Introduction
A new type of bioactive polypeptides of the
neurosecretory hypothalamus, the proline-rich polypeptides (PRPs),
were isolated in 2001 from bovine neurohypophysis neurosecretory
granules (1,2). PRPs are synthesized from a common
precursor, namely neurophysin vasopressin-associated glycoprotein.
The mTORC1 inhibitor proline-rich polypetide 1 (PRP-1; also known
as galarmin) is a short 15-amino acid neuropeptide (sequence,
Ala-Gly-Ala-Pro-Glu-Pro-Ala-Glu-Pro-Ala-Gln-Pro-Gly-Val-Tyr) with
neuroprotective, immunomodulatory (3,4),
antiviral, anti-inflammatory (5),
antibacterial (6), hematopoietic
(7) and antitumor properties
(8-10,11-14).
PRP-1 was identified as a ligand for innate immunity
pattern recognition Toll-like receptors 1,2 and 6, and the mucosal
protein mucin 5B (15). PRP-1
caused significant upregulation of tumor suppressor genes and miRNA
with tumor suppressor function and downregulation of onco-miRNAs in
the human chondrosarcoma JJ012 cell line (11,13).
The antiproliferative effect of PRP-1 was caspase-3-independent and
cytostatic by nature in the bulk human chondrosarcoma cell
population (16) and in triple
negative breast cancer (10). The
antitumor function of PRP-1 was demonstrated to be organ-specific,
since it did not display any effect on glioblastoma (12).
The Ehrlich ascites carcinoma (EAC) is a common
undifferentiated tumor with rapid growth rate and sensitivity to
chemotherapy (17). Ehrlich tumors
originate from spontaneous murine mammary adenocarcinomas and adapt
to ascites form by intraperitoneal (ip) serial passages. The EAC
model is widely used in experimental cancer due to its high
efficiency in producing free neoplastic cells and its accuracy in
terms of survival time (18). The
aim of the present study was in vitro exploration of the
effect of PRP-1 on EAC cells collected from the ascitic fluid of
EAC cell-bearing mice.
Materials and methods
EAC mouse model
The ascitic fluid of [2 to 3-month-old male white
Swiss (SWR/J) mice weighing 20±2 g] with the EAC model was provided
by the Laboratory of Toxicology and Experimental Chemotherapy
(Institute of Fine Organic Chemistry, National Academy of Sciences
of Armenia). Mice were inoculated with EAC-E2G8 tumor cells
(obtained by the Hebei Medical University scholars from the Beijing
Cancer Institute EAC) to produce the EAC model.
The ascitic fluid containing the EAC cells was
obtained from the peritoneal cavity of mice on days 7 (n=10) and 11
(n=10) after tumor growth, and then used for in vitro
experiments at the laboratory of Histochemistry and Functional
Morphology (Institute of Biochemistry after H. Buniatian, NAS
RA).
Culture of cell suspension
The EAC cell suspensions obtained from the
peritoneal cavity of mice (which closely mimic in vivo
conditions) and suspensions containing EAC cells isolated by
centrifugation were used. Ascitic fluid was centrifuged at 300 × g
for 5 min at 18–20°C.
Then, the supernatant was discarded, and the cells
were washed in Hanks' Balanced Salt Solution buffered with
phosphate (pH 7.4) (cat. no. 55037C; Sigma-Aldrich; Merck KGaA).
Subsequently, the cells were re-suspended in Hanks' Balanced Salt
Solution to a concentration of 5×106 cells/ml in
RPMI-1640 medium and grown in tissue culture dishes until ~80%
confluence in RPMI-1640 culture medium (BioloT, Ltd.) containing
10% heat-inactivated fetal bovine serum, 50 U/l penicillin and 1%
L-glutamine. The cell suspensions were incubated at 37°C and 5%
CO2 with constant shaking. Control samples (n=3)
untreated with PRP-1 and experimental samples with single
administration of 0.1 µg/ml PRP-1 (n=3) and 1 µg/ml PRP-1 (n=3)
were cultured for 24 and 72 h in unchanged culture medium. Daily
quantification of the total and viable number of EAC cells was
carried out. Each condition was tested in triplicate.
Tumor cell count
For the culture of EAC cells, 5×106 cells
were obtained from the suspension containing numerous tumor cells,
by diluting it in RPMI-1640 medium. The cells were counted in a
Neubauer chamber (19).
Histological and immunohistological
staining
A light digital microscope (M10; Motic) was used for
histological and immunohistochemical investigations.
Histological staining
Trypan blue (Tr-Bl) staining
The number of viable cells in the suspension was
determined by the method of exclusion with trypan blue (diazo live
dye, at a concentration 0.4%) (20). Using the Tr-Bl staining method, the
percentage of dead and alive cells was calculated after 24 h of
incubation in the control samples and those treated with PRP-1 at
0.1 and 1 µg/ml concentrations.
Haematoxylin and eosin (H&E) staining
EAC suspension smears were fixed in 96% ethanol for
10 min at room temperature; dehydrated by passing through
decreasing concentrations of alcohol baths (96 and 75%) and
distilled water, stained in haematoxylin for 5 min at room
temperature, washed in tap water for ≤5 min, stained in 1% eosin
for 1 min at room temperature, washed in tap water for 2 min,
dehydrated in increasing concentrations of ethanol (75 and 96%),
cleared in xylene two times, and mounted with DPX (cat. no. 06522;
Sigma-Aldrich; Merck KGaA) (21).
Giemsa staining
For staining the smears of the EAC cell suspension,
Giemsa stain was used at a 1:20 ratio. To produce a 1:50 dilution
of Giemsa stain, 1 ml stock solution of Giemsa stain was added to
49 ml phosphate-buffered (pH6.4) solution. The smears were dried in
air and covered in DPX (22).
Papanicolaou staining
A drop of the EAC cell suspension was spread on a
glass slide, dried in air and fixed in 95% ethyl alcohol for 30 min
at room temperature. Slides were then incubated in 70 and 50%
ethanol followed by distilled water, stained in Harris hematoxylin
for 4 min at room temperature, rinsed briefly in warm (20-30°C)
distilled water for 4 min, placed in tap water for 6 min, rinsed in
distilled water, and incubated in 50, 70, 80 and 95% ethanol. Then,
the slides were stained in EA-50 for 2 min at room temperature;
rinsed three times with 95% ethanol, dehydrated in absolute
alcohol, followed by equal parts of absolute alcohol and xylol,
cleared in xylol, and mounted with DPX (23).
Fluorescence microscopy
Determination of the tumor cell apoptosis rate was
performed using the Annexin V-Cy3 apoptosis Detection Kit (product
no. APOAC; Sigma-Aldrich KGaA). The non-fluorescent compound
6-carboxyfluorescein diacetate (6-CFDA) enters viable cells, is
hydrolyzed by esterizes to the fluorescent compound
6-carboxyfluorescein (6-CF) and then retained in the cytoplasm.
Dead cells do not exhibit uptake of 6-CFDA and have an increased
efflux of the free 6-CFDA. The apoptosis assay is based upon double
staining with cyanine 3 (Cy3)-conjugated Annexin V and the vital
dye 6-CFDA. The assay discriminates viable cells
(Cy3/6-CF+) from apoptotic
(Cy3+/6-CF+) and dead (necrotic) cells
(Cy3+/6-CF). There are three possible outcomes: i)
living cells that only stain with 6-CF (green); ii) necrotic cells
that only stain with AnnexinV-Cy3 (red); and iii) cells starting
the apoptotic process, which stain both with Annexin V-Cy3 (red)
and 6-CF (green), resulting in yellow-orange stain (24). A total of 400 cells/sample were
analyzed. Data represent 3 independent experiments (25).
The nuclear counterstain method with DAPI
fluorescent staining dye (cat. no. D1306; Thermo Fisher Scientific,
Inc.) was used to confirm the PRP-1 nuclear localization in the
cultured EAC cells and detection was performed using the ABC
immunohistochemical method.
Immunohistochemical staining
The avidin-biotin complex (ABC) immunohistochemical
method was applied for detection of PRP-1 localization in cancer
cells using antiserum produced at our laboratory (Institute of
Biochemistry NAS, Armenia) against synthetic PRP-1 (26). After rinsing several times in PBS,
the samples were treated with Triton X-100 for 45 min to
permeabilize, 0.3% hydrogen peroxide for 30 min to suppress the
background peroxidase activity, and normal goat serum (1:30; cat
no. S-1000; Vector Laboratories, Inc.) for 45 min to block
non-specific binding of antibodies. Then, the samples were
incubated in primary antiserum [anti-PRP-1; 1:2,000; produced at
the laboratory of Histochemistry and Functional Morphology
(Institute of Biochemistry, NAS, Armenia)] for 24 h at 4°C,
secondary antiserum (biotinylated goat anti-rabbit immunoglobulin;
1:200; cat. no. BP-9100) for 45 min; and streptavidin (1:100; cat.
no. SA-5000; both from Vector Laboratories, Inc.) for 45 min at
room temperature. Immunoreactivity (IR) was revealed by 0.02%
3,3′-diaminobenzidine tetrahydrochloride (DAB) (product no. D5905;
Sigma-Aldrich; Merck KGaA) and 0.6% nickel ammonium sulfate diluted
in 50 mM Tris-HCl buffer (pH 7.6) in the presence of 0.03% hydrogen
peroxide as an oxidant. Both primary and secondary antisera were
diluted in PBS containing 0.1% bovine serum albumin (BSA) (product
no. 05470; Sigma-Aldrich; Merck KGaA) and 0.01% sodium azide. All
incubations, except for the incubation with normal goat serum, were
separated by washes in PBS. The slides were mounted in DPX medium
and the results were analyzed using light microscopy at ×100, ×400
and ×1,000 magnifications. To confirm immunospecificity for PRP-1,
the primary antiserum was replaced by normal goat, phosphate buffer
or PRP-1 antiserum pre-absorbed with synthetic PRP-1, synthesized
at our laboratory (Institute of Biochemistry, NAS, Armenia) for the
negative control experiments.
PRP-1 antiserum production and affinity
chromatography purification
Antiserum production
For immunization 1 to 1.5-year old wild male ~3–4
rabbits [Oryctolagus cuniculus (Linnaeus, 1758)] of the
Californian breed, weighing 2 kg were used. Animal immunization was
performed using pentobarbital (Nembutal; serial no. 71308321 and
registration no. 0285003) anesthesia at a dose of 30–35 mg/kg. The
Institutional Animal Ethics Committee of Buniatian Institute of
Biochemistry of the NAS (IRB 0001621; IORG0009782) provided
approval for the use of the animals. The rabbits woke up normally
and behaved naturally after the procedures which lasted 10–15 min,
were performed. The rabbits were housed in cages (cage model
RBB-S-01) comprised of 2 sections of size, 600×450×450 mm,
separated by a removable partition, one animal in each section.
Rabbits were maintained at a room temperature of 18–22°C and a
relative humidity of 55–65%. Rabbits were fed twice a day, morning
and evening with a daily diet which consisted of: Unlimited access
to hay or grass, a handful of fruits, vegetables or leafy plants
and a small amount of high-quality commercial mix (for rabbits) or
granules (up to 25 g per kg of rabbit body weight). The rabbits
were used once more to obtain a new portion of PRP-1-antiserum with
higher titration.
Antiserum against synthetic PRP-1 was obtained
according to the method by Ambrosius (27). The PRP-1-BSA conjugate, was mixed
with 1 mg/0.2 ml Freund's Complete Adjuvant (product no. F5881;
Sigma-Aldrich; Merck KGaA), until a homogenous emulsion was
achieved (28), and then injected
in equal portions into the lower extremities of rabbits in the
region of both popliteal lymph nodes. Immunization was repeated 1
month later by injecting a freshly prepared emulsion (1 mg
PRP-1-BSA in 1 ml phosphate buffer, pH 7.4) in the following
locations: Into the region of the left popliteal lymph node (0.4
ml), into the auricular vein (0.2 ml) and, intramuscularly on the
right side (0.4 ml). After reimmunization, blood samples were
obtained from the auricular vein on days 7, 9 and 11 from the
rabbits, mixed and kept in the refrigerator (at +4°C). Then 10 ml
of this mixture was lyophilized. The specificity of the antiserum
was tested by immunodiffusion and ELISA (29).
PRP-1 antiserum affinity chromatography
purification
PRP-1 antiserum affinity chromatography purification
was performed using AminoLink™ Plus Immobilization kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions.
