Introduction
Activin is a member of transforming growth factor-β
(TGF-β) superfamily consisting of two inhibin β subunits linked by
disulfide bonds. It is a type of glycoprotein initially isolated
from porcine follicular fluid in 1986 and named for its ability to
stimulate the release of follicle-stimulating hormone (FSH) from
the pituitary. Activin exists in three basic molecular forms
composed of two inhibin β subunits: activin A (βAβA), activin B
(βBβB), and activin AB (βAβB) (1–3). Activin A
is expressed widely in various tissues and cells with strong
bioactivities and is the mostly studied activin (4–6). It first
binds to the type II activin receptors (ActIIRA or ActRIIB) on the
member surface, and then recruits and phosphorylates type I activin
receptors (ActRI). The phosphorylated ActRI activates Smad2 and
Smad3, which form a complex with Smad4 to translocate to the
nucleus. Activin and TGF-β share the same signaling pathway at the
level of Smad2/3/4. Activin A exerts a variety of biological
functions including regulation of hematopoietic cell proliferation,
neuron differentiation, pituitary hormone secretion, and tissue
repair. It is also involved in the process of many diseases, for
example, inflammation, fibrosis and tumorigenesis (7–10).
Activin A has pro- and anti-tumorigenic functions
depending on the tumor type. In breast, liver and colon cancers,
activin signals were revealed to inhibit tumor cell growth. In
addition, tumor tissues were revealed to express decreased levels
of activin A or increased levels of activin antagonists or
demonstrated the downregulation of activin receptors or Smad
proteins (11). In contrast, high
expression of activin A in some tumors is associated with tumor
cell aggressiveness in oral squamous cell carcinoma, esophageal
squamous cell carcinoma, and malignant pleural mesothelioma. In
lung adenocarcinoma, high circulating levels of activin A were also
associated with tumor progression and predicted poor prognosis
(12).
The endoplasmic reticulum (ER) is an organelle
essential for cell survival and normal functions. Various
disturbances in the ER, including ischemia, hypoxia, oxidative
injury, and viral infections, can induce ER stress that first tends
to restore cellular homeostasis (13). However, severe or prolonged ER stress
culminates in cell apoptosis. ER stress is associated with a wide
range of diseases, including neurodegenerative disorders, cancer,
diabetes, as well as many others (14–17). Our
previous study revealed the involvement of activin A in the
apoptosis of myeloma cell line NS-1 cells (18). However, whether activin A can induce
the apoptosis of NS-1 cells via the ER stress pathway is still
unclear. Therefore, in the present study, ER stress-related
proteins such as CHOP, GADD34 and caspase-12 were examined to
investigate the effects of activin A on the apoptosis of NS-1
cells.
Materials and methods
Reagents and antibodies
RPMI-1640 medium and Iscove's modified Dulbecco's
medium (IMDM) were obtained from Gibco; Thermo Fisher Scientific,
Inc. Fetal bovine serum (FBS) was purchased from Biological
Industries Israel Beit-Haemek Ltd. Activin A was obtained from
R&D Systems, Inc. A one-step reverse transcription-polymerase
chain reaction (RT-PCR) kit was provided from Takara Biotechnology
Co., Ltd. Protein extraction kits were purchased from Thermo Fisher
Scientific, Inc. Annexin V-FITC Apoptosis Analysis Kit,
anti-β-tubulin antibody (cat. no. KM9003T) and anti-p-Smad3
antibody (cat. no. SGAP0271) were purchased from Tingjin Sungene
Biotech Co., Ltd. Anti-Smad3 antibody (cat. no. A11471) was
purchased from ABclonal Biotechnology Co., Ltd. Anti-caspase-3
(cat. no. 9662), anti-caspase-12 (cat. no. 2202) and anti-CHOP
(cat. no. 2895) antibodies were purchased from Cell Signaling
Technology, Inc. Anti-GADD34 antibody (cat. no. ab131402) was
purchased from Abcam Co. Horseradish peroxidase-conjugated goat
anti-rabbit IgG antibodies (cat. no. A0545) or anti-mouse IgG
antibodies (cat. no. A3682) were purchased from Sigma-Aldrich
(Merck KGaA).
Cell culture
The mouse myeloma cell line NS-1 was purchased from
the American Type Culture Collection (ATCC) and cultured in
RPMI-1640 medium with 10% FBS in a 5% CO2-humidified
atmosphere at 37°C. Additionally, a second mouse myeloma cell line
SP2/0 (Appendix I) was also used for confirmation. This cell line
was purchased from Beijing BeNa Culture Collection and cultured
with 90% IMDM+10% FBS.
MTT assay for NS-1 cell viability
The NS-1 cells (2×104 cells/well) were
seeded in a 96-well culture plate. Then, the cells were treated
with 0–10 ng/ml activin A for 12 and 24 h, and incubated with 0.5
mg/ml MTT for 4 h. After the supernatant was discarded carefully,
100 µl DMSO per well was added to dissolve the formazan crystals.
Then the absorbance values of the samples were read using a plate
reader (BioTek Instruments, Inc.) at 540 nm to evaluate the cell
viability.
Flow cytometry for cell proliferation
assay
Carboxyfluorescein succinimidyl ester (CFSE) is a
fluorescent dye that can label living cells. It can easily
penetrate the cell membrane and covalently bind with intracellular
proteins in living cells. The labeled fluorescence can be evenly
distributed between two progeny cells, and hence the fluorescence
intensity decreases step by step with cell division. In the present
study, proliferating cells were counted by flow cytometry. In
brief, the NS-1 cells (1×107 cells/ml) in
phosphate-buffered saline (PBS) were treated with 1 µmol/l CFSE in
the dark for 10 min. Then, an equal volume of 5% FBS-RPMI-1640
medium was added to terminate the reaction. The cells were
harvested by centrifugation (150 × g) and resuspended in 5%
FBS-RPMI-1640 medium. The proliferating NS-1 cells in the M2 region
were determined by flow cytometry (BD FACSCalibur; BD Biosciences).
Data were collected and analyzed using Cell Quest software (BD
Biosciences) to obtain the percentage of fluorescent cells.
Giemsa staining
The NS-1 cells (2×104 cells/well) were
seeded in a 96-well culture plate, and treated with 0–10 ng/ml
activin A for 24 h. The cells were washed three times with PBS, and
then incubated with 50 µl of Giemsa for 5 min. After washing twice
with PBS, the cell morphology was observed under an IX71 microscope
and images were captured using a microscope digital camera system
(Olympus Optical Co. Ltd.).
RT-PCR
The NS-1 cells (2×105 cells/well) were
seeded in a 12-well culture plate and incubated with 0–5.0 ng/ml
activin A for 12 h. Then, total RNA was extracted by using TRIzol
reagent in accordance with the manufacturer's protocol (Invitrogen;
Thermo Fisher Scientific, Inc.). The cDNA was amplified by using a
one-step RT-PCR kit according to the manufacturer's protocols
(Takara Biotechnology Co., Ltd.). PCR was performed using the
following reaction conditions: 94°C for 30 sec, 56°C for 20 sec,
72°C for 40 sec (35 cycle) and final extension was 72°C for 10 min.
The primer sequences are listed in Table
I. PCR products were subjected to 2% agarose gel
electrophoresis and stained with ethidium bromide. The specific
bands were visualized using ImageMaster VDS (Pharmacia Biotech; GE
Healthcare). The densitometric quantification of mRNA was
normalized to the internal control GAPDH.
 | Table I.Primer sequences used in RT-PCR. |
Table I.
