Introduction
Endometrial cancer is the most common gynecological
cancer in developed countries (1),
and comprises a range of diseases with distinct genetic and
molecular features (2).
Identification of novel molecular targets may improve
classification and treatment of endometrial cancer.
SRY-box 17 (SOX17) encodes a 414-amino acid protein
member of the SRY-related HMG-box (SOX) transcription factor
superfamily (3). Dysregulation of
SOX17 serves an important role in the development and progression
of numerous types of cancer, including breast cancer (4), gastric cancer (5), lung cancer (6), esophageal cancer (7) and endometrial cancer (8). Low SOX17 protein expression is
correlated with poor prognosis in patients with cancer (9). Conversely, SOX17 expression is
correlated with longer overall survival and it has been reported to
increase sensitivity to cisplatin in endometrial cancer (10). Therefore, SOX17 has been proposed as
a tumor suppressor in endometrial cancer; however, the regulatory
mechanisms underlying SOX17 expression remain unclear in
endometrial cancer.
Epithelial to mesenchymal transition (EMT) is an
important process that leads to cancer metastasis, which is
characterized by loss of epithelial markers, such as E-cadherin,
and an increase in mesenchymal markers, such as fibronectin
(11). MicroRNAs (miRNAs/miRs)
represent a large class of small (~22 nt) RNA molecules, that are
important post-transcriptional regulators (12). miRNAs can regulate the expression of
key target genes, such as SOX17, which are involved in metastasis
of endometrial cancer (13,14). Numerous miRNAs are dysregulated in
endometrial cancer (15) and
participate in EMT (16). For
example, miR-21-5p has been reported to promote the progression of
endometrial cancer (17). However,
the mechanisms underlying the effects of miR-21-5p on progression
of endometrial cancer remain unclear. The present study aimed to
investigate the roles of miR-21-5p and SOX17 in EMT in endometrial
cancer.
Materials and methods
Endometrial cancer samples
The present study was approved by the Ethics
Committee of Jinan Maternity and Child Health Care Hospital (Jinan,
China), and each patient provided written informed consent at the
time of enrollment. A total of 160 postmenopausal women diagnosed
with primary endometrial cancer were recruited between January 2003
and January 2006 at Jinan Maternity and Child Health Care Hospital
(Jinan), China). All patients had not been treated with
preoperative chemotherapy or radiation. The study population had a
median age of 60.5 years (range, 50.0–74.0 years) with a median
body mass index of 23.3 kg/m2 (range, 20.4–38.3
kg/m2). Tumor samples were obtained during surgery
following removal of the necessary amount of endometrial cancer
tissue for routine pathological examination. The corresponding
adjacent normal tissue sample was selected >3 cm away from the
site at which the primary tumor was sampled. Two pathologists
reviewed all tumor tissues and adjacent normal tissues. All tissue
specimens were snap-frozen in liquid nitrogen within 1 h of removal
and were stored at −80°C.
Cell culture
The HEC-1A, HEC-1B, RL95-2 and AN3CA human
endometrial cancer cell lines were purchased from the America Type
Culture Collection. The cell lines were maintained in Eagle's
Minimum Essential Medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% heat-inactivated (56°C, 30 min) fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). Monolayer
cultures were incubated at 37°C in a 95% humidified atmosphere
containing 5% CO2. Cellular morphology was observed
using a light microscope (Leica Microsystems, Inc.).
SOX17 plasmids
SOX17-expressing plasmids and empty vectors (mock,
pcDNA 3.1), and short hairpin RNA (sh)SOX17 and a scramble control
were purchased from Tiangen Biotech Co., Ltd. Human SOX17 was
cloned by reverse transcription-PCR (RT-PCR) from cDNA derived from
adjacent normal tissue samples of patients with endometrial cancer
(forward, 5′-GTTCGGATCCGCCATGAGCAGCCCGGATGCG-3′; reverse,
5′-ATGTGAATTCCACGTCAGGATAGTTGCAGTA-3′), and its sequence was
confirmed by direct sequencing of PCR products. Sequencing analysis
was carried out using the BigDye Terminator v1.1 sequencing kit,
according to manufacturer's protocol (Applied Biosystems; Thermo
Fisher Scientific, Inc.). To generate human SOX17 expression
constructs, the entire encoding region of its cDNA was subcloned in
frame into pcDNA 3.1 (Invitrogen; Thermo Fisher Scientific, Inc.).
Suppression of SOX17 expression was induced using shSOX17:
5′-CGCACGGAAUUCGAACAGUAU-3′. Negative control shRNA
(5′-ACCGAGCAGUACAACGGGAAC-3′; Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used as a control.
miR-21-5p sequences
Pre-miR-21-5p and a control miR, and anti-miR-21-5p
and a scramble miR control were purchased from Ambion; Thermo
Fisher Scientific, Inc. The sequences were as follows:
Pre-miR-27a-3p, 5′-AACAUCAGUCUGAUAAGCUAUU-3′; control miR,
5′-AACCAUUUGAGAGUCAUCAAGA-3′; anti-miR-21-5p,
5′-UCAACAUCAGUCUGAUAAGCUA-3′; scramble miR control,
5′-CAGUACUUUUGUGUAGUACAA-3′.
Transfection
Cell transfection was performed as described
previously (18). For transfection
experiments, HEC-1A and AN3CA cells were cultured in serum-free
medium without antibiotics at 60% confluence for 24 h, and were
then transfected with 0.5 µg SOX17-expressing plasmids or empty
vectors, or 10 nM pre-miR-21-5p or control miR using
FuGENE® HD transfection reagent (Roche Diagnostics),
according to the manufacturer's protocol.
After incubation for 6 h, the medium was removed and
replaced with normal culture medium (serum-free medium without
antibiotics) for 24 h. Subsequently, the MTT assay, RT-qPCR,
RT-quantitative PCR (RT-qPCR), western blotting,
immunocytochemistry and luciferase reporter assay were
performed.
RT-qPCR for mRNA detection
The analysis of mRNA via RT-qPCR was performed as
described previously (18,19). Briefly, total cellular RNA was
extracted from cultured cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and 2 µg total RNA was
reverse transcribed using M-MLV reverse transcriptase (Promega
Corporation), according to the manufacturer's protocol. The PCR
thermocycling conditions were as follows: Denaturation for 30 sec
at 95°C, followed by annealing for 45 sec at 52–58°C depending on
the primers used, and extension for 45 sec at 72°C. Each PCR
reaction was performed for 28–32 cycles. RT-qPCR was performed
using a StepOne™ real-time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and Fast SYBR Green Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Data are shown as
relative expression levels after normalization to GAPDH. The
following primers were used: Fibronectin, forward
5′-TTTTGACAACGGGAAGCATTATCAGATAA-3′, reverse
5′-TGATCAAAACATTTCTCAGCTATTGG-3′; N-cadherin, forward
5′-CACTGCTCAGGACCCAGAT-3′, reverse 5′-TAAGCCGAGTGATGGTCC-3′;
vimentin, forward 5′-CGGGATCCCGCCCTCGTTCGCCTCTTCTC-3′, reverse
5′-CGGAATTCCGATATCGCCTGCCACTGAGTG-3′; E-cadherin, forward
5′-TCAACGATCCTGACCAGCAGTTCG-3′, reverse
5′-GGTGAACCATCATCTGTGGCGATG-3′; and GAPDH, forward
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse
5′-AGCCTTCTCCATGGTGGTGAAGAC-3. Quantification was performed using
the 2−∆∆Cq method (20).