Statistical analysis
Statistical analysis was performed using the
statistical package Statgraphics Centurion 16.2 (StatPoint
Technologies, Inc.). The results were compared with those of the
control, and the Student's t-test was used to estimate the
statistical significance of the data. Data were presented as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Histological (Tr-Bl, H&E, Giemsa, Papanicolaou
and Annexin V-Cy3 apoptosis detection stains) and
immunohistochemical (ABC) methods were used in the present study to
investigate the antitumorigenic effect of PRP-1 in EAC cells. EAC
suspensions, including those obtained from the peritoneal cavity of
mice (since it closely resembles in vivo conditions) and
those containing isolated EAC cells, were used. The effect of 0.1
and 1 µg/ml PRP-1 was studied on the isolated EAC cells of mice on
days 7 and 11 of tumor growth and cultured for 24 and 72 h.
Histological study
Light microscopy and histological staining with
Tr-Bl experiments were used to reveal the percentage of dead and
alive cells after 24 h of incubation in the control samples and
those treated with PRP-1 at the aforementioned concentrations.
Statistical analysis of the tumor growth at 24 h
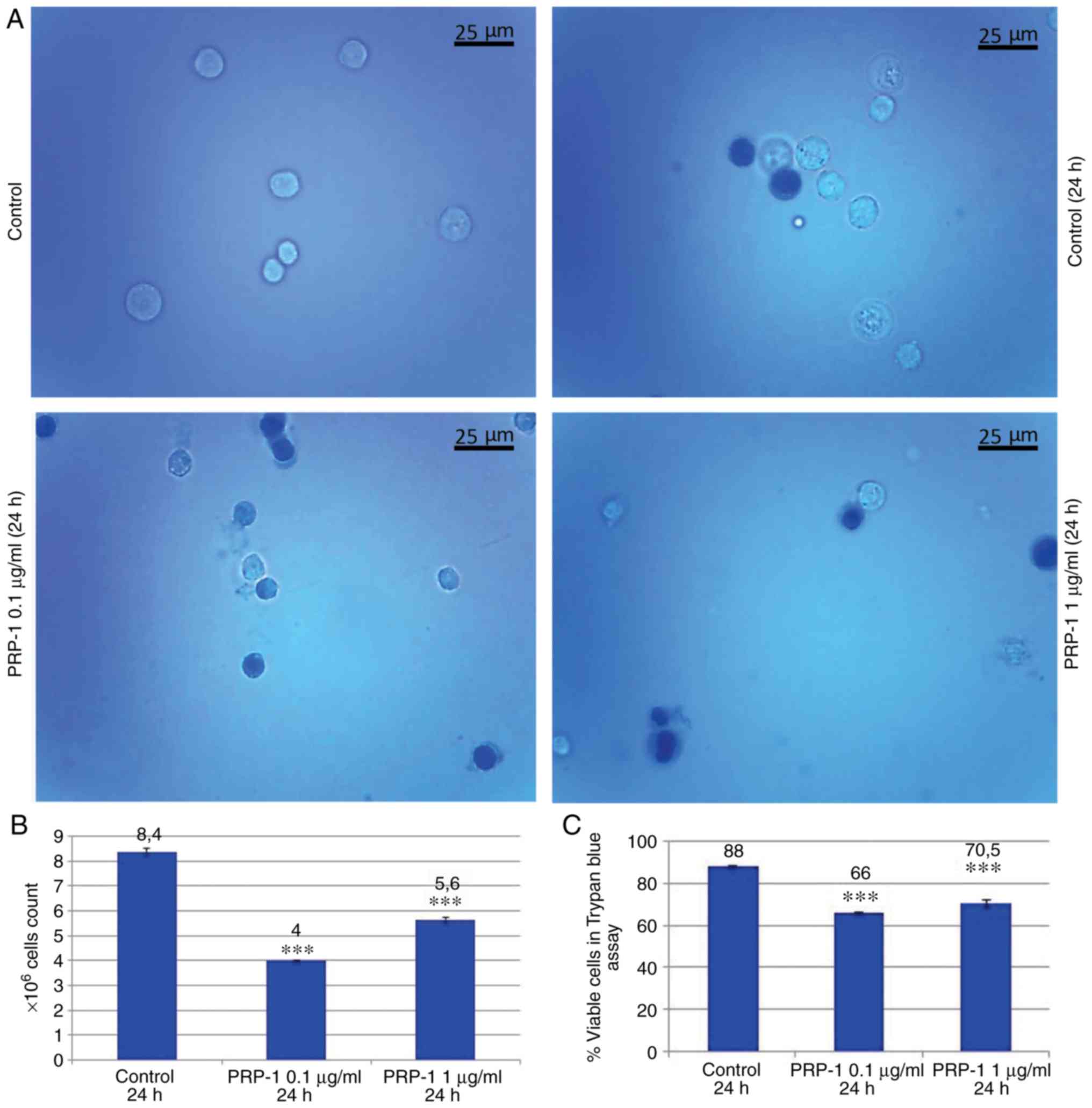
confirmed the PRP-1 antitumorigenic effect on EAC cells. Figs. 1 and 2 revealed a decrease in the number of
total EAC cells when treated with PRP-1 (0 1 and 1 µg/ml),
indicating the anti-proliferative activity of PRP-1 in comparison
with the findings in the 24 h untreated control samples.
Concurrently, the number of viable EAC cells treated with PRP-1 was
decreased compared with that in the control group. On day 7
post-inoculation, the number of total cells was 5×106,
and increased to 8×106, with viable cells comprising 88%
of the cell population, whereas in the 0.1 µg/ml PRP-1-treated
samples, the total number of cells was reduced to 4×106,
where viable cells accounted for 66% of the total population. Thus,
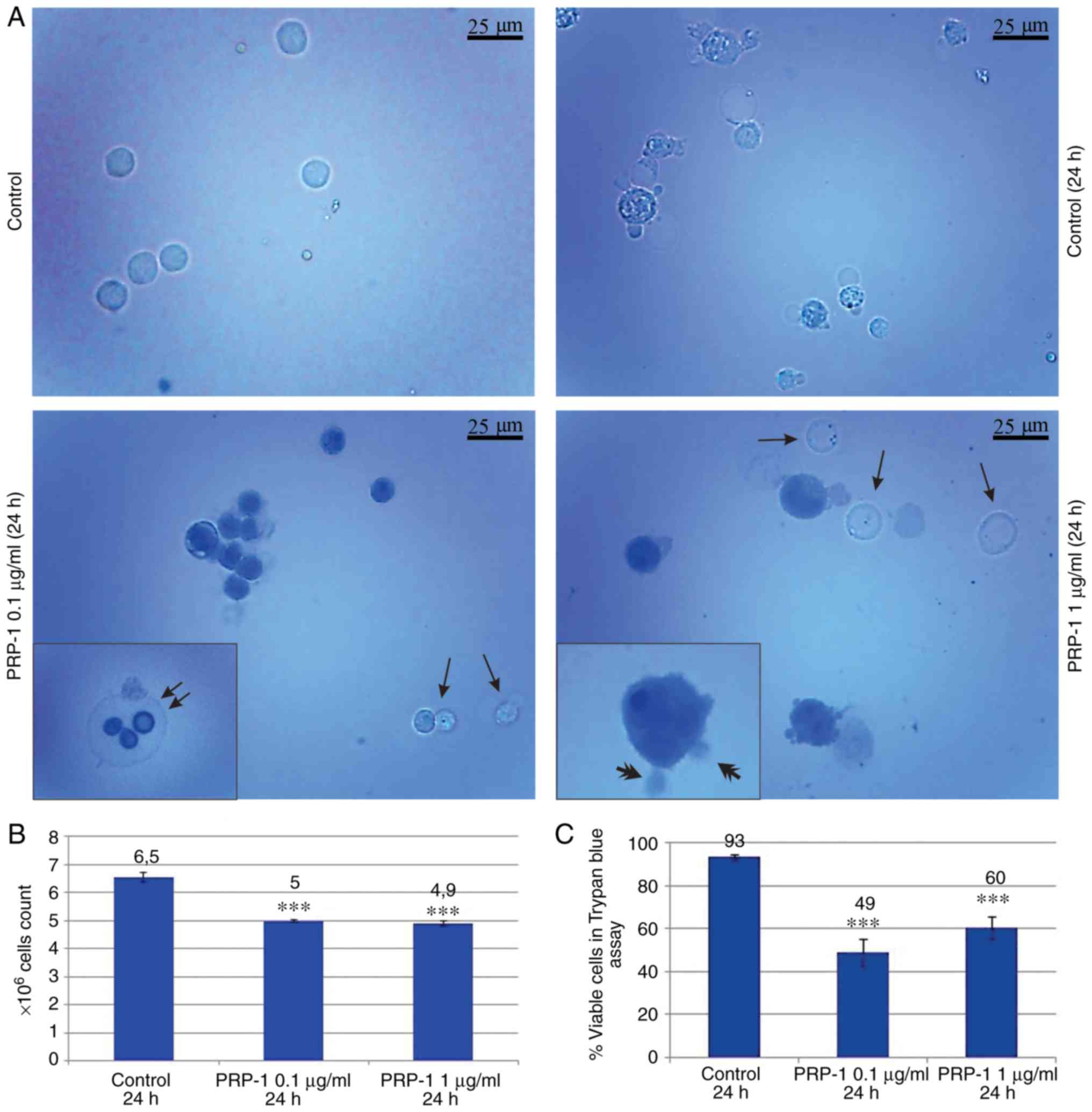
PRP-1 inhibited the growth of viable cells by 22% (Fig. 1B and C). On day 11 post-inoculation,
the total number of cells increased from 5×106 to
6.5×106, of which 93% were viable in the 24 h control,
while in the PRP-1-treated samples, that number was
5×106, with only 49% of viable cells, thus indicating an
inhibition of viable cells by 44% caused by PRP-1 (Fig. 2B and C). The difference between the
effect of 0.1 and 1 µg/ml PRP-1 was clearly visible when comparing
the total number of EAC cells on the 7th day of inoculation
indicating the more effective anti-proliferative action of 0.1
µg/ml PRP-1. Thus, since similar results were obtained with the
11th day inoculation, 0.1 µg/ml PRP-1 was selected for further
use.
