Introduction
Breast cancer is among the most common types of
cancer among women worldwide (1).
The metastatic spread of breast cancer is generally associated with
poor prognosis and increased disease-related mortality (2). The metastasis of breast cancer cells
requires several essential steps, including epithelial-mesenchymal
transition (EMT) (3) and
extracellular matrix degradation (4). Studies over the past decade have
demonstrated that aberrant microRNA (miRNA) expression is closely
associated with a variety of biological processes in breast cancer,
including cell proliferation (5,6),
apoptosis (7,8), metastasis (9,10) and
chemosensitivity (11,12). MicroRNAs (miRNAs) are
single-stranded RNA molecules typically 21–25 nucleotides in
length, that can negatively regulate target gene expression. miRNAs
are evolutionarily conserved, and encoded by endogenous non-coding
genes (13). It is well-known that
miRNAs exert post-transcriptional genetic regulatory effects by
binding to the 3′-untranslated region (UTR) of potential target
mRNAs. Additionally, miRNAs may be utilized as diagnostic and
prognostic biomarkers for breast cancer (14,15).
For example, the levels of miR-214 in the peripheral blood may be
used to detect mammary malignant tumours (16). In another study, miRNA expression
profiling revealed that the expression levels of circulating
miR-92a and miR-21 were correlated with tumour diameter and distant
metastasis (17). The miR-299-5p
gene belongs to a spanning DLK1-DIO3 region, which is one of the
largest miRNA-containing clusters, located in chromosome 14q32
(18). Previously, miR-299 has been
reported to be involved in several carcinogenic processes in
thyroid cancer (19), glioblastoma
(20) and prostate cancer (21). Additionally, it has been reported
that miR-299-5p is downregulated in both the tumour and serum
samples of breast cancer patients (22). However, the biological function of
miR-299-5p in breast cancer remains unclear.
Using bioinformatics analysis (TargetScan and
miRanda), it was predicted that miR-299-5p may bind to a potential
site within the 3′-UTR of serine/threonine kinase 39 (STK39) in the
present study, which provides evidence that miR-299-5p may regulate
STK39 expression. STK39 belongs to the STE20 kinase family
(23) and contains N-terminal
proline and alanine repeats, a kinase catalytic domain, and a
C-terminal region. STK39 is considered to activate p38α
mitogen-activated protein kinase (MAPK), which participates in
regulating various cellular biological activities, such as cell
proliferation, differentiation, cytoskeletal rearrangement and ion
transport (24–26). Previous studies reported that STK39
affects carcinogenesis in several types of cancer and is closely
associated with tumour progression and metastasis, indicating that
STK39 may act as an oncogene (27–29).
However, the effects of miRNAs on the regulation of STK39 have not
been extensively investigated.
In the present study, two human breast cancer cell
lines, MDA-MB-231 and MCF-7, were used to evaluate the effect of
miR-299-5p on cellular biological functions. Using bioinformatics
algorithms, it was investigated whether STK39 is a direct target of
miR-299-5p, and the results were further confirmed by
dual-luciferase reporter assays and immunoblot analysis. The
effects of STK39 knockdown by RNA interference (RNAi) on cancer
cell metastasis were also investigated. The aim of the present
study was to investigate the role of miR-299-5p and its potential
target, STK39, in the regulation of breast cancer biological
functions, and provide evidence that they may be used as novel
targets for breast cancer diagnosis and therapy.
Materials and methods
Breast cancer sample and cell
culture
A total of 30 paired formalin-fixed
paraffin-embedded human breast cancer tissue samples and adjacent
non-cancerous tissue samples were obtained from the Department of
Pathology of The Second Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China) between May 2015 and December 2015. The
patients were aged 35–79 years, with an average age of 59.0±12.7
years. No patients had received radiotherapy or chemotherapy prior
to surgery. All the patients provided informed consent, and the
study protocol was approved by the Ethics Committee of Xi'an
Jiaotong University.
The human breast cancer cell lines, MCF-7 and
MDA-MB-231, and the normal breast epithelial cell line MCF-10A,
were purchased from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). MDA-MB-231 and MCF-7 cells
were cultured in Dulbecco's modified Eagles medium (DMEM)
supplemented with 10% foetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). MCF-10A cells were maintained in DMEM/F12 medium
(HyClone; GE Healthcare Life Sciences) with the addition of 10
µg/ml insulin, 20 ng/ml epidermal growth factor (Sigma-Aldrich;
Merck KGaA) and 5% horse serum (Gibco; Thermo Fisher Scientific,
Inc.). All cells were cultured in a humidified incubator at 37°C
with 5% CO2.
Cell transfection
miR-299-5p mimics, miR-299-5p inhibitor, small
interfering RNAs (siRNAs) of STK39, STK39 overexpression plasmid,
and their respective negative controls were chemically synthesized
by Genepharm Technologies. Prior to transfection, breast cancer
cells were incubated in a 6-well plate at a density of
2×105 cells/well. After a 24-h incubation, cells were
transfected with miRNA mimics or plasmid using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), following the
manufacturer's protocol. All oligonucleotides used in the present
study are listed in Table SI.
Lentivirus infection
The packaged lentivirus for miR-299-5p
overexpression was obtained from GeneChem and is referred to as
LV-miR-299-5p hereafter. The lentiviral vector, LV-miR-ctrl, was
used as a negative control. MDA-MB-231 cells were infected at a
multiplicity of infection of 10 in the presence of 5 µg/ml
polybrene. All steps were performed according to the manufacturer's
instructions.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from each group of breast
cancer cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
cDNA was synthesized from total RNA using the PrimerScript RT
Reagent kit (Takara Bio, Inc.). The amount of 500 ng total RNA was
used for reverse transcription with the following protocol: 42°C
for 15 min and 85°C for 5 sec. Real-time PCR amplification
reactions were conducted on a CFX96 Real Time PCR System (Bio-Rad
Laboratories, Inc.) using SYBR Green Premix Ex Taq II (Takara Bio,
Inc.). The relative expression of genes was calculated using the
2−ΔΔCq method and normalized to the expression of
β-actin (30). For miR-299-5p
quantitative detection, small nuclear RNA (U6) was used as an
internal control. The primer sequences of the target genes used in
the present study are listed in Table
SI. The thermocycling conditions were as follows: 40 cycles
with denaturation at 95°C for 30 sec, and annealing at 60°C for 30
sec.
Wound healing assay
Artificial wounds were created when cells were ~80%
confluent. A sterile 200-µl pipette tip was used to scratch the
cell monolayer. The width of the wound was measured, and images
were captured using an inverted microscope at 0, 24, 48 and 72 h
after wounding. The distance of cell migration was used to quantify
the migration capacity.
Transwell assay
Following cell transfection, breast cancer cells
were collected and suspended in serum-free medium. For the cell
migration assay, a total of 3×104 cells were added into
the upper chamber of Transwell plates, while the lower chamber was
filled with 800 µl DMEM supplemented with 10% FBS. For the cell
invasion assay, the upper surface of the membrane was precoated
with a thin layer of Matrigel (BD Biosciences) and a total of
6×104 cells were added into the upper chamber of the
Transwell plates. After incubation for 24 h, the non-invasive cells
were gently removed from the upper chamber with a cotton swab.
Invading cells that adhered to the bottom of the membrane were
fixed at room temperature with 4% paraformaldehyde and stained with
0.1% crystal violet solution for 30 min. Images were randomly
captured by an inverted microscope (IX73; Olympus Corp.). The
number of invading cells was counted by using at least five fields
for each membrane (magnification, ×200).
Bioinformatics analysis
The TargetScan (http://www.targetscan.org/vert_72) and miRanda
(http://www.microrna.org/) online databases were
employed to predict potential targets and their miR-299-5p binding
sites.
Dual-luciferase reporter assay
The potential binding sites of miR-299-5p in STK39
mRNA 3′-UTR were constructed and cloned between the XhoI
and NotI sites of the psiCHECK-2 dual-luciferase expression
vector (Promega Corporation). psiCHECK2-STK39 wild-type (WT) and
psiCHECK2-STK39 mutant (Mut) vectors were constructed.