RT-qPCR for miRNA detection
The analysis of miRNA expression was performed using
RT-qPCR. Total RNA was isolated from cells or tissues using the
mirVana™ miRNA Isolation kit (cat. no. AM1561; Ambion; Thermo
Fisher Scientific, Inc.). The detection of mature form of miRNAs
was performed using the mirVana™ RT-qPCR miRNA Detection kit
(SYBR-Green) and RT-qPCR Primer Sets (Ambion; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The thermocycling conditions were as follows: One cycle at 50°C for
2 min, one cycle at 95°C 10 min, followed by 40 cycles at 95°C for
15 sec and 60°C for 30 sec, for extension. The following primer
sequences were used: miR-21-5p, forward
5′-TCGCTCGAGATTTTTTTTTATCAAGAGGG-3′, reverse
5′-TCGGCGGCCGCGACAAGAATGAGACTTTAATC-3′; U6, forward
5′-GCTTCGGCAGCACATATACTAAAAT-3′, reverse
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The U6 small nuclear RNA was used as
an internal control. Quantification was performed using the
2−∆∆Cq method (20).
Western blot analysis
Western blot analysis was performed as described
previously (18). Total protein was
prepared using extraction buffer comprising NaCl/Pi
supplemented with 0.5% Triton X-100, 1 mM EDTA, 1 mM phenylmethyl
sulfonyl fluoride and complete protease inhibitors (Roche
Diagnostics). The concentration of each protein lysate was
determined using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). Equal amounts of total protein (50
µg/lane) were separated by 12% SDS/PAGE. Subsequently, samples were
transferred to nitrocellulose membranes and blocked for 60 min at
room temperature in NaCl/Pi containing 5% skim milk
powder (w/v). The membranes were immunoblotted using the following
primary antibodies: Anti-fibronectin (cat. no. ab2413; 1:500;
Abcam), anti-N-cadherin (cat. no. ab18203; 1:500; Abcam),
anti-vimentin (cat. no. ab92547; 1:500; Abcam), anti-E-cadherin
(cat. no. ab40772; 1:500; Abcam), anti-SOX17 (cat. no. ab224637;
1:500; Abcam) and anti-β-actin (cat. no. ab8227 1:500; Abcam)
overnight at 4°C. Subsequently, they were incubated with
IRDye-800-conjugated anti-rabbit secondary antibodies (cat. no.
ab6940, 1:10,000; Abcam) for 30 min at room temperature. The
specific proteins were visualized using the Odyssey™ Infrared
Imaging system (LI-COR Biosciences). β-actin expression was used as
an internal control to confirm equal loading of the protein
samples.
MTT assay
The MTT assay was performed as described previously
(18). The effects of
pre-miR-21-5p, control miR, anti-miR-21-5p, scramble miR control,
SOX17-expressing plasmids, empty vectors, shSOX17 and scramble
control on the proliferation of endometrial cancer cell lines was
assessed by MTT assay (Sigma-Aldrich; Merck KGaA). Briefly, cells
were plated in 96-well plates at a density of 8×103
cells/well in Eagle's Minimum Essential Medium containing 10% FBS
at 37°C in an incubator containing 5% CO2 for 12 h.
Cells were transfected for 48 h. Subsequently, MTT (5 mg/ml) was
added to the wells (20 µl/per well). The plates were then incubated
for 4 h at 37°C, the supernatant was removed and 150 µl dimethyl
sulfoxide was added to each well for 10 min. The absorbance of each
well was then measured using a Synergy™ 4 (BioTek Instruments,
Inc.) at a wavelength of 570 nm, with the reference wavelength set
at 630 nm. Absorbance was directly proportional to the number of
surviving cells.
Bioinformatics analysis
Bioinformatics analysis was performed as described
previously (21). miRanda (August
2010 Release; http://www.microrna.org/microrna/home.do) was used to
identify the target genes of miR-21-5p.
Luciferase reporter assay
Luciferase reporter plasmids containing the wild
type SOX17 mRNA 3′ untranslated region (3′UTR) and mutant SOX17
mRNA 3′UTR were obtained from Tiangen Biotech, Co. Ltd. The
luciferase reporter assay was performed as described previously
(21). For reporter assays,
1×106 cells were transiently transfected with reporter
plasmids (0.1 µg) and 10 nM pre-miR-21-5p or control miR using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Reporter assays were performed 36 h
post-transfection using the Dual-Luciferase® Reporter
Assay system (Promega Corporation), normalized to Renilla
luciferase.
Immunohistochemistry
Immunohistochemistry was performed as described
previously (22). Samples were
incubated with anti-SOX17. (cat. no. ab224637; 1:500; Abcam) for 12
h at 4°C. Subsequently, samples were incubated with
IRDye-800-conjugated anti-rabbit secondary antibodies (cat. no.
ab6940; 1:10,000; Abcam) for 30 min at room temperature. Samples
were observed under a light microscope. Slides were assessed by
quantitative image analysis using the Aperio Image Analysis toolbox
(Leica Biosystems, Inc.). Staining intensity and percentage of
positive nuclei were recorded after manually segmenting the tumor
from adjacent stroma. SOX17 expression levels were
semi-quantitatively classified based on the total scores of
percentage positivity of stained tumour cells and staining
intensity. Namely, the percentage positivity was scored as ‘0’ if
<5% (negative), ‘1’ if 5–30% (sporadic), ‘2’ if 30–70% (focal)
and ‘3’ if >70% (diffuse) of cells were stained, whereas
staining intensity was scored relative to known positive and
negative controls as ‘0’ if no staining, ‘1’ if weakly stained, ‘2’
if moderately stained (intermediate level between strong and weak)
and ‘3’ if strongly stained. The final SOX17 expression score was
defined as follows; ‘SOX17-’ if the sum of the percentage
positivity score and the staining intensity score was 0–1, ‘SOX17
1+’ if the sum was 2–3, ‘SOX17 2+’ if the sum was 4–5 and ‘SOX17
3+’ if the sum was 6. SOX17- and SOX17 1+ were defined as low
expression. SOX17 2+ and SOX17 3+ were defined as high
expression.
Statistical analysis
The results were analyzed using SAS software
(version 9.4; SAS Institute, Inc.). Data are presented as the means
± standard error of the mean of separate experiments (n=3). Samples
were analyzed by two-tailed Student's t-test for the comparison of
two groups, unless otherwise indicated. χ2 tests were
used for comparison of categorical variables. The correlation
between miR-21-5p and SOX17 expression was analyzed by Spearman
correlation (23). Overall survival
was analyzed by Kaplan-Meier methods (24,25).
Survival was compared in terms of miR-21-5p and SOX17 expression by
Kaplan-Meier analysis and log-rank test (two-tailed). P<0.05 was
considered to indicate a statistically significant difference.
Results
Association between miR-21-5p
expression and the clinicopathological features of endometrial
cancer
Normal endometrial samples were used as controls to
determine relative miR-21-5p expression in endometrial cancer
tissues. If the relative value of miR-21-5p expression was ≥1, it
was defined as high expression. If the relative value of miR-21-5p
expression was <1, it was defined as low expression. The
association between miR-21-5p expression and clinicopathological
features is summarized in Table I.
The results demonstrated that miR-21-5p expression was associated
with lymphatic metastasis (P<0.05).
 | Table I.Association between miR-21-5p
expression and the pathological parameters of endometrial
cancer. |
Table I.
Association between miR-21-5p
expression and the pathological parameters of endometrial
cancer.
|
| miR-21-5p
expression |
|
|---|
|
|
|
|
|---|
| Clinical
parameter | Low (%) | High (%) | P-value |
|---|
| Lymphatic
metastasis |
|
|
|
| No | 53 (40) | 79 (60) | <0.05 |
|
Yes | 6 (21) | 22 (79) |
|
| Invasion depth |
|
|
|
|
<1/2 | 40 (39) | 63 (61) | 0.06 |
|
>1/2 | 13 (34) | 25 (66) |
|
| Limited
to endometrium | 6 (32) | 13 (68) |
|
Association between miR-21-5p
expression and overall survival
To detect miR-21-5p expression in endometrial cancer
tissues and adjacent normal tissues, miRNA was isolated from 160
pairs of adjacent normal tissues and endometrial cancer tissues.
