Introduction
Liver cancer is one of the most common malignant
tumors worldwide (1), and may be
treated with several methods, such as surgery (2), radiotherapy (3) and chemotherapy (4). However, a number of patients miss the
opportunity of potentially curative surgical treatment, as the
early symptoms of primary liver cancer are not obvious (5) to enable early diagnosis. The
application of radiotherapy and chemotherapy is also limited due to
their toxicity and side effects. Therefore, it is crucial to
explore the mechanism underlying the occurrence and development of
liver cancer and identify novel treatment options.
With the increasing research interest in gut
microbiota, the association between gut microbiota and tumors has
been gradually elucidated (6,7). The
liver and the gut are connected through the portal vein; therefore,
the liver is not only perfused by abundant blood coming from the
intestine, but is also affected by the gut microbiota, which may
cause chronic inflammation of the liver tissue and increase the
risk of liver cancer (8). It was
reported that imbalance of gut microbiota may cause liver tissue
damage and cirrhosis, thereby promoting the occurrence of liver
cancer (9). It was also observed
that the bacterial metabolite lipopolysaccharide (LPS) promoted
liver tissue inflammation by binding to toll-like receptor (TLR)4,
which increases the risk of liver cirrhosis and liver cancer
(10). The inflammation of liver
tissue also inhibited the function of CD8+ T cells and
promoted liver cancer by promoting the aggregation of
IgA+ (11). Referring to
antibiotics as the major class of antibacterial agents, some
studies explored their potential in antitumor therapy, and it was
reported that bacterial depletion (vancomycin + neomycin +
metronidazole + amphotericin) may inhibit the development of
pancreatic cancer by restoring the function of CD8+ T
cells and promoting the polarization and infiltration of M1-like
macrophages (7). It was also
reported that bacterial depletion may inhibit the activation of
TLR4 to reduce the incidence of liver cancer (9).
Ciprofloxacin, as a quinolone antibiotic, can be
widely used in the treatment of the gastrointestinal tract by
interacting with the topoisomerase II-DNA complexes and inhibiting
the helix rejoining, thereby forming double-stranded DNA breaks and
leading to bacterial death (12).
In addition, ciprofloxacin exerts a certain effect against the
eukaryotic topoisomerase IIα, a DNA gyrase analogue (13). Therefore, it was suggested that
ciprofloxacin may inhibit the development of colorectal, pancreatic
and breast cancer (14–16), among others, through its cytotoxic
action, indicating its potential value in the treatment of
tumors.
However, the occurrence and development of tumors is
a complicated process. In addition to focusing on tumor cells, the
role of the tumor microenvironment must not be overlooked (17,18).
It has been demonstrated that the tumor microenvironment may be
implicated in the poor efficacy of tumor treatment and drug
resistance (19,20).
Tumor-associated macrophages (TAMs) are an important
component of the tumor microenvironment. It was demonstrated that
CD206−CD86+-M1-like macrophages could inhibit
tumor development by enhancing antigen presentation, increasing the
release of pro-inflammatory factors and promoting phagocytosis
(21,22). Although it has been reported that
the use of antibiotics in tumor therapy may promote the conversion
of macrophages to M1-like TAMs (7),
the effect of ciprofloxacin on macrophages remains controversial.
Some studies reported that ciprofloxacin could inhibit the release
of pro-inflammatory factors from macrophages induced by LPS
(23), whereas other studies
demonstrated that ciprofloxacin could promote macrophages to
release tumor necrosis factor (TNF)-α in a non-bacterial-induced
model (24). Therefore, in the
present study, in order to further explore the association between
ciprofloxacin, macrophages and liver cancer, the macrophages were
treated with different concentrations of ciprofloxacin, the
expression of the M1-like macrophage marker CD86 and the M2-like
macrophage marker CD206 was detected, and then the effects of
ciprofloxacin-induced macrophages (MCIP) on tumor cell
proliferation, apoptosis and metastasis of liver cancer were
investigated in vitro and in vivo.
Materials and methods
Specimens and patient data
A total of 30 liver cancer specimens were collected
from the Pathology Archive of the First Affiliated Hospital of
Chongqing Medical University (Chongqing, China) between January
2018 and January 2019, and these 30 liver cancer specimens were
used to detect CD206 expression level by immunohistochemistry. The
protocol of the present study was approved by the Medical Research
Ethics Committee of Chongqing Medical University, and informed
consent was obtained from all the patients.
Immunohistochemical staining
Paraffin sections (7 µm) of tumor tissues were
dewaxed at room temperature, rehydrated and heat-treated for
antigen retrieval with citric acid buffer at 98°C for 20 min. The
sections were then blocked with normal goat serum (C-0005, Bioss)
for 30 min, incubated with primary antibody (CD206 1:3,000, cat.
no. ab252921, Abcam) at 4°C overnight, and were analyzed using an
immunohistochemistry kit (PV-9001, ZSGB-BIO) following standardized
protocol. The sections were counterstained with hematoxylin,
mounted, and coverslipped. Staining intensity (cytoplasm and
membrane) was independently assessed and defined as follows: 0,
negative; 1, weak; 2, moderate; and 3, strong. The percentage of
positive cells was defined as follows: 0, negative; 1, 1–20%; 2,
21–50%; and 3, 51–100%. The immunohistochemical staining score was
calculated as staining intensity × percentage of positive cells and
was defined as follows: 0, negative; 1–4, low; and >4, high.
Cell culture
Human liver cancer Huh7 and HepG2 cell lines were
cultured in RPMI-1640 medium (HyClone; GE Healthcare Life Sciences)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 10 U/ml penicillin-streptomycin at 37°C in a
5% CO2 incubator. When the density of Huh7 and HepG2
cells reached 70–80%, the cells were processed with 0.5% trypsin
and transferred to new dishes. The human monocyte THP-1 cell line
was cultured in RPMI-1640 medium (HyClone; GE Healthcare Life
Sciences) supplemented with 10% FBS at 37°C in a 5% CO2
incubator. A total of 100 ng/ml phorbol-12-myristate-13-acetate
(PMA, Sigma-Aldrich; Merck KGaA, cat. no. P1585) was added to the
culture medium to induce the transformation of 5×106
monocytes into macrophages (M0) for 24 h. LPS (4 µg/ml;
Sigma-Aldrich; Merck KGaA, cat. no. L4391) was added in the culture
medium to induce the conversion of M0 into classical (M1-like)
macrophages for 48 h. Interleukin (IL)-4 (100 ng/ml) and IL-10 (100
ng/ml) were added in the culture medium to induce the conversion of
M0 into non-classical (M2-like) macrophages for 48 h. Various
concentrations of ciprofloxacin (0.5, 1, 2.5, 5 and 10 µg/ml) were
added in the culture medium to induce the conversion of M0 into
ciprofloxacin-induced (MCIP) macrophages; subsequently, the
precipitate was removed by centrifugation at 1,000 × g for 10 min
at room temperature, and the clear supernatant extract of M0 and
MCIP macrophages at 6, 12 and 24 h was collected to make a
conditioned medium.
Western blotting
For the western blot analyses, RIPA buffer
containing protease inhibitors and phosphatase inhibitors (Roche
Diagnostics) was used to prepare whole-cell lysates. Protein (20
µg) from the lysates was separated by 10% SDS-PAGE and transferred
to a PVDF membrane (0.45 µm, EMD Millipore). The membrane was
blocked in 5% BSA (cat. no. A8020, Solarbio Life Science) at 37°C
for 2 h. After overnight incubation at 4°C with primary antibodies
(CD206 1:1,000, cat. no. ab252921, Abcam; Bcl2 1:1,000, cat. no.
