Introduction
Gastric xanthelasma is a benign lesion that was
first described in 1887 (1). It is
characterized by the presence of lipid islands in the gastric
mucosa (2), and infiltration of
lamina propria with foamy macrophages under the microscope
(3). The prevalence of gastric
xanthelasma ranges from 0.018 to 7.7% according to the reported
literature (4–10). The etiology and pathogenesis are
still unclear. Foamy macrophages broken-down to the cell membranes
after mucosal damage to the stomach and abnormality of the lipid
metabolism are the main hypotheses to explain the etiology and
pathogenesis (5,11).
Certain studies have evaluated the correlation
between gastric xanthelasma and numerous factors, such as H.
pylori, atrophic gastritis, intestinal metaplasia, and early
gastric cancer, but there were some conflicting conclusions.
Research by Hori and Sutsumi revealed that gastric xanthelasma was
related to H. pylori (12);
however, research by Yi revealed a negative result concerning H.
pylori (5). Studies by both
Chen et al and Köksal et al found that gastric
xanthelasma was related to atrophic gastritis and intestinal
metaplasia (10,13). Nevertheless, research from Japan
indicated that xanthelasma may be a warning sign for the presence
of early gastric cancer (4,14).
The follow-up strategy varies among patients who
have different risks of gastric cancer. Distinctly understanding
the risk factors of gastric xanthelasma is important to manage
patients who have a high risk of developing gastric cancer.
Therefore, we retrospectively analysed the relationship between
gastric xanthelasma and upper gastrointestinal (GI) endoscopic or
pathological features by performing a cross-sectional study.
Patients and methods
Study design
The present study is a cross-sectional retrospective
study that was performed at the Department of Gastroenterology of
Shanghai Ruijin Hospital North between July 2016 and June 2017. All
of the patients signed an informed consent form before the
procedure. The informed consent forms obtained the consent of
patients to use their clinical data, and assured them that no
identifiable personal information would be disclosed. The present
