Introduction
Although surgical resection, radio chemotherapy and
immunotherapy have considerably advanced, NSCLC still has a high
mortality rate. The 5-year survival rate for NSCLC is only
approximately 15% (1–3). The major challenge in lung cancer
therapy is identifying new targets that can supplement the current
treatment strategies (4), and
exploring the factors affecting NSCLC progression is important for
clinical therapy.
Apoptosis is known as programmed cell death,
resulting in the efficient and orderly removal of damaged cells
(5). Two primary molecular
signaling pathways trigger cell apoptosis: The extrinsic (death
receptor) and the intrinsic (mitochondrial) pathway (6). The intrinsic pathway is initiated by
different stress responses and converges at mitochondria (7). Cytochrome c release from the
mitochondria into the cytoplasm is crucial to initiate the
apoptotic cascade (8). Cytochrome
c binds to IP3 receptors at the endoplasmic
reticulum (ER) after release from the mitochondria, resulting in a
localized increase of calcium (Ca2+) concentration,
which promotes more cytochrome c release (9,10).
When cytochrome c reaches cytotoxic levels in the cytosol,
it will activate cysteine proteases (caspase-3 and caspase-9) and
eventually kill the cells (11,12).
In mammals, oxysterol-binding protein-related
proteins (ORPs) are a 12-member gene family (13). ORP8 is one of the ORPs that locates
to ER-mitochondria contact sites (14). A previous study indicated that ORP8
suppresses the expression of ATP binding cassette transporter A1
(ABCA1) (15). In hepatic cells,
ORP8 modulated lipid homeostasis and sterol regulatory
element-binding protein (SREBP) activity (16). Other functions of ORP8 have been
reported, including inhibition of cell migration (17), regulation of the cell cycle in HepG2
cells (18) and regulation of
phosphatidylinositol-4,5-biphosphate [PtdIns(4,5)P2]
levels at the plasma membrane (19). Notably, ORP8 induced hepatocellular
carcinoma (HCC) cells to ER stress (20) and mediated 25-hydroxycholesterol
(25-OHC) cytotoxicity (21). In
gastric cancer, ORP8 inhibited cell growth and activated apoptosis
(22).
In the present study, it was revealed that ORP8
plays a crucial role in NSCLC tumorigenesis. The overexpression of
ORP8 markedly attenuated the malignant features of NSCLC cells by
affecting cell proliferation, anchorage-independent growth and
apoptosis induction. Furthermore, it was revealed that ORP8 induced
NSCLC apoptosis through the mitochondrial signaling pathway. In
addition, the relationship between ORP8 expression and miR-421
inhibition was assessed. The present findings indicated that ORP8
could be a novel target in NSCLC tumorigenesis.
Materials and methods
Cell lines and reagents
NSCLC cell lines (A549, H1975, HCC827) and normal
lung cell line (NL20) were purchased from the American Type Culture
Collection (ATCC). The cells were maintained in 5% CO2
at 37°C, in pH 7.4 DMEM with 10% FBS, penicillin (100
U/ml)/streptomycin (100 µg/ml) antibiotic mixture. Mito Tracker Red
was obtained from Invitrogen; Thermo Fisher Scientific, Inc.
Hoechst 33342 was purchased from Sigma-Aldrich; Merck KGaA.
Clinical specimens
Tissue specimens from adjacent tissue and matched
primary lung adenocarcinoma tissues (median age was 56 years old)
were collected from 15 patients between September 2017 and
September 2018 at the First Affiliated Hospital of Zhengzhou
University (Henan, China). Samples were immediately stored in
liquid nitrogen. All samples were obtained after written informed
consent was provided from each patient and approval for the study
was obtained from the Ethics Committee of the First Affiliated
Hospital of Zhengzhou University.
Immunohistochemistry
Tissue samples were fixed in 10% (v/v) formaldehyde
at room temperature for 24 h, embedded in paraffin and cut into
5-µm sections. IHC staining was performed according to a previous
protocol (23) using ORP8 (dilution
1:200; product code ab99069; Abcam) antibody. The relative
expression was assessed by the Image-Pro Premier software (v9.0)
program (Media Cybernetics, Inc.).
Gene transfer
Lentiviruses carrying ORP8-cDNA (accession no.
NM_001003712), miR-421 mimic and miR-421 inhibitor were obtained
from Shanghai GenePharma Co., Ltd. The miR-421 mimic sequence was
5′-CGCGGGUUAAUUACAGACAACUA-3′. The miR-421 mimic negative control
(NC) sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′. The miR-421
inhibitor sequence was 5′-GCGCCCAAUUAAUGUCUGUUGAU-3′. The miR-421
inhibitor negative control (NC) sequence was
5′-CAGUACUUUUGUGUAGUACAA-3′. For lentiviral infection,
1×106 cells were resuspended in 100 µl DMEM containing
Polybrene (5 µg/ml) and lentivirus (MOI=10) in a 24-well plate.
After 6 h of infection at 5% CO2 and 37°C, 400 µl DMEM
was added. The infection efficiency was detected using western
blotting or qPCR after 3 days of infection.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from cell lines using TRIzol
reagent with a well-established method. The RNA samples were
reverse-transcribed with the PrimeScript™ RT Reagent kit (DRR047A;
Takara Bio, Inc.). qPCR was performed with the SYBR Advantage qPCR
Premix (Takara Bio, Inc.) kit, following the manufacturer's
protocol. qPCR was detected using the ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, inc.). The qPCR conditions
were 95°C for 2 min followed by 40 cycles at 95°C for 15 sec and
60°C for 40 sec and a final dissociation stage. The relative
expression levels were calculated using the 2−∆∆Cq
method (24), and U6 or actin was
used as the endogenous controls. The primer sequences are listed in
Table I.
 | Table I.The oligonucleotide primers used. |
Table I.
The oligonucleotide primers used.
| Gene | Forward primer
5′-3′ | Reverse primer
5′-3′ |
|---|
| Chop |
GCCTTTCTCCTTTGGGACACTGTCCAGC |
CTCGGCGAGTCGCCTCTACTTCCC |
| Bip |
CCTGGGTGGCGGAACCTTCGATGTG |
CTGGACGGGCTTCATAGTAGACCGG |
| ORP8 |
GAACAGGGAGATTTTGAATCA |
TCCTGTGAGTGGATCAAGTTC |
| Actin |
GGCATCCTCACCCTGAAGTA |
AGGTGTGGTGCCAGATTTTC |
| miR-421 |
CTCACTCACATCAACAGACATTAATT |
TATGGTTGTTCTGCTCTCTGTGTC |
| U6 |
CTCGCTTCGGCAGCACATATACT |
ACGCTTCACGAATTTGCGTGTC |
Western blot analysis
The cells were harvested and lysed in RIPA lysis
buffer (Beyotime Institute of Biotechnology). Protein
concentrations were determined using a BCA kit (Beyotime Institute
of Biotechnology). The amount of protein loaded per lane was 20 µg.