15071, Cell Signaling Technology, Inc.; Bax 1:1,000, cat. no. 2774,
Cell Signaling Technology, Inc.; p-AKT 1:1,000, cat. no. 4060, Cell
Signaling Technology, Inc.; AKT 1:1,000, cat. no. 4685, Cell
Signaling Technology, Inc.; CD86 1:1,000, YM0137, Immunoway; and
β-actin 1:1,000, TA-09, ZSGB-BIO), the membranes were washed 3
times with 0.1% TBST (5 min per wash). After incubating with
horseradish peroxidase-conjugated anti-mouse/rabbit IgG (1:3,000,
cat. no. ZDR-5307/5306, ZSGB-BIO) at 37°C for 1 h, the membranes
were examined by enhanced chemiluminescence (cat. no. P0018AS,
Beyotime Institute of Biotechnology).
RT-quantitative-PCR (RT-qPCR)
analysis
Total RNA was extracted from Huh7 or HepG2 cells by
TRIzol (TaKaRa Bio) and PrimeScriptTM RT Master Mix transcription
kit (TaKaRa Bio) was used to reverse-trasncribe RNA into cDNA. The
reverse transcription conditions were as follows: i) 37°C for 15
min; ii) 85°C for 5 sec. qPCR was performed in a CFX-connect fast
real-time PCR system (Bio-Rad Laboratories, Inc.). The sample
mixture was composed of 5 µl SYBR (TaKaRa Bio), 0.8 µl primers, 1
µl cDNA, and 3.2 µl ddH2O. The thermocycling conditions
were as follows: i) 95°C for 10 min; ii) 95°C for 20 sec; iii) 56°C
for 10 sec; iv) the temperature was reduced by 3°C/cycle and steps
ii-iv were repeated for 35 cycles; v) 95°C for 20 sec; and vi) 55°C
for 20 sec.
The sequences of the primers used were as follows:
TNF-α: Forward, 5′-GCCAACGGCATGGATCTCAA-3′ and reverse,
5′-CAGCCTTGTCCCTTGAAGAGAAC-3′; IL-1β: Forward,
5′-GAAATGATGGCTTATTACAGTGGC-3′ and reverse,
5′-TAGTGGTGGTCGGAGATTCGTAG-3′.
MTT
Huh7 and HepG2 cells (2×103/well) were
inoculated into a 6-well plate and maintained in an incubator at
37°C with 5% CO2 for ≤4 days. Cells in each well were
treated with 10 µl MTT (5 mg/ml) for 4 h at 37°C. The medium was
removed, and cells were lysed with 200 µl DMSO for 30 min. Cell
viability was measured at 492 nm using an ELISA plate reader
(BioTek Instruments, Inc.).
Crystal violet staining
Huh7 and HepG2 cells (3×104/well) were
inoculated into a 24-well plate and the conditioned medium was
added in each well for 48 h. The medium was removed and 500 µl 4%
paraformaldehyde was added in each well at room temperature for 30
min, then cells were dyed by 500 µl 1% crystal violet solution at
room temperature for 30 min. The cells were lysed with 200 µl
glacial acetic acid and measured at 592 nm using an ELISA plate
reader (BioTek Instruments, Inc.).
Hoechst staining
Huh7 and HepG2 cells (5×104/well) were
inoculated into a 6-well plate, and treated with conditioned medium
for 48 h, fixed with 4% paraformaldehyde and stained with 10 ng/ml
Hoechst 33258 solution (Solarbio, cat. no. C0021). Increased
condensation of chromatin was observed in apoptotic cells.
Apoptotic cells were captured with an inverted fluorescence
microscope (Nikon 80i; Nikon Corporation) in 3 random fields per
experiment (magnification, ×100).
Wound healing test
When the cell density reached >80% in the 6-well
plate, conditioned medium with 1% FBS of M0 or MCIP was added in
the 6-well plate. The cells were scratched with a small 10-µl
pipette tip and washed with PBS 3 times (30 sec/per wash) before
being observed and photographed under a microscope (Nikon 80i) at 0
and 24 h (magnification, ×100). Then, the wound width was recorded
at the same observation point. The mean wound width of multiple
observation points was calculated, and the wound healing rate of
each group was obtained.
Transwell assay
Transwell inserts (24-well, with an 8.0-µm pore
polycarbonate membrane) were purchased from Corning, Inc. (cat. no.
3422). Matrigel (50 µl; cat. no. 356234, Corning, Inc.) was used in
the invasion experiment; the volume ratio of Matrigel and culture
medium was 1:5. M0 or MCIP conditioned medium (400 µl) with 10% FBS
containing 40,000 Huh7 cells or 40,000 HepG2 cells was added to
each upper chamber, and 700 µl RPMI-1640 medium containing 15% FBS
was added to the lower chamber. Four parallel controls were set up
in each group. After 24 h, the cells on the lower side of the
chamber membrane were fixed for 20 min with 500 µl 4%
paraformaldehyde at room temperature. The cells were stained with
500 µl 1% crystal violet solution for 30 min at room temperature.
After washing and drying, the cells were counted under a microscope
(Nikon 80i) at a magnification of ×100. At least 10 visual fields
were observed in each chamber, and the mean values were obtained
after counting.
In vivo experiment
A total of 14 specific pathogen-free male BALB/c
nude mice, aged 6 weeks and weighing 20±1 g, were purchased from
HFK Bioscience Co. Ltd. The nude mice were randomly divided into
two groups (Huh7 group, n=5; Huh7 + MCIP group, n=9). Huh7 cells
(5×106) with or without MCIP (1×107) were
suspended in 100 µl PBS, and then inoculated into the subcutaneous
region of the right upper armpit of the nude mice. The tumors were
resected 3 weeks after implantation. The length (a) and width (b)
of each tumor were measured, and the tumor volume (V) was
calculated as follows: V = a × b2 × π/6. The
experimental procedures, which ensured the safety of practitioners
in laboratory animal projects and conformed to ethical standards
and international practices, were approved by the Animal
Experimental Ethics Committee of Chongqing Medical University. The
animals were fed a standard laboratory diet with free access to
food and water, and were kept in a room at controlled temperature
(22±1°C) and humidity (65-70%), with a 12-h light-dark cycle. After
the study, all animals were anaesthetized by isoflurane inhalation
(1.5–2%) and then euthanized by cervical dislocation. All animal
experiments conformed to the guidelines from Directive 2010/63/EU
of the European Parliament on the protection of animals used for
scientific purposes.
Statistical analysis
SPSS 16.0 statistical software (SPSS, Inc.) was used
to analyze the experimental results. Each experiment was repeated
three times. The differences between groups were evaluated by
t-test or Bonferroni-corrected t-test. P<0.05 was considered to
indicate statistically significant differences.
Results
Ciprofloxacin promotes
CD86+CD206− macrophage polarization
Among macrophages, which are an important component
of the tumor microenvironment, non-classical (M2-like) macrophages
can promote tumors, while classical (M1-like) macrophages can
inhibit tumors. In accordance with existing reports, the
immunohistochemistry examination demonstrated that the expression
of CD206, a marker of M2-like macrophages, was higher in the
low-differentiation group compared with that in the
high-differentiation group (Fig.
1A).
