Introduction
Gastric cancer (GC) is the fourth most commonly
diagnosed type of cancers and is the third highest cause of
cancer-associated death worldwide, thereby making it a severe
public health concern (1). GC most
frequently occurs in East Asia and is the second most deadly cancer
in China according to recent statistics (2). The multifactorial pathogenesis of GC
involves the genetic and epigenetic alterations of oncogenes,
tumor-suppressor genes and growth factors involved in GC
development (3). Poor early screening
results in delayed diagnosis, and currently there are no sufficient
therapeutic methods available that can reduce its high death
probability. At present, identifying potential molecular targets of
the metastatic process is critical for the identification and
therapy of GC.
MicroRNAs (miRNAs/miRs), 18- to 22-nucleotide-long
non-coding RNAs, are pivotal in post-transcriptional regulation,
and mainly bind to the miRNA recognizing elements in the
3′-untranslated region (UTR) to silence the target mRNA and
decrease the expression of the corresponding protein (4). miRNAs modulate the expression of their
target genes in various diseases including cancer and thereby
regulate multitudinous physiological and pathological processes
(including occurrence, differentiation, stress response, death and
proliferation) (5–9). miRNAs have been reported to cause or
suppress tumors, and various miRNAs can be abnormally expressed in
different types of cancer, indicating that miRNAs could potentially
be applied as cancer diagnostic and treatment strategies (10). Dysregulated miRNAs have been observed
in GC and participate in the diffusion, apoptosis, movement and
invasion of GC cells by regulating different tumor-related target
genes (11–13). The expression of miR-183 was found to
be elevated in colon cancer, synovial sarcoma and GC, and this
dysregulation was also observed in the corresponding tumor-derived
cell lines. For example, miR-183 potentially produces tumors by
regulating the tumor-suppressor genes EGR1 and PTEN,
and the breakdown of this essential miRNA modulation system is
probably pivotal to the development of a diverse range of tumors,
including colon cancer and synovial sarcoma (14). Previous studies have revealed that
miR-183 may function as an oncogene by regulating GC cell
proliferation, apoptosis and metastasis, and the oncogenic effect
of miR-183 may be via directly targeting PDCD4 (15). In addition, miR-183 is downregulated
in GC cells and tissues, and inhibits GC cell proliferation and
invasion by targeting Bmi-1. Therefore, targeting miR-183
may be a potential therapeutic strategy in GC patients (16). At present, to the best of our
knowledge, there have been no reports regarding the roles of
tropomyosin (TPM) in GC, but its receptor kinase TPM- related
receptor kinase B (TrkB) has been shown to promote cell
proliferation and invasion, and is associated with the poor
prognosis of various malignancies (17). However, TrkA expression is associated
with tumor progression and poor survival, and is an independent
predictor of poor outcomes in patients with GC (18). In addition, the roles of miR-183 in GC
progression are still not completely understood. The aim of the
present study was to clarify the influence of miR-183-5p.1 on GC
motility as well as the underlying mechanisms.
Materials and methods
Sample collection
GC samples were collected from 24 GC patients who
underwent surgical resection between January 2015 and April 2017 at
Shanghai General Hospital, Shanghai Jiaotong University School of
Medicine. Patients were aged between 42 and 72 years, with a median
of 56 years. All patients gave written informed consent and all
protocols were approved by the Ethics Committees of Shanghai
General Hospital, Shanghai Jiaotong University School of Medicine
(No. 2018KY012).
Cell culture
GES-1 cells and the human GC cell lines MKN-7, AGS
and HGC-27 (Cell Resource Center of Shanghai Academy of Sciences,
Chinese Academy of Sciences) were cultured in wells or flasks at
37°C under 5% CO2 in Dulbecco's modified Eagle's medium
(DMEM; HyClone; GE Healthcare Life Sciences) containing 100 µg/ml
streptomycin, 100 U/ml penicillin, and 10% (v/v) fetal bovine serum
(FBS; HyClone; GE Healthcare Life Sciences). Cell morphology was
observed under an inverted microscope.
Reverse transcription-quantitative PCR
(RT-qPCR)
All primers for miR-183-5p.1 and TPM1 were
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.). Total
RNA isolated using TRIzol reagent (Thermo Fisher Scientific, Inc.)
was quantified spectrophotometrically. The samples were analyzed on
an ABI 7500 RT-PCR device (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol and
GAPDH and U6 genes were used as the internal controls. The primers
used for PCR were as follows: TPM1 forward primers,
5′-GCCGACGTAGCTTCTCTGAAC-3′ and reverse,
5′-TTTGGGCTCGACTCTCAATGA-3′; GAPDH forward primers,
5′-ATTCCATGGCACCGTCAAGGCTGA-3′ and reverse,
5′-TTCTCCATGGTGGTGAAGACGCCA-3′. miR-183-5p.1 RT primer,
5′-GCGAGCACAGAATTAATACGACTCACTATAGG-3′; miR-183-5p.1 forward,
5′-TATGGCACTGGTAGAATTCACT-3′ and reverse,
5′-GCGAGCACAGAATTAATACGAC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′; and GAPDH forward,
5′-CTCAGACACCATGGGGAAGGTGA-3′ and reverse,
5′-ATGATCTTGAGGCTGTTGTCATA-3′. All results were quantified using
the 2−ΔΔCq method as previously described (19).
Transfection of miR-183-5p.1
oligonucleotides
The miR-183-5p.1 oligonucleotides were provided by
Tiangen Biotech Co., Ltd. GES-1, MKN-7, AGS and HGC-27 cells seeded
in 6-well plates (Corning Incorporated) at 50% confluence were
incubated overnight and then transfected with 100 nM miRNA with
Lipofectamine 2000™ (Invitrogen; Thermo Fisher Scientific, Inc.)
for 48 h. miR-183-5p.1 expression was then quantified by
RT-qPCR.
Luciferase reporter assay
The target genes of miR-183-5p.1 were predicted by
TargetScan (www.targetscan.org/vert_71/) and confirmed by miRBase
(www.mirbase.org) or miRecords (mirecords.biolead.org/). A potential target gene,
TPM1, was identified. Based on the sequence of TPM1
(National Center for Biotechnology Information Genbank), the 3′-UTR
was transformed via amplification and cloning into a specific
vector TPM1-UTR-pISo, which was used to build the Luciferase
reporter plasmids of WT-TPM1 and MUT-TPM1 mRNAs. After 24 h of
culture, the AGS cells were co-transfected with miR-183-5p.1
inhibitors and mimics using the transfection reagent. The relative
luciferase activity of TPM1 was detected via dual-luciferase
reporter experiments.
Methyl thiazolyl tetrazolium (MTT)
assays
The co-transfected GES-1, MKN-7, AGS, and HGC-27
cells were plated on 96-well plates (5×103 cells/well)
for 0, 12, 24, 48 or 72 h of culture. After 4 h of treatment with
20 µl of 5 mg/ml sterilized MTT (Sigma-Aldrich; Merck KGaA) at
37°C, the incubation medium was changed to 150 µl of dimethyl
sulfoxide. The absorbance at 490 and 540 nm was detected via an
enzyme-linked immunosorbent assay reader (BioTek Instruments,
Inc.). Experiments were conducted in triplicate.
Flow cytometry
GES-1, MKN-7, AGS and HGC-27 cells co-transfected
with miR-183-5p.1 mimics and miR-183-5p.1 inhibitors for 24 h were
detected using an Annexin V-FITC Apoptosis kit (BD Biosciences;
cat. no. 559763), according to the manufacturer's protocols.
Briefly, transfected cells were washed with PBS twice and
re-suspended in 100 µl 1X binding buffer (BD Biosciences; cat. no.
