Introduction
Epigallocatechin-3-gallate (EGCG) is isolated from
green tea, which originated in China, and belongs to a class of
catechuic monomers (1). Numerous
studies have reported that EGCG exhibits anticancer activity
against various types of cancer, including cervical, prostate,
breast, colorectal, esophageal and lung cancer (2–7). EGCG
exerts its anticancer activity by suppressing cell proliferation,
migration and invasion, and by inducing apoptosis in lung cancer
cells (8–11). A number of studies on the anticancer
activity of EGCG focus on lung cancer cells (12–16);
however, few studies (17,18) focus on ovarian cancer cells.
Ovarian cancer is a prevalent gynecological
malignancy, which severely threatens women's health (19). Ovarian cancer occurs in women with a
prevalence ~15,000 per 100,000 individuals worldwide (20). Due to a lack of specific symptoms in
the early stages, 60–70% of patients with ovarian cancer are
diagnosed at an advanced stage, and their 5-year survival rate is
~40% (21,22). Standard therapy strategies for
advanced-stage ovarian cancer are primary debulking surgery
combined with platinum and paclitaxel chemotherapy (23,24).
Although chemotherapy can increase the median survival of ovarian
cancer, the toxicity and drug resistance causes the failure of
chemotherapy and recurrence of the tumor (25–27).
Thus, new drugs with low toxicity and high efficacy to treat
ovarian cancer are urgently required.
The PTEN/AKT/mTOR pathway is involved in the
progression of ovarian cancer and is activated in <70% of
ovarian cancer cases, which makes this pathway crucial in ovarian
cancer therapy (28,29). A previous bioinformatics analysis
has highlighted the potential use of EGCG in ovarian cancer
treatment (30), but these findings
lacked experiment data support. Thus, the aim of the present study
was to investigate the molecular mechanism of EGCG as well as its
anticancer activity in SKOV3 cells and a xenograft model, and to
support clinical application of EGCG in the treatment of ovarian
cancer.
Materials and methods
Cell culture and treatment
Ovarian cancer cell lines SKOV3, CAOV-3 and
NIH-OVCAR-3 were obtained from the Kunming Cell Bank, Conservation
Genetics, the Chinese Academy of Sciences. EGCG was purchased from
Dalian Meilun Biotechnology Co., Ltd. The lung cancer cell line
A549 and human retinal pigment epithelium (RPE) cell line were
obtained from the Shanghai Cell Resource Center, the Chinese
Academy of Biological Sciences. SKOV3 cells were maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.), whereas
the other cell lines were maintained in DMEM medium supplemented
with 10% fetal bovine serum (both Invitrogen; Thermo Fisher
Scientific, Inc.) and 1% antibiotic solution (100 U/ml penicillin
and 100 µg/ml streptomycin) under humidified conditions with 5%
CO2 at 37°C. EGCG was dissolved to different
concentrations (0, 5, 10, 20, 40 and 80 µg/ml) in RPMI-1640 medium;
VO-Ohpic trihydrate (VO-Ohpic; Sigma-Aldrich; Merck KGaA) was
dissolved in DMSO (Xilong Chemical Industry, China) and diluted to
0.1 µM with RPMI-1640 medium.
Cell viability assay
The viability of SKOV3, A549, CAOV-3 and NIH-OVCAR-3
cells was measured by MTT assay. First, a total of 3×103
cells were seeded in 96-well plates and treated with different
concentrations of EGCG (0, 5, 10, 20, 40 and 80 µg/ml) for 24, 48,
72 h at 37°C. Subsequently, 20 µl MTT solution (5 mg/ml) was added
to the cells and incubated for another 4 h at 37°C. Finally, 150 µl
DMSO was used to dissolve the formazan complex, and the optical
density was measured at 490 nm using a microplate reader (Tecan
Group, Ltd.).
Cell colony forming assay
SKOV3 cells (5×102) were seeded into
6-well plates. Following treatment with different concentrations of
EGCG (0, 5, 10, 20, 40 µg/ml), the cells were cultured for another
10–12 days at 37°C with 5% CO2. Subsequently, the
colonies were fixed with absolute methyl alcohol at 25°C for 20 min
and stained with Giemsa solution at 25°C for 30 min. Finally, the
number of colonies containing >50 cells were counted under a
light microscope.
Apoptosis analysis by flow
cytometry
Following EGCG treatment for 48 h at 37°C, SKOV3
cells were collected, washed twice with ice-cold PBS and suspended
in 100 µl 1X binding buffer (BD Biosciences). The cells were
stained using an Annexin V-FITC apoptosis detection kit (BD
Biosciences) by incubation with 5 µl Annexin V-FITC and propidium
iodide for 30 min in the dark, followed by the addition of another
100 µl 1X binding buffer and filtration with 300 mesh. Early and
late apoptosis was determined using BD Accuri C6 Plus flow
cytometer (BD Biosciences), and the results were analyzed by
FlowJo-V10 software (FlowJo LLC).
Reverse transcription-quantitative PCR
(RT-qPCR)
Following EGCG treatment for 48 h at 37°C, total RNA
was extracted from SKOV3 cells using TRIzol® reagent
(Tiangen Biotech Co., Ltd.) and reverse-transcribed into cDNA using
a GoScript™ Reverse Transcription Mix (Promega, Biotech Co., Ltd,
Beijing) at 42°C for 20 min and 90°C for 5 min. QPCR analysis was
performed using UltraSYBR Mixture (CW Bio) on the ABI 7500 Fast
Real-Time PCR Detection system (Thermo Fisher Scientific, Inc.).
The thermocycling conditions for were as follows: 95°C for 30 sec,
40 cycles of 95°C for 5 sec and 60°C for 30 sec, followed by 95°C
for 15 sec, 60°C for 1 min, 95°C for 15 sec and 50°C for 30 sec.
The primer sequences used in this study are presented in Table I. The relative mRNA expression
levels were analyzed using the 2−ΔΔCq method (31).
 | Table I.Sequences of forward and reverse
primers used in reverse transcription-quantitative PCR. |
Table I.
Sequences of forward and reverse
primers used in reverse transcription-quantitative PCR.
| Gene | Sequences
(5′→3′) |
|---|
| Bcl-2 | F:
GCCACTTACCTGAATGACCACC |
|
| R:
AACCAGCGGTTGAAGCGTTCCT |
| Bax | F:
AGACACCTGAGCTGACCTTGGAG |
|
| R:
GTTGAAGTTGCCATCAGCAAACA |
| Caspase-3 | F:
AGAACTGGACTGTGGCATTGAG |
|
| R:
GCTTGTCGGCATACTGTTTCAG |
| AKT | F:
AGAACCTCATRCTGGACAA |
|
| R:
CTCATGGTCCTGGTTGTAGA |
| PTEN | F:
CAGTAGAGGAGCCGTCAAATC |
|
| R:
CAGAGTCAGTGGTGTCAGAATATC |
| mTOR | F:
TCCGAGAGATGAGTCAAGAGG |
|
| R:
CACCTTCCACTCCTATGAGGC |
| β-actin | F:
AAAGACCTGTACGCCAACAC |
|
| R:
GTCATACTCCTGCTTGCTGAT |
Western blot assay
Following treatment with EGCG for 48 h at 37°C,
cells were lysed in RIPA buffer with 2 µg/ml aprotinin, 5 µg/ml
leupeptin, 1 µg/ml pepstatin, 15 mM DTT and 1 mM PMSF. The lysates
were centrifuged at 9,180 × g for 25 min at 4°C. The protein
concentrations were measured using a Bicinchoninic Acid (BCA)
Protein Quantitation kit (Beyotime Institute of Biotechnology). The
protein samples (30 µg/lane) were isolated by 8 or 10% SDS-PAGE and
electro-transferred to nitrocellulose membranes. The membranes were
blocked with 5% (w/v) skimmed milk in PBS + 0.1% Tween-20 (PBST)
for 2 h, followed by incubation with primary antibodies against Bax
(cat. no. ab182733; 1:2,000; Abcam), Bcl-2 (cat. no. ab182858;
1:2,000; Abcam), total caspase-3 (cat. no. ab32351; 1:2,000;
Abcam), PTEN (cat. no. ab32199; 1:2,000; Abcam),
phosphoinositide-dependent kinase-1 (cat. no. WL00707; PDK1;
1:1,000; Wanleibio Co., Ltd.), AKT (cat. no. ab18785; 1:2,000;
Abcam), phosphor (p)-AKT (cat. no. WLP001a; Ser473; 1:1,000;
Wanleibio Co., Ltd.), mTOR (cat. no. ab32028; 1:2,000; Abcam),
p-mTOR (cat. no. ab137133; 1:2,000; Abcam) and β-actin (cat. no.
