Introduction
Malignant gliomas, especially glioblastomas, are the
most frequent and devastating primary tumors of the central nervous
system (1,2). Despite improvements in multimodality
treatments consisting of combinations of surgical resection,
irradiation, and chemotherapy, the prognosis for glioblastoma
patients remains dismal (3,4). Novel treatment strategies for patients
with glioblastomas are thus urgently required.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a member of the tumor necrosis factor (TNF)
family, and induces apoptosis in cancer cells by binding to its
receptors, death receptor 4 (DR4) and DR5 (5–9). Although
DR4 and DR5 are highly expressed in cancer cells, such expression
is limited in normal cells (10,11). TRAIL
has thus been expected to represent one of the most promising
agents for use in cancer treatment (5–8). In a
study employing malignant glioma specimens, it was demonstrated
that the expression of DR4 was 75% and that of DR5 was 95%
(12). However, several
investigations have indicated that most glioma cell lines display
resistance to TRAIL-induced apoptosis (12,13).
Recently, various combination treatments with other drugs as a
sensitizer have been attempted and have yielded promising results
(14–18). To overcome the resistance to
TRAIL-induced apoptosis and to improve the efficacy of TRAIL-based
therapies, identification of ideal agents for combination treatment
is important for achieving rational clinical treatment in
glioblastoma patients.
Human interferon-β (IFN-β) is a cytokine which
belongs to the type I IFNs (19). It
was originally identified as an antiviral agent, and has been
revealed to exhibit pleiotropic antitumor activities including an
anti-angiogenic activity, immunomodulatory activity, growth
inhibition, and apoptosis induction (20,21). In
addition to such multiple functions of IFN-β against human
neoplasias, it can act as a drug sensitizer enhancing the antitumor
effect when administered in combination with other agents in the
treatment of malignant gliomas (22–26). IFN-β
binds to the cell membrane receptor, interferon-α/β receptor
(IFNAR), and acts by enhancing the expression of IFN-stimulated
genes (ISGs) through the signal induction of the Janus kinase
(JAK)/signal transducer and activator of the transcription (STAT)
signaling pathway. To date, over 300 ISGs have been identified,
including genes such as TRAIL and Fas, suggesting a strong
involvement with exogenous apoptosis. Furthermore, several recent
studies have demonstrated that the apoptotic activity of IFN
depends partly on the TRAIL signaling pathway (27,28).
The aforementioned findings suggest that favorable
therapeutic interactions could occur between TRAIL and IFN-β.
Therefore, in the present study, the potential sensitizing effects
of IFN-β towards TRAIL-induced apoptosis was investigated in
malignant glioma cells aiming to provide an experimental basis for
rational clinical treatments in glioblastoma patients.
Materials and methods
Cell lines, culture conditions and
materials
Human malignant glioma cell lines A-172 (cell no.
JCRB0228; lot no. 021999), AM-38 (cell no. IFO50492; lot no.
12082003), T98G (cell no. IFO50303; lot no. 1007), U-251MG (cell
no. IFO50288; lot no. 12132002), and YH-13 (cell no. IFO50493; lot
no. 1164) were purchased from Health Science Research Resources
Bank (Sennan, Osaka, Japan). U-87MG (glioblastoma of unknown
origin; cat. no. HTB-14; lot no. 2497162) and U-138MG (cat. no.
HTB-16; lot no. 1104428) were purchased from the American Type
Culture Collection (Manassas, VA, USA). In a previous study, we
confirmed that O6-methylguanine-DNA
methyltransferase (MGMT, a key factor of alkylating agents) is
expressed in T98G, U-138MG and YH-13 cells by real-time RT-PCR and
western blot analysis (29).
Consistent with an earlier study (30), it was also confirmed that T98G (237
Met→Ile) and U-251MG (273 Arg→His) have a point mutation in the
p53 gene (data not shown).
Cells were cultured in Dulbecco's modified Eagle's
minimum essential medium (DMEM; Nissui Pharmaceutical Co., Ltd.)
supplemented with 10% fetal calf serum (FCS; Life Technologies;
Thermo Fisher Scientific, Inc.) using plastic culture flasks
(Corning, Inc.) in a 37°C-humidified incubator with 5%
CO2. Natural-type IFN-β (Toray Industries, Inc.) and
TRAIL (Wako Pure Chemical Industries, Ltd.) were employed for the
experiments.
Cell viability analysis
Cells were seeded at 1×104 cells/well in
24-well plates. After 24 h of attachment, the cells were further
incubated with fresh medium containing TRAIL, and/or IFN-β for 72
h. To determine the cell viability, the surviving cells in each
well were counted using a Coulter Counter (Coulter Counter Z1;
Beckman Coulter, Inc.) after confirming the presence of living
cells with 0.45% trypan blue solution (Sigma-Aldrich; Merck KGaA).
The experiments were repeated 6 times at each concentration.
Further treatment conditions were set with TRAIL at
1 ng/ml and IFN-β at 10 IU/ml, since the cell growth inhibitory
effect was significant when TRAIL was at 1 ng/ml or more, and 10
IU/ml of IFN-β represents a clinically relevant concentration
(26,31). In phase II RCS for non-small cell
carcinoma and B cell lymphoma, 8 mg/kg of TRAIL was administered,
and its blood concentration reached about 80 µg/ml (32,33). The
dose of TRAIL (1 ng/ml) employed in the following experiments was
thus considered to be a low clinical dose.
Since U-138MG displayed a marked antitumor effect at
a small amount (0.1 and 1 ng/ml) of TRAIL when used in combination
with 10 IU/ml of IFN-β, these cells were employed in the following
experiments.
Analysis of apoptosis by flow
cytometry
Cells were seeded at 1×106 cells/well in
6-well plates (Corning, Inc.) and cultured for 24 h. Subsequently,
the cells were further incubated with fresh medium (control),
medium containing TRAIL (1 ng/ml) and/or IFN-β (10 IU/ml) for 72 h.
The cells were washed with phosphate-buffered saline (PBS) and
collected using trypsin-EDTA solution. After suspension with 100 µl
binding buffer, 5 µl of Annexin V Alexa Fluor 488 conjugate
(Invitrogen; Thermo Fisher Scientific, Inc.) and 10 µl of propidium
iodide solution (PI; Miltenyi Biotec, Inc.) were added, and the
cells were incubated at room temperature for 15 min. Stained cells
were analyzed with a fluorescence-activated cell sorter
(FACS)-Calibur flow cytometer (BD Biosciences). The experiments
were repeated 3 times to confirm reproducibility.
Western blot analysis
Proteins were isolated from 1×107 cells
using RIPA buffer (Wako Pure Chemical Industries, Ltd.)
supplemented with protease inhibitor complex mix (Roche
Diagnostics). The protein concentrations were determined using a
Pierce BCA protein assay kit (Thermo Fisher Scientific, Inc.). A
total of 50 µg of protein was separated by 12% sodium dodecyl
sulfate (SDS)-polyacrylamide gel electrophoresis (TEFCO, Inc.) and
transferred onto nitrocellulose membranes (GE Healthcare) for 30
min at 15 V employing Bio-Rad Trans Blot (Bio-Rad Laboratories,
Inc.). The membranes were blocked with 1% skimmed milk dissolved in
washing buffer (PBS + 0.1% Tween-20) for 60 min at room
temperature. The membranes were incubated with primary antibodies
diluted according to the manufacturer's instructions at 4°C
overnight (anti-caspase-3 rabbit mAb, cat. no. 9665; 1:1,000
dilution; anti-caspase-8 mouse mAb cat. no. 9746; 1:1,000 dilution;
and anti-caspase-9 mouse mAb, cat. no. 9508; 1:1,000 dilution)
(Cell Signaling Technology, Inc.). Anti-β-actin mouse mAb (cat. no.