559763), then incubated for 15 min with Annexin-V/PI (each 5 µl; BD
Biosciences; cat. no. 559763) at 37°C in the dark. Finally, 400 µl
1X binding buffer was added to each tube and analyzed on a FACS
Canto II flow cytometer (BD Biosciences) using FlowJo 7.6 software
(Tree Star, Inc.).
Transwell assays
Cell migration and invasion were evaluated in
12-well Transwell chambers with 8.0-µm PET film pores (Corning
Inc.). Firstly, the lower chamber was filled with 600 µl of medium
with 10% FBS as the attracting agent. Then to the upper chamber 100
µl of serum-free DMEM with 1×105 cells was added, and
then incubated for 48 h. The transmigrating cells, after fixation
in methanol, staining with 0.2% gentian violet (m/v; Sigma-Aldrich;
Merck KGaA) and washing with PBS, were photographed under an
upright microscope, and the number of cells was counted.
Western blot analysis
After lysis in a radioimmunoprecipitation assay
buffer with Roche protease inhibitor cocktail (Roche Diagnosis),
total proteins were detected via the bicinchoninic acid method. The
supernatant of the cell lysates containing 50 µg of protein were
run on 10% SDS-PAGE and then transferred to polyvinylidene
difluoride membranes (EMD Millipore). Membranes were cultured with
primary antibodies raised against TPM1 (Invitrogen; Thermo Fisher
Scientific, Inc.; dilution 1:1,000; cat. no. PA5-29846), TPM2
(Invitrogen; Thermo Fisher Scientific, Inc.; dilution 1:1,000; cat.
no. PA5-22012), TPM3 (Invitrogen; Thermo Fisher Scientific, Inc.;
dilution 1:1,200; cat. no. PA5-29005), Bcl-2 (Invitrogen; Thermo
Fisher Scientific, Inc.; dilution 1:1,200; cat. no. 13-8800), P53
(Invitrogen; Thermo Fisher Scientific, Inc.; dilution 1:1,000; cat.
no. MA5-12554) and GAPDH (Invitrogen; Thermo Fisher Scientific,
Inc.; dilution 1:2,000; cat. no. PA1-987-HRP), and Goat anti-Rabbit
IgG (H+L) Cross-Adsorbed Secondary Antibody, horseradish peroxidase
(HRP)-conjugated (Invitrogen; Thermo Fisher Scientific, Inc.;
dilution 1:2,000; cat. no. G-21234) or Goat anti-Mouse IgG (H+L)
Cross-Adsorbed Secondary Antibody, HRP-conjugated (Invitrogen;
Thermo Fisher Scientific, Inc.; dilution 1:2,000; cat. no. G-21040)
raised against the primary antibodies. Blots were analyzed using
the enhanced chemiluminescence matrix. Band intensity was assessed
with ImageJ 1.45 software (National Institutes of Health, Bethesda,
MD, USA).
Immunofluorescence analysis
The apoptosis of AGS cells transfected with
miR-183-5p.1 oligonucleotides was analyzed using a Hoechst 33258
detection kit (Beyotime Institute of Biotechnology; cat. no. C1011)
and TUNEL assay (Beyotime Institute of Biotechnology; cat. no.
C1089) according to the manufacturer's protocols. After
transfection, cells were washed with PBS twice and incubated with
Hoechst 33258 reagent (50 µl) for 60 min at 37°C in the dark, and