The protein samples were separated by 10% SDS-PAGE electrophoresis
and transferred to polyvinylidene difluoride (PVDF) membranes
(Bio-Rad Laboratories, Inc.). Membranes were blocked with bovine
serum albumin (5%) for 1 h at room temperature with gentle shaking,
incubated with the primary antibodies (1:1,000) at 4°C for 12–16 h,
and then incubated with the HRP-labeled secondary antibodies (goat
anti-mouse 1:1,000 and goat anti-rabbit 1:3,000) for 1 h at 25°C.
Finally, the membranes were detected by enhanced chemiluminescence
(ECL) plus western blot detection reagents (Thermo Fisher
Scientific, Inc.). Proteins were quantified by a gel imager (Tanon
5200; Tanon Science and Technology). The corresponding primary
antibodies were as follows: ORP8 (product code ab99069; Abcam),
actin (product no. 3700), caspase-3 (product no. 14220), caspase-9
(product no. 9502), cytochrome c (product no. 12963), COX 4
(product no. 4844; all from Cell Signaling Technology, Inc.). The
HRP-conjugated secondary antibodies (product nos. 7076 and 7074)
were obtained from Cell Signaling Technology, Inc.
MTS assay
To detect cell proliferation, H1975, A549 and HCC827
cells (1×103 cells/well) stably expressing a control or
ORP8 were seeded in 96-well plates. For each well, 20 µl of the MTS
solution (Promega Corp.) was added. After 1 h of incubation, 25 µl
of 10% SDS solution was added, and the absorbance was detected at
492 and 690 nm.
Anchorage-independent growth
Cells (8×103/ml/well) were suspended and
added to the upper layer of solidified 1 ml DMEM/10% FBS/0.3% agar
with 2 ml of DMEM/10% FBS/0.6% in the bottom layer of the agar in
each well of a 6-well plate. After maintenance for 10–14 days in 5%
CO2 at 37°C, the colonies were counted using the
Image-Pro Plus software (version 6.2; Media Cybernetics, Inc.). The
diameter of each colony was >25 µm.
Hoechst 33342 staining
Cell apoptosis analysis was performed with Hoechst
33342 staining in H1975, A549, and HCC827 cells. Cells
(1×106) were collected, washed with cold PBS, stained
with Hoechst 33342 (20 µg/ml) at 37°C for 10 min, resuspended in 20
µl PBS, and immediately imaged using an Olympus BX53 fluorescence
microscope (Olympus Corp.) at an ×400 magnification. The number of
apoptotic cells in each group were counted in 10 random fields with
>500 cells.
Mitochondrial isolation
H1975 and A549 cells (1×107) were
collected and washed with PBS, and cytochrome c release was
determined using a mitochondrial isolation kit (Beyotime Institute
of Biotechnology), according to the manufacturer's protocol.
Assessment of the intracellular
cytochrome c distribution
To detect the intracellular cytochrome c
distribution, confocal microscopy was used as previously described
(25). Cells were incubated with
Mito Tracker dye (Mito Tracker Red; Molecular Probes; Thermo Fisher
Scientific, Inc.) for 10–15 min in an incubator in the dark. Then
the slides were fixed with 4% formaldehyde at room tempetature for
30 min. The fixed slides were stained with an anti-cytochrome
c antibody at 4°C for 12–16 h, then with a FITC-labeled
antibody at 37°C for 30 min. The cytochrome c antibody
(product no. 12963) was purchased from Cell Signaling Technology,
the FITC-labeled antibody (product no. A-21202) was purchased from
Thermo Fisher Scientific, Inc. Images were obtained using different
excitation filters and merged.
Dual-luciferase reporter assay
The potential targets of miR-421 were predicted by
miRanda (http://www.microrna.org/microrna/home.do), miRDB
(http://mirdb.org/), miRWalk (http://mirwalk.umm.uni-heidelberg.de/) and TargetScan
(http://www.targetscan.org/vert_72/).
The 3′-UTR segments of miR-421 binding sites of ORP8 were inserted
into the Dual-Glo luciferase assay system plasmid, pGLO (Promega
Corporation). pGLO vectors and mimic-miR-421 were co-transfected
into cells. Then, the cells were subjected to luciferase activity
detection with a luciferase assay kit (Promega Corporation)
according to the manufacturer's protocol. Renilla
fluorescence was used as the internal standard.
Statistical analysis
The results are presented as the mean ± standard
deviation (SD) of 3 independent experiments, and each dose or
treatment was tested in triplicate. For the statistical analyses,
all data were analyzed by Student's t-test for pairwise comparisons
or one-way analysis of variance (ANOVA) followed by Bonferroni test
for multivariate analysis. Differences were considered to be
statistically significant when the P-value was <0.05.
Results
ORP8 is expressed at low levels in
human NSCLC
NSCLC is the most common type of lung cancer, and
numerous oncogenes are associated with this disease. ORP8 is a
member of the oxysterol-binding protein-related protein (ORP)
family of proteins that are expressed in the brain, liver, lung,
spleen, and kidney in mice (15).
However, ORP8 expression in human NSCLC has not been well defined.
