Introduction
Ovarian cancer (OC) remains a devastating
gynecological malignancy in developed countries (1). The high mortality rate of this tumor is
mainly due to the fact that the majority of the patients (75%)
present at an advanced stage and have already developed extensive
peritoneal metastases (2). Patients
often have non-specific pelvic or abdominal symptoms. Despite
improvements in surgical techniques and treatment strategies for OC
patients in recent years, the prognosis remains poor (3). Thus, it is crucial to explore novel
targets for OC therapy.
The Hippo signaling pathway has been identified as a
tumor suppressor pathway that is involved in cell death, cell
proliferation and tissue growth (4).
Yes-associated protein (YAP), a direct downstream effector of the
Hippo pathway, is a key transcriptional co-activator that interacts
with the TEA domain family member (TEAD) to regulate the expression
of target genes (5,6). Recent advances have revealed the
upregulation of YAP in several types of cancer, such as breast
cancer (7), hepatocellular carcinoma
(HCC) (8) and lung adenocarcinoma
(9), and have identified YAP as an
oncogene in OC (10,11). Moreover, it was demonstrated that YAP
could form autocrine loops with the ERBB pathway to regulate OC
initiation and progression (11).
However, the effect and specific mechanism of targeting YAP in OC
progression requires further investigation.
The aim of the present study was to determine the
expression of YAP in OC tissues and its correlation with OC
prognosis, as well as elucidate the underlying mechanism, hoping to
uncover a novel approach to OC therapy.
Materials and methods
Cell culture
The human ovarian cancer cell lines ES-2 (cat. no.
CRL-1978) and SK-OV-3 (cat. no. HTB-77), were purchased from the
American Type Culture Collection (ATCC). All cell lines were tested
every 6 months, or new ATCC cell lines were obtained every 6
months. SK-OV-3 and ES-2 were maintained in McCoy's 5A medium
supplemented with 10% fetal bovine serum and 1% antibiotic mixture
(10,000 U/ml penicillin and 10 mg/ml streptomycin) and cultured
with 5% CO2 at 37°C.
Reagents and antibodies
Negative control (siControl) and YAP-1-specific
(siYAP-1, siYAP-2) siRNA were purchased from Invitrogen; Thermo
Fisher Scientific, Inc. (sequences: siYAP-1:
5′-CAGCAGAAUAUGAUGAACUCGGCUU-3′; siYAP-2:
5′-GGAAGGAGAUGGAAUGAACAUAGAA-3′). Peptide 17 was purchased from
Selleck Chemicals. YAP, phospho-Akt (Ser473), phospho-PI3K p85
(Tyr458)/p55 (Tyr199), mTOR, and Akt2 antibody were obtained from
Cell Signaling Technology. The GSK3-β and GAPDH antibodies were
obtained from Proteintech Group, Inc. The Ki-67, PI3K p85 beta,
phospho-GSK3-β (Ser9), and phospho-mTOR (Ser2448) antibodies were
obtained from Abcam. Matrigel was purchased from BD Biosciences.
The secondary antibodies were horseradish peroxidase
(HRP)-conjugated goat anti-rabbit and anti-mouse IgG (1:5,000;
Antgene). The complete information on all the antibodies used in
the present study is provided in supplementary Table SI.
Migration assay
Transwell plates (8-µm pore size; Corning, Inc.)
were used for the migration assay as described previously (12). Cells transfected with siControl or
siYAP or treated with peptide 17 (250 nM) were harvested and
re-suspended with serum-free McCoy's 5A and seeded at a cell
density of 2×104 into the upper chamber. Complete media
(500 µl) were added to the bottom chambers. The cells were
incubated at 37°C for 24 h. After 1 day of incubation, the cells
that migrated to the lower surface of the membrane were fixed with
4% paraformaldehyde for 15 min, then washed with phosphate-buffered
saline (PBS) for 3 times and stained with 0.05% crystal violet
solution for 15 min. Non-migrating cells on the top surface of the
filter were carefully wiped off with a cotton swab, and the
migrating cells were quantified by counting five random fields
using a phase contrast microscope.
Invasion assay
In vitro cell invasion was investigated using
a Transwell assay (8.0 µm, Corning, Inc.) coated with basement
membrane Matrigel (BD Biosciences). Cells transfected with
siControl or siYAP or treated with peptide 17 (250 nM) were
harvested and seeded at a cell density of 2×104 into the
upper chamber. Complete media (500 µl) were added to the bottom
chambers. After 48 h of incubation, non-invading cells on the top
surface of the filter were carefully wiped off with a cotton swab,
and the invading cells were quantified by counting five random
fields using a phase contrast microscope.
Wound healing assay
Cells transfected with siControl or siYAP or treated
with peptide 17 (250 nM) were seeded in 6-well plates in
triplicate. After the cells had reached ~100% confluence, the
monolayers were manually scratched using a sterile 10-µl
pipette tip and cultured for 24 h. The area of the scratch was
photographed under a microscope (Olympus Corporation) and measured
using ImageJ software (National Institutes of Health). The results
were expressed as a percentage of the original area.
Cell viability assay
Cell viability was determined using a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) at defined
intervals. Cells transfected with siControl or siYAP
(4×103 cells per well) were placed in 96-well plates in
triplicate and incubated for 72 h. After treatment, cell viability
was detected using the CCK-8 assay according to the manufacturer's
instructions. After incubating with CCK-8 dye at 37°C for 2 h in
the dark, the absorbance value was measured by SpectraMax190
(Molecular Devices, LLC) at a wavelength of 450 nm.
EdU assay
The EdU assay was carried out using the Cell-Light™
EdU imaging detecting kit according to the manufacturer's
instructions (RiboBio).
Immunohistochemistry (IHC)
Normal ovaries, normal fallopian tubes and OC
tissues were collected from patients undergoing surgery at the
Department of Gynecological Oncology of Tongji Hospital (Wuhan,
China). IHC staining was conducted as previously described
(12,13).
