrates
that Ku80 serves a critical role in the development of TC.
Cancer occurrence is a complex process. The mutation
or ectopicity of related genes in a pathway can lead to the
proliferation or apoptosis of tumor cells. Common mutations include
those in BRAF, RET, RAS, PTEN and RET/PTC (18). The RET proto-oncogene, located on the
long arm of chromosome 10 (10q11.2), encodes a 150-kD receptor
tyrosine kinase that was discovered in 1985 in experiments
involving lymphoma DNA extracts; transfection into NIH3T3 mouse
fibroblast cells results in malignant transformation (19,20).
Subsequent analysis of human tumors has revealed that the RET/TC
fusion protein is a characteristic alteration found in ~20% of
patients with PTC (21). Oncogenes of
the RET/PTC family, particularly RET/PTC1 and RET/PTC3, serve key
roles in the development of several PTCs, particularly pediatric
PTC and those resulting from exposure to ionizing radiation
(22). The low RET/PTC oncogene
expression observed in certain benign thyroid diseases suggests
that the local cellular microenvironment may be important in
regulating RET/PTC oncogenic activity. Based on previous reports,
the present study assessed the gene expression levels of RET/TC
remodeling in TC, normal and HT tissues by RT-qPCR and IHC
analyses. Compared with levels in the normal control group, the HT
and TC samples had significantly higher expression levels of
RET/TC. However, its expression was lower in HT samples than that
in TC samples, suggesting that the protein expression of RET in HT
may be an early precursor of TC, and may be involved in the
subsequent development of TC.
NF-κB consists of heterodimers of various members of
the Rel family, including p50, c-Rel, v-rel, p52, p-65 (RelA) and
Rel B (23). It is constitutively
activated in several types of cancer (24,25). As
NF-κB regulates numerous genes associated with cell transformation,
survival, proliferation, invasion, metastasis and inflammation, the
constitutive activation of NF-κB in cancer cells is key in various
aspects of tumor progression (26).
Numerous genes necessary for tumor growth have NF-κB-binding sites
and are targeted by this transcription factor (27). The present study found that, compared
with levels in normal thyroid tissues, the expression levels of
NF-κB in the diseased thyroid tissues were elevated, with RET
protein expression observed in HT and TC tissues. In addition,
NF-κB was found to undergo nuclear transcription and activation in
TC cells, suggesting that the protein expression of RET may cause
HT cancer to develop into TC by activating NF-κB.
In conclusion, the present study demonstrated that
the expression of Ku80 was significantly increased in TC, which
exhibited high expression levels of RET/TC and NF-KB. In addition,
the expression of Ku80 was shown to be positively correlated with
the levels of RET/TC and NF-KB. Furthermore, Ku80 knockdown
significantly reduced proliferation, invasion and tumor formation
in TC cells, and markedly increased the apoptotic rate. It was also
shown that Ku80 is involved in the pathogenesis of TC. Finally,
Ku80 knockdown significantly downregulated MAPK signaling
pathway-related molecules in addition to proliferation and
metastasis-associated proteins, including EGFR, p-ERK1/2,
p-SAPK/JNK, p-Akt, p-TAK1, PCNA, Ki-67, p-Rb, HIF-1a, survivin,
vimentin, CD45, p-HSP27 and p-Smad2. These findings suggest that
the increased expression of Ku80 is closely related to the
occurrence and development of TC. Upregulating Ku80 modulates the
transcription of a variety of genes which promote cell
proliferation, invasion and tumor formation, and inhibit apoptosis
via the MAPK pathway. The present study provides a theoretical
basis for TC targeted therapy. However, the work was limited by the
absence of mechanistic investigation; screening for possible
molecules was only performed through gene chip technology and the
results were not confirmed. Further investigation is required to
determine which molecules regulate the function of Ku80.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81372857), the Science and
Technology Research and Development Program of Shaanxi Province
(grant no. 2008K09-09), the Xi'an Science and Technology Program
[grant no. SF09027(9)] and the Xi'an Science and Technology Program
[grant no. 2016047SF/YX039(3)].
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YF and JL performed the experiments, participated in
data collection and drafted the manuscript. WW, HF, YD and NL
performed the statistical analysis and participated in study
design. YZ, JY and JW helped draft the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committees of Xi'an Central Hospital (LP2018-08-27).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IHC
|
immunohistochemistry
|
|
TC
|
thyroid carcinoma
|
|
NF-κB
|
nuclear factor-κB
|
|
NJEJ
|
non-homologous end-joining
|
|
EGFP
|
enhanced green fluorescent protein
|
|
OD
|
optical density
|
|
TBS-T
|
TBS-Tween 20
|
References
|
1
|
Liu S, Semenciw R, Ugnat AM and Mao Y:
Increasing thyroid cancer incidence in Canada, 1970–1996: time
trends and age-period-cohort effects. Br J Cancer. 85:1335–1339.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Homayouni M, Mohammad Arabzadeh SA, Nili
F, Razi F and Amoli MM: Evaluation of the presence of Epstein-Barr
virus (EBV) in Iranian patients with thyroid papillary carcinoma.
