Introduction
The incidence and mortality rates of colorectal
cancer (CRC) vary around the world (1,2). Early
stage CRC commonly shows limited clinical signs, and patients are
often diagnosed at the metastatic stage, rendering therapy
difficult (3). Therefore, early
diagnosis and treatment of CRC remains challenging for patients and
surgeons. In 2016, CRC ranked fourth and second among the most
frequently diagnosed and the deadliest malignancies, respectively,
in the USA (4–7). According to the American Cancer
Society, there were approximately 13,450 newly diagnosed cancer
patients in the US in 2016, 30% of whom presented with CRC
(8). In 2015, 376,000 new CRC cases
were diagnosed in China, with 191,000 succumbing to the malignancy
(9). National polyp screening
programs constitute an early diagnosis tool, which can markedly
improve CRC prognosis (10–14). Early diagnosis and treatment of CRC
is becoming increasingly important to surgeons. Surgery remains the
principal therapeutic option for loco-regional CRC. At present,
robotic and laparoscopic surgeries are performed for CRC, with
improve patient outcome in comparison with traditional surgical
techniques (15–17). However, patient prognosis is not
significantly enhanced. In order to improve the prognosis of CRC
patients, surgeons and pathologists have made unremitting efforts
to examine the prognostic values of various tumor markers. The
American Joint Committee on Cancer (AJCC) tumor-node-metastasis
(TNM) staging system provides a universal modality and guides
clinical treatment (18–21). Based on the original version, AJCC-8
(American Joint Committee on Cancer 8 edition) provides improved
guidance for the individualized treatment of CRC patients and more
effective treatment of patients with IVC peritoneal metastases.
Tumor invasion and metastasis of CRC result from
well-coordinated events involving many intracellular and
extracellular factors (22–24). While many factors affect prognosis
in CRC (25,26), Ki67 is broadly employed in
pathological analyses to evaluate cell proliferation in various
malignancies (27–30). Although Ki67 is expressed in benign
tumors, its levels are very low; however, it is found at high
levels in multiple malignant lesions, and tightly associated with
distant metastasis, resulting in poor patient prognosis. The
prognostic value of Ki67 has been assessed in various types of
cancers, particularly brain, neuroendocrine, and lymphoid tissue
malignancies, and its levels are commonly utilized to grade tumors
(31). Nevertheless, its prognostic
and predictive roles remain debatable mostly as standard
quantification techniques for Ki67 are in existence (32). Ki67 expression is usually examined
as a percentage, which is closely related to the pathologist's
clinical experience.
Hashimoto et al assessed the rate and
clinical significance of fascin expression in association with CRC
progression and cancer cell proliferation based on Ki67 (33). Most often, Ki67 is assessed visually
by pathologists although no consensus is available concerning the
specific regions to score (34).
Meanwhile, whether automated techniques could yield suitable
accuracy and prognostic power for Ki67 is not known. Indeed,
head-to-head comparisons between scores from automated and
pathologist-based techniques in terms of prognostic value have been
rarely reported, and discrepant findings in breast cancer have been
obtained (35–37). Previous reports (38,39)
discussed the role of Ki67 expression in lung and breast cancers,
examining ways to define the cutoff of Ki67 expression. Similar
questions remain for CRC. How to grade Ki67 expression remains
therefore an open question. A 20% cutoff has been reported
(38). Nonetheless, a previous
meta-analysis assessing various cut-off levels of Ki67 in regards
to prognosis suggested a visual cut-off >25% to provide a higher
discriminatory power in mortality risk compared with the remaining
cut-off points evaluated (39).
Signal intensity scores were 0 (negative), 1 (weak), 2 (moderate)
and 3 (strong); positivity extent was scored as 0 (<5%), 1
(5–25%), 2 (>25–50%, 3 (<50–75%) and 4 (>75%). Both
sub-scores were multiplied to yield the final score, which was
considered to be positive if >5 (40). Can a suitable cutoff increase the
prognostic value of Ki67 expression in colorectal cancer? This is
the starting point of the present research; as not many studies
have been reported. Some scholars hold opposite views on the
relationship between Ki67 expression and prognosis in CRC,
suggesting that high Ki67 expression instead reflects better
prognosis (41). Other studies have
reported that mean Ki67 expression is higher in p53-positive cases,
and Ki67 and p53 are not correlated to clinical and pathological
parameters (42). Whether Ki67
expression is related to clinicopathological indicators and
prognosis remains controversial. Meanwhile, the cutoffs vary, and
the outcomes are rather controversial among previous studies.
Therefore, we analyzed the associations of Ki67 expression with
clinicopathological parameters and the prognosis of CRC patients in
this study.
Here we divided cases into four grades based on 25%
intervals of Ki67 immunohistochemical signals. Associations of Ki67
expression levels with clinicopathological factors and CRC
prognosis were analyzed. Prognosis in CRC was also analyzed based
on Ki67 expression according to 5-year disease-free survival (DFS)
and overall survival (OS) in and out of the AJCC-8
stratification.
Patients and methods
Patients
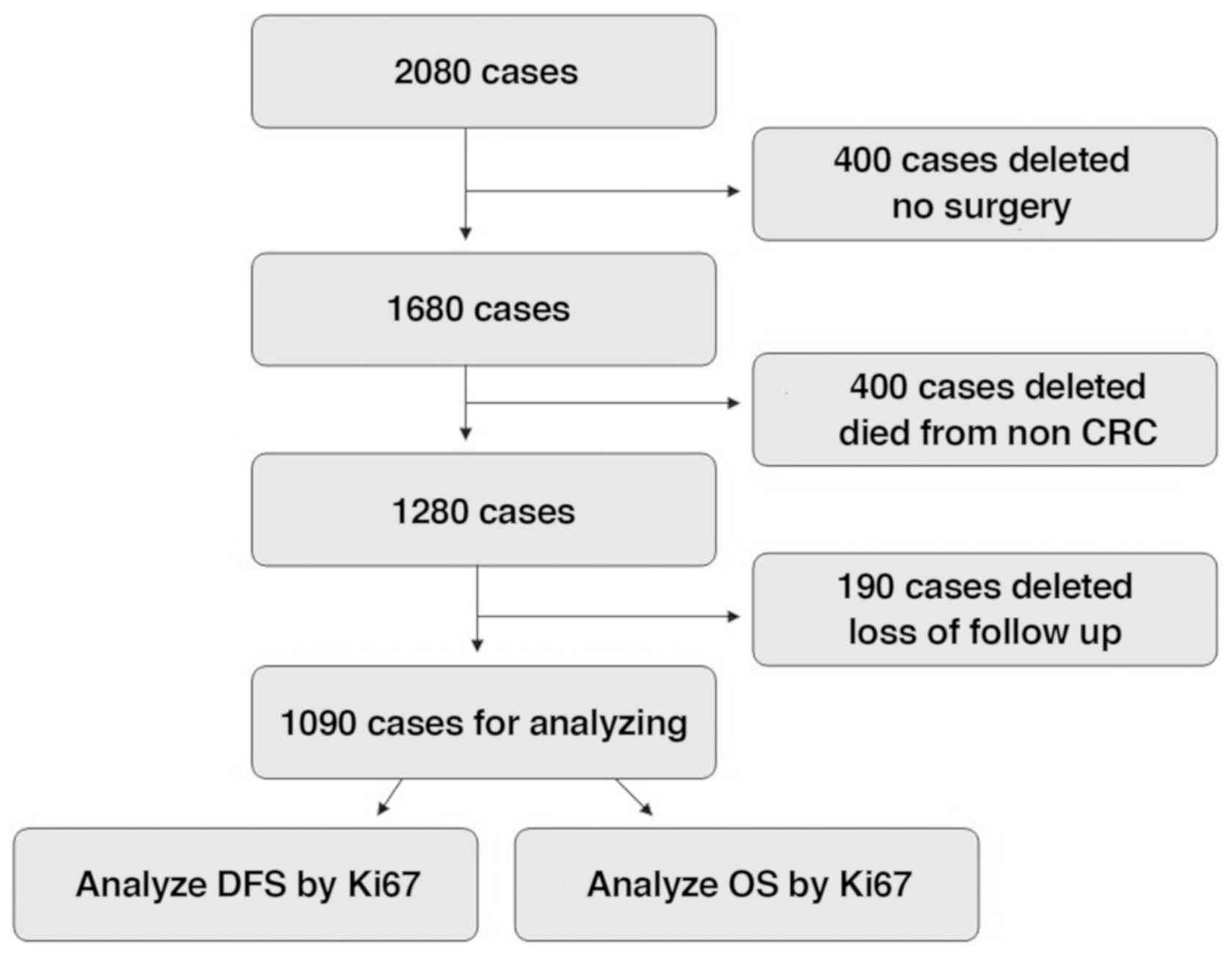
In total, 2,080 CRC cases were enrolled at Huzhou
Central Hospital between January 2006 and December 2012. A total of
400 cases did not undergo surgery, 400 succumbed to non-CRC causes,
and 190 were lost to follow-up and thus were excluded from the
present study. Therefore, 1,090 cases (stage 0 to stage IV) were
involved in the final analysis. Inclusion criteria were CRC
diagnosis by colonoscopy, computed tomography and pathology; no
pre-surgical adjuvant therapy, radical surgery and normal lymph
nodes harvested; other organ metastases found before or during
surgery, and combined resection to achieve R0 resection; complete
postoperative clinical and pathological data; postoperative routine
immunohistochemical and pathological analyses; post-surgical
chemotherapy based on the National Comprehensive Cancer Network
(NCCN) guidelines; adenocarcinoma by pathological diagnosis;
complete follow-up data, including recurrence and metastasis at
follow-up. Exclusion criteria included severe heart, brain, liver
or lung disease which may influence tolerance to surgery; non-CRC
parameters causing death, interstitial or neuronal tumor, lymphoma,
melanoma and other non-adenocarcinomas concomitant with CRC
(Fig. 1).