TA-09; 1:500; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd.) diluted in primary antibody diluent (Beyotime Biotechnology)
overnight at 4°C. The membranes were washed for 7 min with PBST
three times and incubated with horseradish peroxidase-conjugated
goat anti-rabbit or anti-mouse IgG secondary antibodies (cat. nos.
31430 and 31460; 1:5,000; Thermo Fisher Scientific, Inc.) for 1 h
at 25°C. Finally, the protein signals were detected using an X-ray
film. The optical density of the protein bands was measured using
ImageJ V1.8.0 software (National Institutes of Health).
Ovarian cancer xenograft model
Female BALB/c nude mice (4–5 weeks old) were
purchased from Hunan SJA Laboratory Animal Co., Ltd. A total of
1×107 SKOV3 cells in 200 µl PBS were injected
subcutaneously into the right flanks of the mice. Once the tumor
volume reached 50 mm3, the animals were randomized into
five groups (n=7 per group). The mice in the control group were
administered normal saline; the positive control group were
administered 5 mg/kg paclitaxel; and the mice in the experimental
groups were administered 10, 30 or 50 mg/kg EGCG. EGCG and saline
were administered every day, and paclitaxel was administered twice
a week. Tumor volume was calculated using the following formula:
Volume=length × width2/2. Following treatment for 21
days, the mice were euthanized, and tumor tissues were collected
and maintained in a −80°C deep freezer until further analysis.
Ethical approval for the use of animals was obtained prior to the
start of this study from the Institutional Animal Care and Use
Committee of Guilin Medical University (Guilin, China), and all the
animals used in the experiments were treated humanely.
Hematoxylin and eosin (HE)
staining
Livers from nude mice were collected and immersed in
a formaldehyde solution (37-40% formaldehyde/PBS, 1:9) at 4°C for
24 h. After fixation, liver samples were dehydrated in 70, 80, 90
and 100% alcohol, cleared in pure benzene and embedded in paraffin.
Then, 3-µm sections were cut and mounted onto slides, followed by
10-min dewaxing with fresh xylene for three times. Subsequently,
the sections were placed in 100, 95, 85 and 75% alcohol for 5 min
and stained with hematoxylin (Beijing Solarbio Science &
Technology Co., Ltd.) at 25°C for 15 min. The sections were
differentiated with hydrochloric alcohol, then dehydrated in 75,
85, 95 and 100% alcohol for 5 min. After dehydration, the sections
were stained with eosin (Solarbio Biotechnology Company, Shanghai,
China) at 25°C for 15 sec and placed in fresh xylene for 5 min.
Finally, the sections were sealed with neutral gum. Morphological
changes of liver tissue were observed under a light microscope.
Statistical analysis
The data are presented as the mean ± SD of at least
three independent experiments. All data were analyzed by SPSS
version 17.0 (SPSS, Inc.), and one-way ANOVA followed by Tukey's
post hoc test was used to assess the statistical significance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
EGCG inhibits cancer cell
proliferation
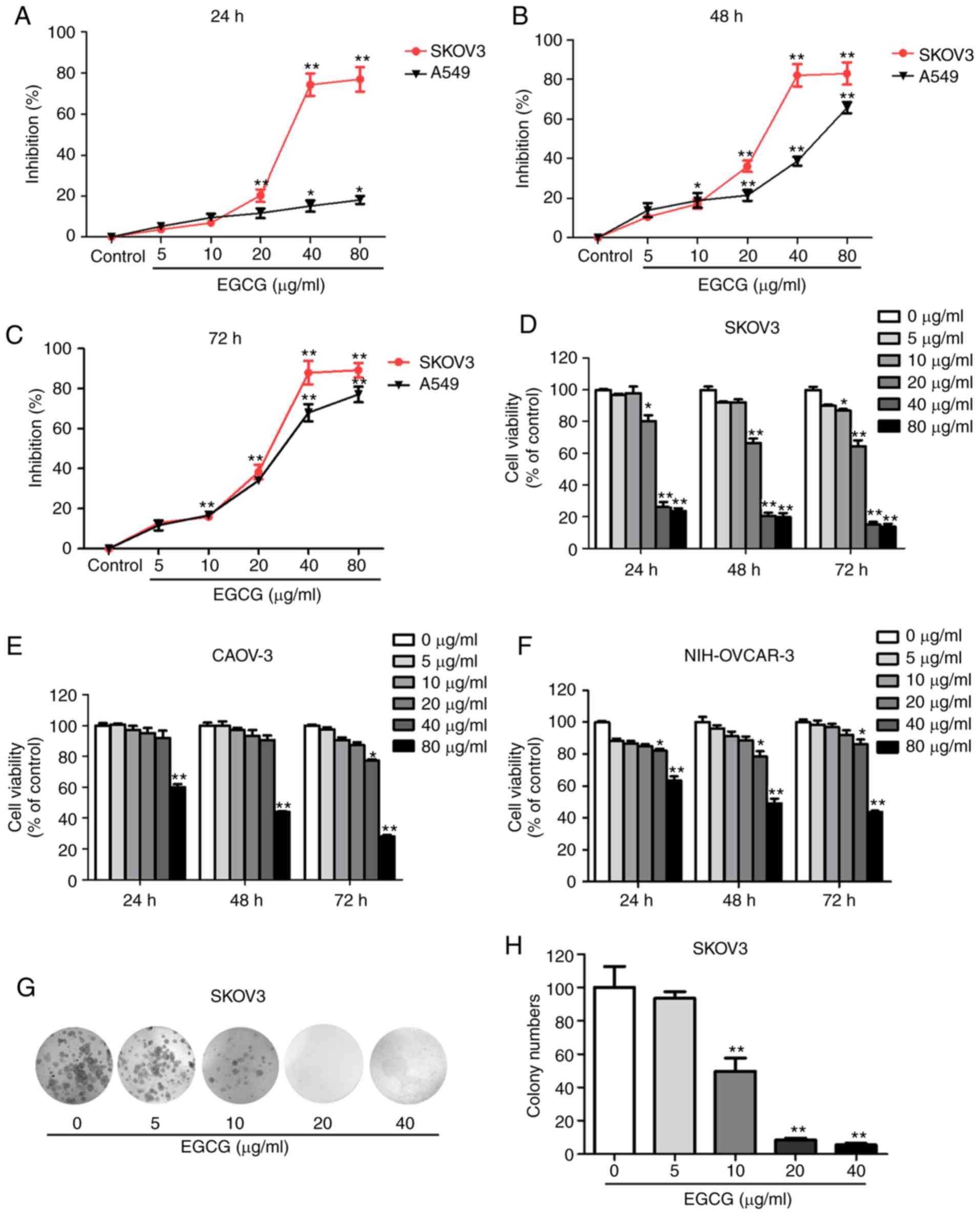
To determine the EGCG-mediated proliferation
inhibition in SKOV3 and A549 cells, cell viability was examined at
24, 48 and 72 h following treatment with a range of EGCG
concentrations. As presented in Fig.
1A-C, EGCG exhibited a significant proliferation inhibition on
SKOV3 cell and A549 cells. In addition, CAOV-3 and NIH-OVCAR-3 cell
lines were used to investigate the EGCG-mediated proliferation
inhibition. The results demonstrated that EGCG inhibited SKOV3,
CAOV-3 and NIH-OVCAR-3 cell proliferation in a dose- and
time-dependent manner (Fig. 1D-F).
Among the four cell lines, SKOV3 exhibited the lowest
IC50 values, suggesting that it was more sensitive to
EGCG compared with the other three cell lines (Table II). In addition, to detect the
toxicity of EGCG to normal cells, EGCG was used to treat normal
human RPE cells; the results demonstrated that 40 µg/ml EGCG had a
small effect on the proliferation of RPE cells (Fig. S1A). In addition, to further confirm
the proliferation inhibition of EGCG in SKOV3 cells, a colony
formation assay was conducted. Compared with the control group,
EGCG treatment significantly decreased SKOV3 cell colony formation
(Fig. 1G and H).
 | Table II.IC50 of
epigallocatechin-3-gallate in four cancer cell lines. |
Table II.
