Introduction
Breast cancer is the most common malignant tumor
affecting women's health worldwide. Due to the metastasis of breast
cancer cells and sensitivity to chemotherapy, the incidence of
breast cancer with approximately 1,7000,000 new cases each year
remains alarmingly high, and the prognosis after surgery is poor
(1). Most tumors are difficult to
cure due to distant metastases, and some patients experience tumor
recurrence after treatment. More than 90% of breast cancer-related
deaths are associated with tumor cell metastasis, and metastatic
sites usually include the bone, brain, liver and lung (2). Previous findings have shown that 54
genes, including MMP1, CXCL1 and PTGS2, are markers
and mediate the metastasis of breast cancer cells to the lungs
(3). Cancer stem cell-like cells
(CSCs) promote tumor metastasis. Recent findings have shown that
increasing the CD44v/CD44s ratio in breast cancer cells by
regulating the expression of epithelial splicing regulatory protein
1 (ESRP1) leads to the promotion of lung metastasis without
influencing cancer cell stemness (4). In addition, it has been reported that
epithelial-mesenchymal transition (EMT) is associated with breast
cancer metastasis (5).
EMT is a cellular process in which cells lose their
epithelial characteristics and acquire mesenchymal features, which
enable the cells to migrate more efficiently and invade the
underlying mesenchyme (6). In
cancer, EMT is associated with tumorigenesis, invasion, metastasis
and resistance to chemotherapy (7).
EMT can alter the expression of adhesion factors, enhance the
migration and invasion of tumor cells, and ultimately transfer
tumors away from the primary site to adjacent or distal organs. The
growth and metastasis of primary tumors is controlled by the
regulation of TGF-β1, which induces EMT-related changes in cells
(8). TGF-β1 promotes tumorigenesis
in the tumor microenvironment. Clinical data have shown that TGF-β1
protects NIH3T3 fibroblasts (CAFs) from starvation-induced growth
inhibition, mitochondrial damage and cell apoptosis (9).
EMT can induce cancer metastasis by promoting tumor
malignancy, reprogramming cancer metabolism, and disrupting the
extracellular matrix (10).
Accumulating evidence has demonstrated that aberrant cancer
metabolism can induce EMT through multiple pathological pathways
(11). Most studies have focused on
the relationship between glucose metabolism and EMT. Changes in
lipid metabolism during EMT have also been reported (10).
The aim of the present study was to explore changes
in fatty acid metabolism and regulatory mechanisms of
TGF-β1-induced EMT in breast cancer cells, which is of great
significance for the clinical treatment of breast cancer.
Materials and methods
Reagents
Recombinant human TGF-β1 was purchased from R&D
Systems. For in vitro cell studies, TGF-β1 was reconstituted
at 20 µg/ml in sterile 4 mM HCl containing 1 mg/ml human or bovine
serum albumin and stored at −20°C. Then, the solution was further
diluted in fresh medium for cell experiments. Primary antibodies
against NADH-ubiquinone oxidoreductase chain 1 (ND1, 1:1,000; Anbo
Biotechnology, Inc.), mitochondrial transcription factor A (TFAM,
1:500; Cell Signaling Technology, Inc.), pyruvate dehydrogenase
(PDH, 1:500; CST), carnitine palmitoyl transferase 1A (CPT1A;
1:500; CST), fatty acid synthase (FASN; 1:500; CST), E-cadherin
(1:500; CST), vimentin (1:500; CST), Snail (1:500; CST), HIF-1α
(1:1,000), NADH:ubiquinone oxidoreductase subunit B8 (NDUFB8,
1:1,000; Abcam), lactate dehydrogenase (LDH, 1:1,000; Abcam), and
GAPDH (1:1,000; Abcam) were used in this study.
Patients and tissue samples
Breast cancer samples and matching normal tissues
were obtained from 20 patients with breast cancer (19 females and 1
male; age range, 30.0-68.0 years) at the Thyroid and Breast Surgery
of the First People's Hospital of Dali Autonomous Prefecture
(Yunnan, China), between September, 2017 and December, 2018. The
fresh tumor tissue specimens were excised from the tumors, and
their corresponding adjacent normal tissues were taken ≥3 cm from
the tumor edge. All specimens were stabilized by snap-freezing
immediately in cryovials, immersed in liquid nitrogen and stored at
−80°C until analysis. All the patients were diagnosed for the first
time and did not have a treatment history of chemotherapy or
radiotherapy prior to surgery.
The study was approved by the Medical Ethics
Committee of Dali University (no. 2016-10), and all patients signed
an informed consent form.
Cell lines and cell culture
Human breast cancer MCF-7 cells were obtained from
the Kunming Cell Bank of the Chinese Academy of Science (Kunming,
China). The cell lines were cultured in high glucose DMEM (Gibco,
Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum
(Biological Industries) and 1% penicillin-streptomycin (Gibco) at
37°C in a humidified incubator with 5% CO2. The
experiments were performed with cells in the logarithmic phase, and
the cells were MAP-tested and free of mycoplasma contamination. To
investigate the effect of metabolic reprogramming during
TGF-β1-induced EMT, MCF-7 cells were treated with 2 ng/ml TGF-β1 at
different times (0, 3, 6 and 9 days) and then further cultured
withdrawal TGF-β1 for 3, 6 and 9 days. After different treatments,
the cells were observed and photographed for morphological
alterations under an inverted light microscope.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from treated MCF-7 cells at
different times using TRIzol® reagent (Solarbio)
according to the manufacturer's protocols and then quantified. The
RNA (2 µg) was used to generate first-strand DNA using M-MLV
reverse transcriptase (Promega) at 42°C for 15 min. For standard
PCR, products were visualized using 1% agarose gels. All reactions
were performed in triplicate on an ABI Prism 7300 Sequence
Detection System (Applied Biosystems, Thermo Fisher Scientific,
Inc.) using SYBR-Green (Takara Bio, Inc.) fluorescent dye, and the
data were normalized to β-actin mRNA levels. PCR was performed
under the following conditions: 94°C for 2 min, followed by 40
cycles of 94°C for 30 sec, and 58°C for 40 sec. The primer
sequences used are listed in Table
I.
 | Table I.Primer sequences used in RT-qPCR. |
Table I.