Subsequently, 293T cells were co-transfected with
psiCHECK2-STK39-WT or psiCHECK2-STK39-Mut along with miR-299-5p
mimics or miR-negative control. After 48 h of co-transfection,
luciferase activity was detected using the Dual-Luciferase Reporter
Assay System (Promega Corporation). Renilla luciferase
activity was used for normalization.
Western blot assay
After 48 h of transfection, transfected breast
cancer cells were lysed using RIPA lysis buffer (Beyotime Institute
of Biotechnology) supplemented with a protease inhibitor cocktail
(Roche Diagnostics). The protein concentration of each lysate was
detected using a BCA assay kit (Beyotime Institute of
Biotechnology). Equal amounts of cellular proteins (40 µg/lane)
were separated by 10% SDS-PAGE and subsequently transferred to PVDF
membranes (EMD Millipore). After blocking in 5% non-fat dry milk
for 1 h, the membranes were incubated with diluted primary
antibodies at 4°C overnight. After washing with Tris-buffered
saline supplemented with 0.1% Tween-20, the membranes were
incubated with horseradish peroxidase-conjugated (HRP) secondary
antibodies (diluted in 1:20,000; cat no. 111-035-003 or
115-035-003; Jackson ImmunoResearch Laboratories, Inc.) for 1 h at
room temperature. The washed membranes were incubated with ECL
Western HRP Substrate (EMD Millipore) for chemiluminescence
detection. The levels of β-actin were used to normalize the
relative expression of proteins, and the protein band intensity was
analysed using ImageJ software (version 1.48; National Institutes
of Health). The primary antibodies used were as follows: STK39
(also known as SPAK) (diluted 1:2,000, product code ab128894),
matrix metallopeptidase (MMP)-2 (diluted 1:2,000; ab92536), and
MMP-9 (diluted 1:2,000; product code ab76003; all from Abcam),
E-cadherin (diluted 1:1,000, product no. 3195) and N-cadherin
(diluted 1:1,000; product no. 13116; both from Cell Signaling
Technology Inc.), vimentin (diluted 1:1,000; product code ab92547;
Abcam) and β-actin (diluted in 1:1,000; cat. no. sc47778; Santa
Cruz Biotechnology, Inc.).
Xenograft assay
MDA-MB-231 cells stably overexpressing miR-299-5p
were generated. Subsequently, MDA-MB-231 cells were suspended in
phosphate-buffered saline at a density of 2×106
cells/ml. Female BALB/c nude mice (aged 4–5 weeks and weighing
18–20 g, purchased from Beijing Vital River Laboratory Animal
Technology Co.) were injected with 0.1 ml of the cell suspension
via the tail vein (n=10/group). All mice were euthanized by
isoflurane after 7 weeks. The organs with metastatic foci were
subjected to haematoxylin-eosin staining. All animal experimental
procedures were approved by the Institutional Animal Care and Use
Committee of Xi'an Jiaotong University.
Immunohistochemistry
Paraffin sections (4-µm) were deparaffinized with
xylene and rehydrated through a graded ethanol series. Endogenous
peroxidase was blocked with 3% hydrogen peroxide for 5 min at room
temperature, and then antigen retrieval [in 110°C citrate buffer
(pH 6.0) for 2 min] and blocking were performed. The sections were
incubated with anti-STK39 antibody (diluted in 1:100) at 4°C
overnight. Subsequently, the sections were incubated with
HRP-conjugated secondary antibody (Jackson ImmunoResearch
Laboratories, Inc.). Detection was performed using
3,3′-diaminobenzidine and haematoxylin. For each sample, the
percentage of positive cells was counted to evaluate the expression
of STK39.
Statistical analysis
Data are presented as the mean ± standard error of
mean. All data were pooled from at least three independent
experiments. Differences between two groups were analysed using the
Student's t-test and differences among multiple groups were
analysed using one-way ANOVA followed by Dunnett's post hoc test.
The association between miR-299-5p or STK39 expression and
clinicopathological characteristics of breast cancer patients was
analysed using Fisher's exact probabilities test. All tests were
two-sided, and P<0.05 was considered to indicate statistically
significant differences. All statistical calculations were
performed using SPSS 17.0 (SPSS Inc.), and all graphs were drawn
with GraphPad Prism 5.0 (GraphPad Software, Inc.).
Results
miR-299-5p is downregulated in breast
cancer clinical samples and cell lines
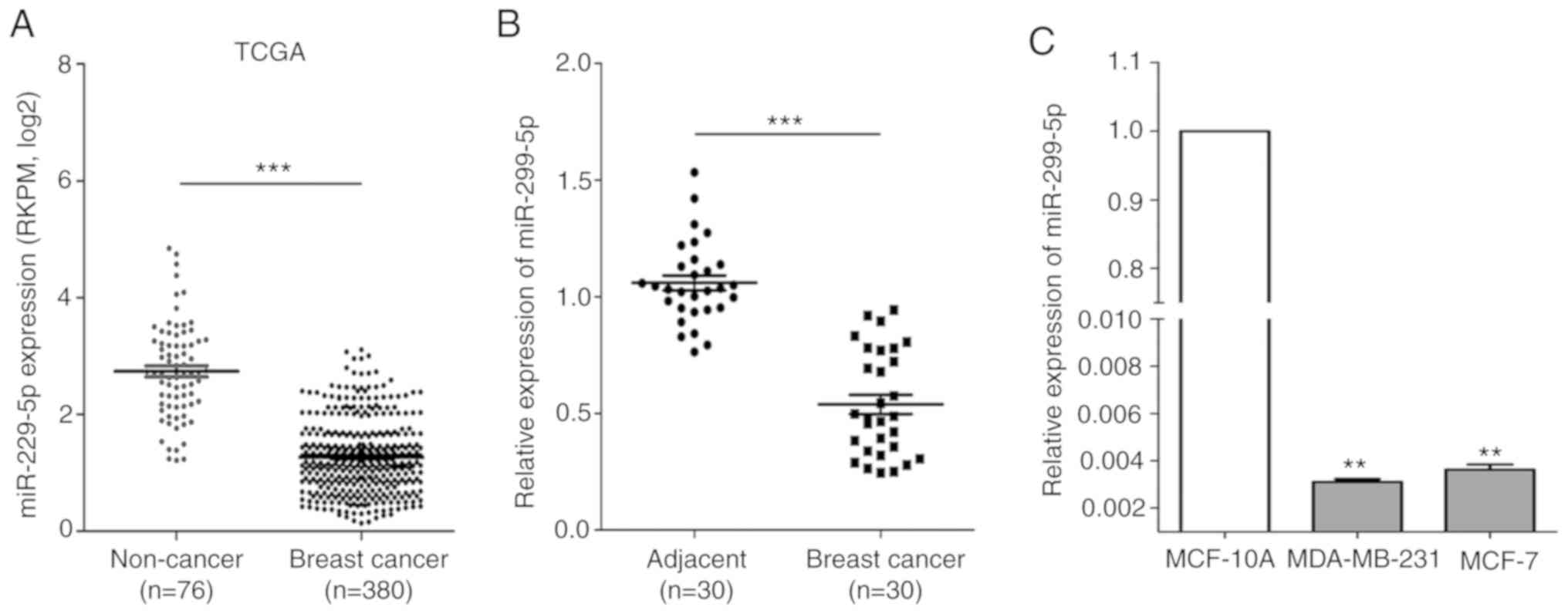
By searching The Cancer Genome Atlas (TCGA) database
(https://cancergenome.nih.gov/), it was
observed that miR-299-5p expression was significantly decreased in
breast cancer tissues (n=380, P<0.001) compared with that in
non-cancerous tissues (n=76) (Fig.
1A). miR-299-5p expression was evaluated in 30 pairs of human
breast cancer tissue and adjacent non-cancerous tissue samples
using RT-qPCR. The association between miR-299-5p expression and
clinicopathological characteristics is presented in Table I. Decreased expression of miR-299-5p
was revealed to be significantly correlated with lymph node
metastasis (P=0.023). Compared with adjacent non-cancerous tissues,
miR-299-5p was significantly downregulated in breast cancer tissues
(Fig. 1B). The expression of
miR-299-5p was also assessed in two different breast cancer cell
lines and in normal human mammary epithelial cells using RT-qPCR.
The results indicated that miR-299-5p was markedly downregulated in
both breast cancer cell lines (Fig.
