Introduction
As one of the major cancers, renal cancer has a high
incidence and mortality rate of approximately 273,518 and 116,368
worldwide, 32,508 and 10,675 in China, and 65,150 and 13,680 in the
US, respectively (1). Renal cell
carcinoma (RCC) accounts for 90% of all renal tumors, of which 75%
are clear cell RCC (ccRCC) and 25% are non-clear cell carcinomas
comprising papillary RCC, chromophobe RCC and oncocytoma RCC
(2). Although the 5-year survival
rate of local RCC patients is as high as 65 to 93% and as high as
47 to 77% in stage 1 and stage 2 patients, respectively,
approximately 25–30% of patients with advanced disease have a poor
prognosis (i.e., 5-year survival rates ranging from 34 to 80% and
from 2 to 20% in patients with stage 3 and stage 4, respectively)
(3,4). Several molecularly targeted drugs,
including sunitinib, sorafenib and temsirolimus, which mainly
target the vascular endothelial growth factor (VEGF) and mammalian
target of rapamycin (mTOR) signaling pathways aberrantly activated
due to a deficiency in the tumor-suppressor gene von Hippel-Lindau
(VHL) in most cases of ccRCC, were recently developed to
treat advanced renal cancer (5).
Although there has been a significant increase in treatment
regimens for advanced RCC, a sustained complete response is
infrequent (6).
Anaplastic lymphoma kinase (ALK) is a receptor
tyrosine kinase that was first discovered as a fusion gene of
nucleophosmin (NPM1) in anaplastic large-cell lymphoma
(ALCL) (7). Since then, various
ALK fusion genes mediated by translocation have been
identified in multiple malignancies, including inflammatory
myofibroblastic tumor (IMT), non-small cell lung cancer (NSCLC) and
ovarian cancer (8–10). In the scope of the kinase domain,
activating mutations with ALK have also been identified in
neuroblastoma (11–14) and anaplastic thyroid cancer
(15). In addition, amplification
of the ALK gene has been discovered in neuroblastoma,
inflammatory breast cancer (16),
and esophageal cancer (17).
Although these ALKomas appear in various organs, they share
activated ALK with the activity of ALK kinase, which is needed for
tumor maintenance (18). Therefore,
an aberration in ALK could be used therapeutically as an ‘Achilles
heel’ for tumors. Indeed, it has been reported that there is
significant clinical efficacy with ALK inhibitors for NSCLC, ALCL
and IMT with ALK fusions (19–22),
including crizotinib and ceritinib, ALK-targeting small-molecule
tyrosine kinase inhibitors (TKIs). The above have already been
approved by the FDA and can be useful for treating NSCLCs positive
for ALK rearrangement (23,24). The above findings illustrate that an
ALK fusion associated with an oncogene would be one of the most
hopeful targets in cancer therapy.
Regarding renal cancer, the ALK fusion gene
has also been found in renal medullary carcinoma (RMC) with sickle
cell traits and RCC of the unclassified and papillary subtypes
(4,25–30);
specifically, the VCL-ALK fusion gene was found in 3
patients with RMC. RMC mostly affects young individuals and is
associated with poor outcomes, but the finding of VCL-ALK
has enhanced the possibility of an effective treatment for patients
with an ALK inhibitor. Moreover, the TPM3-ALK or
EML4-ALK (E2; A20 variant 5a) fusion has also been detected
in a single case each of RCC (4).
Despite sporadic reports in renal cancer, the
presence of an ALK fusion has not been found in ccRCC. Herein, we
screened 87 patients with ccRCC by immunohistochemistry using a
newly developed highly sensitive anti-ALK antibody and detected 4
patients positive for the ALK protein, among which 2 patients were
further confirmed as having the EML4-ALK fusion gene by
RT-PCR and FISH.
Materials and methods
Tissues
We examined 24 and 63 renal tumor tissues from ccRCC
patients who had undergone surgery at the General Hospital of the
Chinese PLA, Beijing between April 2008 and July 2010 and Changhai
Hospital affiliated with the Second Military Medical University,
Shanghai between May 2008 and December 2010, respectively. The
demographic information of the patients is documented in Table I. Surgically removed tumor specimens
were fixed in 10% neutralized formalin and embedded in paraffin for
routine histopathological examination. For the 4 cases positive for
anti-ALK immunohistochemistry, total RNA was extracted from the
corresponding snap-frozen specimen, and purified using RNeasy Mini
kit (Qiagen, China). Our study was approval by the Ethics Committee
of the General Hospital of the Chinese PLA and Changhai Hospital
affiliated to the Second Military Medical University. Frozen normal
adult human renal tissues were purchased from Outdo Biotech Co.,
Ltd., the use of which did not require Institutional Review Board
(IRB) approval.
 | Table I.Clinicopathological characteristics
of the patients with ccRCC in the present study. |
Table I.
Clinicopathological characteristics
of the patients with ccRCC in the present study.
| Variable/group | Data |
|---|
| Age (years) |
|
|
Median | 56 |
|
Range | 28–79 |
| Sex, n (%) |
|
|
Male | 62 (71.2) |
|
Female | 25 (28.8) |
| Stagea, n (%) |
|
| I | 50 (57.5) |
| II | 22 (25.3) |
|
III | 10 (11.5) |
| IV | 5 (5.7) |
| Total | 87 (100) |
Cell culture
The human renal proximal tubal cell (PTC) line HK2
was obtained from the Cell Collection of the Chinese Academy of
Medical Sciences (Beijing, China). The H2228 lung cancer cell line
with confirmed EML4-ALK rearrangement (9) was purchased from ATCC. Cells were
cultured in complete DMEM (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% foetal bovine serum (FBS) and 1%
penicillin/streptomycin/amphotericin B. The culture was carried out
in a humidified 5% CO2 environment at 37°C. The cells
were washed with phosphate-buffered saline (PBS), detached with
0.25% trypsin/0.2% EDTA, and plated at 30,000-40,000
cells/cm2 when the cells reached 70–80% confluence. The
culture medium was changed one time every 2 days.
Immunohistochemistry
Immunohistochemical staining was conducted on
formalin-fixed, paraffin-embedded (FFPE) tissue sections (4-µm
thick) as previously described (31). Briefly, the slides were heated for 3
min in a pressure cooker (100°C) for antigen retrieval at a
concentration of 0.01 mol/l Tris-EDTA buffer at pH 8.0 after
deparaffinization and rehydration. Under room temperature
conditions, all further steps were conducted in a hydrated chamber.
Endogenous peroxidase activity was quenched with 3% hydrogen
peroxide for 30 min, and for further study, our precultured slides
were incubated with 20% normal goat serum at the concentration of
50 mmol/l Tris-HCl at pH 7.4. A rabbit monoclonal anti-human ALK
antibody from clone D5F3 (cat. #3633; Cell Signaling Technology)
was added at a 1:250 dilution in Dako diluent and incubated for 12
h. Next, the slides were washed with Tris-HCl and visualized with a
horseradish peroxidase-conjugated anti-rabbit EnVision+ Kit (Dako).