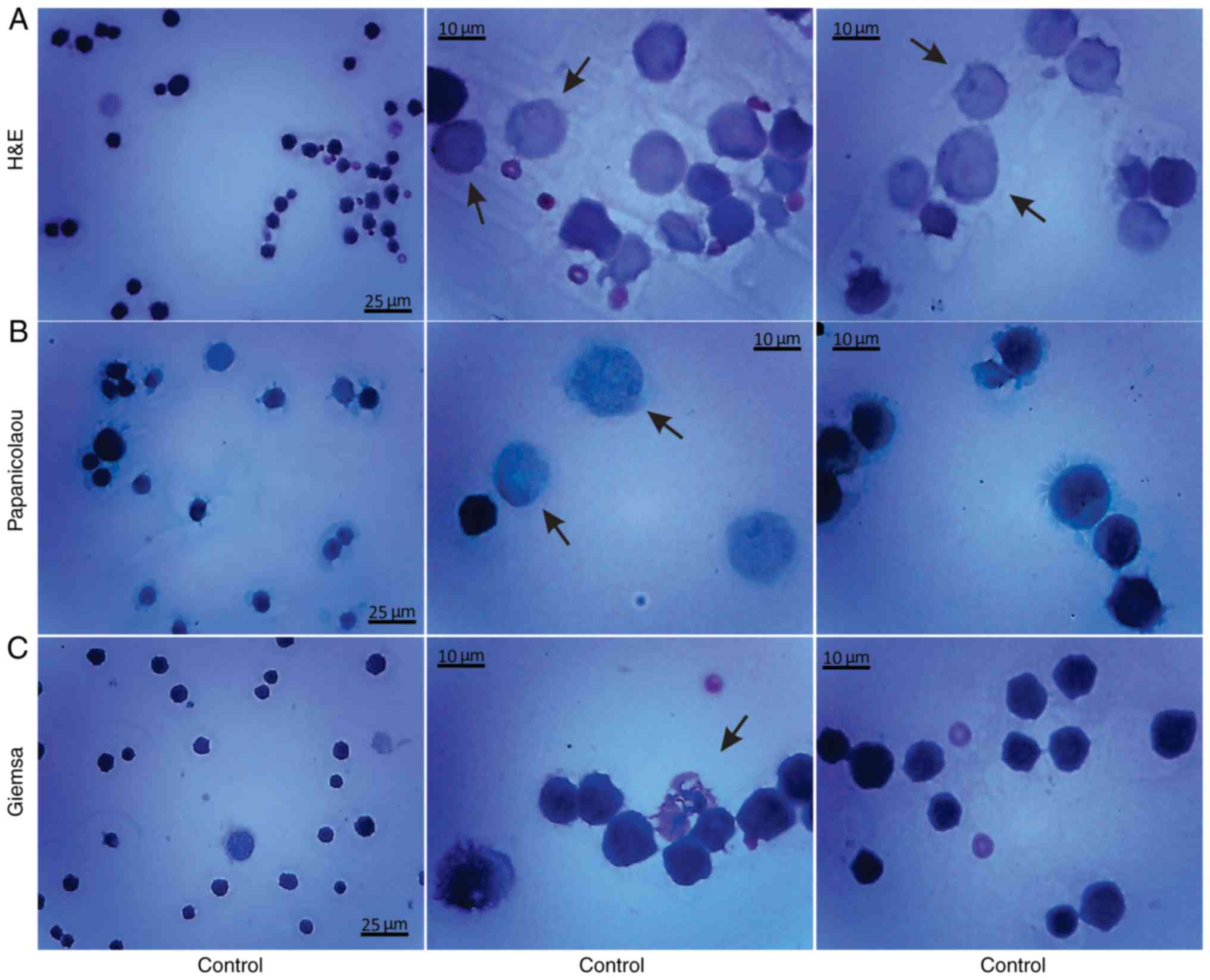
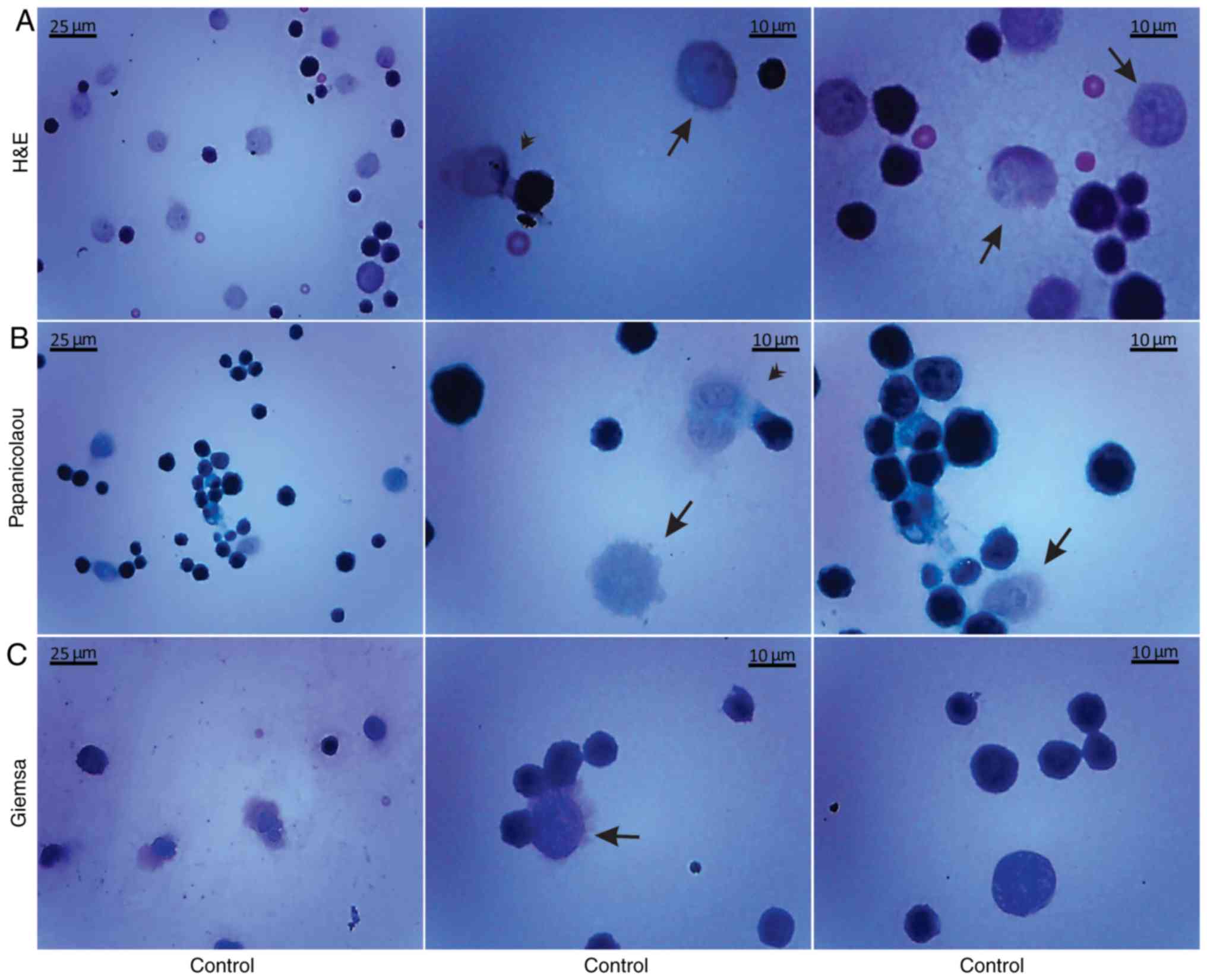
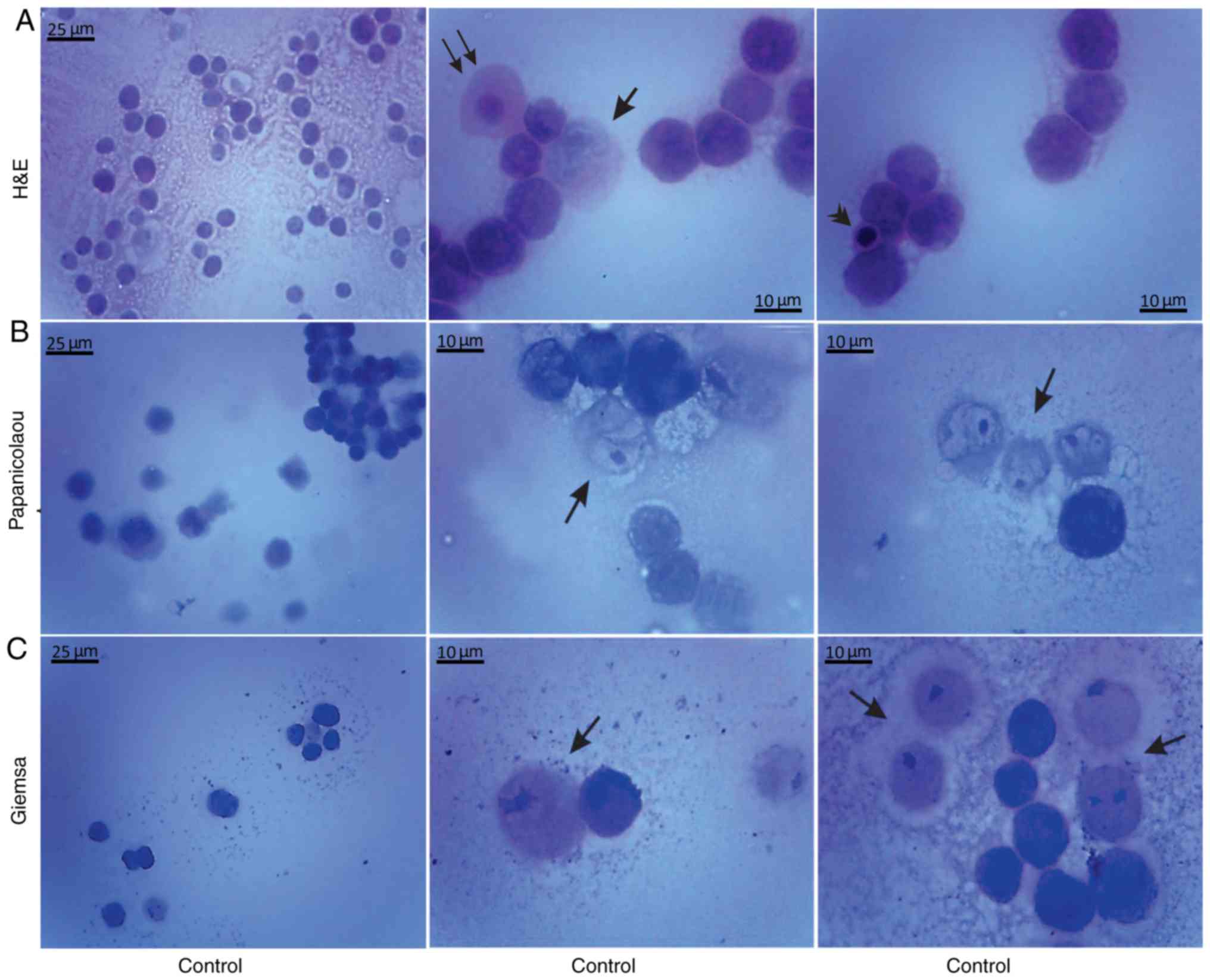
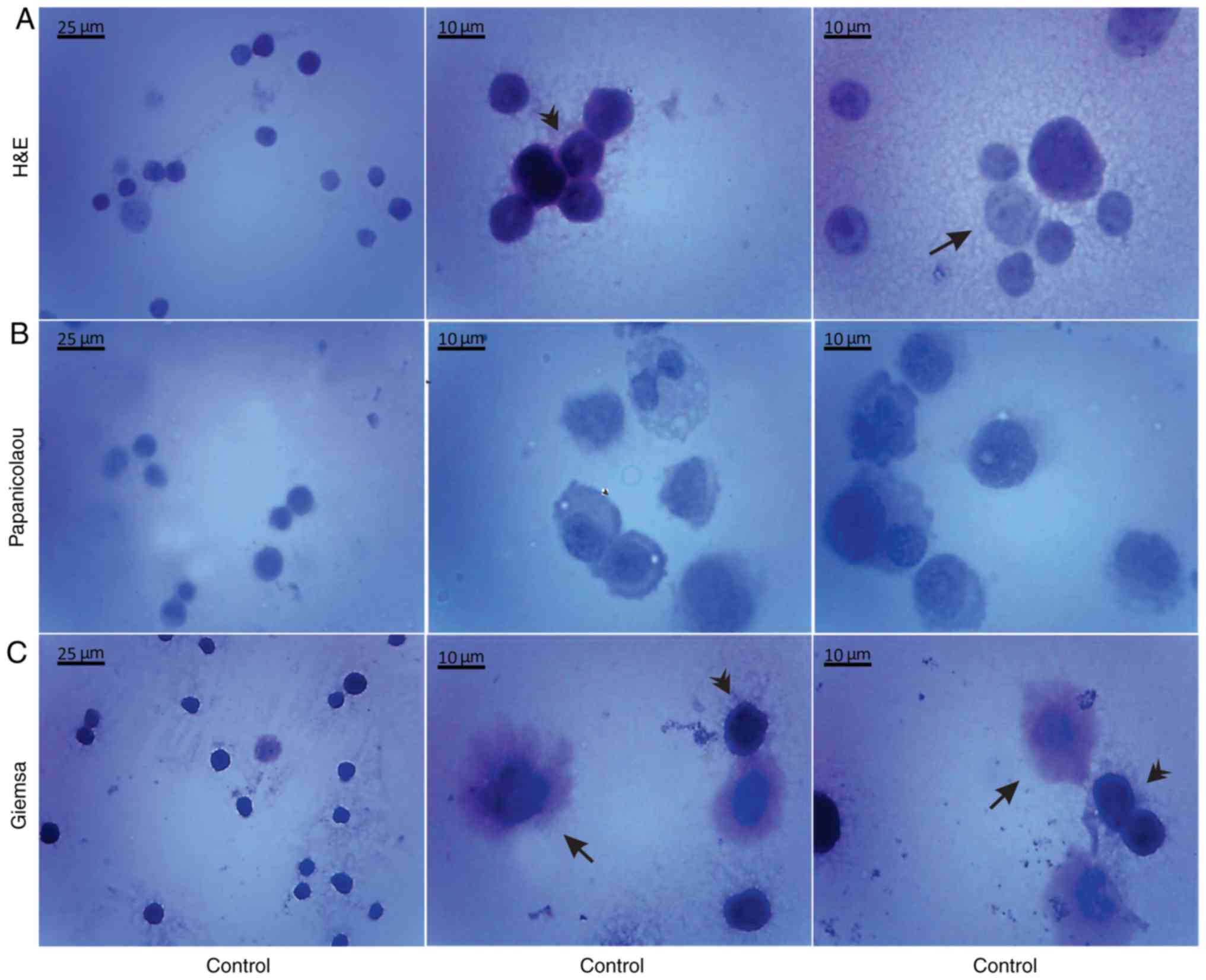
Histological methods with H&E, Papanicolaou and
Giemsa staining were applied to examine the morphological
characteristics of the non-cultured control EAC cells obtained from
the peritoneal cavity of mice on days 7 and 11 of tumor growth
(Figs. 3 and 4). Small erythrocytes not containing
nuclei, and macrophages were observed next to EAC cells.