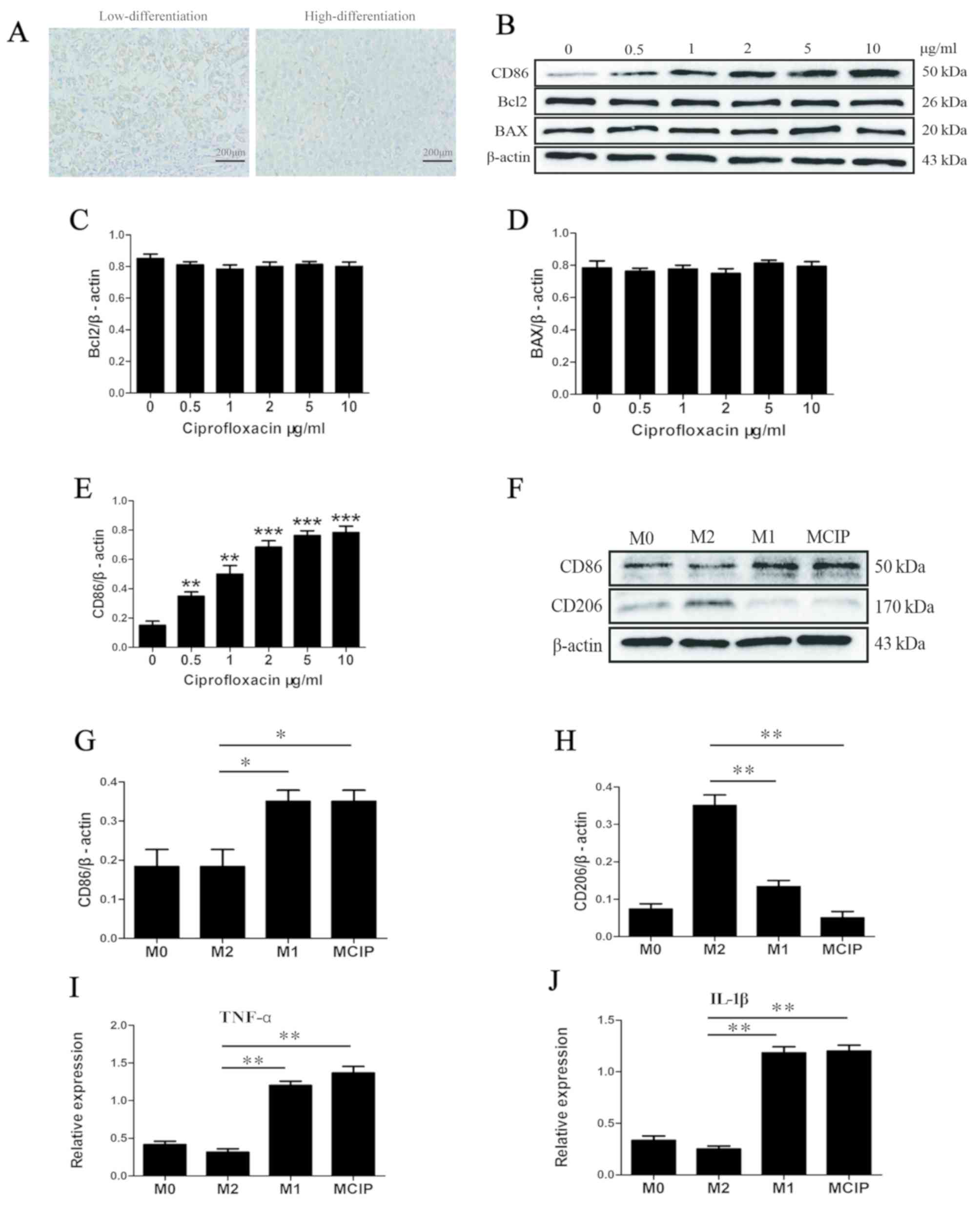 | Figure 1.Ciprofloxacin promotes
CD86+CD206− macrophage polarization. (A) The
expression of CD206 in highly and poorly differentiated liver
cancer tissues by immunohistochemistry (low differentiation, n=15;
high differentiation, n=15). (B) The protein levels of CD86, Bcl2
and BAX were detected by western blotting in macrophages after
treatment with different concentrations of ciprofloxacin.
Densitometry analysis was used to quantify the expression of (C)
Bcl2, (D) BAX and (E) CD86 (*P<0.05 **P<0.005 ***P<0.001
vs. 0 µg/ml group). (F) The protein levels of CD86 and CD206 were
detected by western blotting in M0 macrophages (M0), M1-like
macrophages (M1), M2-like macrophages (M2) and
ciprofloxacin-induced macrophages (MCIP). Densitometry analysis was
used to quantify the expression of (G) CD86 (*P<0.05 vs. M2
group) and (H) CD206 (**P<0.005 vs. M2 group). (I) The mRNA
level of TNF-α was detected by quantitative PCR in the M0, M1, M2
and MCIP groups (**P<0.005 vs. M2 group). (J) The mRNA level of
IL-1β was detected by quantitative PCR in the M0, M1, M2 and MCIP
groups (**P<0.005 vs. M2 group). TNF tumor necrosis
factor; IL, interleukin. |
To investigate the association between ciprofloxacin
and macrophage polarization, M0 macrophages were exposed to
ciprofloxacin (MCIP) at various concentrations. The western blot
analysis revealed that the M1-like macrophage-related marker CD86
was upregulated (Fig. 1B and E),
while the protein levels of Bcl2 and BAX were not affected by
ciprofloxacin (Fig. 1B-D);
accordingly, in the subsequent experiments, 2 µg/ml ciprofloxacin
was used to treat macrophages. When using M1-like and M2-like
macrophages as a reference, it was observed that CD86 was
upregulated in M0 by ciprofloxacin treatment; by contrast, the
M2-like macrophage-related marker CD206 was downregulated (Fig. 1F-H). Subsequently, the expression of
cytokines was investigated and it was observed that the
pro-inflammatory factors TNF-α and IL-1β were upregulated in
M1-like and MCIP macrophages (Fig. 1I
and J); by contrast, these cytokines were downregulated in
M2-like macrophages.
Effect of MCIP on the proliferation
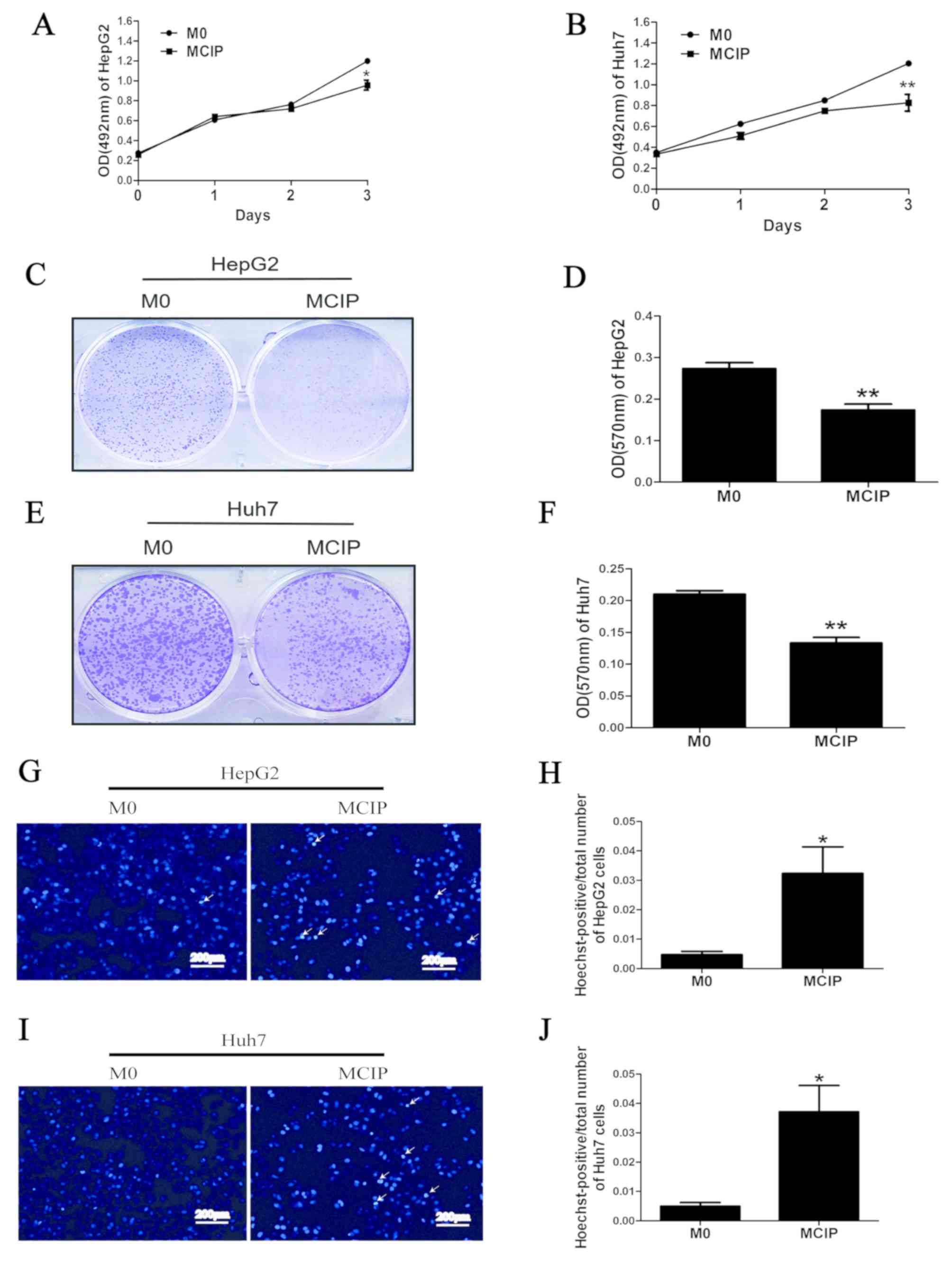
and apoptosis of liver cancer cells
It was observed in previous experiments that
ciprofloxacin increased the number of
CD86+CD206− macrophages, but it was uncertain
whether this group of macrophages exerted a tumor-suppressive
effect similar to that of M1-like macrophages; therefore, the
following experiment was conducted: The conditioned medium of MCIP
was collected to treat HepG2 and Huh7 cells. MTT assay demonstrated
that the OD values did not differ significantly between the groups
on days 0, 1 and 2. However, on day 3, the OD values of HepG2
(Fig. 2A) and Huh7 (Fig. 2B) cells in the MCIP conditioned
medium group were significantly lower compared with those in the
control group. The results of crystal violet staining revealed that
the number of HepG2 (Fig. 2C and D)
and Huh7 (Fig. 2E and F) cells was
lower in the MCIP group compared with the M0 group. These results
demonstrated that macrophages inhibited the proliferation of liver
cancer cells to a greater extent following ciprofloxacin treatment.