The sections were then counterstained by using haematoxylin and
mounted. ALK-rearranged NSCLC patients served as positive controls
and were contained in each staining batch. For the blank control,
the same incubation steps were performed, except IgG serum was
substituted for the primary antibody (ALK). For CAIX, ABC, CD10,
c-Kit, p53, p16, and Ki-67 staining, primary antibodies were
anti-ABC clone W6/32 (cat. no. M073601-2; Dako), anti-p53 DO-7
(cat. no. IS61630-2; Dako), anti-CD10 clone 56C6 (cat. no.
IS64830-2; Dako), anti-Ki67 clone MIB-1 (cat. no. IS62630-2; Dako)
and rabbit polyclonal antibody against human c-Kit (cat. no.
A450229-2; Dako), and anti-CAIX clone 2D3 (cat. no. ab107257;
Abcam), and anti-p16 clone EPR1473 (cat. no. ab108349; Abcam).
Deparaffined sections were incubated with primary antibodies at a
1:20 dilution for ABC and p53, 1:100 dilution for CD10 and p16, and
1:80 dilution for c-Kit, CAIX and Ki-67, at 4°C, overnight. After
washing in PBS, the sections were visualized and counterstained as
described above. PBS substituted for the primary antibody was used
as the negative control.
ALK fusion analysis by rapid
amplification of cDNA ends (RACE)-coupled PCR sequencing
We adopted the method of RACE-coupled PCR sequencing
to detect the fusion partner of ALK as described previously
(32). Briefly, an ALK
gene-specific primer was applied to reverse transcribe RNAs into
cDNAs. Next, cDNAs were purified and further subjected to
polycytidine (poly-C) tailing. Two PCRs were carried out to amplify
the target cDNA fragments spanning exon 20 of ALK and its upstream
sequence, which may include the transcript sequence of any gene
fused to ALK. Then, the resultant PCR products were purified and
sequenced using the M13 sequencing primer. Target sequences of
interest were aligned with the ALK reference sequence (NM_004304.3)
to confirm its fusion with another gene.
RT-PCR and sequencing
Reverse transcription polymerase chain reaction
(RT-PCR) was carried out with total RNA extracted from frozen tumor
tissues by the use of TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Reverse transcription was conducted with a
RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher
Scientific, Inc.). Simultaneously, PCR was carried out with primers
specific to EML4-ALK (EML4 exon 2-ALK exon 20) and GAPDH, as
follows: Forward, 5′-TGTGCTCTGAACAGGACGAACT-3′ and reverse,
5′-GCCTCCACTGGTGACAAACTC-3′ (1,399 bp for EML4-ALK variant 1 and
575 bp for EML4-ALK variant 3); and forward,
5′-CCATGTTCGTCATGGGTGTGAACCA-3′ and reverse,
5′-GCCAGTAGAGGCAGGGATGATGTTC-3′ (250 bp). Direct sequencing was
carried out on the PCR products by the use of forward and reverse
PCR primers.
Fluorescence in situ
hybridization
Fluorescence in situ hybridization (FISH)
analysis was conducted on 4-µm thick FFPE tissue sections of 2
patients with ccRCC positive for ALK expression as determined by
IHC using the Vysis LSI ALK dual colour break-apart probe (Abbott
Molecular). Next, hybridized slides were stained with
4′,6-diamidino-2-phenylindole and measured on an FV1000 confocal
laser scanning microscope at ×400 magnification (Olympus, Tokyo,
Japan).
EML4-ALK expression in HK2 cells
The retroviral vector pMXS expressing the
bicistronic EML4-ALK fusion gene and mouse CD8 was the gift of Dr
Hiroyuki Mano of Jichi Medical University (9). The retrovirus was prepared by
transiently cotransfecting three plasmids (pMXS or pMXS-EML4-ALK,
psPAX2, and pMD2.G), as previously described (31). The resultant virus was used to
infect HK2 cells, and then cells stably expressing the control or
the EML4-ALK fusion gene were obtained by flow cytometry by using a
mouse CD8 marker. EML4-ALK expression was confirmed by western
blotting with an ALK-specific antibody (D5F3).
Colony formation assays, proliferation
assays and Annexin V/PI staining
All assays were performed as previously described
(31). Briefly, cells were plated
in triplicate wells of 6-well dishes at a low density (150
cells/well) and cultured under normal conditions without
perturbation. After 10 days, the colonies were washed with PBS and
stained with crystal violet (0.5% w/v in 25% methanol). Stained
plates were rinsed in ddH2O and allowed to dry at room
temperature. The plates were photographed, and colonies were
counted using ImageJ 1.8.0 software (NIH; National Institutes of
Health, Bethesda, MD, USA). For the proliferation assay, cells
(3×103 per well) were cultured in 6-well plates in
medium containing complete supplements. Cell proliferation was
detected on a hemocytometer by cell counting every 3 days. For
Annexin V/PI staining, cells (1×105 per well) were
cultured in 6-well plates with or without serum for 24 h and
subjected to Annexin V/PI staining on the basis of the
manufacturer's instructions (Vazyme, Nanjing, China) and
immediately analyzed by flow cytometry.
Xenograft model in nude mice
Animal care and protocols were reviewed and approved
by the Institutional Animal Care and Use Committee of the General
Hospital of the Chinese PLA. Forty 5–6 week-old female BALB/c nude
mice with initial weight of ~20 g were obtained from Weitonglihua
Biotechnology (Beijing, China) and maintained on a 12-h light-dark
cycle in a temperature-controlled high barrier facility with free
access to food and water and treated under specific pathogen-free
conditions at the Animal Centre of the General Hospital of the
Chinese PLA. The xenograft model was established by subcutaneously
injecting HK2 cells stably expressing control or EML4-ALK
(3×106 cells per mouse) into the right flanks of mice.
When the xenograft reached 100–150 mm3 (control HK2) or
250–350 mm3 (EML4-ALK HK2), animals were randomly
separated into two groups (5 mice per group) and intraperitoneally
injected with vehicle (DMSO; from Sigma-Aldrich; Merck KGaA) or
crizotinib (250 mg/kg reconstituted in DMSO; from Selleck) twice
weekly for 2 weeks. Mice were weekly monitored for tumou growth by
using a calliper for 30 days, and then euthanized by cervical
dislocation with tumor harvested for imaging when they seemed
moribund or their tumors reached 15 mm in diameter. Tumor volume
(V) was calculated according to the following formula: V
(mm3) = length × width2/2.