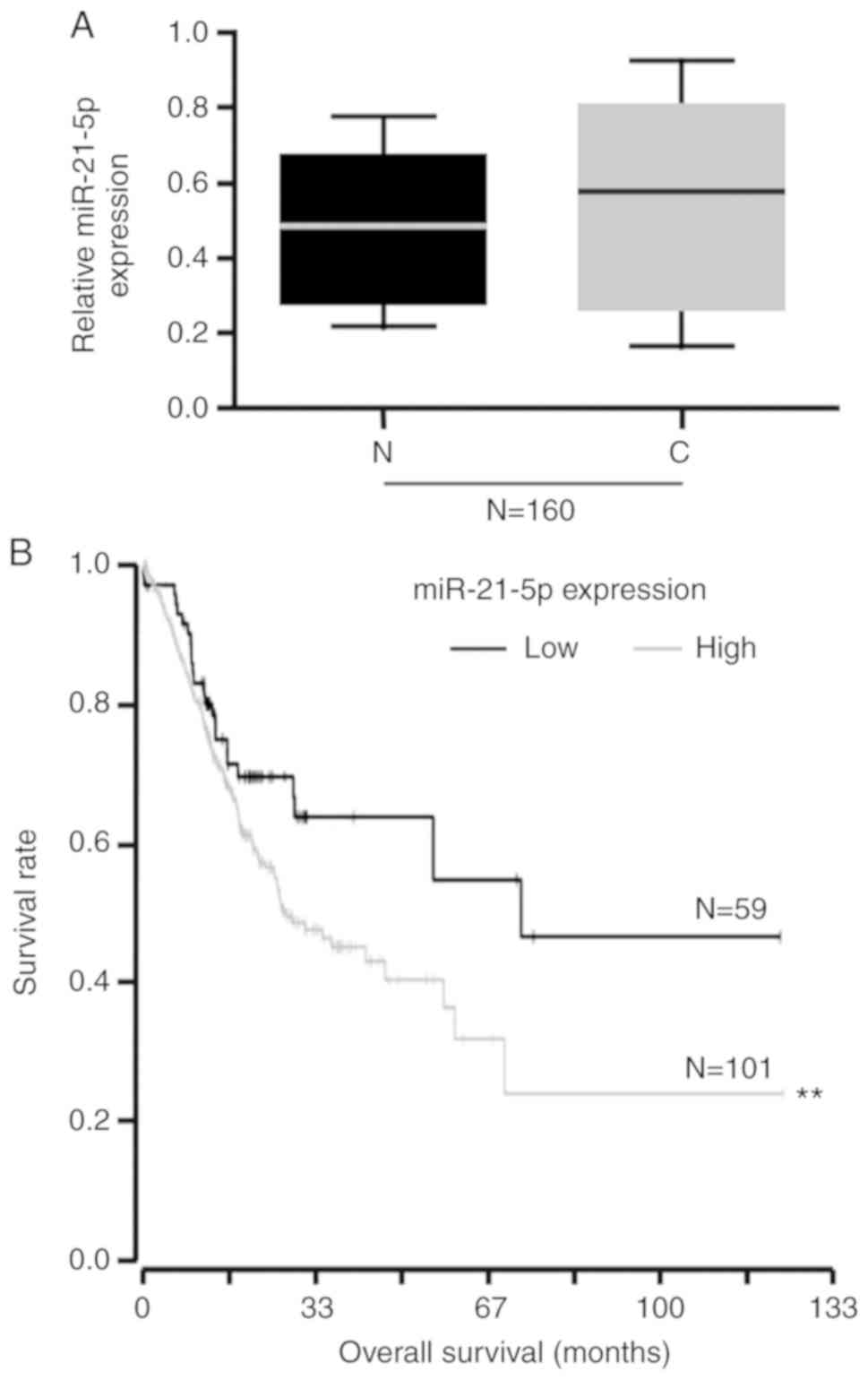
RT-qPCR was then performed. The results indicated that miR-21-5p
expression was not significantly altered in endometrial cancer
tissues compared with in normal tissues (Fig. 1A). Kaplan-Meier curves were applied
to evaluate overall survival of the 160 patients with primary
endometrial cancers, stratified based on tumor expression of
miR-21-5p. There was a significant difference between the two
overall survival curves; survival among patients with high
miR-21-5p expression was much poorer than survival among patients
with low miR-21-5p expression (Fig.
1B).
Overexpression of miR-21-5p promotes
EMT in endometrial cancer cell lines
To examine miR-21-5p expression in HEC-1A, HEC-1B,
RL95-2 and AN3CA endometrial cancer cell lines, RT-qPCR was
conducted. miR-21-5p expression was highest in AN3CA cells and was
lowest in HEC-1A cells (Fig. 2A).
HEC-1A cells were selected to study the overexpression of
miR-21-5p, since endogenous miR-21-5p was very low in this cell
line. AN3CA cells were selected to study miR-21-5p knockdown, since
endogenous miR-21-5p was very high in this cell line. To determine
whether miR-21-5p can promote EMT, HEC-1A cells were transfected
with control miR (mock) or pre-miR-21-5p. The results demonstrated
that miR-21-5p expression was increased by pre-miR-21-5p in HEC-1A
cells (Fig. 2B); this increase in
miR-21-5p induced visible alterations in HEC-1A cell morphology
(Fig. 2C). To confirm that these
alterations were associated with EMT, RT-qPCR and western blotting
were conducted to examine the expression of epithelial and
mesenchymal markers in HEC-1A cells transfected with control miR or
pre-miR-21-5p. The results demonstrated that E-cadherin was
significantly decreased, whereas fibronectin, N-cadherin and
vimentin were increased by pre-miR-21-5p in HEC-1A cells (Fig. 2D and E). In addition, the MTT assay
was used to determine whether miR-21-5p could affect proliferation
of HEC-1A cells; overexpression of miR-21-5p promoted proliferation
of HEC-1A cells (Fig. 2F).