1C).
 | Table I.Correlation between miR-299-5p
expression and clinicopathological characteristics of breast cancer
patients. |
Table I.
Correlation between miR-299-5p
expression and clinicopathological characteristics of breast cancer
patients.
|
|
| miR-299-5p
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Patients
(n=30) | Low (n=19) | High (n=11) | P-value |
|---|
| Age (years) |
|
|
| 0.707 |
|
<60 | 16 | 11 | 5 |
|
|
≥60 | 14 | 8 | 6 |
|
| Tumor size
(cm) |
|
|
| 0.238 |
|
<2 | 11 | 5 | 6 |
|
| ≥2 | 19 | 14 | 5 |
|
| Lymph node
metastasis |
|
|
| 0.023a |
|
Negative | 13 | 5 | 8 |
|
|
Positive | 17 | 14 | 3 |
|
| TNM stage |
|
|
| 0.708 |
|
I+II | 17 | 10 | 7 |
|
|
III+IV | 13 | 9 | 4 |
|
miR-299-5p inhibits breast cancer cell
migration and invasion
To explore the function of miR-299-5p in breast
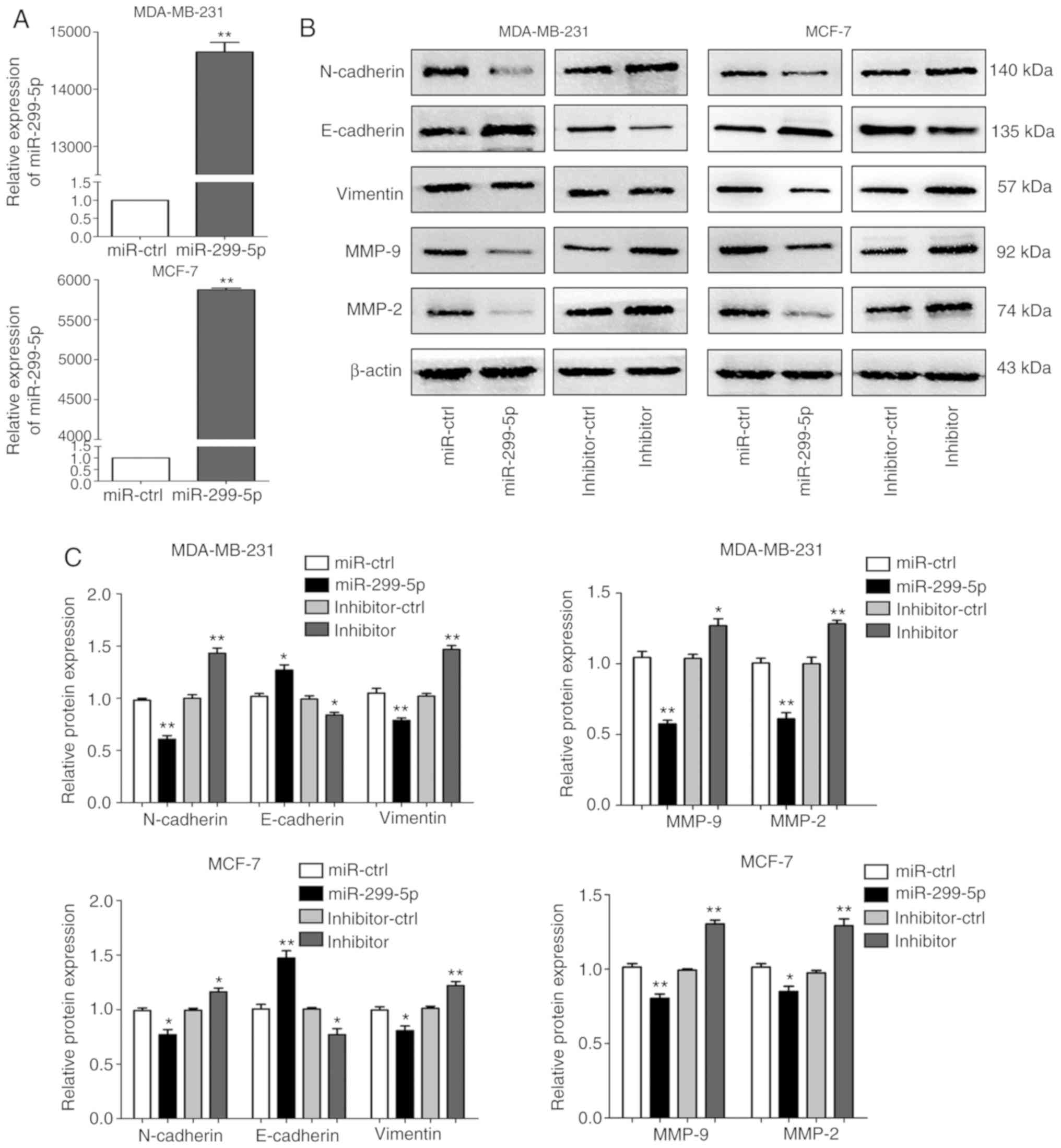
cancer, MDA-MB-231 and MCF-7 cell lines were transfected with
miR-299-5p mimics or negative control (miR-ctrl). The expression
levels of miR-299-5p in the two cell lines were significantly
increased following transfection (Fig.
2A). However, the cell proliferation and apoptosis assays
demonstrated that miR-299-5p exerted no effect on cell
proliferation and apoptosis (Fig.
S1). In addition, the in vivo experiment also
demonstrated this point (Fig.
S2).
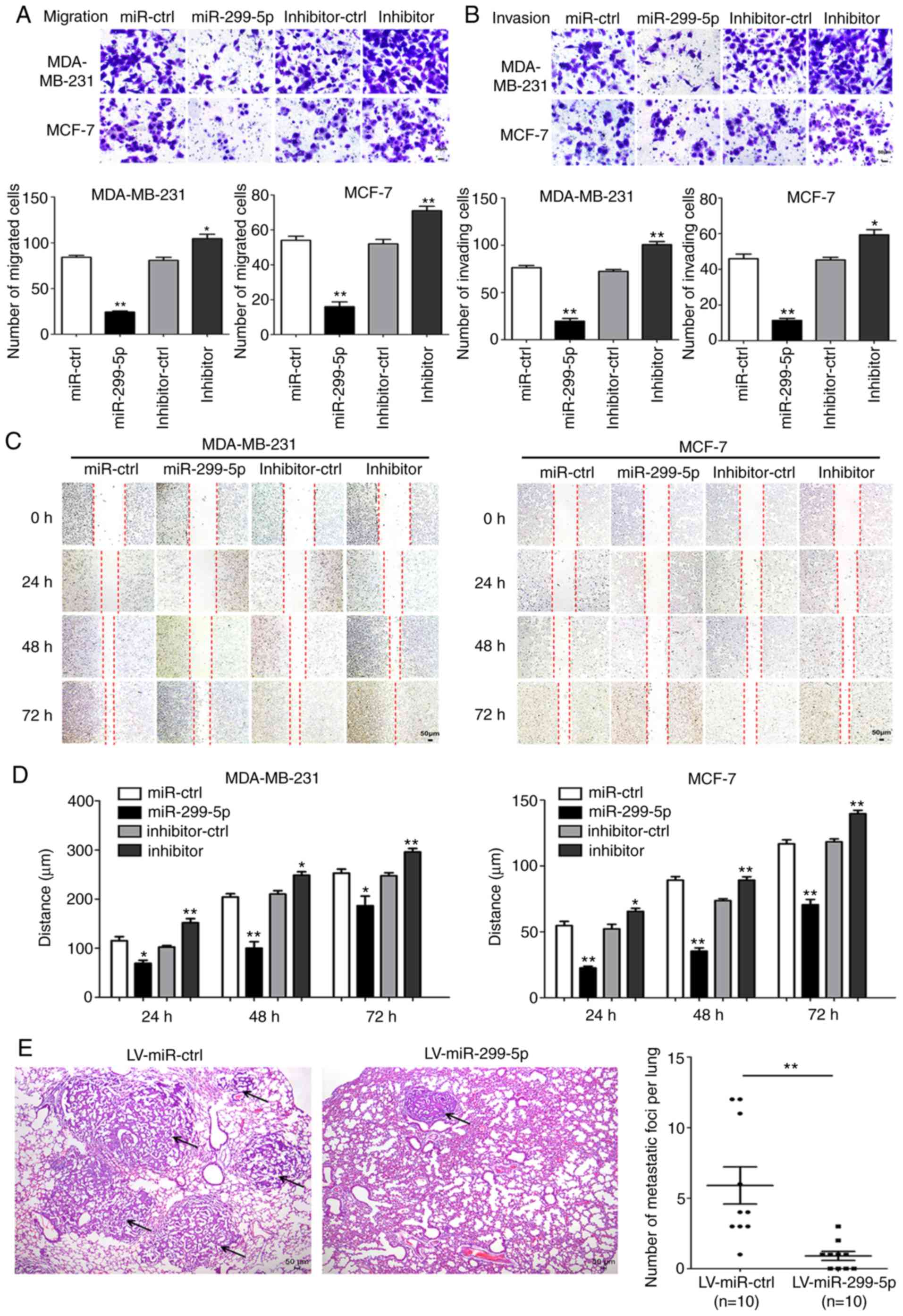
Next, the effects of miR-299-5p on cell migration
and invasion were detected. Transwell and wound healing assays
demonstrated that increased expression of miR-299-5p led to a
marked decrease in the capacity for migration and invasion. By
contrast, the suppression of miR-299-5p with a miRNA inhibitor
promoted cancer cell migration and invasion (Fig. 3A and B). Wound healing assays
yielded consistent results (Fig. 3C and
D). Following transfection with miR-299-5p mimics and
inhibitor, the expression of markers for EMT and extracellular
matrix degradation was assessed, including E-cadherin, N-cadherin,
vimentin, MMP-2 and MMP-9. Overexpression of miR-299-5p decreased
the expression of N-cadherin, vimentin, MMP-2 and MMP-9 in
MDA-MB-231 and MCF-7 cells. The expression of E-cadherin was
markedly upregulated in the two cell lines (Fig. 2B and C). By contrast, miR-299-5p
inhibitor exhibited the opposite results. These results indicated
that miR-299-5p may inhibit breast cancer cell migration and
invasion via regulation of EMT and extracellular matrix
degradation.