Primer sequences used in RT-PCR.
| Gene | Primer | Sequence
(5′-3′) | Fragment size
(bp) | T (°C) |
|---|
| ActRIIA | F |
ATTGGCCAGCATCCATCTCTTG | 295 | 56 |
|
| R |
GCCACCATCATAGACTAGATTC |
|
|
| ActRIIB | F |
TGCTGAAGAGCGACCTCAC | 535 | 58 |
|
| R |
AGCAGGTCCACATTGGTGAC |
|
|
| Smad3 | F |
CTCCTACTACGAGCTGAACCA | 572 | 58 |
|
| R |
AAGACACACTGGAACAGCGGA |
|
|
| Caspase-3 | F |
TGGTGATGAAGGGGTCATTTATG | 105 | 56 |
|
| R |
TTCGGCTTTCCAGTCAGACTC |
|
|
| Caspase-12 | F |
TGCTGACAGCTCCTCATGGAC | 342 | 56 |
|
| R |
ATGTGCTGTCTGAGGACTGGTG |
|
|
| CHOP | F |
TAGCTTGGCTGACAGAGGAG | 331 | 56 |
|
| R |
GTTCATGCTTGGTGCAGGCT |
|
|
| p53 | F |
CCTCCAGAAGATATCCTGCCAT | 275 | 56 |
|
| R |
CACATAACAGACTTGGCTGTCC |
|
|
| p21 | F |
GCCTTGTCGCTGTCTTGCACT | 297 | 56 |
|
| R |
GAGAGGGCAGGCAGCGTATATA |
|
|
| GAPDH | F |
GATTGTTGCCATCAACGACC | 372 | 56 |
|
| R |
GTGCAGGATGCATTGCTGAC |
|
|
Flow cytometry for cells apoptosis
assay
The NS-1 cells (1×106 cells/well) were
seeded in a 12-well culture plate. They were treated with 0–10
ng/ml activin A for 24 h, collected, and re-suspended in 100 µl of
FACS buffer followed by the addition of 1 µl FITC-Annexin V and 1
µl 7-AAD for 5 min in the dark. Then, the labeled cells were
analyzed by flow cytometry (BD FACSCalibur). Data were collected
and analyzed using the Cell Quest software (BD Biosciences) to
obtain the percentage of fluorescent cells.
Western blotting
The NS-1 cells (1×106 cells/well) were
treated with activin A for 12 h, harvested, and lysed in protein
lysis buffer (M-PER; Thermo Fisher Scientific, Inc.). Then the
protein concentration was determined by the Pierce BCA Protein
Assay (Thermo Fisher Scientific, Inc.). The proteins (30 µg each
sample) were separated by electrophoresis with 10% SDS-PAGE gel and
transferred onto a polyvinylidene difluoride membrane. Then the
membrane was blocked with 2% BSA for 1 h at room temperature and
incubated in primary antibodies against CHOP (1:1,000 dilution),
caspase-3 (1:1,000 dilution), caspase-12 (1:500 dilution), GADD34
(1:500 dilution), and β-tubulin (1:1,000) at 4°C overnight,
respectively. Following washing with 1X TBST with 0.1% Tween-20
three times, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG antibodies (1:160,000)
or anti-mouse IgG antibodies (1:40,000) for 1 h at room
temperature. Finally, the labeled proteins were detected by
chemiluminescence (ECLPlus; Amersham Pharmacia Biotech; GE
Healthcare) and analyzed using ImageJ software (v1.43; National
Institutes of Health). The protein levels were normalized to
β-tubulin.
Establishment of tumor-bearing mice
with NS-1 cells
Male Balb/c mice (18–20 g) provided by Vital River
Laboratories Technology Co. Ltd. were housed in a
temperature-controlled room with a 12-h light/dark cycle. All
animal experiments were conducted in accordance with the Jilin
University guidelines for the care and use of animals. A laboratory
animal ethics review form was approved by the Laboratory Animal
Ethics Committee of the College of Basic Medical Sciences of Jilin
University (No. 2018-018). The NS-1 cells in the logarithmic growth
phase were resuspended in saline. Then, 2.0×106 NS-1
cells in 100 µl of saline were inoculated on the back of the mouse
via subcutaneous injection and the tumor size was measured every
day until the volume was ~105±25 mm3. The tumor-bearing
mice with NS-1 cells were randomly divided into control and activin
A groups. Furthermore, 20 ng activin A in 1 µl of saline was
injected with a microsyringe into each tumor center of mice in the
activin A group, and the same volume of saline was injected in the
control group. Before injection, a cotton ball fully soaked with
0.5 ml ether was placed into an anesthesia box, then the mouse was
quickly placed into the anesthesia box for ~30 to 60 sec in order
to relieve mouse suffering and distress. The administration was
repeated once 12 h later. The tumor was measured in the following
six consecutive days. On the sixth day, all the mice were
sacrificed by anesthesia with intraperitoneal injection of 3%
pentobarbital sodium overdose at 90 mg/kg until the collection of
the blood from the heart and when breathing and a heartbeat
disappeared. The lethal dose was in general approximately three
times the anesthetic dose. Then the tumors were dissected, and the
volume of the tumors was measured. Activin A was injected directly
into tumors. It was observed that after injection of activin A, the
volume of the tumors had significantly decreased. However, the
volume of the tumors in the control group grew too fast, and as
time progressed, the tumors in the control group began to ulcer and
rupture. Therefore, only 6 days were observed.
Smad3 overexpression in NS-1
cells
The NS-1 cells were transfected with
Smad3-expressing plasmid pcDNA-Smad3 and control empty plasmid
pcDNA3, respectively. Briefly, the pcDNA-Smad3 plasmids or control
pcDNA3 plasmids (0.3 µg) were enfolded with Lipofectamine 2000
(1:2) according to the manufacturer's protocol and then transfected
into NS-1 cells.
Statistical analysis
Data were repeated at least three times. All results
were expressed as the means ± standard deviation (SD). The data
were analyzed using Student's t-test for comparison of two
groups and ANOVA with post hoc Dunnett's test for multiple group
comparisons. Statistical analysis was performed using the software
SPSS17.0 (SPSS, Inc.). Data were considered as statistically
significant at P<0.05.