Statistical analysis
Statistical analyses were carried out with GraphPad
Prism (version 5.04, GraphPad Software, Inc.). The results are
presented as the mean ± SD obtained from at least three independent
experiments. Differences in groups were determined by two-way ANOVA
with multiple comparisons and Bonferroni correction or two-tailed
paired Student's t-test with P<0.05 considered to indicate a
statistically significant result.
Results
Detection of ALK protein expression in
ccRCC tumor samples by IHC
We screened ALK protein expression in the FFPE
specimens of 87 pathologically defined ccRCCs by IHC with a highly
sensitive rabbit anti-human ALK monoclonal antibody, D5F3, which
was reported to possess 100% sensitivity and 99% specificity in the
measurement of ALK protein expression from NSCLC tumor samples in a
previous study (33). We first
optimized standard immunohistochemical staining using known
ALK-rearranged and ALK-germline NSCLC tumor samples, and each case
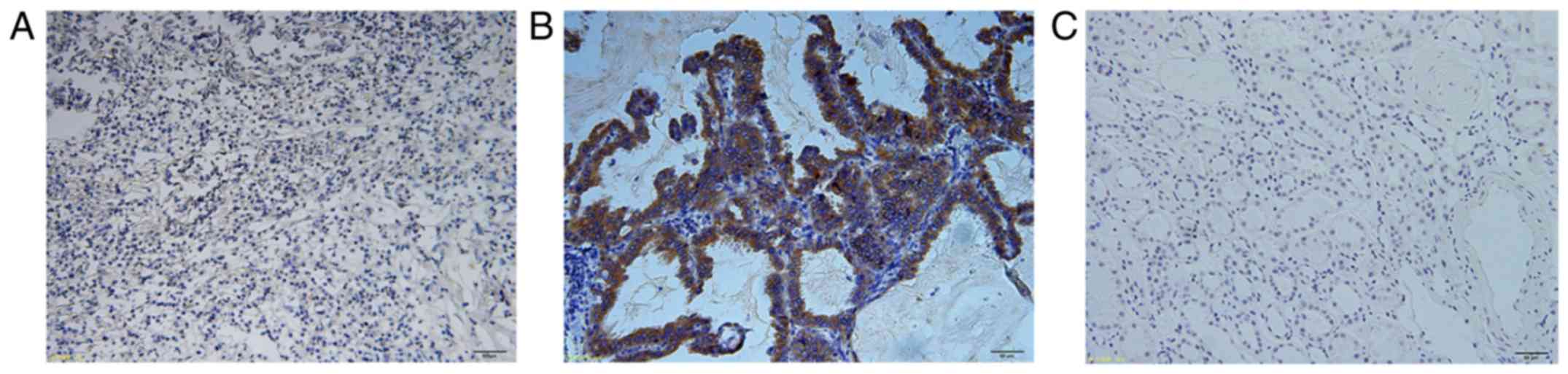
was confirmed by FISH. As shown in Fig.
1A and B, the D5F3 antibody displayed robust tumor-specific
staining in ALK-rearranged tumors and essentially no tissue
staining in ALK-germline tumors using a wide scope of antibody
dilutions and antigen retrieval methods (data not shown). There is
also a lack of staining in normal adult human renal tissue which is
known to not express ALK (18),
further validating the staining specificity of this antibody
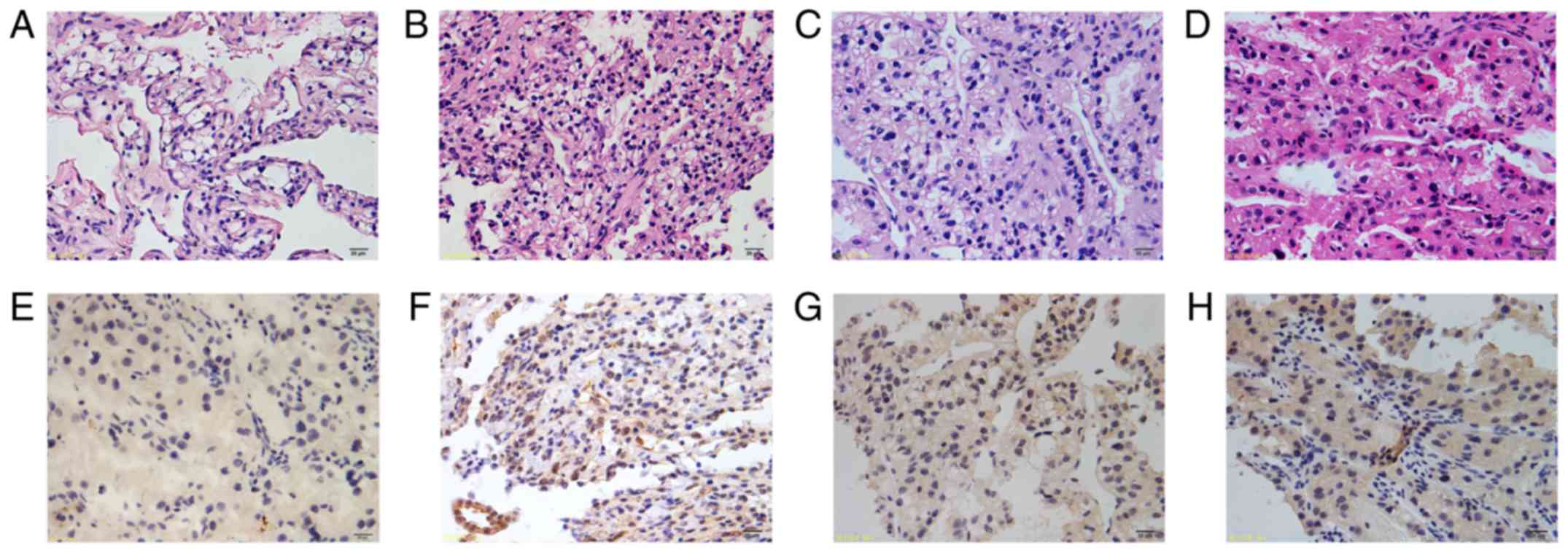
(Fig. 1C). Then, ccRCC tissue
sections were immunostained for ALK expression using the same
protocol, and 4 positive samples with middle-level staining were
detected (sample 416, Fig. 2A and
E; sample 413, Fig. 2B and F;