To investigate the effect of MCIP on the apoptosis of liver cancer
cells, Hoechst staining was used, and it demonstrated typical
apoptotic changes of the nuclei of HepG2 (Fig. 2G and H) and Huh7 (Fig. 2I and J) cells in the MCIP
conditioned medium group, and that the numbers of HepG2 and Huh7
cells in the MCIP conditioned medium group were lower compared with
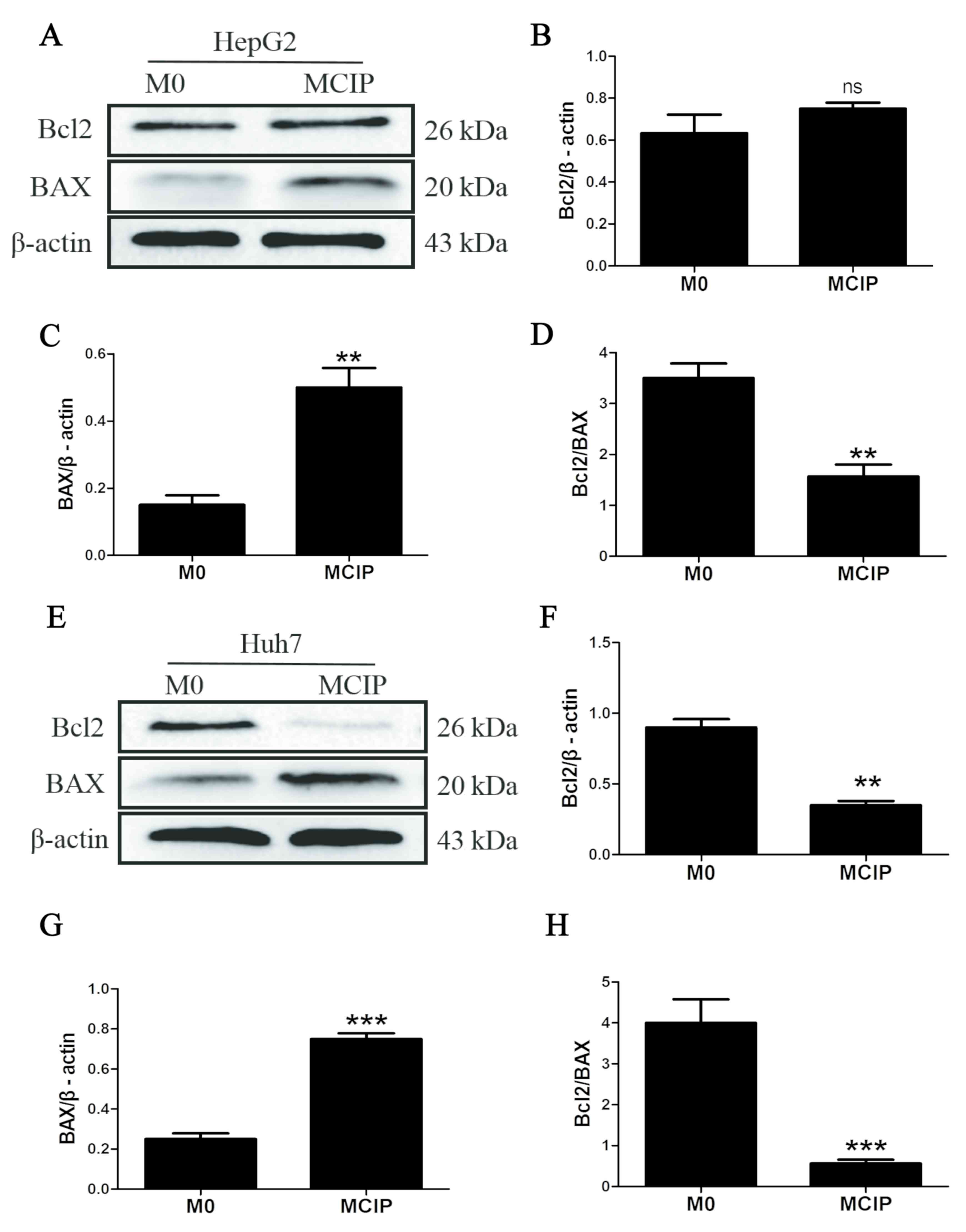
those in the control group. To compare with the results of previous
studies, it was reported that the protein levels of Bcl2 were
downregulated in Huh7 cells (Fig. 3E
and F) and were unaffected in HepG2 cells (Fig. 3A and B), while BAX was upregulated
in Huh7 (Fig. 3E and G) and HepG2
(Fig. 3A and C) cells. However, the
Bcl2/BAX ratio was decreased in HepG2 (Fig. 3D) and Huh7 (Fig. 3H) cells following treatment with
MCIP conditioned medium.