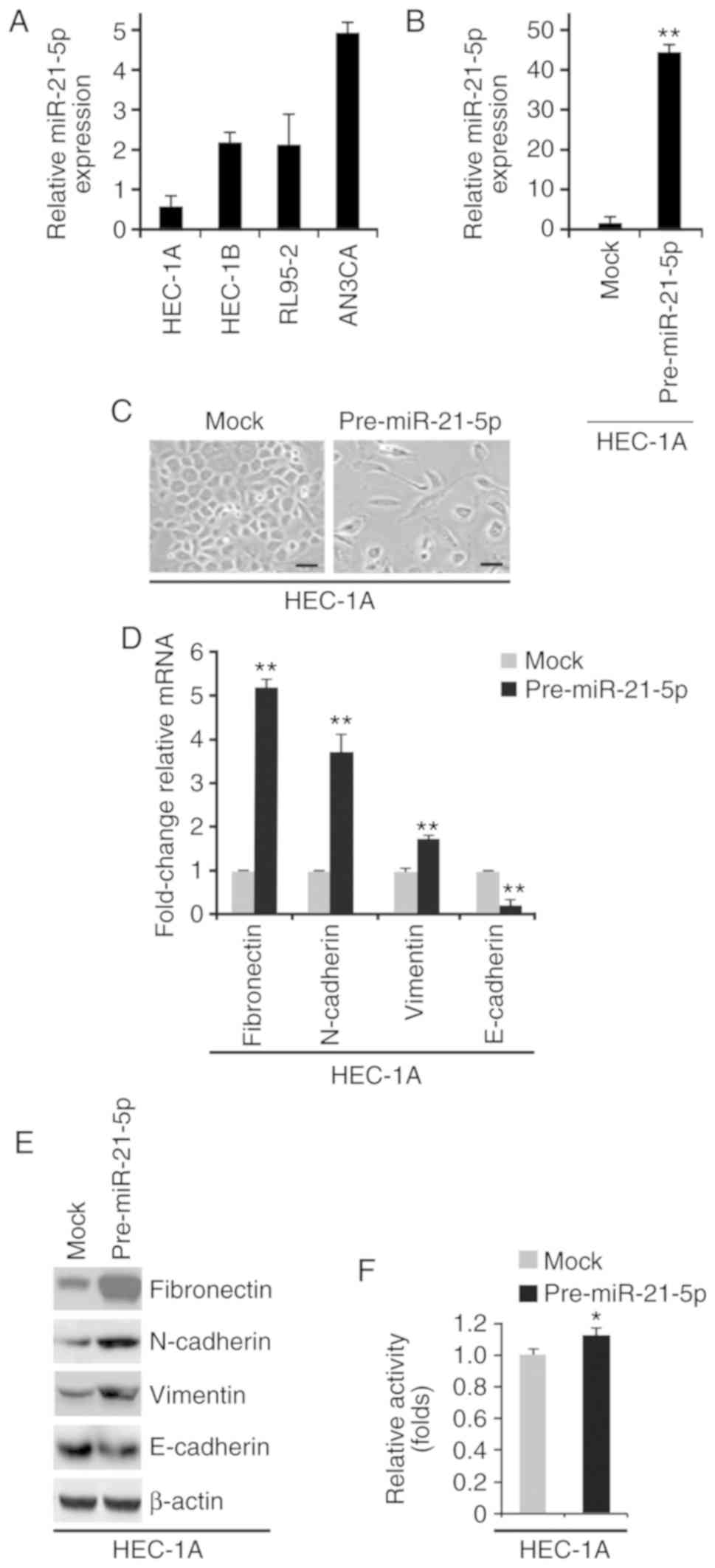 | Figure 2.miR-21-5p promotes epithelial to
mesenchymal transition in endometrial cancer. (A) RT-qPCR analysis
of miR-21-5p in HEC-1A, HEC-1B, AN3CA and RL95-2 cells. (B) RT-qPCR
analysis of miR-21-5p in HEC-1A cells transfected as indicated
(n=3). (C) HEC-1A cells were transfected as indicated, and images
of the cells were captured. Scale bars, 50 µm n=3. (D) RT-qPCR
analysis of fibronectin, N-cadherin, vimentin and E-cadherin in
HEC-1A cells transfected as indicated (n=3). (E) Western blot
analysis of fibronectin, N-cadherin, vimentin and E-cadherin in
HEC-1A cells transfected as indicated (n=3). (F) MTT assay of
HEC-1A cells transfected as indicated (n=3). *P<0.05 and
**P<0.01 vs. the Mock group. miR-21-5p, microRNA-21-5p; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
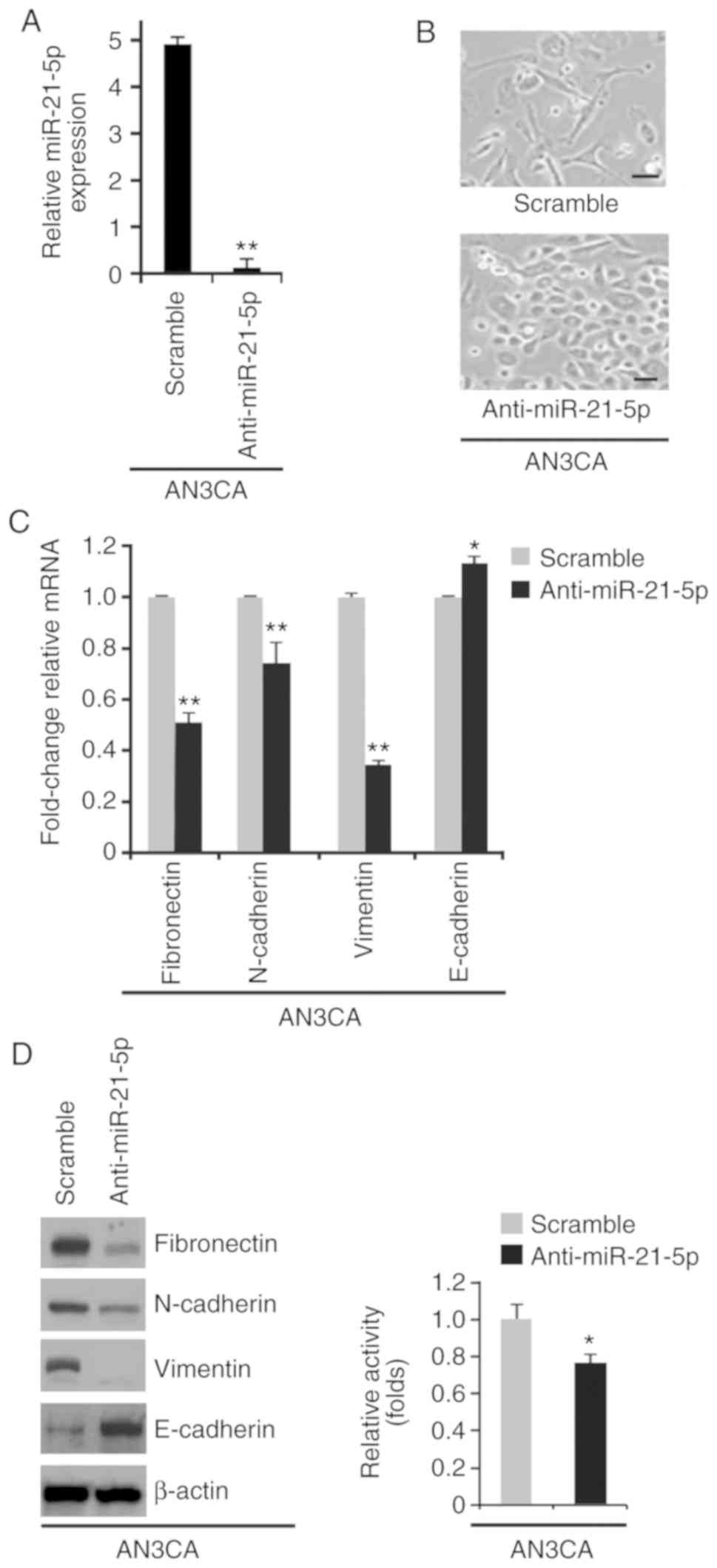
miR-21-5p knockdown promotes
mesenchymal to epithelial transition (MET) in endometrial cancer
cell lines
The present study also investigated the effects of
an inhibitor of miR-21-5p, anti-miR-21-5p. A total of 48 h
post-transfection, miR-21-5p expression was determined by RT-qPCR;
miR-21-5p expression was downregulated by anti-miR-21-5p in AN3CA
cells (Fig. 3A). Silencing
miR-21-5p promoted visible alterations in AN3CA cell morphology
(Fig. 3B). To further study the
role of anti-miR-21-5p in MET, RT-qPCR and western blotting were
used to examine the expression levels of epithelial and mesenchymal
markers in AN3CA cells transfected with anti-miR-21-5p or a
scramble miR control. E-cadherin expression was increased, whereas
fibronectin, N-cadherin and vimentin expression were decreased by
anti-miR-21-5p (Fig. 3C and D). The
MTT assay was used to determine whether anti-miR-21-5p could affect
proliferation of AN3CA cells; silencing miR-21-5p inhibited
proliferation of AN3CA cells (Fig.