study was approved by the Institutional Review Board of Jiading
Hospital of Traditional Chinese Medicine (Shanghai, China)
(approval no. 2017005).
Patients
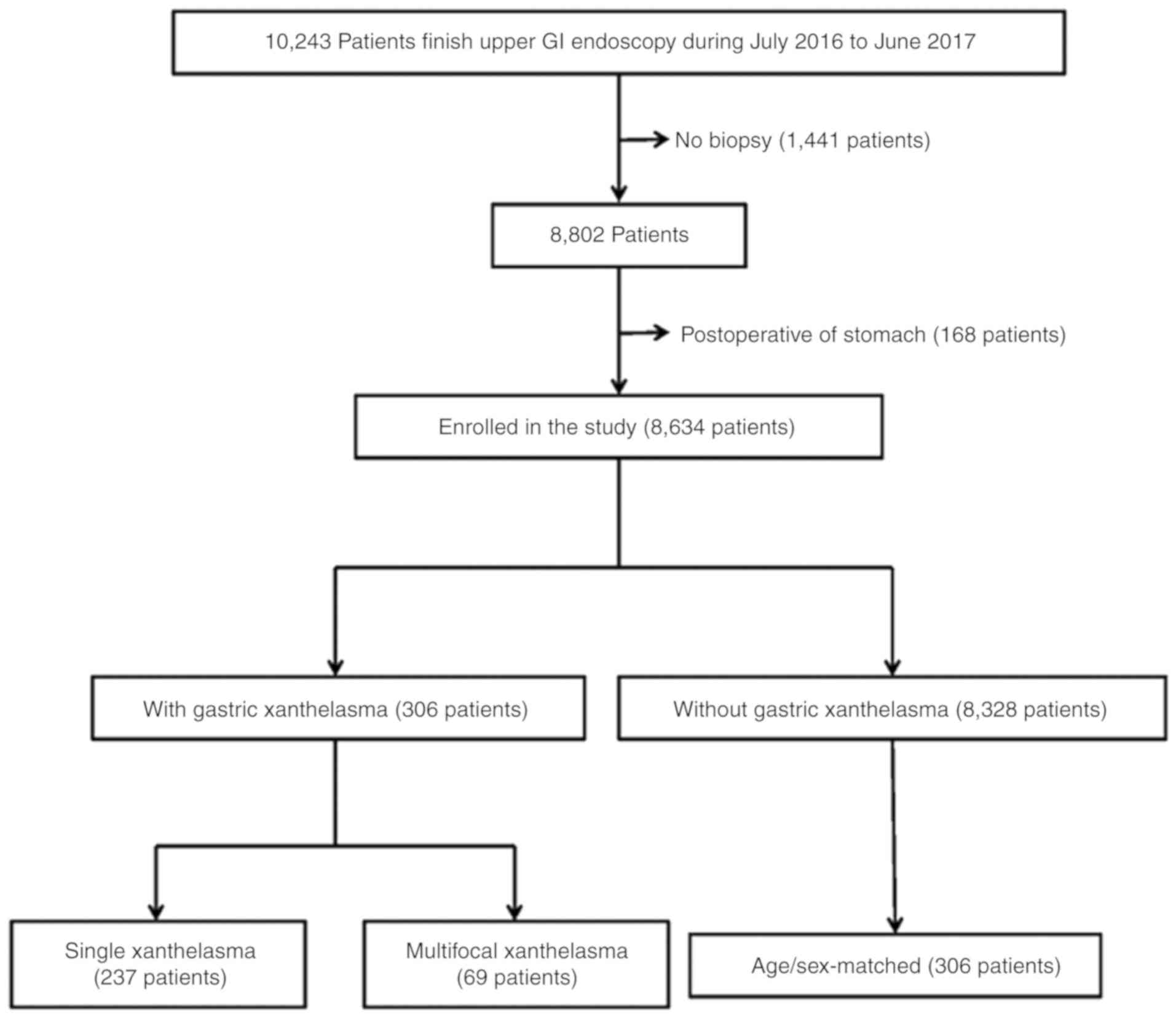
A total of 10,243 patients underwent upper GI
endoscopic examinations. The patients who did not have a stomach
biopsy pathological result and who had previously undergone
gastrectomy were excluded from this study. The following factors
were examined retrospectively: Age, sex, endoscopy findings, and
pathological results. Finally, 8,634 patients were enrolled in the
study, as presented in Fig. 1. To
exclude the influence of age and sex, controls matched with the
patients in terms of age and sex were also analyzed.
Endoscopic procedure
Upper GI endoscopic examination was performed using
pan-endoscopes (model name: Fujinon VP-4450HD-EG-590WR; Fujifilm)
equipped with the Picture Archiving and Communication System
(PacsVideo diagnostic work station, Neusoft Group Co., Ltd.). All
of the examinations were performed by 11 experienced endoscopists,
who carefully observed the esophagus, the entire stomach, and the
duodenum. The endoscopy findings, including peptic ulcer, bile
reflux, gastric polyp, and gastric xanthelasma, were recorded. Two
non-targeted biopsies in the antrum were routine biopsies.
Additional biopsies were collected where lesions were found. The
diagnosis of gastric xanthelasma was based on endoscopy and
histopathologic examination of biopsy samples. Anatomic locations
of all xanthelasmas were recorded during the procedure.
Histologic evaluation
The biopsy samples were fixed in 4% formalin,
embedded in paraffin in room temperature, and were performed on
4-µm sections. The histopathological changes of specimens were
stained by hematoxylin-eosin staining and were observed under light
microscope at ×400 magnification (Olympus BX-51; Olympus).
Histological diagnosis was independently performed by two
experienced pathologists. The pathological results, including
simple atrophy, intestinal metaplasia, H. pylori, dysplasia,
and gastric cancer, were recorded according to the updated Sydney
system (15).