Follow-up
Routine follow-up was carried out in the outpatient
clinic two weeks post-operation, at 3- and 6-month intervals for
the first and second years, respectively, and yearly for the
remaining 3 years. Phone calls and mail were also used for
follow-up. During the follow-up period, the patient statuses
included i) death, censored and ii) death and recurrence and
censored.
Ethics statement
The current trial followed the 2008 Declaration of
Helsinki, and had approval from the Ethics Committee of Huzhou
Central Hospital (Huzhou, Zhejiang, China). All patients provided
signed informed consent for the use of their tissue samples for
Ki67 immunohistochemistry immunoassay and medical records for
research.
Detection of tissue Ki67
Immunohistochemistry was performed by the Envision
two-step method [cat. no. ZM-0166 (Beijing Zhongshang Jinqiao Co.);
K5007 (Dako)]. The primary antibody was raised against Ki67 (cat.
no. ZM-0166, 1:200 dilution) and K5007 (Dako; no dilution) was used
as the secondary antibody. The steps included: i) Dewaxing with hot
water; ii) antigen repair under high pressure citric with acid at
pH 6.0; iii) hydrogen peroxide blocking of endogenous peroxidase;
iv) primary antibody incubation at 37°C for 30 min; v) secondary
antibody incubation at 37°C for 15 min; vi) DAB staining at 22°C
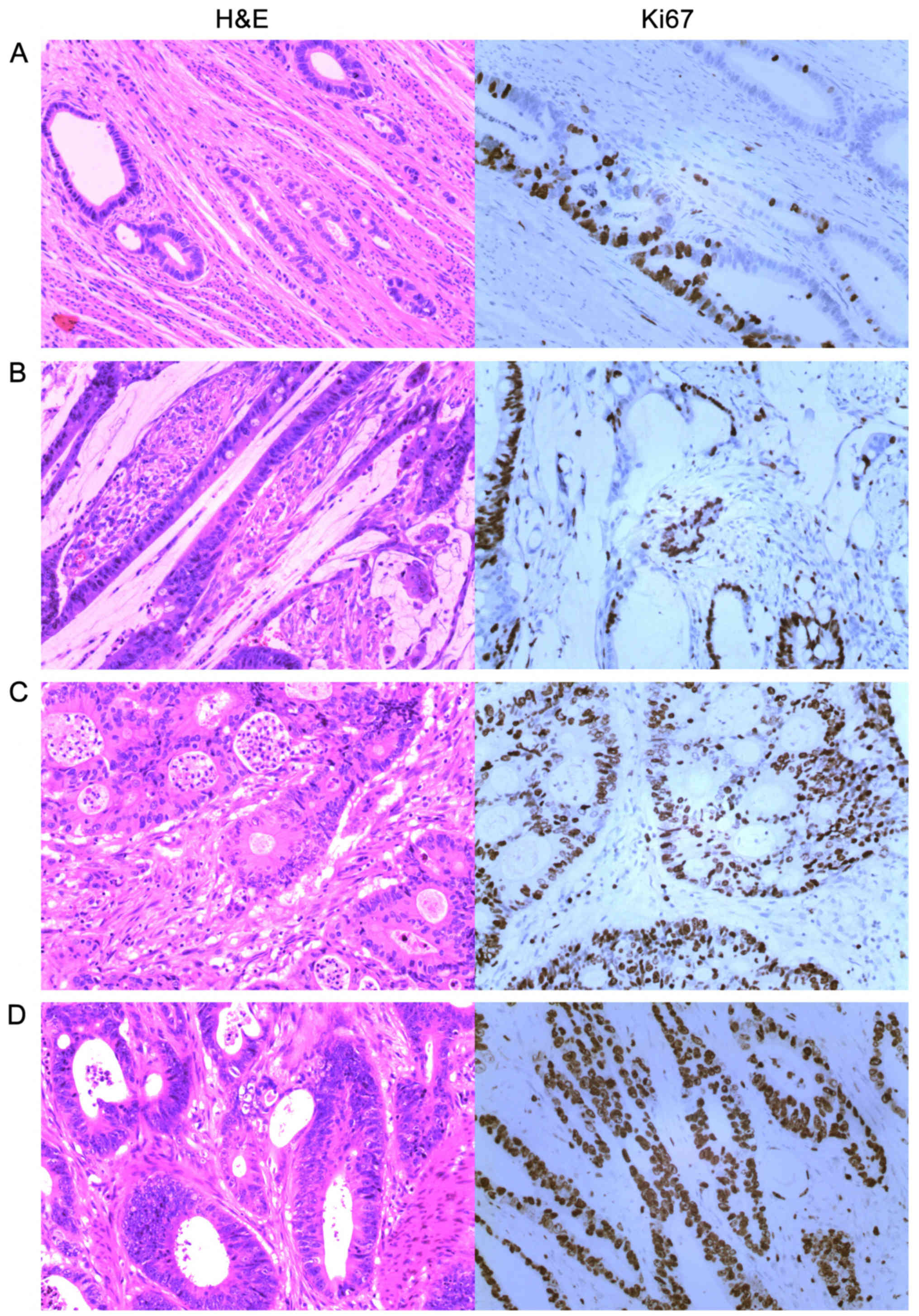
for 5 min; vii) dehydration and mounting. We compared conventional
hematoxylin and eosin (H&E) staining with Ki67 DBA
immunostaining and defined + as >0 and ≤25%; ++ as >25 and
≤50%; +++ as >50 and ≤75%; and ++++ as >75% (Fig. 2).
Surgical methods
According to lesion location and the principle of
malignant tumor resection, the following resection methods were
used: Right hemicolectomy (RHC); left hemicolectomy specimen (LHC),
with the excised portion including the left colon and the
descending colon; Hartmann (HO), with 5 cm tissue distal to the
tumor removed and the distal colorectal segment closed, the
proximal end removed (10 cm), and the proximal end taken for
fistula; anterior rectal resection specimen (AR), with the extent
of resection involving the sigmoid colon and part of the rectum;
anterior rectal perineal resection specimen (APR), with the scope
of resection involving the sigmoid colon, whole rectum and anal
canal and perineum.
Statistical analysis
SPSS 21.0 (IBM Corp.) was employed for data
analysis. Clinicopathological measurement data among groups with
different Ki67 expression patterns were assessed by one-way
analysis of variance (ANOVA); count data were analyzed by Crosstabs
and the Pearson's χ2 test. Bivariate correlation
analysis was performed to assess clinical and pathological
indicators with significant significance in Ki67 expression.