then washed with PBS twice. After this, cells were stained with
TUNEL solution (50 µl) for 60 min at 37°C in the dark and washed
with PBS twice. Images were captured and the cells were counted
using an Olympus CKX53 inverted fluorescence microscope
(magnification, ×200). The immunostained sections were evaluated by
two independent pathologists.
Statistical analysis
All data were analyzed with SPSS 16.0 (SPSS, Inc.).
Statistical analysis was conducted via Student's t-test and one-way
analysis of variance with Tukey's post hoc test. The plotting of
graphs was conducted on GraphPad Prism 5.0 (GraphPad Software,
Inc.). Results are expressed as the mean ± standard error of mean.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-183-5p.1 and TPM1 expression in GC
tissues or cells
RT-qPCR revealed that miR-183-5p.1 expression was
significantly increased in GC tissues when compared with that noted
in the normal or adjacent tissues (Fig.
1A), and increased in MKN-7, AGS and HGC-27 cells when compared
with GES-1 cells (Fig. 1B). Notably,
TPM1 mRNA expression was decreased in the GC tissues when compared
with that in normal or adjacent tissues (Fig. 1C) and in MKN-7, AGS and HGC-27 cells
compared with that noted in the GES-1 cells (Fig. 1D). The TPM1 protein expression in AGS,
MKN-7 and HGC-27 cells (Fig. 1E-J)
was consistent with that observed at the mRNA level.
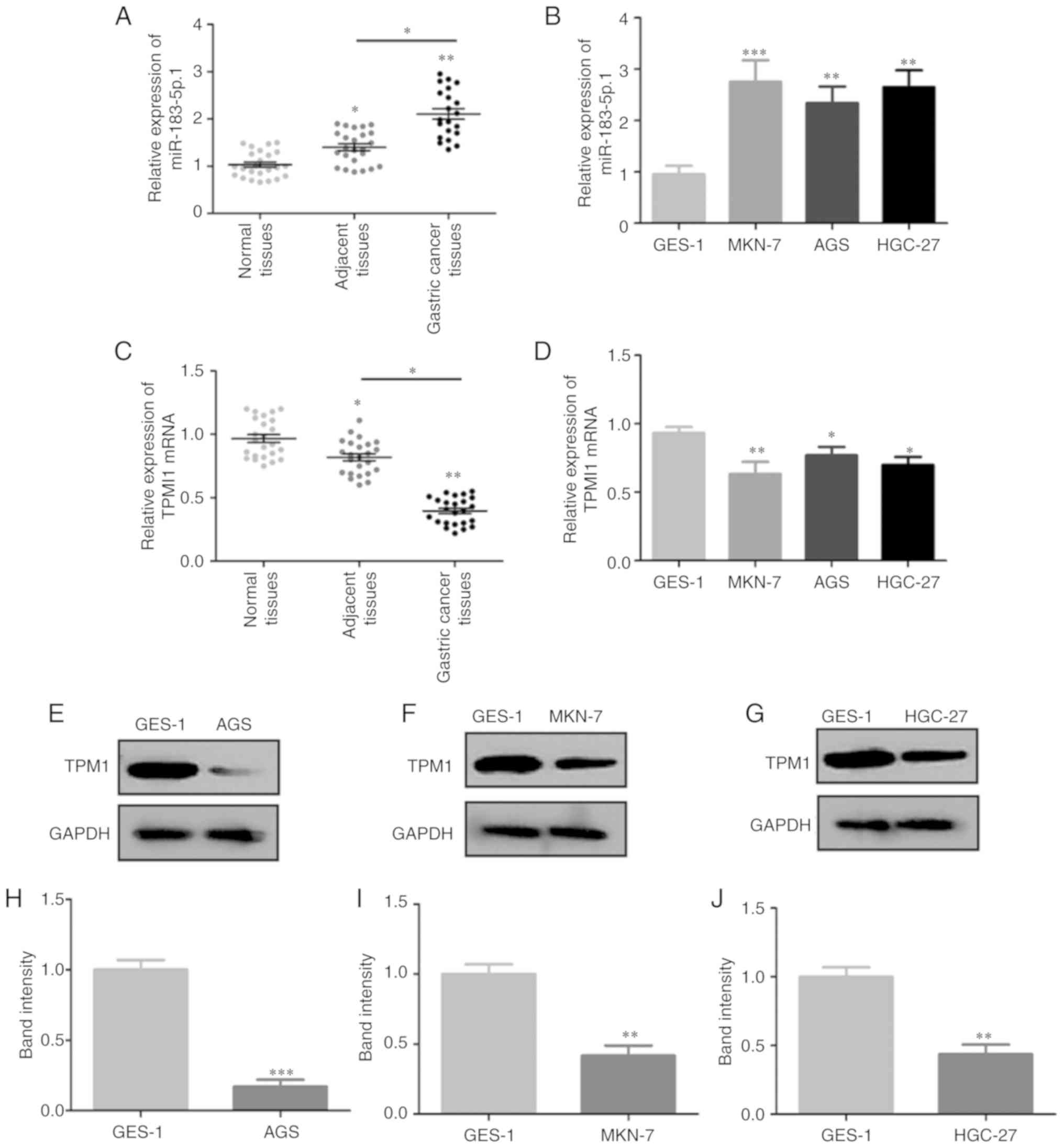 | Figure 1.Expression of miR-183-5p.1 and TPM1
in GC cells and tissues. miR-183-5p.1 levels in (A) normal tissues,
adjacent tissues, GC tissues and (B) normal gastric epithelial
cells (GES-1) and GC cell lines (MKN-7, AGS, HGC-27) were
determined by RT-qPCR. TPM1 mRNA expression in (C) normal tissues,
adjacent tissues, GC tissues and (D) GES-1, MKN-7, AGS, HGC-27
cells as quantified by RT-qPCR. (E) TPM1 protein expression in
GES-1 and AGS cells was determined by western blotting and (H)
statistically analyzed. (F) TPM1 protein expression in GES-1 and
MKN-7 cells was determined by western blotting and (I)
statistically analyzed. (G) TPM1 protein expression in GES-1 and
HGC-27 cells was determined by western blotting and (J)
statistically analyzed. Results are presented as the mean ±
standard error of the mean (n=3) *P<0.05, **P<0.01,
***P<0.001. GC, gastric cancer; RT-qPCR, reverse
transcription-quantitative PCR; TPM1, tropomyosin 1; miR,
microRNA. |
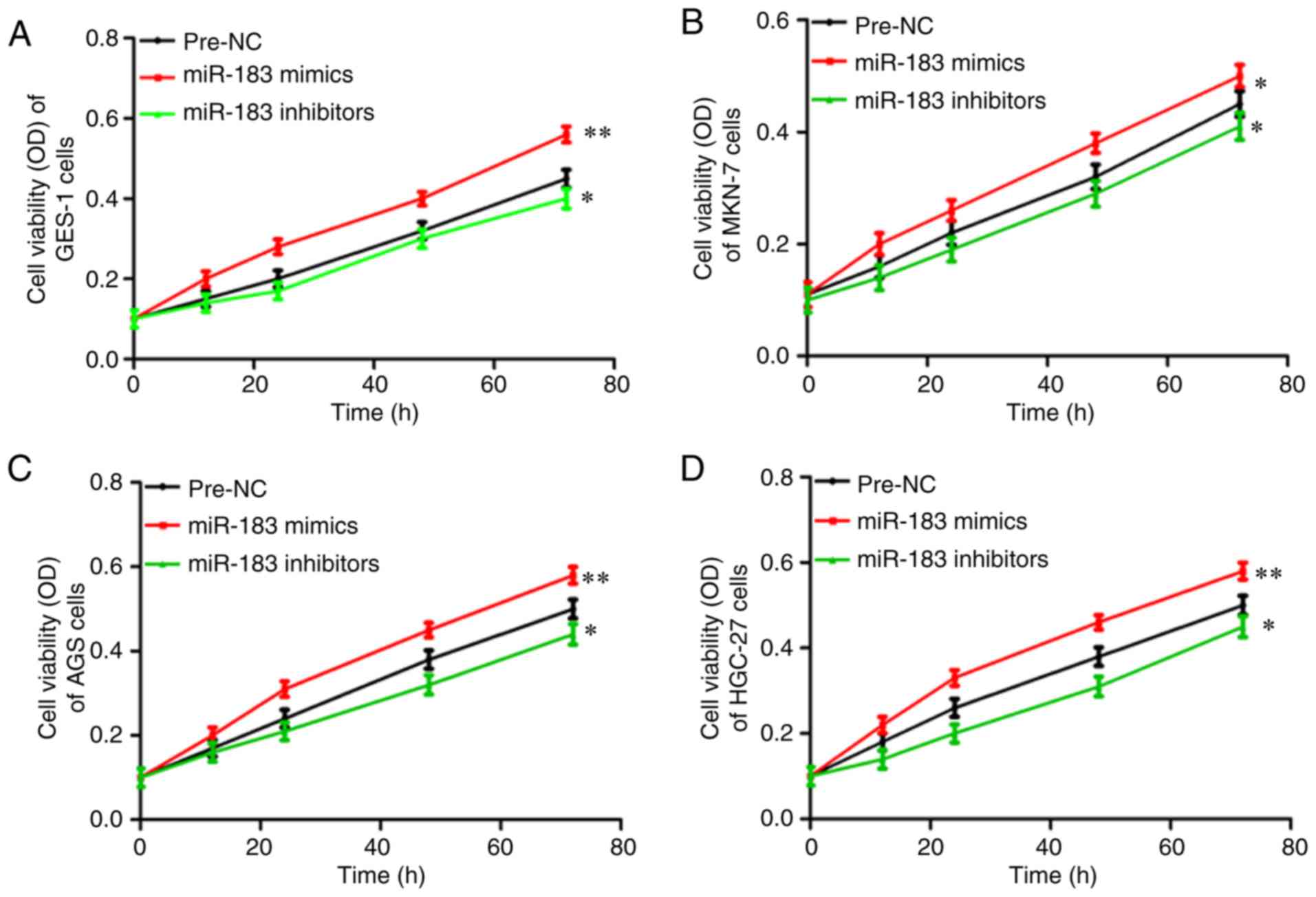
miR-183-5p.1 modulates the viability
of GC cells
The roles of miR-183-5p.1 in regulating cancer were
assessed through MTT assays to investigate the viability of the
transfected GES-1 (Fig. 2A), MKN-7
(Fig. 2B), AGS (Fig. 2C) and HGC-27 cells (Fig. 2D). The viability of the MKN-7, AGS and
HGC-27 cells was significantly enhanced via culture with the
miR-183-5p.1 mimics, but was significantly inhibited by
miR-183-5p.1 inhibitors.