3E).
miR-21-5p regulates SOX17 expression
in endometrial cancer
To screen target genes of miR-21-5p, the online
software miRanda (http://www.microrna.org/microrna/home.do) was used. A
large number of target genes were identified, including SOX17.
Recently, SOX17 has been reported to act as a tumor suppressor gene
in endometrial cancer (8).
Therefore, the present study focused on this gene. miR-21-5p target
sites were detected on the 3′-untranslated region (3′UTR) of SOX17,
and identical sequences were revealed among different species
(Fig. 4A).
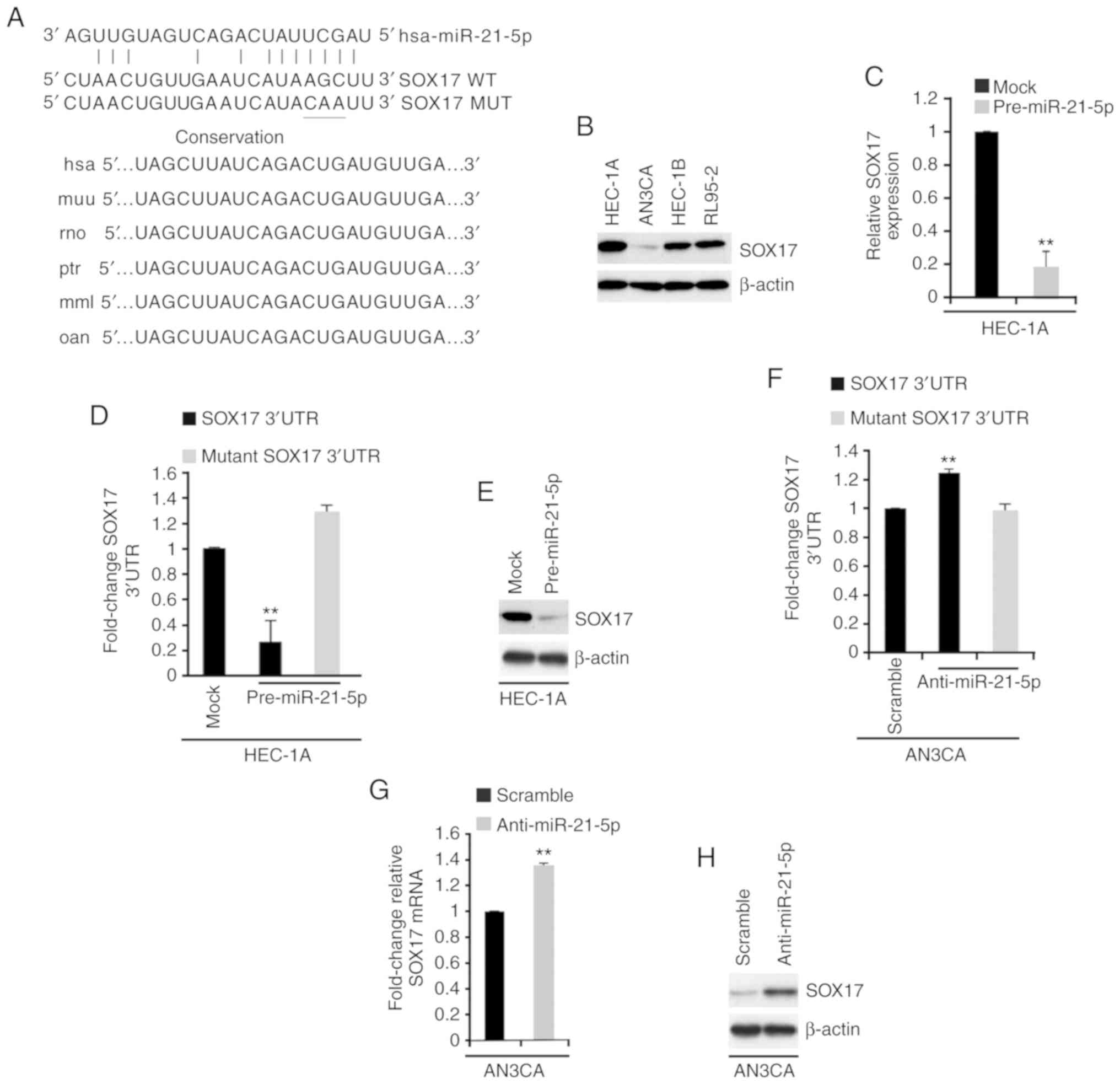 | Figure 4.miR-21-5p regulates SOX17 expression
in endometrial cancer. (A) Predicted binding sites of miR-21-5p and
SOX17, as determined using the miRanda algorithm. Conservation of
miR-21-5p targeting sites in the 3′UTR of SOX17 (WT), and the MUT
sequence that abrogates miR-21-5p binding to SOX17 mRNA. (B)
Western blotting of SOX17 in HEC-1A, AN3CA, HEC-1B and RL95-2 cells
(n=3). (C) RT-qPCR analysis of SOX17 in HEC-1A cells transfected as
indicated (n=3). **P<0.01 vs. the Mock group. (D) Luciferase
reporter assay in HEC-1A cells transfected as indicated (n=3).
**P<0.01 vs. mutant SOX17 3′UTR. (E) Western blot analysis of
SOX17 in HEC-1A cells transfected as indicated (n=3). (F)
Luciferase reporter assay in AN3CA cells transfected as indicated
(n=3). (G) RT-qPCR analysis of SOX17 mRNA in AN3CA cells
transfected as indicated (n=3). (H) Western blot analysis of SOX17
protein in AN3CA cells transfected as indicated (n=3). **P<0.01
vs. the Scramble group. 3′UTR, 3′-untranslated region; miR-21-5p,
microRNA-21-5p; MUT, mutant; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; SOX17,
SRY-box 17; WT, wild type. |
To identify SOX17 expression in endometrial cancer
cell lines, western blotting was performed. The results
demonstrated that SOX17 protein expression was highest in HEC-1A
cells and lowest in AN3CA cells (Fig.
4B). To determine the role of miR-21-5p in regulating SOX17
expression, RT-qPCR was conducted to examine SOX17 mRNA expression
in HEC-1A cells transfected with control miR (mock) or
pre-miR-21-5p. The results demonstrated that pre-miR-21-5p
inhibited SOX17 mRNA expression in HEC-1A cells (Fig. 4C). To investigate the direct
regulation of SOX17 by miR-21-5p, a luciferase reporter assay was
performed. Wild type SOX17 3′UTR was introduced into HEC-1A cells.
The results revealed that the luciferase activity of wild type
plasmids was suppressed by pre-miR-21-5p (Fig. 4D). Subsequently, three bases in the
predicted sites were mutated (Fig.
4A), and mutant SOX17 3′UTR was introduced into HEC-1A cells.
As expected, the luciferase activity of mutant reporters was not be
affected by miR-21-5p in HEC-1A cells (Fig. 4D). In addition, western blotting was
conducted to determine SOX17 protein expression in HEC-1A cells
transfected with control miR or pre-miR-21-5p; SOX17 protein
expression was also decreased by pre-miR-21-5p (Fig. 4E).
The present study also investigated whether
silencing miR-21-5p could regulate SOX17 expression in AN3CA cells.
AN3CA cells were transfected with anti-miR-21-5p or a scramble miR
control. A total of 48 h post-transfection, miR-21-5p expression
was determined by RT-qPCR, and miR-21-5p expression was decreased
by anti-miR-21-5p (Fig. 3A). A
luciferase reporter assay was subsequently performed to examine
whether anti-miR-21-5p could regulate the activity of SOX17 3′UTR
in AN3CA cells. The results demonstrated that silencing miR-21-5p
promoted SOX17 3′UTR activity in AN3CA cells (Fig. 4F). RT-qPCR and western blotting were
used to examine the mRNA and protein expression levels of SOX17 in
AN3CA cells transfected with a scramble miR control and
anti-miR-21-5p. Silencing miR-21-5p promoted SOX17 mRNA and protein
expression (Fig. 4G and H).