013-24553; 1:2,000 dilution; Wako Pure Chemical Industries, Ltd.)
was utilized as a loading control. Anti-mouse or anti-rabbit IgG
(cat. no. A4416; 1:5,000 dilution; Sigma-Aldrich; Merck KGaA, cat.
no. 7074; 1:5,000 dilution; Cell Signaling Technology, Inc.,
respectively) was employed as the secondary antibody for 60 min at
room temperature. The band patterns were analyzed using ImageQuant
LAS-4000 after treatment with ECL Prime Western Blotting Detection
Reagent (both from GE Healthcare). The same experiments were
repeated 3 times to confirm reproducibility.
Real-time reverse transcription-PCR
analysis
RNA extraction was performed using an RNeasy Mini
kit (Qiagen, Inc.). Relative mRNA expression was evaluated by
real-time reverse transcription-PCR (qRT-PCR) employing a StepOne
Real-time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and a One-Step qPCR kit (SYBR® Green Real-time PCR
Master Mix (Toyobo Life Science) according to the manufacturers'
instructions. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA
was utilized as an internal control.
The following primers were used in this experiment
(17,34–36): GAPDH
forward, 5′-CAGAACATCATCCCTGCCTCT-3′ and reverse,
5′-GCTTGACAAAGTGGTCGTTGAG-3′; DR4 forward,
5′-TGTACGCCTGGAGTGACAT-3′ and reverse, 5′-CACCAACAGCAACGGAACAA-3′;
DR5 forward, 5′-CAGGTGTCAACATGTTGTCC-3′ and reverse,
5′-ATCGAAGCACTGTCTGAGAG-3′; cellular FLICE inhibitory protein
(c-FLIP) forward, 5′-CGGACTATAGAGTGCTGATGG-3′ and reverse,
5′-GATTATCAGGCAGATTCCTAG-3′; p53 forward,
5′-GGCCCACTTCACCGTACTAA-3′ and reverse, 5′-CTGGTTTCAAGGCCAGATGT-3′;
and B-cell lymphoma 2-associated × protein (Bax) forward,
5′-TTTGCTTCAGGGTTTCATCC-3′ and reverse, 5′-CAGTTGAAGTTGCCGTCAGA-3′.
The thermocycling conditions were 90°C for 30 sec, 61°C for 20 min,
and 95°C for 1 min, followed by 40 cycles at 95°C for 15 sec, 55°C
for 15 sec and 74°C for 45 sec. The expression levels were
calculated employing the following equations by comparing the
threshold cycle (CT): ∆Cq = CT of DR4, DR5, FAS, p53, Bax, or
c-FLIP-CT of GAPDH, ∆∆Cq (target cell line)-∆Cq (reference cell
line), and ratio = 2−∆∆Cq (37). The experiments were repeated 3 times
under each condition.
Influence of DR5 blocking antibody on
the antitumor effect of combined treatment with TRAIL and
IFN-β
In order to evaluate the involvement of DR5 in the
antitumor effect of TRAIL in combination with IFN-β, the
anti-apoptotic effect of administering DR5 blocking antibody was
examined. U-138MG cells were seeded at 5×104 cells/well
in 24-well plates. After 24 h of attachment, the cells were further
incubated with fresh medium containing 10 ng/ml DR5 blocking
antibody (Recombinant Human TRAIL R2/TNFRSF10B Fc Chimera Protein;
R&D Systems, Inc.), or 1 ng/ml TRAIL and 10 IU/ml IFN-β
without/with DR5 blocking antibody (2.5, 5 and 10 ng/ml) for 72 h.
The surviving cells in each well were then counted using a Coulter
Counter (Coulter Counter Z1; Beckman Coulter, Inc.) after
confirming the presence of living cells with 0.45% trypan blue
solution (Sigma-Aldrich; Merck KGaA). The experiments were repeated
6 times at each concentration.
Statistical analysis
The experiments were repeated at least 3 times under
each condition. Where the same experiment was performed more than 6
times, the mean value and standard error (SE) were calculated and
utilized. Mann-Whitney's U test was carried out to compare data
between pairs of groups. The Tukey-Kramer test was conducted to
perform comparisons of three or more groups, and when there was a
significant difference, Student's t-test was employed as a
subsequent test. For the data analyses, the statistical software
SPSS (version 21.0: IBM Corp.) was used.
Results
Antitumor effect of TRAIL and IFN-β in
malignant glioma cells
To evaluate the antitumor effect of TRAIL and IFN-β,
the 7 malignant glioma cell lines (A-172, AM-38, T98G, U-87MG,
U-138MG, U-251MG and YH-13) were treated with 0–1,000 ng/ml of
TRAIL or 0–1,000 IU/ml of IFN-β for 72 h, and then assessed by
counting the viable cells in the media. In all cell lines, both
TRAIL and IFN-β caused a decrease in cell viability in a
dose-dependent manner as revealed in Fig.
1.
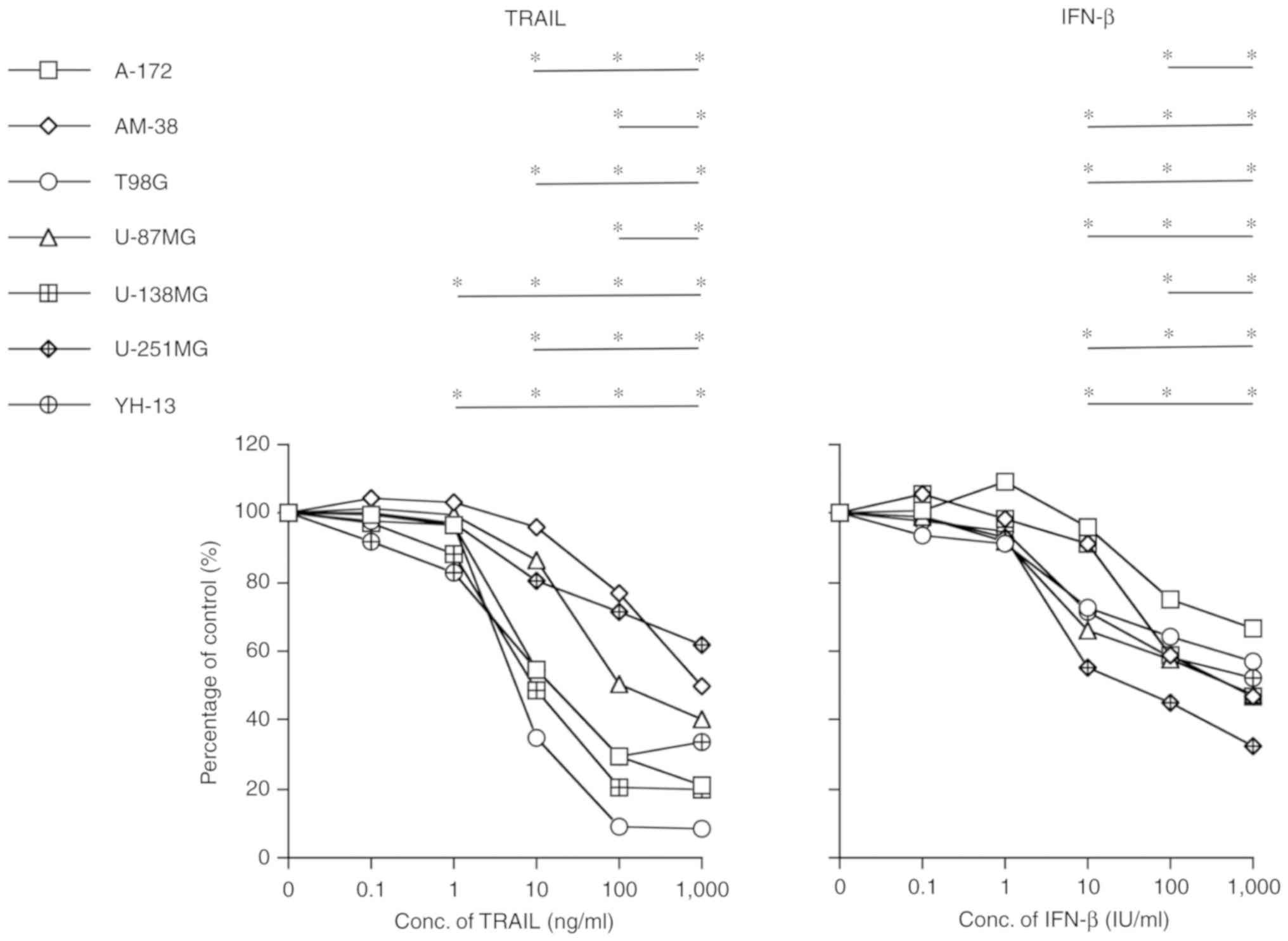 | Figure 1.Antitumor effects of TRAIL (0–1,000
ng/ml) or IFN-β (0–1,000 IU/ml) against 7 types of malignant glioma
cell lines (A-172, AM-38, T98G, U-87MG, U-138MG, U-251MG and
YH-13). Both TRAIL (left) and IFN-β (right) exhibited a cell growth
inhibitory effect in a dose-dependent manner in all cell lines at
72 h. Data are expressed as a percentage of the control. Compared
to the no treatment group and the treatment groups, *P<0.05.