sample 405, Fig. 2C and G; sample
384 Fig. 2D and H).
Identification of the 5′ ALK fusion
partner and EML4-ALK expression
We performed 5′-RACE assays to determine whether
samples with ALK expression detected by IHC expressed the wild-type
or fusion ALK gene. The first round of 5′-RACE did not yield
products that could be visualized on the gel; however, an
approximate 1,800-bp band was obvious in the nested reaction of
samples 405 and 413. The nested reaction included a portion of ALK
cDNA (exon 20) immediately preceded with a non-ALK sequence, which
was confirmed by sequencing analysis of the purified and cloned
products. Furthermore, BLASTN analysis indicated that the non-ALK
sequence reached 100% and was identical to a portion of EML4 mRNA
(NCBI reference number: NM_3373) that included exons 1 through 13
of the gene. Confirmatory RT-PCR conducted on the independently
synthesized tumor cDNA with EML4 exon 2 forward and ALK exon 20
reverse primers produced a predicted 1399-bp product (Fig. 3A), the sequencing of which confirmed
the breakpoint (Fig. 3B). This
EML4-ALK product is named variant 1 (E13; A20). To determine the
genomic rearrangement, we performed ALK-split FISH assays. As shown
in Fig. 3C, FISH demonstrated that
30 and 24% of scored nuclei in samples 405 and 413, respectively,
displayed split ALK signals, in accordance with a rearrangement. We
did not obtain appreciable bands from samples 416 or 384 by
5′-RACE; additionally, RT-PCR with the primers used to amplify
known ALK fusion genes, including EML4-ALK, VCL-ALK, TMP3-ALK,
NPM1-ALK, TFG-ALK, ATIC-ALK and CLTC-ALK, showed no
specific bands (data not shown).
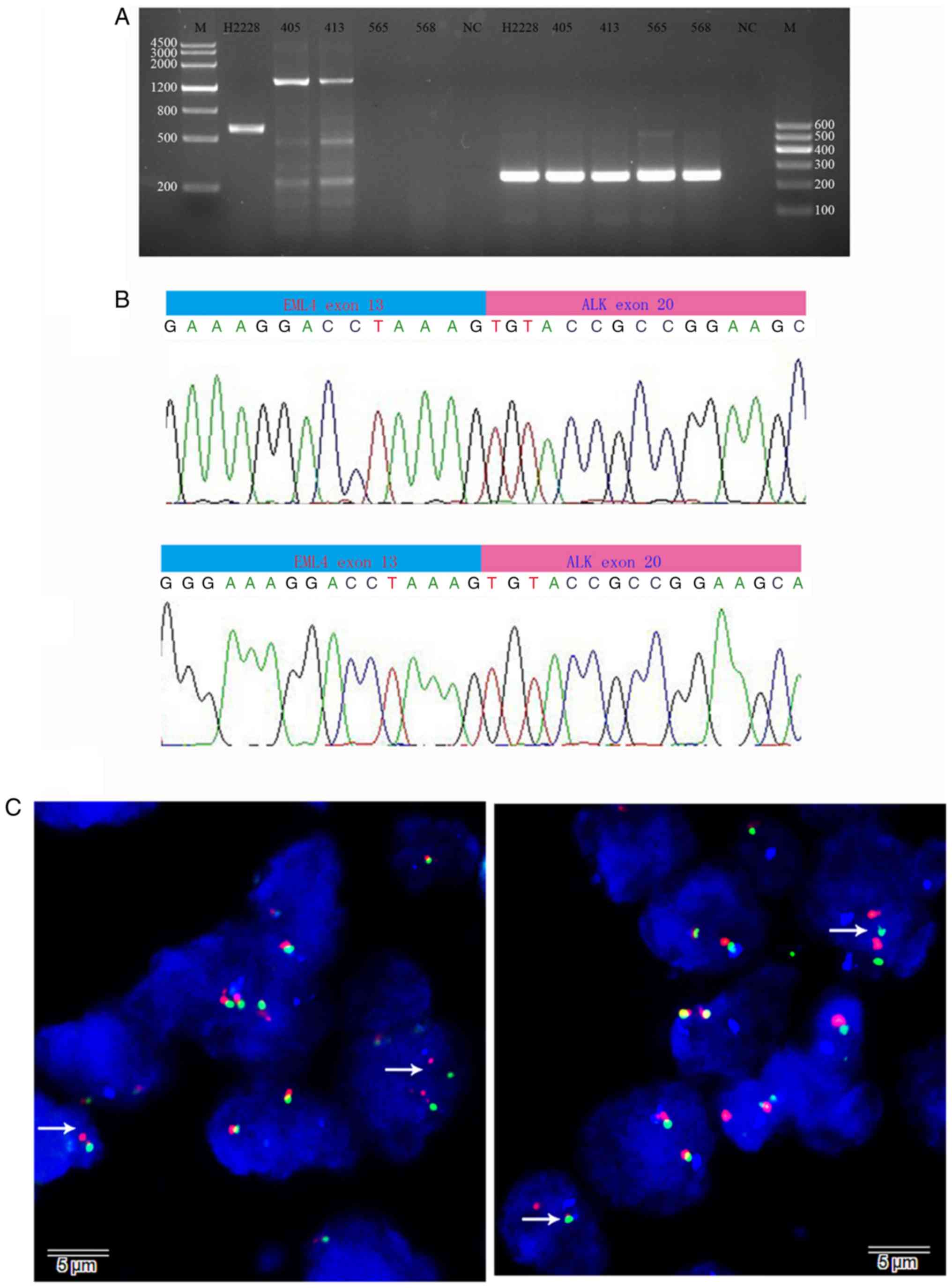 | Figure 3.Identification of the 5′ ALK fusion
partner and EML4-ALK expression. (A) Electrophoresis of
products from the RT-PCR analysis of tumor cDNA with the EML4 exon
2 forward and ALK exon 20 reverse primers shows an expected
1,399-bp product (column 3, sample 405; column 4, sample 413). In
column 2, cDNA from H2228 lung cancer cells containing a known
EML4-ALK fusion gene (variant 3) was amplified by the same
primers, which showed an expected 599-bp product. In columns 5 and
6, samples 565 and 568, with negative ALK expression by IHC, were
included as the negative control. In columns 8–12, GAPDH was
amplified as a positive control (250 bp), and in columns 3 and 7, a
negative control reaction without cDNA was run. In columns 1 and
14, DNA molecular markers are shown. (B) Sequencing results from
samples 405 (upper panel) and 413 (lower panel) show the sequence
of the EML4-ALK fusion breakpoint; the DNA base on EML4 exon
13 (blue) is immediately followed by the DNA base on ALK exon 20
(pink). (C) FISH analysis with the Vysis LSI ALK dual colour
break-apart probe confirmed the presence of ALK rearrangement. The
white arrow denotes split red-green signals indicative of ALK
rearrangement, while touching red-green signals are not indicative
of an ALK rearrangement. EML4-ALK, echinoderm
microtubule-associated protein-like 4/anaplastic lymphoma kinase;
FISH, fluorescence in situ hybridization. |
Case report and pathological
findings
Samples 405 and 413 were obtained from 38- and
59-year-old male patients, respectively, with ccRCC of Fuhrman
grade II diagnosed in March 2012. Patient 405 had a local tumor 4
cm in diameter on his right kidney, and his tumor tissue presented
gland ducts and an acinar arrangement, cuboidal cells, a
translucent cytoplasm, a round nucleus, a nucleolus partially
visible under low magnification, and blood sinus dilatation and
congestion (Fig. 4A). After
surgery, he remains in good health, with no evidence of disease at
present. Patient 413 presented with a tumor 4.5 cm in diameter on
his left kidney, with a cancer embolus noted in the blood vessels
around the tumor that invaded the renal capsule. His tumor tissue
showed a group-like arrangement with rounded and polygonal cancer
cells, a translucent cytoplasm, a small round nucleus and vaguely
visible nucleoli, and rich vascularity in the interstitial space
(Fig. 4B). After surgery, he
remained in good health until December 2015, when a routine
examination showed that he had a new small tumor 0.4 cm in diameter
on the right kidney, and he is now under intensive observation
without further treatment. According to IHC, these two neoplasms
were positive for ABC, CAIX and CD10 (Fig. 4) and negative for c-KIT, p53 and
p16, with a relatively low Ki-67 index (5% staining; data not
shown).