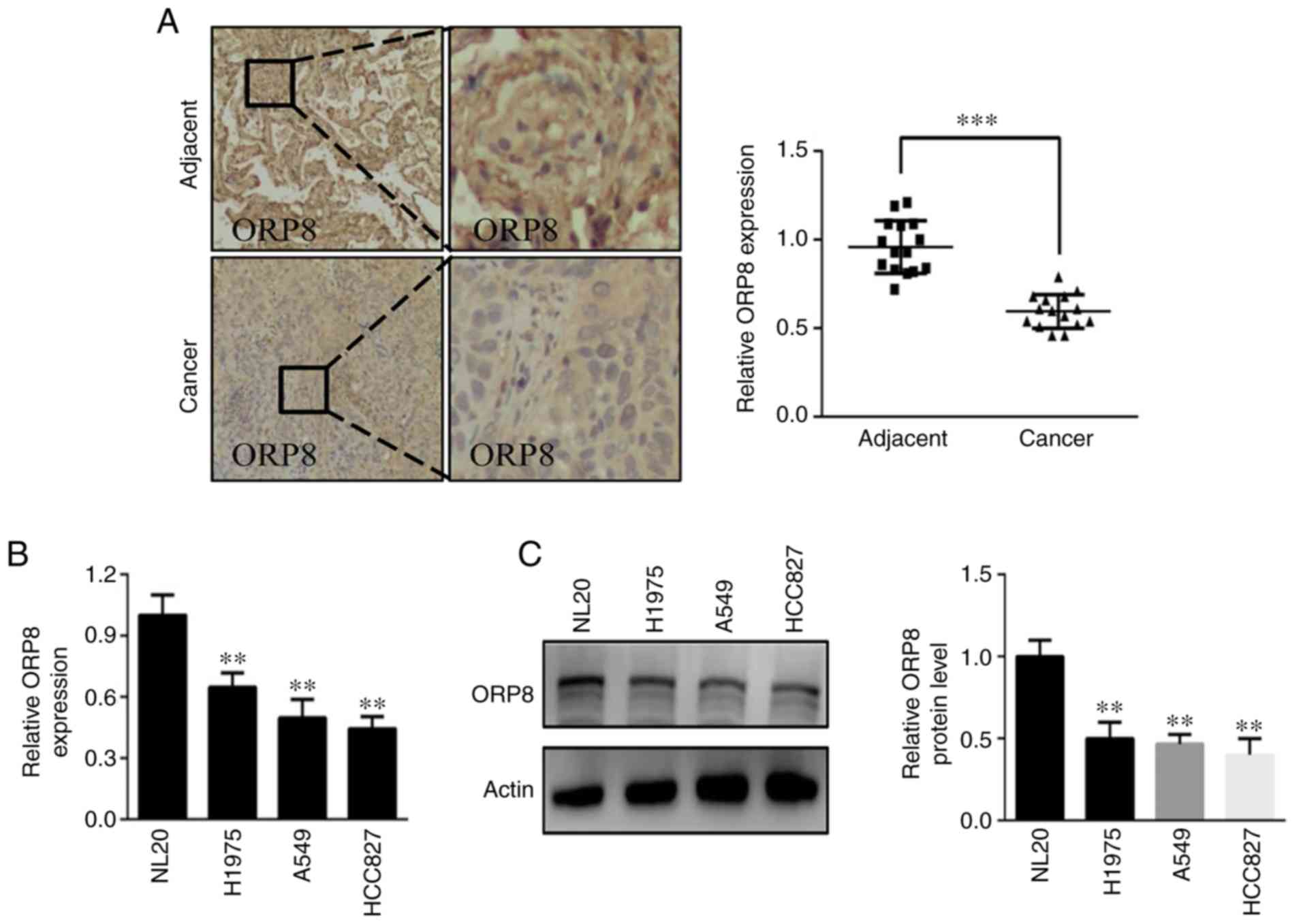
Initially, the expression of ORP8 was investigated in 15-paired
NSCLC specimens and lung cancer cell lines. The results indicated
that ORP8 was expressed at lower levels in the NSCLC tissues than
in the adjacent non-tumor tissues (Fig.
1A). Similarly, ORP8 expression was downregulated in several
NSCLC cell lines (HCC827, A549 and H1975) compared with that of the
normal NL20 lung cell line both at the mRNA and protein levels
(Fig. 1B and C). These results
indicated that ORP8 downregulation may promote NSCLC
progression.
ORP8 overexpression in human NSCLC
cells reduces their tumorigenic properties
Aberrant cell proliferation is a hallmark of cancer,
and the elimination of uncontrolled cell differentiation
contributes to cancer treatment (26). In the present study, it was
hypothesized that ORP8 may play a crucial role in cell growth. To
assess the function of ORP8 in NSCLC, stable ORP8-overexpressing
cells were generated. The overexpression efficiency was verified in
H1975, A549 and HCC827 cells using western blotting (Figs. 2A and S1A and B). MTS and anchorage-independent
cell growth assays were investigated to evaluate the effect of ORP8
expression. The results revealed that ORP8 overexpression inhibited
proliferation in H1975, A549 and HCC827 cells (Fig. 2B, D and F). In addition, colony