Gene expression and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from cells or tissues using
TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. NanoDrop (Thermo Fisher
Scientific, Inc.) was used to assess the quality and quantity of
the total RNA. Total RNA (1 µg) was used to synthesize cDNA
using M-MLV reverse transcriptase (Takara Bio, Inc.). RT-qPCR
detection was performed using the Bio-Rad CFX96 system with
SYBR-Green to determine the expression level of mRNA of interest
(Bio-Rad Laboratories, Inc.). The primers used were as follows:
GAPDH forward, 5′-ATGGAAATCCCATCACCATCTT-3′ and reverse,
5′-CGCCCCACTTGATTTTGG-3′; YAP forward, 5′-TAGCCCTGCGTAGCCAGTTA-3′
and reverse, 5′-TCATGCTTAGTCCACTGTCTGT-3′. The comparative Cq
method was used to calculate the relative mRNA expression levels.
The expression level of GAPDH was used as a loading control.
Western blot analysis
After washing twice with cold PBS, the cells were
lysed with radioimmunoprecipitation assay (RIPA) lysis buffer
(Beyotime Institute of Biotechnology) containing 1X protease
inhibitor cocktail (Roche Diagnostics). Proteins were separated and
electrophoresed on 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis gels, transferred to nitrocellulose membranes by a
wet transfer apparatus, and then blocked with TBST buffer (25
mmol/l Tri-HCl, pH 7.5, 137 mmol/l NaCl, 2.7 mmol/l KCl and 0.05%
Tween-20) with 5% bovine serum albumin for 1 h at 37°C. The blots
were probed with the indicated antibodies in blocking buffer
overnight at 4°C. GAPDH was used as the internal control. After the
membranes were washed with TBST 3 times for 20 min each time,
HRP-linked secondary antibody (Antgene) was applied to the membrane
for 60 min. The protein was finally detected using enhanced
chemiluminescence (ECL; Bio-Rad Laboratories, Inc.). Band density
was measured by ImageJ software (National Institutes of Health) and
normalized to GAPDH.
Mouse experiments
Six-week-old female BALB/c-nu mice (n=10 weight:
17–19 g) were obtained from the Shanghai Animal Experimental
Center. All mice were housed under pathogen-free conditions in the
Animal Research Center of Tongji Hospital at 22°C with 12-h
light/dark cycles and free access to water and food. SK-OV3-ip3-luc
(1×106) cells were injected orthotopically under the
ovarian bursa. One week after surgery, the mice were randomized
into the control (saline) and treatment (peptide 17) experimental
groups (n=5 per group). Peptide 17 was administered i.p. (0.2
mg/kg) daily and saline was used as control. The mice were
anesthetized with pentobarbital sodium (75 mg/kg) after 28 days of
treatment, and imaged with the IVIS SPECTRUM system (Caliper;
Xenogen) 10 min after i.p. injection of 100 mg/kg D-luciferin
substrate. Living Image software version 4.3.1 (Caliper; Xenogen)
was used for the analysis. The tumor-bearing mice were sacrificed
by cervical dislocation, and the tumors were removed for
assessment.
Colony formation
Cells transfected with siControl or siYAP were
seeded in 6-well plates and incubated at 37°C for 10 days. For the
peptide 17 experiment, cells were plated in 6-well plates and
treated with peptide 17 (250 nM) for 10 days; the medium was
replaced every 48 h to ensure constant levels of the drug. The
cells were stained with 0.1% (w/v) crystal violet solution.
Data availability
The Kaplan-Meier plotter tool (http://kmplot.com/analysis/) was used to generate
survival curves combining YAP1 (Affymetrix probe 224895_at) mRNA
data from all public ovarian cancer datasets (14).
Statistical analysis
All data analyses in the present study were
performed using GraphPad Prism 6 (GraphPad Software, Inc.). Data
are expressed as the means ± standard deviation from at
least 3 independent experiments. Two experimental groups were
compared using a paired Student's t-test for paired data or a
Student's t-test for unpaired data. Analysis of variance was used
to compare differences among multiple groups (post hoc test:
Turkey's multiple comparisons test). Kruskal-Wallis test (post hoc
test: Dunn's multiple comparisons test) was used to compare
differences in YAP expression among normal ovaries, normal
fallopian tubes, primary tumors and metastases. P-values <0.05
were considered to indicate statistically significant
differences.
Results
YAP is significantly upregulated in
human OC tissues and is correlated with poor prognosis
To explore the role of YAP in OC, YAP expression was
first determined in normal ovaries, normal fallopian tubes and
paired primary tumors and metastases of epithelial ovarian cancer
(EOC) by IHC. The information on different tissues is provided in
the supplementary Table SII.
Compared with normal ovaries and normal fallopian tubes, YAP
expression was significantly higher in OC primary tumors and
metastases (Fig. 1A and B) (primary
vs. normal ovary, P=0.0051; primary vs. normal fallopian tube,
P=0.0371; metastases vs. normal ovary: P<0.0001; metastases vs.
normal fallopian tube, P<0.0001). Notably, higher nuclear YAP
levels were observed in metastases compared with primary tumors
(Fig. 1C). Furthermore, Kaplan-Meier
plot analysis of YAP expression in OC was performed to determine
whether YAP is correlated with prognosis in OC patients. The result
indicated an association of high YAP expression with poor
progression-free survival (PFS) in OC patients (Fig. 1D). Thus, these results confirmed that
YAP is upregulated in OC tissues and is correlated with patient
survival, suggesting that YAP may affect OC progression.