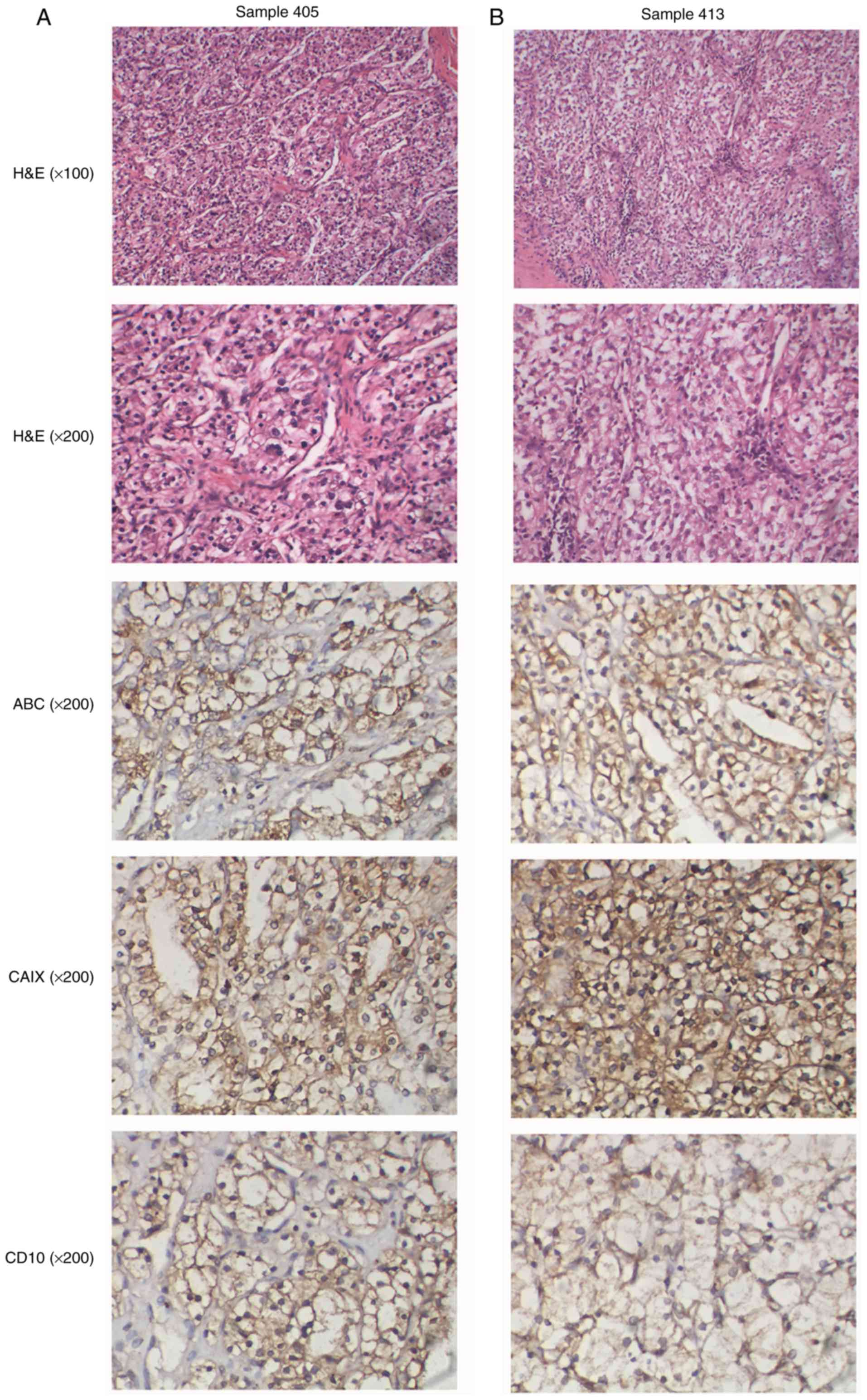 | Figure 4.Histological and immunohistochemical
analyses of ALK-rearranged cases. (A) Sample 405; this patient's
tumor tissue presents gland ducts and an acinar arrangement,
cuboidal cells, a translucent cytoplasm, a round nucleus, nucleolus
partially visible under low magnification, and blood sinus
dilatation and congestion. (B) Sample 413; this patient tumor
tissue shows a group-like arrangement with rounded and polygonal
cancer cells, a translucent cytoplasm, a small round nucleus and
vaguely visible nucleoli, and rich vascularity in the interstitial
space. These two neoplasms were positive for carbonic anhydrase 9
(CAIX), HLA class I (ABC) and cluster of differentiation 10 (CD10).
ALK, anaplastic lymphoma kinase. |
Functional effect of the EML4-ALK
fusion gene in renal epithelial cells
To further study the effect of EML4-ALK expression
on renal carcinogenesis, we carried out correlative research on the
biological effects of EML4-ALK. First, we steadily expressed
EML4-ALK by the use of retrovirus transduction in HK2 cells, a
deathless human renal proximal tubal epithelial cell line lacking
endogenous EML4-ALK expression (Fig.
5A). EML4-ALK expression in HK2 cells enhanced cell colony
formation and cell proliferation in vitro (Fig. 3B and C). Furthermore, EML4-ALK
expression protected HK2 cells from serum starvation-induced
apoptosis (Fig. 5D). In addition,
we evaluated the protumorigenic effect of EML4-ALK expression in
vivo. As shown in Fig. 5E,
EML4-ALK expression promoted the outgrowth of HK2 cell-derived
xenografts in nude mice (232±164.6 vs. 1,311.2±1,261.6
mm3 for Control/Vehicle vs EML4-ALK/Vehicle,
P<0.001), consistent with the transforming activity of this
fusion gene previously reported (9); more importantly, the inhibition of ALK
activity with the small-molecule TKI crizotinib completely
abolished the protumor effect of EML4-ALK expression in this
context (357.25±262.5 vs. 440.75±110.4 mm3 for
Control/Crizotinib vs. EML4-ALK/Crizotinib, P>0.05), indicating
the therapeutic potential of ALK-targeting TKIs in ccRCC with ALK
rearrangement.