Primer sequences used in RT-qPCR.
| Target gene | Primer | Nucleotide
sequence |
|---|
|
E-cadherin | F |
5′-GAACGCATTGCCACATACACT-3′ |
|
| R |
5′-TTCCATGACAGACCCCTTAAA-3′ |
|
N-cadherin | F |
5′-TGGAGACATTGGGGACTTC-3′ |
|
| R |
5′-TGCTCACCACCACTACTTG-3′ |
| Snail1 | F |
5′-ATCCTCAACCCCACCGCCT-3′ |
|
| R |
5′-GCCTTTCCCACTGTCCTCA-3′ |
| FASN | F |
5′-TGGGAGGAGTGTAAACAG-3′ |
|
| R |
5′-GAAGTAGGAGTGGAAGGC-3′ |
| CPT1 | F |
5′-TCTTGGGGTGATGGTGTG-3′ |
|
| R |
5′-GTCAATAGTGAGGGTTTT-3′ |
| ACC | F |
5′-TGTGCTGGTCTACATTCCTCC-3′ |
|
| R |
5′-TGATTTCTACCGTCCCTTCTG-3′ |
| CD36 | F |
5′-CCGTGACATCAAGGAGAAGC-3′ |
|
| R |
5′-TACCGCAAGATTCCATACCC-3′ |
| β-actin | F |
5′-CATGTACGTTGCTATCCAGGC-3′ |
|
| R |
5′-CTCCTTAATGTCACGCACGAT-3′ |
Determination of mtDNA content
Total genomic DNA was isolated from 2×106
TGF-β1-treated MCF-7 cells and at different times resuspended in
200-µl PBS using a Tissue DNA kit (Omega) according to the
manufacturer's protocol. mtDNA content was detected by RT-qPCR
using ABI Prism 7300 Sequence Detection Systems according to the
manufacturer's protocol (Applied Biosystems). RT-qPCR was conducted
following the standard protocol using SYBR Premix Ex Taq (Takara):
1 cycle at 95°C for 2 min, 40 cycles at 95°C for 30 sec, and 58°C
for 40 sec, followed by melt curve analysis. The D-loop content was
normalized by 18S rDNA. Specific primers were used to amplify the
fragment of the mitochondrial D-loop region:
5′-GGGGAAGCAGATTTGGGTAC-3′ (forward) and 5′-AGGGTGGGTAGGTTTGTTGG-3′
(reverse), and the fragment of the nuclear genomic 18S rDNA gene
region: 5′-CAGGAAGGAAGGCTGGAAG-3′ (forward) and
5′-CGGGAAATCGTGCGTGAC-3′ (reverse).
Immunohistochemical (IHC)
staining
Immunohistochemistry was performed using a
horseradish peroxidase (HRP) detection system (Beyotime Institute
of Biotechnology). The breast cancer and non-cancerous breast
tissue samples were immediately fixed in 10% neutral-buffered
formalin at room temperature for 24–48 h, and subsequently embedded
in paraffin. The paraffin-embedded tissues were sectioned
continuously at 4 µm. The slides were heated at 60°C for
deparaffination for 1 h, and endogenous peroxidase was blocked by
immersion in hydrogen peroxide for 10 min, followed by microwave
pretreatment for antigen retrieval. Then, the slides were washed in
PBS and incubated with E-cadherin and vimentin antibodies (1:1,000;
Cell Signaling Technology, Inc.) at 37°C for 1 h. The tissue
sections were washed and incubated for 15 min at room temperature
with preabsorbed biotinylated secondary antibodies specific for the
types of primary antibodies used. The staining was visualized using
3,3′-diaminobenzidine (DAB) substrate-chromogen (Zymed
Laboratories), and the slides were counterstained with hematoxylin.
At high microscopy magnification (×400), five representative fields
of view were selected, and the proportion of positively staining
cells was counted in each visual field. The staining intensity
scores were assigned as follows: 0 (negative), 1 (weak), 2
(moderate) and 3 (strong). The scores for the percentage of
positive tumor cells were determined as follows: 1 (0-25%), 2
(26-50%), 3 (51-75%) and 4 (76-100%). The immunoreactive score
(IRS) of each section was calculated by the product of the staining
intensity and the percentage of tumor cells. According to the IRS,
the staining patterns were divided into three classes: weak (IRS:
0–2), moderate (IRS: 2–4), and strong (IRS: 4–6).
Adenosine triphosphate
measurements
A total of 3×105 cells were collected and
resuspended in PBS. Then, 120 µl of lysis buffer from ATP detection
kit was added to the cells and incubated on ice for 30 min at 4°C.
The cell lysate was centrifuged at 1,000 × g for 10 min at 4°C,
after which ATP levels in the supernatant were measured using an
ATP detection kit (Beyotime). Bioluminescence was assessed on a
LUMAT LB9507 (EG&G Berthold Technologies Inc.) and an ATP assay
kit (Beyotime) was used to measure ATP levels in the supernatant.
The relative light units (RLUs) were measured with a Pi-l02 ATP
fluorometer (Hygiena), and a standard curve was calculated to
determine the ATP concentration (nmol/l). ATP content was
normalized by using a BCA protein assay kit (Dingguo).
Matrigel invasion assay
Cell invasion was evaluated in Transwell chambers
coated with 50 µl of Matrigel (BD Biocoat) in serum-free DMEM at a
dilution of 1:4. The upper and lower chambers were separated by
polycarbonate membranes with 8-µm pores (24-well inserts; Corning).
MCF-7 cells were pretreated with TGF-β1 (2 ng/ml) for 24 h. Then,
the cells were collected and resuspended in serum-free DMEM and
5×104 cells were seeded in the upper chamber. The lower
chambers contained 600 µl of DMEM with 10% FBS as a
chemoattractant. After incubating the cells for 24 h in a 37°C
incubator, cells on the upper surface were removed with a cotton
swab, and the invaded cells were fixed and stained with 0.5%
crystal violet for 15 min and counted in five random fields at ×400
magnification.
Western blot analysis
Cells were washed with ice-cold PBS and lysed with
cell lysis buffer (Beyotime) containing protease inhibitors
(Beyotime), and protein concentrations were quantified with an
enhanced BCA protein assay kit (Dingguo). The total protein
concentration of the extracted sample was adjusted to 2 µg/ml.
Total protein (20 µg) were separated by 12% SDS-PAGE, and
immunoblotting was performed on polyvinylidene fluoride (PVDF)
membranes (Millipore). The membrane was blocked with 5% fat-free
milk in PBS for 2 h at room temperature and then incubated with
primary antibodies at 4°C overnight. After 3 washes with
Tris-buffered saline with Tween-20 (TBST), the PVDF membranes were
incubated with secondary antibodies conjugated with HRP (1:1,000,
Beyotime) for 2 h at room temperature. The antigen-antibody
complexes were visualized with Immobilon Western Chemiluminescent
HRP Substrate (Millipore). Then, the blots were imaged with a Tanon
5200 chemiluminescence imaging system.
Statistical analysis
Results are presented as the mean ± standard error
(means ± SE) obtained from at least three independent experiments.
Statistical significance was determined by the independent paired
t-test or one-way analysis of variance with Dunnett's post hoc
analysis using SPSS software (version 19.0; IBM Corp.). Pearson's
correlation analysis was used to analyze the association of protein
expression in breast cancer tissues. In all the statistical
analyses, P<0.05 was considered to indicate a statistically
significant difference and all P-values were two-sided.