formation was reduced in ORP8-overexpressing cells (Fig. 2C, E and G), which indicated that
increased ORP8 expression reduced cell transformation.
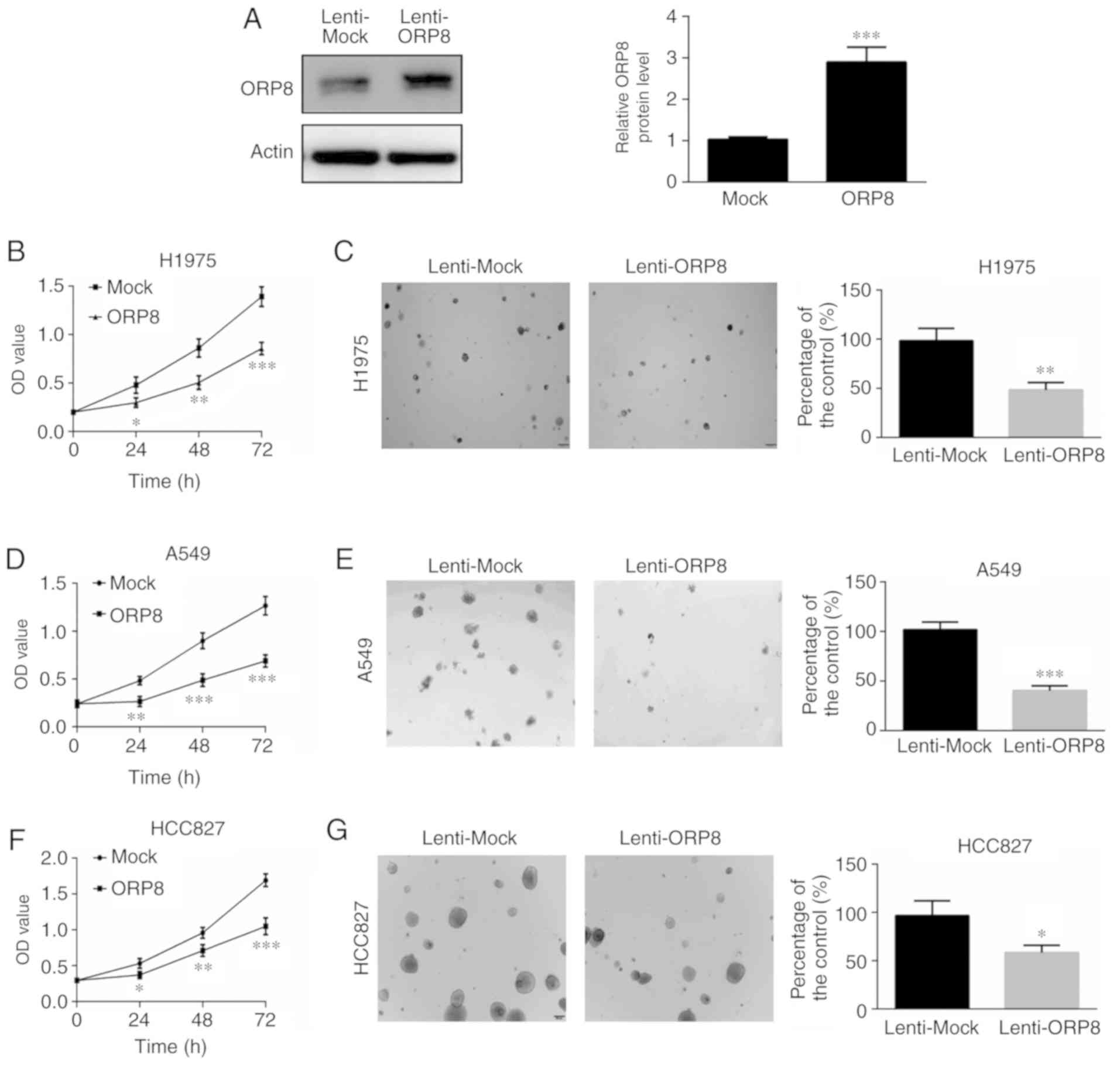 | Figure 2.ORP8 overexpression inhibits H1975,
A549 and HCC827 cell growth. (A) H1975 cells were infected with a
lentivirus carrying ORP8 cDNA, and ORP8 overexpression efficiency
was detected using western blotting. (B, D and F) Overexpression of
ORP8 decreased the proliferation of H1975, A549 and HCC827 lung
cancer cells. Cell growth was determined at 24, 48, and 72 h using
an MTS assay. (C, E and G) Overexpression of ORP8 reduced the
anchorage independent growth of H1975, A549 and HCC827 cells.
Colonies were detected using a microscope and the Image Pro Plus
(v.6) software. The data represent the mean ± SD of three
individual experiments (*P<0.05, **P<0.01, and ***P<0.001,
n=3). ORP8, oxysterol-binding protein-related protein 8. |
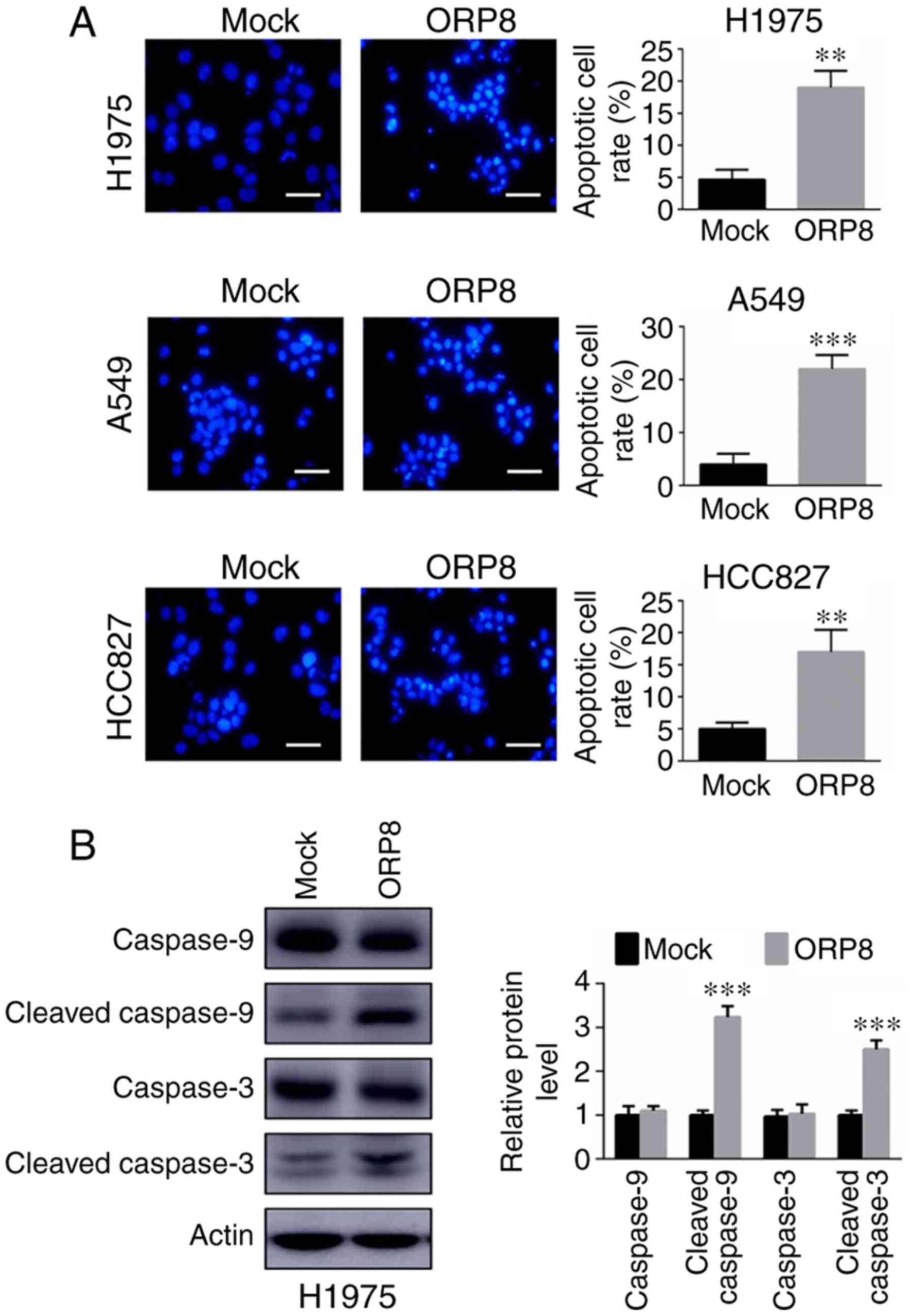
During the progression of transformation, cell
growth is regulated not only by proliferation but also by cell
death or apoptosis. Studies have revealed that ORP8 plays a key
role in coordinating apoptosis in liver and gastric cancer
(20,22). In the present study, the apoptosis
assay results indicated that overexpression of ORP8 resulted in
increased apoptosis in H1975, A549 and HCC827 lung cancer cell
lines stained with Hoechst 33342 (Fig.
