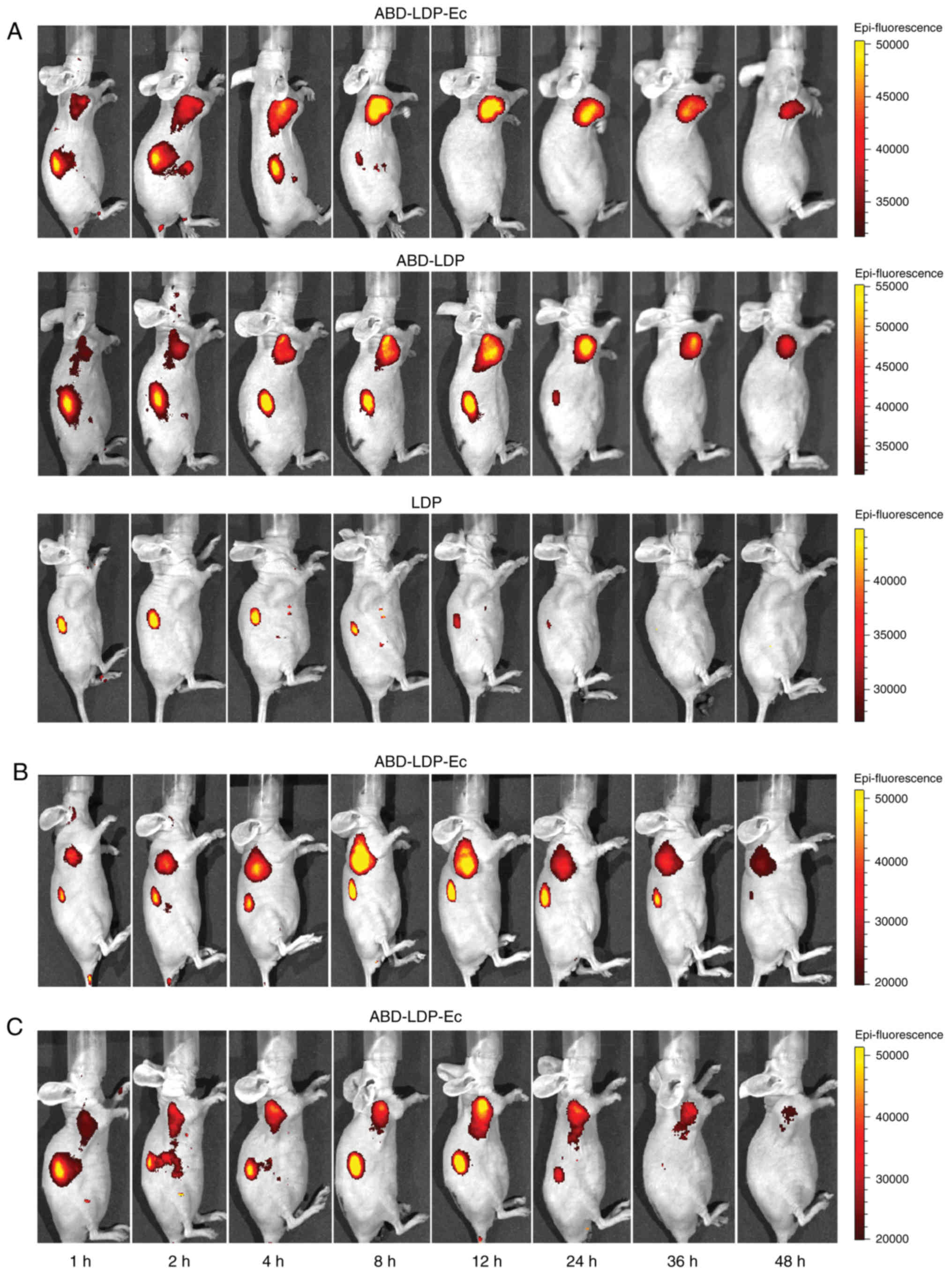
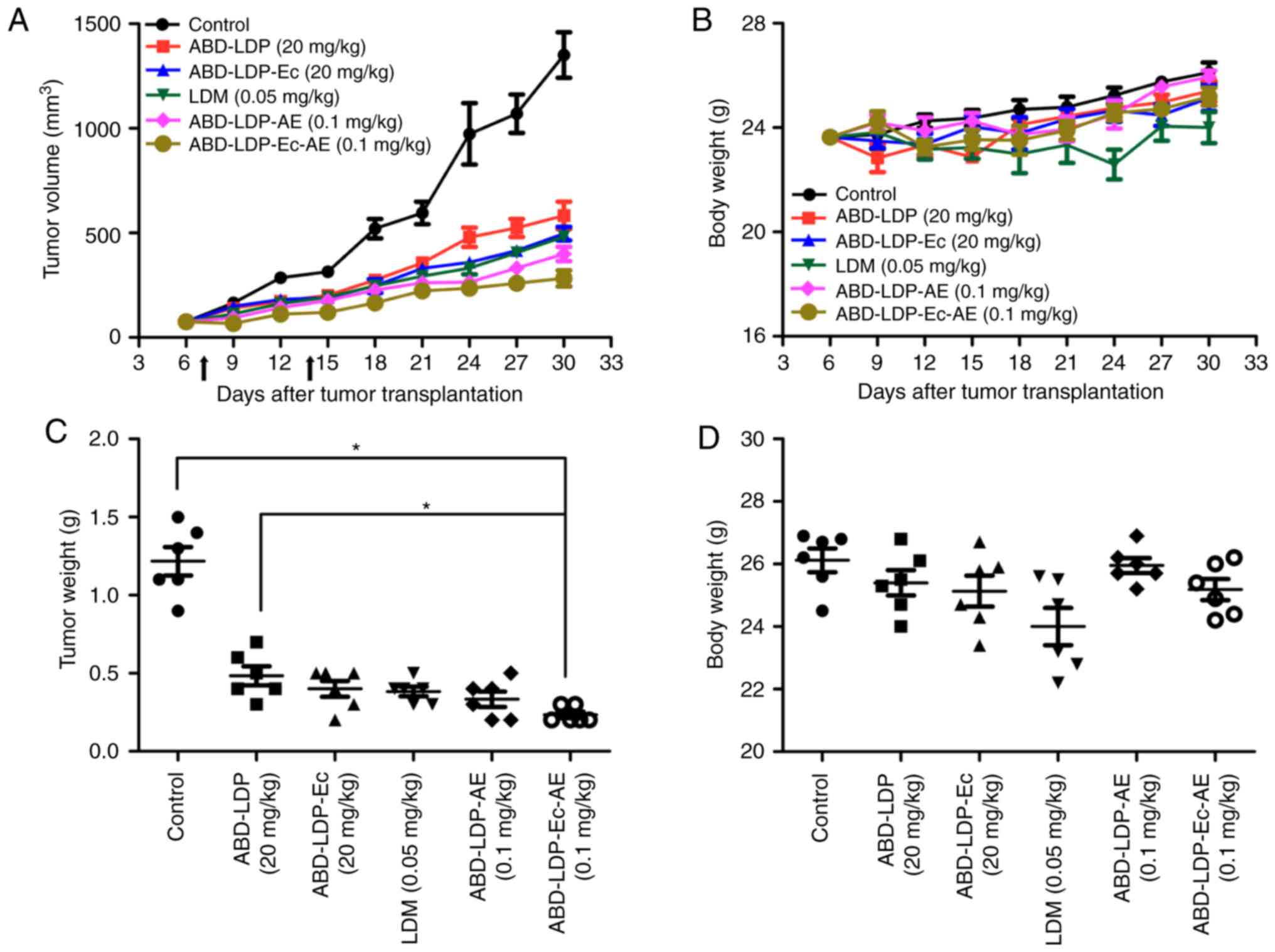
|
1
|
Carter PJ and Senter PD: Antibody-drug
conjugates in cancer therapy. Cancer J. 14:154–169. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng Q and Tong R: Anticancer
nanoparticulate polymer drug conjugate. Bioeng Trans Med.
1:277–296. 2016. View Article : Google Scholar
|
|
3
|
Ma P and Mumper RJ: Paclitaxel
nano-delivery systems: A comprehensive review. J Nanomed
Nanotechnol. 4:10001642013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakhaei E, Kim CW, Funamoto D, Sato H,
Nakamura Y, Kishimura A, Mori T and Katayama Y: Design of a ligand
for cancer imaging with long blood circulation and an enhanced
accumulation ability in tumors. Medchemcomm. 8:1190–1195. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei Y, Wang Y, Xia D, Guo S, Wang F, Zhang
X and Gan Y: Thermosensitive liposomal codelivery of hsa-paclitaxel
and hsa-ellagic acid complexes for enhanced drug perfusion and
efficacy against pancreatic cancer. ACS Appl Mater Interfaces.