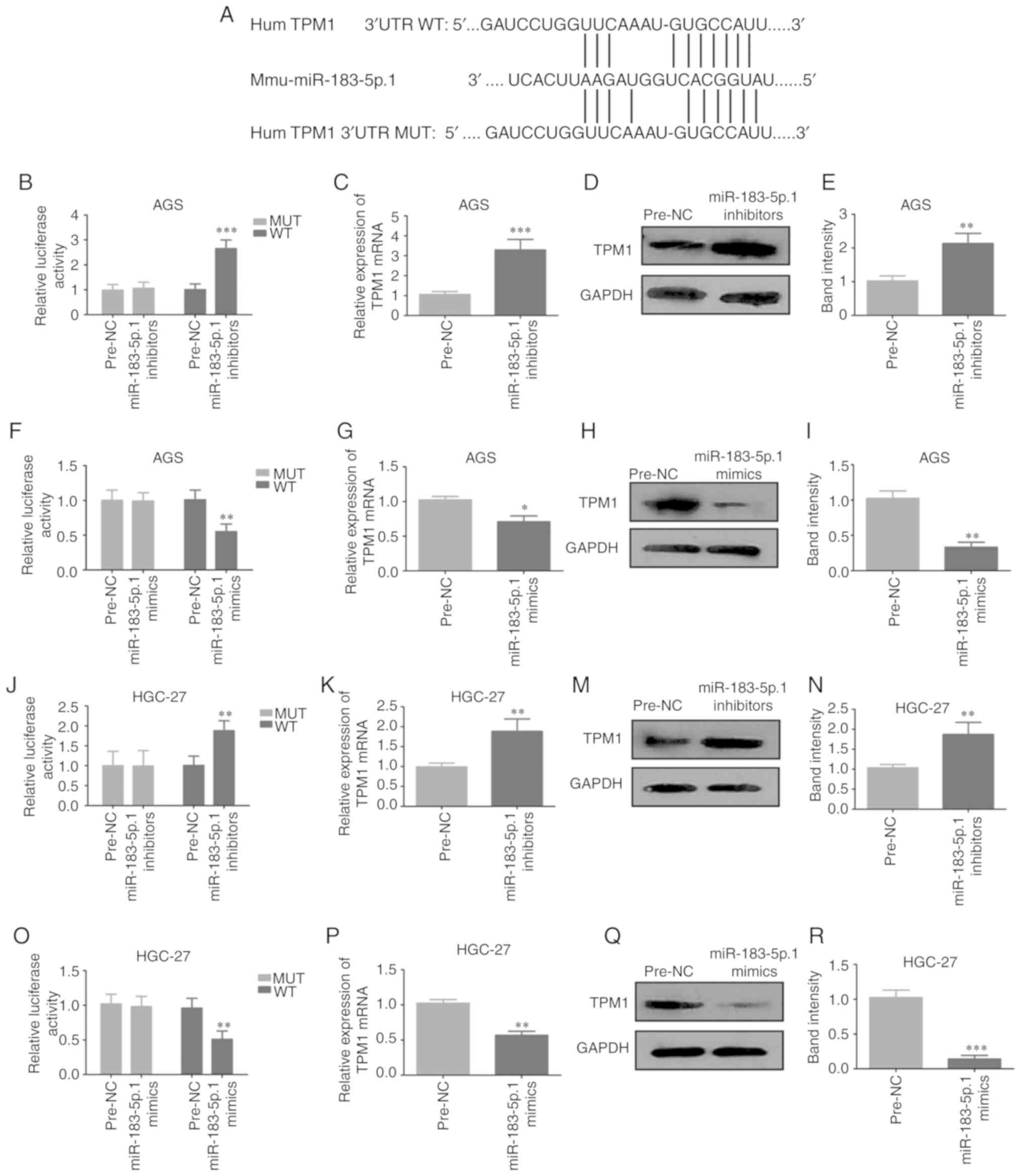
miR-183-5p.1 targets the TPM1 gene
directly in GC
To determine the biofunctions of miR-183-5p.1 in GC,
the present study identified the potential target genes of
miR-183-5p.1 that were dysregulated in GC using three predictive
algorithms: TargetScan, PicTar and MicroCosm. TargetScan showed
that TPM1 was a key target gene of miR-183-5p.1, and that
miR-183-5p.1 was bound to the 3′UTR of TPM1 (Fig. 3A). The relationship between
miR-183-5p.1 and TPM1 was validated through Luciferase reporter
assay. Plasmids of TPM1-UTR-pISo (WT) and Mu-TPM1-UTR-pIS0 (MUT)
were established and separately transfected into AGS and HGC-27
cells. It was demonstrated that miR-183-5p.1 inhibitors markedly
intensified the luciferase activity of TPM1-UTR-pISo (WT; Fig. 3B and J). Furthermore, transfection
with miR-183-5p.1 inhibitors markedly elevated TPM1 expression in
AGS cells (Fig. 3C-E) and HGC-27
cells (3K-N) at the mRNA and protein levels. Additionally,
transfection with miR-183-5p.1 mimics significantly weakened the
luciferase activity of TPM1-UTR-pISo (WT; Fig. 3F and O) and TPM1 expression in the AGS
cells (Fig. 3G-I) and HGC-27 cells
(3P-R). Taken together the results indicate that TPM1 is one of the
target genes of miR-183-5p.1.
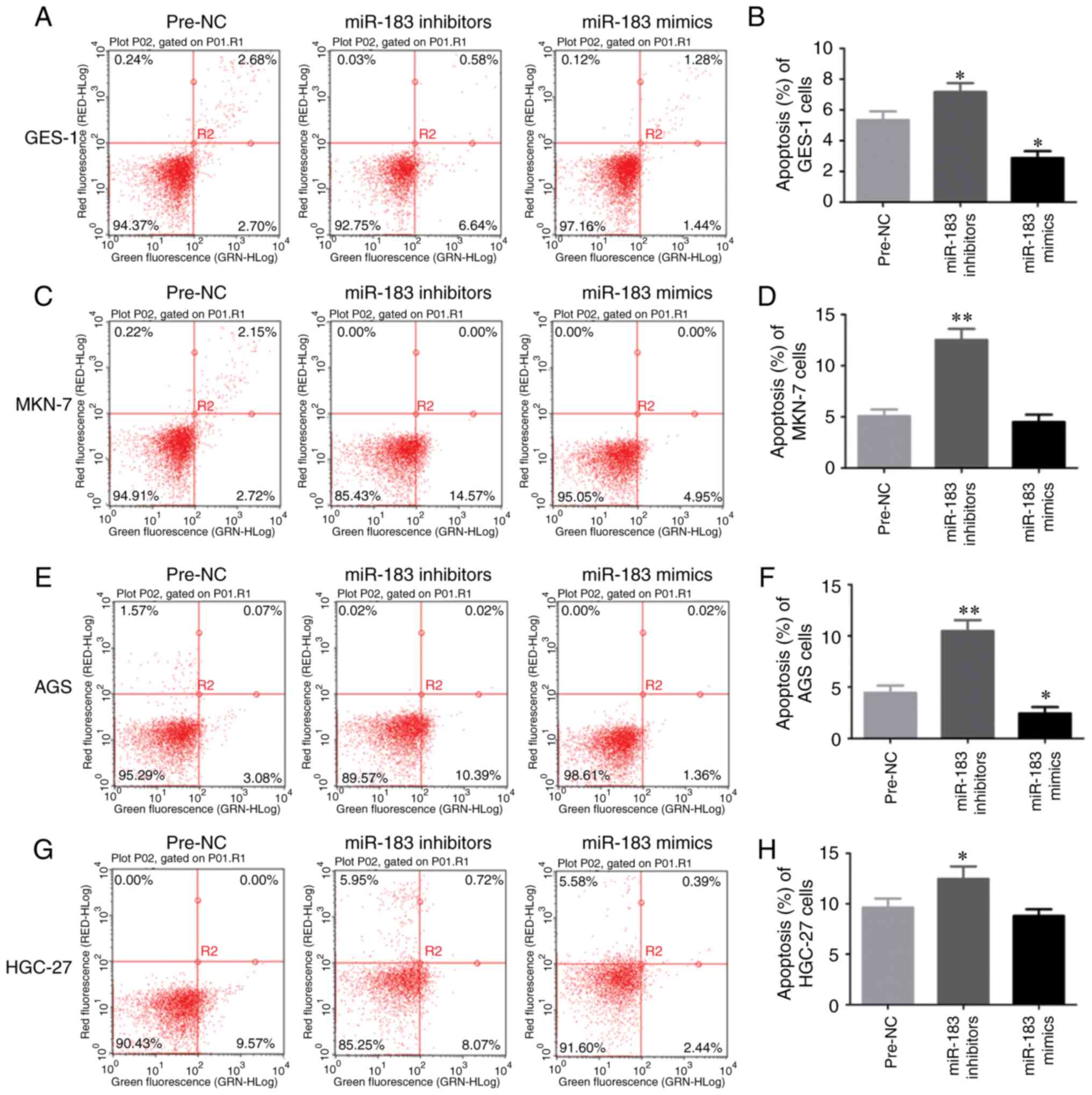
Apoptosis of GC cells is regulated by
miR-183-5p.1
To investigate the function of miR-183-5p.1 in the
early and the late apoptosis of GC cells, GES-1, MKN-7, AGS and
HGC-27 cell lines were analyzed by flow cytometry. Knockdown of
miR-183-5p.1 enhanced the early and the late apoptosis of GES-1
cells (Fig. 4A and B). In addition,
the flow cytometry results revealed that the early and the late
death of MKN-7 (Fig. 4C and D), AGS
(Fig. 4E and F) and HGC-27 (Fig. 4G and H) cells was promoted by the
knockdown of miR-183-5p.1, while the early and the late apoptosis
of GES-1 (Fig. 4A and B) and AGS
cells (Fig. 4E and F) was
significantly inhibited by the overexpression of miR-183-5p.1.
