Introduction
Gliomas are the most common neoplasm of the central
nervous system in humans. Glioblastoma multiforme (GBM) is one of
the most serious types of gliomas. Although significant progress
has been made in the development of novel therapies for the
treatment of this disease, the survival rate of glioma patients
remains considerably low. The median survival time in patients with
newly diagnosed GBM is frequently less than 15 months (1,2).
Approximately 77,000 new glioblastoma cases are diagnosed annually
in the United States and Europe (3). The 5-year survival rate of patients is
~5% (4). Despite maximal tumor
resection, high-dose radiation and temozolomide (TMZ) chemotherapy
that are currently used in clinical treatment, the prognosis of GBM
has not improved significantly. The majority of low-grade glioma
(LGG) cases will develop into GBM with conventional therapy.
Therefore, it is urgent to explore a new effective and feasible
treatment for GBM and LGG.
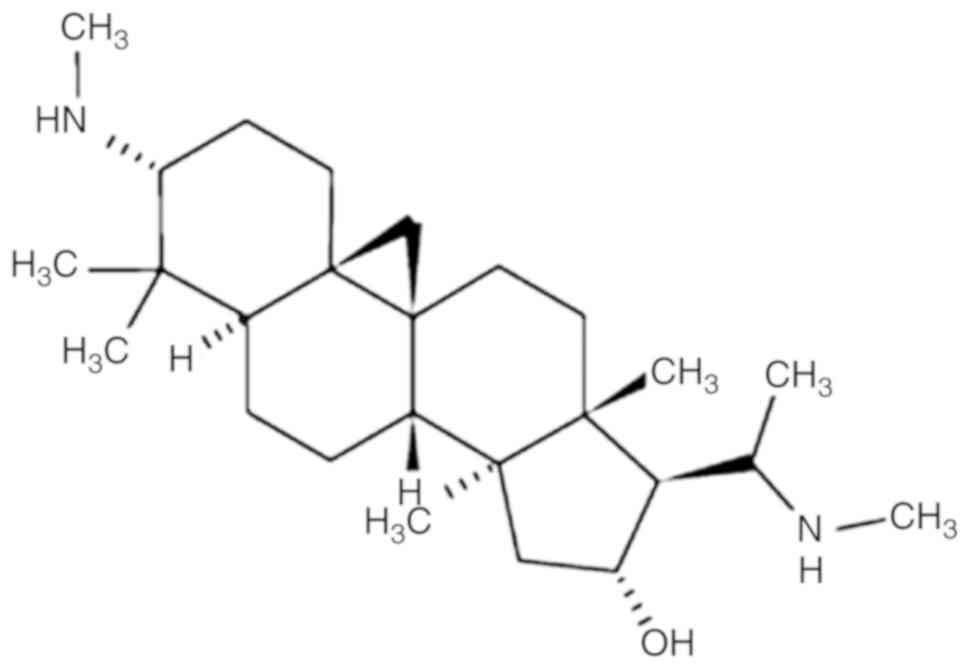
Cyclovirobuxine D (CVB-D) is the main active
component of the traditional Chinese medicine Buxus
microphylla and has demonstrated a definitive therapeutic
effect on various cardiovascular diseases (5,6). The
mechanism of action of CVB-D has been recently studied (7–13).
CVB-D [molecular formula: C26-H46-N2-O; molecular weight: 402.662;
chemical name:
9,19-cyclopregnan-16-ol,4,4,14-trimethyl-3,20-bis(methylamino)-,(3β,5α,16α,20S)-]
is a triterpenoid alkaloid (Fig. 1)
extracted from the traditional Chinese medicine Buxus
microphylla. This extract has also been investigated for its
antitumor effect against breast cancer (13). It is interesting to note that CVB-D
can cross the blood-brain barrier (BBB), suggesting that it can
have higher efficacy against GBM and LGG treatment than other
anti-cancer compounds (14).
However, whether and how CVB-D affects GBM and LGG growth remains
unknown. In view of these findings, the aim of the present study
was to investigate the effects of CVB-D on human GBM and LGG
cells.
Materials and methods
Materials
CVB-D (C26H46N2O,
FW 402.66, purity 66; YuanYe Biotechnology Co., Ltd.), the
Rhodamine 123 (Rh123) kit (BB-41051-1), the Annexin V-FITC/PI
apoptosis test kit (BB-4101-2) and the cell cycle test kit
(BB-4104-2) were purchased from Shanghai BestBio Biotechnology Co.,
Ltd. Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Molecular Technologies, Inc. Fetal bovine serum (100 kU/l) and
penicillin and streptomycin (100 mg/l) were purchased from
Gibco-BRL Invitrogen; Thermo Fisher Scientific, Inc. The BCA
Protein Assay Kit (P0010), and the reagent kits for the protein
measurements were purchased from Beyotime Institute of
Biotechnology. Bax (cat no. ab69643), Bcl-2 (cat. no. ab32124),
caspase-3 (cat. no. ab32351) and cleaved caspase-3 (cat. no.
9664S), beta-actin (cat. no. 4970S) antibodies were purchased from
Cell Signaling Technology and Abcam, Inc. Horseradish
peroxidase-conjugated anti-rabbit (cat. no. ab44171) and anti-mouse
(cat. no. ab21172) antibodies were obtained from Bioworld
Technology, Inc. Immobilon western chemiluminescent HRP Substrate
and PVDF membranes were obtained from EMD Millipore. The Hoechst
33342 staining kit (C1028) was purchased from Beyotime Institute of
Biotechnology.
Cell culture and cell lines
The GBM cell line T98G and the LGG cell line Hs683
were obtained from ATCC. The cells were cultured in high glucose
DMEM medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) at 37°C under an atmosphere of 5% CO2 and 95% air.
In addition, high glucose DMEM supplemented with 2% FBS was used in
the scratch testing. The medium (half of the total volume) was
changed every 3 days during the culture period.
Cell viability assay and colony
formation ability
Cell proliferation of T98G and Hs683 cells was
monitored by CCK-8 assay. The cells were plated in a 96-well plate
at a concentration of 1×106 cells/ml. The cells were
pretreated with various concentrations of CVB-D (0, 15, 30, 60, 120
and 240 µmol/l) for 24, 48 and 72 h. Almost all cells reduced cell
viability when treated with 240 µmol/l for 72 h, which was the
highest concentration and longest time-scale threshold in the
experiment. Each group included 6 replicates and was assessed in 3
different experiments. The 96-well plate was washed with PBS (0.01
M) and 90 µl of serum-free medium was mixed with 10 µl CCK-8
solution and added to the wells. The plate was incubated at 37°C
without light. Following incubation for 1–2 h, the absorbance was
monitored at 450 nm using a microplate reader (Tecan Group, Ltd.).
The colony formation ability of T98G and Hs683 cells was analyzed
following CVB-D treatment (0, 5, 10, 20, 40 and 80 µmol/l). It was
determined that few cells floated when treated with 80 µmol/l,
which the highest threshold concentration for 24 h, and had no
effect on colony formation results. The cells were plated in 6-well
plates with 500 cells/well. The cells were pretreated with various
concentrations of CVB-D (0, 5, 10, 20, 40 and 80 µmol/l) for 24 h.