Results
TGF-β1 induces EMT in MCF-7 cells
Findings have shown that TGF-β1 induces EMT in lung
cancer cells (12). To investigate
the changes in energy metabolism during EMT in breast cancer cells,
we constructed a TGF-β1-induced EMT MCF-7 cell model. RT-qPCR was
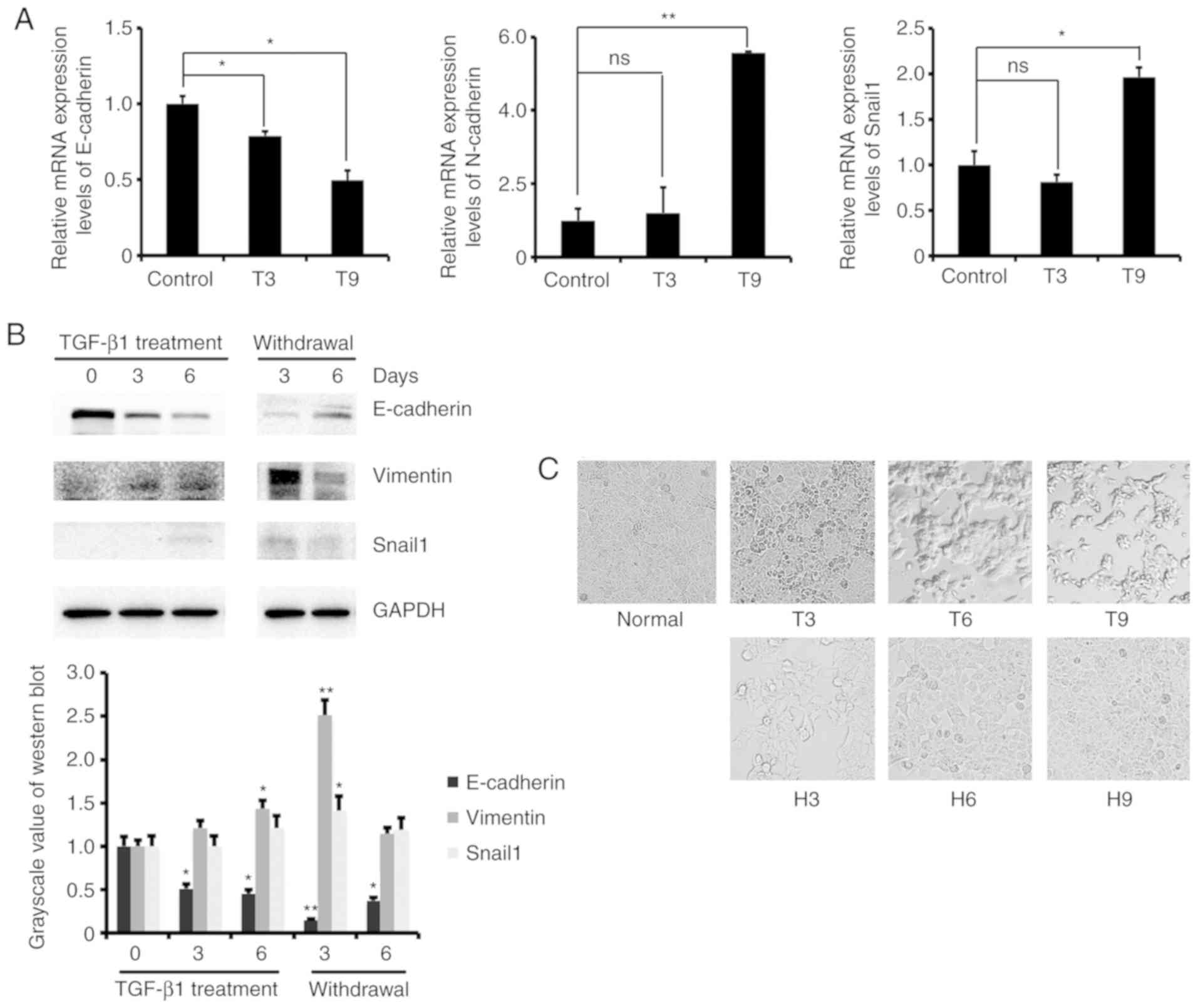
initially used to detect the mRNA expression levels of EMT marker
genes in MCF-7 cells after 3 and 9 days of treatment with 2 ng/ml
TGF-β1. The results showed that the mRNA expression level of
E-cadherin decreased, and the expression of N-cadherin and Snail1
increased (Fig. 1A). We also
examined the protein expression levels of EMT marker genes in MCF-7
cells treated with TGF-β1 for 0, 3, and 6 days and further cultured
them withdrawal TGF-β1 for 3 and 6 days. The results showed that
the protein expression level of E-cadherin decreased and N-cadherin
and Snail1 increased after TGF-β1 treatment. The opposite results
were obtained after removal of TGF-β1 (Fig. 1B). These results indicated that
TGF-β1 regulates the expression of EMT marker genes to promote the
transformation of MCF-7 cells from epithelial to mesenchymal cells.
In addition, we observed changes in cell morphology during the
induction of EMT by TGF-β1. As the induction time increased, the
cells changed from a round shape to a long strip shape, and the
cell adherence ability was reduced. After switching to normal
medium, the original adherence ability of the cells was restored
(Fig. 1C). These results confirmed
that low concentrations of TGF-β1 induced MCF-7 cells to transform
from epithelial to mesenchymal cells.