Results
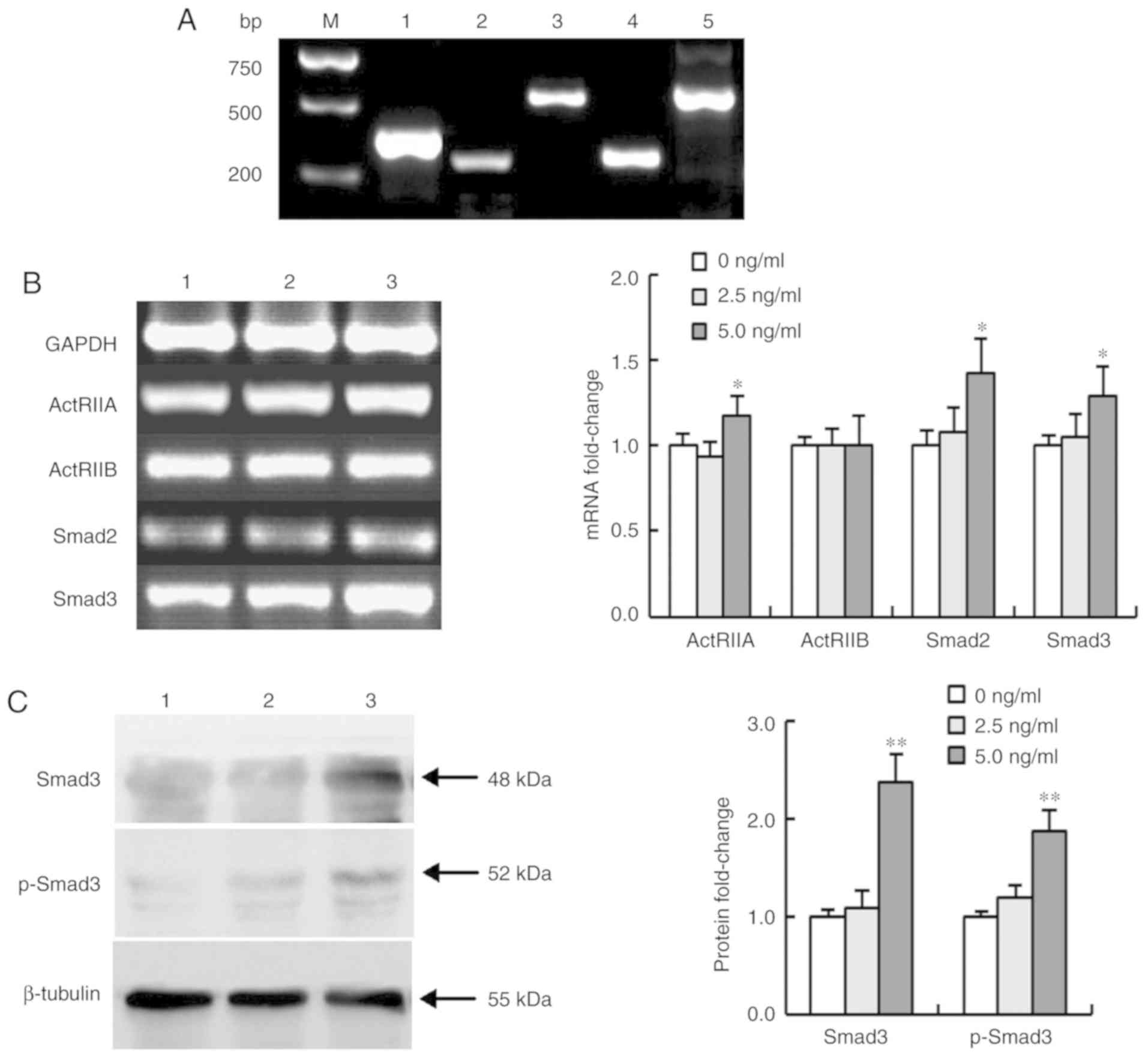
Activin A affects the expression of
ActRIIs and Smads in NS-1 cells
Activin A first binds to activin type II receptors
(ActRIIA or ActRIIB) and then activates downstream signaling
molecules Smad2 and Smad3. To confirm whether activin A exerted an
effect on NS-1 cells, the mRNA expression of ActRIIs and Smads were
examined in NS-1 cells after treated with activin A. The results
revealed that not only was the mRNA expression of ActRIIA, ActRIIB,
Smad2 and Smad3 detectable in NS-1 cells, but ActRIIA, Smad2 and
Smad3 were also upregulated in NS-1 cells treated with activin A
for 12 h. Conversely, activin A did not alter the mRNA expression
of ActRIIB. In addition, the protein levels of Smad3 and p-Smad3
were increased (Fig. 1), confirming
that activin A may exert an effect on NS-1 cells via the
ActRIIA/Smad3 signaling pathway.
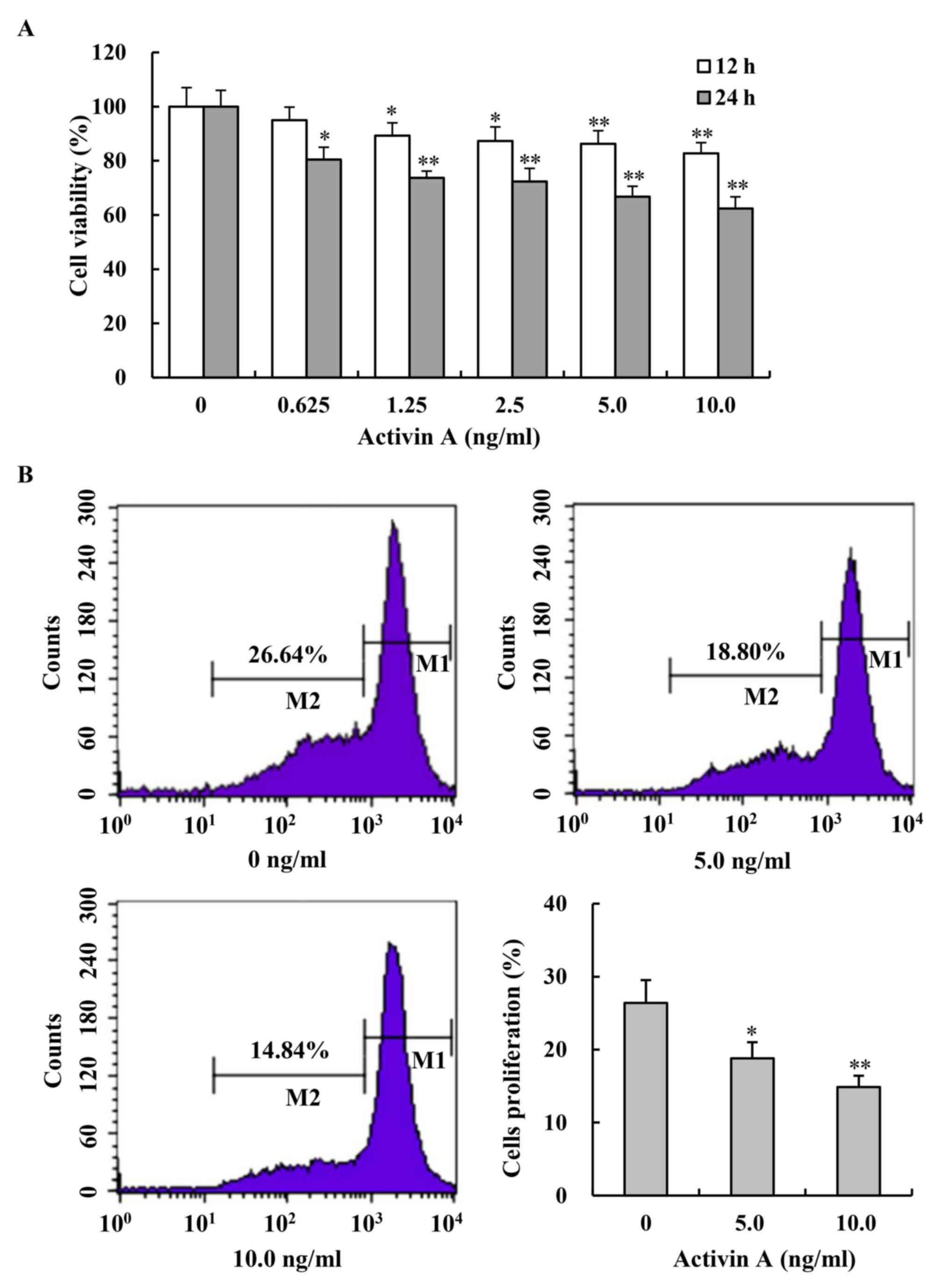
Activin A inhibits the viabilities and
proliferation of NS-1 cells
To demonstrate the effect of activin A on NS-1
cells, cell viability was first examined by MTT assay. After
treatment with activin A for 12 and 24 h, the viability of NS-1
cells was inhibited in a dose-dependent manner, compared with
untreated NS-1 cells in the control group (Fig. 2A). Then, the proliferation of NS-1
cells was further evaluated by flow cytometry after the cells were
labeled with CFSE and treated with activin A for 24 h. The results
revealed that the percentage of proliferating cells (M2)
significantly decreased with the treatment of activin A, compared
with that in the control group (Fig.
2B). Additionally, the second mouse myeloma cell line SP2/0
(Appendix I) was used to verify the findings, and it was revealed
that activin A also inhibited the viability and proliferation of
SP2/0 cells (Fig. S1). These results
indicated that activin A could suppress NS-1 cell
proliferation.