Macrophages exhibiting phagocytosis were also observed, located
mainly in close contact with EAC cells.
Morphological evaluation of the isolated, by
centrifugation, non-cultured control EAC cells was performed by the
aforementioned histological methods on days 7 (Fig. 5) and 11 (Fig. 6) of tumor growth. The isolated
control EAC cells exhibited a typical tumor morphology, i.e. linked
with each other by cytoplasmic bridges, and centrally-located large
nuclei in different stages of mitosis were detected. In addition,
normoblasts, the apoptotic bodies, as well as macrophages were
revealed, located in close proximity to strongly-stained EAC cells.
Notably, EAC cells on the 11th day of tumor growth markedly
differed from tumor cells on the 7th day after inoculation, since
they were larger in size and exhibited pathological mitosis.
To confirm the antitumorigenic effect of PRP-1,
histological examination with H&E staining was further
conducted, which is widely used in medical diagnosis and cancer
detection.
PRP-1-induced morphological features of EAC cells
(Figs. 7 and 8) indicated the apoptotic nature of PRP-1
as well, manifested by cell shrinkage, membrane blebbing, nuclear
condensation (pyknosis) and fragmentation (karyorrhexis) as well as
by a predominant presence of apoptotic bodies (30–35).
There are indications that choice of program of autodestruction
occurs before initiation of the irreversible phase of cell response
to a lethal signal. The hallmark of apoptosis, externalization of
phosphatidylserine, that designates a cell with an ‘eat me’
message, is the earliest feature of apoptosis triggering (36,37).
In necrotic cells this feature is usually registered after plasma
membrane destruction; therefore, necrotizing cells are not
recognized by phagocytes and they cannot be digested until their
intracellular contents are spilled into the extracellular space
(38). Thus, statistical analysis
of the number of apoptotic and necrotic cells was based on the
certain aforementioned morphological features, i.e. the nuclear
lysis, activated loss of cell membrane integrity and an
uncontrolled release of products of cell death into the
extracellular space caused by necrosis (39,40),
and the cell shrinkage, membrane blebbing and predominant presence
of apoptotic bodies induced by apoptosis (41).
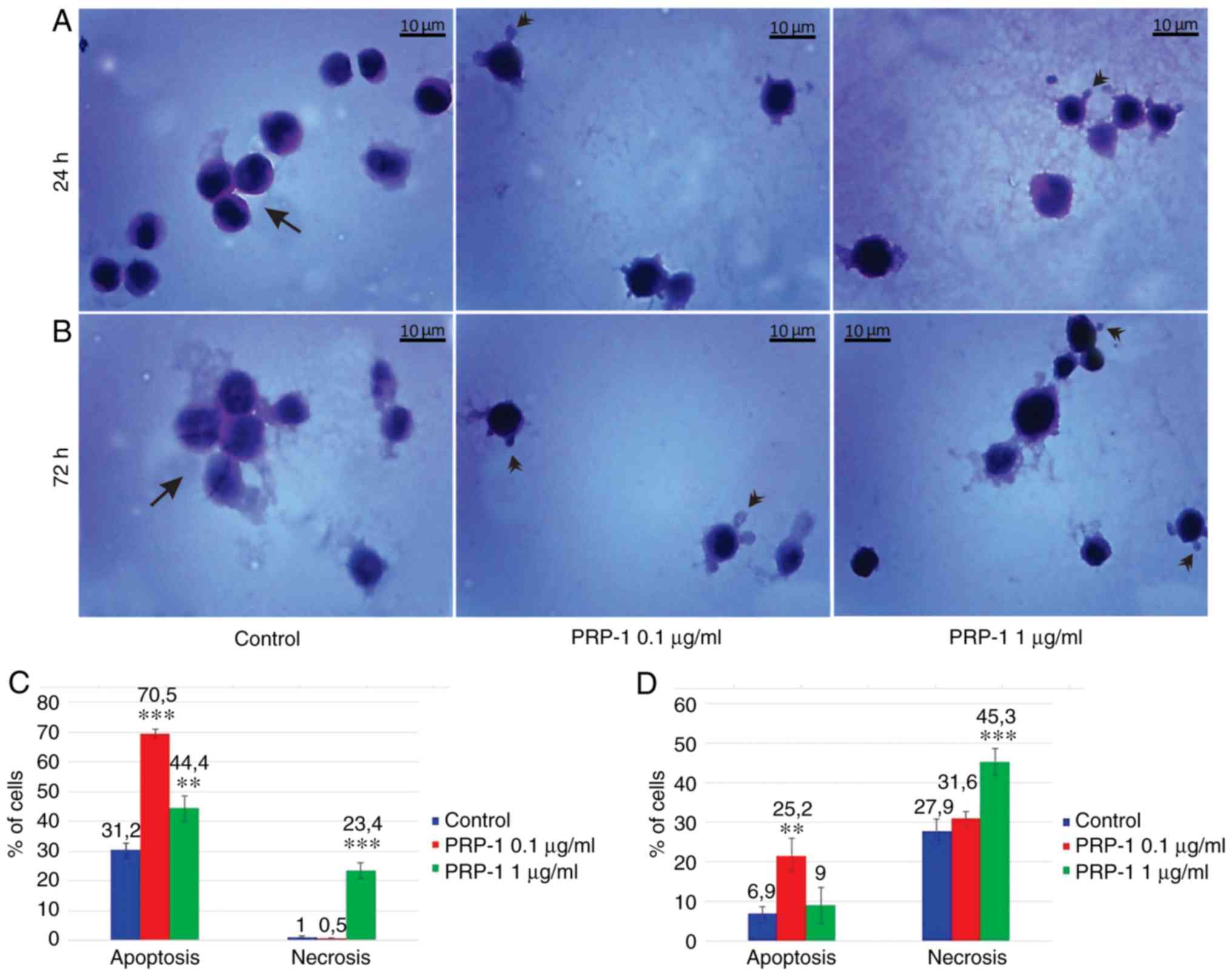
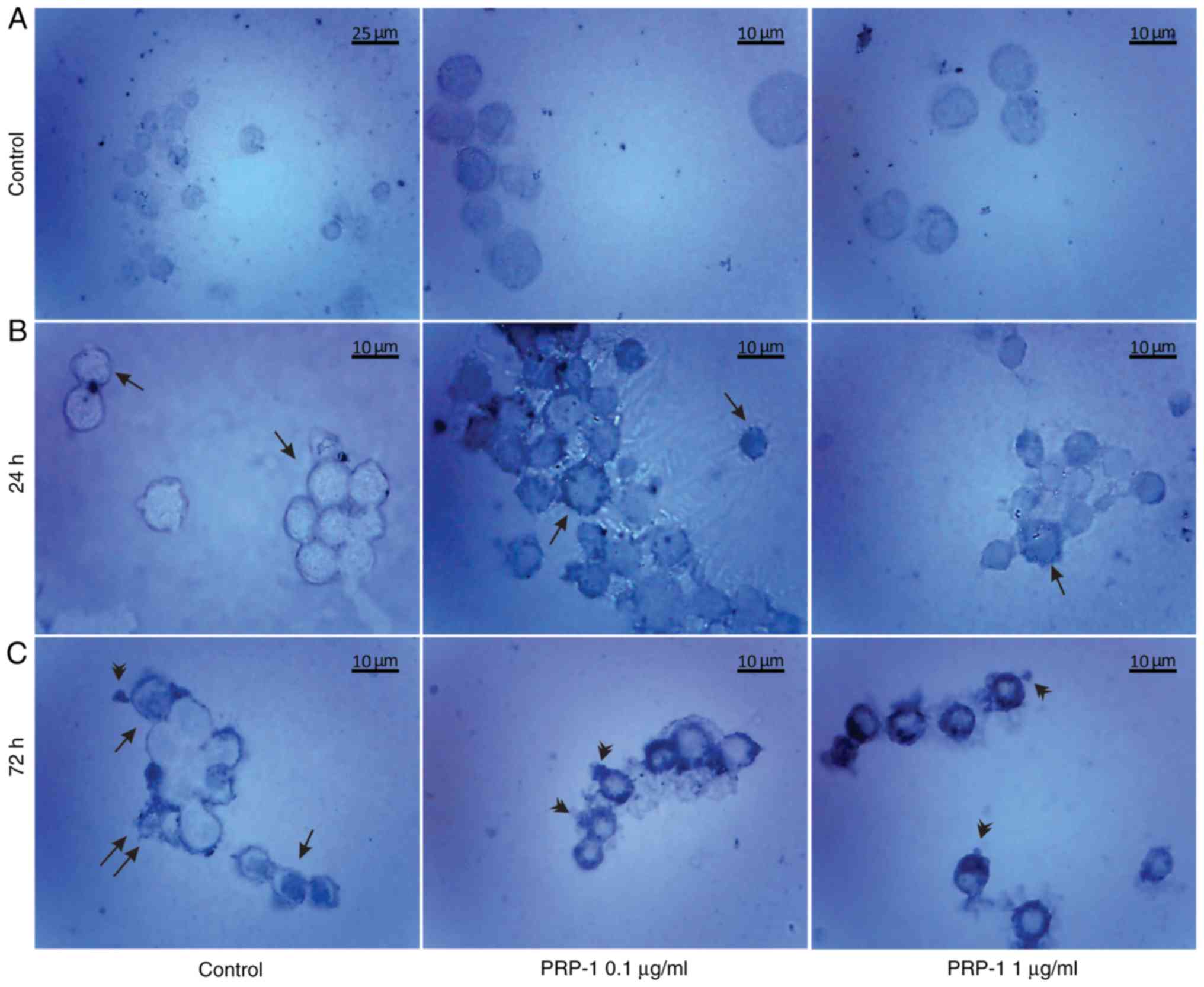
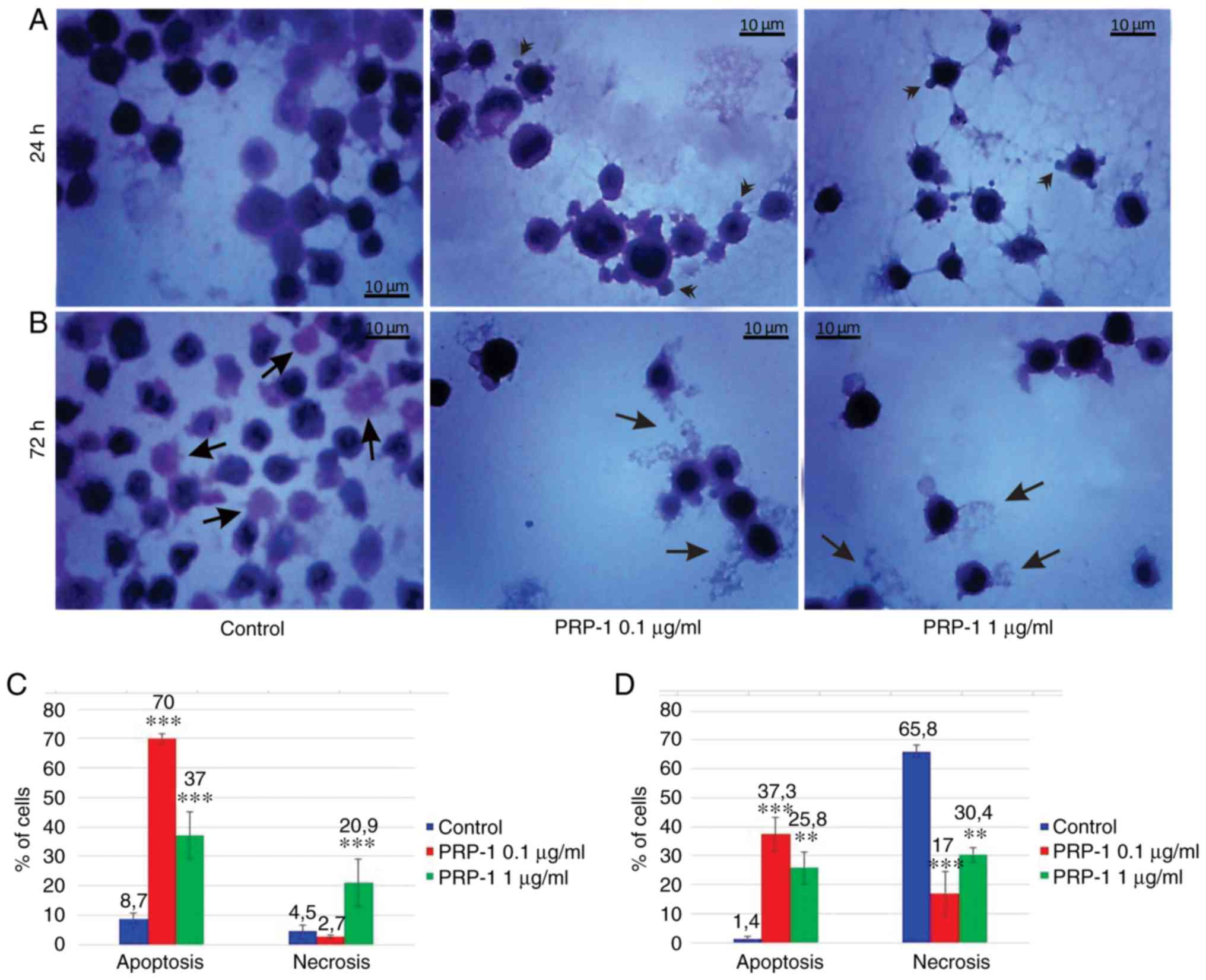 | Figure 7.Histological evaluation of the
hypothalamic PRP-1 effect on mouse-isolated EAC cells on day 7 of
tumor growth by H&E staining. Morphological changes of tumor
cells (A) 24 and (B) 72 h after culture. (A) In control samples,
numerous EAC cells linked with each other were detected at 24 h,
whereas a decreased number of cells was observed with both doses of
PRP-1. In the experimental samples, PRP-1-induced morphological
changes were similar for both the two time-points of culture.