To further validate the tumour suppressive effect of
miR-299-5p in vivo, miR-299-5p-expressing MDA-MB-231 cells
and control MDA-MB-231 cells were injected into the tail veins of
nude mice. The mean number of pulmonary metastatic foci was
significantly reduced in mice injected with miR-299-5p-expressing
cells compared with the control group (Fig. 3E). The maximum diameter of
metastatic foci in the LV-miR-ctrl group was 0.36 cm, and in the
LV-miR-299-5p group it was 0.11 cm. These findings indicated that
miR-299-5p may act as a tumour suppressor in breast cancer by
inhibiting breast cancer metastasis in vitro and in
vivo.
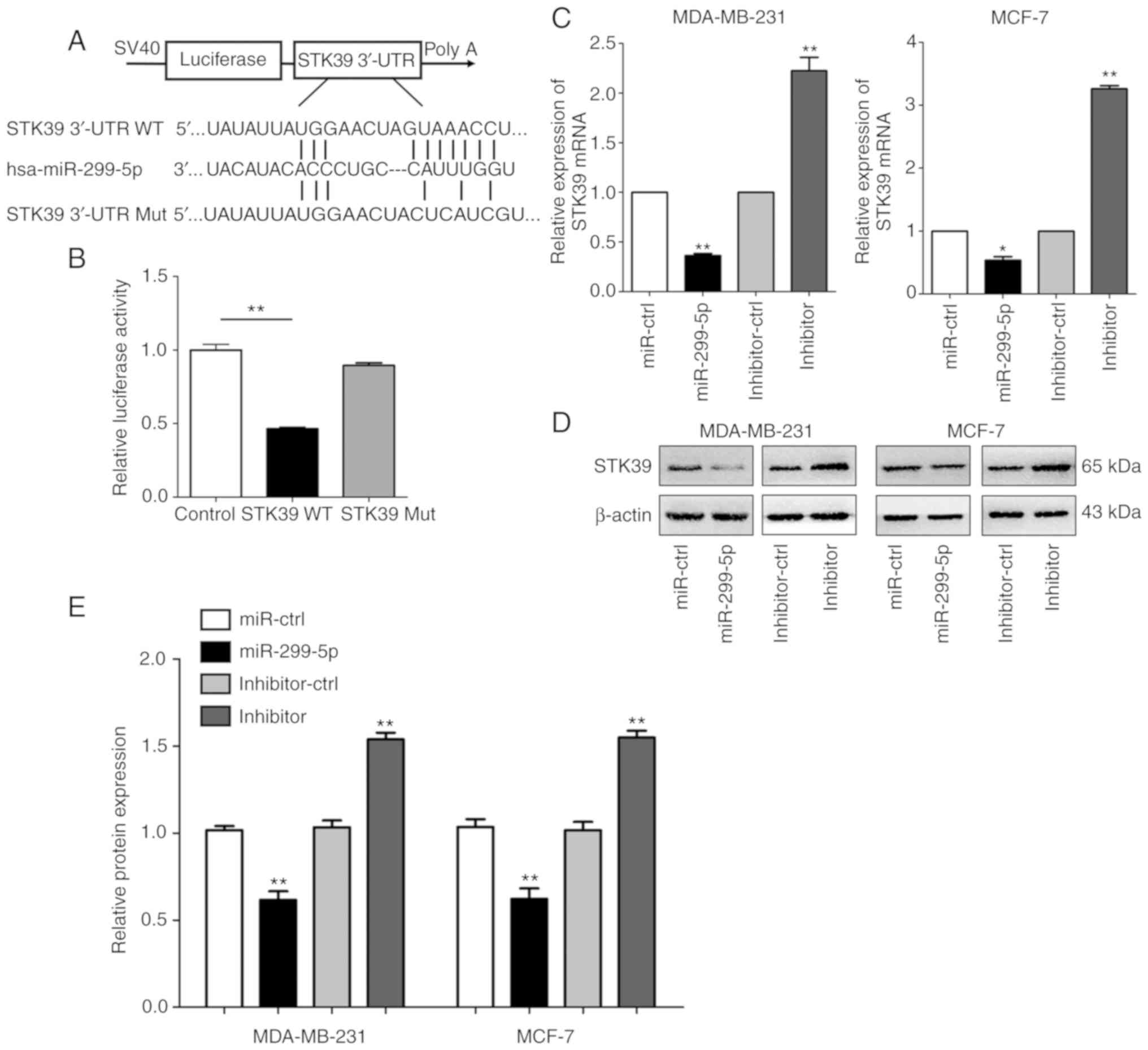
STK39 is a direct target of miR-299-5p
in breast cancer
To investigate the underlying molecular mechanism
through which miR-299-5p suppresses breast cancer cell metastasis,
the TargetScan (http://www.targetscan.org/vert_72) and miRanda
(http://www.microrna.org/) prediction databases
were searched to identify miR-299-5p target genes. We were able to
determine that the 3′-UTR of STK39 mRNA contains putative binding
sites for miR-299-5p. To validate the association between
miR-299-5p and STK39, STK39-WT and STK39-Mut 3′-UTR fragments were
cloned into the psiCHECK-2 dual-luciferase reporter vector
(Fig. 4A). miR-299-5p mimics and
STK39-WT- or STK39-Mut-3′-UTR vectors were co-transfected into 293T
cells. miR-299-5p significantly reduced the relative luciferase
activity of the STK39-WT-3′-UTR vector in 293T cells, but did not
affect the luciferase activity of the STK39-Mut-3′-UTR vector,
indicating that miR-299-5p binds directly to 3′-UTR of STK39
(Fig. 4B). This result revealed
that miR-299-5p exerts its effect by directly targeting the 3′-UTR
of STK39. Moreover, the mRNA and protein levels of STK39 were
revealed to be inversely correlated with miR-299-5p expression
(Fig. 4C-E).