9:25138–25151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Byeon HJ, Min SY, Kim I, Lee ES, Oh KT,
Shin BS, Lee KC and Youn YS: Human serum albumin-TRAIL conjugate
for the treatment of rheumatoid arthritis. Bioconjug Chem.
25:2212–2221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sheffield WP, Eltringham-Smith LJ and
Bhakta V: Fusion to human serum albumin extends the circulatory
half-life and duration of antithrombotic action of the kunitz
protease inhibitor domain of protease nexin 2. Cell Physiol
Biochem. 45:772–782. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Château M, Holst E and Björck L:
Protein PAB, an albumin-binding bacterial surface protein promoting
growth and virulence. J Biol Chem. 271:26609–26615. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jonsson A, Dogan J, Herne N, Abrahmsén L
and Nygren PA: Engineering of a femtomolar affinity binding protein
to human serum albumin. Protein Eng Des Sel. 21:515–527. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nilvebrant J and Hober S: The
albumin-binding domain as a scaffold for protein engineering.
Comput Struct Biotechnol J. 6:e2013030092013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cantante C, Lourenco S, Morais M, Leandro
J, Gano L, Silva N, Leandro P, Serrano M, Henriques AO, Andre A, et
al: Albumin-binding domain from Streptococcus zooepidemicus protein
Zag as a novel strategy to improve the half-life of therapeutic
proteins. J Biotechnol. 253:23–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo R, Guo W, Cao L, Liu H, Liu J, Xu H,
Huang W, Wang F and Hong Z: Fusion of an albumin-binding domain
extends the half-life of immunotoxins. Int J Pharm. 511:538–549.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adabi E, Saebi F, Moradi Hasan-Abad A,
Teimoori-Toolabi L and Kardar GA: Evaluation of an albumin-binding
domain protein fused to recombinant human il-2 and its effects on
the bioactivity and serum half-life of the cytokine. Iran Biomed J.
21:77–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jonckheere N, Vasseur R and Van Seuningen
I: The cornerstone K-RAS mutation in pancreatic adenocarcinoma:
From cell signaling network, target genes, biological processes to
therapeutic targeting. Crit Rev Oncol Hematol. 111:7–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schönleben F, Qiu W, Bruckman KC, Ciau NT,
Li X, Lauerman MH, Frucht H, Chabot JA, Allendorf JD, Remotti HE
and Su GH: BRAF and KRAS gene mutations in intraductal papillary
mucinous neoplasm/carcinoma (IPMN/IPMC) of the pancreas. Cancer
Lett. 249:242–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fitzgerald TL, Lertpiriyapong K, Cocco L,
Martelli AM, Libra M, Candido S, Montalto G, Cervello M, Steelman
L, Abrams SL and McCubrey JA: Roles of EGFR and KRAS and their
downstream signaling pathways in pancreatic cancer and pancreatic
cancer stem cells. Adv Biol Regul. 59:65–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Waters AM and Der CJ: KRAS: The critical
driver and therapeutic target for pancreatic cancer. Cold Spring
Harb Perspect Med. 8(pii): a0314352018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Commisso C, Davidson SM, Soydaner-Azeloglu
RG, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S,
Grabocka E, Nofal M, Drebin JA, et al: Macropinocytosis of protein
is an amino acid supply route in Ras-transformed cells. Nature.
497:633–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Sheng W, Wang Y, Li L, Li Y, Zhang
S, Liu X, Chen S and Zhen Y: A macropinocytosis-intensifying
albumin domain-based scfv antibody and its conjugate directed
against k-ras mutant pancreatic cancer. Mol Pharm. 15:2403–2412.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tai CJ, Huang MT, Wu CH, Wang CK, Tai CJ,
Chang CC, Hsieh CI, Chang YJ, Wu CJ, Kuo LJ, et al: Combination of
two targeted medications (bevacizumab plus cetuximab) improve the
therapeutic response of pancreatic carcinoma. Medicine (Baltimore).