IC50 of
epigallocatechin-3-gallate in four cancer cell lines.
|
| IC50,
µg/ml |
|---|
|
|
|
|---|
| Cell line | 24 h | 48 h | 72 h |
|---|
| SKOV3 |
34.58 |
26.07 | 22.04 |
| NIH-OVCAR-3 | 349.62 | 118.82 | 82.19 |
| CAOV-3 | 410.81 | 123.67 | 57.64 |
| A549 |
72.61 |
56.67 | 29.24 |
EGCG induces apoptosis in SKOV3
cells
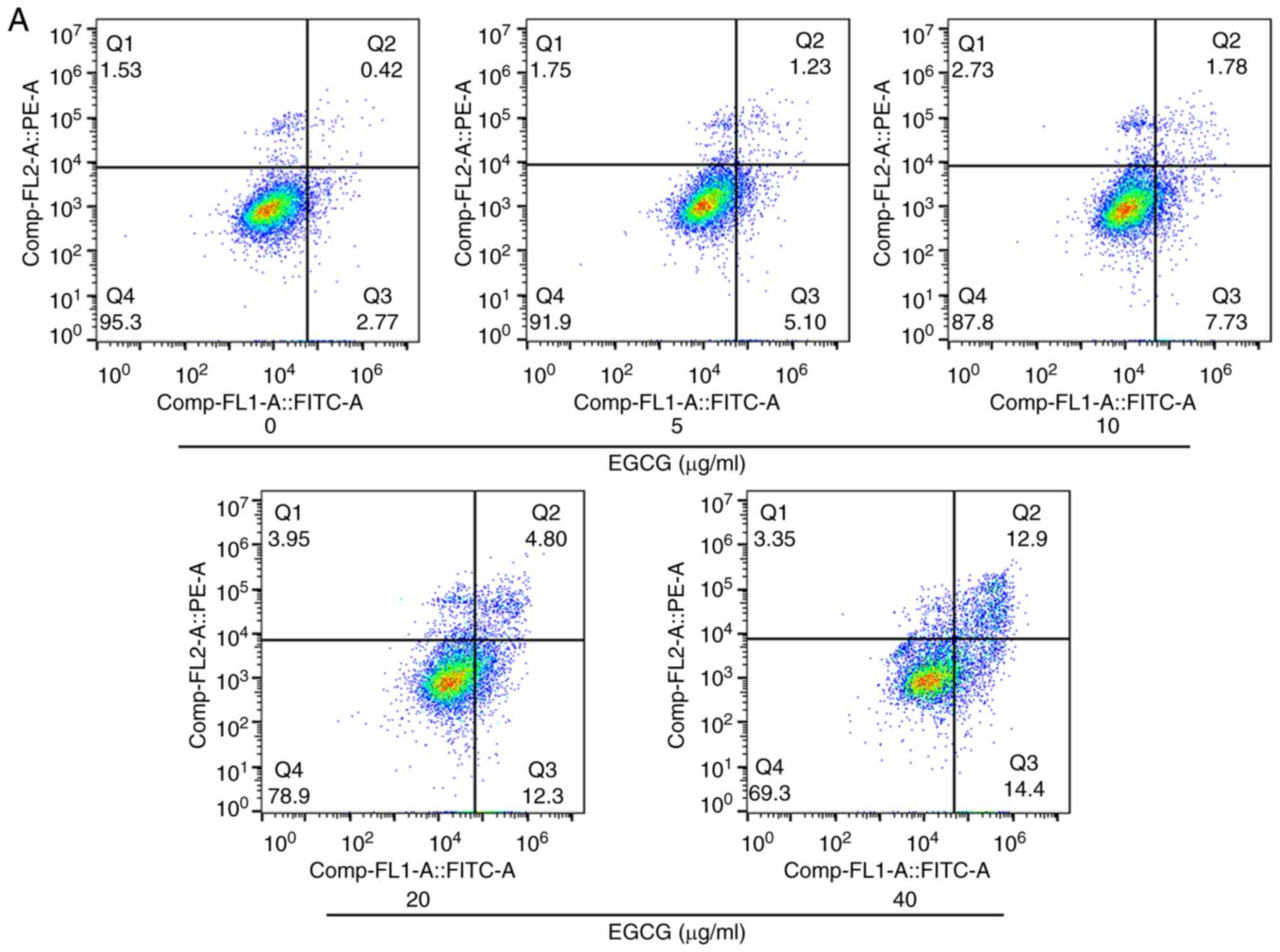
The apoptotic rates of EGCG-treated cells were
examined by flow cytometry. As presented in Fig. 2A and B, the apoptotic rates in the
5, 10, 20 and 40 µg/ml EGCG treatment groups increased to 6.33,
9.51, 17.10 and 27.30%, respectively, compared with the 3.19% in
the control group. Increasing doses of EGCG induced higher rates of
SKOV3 cells apoptosis (Fig. 2B).
However, the apoptotic rate of 40 µg/ml EGCG was only 5% in human
normal RPE cells (Fig. S1B and C).
The RT-qPCR results suggested that EGCG increased the mRNA
expression of Bax and caspase-3, and decreased the expression of
Bcl-2 compared with the control cells (Fig. 2C). Similarly, western blotting
results demonstrated that EGCG treatment upregulated the protein
expression of Bax and caspase-3 and downregulated the expression of
Bcl-2 in SKOV3 cells compared with the untreated control (Fig. 2D and E). Taken together, these
results indicated that EGCG promoted SKOV3 cell apoptosis and
regulated the expression of apoptosis-related factors.
EGCG inhibits the activation of the
PTEN/AKT/mTOR signaling pathway
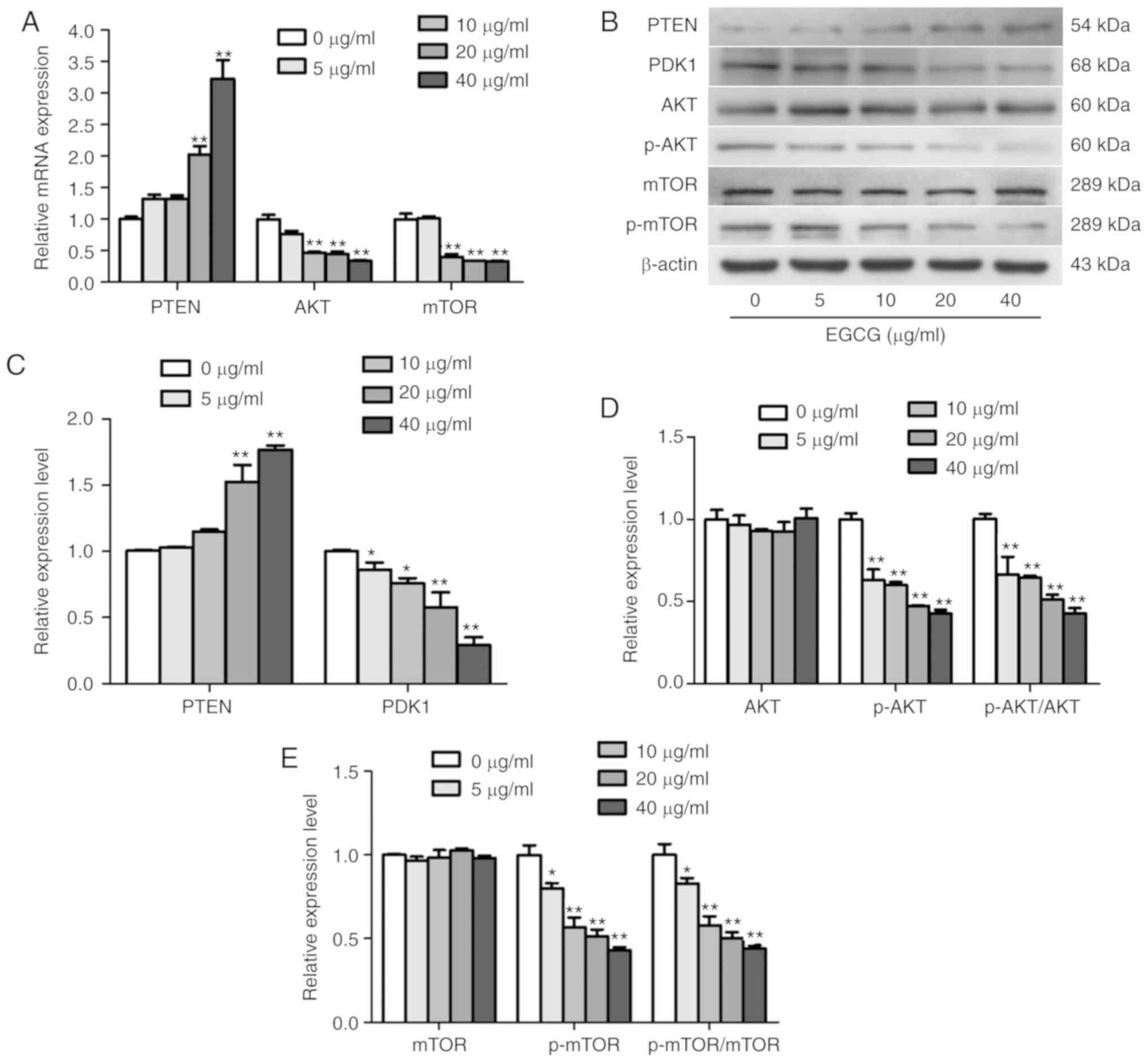
The RT-qPCR and western blotting results revealed
that EGCG upregulated the expression of PTEN. Thus, the expression
levels of PDK1, AKT and mTOR were also determined in SKOV3 cells.