Five-year DFS and OS were analyzed by the Kaplan-Meier method and
the Breslow test. Survival rates were equally assessed by
multivariable Cox's regression according to various clinical,
pathological, and biochemical parameters, which were analyzed in
our previous studies (21,26). Patient statuses were divided into
two: i) Only death was considered an event, and other parameters
were censored for OS analysis; ii) death and recurrence were
considered events, and other parameters were censored for DFS
analysis. Weighted analysis and the non-parametric Chi-square test
were applied to compare DFS and OS under different Ki67 levels.
Results
General data
A total of 1,090 patients of the 2,080 enrolled CRC
cases were evaluated (52.4%), including 550 men (50.5%) and 540
women (49.5%). According to Ki67 expression (+, ++, +++ and ++++),
the entire patient population consisted of 61 (11.1%), 141 (25.6%),
202 (36.7%) and 146 (26.5%) male patients, and 70 (13%), 144
(26.7%), 195 (36.1%) and 131 (24.3%) females, respectively. The
mean age was 62.26 years (range, 17–89).
Clinicopathological properties of the
various groups based on Ki67 expression
According to Ki67 expression (from low to high),
sex, age, American Society of Anesthesiologists (ASA) stage,
location, surgical method, operation time, invasive depth, tumor
differentiation, tumor size, AJCC-8 stage, the number of lymph
nodes harvested, the number of positive lymph nodes, complications,
and chemotherapy status were assessed. By single factor ANONA and F
test, there were no significant differences in age, operation time
and the number of lymph nodes harvested. However, there were
significant differences noted in regards to tumor size and the
number of positive lymph nodes. Regarding measurement variations,
from Ki67+ to Ki67++++, mean and standard deviations were as
follows: Age, 61.64±15.80, 62.79±14.33, 62.21±14.28 and 62.07±14.31
(P=0.880); operation time (min), 153.6±34.8, 151.2±38.6, 155.0±33.2
and 154.1±33.2 (P=0.568); number of lymph nodes harvested,
14.04±1.9, 14.17±1.8, 14.25±1.8 and 14.19±1.9 (P=0.727); tumor size
(cm), 3.58±1.1, 3.38±1.2, 3.74±0.9 and 3.79±0.9 (P<0.001);
number of positive lymph nodes, 0.25±0.9, 0.61±1.4, 2.45±2.2 and
2.86±2.5 (P<0.001). From Ki67(+) to Ki67(++++) significant
differences were found in count variables such as invasive depth,
tumor differentiation, AJCC-8 stage and chemotherapy status
(P<0.001; P<0.001; P=0.003 and P=0.005, respectively).
However, no statistical differences were found in sex, ASA stage,
location, surgical method, and complications (P=0.684, P=0.860,
P=0.439, P=0.768 and P=0.587, respectively). Details are shown in
Table I.
 | Table I.Association of the
clinicopathological features and Ki67 expression in all involved
CRC cases. |
Table I.
Association of the
clinicopathological features and Ki67 expression in all involved
CRC cases.
|
| N | Ki67+ | Ki67++ | Ki67+++ | Ki67++++ | P-value |
|---|
| Sex, n (%) |
|
|
|
|
| 0.684 |
|
Male | 550 | 61 (46.6) | 141 (49.5) | 202 (50.9) | 146 (52.7) |
|
|
Female | 540 | 70 (53.4) | 144 (50.5) | 195 (49.1) | 131 (47.3) |
|
| Mean age
(years) | 1,090 | 61.64±15.80 | 62.79±14.33 | 62.21±14.28 | 62.07±14.31 | 0.880 |
| ASA stage, n
(%) |
|
|
|
|
| 0.860 |
| I | 797 | 94 (71.8) | 212 (74.4) | 293 (73.8) | 198 (71.3) |
|
| II | 264 | 33 (25.2) | 64 (22.5) | 93 (23.4) | 74 (26.7) |
|
|
III | 29 | 4 (3.1) | 9 (3.2) | 11 (2.8) | 5 (1.8) |
|
| Location, n
(%) |
|
|
|
|
| 0.439 |
|
Ileocecum | 73 | 11 (8.4) | 20 (7.0) | 24 (6.0) | 18 (6.5) |
|
| Right
colon | 95 | 4 (3.1) | 31 (10.9) | 41 (10.3) | 19 (6.9) |
|
|
Transverse colon | 174 | 25 (19.1) | 42 (14.7) | 60 (15.1) | 47 (17.0) |
|
| Left
colon | 206 | 24 (18.3) | 57 (20.0) | 75 (18.9) | 50 (18.1) |
|
| Sigmoid
colon | 108 | 16 (12.2) | 20 (7.0) | 40 (10.1) | 32 (11.6) |
|
|
Rectum | 434 | 51 (38.9) | 115 (40.4) | 157 (39.5) | 111 (40.1) |
|
| Surgical method, n
(%) |
|
|
|
|
| 0.768 |
|
RHC | 207 | 21 (16.0) | 58 (20.4) | 80 (20.2) | 48 (17.3) |
|
|
LHC | 431 | 55 (42.0) | 108 (37.9) | 155 (39.0) | 113 (40.8) |
|
| HO | 24 | 3 (2.3) | 7 (2.5) | 6 (1.5) | 8 (2.9) |
|
| AR | 327 | 43 (32.8) | 91 (31.9) | 112 (28.2) | 81 (29.2) |
|
|
APR | 101 | 9 (6.9) | 21 (7.4) | 44 (11.1) | 27 (9.7) |
|
| Operation time
(min) | 1,090 | 153.6±34.8 | 151.2±38.6 | 155.0±33.2 | 154.1±33.2 | 0.568 |
| Invasive depth, n
(%) |
|
|
|
|
|
<0.001a |
| Tis and
T1 | 127 | 24 (18.3) | 53 (18.6) | 31 (7.8) | 19 (6.9) |
|
| T2 | 210 | 5 (3.8) | 66 (23.2) | 112 (28.2) | 27 (9.7) |
|
| T3 | 421 | 78 (59.5) | 92 (32.3) | 132 (33.2) | 119 (43.0) |
|
| T4 | 332 | 24 (18.3) | 74 (6.0) | 122 (30.7) | 112 (40.4) |
|
| Differentiation, n
(%) |
|
|
|
|
|
<0.001a |
|
Well | 194 | 58 (44.3) | 97 (34.0) | 30 (7.6) | 9 (3.2) |
|
|
Moderate | 696 | 69 (52.7) | 177 (62.1) | 282 (71.0) | 168 (60.6) |
|
| Poor or
undifferentiation | 200 | 4 (3.1) | 11 (3.9) | 85 (21.4) | 100 (36.1) |
|
| Tumor size
(cm) | 1,090 | 3.58±1.1 | 3.38±1.2 | 3.74±0.9 | 3.79±0.9 |
<0.001a |
| AJCC-8, n (%) |
|
|
|
|
| 0.003a |
| 0 | 16 | 5 (3.8) | 11 (3.9) | 0 (0) | 0 (0) |
|
| I | 131 | 17 (13.0) | 85 (29.8) | 28 (7.1) | 1 (0.4) |
|
| II | 225 | 93 (71.0) | 112 (39.3) | 12 (3.0) | 8 (2.9) |
|
|
III | 663 | 14 (10.7) | 75 (26.3) | 323 (81.4) | 25 (90.6) |
|
| IV | 55 | 2 (1.5) | 2 (0.7) | 34 (8.6) | 17 (6.1) |
|
| No. of lymph nodes
harvested | 1,090 | 14.04±1.9 | 14.17±1.8 | 14.25±1.8 | 14.19±1.9 | 0.727 |
| No of positive
lymph nodes | 1,090 | 0.25±0.9 | 0.61±1.4 | 2.45±2.2 | 2.86±2.5 |
<0.001a |
| Complications |
|
|
|
|
| 0.587 |
|
Yes | 104 | 13 (9.9) | 28 (9.8) | 32 (8.1) | 31 (11.2) |
|
| No | 986 | 118 (90.1) | 257 (90.2) | 365 (91.9) | 246 (88.8) |
|
| Chemotherapy, n
(%) |
|
|
|
|
| 0.005a |
|
Yes | 895 | 107 (81.7) | 189 (66.3) | 343 (86.4) | 256 (92.4) |
|
| No | 195 | 24 (18.3) | 96 (33.7) | 54 (13.6) | 21 (7.6) |
|
Associations of Ki67 with
clinicopathological features showing significant differences
We further analyzed the associations of
clinicopathological indices which showed significant differences
based on Ki67 expression. Spearman rho coefficients of invasive
depth, tumor differentiation, tumor size, AJCC-8 stage, the number
of positive lymph nodes and chemotherapy status were 0.170 (95% CI
0.113–0.225, P<0.001), 0.456 (95% CI 0.411–0.500, P<0.001),
0.122 (95% CI −0.063–0.181, P<0.001), 0.195 (95% CI 0.138–0.254,
P<0.001), 0.514 (95% CI 0.468–0.558) and −0.201 (95% CI
−0.253–0.148, P<0.001), respectively, as summarized in Table II.
 | Table II.Significant correlations between Ki67
and clinicopathological features of the CRC cases. |
Table II.