TRAIL, tumor necrosis factor-related apoptosis-inducing ligand;
IFN-β, interferon-β. |
Antitumor effect of a combination of
TRAIL and IFN-β
To assess whether or not combined treatment with
TRAIL and IFN-β could exert a synergistic effect in the 7 malignant
glioma cell lines, cells were treated with various concentrations
of TRAIL (0–1,000 ng/ml) or TRAIL with 10 IU/ml of IFN-β for 72 h.
As revealed in Fig. 2, combined
treatment with TRAIL and IFN-β revealed an additive cell growth
inhibitory effect in the A-172, AM-38, T98G, U-138MG, and U-251MG
cell lines, whereas no significant additive effect was observed in
the U-87MG and YH-13 cell lines.
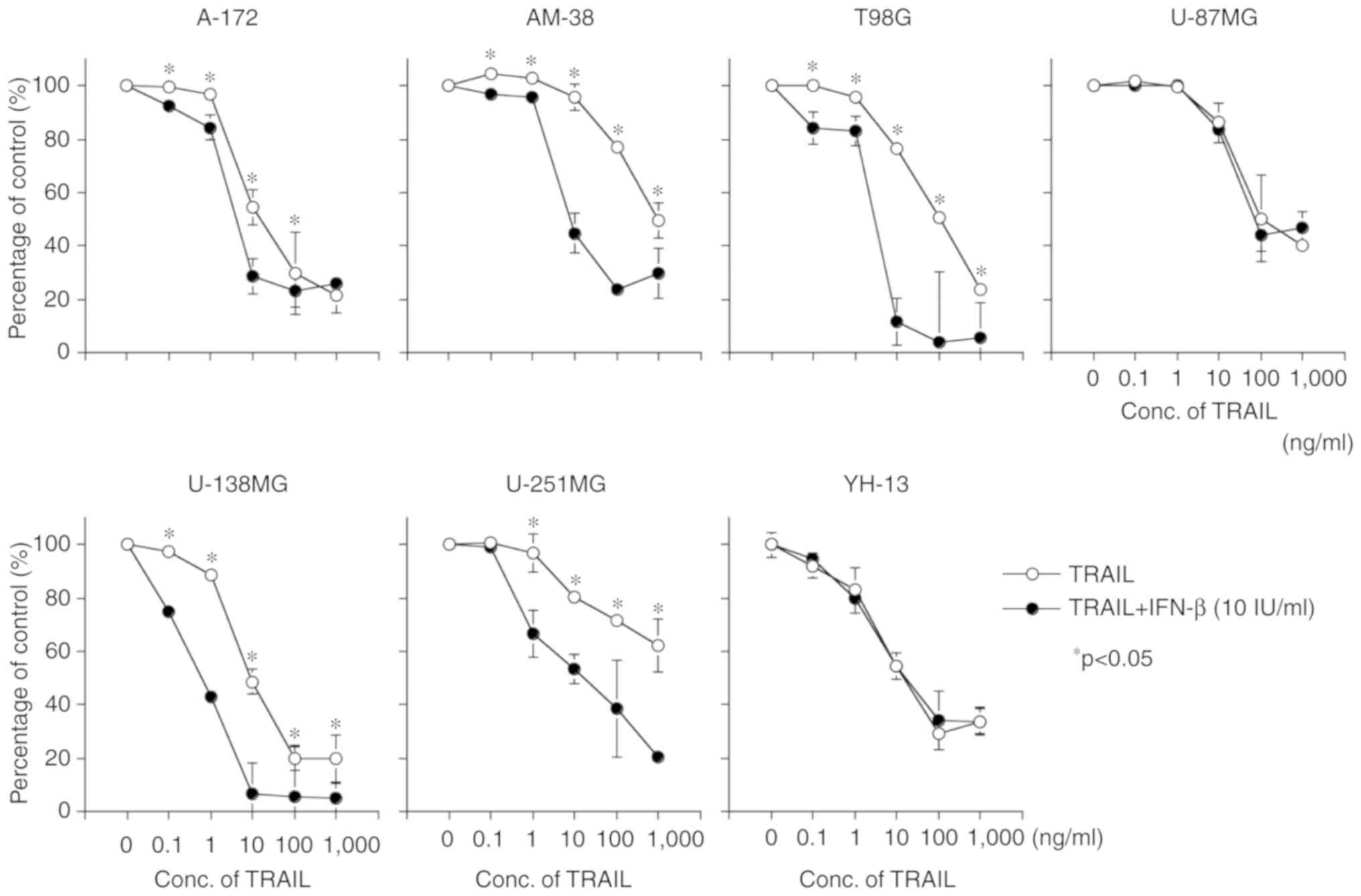 | Figure 2.Cell growth inhibitory effect of a
combination of TRAIL and IFN-β on malignant glioma cell lines.
Seven malignant glioma cell lines (A-172, AM-38, T98G, U-87MG,
U-138MG, U-251MG and YH-13) were exposed to medium containing
various concentrations of TRAIL (0–1,000 ng/ml) without IFN-β (○)
or with 10 IU/ml IFN-β (•) for 72 h. The combined treatment with
TRAIL and IFN-β revealed an additive cell growth inhibitory effect
in A-172, AM-38, T98G, U-138MG, and U-251MG, whereas it did not do
so in U-87MG and YH-13. Data are presented as the means ± SE
(standard error). *P<0.05. TRAIL, tumor necrosis factor-related
apoptosis-inducing ligand; IFN-β, interferon-β. |
These results did not appear to be related to the
MGMT or the p53 status in the case of TRAIL or TRAIL and
IFN-β sensitivity. Because the additive cell growth inhibitory
effect of combined treatment with TRAIL and IFN-β was observed with
T98G and U-138MG, but not with YH-13, although these three cell
lines are MGMT positive. In addition, the additive cell growth
inhibitory effect of combined treatment with TRAIL and IFN-β was
observed with the A-172, AM-38, T98G, U-138MG and U-251MG cell
lines, although A-172, AM-38, U-87MG, U-138MG and YH-13 are
wild-types of p53 status, in contrast T98G and U-251MG are
mutant types of p53 status.