Subsequently, the medium was changed in the culture for the next 10
days. Finally, the cells were permeabilized with 4%
polyformaldehyde (10 min at room temperature) and stained with
crystal violet (10 min, at room temperature, 0.05% w/v). The
software GraphPad Prism 8 (GraphPad Software, Inc.) was used to
perform statistical analysis of cell viability results.
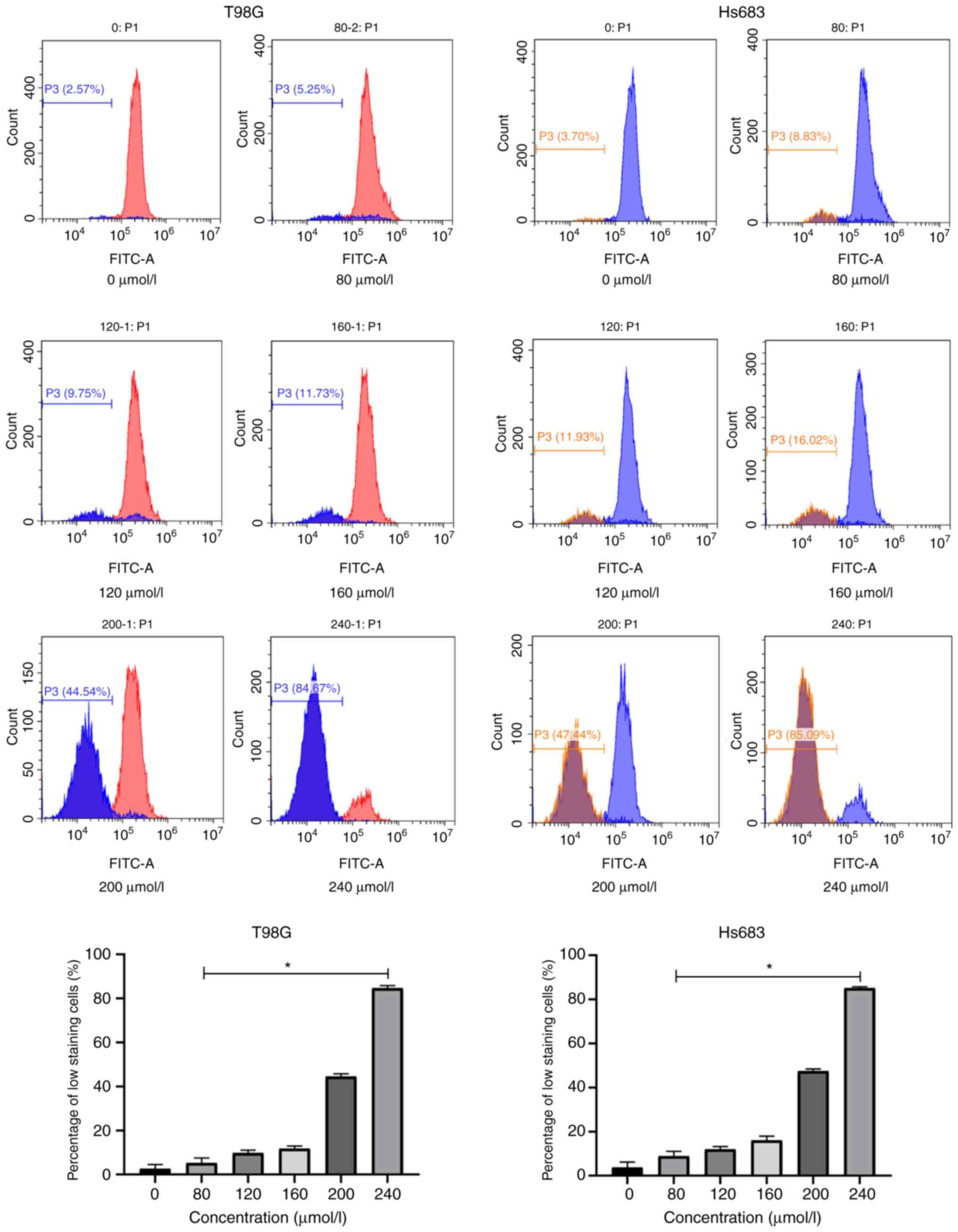
Capture of mitochondrial membrane
potential (MMP or ΔΨm)
MMP was assessed using fluorescent Rh123 dye using 6
different concentrations of CVB-D (0, 40, 80, 120, 160 and 200
µmol/l) for 6 h. Few cells floated and an evident trend was
captured when cells were treated with 200 µmol/l CVB-D for 6 h,
thus, this was determined as our highest concentration and
time-scale threshold for the Rh123 staining experiment. Rh123 was
added to the cultures according to the manufacturer's instructions.
The images were captured by a flow cytometry (Beckman Coulter). The
change in low staining of FITC+ cells corresponded to
the MMP change of the cells. The software GraphPad Prism 8 was used
to perform statistical analysis of the results.
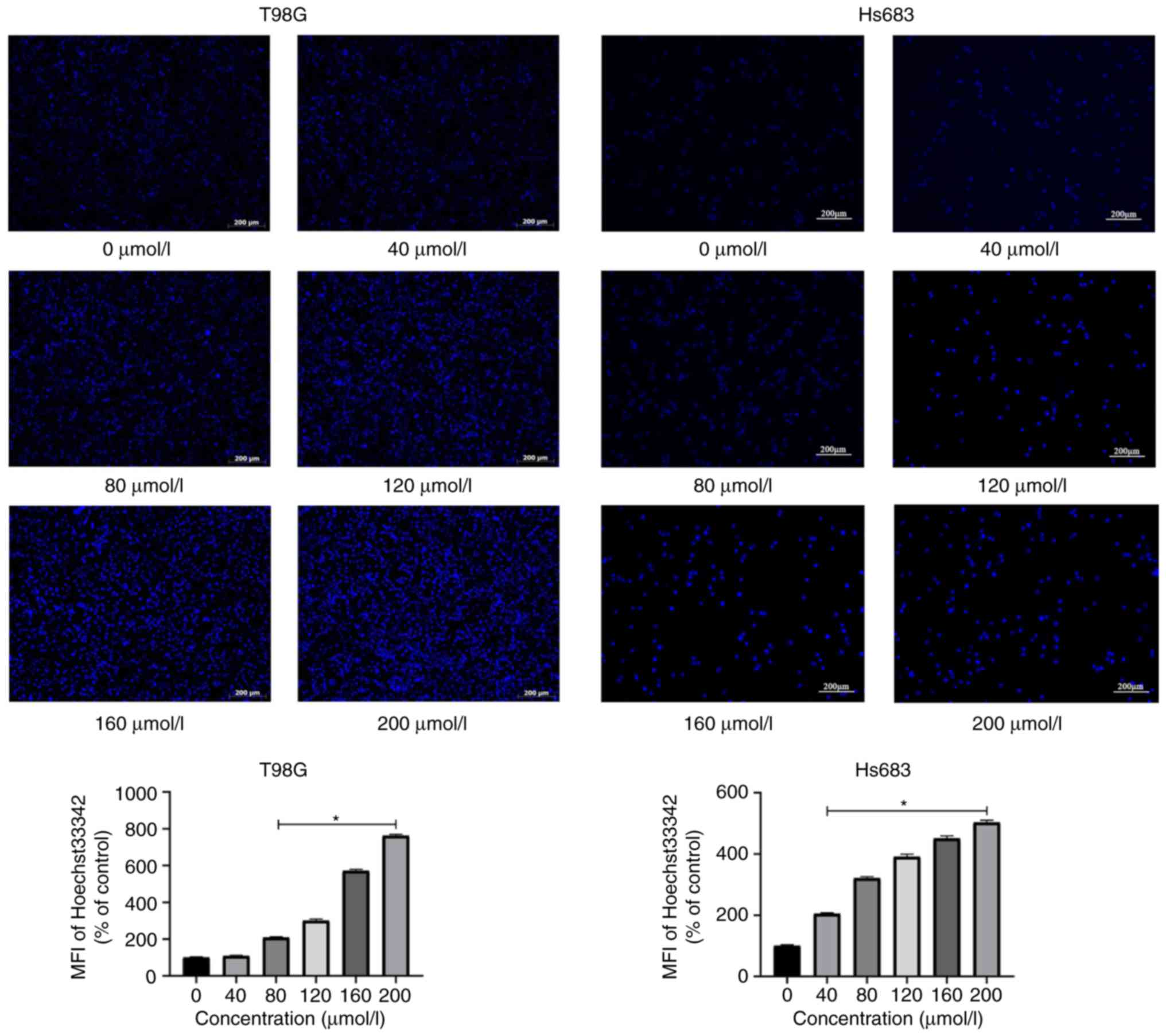
Hoechst staining
A total of 6 different concentrations CVB-D (0, 40,
80, 120, 160 and 200 µmol/l) were added to the cells in 6-well
plates for 6 h. Few cells floated and an evident trend was captured
when cells were treated with 200 µmol/l CVB-D for 6 h, thus, this