Statistical analysis
SPSS 19.0 (IBM Corp.) statistical package for
Windows was used for the statistical analysis. Data concerning age
were expressed as the mean ± SD. Differences in age between the two
groups were analyzed by the independent samples t-test or the
Mann-Whitney U test if the data were not parametric. The Chi-square
test was used to compare categorical variables in different groups,
and the Fisher's exact test was used when low anticipated cell
counts were noted. Both univariate analysis and binary logistic
regression were used to analyze the association of gastric
xanthelasma with other factors. All of the calculated P-values were
two-tailed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characteristics of patients with and
without gastric xanthelasma
Upon examination of the 8,634 patients, a total of
306 (3.54%) patients had xanthelasma. According to the presence of
gastric xanthelasma, the patients were divided into the following
two groups: With xanthelasma and without xanthelasma. The clinical
characteristics of the two groups are summarized in Table I. The average age of patients with
xanthelasma was 55.76 years, which was higher than that of those
without xanthelasma (P<0.0001). The frequency of duodenal ulcer
in the group with xanthelasma was less than that in the group
without xanthelasma (6.5 vs. 10.7%, P=0.020). The frequency of
atrophy in patients with xanthelasma was significantly higher than
those without xanthelasma (30.1 vs. 18.9%, OR 1.839, 95% CI
1.432-2.362, P<0.0001). The frequency of intestinal metaplasia
in patients with xanthelasma was also significantly higher than
that in those without xanthelasma (59.2 vs. 30.5%, OR 3.296, 95% CI
2.612-4.159, P<0.0001). However, there were no significant
differences in sex, gastric ulcer, bile reflux, gastric polyp,
H. pylori, dysplasia, and gastric cancer between the two
groups.
 | Table I.Basic characteristics of the study
population with and without gastric xanthelasma. |
Table I.
Basic characteristics of the study
population with and without gastric xanthelasma.
| Characteristics | With xanthelasma
(n=306) n (%) | Without xanthelasma
(n=8,328) n (%) | OR | 95% CI | P-value |
|---|
| Age (years) | 55.76±11.18 | 49.17±13.58 |
|
|
<0.0001a |
| Sex |
|
| 0.860 | 0.684-1.081 | 0.201b |
| Male | 164 (53.6%) | 4,150 (49.8%) |
|
|
|
|
Female | 142 (46.4%) | 4,178 (50.2%) |
|
|
|
| Gastric ulcer |
|
| 0.761 | 0.402-1.442 | 0.470b |
|
Present | 10 (3.3%) | 354
(4.3%) |
|
|
|
|
Absent | 296 (96.7%) | 7,974 (95.7%) |
|
|
|
| Duodenal ulcer |
|
| 0.584 | 0.369-0.923 | 0.018b |
|
Present | 20 (6.5%) | 891
(10.7%) |
|
|
|
|
Absent | 286 (93.5%) | 7,437 (89.3%) |
|
|
|
| Bile reflux |
|
| 0.857 | 0.561-1.309 | 0.542b |
|
Present | 24 (8.9%) | 752
(9.3%) |
|
|
|
|
Absent | 282 (91.1%) | 7,576 (90.7%) |
|
|
|
| Gastric polyp |
|
| 1.178 | 0.811-1.712 | 0.363b |
|
Present | 32
(10.5%) | 751
(9.0%) |
|
|
|
|
Absent | 274 (89.5%) | 7,577 (91.0%) |
|
|
|
| Atrophy |
|
| 1.839 | 1.432-2.362 |
<0.0001b |
|
Present | 92
(30.1%) | 1,578 (18.9%) |
|
|
|
|
Absent | 214 (69.9%) | 6,750 (81.1%) |
|
|
|
| Intestinal
metaplasia |
|
| 3.296 | 2.612-4.159 |
<0.0001b |
|
Present | 181 (59.2%) | 2,542 (30.5%) |
|
|
|
|
Absent | 125 (40.8%) | 5,786 (69.5%) |
|
|
|
| H. pylori |
|
| 1.018 | 0.795-1.305 | 0.899b |
|
Present | 93
(30.4%) | 2,499 (30.0%) |
|
|
|
|
Absent | 213 (69.6%) | 5,829 (70.0%) |
|
|
|
| Dysplasia |
|
| 0.997 | 0.996-0.998 | 0.9999b |
|
Present | 0 (0) | 27
(0.3%) |
|
|
|
|
Absent | 306 (100%) | 8,301 (99.7%) |
|
|
|
| Gastric cancer |
|
| 1.112 | 0.407-3.043 | 0.836b |
|
Present | 4
(1.3%) | 98
(1.2%) |
|
|
|
|
Absent | 302 (98.7%) | 8,230 (98.8%) |
|
|
|
The significantly different factors were evaluated
by binary logistic analysis. The results revealed that age and
intestinal metaplasia remained significantly related to gastric
xanthelasma (P<0.0001; Table
II).
 | Table II.Binary logistic analysis of factors
related to gastric xanthelasma. |
Table II.