Detection of apoptosis by flow
cytometry and western blotting
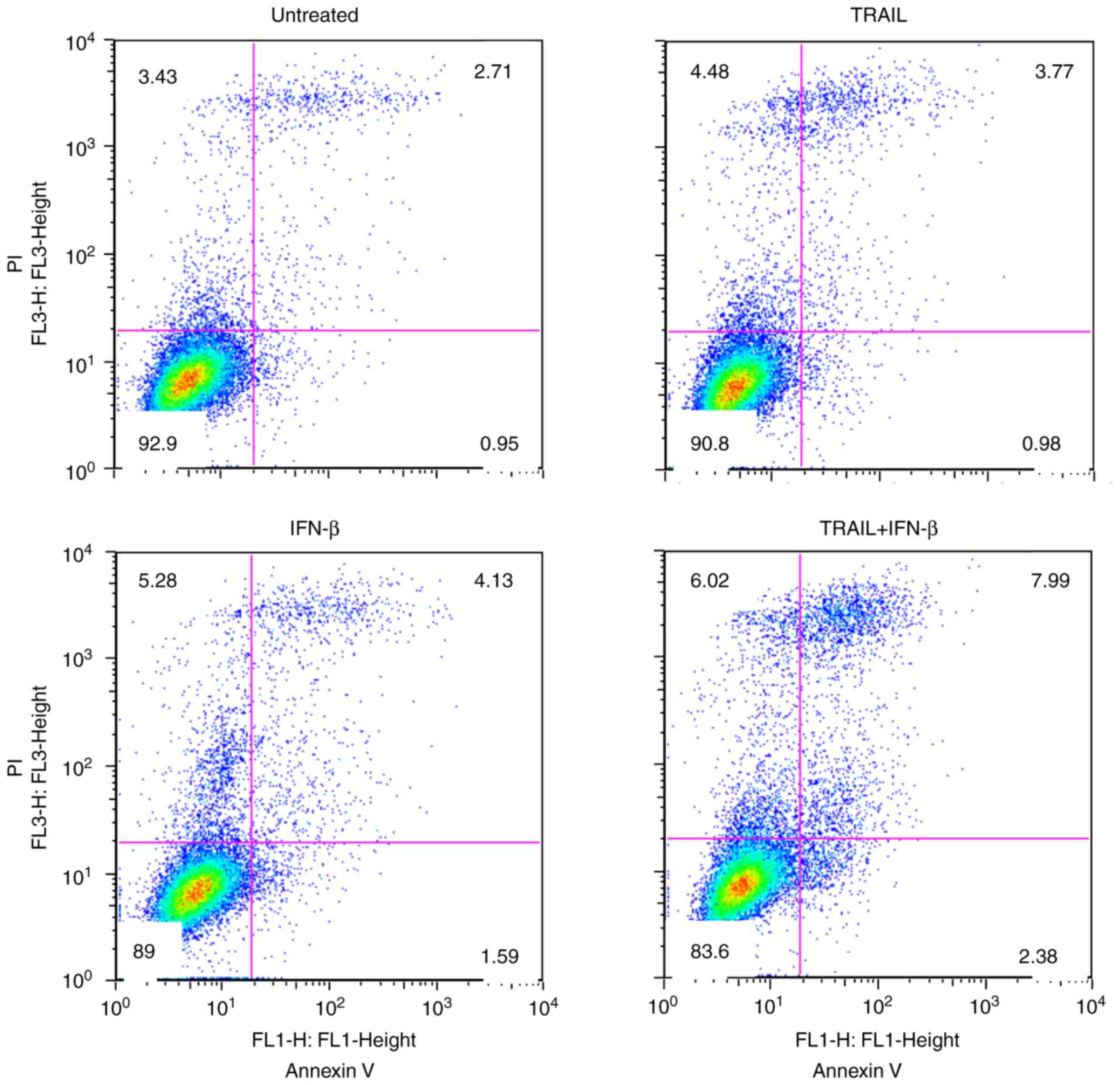
To assess the apoptosis induced by TRAIL and IFN-β,
flow cytometry with Annexin V/PI double staining was performed
(Annexin V-positive, early-stage apoptosis; Annexin V/PI-positive,
late-stage apoptosis). As revealed in Fig. 3, combined treatment with TRAIL (1
ng/ml) and IFN-β (10 IU/ml) induced significant apoptosis after 72
h in U-138MG cells. The proportion of Annexin V/PI positive cells
following combined treatment with TRAIL and IFN-β (mean = 7.99%)
was higher than that for each single agent (means: Untreated,
2.71%; TRAIL, 3.77%; and IFN-β, 4.13%, respectively).
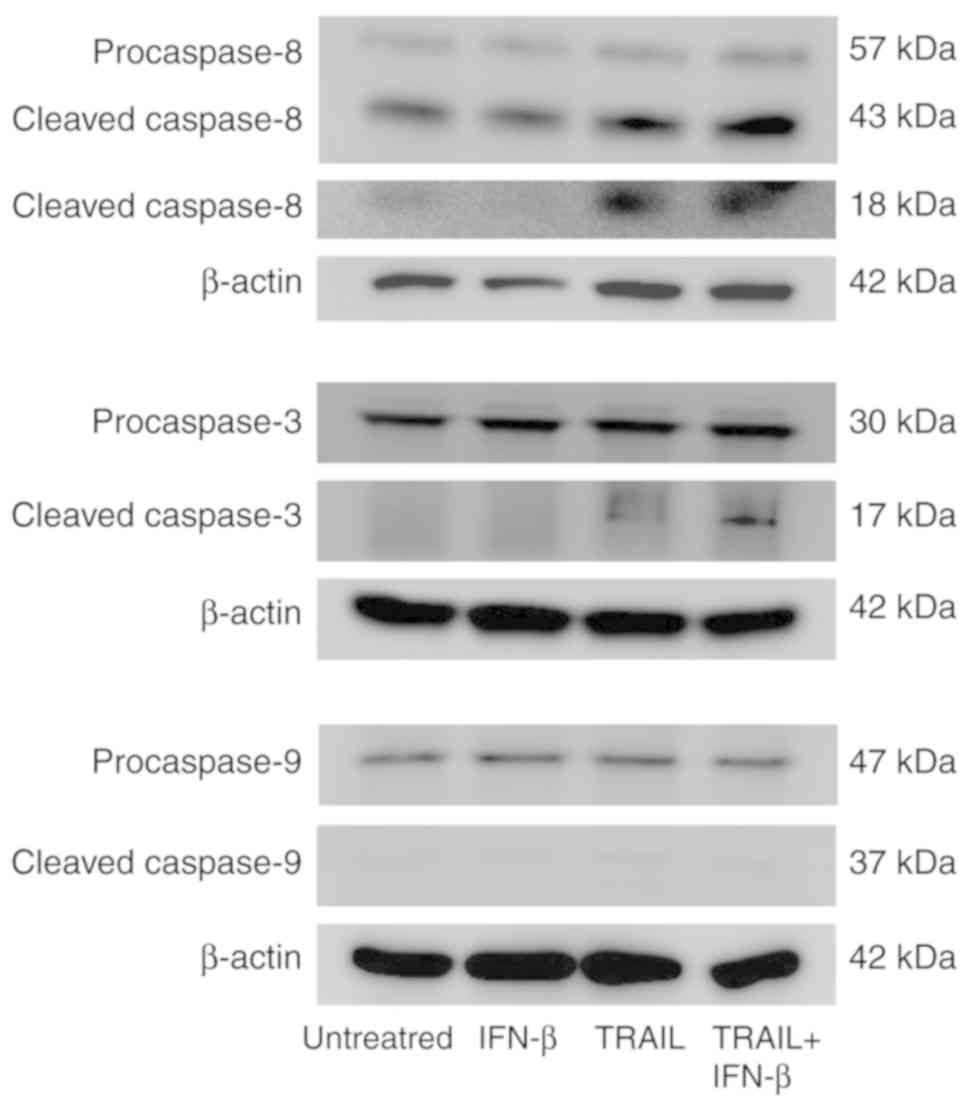
Furthermore, to evaluate the underlying mechanisms
of the apoptotic effect of TRAIL and IFN-β, the protein expression
of caspase-3 (an effector caspase), caspase-8 (extrinsic apoptotic
pathway), and caspase-9 (intrinsic mitochondrial pathway) were
evaluated by western blot analysis. As revealed in Fig. 4, following 24 h of combined treatment
with TRAIL and IFN-β, the protein expression levels of cleaved
caspase-8 and cleaved caspase-3 were revealed to be more marked
when compared to those following treatment with TRAIL or IFN-β
alone. However, as regards the protein expression of caspase-9 and
cleaved caspase-9, no difference was noted among the treatments.