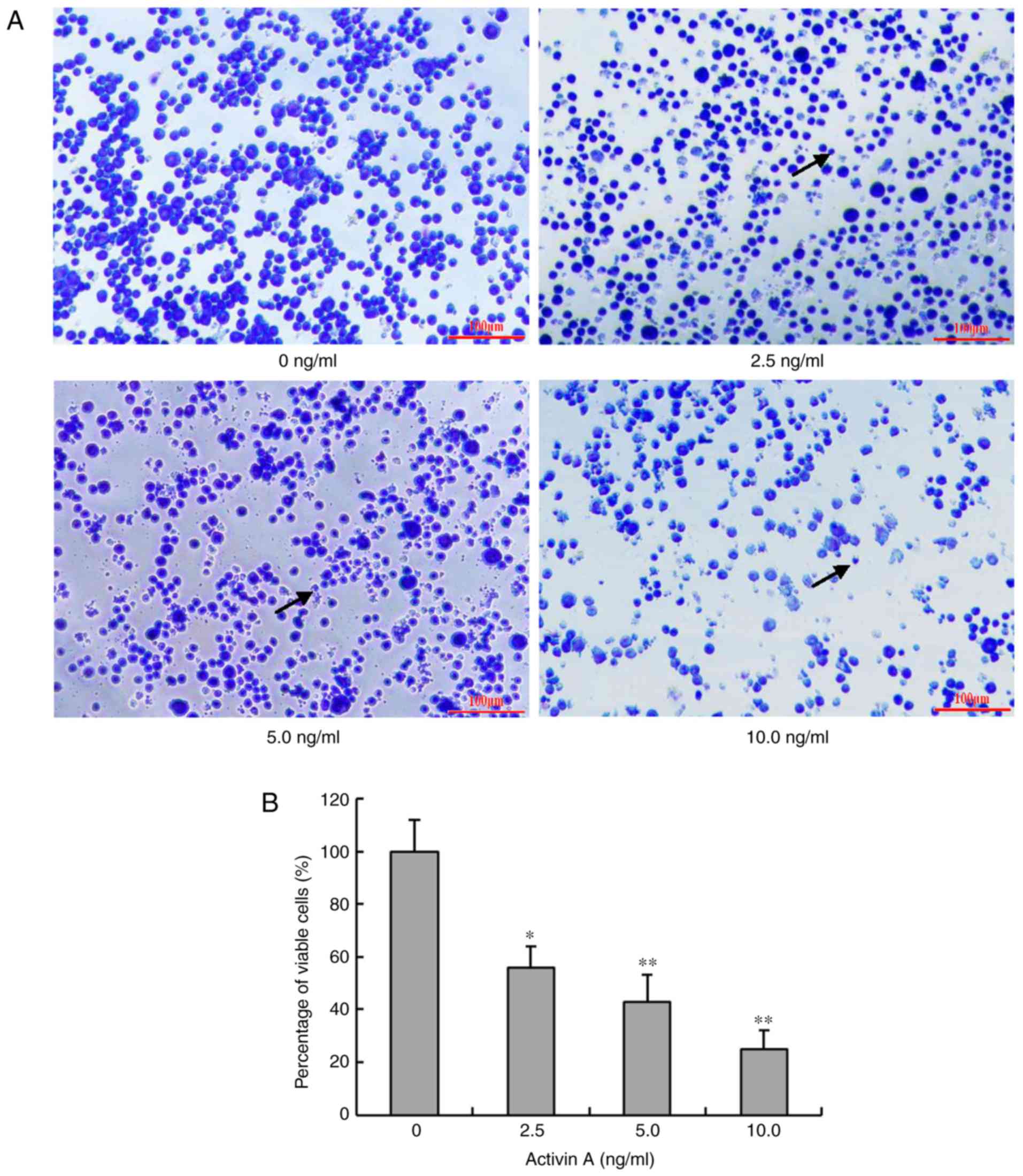
Activin A alters the morphology of
NS-1 cells
In order to further evaluate the effect of activin
A, the morphological changes of NS-1 cells were examined by Giemsa
staining after treatment with activin A for 24 h. In the control
group, the cells were identical in size and the nuclear membrane
was smooth. However, the morphology of cells treated with activin A
was evidently different in size and shape. The cells shrunk and the
number of cells with chromatin condensation was markedly increased
(Fig. 3A). The percentage of viable
cells also decreased with the treatment of activin A (Fig. 3B). These data indicated that activin A
may induce NS-1 cell apoptosis. According to Fig. 3, a greater effect of reduction of cell
viability was observed than Fig. 2A.
Cells in Fig. 3 were stained by
Giemsa. Some cells lost the ability of adhesion although they were
not dead. These cells detached from the culture plate when they
were washed with PBS repeatedly. Therefore, the ratio of cell
viability by MTT detection was different from that of Giemsa
staining living cells.
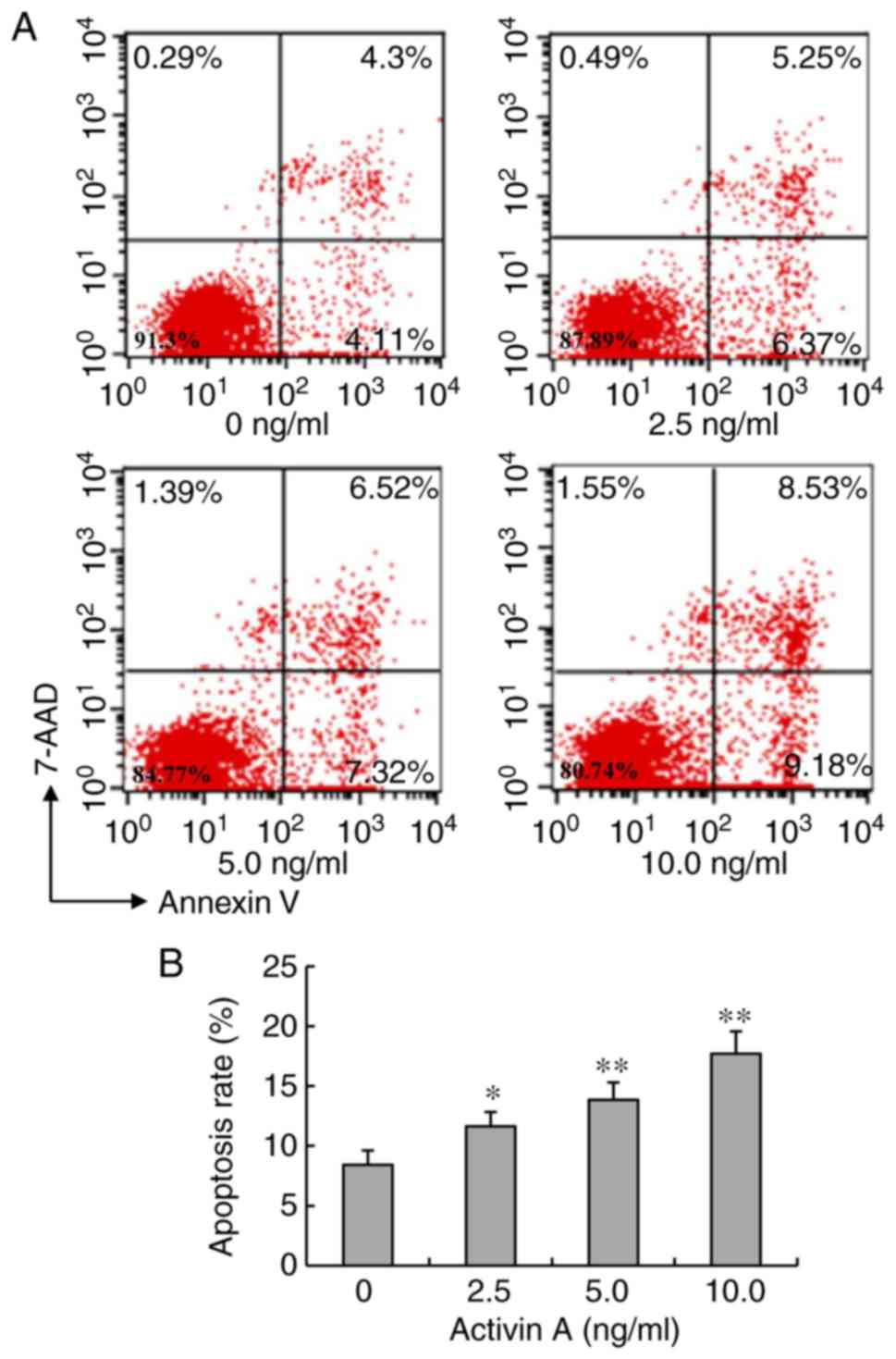
Activin A increases the apoptotic
ratio of NS-1 cells
Chromatin condensation is one of the hallmarks of
apoptosis. Therefore, the apoptotic ratio of NS-1 cells stained
with FITC-Annexin V and 7-AAD was further examined by flow
cytometry. After treatment with activin A for 24 h, the apoptotic
ratio of NS-1 cells significantly increased compared with that in
the control group (Fig. 4). These
data further confirmed that activin A induced NS-1 cell
apoptosis.