The results demonstrated that EGCG reduced the mRNA levels of AKT
and mTOR, and reduced the protein expression of PDK1, p-AKT and
p-mTOR, whereas no changes were observed in the total AKT and mTOR
protein levels (Fig. 3). Taken
together, these results demonstrated that EGCG modulated the
activation of the PTEN/AKT/mTOR pathway in SKOV3 cells.
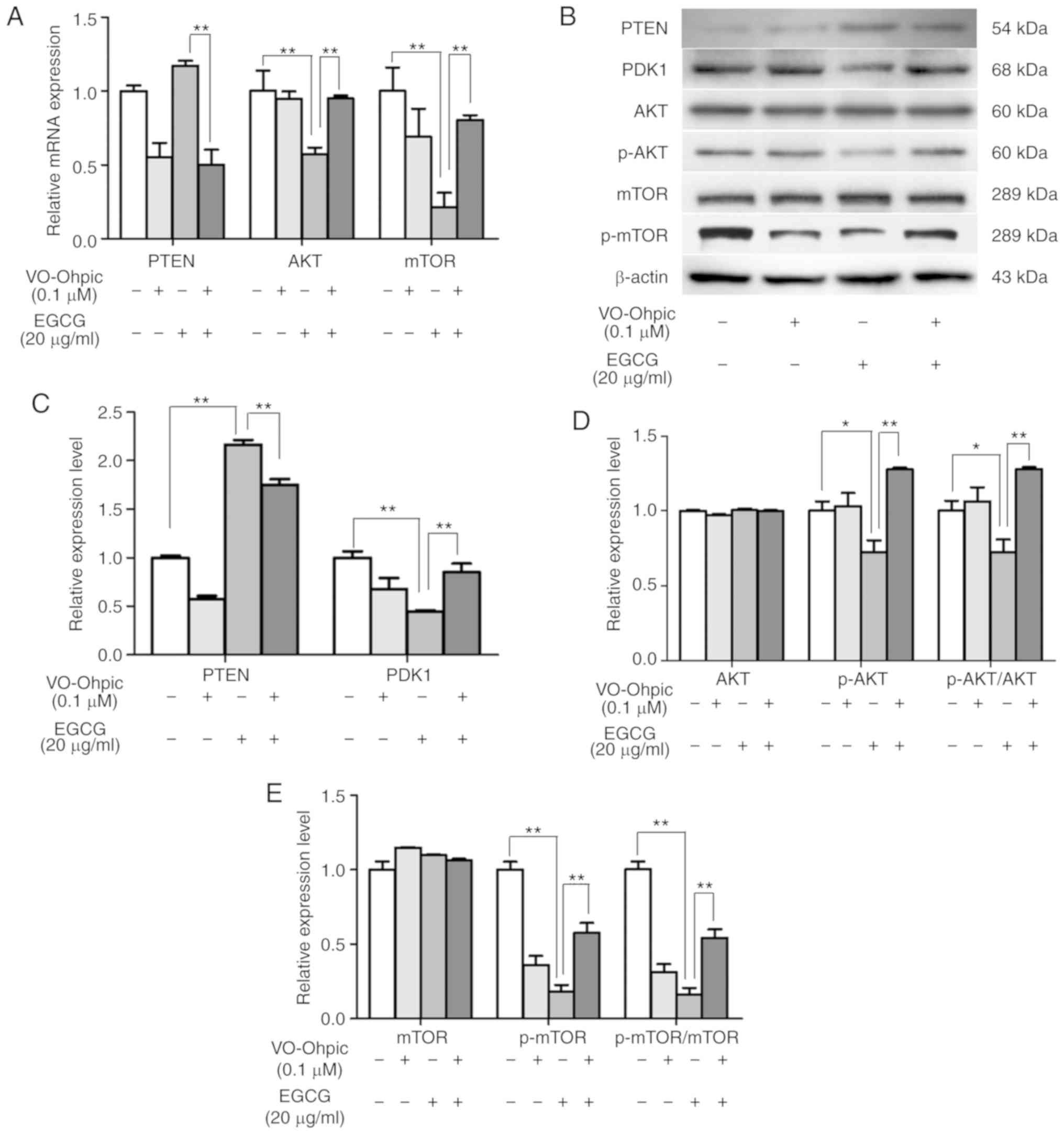
VO-Ohpic trihydrate reverses the
antitumor effects of EGCG
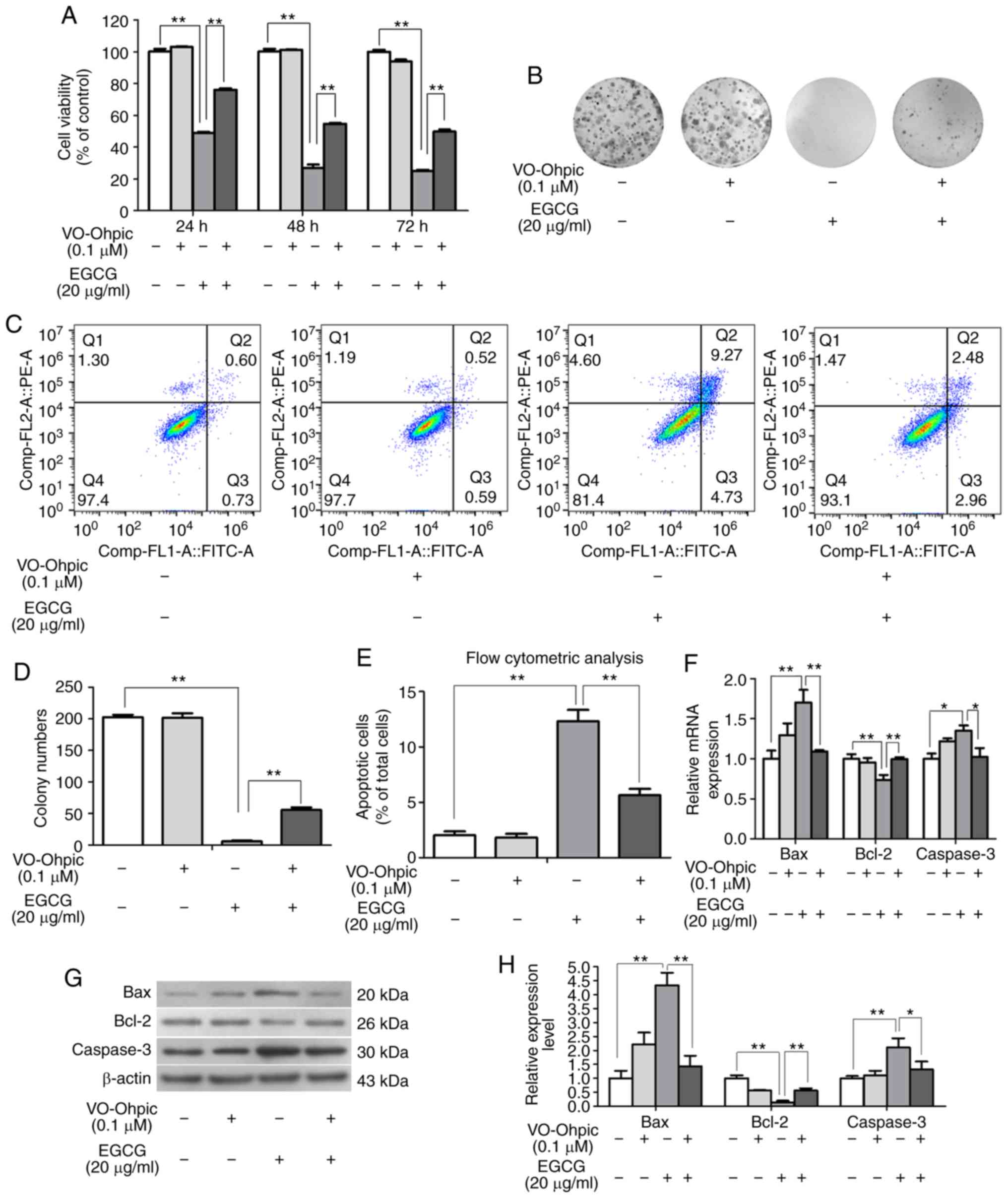
To confirm the effects of EGCG on the PTEN/AKT/mTOR
pathway, VO-Ohpic was used to determine whether the effects of EGCG
on SKOV3 cells were altered. First, the effect on proliferation was
detected by the MTT and colony formation assays. As presented in
Fig. 4A, 0.1 µM VO-Ohpic alone did
not affect the cell viability. The viability of cells in the EGCG
and VO-Ohpic co-treatment group was higher compared with that of
cells in the EGCG group, indicating that VO-Ohpic partly rescued
the antiproliferative effect of EGCG in SKOV3 cells (Fig. 4B and D). Second, the flow cytometry
results indicated that the apoptotic rate of control group was
1.33% and that of the VO-Ohpic group was 1.11%, whereas the
apoptotic rate of EGCG group was 14.00%, which was significantly
higher compared with that of VO-Ohpic treated group. The apoptotic
rate of SKOV3 cells was 5.44% in the EGCG and VO-Ohpic co-treatment
group, which was 8.56% lower compared with the EGCG group (Fig. 4C and E). The mRNA and protein