Decreased cell proliferation,
increased cell invasion and ATP levels during TGF-β1-induced
EMT
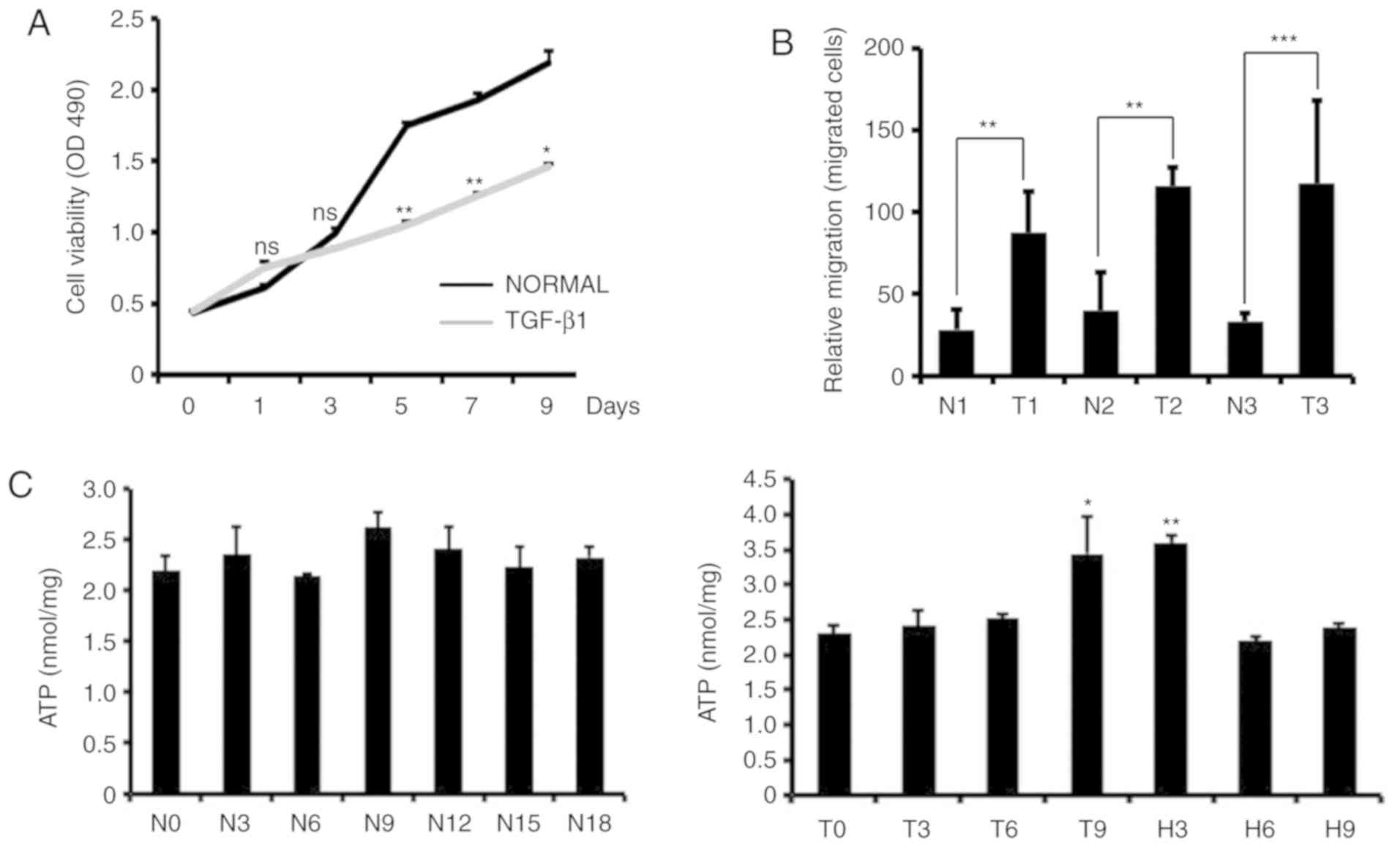
Previous findings have shown that TGF-β1 affects
cell proliferation, differentiation and EMT (13). We examined changes in cell
proliferation, invasion and intracellular ATP levels in
TGF-β1-treated MCF-7 cells. We used MTS to detect the proliferative
capacity of the cells, and the results showed that the addition of
TGF-β1 to MCF-7 cells significantly reduced the growth rate of the
cells (Fig. 2A). Through cell
invasion experiments, we found that TGF-β1-treated MCF-7 cells were
more invasive than control cells (Fig.
2B). We also measured intracellular ATP levels in MCF-7 cells
at different times after TGF-β1-induced EMT and TGF-β1 removal. We
found that there was no significant increase in the intracellular
ATP content after TGF-β1 treatment for 3 or 6 days. ATP levels
increased significantly on the 9th day of TGF-β1 treatment, while
the ATP content decreased after the removal of TGF-β1, and the
intracellular ATP content did not change significantly with
increasing culture time in the control cells (Fig. 2C).
Increased fatty acid oxidation and
decreased fatty acid synthesis in TGF-β1-induced EMT MCF-7
cells
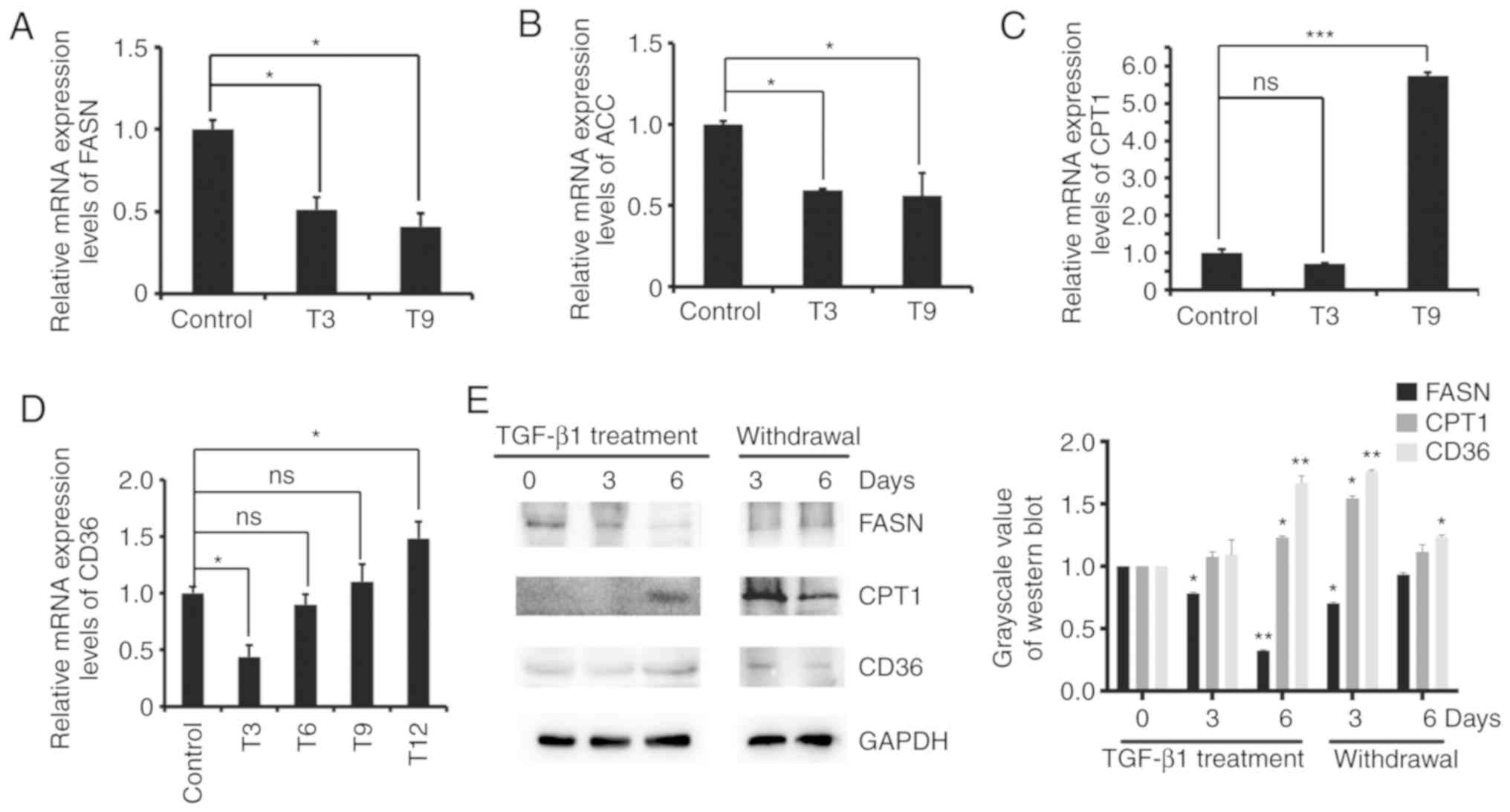
Previous data showed that MCF-7 cells have increased
ATP content during EMT, indicating that the metabolic pathway may
be changed during EMT. We explored the relationship between EMT and
lipid metabolism. We examined the mRNA and protein expression of
lipid metabolism-related genes in MCF-7 cells after TGF-β1-induced
EMT. The RT-qPCR results showed that the expression levels of CPT1
and CD36 mRNA increased after TGF-β1 treatment of MCF-7 cells,
while the mRNA expression levels of FASN and ACC decreased
(Fig. 3A-D). Western blot analysis
revealed that the protein expression levels of CPT1 and CD36
increased, and the protein expression levels of FASN decreased
(Fig. 3E). These results indicated
that cellular fatty acid β-oxidation increased and fatty acid
synthesis decreased in MCF-7 cells following EMT.