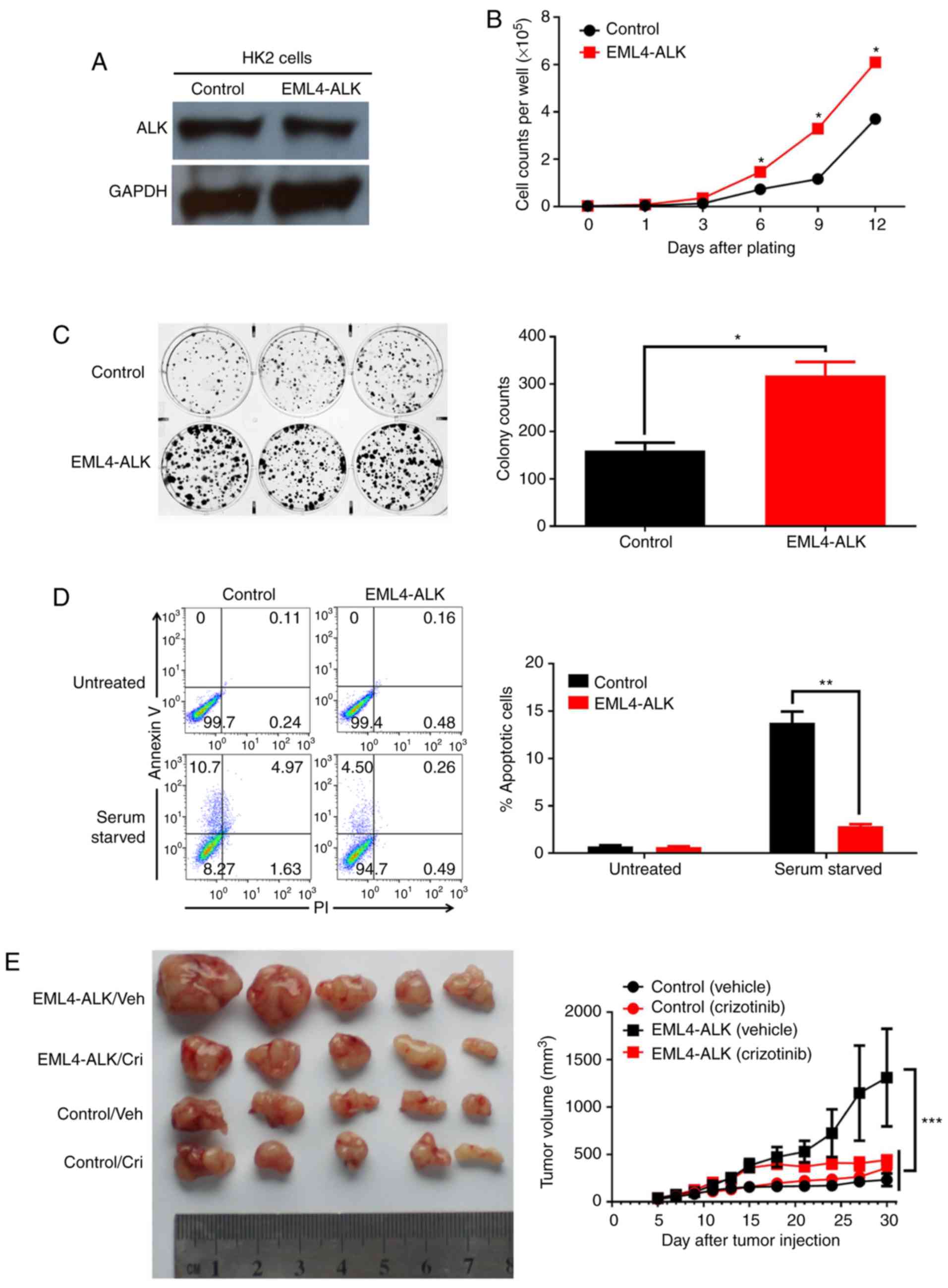 | Figure 5.EML4-ALK overexpression has a
protumorigenic effect on normal renal epithelial cells. (A)
EML4-ALK expression in HK2 cells stably infected with a control or
EML4-ALK-carrying retrovirus determined by western blot analysis.
In vitro (B) proliferation and (C) clonogenic colony
formation assays. HK2 cells stably expressing the control or
EML4-ALK retrovirus were plated into 6-well plates and cultured for
10 or 12 days, and cell counts were performed every 3 days (B) and
colonies were imaged (C, left) and counted at the end of the
experiment (C, right). The data are expressed as the mean ± SD of
triplicate samples. (D) HK2 cells stably expressing the control or
EML4-ALK retrovirus were plated into 6-well plates with (untreated)
or without serum (serum starved) for 24 h, and cell apoptosis was
analyzed by Annexin V/PI staining and flow cytometry. The data are
expressed as the mean ± SD of triplicate samples. (E) Tumors were
established by subcutaneously injecting HK2 cells stably expressing
the control or EML4-ALK retrovirus into the right flanks of nude
mice. When the xenograft reached 100–150 mm3 (control
HK2) or 250–350 mm3 (EML4-ALK HK2), animals were
randomly divided into two groups and intratumorally injected with
vehicle (DMSO) or crizotinib (250 mg/kg) twice weekly for 2 weeks.
Tumor formation was monitored, harvested tumors were imaged (E,
left), and their volume was calculated (E, right). Each time point
represents the mean tumor volume for each group. Data are
representative of 2–3 independent experiments. *P<0.05,
**P<0.01, ***P<0.001, paired Student's t-test (for B, C and
D) or two-way ANOVA with multiple comparisons corrected with the
Bonferroni method (for E). EML4-ALK, echinoderm
microtubule-associated protein-like 4/anaplastic lymphoma
kinase. |
Discussion
An anaplastic lymphoma kinase (ALK) rearrangement is
a key molecular event resulting in the emergence of functional
ALK fusion genes capable of driving carcinogenesis, and
approximately 30 ALK fusion genes with different partners
have been identified in a plethora of tumor types (18,34).
Importantly, the targeted inhibition of ALK fusion genes by
small-molecule tyrosine kinase inhibitors (TKIs) has demonstrated
an impressive clinical outcome in treating cancer patients with ALK
rearrangements confirmed by RT-PCR, immunohistochemistry (IHC)
and/or fluorescence in situ hybridization (FISH) (18,34).
Thus, the identification of ALK rearrangement events and the
elucidation of their functional effects have always been of
clinical significance. In the present study, we screened ALK
expression in clear cell renal cell carcinoma (ccRCC) patients via
routine IHC techniques and found 4 patients with positive ALK
staining that were verified to have the EML4-ALK
rearrangement by RT-PCR and FISH techniques.