Significant correlations between Ki67
and clinicopathological features of the CRC cases.
| Ki67 | Spearman rho | 95% CI
(lower-upper) | P-value |
|---|
| Invasive depth | 0.170 | 0.113–0.225 |
<0.001a |
| Tumor
differentiation | 0.456 | 0.411–0.500 |
<0.001a |
| Tumor size | 0.122 | −0.063–0.181 |
<0.001a |
| AJCC-8 stage | 0.195 | 0.138–0.254 |
<0.001a |
| No of positive
lymph nodes | 0.514 | 0.468–0.558 |
<0.001a |
| Chemotherapy
status | −0.201 | −0.253–0.148 |
<0.001a |
Five-year DFS and OS by Ki67
expression in AJCC-8 stratification
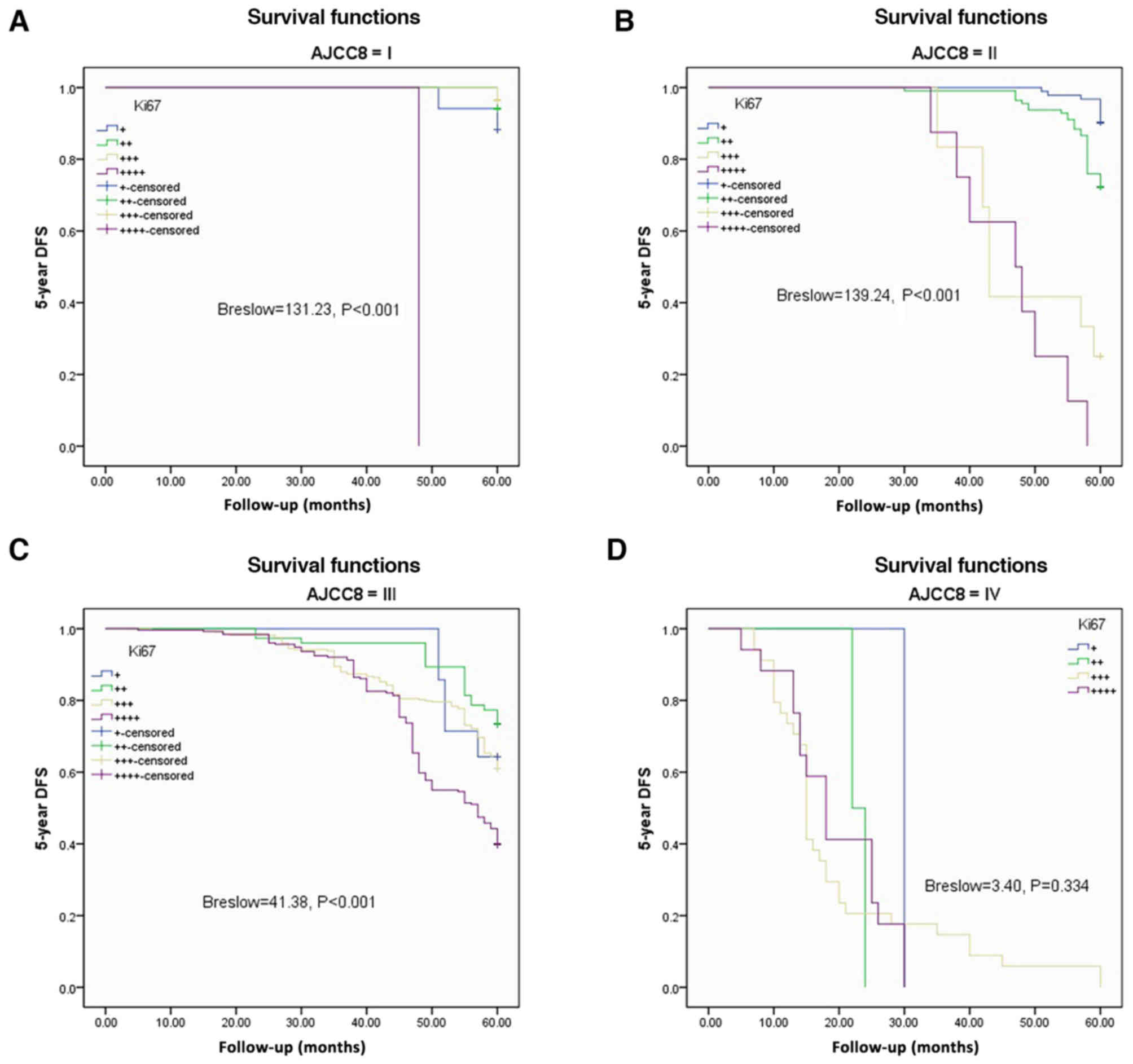
The Kaplan-Meier method and Breslow test were
applied to assess DFS and OS based on Ki67 expression in the AJCC-8
stratification. There were significant differences in DFS among the
various Ki67 expression groups from AJCC-8=I to AJCC-8=III (all
P<0.001), but no statistical significance at AJCC-8=IV (P=0.334;
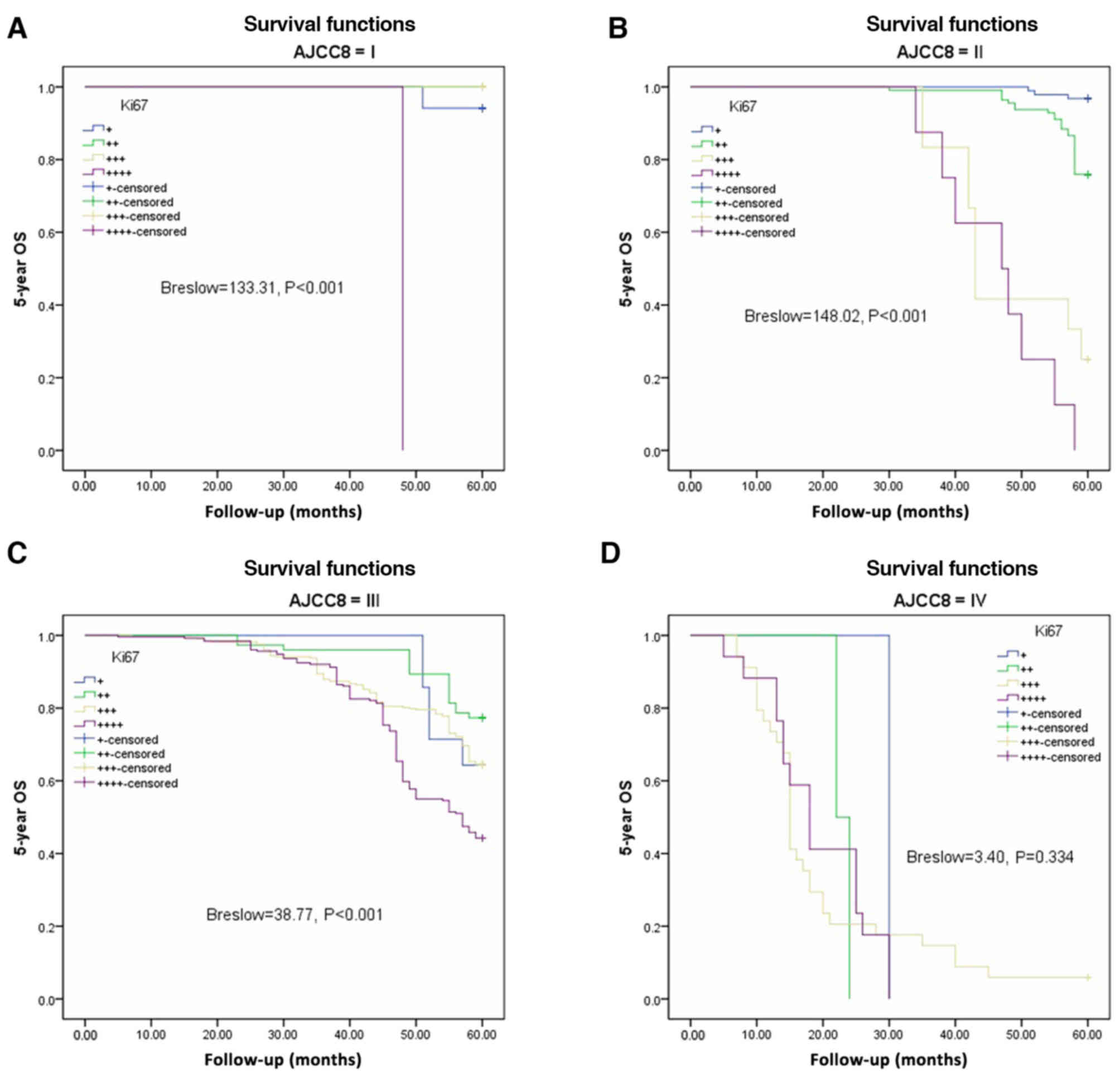
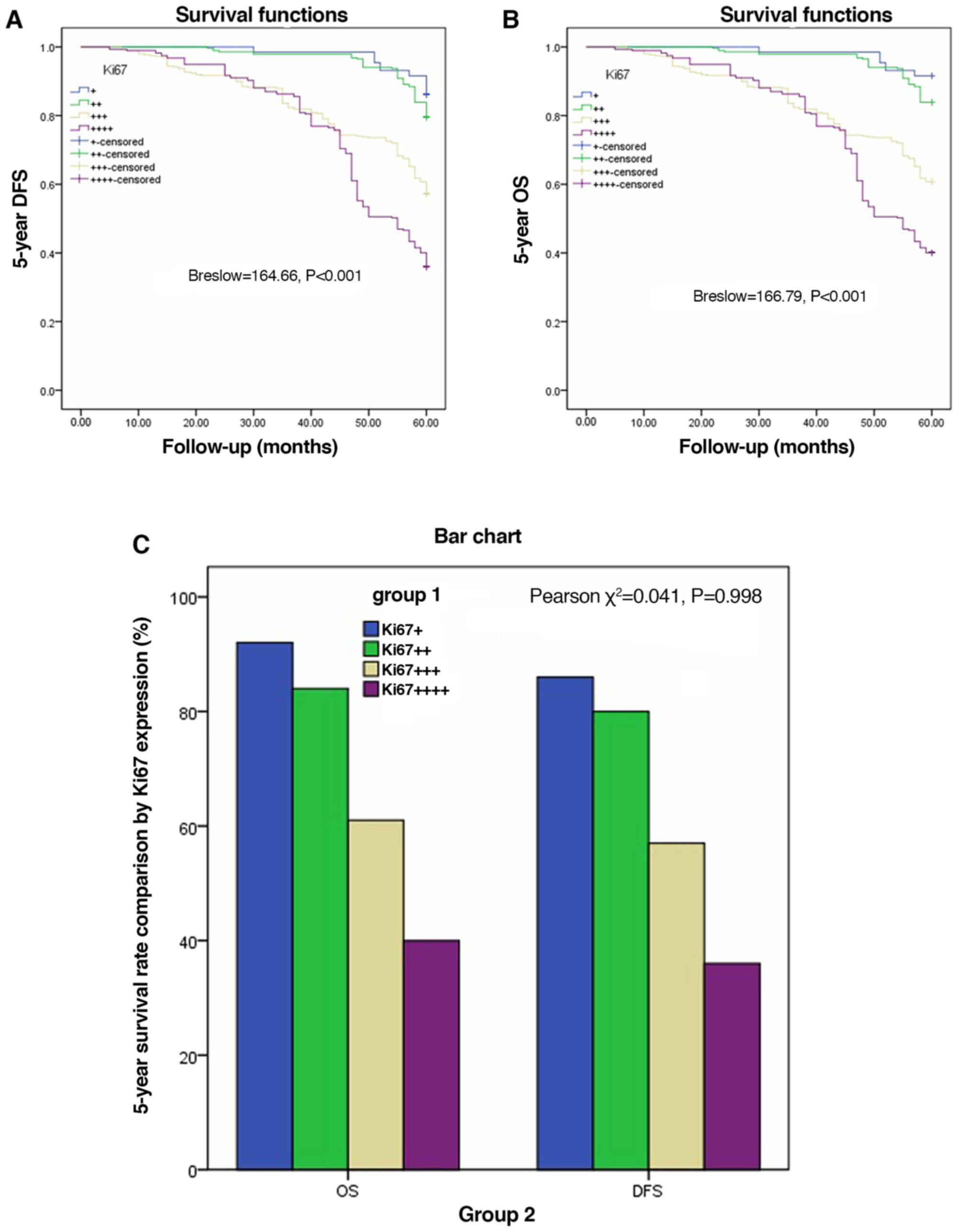
Fig. 3A-D). There were significant
differences in OS among the different Ki67 expression groups from
AJCC-8=I to AJCC-8=III (all P<0.001), but no statistical
significance at AJCC-8=IV (P=0.334; Fig. 4A-D). DFS and OS survival rates were
assessed based on Ki67 expression and AJCC-8 stratification.
Table III documents the DFS and
OS survival rates at different Ki67 levels in the AJCC-8
stratification. There were no patients with Ki67+++ or Ki67++++ at
stage 0, not only in DFS but also in OS. Table IV shows a comparison of DFS and OS
at different Ki67 expression levels. There were no significant
differences among all columns (χ2=0.202, P=0.653;