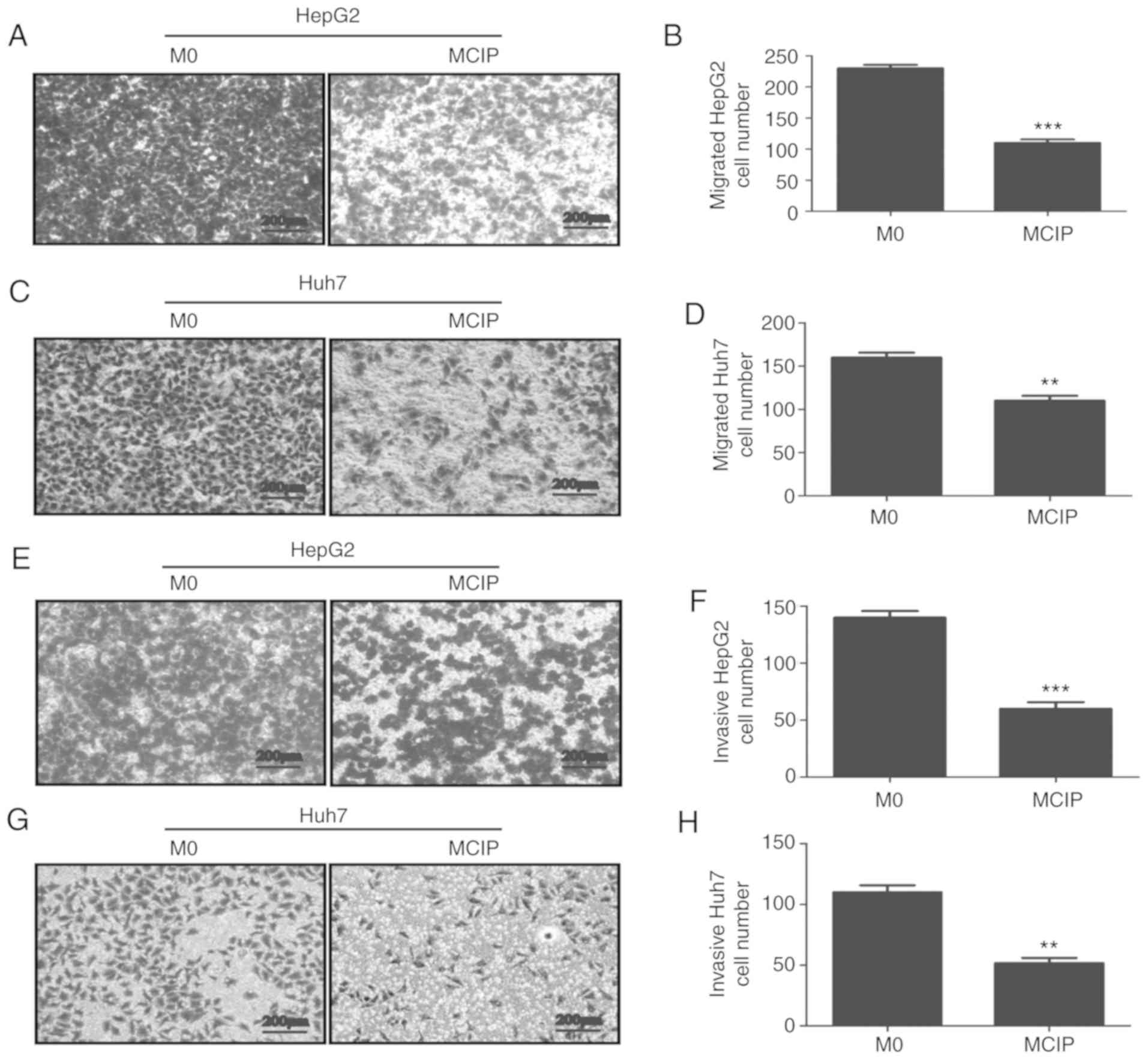
Effect of MCIP on the migration and
invasion of liver cancer cells
To explore the role of MCIP in metastasis, the wound
healing test was used. It revealed that the migration abilities of
HepG2 (Fig. 4I and J) and Huh7
(Fig. 4K and L) cells were
inhibited by treatment with MCIP conditioned medium. To verify the
effect of MCIP on the migration abilities of HepG2 and Huh7 cells,
Transwell assays without Matrigel revealed that the transmembrane
numbers of HepG2 (Fig. 4A and B)
and Huh7 (Fig. 4C and D) cells were
lower in the MCIP conditioned group compared with those in the
control group. The Transwell assay with Matrigel was used to verify
the effect of MCIP on the invasion abilities of HepG2 (Fig. 4E and F) and Huh7 (Fig. 4G and H). The results revealed that
the transmembrane numbers of Huh7 and HepG2 cells were lower in the
MCIP conditioned group compared with those in the control
group.
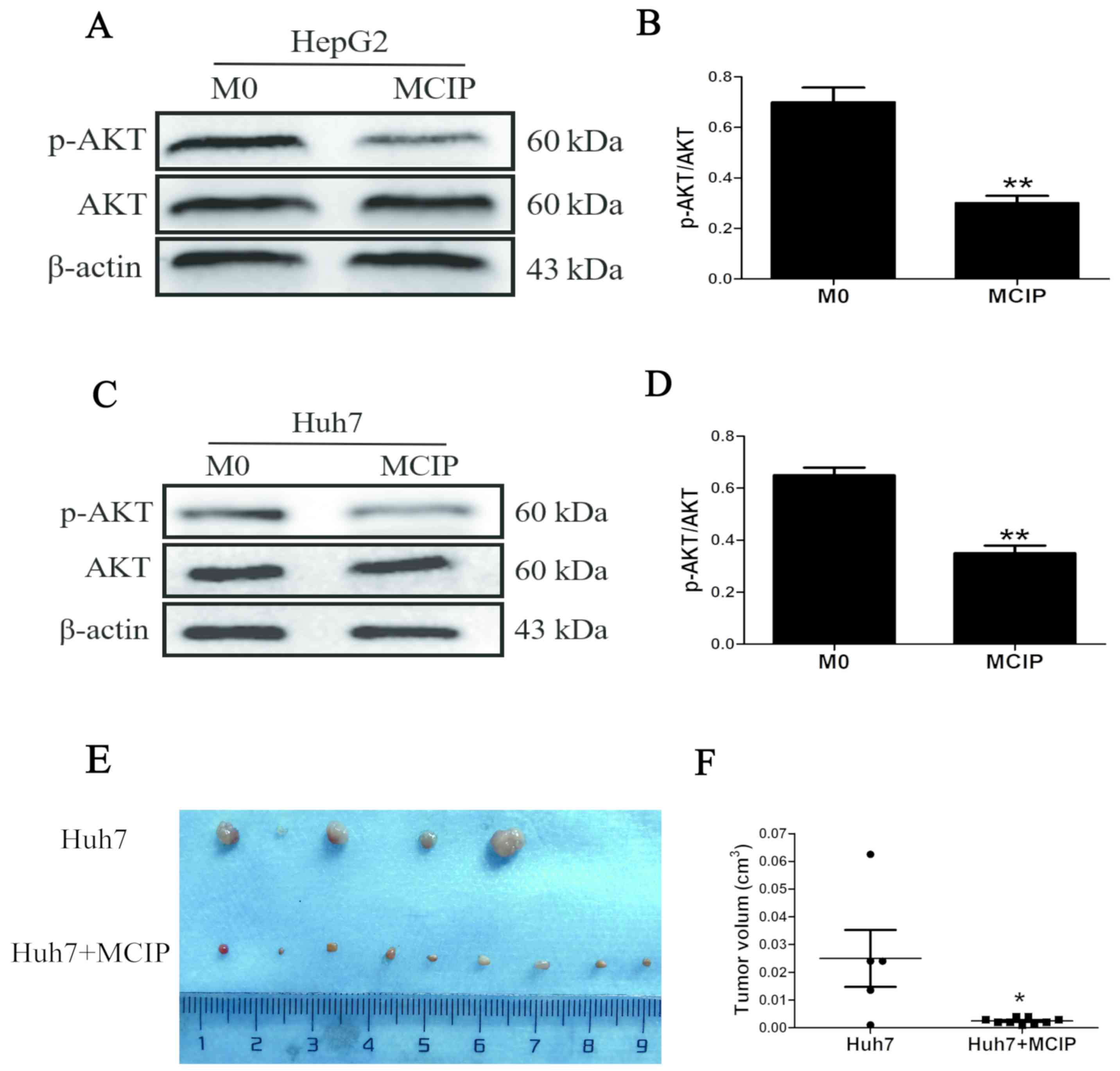
Effect of the phosphatidylinositol
3-kinase (P13K)/AKT signaling pathway on the regulation of liver
cancer cells by MCIP
PI3K/AKT has been proven to play a key role in the
pathogenic process of tumors. To verify whether this signaling
pathway was involved in regulating liver cancer, western blotting
was used. The results revealed that the level of phosphorylated AKT
(p-AKT) was downregulated in HepG2 (Fig. 5A) and Huh7 (Fig. 5C) cells by adding MCIP conditioned
medium compared with the control group, whereas the total AKT level
did not differ between the MCIP conditioned medium and control
groups (Fig. 5A and C). Therefore,
the p-AKT/AKT ratio was decreased in HepG2 (Fig. 5B) and Huh7 (Fig. 5D) cells after treatment with MCIP
conditioned medium.