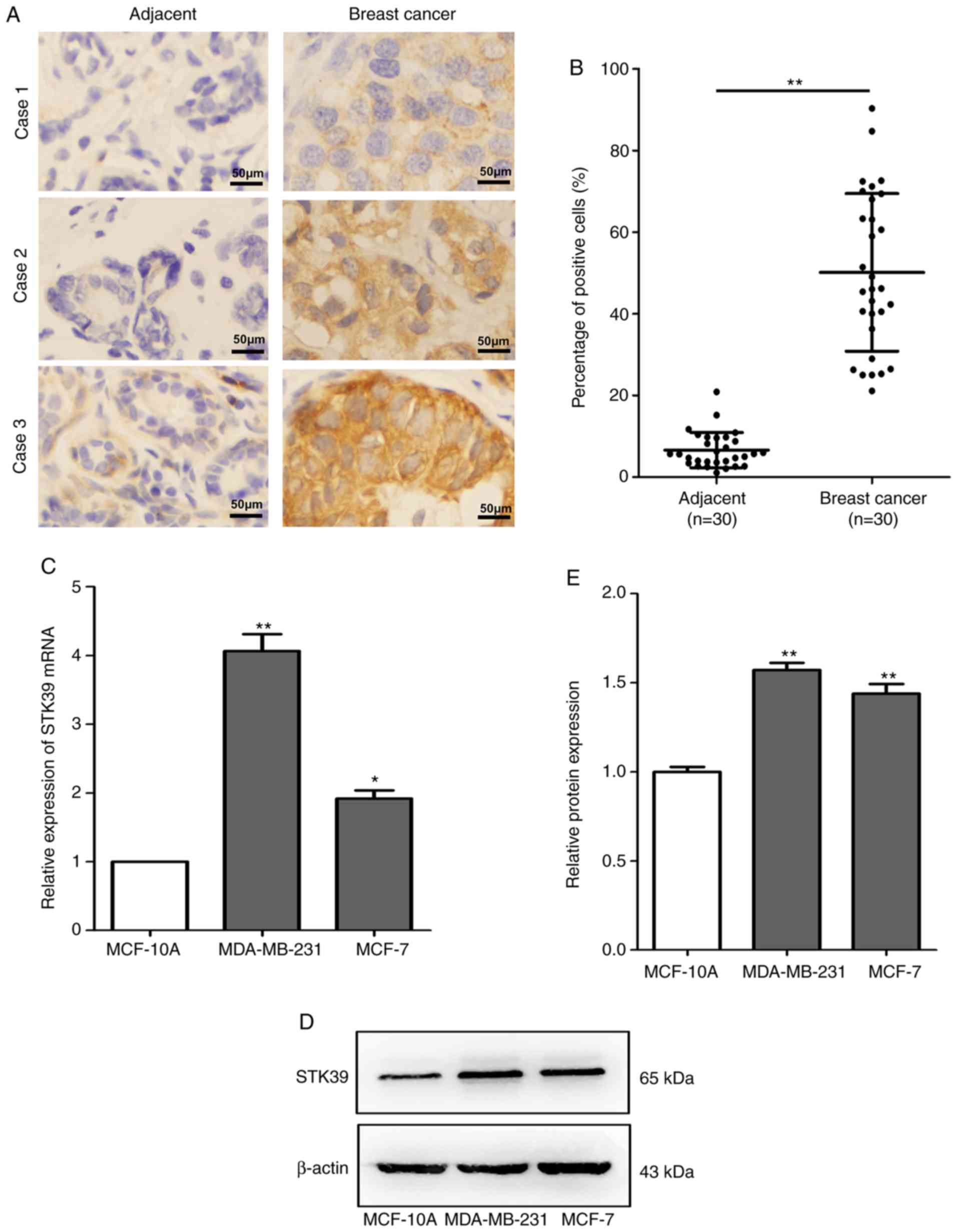
STK39 is upregulated in breast cancer
tissues and cell lines
To determine whether STK39 is associated with
miR-299-5p-mediated inhibition of breast cancer metastasis, STK39
expression was assessed in 30 pairs of human breast cancer tissue
and adjacent non-cancerous tissue samples using
immunohistochemistry. The association between STK39 expression and
clinicopathological characteristics is presented in Table II. Increased expression of STK39
was revealed to be significantly correlated with lymph node
metastasis (P=0.020). However, no association was observed between
STK39 levels and age, tumour size, or TNM stage. Compared with
non-cancerous tissues, STK39 was significantly upregulated
(Fig. 5A and B) in breast cancer
tissues. Moreover, the expression of STK39 at the mRNA and protein
levels was also increased in breast cancer cell lines compared with
that in normal breast epithelial cells (Fig. 5C-E).
 | Table II.Correlation between STK39 expression
and clinicopathological characteristics of breast cancer
patients. |
Table II.
Correlation between STK39 expression
and clinicopathological characteristics of breast cancer
patients.
|
|
| STK39
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Patients
(n=30) | Low (n=9) | High (n=21) | P-value |
|---|
| Age (years) |
|
|
| 0.999 |
|
<60 | 16 | 5 | 11 |
|
|
≥60 | 14 | 4 | 10 |
|
| Tumor size
(cm) |
|
|
| 0.687 |
|
<2 | 11 | 4 | 7 |
|
| ≥2 | 19 | 5 | 14 |
|
| Lymph node
metastasis |
|
|
| 0.020a |
|
Negative | 13 | 7 | 6 |
|
|
Positive | 17 | 2 | 15 |
|
| TNM stage |
|
|
| 0.691 |
|
I+II | 17 | 6 | 11 |
|
|
III+IV | 13 | 3 | 10 |
|
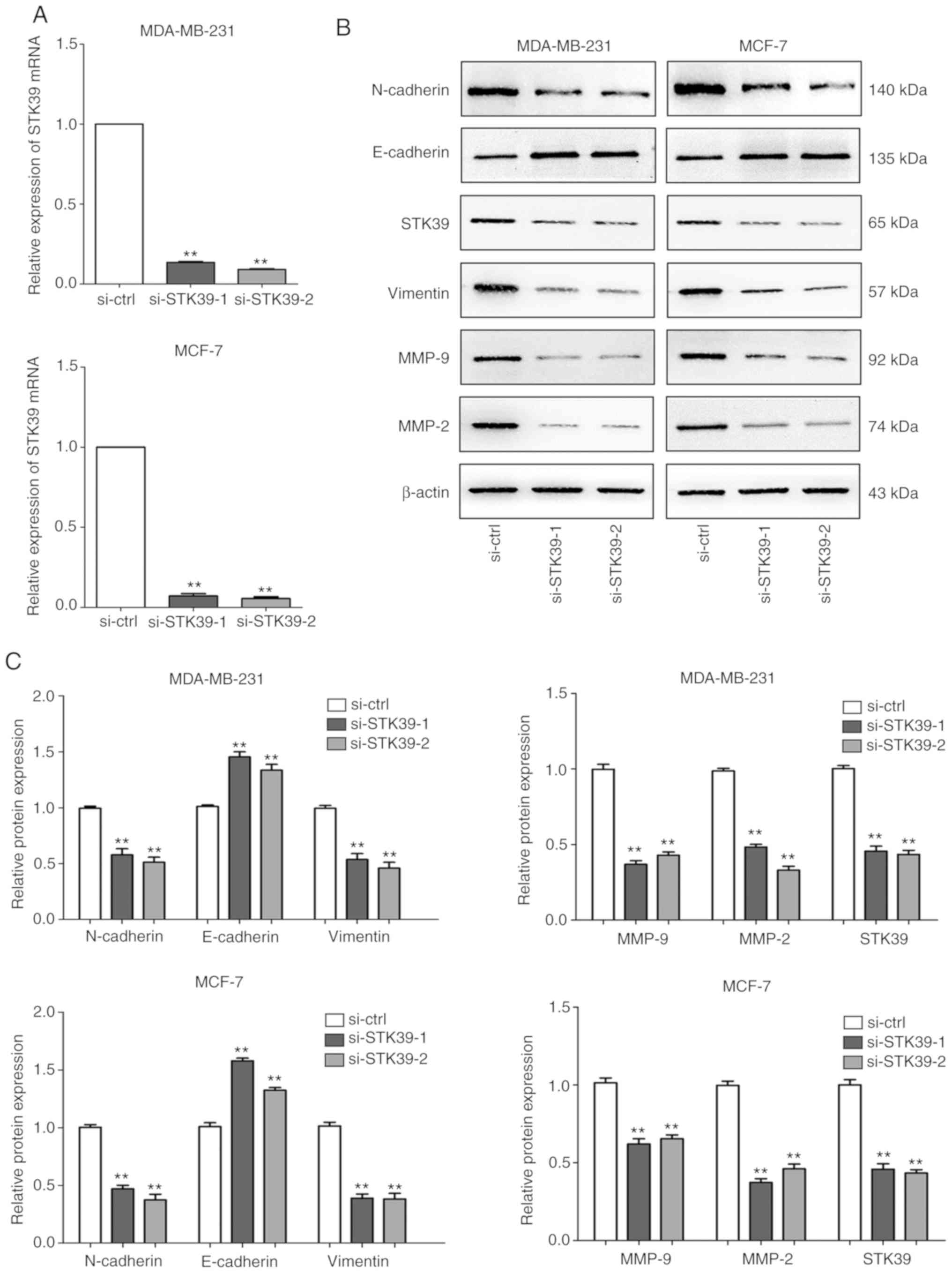
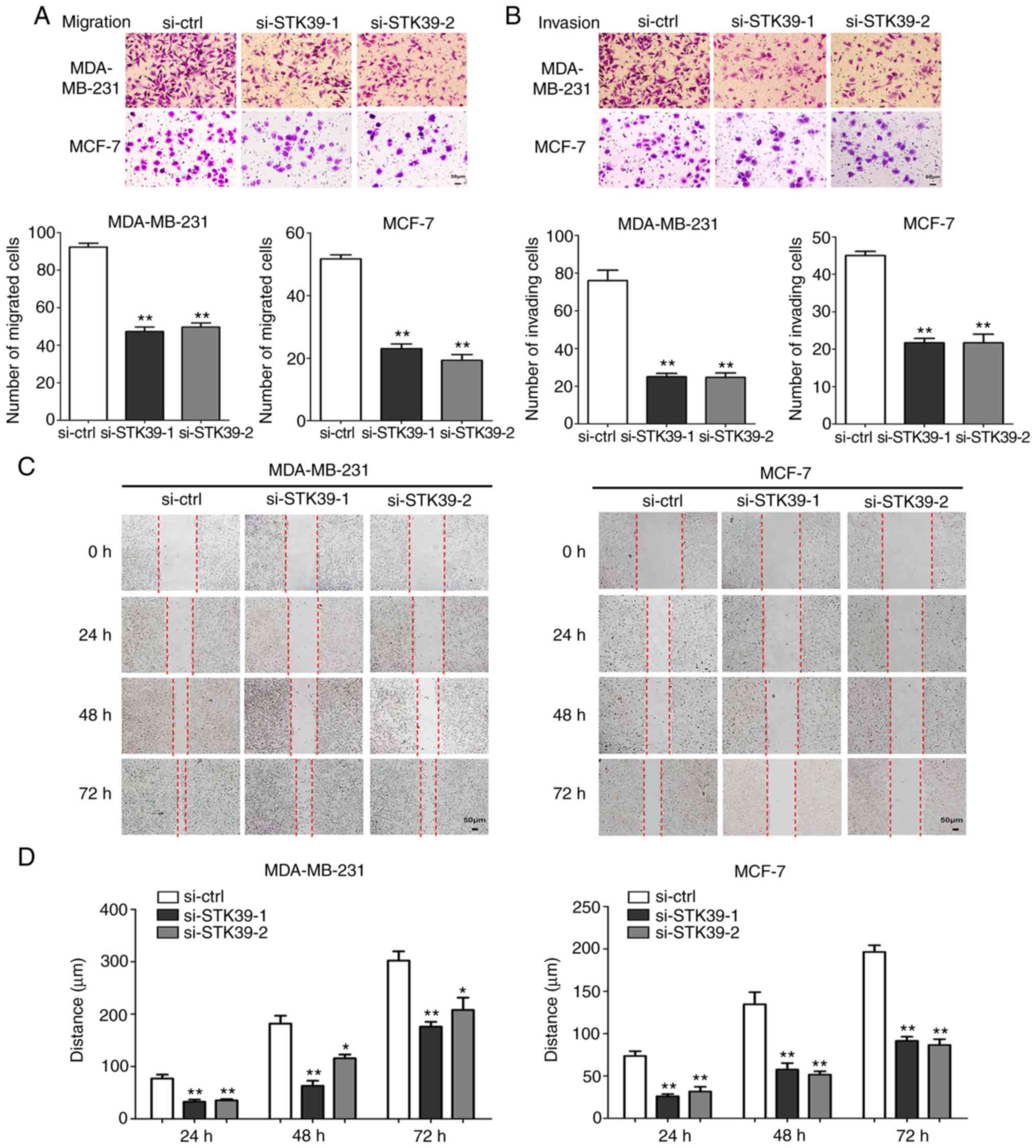