was determined as our highest concentration and time-scale
threshold for the Hoechst 33342 staining experiment. Hoechst 33342
was added to the cultures according to the manufacturer's
instructions. The images were captured by a fluorescent microscope
(Zeiss GmbH). The change in brightness corresponded to the
percentage of apoptotic cells. The software Image-Pro Plus 6.0
(Media Cybernetics, Inc.) was used to measure the mean fluorescence
intensity (MFI) of figures.
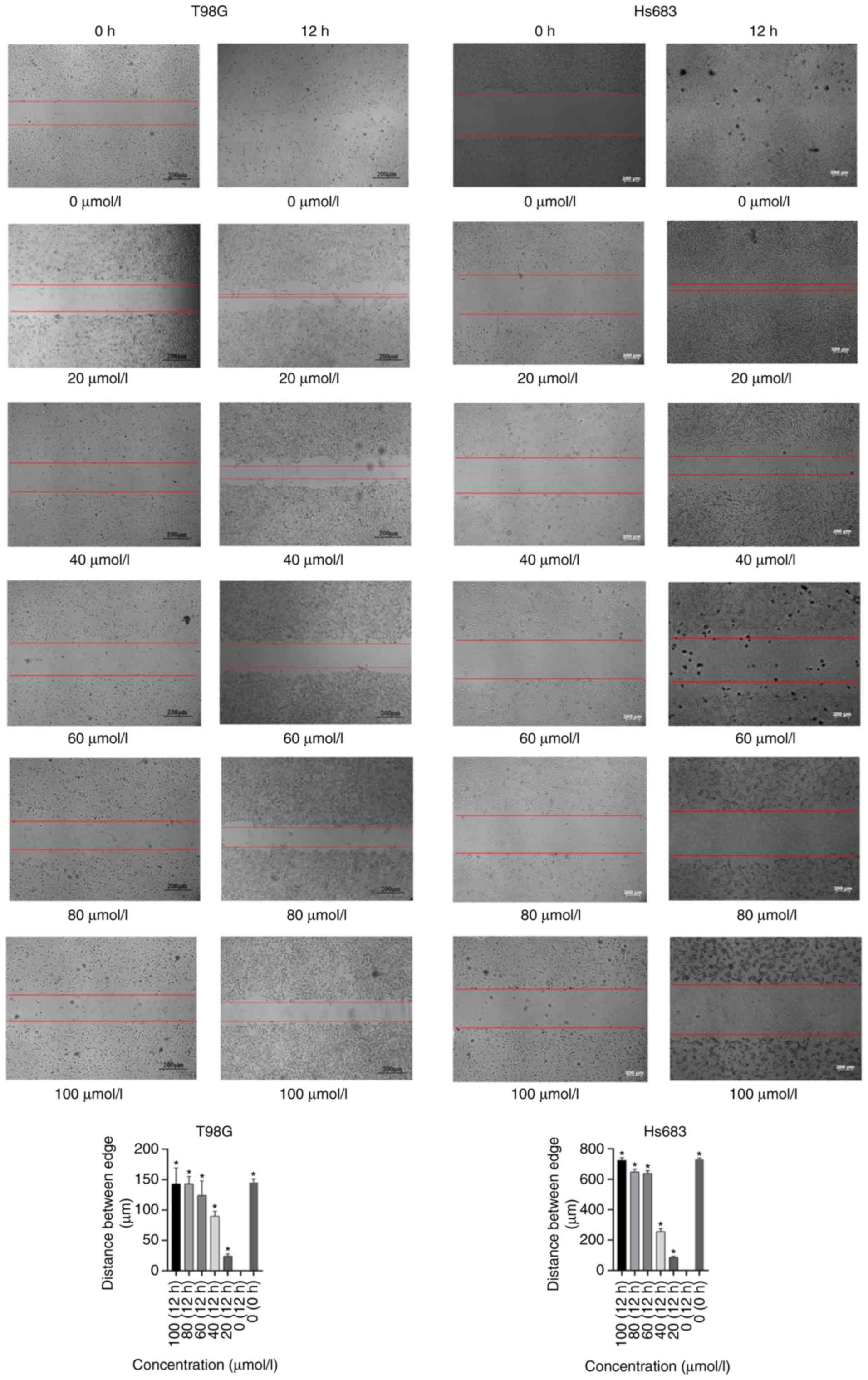
Scratch testing
A total of 6 different concentrations CVB-D (0, 20,
40, 60, 80 and 100 µmol/l) were added to the cells in a 6-well
plate with 2% FBS-DMEM culture medium for 12 h after the scratch
wound was made. Few cells float and an evident trend was captured
when cells were treated with 100 µmol/l CVB-D for 12 h, thus, this
was determined as the highest concentration and time-scale
threshold for the scratch experiment. A high concentration and a
long culture duration increase the number of cells floating which
affects the experimental results. Subsequently, the images were
captured by a bright field view of the microscope (Zeiss GmbH). The
distances between the the two edges of the scratched cells
corresponded to the migration of GBM and LGG cells. The software
ImageJ (version 1.50i; National Institutes of Health) was used to
do assess the distance between the edges.