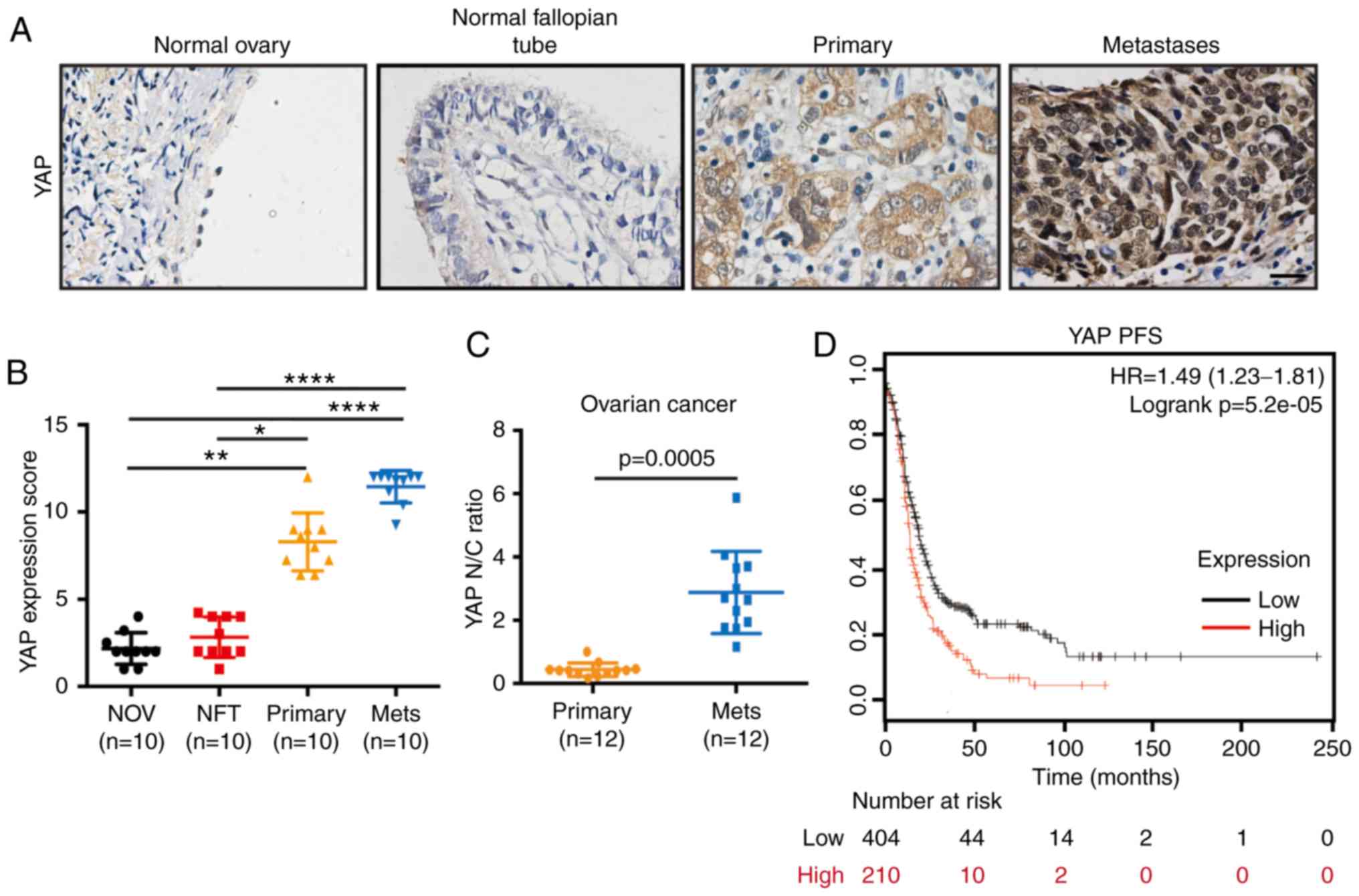 | Figure 1.YAP is highly expressed in OC and is
correlated with poor patient prognosis. (A) Representative YAP IHC
image from analyses of human ovarian samples including normal
ovaries, normal fallopian tubes, paired primary tumors and
metastases of epithelial OC patients. Scale bar, 20 µm. (B) YAP
expression scores of the above IHC-stained tissues: Normal ovaries
(NOV), normal fallopian tubes (NFT), primary tumors (Primary), and
metastases (Mets) (n=10). (Kruskal-Wallis test, *P<0.05,
**P<0.01, ****P<0.0001. (C) Distribution of nuclear (N) to
cytoplasmic (C) ratio (N/C ratio) of YAP staining in paired primary
tumors (Primary) and metastases (Mets) of OC (n=12). Geometric mean
for N/C ratio for each category was calculated. (D)
Progression-free survival (PFS) curve in OC patients with low or
high expression levels of YAP. OC, ovarian cancer; IHC,
immunohistochemistry; YAP, Yes-associated protein. |
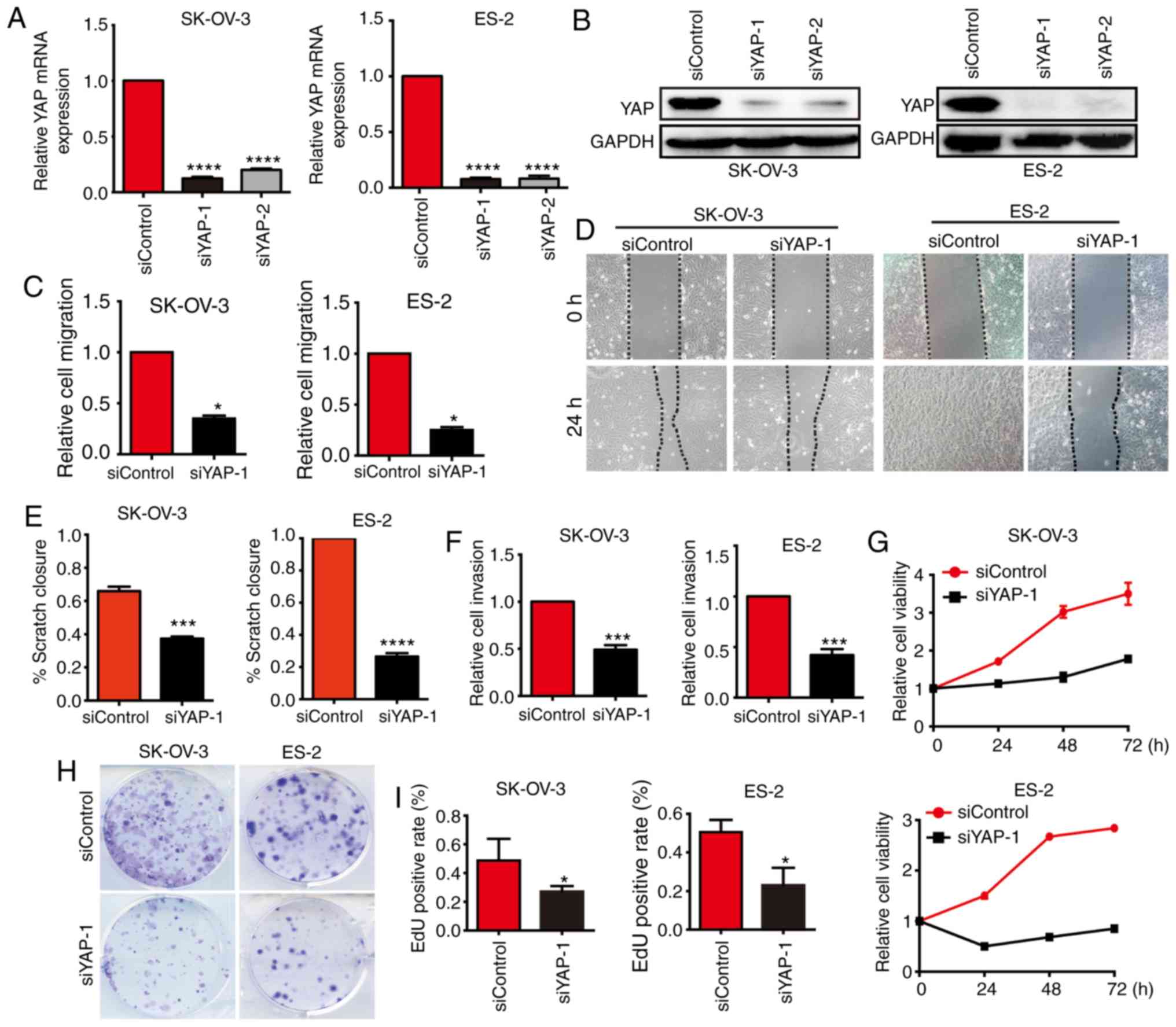
YAP silencing inhibits the malignant
behavior of OC cells
To further explore the function of YAP in OC, two
siRNAs targeting YAP and siControl were employed to transfect
SK-OV-3 and ES-2 cells. qPCR and western blot analyses demonstrated
that transfection of siYAP-1 and siYAP-2 efficiently inhibited YAP
expression at both the mRNA and protein levels, particularly
siYAP-1 (Fig. 2A and B). Transwell
assays indicated that the migratory ability of both SK-OV-3 and
ES-2 cells transfected with siYAP was markedly inhibited compared
with cells transfected with siControl (Fig. 2C). Consistently, wound healing assays
also confirmed that silencing of YAP diminished the migration of OC
cells (Fig. 2D and E). We also
demonstrated that YAP silencing inhibited the invasion of OC cells,