detection results demonstrated that VO-Ohpic reversed the effects
of EGCG on Bax, caspase-3 and Bcl-2 expression levels in SKOV3
cells (Fig. 4F-H). These results
demonstrated that VO-Ohpic partly rescued the proapoptotic effects
of EGCG. In addition, the EGCG-induced changes in the expression
levels of PTEN, PDK1, AKT, p-AKT and p-mTOR were reversed by
VO-Ohpic (Fig. 5). These results
suggested that EGCG exerted its antiproliferative and proapoptotic
effects by regulating the PTEN/AKT/mTOR signaling pathway in SKOV3
cells.
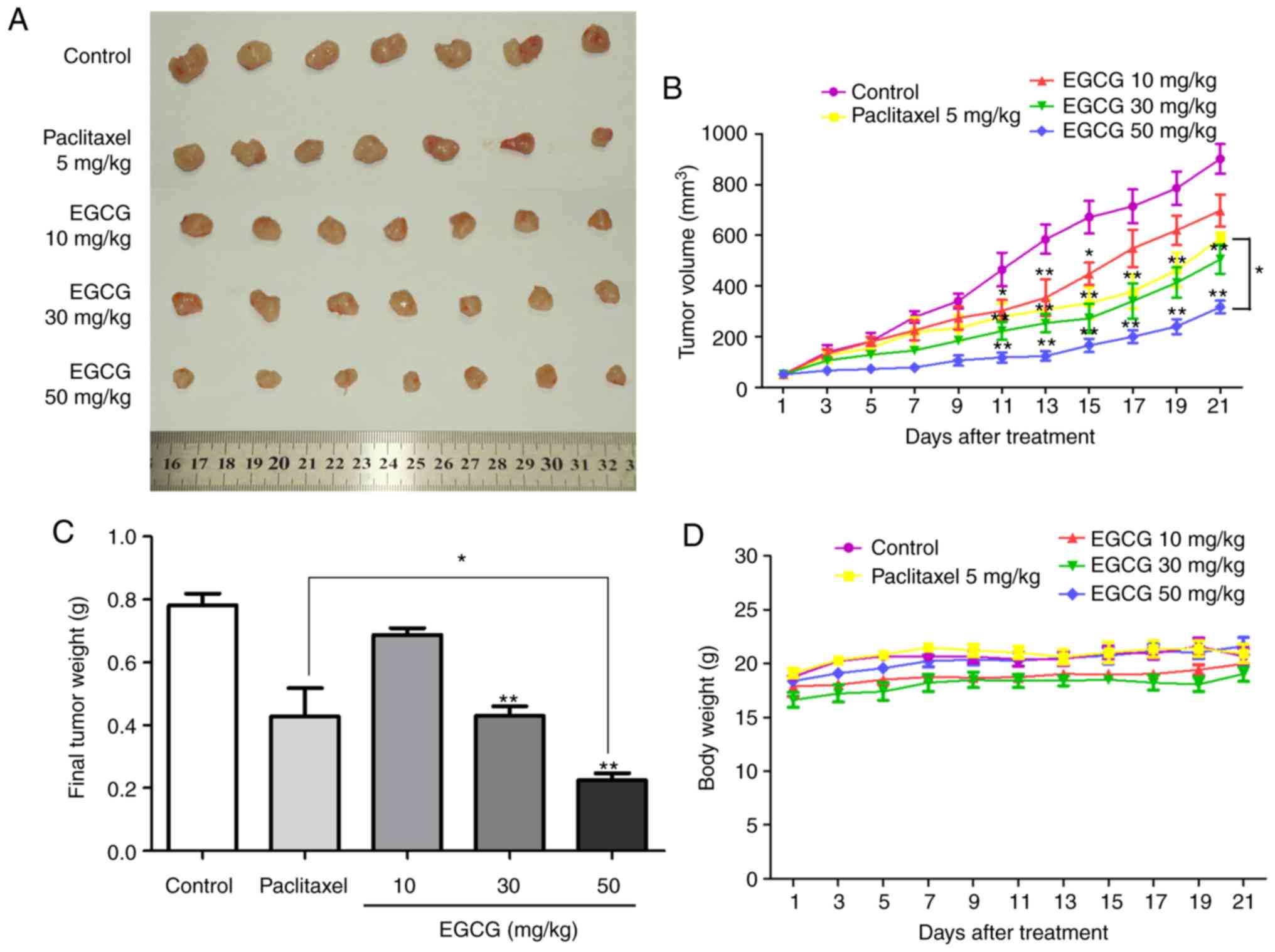
EGCG suppresses xenograft ovarian
tumor growth in vivo
To investigate the antitumor effect of EGCG on
ovarian cancer in vivo, a xenograft tumor model was
established in BALB/c nude mice. As presented in Fig. 6A and B, EGCG significantly
suppressed tumor growth in vivo. In addition, the mean tumor
volume in the 50 mg/kg EGCG treatment group was lower compared with
that in the 5 mg/kg paclitaxel group (Fig. 6B). Compared with normal saline
treatment, 50 mg/kg EGCG significantly decreased the tumor weight
at the end of the experiment by 71.25%, whereas paclitaxel
decreased it by 39.62% (Fig. 6C).
In addition, EGCG-treated mice exhibited a high tolerance and did
not experience significant loss of body weight (Fig. 6D). In addition, the HE staining
results revealed that EGCG exerted limited effects on the mouse
liver (Fig. S2), which was
consistent with previous studies (32,33).