95:e32592016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moon do C, Lee HS, Lee YI, Chung MJ, Park
JY, Park SW, Song SY, Chung JB and Bang S: Concomitant statin use
has a favorable effect on gemcitabine-erlotinib combination
chemotherapy for advanced pancreatic cancer. Yonsei Med J.
57:1124–1130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shao RG and Zhen YS: Enediyne anticancer
antibiotic lidamyin: Chemistry, biology and pharmacology.
Anticancer Agents Med Chem. 30:121–131. 2008.
|
|
25
|
Guo XF, Zhu XF, Shang Y, Zhang SH and Zhen
YS: A bispecific enediyne-energized fusion protein containing
ligand-based and antibody-based oligopeptides against epidermal
growth factor receptor and human epidermal growth factor receptor 2
shows potent antitumor activity. Clin Cancer Res. 16:2085–2094.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Hu L, Zhao CY, Zhang SH, Wang R, Li
Y, Shao RG and Zhen YS: The recombinant and reconstituted novel
albumin-lidamycin conjugate shows lasting tumor imaging and
intensively enhanced therapeutic efficacy. Bioconjug Chem.
29:3104–3112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheng W, Shang Y, Li L and Zhen Y: An
EGFR/CD13 bispecific fusion protein and its enediyne-energized
analog show potent antitumor activity. Anticancer Drugs. 25:82–91.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang YH, Shang BY and Zhen YS: Antitumor
efficacy of lidamycin on hepatoma and active moiety of its
molecule. World J Gastroenterol. 11:3980–3984. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van de Poll ML, van Vugt MJ, Lenferink AE
and van Zoelen EJ: Indentification of the minimal requirement for
binding to the human epidermal growth factor (EGF) receptor using
chimeras of human EGF and EGF repeat of Drosophila Notch. J Bio
Chem. 273:16075–16081. 1998. View Article : Google Scholar
|
|
30
|
Du Y, Shang BY, Sheng WJ, Zhang SH, Li Y,
Miao QF and Zhen YS: A recombinantly tailored β-defensin that
displays intensive macropinocytosis-mediated uptake exerting potent
efficacy against K-Ras mutant pancreatic cancer. Oncotarget.
7:58418–58434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka KI, Shimoda M, Chuang VTG, Nishida
K, Kawahara M, Ishida T, Otagiri M, Maruyama T and Ishima Y:
Thioredoxin-albumin fusion protein prevents copper enhanced
zinc-induced neurotoxicity via its antioxidative activity. Int J
Pharm. 535:140–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wan A, Miao Y, Peng L, Cai Y, Chen Y, He
Y, Yang J, Jin J and Li H: Binding and biologic characterization of
recombinant human serum albumin-eTGFBR2 fusion protein expressed in
CHO cells. Bioengineered. 8:600–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kruth HS, Jones NL, Huang W, Zhao B, Ishii
I, Chang J, Combs CA, Malide D and Zhang WY: Macropinocytosis is
the endocytic pathway that mediates macrophage foam cell formation
with native low density lipoprotein. J Biol Chem. 280:2352–2360.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sallusto F, Cella M, Danieli C and
Lanzavecchia A: Dendritic cells use macropinocytosis and the
mannose receptor to concentrate macromolecules in the major
histocompatibility complex class II compartment: Downregulation by
cytokines and bacterial products. J Exp Med. 182:389–400. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Recouvreux MV and Commisso C:
Macropinocytosis: A metabolic adaptation to nutrient stress in
cancer. Front Endocrinol (Lausanne). 8:2612017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakase I, Niwa M, Takeuchi T, Sonomura K,
Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, et
al: Cellular uptake of arginine-rich peptides: Roles for
macropinocytosis and actin rearrangement. Mol Ther. 10:1011–1022.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Veltman DM, Williams TD, Bloomfield G,
Chen BC, Betzig E, Insall RH and Kay RR: A plasma membrane template
for macropinocytic cups. Elife. 5(pii): e200852016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tisdale EJ, Shisheva A and Artalejo CR:
Overexpression of atypical protein kinase C in HeLa cells
facilitates macropinocytosis via Src activation. Cell Signal.
26:1235–1242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cullis J, Siolas D, Avanzi A, Barui S,
Maitra A and Bar-Sagi D: Macropinocytosis of Nab-paclitaxel drives
macrophage activation in pancreatic cancer. Cancer Immunol Res.
5:182–190. 2017. View Article : Google Scholar : PubMed/NCBI
|