Apoptotic membrane blebbing and apoptotic bodies (arrowheads),
smaller and round-shaped cells with eosinophilic cytoplasm and
condensed nuclei (pyknosis), and loss of reticular extensions and
contacts with adjacent cells were observed. (B) Necrotic EAC cells
containing no nuclei (karyolysis) or cells with lost membrane
integrity (arrows) were mainly presented in the control samples
after 72 h of culture, whereas few necrotic cells with lost plasma
membrane integrity and released cell death products (arrows) were
detected in the samples treated with PRP-1 for 72 h. Statistical
data regarding the PRP-1 (0.1 and 1 µg/ml) effect on the apoptosis
and necrosis in tumor cells treated for (C) 24 h and (D) 72 h were
presented according to the H&E exclusion test in comparison
with the findings in the untreated control cells. Data are
presented as the mean ± standard deviation (n=3), and represent ≥3
independent experiments. **P<0.01; ***P<0.001, significant
difference compared to (C) the control at 24 h and (D) the control
at 72 h. EAC, Ehrlich ascites carcinoma; PRP-1, proline-rich
polypeptide 1; H&E, hematoxylin and eosin. |
In the 7th day inoculated mice EAC cells, PRP-1
induced apoptosis by 70% (0.1 µg/ml) and 37% (1 µg/ml) at the 24 h
culture, in comparison with the control samples (8.7%). At the 72 h
culture, PRP-induced apoptosis by 37.3% (0.1 µg/ml) and 25.8% (1
µg/ml) compared to 1.4% apoptosis in the control samples (Fig. 7). In the control EAC cells of the
11th-day inoculated mice (Fig. 8),
cultured for 24 h, apoptosis was 31.2%, whereas in the experimental
samples of 0.1 and 1 µg/ml PRP-1, apoptosis was increased to 70.5
and 44.4%, respectively. At the 72 h culture, apoptosis was 6.9% in
the control group, against 25.2% for the 0 1 µg/ml PRP-1 group and
9% for the 1 µg/ml PRP-1 group.
On the 7th day post-inoculation a statistically
significant increase in the number of necrotic cells was observed
after the 24 h 1 µg/ml PRP-1 administration (20.9%) compared to the
control (4.5%), whereas no differences were observed between the
control and the 0.1-µg/ml PRP-1 (2.7%) samples. The statistically
significant decrease of the number of necrotic cells was observed
for the 72 h treatment with 0.1 µg/ml PRP-1 (17%) in comparison
with both control samples (65.8%) and cells exposed to 1 µg/ml
PRP-1 (30.4%).
On the 11th day post-inoculation, a statistically
significant increase of the number of necrotic cells was also
revealed after the 24 h (23.5%) culture with 1 µg/ml PRP-1 compared
to those in the control samples (1%) and experimental samples
exposed to 0.1 µg/ml PRP-1 (0.5%). Thus, taking into account the
similar data obtained in the control samples, as well as the
samples treated with 1 µg/ml PRP-1, further experiments were
carried out for the cells with 0.1 µg/ml PRP-1.
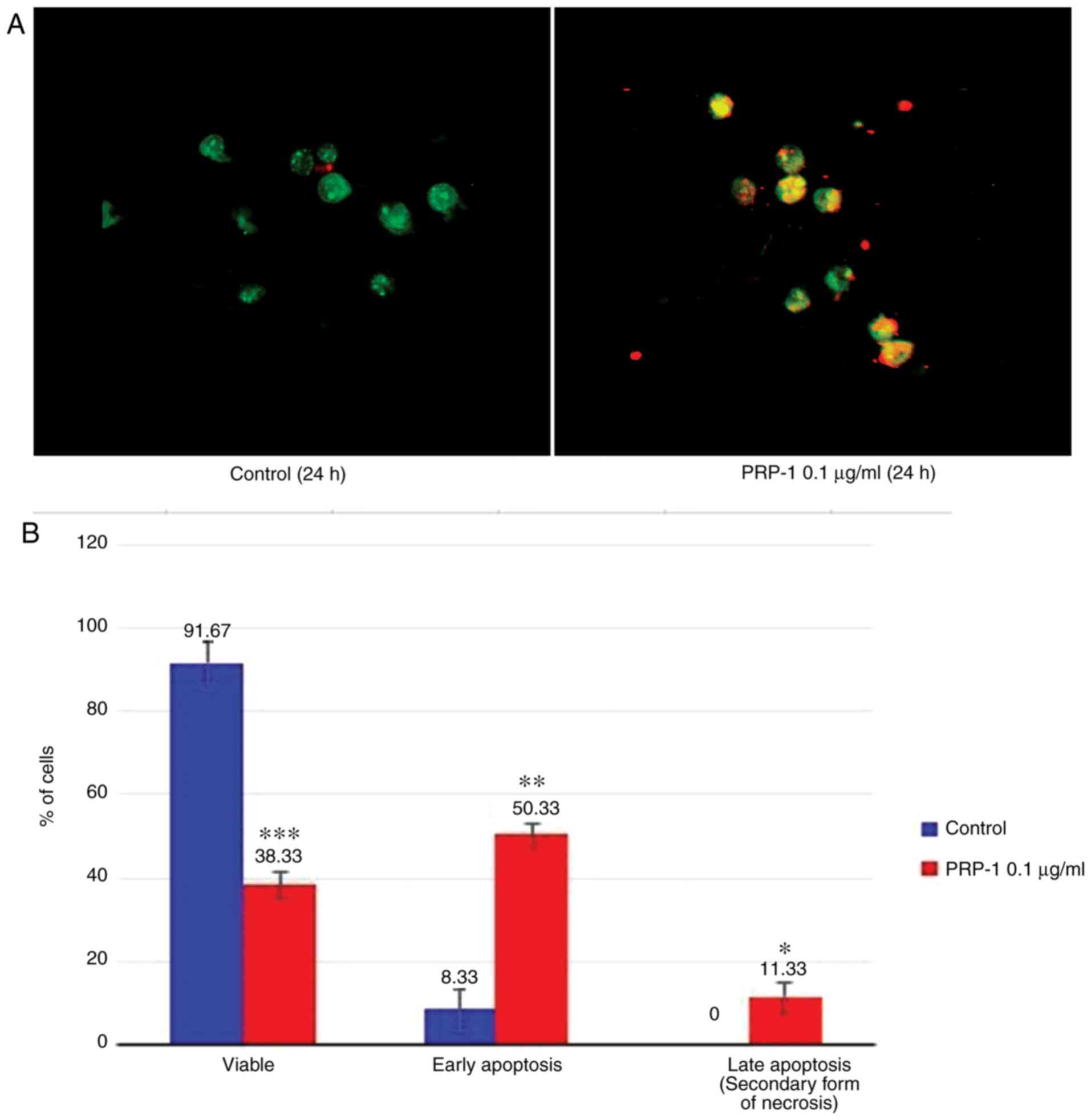
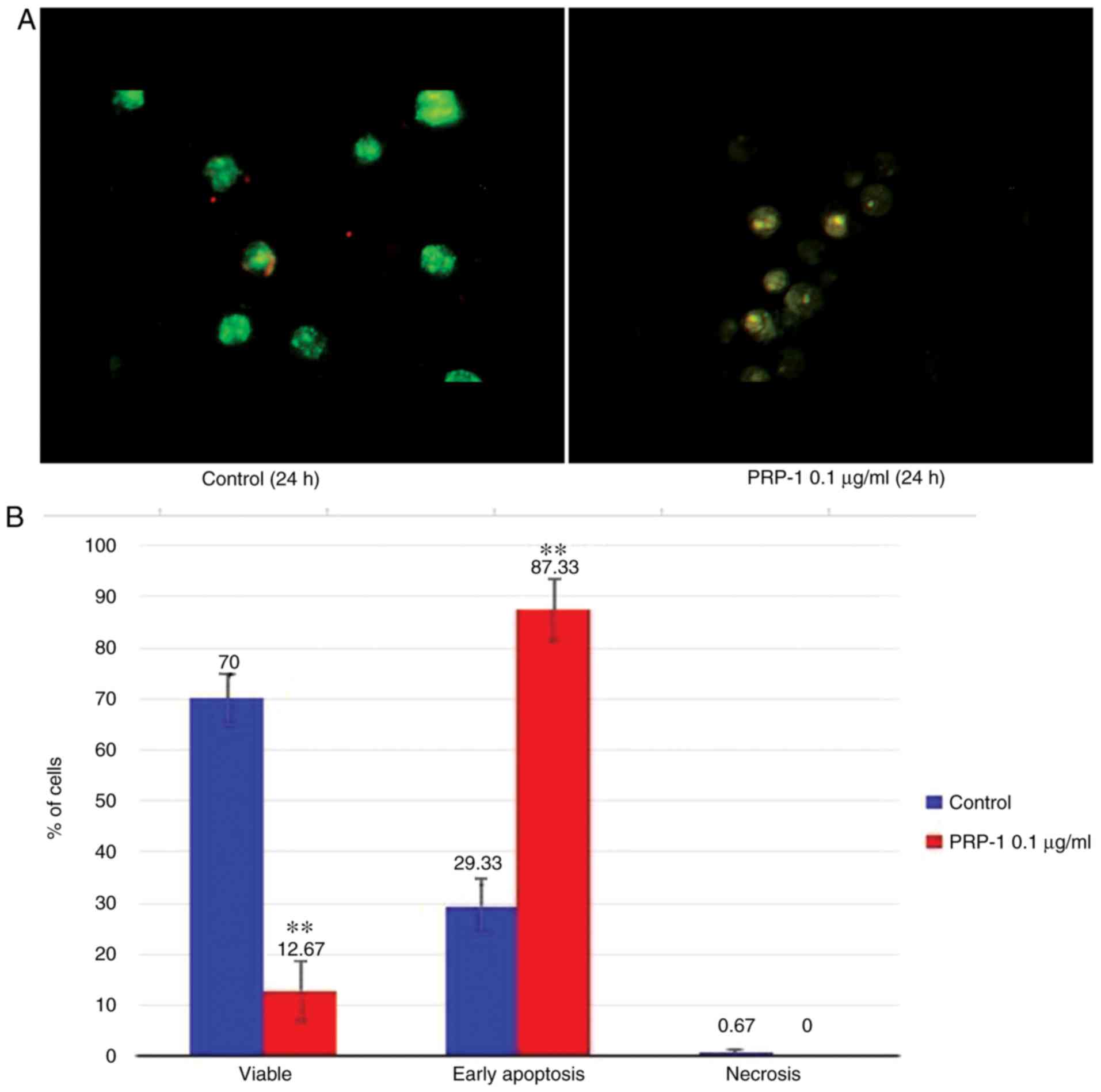
Fluorescence microscopy
The apoptosis-related morphological changes were
monitored with histological methods (Tr-Bl and H&E staining).
The data of the statistical analysis observed in EAC cells treated
with 0.1 µg/ml PRP-1 was confirmed by fluorescence microscopy with
Annexin V-Cy3 staining (Figs. 9A
and 10A) in comparison to
non-treated control samples at 24 h.