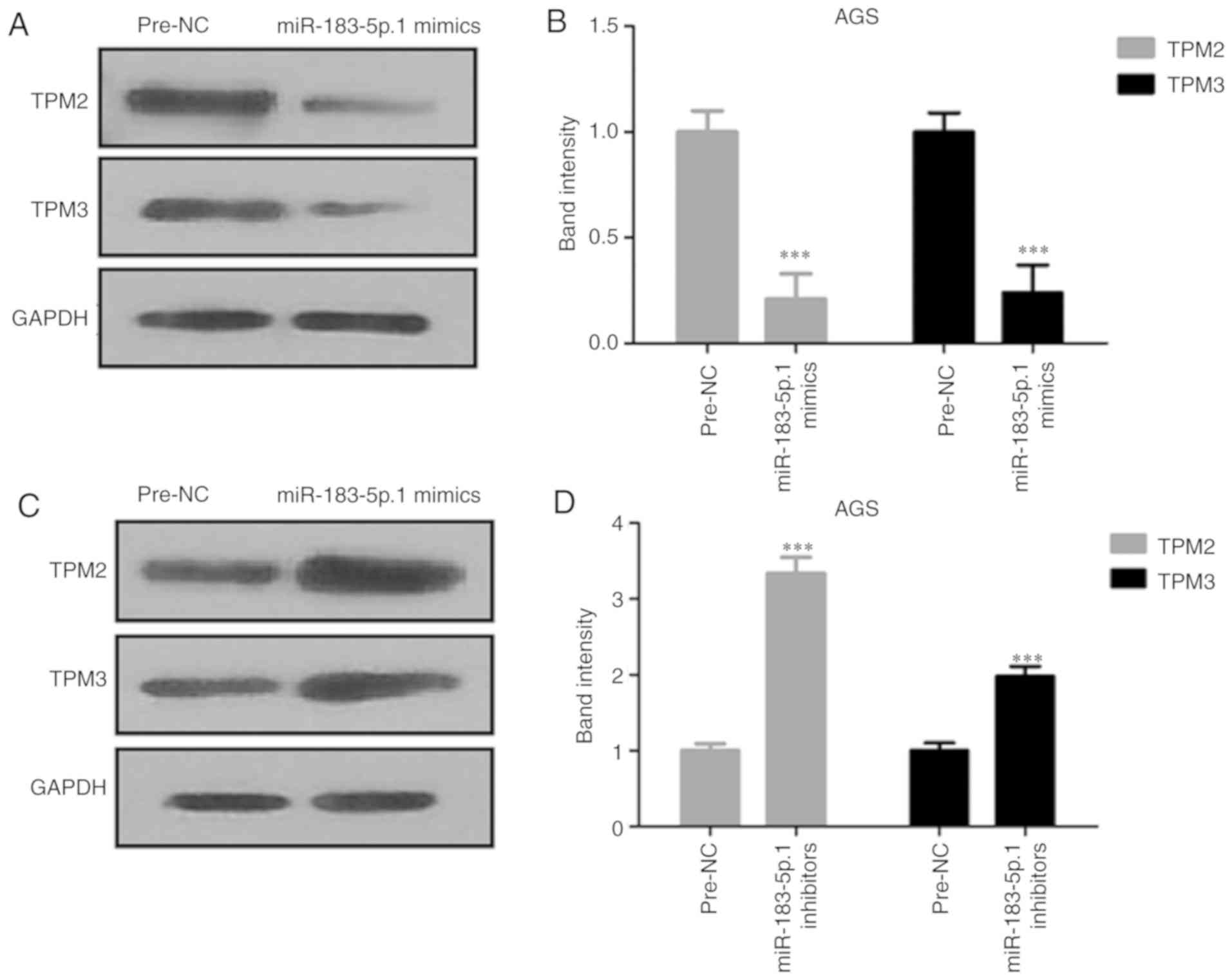
Expression of TPM2 and TPM3 is
affected by miR-183-5p.1
The protein expression of TPM2 and TPM3 was analyzed
by western blot analysis. The expression of TPM2 and TPM3 proteins
were decreased in AGS cells after transfection with miR-183-5p.1
mimics (Fig. 5A and B), and
significantly increased after the knockdown of miR-183-5p.1
(Fig. 5C and D).
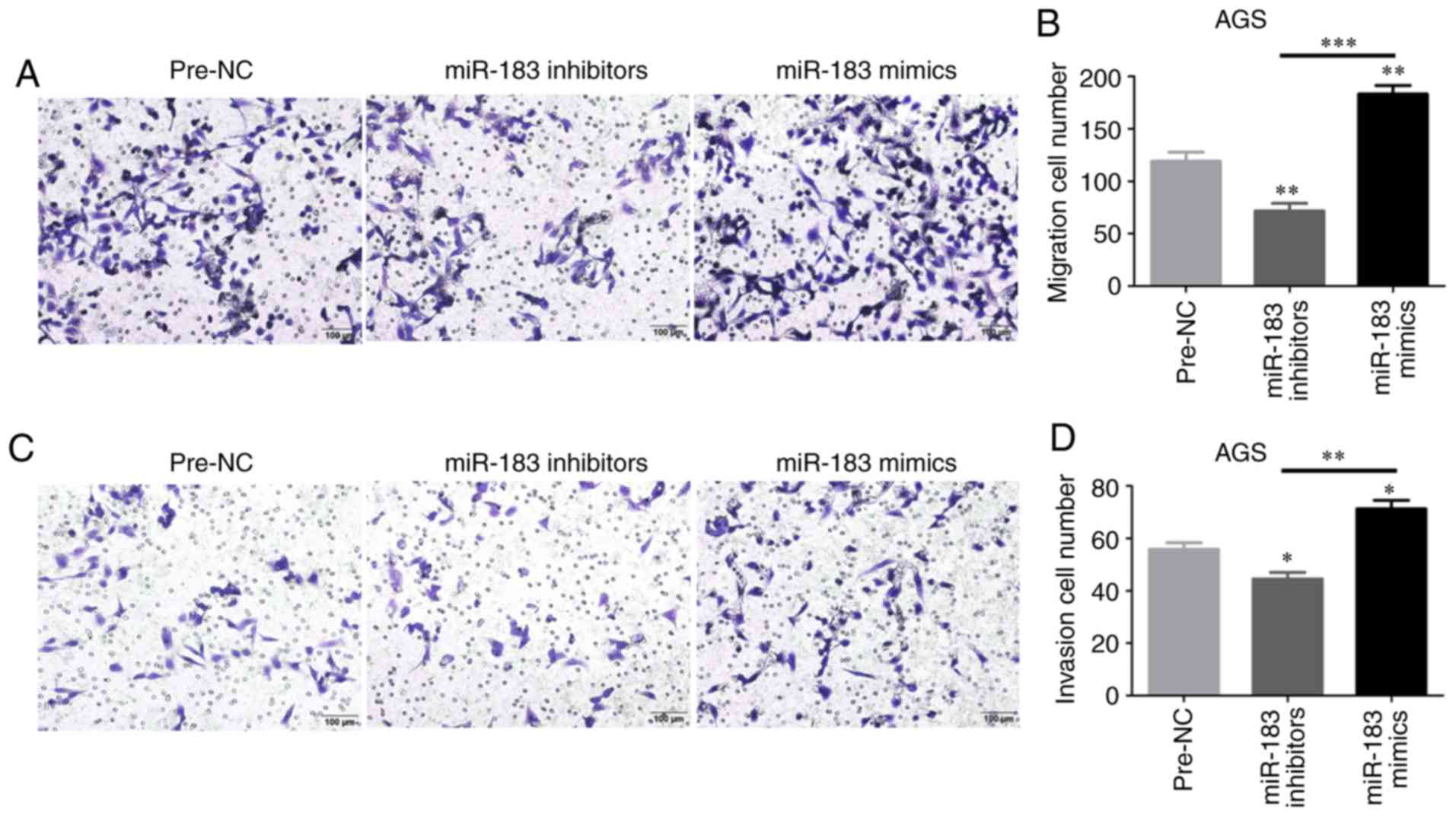
Cell migration and invasion are
promoted by miR-183-5p.1
The regulatory effects of miR-183-5p.1 on GC cell
migration and invasion were evaluated through Transwell assays. The
migration of AGS cells was enhanced in the mimics groups at 24 h
(Fig. 6A and B), but was weakened in
the inhibitor groups. In addition, the invasion of AGS cells was
validated by Transwell assays (Fig. 6C
and D). These results indicated that miR-183-5p.1 could
effectively promote GC cell migration and invasion, and that the
frequent metastasis of GC may be explained by the mechanism that
miR-183-5p.1 mimics stimulate the upregulated bio-properties of GC
cells.