χ2=0.098, P=0.755; χ2=0.136, P=0.713 and
χ2=0.211 P=0.646 respectively). DFS and OS showed
statistical differences only between Ki67 expression groups
(Breslow=164.66, P<0.001; Brelow=166.79, P<0.001), indicating
elevated Ki67 expression was associated with poorer prognosis.
Details are shown in Table IV and
Fig. 5A and B. Crosstabs analysis
showed that there were no significant differences between DFS and
OS at different Ki67 expression levels (χ2=0.041,
P=0.098; Fig. 5C).
 | Table III.Analysis of the 5-year DFS and OS by
Ki67 expression according to AJCC-8 stage. |
Table III.
Analysis of the 5-year DFS and OS by
Ki67 expression according to AJCC-8 stage.
|
|
| N | + | ++ | +++ | ++++ |
|---|
| DFS | 0 | 16 | 100 | 100 | – | – |
|
| I | 131 | 88.2 | 94.1 | 96.4 | 0 |
|
| II | 225 | 90.3 | 72.3 | 25.0 | 0 |
|
| III | 663 | 64.3 | 73.3 | 61.0 | 39.8 |
|
| IV | 55 | 0 | 0 | 0 | 0 |
| OS | 0 | 16 | 100 | 100 | – | – |
|
| I | 131 | 94.1 | 100 | 100 | 0 |
|
| II | 225 | 96.8 | 75.9 | 25.0 | 0 |
|
| III | 663 | 64.3 | 77.3 | 64.4 | 44.2 |
|
| IV | 55 | 0 | 0 | 5.9 | 0 |
 | Table IV.Comparison of the 5-year DFS and OS
by Ki67 expression (%). |
Table IV.
Comparison of the 5-year DFS and OS
by Ki67 expression (%).
|
| N | + | ++ | +++ | ++++ | Breslow | P-value |
|---|
| DFS | 1,090 | 86.3 | 79.6 | 57.2 | 36.1 | 164.66 | <0.001 |
| OS | 1,090 | 91.6 | 83.9 | 60.7 | 40.1 | 166.79 | <0.001 |
| χ2 |
| 0.202 | 0.098 | 0.136 | 0.211 |
|
|
| P-value |
| 0.653 | 0.755 | 0.713 | 0.646 |
|
|
Multivariable analysis of CRC
prognostic factors
To identify independent predictive factors of CRC
prognosis, Cox proportional hazard model analysis was performed.