Effect of MCIP on Huh7 cells in
vivo
To demonstrate the effect of MCIP on liver cancer
cells in vivo, the subcutaneous tumorigenicity test was
used. Huh7 cells were used, as the tumorigenesis rate of Huh7 cells
was higher compared with that of HepG2 cells. Huh7 cells
(5×106) were injected into the armpit of nude mice
together with 1×107 ciprofloxacin-treated macrophages
(MCIP) to construct a subcutaneous tumor model. As expected, the
tumor size of the Huh7 + MCIP group was smaller compared with that
of the Huh7 group (Fig. 5E and F).
The results of the in vivo experiments were consistent with
those of the in vitro experiments.
Discussion
Liver cancer is one of the most common malignant
tumors worldwide. The annual death toll of patients with liver
cancer is reported to be as high as 745,000. The occurrence and
development of liver cancer is a complex process involving multiple
factors. Due to the insidious symptoms at the early stages of liver
cancer, the majority of the patients miss the opportunity for
surgical treatment. Moreover, the efficacy of other conventional
treatments, such as chemotherapy, radiotherapy and molecular
targeted drugs, has also been limited in clinical application due
to the associated toxic side effects and drug resistance (25–28).
Consequently, the development of preventive or therapeutic
strategies adopting novel mechanisms, such as the application of
antibiotics targeting liver cancer-related immunity mechanisms, is
imminent.
Ciprofloxacin belongs to the class of quinolone
antibiotics (29). Its main
mechanism of antibacterial activity is to inhibit bacterial DNA
replication and division by acting on the topoisomerase II and
topoisomerase IV of bacteria. However, mammalian topoisomerase II
is also one of the targets of certain antitumor drugs (30). Due to the similarities in the DNA
synthesis mechanism for topoisomerase II between mammals and
bacteria, quinolone antibiotics have also been investigated in the
field of antitumor research. It was demonstrated that ciprofloxacin
could induce apoptosis of tumor cells through its cytotoxic action
(14–16).
Although numerous studies have investigated the
direct effects of ciprofloxacin on tumors, there are only few
studies on the effects of ciprofloxacin on the components of the
tumor microenvironment, such as TAMs. In the present study, it was
observed that ciprofloxacin at 0.5, 1, 2.5, 5 and 10 µg/ml promoted
the expression of CD86, which was highly expressed in M1-like TAMs
(31,32), while the expressions of IL-1β and
TNF-α were also increased; conversely, the expression of the CD206,
which was highly expressed in M2-like TAMs (33–35),
was reduced. TAMs are a complex group, and each member exhibits
various biological characteristics and functions. Some studies
confirmed that M1-like TAMs had strong phagocytic and
antigen-presenting abilities, and secreted a large number of
pro-inflammatory factors, which contributed to bacterial
elimination and antitumor immunity (36,37);
on the contrary, M2-like TAMs exhibited reduced phagocytic and
antigen-presenting abilities, and could secrete anti-inflammatory
factors to suppress the immune response and promote tumor
progression (33,38,39).
It was observed herein that ciprofloxacin promoted the expression
of CD86 and inhibited the expression of CD206. This result
suggested that ciprofloxacin may be involved in the regulation of
M0 TAMs to CD86+CD206−-M1-like macrophages.
Accordingly, in order to elucidate whether the macrophages treated
with ciprofloxacin promote tumor inhibition, in-depth investigation
and analysis were conducted in this study.
Due to the regulatory interaction between tumor
cells and TAMs (40), in order to
avoid the interference of tumor cells in MCIP, the method of
co-culture was excluded, and MCIP conditioned medium was prepared
to verify the MCIP function. It was demonstrated that the MCIP
conditioned medium could inhibit the proliferation of liver cancer
cells by MTT assay, while crystal violet staining, Hoechst staining
and the Bcl2/BAX ratio demonstrated that the addition of MCIP
conditioned medium could reduce the number of liver cancer cells
and promote cell apoptosis. In addition, the caspase pathway and
the presence of phosphatidylserine on the exterior surface of the
plasma membrane may also be used to verify the effect of
ciprofloxacin on cell apoptosis; therefore, the mechanism through
which MCIP conditioned medium regulates apoptosis of liver cancer
cells required further investigation. Transwell and wound healing
experiments revealed that the addition of MCIP conditioned medium
inhibited the migration and invasion of liver cancer cells, and
downregulated the level of p-AKT. It was also observed in
vivo that the subcutaneous tumors of nude mice that had
received MCIP treatment were smaller. In summary, MCIP conditioned
medium inhibited the proliferation, migration and invasion of liver
cancer cells, as well as subcutaneous tumor formation, and promoted
apoptosis. However, due to the limitation of conditioned medium
preparation in this study, conditioned medium containing 1% FBS was
used for the wound healing experiments. Therefore, in order to
exclude the interference of proliferation, in addition to the
Transwell assay, more experiments are required to verify the effect
of MCIP on the migration and invasion of liver cancer cells.
Furthermore, the composition of the conditioned medium is
complicated, and the contributions made by specific cytokines
towards the overall regulatory function require follow-up
experiments to verify.
The occurrence and development of tumors is a
complex process, and the effect of the tumor microenvironment is
suggested to be significant, particularly in promoting tumors and
inhibiting the effects of drug treatment (19,20).
It was previously confirmed that M2-like TAMs promote tumor
angiogenesis by secreting vascular endothelial growth factor
(41), degrade extracellular matrix
by inducing the secretion of matrix metallopeptidases, promote
vascular endothelial cell migration and facilitate tumor cell
metastasis (42,43). Therefore, the investigation of
macrophages as a therapeutic target may be of considerable value in
tumor immunotherapy. It was observed in the present study that
ciprofloxacin could increase the number of
CD86+CD206− macrophages and inhibit the
progression of liver cancer, which further elucidated the mechanism
and potential of ciprofloxacin in the treatment of liver cancer.
The results revealed that ciprofloxacin-induced macrophages (MCIP)
highly expressed CD86, TNF-α and IL-1β; however, in other
experiments, it was confirmed that MCIP could regulate the
proliferation, metastasis and apoptosis of liver cancer, which was
similar to the functions of M1-like TAMs. However, the
classification of TAMs is complicated. In addition to the classical
CD86 and CD206, CD163, CD204, CD200R and some cytokines may also be
used for the identification of M1-like and M2-like macrophages
(44,45). Although increased expression levels
of CD86, TNF-α and IL-1β were observed, the decreased expression of
CD206 in macrophages after treatment with ciprofloxacin and MCIP
also exerted some tumor-inhibitory effects. However, other studies
reported that ciprofloxacin could promote the expression of TNF-α
and did not affect the expression of IL-1β in animal models
(46). The changes in these
cytokines were not consistent with the classical model of
LPS-induced M1-like macrophage polarization (47). Thus, although MCIP overexpressed
CD86 with low expression of CD206 and exhibited the similar
tumor-suppressive trend as M1-like TAMs, the identification of
macrophage types prone to MCIP requires more indicators for
validation.
It was previously reported that ciprofloxacin could
inhibit the secretion of TNF-α and IL-1β from microglia and other
cells following stimulation by LPS (23), but other studies reported that
ciprofloxacin could promote the expression of TNF-α and reduce the
expression of IL-10 (48), which
suggested that ciprofloxacin played different roles in different
disease models and different types of immune cells. In order to
investigate the effect of ciprofloxacin on TAMs in our experiments,
an experimental model of THP-1 monocytes transforming into
macrophages was induced by the addition of PMA (49,50).
Therefore, in the follow-up study, extraction of primary TAMs will
be planned to explore the regulation on macrophage function by
ciprofloxacin. In order to explore the effect of MCIP on tumor
development, MCIP conditioned medium was adopted to observe the
effect of MCIP on liver cancer cell proliferation, apoptosis,
migration and invasion, while the effect on tumor occurrence
resulting from the interaction between oncogenic cells and the
microenvironment was investigated. Although it has been reported
that tumor cells could regulate the transformation of TAMs to
M2-like TAMs through various pathways, in our experiments, only the
effects of MCIP on tumors were explored, whereas the possible
regulatory effects of tumor cells on MCIP were not. Therefore, in
the follow-up study, the experimental design should take into
consideration both aspects of tumor cell-MCIP interactions, to
further elucidate the association between ciprofloxacin, TAMs and
liver cancer.