as assessed by Transwell assays (Fig.
2F). Cell viability assay demonstrated that silencing of YAP
inhibited the proliferation of OC cells (Fig. 2G). Similarly, colony formation assays
confirmed that the colony-forming ability of OC cells was reduced
upon transfection with siRNA of YAP (Fig.
2H). The EdU assay also supported these conclusions (Fig. 2I). Thus, the findings demonstrated
that silencing of YAP diminished the migration, invasion and
proliferation of OC cells, indicating that YAP may be a potential
target for the treatment of OC.
YAP silencing regulates the
PI3K/Akt/mTOR pathway
To further explore the mechanism through which
silencing of YAP inhibits the malignant behavior of OC cells, we
evaluated the activation of the PI3K/Akt/mTOR pathway, a key
signaling pathway involved in tumor progression. The
phosphorylation status of PI3K, Akt and mTOR, the key signaling
proteins of the PI3K/Akt/mTOR pathway, was assessed by western
blotting. Compared with siControl-transfected cells, the
phosphorylation of PI3K was significantly lower in
siYAP-transfected SK-OV-3 and ES-2 cells (Fig. 3A and B). Similarly, downregulation of
YAP expression decreased the phosphorylation of Akt and mTOR in
both types of OC cells (Fig. 3A, C and
D). Moreover, the activation of GSK-3β was assessed in both
cell lines, since glycogen synthase kinase (GSK)-3β is another
downstream regulator of the PI3K/Akt pathway. However, no
significant changes in the phosphorylation status of GSK-3β were
observed between siControl-transfected and siYAP-transfected cells
(Fig. 3A and E). These results
indicate that silencing of YAP may inhibit the activation of the
PI3K/Akt/mTOR pathway, suggesting that this be the mechanism
underlying YAP-regulated OC progression.