Increased OXPHOS activity during
TGF-β1-induced EMT
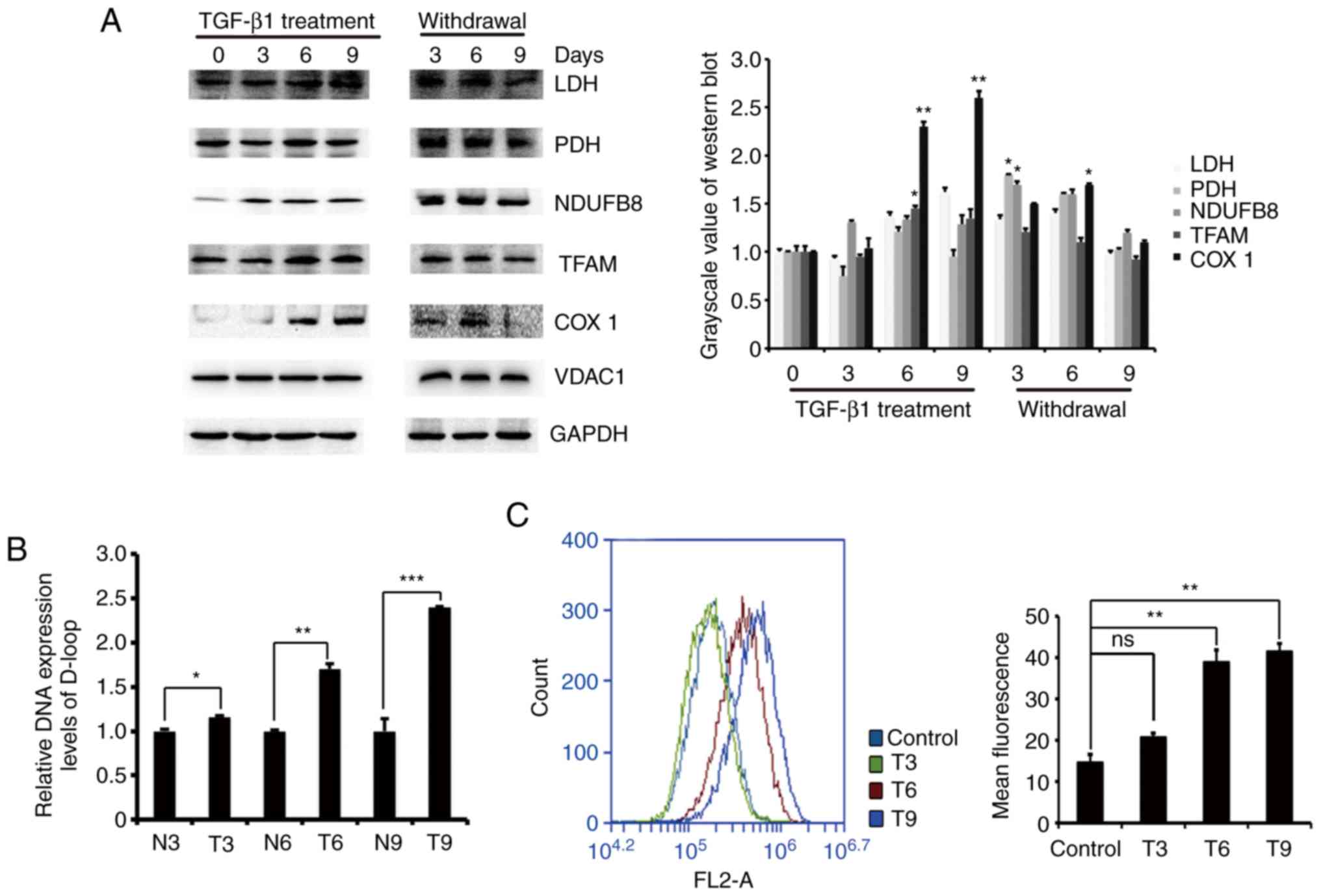
We examined changes in the cellular glucose
metabolism pathway and OXPHOS activity. We examined the enzymes
lactate dehydrogenase (LDH) and pyruvate dehydrogenase (PDH), which
are associated with glycolysis and the tricarboxylic acid (TCA)
cycle, and the expression of mitochondrial oxidative
phosphorylation-related proteins NDUFB8, COXI, and TFAM by western
blotting. The results showed that the protein expression of LDH and
PDH increased after TGF-β1 treatment of MCF-7 cells, and the
protein expression of NDUFB8, COXI and TFAM also increased compared
to that in the control. However, the protein expression decreased
after removal of TGF-β1 (Fig. 4A).
This indicates that glycolysis and the TCA cycle were enhanced.
Subsequently, we examined changes in mtDNA copy number in MCF-7
cells during EMT. The gene content of the mitochondrial D-loop
region and the 18S RNA gene were amplified by RT-qPCR, and it was
found that the ratio of the mitochondrial D-loop region gene to the
18S RNA gene was significantly increased compared with that of the
control cells (Fig. 4B).
Fluorescent staining of mitochondrial ROS and flow cytometry
analysis revealed an increase in mitochondrial ROS levels after
TGF-β1 treatment (Fig. 4C). These
results indicated that intracellular mitochondrial OXPHOS activity
increased in TGF-β1-induced EMT.