The effect of combined treatment with TRAIL and IFN-β in U-138MG
may thus be due to promotion of apoptosis through the exogenous
apoptotic pathway.
Expression of apoptosis-related
genes
To further elucidate the role of IFN-β in combined
treatment with TRAIL and IFN-β, the mRNA expression levels of
apoptosis-related genes were evaluated by real-time qRT-PCR. The
mRNA expression levels of DR4, DR5, Fas, p53, Bax and
c-FLIP were determined following 4 h of IFN-β treatment in
U-138MG cells. Significant upregulation of DR5 (mean:
2.97±0.23-fold), Fas (mean: 1.65±0.11-fold), and p53
(mean: 1.50±0.11-fold) was detected; however, no significant
changes in DR4 (mean: 1.07±0.01-fold), Bax (mean:
1.17±0.11-fold), and c-FLIP (mean: 0.89±0.23-fold) were
revealed (Fig. 5).
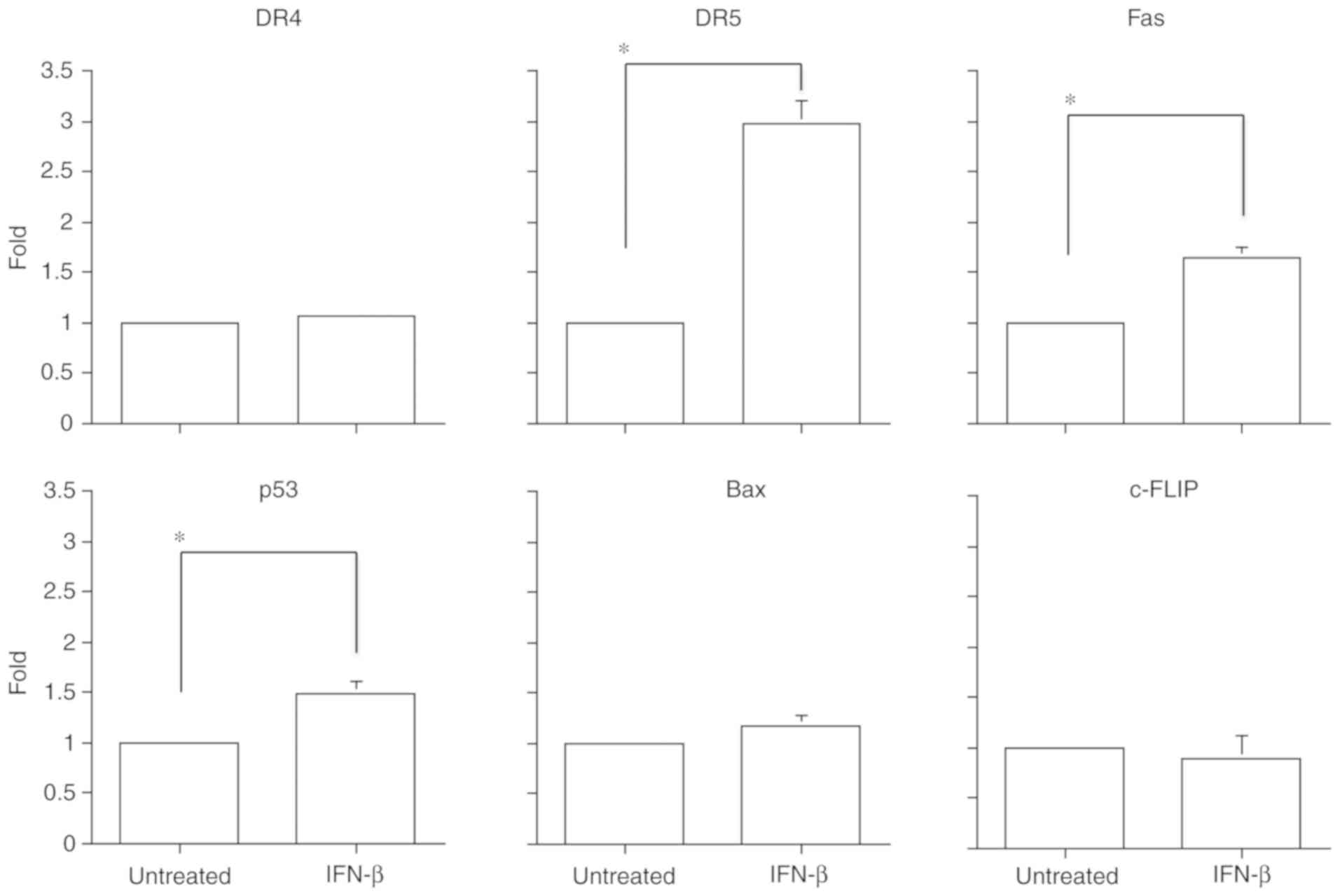 | Figure 5.Apoptosis-related mRNA expression
levels of DR4, DR5, Fas, p53, Bax, and c-FLIP
following IFN-β treatment as analyzed by real-time qRT-PCR.