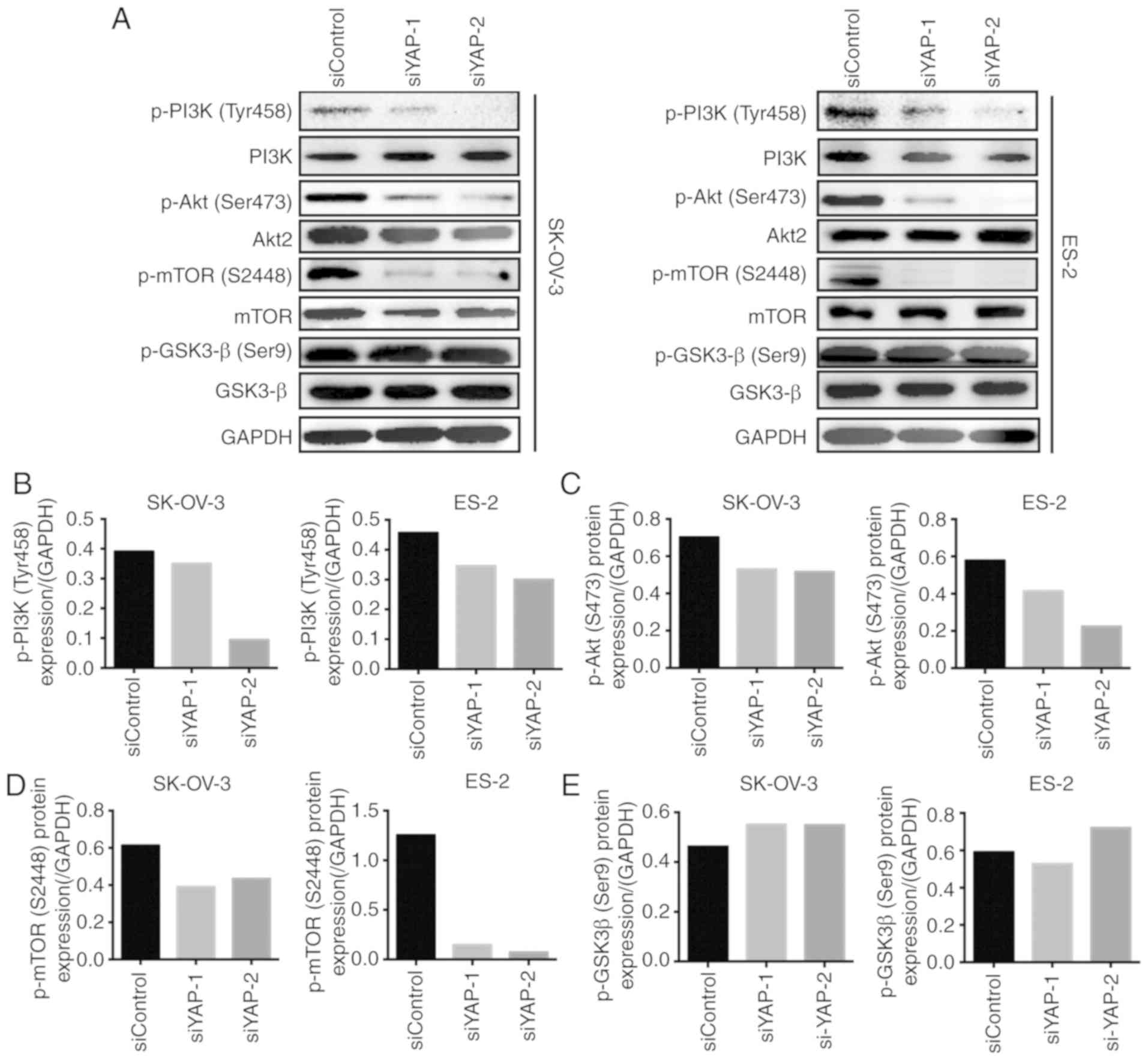 | Figure 3.YAP silencing regulates the
PI3K/AKT/mTOR pathway. (A) Western blot analyzed the expression of
phospho-PI3K (Tyr458), phospho-Akt (Ser473), phospho-mTOR (S2448),
phospho-GSK3-β (Ser9), PI3K, mTOR, Akt2, and GSK3-β in SK-OV-3 and
ES-2 cells transfected with siYAP-1, siYAP-2, or siControl. GAPDH
was used as a loading control. (B) Quantification of phospho-PI3K
(Tyr458) relative to GAPDH in SK-OV-3 and ES-2 cells transfected
with siYAP-1, siYAP-2, or siControl. (C) Quantification of
phospho-Akt (Ser473) relative to GAPDH in SK-OV-3 and ES-2 cells
transfected with siYAP-1, siYAP-2, or siControl. (D) Quantification
of phospho-mTOR (S2448) relative to GAPDH in SK-OV-3 and ES-2 cells
transfected with siYAP-1, siYAP-2, or siControl. (E) Quantification
of phospho-GSK3-β (Ser9) relative to GAPDH in SK-OV-3 and ES-2
cells transfected with siYAP-1, siYAP-2, or siControl. YAP,
Yes-associated protein; GSK, glycogen synthase kinase. |
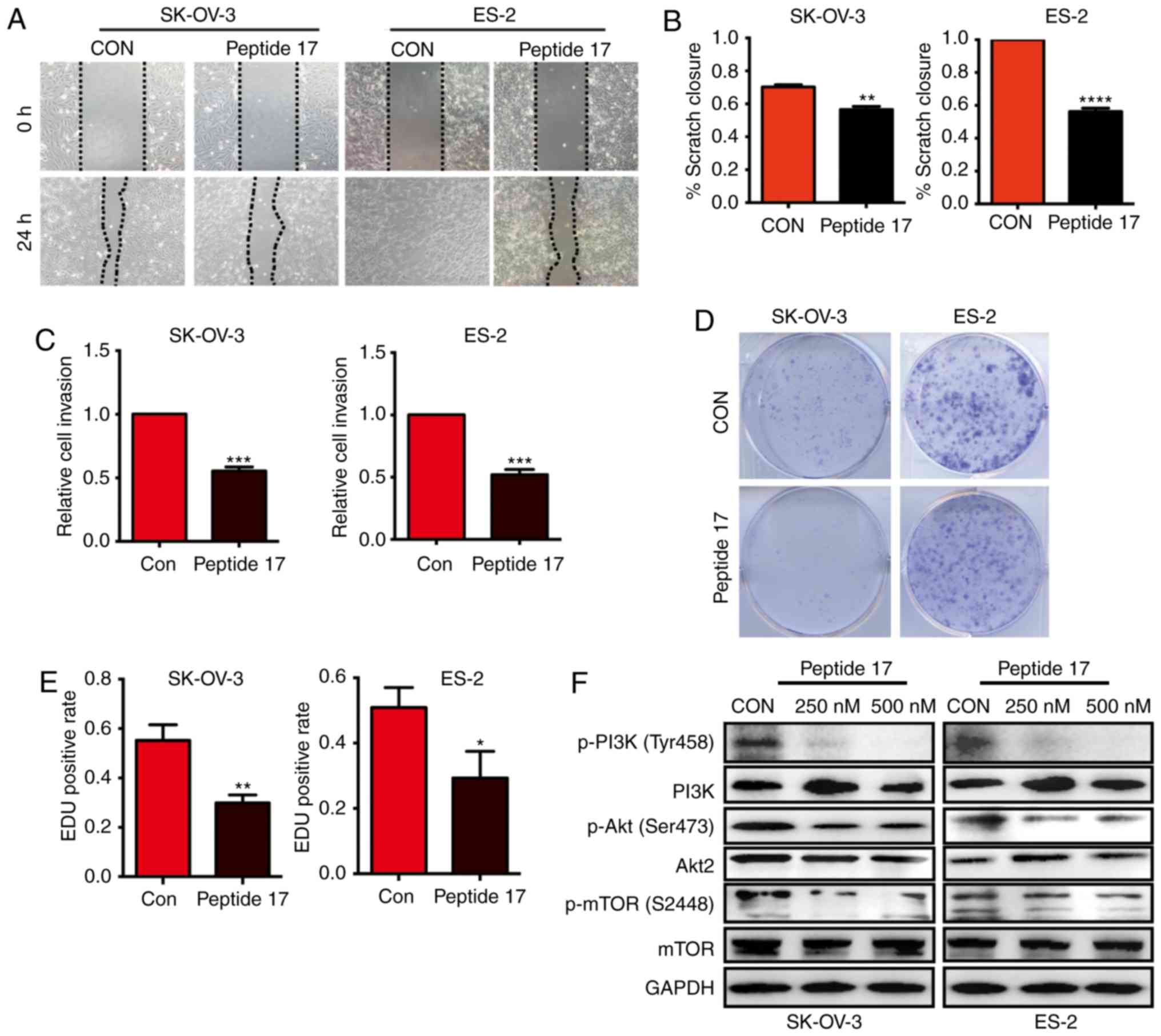
Peptide 17 inhibits the malignant
behavior of OC cells and modulates the PI3K/Akt/mTOR pathway
As YAP silencing significantly inhibited the
malignant behavior of OC cells, we next investigated whether
peptide 17, a YAP inhibitor, exerted the same effect. Wound healing