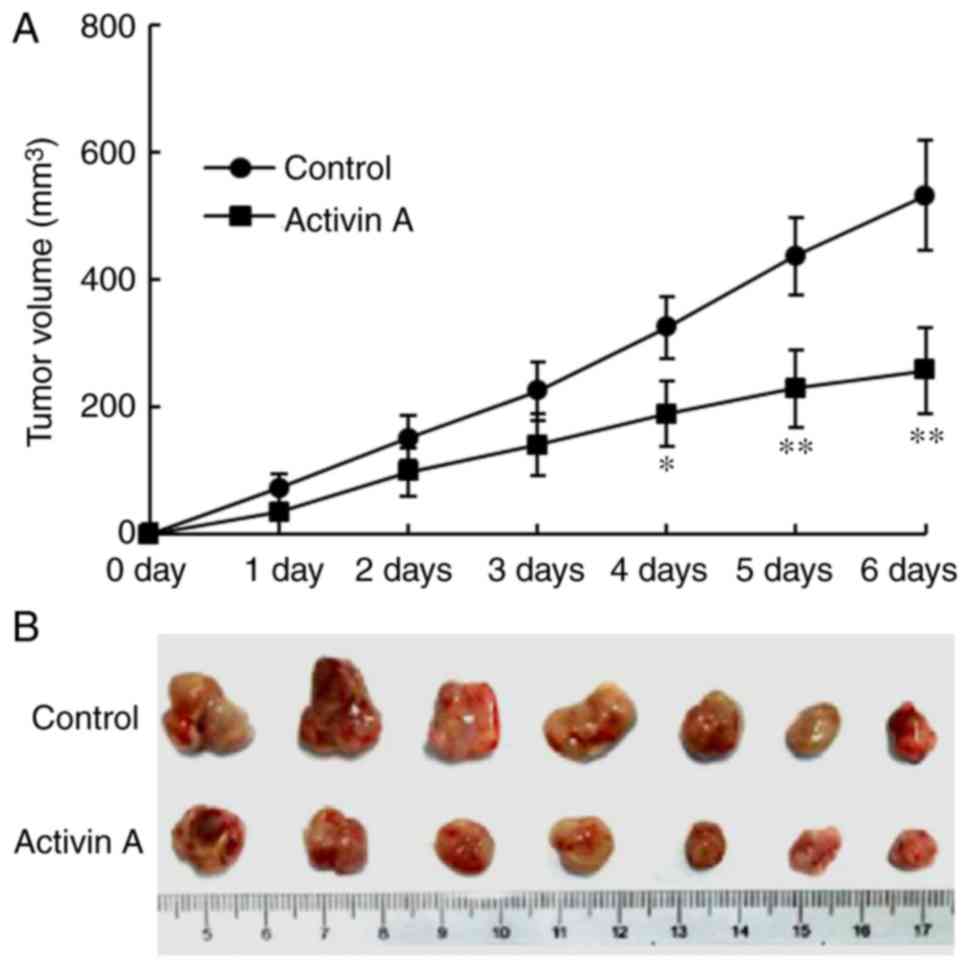
Activin A inhibits the growth of solid
tumors of NS-1 cells in mice
After the solid tumors of NS-1cells were formed in
mice, exogenous activin A was injected into the solid tumors and
the same volume of saline was injected in the control group. Then,
the volume of the tumors was assessed for the following 6 days. The
results revealed that the volume of the tumors treated with
exogenous activin A was significantly reduced with time, compared
with that in the control group (Fig.
5A). On the sixth day, the tumors were dissected. The gross
morphology of solid tumors is presented in Fig. 5B. Although there was no significant
difference in the body weight of the mice between the control group
and activin A group, the ratio of tumor weight/body weights of mice
treated with exogenous activin A was significantly lower than that
in the control group (Fig. S2).
These data revealed that activin A inhibited the growth of solid
tumors of NS-1 cells in vivo in mice.
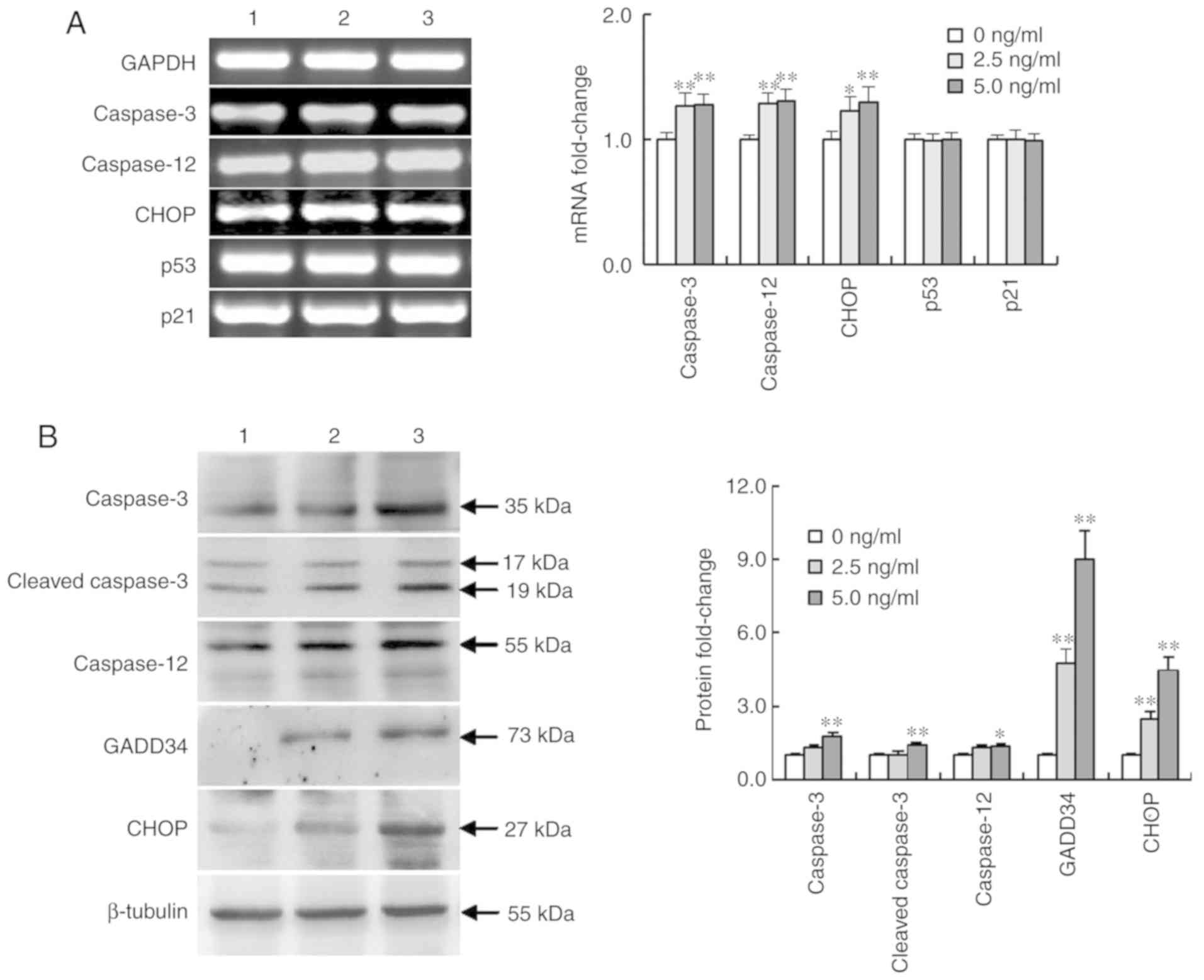
Activin A influences the expression of
apoptosis-related genes in NS-1 cells
To assess whether activin A promoted NS-1 cell
apoptosis via the ER stress pathway, the expression of certain
apoptosis-related genes was examined after treatment with activin A
for 12 h. The results revealed that the mRNA expression of
caspase-3, caspase-12 and CHOP was significantly upregulated,
whereas no change was observed in the mRNA expression of p53 and
p21 (Fig. 6A). Furthermore, western
blotting results revealed that activin A significantly upregulated
the protein expression of CHOP, caspase-3, cleaved-caspase-3,
caspase-12 and GADD34 (Fig. 6B).