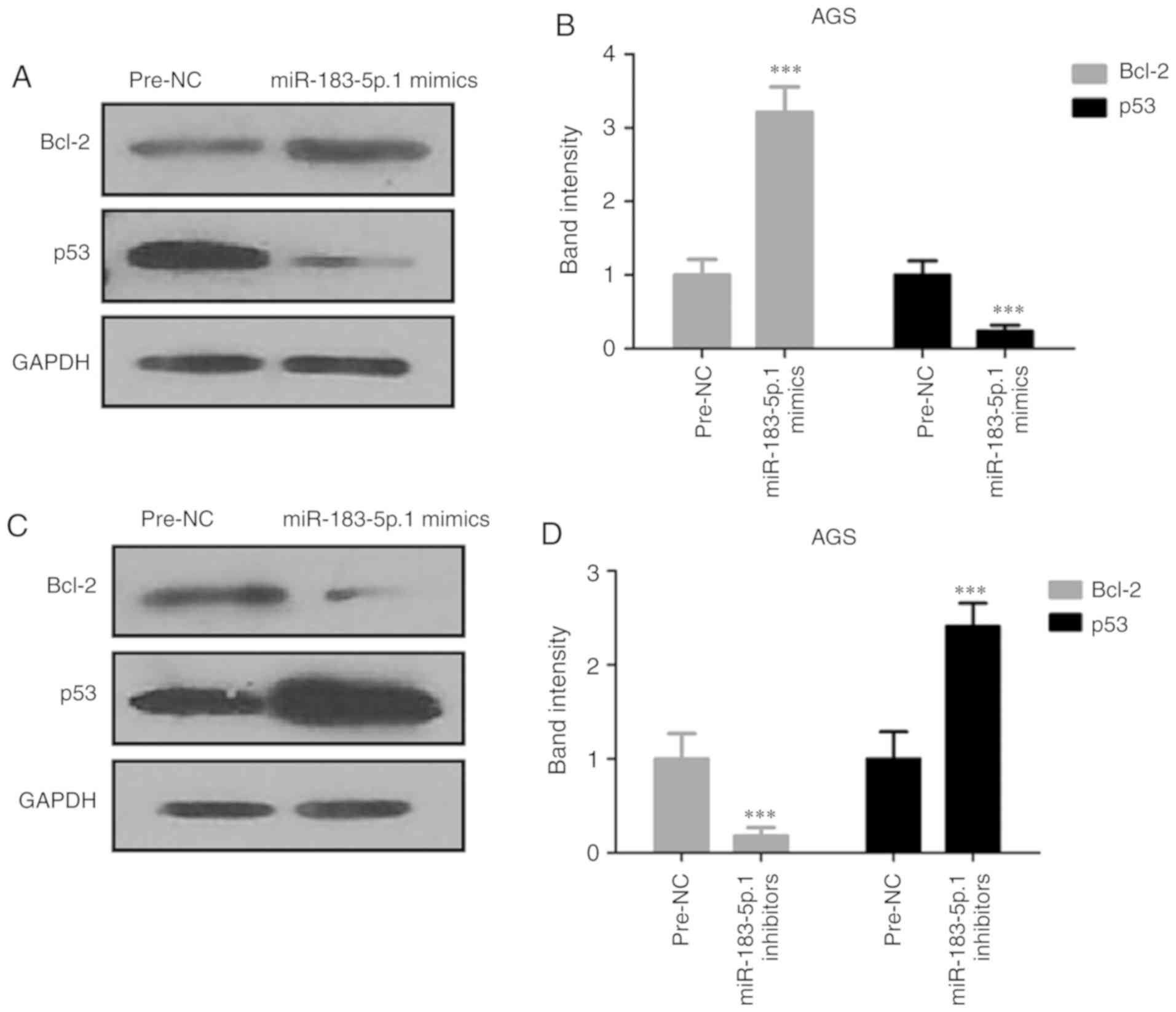
Expression of Bcl-2 and p53 in AGS
cells
Since miR-183-5p.1 was found to be capable of
regulating the migration and invasion of tumor cells through TPM1
protein, the present study detected the expression of
apoptosis-related proteins Bcl-2 and p53 through western blotting
(Fig. 7A-D). The experiments
demonstrated that p53 was significantly increased and Bcl-2 was
significantly decreased in the miR-183-5p.1-knockdown cells.
However, miR-183-5p.1 overexpression had the opposite effect on the
expression of Bcl-2 and p53. These results indicated that the
overexpression of miR-183-5p.1 suppressed the death of AGS
cells.