assays indicated that peptide 17 markedly impaired the migration of
SK-OV-3 and ES-2 cells compared with the control group (Fig. 4A and B). Peptide 17 also inhibited the
invasive ability of both SK-OV-3 and ES-2 cells, as evidenced by
Transwell assays (Fig. 4C). The
colony formation assay demonstrated that peptide 17 significantly
inhibited colony formation by both SK-OV-3 and ES-2 cells (Fig. 4D). Similar results were also obtained
by the EdU assay (Fig. 4E). These
results demonstrated that peptide 17 inhibited the malignant
behavior of OC cells. Considering that silencing YAP inhibited
activation of the PI3K/Akt/mTOR pathway, we further investigated
whether peptide 17 exerted a similar effect. Western blot analysis
demonstrated that phosphorylation of PI3K, Akt and mTOR was lower
in cells treated with peptide 17 compared with the control group
(Fig. 4F). These results demonstrated
that peptide 17 markedly attenuated the malignant behavior of OC
cells and modulated the PI3K/Akt/mTOR pathway, suggesting that
peptide 17 may be a potential therapeutic strategy for OC.
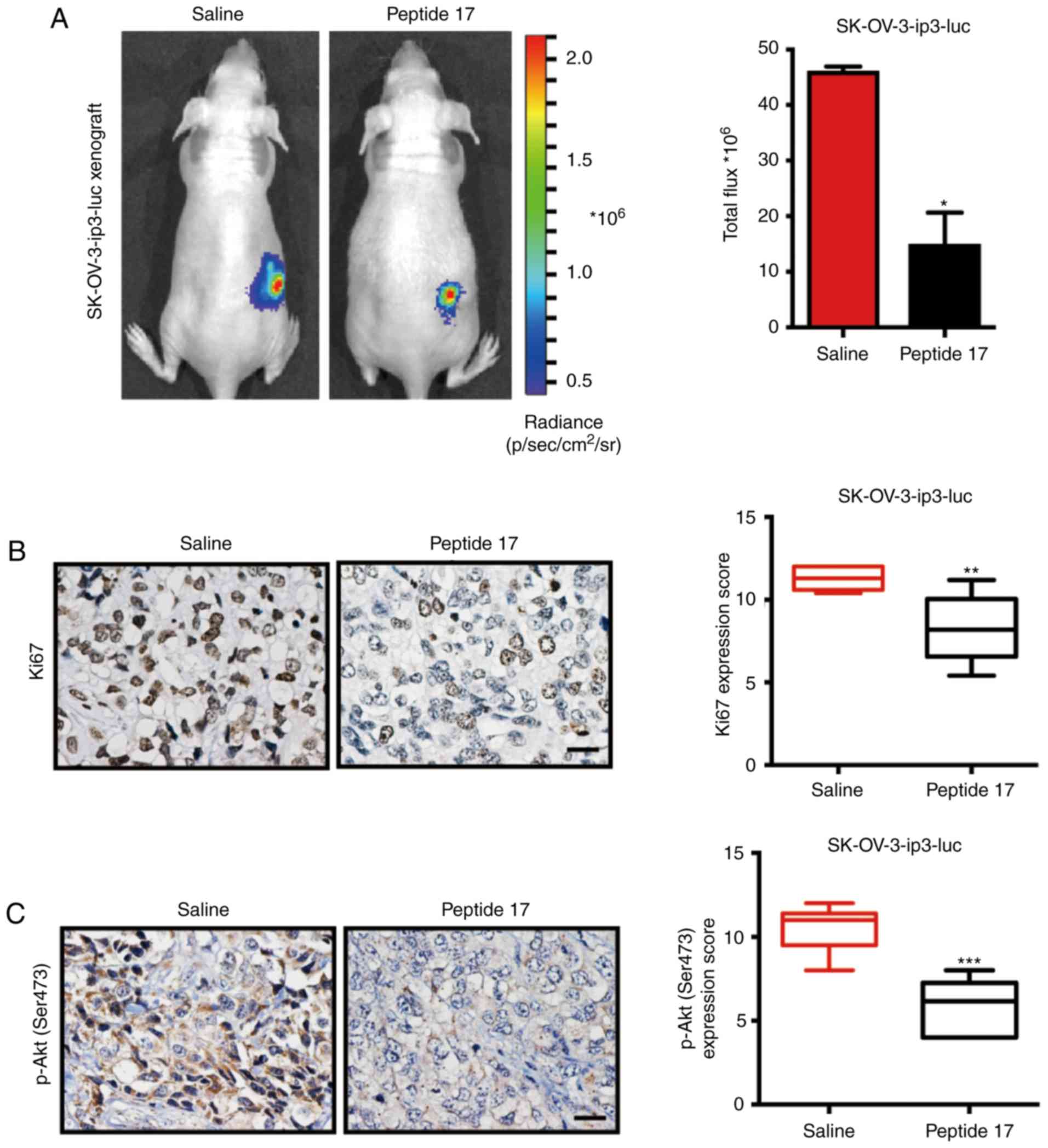
Peptide 17 restrains OC progression in
vivo
Next, to verify whether inhibition of YAP with
peptide 17 restrains OC progression in vivo, an orthotopic
ovarian cancer model utilizing SK-OV-3-ip3-luc cells was employed.