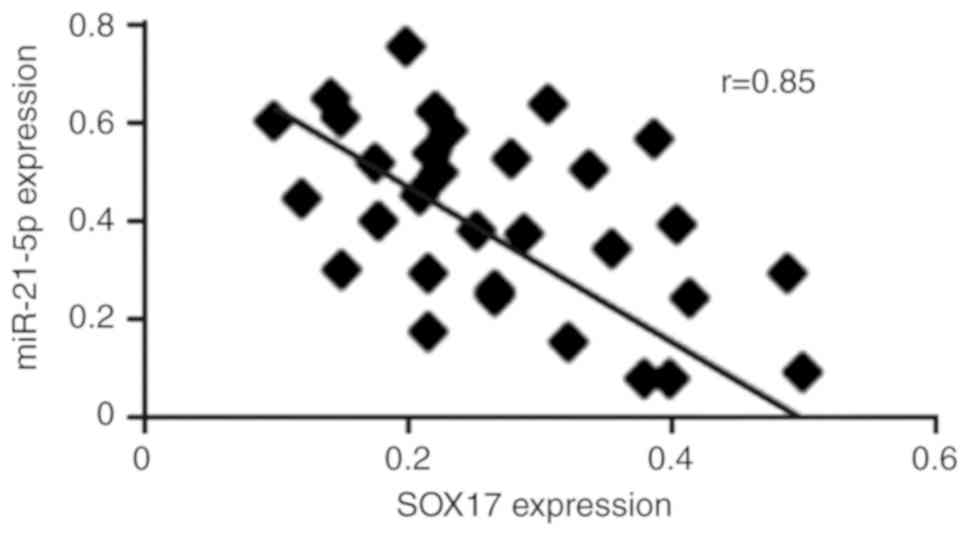
SOX17 expression is negatively
correlated with miR-21-5p expression in endometrial cancer
To investigate the association between SOX17 mRNA
expression and miR-21-5p expression in endometrial cancer, RT-qPCR
was used to examine SOX17 and miR-21-5p expression in 30
endometrial cancer tissues [there is an 80% power to detect a
moderate (r≥0.4) correlation between SOX17 and miR-21-5p
expression, when sample size is 30]. The results demonstrated that
SOX17 mRNA was negatively correlated with miR-21-5p expression
(r=0.85, P<0.01; Fig. 5).
SOX17 regulates EMT in endometrial
cancer cell lines
To determine the role of SOX17 in endometrial
cancer, AN3CA cells were transfected with SOX17-expressing plasmids
or empty vectors (mock). SOX17 protein was significantly increased
by SOX17-expressing plasmids (Fig.
6A). The increase in SOX17 expression induced visible
alterations in AN3CA cell morphology (Fig. 6B). To confirm that these visible
alterations in cell morphology were associated with EMT, western
blot analysis was conducted to determine the expression levels of
epithelial and mesenchymal markers in AN3CA cells transfected with
SOX17-expressing plasmids or empty vectors. E-cadherin was markedly
increased, whereas fibronectin, N-cadherin and vimentin were
decreased by SOX17 (Fig. 6C).
Furthermore, SOX17 overexpression inhibited proliferation of AN3CA
cells (Fig. 6D). The present study
also determined the effects of an inhibitor of SOX17, shSOX17. A
total of 48 h post-transfection, SOX17 expression was examined by
western blotting. shSOX17 inhibited SOX17 protein expression in
HEC-1A cells (Fig. 6E) and
silencing SOX17 promoted visible alterations in HEC-1A cell
morphology (Fig. 6F). To analyze
the role of SOX17 in EMT, western blotting was performed to
determine the expression levels of epithelial and mesenchymal
markers in HEC-1A cells transfected with shSOX17 or a scramble
control. E-cadherin was decreased, whereas fibronectin, N-cadherin
and vimentin were increased by shSOX17 in the cells (Fig. 6G). Cell proliferation was determined
by MTT assay; silencing SOX17 promoted proliferation of HEC-1A
cells (Fig. 6H).
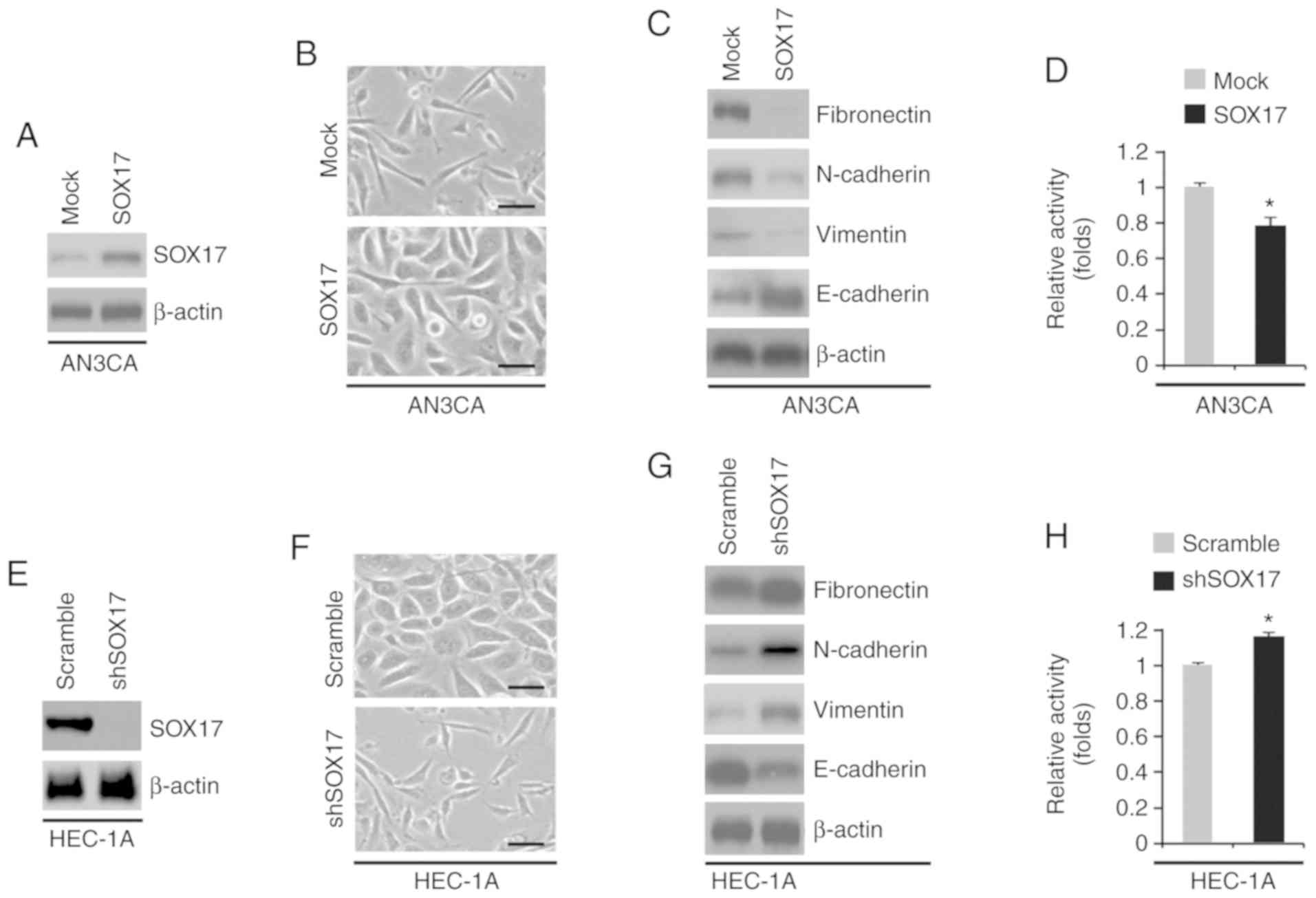 | Figure 6.SOX17 regulates EMT in endometrial
cancer. (A) Western blot analysis of SOX17 in AN3CA cells
transfected as indicated (n=3). (B) AN3CA cells were transfected as
indicated, and images of the cells were captured. Scale bars, 50
µm. n=3. (C) Western blot analysis of fibronectin, N-cadherin,
vimentin and E-cadherin in AN3CA cells transfected as indicated
(n=3). (D) MTT assay of AN3CA cells transfected as indicated (n=3).