Previous studies screened 1,714 patients with renal
cancers, including renal medullary carcinoma (RMC), ccRCC and
papillary RCC, and identified 8 ALK rearrangement-positive patients
among which two groups were established (4,25–30):
three cases of VCL-ALK-rearranged paediatric tumors with
sickle cell traits and 5 cases of adult tumors with a papillary
architecture and an alternative ALK fusion partner (e.g.,
TPM4 or ELM4). Our identification of the
EML4-ALK fusion gene in 2 patients with ccRCC constitutes
the third group. Current data from 6 previous studies and ours on
10 ALK-rearranged tumors among 1,801 representative RCCs
with main morphologies, which were observed in different
ethnicities of pediatric and adult patients (Table II), demonstrate a very low overall
frequency (0.55%) of ALK rearrangement in RCC. Currently, no
consistent pathological features of all ALK-rearranged RCCs have
been revealed except for the presence of a non-specific
eosinophilic cytoplasm (35). Smith
et al summarized the distinct features of ALK-rearranged RMC
patients: sickle-cell traits, an age of approximately 9.3 years,
polygonal spindle-shaped cells with obvious cytoplasmic vacuoles, a
small Ki-67 index and pristine INI-1 (27). However, no distinct features were
noted for patients with ALK-rearranged RCC other than RMC; thus, as
mentioned by Debelenko, it appears that tumor histology cannot be
used to predict ALK rearrangement and that ALK-rearranged
RCC represents an individual pathologic subtype would also be
impractical (35).
 | Table II.Clinicopathologic characteristics of
the ALK-rearranged renal cancer cases. |
Table II.
Clinicopathologic characteristics of
the ALK-rearranged renal cancer cases.
| Authors | No. of cases | No. of
ALK+ cases | Age
(years)/sex | Patient
ethnicity | Sickle cell
trait | Fuhrman grade | Tumor
morphology | Fusion partner | Follow-up | (Refs.) |
|---|
| Debelenko et
al | 6 | 1 | 16/M |
African-American | Yes | III |
Unclassifieda | VCL | 9 months, NED | (25) |
| Marino-Enriquez
et al | 1 | 1 | 6/M |
African-American | Yes | II |
Medullarya | VCL | 21 months, NED | (26) |
| Smith et
al | 1 | 1 | 6/M |
African-American | Yes | Unknown |
Unclassifieda | VCL | 19 months, NED | (27) |
| Sugawara et
al | 343 | 2 | 36/F; | Japanese | No | I | Unclassified | TPM3 | 2 years, NED | (4) |
|
|
|
| 53/F | Japanese | No | I | Papillary | EML4 | 3 years, NED |
|
| Sukov et
al | 534 | 2 | 61/M; | Unknown | No | I | Papillary | Unknown, | 4 years, DOD; | (28) |
|
|
|
| 59/M | Unknown | No | I | Papillary | not VCL | 1.4 years, DOD |
|
| Lee et
al | 829 | 1 | 44/M | Korean | No | III | Papillary | Unchecked | 12 years, NED | (30) |
| Present study | 87 | 2 | 38/M; | Chinese | No | II | Clear cell | EML4 | 3.8 years, NED |
|
|
|
|
| 59/M | Chinese | No | II | Clear cell | EML4 | 3.8
yearsb |
|
|
| 1,801 | 10 (0.55%) |
|
|
|
|
|
|
|
|
Although it has been well documented that ALK
fusion genes confer transforming activity in multiple cancers
(18), including non-small cell
lung cancer (NSCLC) and anaplastic large-cell lymphoma (ALCL)
(7,9), it has never been determined whether
the same is true in the setting of RCC. Using overexpression
experiments in the immortalized renal epithelial cell line HK2, we
first showed that ELM4-ALK expression significantly promoted the
malignant behaviors of otherwise normal cells in vitro,
conferring a tumorigenic effect in vivo. More importantly,
the ALK-targeted TKI crizotinib nearly completely suppressed the
protumour effect of ELM4-ALK in the HK2 ×enograft model,
preliminarily validating the effectiveness of ALK-targeted therapy
in treating ALK-rearranged RCC. However, one limitation of our
findings is that we cannot rule out the influence and contribution
of the host on the final analysis. Certainly, it would be more
clinically relevant if the efficacy of ALK-targeted TKIs is tested
in xenografts from tumor tissues of ALK-rearranged RCC
patients.
In summary, we identified two patients with
ALK rearrangement among 87 Chinese ccRCC patients.
Furthermore, the EML4-ALK (E13: A20 variant 1) fusion gene
was revealed in these two patients, whose protumor effect and its
effectiveness as a therapeutic target were tested in an animal
xenograft model. There is no clinical trials or case report of an
ALK inhibitor in treating ALK-rearranged RCC to date; however,
ALK-RCC has been recently proposed and incorporated into the recent
World Health Organisation Classification of Renal Tumours as a
provisional entity (36); as
ALK-targeted TKIs have shown impressive therapeutic efficacy in
treating patients with ALK rearrangement in NSCLC and other
cancers, it is possible that ALK inhibitor therapy will provide
great benefit for patients with advanced stage ALK-rearranged ccRCC
in the near future.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (nos. 81372528 and 81672274) and the
National High Technology Research and Development Program (‘863’
Program) of China (no. 2014AA020704).
Availability of data and materials
The datasets and certain material used and/or
analyzed during the present study are available from the
corresponding author on reasonable request.
Authors' contributions
WC and HW conceived the study. WC, WL and BB
performed the experiments. WC and HW analyzed the data and prepared
the manuscript. All authors read and approved the final manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Human tissues were provided by the Department of
Pathology, General Hospital of Chinese PLA (Beijing) and Changhai
Hospital affiliated to Second Military Medical University
(Shanghai) following approval by the Ethics Committee of the
General Hospital of Chinese PLA and Changhai Hospital affiliated to
the Second Military Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lopez-Beltran A, Carrasco JC, Cheng L,
Scarpelli M, Kirkali Z and Montironi R: 2009 update on the
classification of renal epithelial tumors in adults. Int J Urol.
16:432–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rini BI, Campbell SC and Rathmell WK:
Renal cell carcinoma. Curr Opin Oncol. 18:289–296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugawara E, Togashi Y, Kuroda N, Sakata S,
Hatano S, Asaka R, Yuasa T, Yonese J, Kitagawa M, Mano H, et al:
Identification of anaplastic lymphoma kinase fusions in renal
cancer: Large-scale immunohistochemical screening by the
intercalated antibody-enhanced polymer method. Cancer.
118:4427–4436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pécuchet N, Fournier LS and Oudard S: New
insights into the management of renal cell cancer. Oncology.