3A). The protein levels of cleaved caspase-3 and caspase-9 were
analyzed using western blot analysis. The cleavage of caspases was
significantly enhanced, indicating that apoptosis was induced in
ORP8-overexpressing cells (Figs. 3B
and S1C). Overall, the present
data revealed that ORP8 was involved in modulating cell survival
and apoptosis.
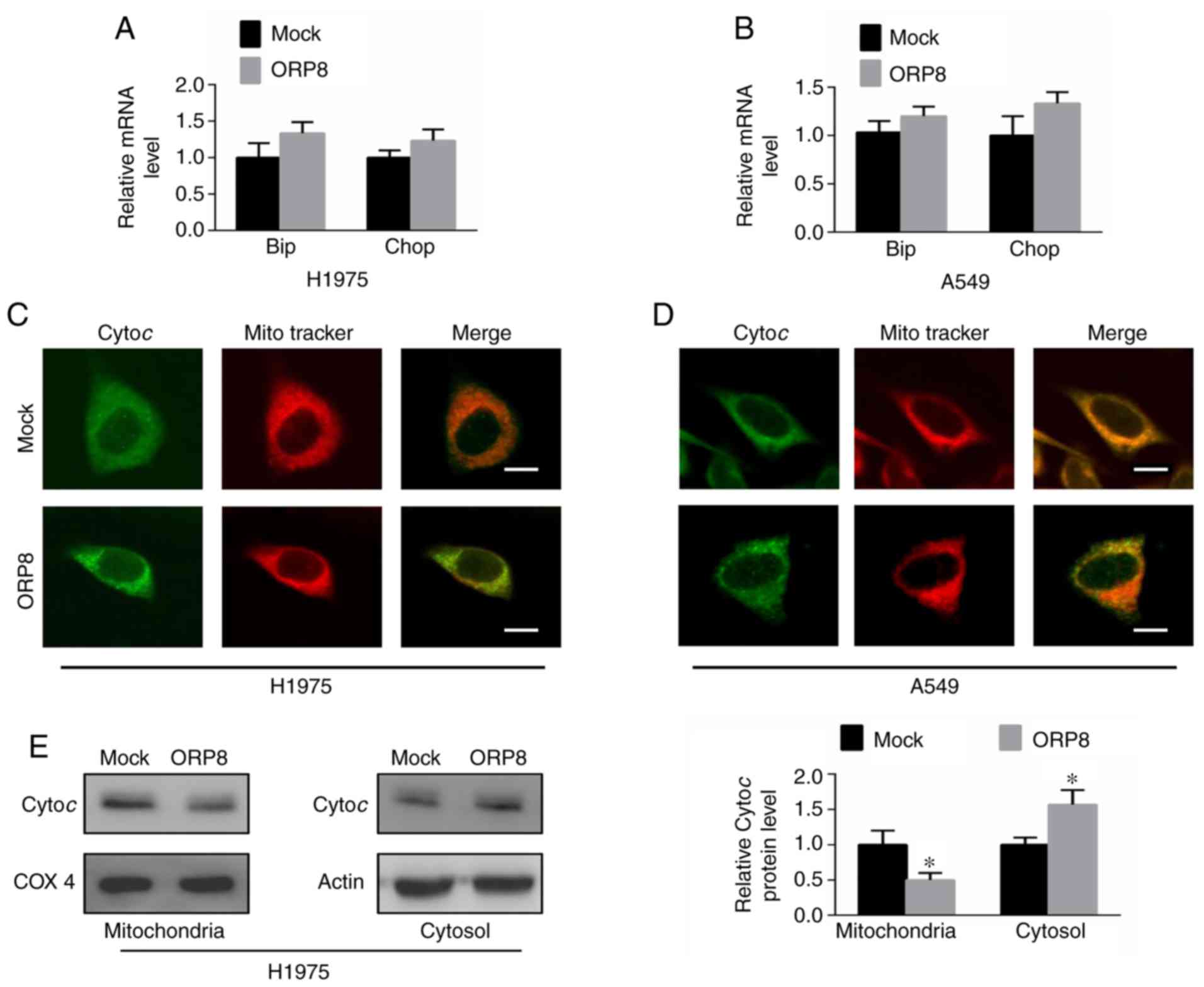
ORP8 overexpression releases
cytochrome c from mitochondria
In liver cancer cells and gastric cancer cells,
studies have shown that ORP8 overexpression could induce ER stress
responses (20–22) and that ORP8 localizes to
ER-mitochondria contact sites (14). To determine whether ORP8
overexpression induced ER stress in NSCLC cells, the mRNA levels of
Chop and Bip, which are the central components of ER stress
responses, were detected (27).
Results revealed that the expression of Chop and Bip was barely
altered at both the mRNA and protein levels in H1975 and A549 cells
(Figs. 4A and B and S2A and B). Mitochondria are the
intracellular organelles that have critical roles in the apoptotic
pathway (28), and the release of
cytochrome c from the mitochondria into the cytoplasm is
crucial to initiate the apoptotic cascade (8). Thus, to better understand the role of
ORP8 in inducing cell apoptosis, cytochrome c release from
mitochondria was assessed. Immunofluorescence staining was employed
to observe the localization of cytochrome c relative to
mitochondria. The present results revealed that cytochrome c
co-localized with the mitochondrial marker (Mito Tracker) in the
control H1975 and A549 cells (Fig. 4C
and D, upper images), while cytosolic cytochrome c was
increased in the ORP8-overexpressing cells (Fig. 4C and D, lower images). Western blot
analysis similarly revealed that cytosolic cytochrome c
levels were significantly enhanced after ORP8 overexpression in
H1975, A549 and HCC827 cells (Figs.
4E and S2C and D). These data
indicated that ORP8 upregulation promoted the release of cytochrome
c from mitochondria, which induced cancer cell
apoptosis.