Flow cytometric assessment for
apoptosis
T98G and Hs683 cells were seeded in 6-well plates
and pretreated with 0, 80, 120, 160, 200 and 240 µmol/l CVB-D for
24 h. The highest concentration and longest time-scale were
determined by cell viability experiment. Cells had <50%
viability when treated with 240 µmol/l CVB-D for 24 h and an
evident trend of apoptosis was captured. The induction of apoptosis
was assessed by Annexin V-FITC/PI apoptosis test. The cell cycle
analysis was performed by seeding cells in a 6-well plate and
pretreating them with 0, 80, 160 and 240 µmol/l CVB-D for 24 h. The
highest concentration and longest time-scale were determined by
cell viability experiment. Cell had <50% viability when treated
with 240 µmol/l CVB-D for 24 h and evident trend of the cell cycle
was captured. The two tests were carried out in accordance with the
manufacturer's instructions. The ModFit software (version 4.1) was
used for cell cycle analysis. The CytExpert software (version 2.0)
was used for detection of apoptosis and cell cycle progression. The
software GraphPad Prism 8 was used to perform statistical analysis
of the results.
Western blot analysis
All cells were subcultured in 6-well plates. The
cells were incubated initially for 24 h and subsequently treated
with 0, 50, 100 and 200 µmol/l CVB-D for an additional 24 h. Since
cell lysis had evident reduced protein collection when treated with
240 µmol/l CVB-D for 24 h, the highest concentration and longest
time-scale was determined as 200 µmol/l. The concentration ratio
2:1 was used for grouping and an obvious trend was captured in the
western blot experiment. The cells were subsequently washed with
ice-cold PBS and lysed in RIPA buffer with 1X PMSF (Beyotime
Institute of Biotechnology). The protein concentration was measured
with the BCA method. SDS-polyacrylamide gels (10%) were used for
electrophoresis. Following electrophoresis, the proteins were
transferred to PVDF membranes and blocked in 5% skimmed milk in
TBST for 1 h. The membranes were probed with primary antibodies for
β-actin, Bax, Bcl-2, caspase-3 and cleaved caspase-3 (all 1:1,000;
all from Cell Signaling Technology, Inc.) at 4°C overnight. The
membranes were subsequently washed and incubated with secondary
antibodies for 1 h [1:10,000, goat anti-mouse IgG (H+L) HRP,