Although a number of studies confirmed that
ciprofloxacin may be used to inhibit the development of a wide
range of tumors, ciprofloxacin cannot differentiate between tumor
cells and normal tissue, as it targets topoisomerases of all
eukaryotes (51). While
ciprofloxacin directly acts on tumor cells, it may also damage
normal cells, inducing certain adverse reactions. In addition, drug
resistance is also an unavoidable limitation of this antibiotic
(52). It was observed in the
experiment that ciprofloxacin-induced MCIP exerted a certain
inhibitory effect on tumors, but similar to M1-like macrophages,
their inhibitory effects on cells were not cell-specific. It was
reported that M1-like macrophage overactivation may be associated
with cardiovascular (53),
autoimmune (54) and metabolic
(55) diseases, so extensive
investigation is still required before the clinical application of
ciprofloxacin in antitumor therapy. More in-depth research is
required to verify the effect of ciprofloxacin on macrophage
polarization and to further explore its specific regulatory
mechanisms.
In summary, the present study further elucidated the
biological mechanism underlying the potential applicability of
ciprofloxacin in antitumor therapy and indicated new potential
targets for the treatment of liver cancer.
Acknowledgements
The authors wish to thank the Ministry of Education
Key Laboratory of Diagnostic Medicine (Chongqing Medical
University) for providing the experimental platform, and the First
Affiliated Hospital of Chongqing Medical University for providing
the liver cancer samples.
Funding
The present study was supported by the Natural
Science Foundation of China (grant no. NSFC 81672103). The funders
played no part in the study design, data collection and analysis,
decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets generated during and/or analyzed during
the present study are available from the corresponding author on
reasonable request.
Authors' contributions
QS conceived and supervised the study. MTF obtained
most data and wrote the manuscript. SCC was responsible for
collating experimental data. YGW, MJB, LQA, JHW and MYZ were
responsible for data analysis. XL provided the liver cancer
specimens. YJJ, XWW and GGH designed the methods of the
experiments. MHZ and BC were responsible for checking the
manuscript. All authors participated in data interpretation. All
the authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical
Research Ethics Committee of Chongqing Medical University, and
written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AKT
|
serine/threonine kinase
|
|
BSA
|
bovine serum albumin
|
|
ddH2O
|
double-distilled water
|
|
EP
|
Eppendorf
|
|
FBS
|
fetal bovine serum
|
|
PBS
|
phosphate-buffered saline
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
PVDF
|
polyvinylidene fluoride
|
|
RT
|
reverse transcription
|
|
PCR
|
polymerase chain reaction
|
|
SDS
|
sodium dodecyl sulfate
|
|
TBST
|
Tris-buffered saline
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eggert T and Greten TF: Current standard
and future perspectives in non-surgical therapy for hepatocellular
carcinoma. Digestion. 96:1–4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohri N, Dawson LA, Krishnan S, Seong J,
Cheng JC, Sarin SK, Kinkhabwala M, Ahmed MM, Vikram B, Coleman CN
and Guha C: Radiotherapy for hepatocellular carcinoma: New
indications and directions for future study. J Natl Cancer Inst.
108:djw1332016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikeda M, Morizane C, Ueno M, Okusaka T,
Ishii H and Furuse J: Chemotherapy for hepatocellular carcinoma:
Current status and future perspectives. Jpn J Clin Oncol.
48:103–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim E, Lisby A, Ma C, Lo N, Ehmer U, Hayer
KE, Furth EE and Viatour P: Promotion of growth factor signaling as
a critical function of β-catenin during HCC progression. Nat
Commun. 10:19092019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geller LT, Barzily-Rokni M, Danino T,
Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee
K, et al: Potential role of intratumor bacteria in mediating tumor
resistance to the chemotherapeutic drug gemcitabine. Science.
357:1156–1160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pushalkar S, Hundeyin M, Daley D,
Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres
LE, et al: The pancreatic cancer microbiome promotes oncogenesis by
induction of innate and adaptive immune suppression. Cancer Discov.
8:403–416. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu
H, Zhai B, Tan YX, Shan L, Liu Q, et al: Profound impact of gut
homeostasis on chemically-induced pro-tumorigenic inflammation and
hepatocarcinogenesis in rats. J Hepatol. 57:803–812. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu LX and Schwabe RF: The gut microbiome
and liver cancer: Mechanisms and clinical translation. Nat Rev
Gastroenterol Hepatol. 14:527–539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dapito DH, Mencin A, Gwak GY, Pradere JP,
Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A,
Bataller R, et al: Promotion of hepatocellular carcinoma by the
intestinal microbiota and TLR4. Cancer Cell. 21:504–516. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shalapour S, Lin XJ, Bastian IN, Brain J,
Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, et
al: Inflammation-induced IgA+ cells dismantle anti-liver cancer
immunity. Nature. 551:340–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aldred KJ, Kerns RJ and Osheroff N:
Mechanism of quinolone action and resistance. Biochemistry.
53:1565–1574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hangas A, Aasumets K, Kekalainen NJ,
Paloheinä M, Pohjoismäki JL, Gerhold JM and Goffart S:
Ciprofloxacin impairs mitochondrial DNA replication initiation
through inhibition of Topoisomerase 2. Nucleic Acids Res.
46:9625–9636. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herold C, Ocker M, Ganslmayer M, Gerauer
H, Hahn EG and Schuppan D: Ciprofloxacin induces apoptosis and
inhibits proliferation of human colorectal carcinoma cells. Br J
Cancer. 86:443–448. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beberok A, Wrzesniok D, Rok J, Rzepka Z,
Respondek M and Buszman E: Ciprofloxacin triggers the apoptosis of
human triple-negative breast cancer MDA-MB-231 cells via the
p53/Bax/Bcl-2 signaling pathway. Int J Oncol.V. 52:1727–1737.
2018.
|
|
16
|
Yadav V, Varshney P, Sultana S, Yadav J
and Saini N: Moxifloxacin and ciprofloxacin induces S-phase arrest
and augments apoptotic effects of cisplatin in human pancreatic
cancer cells via ERK activation. BMC Cancer. 15:5812015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bottcher JP, Bonavita E, Chakravarty P,
Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E,
Zelenay S and Reis e Sousa C: NK cells stimulate recruitment of
cDC1 into the tumor microenvironment promoting cancer immune
control. Cell. 172:1022–1037 e14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su S, Chen J, Yao H, Liu J, Yu S, Lao L,
Wang M, Luo M, Xing Y, Chen F, et al:
CD10+GPR77+ cancer-associated fibroblasts
promote cancer formation and chemoresistance by sustaining cancer
stemness. Cell. 172:841–856 e16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huelsken J and Hanahan D: A subset of
cancer-associated fibroblasts determines therapy resistance. Cell.
172:643–644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dalton HJ, Pradeep S, McGuire M,
Hailemichael Y, Ma S, Lyons Y, Armaiz-Pena GN, Previs RA, Hansen
JM, Rupaimoole R, et al: Macrophages facilitate resistance to
anti-VEGF therapy by altered VEGFR expression. Clin Cancer Res.
23:7034–7046. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chavez-Galan L, Olleros ML, Vesin D and
Garcia I: Much more than M1 and M2 macrophages, there are also
CD169(+) and TCR(+) macrophages. Front Immunol.