Significant upregulation of DR5, Fas, and p53 was
observed in the case of IFN-β (10 IU/ml) treatment for 4 h in
U-138MG cells. However, there were no differences in DR4,
Bax, and c-FLIP following 4 h of IFN-β treatment in
U-138MG cells. Data are presented as the means ± SE (standard
error). *P<0.05 (Student's t-test). DR4, death receptor 4; DR5,
death receptor 5; c-FLIP, cellular FLICE inhibitory protein;
Bax, B-cell lymphoma 2-associated × protein; IFN-β,
interferon-β. |
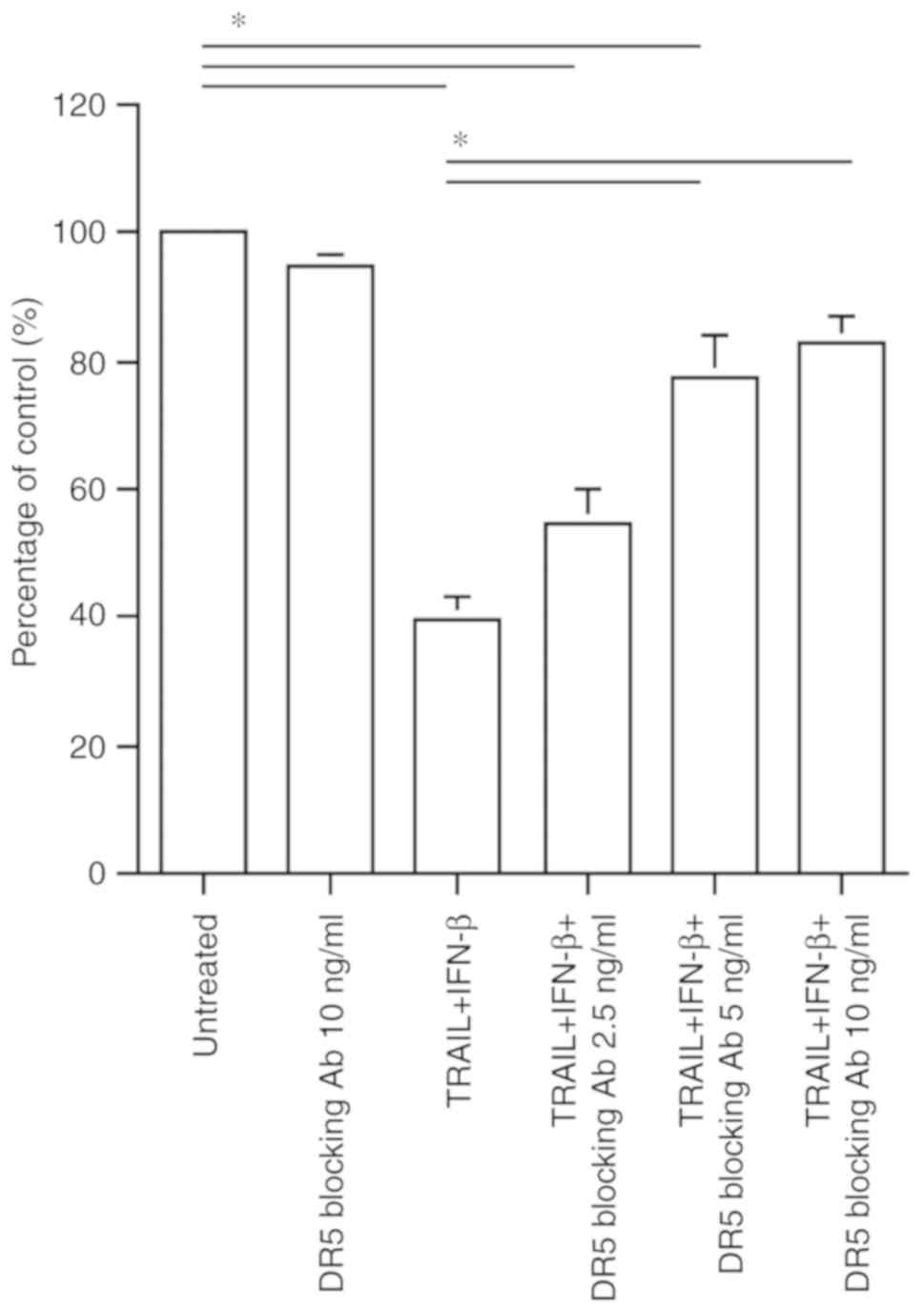
Apoptosis inhibition by DR5 blocking
antibody
To confirm that the antitumor effect of combined
treatment with TRAIL and IFN-β in malignant glioma cells is
dependent on DR5, the number of surviving cells was counted after
treatment for 72 h with DR5 blocking antibody (2.5, 5 or 10 ng/ml).
The DR5 blocking antibody alone displayed no significant effect on
U-138MG cells, and combined treatment with TRAIL (1 ng/ml) and
IFN-β (10 IU/ml) exhibited a significant cell growth inhibitory
effect (mean: 39.3±10.4% when compared to no treatment). The DR5
blocking antibody caused a significant attenuation of the
inhibitory effect on cell proliferation in a
concentration-dependent manner in U-138MG; the viable cells when
2.5, 5, or 10 ng/ml of DR5 blocking antibody was added to TRAIL and
IFN-β combined treatment amounted to 54.8±9.0, 77.4±8.5 and
82.8±5.1%, respectively, as compared to the untreated case
(Fig. 6).
Discussion
TRAIL has been considered to represent a promising
agent for malignant glioma treatment, due to its ability to induce
apoptosis of cancer cells without affecting normal cells (10,11).
However, several studies have suggested that most glioma cells
display resistance to TRAIL-induced apoptosis (12,13), and
to overcome this resistance is a critical issue. Among various
investigations conducted, it has been reported that TRAIL-induced
apoptosis can be enhanced by combination with other drugs or by
irradiation (14–18,38).
Combined treatment with TRAIL sensitizers is regarded as a
promising strategy to overcome TRAIL resistance, and several
attempts have been made to identify such TRAIL sensitizers
(14–18).
In the present study, TRAIL exhibited a
dose-dependent antitumor effect in all of 7 types of malignant
glioma cell lines, although the intensity of the effect varied
among the cell lines. This finding is consistent with those
obtained in previous studies and suggests that
sensitivity/resistance to TRAIL differs among malignant gliomas
(39–41). Furthermore, the combination treatment
with TRAIL and IFN-β in 5 of the 7 malignant glioma cell lines
demonstrated a more significant antitumor effect than TRAIL alone.
To the best of our knowledge, this is the first time that IFN-β
enhancement of TRAIL-induced apoptosis has been observed in
particular malignant glioma cells. In the future, one important
issue requiring examination will be the antitumor effect occurring
after application of TRAIL and IFN-β in combination with TMZ, which
is the current standard therapeutic agent for malignant gliomas. In
addition, once sufficient data have been gathered, the
morphological changes of the cells associated with TRAIL, IFN-β,
and combined treatment will require investigation.
Higuchi and Hashida (31) reported that the plasma concentration
levels of IFN-β were 40 and 96 IU/ml following intravenous
administration of 3×106 and 6×106 IU IFN-β,
respectively, when administered in 60 min. On this basis, 10 IU/ml
is considered a clinically relevant concentration of IFN-β.
Furthermore, in phase II RCS for non-small cell lung cancer and B
cell lymphoma, 8 mg/kg of TRAIL was administered, and its plasma
concentration reached ~80 mg/ml (32,33). The
concentration of TRAIL used in the present study is therefore
considered to be clinically low. In particular, in A-172, AM-38,
T98G, and U-138MG cells, a cell growth inhibitory effect was also
significantly observed even at a small amount (0.1 and 1 ng/ml) of
TRAIL with 10 IU/ml of IFN-β, suggesting that IFN-β represents a
good candidate for use in combination with TRAIL-based treatment
regimens for malignant gliomas. However, based on the present
study, we do not have sufficient data to elucidate the exact
differences in sensitivity to each drug between each cell line.
Furthermore, the detailed mechanisms underlying such differences
remain unidentified in literature, and this presents our next topic
of research. In the present study, the focus was on evaluating in
more detail how the combined treatment with TRAIL and IFN-β can
provide a more marked antitumor effect.
Following combined treatment with TRAIL (1 ng/ml)
and IFN-β (10 IU/ml), FACS analysis indicated that late-phase
apoptosis or necrosis was increased (42), when compared to that with TRAIL or
IFN-β alone in U-138MG cells. Furthermore, it was revealed by
western blotting that caspase-8 and caspase-3, which are involved
in the extrinsic apoptotic pathways (17,34–46), were
cleaved by combined treatment with TRAIL and IFN-β, whereas
caspase-9 which is involved in the intrinsic apoptotic pathway
exhibited no cleavage. The antitumor effect of combined treatment
with TRAIL and IFN-β may thus be enhanced via an extrinsic
apoptotic system in U-138MG cells.