Statistically significant differences between the
control and experimental samples in terms of PRP-1-induced
apoptosis were observed in EAC cells, as depicted in Figs. 9B and 10B. It was revealed that 24 h of
incubation with 0.1 µg/ml PRP-1 induced a statistically significant
number of early apoptotic cells reaching 50.33% and late apoptotic
cells (11.33%) on day 7 post-inoculation (Fig. 9B).
According the literature data, a secondary form of
necrosis has been demonstrated to occur in late apoptotic cells
where apoptotic bodies undergo secondary necrotic changes and turn
to detritus, taking mainly place in vitro when phagocytosis
does not occur because of the absence of macrophages (38). Concomitantly, 53.34% inhibition of
the viable number of cells caused by PRP-1 was observed.
On day 11 post-inoculation, incubation with 0.1
µg/ml PRP-1 caused a 58% increase in apoptotic cells and a 57.33%
decrease in viable cells (Fig.
10B).
Immunohistochemical study
Using a PRP-1-antiserum, an immunohistochemical
study was carried out to detect PRP-1 localization in the EAC cells
and to examine PRP-1-triggered cell death (apoptosis/necrosis) on
days 7 and 11 of tumor growth (Figs.
11 and 12). The results
obtained detected no PRP-1-immunoreactivity (PRP-1-IR) in the
control non-cultured EAC cells. No intracellular PRP-1-IR was
detected in control EAC cells as well, after 24 h of culture,
whereas the plasma membrane exhibited weak PRP-1-IR in the form of
a narrow ring. In the experimental samples, the sub-membrane
cytoplasm with dense PRP-1-IR was detected in the tumor cells
exposed to 0.1 µg/ml PRP-1. However, on the 7 and 11th days of
tumor growth, at 72 h after EAC cell culture, nuclear, as well as
dense cytoplasmatic IR for PRP-1 was detected in tumor cells both
in the untreated control and PRP-1-treated samples. Morphological
changes of cells undergoing death-related processes (apoptosis and
necrosis) were clearly observed, including release of PRP-1-Ir
intracellular contents from necrotic cells into the extracellular
space, which was detected predominantly in the control samples,
while PRP-1-immunoreactive (PRP-1-Ir) apoptotic cells with plasma
blebs and apoptotic bodies were revealed mainly in the experimental
samples.
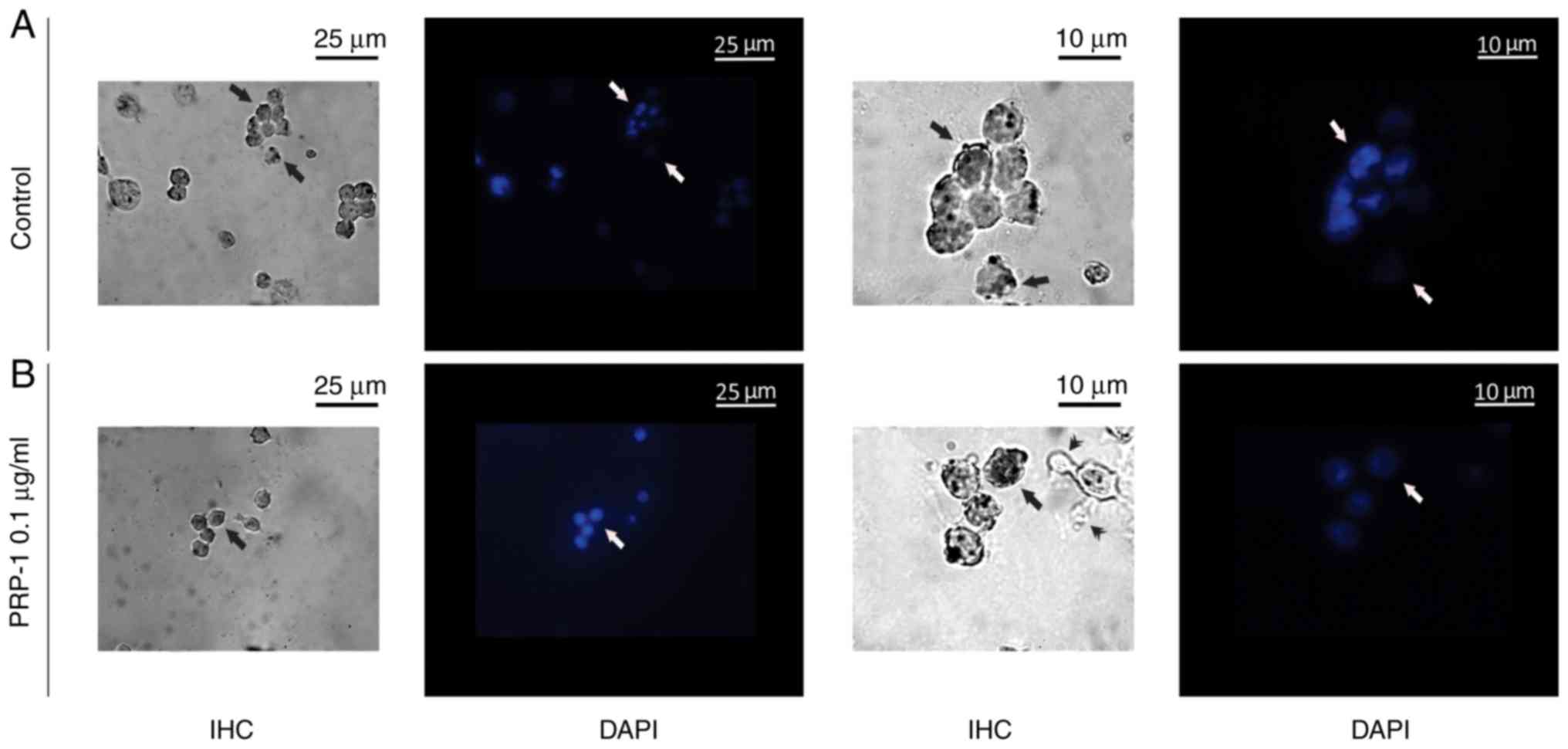
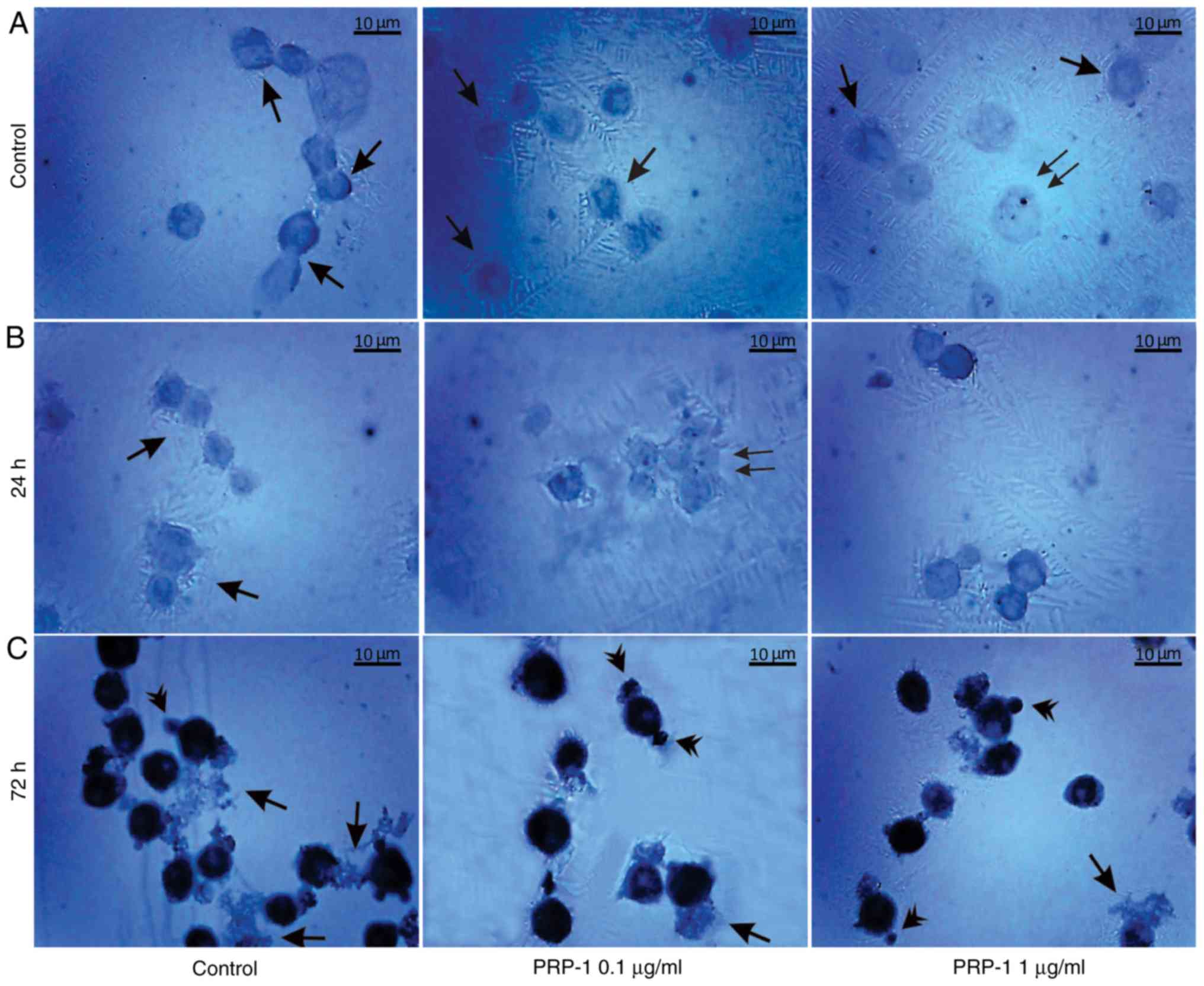 | Figure 12.Immunohistochemical localization of
the hypothalamic PRP-1 in cultured mouse EAC cells on the 11th day
of tumor growth according to the ABC immunohistochemical method.