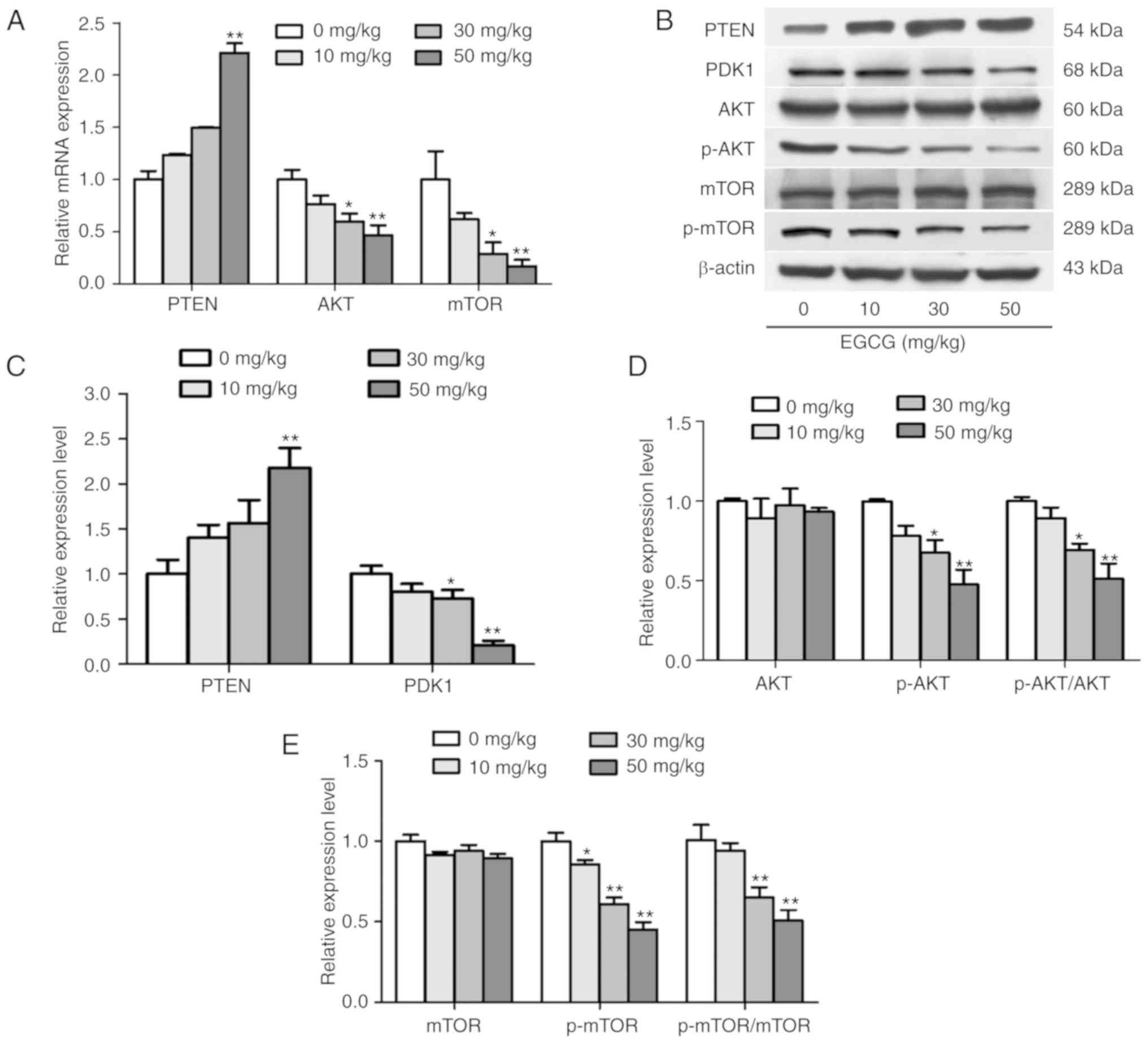
Furthermore, the activation of the PTEN/AKT/mTOR pathway was
detected in tumor tissues. As presented in Fig. 7, mRNA and protein expression assays
revealed that EGCG decreased the expression levels of AKT and mTOR,
as well as increased the expression levels of PTEN in tumor tissues
compared with those in the control group. These results were
consistent with the in vitro assay results. Taken together,
the results demonstrated that EGCG substantially suppressed tumor
growth in mouse ovarian cancer xenografts, and the anticancer
activity of EGCG in the xenograft tumors was partially associated
with the regulation of the PTEN/AKT/mTOR pathway.
Discussion
Ovarian cancer is a common malignant gynecologic
cancer, and patients typically present with advanced disease at the
time of diagnosis due to a lack of early symptoms (34). In the past 10 years, therapeutic
methods and drugs for ovarian cancer have been continuously
developed, but the overall development is slow and the mortality of
ovarian cancer is still increasing (35,36).
Exploring novel therapeutic drugs is essential for the treatment of
ovarian cancer. EGCG has been demonstrated to possess anticancer
bioactivity, which has attracted attention; EGCG has demonstrated
cancer preventive activity in various types of human cancer,
including lung, oral cavity and esophageal cancers (37,38).
Numerous studies have been conducted on the effects of EGCG on lung
cancer (39–42), and it had been reported that oral
administration of EGCG was feasible and safe to patients with
advanced lung cancer (43).
However, the present study demonstrated that EGCG exerted a
stronger proliferation inhibition on ovarian cancer SKOV3 cells
compared with that on lung cancer A549 cells, although few studies
(44,45) have focused on the effects of EGCG on
ovarian cancer. Thus, it was meaningful and worthy to study the
effects of EGCG on ovarian cancer and explore the underlying
molecular mechanism. In the present study, the MTT assay results
revealed that EGCG inhibited SKOV3, CAOV-3 and NIH-OVCAR-3 cell
proliferation; SKOV3 was the most sensitive to EGCG treatment among
the four tested cell lines, and thus SKOV3 cells were selected as
the research object of this study. In addition, MTT assay results
revealed that 40 µg/ml EGCG exerted a limited effect on human
retinal pigment epithelium (RPE) cell viability (Fig. S1A). In the flow cytometry analysis,
the apoptotic rate of SKOV3 cells in the 40 µg/ml EGCG treatment
group reached 27.3%, whereas that in RPE cells was only 5%
(Fig. S1B and C). Additionally,
EGCG increased the expression of Bax and caspase-3, and decreased
the expression of Bcl-2 in SKOV3 cells compared with the untreated
control group. These results indicated that EGCG exhibited
anticancer effects on ovarian cancer cells, but limited
cytotoxicity to normal cells.
PTEN prevents PDK1-mediated phosphorylation of AKT
by converting PIP3 to PIP2, and further inhibits the
phosphorylation of mTOR (46).
Upregulation of PTEN suppresses cell proliferation and promotes
apoptosis, which is associated with its negative regulation of the
AKT/mTOR pathway (47). Abnormal
activation of the AKT/mTOR pathway has been observed in various
types of cancer, including ovarian cancer (48–51).
Certain molecules targeting this pathway, including AKT inhibitor
MK-2206 (52), mTOR inhibitor
AZD8055 (53) and dual PI3K/mTOR
inhibitor PF-04691502 (54), have
been used for cancer treatment. Multiple studies have demonstrated
that the AKT/mTOR pathway serves a prominent role in ovarian cancer
tumorigenesis, proliferation and progression (55–57).
In the bioinformatics analysis performed by Shen et al
(58), AKT was also identified as a
target protein in ovarian cancer, but it was not verified if EGCG
exerted anti-ovarian cancer effect by targeting AKT. Therefore, the
present study evaluated the expression of PTEN, PDK1, AKT and mTOR
in ovarian cancer cells after EGCG treatment. The results suggested
that the PTEN/AKT/mTOR pathway was involved in anti-ovarian cancer
activity of EGCG. In addition, the PTEN inhibitor VO-Ohpic reversed
the effects of EGCG on the proliferation inhibition, apoptosis
induction and the PTEN/AKT/mTOR pathway activation in ovarian
cancer cells. These results demonstrated that EGCG exerted
anticancer effects in SKOV3 cells through the PTEN/AKT/mTOR
pathway.
To further confirm the role of EGCG in the
proliferation inhibition of ovarian cancer, an in vivo
experiment was performed in the present study, which demonstrated
that EGCG significantly decreased tumor growth in nude mice
compared with the control group, and the mean tumor volume in the
50 mg/kg EGCG group was markedly attenuated compared with those in
the control and 5 mg/kg paclitaxel groups. EGCG-treated mice
exhibited high tolerance and did not experience significant loss of
body weight.
Paclitaxel is the first-line drug for ovarian cancer
treatment; standard initial therapy for ovarian cancer is
platinum/paclitaxel combination chemotherapy (59). The in vivo results of the
present study demonstrated that 50 mg/kg EGCG treatment exhibited
stronger growth suppression on ovarian cancer cells compared with 5
mg/kg paclitaxel, indicating that EGCG may be a potential
therapeutic agent for ovarian cancer. In addition, EGCG treatment
resulted in an inhibition of the PTEN/AKT/mTOR pathway in nude
mice. These results suggested that EGCG exerted anti-ovarian cancer
effects in vivo via the PTEN/AKT/mTOR pathway.