Binary logistic analysis of factors
related to gastric xanthelasma.
|
Characteristics | OR | 95% CI | P-value |
|---|
| Age | 1.027 | 1.017-1.037 | <0.0001 |
| Duodenal ulcer | 0.650 | 0.410-1.032 | 0.068 |
| Atrophy | 0.945 | 0.720-1.240 | 0.683 |
| Intestinal
metaplasia | 2.700 | 2.090-3.487 | <0.0001 |
Comparison between patients with
gastric xanthelasma and age/sex-matched controls without gastric
xanthelasma
To eliminate the statistical influence of age, 306
patients without xanthelasma were matched in terms of age and sex
with patients with xanthelasma. The differences in duodenal ulcer,
atrophy, and intestinal metaplasia between these subgroups were
analyzed. The Chi-square test revealed that between the two groups,
there was no difference in the presence of duodenal ulcer
(P=0.872), but there were significant differences in the presence
of atrophy (OR 1.763, 95% CI 1.214-2.560, P=0.003) and intestinal
metaplasia (OR 2.508, 95% CI 1.811-3.474, P<0.0001) (Table III). Furthermore, binary logistic
analysis revealed that intestinal metaplasia was significantly
related to gastric xanthelasma (OR 2.338, 95% CI, 1.659-3.297,
P<0.0001), while atrophy was not related to gastric xanthelasma
(P=0.221; Table IV).
 | Table III.Comparison between patients with
gastric xanthelasma and age/sex-matched controls without gastric
xanthelasma. |
Table III.
Comparison between patients with
gastric xanthelasma and age/sex-matched controls without gastric
xanthelasma.
|
Characteristics | With xanthelasma
(n=306) n (%) | Without xanthelasma
(n=306) n (%) | OR | 95% CI | χ2 | P-value |
|---|
| Duodenal ulcer |
|
| 0.949 | 0.503-1.789 | 0.026 | 0.872a |
|
Present | 20 (6.5) | 21 (6.9) |
|
|
|
|
|
Absent | 286 (93.5) | 285 (93.1) |
|
|
|
|
| Atrophy |
|
| 1.763 | 1.214-2.560 | 8.963 | 0.003a |
|
Present | 92
(30.1) | 60
(19.6) |
|
|
|
|
|
Absent | 214 (69.9) | 246 (80.4) |
|
|
|
|
| Intestinal
metaplasia |
|
| 2.508 | 1.811-3.474 | 31.174 |
<0.0001a |
|
Present | 181 (59.2) | 112 (36.6) |
|
|
|
|
|
Absent | 125 (40.8) | 194 (63.4) |
|
|
|
|
 | Table IV.Binary logistic analysis of factors
related to gastric xanthelasma. |
Table IV.
Binary logistic analysis of factors
related to gastric xanthelasma.
|
Characteristics | OR | 95% CI | P-value |
|---|
| Atrophy | 1.284 | 0.861-1.916 | 0.221 |
| Intestinal
metaplasia | 2.338 | 1.659-3.297 | <0.0001 |
Comparison of basic characteristics
between patients with single and multifocal xanthelasma
A subgroup analysis compared the basic
characteristics between patients with single and multifocal
xanthelasma. The average ages of patients with single and
multifocal xanthelasma were 55.20 and 57.99 years, respectively,
which revealed no significant difference (P=0.074). Other factors,
including sex, atrophy, intestinal metaplasia, H. pylori,
and gastric cancer also exhibited no differences (P>0.05;
Table V).
 | Table V.Basic characteristics of the study
population with single and multifocal xanthelasma. |
Table V.
Basic characteristics of the study
population with single and multifocal xanthelasma.
|
Characteristics | Single xanthelasma
(n=237) n (%) | Multifocal
xanthelasma (n=69) n (%) | χ2 | P-value |
|---|
| Age (years) | 55.20±11.62 | 57.99±10.43 |
| 0.074a |
|
Sex |
|
| 1.216 | 0.270b |
|
Male | 123 (51.9%) | 41 (59.4%) |
|
|
|
Female | 114 (48.1%) | 28 (40.6%) |
|
|
| Atrophy |
|
| 1.052 | 0.305b |
|
Present | 64
(27.0%) | 23 (33.3%) |
|
|
|
Absent | 173 (73.0%) | 46 (66.7%) |
|
|
| Intestinal
metaplasia |
|
| 0.630 | 0.427b |
|
Present | 135 (57.0%) | 43 (62.3%) |
|
|
|
Absent | 102 (43.0%) | 26 (37.7%) |
|
|
| H.