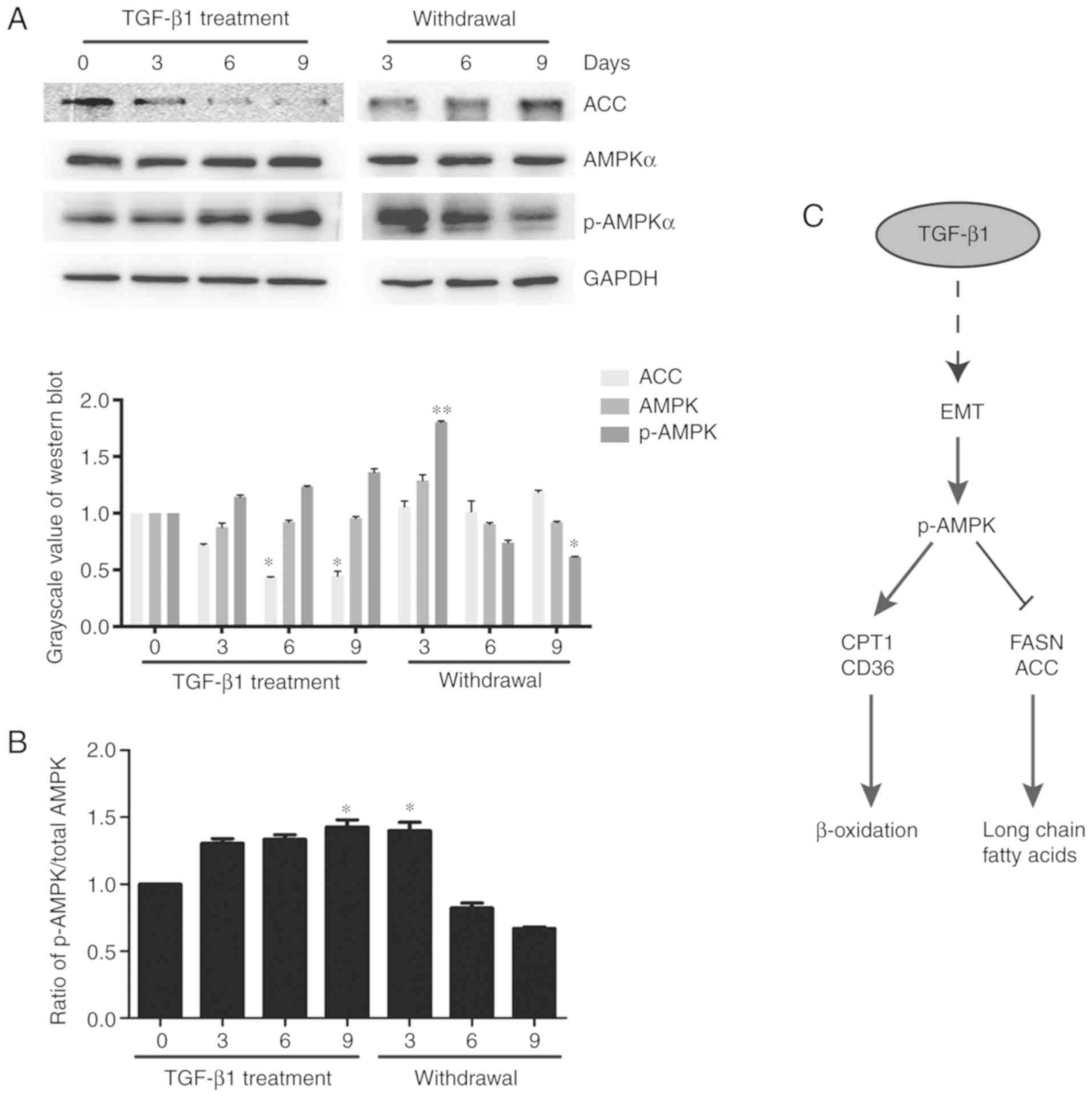
The above research shows that fatty acid oxidation,
oxidative phosphorylation activity and ATP levels are increased in
MCF-7 cells undergoing TGF-β1-induced EMT. It has been reported
that AMPK is an energy receptor for tumor progression; in addition,
the AMPK pathway also plays an important role in lipid metabolism
regulation (14). Our study found
that p-AMPK protein did not change before 6 days of
TGF-β1-treatment, but p-AMPK levels in treated cells were
significantly higher than those in control cells on the 9th day.
Moreover, the expression of ACC was decreased (Fig. 5A). The ratio of p-AMPK to total AMPK
also showed an increase in p-AMPK expression level at 9 days
(Fig. 5B). Therefore, we
hypothesized that TGF-β1 induces EMT in MCF-7 cells and that AMPK
is activated. In addition, p-AMPK inhibited the expression of FASN
and ACC, increased the expression of CPT1 and CD36, and enhanced
fatty acid β-oxidation, thereby, producing a large amount of ATP to
facilitate breast cancer cell metastasis (Fig. 5C).
Increased fatty acid synthesis in
epithelial cells and increased fatty acid oxidation in mesenchymal
cells in breast cancer tissue
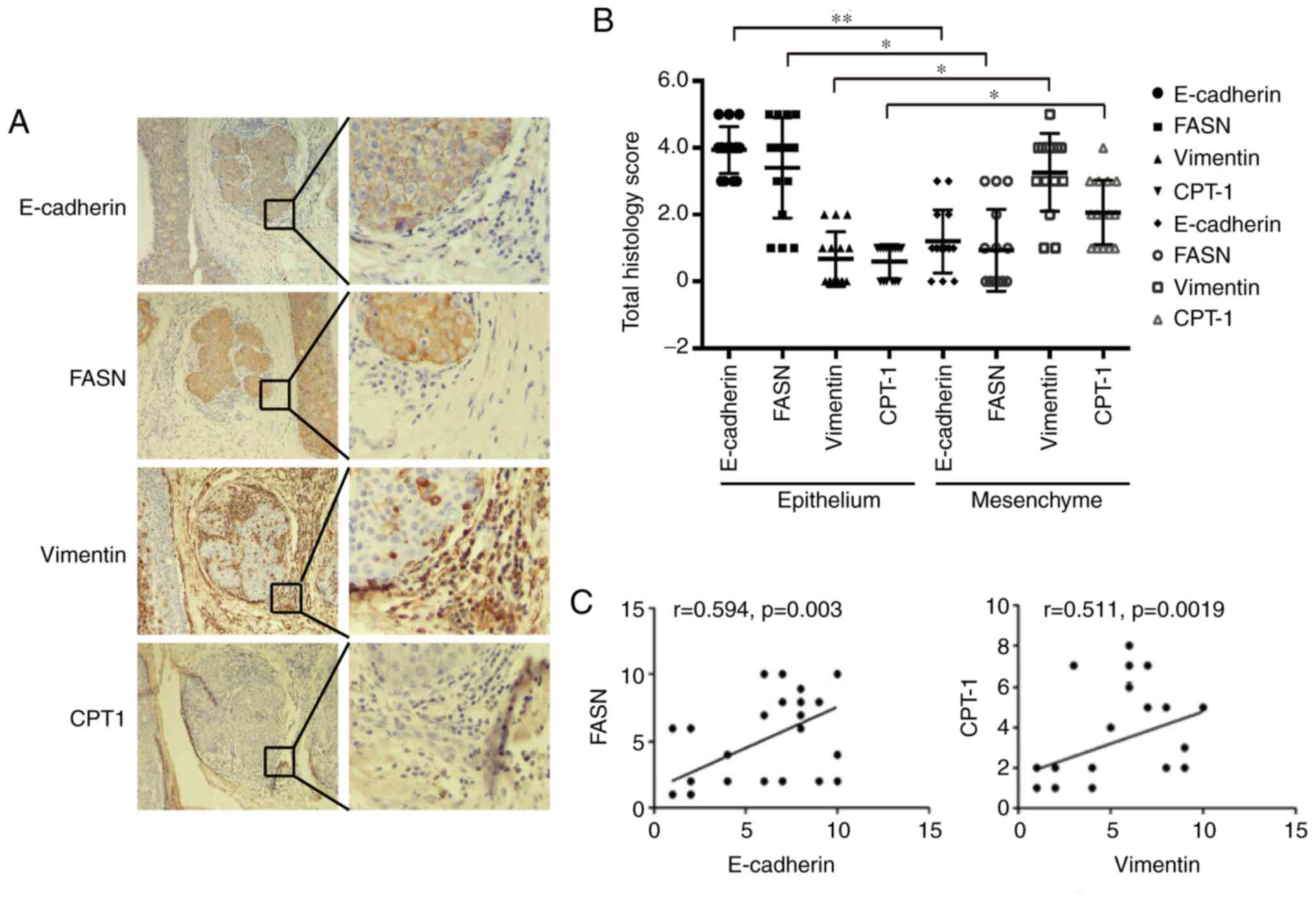
Cellular experiments showed an increase in fatty
acid β-oxidation and a decrease in fatty acid synthesis in the EMT
of MCF-7 cells; thus, we examined metabolic pathway changes in
breast cancer tissue. We used the epithelial cell marker E-cadherin
and the mesenchymal cell marker vimentin for immunohistochemical
staining in breast cancer pathological samples. The results showed
that E-cadherin was expressed in epithelial cells, and vimentin was
expressed in mesenchymal cells. Then, we examined the expression of
FASN and CPT1 in epithelial and mesenchymal cells. The results
showed that FASN was highly expressed in epithelial cells, and
there were high CPT1 expression levels in interstitial cells. At
the same time, the Pearson's correlation analysis shows that
E-cadherin is significantly positively correlated with FASN
expression (P<0.05, R>0) and vimentin is positively
correlated with CPT1 expression (P<0.05, R>0; Fig. 6A-C). These results indicated that
epithelial and mesenchymal cells have different lipid metabolic
pathways in breast cancer tissues. Epithelial cells mainly undergo
fat synthesis, while mesenchymal cells mainly undergo lipolysis in
breast cancer tissues, suggesting that mesenchymal cells need to
obtain more energy through lipolysis to support cell breast cancer
metastasis in breast cancer tissues.
Discussion
Reprogrammed metabolism has been identified as a
hallmark of cancer cells (15).
Most tumor cells are in a state of hypoxia and nutrient deficiency,
and tumor cells must adapt their metabolism to survive and
proliferate in harsh microenvironments (16). Cancer cells may use glucose, ketone
bodies, glutamine and lactic acid as fuel sources. Lipids are also
used as an important energy source in some tumor tissues (17). Chen et al, reported that the
lipid metabolism activity of ovarian cancer cells is enhanced in
ascites microenvironment culture (18). Lipids affect the ability of cancer
stem cells (CSCs) to self-renew (19). In our study, TGF-β1 induced EMT in
MCF-7 cells, and we detected an increase in CPT1 and CD36
expression at 9 and 12 days after TGF-β1 treatment. A significant
increase in cellular ATP levels was also detected at 9 days after
TGF-β1 treatment. The results showed that the process of
TGF-β1-induced EMT promoted the increase of fatty acid oxidation
pathway to provide energy for breast cancer metastasis. Therefore,
lipid metabolism plays an important role in the occurrence and
metastasis of cancer.
Cancer metastasis is a prominent feature of cancer
cells and is responsible for most cancer-associated mortality.