Knockdown of STK39 inhibits breast
cancer cell migration and invasion
To further validate the role of STK39 in breast
cancer, siRNAs were used to perform STK39 loss-of-function
experiments. As revealed in Fig. 6A and
B, following transfection, siRNA-mediated depletion of STK39 in
MDA-MB-231 and MCF-7 cells reduced the levels of STK39 mRNA and
protein.
Cell migration and invasion abilities after STK39
knockdown were next examined. As revealed in Fig. 7A and B, MDA-MB-231 and MCF-7 cell
migration and invasion were markedly inhibited in Transwell assays.
In addition, the results of the wound healing assays were
consistent (Fig. 7C and D).
Furthermore, immunoblot assays revealed that the knockdown of STK39
led to increased expression of E-cadherin and decreased expression
of vimentin, N-cadherin, MMP-2, and MMP-9 (Fig. 6B and C).
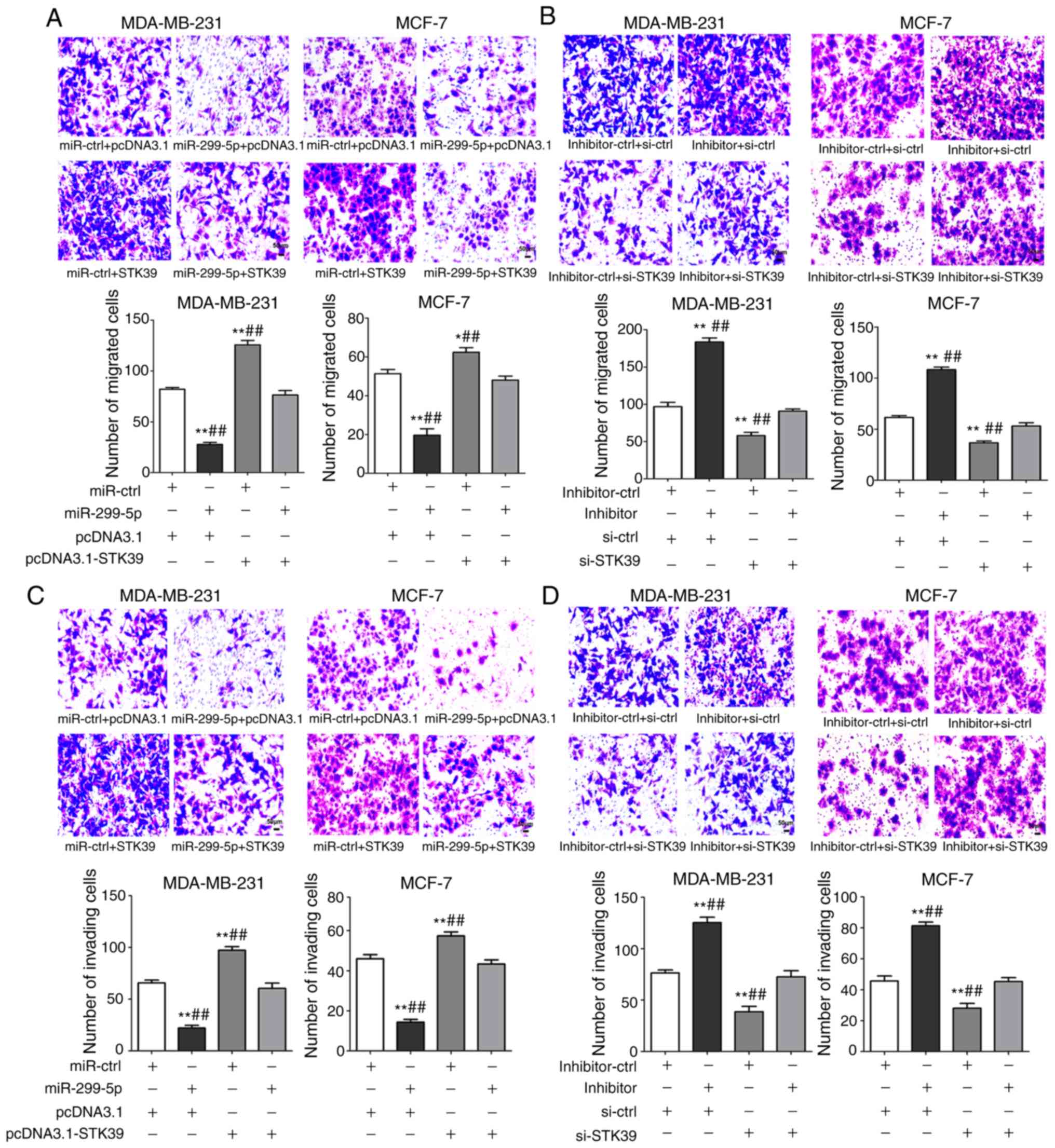
STK39 reverses the effects of
miR-299-5p on breast cancer cell lines
To confirm that STK39 is a functional target gene of
miR-299-5p, rescue experiments in breast cancer cell lines were
performed. MDA-MB-231 and MCF-7 cells were co-transfected with
STK39-overexpression plasmid and miR-299-5p mimics. To ensure the
efficiency of co-transfection, the levels of miR-299-5p and STK39
were evaluated after transfection. As revealed in Fig. 8A and B, STK39 overexpression reduced
the tumour-suppressing effects of miR-299-5p on cell migration, and
STK39 knockdown limited the effect of the miR-299-5p inhibitor on
cell migration. Similar effects were also observed on cell invasion
(Fig. 8C and D). Collectively,
these results provide evidence that STK39 downregulation is
indispensable for the tumour-suppressive role of miR-299-5p in
breast cancer metastasis.