(A) EAC control cells before culture, where PRP-1 was localized in
the cell membrane, cytoplasm (arrows) and nucleoli (double arrows)
of certain tumor cells. PRP-1-IR in the tumor cells (B) 24 h and
(C) 72 h after their culture. (B) In the untreated control samples
at 24 h, weak PRP-1-IR was mainly observed in the perinuclear zone
of cell cytoplasm. In the experimental samples exposed to 0.1 µg/ml
PRP-1 for 24 h, PRP-1-IR was observed in the cells nucleoli (double
arrows). (C) At 72 h after EAC cell culture, dense cytoplasmatic IR
for PRP-1 was detected in tumor cells both in the control and
PRP-1-treated samples. Morphological changes of cells undergoing
death-related processes (apoptosis and necrosis) were clearly
observed, including release of PRP-1-Ir intracellular contents from
necrotic cells into the extracellular space, which was detected
predominantly in the control samples (arrows), while PRP-1-Ir
plasma blebs and apoptotic bodies (arrowheads) were revealed mainly
in the experimental samples. PRP-1, proline-rich polypeptide 1;
EAC, Ehrlich ascites carcinoma; ABC, avidin-biotin complex;
PRP-1-IR, PRP-1-immunoreactivity; PRP-1-Ir,
PRP-1-immunoreactive. |
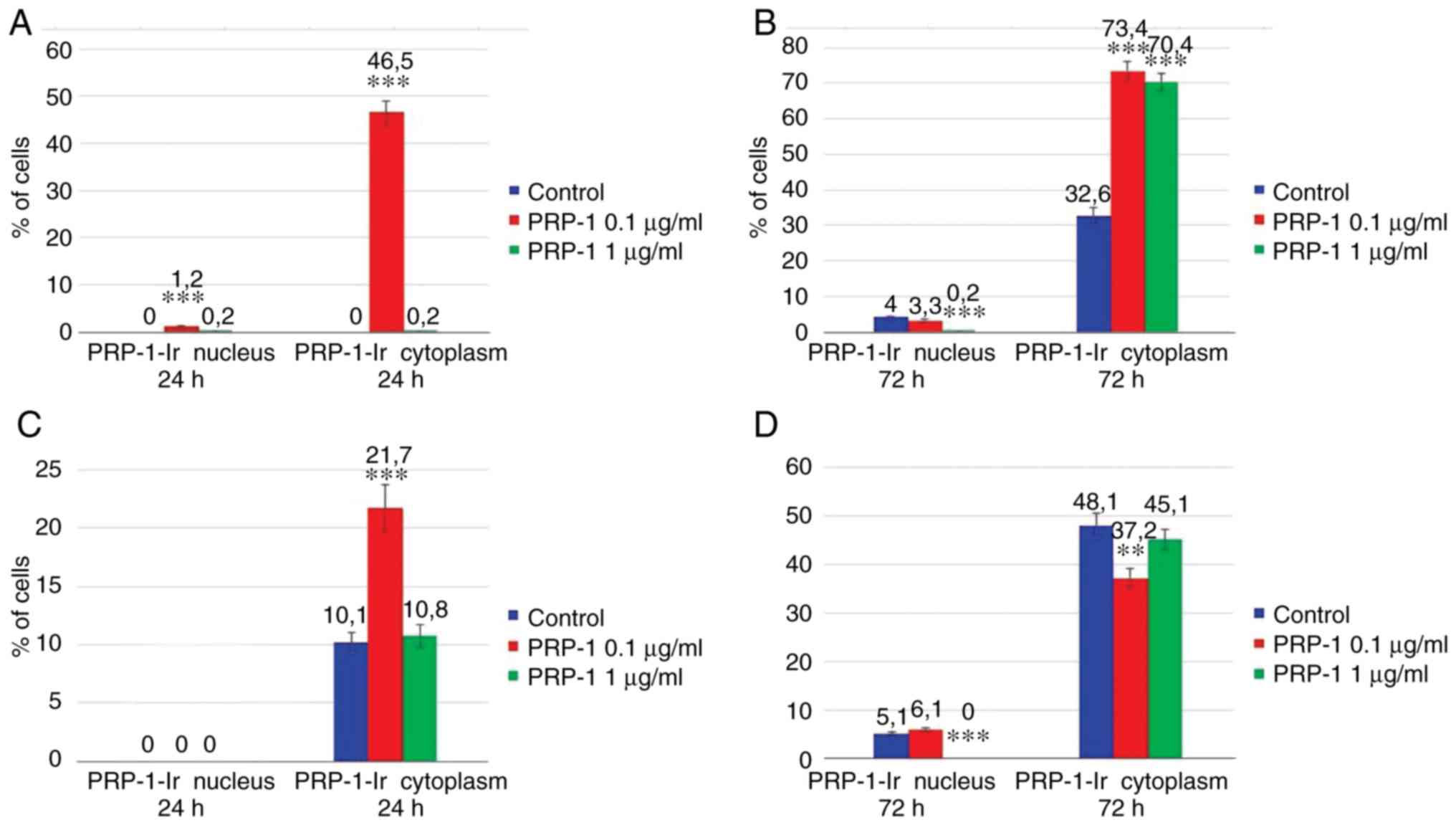
Data of the statistical analysis on the PRP-1
cellular localization is presented in the Fig. 13. Thus, on the 7th day of
inoculation, in the experimental samples exposed to 24 h 0.1 µg/ml
PRP-1 (Fig. 13A) the sub-membrane
cytoplasm with dense PRP-1-IR was detected in 46.5% of tumor cells
in contrast to control cells. After 72 h of culture, nuclear
localization of PRP-1 was detected in ~4% of the control cells and
in 3.3% of 0.1 µg/ml PRP-1-treated tumor cells. The number of cells
with cytoplasmic reactivity constituted 70.4 and 73.4% in the 1 and
0.1 µg/ml PRP-1 groups, respectively, in contrast to 32.6% in the
control cells (Fig. 13B).
On the 11th day of inoculation in the untreated
control samples at 24 h PRP-1-IR was mainly observed in the
cytoplasmic perinuclear zone in ~10.1% of cells (Fig. 13C). At 72 h after EAC cell culture,
strong cytoplasmatic IR for PRP-1 was observed in tumor cells both
in the control (48.1%) and PRP-1-treated (~40%) samples (Fig. 13D).
A nuclear counterstain method with DAPI fluorescent
staining applied, confirmed the immunohistochemically detected
PRP-1 nuclear localization in the cultured EAC cells both in the
control and 0.1 µg/ml PRP-1-treated samples (Fig. 14).
Discussion
The cytostatic or antiproliferative effect of the
hypothalamic PRP-1 in the human chondrosarcoma JJ012 cell line was
previously demonstrated after PRP-1 treatment compared with
chondrocyte culture, indicating that PRP-1 selectively targets
malignant sarcoma cells and not benign cells (11). Unlike in chondrosarcoma, in
glioblastoma, PRP-1 does not have any inhibitory activity on cell
proliferation (12). The objective
of the present morphological study was to investigate the
antitumorigenic effect of PRP-1 against EAC cells to elucidate the
underlying molecular mechanisms of action
(cytostatic/antiproliferative or cytotoxic/apoptotic) on the
aforementioned tumor cells.
In the present study data obtained by histological
method with Tr-Bl staining revealed an inhibitory effect of PRP-1
on the number of tumor cells and their viability observed 24 h
after a single administration of PRP-1 in comparison with
non-treated control samples. For example, on day 7
post-inoculation, the number of total cells was 5×106,
and increased to 8×106, with viable cells comprising
88%, whereas in 0.1 µg/ml PRP-1-treated samples, the total number
of cells decreased to 4×106, 66% of which were viable
cells. Thus, PRP-1 inhibited the growth of viable cells by 22%. On
day 11 post-inoculation, the total number of cells increased from
5×106 to 6.5×106, of which 93% were viable
cells in the 24 h control. It is notable that there were no
significant differences between the total number of EAC cells
(5×106) exposed to 0.1 and 1 µg/ml PRP-1. As for the
viable cells, in comparison with the percentage of inhibition
observed in the control group, 0.1 µg/ml PRP-1 inhibited the viable
cells by 49%. The results of different types of in vitro
experiments carried out by our group, such as biochemical,
immunological, morphological investigations confirmed that lower
doses of PRP-1 were more effective than higher doses which was
distinctive of several neuropeptides (10).
Histological methods with H&E, Papanicolaou and
Giemsa staining were applied in order to examine the morphological
features of the EAC control cells of mice on the 7 and 11th days of
tumor growth. Staining with Papanicolaou revealed that EAC cells on
the 11th day of tumor growth were markedly different from the tumor
cells on the 7th day, as they were larger in size and exhibited
pathological mitosis. Cells resembling macrophages were notably
distinguished by strong staining with Giemsa.
Since histological methods with H&E staining are
widely used in medical diagnosis and cancer detection, H&E
staining was selected to study the morphological features of the
control and PRP-1-exposed EAC cells, and to elucidate the
underlying mechanisms of PRP-1 antitumor activity.
Based on the data regarding the cell shrinkage,
membrane blebbing, chromosome condensation (pyknosis), nuclear
fragmentation (karyorrhexis), as well as a predominant presence of
apoptotic bodies (30–35), the apoptotic nature of PRP-1 was
confirmed.
Conventional screening models for anticancer agents
are geared toward the selection of cytotoxic drugs. It is highly
desirable to have compounds that can cause cancer cell death via
apoptosis, whereas the importance of cytostatic drugs, particularly
mTORC1 inhibitors, cannot be denied. In contrast to apoptosis, the
membrane integrity is lost due to necrotic cellular death,
accompanied by an uncontrolled release of products of cell death
into the extracellular space (39–41).
Data of the statistical analysis on the number of necrotic and
apoptotic cells were included in the present study.
Typical morphological features of apoptotic cells
can be observed using inverted phase contrast and fluorescence
microscopy. In the present study, morphological data from the
detection of apoptotic tumor cells indicated the apoptotic nature
of PRP-1 in EAC cells. To verify this observation, a series of
experiments were performed which focused on the determination of
apoptosis in cultured tumor cells using an Annexin V-Cy3 apoptosis
detection kit. Analysis of apoptosis by the Annexin V-Cy3 in the 7
day inoculated mice EAC-cultured cells exposed to 0.1 µg/ml
hypothalamic PRP-1 for 24 h revealed early apoptotic cells, as well
as late apoptotic cells, containing and surrounded by fragments of
necrotic nuclei, in contrast to the numerous viable tumor cells
detected in the untreated control samples. A secondary form of
necrosis is known to occur in late apoptotic cells where apoptotic
bodies undergo secondary necrotic changes and turn to detritus,
taking mainly place in vitro when phagocytosis does not
occur due to the absence of macrophages (38).
On day 11 of tumor growth after treatment with
PRP-1, the cell plasma membrane and certain intracellular
components manifested signs of early-stage apoptosis. Thus, the
apoptotic effect of PRP-1 on EAC-cultured cells of the 7 day
inoculated mice revealed a significant difference between the
control and experimental samples, since PRP-1 induced a decrease in
viable number of cells, and an increase the early and late
apoptotic number of cells.
Apoptosis experiments indicated that 24 h of culture
with PRP-1 induced a statistically significant increase in the
number of early apoptotic cells, reaching 50.33% on day 7
post-inoculation, whereas day 11 post-inoculation, a 58.33%
increase in the number of early apoptotic cells was detected. In
conclusion, the PRP-1 effect was cellular-context dependent, and in
EAC cells acted as a cytotoxic agent, causing programmed cell death
type I apoptosis.
A series of experiments which aimed to elucidate the
possible participation of PRP-1 in antitumorigenic processes were
carried out by detecting the immunohistochemical localization of
PRP-1 in the control and experimental EAC cells using an antibody
against the synthetic hypothalamic polypeptide of interest.
On the 7th day of tumor growth, no PRP-1-IR was
detected in the mice non-cultured control EAC cells. In the
untreated control EAC cells cultured for 24 h, no intracellular
PRP-1-IR was detected, but the PRP-1-Ir cell membrane (21%) was
demonstrated in the form of a narrow ring. In the experimental
samples exposed to 0.1 µg/ml PRP-1, strong PRP-1-IR was observed in
the cytoplasm (46.5%) and nucleoli (10%) of the total number of
tumor cells.
In the control cells, as well as in the
PRP-1-treated samples after 72 h of incubation, nuclear
localization of PRP-1 was detected in 3.3–4% of tumor cells, which
was statistically not significant. However, the number of cells
exposed to PRP-1 with cytoplasmic PRP-1-IR constituted 73.4%, in
contrast to 32.6% of the control cells. Notably, dense PRP-1-IR was
noticed in 25% of apoptotic cells with the membrane blebbing, in
contrast to 4% of the untreated control cells.
Immunohistochemically-detected PRP-1 nuclear
localization in the cultured EAC cells both in the control and
PRP-1-treated samples was confirmed by the nuclear counterstain
method with DAPI fluorescent staining. In mice inoculated for 11
days, PRP-1-IR was different in control-EAC cells before their
culture compared with that of cells after 7 days of tumor growth.
In certain EAC cells, weak positivity for PRP-1 was observed in the
cell membrane (15%) and the perinuclear zone of the cytoplasm (9%).
In the experimental samples exposed to 0.1 µg/ml PRP-1 for 24 h,
PRP-1-IR was observed in the nucleoli of the cultured tumor cells.
Morphological changes of cells undergoing death-related processes
(apoptosis and necrosis) were clearly observed 72 h after EAC cell
culture, as manifested by markedly dense cytoplasmatic IR for PRP-1
both in the control (48%) and PRP-1-treated (~40%) samples.
PRP-1-Ir cell membrane blebs and apoptotic bodies were observed in
close contact to EAC cells mainly in the experimental samples,
indicating that apoptosis was involved in the underlying mechanisms
of PRP-1 antitumorigenic action. PRP-1-IR was detected in the
nucleus and cytoplasm of EAC cells cultured for 72 h in both
control (untreated) and exiperimental (PRP-1-treated) samples,
which may be explained by the possible biosynthesis of endogenous
PRP-1 in the studied cancer cells. This hypothesis is in agreement
with previous immunohistochemical data on the detection of PRP-1 in
the nuclei of neural cells of labyrinthectomized rats (42), motoneurons of spinal cord
hemisectioned rats in response to cobra venom administration
(43) and bone marrow-derived
immune system cells of immobilized rats (44).
The time-dependent quantity of PRP-1 expression in
the blood samples of PRP-1-injected rats was demonstrated
previously (45). Thus, PRP-1 has
been quantified in intact (normal) rat blood serum samples, which
was estimated to be ~1.78 ng/ml, with a significant increase in
PRP-1 concentration (36.76 ng/ml) in blood samples at 5 h
post-PRP-1 i/p injection. However, the peptide concentration in the
blood decreased at day 2 post-injection to 3.111 ng/ml, similar to
that found in the control, which was explained by proteolytic
breakdown of the peptide. This assumption has been based on the
recently revealed data indicating PRP-1 as a new natural substrate
for DPPs (46) that are widely
expressed on the surface of the lymphocytes in the blood plasma,
substrates of which are chemokines, hormones, neuropeptides, and
growth factors (47,48).
In summary, the present immunohistochemical results
revealed PRP-1 localization in the nucleus and cytoplasm of both
untreated control and PRP-1-exposed experimental EAC cells cultured
for 72 h, which can be indicative of endogenous PRP-1 synthesis.