product code ab21172; 1:10,000, goat anti-rabbit IgG (H+L) HRP,
product code ab44171] according to the source of primary
antibodies. The membranes were subsequently visualized by
chemiluminescent ECL reagent (EMD Millipore). The software ImageJ
(version 1.50i) was used for densitometric analysis of protein
expression and statistical analysis by SPSS 22 software (IBM
Corp.).
Statistical analysis
The unpaired Student's t-test was used to determine
the differences between the two groups. The data were presented as
the mean ± standard error of mean (SEM). One way ANOVA followed by
Bonferroni's post hoc test were used to perform multi-group data
comparisons using GraphPad Prism 8 and SPSS 22 software. P<0.05
was used to indicate a statistically significant difference.
Results
CVB-D reduces cell viability and
colony formation ability of GBM and LGG cells
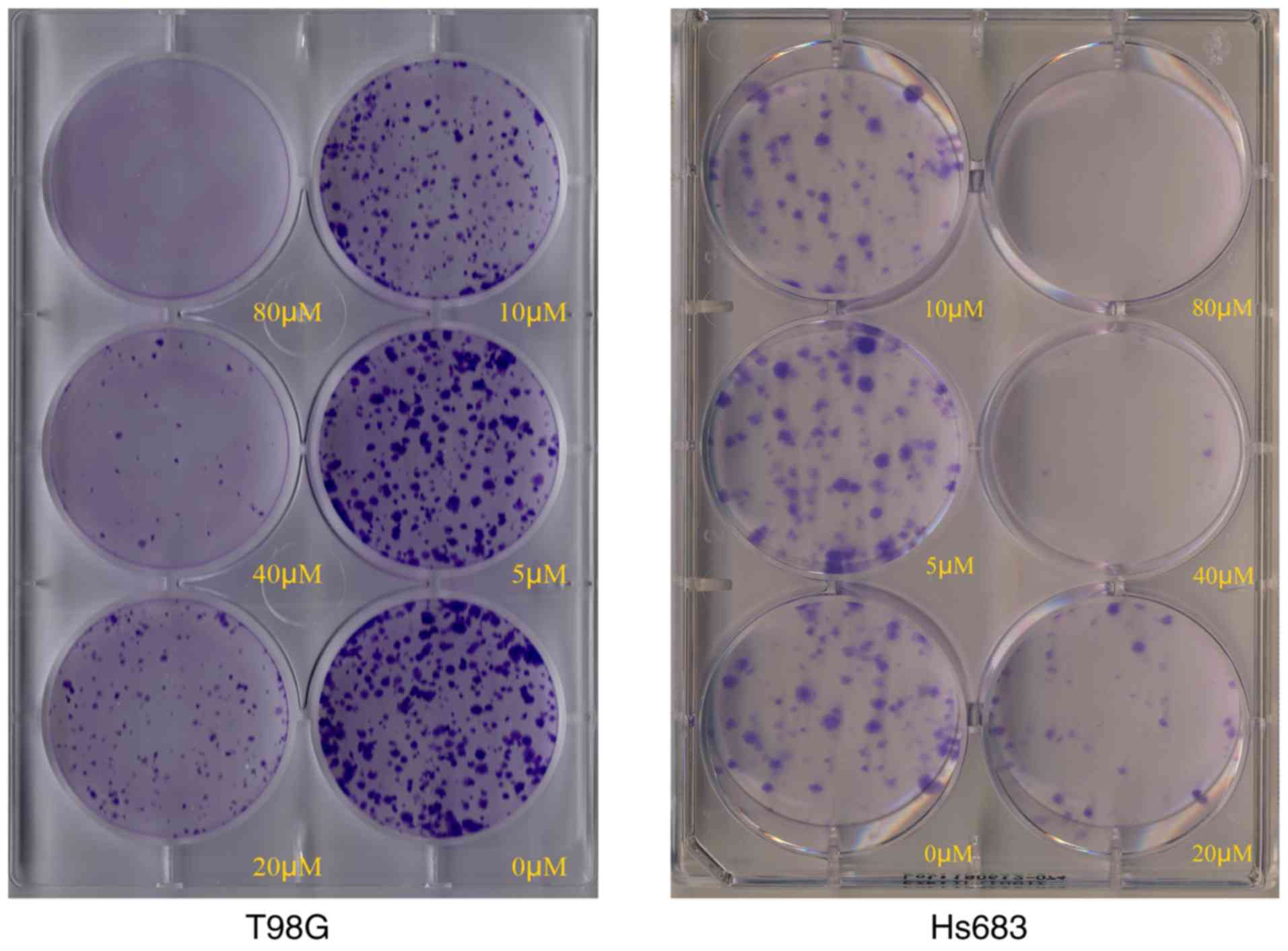
Crystal violet staining indicated that the number of
colonies of CVB-D-treated T98G and Hs683 cells were markedly
decreased compared with those noted in untreated cells (Fig. 2). Subsequently, various
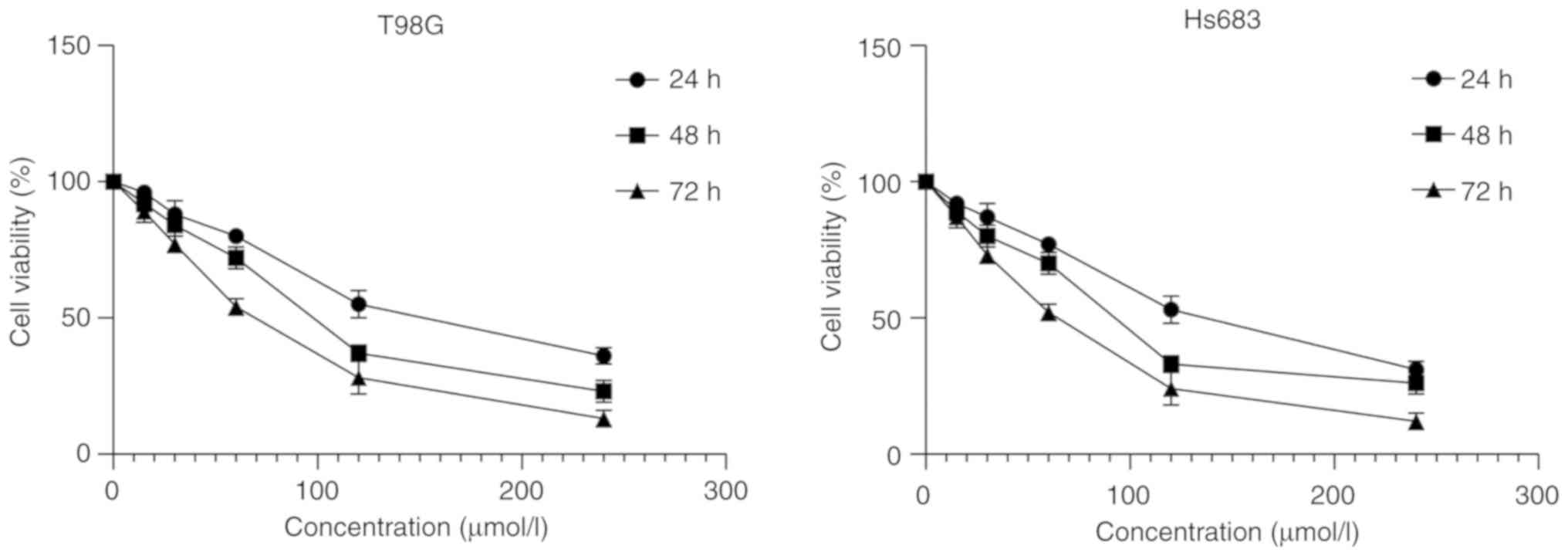
concentrations of CVB-D were used to treat the cells. Following
incubation with 0, 15, 30, 60, 120 and 240 µmol/l CVB-D for 24, 48
and 72 h, the viability of the cells was assessed using a CCK-8
kit. The results indicated that the cell viability was reduced in a
concentration- and time-dependent manner (Fig. 3). All dosing groups exhibited
statistical significant differences compared with the control group
(data not shown).
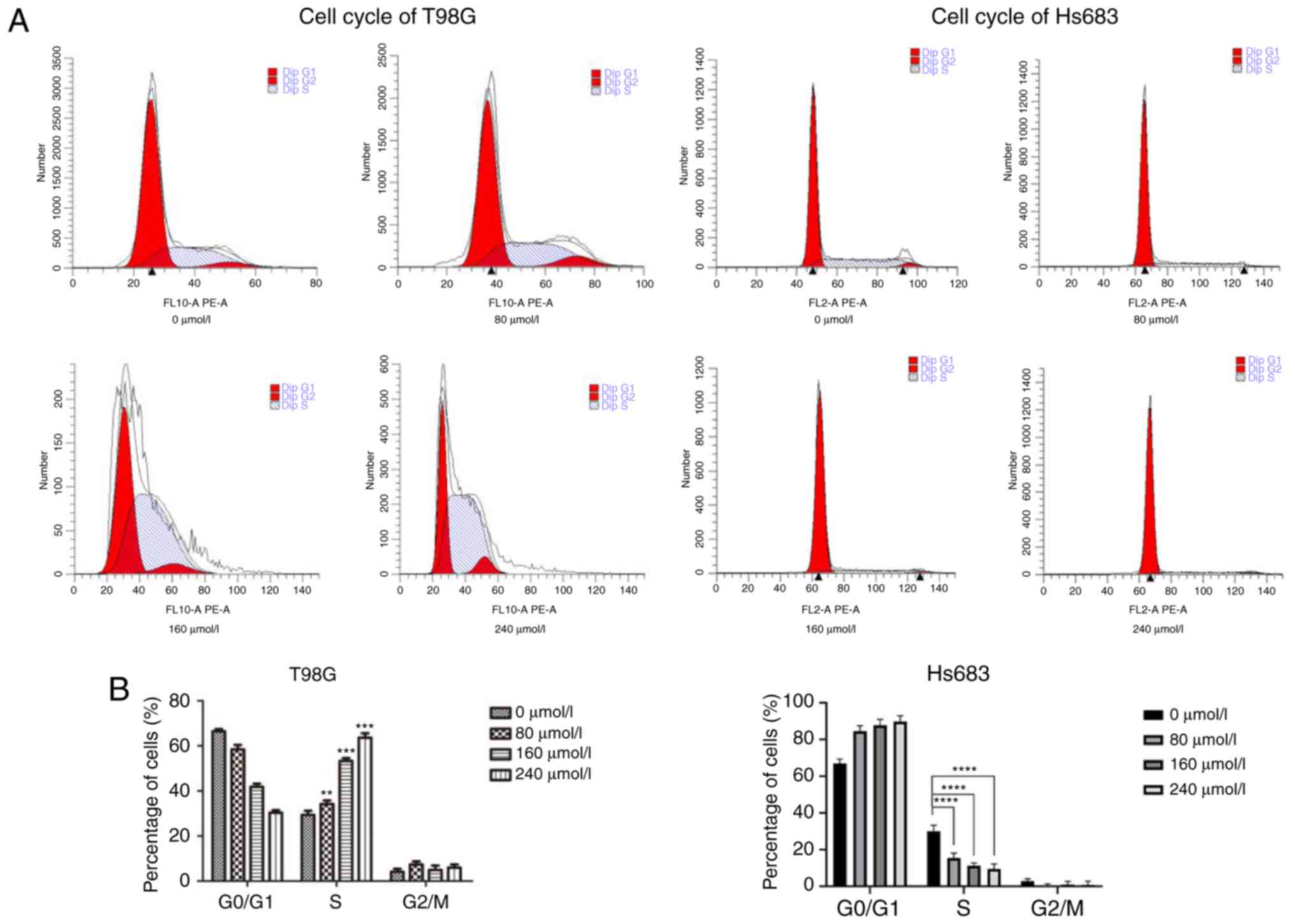
CVB-D arrests cells at the S and
G0/G1 phase
The cell cycle plays an important role in cancer
cell proliferation. Therefore, the cell cycle of CVB-D pretreated
GBM cells was assessed by flow cytometry (Fig. 4). Subsequently, treatment of GBM
cells with different concentrations of CVB-D arrested the cells at
the S phase. Concomitantly, the number of cells in the other two
populations was decreased. LGG cells were arrested at the
G0/G1 phase according to the concentration of
CVB-D used. In addition, the number of cells in the other two
populations were both decreased. These phenomena indicated that the
effects of CVB-D on the cell cycle were concentration-dependent,
which may be used as a strategy for tumor therapy.