ORP8 is a target of miR-421
Studies have revealed that upregulated miR-421
expression is associated with poor prognosis in NSCLC (29). Therefore, it was assessed whether
the downregulation of ORP8 in NSCLC was due to miR-421
dysregulation. Notably, miR-421 was revealed to target ORP8 by
using four different analytical programs (Fig. 5A). In addition, miR-421 was
upregulated in NSCLC cells, compared with NL20 cells, in contrast
to ORP8 downregulation in NSCLC (Fig.
5B). When cells were infected with miR-421 mimic or inhibitor,
miR-421 expression was significantly increased or decreased,
respectively (Fig. S3A and B).
Moreover, ORP8 protein expression was significantly increased in
H1975 and A549 cells treated with the miR-421 inhibitor (Figs. 5C and S3C), whereas ORP8 expression was
significantly inhibited in H1975 and A549 cells treated with a
miR-421 mimic (Figs. 5D and
S3D). Moreover, H1975 cells were
transfected with the mutant (MUT) and wild-type (WT) 3′-UTRs of
ORP8. The luciferase activity was inhibited by miR-421 when
transfected with the WT 3′-UTR, however, the activity was restored
by the MUT 3′-UTR transfection (Fig.
5E). In addition, the inhibition of cell proliferation with
ORP8 overexpression was rescued by the miR-421 mimic (Fig. 5F). These data indicated thatmiR-421
could be a potential target for ORP8 downregulation in NSCLC
patients.
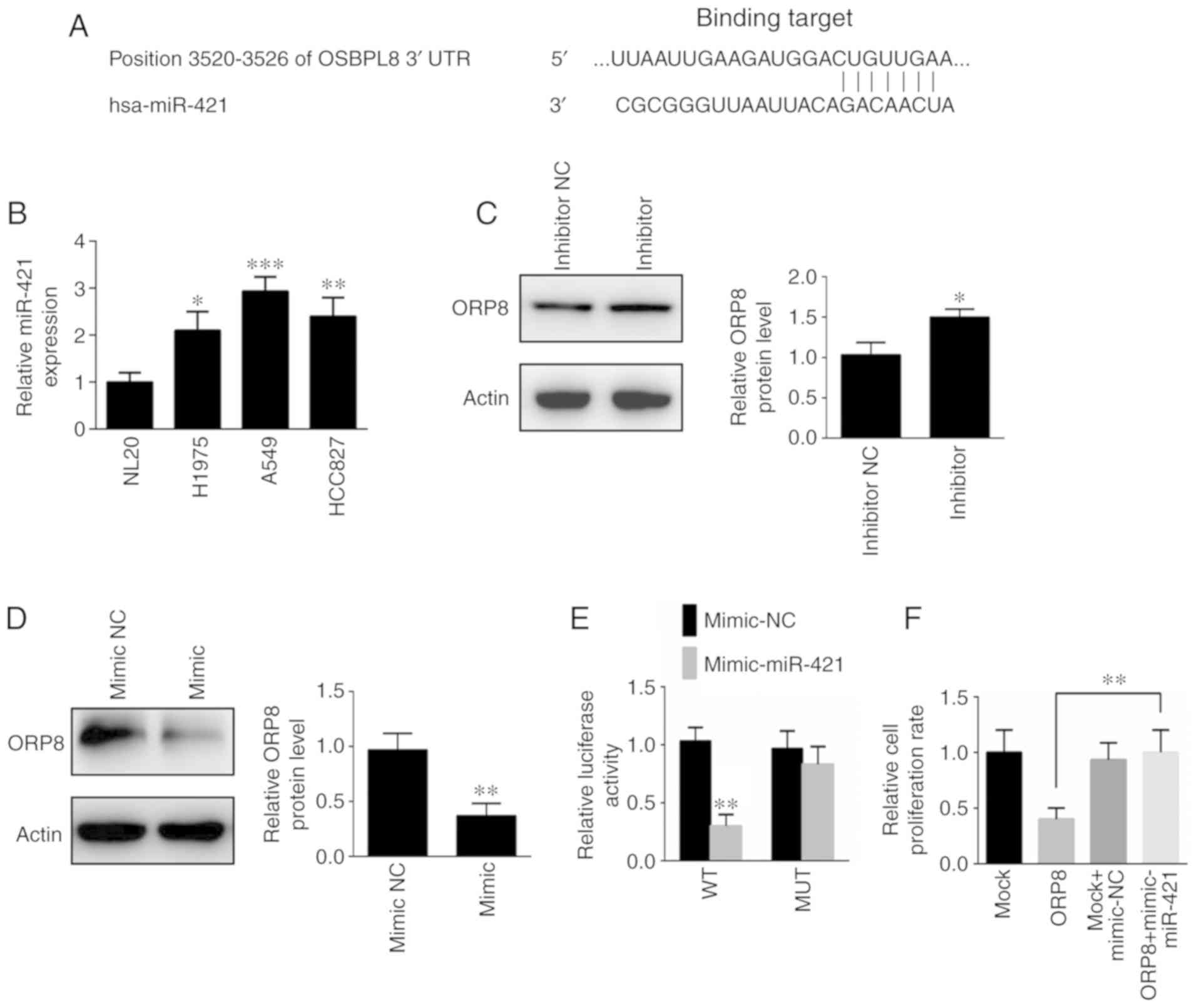 | Figure 5.ORP8 is a target of miR-421. (A) Four
software packages (miRanda, miRDB, miRWalk, Targetscan) were used
to predict miR-421 targeting of ORP8. (B) The mRNA level of miR-421
was detected by qPCR in NSCLC cell lines (H1975, A549, and HCC827)
and a normal lung cell line (NL20). (C) H1975 cells infected with
an inhibitor NC or an inhibitor of miR-421, and the protein level
of ORP8 was detected by western blotting. (D) H1975 cells infected
with a mimic NC or miR-421 mimic, and the protein level of ORP8 was
detected by western blotting. (E) The luciferase activity between
miR-421 and the ORP8-3′-UTR was evaluated using a luciferase
reporter assay. (F) H1975 cells were infected with a lentivirus
carrying ORP8 cDNA or an inhibitor of miR-421, and cell growth was
determined at 72 h using the MTS assay. The data represent the mean
± SD of three individual experiments (*P<0.05, **P<0.01 and
***P<0.001, n=3). ORP8, oxysterol-binding protein-related
protein 8; NSCLC, non-small cell lung cancer. |
Discussion
NSCLC still has a high mortality rate. Targeted
therapy, which depends on activated oncogenes and downstream
signaling cascades, has emerged as an impressive approach for
NSCLC. The present study demonstrated that ORP8 has a key role in
NSCLC and maybe a potential therapeutic target for NSCLC.