These data indicated the involvement of the ER stress pathway
proteins in activin A-induced NS-1 cell apoptosis.
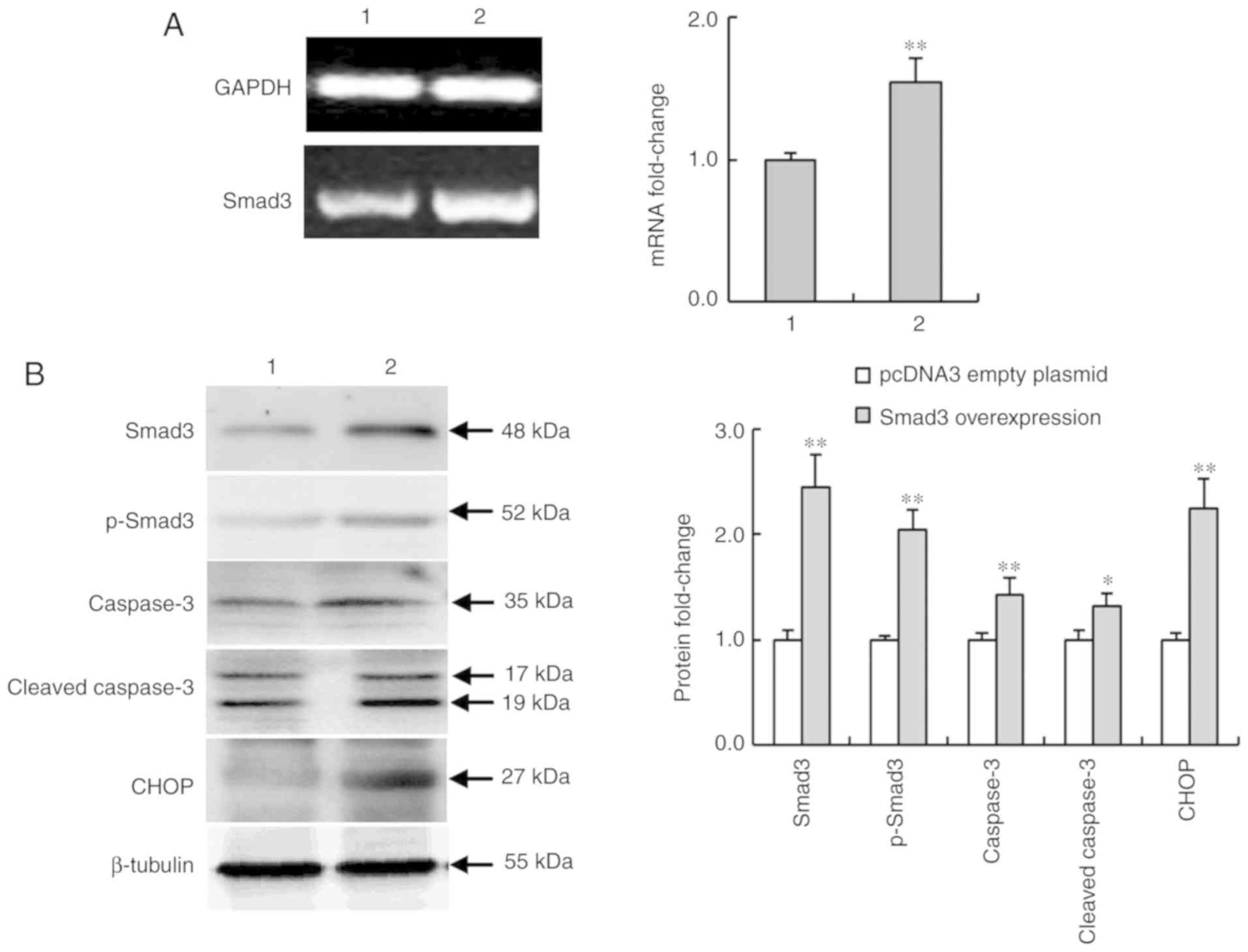
Smad3-overexpression regulates the
expression of apoptosis-related proteins in NS-1 cells
Smad3 plays an important role in activin signaling
transduction. In Fig. 1 it was
revealed that activin A promoted Smad3 expression. Thus, the role
of Smad3 in NS-1 cells was further investigated. Fig. 7A and B revealed that the mRNA and
protein expression of Smad3 were overexpressed in NS-1 cells
transfected with Lipofectamine 2000. In addition, the level of
p-Smad3 was also increased. Furthermore, the results revealed that
Smad3 overexpression significantly increased the expression of
caspase-3, cleaved-caspase-3 and CHOP protein of the ER stress
pathway, compared with the pcDNA3 empty plasmid control group
(Fig. 7B). These data further
confirmed the involvement of CHOP in activin A-induced apoptosis of
myeloma NS-1 cells.
Discussion
Activin A binds with high affinity to activin type
II receptors, which recruit type I receptors and are necessary for
the activation of Smad2/3 signaling. It is of greater clinical
significance to study human tumors. However, tumors can also occur
in animals, thus the effect of activin A was first analyzed on
mouse NS-1 cells. The present study revealed the expression of
ActRIIs and Smad2/3 in NS-1 cells. Activin A first binds with
either ActRIIA or ActRIIB, however not both receptors are increased
in one cell at the same time. In addition, in the present study,
activin A induced the increase in the mRNA expression of ActRIIA,
Smad2 and Samd3 but not ActRIIB. Activin A also increased the
levels of Smad3 and p-Smad3 proteins. These results imply that
activin A may play an important role in NS-1 cells by promoting
ActRII/Smad3 signaling.