84:22–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singer EA, Gupta GN and Srinivasan R:
Targeted therapeutic strategies for the management of renal cell
carcinoma. Curr Opin Oncol. 24:284–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morris SW, Kirstein MN, Valentine MB,
Dittmer KG, Shapiro DN, Saltman DL and Look AT: Fusion of a kinase
gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's
lymphoma. Science. 263:1281–1284. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lawrence B, Perez-Atayde A, Hibbard MK,
Rubin BP, Dal Cin P, Pinkus JL, Pinkus GS, Xiao S, Yi ES, Fletcher
CD and Fletcher JA: TPM3-ALK and TPM4-ALK oncogenes in inflammatory
myofibroblastic tumors. Am J Pathol. 157:377–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren H, Tan ZP, Zhu X, Crosby K, Haack H,
Ren JM, Beausoleil S, Moritz A, Innocenti G, Rush J, et al:
Identification of anaplastic lymphoma kinase as a potential
therapeutic target in ovarian cancer. Cancer Res. 72:3312–3323.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mossé YP, Laudenslager M, Longo L, Cole
KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P,
et al: Identification of ALK as a major familial neuroblastoma
predisposition gene. Nature. 455:930–935. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Janoueix-Lerosey I, Lequin D, Brugières L,
Ribeiro A, de Pontual L, Combaret V, Raynal V, Puisieux A,
Schleiermacher G, Pierron G, et al: Somatic and germline activating
mutations of the ALK kinase receptor in neuroblastoma. Nature.
455:967–970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
George RE, Sanda T, Hanna M, Fröhling S,
Luther W II, Zhang J, Ahn Y, Zhou W, London WB, McGrady P, et al:
Activating mutations in ALK provide a therapeutic target in
neuroblastoma. Nature. 455:975–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Takita J, Choi YL, Kato M, Ohira
M, Sanada M, Wang L, Soda M, Kikuchi A, Igarashi T, et al:
Oncogenic mutations of ALK kinase in neuroblastoma. Nature.
455:971–974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murugan AK and Xing M: Anaplastic thyroid
cancers harbor novel oncogenic mutations of the ALK gene. Cancer
Res. 71:4403–4411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tuma RS: ALK gene amplified in most
inflammatory breast cancers. J Natl Cancer Inst. 104:87–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schoppmann SF, Streubel B and Birner P:
Amplification but not translocation of anaplastic lymphoma kinase
is a frequent event in oesophageal cancer. Eur J Cancer.
49:1876–1881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mano H: ALKoma: A cancer subtype with a
shared target. Cancer Discov. 2:495–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwak EL, Bang YJ, Camidge DR, Shaw AT,
Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, et al:
Anaplastic lymphoma kinase inhibition in non-small-cell lung
cancer. N Engl J Med. 363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gambacorti-Passerini C, Messa C and
Pogliani EM: Crizotinib in anaplastic large-cell lymphoma. N Engl J
Med. 364:775–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mossé YP, Lim MS, Voss SD, Wilner K,
Ruffner K, Laliberte J, Rolland D, Balis FM, Maris JM, Weigel BJ,
et al: Safety and activity of crizotinib for paediatric patients
with refractory solid tumours or anaplastic large-cell lymphoma: A
Children's Oncology Group phase 1 consortium study. Lancet Oncol.
14:472–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Butrynski JE, D'Adamo DR, Hornick JL, Dal
Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ,
Ramaiya N, et al: Crizotinib in ALK-rearranged inflammatory
myofibroblastic tumor. N Engl J Med. 363:1727–1733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong KM, Noonan S, O'Bryant C and Jimeno
A: Alectinib for the treatment of ALK-positive stage IV non-small
cell lung cancer. Drugs Today (Barc). 51:161–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loong HH, Mok K, Leung LK and Mok TS:
Crizotinib in the management of advanced-stage non-small-cell lung
cancer. Future Oncol. 11:735–745. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Debelenko LV, Raimondi SC, Daw N,
Shivakumar BR, Huang D, Nelson M and Bridge JA: Renal cell
carcinoma with novel VCL-ALK fusion: New representative of
ALK-associated tumor spectrum. Mod Pathol. 24:430–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mariño-Enríquez A, Ou WB, Weldon CB,
Fletcher JA and Pérez-Atayde AR: ALK rearrangement in sickle cell
trait-associated renal medullary carcinoma. Genes Chromosomes
Cancer. 50:146–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith NE, Deyrup AT, Mariño-Enriquez A,
Fletcher JA, Bridge JA, Illei PB, Netto GJ and Argani P: VCL-ALK
renal cell carcinoma in children with sickle-cell trait: The eighth
sickle-cell nephropathy? Am J Surg Pathol. 38:858–863. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sukov WR, Hodge JC, Lohse CM, Akre MK,
Leibovich BC, Thompson RH and Cheville JC: ALK alterations in adult
renal cell carcinoma: Frequency, clinicopathologic features and
outcome in a large series of consecutively treated patients. Mod
Pathol. 25:1516–1525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hodge JC, Pearce KE and Sukov WR: Distinct
ALK-rearranged and VCL-negative papillary renal cell carcinoma
variant in two adults without sickle cell trait. Mod Pathol.
26:604–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee C, Park JW, Suh JH, Nam KH and Moon
KC: ALK-positive renal cell carcinoma in a large series of
consecutively resected Korean renal cell carcinoma patients. Korean
J Pathol. 47:452–457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie S, Li Y, Li X, Wang L, Yang N, Wang Y
and Wei H: Mer receptor tyrosine kinase is frequently overexpressed
in human non-small cell lung cancer, confirming resistance to
erlotinib. Oncotarget. 6:9206–9219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Zhang S, Yang X, Yang J, Zhou Q,
Yin L, An S, Lin J, Chen S, Xie Z, et al: Fusion of EML4 and ALK is
associated with development of lung adenocarcinomas lacking EGFR
and KRAS mutations and is correlated with ALK expression. Mol
Cancer. 9:1882010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mino-Kenudson M, Chirieac LR, Law K,
Hornick JL, Lindeman N, Mark EJ, Cohen DW, Johnson BE, Jänne PA,
Iafrate AJ and Rodig SJ: A novel, highly sensitive antibody allows
for the routine detection of ALK-rearranged lung adenocarcinomas by
standard immunohistochemistry. Clin Cancer Res. 16:1561–1571. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roskoski R Jr: Anaplastic lymphoma kinase
(ALK): Structure, oncogenic activation, and pharmacological
inhibition. Pharmacol Res. 68:68–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Debelenko LV: Reply to ‘Distinct
ALK-rearranged and VCL-negative papillary renal cell carcinoma
variants in two adults without sickle cell trait’. Mod Pathol.
26:605–607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuroda N, Sugawara E, Kusano H, Yuba Y,
Yorita K and Takeuchi K: A review of ALK-rearranged renal cell
carcinomas with a focus on clinical and pathobiological aspects.
Pol J Pathol. 69:109–113. 2018. View Article : Google Scholar : PubMed/NCBI
|