Sex, invasive depth, lymph node metastasis, tumor differentiation,
AJCC-8 stage, chemotherapy status and Ki67 were included in the
model. Regarding sex and chemotherapy status, hazard ratios (HRs)
(95% CIs) for Female/Male and No/Yes were 0.976 (0.796–1.198) and
0.986 (0.522–1.862), respectively, which were not significant
(P=0.819 and P=0.964, respectively). HRs (95% CIs) for invasive
depth at T2/Tis and T1, T3/Tis and T1, T4/Tis and T1 were 1.5336
(0.855–2.748), 1.845 (1.034–3.290) and 1.331 (0.746–2.376),
respectively, showing statistically significant differences
(P=0.03). HRs (95% CIs) for lymph node metastasis at N1/N0 and
N2/N0 were 0.909 (0.616–1.342) and 1.690 (1.168–2.446)
(P<0.001). Regarding differentiation (moderate/well, poor or
no/well), AJCC-8 stage (I/0, II/0, III/0 and IV/0), Ki67 expression
(++/+, +++/+ and ++++/+) HRs (95% CIs) were, respectively, 1.677
(1.036–2.715), 6.443 (3.883–10.756) and 134.375 (0–1.173E+29);
2,015.297 (0–1.736E+30), 1,098.443 (0–9.461E+30) and 30582.466
(0–2.637E+31); and 2.59 (1.327–50055), 4.732 (2.275–9.843) and
6.762 (3.226–14.174), showing significant differences (all
P<0.001; Table V).
 | Table V.Multivariate analysis of prognosis
for CRC using OS. |
Table V.
Multivariate analysis of prognosis
for CRC using OS.
| Factors | HR (95% CI) | P-value |
|---|
| Sex |
| 0.819 |
|
Female/Male | 0.976
(0.796–1.198) |
|
| Invasive depth |
| 0.030a |
| T2/Tis
andT1 | 1.5336
(0.855–2.748) |
|
| T3/Tis
and T1 | 1.845
(1.034–3.290) |
|
| T4/Tis
and T1 | 1.331
(0.746–2.376) |
|
| Lymph node
metastasis |
|
<0.001a |
|
N1/N0 | 0.909
(0.616–1.342) |
|
|
N2/N0 | 1.690
(1.168–2.446) |
|
|
Differentiation |
|
<0.001a |
|
Moderate/well | 1.677
(1.036–2.715) |
|
| Poorly
or undifferentiation/well | 6.443
(3.883–10.756) |
|
| AJCC-8 stage |
|
<0.001a |
|
I/0 | 134.375
(0–1.173E+29) |
|
|
II/0 | 2,015.297
(0–1.736E+30) |
|
|
III/0 | 1,098.443
(0–9.461E+30) |
|
|
IV/0 | 3,0582.466
(0–2.637E+31) |
|
| Chemotherapy
status |
| 0.964 |
|
No/Yes | 0.986
(0.522–1.862) |
|
| Ki67 |
|
<0.001a |
|
++/+ | 2.59
(1.327–50055) |
|
|
+++/+ | 4.732
(2.275–9.843) |
|
|
++++/+ | 6.762
(3.226–14.174) |
|
Discussion
In the present study, we initially considered
whether to include stage 0. Some Ki67 data were not available in
this period, which inevitably resulted in that survival classified
by Ki67 was not calculated. According to AJCC-8 (American Joint
Committee on Cancer 5th edition) and later versions, colorectal
cancer CRC) stage 0 refers to TisN0M0. This refers to carcinoma
in situ, which is localized within the epithelium or
infiltrates the lamina propria. Although it is not invasive cancer,
it is invasive. We believe that stage 0 tumors are within the
mucosa, but not invasive malignant tumors because invasive
malignancies usually refer to advanced cancer. Therefore, we
included stage 0 in this study. With the increasing attention paid
to early CRC detection, this study found that stages 0-II only
accounted for 34.12% of all cases, and most cases were stage III in
the same period, accounting for 60%. Therefore, the diagnosis and
treatment of CRC still requires further investigation to improve
prognosis. As published in previous reports (10–14),
national polyp screening programs could improve CRC prognosis
drastically. Research and application of many tumor markers improve
the early detection rate of tumors as well as patient prognosis
(43–45). Ki67 expression is a tumor marker
that has been used for a long time in clinical practice, but its
classification criteria and relationship with prognosis remain
controversial (39,46,47).
What role does Ki67 expression play in the prognosis of colorectal
cancer? Melling et al (41)
considered that high Ki67 has a good prognostic value for CRC,
contrasting with Luo et al (40). Their results showed that high Ki67
expression is associated with low tumor stage and nodal status, but
not with tumor grade, histological tumor type or tumor
localization, representing an independent predictor of favorable
survival; these findings strongly argue for a clinical utility of
Ki67 immunostaining as an independent prognostic biomarker in CRC.
This study showed that high Ki67 expression was associated not only
with tumor stage (AJCC-8), tumor size and nodal status, but also
with tumor differentiation, tumor invasive depth, and chemotherapy
status, which were not discussed in Meling et al (41). We used 25% as a cutoff for Ki67
expression and different study methods such as in and out AJCC-8
stratification, which may explain the discrepancy. The above
findings indicate that it is ideal to use 25% as a cutoff for Ki67
expression.
The nuclear protein Ki67 was first described in
Hodgkin lymphoma-derived cells (48). It is expressed throughout cell
division, but is highly suppressed in resting cells (G0 phase)
(49,50). Ki67 staining is broadly utilized
clinically as an index of cell proliferation, although its
functions and dynamics are poorly understood. Miller et al
(51) tracked Ki67 amounts in
single cells without external stimuli, and demonstrated that it
accumulates only in the S, G2, and M phases, with continuous
degradation in G1 and G0. Ki67 expression is commonly utilized in
oncology as a proliferation indicator. Here we explored the
association of Ki67 expression with CRC.
Forones et al (42) hypothesized that Ki67 and P53 are not
correlated with clinical and pathologic parameters. This study
showed differences in tumor invasive depth based on the Ki67
amounts, and elevated Ki67 expression was associated with increased
invasive depth. This may be because tumor invasion and metastasis
result from highly coordinated events involving many intracellular
and extracellular factors (18,19).
Another reason may be that higher Ki67 amounts are associated with
poorer tumor differentiation and high AJCC grade, as well as
elevated positive lymph node rate, corroborating this study.
We assessed the associations of clinicopathological
features with Ki67 expression, and the results showed strong
correlations, which require confirmation by molecular and genetic
studies. We also showed that the surgical method was not related to
Ki67 expression, demonstrating that cancer location in CRC is not
associated with Ki67 expression. In this study, all tumor cases
were adenocarcinomas. The associations of other types of cancer,
such as melanoma, carcinoid, malignant stoma tumor and
neurofibroma, with Ki67 expression, were not covered in this work.
This is a flaw in the present study. We expect relevant studies to
be performed.
One of the highlights of this work is that DFS and
OS were analyzed based on Ki67 expression in and out of the AJCC-8
stratification. Higher Ki67 expression levels reflected poorer DFS
and OS out of the AJCC-8 stratification with statistical
significance.
Feng et al (52) reported that elevated Ki67 expression
is associated with poorer prognosis in breast cancer. Shin et
al (53) pointed out that high
Ki67 reflects poor prognosis in CRC. This suggests that the gene
encoding Ki67 has a similar function in tumors. However, it is
puzzling that analysis based on the AJCC-8 stratification revealed
comparable DFS and OS for different Ki67 levels in stage IV cases.
In general, Ki-67 is closely related to RNA transcription, and
shows high expression levels during cell division and
proliferation, reflecting the activity of cell division and
increasing the risk of tumor invasion and metastasis, which worsen
patient prognosis (54). We believe
that patients with stage IV disease have poorer prognosis, and
adverse factors other than Ki67 expression may play additional
roles, such as surgery, chemotherapy, patient physical status, and
genetic factors (55–60). In the present study, cases with
stage IV disease were limited in number, which could have caused a
bias. We look forward to undergoing future research to assess
patients with stage IV CRC and Ki67 expression. Finally, the
present study demonstrated that Ki67 expression is an independent
risk factor for poor prognosis in CRC by multivariate analysis and
Cox regression.
Mucinous vs. non-mucinous lesions have different
molecular mechanisms (61,62), but this issue was not investigated
in this study. We used the Broder classification of
clinicopathological features to analyze Ki67 expression. Some
limitations may exist, including no analysis of the marker P53.
In conclusion, a cutoff of 25% is a good
classification tool. In such classification, high Ki67 amounts are
closely associated with poor prognosis in CRC and independently
predict prognosis in the AJCC-8 stratification.