Epithelial-mesenchymal transition (EMT) plays an essential role in
the initiation and development of cancer metastasis. Studies showed
that the acidic microenvironment derived from cancer cell
metabolism induces the EMT phenotype and cancer metastasis
(20,21). Glycolytic enzymes (PKM2, LDHA)
promoted the mesenchymal transition of cancer cells by regulating
the expression of transcriptional factors (22,23).
In addition, EMT conversely regulates cancer metabolism (24,25).
However, few studies are available on fatty metabolism during EMT.
In the present study, we detected changes in the expression of
fatty metabolism-related enzymes during TGF-β1-induced EMT. The
results found that the expression of FASN and ACC decreased during
EMT. While the expression of CPT1 and CD36 decreased after 3 days
of TGF-β1 treatment, the expression levels were significantly
higher in CPT1 in T9 and in CD36 in T12 than in the control. At the
same time, ATP levels also increased significantly at 9 days of
TGF-β1 treatment. We demonstrated that in the first 3 days of
TGF-β1 induced EMT, both the fatty acid anabolism and catabolism
pathways were reduced, while in the later stage of EMT, the
cellular catabolism of fatty acids was significantly enhanced.
AMP-activated protein kinase (AMPK) is a conserved
sensor of cellular energy change that is expressed ubiquitously in
eukaryotic cells (26). AMPK
responds to changes in intracellular adenine nucleotide levels and
is activated by an increase in the ratio of AMP/ADP relative to ATP
(27). Furthermore, AMPK is very
important in breast cancer glycolysis and glycogen catabolism.
Findings have shown that, the glycolysis inhibitor 2-deoxyglucose
(2-DG) activates the expression of AMPK by decreasing the ATP
content, thus contributing to the recycling of ATP in MCF-7 cells
(28). In addition, it has been
reported that p-AMPK regulates the fatty acid metabolism pathway.
When the ATP level is low, AMPK is activated, and p-AMPK inhibits
ACC expression (29,30). In the present study, we detected
that p-AMPK increased significantly at 9 days of TGF-β1 treatment.
Thus, changes in energy levels occur during TGF-β1-induced EMT in
MCF-7 cells. At the later stages of epithelial to mesenchymal
transition, ATP was consumed, ATP content decreased, p-AMPK was
activated, and fatty acid oxidation and oxidative phosphorylation
were promoted. ATP was produced, and cells provided energy for the
distant metastasis of cancer. However, whether the energy level
during breast cancer EMT is a signal to regulate lipid metabolism
needs further verification. Further studies should investigate the
effect of ATP level in the regulation of fatty acid oxidation
during EMT in breast cancer including the AMPK inhibitor
experiment.
In conclusion, to the best of our knowledge, this
study has shown, for the first time, that fatty acid β-oxidation
and oxidative phosphorylation may be regulated by the p-AMPK
pathway during EMT. Fatty acids act as an energy source to promote
breast cancer cell metastasis. The results of the present study are
important for understanding the relationship between cancer
metabolism and metastasis during EMT in breast cancer cells.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National
Nature Science Foundation of China (grant nos. 31760331, 31260276,
31601155, 81860531, 81660583, 81560458 and 81760507), the Science
and Technology Innovation Team of Yunnan Province (2011CI123) and
Dali University (ZKPY2019308), the Reserve Talents of Young and
Middle-aged Academic and Technical Leaders of Yunnan Province
(grant no. 2017HB077), the Top Young Talents of Ten Thousand
Talents Plan of Yunnan Province (grant no. 2019), and the Key
Project of Science and Technology Department of Yunnan Province
(grant no. 2020).