ORP8 is an ORP family member that plays an important
role in several signaling pathways. For instance, ORP8 regulates
calcium signaling in specific cell compartments (30). ORP8 localizes to ER-mitochondria
contact sites and is involved in mitochondrial function (14). ORP8 induces HCC cell ER stress
(20), and the overexpression of
ORP8 significantly increases the ER stress response induced by
25-OHC (21). Although ORP8 plays
important roles in multiple signaling pathways and cancer
development (31), its biological
functions in NSCLC remain unclear. The present study was designed
to study the function of ORP8 in NSCLC tumorigenesis.
The present data indicated that ORP8 was
downregulated in NSCLC, both in cell lines and tissues. For
functional study, stable ORP8-overexpressing H1975, A549 and HCC827
lung cancer cell lines were used. It was demonstrated that ORP8
overexpression inhibited cell growth and induced cell apoptosis in
NSCLC. These results indicated that ORP8 overexpression markedly
attenuated malignant features of NSCLC cells, and thus, an
understanding of the mechanisms by which ORP8 regulates lung cancer
development is required.
During tumor viability or progression, apoptosis
plays a crucial role. A lack of apoptosis could lead to tumor
development. Thus, inducing cell apoptosis could be a strategy for
an oncotherapy approach (32).
Disruption of the ER normal function induces a stress response,
also known as an unfolded protein response (UPR), which initially
compensates the damage of cells (33,34).
When limiting the protein folding capacity of the ER, ER stress
causes the aberrant accumulation of misfolded and unfolded proteins
(35,36). If the defensive UPR fails to deal
with the misfolded proteins, ER stress will induce cell apoptosis
(37). Evidence has indicated that
ORP8 overexpression induces the ER stress response (20–22).
Therefore, it was hypothesized that ER stress is induced by ORP8
overexpression in NSCLC cells. However, ER stress was not evident
in H1975 and A549 cells. These data indicated that ER stress may
not be the main mechanism for inducing apoptosis in NSCLC cells
overexpressing ORP8.
The mitochondrial pathway induces cell apoptosis by
decreasing mitochondrial membrane potential, increasing membrane
permeability and decreasing ATP synthesis (38–40).
Then, cytochrome c release from mitochondria into the
cytoplasm could activate caspase-9. This cascade further activates
caspase-3 and causes cell apoptosis (41–43). A
previous study revealed that ORP8 localized to ER-mitochondria
contact sites and was involved in mitochondrial function (14). Therefore, to better understand the
role by which ORP8 regulates lung cancer apoptosis, cytochrome
c release from mitochondria was evaluated. The present data
revealed that cytosolic cytochrome c levels were
significantly enhanced after ORP8 overexpression in H1975, A549 and
HCC827 cells. Therefore, it was concluded that ORP8 overexpression
induced mitochondrial-associated apoptosis by affecting the
subcellular localization of cytochrome c in NSCLC cells.
Dysregulated miRNA expression has been implicated in
cancer development (44). Four
different analytical programs were used to reveal that miR-421
could target ORP8. Previous research has revealed that miR-421
expression is upregulated and associated with poor prognosis in
non-small-cell lung cancer (29).
The present experiments revealed the role of miR-421 by determining
that it targets ORP8, indicating that the upregulated miR-421
expression led to the reduction of ORP8 expression in NSCLC
cells.
Overall, the present data demonstrated that ORP8
regulated cytochrome c release from mitochondria and plays a
critical role in human NSCLC carcinogenesis. ORP8 was revealed to
be expressed at low levels in human NSCLC and inhibit cell growth
and induce cell apoptosis. ORP8 downregulated expression in NSCLC
coincided with the increased expression of miR-421, and increased
expression of miR-421 decreased ORP8 expression aiding in the
maintenance of the proliferative potential. The present results
indicated that ORP8 may be a candidate molecular target for NSCLC
treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical
Science and Technology Project of Henan Province (2018020007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ, JiwL and JC conceived and designed the
experiments. JiwL, ZL, XF, JinL and HL performed the experiments.
JiwL, JC, SW and MZ analyzed the data. MZ, JiwL and SW contributed
the reagents. JiwL and JC wrote the manuscript. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Written informed consent for the research was
obtained from each patient. This retrospective study was approved
by the Ethics Committee of The First Affiliated Hospital of
Zhengzhou University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Trinidad López C, Souto Bayarri M, Oca
Pernas R, Delgado Sánchez-Gracián C, González Vázquez M, Vaamonde
Liste A, Tardáguila De La Fuente G and De La Fuente Aguado J:
Characteristics of computed tomography perfusion parameters in
non-small-cell-lung-cancer and its relationship to histology, size,
stage an treatment response. Clin Imaging. 50:5–12. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katlic MR, Facktor MA, Berry SA, McKinley
KE, Bothe A Jr and Steele GD Jr: ProvenCare lung cancer: A
multi-institutional improvement collaborative. CA Cancer J Clin.
61:382–396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janku F, Stewart DJ and Kurzrock R:
Targeted therapy in Non-small-cell lung cancer-is it becoming a
reality? Nat Rev Clin Oncol. 7:401–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skulachev VP: Cytochrome c in the
apoptotic and antioxidant cascades. FEBS Lett. 423:275–280. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Breckenridge DG, Stojanovic M, Marcellus
RC and Shore GC: Caspase cleavage product of BAP31 induces
mitochondrial fission through endoplasmic reticulum calcium
signals, enhancing cytochrome c release to the cytosol. J Cell
Biol. 160:1115–1127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boehning D, Patterson RL, Sedaghat L,
Glebova NO, Kurosaki T and Snyder SH: Cytochrome c binds to
inositol (1,4,5) trisphosphate receptors, amplifying
calcium-dependent apoptosis. Nat Cell Biol. 5:1051–1061. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang X and Wang X: Cytochrome C-mediated
apoptosis. Annu Rev Biochem. 73:87–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa I, Nakata M, Kawabata S and
Hamada S: Cytochrome c-mediated caspase-9 activation triggers
apoptosis in Streptococcus pyogenes-infected epithelial cells. Cell
Microbiol. 3:395–405. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lehto M, Laitinen S, Chinetti G, Johansson
M, Ehnholm C, Staels B, Ikonen E and Olkkonen VM: The OSBP-related
protein family in humans. J Lipid Res. 42:1203–1213.