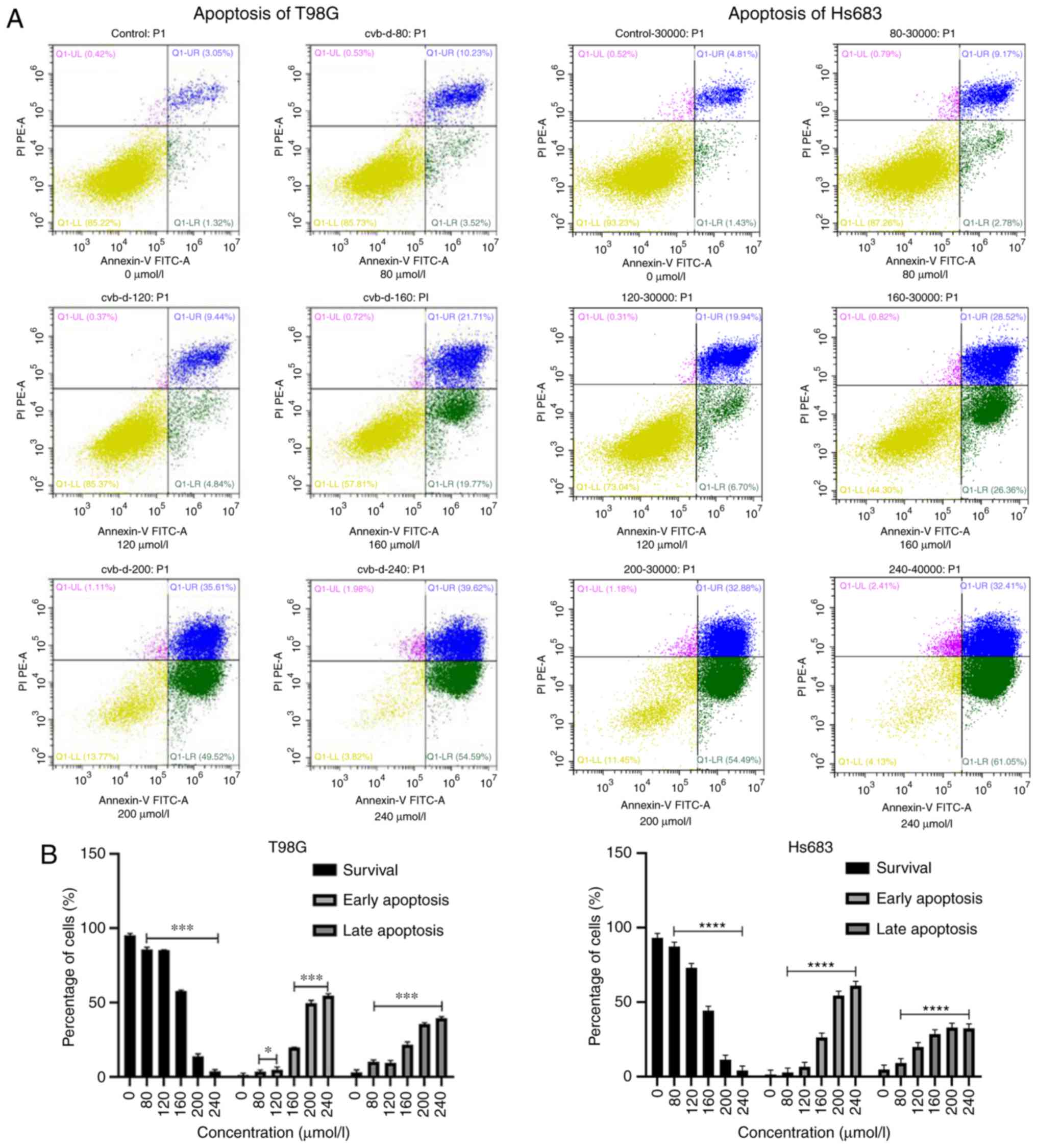
CVB-D induces cell apoptosis by a
mitochondrial-dependent pathway
CVB-D pretreated GBM and LGG cells were stained by
PI and Annexin V-FITC dyes. The results indicated that the
surviving cells were PI−/Annexin V−.
PI−/Annexin V+ and PI+/Annexin
V+ cells represented the two phases of cell apoptosis.
Early and late apoptotic cells were labeled as
PI−/Annexin V+ and PI+/Annexin
V+, respectively. The number of surviving cells was
decreased following an increase in the treatment of the cells by
CVB-D (Fig. 5). Subsequently,
Hoechst staining was used for the observation of the morphological
characteristics of the apoptotic GBM and LGG cells. Each Hoechst
staining group was monitored by fluorescence microscopy (Fig. 6). Concomitantly, Rh123 staining was
used to confirm the induction of cell apoptosis by the
mitochondrial-dependent pathway (Fig.
7).
CVB-D significantly inhibits tumor
cell migration
Initially, various concentrations of CVB-D (0, 20,
40, 60, 80 and 100 µmol/l) were used for 12 h pretreatment of the
cells and cell migration was assessed by light microscopy. The
results indicated that CVB-D significantly inhibited tumor cell
migration of GBM and LGG cells. Significant differences were noted
in all the CVB-D groups compared with the control samples (Fig. 8).