TRAIL induces apoptosis of cancer cells by binding
to its receptors DR4 and DR5 (9). In
malignant glioma cells, there have been indications that cisplatin
and irradiation may upregulate the expression of DR5 (38,47).
Conversely, in malignant glioma cells, IFN-β has been revealed to
upregulate the expression of p53 (24). Furthermore, it has been suggested that
p53 promotes the expression of DR5 (48). In the present study, it was
demonstrated and confirmed that the quantitative p53 and
DR5 mRNA levels were upregulated by IFN-β (10 IU/ml),
however, no significant upregulation was observed for DR4 in
U-138MG cells. In the future, it is surmised that it will also be
necessary to investigate the protein levels produced by such
apoptosis-related genes following IFN-β treatment. In addition, as
revealed in Fig. 6, the antitumor
effect elicited by the combined treatment with TRAIL and IFN-β was
significantly attenuated depending on the concentration of DR5
blocking antibody. These findings indicated that upregulation of
DR5 via p53 by IFN-β may play an important role in the enhanced
antitumor effect of combined treatment with TRAIL and IFN-β in
U-138MG, although the investigation of the effect of DR5 blocking
antibody with TRAIL or INF-β treatment alone is required. Moreover,
Fas, which is a receptor that induces apoptosis (extrinsic
apoptotic pathway) (46), was also
associated with increased expression levels of quantitative mRNA by
IFN-β in the present study. These circumstances were considered to
represent part of the mechanism whereby the antitumor effect was
enhanced by employing IFN-β in combination with TRAIL.
Finally, c-FLIP is expressed in tumor cells to a
certain level, and competes with caspase-8 to inhibit apoptosis by
binding to Fas-associated death domain (49,50). It
has been reported that various drugs lower the expression of
c-FLIP, and the induction of apoptosis by TRAIL could be
enhanced (49–53). It was therefore investigated whether
the expression of c-FLIP was decreased by the administration
of IFN-β. However, in the present study, no significant decrease in
quantitative c-FLIP mRNA level by IFN-β was observed in
U-138MG cells.
In conclusion, in the present study, it was
demonstrated that combined treatment with a clinically relevant
concentration of TRAIL and IFN-β produced a significantly enhanced
antitumor effect in malignant glioma cells as compared to that
achieved when either agent was used alone, and this may be
partially dependent on DR5 through the extrinsic pathway of
apoptosis. Although the present findings were not sufficient to
yield a conclusive theory of the mechanism of action, and in
vivo experiments will also need to be undertaken in the future,
the data did provide an experimental basis to suggest that combined
treatment with TRAIL and IFN-β could offer a new therapeutic
strategy for malignant gliomas.
Acknowledgements
The authors are grateful to Mr. Hiroyuki Satake and
Mr. Nobuo Miyazaki, Toray Industries Inc. (Tokyo, Japan) for their
invaluable discussions. Some parts of this study have been
incorporated within a Japanese-language thesis submitted for Sodai
Yoshimura's Ph.D. degree at Nihon University School of Medicine
(Tokyo, Japan).
Funding
The present study was supported in part by
Grants-in-Aid for Scientific Research from the Japan Society for
the Promotion of Science (grant no. 16K10772) and in part by a
grant from the Health Sciences Research Institute, Inc. (Yokohama,
Japan) for the Division of Companion Diagnostics, Department of
Pathology and Microbiology, Nihon University School of Medicine
(Tokyo, Japan).
Availability of data and materials
All data related to this study are included in this
article.
Authors' contributions
SYo and ES developed the experimental design,
performed most of the experiments and analysis, and drafted the
basic manuscript. YH and SYa were involved in the conception and
design of the study, undertook part of the experiments, analyzed
the data, and contributed to the writing of the draft manuscript.
KS also conducted some experiments and analyzed the data. TU, TN,
HH, and YK were also involved in the conception and design of the
study and proofread the manuscript. AY contributed to the
experimental design and the writing of the manuscript. All authors
have read and approved the final manuscript and agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests with regard to the subjects discussed in this study. TU
received funds for other research projects not related to this
study from the Ministry of Education, Culture, Sports, Science and
Technology, Japan, the Ministry of Economy, Trade and Industry,
Japan and the Human Frontier Science Program, while AY received
research funds for another research project from Medtronic Japan
Co., Ltd.
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Report of brain tumor registry of Japan
(2015–2017). Neuro Med Chir (Tokyo). 59 (Suppl):S1–S81. 2019.
|
|
3
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: Genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ashkenazi A, Holland P and Eckhardt SG:
Ligand-based targeting of apoptosis in cancer: The potential of
recombinant human apoptosis ligand 2/tumor necrosis factor-related
apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol.
26:3621–3630. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naoum GE, Buchsbaum DJ, Tawadros F,
Farooqi A and Arafat WO: Journey of TRAIL from bench to bedside and
its potential role in immuno-oncology. Oncol Rev. 11:3322017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Karstedt S, Montinaro A and Walczak H:
Exploring the TRAILs less travelled: TRAIL in cancer biology and
therapy. Nat Rev Cancer. 17:352–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suliman A, Lam A, Datta R and Srivastava
RK: Intracellular mechanisms of TRAIL: Apoptosis through
mitochondrial-dependent and -independent pathways. Oncogene.
20:2122–2133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagane M, Huang HJ and Cavenee WK: The
potential of TRAIL for cancer chemotherapy. Apoptosis. 6:191–197.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuijlen JM, Bremer E, Mooij JJ, den Dunnen
WF and Helfrich W: Review: On TRAIL for malignant glioma therapy?
Neuropathol Appl Neurobiol. 36:168–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hawkins CJ: TRAIL and malignant glioma.
Vitam Horm. 67:427–452. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shang Z and Zhang L: Digitoxin increases
sensitivity of glioma stem cells to TRAIL-mediated apoptosis.
Neurosci Lett. 653:19–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan M, Bi Y, Qazi JI, Fan L and Gao H:
Evodiamine sensitizes U87 glioblastoma cells to TRAIL via the death
receptor pathway. Mol Med Rep. 11:257–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Badr CE, Wurdinger T, Nilsson J, Niers JM,
Whalen M, Degterev A and Tannous BA: Lanatoside C sensitizes
glioblastoma cells to tumor necrosis factor-related
apoptosis-inducing ligand and induces an alternative cell death
pathway. Neuro Oncol. 13:1213–1224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA,
Lim JH, Kwon TK and Choi KS: Monensin, a polyether ionophore
antibiotic, overcomes TRAIL resistance in glioma cells via
endoplasmic reticulum stress, DR5 upregulation and c-FLIP
downregulation. Carcinogenesis. 34:1918–1928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calzolari A, Saulle E, De Angelis ML,
Pasquini L, Boe A, Pelacchi F, Ricci-Vitiani L, Baiocchi M and
Testa U: Salinomycin potentiates the cytotoxic effects of TRAIL on
glioblastoma cell lines. PLoS One. 9:e944382014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borden EC, Sen GC, Uze G, Silverman RH,
Ransohoff RM, Foster GR and Stark GR: Interferons at age 50: Past,
current and future impact on biomedicine. Nat Rev Drug Discov.
6:975–990. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Isaacs A and Lindenmann J: Virus
interference. I. The interferon. Proc R Soc Lond B Biol Sci.