*P<0.05 vs. the Mock group. (E) Western blot analysis of SOX17
in HEC-1A cells transfected as indicated (n=3). (F) HEC-1A cells
were transfected as indicated, and images of the cells were
captured. Scale bars, 50 µm. n=3. (G) Western blot analysis of
fibronectin, N-cadherin, vimentin and E-cadherin in HEC-1A cells
transfected as indicated (n=3). (H) MTT assay of HEC-1A cells
transfected as indicated (n=3). *P<0.05 vs. the Scramble group.
miR-21-5p, microRNA-21-5p; shSOX17, short hairpin RNA-SOX17; SOX17,
SRY-box 17. |
Association between SOX17 expression
and clinicopathological features in gastric cancer
The association between SOX17 expression and
clinicopathological features was summarized in Table II. The results demonstrated that
SOX17 expression was not associated with lymphatic metastasis
(P>0.05) and invasion depth (P>0.05).
 | Table II.Association between SOX17 expression
and pathological parameters of endometrial cancer. |
Table II.
Association between SOX17 expression
and pathological parameters of endometrial cancer.
|
| SOX17
expression |
|
|---|
|
|
|
|
|---|
| Clinical
parameter | Low (%) | High (%) | P-value |
|---|
| Lymphatic
metastasis |
|
| 0.12 |
| No | 93 (51) | 44 (49) |
|
|
Yes | 16 (50) | 7
(50) |
|
| Invasion depth |
|
| 0.10 |
|
<1/2 | 72 (68) | 34 (32) |
|
|
>1/2 | 27 (68) | 12 (32) |
|
| Limited
to endometrium | 10 (67) | 5
(33) |
|
Association between SOX17 expression
and overall survival
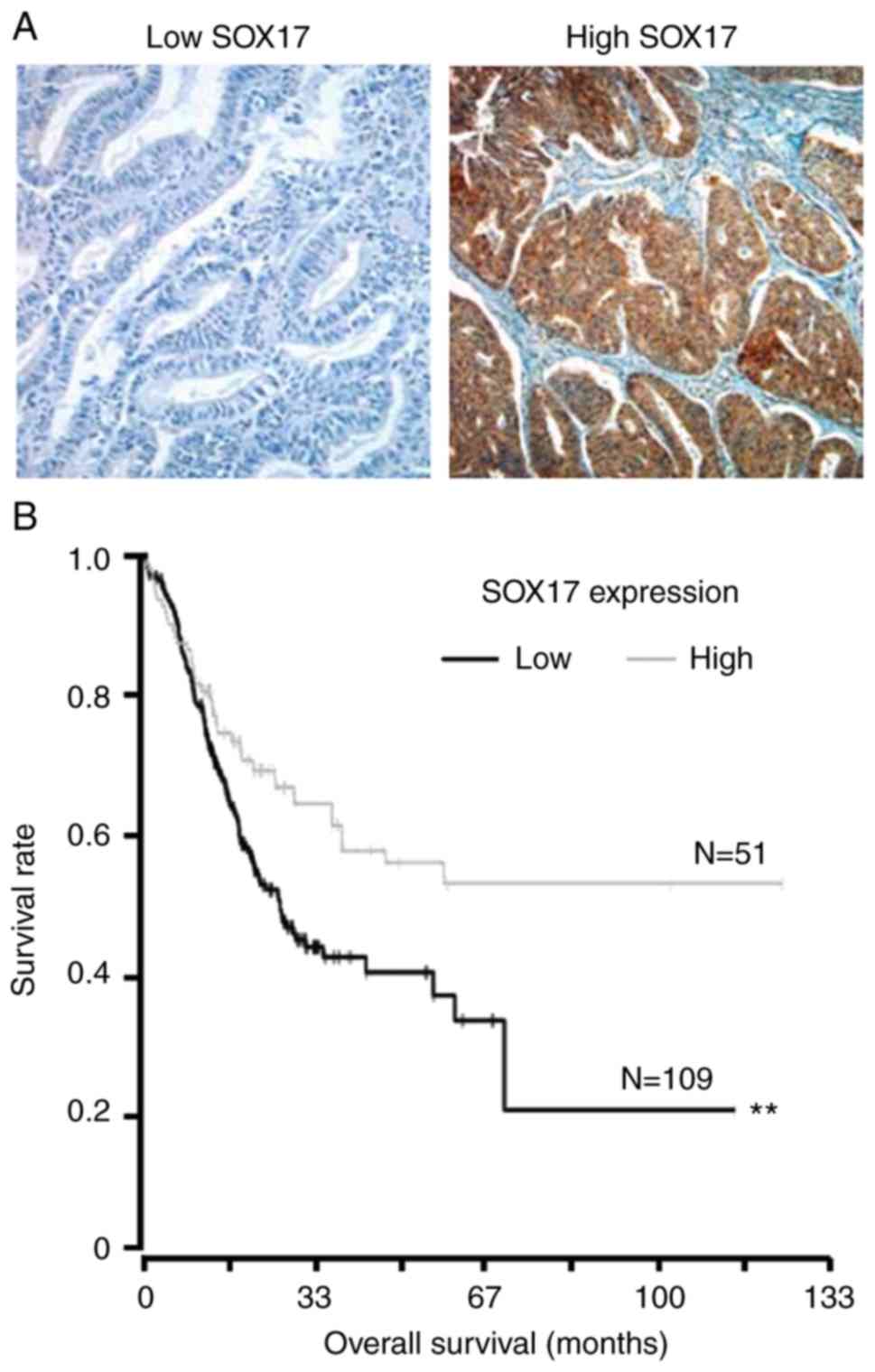
To identify SOX17 expression in endometrial cancer
tissues, immunohistochemistry was performed. Kaplan-Meier curves
were applied to assess the overall survival for 160 patients with
primary endometrial cancers, stratified based on tumor SOX17
expression. Representative images of immunohistochemistry staining
are shown in Fig. 7A. There was a
significant difference between the two overall survival curves;
survival among patients with low-level SOX17 expression was much
poorer than survival among patients with high-level SOX17
expression (Fig. 7B).
Discussion
The primary aim of the present study was to
investigate the role of miR-21-5p and SOX17 in endometrial cancer.
This study is the first, to the best of our knowledge, to
demonstrate that miR-21-5p may promote EMT by targeting SOX17 and
is correlated with poor survival in patients with endometrial
cancer.
The present study revealed that miR-21-5p expression
was highest in AN3CA cells and lowest in HEC-1A cells. HEC-1A cells
are derived from moderately differentiated endometrial cancer,
whereas AN3CA cells are derived from undifferentiated endometrial
cancer (26). Therefore, miR-21-5p
may be associated with poor differentiation. EMT is a critical
developmental program whereby epithelial cells obtain mesenchymal
characters (27,28), and it is a crucial step during
endometrial cancer metastasis (27,28).