pylori |
|
| 1.472 | 0.225b |
|
Present | 71
(30.0%) | 26 (37.7%) |
|
|
|
Absent | 166 (70.0%) | 43 (62.3%) |
|
|
| Gastric cancer |
|
|
| 0.9999c |
|
Present | 3
(1.3%) | 1 (1.4%) |
|
|
|
Absent | 234 (98.7%) | 68 (98.6%) |
|
|
Comparison of localization
characteristics between gastric xanthelasma patients with and
without intestinal metaplasia
The locations of gastric xanthelasma were recorded
as cardia, fundus, corpus, angle, and antrum of the stomach.
Subgroup analysis compared localization characteristics between
gastric xanthelasma patients with and without intestinal
metaplasia; however, there were no significant differences in
localization characteristics (P>0.05; Table VI).
 | Table VI.Location characteristics of gastric
xanthelasma with or without intestinal metaplasia. |
Table VI.
Location characteristics of gastric
xanthelasma with or without intestinal metaplasia.
| Location | With intestinal
metaplasia (n=181) | Without intestinal
metaplasia (n=125) |
χ2 | P-value |
|---|
| Cardia |
|
|
| 0.479a |
|
Present | 6 | 2 |
|
|
|
Absent | 175 | 123 |
|
|
| Fundus |
|
| 0.287 | 0.592b |
|
Present | 9 | 8 |
|
|
|
Absent | 172 | 117 |
|
|
| Corpus |
|
| 0.153 | 0.696b |
|
Present | 26 | 16 |
|
|
|
Absent | 155 | 109 |
|
|
| Angle |
|
| 1.320 | 0.251b |
|
Present | 11 | 12 |
|
|
|
Absent | 170 | 113 |
|
|
| Antrum |
|
| 3.425 | 0.064b |
|
Present | 79 | 68 |
|
|
|
Absent | 102 | 57 |
|
|
Discussion
The prevalence of gastric xanthelasma was 3.54% in
our study, which is consistent with that in the reported literature
and is higher than a prevalence of 0.8% reported from China in 1989
and 2017 (10). The present study
revealed that gastric xanthelasma was associated with age, duodenal
ulcer, atrophy, and intestinal metaplasia using univariate
analysis. Binary logistic analysis revealed that gastric
xanthelasma was significantly associated with age and intestinal
metaplasia. After adjusting for age, xanthelasma remained
associated with intestinal metaplasia. Number and location of
gastric xanthelasma exhibited no difference for intestinal
metaplasia.
Previous research has indicated that gastric
xanthelasma had a strong correlation with atrophy (16,17),
intestinal metaplasia and early gastric cancer (18,19).
Furthermore, in a previous study, 44.4-76.0% of gastric xanthelasma
patients were diagnosed as having atrophy (14). Isomoto et al found that
gastric xanthelasma patients had more severe atrophy than controls
(17). The present study revealed
that 30.1% of gastric xanthelasma patients had atrophy, and this
rate was lower than that reported in the literature. Moreover, in
previous studies, 13.3-48.9% of gastric xanthelasma patients were
diagnosed as having intestinal metaplasia (18,19).
However, the present data revealed that 59.2% of gastric
xanthelasma patients had intestinal metaplasia, which is the
highest ever reported. Furthermore, in previous research,
14.0-20.1% of gastric xanthelasma patients were diagnosed as having
early gastric cancer, and 47.6-72.5% of early gastric cancer
patients were observed to have gastric xanthelasma (16). However, the present research
indicated that gastric xanthelasma patients showed no significant
increase in early gastric cancer when compared to those without
gastric xanthelasma, which is consistent with the result reported
by Köksal et al (13).
Notably, binary logistic analysis revealed that
gastric xanthelasma was an independent risk factor for intestinal
metaplasia, not for atrophy and early gastric cancer, which is
inconsistent with the literature (16). Sekikawa et al conducted
research on the association beween gastric xanthelasma and other
endoscopic examinations results, including atrophy, gastric ulcer,
duodenal ulcer, reflux esophagitis, fundic gland polyp, and gastric
cancer, but they did not analyze the association between gastric
xanthelasma and pathological results of gastric mucosa (14). Two researchers completed the
analysis of the relationship between gastric xanthelasma and
pathological results of gastric mucosa; however, multivariate
correlation analysis was not performed, and the authors concluded
that gastric xanthelasma is a warning marker of multifocal atrophic
gastritis and advanced intestinal metaplasia (13). The present univariate analysis
revealed that gastric xanthelasma was significantly associated to
both atrophy and intestinal metaplasia, while binary logistic
analysis revealed that atrophy was not associated to gastric
xanthelasma. This indicated that atrophy was not an independent
factor for gastric xanthelasma.