In summary, the results of the present study
suggested that EGCG exerted stronger proliferation inhibition on
SKOV3 cells compared with A549 cells, and the PTEN/AKT/mTOR
signaling pathway was involved in the anti-ovarian cancer effects
of EGCG in vitro and in vivo. However, future
analysis of PTEN or AKT overexpression and blood test (detection of
liver- or heart-related enzymes ALT, AST and CK) after EGCG
treatment in nude mice will be required to support the potential
application of EGCG in ovarian cancer therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 31460229, 81760443 and
81760663), the Guangxi Natural Science Foundation (grant no.
2017GXNSFDA198029), the Small Talent Highland Fund in Guangxi
(grant no. 201707) and The Scientific Research and Technology
Development Plan of Guilin (grant no. 20170109-38).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC, JW, MLF and JLQ designed the study. JLQ, MLF,
MJH and DY performed the experiments. JLQ and JW wrote the
manuscript. HWL, XML and XG reviewed and revised the manuscript.
FXH and HPL conducted data analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Ethical approval for the use of animals was obtained
prior to the start of this study from the Institutional Animal Care
and Use Committee of Guilin Medical University (Guilin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan N and Mukhtar H: Tea polyphenols in
promotion of human health. Nutrients. 11(pii): E392018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang YQ, Lu JL, Liang YR and Li QS:
Suppressive effects of EGCG on cervical cancer. Molecules. 23(pii):
E23342018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang LX, Shi YL, Zhang LJ, Wang KR, Xiang
LP, Cai ZY, Lu JL, Ye JH, Liang YR and Zheng XQ: Inhibitory effects
of (−)-Epigallocatechin-3-gallate on esophageal cancer. Molecules.
24(pii): E9542019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanna V, Singh CK, Jashari R, Adhami VM,
Chamcheu JC, Rady I, Sechi M, Mukhtar H and Siddiqui IA: Targeted
nanoparticles encapsulating (−)-epigallocatechin-3-gallate for
prostate cancer prevention and therapy. Sci Rep. 7:415732017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong OY, Noh EM, Jang HY, Lee YR, Lee BK,
Jung SH, Kim JS and Youn HJ: Epigallocatechin gallate inhibits the
growth of MDA-MB-231 breast cancer cells via inactivation of the
beta-catenin signaling pathway. Oncol Lett. 14:441–446. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flores-Perez A, Marchat LA, Sánchez LL,
Romero-Zamora D, Arechaga-Ocampo E, Ramírez-Torres N, Chávez JD,
Carlos-Reyes Á, Astudillo-de la Vega H, Ruiz-García E, et al:
Differential proteomic analysis reveals that EGCG inhibits HDGF and
activates apoptosis to increase the sensitivity of non-small cells
lung cancer to chemotherapy. Proteomics Clin Appl. 10:172–182.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ying L, Yan F, Williams BR, Xu P, Li X,
Zhao Y, Hu Y, Wang Y, Xu D and Dai J:
(−)-Epigallocatechin-3-gallate and EZH2 inhibitor GSK343 have
similar inhibitory effects and mechanisms of action on colorectal
cancer cells. Clin Exp Pharmacol Physiol. 45:58–67. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dhatwalia SK, Kumar M and Dhawan DK: Role
of EGCG in containing the progression of lung tumorigenesis-a
multistage targeting approach. Nutr Cancer. 70:334–349. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi J, Liu F, Zhang W, Liu X, Lin B and
Tang X: Epigallocatechin-3-gallate inhibits nicotine-induced
migration and invasion by the suppression of angiogenesis and
epithelial-mesenchymal transition in non-small cell lung cancer
cells. Oncol Rep. 33:2972–2980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Wang XQ, Zhang Q, Zhu JY, Li Y,
Xie CF, Li XT, Wu JS, Geng SS, Zhong CY and Han HY:
(−)-Epigallocatechin-3-Gallate inhibits colorectal cancer stem
cells by suppressing Wnt/β-catenin pathway. Nutrients. 9(pii):
E5722017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Li P, Qu Z, Xiong W, Liu A and
Zhang S: Advances in the antagonism of Epigallocatechin-3-gallate
in the treatment of digestive tract tumors. Molecules. 24(pii):
E17262019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rawangkan A, Wongsirisin P, Namiki K, Iida
K, Kobayashi Y, Shimizu Y, Fujiki H and Suganuma M: Green tea
catechin is an alternative immune checkpoint inhibitor that
inhibits PD-L1 expression and lung tumor growth. Molecules.
23(pii): E20712018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma YC, Li C, Gao F, Xu Y, Jiang ZB, Liu JX
and Jin LY: Epigallocatechin gallate inhibits the growth of human
lung cancer by directly targeting the EGFR signaling pathway. Oncol
Rep. 31:1343–1349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Sun P, Wang Q, Zhang P, Wang Y, Zi
C, Wang X and Sheng J: (−)-Epigallocatechin-3-gallate derivatives
combined with cisplatin exhibit synergistic inhibitory effects on
non-small-cell lung cancer cells. Cancer Cell Int. 19:2662019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng J, Chang C, Chen Y, Bi F, Ji C and
Liu W: EGCG overcomes gefitinib resistance by inhibiting autophagy
and augmenting cell death through targeting ERK phosphorylation in
NSCLC. Onco Targets Ther. 12:6033–6043. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng P, Hu C, Xiong Z, Li Y, Jiang J, Yang
H, Tang Y, Cao L and Lu R: Epigallocatechin-3-gallate-induced
vascular normalization in A549-cell xenograft-bearing nude mice:
Therapeutic efficacy in combination with chemotherapy. Cancer Manag
Res. 11:2425–2439. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan C, Yang J, Shen L and Chen X:
Inhibitory effect of Epigallocatechin gallate on ovarian cancer
cell proliferation associated with aquaporin 5 expression. Arch
Gynecol Obstet. 285:459–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Chang Z, Fan Q and Wang L:
Epigallocatechin-3-gallate inhibits the proliferation and migration
of human ovarian carcinoma cells by modulating p38 kinase and
matrix metalloproteinase-2. Mol Med Rep. 9:1085–1089. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Padmakumar S, Parayath NN, Nair SV, Menon
D and Amiji MM: Enhanced anti-tumor efficacy and safety with
metronomic intraperitoneal chemotherapy for metastatic ovarian
cancer using biodegradable nanotextile implants. J Control Release.
305:29–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mahalaxmi I, Devi SM, Kaavya J, Arul N,
Balachandar V and Santhy KS: New insight into NANOG: A novel
therapeutic target for ovarian cancer (OC). Eur J Pharmacol.
852:51–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pisanic TR II, Cope LM, Lin SF, Yen TT,
Athamanolap P, Asaka R, Nakayama K, Fader AN, Wang TH, Shih IM and
Wang TL: Methylomic analysis of ovarian cancers identifies
tumor-specific alterations readily detectable in early precursor
lesions. Clin Cancer Res. 24:6536–6547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chuffa LGD, Reiter RJ and Lupi LA:
Melatonin as a promising agent to treat ovarian cancer: Molecular
mechanisms. Carcinogenesis. 38:945–952. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Madariaga A, Lheureux S and Oza A:
Tailoring ovarian cancer treatment: Implications of BRCA1/2
mutations. Cancers. 11(pii): E4162019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roane BM, Arend RC and Birrer MJ: Review:
Targeting the transforming growth Factor-beta pathway in ovarian
cancer. Cancers (Basel). 11(pii): E6682019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomao F, Marchetti C, Romito A, Di Pinto
A, Di Donato V, Capri O, Palaia I, Monti M, Muzii L and Benedetti
Panici P: Overcoming platinum resistance in ovarian cancer
treatment: From clinical practice to emerging chemical therapies.
Expert Opin Pharmacother. 18:1443–1455. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Feng Y, Wang XY, Zhang YN, Yuan
CN, Zhang SF, Shen YM, Fu YF, Zhou CY, Li X, et al: The inhibition
of UBC13 expression and blockage of the DNMT1-CHFR-Aurora A pathway
contribute to paclitaxel resistance in ovarian cancer. Cell Death
Dis. 9:932018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yap TA, Carden CP and Kaye SB: Beyond
chemotherapy: Targeted therapies in ovarian cancer. Nat Rev Cancer.
9:167–181. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gasparri M, Bardhi E, Ruscito I, Papadia
A, Farooqi AA, Marchetti C, Bogani G, Ceccacci I, Mueller MD and
Benedetti Panici P: PI3K/AKT/mTOR pathway in ovarian cancer
treatment: Are we on the right track? Geburtshilfe Frauenheilkd.