Acknowledgements
We are grateful to Professors Liqing Li, Qiang Yan
and Zhihong Ma for critically revising this manuscript, as well as
to Professor Jingliang Ping and Wei Xu for carrying out the
pathological analyses. Trial registration: Chinese Clinical Trial
Registry ChiCTR20190404 Registered 4 April 2019 Retrospectively
registered http://:www.chictrorgcnindexaspx.
Funding
No current external funding sources sponsored this
study, which was funded by Project 2018C37090 to GT.
Availability of data and materials
The corresponding author will provide all data upon
reasonable request.
Authors' contributions
GT, GZ, JL and ZZ conceived and designed the study.
GT and YC performed the procedures of data collection and
statistical analysis and wrote the initial manuscript. PN and XX
were involved in the conception of the study and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current trial followed the 2008 Declaration of
Helsinki, and had approval from the Ethics Committee of Huzhou
Central Hospital (Huzhou, Zhejiang, China). All patients provided
signed informed consent for the use of their tissue samples for
Ki67 immunohistochemistry immunoassay and medical records for
research.
Patient consent for publication
Not applicable.
Competing interests
All authors declare no competing interests regarding
the present study.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen ST, Wu MC, Hsu TC, Yen DW, Chang CN,
Hsu WT, Wang CC, Lee M, Liu SH and Lee CC; Health Economics and
Outcome Research Group, National Taiwan University Hospital, :
Comparison of outcome and cost among open, laparoscopic, and
robotic surgical treatments for rectal cancer: A propensity score
matched analysis of nationwide inpatient sample data. J Surg Oncol.
117:497–505. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ali I, Lone MN, Al-Othman ZA, Al-Warthan A
and Sanagi MM: Heterocyclic scaffolds: Centrality in anticancer
drug development. Curr Drug Targets. 16:711–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ali I, Wani WA, Haque A and Saleem K:
Glutamic acid and its derivatives: Candidates for rational design
of anticancer drugs. Future Med Chem. 5:961–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ali I, Haque A, Saleem K and Hsieh MF:
Curcumin-I Knoevenagel's condensates and their Schiff's bases as
anticancer agents: Synthesis, pharmacological and simulation
studies. Bioorg Med Chem. 21:3808–3820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ali I, Wani WA, Saleem K and Haque A:
Platinum compounds: A hope for future cancer chemotherapy.
Anticancer Agents Med Chem. 13:296–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buskermolen M, Cenin DR, Helsingen LM,
Guyatt G, Vandvik PO, Haug U, Bretthauer M and Lansdorp-Vogelaar I:
Colorectal cancer screening with faecal immunochemical testing,
sigmoidoscopy or colonoscopy: A microsimulation modelling study.
BMJ. 367:l5383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Obaro AE, Burling DN and Plumb AA: Colon
cancer screening with CT colonography: Logistics,
cost-effectiveness, efficiency and progress. Br J Radiol.
91:201803072018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Digoras I, Arrospide A, Portillo I,
Arana-Arri E, Martínez-Indart L, Mar J, de Koning HJ, Lastra R,
Soto-Gordoa M, van der Meulen M and Lansdorp-Vogelaar I: Evaluation
of the colorectal cancer screening Programme in the Basque Country
(Spain) and its effectiveness based on the Miscan-colon model. BMC
Public Health. 18:782017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arana-Arri E, Idigoras I, Uranga B, Pérez
R, Irurzun A, Gutiérrez-Ibarluzea I, Fraser CG and Portillo I;
EUSKOLON Group, : Population-based colorectal cancer screening
programmes using a faecal immunochemical test: Should faecal
haemoglobin cut-offs differ by age and sex? BMC Cancer. 17:5772017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
European Colorectal Cancer Screening
Guidelines Working Group, ; von Karsa L, Patnick J, Segnan N, Atkin
W, Halloran S, Lansdorp-Vogelaar I, Malila N, Minozzi S, Moss S, et
al: European guidelines for quality assurance in colorectal cancer
screening and diagnosis: Overview and introduction to the full
supplement publication. Endoscopy. 45:51–59. 2013.PubMed/NCBI
|
|
15
|
Park JS, Kang H, Park SY, Kim HJ, Woo IT,
Park IK and Choi GS: Long-term oncologic after robotic versus
laparoscopic right colectomy: A prospective randomized study. Surg
Endosc. 33:2975–2981. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JC, Lee JL, Yoon YS, Kim CW, Park IJ
and Lim SB: Robotic left colectomy with complete mesocolectomy for
splenic flexure and descending colon cancer, compared with a
laparoscopic procedure. Int J Med Robot. 14:e19182018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de'Angelis N, Abdalla S, Bianchi G, Memeo
R, Charpy C, Petrucciani N, Sobhani I and Brunetti F: Robotic
versus laparoscopic colorectal cancer surgery in elderly patients:
A propensity score match analysis. J Laparoendosc Adv Surg Tech A.
28:1334–1345. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology (NCCN
Guidelines®): Colon Cancer, 2016. Available from: URL:.
https://www.nccn.org/professionals/physician_gls/default.aspx
|
|
19
|
Practice Guidelines in Oncology: (NCCN
Guidelines®): Rectal Cancer, 2016. Available from: URL.
https://www.nccn.org/professionals/physician_gls/default.aspx
|
|
20
|
Yao HW, Wu HW and Liu YH: From traditional
population-based approach to individualized precision medicine: The
interpretation of update on The AJCC Colorectal Cancer Staging
System, Eighth Edition. Zhonghua Wai Ke Za Zhi. 55:24–27. 2017.(In
Chinese). PubMed/NCBI
|
|
21
|
Tong GJ, Zhang GY, Liu J, Zheng ZZ, Chen
Y, Niu PP and Xu XT: Comparison of the eighth version of the
American Joint Committee on Cancer manual to the seventh version
for colorectal cancer: A retrospective review of our data. World J
Clin Oncol. 9:148–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo W and Giancotti FG: Integrin
signalling during tumour progression. Nat Rev Mol Cell Biol.
5:816–826. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiang Z, Mi Y, Jing H, Li L, Jiong SHI,
Baorui L and Xiaoping Q: Expressions of C-MET, COX-2, MSS and Ki-67
in colorectal cancer and correlations with prognosis. J Prev Med
Chin PLA. 36:598–601. 2018.9 (In Chinese).
|
|
26
|
Tong G, Xu W, Zhang G, Liu J, Zheng Z,
Chen Y, Niu P and Xu X: The role of tissue and serum
carcinoembryonic antigen in stages I to III of colorectal cancer-A
retrospective cohort study. Cancer Med. 7:5327–5338. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tian Y, Ma Z, Chen Z, Li M, Wu Z, Hong M,
Wang H, Svatek R, Rodriguez R and Wang Z: Clinicopathological and
prognostic value of Ki-67 expression in bladder cancer: A
systematic review and meta-analysis. PLoS One. 11:e01588912016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arihiro K, Oda M, Ohara M, Kadoya T, Osaki
A, Nishisaka T, Shiroma N and Kobayashi Y: Comparison of visual
assessment and image analysis in the evaluation of Ki-67 expression
and their prognostic significance in immunohistochemically defined
luminal breast carcinoma. Jpn J Clin Oncol. 46:1081–1087. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clay V, Papaxoinis G, Sanderson B, Valle
JW, Howell M, Lamarca A, Krysiak P, Bishop P, Nonaka D and Mansoor
W: Evaluation of diagnostic and prognostic significance of Ki-67
index in pulmonary carcinoid tumours. Clin Transl Oncol.