Researchers from Japan revealed that gastric
xanthelasma was an independent risk factor for early gastric cancer
(16). However, the present study
did not derive the same conclusion. There are some possible reasons
for this discrepancy. First, the detection rate of early gastric
cancer was 61.7% in 2008, according to the nationwide
population-based data in Japan (20); however, this rate is lower than 10%
in China (21). Second, image
enhanced endoscopy (IEE), such as magnifying endoscopy with
narrow-band imaging, is generally used in Japan; however, IEE is
not prevalently used in China (22).
Moreover, 30% of patients were diagnosed as having
H. pylori infection by pathologists in our study, but there
was no difference between patients with and without gastric
xanthelasma. A study from South Korea presented the same conclusion
concerning H. pylori (5),
however two researchers in the 1990s found that gastric xanthelasma
was closely associated with H. pylori (12,17).
In the present study it was also revealed the number
and location of gastric xanthelasma exhibited no difference for
intestinal metaplasia. However, research by Köksal et al
revealed that multiple xanthelasma patients had a higher
probability of being diagnosed as having intestinal metaplasia than
those with single xanthelasmas, and the location of gastric
xanthelasma exhibited no difference (13).
While the present research is single-center and
retrospective, the data are integral and credible. The present
study, however has some limitations. First, a single-center study
may lack representation; therefore, multicenter research is
required. Second, in the present research, H. pylori
infection was based on pathology diagnosis and we did not have
complete data of other tests, which may have caused bias while
analyzing the relationship between gastric xanthelasma and H.
pylori infection. Third, due to only two non-targeted biopsies
in our routine biopsy, the OLGA and OLGIM systems could not be used
to evaluate the correlation between gastric xanthelasma and the
grade of atrophy and intestinal metaplasia.
In summary, gastric xanthelasma was associated with
age and intestinal metaplasia. Age- and sex-matched analysis
revealed that gastric xanthelasma was significantly associated with
intestinal metaplasia. This indicates that gastric xanthelasma may
be an independent endoscopic warning sign of intestinal metaplasia.
Therefore, when gastric xanthelasma is encountered during an
endoscopic procedure, more attention should be paid since it may
indicate intestinal metaplasia of the gastric mucosa.
Acknowledgements
We would like to thank Professor Meiyu Shi from
Shanghai University of Traditional Chinese Medicine for
biostatistics support.
Funding
The present study was supported by the Chinese
Medicine Key Disciplines of Jiading District (grant no.
2017-ZYZDZK-01) and the Shanghai Health and Wellness Commission
Fund (grant no. 201640231).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
DX and PC conceived and designed the study. XY, YW
and PC provided administrative support and study patients. DX
collected and assembled the data. DX and XT performed data analysis
and interpretation. All of the authors contributed to the writing
and final approval of the manuscript and agree to be accountable
for all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All of the patients signed an informed consent form
before the procedure. The informed consent forms obtained the
consent of patients to use their clinical data, and assured them
that no identifiable personal information would be disclosed. The
present study was approved by the Institutional Review Board of
Jiading Hospital of Traditional Chinese Medicine (Shanghai, China)
(approval no. 2017005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khachaturian T, Dinning JP and Earnest DL:
Gastric xanthelasma in a patient after partial gastrectomy. Am J
Gastroenterol. 93:1588–1589. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kimura K, Hiramoto T and Buncher CR:
Gastric xanthelasma. Arch Pathol. 87:110–117. 1969.PubMed/NCBI
|
|
3
|
Coates AG, Nostrant TT, Wilson JA, Dobbins
WO III and Agha FP: Gastric xanthomatosis and cholestasis. A causal
relationship. Dig Dis Sci. 31:925–928. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sekikawa A, Fukui H, Maruo T, Tsumura T,
Kanesaka T, Okabe Y and Osaki Y: Gastric xanthelasma may be a
warning sign for the presence of early gastric cancer. J
Gastroenterol Hepatol. 29:951–956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yi SY: Dyslipidemia and H pylori in
gastric xanthomatosis. World J Gastroenterol. 13:4598–4601. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gencosmanoglu R, Sen-Oran E,
Kurtkaya-Yapicier O and Tozun N: Xanthelasmas of the upper
gastrointestinal tract. J Gastroentero. 39:215–219. 2004.