2001.PubMed/NCBI
|
|
14
|
Galmes R, Houcine A, van Vliet AR,
Agostinis P, Jackson CL and Giordano F: ORP5/ORP8 localize to
endoplasmic reticulum-mitochondria contacts and are involved in
mitochondrial function. EMBO Rep. 17:800–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan D, Mäyränpää MI, Wong J, Perttilä J,
Lehto M, Jauhiainen M, Kovanen PT, Ehnholm C, Brown AJ and Olkkonen
VM: OSBP-related protein 8 (ORP8) suppresses ABCA1 expression and
cholesterol efflux from macrophages. J Biol Chem. 283:332–340.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou T, Li S, Zhong W, Vihervaara T,
Béaslas O, Perttilä J, Luo W, Jiang Y, Lehto M, Olkkonen VM and Yan
D: OSBP-related protein 8 (ORP8) regulates plasma and liver tissue
lipid levels and interacts with the nucleoporin Nup62. PLoS One.
6:e210782011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Béaslas O, Vihervaara T, Li J, Laurila PP,
Yan D and Olkkonen VM: Silencing of OSBP-related protein 8 (ORP8)
modifies the macrophage transcriptome, nucleoporin p62
distribution, and migration capacity. Exp Cell Res. 318:1933–1945.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong W, Zhou Y, Li J, Mysore R, Luo W, Li
S, Chang MS, Olkkonen VM and Yan D: OSBP-related protein 8 (ORP8)
interacts with Homo sapiens sperm associated antigen 5 (SPAG5) and
mediates oxysterol interference of HepG2 cell cycle. Exp Cell Res.
322:227–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghai R, Du X, Wang H, Dong J, Ferguson C,
Brown AJ, Parton RG, Wu JW and Yang H: ORP5 and ORP8 bind
phosphatidylinositol-4, 5-biphosphate (PtdIns(4,5)P2)
and regulate its level at the plasma membrane. Nat Commun.
8:7572017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong W, Qin S, Zhu B, Pu M, Liu F, Wang
L, Ye G, Yi Q and Yan D: Oxysterol-binding protein-related protein
8 (ORP8) increases sensitivity of hepatocellular carcinoma cells to
Fas-mediated apoptosis. J Biol Chem. 290:8876–8887. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Zheng X, Lou N, Zhong W and Yan D:
Oxysterol binding protein-related protein 8 mediates the
cytotoxicity of 25-hydroxycholesterol. J Lipid Res. 57:1845–1853.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo X, Zhang L, Fan Y, Zhang D, Qin L,
Dong S and Li G: Oxysterol-binding Protein-related protein 8
inhibits gastric cancer growth through induction of ER stress,
inhibition of wnt signaling, and activation of apoptosis. Oncol
Res. 25:799–808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeung YT, Yin S, Lu B, Fan S, Yang R, Bai
R, Zhang C, Bode AM, Liu K and Dong Z: Losmapimod overcomes
gefitinib resistance in non-small cell lung cancer by preventing
tetraploidization. EBioMedicine. 28:51–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun L, Xie P, Wada J, Kashihara N, Liu FY,
Zhao Y, Kumar D, Chugh SS, Danesh FR and Kanwar YS: Rap1b GTPase
ameliorates glucose-induced mitochondrial dysfunction. J Am Soc
Nephrol. 19:2293–2301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim I, Xu W and Reed JC: Cell death and
endoplasmic reticulum stress: Disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 7:1013–1030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krajewski S, Krajewska M, Ellerby LM,
Welsh K, Xie Z, Deveraux QL, Salvesen GS, Bredesen DE, Rosenthal
RE, Fiskum G and Reed JC: Release of caspase-9 from mitochondria
during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci
USA. 96:5752–5757. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Cui X, Li Y, Zhang T and Li S:
Upregulated expression of miR-421 is associated with poor prognosis
in non-small-cell lung cancer. Cancer Manag Res. 10:2627–2633.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pulli I, Lassila T, Pan G, Yan D, Olkkonen
VM and Törnquist K: Oxysterol-binding protein related-proteins
(ORPs) 5 and 8 regulate calcium signaling at specific cell
compartments. Cell Calcium. 72:62–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du X, Turner N and Yang H: The role of
oxysterol-binding protein and its related proteins in cancer. Semin
Cell Dev Biol. 81:149–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cui Z, Lin D, Cheng F, Luo L, Kong L, Xu
J, Hu J and Lan F: The role of the WWOX gene in leukemia and its
mechanisms of action. Oncol Rep. 29:2154–2162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sozen E, Karademir B and Ozer NK: Basic
mechanisms in endoplasmic reticulum stress and relation to
cardiovascular diseases. Free Radic Biol Med. 78:30–41. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji C: Advances and new concepts in
alcohol-induced organelle stress, unfolded protein responses and
organ damage. Biomolecules. 5:1099–1121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hiramatsu N, Chiang WC, Kurt TD, Sigurdson
CJ and Lin JH: Multiple mechanisms of unfolded protein
response-induced cell death. Am J Pathol. 185:1800–1808. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heo JH, Han SW and Lee SK: Free radicals
as triggers of brain edema formation after stroke. Free Radic Biol
Med. 39:51–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sanderson TH, Reynolds CA, Kumar R,
Przyklenk K and Hüttemann M: Molecular mechanisms of
ischemia-reperfusion injury in brain: Pivotal role of the
mitochondrial membrane potential in reactive oxygen species
generation. Mol Neurobiol. 47:9–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Siesjö BK, Elmér E, Janelidze S, Keep M,
Kristián T, Ouyang YB and Uchino H: Role and mechanisms of
secondary mitochondrial failure. Acta Neurochir Suppl. 73:7–13.
1999.PubMed/NCBI
|
|
41
|
Nita DA, Nita V, Spulber S, Moldovan M,
Popa DP, Zagrean AM and Zagrean L: Oxidative damage following
cerebral ischemia depends on reperfusion-a biochemical study in
rat. J Cell Mol Med. 5:163–170. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zuo W, Zhang S, Xia CY, Guo XF, He WB and
Chen NH: Mitochondria autophagy is induced after hypoxic/ischemic
stress in a Drp1 dependent manner: The role of inhibition of Drp1
in ischemic brain damage. Neuropharmacology. 86:103–115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yuan J and Yankner BA: Apoptosis in the
nervous system. Nature. 407:802–809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sassen S, Miska EA and Caldas C: MicroRNA:
Implications for cancer. Virchows Arch. 452:1–10. 2008. View Article : Google Scholar : PubMed/NCBI
|