Consistent with the in vitro results, mice treated with
peptide 17 developed significantly smaller tumors compared with
mice treated with saline (Fig. 5A).
To better understand these results, the tumors were removed at the
end of the experiment, and further experiments were conducted.
Subsequently, tumors from peptide 17-treated mice exhibited
increased Ki-67 staining, as evidenced by IHC, compared with those
in the saline group (Fig. 5B),
indicating that peptide 17 inhibited the growth of OC in
vivo. Since it was determined that peptide 17 could regulate
the activation of the PI3K/Akt/mTOR signaling pathway in OC cells,
phospho-Akt (Ser473) and phospho-mTOR (S2448) expression were
detected in the obtained tumor sections. Mechanistically, IHC
analysis revealed significantly diminished staining of phospho-Akt
(Ser473) (Fig. 5C) and phospho-mTOR
(S2448) in peptide 17-treated mice, compared with the saline group
(Fig. 5D). As shown by western blot
analysis, the expression of phospho-PI3K (Tyr458), phospho-Akt
(Ser473) and phospho-mTOR (S2448) was found to be markedly impaired
in tumors from peptide 17-treated mice compared with those from
saline-treated mice (Fig. 5E-F).
Thus, these data demonstrated that peptide 17 significantly
attenuated OC progression through inhibition of the PI3K/Akt/mTOR
signaling pathway.
Discussion
In the present study, the expression of YAP in OC
was determined, and a correlation between YAP expression and OC
prognosis was identified. Furthermore, YAP silencing was shown to
inhibit malignant phenotypes of OC cells through regulation of the
PI3K/Akt/mTOR pathway. Of note, peptide 17, a YAP inhibitor,
exerted a significant inhibitory effect on OC progression. Thus,
the findings of the present study emphasized that targeting YAP
suppresses OC progression through regulation of the PI3K/Akt/mTOR
pathway, indicating the potential clinical value of peptide 17 in
OC treatment.
The Hippo/YAP pathway plays a crucial role in
development, growth and organogenesis, and dysregulation of this
pathway has been associated with tumor progression (15,16). Some
studies have also found that the Hippo/YAP pathway may be involved
in the initiation and progression of ovarian cancer (10,17). Our
study further indicated that targeting YAP with siRNA or YAP
inhibitor significantly attenuated OC progression in vitro
and in vivo through reducing activation of the PI3K/Akt/mTOR
pathway. Although the connection between YAP and the PI3K/AKT/mTOR
pathway has previously been demonstrated in other cancers (18,19), these
results of the present study depicted the regulation between YAP
and the PI3K/AKT/mTOR pathway in OC.
Currently, therapy with small molecule inhibitors is
an important therapeutic strategy for tumor progression. For
example, based on the key role of poly [adenosine diphosphate
(ADP)-ribose] polymerase (PARP) in DNA damage repair, PARP
inhibitors have been approved by the U.S. Food and Drug
Administration as monotherapy for patients with several different
types of cancer (20,21). In addition, lung cancers with
activating EGFR mutations exhibit a notable response to treatment
with EGFR tyrosine kinase inhibitors (22). With stronger binding affinity to TEAD1
rather than the YAP protein, peptide 17 is identified as a YAP-TEAD
protein-protein interaction inhibitor (23), which can attenuate the oncogenic
function of YAP and may thus be of value in the treatment of
YAP-involved cancers (23,24). In the present study, it was confirmed
that peptide 17 markedly attenuated OC progression both in
vitro and in vivo. As a small molecular inhibitor,
peptide 17 exhibited strong potential for OC therapy in the
clinical setting. Further experiments should be performed to
investigate the safety of peptide 17.
In summary, these findings of the present study
highlighted the effectiveness of targeting YAP in restraining OC
progression, and revealed that the underlying mechanism may be
regulation of the PI3K/Akt/mTOR pathway. Of note, peptide 17 may be
recommended as a therapeutic strategy against OC progression,
providing novel options for OC management.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the ‘973’
Program of China (grant no. 2015CB553903 to Ding Ma and Junbo Hu),
the National Science-Technology Supporting Plan Projects (grant no.