Pathol Res Pract. 213:854–856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM and
Schlumberger M: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lieber MR, Ma Y, Pannicke U and Schwarz K:
The mechanism of vertebrate nonhomologous DNA end joining and its
role in V(D)J recombination. DNA Repair (Amst). 3:817–826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bertinato J, Tomlinson JJ, Schild-Poulter
C and Hache RJ: Evidence implicating Ku antigen as a structural
factor in RNA polymerase II-mediated transcription. Gene.
302:53–64. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gullo C, Au M, Feng G and Teoh G: The
biology of Ku and its potential oncogenic role in cancer. Biochim
Biophys Acta. 1765:223–234. 2006.PubMed/NCBI
|
|
7
|
Capes-Davis A, Reid YA, Kline MC, Storts
DR, Strauss E, Dirks WG, Drexler HG, MacLeod RA, Sykes G, Kohara A,
et al: Match criteria for human cell line authentication: Where do
we draw the line? Int J Cancer. 132:2510–2519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui H, Lan X, Lu S, Zhang F and Zhang W:
Bioinformatic prediction and functional characterization of human
KIAA0100 gene. J Pharm Anal. 7:10–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gomes BC, Silva SN, Azevedo AP, Manita I,
Gil OM, Ferreira TC, Limbert E, Rueff J and Gaspar JF: The role of
common variants of non-homologous end-joining repair genes XRCC4,
LIG4 and Ku80 in thyroid cancer risk. Oncol Rep. 24:1079–1085.
2010.PubMed/NCBI
|
|
11
|
Neely RJ, Brose MS, Gray CM, McCorkell KA,
Leibowitz JM, Ma C, Rothstein JL and May MJ: The RET/PTC3 oncogene
activates classical NF-κB by stabilizing NIK. Oncogene. 30:87–96.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim JW, Kim H and Kim KH: Expression of
Ku70 and Ku80 mediated by NF-kappa B and cyclooxygenase-2 is
related to proliferation of human gastric cancer cells. J Biol
Chem. 277:46093–46100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erdogdu IH, Yumrutas O, Ozgur Cevik M,
Bozgeyik I, Erdogdu M, Inan HM and Bagis H: Differential expression
of PIWIL2 in papillary thyroid cancers. Gene. 649:8–13. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stewart BW and Kleihues P: World Cancer
ReportIARC Press; Lyon: 2003
|
|
15
|
Williams D: Radiation carcinogenesis:
Lessons from Chernobyl. Oncogene. 27 (Suppl 2):S9–S18. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dobbs TA, Tainer JA and Lees-Miller SP: A
structural model for regulation of NHEJ by DNA-PKcs
autophosphorylation. DNA Repair (Amst). 9:1307–1314. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tuteja R and Tuteja N: Ku autoantigen: A
multifunctional DNA-binding protein. Crit Rev Biochem Mol Biol.
35:1–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Zhang B, Zhao Y, Chen P, Ji M, Hou
P and Shi B: Association of BRAFV600E mutation with
clinicopathological features of papillary thyroid carcinoma: A
study on a Chinese population. Int J Clin Exp Pathol. 7:6922–6928.
2014.PubMed/NCBI
|
|
19
|
Ishizaka Y, Itoh F, Tahira T, Ikeda I,
Sugimura T, Tucker J, Fertitta A, Carrano AV and Nagao M: Human ret
proto-oncogene mapped to chromosome 10q11.2. Oncogene. 4:1519–1521.
1989.PubMed/NCBI
|
|
20
|
Takahashi M, Ritz J and Cooper GM:
Activation of a novel human transforming gene, ret, by DNA
rearrangement. Cell. 42:581–588. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nikiforov YE: RET/PTC rearrangement in
thyroid tumors. Endocr Pathol. 13:3–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prescott JD and Zeiger MA: The RET
oncogene in papillary thyroid carcinoma. Cancer. 121:2137–2146.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thanos D and Maniatis T: NF-kappa B: A
lesson in family values. Cell. 80:529–532. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amit S and Ben-Neriah Y: NF-kappaB
activation in cancer: A challenge for ubiquitination- and
proteasome-based therapeutic approach. Semin Cancer Biol. 13:15–28.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gupta SC, Sundaram C, Reuter S and
Aggarwal BB: Inhibiting NF-κB activation by small molecules as a
therapeutic strategy. Biochim Biophys Acta. 1799:775–787. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uwagawa T and Yanaga K: Effect of NF-κB
inhibition on chemoresistance in biliary-pancreatic cancer. Surg
Today. 45:1481–1488. 2015. View Article : Google Scholar : PubMed/NCBI
|