View Article : Google Scholar
|
|
7
|
Petrov S, Churtchev J, Mitova R, Boyanova
L and Tarassov M: Xanthoma of the stomach-some morphometrical
peculiarities and scanning electron microscopy.
Hepatogastroenterology. 46:1220–1222. 1999.PubMed/NCBI
|
|
8
|
Chen YS, Lin JB, Dai KS, Deng BX, Xu LZ,
Lin CD and Jiang ZG: Gastric xanthelasma. Chin Med J (Engl).
102:639–643. 1989.PubMed/NCBI
|
|
9
|
Terruzzi V, Minoli G, Butti GC and Rossini
A: Gastric lipid islands in the gastric stump and in non-operated
stomach. Endoscopy. 12:58–62. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, He XJ, Zhou MJ and Li YM: Gastric
xanthelasma and metabolic disorders: A large retrospective study
among Chinese population. World J Gastroenterol. 23:7756–7764.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lechago J: Lipid islands of the stomach:
An insular issue? Gastroenterology. 110:630–632. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hori S and Tsutsumi Y: Helicobacter
pylori infection in gastric xanthomas: Immunohistochemical
analysis of 145 lesions. Pathol Int. 46:589–593. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Köksal AŞ, Suna N, Kalkan İH, Eminler AT,
Sakaoğulları ŞZ, Turhan N, Saygılı F, Kuzu UB, Öztaş E and Parlak
E: Is gastric xanthelasma an alarming endoscopic marker for
advanced atrophic gastritis and intestinal metaplasia? Dig Dis Sci.
61:2949–2955. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sekikawa A, Fukui H, Sada R, Fukuhara M,
Marui S, Tanke G, Endo M, Ohara Y, Matsuda F, Nakajima J, et al:
Gastric atrophy and xanthelasma are markers for predicting the
development of early gastric cancer. J Gastroenterol. 51:35–42.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis. The updated Sydney
System. International Workshop on the Histopathology of Gastritis,
Houston 1994. Am J Surg Pathol. 20:1161–1181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitamura S, Muguruma N, Okamoto K,
Tanahashi T, Fukuya A, Tanaka K, Fujimoto D, Kimura T, Miyamoto H,
Bando Y, et al: Clinicopathological assessment of gastric xanthoma
as potential predictive marker of gastric cancer. Digestion.
96:199–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Isomoto H, Mizuta Y, Inoue K, Matsuo T,
Hayakawa T, Miyazaki M, Onita K, Takeshima F, Murase K, Shimokawa I
and Kohno S: A close relationship between Helicobacter
pylori infection and gastric xanthoma. Scand J Gastroenterol.
34:346–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arévalo F and Cerrillo G: Gastric xantoma:
Histological findings and clinico endoscopic characteristics in the
‘Hospital Nacional 2 de Mayo’ (1999–2005). Rev Gastroenterol Peru.
25:268–271. 2005.(In Spanish). PubMed/NCBI
|
|
19
|
Moretó M, Ojembarrena E, Zaballa M, Tánago
JG, Ibáñez S and Setién F: Retrospective endoscopic analysis of
gastric xanthelasma in the non-operated stomach. Endoscopy.
17:210–211. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
The Editorial Board of the Cancer
Statistics in Japan, . Cancer Statistics in Japan 2016. In:
Foundation for promotion of cancer research; Tokyo: pp. 922017
|
|
21
|
Du YQ, Cai QC, Liao Z, Fang J and Zhu CP:
China experts consensus on the protocol of early gastric cancer
screening (2017, Shanghai). Chin J Digest. 38:87–92. 2018.
|
|
22
|
Yao K, Uedo N, Muto M and Ishikawa H:
Development of an e-learning system for teaching endoscopists how
to diagnose early gastric cancer: Basic principles for improving
early detection. Gastric cancer. 20 (Suppl 1):S28–S38. 2017.
View Article : Google Scholar
|