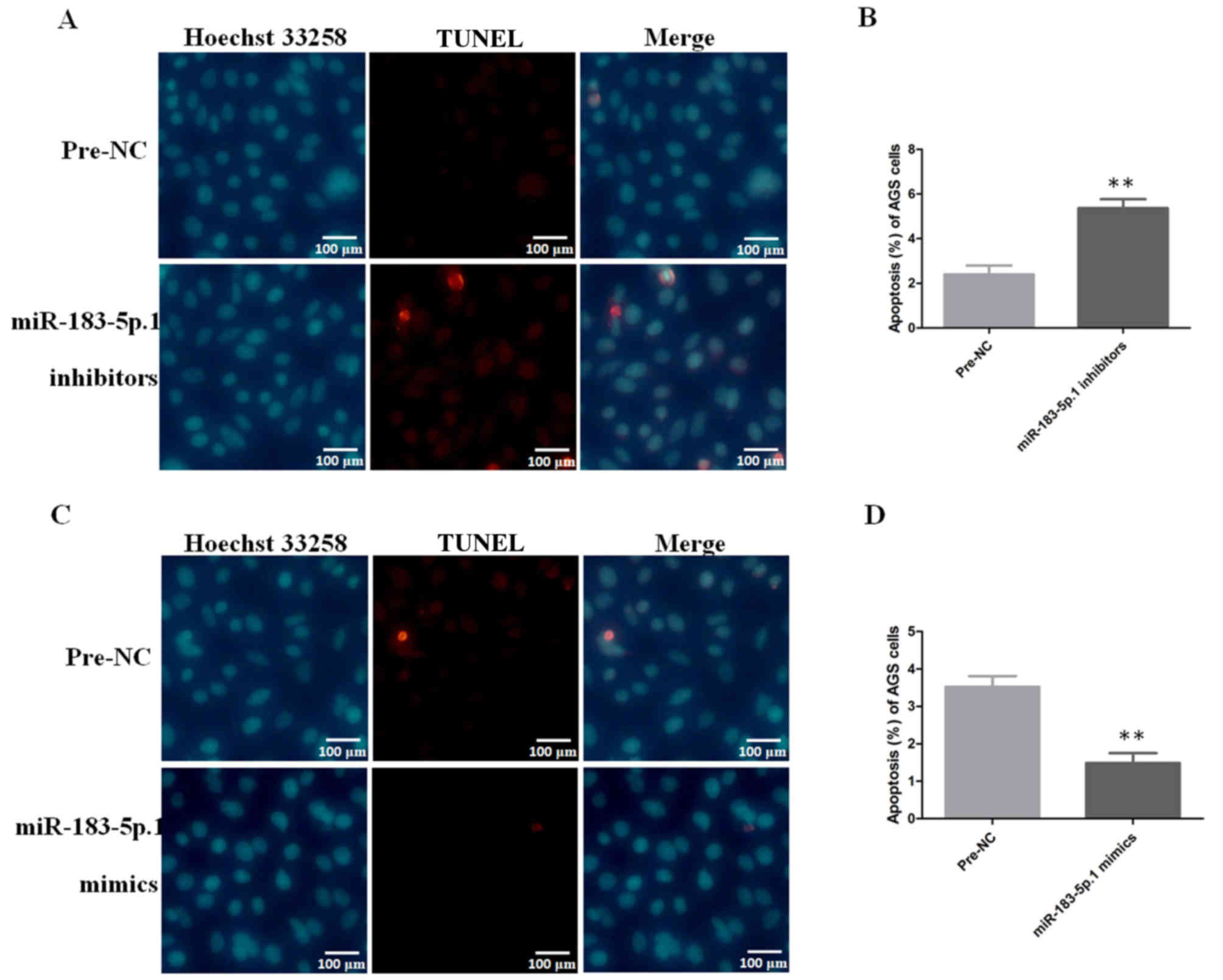
Apoptotic analysis of AGS cells by
immunofluorescence
After AGS cells were transfected with miR-183-5p.1
mimics and miR-183-5p.1 inhibitors, the apoptosis of AGS cells was
detected using Hoechst 33258 and TUNEL detection kits. The results
revealed that the apoptosis of AGS cells was induced significantly
(Fig. 8A and B) in the AGS cells
transfected with miR-183-5p.1 inhibitors, while the overexpression
of miR-183-5p.1 suppressed the apoptosis of the AGS cells (Fig. 8C and D).
Discussion
miR-183, one of the developmentally conserved miRNAs
(including miR-96/miR-182) that is located on human chromosome
7q32.3, may play key cellular roles in tumorigenesis (20). miR-183 is abnormally upregulated in
leukemia and hepatic cancers as well as other types of cancer
(21–24). However, miR-183 expression was
decreased and was inversely related to the metastasis of breast and
lung cancers (25). Furthermore,
miR-183 downregulation has been linked with metastatic lung cancer,
and its excessive expression was found to suppress the invasion of
cancer cells (26), indicating that
miR-183 may be involved in cancer occurrence, migration or spread
and play an antitumor role. The present study revealed that
miR-183-5p.1 expression was markedly increased and both TPM1 mRNA
and protein expression were significantly decreased in GC tissues
and cell lines. However, the dual-luciferase reporter analysis
indicated that miR-183-5p.1 targets the 3′UTR of TPM1.
TPMs, a large group of actin-connecting proteins,
have ~40 different isoforms that are critical in diverse
actin-based procedures (27). TPMs
are expressed in non-muscle cells, irrespective of their molecular
mass, including TPM1, TPM2 and TPM3 (28). TPMs as a tumor-associated protein
family have been investigated widely and play critical roles in
stress fiber modulation and actin cytoskeleton modification, which
are closely associated with tumor-specific variations in actin
filament aggregation. A growing body of evidence has indicated that
the migratory and invasion ability of tumor cells is enhanced by
the destruction of stress fibers and by TPM-mediated relevant
adhesive structures (29–32). The present study also investigated the
influence of miR-183-5p.1 and TPM1 knockdown on AGS cells. TPM1
expression in AGS cells after transfection with miR-183-5p.1 mimics
and inhibitors markedly decreased and increased in vitro at
the mRNA and protein levels, respectively. However, the
transfection of miR-183-5p.1 inhibitors increased the expression of
TPM2 and TPM3, and the trend in expression alterations was the
opposite after transfection with miR-183-5p.1 mimics. TPM2 and TPM3
are isoforms of TPM1 and are coded by the same gene TPM. We
used three software programs, TargetScan, miRBase and miRecords, to
predict the targets of miR-183-5p.1, and found that miR-183-5p.1
could bind sites in TPM1 and TPM3. But based on the pros and cons
of software forecasting, we investigated TPM1 in this study. In
addition, in the present study, the expression levels of TPM2 and
TPM3 proteins were also affected by the abnormal expression of
miR-183-5p.1. We believe that TPM2 and TPM3 are the targets of our
follow-up study. Moreover, these specific impact mechanisms still
need to be investigated in depth in subsequent research.
Recent studies have demonstrated that TPM1 as a
critical antitumor gene is downregulated in various solid tumors,
including breast cancer, colon cancer and urinary bladder carcinoma
(33–35). Recent research has confirmed the
antitumor role of TPM1 in breast cancer cells (36). TPM1 is necessary for the formation of
stress fibers, and reduction in cell motility and migration.
Activation of the Ras-ERK signaling pathway inhibits the TGF-β
induction of stress fibers by suppressing the expression of TPM,
leading to a more motile and invasive phenotype (37). In addition, TPM1 overexpression was
found to induce the apoptosis and inhibit the invasion of renal
cancer cells (38). Although a number
of experiments have indicated the tumor-suppressor role of TPM1 in
various tumor types, the underlying mechanism of its
tumor-suppressor gene functions is still unknown.
The present study discovered that transfection with
miR-183-5p.1 inhibitors largely weakened the survival of AGS cells
and promoted apoptosis, but the opposite results were observed
after transfection with miR-183-5p.1 mimics. Similarly, increased
AGS cell migration and invasion after transfection with
miR-183-5p.1 mimics was significantly promoted by the concurrent
siRNA-mediated knockdown of TPM1. According to the above results,
we speculate that miR-183-5p.1 targets TPM1 to enhance cell
survival, motion and invasion, and reduces cell death in GC cells.
Notably, miR-183 was significantly decreased in GC cells and
inhibited the invasion of GC indicating that miR-183 may act as a
tumor suppressor in GC, partially at least via regulation of Ezrin
as previously reported (39). We
assessed different targets of miR-183, but these two target genes
TPM1 and Ezrin play different roles in GC. In addition miR-183 was
investigated by Cao et al (39), but in our study we studied the
function of miR-183-5p.1 although they both belong to miR-183
family. However, these arguments warrant further in-depth research
for confirmation.
Further analysis of apoptotic signaling proteins
revealed that Bcl-2 and P53 participated in the effects of
miR-183-5p.1 and TPM1 on AGS cells, suggesting that the inhibitory
effect of miR-183-5p.1 on TPM1 expression may be associated with
the activation of apoptotic signaling pathways. However, the
present study did not identify which apoptosis-related gene was
regulated by miR-183-5p.1 or TPM1, and also did not determine the
mechanism of miR-183-5p.1 and TPM underlying the regulation of
apoptosis-related genes. The apoptosis-related genes involved in
the regulation of miR-183-5p.1 and TPM should be investigated in
the future, and the mechanisms underlying miR-183-5p.1 and TPM
regulation of apoptosis-related genes should also be further
investigated.