Discussion
The carcinogenesis and metastasis of breast cancer
is a multifactorial process that includes expression changes in
various oncogenes and tumour suppressors. Accordingly, numerous
cancer-related genes and their biological functions have been
identified, and the regulatory effects of thousands of unique
non-coding RNAs are currently being investigated (31). Specifically, miRNAs are known to be
widely involved in cancer development and progression, and several
studies have suggested that miRNAs are dysregulated in various
cancers (32), and regulate diverse
biological functions in tumour cells.
The majority of studies on miR-299-5p have
identified it as a tumour suppressor that is downregulated in
different type of cancers (19–21).
van Schooneveld et al observed that miR-299-5p was
downregulated in both breast cancer tissue and serum samples
compared with healthy individuals. They compared the expression
level of miR-299-5p and other miRNAs in serum from patients with
metastatic breast cancer receiving treatment, patients with
untreated metastatic breast cancer, and healthy individuals, and
found that the lowest expression value of miR-299-5p was observed
in patients with metastatic breast cancer, whereas the expression
level returned to normal with treatment. Moreover, the expression
level of miR-299-5p exhibited a negative association with patient
age at diagnosis (22). In the
present study, miR-299-5p was revealed to be significantly
downregulated in breast cancer tissues and cell lines, which was
consistent with the results from clinical samples. These data
indicated that miR-299-5p inhibited breast cancer cell migration
and invasion, whereas it was not involved in cell proliferation and
apoptosis. The restoration of miR-299-5p expression inhibited cell
migration and invasion in breast cancer cells, while miR-299-5p
inhibition promoted cell migration and invasion.
Previous studies have reported that the targets of
miR-299-5p, include RAD21 (33) and
osteopontin (34), which increase
the expression of mesenchymal markers in EMT (35). Using bioinformatics analysis, STK39
was predicted to be a direct target of miR-299-5p. STK39 (also
referred to as SPAK) is a member of the SPS1 subfamily of STE20
kinases (23). In previous studies,
STK39 was demonstrated to activate the p38 MAPK pathway, which
indicates that STK39 is involved in cellular stress responses
(26). In addition, STK39 has been
revealed to play a critical role in the regulation of ionic and
osmotic cellular homeostasis (36,37).
Recent studies have demonstrated that STK39 has carcinogenic
functions in several cancer types (27–29).
Single nucleotide polymorphisms of STK39 are correlated with
prognosis in early-stage non-small cell lung cancer (38). Stanton et al identified STK39
as an early-stage antigen in breast cancer, and its overexpression
in women with breast cancer predicted worse prognosis (39). In another study, the gene expression
of STK39 was upregulated in approximately half of patients with
ductal carcinoma in situ and invasive ductal carcinomas
compared with normal breast tissue. Furthermore, the autoantibody
of STK39 provided a feasible tool for human breast cancer
diagnostics (40). The results of
the present bioinformatics analysis and dual-luciferase assays
demonstrated that miR-299-5p directly targeted STK39. To the best
of our knowledge, this is the first study to demonstrate that
miR-299-5p targets STK39 in breast cancer. It was observed that the
upregulation of STK39 promoted breast cancer cell migration and
invasion and demonstrated that STK39 may be involved in the EMT
process and MMP expression during breast cancer metastasis.
Breast cancer is a heterogeneous disease
characterized by diverse tumour biology, disparate response to
therapeutics, and wide variations in the expression of hormone
receptors and epidermal growth factor receptor (41). The established breast cancer cell
lines belong to different subtypes of human breast cancer, which
may lead to differences in biological behaviours. The
triple-negative breast cancer cell line, MDA-MB-231, exhibits a
high propensity to metastasize. MCF-7 is a hormone receptor
positive, luminal epithelium-like, non-aggressive tumour cell line.
In the present study, both cell lines produced similar results when
subjected to miR-299-5p and STK39 functional experiments. Thus, the
effect of miR-299-5p and STK39 on breast cancer is consistent in
different biological contexts.
In conclusion, the present study provided evidence
that miR-299-5p acts as an anti-tumour miRNA in breast cancer.
miR-299-5p inhibited the migration and invasion capabilities of
breast cancer cells by directly targeting a novel target gene,
STK39. STK39 may play a role in the EMT process and in the
expression of MMPs, both of which facilitate cell metastasis in
breast cancer. As a novel biomarker, miR-299-5p not only evaluates
the efficacy of the treatment in metastatic breast cancer, but also
takes part in the metastasis of breast cancer, which facilitates
its potential application in diagnosis and therapy. The findings of
the present study demonstrated that the association between
miR-299-5p and STK39 regulates breast cancer metastasis and
highlights that it may lead to the development of new diagnostic
and therapeutic approaches to breast cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JZ conceived and designed the study. CL and AW
performed the experiments. CL, YC and YL analyzed the data. CL
wrote the manuscript. YC, HZ and JZ reviewed and edited the
manuscript. All authors read and approved the final manuscript and
agreed to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The experiment was approved by the Ethics Committee
of Xi'an Jiaotong University and all patients provided informed
consent. All experimental procedures involving the use of animals
were approved by the Institutional Animal Care and Use Committee of
Xi'an Jiaotong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cardoso F, Costa A, Senkus E, Aapro M,
André F, Barrios CH, Bergh J, Bhattacharyya G, Biganzoli L, Cardoso
MJ, et al: 3rd ESO-ESMO International Consensus Guidelines for
Advanced Breast Cancer (ABC 3). Ann Oncol. 28:31112017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaffer CL, San Juan BP, Lim E and
Weinberg RA: EMT, cell plasticity and metastasis. Cancer Metastasis
Rev. 35:645–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pickup MW, Mouw JK and Weaver VM: The
extracellular matrix modulates the hallmarks of cancer. EMBO Rep.
15:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie F, Hosany S, Zhong S, Jiang Y, Zhang
F, Lin L, Wang X, Gao S and Hu X: MicroRNA-193a inhibits breast
cancer proliferation and metastasis by downregulating WT1. PLoS
One. 12:e01855652017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xue Y, Xu W, Zhao W, Wang W, Zhang D and
Wu P: miR-381 inhibited breast cancer cells proliferation,
epithelial-to-mesenchymal transition and metastasis by targeting
CXCR4. Biomed Pharmacother. 86:426–433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gong Y, He T, Yang L, Yang G, Chen Y and
Zhang X: The role of miR-100 in regulating apoptosis of breast
cancer cells. Sci Rep. 5:116502015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Breunig C, Pahl J, Küblbeck M, Miller M,
Antonelli D, Erdem N, Wirth C, Will R, Bott A, Cerwenka A and
Wiemann S: MicroRNA-519a-3p mediates apoptosis resistance in breast
cancer cells and their escape from recognition by natural killer
cells. Cell Death Dis. 8:e29732017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang G, Zhang W, Li B, Stringer-Reasor E,
Chu C, Sun L, Bae S, Chen D, Wei S, Jiao K, et al: MicroRNA-200c
and microRNA-141 are regulated by a FOXP3-KAT2B axis and associated
with tumor metastasis in breast cancer. Breast Cancer Res.
19:732017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Samaeekia R, Adorno-Cruz V, Bockhorn J,
Chang YF, Huang S, Prat A, Ha N, Kibria G, Huo D, Zheng H, et al:
miR-206 inhibits stemness and metastasis of breast cancer by
targeting MKL1/IL11 pathway. Clin Cancer Res. 23:1091–1103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Wang YW, Xing AY, Xiang S, Shi DB,
Liu L, Li YX and Gao P: Suppression of SPIN1-mediated PI3K-Akt
pathway by miR-489 increases chemosensitivity in breast cancer. J
Pathol. 239:459–472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue J, Chi Y, Chen Y, Huang S, Ye X, Niu
J, Wang W, Pfeffer LM, Shao ZM, Wu ZH and Wu J: MiRNA-621
sensitizes breast cancer to chemotherapy by suppressing FBXO11 and
enhancing p53 activity. Oncogene. 35:448–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nassar FJ, Nasr R and Talhouk R: MicroRNAs
as biomarkers for early breast cancer diagnosis, prognosis and
therapy prediction. Pharmacol Ther. 172:34–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwarzenbach H, Milde-Langosch K,
Steinbach B, Muller V and Pantel K: Diagnostic potential of
PTEN-targeting miR-214 in the blood of breast cancer patients.
Breast Cancer Res Treat. 134:933–941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Si H, Sun X, Chen Y, Cao Y, Chen S, Wang H
and Hu C: Circulating microRNA-92a and microRNA-21 as novel
minimally invasive biomarkers for primary breast cancer. J Cancer
Res Clin Oncol. 139:223–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benetatos L, Hatzimichael E, Londin E,
Vartholomatos G, Loher P, Rigoutsos I and Briasoulis E: The
microRNAs within the DLK1-DIO3 genomic region: Involvement in
disease pathogenesis. Cell Mol Life Sci. 70:795–814. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, He L, Sun W, Qin Y, Dong W, Zhang
T, Zhang P and Zhang H: miRNA-299-5p regulates estrogen receptor
alpha and inhibits migration and invasion of papillary thyroid
cancer cell. Cancer Manag Res. 10:6181–6193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng Y, He X, Chen H, Duan H, Shao B, Yang
F, Li H, Yang P, Zeng Y, Zheng J, et al: Inhibition of
microRNA-299-5p sensitizes glioblastoma cells to temozolomide via
the MAPK/ERK signaling pathway. Biosci Rep. 38:2018. View Article : Google Scholar
|
|
21
|
Formosa A, Markert EK, Lena AM, Italiano
D, Finazzi-Agro' E, Levine AJ, Bernardini S, Garabadgiu AV, Melino
G and Candi E: MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c,
miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p,
mapped to the 14q32.31 locus, regulate proliferation, apoptosis,
migration and invasion in metastatic prostate cancer cells.
Oncogene. 33:5173–5182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Schooneveld E, Wouters MC, Van der
Auwera I, Peeters DJ, Wildiers H, Van Dam PA, Vergote I, Vermeulen
PB, Dirix LY and Van Laere SJ: Expression profiling of cancerous
and normal breast tissues identifies microRNAs that are
differentially expressed in serum from patients with (metastatic)
breast cancer and healthy volunteers. Breast Cancer Res.
14:R342012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gallolu Kankanamalage S, Karra AS and Cobb
MH: WNK pathways in cancer signaling networks. Cell Commun Signal.
16:722018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gagnon KB and Delpire E: Molecular
physiology of SPAK and OSR1: Two Ste20-related protein kinases
regulating ion transport. Physiol Rev. 92:1577–1617. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Delpire E and Gagnon KB: SPAK and OSR1:
STE20 kinases involved in the regulation of ion homoeostasis and
volume control in mammalian cells. Biochem J. 409:321–331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alessi DR, Zhang J, Khanna A, Hochdorfer
T, Shang Y and Kahle KT: The WNK-SPAK/OSR1 pathway: Master
regulator of cation-chloride cotransporters. Sci Signal. 7:re32014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, Zhu W, Xiong L, Yu X, Chen X and Lin
Q: Role of high expression levels of STK39 in the growth, migration
and invasion of non-small cell type lung cancer cells. Oncotarget.
7:61366–61377. 2016.PubMed/NCBI
|
|
28
|
Huang T, Zhou Y, Cao Y, Tao J, Zhou ZH and
Hang DH: STK39, overexpressed in osteosarcoma, regulates
osteosarcoma cell invasion and proliferation. Oncol Lett.
14:4599–4604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Q, Zhu Y, Liu L, Wang H, Jiang S, Hu
X and Guo J: STK39 blockage by RNA interference inhibits the
proliferation and induces the apoptosis of renal cell carcinoma.
Onco Targets Ther. 11:1511–1519. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Romano G, Veneziano D, Acunzo M and Croce
CM: Small non-coding RNA and cancer. Carcinogenesis. 38:485–491.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan M, Xu H, Waddell N, Shield-Artin K,
Haviv I; kConFab authors, ; McKay MJ and Fox SB: Enhanced RAD21
cohesin expression confers poor prognosis in BRCA2 and BRCAX, but
not BRCA1 familial breast cancers. Breast Cancer Res. 14:R692012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shevde LA, Metge BJ, Mitra A, Xi Y, Ju J,
King JA and Samant RS: Spheroid-forming subpopulation of breast
cancer cells demonstrates vasculogenic mimicry via hsa-miR-299-5p
regulated de novo expression of osteopontin. J Cell Mol Med.
14:1693–1706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong Q, Zhu X, Dai C, Zhang X, Gao X, Wei
J, Sheng Y, Zheng Y, Yu J, et al: Osteopontin promotes
epithelial-mesenchymal transition of hepatocellular carcinoma
through regulating vimentin. Oncotarget. 7:12997–13012. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J, Karimy JK, Delpire E and Kahle
KT: Pharmacological targeting of SPAK kinase in disorders of
impaired epithelial transport. Expert Opin Ther Targets.
21:795–804. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou X, Naguro I, Ichijo H and Watanabe K:
Mitogen-activated protein kinases as key players in osmotic stress
signaling. Biochim Biophys Acta. 1860:2037–2052. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang YT, Heist RS, Chirieac LR, Lin X,
Skaug V, Zienolddiny S, Haugen A, Wu MC, Wang Z, Su L, et al:
Genome-wide analysis of survival in early-stage non-small-cell lung
cancer. J Clin Oncol. 27:2660–2667. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stanton SE, Gad E, Corulli LR, Lu H and
Disis ML: Tumor-associated antigens identified early in mouse
mammary tumor development can be effective vaccine targets.
Vaccine. 37:3552–3561. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mao J, Ladd J, Gad E, Rastetter L, Johnson
MM, Marzbani E, Childs JS, Lu H, Dang Y, Broussard E, et al: Mining
the pre-diagnostic antibody repertoire of TgMMTV-neu mice to
identify autoantibodies useful for the early detection of human
breast cancer. J Transl Med. 12:1212014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rivenbark AG, O'Connor SM and Coleman WB:
Molecular and cellular heterogeneity in breast cancer: Challenges
for personalized medicine. Am J Pathol. 183:1113–1124. 2013.
View Article : Google Scholar : PubMed/NCBI
|