2015BAI13B05), the National Key Research and Development Program of
China (grant no. 2016YFC0902901), the National Science Foundation
of China (grant nos. 81372801 and 81572570), the National Science
and Technology Major Sub-Project (grant no. 2018ZX10301402-002),
and the Technical Innovation Special Project of Hubei Province
(grant no. 2018ACA138). These grants provided financial supports
for the study but did not impose restrictions on the design of the
study, collection, analysis and interpretation of data, or writing
of the manuscript.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XW conceived and designed the experiments. YJ, HL,
JM, QH and YM performed the experiments. CS provided assistance
with statistical analyses. XL, SX and XY conducted the
bioinformatics analysis. XW and QG wrote the paper. ZY and TJ
provided assistance with revising the manuscript. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Human tissues were donated for research purposes by
patients undergoing surgery at the Department of Obstetrics and
Gynecology, Tongji Hospital, Huazhong University of Science and
Technology, after obtaining written informed consent of the
patients and the authorization of the Ethics Committee of Tongji
Hospital (TJ-IRB20181103). All mouse experiments were conducted in
accordance with a protocol approved by the Ethics Committee of
Tongji Hospital, Tongji Medical College, Huazhong University of
Science and Technology (Institutional Review Board Approval of
Experimental Animals: IRB ID: TJ-A20160105).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no financial or
non-financial competing interests.
References
|
1
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz PE: Current diagnosis and
treatment modalities for ovarian cancer. Cancer Treat Res.
107:99–118. 2002.PubMed/NCBI
|
|
4
|
Basu-Roy U, Bayin NS, Rattanakorn K, Han
E, Placantonakis DG, Mansukhani A and Basilico C: Sox2 antagonizes
the Hippo pathway to maintain stemness in cancer cells. Nat Commun.
6:64112015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu AM, Xu MZ, Chen J, Poon RT and Luk JM:
Targeting YAP and Hippo signaling pathway in liver cancer. Expert
Opin Ther Targets. 14:855–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pegoraro S, Ros G, Ciani Y, Sgarra R,
Piazza S and Manfioletti G: A novel HMGA1-CCNE2-YAP axis regulates
breast cancer aggressiveness. Oncotarget. 6:19087–19101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu H, Wei L, Fan F, Ji S, Zhang S, Geng J,
Hong L, Fan X, Chen Q, Tian J, et al: Integration of Hippo
signalling and the unfolded protein response to restrain liver
overgrowth and tumorigenesis. Nat Commun. 6:62392015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Y, Zhang W, Han X, Li F, Wang X, Wang
R, Fang Z, Tong X, Yao S, Li F, et al: YAP inhibits squamous
transdifferentiation of Lkb1-deficient lung adenocarcinoma through
ZEB2-dependent DNp63 repression. Nat Commun. 5:46292014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, George J, Deb S, Degoutin JL,
Takano EA, Fox SB, Bowtell DD and Harvey KF; AOCS Study Group, :
The Hippo pathway transcriptional co-activator, YAP, is an ovarian
cancer oncogene. Oncogene. 30:2810–2822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He C, Lv X, Hua G, Lele SM, Remmenga S,
Dong J, Davis JS and Wang C: YAP forms autocrine loops with the
ERBB pathway to regulate ovarian cancer initiation and progression.
Oncogene. 34:6040–6054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei X, Liu Y, Gong C, Ji T, Zhou X, Zhang
T, Wan D, Xu S, Jin P, Yang X, et al: Targeting leptin as a
therapeutic strategy against ovarian cancer peritoneal metastasis.
Anticancer Agents Med Chem. 17:1093–1101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji T, Gong D, Han Z, Wei X, Yan Y, Ye F,
Ding W, Wang J, Xia X, Li F, et al: Abrogation of constitutive
Stat3 activity circumvents cisplatin resistant ovarian cancer.
Cancer Lett. 341:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gyorffy B, Lanczky A and Szallasi Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovarian-cancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Pan P, Wang Z, Zhang Y, Xie P,
Geng D, Jiang Y, Yu R and Zhou X: β-catenin-mediated YAP signaling
promotes human glioma growth. J Exp Clin Cancer Res. 36:1362017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma Y, Yang Y, Wang F, Wei Q and Qin H:
Hippo-YAP signaling pathway: A new paradigm for cancer therapy. Int
J Cancer. 137:2275–2286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hua G, Lv X, He C, Remmenga SW, Rodabough
KJ, Dong J, Yang L, Lele SM, Yang P, Zhou J, et al: YAP induces
high-grade serous carcinoma in fallopian tube secretory epithelial
cells. Oncogene. 35:2247–2265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang EY, Cheng JC, Thakur A, Yi Y, Tsai SH
and Hoodless PA: YAP transcriptionally regulates ErbB2 to promote
liver cell proliferation. Biochim Biophys Acta Gene Regul Mech. Jul
17–2018.(Epub ahead of print) doi: 10.1016/j.bbagrm.2018.07.004.
View Article : Google Scholar
|
|
19
|
Liu M, Lin Y, Zhang XC, Tan YH, Yao YL,
Tan J, Zhang X, Cui YH, Liu X, Wang Y and Bian XW: Phosphorylated
mTOR and YAP serve as prognostic markers and therapeutic targets in
gliomas. Lab Invest. 97:1354–1363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tutt A, Robson M, Garber JE, Domchek SM,
Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler
RK, et al: Oral poly(ADP-ribose) polymerase inhibitor olaparib in
patients with BRCA1 or BRCA2 mutations and advanced breast cancer:
A proof-of-concept trial. Lancet. 376:235–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dowell JE and Minna JD: EGFR mutations and
molecularly targeted therapy: A new era in the treatment of lung
cancer. Nat Clin Prac Oncol. 3:170–171. 2006. View Article : Google Scholar
|
|
23
|
Zhang Z, Lin Z, Zhou Z, Shen HC, Yan SF,
Mayweg AV, Xu Z, Qin N, Wong JC, Zhang Z, et al: Structure-Based
design and synthesis of potent cyclic peptides inhibiting the
YAP-TEAD protein-protein interaction. ACS Med Chem Lett. 5:993–998.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Z, Hu T, Xu Z, Lin Z, Zhang Z, Feng
T, Zhu L, Rong Y, Shen H, Luk JM, et al: Targeting Hippo pathway by
specific interruption of YAP-TEAD interaction using cyclic YAP-like
peptides. FASEB J. 29:724–732. 2015. View Article : Google Scholar : PubMed/NCBI
|