19:579–586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berlin A, Castro-Mesta JF, Rodriguez-Romo
L, Hernandez-Barajas D, González-Guerrero JF, Rodríguez-Fernández
IA, González-Conchas G, Verdines-Perez A and Vera-Badillo FE:
Prognostic role of Ki-67 score in localized prostate cancer: A
systematic review and meta-analysis. Urologic Oncology, Seminars
and Original Investigations. Elsevier; Amsterdam: 2017, View Article : Google Scholar
|
|
31
|
Niazi MKK, Pennell M, Elkins C, Hemminger
J, Jin M, Kirby S, Kurt H, Miller B, Plocharczyk E and Roth R:
Entropy based quantification of Ki-67 positive cell images and its
evaluation by a reader study. SPIE Medical Imaging. International
Society for Optics and Photonics; Bellingham: pp. p86760I2013
|
|
32
|
Liu Y, Yin W, Yan T, Du Y, Shao Z and Lu
J: The clinical significance of Ki-67 as a marker of prognostic
value and chemosensitivity prediction in hormone-receptor-positive
breast cancer: A meta-analysis of the published literature. Curr
Med Res Opin. 29:1453–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashimoto Y, Skacel M, Lavery IC,
Mukherjee AL, Casey G and Adams JC: Prognostic significance of
fascin expression in advanced colorectal cancer: An
immunohistochemical study of colorectal adenomas and
adenocarcinomas. BMC Cancer. 6:2412006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dowsett M, Nielsen TO, A'Hern R, Bartlett
J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et
al: Assessment of Ki67 in breast cancer: Recommendations from the
International Ki67 in Breast Cancer working group. J Natl Cancer
Inst. 103:1656–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Konsti J, Lundin M, Joensuu H, Lehtimäki
T, Sihto H, Holli K, Turpeenniemi-Hujanen T, Kataja V, Sailas L,
Isola J and Lundin J: Development and evaluation of a virtual
microscopy application for automated assessment of Ki-67 expression
in breast cancer. BMC Clin Pathol. 11:32011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mohammed ZM, McMillan DC, Elsberger B,
Going JJ, Orange C, Mallon E, Doughty JC and Edwards J: Comparison
of visual and automated assessment of Ki-67 proliferative activity
and their impact on outcome in primary operable invasive ductal
breast cancer. Br J Cancer. 106:383–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Klauschen F, Wienert S, Schmitt WD, Loibl
S, Gerber B, Blohmer JU, Huober J, Rüdiger T, Erbstößer E, Mehta K,
et al: Standardized Ki67 diagnostics using automated
scoring-Clinical validation in the GeparTrio Breast Cancer Study.
Clin Cancer Res. 21:3651–3657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei DM, Chen WJ, Meng RM, Zhao N, Zhang
XY, Liao DY and Chen G: Augmented expression of Ki-67 is correlated
with clinicopathological characteristics and prognosis for lung
cancer patients: An up-dated systematic review and meta-analysis
with 108 studies and 14,732 patients. Respir Res. 19:1502018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Petrelli F, Viale G, Cabiddu M and Barni
S: Prognostic value of different cut-off levels of Ki-67 in breast
cancer: A systematic review and meta-analysis of 64,196 patients.
Breast Cancer Res Treat. 153:477–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luo ZW, Zhu MG, Zhang ZQ, Ye FJ, Huang WH
and Luo XZ: Increased expression of Ki-67 is a poor prognostic
marker for colorectal cancer patients: A meta analysis. BMC Cancer.
19:1232019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Melling N, Kowitz CM, Simon R, Bokemeyer
C, Terracciano L, Sauter G, Izbicki JR and Marx AH: High Ki67
expression is an independent good prognostic marker in colorectal
cancer. J Clin Pathol. 69:209–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Forones NM, Oshima C, Nanogaki S, Tanaka M
and Barbosa V: Determination of proliferative activity using Ki67
and expression of p53 in colorectal cancer. Arq Gastroenterol.
36:122–126. 1999.(In Portuguese). PubMed/NCBI
|
|
43
|
Bacher JW, Flanagan LA, Smalley RL, Nassif
NA, Burgart LJ, Halberg RB, Megid WM and Thibodeau SN: Development
of a fluorescent multiplex assay for detection of MSI-High tumors.
Dis Markers. 20:237–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Geiersbach KB and Samowitz WS:
Microsatellite instability and colorectal cancer. Arch Pathol Lab
Med. 135:1269–1277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Iacopetta B and Watanabe T: Predictive
value of microsatellite instability for benefit from adjuvant
fluorouracil chemotherapy in colorectal cancer. Gut. 55:1671–1672.
2006.PubMed/NCBI
|
|
46
|
Abubakar M, Howat WJ, Daley F, Zabaglo L,
McDuffus LA, Blows F, Coulson P, Raza Ali H, Benitez J, Milne R, et
al: High-throughput automated scoring of Ki67 in breast cancer
tissue microarrays from the Breast Cancer Association Consortium. J
Pathol Clin Res. 2:138–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Abubakar M, Orr N, Daley F, Coulson P, Ali
HR, Blows F, Benitez J, Milne R, Brenner H, Stegmaier C, et al:
Prognostic value of automated KI67 scoring in breast cancer: A
centralised evaluation of 8088 patients from 10 study groups.
Breast Cancer Res. 18:1042016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a human
nuclear antigen associated with cell proliferation. Int J Cancer.
31:13–20. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715.
1984.PubMed/NCBI
|
|
50
|
Bullwinkel J, Baron-Lühr B, Lüdemann A,
Wohlenberg C, Gerdes J and Scholzen T: Ki-67 protein is associated
with ribosomal RNA transcription in quiescent and proliferating
cells. J Cell Physiol. 206:624–635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Miller I, Min M, Yang C, Tian C, Gookin S,
Carter D and Spencer SL: Ki67 is a graded rather than a binary
marker of proliferation versus quiescence. Cell Rep.
24:1105–1112.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Feng X, Li H, Kornaga EN, Dean M,
Lees-Miller SP, Riabowol K, Magliocco AM, Morris D, Watson PH,
Enwere EK, et al: Low Ki67/high ATM protein expression in malignant
tumors predicts favorable prognosis in a retrospective study of
early stage hormone receptor positive breast cancer. Oncotarget.
7:85798–85812. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shin IY, Sung NY, Lee YS, Kwon TS, Si Y,
Lee YS, Oh ST and Lee IK: The expression of multiple proteins as
prognostic factors in colorectal cancer: Cathepsin D, p53, COX-2,
epidermal growth factor receptor, C-erbB-2, and Ki-67. Gut Liver.
8:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Scopa CD, Tsamandas AC, Zolota V,
Kalofonos HP, Batistatou A and Vagianos C: Potential role of bcl-2
and ki-67 expression and apoptosis in colorectal carcinoma: A
clinicopathologic study. Dig Dis Sci. 48:1990–1997. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
De Roock W, Claes B, Bernasconi D, De
Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V,
Papamichael D, Laurent-Puig P, et al: Effects of KRAS, BRAF, NRAS,
and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy
in chemotherapy-refractory metastatic colorectal cancer: A
retrospective consortium analysis. Lancet Oncol. 11:753–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Aprile G, Macerelli M, De Maglio G,
Pizzolitto S and Fasola G: The relevance of BRAF and extended ras
mutational analyses for metastatic colorectal cancer patients. OA
Mol Oncol. 1:72013.
|
|
57
|
Kislitsin D, Lerner A, Rennert G and Lev
Z: K-ras mutations in sporadic colorectal tumors in Israel: Unusual
high frequency of codon 13 mutations and evidence for
nonhomogeneous representation of mutation subtypes. Dig Dis Sci.
47:1073–1079. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fransén K, Klintenäs M, Osterström A,
Dimberg J, Monstein HJ and Söderkvist P: Mutation analysis of the
BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas.
Carcinogenesis. 25:527–533. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wong R and Cunningham D: Using predictive
biomarkers to select patients with advanced colorectal cancer for
treatment with epidermal growth factor receptor antibodies. J Clin
Oncol. 26:5668–5670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sartore-Bianchi A, Martini M, Molinari F,
Veronese S, Nichelatti M, Artale S, Di Nicolantonio F, Saletti P,
De Dosso S, Mazzucchelli L, et al: PIK3CA mutations in colorectal
cancer are associated with clinical resistance to EGFR-targeted
monoclonal antibodies. Cancer Res. 69:1851–1857. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shia J, Schultz N, Kuk D, Vakiani E,
Middha S, Segal NH, Hechtman JF, Berger MF, Stadler ZK, Weiser MR,
et al: Morphological characterization of colorectal cancers in The
Cancer Genome Atlas reveals distinct morphology-molecular
associations: Clinical and biological implications. Mod Pathol.
30:599–609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Song GA, Deng G, Bell I, Kakar S,
Sleisenger MH and Kim YS: Mucinous carcinomas of the colorectum
have distinct molecular genetic characteristics. Int J Oncol.
26:745–750. 2005.PubMed/NCBI
|