147:258–267. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vannucchi S, Chiantore MV, Mangino G,
Percario ZA, Affabris E, Fiorucci G and Romeo G: Perspectives in
biomolecular therapeutic intervention in cancer: From the early to
the new strategies with type I interferons. Curr Med Chem.
14:667–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshida J, Kajita Y, Wakabayashi T and
Sugita K: Long-term follow-up results of 175 patients with
malignant glioma: Importance of radical tumor resection and
postoperative adjuvant therapy with interferon, ACNU and radiation.
Acta Neurochir (Wien). 127:55–59. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Watanabe T, Katayama Y, Yoshino A, Komine
C, Yokoyama T and Fukushima T: Treatment of low-grade diffuse
astrocytomas by surgery and human fibroblast interferon without
radiation therapy. J Neurooncol. 61:171–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Natsume A, Ishii D, Wakabayashi T, Tsuno
T, Hatano H, Mizuno M and Yoshida J: IFN-beta down-regulates the
expression of DNA repair gene MGMT and sensitizes resistant glioma
cells to temozolomide. Cancer Res. 65:7573–7579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watanabe T, Katayama Y, Yoshino A, Fukaya
C and Yamamoto T: Human interferon beta, nimustine hydrochloride,
and radiation therapy in the treatment of newly diagnosed malignant
astrocytomas. J Neurooncol. 72:57–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshino A, Ogino A, Yachi K, Ohta T,
Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N and Sano
E: Effect of IFN-beta on human glioma cell lines with temozolomide
resistance. Int J Oncol. 35:139–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Der SD, Zhou A, Williams BR and Silverman
RH: Identification of genes differentially regulated by interferon
alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad
Sci USA. 95:15623–15628. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chawla-Sarkar M, Lindner DJ, Liu YF,
Williams BR, Sen GC, Silverman RH and Borden EC: Apoptosis and
interferons: Role of interferon-stimulated genes as mediators of
apoptosis. Apoptosis. 8:237–249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshino A, Ogino A, Yachi K, Ohta T,
Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N, Sano E
and Tsumoto K: Gene expression profiling predicts response to
temozolomide in malignant gliomas. Int J Oncol. 36:1367–1377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wischhusen J, Naumann U, Ohgaki H,
Rastinejad F and Weller M: CP-31398, a novel p53-stabilizing agent,
induces p53-dependent and p53-independent glioma cell death.
Oncogene. 22:8233–8245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Higuchi Y and Hashida M: Pharmacokinetics
of interferon. Clinic All-Round. 52:2499–2505. 2003.(In
Japanese).
|
|
32
|
Soria JC, Márk Z, Zatloukal P, Szima B,
Albert I, Juhász E, Pujol JL, Kozielski J, Baker N, Smethurst D, et
al: Randomized phase II study of dulanermin in combination with
paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell
lung cancer. J Clin Oncol. 29:4442–4451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheah CY, Belada D, Fanale MA, Janikova A,
Czucman MS, Flinn IW, Kapp AV, Ashkenazi A, Kelley S, Bray GL, et
al: Dulanermin with rituximab in patients with relapsed indolent
B-cell lymphoma: An open-label phase 1b/2 randomised study. Lancet
Haematol. 2:e166–e174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ray P, Guha D, Chakraborty J, Banerjee S,
Adhikary A, Chakraborty S, Das T and Sa G: Crocetin exploits
p53-induced death domain (PIDD) and FAS-associated death domain
(FADD) proteins to induce apoptosis in colorectal cancer. Sci Rep.
6:329792016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Edagawa M, Kawauchi J, Hirata M, Goshima
H, Inoue M, Okamoto T, Murakami A, Maehara Y and Kitajima S: Role
of ATF3 for ER stress-induced sensitization of p53-deficient human
colon cancer cells to TRAIL-mediated apoptosis through upregulation
of DR5 by zerumbone and celecoxib. J Biol Chem. 289:21544–21561.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Earel JK Jr, VanOosten RL and Griffith TS:
Histone deacetylase inhibitors modulate the sensitivity of tumor
necrosis factor-related apoptosis-inducing ligand-resistant bladder
tumor cells. Cancer Res. 66:499–507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu J, Gao Q, Xie T, Liu Y, Luo L, Xu C,
Shen L, Wan F, Lei T and Ye F: Synergistic effect of TRAIL and
irradiation in elimination of glioblastoma stem-like cells. Clin
Exp Med. 18:399–411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Röhn TA, Wagenknecht B, Roth W, Naumann U,
Gulbins E, Krammer PH, Walczak H and Weller M: CCNU-dependent
potentiation of TRAIL/Apo2L-induced apoptosis in human glioma cells
is p53-independent but may involve enhanced cytochrome c release.
Oncogene. 20:4128–4137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hao C, Beguinot F, Condorelli G, Trencia
A, Van Meir EG, Yong VW, Parney IF, Roa WH and Petruk KC: Induction
and intracellular regulation of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) mediated apoptosis in human
malignant glioma cells. Cancer Res. 61:1162–1170. 2001.PubMed/NCBI
|
|
41
|
Xiao C, Yang BF, Asadi N, Beguinot F and
Hao C: Tumor necrosis factor-related apoptosis-inducing
ligand-induced death-inducing signaling complex and its modulation
by c-FLIP and PED/PEA-15 in glioma cells. J Biol Chem.
277:25020–25025. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rieger AM, Nelson KL, Konowalchuk JD and
Barreda DR: Modified annexin V/propidium iodide apoptosis assay for
accurate assessment of cell death. J Vis Exp. 24:2011.
|
|
43
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Green DR: Apoptotic pathways: Paper wraps
stone blunts scissors. Cell. 102:1–4. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ding L, Yuan C, Wei F, Wang G, Zhang J,
Bellail AC, Zhang Z, Olson JJ and Hao C: Cisplatin restores TRAIL
apoptotic pathway in glioblastoma-derived stem cells through
up-regulation of DR5 and down-regulation of c-FLIP. Cancer Invest.
29:511–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu GS, Burns TF, McDonald ER III, Jiang W,
Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, et al:
KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor
gene. Nat Genet. 17:141–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang Y, Yang X, Xu T, Kong Q, Zhang Y,
Shen Y, Wei Y, Wang G and Chang KJ: Overcoming resistance to
TRAIL-induced apoptosis in solid tumor cells by simultaneously
targeting death receptors, c-FLIP and IAPs. Int J Oncol.
49:153–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Min KJ, Um HJ, Seo SU, Woo SM, Kim S, Park
JW, Lee HS, Kim SH, Choi YH, Lee TJ and Kwon TK: Angelicin
potentiates TRAIL-induced apoptosis in renal carcinoma Caki cells
through activation of caspase 3 and down-regulation of c-FLIP
expression. Drug Dev Res. 79:3–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lemke J, von Karstedt S, Abd El Hay M,
Conti A, Arce F, Montinaro A, Papenfuss K, El-Bahrawy MA and
Walczak H: Selective CDK9 inhibition overcomes TRAIL resistance by
concomitant suppression of cFlip and Mcl-1. Cell Death Differ.
21:491–502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Woo SM, Min KJ, Seo BR and Kwon TK: YM155
sensitizes TRAIL-induced apoptosis through cathepsin S-dependent
down-regulation of Mcl-1 and NF-κB-mediated down-regulation of
c-FLIP expression in human renal carcinoma Caki cells. Oncotarget.
7:61520–61532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Irmler M, Thome M, Hahne M, Schneider P,
Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C,
et al: Inhibition of death receptor signals by cellular FLIP.
Nature. 388:190–195. 1997. View
Article : Google Scholar : PubMed/NCBI
|