6:2632015.PubMed/NCBI
|
|
22
|
Huang YK, Wang M, Sun Y, Di Costanzo N,
Mitchell C, Achuthan A, Hamilton JA, Busuttil RA and Boussioutas A:
Macrophage spatial heterogeneity in gastric cancer defined by
multiplex immunohistochemistry. Nat Commun. 10:39282019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zusso M, Lunardi V, Franceschini D,
Pagetta A, Lo R, Stifani S, Frigo AC, Giusti P and Moro S:
Ciprofloxacin and levofloxacin attenuate microglia inflammatory
response via TLR4/NF-kB pathway. J Neuroinflammation. 16:1482019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shaki F, Ashari S and Ahangar N: Melatonin
can attenuate ciprofloxacin induced nephrotoxicity: Involvement of
nitric oxide and TNF-α. Biomed Pharmacother. 84:1172–1178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bray F, Ferlay J, Laversanne M, Brewster
DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E,
Swaminathan R, Antoni S, et al: Cancer incidence in five
continents: Inclusion criteria, highlights from Volume X and the
global status of cancer registration. Int J Cancer. 137:2060–2071.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Broutier L, Mastrogiovanni G, Verstegen
MM, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R,
Sidorova O, Gaspersz MP, et al: Human primary liver cancer-derived
organoid cultures for disease modeling and drug screening. Nat Med.
23:1424–1435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Malenstein H, Dekervel J, Verslype C,
Van Cutsem E, Windmolders P, Nevens F and van Pelt J: Long-term
exposure to sorafenib of liver cancer cells induces resistance with
epithelial-to-mesenchymal transition, increased invasion and risk
of rebound growth. Cancer Lett. 329:74–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang JD, Hainaut P, Gores GJ, Amadou A,
Plymoth A and Roberts LR: A global view of hepatocellular
carcinoma: Trends, risk, prevention and management. Nat Rev
Gastroenterol Hepatol. 16:589–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang GF, Liu X, Zhang S, Pan B and Liu
ML: Ciprofloxacin derivatives and their antibacterial activities.
Eur J Med Chem. 146:599–612. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Delgado JL, Hsieh CM, Chan NL and Hiasa H:
Topoisomerases as anticancer targets. Biochem J. 475:373–398. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Q, Zhang Y, Liu S, Liu Y, Yang X, Liu
G, Shimizu T, Ikenaka K, Fan K and Ma J: Cathepsin C promotes
microglia M1 polarization and aggravates neuroinflammation via
activation of Ca2+-dependent PKC/p38MAPK/NF-KB pathway.
J Neuroinflammation. 16:102019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian L, Li W, Yang L, Chang N, Fan X, Ji
X, Xie J, Yang L and Li L: Cannabinoid receptor 1 participates in
liver inflammation by promoting M1 macrophage polarization via
rhoA/NF-KB p65 and ERK1/2 pathways, respectively, in mouse liver
fibrogenesis. Front Immunol. 8:12142017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nawaz A, Aminuddin A, Kado T, Takikawa A,
Yamamoto S, Tsuneyama K, Igarashi Y, Ikutani M, Nishida Y, Nagai Y,
et al: CD206(+) M2-like macrophages regulate systemic glucose
metabolism by inhibiting proliferation of adipocyte progenitors.
Nat Commun. 8:2862017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji J, Xue TF, Guo XD, Yang J, Guo RB, Wang
J, Huang JY, Zhao XJ and Sun XL: Antagonizing peroxisome
proliferator-activated receptor ү facilitates M1-to-M2 shift of
microglia by enhancing autophagy via the LKB1-AMPK signaling
pathway. Aging Cell. 17:e127742018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jaiswal A, Reddy SS, Maurya M, Maurya P
and Barthwal MK: MicroRNA-99a mimics inhibit M1 macrophage
phenotype and adipose tissue inflammation by targeting TNFα. Cell
Mol Immunol. 16:495–507. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hirayama D, Iida T and Nakase H: The
phagocytic function of macrophage-enforcing innate immunity and
tissue homeostasis. Int J Mol Sci. 19:E922017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huber-Ruano I, Raventos C, Cuartas I,
Sánchez-Jaro C, Arias A, Parra JL, Wosikowski K, Janicot M and
Seoane J: An antisense oligonucleotide targeting TGF-β2 inhibits
lung metastasis and induces CD86 expression in tumor-associated
macrophages. Ann Oncol. 28:2278–2285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Zhang S, Wang Q and Zhang X:
Tumor-recruited M2 macrophages promote gastric and breast cancer
metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol
Oncol. 10:362017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haque A, Moriyama M, Kubota K, Ishiguro N,
Sakamoto M, Chinju A, Mochizuki K, Sakamoto T, Kaneko N, Munemura
R, et al: CD206+ tumor-associated macrophages promote
proliferation and invasion in oral squamous cell carcinoma via EGF
production. Sci Rep. 9:146112019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in.development, homeostasis, and disease.
Nature. 496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song Y, Tang C and Yin C: Combination
antitumor immunotherapy with VEGF and PIGF siRNA via systemic
delivery of multi-functionalized nanoparticles to tumor-associated
macrophages and breast cancer cells. Biomaterials. 185:117–132.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao
YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, et al:
Tumor-associated macrophage-induced invasion and angiogenesis of
human basal cell carcinoma cells by cyclooxygenase-2 induction. J
Invest Dermatol. 129:1016–1025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu C, Rong D, Zhang B, Zheng W, Wang X,
Chen Z and Tang W: Current perspectives on the immunosuppressive
tumor microenvironment in hepatocellular carcinoma: Challenges and
opportunities. Mol Cancer. 18:1302019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sica A, Erreni M, Allavena P and Porta C:
Macrophage polarization in pathology. Cell Mol Life Sci.
72:4111–4126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kubota K, Moriyama M, Furukawa S, Rafiul
HASM, Maruse Y, Jinno T, Tanaka A, Ohta M, Ishiguro N, Yamauchi M,
et al: CD163+CD204+ tumor-associated
macrophages contribute to T cell regulation via interleukin-10 and
PD-L1 production in oral squamous cell carcinoma. Sci Rep.
7:17552017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lopez Nadal A, Peggs D, Wiegertjes GF and
Brugman S: Exposure to antibiotics affects saponin
immersion-induced immune stimulation and shift in microbial
composition in zebrafish larvae. Front Microbiol. 9:25882018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Orihuela R, McPherson CA and Harry GJ:
Microglial M1/M2 polarization and metabolic states. Br J Pharmacol.
173:649–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Anuforom O, Wallace GR, Buckner MM and
Piddock LJ: Ciprofloxacin and ceftriaxone alter cytokine responses,
but not Toll-like receptors, to Salmonella infection in vitro. J
Antimicrob Chemother. 71:1826–1833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Genin M, Clement F, Fattaccioli A, Raes M
and Michiels C: M1 and M2 macrophages derived from THP-1 cells
differentially modulate the response of cancer cells to etoposide.
BMC Cancer. 15:5772015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang F, Parayath NN, Ene CI, Stephan SB,
Koehne AL, Coon ME, Holland EC and Stephan MT: Genetic programming
of macrophages to perform anti-tumor functions using targeted mRNA
nanocarriers. Nat Commun. 10:39742019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Oliphant CM and Green GM: Quinolones: A
comprehensive review. Am Fam Physician. 65:455–464. 2002.PubMed/NCBI
|
|
52
|
Buckner MMC, Ciusa ML and Piddock LJV:
Strategies to combat antimicrobial resistance: Anti-plasmid and
plasmid curing. FEMS Microbiol Rev. 42:781–804. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Y, Zhang Y, Wang Z, Zhang J, Qiao RR,
Xu M, Yang N, Gao L, Qiao H, Gao M and Cao F: Optical/MRI
dual-modality imaging of M1 macrophage polarization in
atherosclerotic plaque with MARCO-targeted upconversion
luminescence probe. Biomaterials. 219:1193782019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Di Benedetto P, Ruscitti P, Vadasz Z,
Toubi E and Giacomelli R: Macrophages with regulatory functions, a
possible new therapeutic perspective in autoimmune diseases.
Autoimmun Rev. 18:1023692019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lumeng CN, Bodzin JL and Saltiel AR:
Obesity induces a phenotypic switch in adipose tissue macrophage
polarization. J Clin Invest. 117:175–184. 2007. View Article : Google Scholar : PubMed/NCBI
|