Availability of data and materials
The datasets used or analyzed in the present study
are available from the corresponding author upon reasonable
request.
Authors' contributions
QQL, MY and WX conceived and designed the
experiments. QQL, HYH, SA, XXY, TL, LY and ZYF performed the
experiments. ZQZ, LD, QHC and JL were responsible for the
collection and assembly of data. QQL and SA analyzed the data and
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures were conducted in accordance with
standard guidelines for the Study of Ethics Committee of Yunnan
University (Kunming, China), and written informed consent was
obtained. Prior to study inclusion, written informed consent was
obtained from all the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anastasiadi Z, Lianos GD, Ignatiadou E,
Harissis HV and Mitsis M: Breast cancer in young women: An
overview. Updates Surg. 69:313–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medeiros B and Allan AL: Molecular
mechanisms of breast cancer metastasis to the lung: Clinical and
experimental perspectives. Int J Mol Sc. 20:22722019. View Article : Google Scholar
|
|
3
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu J, Li G, Zhang P, Zhuang X and Hu G: A
CD44v+ subpopulation of breast cancer stem-like cells
with enhanced lung metastasis capacity. Cell Death Dis.
8:e26792017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu X, Yan Q, Wang Y and Dong X: NTN4 is
associated with breast cancer metastasis via regulation of
EMT-related biomarkers. Oncol Rep. 37:449–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pastushenko I, Brisebarre A, Sifrim A,
Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D,
Moers V, Lemaire S, et al: Identification of the tumour transition
states occurring during EMT. Nature. 556:463–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ungefroren H, Witte D and Lehnert H: The
role of small GTPases of the Rho/Rac family in TGF-β-induced EMT
and cell motility in cancer. Dev Dyn. 247:451–461. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu FL, Mo EP, Yang L, Du J, Wang HS,
Zhang H, Kurihara H, Xu J and Cai SH: Autophagy is involved in
TGF-β1-induced protective mechanisms and formation of
cancer-associated fibroblasts phenotype in tumor microenvironment.
Oncotarget. 7:4122–4141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang R and Zong X: Aberrant cancer
metabolism in epithelial-mesenchymal transition and cancer
metastasis: Mechanisms in cancer progression. Crit Rev Oncol
Hematol. 115:13–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen XL, Lei L, Hong LL and Ling ZQ:
Potential role of NDRG2 in reprogramming cancer metabolism and
epithelial-to-mesenchymal transition. Histol Histopathol.
33:655–663. 2018.PubMed/NCBI
|
|
12
|
Tan TZ, Miow QH, Miki Y, Noda T, Mori S,
Huang RY and Thiery JP: Epithelial-mesenchymal transition spectrum
quantification and its efficacy in deciphering survival and drug
responses of cancer patients. EMBO Mol Med. 6:1279–1293. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Syed V: TGF-β Signaling in Cancer. J Cell
Biochem. 117:1279–1287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim I and He YY: Targeting the
AMP-activated protein kinase for cancer prevention and therapy.
Front Oncol. 3:1752013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiarugi P and Cirri P: Metabolic
exchanges within tumor microenvironment. Cancer Lett. 380:272–280.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gouirand V, Guillaumond F and Vasseur S:
Influence of the tumor microenvironment on cancer cells metabolic
reprogramming. Front Oncol. 8:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng S, Wang G, Wang Y, Cai L, Qian K, Ju
L, Liu X, Xiao Y and Wang X: Fatty acid oxidation inhibitor
etomoxir suppresses tumor progression and induces cell cycle arrest
via PPARγ-mediated pathway in bladder cancer. Clin Sci (Lond).
133:1745–1758. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen RR, Yung MMH, Xuan Y, Zhan S, Leung
LL, Liang RR, Leung THY, Yang H, Xu D, Sharma R, et al: Targeting
of lipid metabolism with a metabolic inhibitor cocktail eradicates
peritoneal metastases in ovarian cancer cells. Commun Biol.
2:2812019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Condello S, Thomes-Pepin J, Ma X,
Xia Y, Hurley TD, Matei D and Cheng JX: Lipid desaturation is a
metabolic marker and therapeutic target of ovarian cancer stem
cells. Cell Stem Cell. 20:303–314.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klein M, Seeger P, Schuricht B, Alper SL
and Schwab A: Polarization of Na(+)/H(+) and Cl(−)/HCO (3)(−)
exchangers in migrating renal epithelial cells. J Gen Physiol.
115:599–608. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lagana A, Vadnais J, Le PU, Nguyen TN,
Laprade R, Nabi IR and Noël J: Regulation of the formation of tumor
cell pseudopodia by the Na(+)/H(+) exchanger NHE1. J Cell Sc.
113:3649–3662. 2000.
|
|
22
|
Fan FT, Shen CS, Tao L, Tian C, Liu ZG,
Zhu ZJ, Liu YP, Pei CS, Wu HY, Zhang L, et al: PKM2 regulates
hepatocellular carcinoma cell epithelial-mesenchymal transition and
migration upon EGFR activation. Asian Pac J Cancer Prev.
15:1961–1970. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arseneault R, Chien A, Newington JT,
Rappon T, Harris R and Cumming RC: Attenuation of LDHA expression
in cancer cells leads to redox-dependent alterations in
cytoskeletal structure and cell migration. Cancer Lett.
338:255–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Wang X, Zhang J, Lam EK, Shin VY,
Cheng AS, Yu J, Chan FK, Sung JJ and Jin HC: Warburg effect
revisited: An epigenetic link between glycolysis and gastric
carcinogenesis. Oncogene. 29:442–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang L, Hou Y, Yuan J, Tang S, Zhang H,
Zhu Q, Du YE, Zhou M, Wen S, Xu L, et al: Twist promotes
reprogramming of glucose metabolism in breast cancer cells through
PI3K/AKT and p53 signaling pathways. Oncotarget. 6:25755–25769.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hardie DG, Schaffer BE and Brunet A: AMPK:
An energy-sensing pathway with multiple inputs and outputs. Trends
Cell Biol. 26:190–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carling D: AMPK signalling in health and
disease. Current opinion in cell biology. 45:31–37. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y, Sarkissyan M, McGhee E, Lee S and
Vadgama JV: Combined inhibition of glycolysis and AMPK induces
synergistic breast cancer cell killing. Curr Opin Cell Biol.
151:529–539. 2015.
|
|
29
|
Liu X, Chhipa RR, Nakano I and Dasgupta B:
The AMPK inhibitor compound C is a potent AMPK-independent
antiglioma agent. Mol Cancer Ther. 13:596–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mauro L, Naimo GD, Gelsomino L, Malivindi
R, Bruno L, Pellegrino M, Tarallo R, Memoli D, Weisz A, Panno ML
and Andò S: Uncoupling effects of estrogen receptor alpha on
LKB1/AMPK interaction upon adiponectin exposure in breast cancer.
FASEB J. 32:4343–4355. 2018. View Article : Google Scholar : PubMed/NCBI
|