In conclusion, TPM1 functions as an antitumor gene
in GC. Determination of the function of miR-183-5p.1 in GC suggests
an important method with which to identify potential markers of GC
metastasis and efficient molecular targets for GC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All results and data generated or analyzed during
the present study are included in this published article or are
available from the corresponding author on reasonable request.
Authors' contributions
JL and ZC conceived and designed all experiments in
this study. JS, JL and HY performed the experiments and analyzed
all data. JL and ZC drafted and revised this article. All authors
have read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Shanghai General Hospital, Shanghai Jiaotong
University School of Medicine. Written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Otani K, Li X, Arakawa T, Chan FK and Yu
J: Epigenetic-mediated tumor suppressor genes as diagnostic or
prognostic biomarkers in gastric cancer. Expert Rev Mol Diagn.
13:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu S, Huang S, Ding J, Zhao Y, Liang L,
Liu T, Zhan R and He X: Multiple microRNAs modulate p21Cip1/Waf1
expression by directly targeting its 3′untranslated region.
Oncogene. 29:2302–2308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carleton M, Cleary MA and Linsley PS:
MicroRNAs and cell cycle regulation. Cell Cycle. 6:2127–2132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boehm M and Slack FJ: MicroRNA control of
lifespan and metabolism. Cell Cycle. 5:837–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baltimore D, Boldin MP, O'Connell RM, Rao
DS and Taganov KD: MicroRNAs: New regulators of immune cell
development and function. Nat Immunol. 9:839–845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu KW, Wang AM, Ping YH, Huang KH, Huang
TT, Lee HC, Lo SS, Chi CW and Yeh TS: Downregulation of tumor
suppressor MBP-1 by microRNA-363 in gastric carcinogenesis.
Carcinogenesis. 35:208–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song JH and Meltzer SJ: MicroRNAs in
pathogenesis, diagnosis, and treatment of gastroesophageal cancers.
Gastroenterology. 143:35–47.e2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sarver AL, Li L and Subramanian S:
MicroRNA miR-183 functions as an oncogene by targeting the
transcription factor EGR1 and promoting tumor cell migration.
Cancer Res. 70:9570–9580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Deng L, Zhi Q, Meng Q, Qian A, Sang
H, Li X and Xia J: MicroRNA-183 functions as an oncogene by
regulating PDCD4 in gastric cancer. Anticancer Agents Med Chem.
16:447–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu L, Li Y, Yan D, He J and Liu D:
MicroRNA-183 inhibits gastric cancer proliferation and invasion via
directly targeting Bmi-1. Oncol Lett. 8:2345–2351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka K, Shimura T, Kitajima T, Kondo S,
Ide S, Okugawa Y, Saigusa S, Toiyama Y, Inoue Y, Araki T, et al:
Tropomyosin-related receptor kinase B at the invasive front and
tumour cell dedifferentiation in gastric cancer. Br J Cancer.
110:2923–2934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamiya A, Inokuchi M, Otsuki S, Sugita H,
Kato K, Uetake H, Sugihara K, Takagi Y and Kojima K: Prognostic
value of tropomyosin-related kinases A, B, and C in gastric cancer.
Clin Transl Oncol. 18:599–607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pierce ML, Weston MD, Fritzsch B, Gabel
HW, Ruvkun G and Soukup GA: MicroRNA-183 family conservation and
ciliated neurosensory organ expression. Evol Dev. 10:106–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bandrés E, Cubedo E, Agirre X, Malumbres
R, Zárate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzó M and
García-Foncillas J: Identification by real-time PCR of 13 mature
microRNAs differentially expressed in colorectal cancer and
non-tumoral tissues. Mol Cancer. 5:292006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Motoyama K, Inoue H, Takatsuno Y, Tanaka
F, Mimori K, Uetake H, Sugihara K and Mori M: Over- and
under-expressed microRNAs in human colorectal cancer. Int J Oncol.
34:1069–1075. 2009.PubMed/NCBI
|
|
23
|
Agirre X, Jiménez-Velasco A, San
José-Enériz E, Garate L, Bandrés E, Cordeu L, Aparicio O, Saez B,
Navarro G, Vilas-Zornoza A, et al: Down-regulation of hsa-miR-10a
in chronic myeloid leukemia CD34+ cells increases
USF2-mediated cell growth. Mol Cancer Res. 6:1830–1840. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ladeiro Y, Couchy G, Balabaud C,
Bioulac-Sage P, Pelletier L, Rebouissou S and Zucman-Rossi J:
MicroRNA profiling in hepatocellular tumors is associated with
clinical features and oncogene/tumor suppressor gene mutations.
Hepatology. 47:1955–1963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Macedo T, Silva-Oliveira RJ, Silva VAO,
Vidal DO, Evangelista AF and Marques MMC: Overexpression of mir-183
and mir-494 promotes proliferation and migration in human breast
cancer cell lines. Oncol Lett. 14:1054–1060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang G, Mao W and Zheng S: MicroRNA-183
regulates Ezrin expression in lung cancer cells. FEBS Lett.
582:3663–3668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gunning PW, Hardeman EC, Lappalainen P and
Mulvihill DP: Tropomyosin-master regulator of actin filament
function in the cytoskeleton. J Cell Sci. 128:2965–2974. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zare M, Jazii FR, Soheili ZS and
Moghanibashi MM: Downregulation of tropomyosin-1 in squamous cell
carcinoma of esophagus, the role of Ras signaling and methylation.
Mol Carcinog. 51:796–806. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Redwood C and Robinson P:
Alpha-tropomyosin mutations in inherited cardiomyopathies. J Muscle
Res Cell Motil. 34:285–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Perry SV: Vertebrate tropomyosin:
Distribution, properties and function. J Muscle Res Cell Motil.
22:5–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pawlak G and Helfman DM: Cytoskeletal
changes in cell transformation and tumorigenesis. Curr Opin Genet
Dev. 11:41–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Helfman DM, Flynn P, Khan P and Saeed A:
Tropomyosin as a regulator of cancer cell transformation. Adv Exp
Med Biol. 644:124–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bharadwaj S and Prasad GL: Tropomyosin-1,
a novel suppressor of cellular transformation is downregulated by
promoter methylation in cancer cells. Cancer Lett. 183:205–213.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raval GN, Bharadwaj S, Levine EA,
Willingham MC, Geary RL, Kute T and Prasad GL: Loss of expression
of tropomyosin-1, a novel class II tumor suppressor that induces
anoikis, in primary breast tumors. Oncogene. 22:6194–6203. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pawlak G, McGarvey TW, Nguyen TB,
Tomaszewski JE, Puthiyaveettil R, Malkowicz SB and Helfman DM:
Alterations in tropomyosin isoform expression in human transitional
cell carcinoma of the urinary bladder. Int J Cancer. 110:368–373.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dube S, Yalamanchili S, Lachant J, Abbott
L, Benz P, Mitschow C, Dube DK and Poiesz BJ: Expression of
tropomyosin 1 gene isoforms in human breast cancer cell lines. Int
J Breast Cancer. 2015:8594272015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bakin AV, Safina A, Rinehart C, Daroqui C,
Darbary H and Helfman DM: A critical role of tropomyosins in
TGF-beta regulation of the actin cytoskeleton and cell motility in
epithelial cells. Mol Biol Cell. 15:4682–4694. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Guan J, Lu Z, Jin J, Cai Y, Wang C
and Wang F: Clinical and tumor significance of tropomyosin-1
expression levels in renal cell carcinoma. Oncol Rep. 33:1326–1334.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao LL, Xie JW, Lin Y, Zheng CH, Li P,
Wang JB, Lin JX, Lu J, Chen QY and Huang CM: miR-183 inhibits
invasion of gastric cancer by targeting Ezrin. Int J Clin Exp
Pathol. 7:5582–5594. 2014.PubMed/NCBI
|