Activin A plays a pro- or anti-tumor role according
to the tumor type (11). Circulating
activin A levels are elevated in patients with advanced multiple
myeloma, and MSCs and osteoblasts are the main sources of activin A
(19,20). Previous studies have revealed that
activin A induces apoptosis in mouse myeloma NS-1 cells via the
mitochondrial pathway by increasing the expression of cleaved
caspase-3 and Cyt c proteins and the ratio of Bax/Bcl-2
(18). However, whether activin A can
induce NS-1 cell apoptosis via the ER stress pathway is still
unclear. In the present study, it was revealed that activin A
inhibited the viability and proliferation of NS-1 cells using MTT
assays and CSFE staining. Hence, the antitumor effect of activin A
on NS-1cells in vivo was examined. This study demonstrated
that activin A inhibited the growth of solid tumors of NS-1 cells
in mice. The aforementioned findings indicated that activin A could
directly exert a suppressive effect on the proliferation of myeloma
cells.
Apoptosis is recognized as a programmed cell death
involving complex signaling cascades and stress responses. It is
closely correlated with tumor cell proliferation and drug
resistance in tumor therapy (21),
and can occur through three major pathways: The Fas death receptor
pathway, mitochondrial pathway, and ER stress pathway (22–24). The
ER is a membranous compartment present in eukaryotic cells, which
controls the synthesis, folding, and trafficking of proteins to be
secreted, as well as calcium storage and synthesis of membranes.
Previous studies reported that activin A can induce B-cell
apoptosis associated with Smad3 (25), and induce NS-1 cell apoptosis mediated
by the mitochondrial pathway (18).
However, whether the ER stress pathway is involved in activin
A-induced NS-1 cell apoptosis is still unclear.
Apoptosis induced by ER stress is accompanied by the
induction of pro-apoptotic CHOP (26,14).
CHOP-induced apoptosis in ER stress has been involved in numerous
human metabolic diseases. As a key marker of ongoing ER stress,
CHOP not only leads to the downregulation of the anti-apoptotic
protein Bcl-2 expression, but also can exacerbate ER stress by
inducing the expression of GADD34 involved in a pro-apoptotic
mechanism (28,29). The present study revealed an
upregulation of the mRNA and protein expression of CHOP in NS-1
cells after treatment with activin A, and an increase of GADD34.
Cell death triggered by CHOP-mediated apoptosis is also closely
correlated with the suppression of cell cycle regulator protein 21
(p21), which inhibited the cell cycle in the G1 phase. Tumor
suppressor p53 (p53) upregulates p21 to suppress the cell cycle at
the G1 phase (30–32). However, no effect of activin A on the
expression of p21 and p53 was observed in the present study. Smad3
has been reported to alter the expression of p21. However, activin
A may also induce activation of non-Smad3 dependent pathways. Smad3
induces changes in p21 expression in other cells, while in NS-1
cells, there is no significant change in p21 expression after
activin A treatment. Therefore, p21 expression was not detected in
NS-1 cells in subsequent experiments. These results indicated that
activin A elicited a downstream apoptosis response mediated by
CHOP/GADD34 signaling. The ER pathway contains many proteins,
however CHOP and GADD34 are important proteins in this pathway.
Therefore, the results of this study are only a first step in
suggesting that activin A may induce NS-1 cell apoptosis via the ER
stress pathway and more studies are required with further
experiments.
Chronic or excessive ER stress can also lead to the
activation of caspase-dependent apoptosis. Caspase-12 is an ER
protease typically activated under ER stress conditions. Previous
studies revealed the role of caspase-12 in ER stress-induced cell
death (33–35). Caspase-12 activation was also detected
in NS-1 cells treated with activin A, indicating that the induction
of apoptosis caused by activin A was also mediated by the
upregulation of caspase-12. Furthermore, caspase-12 can activate
caspase-3 to induce apoptosis directly (36). We previously demonstrated that activin
A increased the expression of cleaved caspase-3 protein in NS-1
cells (18). In the present study,
activin A enhanced the expression of both caspase-3 and cleaved
caspase-3, which further confirmed that the activation of
caspases-3 is involved in activin A-induced NS-1 cell
apoptosis.
The activin A signaling pathway is mediated by the
serine/threonine kinase receptor on the cell surface and
intracellular Smad proteins. Activin A binds to ActRIIA/IIB, which
recruits ActRI to form a signal-transducing heterodimer, leading to
the phosphorylation of cytoplasmic Smad2/3 proteins. The activated
Smad2/3 associates with Smad4, which translocates into the nucleus
and drives downstream transcriptional targets (37). A number of studies have revealed the
involvement of ActRII/Smad3 signaling in the pathophysiological
processes of various diseases. Therefore, the levels of ActRIIA/IIB
and Smads were assessed after treatment with activin A. The RT-PCR
results revealed that the mRNA expression of ActRIIA and Smad3 and
the protein levels of Smad3 and p-Smad3 were increased in NS-1
cells treated with activin A. A high concentration of activin A
could induce cardiomyocyte apoptosis via the ER stress pathway by
enhancing the expression of CHOP, and the apoptotic rates were
significantly reduced by Smad3 inhibitor (38). Smad3/ATF4 signaling was revealed to be
involved in ER stress-induced apoptosis of brown adipocytes in
vivo and in vitro (39).
Therefore, the present study further explored the relationship
between Smad3 and CHOP. It was revealed that Smad3 overexpression
could promote the expression of pro-apoptotic proteins CHOP,
caspase-3 and p-Smad3, indicating that activin A inhibited NS-1
cell proliferation and promoted apoptosis via Smad3/CHOP
signaling.
In conclusion, the activation of activin A/Smad3
signaling may inhibit NS-1 cell proliferation and induce apoptosis
by activating CHOP in the ER stress pathway. Therefore, these
results supported the therapeutic implications on targeting activin
A-induced apoptosis via CHOP signaling for multiple myeloma
therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Basic
Research Program of China (2015CB943300), the Health Commission
Foundation and the International Cooperation Foundation of Jilin
Province, China (20180414040GH, 2018J063 and 2016J066), and the
Foundation of Bethune Project of Jilin University (2018B05).
Availability of data and materials
The datasets described in this study are available
from the corresponding author on reasonable request.
Authors' contributions
ZL designed the study and reviewed the manuscript.
JG was responsible for writing the manuscript and analyzing the
data. XC contributed to the study design and performed the
statistical analysis. HS contributed the animal model experiments.
JL, YS, YZ and FL performed the experiments and analyzed the data.
YY cultured the cell lines. All authors have read and approved the
final manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the Jilin University guidelines for the care and use of
animals. A laboratory animal ethics review form was approved by the
Laboratory Animal Ethics Committee of the College of Basic Medical
Sciences of Jilin University (No. 2018-018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Ling N, Ying SY, Ueno N, Shimasaki S, Esch
F, Hotta M and Guillemin R: Pituitary FSH is released by a
heterodimer of the beta-subunits from the two forms of inhibin.
Nature. 321:779–782. 1986. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woodruff TK, Besecke LM, Groome N, Draper
LB, Schwartz NB and Weiss J: Inhibin A and inhibin B are inversely
correlated to follicle stimulating hormone, yet are discordant
during the follicular phase of the rat estrous cycle, and inhibin A
is expressed in a sexually dimorphic manner. Endocrinology.
137:5463–5467. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thompson TB, Cook RW, Chapman SC,
Jardetzky TS and Woodruff TK: Beta A versus beta B: Is it merely a
matter of expression? Mol Cell Endocrinol. 225:9–17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi Y, Ge J, Ma C, Wu N, Cui X and Liu Z:
Activin A regulates activation of mouse neutrophils by Smad3
signalling. Open Biol. 7:1603422017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoda MA, Rozsas A, Lang E, Klikovits T,
Lohinai Z, Torok S, Berta J, Bendek M, Berger W, Hegedus B, et al:
High circulating activin A level is associated with tumor
progression and predicts poor prognosis in lung adenocarcinoma.