Notably, our group previously reported the detection of PRP-1
receptors in the nuclei of cancer cells (15). Further in vitro and in
vivo experiments are required to identify the involvement of
particular caspases in apoptosis and to elucidate other
PRP-1-triggered molecular pathways leading to cancer cell
death.
Acknowledgements
Authors would like to thank the Miami Center of
Orthopaedic Research and Education (Miami, FL, USA). Ehrlich
Ascites Carcinoma (EAC) cells were kindly provided by Senior
Researcher Hrachya Stepanyan from the laboratory of Toxicology and
Experimental Chemotherapy, Institute of Fine Organic Chemistry,
NAS, Armenia.
Funding
The present study was supported in part by a gift
from Ratcliffe Foundation to the Miami Center of Orthopaedic
Research and Education and the Buniatian Institute of Biochemistry
NAS of the Republic of Armenia.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SA, IS, NT, NK, TD and KG were involved in the study
design and contributed to the preparation of the manuscript and
data analysis. AS and RA were in charge of the fluorescence
microscopy, statistical and experimental data analysis at Yerevan
State University. GC and SC contributed to the peptide synthesis.
All authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The Institutional Animal Ethics Committee of the
Institute of Biochemistry after H.Buniatian, NAS, RA (IRB 0001621;
IORG0009782) provided approval for the use of the animals in the
present study.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Galoyan A: Neurochemistry of brain
neuroendocrine immune system: Signal molecules. Neurochem Res.
25:9–10. 2000. View Article : Google Scholar
|
|
2
|
Markossian KA, Gurvitz BY and Galoyan AA:
Isolation and chemical identification of new peptides from
neurоsecretory granules of hypothalamus. Neurokhimiya. 16:22–25.
1999.
|
|
3
|
Davtyan TK, Manukyan HA, Mkrtchyan NR,
Avetisyan SA and Galoyan AA: Hypothalamic proline-rich polypeptide
is a regulator of oxidative burst in human neutrophils and
monocyte. Neuroimmunomodulation. 12:270–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galoyan AA, Sarkissian JS, Sulkhanyan RM,
Chavushyan VA, Avetisyan ZA, Avakyan ZE, Gevorgyan AJ, Abrahamyan
DO and Grigorian YKh: PRP-1 protective effect against central and
peripheral neurodegeneration following n. ischiadicus transection.
Neurochem Res. 30:487–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galoian K, Luo S, Qureshi A, Patel P,
Price R, Morse A, Chailyan G, Abrahamyan S and Temple HT: Proline
rich polypeptide-1 and inflammatory pathway signaling in
chondrosarcoma. Mol Clin Oncol. 5:618–624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Durgaryan AA, Matevosyan MB, Seferyan TY,
Sargsyan MA, Grigoryan SL, Galoian KA and Galoyan AA: The
protective and immunomodulatory effects of hypothalamic
proline-rich polypeptide galarmin against methicillin-resistant
Staphylococcus aureus infection in mice. Eur J Clin
Microbiol Infect Dis. 3:2153–2165. 2012. View Article : Google Scholar
|
|
7
|
Galoyan AA, Korochkin LI, Rybalkina EJ,
Pavlova GV, Saburina IN, Zaraiski EI, Galoyan NA, Davtyan TK,
Bezirganyan KB and Revishchin AV: Hypothalamic proline-rich
polypeptide enhances bone marrow colony-forming cell proliferation
and stromal progenitor cell differentiation. Cell Transplant.
17:1061–1066. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galoyan KA, Scully SP and Galoyan AA:
Myc-Oncogene inactivating effect by proline rich polypeptide
(PRP-1) in chondrosarcoma. Neurochem Res. 34:379–385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galoian K, Temple TH and Galoyan A:
Cytostatic effect of the hypothalamic cytokine PRP-1 is mediated by
its inhibition of mTOR and cMyc in high grade metastatic
chondrosarcoma. Neurochem Res. 36:812–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galoian KA, Temple HT and Galoyan AA:
Cytostatic effect of novel mTOR inhibitor, PRP-1 in MDA231 (ER-)
breast carcinoma cell line. The cytokine inhibits mesenchymal
tumors. Tumor Biol. 32:745–751. 2011. View Article : Google Scholar
|
|
11
|
Galoian KA, Guettouche T, Issac B, Qureshi
A and Temple HT: Regulation of onco and tumor suppressor miRNAs by
mTORC1 inhibitor PRP-1 in human chondrosarcoma. Tumour Biol.
35:2335–2341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galoian K, Qureshi A, Dippolito G,
Schiller PC, Molinari M, Johnstone AL, Brothers SP, Paz AC and
Temple HT: Epigenetic regulation of embryonic stem cell marker
miR302C in human chondrosarcoma as determinant of antiproliferative
activity of proline rich polypeptide-1. Lnt J Oncol. 47:465–472.
2015. View Article : Google Scholar
|
|
13
|
Galoian K, Qureshi A, Wideroff G and
Temple HT: Restoration of desmosomal junction protein expression
and inhibition of H3K9-specific histone demethylase activity by
cytostatic proline-rich polypeptide-1 leads to suppression of
tumorigenic potential in human chondrosarcoma cells. Mol Clin
Oncol. 3:171–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chailakhyan RK, Gerasimov YV, Chailakhyan
MR and Galoyan AA: Proline-rich hypothalamic polypeptide has
opposite effects on the proliferation of human normal bone marrow
stromal cells and human giant-cell tumour stromal cells. Neurochem
Res. 35:934–939. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galoian K, Abrahamyan S, Chailyan G,
Qureshi A, Patel P, Metser G, Moran A, Sahakyan I, Tumasyan N, Lee
A, et al: Toll like receptors TLR1/2, TLR6 and MUC5B as binding
interaction partners with cytostatic proline rich polypeptide 1 in
human chondrosarcoma. Int J Oncol. 52:139–154. 2018.PubMed/NCBI
|
|
16
|
Galoian K, Scully S, McNamara G, Flynn P
and Galoyan A: Antitumorigenic effect of brain proline rich
polypeptide-1 in human chondrosarcoma. Neurochem Res. 34:2117–2121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jaganathan SK, Mondhe D, Wani ZA, Pal HC
and Mandal M: Effect of honey and eugenol on Ehrlich ascites and
solid carcinoma. J Biomed Biotechnol. 9891632010.PubMed/NCBI
|
|
18
|
Frajacomo FT, de Souza Padilha C,
Marinello PC, Guarnier FA, Cecchini R, Duarte JA and Deminice R:
Solid Ehrlich carcinoma reproduces functional and biological
characteristics of cancer cachexia. Life Sci. 162:47–53. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mondal A, Singha T, Maity TK and Pal D:
Evaluation of antitumor and antioxidant activity of
melothriaheterophylla (Lour.). Cogn. Indian J Pharm Sci.
75:515–522. 2013.PubMed/NCBI
|
|
20
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol. 111:A3B1–A3B3. 2015.
View Article : Google Scholar
|
|
21
|
Fischer A, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc pdb.prot4986. 2008.
|
|
22
|
Redginal H: Giemsa preparation for
staining blood films. Science. 86:5481937. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deshpande AK, Bayya P and Veeragandham S:
Comparative study of Papanicolaou stain (PAP) with rapid economic
acetic acid papanicolaou stain (REAP) in cervical cytology. J
Evolution of Med Dental Sci. 4:7089–7095. 2015. View Article : Google Scholar
|
|
24
|
Farinacci M: Improved apoptosis detection
in ovine neutrophils by annexin V and carboxyfluorescein diacetate
staining. Cytotechnology. 54:149–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szkaradek N, Sypniewski D, Waszkielewicz
AM, Gunia-Krzyżak A, Galilejczyk A, Gałka S, Marona H and Bednarek
I: Synthesis and in vitro evaluation of the anticancer potential of
new aminoalkanol derivatives of xanthone. Anticancer Agents Med
Chem. 16:1587–1604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu SM, Roine L and Farger H: Use of
avidin-biotin-peroxidasee (ABC) in immunoperoxidase technioques:
comparison between ABC and unlabelled antibody (PAP) procedures. J
Histochem Cytochem. 29:577–580. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ambrosius X: Obtaining of antisera from
different animals. Immunological methods. Frimel HG: pp. 14–15.
1987
|
|
28
|
Bret-Dibat JL, Zouaoui D, Déry O, Zerari
F, Grassi J, Maillet S, Conrath M and Couraud JY: Antipeptide
polyclonal antibodies that recognize a substance P-binding site in
mammalian tissues: A biochemical and immunocytochemical study. J
Neurochem. 63:333–343. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Engvall E: Enzyme immunoassay ELISA and
EMIT. Methods Enzymol. 70:419–439. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kasper DL and Zaleznik DF: Gas gangrene,
antibiotic associated colitis, and other Clostridial infections. In
Stone RM. Harrison's principles of internal medicine
self-assessment and board review. Med Pub Div. 922–927. 2001.
|
|
31
|
Andrade R, Crisol L, Prado R, Boyano MD,
Arluzea J and Arechaga J: Plasma membrane and nuclear envelope
integrity during the blebbing stage of apoptosis: A time-lapse
study. Biol Cell. 102:25–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shimizu T, Maeno E and Okada Y:
Prerequisite role of persistent cell shrinkage in apoptosis of
human epithelial cells. Sheng Li Xue Bao. 59:512–516.
2007.PubMed/NCBI
|
|
33
|
Somasekharan SP, Koc M, Morizot A, Micheau
O, Sorensen PH, Gaide O, Andera L and Martinou JC: TRAIL promotes
membrane blebbing, detachment and migration of cells displaying a
dysfunctional intrinsic pathway of apoptosis. Apoptosis.
18:324–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagata S: Apoptotic DNA fragmentation. Exp
Cell Res. 256:12–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagata S, Nagase H, Kawane K, Mukae N and
Fukuyama H: Degradation of chromosomal DNA during apoptosis. Cell
Death Differ. 10:108–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fadok VA, Bratton DL and Henson PM:
Phagocyte receptors for apoptotic cells: Recognition, uptake and
consequences. J Clin Invest. 108:957–962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Henson PM, Bratton DL and Fadok VA:
Apoptotic cell removal. Curr Biol. 11:R795–R805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shacter E, Williams JA, Hinson RM,
Senturker S and Lee YJ: Oxidative stress interferes with cancer
chemotherapy: Inhibition of lymphoma cell apoptosis and
phagocytosis. Blood. 96:307–313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Niles AL, Moravec RA and Riss TL: In vitro
viability and cytotoxicity testing and same-well multi-parametric
combinations for high throughput screening. Curr Chem Genomics.
3:33–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Proskuryakov SY, Konoplyannikov AG and
Gabai VL: Necrosis: A specific form of programmed cell death? Exp
Cell Res. 283:1–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Classification of cell death:
Recommendations of the nomenclature committee on cell death. Cell
Death Differ. 16:3–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tumasyan N, Sahakyan I, Kocharyan N,
Galoyan K and Abrahamyan S: Effect of the physiologically active
compounds on rat brain plasticity under the stress. Materials of
the XIV International scientific ecological. Conference. 240–242.
2016.
|
|
43
|
Abrahamyan SS, Meliksetyan IB, Chavushyan
VA, Aloyan ML and Sarkissian JS: Protective action of snake venom
Naja Naja Oxiana at spinal cord hemisection. Ideggyogy Sz.
60:148–153. 2007.PubMed/NCBI
|
|
44
|
Abrahamyan SS, Sahakyan IK, Meliksetyan
IB, Tumasyan NV, Badalyan BY and Galoyan AA: Histochemical and
immunohistochemical study of morphofunctional state of brain and
bone marrow cell structures of rats under stress. N Armenian Med J.
5:60–68. 2011.
|
|
45
|
Abrahamyan SS, Davtyan TK, Khachatryan AR,
Tumasyan NV, Sahakyan IK, Harutyunyan HA, Chailyan SG and Galoyan
AA: Quantification of the hypothalamic proline rich polypeptide-1
in rat blood serum. Neirokhimiya. 31:47–53. 2014.
|
|
46
|
Antonyan АA, Sharoyan SG, Mardanyan SS and
Galoyan AA: Proline-rich cytokine from neurosecretory granules: A
new natural substrate for dipeptidyl peptidase IV. Neurochem Res.
36:34–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mentlein R: Proline residues in the
maturation and degradation of peptide hormones and neuropeptides.
FEBS Lett. 234:251–256. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yaron A and Naider F: Proline-dependent
structural and biological properties of peptides and proteins. Crit
Rev Biochem Mol Biol. 28:31–81. 1993. View Article : Google Scholar : PubMed/NCBI
|