77:1095–1103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mabuchi S, Kuroda H, Takahashi R and
Sasano T: The PI3K/AKT/mTOR pathway as a therapeutic target in
ovarian cancer. Gynecol Oncol. 137:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xinqiang S, Mu Z, Lei C and Mun LY:
Bioinformatics analysis on molecular mechanism of green tea
compound Epigallocatechin-3-gallate against ovarian cancer. Clin
Transl Sci. 10:302–307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo KW, Wei C, Lung WY, Wei XY, Cheng BH,
Cai ZM and Huang WR: EGCG inhibited bladder cancer SW780 cell
proliferation and migration both in vitro and in vivo via
down-regulation of NF-κB and MMP-9. J Nutr Biochem. 41:56–64. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan H, Chen J, Shen K, Wang X, Wang P, Fu
G, Meng H, Wang Y and Jin B: Mitochondrial modulation by
Epigallocatechin 3-Gallate ameliorates cisplatin induced renal
injury through decreasing oxidative/nitrative stress, inflammation
and NF-kB in mice. PLoS One. 10:e01247752015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Yang R, Zhao L, Zhang X, Xu T and
Cui M: Basing on uPAR-binding fragment to design chimeric antigen
receptors triggers antitumor efficacy against uPAR expressing
ovarian cancer cells. Biomed Pharmacother. 117:1091732019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Russell MR, Graham C, D'Amato A,
Gentry-Maharaj A, Ryan A, Kalsi JK, Whetton AD, Menon U, Jacobs I
and Graham RLJ: Diagnosis of epithelial ovarian cancer using a
combined protein biomarker panel. Br J Cancer. 121:483–489. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yarmolinsky J, Relton CL, Lophatananon A,
Muir K, Menon U, Gentry-Maharaj A, Walther A, Zheng J, Fasching P,
Zheng W, et al: Appraising the role of previously reported risk
factors in epithelial ovarian cancer risk: A Mendelian
randomization analysis. PLoS Med. 16:e10028932019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang CS and Hong J: Prevention of chronic
diseases by tea: Possible mechanisms and human relevance. Annu Rev
Nutr. 33:161–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, He Y, Wu X, Zhao G, Zhang K, Yang
CS, Reiter RJ and Zhang J: Melatonin and
(−)-Epigallocatechin-3-Gallate: Partners in fighting cancer. Cells.
8(pii): E7452019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang L, Xie J, Gan R, Wu Z, Luo H, Chen
X, Lu Y, Wu L and Zheng D: Synergistic inhibition of lung cancer
cells by EGCG and NF-κB inhibitor BAY11-7082. J Cancer.
10:6543–6556. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bhardwaj V and Mandal AKA: Next-Generation
sequencing reveals the role of Epigallocatechin-3-gallate in
regulating putative novel and known microRNAs which target the MAPK
pathway in non-small-cell lung cancer A549 cells. Molecules.
24(pii): E3682019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu C, Jiao Y, Xue J, Zhang Q, Yang H, Xing
L, Chen G, Wu J, Zhang S, Zhu W and Cao J: Metformin sensitizes
non-small cell lung cancer cells to an Epigallocatechin-3-Gallate
(EGCG) treatment by suppressing the Nrf2/HO-1 signaling pathway.
Int J Biol Sci. 13:1560–1569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li M, Li JJ, Gu QH, An J, Cao LM, Yang HP
and Hu CP: EGCG induces lung cancer A549 cell apoptosis by
regulating Ku70 acetylation. Oncol Rep. 35:2339–2347. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao H, Zhu W, Xie P, Li H, Zhang X, Sun
X, Yu J and Xing L: A phase I study of concurrent chemotherapy and
thoracic radiotherapy with oral epigallocatechin-3-gallate
protection in patients with locally advanced stage III
non-small-cell lung cancer. Radiother Oncol. 110:132–136. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tian M, Tian D, Qiao X, Li J and Zhang L:
Modulation of Myb-induced NF-kB-STAT3 signaling and resulting
cisplatin resistance in ovarian cancer by dietary factors. J Cell
Physiol. 234:21126–21134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen H, Landen CN, Li Y, Alvarez RD and
Tollefsbol TO: Epigallocatechin gallate and sulforaphane
combination treatment induce apoptosis in paclitaxel-resistant
ovarian cancer cells through hTERT and Bcl-2 down-regulation. Exp
Cell Res. 319:697–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pérez-Ramírez C, Cañadas-Garre M, Molina
MÁ, Faus-Dáder MJ and Calleja-Hernández MÁ: PTEN and PI3K/AKT in
non-small-cell lung cancer. Pharmacogenomics. 16:1843–1862. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen Q, Weng HY, Tang XP, Lin Y, Yuan Y,
Li Q, Tang Z, Wu HB, Yang S, Li Y, et al: ARL4C stabilized by
AKT/mTOR pathway promotes the invasion of PTEN-deficient primary
human glioblastoma. J Pathol. 247:266–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu JL, Wang ZW, Hu LM, Yin ZQ, Huang MD,
Hu ZB, Shen HB and Shu YQ: Genetic variants in the
PI3K/PTEN/AKT/mTOR pathway predict Platinum-based chemotherapy
response of advanced non-small cell lung cancers in a Chinese
population. Asian Pac J Cancer Prev. 13:2157–2162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin YT, Wang HC, Hsu YC, Cho CL, Yang MY
and Chien CY: Capsaicin induces autophagy and apoptosis in human
nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR
pathway. Int J Mol Sci. 18(pii): E13432017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen J, Zhao KN, Li R, Shao R and Chen C:
Activation of PI3K/Akt/mTOR pathway and dual inhibitors of PI3K and
mTOR in endometrial cancer. Curr Med Chem. 21:3070–3080. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu HY, Zhang YY, Zhu BL, Feng FZ, Yan H,
Zhang HY and Zhou B: miR-21 regulates the proliferation and
apoptosis of ovarian cancer cells through PTEN/PI3K/AKT. Eur Rev
Med Pharmacol Sci. 23:4149–4155. 2019.PubMed/NCBI
|
|
52
|
Yap TA, Yan L, Patnaik A, Tunariu N,
Biondo A, Fearen I, Papadopoulos KP, Olmos D, Baird R, Delgado L,
et al: Interrogating two schedules of the AKT inhibitor MK-2206 in
patients with advanced solid tumors incorporating novel
pharmacodynamic and functional imaging biomarkers. Clin Cancer Res.
20:5672–5685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pétigny-Lechartier C, Duboc C and Jebahi
A: The mTORC1/2 inhibitor AZD8055 strengthens the efficiency of the
MEK inhibitor trametinib to reduce the Mcl-1/[Bim and Puma] ratio
and to Sensitize Ovarian Carcinoma cells to ABT-737. Mol Cancer
Ther. 16:102–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wainberg ZA, Alsina M, Soares HP, Braña I,
Britten CD, Del Conte G, Ezeh P, Houk B, Kern KA, Leong S, et al: A
Multi-Arm phase I study of the PI3K/mTOR inhibitors PF-04691502 and
Gedatolisib (PF-05212384) plus Irinotecan or the MEK inhibitor
PD-0325901 in advanced cancer. Target Oncol. 12:775–785. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Biswas R, Ahn JC and Kim JS: Sulforaphene
synergistically sensitizes cisplatin via enhanced mitochondrial
dysfunction and PI3K/PTEN modulation in ovarian cancer cells.
Anticancer Res. 35:3901–3908. 2015.PubMed/NCBI
|
|
56
|
Cai J, Xu L, Tang H, Yang Q, Yi X, Fang Y,
Zhu Y and Wang Z: The role of the PTEN/PI3K/Akt pathwayon prognosis
in epithelial ovarian cancer: A meta-analysis. Oncologist.
19:528–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer:
Biological and therapeutic significance. Semin Cancer Biol.
59:147–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shen J, Yu S, Sun X, Yin M, Fei J and Zhou
J: Identification of key biomarkers associated with development and
prognosis in patients with ovarian carcinoma: Evidence from
bioinformatic analysis. J Ovarian Res. 12:1102019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Steffensen KD, Smoter M, Waldstrøm M,
Grala B, Bodnar L, Stec R, Szczylik C and Jakobsen A: Resistance to
first line platinum paclitaxel chemotherapy in serous epithelial
ovarian cancer: The prediction value of ERCC1 and Tau expression.
Int J Oncol. 44:1736–1744. 2014. View Article : Google Scholar : PubMed/NCBI
|