Oncotarget. 7:13388–13399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei Q, Liu H, Liu M, Yang C, Yang J, Liu Z
and Yang P: Ramipril attenuates left ventricular remodeling by
regulating the expression of activin A-follistatin in a rat model
of heart failure. Sci Rep. 6:336772016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jana A, Krett NL, Guzman G, Khalid A,
Ozden O, Staudacher JJ, Bauer J, Baik SH, Carroll T, Yazici C and
Jung B: NFkB is essential for activin-induced colorectal cancer
migration via upregulation of PI3K-MDM2 pathway. Oncotarget.
8:37377–37393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arber C, Precious SV, Cambray S,
Risner-Janiczek JR, Kelly C, Noakes Z, Fjodorova M, Heuer A,
Ungless MA, Rodríguez TA, et al: Activin A directs striatal
projection neuron differentiation of human pluripotent stem cells.
Development. 142:1375–1386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Kretser DM, O'Hehir RE, Hardy CL and
Hedger MP: The roles of activin A and its binding protein,
follistatin, in inflammation and tissue repair. Mol Cell
Endocrinol. 359:101–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li N, Cui X, Ge J, Li J, Niu L, Liu H, Qi
Y, Liu Z and Wang Y: Activin A inhibits activities of
lipopolysaccharide-activated macrophages via TLR4, not of TLR2.
Biochem Biophys Res Commun. 435:222–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeruss JS, Sturgis CD, Rademaker AW and
Woodruff TK: Down-regulation of activin, activin receptors, and
smads in high-grade breast cancer. Cancer Res. 63:3783–3790.
2003.PubMed/NCBI
|
|
12
|
Loomans HA and Andl CD: Intertwining of
activin A and TGFβ signaling: Dual roles in cancer progression and
cancer cell invasion. Cancers (Basel). 7:70–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rutkowski DT and Kaufman RJ: A trip to the
ER: Coping with stress. Trends Cell Biol. 14:20–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doyle KM, Kennedy D, Gorman AM, Gupta S,
Healy SJ and Samali A: Unfolded proteins and endoplasmic reticulum
stress in neurodegenerative disorders. J Cell Mol Med.
15:2025–2039. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Catena V, Bruno T, De Nicola F, Goeman F,
Pallocca M, Iezzi S, Sorino C, Cigliana G, Floridi A, Blandino G
and Fanciulli M: Deptor transcriptionally regulates endoplasmic
reticulum homeostasis in multiple myeloma cells. Oncotarget.
7:70546–70558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clark AL and Urano F: Endoplasmic
reticulum stress in beta cells and autoimmune diabetes. Curr Opin
Immunol. 43:60–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Qi Y, Zhao Y, Sun H, Ge J and Liu
Z: Activin A induces apoptosis of mouse myeloma cells via the
mitochondrial pathway. Oncol Lett. 15:2590–2594. 2018.PubMed/NCBI
|
|
19
|
Garcia-Gomez A, Sanchez-Guijo F, Del
Cañizo MC, San Miguel JF and Garayoa M: Multiple myeloma
mesenchymal stromal cells: Contribution to myeloma bone disease and
therapeutics. World J Stem Cells. 6:322–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Terpos E, Kastritis E, Christoulas D,
Gkotzamanidou M, Eleutherakis-Papaiakovou E, Kanellias N,
Papatheodorou A and Dimopoulos MA: Circulating activin-A is
elevated in patients with advanced multiple myeloma and correlates
with extensive bone involvement and inferior survival; no
alterations post-lenalidomide and dexamethasone therapy. Ann Oncol.
23:2681–2686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Tumorigenic and immunosuppressive effects of
endoplasmic reticulum stress in cancer. Cell. 168:692–706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernández A, Ordóñez R, Reiter RJ,
González-Gallego J and Mauriz JL: Melatonin and endoplasmic
reticulum stress: Relation to autophagy and apoptosis. J Pineal
Res. 59:292–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wachmann K, Pop C, van Raam BJ, Drag M,
Mace PD, Snipas SJ, Zmasek C, Schwarzenbacher R, Salvesen GS and
Riedl SJ: Activation and specificity of human caspase-10.
Biochemistry. 49:8307–8315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su J, Zhou L, Xia MH, Xu Y, Xiang XY and
Sun LK: Bcl-2 family proteins are involved in the signal crosstalk
between endoplasmic reticulum stress and mitochondrial dysfunction
in tumor chemotherapy resistance. BioMed Res Int. 2014:2343702014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamakawa N, Tsuchida K and Sugino H: The
ras GAP-binding protein, Dok-1, mediates activin signaling via
serine/threonine kinase receptors. EMBO J. 21:1684–1694. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong Y, Kalueff AV and Song C:
N-methyl-d-aspartate receptor-mediated calcium overload and
endoplasmic reticulum stress are involved in
interleukin-1beta-induced neuronal apoptosis in rat hippocampus. J
Neuroimmunol. 307:7–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mc Cullough KD, Martindale JL, Klotz LO,
Aw TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang J, Fairbrother W and Reed JC:
Therapeutic targeting of Bcl-2 family for treatment of B-cell
malignancies. Expert Rev Hematol. 8:283–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mihailidou C, Papazian I, Papavassiliou AG
and Kiaris H: CHOP-dependent regulation of p21/waf1 during ER
stress. Cell Physiol Biochem. 25:761–766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu YM, Chen ZJ, Jiang GM, Zhang KS, Liu Q,
Liang SW, Zhou Y, Huang HB, Du J and Wang HS: Inverse agonist of
estrogen-related receptor α suppresses the growth of triple
negative breast cancer cells through ROS generation and interaction
with multiple cell signaling pathways. Oncotarget. 7:12568–12581.
2016.PubMed/NCBI
|
|
32
|
Kapur A, Felder M, Fass L, Kaur J,
Czarnecki A, Rathi K, Zeng S, Osowski KK, Howell C, Xiong MP, et
al: Modulation of oxidative stress and subsequent induction of
apoptosis and endoplasmic reticulum stress allows citral to
decrease cancer cell proliferation. Sci Rep. 6:275302016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duclos C, Lavoie C and Denault JB:
Caspases rule the intracellular trafficking cartel. FEBS J.
284:1394–1420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
García de la Cadena S and Massieu L:
Caspases and their role in inflammation and ischemic neuronal
death. Focus on caspase-12. Apoptosis. 21:763–777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu XS, Qiao YB, Li Y, Yang B, Chen MB and
Xing CG: Preclinical study of cinobufagin as a promising
anti-colorectal cancer agent. Oncotarget. 8:988–998.
2017.PubMed/NCBI
|
|
36
|
Nakagawa T and Yuan J: Cross-talk between
two cysteine protease families. Activation of caspase-12 by calpain
in apoptosis. J Cell Biol. 150:887–894. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyazawa K, Shinozaki M, Hara T, Furuya T
and Miyazono K: Two major Smad pathways in TGF-beta superfamily
signaling. Genes Cells. 7:1191–1204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu M, Mao C, Li J, Han F and Yang P:
Effects of the activin A-follistatin system on myocardial cell
apoptosis through the endoplasmic reticulum stress pathway in heart
failure. Int J Mol Sci. 18:E3742017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Z, Gu H, Gan L, Xu Y, Feng F, Saeed M
and Sun C: Reducing Smad3/ATF4 was essential for Sirt1 inhibiting
ER stress-induced apoptosis in mice brown adipose tissue.
Oncotarget. 8:9267–9279. 2017.PubMed/NCBI
|