The present study revealed that miR-21-5p promoted EMT, whereas
silencing miR-21-5p reversed EMT in endometrial cancer cells.
miR-21-5p expression is dysregulated in endometrial cancer
(15) and promotes the progression
of endometrial cancer (17).
Consistent with a previous report (17), the present study observed that
miR-21-5p expression was inversely correlated with overall
survival.
SOX17 protein expression was highest in HEC-1A cells
and lowest in AN3CA cells, thus indicating that SOX17 protein
expression may be associated with better differentiation. In a
previous study, overexpression of SOX17 inhibited proliferation and
promoted apoptosis of HEC-1B cells, and inhibited growth of
endometrial cancer in animal models (8). Consistent with this previous report
(8), the present results
demonstrated that SOX17 inhibited proliferation of AN3CA cells.
Furthermore, the present study is the first, to the best of our
knowledge, to indicate that overexpression of SOX17 may inhibit EMT
in endometrial cancer cell lines.
SOX17 expression is correlated with longer
recurrence-free survival in endometrial cancer (8). The present study indicated that tumor
SOX17 expression was correlated with improved overall survival in
Chinese patients, which is in line with a previous report (8). These results suggested that SOX17 may
be a candidate tumor suppressor gene in endometrial cancer.
In conclusion, SOX17 was revealed to function as a
candidate tumor suppressor gene in the progression of endometrial
cancer by regulating EMT and proliferation. Furthermore, the
present study suggested that miR-21-5p may be a functional target
for endometrial cancer therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by Jinan Maternity
and Child Health Care Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and YH performed the majority of the experimental
work, initially conceived the study and wrote a draft of the
manuscript. QL performed the remainder of the experimental work.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Maternity and Child Health Care Hospital (Jinan,
China), and each patient provided written informed consent at the
time of enrollment.
Patient consent for publication
Consent for publication was obtained from each
patient.
Competing interests
All authors declare that there are no competing
interests.
References
|
1
|
Janda M, Gebski V, Davies LC, Forder P,
Brand A, Hogg R, Jobling TW, Land R, Manolitsas T, Nascimento M, et
al: Effect of total laparoscopic hysterectomy vs total abdominal
hysterectomy on disease-free survival among women with stage I
endometrial cancer: A randomized clinical trial. JAMA.
317:1224–1233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamachi Y and Kondoh H: Sox proteins:
Regulators of cell fate specification and differentiation.
Development. 140:4129–4144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA. org resource: Targets and expression.
Nucleic Acids Res. 36:D149–D153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye YW, Wu JH, Wang CM, Zhou Y, Du CY,
Zheng BQ, Cao X, Zhou XY, Sun MH and Shi YQ: Sox17 regulates
proliferation and cell cycle during gastric cancer progression.
Cancer Lett. 307:124–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin D, Jia Y, Yu Y, Brock MV, Herman JG,
Han C, Su X, Liu Y and Guo M: SOX17 methylation inhibits its
antagonism of Wnt signaling pathway in lung cancer. Discov Med.
14:33–40. 2012.PubMed/NCBI
|
|
7
|
Jia Y, Yang Y, Zhan Q, Brock MV, Zheng X,
Yu Y, Herman JG and Guo M: Inhibition of SOX17 by microRNA 141 and
methylation activates the WNT signaling pathway in esophageal
cancer. J Mol Diagn. 14:577–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Bao W, Wang K, Lu W, Wang H, Tong
H and Wan X: SOX17 is a tumor suppressor in endometrial cancer.
Oncotarget. 7:76036–76046. 2016.PubMed/NCBI
|
|
9
|
Kuo I, Wu CC, Chang JM, Huang YL, Lin CH,
Yan JJ, Sheu BS, Lu PJ, Chang WL, Lai WW and Wang YC: Low SOX17
expression is a prognostic factor and drives transcriptional
dysregulation and esophageal cancer progression. Int J Cancer.
135:563–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Jiang F, Bao W, Zhang H, He X,
Wang H and Wan X: SOX17 increases the cisplatin sensitivity of an
endometrial cancer cell line. Cancer Cell Int. 16:292016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vonlanthen S, Heighway J, Altermatt H,
Gugger M, Kappeler A, Borner MM, van Lohuizen M and Betticher DC:
The bmi-1 oncoprotein is differentially expressed in non-small cell
lung cancer and correlates with INK4A-ARF locus expression. Br J
Cancer. 84:1372–1376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Browne G, Taipaleenmäki H, Stein GS, Stein
JL and Lian JB: MicroRNAs in the control of metastatic bone
disease. Trends Endocrinol Metab. 25:320–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mei Z, Zhou L, Zhu Y, Jie K, Fan D, Chen
J, Liu X, Jiang L, Jia Q and Li W: Interleukin-22 promotes
papillary thyroid cancer cell migration and invasion through
microRNA-595/Sox17 axis. Tumour Biol. 37:11753–11762. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Myatt SS, Wang J, Monteiro LJ, Christian
M, Ho KK, Fusi L, Dina RE, Brosens JJ, Ghaem-Maghami S and Lam EW:
Definition of microRNAs that repress expression of the tumor
suppressor gene FOXO1 in endometrial cancer. Cancer Res.
70:367–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong P, Kaneuchi M, Watari H, Hamada J,
Sudo S, Ju J and Sakuragi N: MicroRNA-194 inhibits epithelial to
mesenchymal transition of endometrial cancer cells by targeting
oncogene BMI-1. Mol Cancer. 10:992011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin X, Yan L, Zhao X, Li C and Fu Y:
MicroRNA-21 overexpression contributes to cell proliferation by
targeting PTEN in endometrioid endometrial cancer. Oncol Lett.
4:1290–1296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li
YQ, Yan TB, Sun XG, Hu P and Zhang TC: Estrogen receptor α mediates
proliferation of breast cancer MCF-7 cells via a
p21/PCNA/E2F1-dependent pathway. FEBS J. 281:927–942. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang P, Zhang L, Zhang J and Xu G:
MicroRNA-124-3p inhibits cell growth and metastasis in cervical
cancer by targeting IGF2BP1. Exp Ther Med. 15:1385–1393.
2018.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Junn E, Lee KW, Jeong BS, Chan TW, Im JY
and Mouradian MM: Repression of alpha-synuclein expression and
toxicity by microRNA-7. Proc Natl Acad Sci USA. 106:13052–13057.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang SY, Caamano J, Cooper F, Guo X and
Klein-Szanto AJ: Immunohistochemistry of cyclin D1 in human breast
cancer. Am J Clin Pathol. 102:695–698. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hauke J and Kossowski T: Comparison of
values of Pearson's and Spearman's correlation coefficients on the
same sets of data. Quaestiones Geographicae. 30:87–93. 2011.
View Article : Google Scholar
|
|
24
|
Metz CE: Basic principles of ROC analysis.
Semin Nucl Med. 8:283–298. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zweig MH and Campbell G:
Receiver-operating characteristic (ROC) plots: A fundamental
evaluation tool in clinical medicine. Clin Chem. 39:561–577. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao J, Niwa K, Sun W, Takemura M, Lian Z,
Onogi K, Seishima M, Mori H and Tamaya T: Non-steroidal
anti-inflammatory drugs inhibit cellular proliferation and
upregulate cyclooxygenase-2 protein expression in endometrial
cancer cells. Cancer Sci. 95:901–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsuji T, Ibaragi S and Hu GF:
Epithelial-mesenchymal transition and cell cooperativity in
metastasis. Cancer Res. 69:7135–7139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gavert N and Ben-Ze'ev A:
Epithelial-mesenchymal transition and the invasive potential of
tumors. Trends Mol Med. 14:199–209. 2008. View Article : Google Scholar : PubMed/NCBI
|