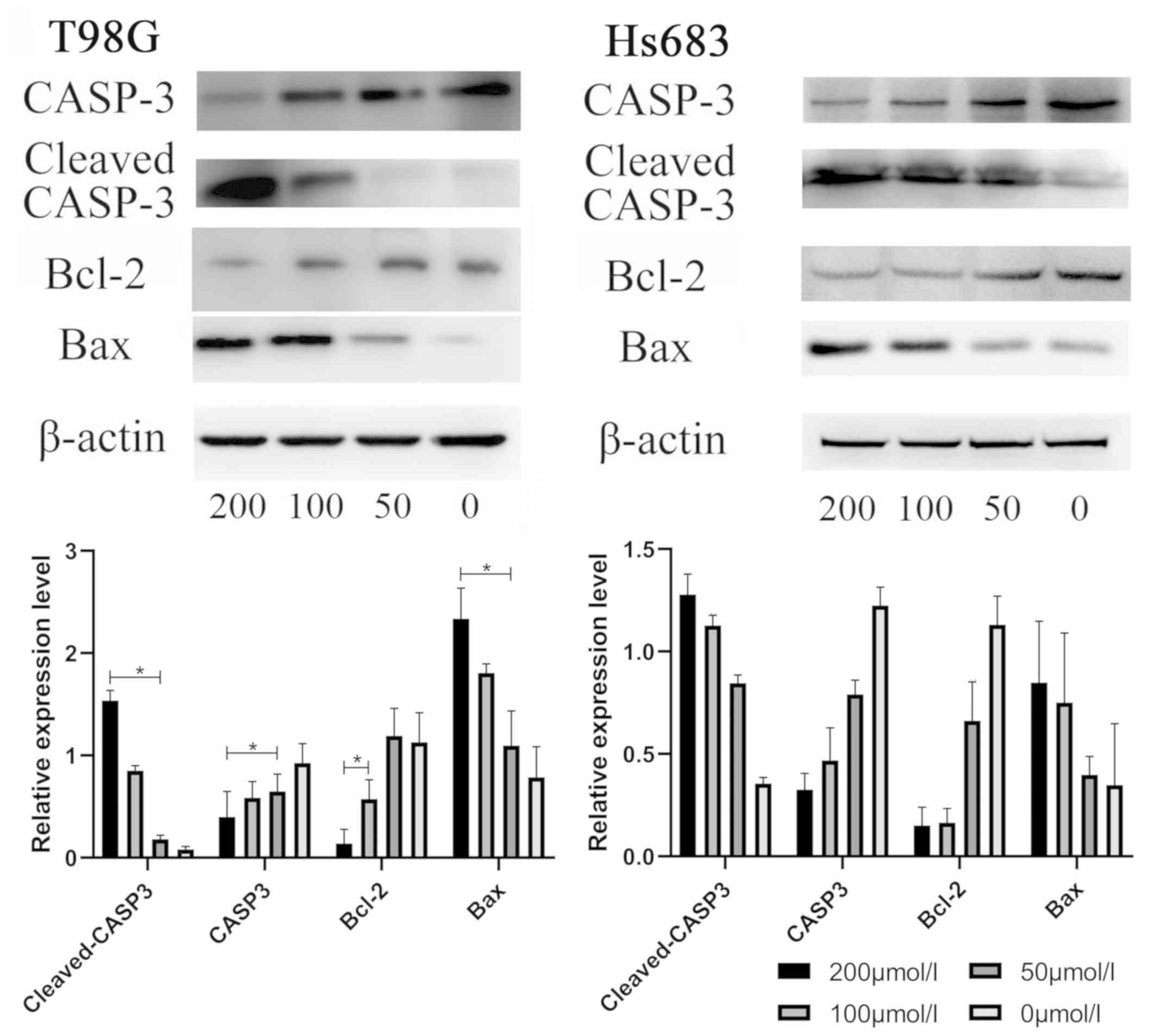
CVB-D induces the expression levels of
apoptosis-associated proteins in GBM and LGG cells
The expression levels of the proteins associated
with cell apoptosis were examined by western blotting. The results
indicated that the expression levels of Bax and cleaved caspase-3
were increased. In contrast to these findings, the expression
levels of caspase-3 and Bcl-2 were decreased in CVB-D-pretreated
T98G and Hs683 cells in a concentration-dependent manner. These
results indicated that the apoptotic process was regulated by
increased expression in the levels of the apoptosis-associated
proteins. The ratios of Bax/Bcl-2 and cleaved caspase-3 were
increased suggesting the induction of apoptosis in T98G and Hs683
cells (Fig. 9).
Discussion
Human brain glioblastoma is a malignant tumor, which
usually recurs from the surgical resection area and is
characterized by the tumor infiltration or the transfer of tumor
cells along the white matter fiber bundle. The main treatment
strategy involves the inhibition of glioblastoma cell migration.
Previous studies have revealed that its average incidence rate is
3.19/100,000 in the population, whereas the 5-year survival rate of
GBM patients is estimated to ~5% (4,15).
Conventional surgery and radiotherapy/chemotherapy do not
effectively prolong the survival of patients with GBM. These
patients do not often survive from postoperative tumor recurrence
and their median survival rate is estimated to ~15 months (15). Therefore, it is important to
identify and develop new effective strategies for the treatment of
GBM.
CVB-D is the main active component of the
traditional Chinese medicine Buxus microphylla, which has
been used to treat cardiovascular diseases (12). One of the main obstacles in the
treatment of brain tumors is the inability of the chemotherapeutic
drugs to cross the BBB. Therefore, although several novel drugs
have been successfully applied in the treatment of various types of
tumors their efficacy is considerably low in brain tumors, notably
in GBM and LGG. The antitumor effect of CVB-D has been investigated
in breast and gastric cancers (13,16).
However, it is uncertain whether it is effective in the treatment
of GBM and LGG. Previous studies have revealed that cell apoptosis
can be induced by a variety of drugs and physical and chemical
factors (17–19). Moreover, a previous study
demonstrated that CVD-B exhibited antitumor effects via the
Akt/mTOR pathway (13), whereas it
has also been revealed that the Akt/mTOR signaling pathway is
associated with various physiological processes including cell
growth, proliferation and apoptosis (20–22). A
recent study developed a novel drug delivery system for CVB-D,
which can in theory enhance the efficacy of CVB-D therapy (14).
CVB-D affects the expression of the family members
of cysteine-containing aspartate-specific proteases (caspases),
which contain several proteins that play a key role in the cellular
process of apoptosis. Caspase-3 is one of the most important
members of this protein family, which has been reported to be the
key executor of cell apoptosis. Upon activation of caspase enzymes
by the external apoptotic signals, the apoptotic cascade is
activated. Subsequently, the apoptosis-signaling pathway of the
cells is activated by the interaction of caspases with several
other proteases. In the present study, it was demonstrated that
CVB-D induced apoptosis via upregulation of apoptosis-associated
proteins. Future studies may address the investigation of the gene
expression profile of CVB-D-induced apoptotic cells.
The data reported in the present study revealed that
the GBM and LGG cell migration and cell cycle progression were
inhibited following treatment of the cells with a low concentration
of CVB-D. This strategy can be applied as a potential adjuvant
therapy in GBM or LGG patients that are treated with
temozolamide.
In addition, CVB-D could regulate calcium levels
inside the cells (23). Previous
studies have suggested that calcium levels play an important role
in tumor growth, apoptosis and differentiation (24–26).
The hypothesis that CVB-D can promote tumor apoptosis by affecting
calcium ion channels can be further explored in future studies and
can aid the investigation of the antitumor activity of this
compound.
The present study, to the best of our knowledge, is
the first to reveal the antitumor activity of CVB-D in human GBM
and LGG. The results demonstrated that CVB-D inhibited cell
proliferation and tumor migration, while it induced apoptosis via
the mitochondrial-dependent pathway. CVB-D is a main component of
the traditional Chinese medicine Buxus microphylla and
exhibits potent antitumor effects against GBM and LGG. This
evidence can offer insight in the treatment of this type of
disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant. no. 31360258); Special
fund for science and technology development of Guangdong Province
(no. 2016A020215036); natural Science Foundation of Guangdong
Province (nos. 2015A030313077, 2015A030313047 and 2017A030310192);
project of Educational Commission of Guangdong Province
(2018GkQNCX085); Science and Technology Program of Jiangmen
(2019E021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, KG and JX designed the study. LZ performed the
experiments and HT wrote the manuscript. HT, FW, SO, TW, YF helped
to performed the experiments and collected the data, HT and FW
participated in the statistical analysis. All authors read and
approved the final manuscript. All authors have read and approved
the final manuscript and agree to be accountable for all aspects of
the research in ensuring that the accuracy or integrity of any part
of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Imperato JP, Paleologos NA and Vick NA:
Effects of treatment on long-term survivors with malignant
astrocytomas. Ann Neurol. 28:818–822. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ashok AR, Pouratian N, Sherman J, Ahmed G
and Shaffrey ME: Advances in brain tumor surgery. Neurol Clin.
25:975–1003. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shan P, Mao RB, Xu JM and Li JX: The
beneficial effects of cyclovirobuxine D (CVBD) in coronary heart
disease. A double blind analysis of 110 cases. J Tradit Chin Med.
4:15–19. 1984.PubMed/NCBI
|
|
5
|
Grossini E, Battaqlia A, Brunelleschi S,
Mary DA, Molinari C, Viano I and Vacca G: Coronary effects of
cyclovirobuxine D in anesthetized pigs and in isolated porcine
coronary arteries. Life Sci. 65:59–65. 1999. View Article : Google Scholar
|
|
6
|
Yu B, Fang TH, Lü GH, Xu HQ and Lu JF:
Beneficial effect of cyclovirobuxine D on heart failure rats
following myocardial infarction. Fitoterapia. 82:868–877. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grossini E, Avanzi G, Gallicchio M,
Molinari C, Vacca G and Bellomo G: Regulation of Ca2+
Movements by cyclovirobuxine D in ECV304 endothelial cells.
Pharmacol Res. 52:154–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen QW, Shan HL, Sun HL, Wang H and Yang
BF: Effects of cyclovirobuxine D on intracellular Ca2+
and L-type Ca2+ current in rat ventricular
cardiomyocytes. Yao Xue Xue Bao. 39:500–503. 2004.(In Chinese).
PubMed/NCBI
|
|
9
|
Hu D, Liu X, Wang Y and Chen S:
Cyclovirobuxine D ameliorates acute myocardial ischemia by
K(ATP)channel opening, nitric oxide release and anti-thrombosis.
Eur J Pharmacol. 569:103–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yue Y, Liu R, Liu J, Dong Q and Fan J:
Experimental and theoretical investigation on the interaction
between cyclovirobuxine D and human serum albumin. Spectrochim Acta
A Mol. Biomol. Spectrosc. 128:552–558. 2014.
|
|
11
|
Guo Q, Guo J, Yang R, Peng H, Zhao J, Li L
and Peng S: Cyclovirobuxine D attenuates doxorubicin-induced
cardiomyopathy by suppression of oxidative damage and mitochondrial
biogenesis impairment. Oxid Med Cell Longev. 2015:1519722015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Sun D, Gao S, Gao Y, Ye J and Liu P:
Cyclovirobuxine D induces autophagy-associated cell death via the
Akt/mTOR pathway in MCF-7 human breast cancer cells. J Pharmacol
Sci. 125:74–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burri SH, Gondi V, Brown PD and Mehta MP:
The evolving role of tumor treating fields in managing
glioblastoma: Guide for Oncologists. Am J Clin Oncol. 41:191–196.
2018.PubMed/NCBI
|
|
14
|
Thakkar JP, Dolecek TA, Horbinski C,
Ostrom QT, Lightner DD, Barnholtz-Sloan JS and Villano JL:
Epidemiologic and molecular prognostic review of glioblastoma.
Cancer Epidemiol Biomarkers Prev. 23:1985–1996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Tan Z, Chen J and Dong C:
Cyclovirobuxine D inhibits cell proliferation and induces
mitochondria-mediated apoptosis in human gastric cancer cells.
Molecules. 20:20659–20668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao W, Li X, Wang J, Wang C, Jia Y, Yuan
S, Huang Y, Shi Y and Tong Z: Decreasing eukaryotic initiation
factor 3C (EIF3C) suppresses proliferation and stimulates apoptosis
in breast cancer cell lines through mammalian target of rapamycin
(mTOR) pathway. Med Sci Monit. 23:4182–4191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin J, Feng J, Yang H, Yan Z, Li Q, Wei L,
Lai Z, Jin Y and Peng J: Scutellaria barbata D. Don inhibits
5-fluorouracil resistance in colorectal cancer by regulating
PI3K/AKT pathway. Oncol Rep. 38:2293–2300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan J, Shen W, Shi W, Chen X, Sun D, Xu C,
Yan Q, Cheng H, Lai Y and Ji H: ONTD induces growth arrest and
apoptosis of human hepatoma Bel-7402 cells though a peroxisome
proliferator-activated receptor γ-dependent pathway. Toxicol In
Vitro. 45:44–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S, Fisher RC, Sign S, Molina LA,
Shenoy AK, Lopez MC, Baker HV, Koomen JM, Chen Y, Gittleman H, et
al: Inhibition of PI3K/Akt/mTOR signaling in PI3KR2-overexpressing
colon cancer stem cells reduces tumor growth due to apoptosis.
Oncotarget. 8:50476–50488. 2016.PubMed/NCBI
|
|
20
|
Amini-Farsani Z, Sangtarash MH, Shamsara M
and Teimori H: MiR-221/222 promote chemoresistance to cisplatin in
ovarian cancer cells by targeting PTEN/PI3K/AKT signaling pathway.
Cytotechnology. 70:203–213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zahed Panah M, Nikbakht M, Sajjadi SM,
Rostami S, Norooznezhad AH, Kamranzadeh Fumani H, Ghavamzadeh A and
Mohammadi S: Anti-apoptotic effects of osteopontin via the
up-regulation of AKT/mTOR/β-catenin loop in acute myeloid leukemia
cells. Int J Hematol Oncol Stem Cell Res. 11:148–157.
2017.PubMed/NCBI
|
|
22
|
Yu B, Ruan M, Zhou L, Xu L and Fang T:
Influence of cyclovirobuxine D on intracellular [Ca2+]
regulation and the expression of the calcium cycling proteins in
rat myocytes. Fitoterapia. 83:1653–1665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen YF, Chen YT, Chiu WT and Shen MR:
Remodeling of calcium signaling in tumor progression. J Biomed Sci.
20:232013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stewart TA, Yapa KT and Monteith GR:
Altered calcium signaling in cancer cells. Biochim Biophys Acta.
1848:2502–2511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Macià A, Herreros J, Martí RM and Cantí C:
Calcium channel expression and applicability as targeted therapies
in melanoma. Biomed Res Int. 2015:5871352015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei H, Liu T, Jiang N, Zhou K, Yang K,
Ning W and Yu Y: A novel delivery system of Cyclovirobuxine D for
brain targeting: Angiopep-conjugated polysorbate 80-coated
liposomes via intranasal administration. J Biomed Nanotechnol.
14:1252–1262. 2018. View Article : Google Scholar : PubMed/NCBI
|