Introduction
Pancreatic cancer is the one of the major lethal
malignant neoplasms; it has a high incidence rate worldwide, and it
can cause long hospital stays and high costs (1). The estimation of the 5-year survival
rate for pancreatic cancer is approximately 5% (2). Certain risk factors leading to
pancreatic cancer have been identified, such as cigarette smoking,
positive family history, genetics, diabetes mellitus, obesity,
dietary factors, alcohol use as well as physical inactivity
(3).
Over the past several decades, emerging research has
focused on identifying the underlying mechanisms of pancreatic
cancer (4,5). However, to date, the investigations of
the specific mechanisms, are not satisfactory (1,2,6,7).
Therefore, there is an urgent need to improve the understanding of
pancreatic cancer.
Centromere protein M (CENPM) has been reported as a
novel biomarker of hepatocellular carcinoma (8), melanoma (9) and bladder cancer (10). However, the function of CENPM has
not been studied in pancreatic cancer. The CENPM gene (11,12)
encodes the centromere protein M. CENPM is recruited by CENPA
nucleosomes and is assembled into CENPA nucleosome-associated
complex (NAC) (13). CENPA NAC
plays important roles in kinetochore assembly, mitotic progression
regulation and chromatin complex formation (14). As is commonly known, the
proliferation of cancer cells is closely related to mitosis and
chromosome separation (12). Thus,
the CENPM gene was selected as the target of our research.
In the present study, it was revealed that CENPM
expression was upregulated in both pancreatic carcinoma and
pancreatic ductal adenocarcinoma compared with normal tissues (data
from the ‘Pei Pancreas’ and ‘Grutzmann Pancreas’ databases)
(15,16). Whether the capacity of migration and
invasion of pancreatic cancer would be influenced by CENPM is
unknown. Hence, PANC-1 and CFPAC-1 were selected to determine the
mechanism of CENPM in the proliferation and metastasis of
pancreatic cancer.
Materials and methods
Bioinformatics analyses of CENPM
The expression data for the CENPM gene in pancreatic
cancer patients were downloaded from the Oncomine online database
(https://www.oncomine.org/). This
database is a cancer microarray platform and can provide
translational bioinformatics services (17). Oncomine gene expression array
datasets include 715 independent datasets and >80,000 samples.
The datasets extracted from Oncomine were the Pei Pancreas and
Grutzmann Pancreas datasets (15,16).
In the Pei Pancreas datasets, there were 16 normal and 36
pancreatic carcinoma samples. In the Grutzmann Pancreas datasets,
there were 11 normal and 14 pancreatic ductal adenocarcinoma
samples. Patient overall survival analysis and disease-free
survival analyses were extracted from Gene Expression Profiling
Interactive Analysis (GEPIA, http://gepia.cancer-pku.cn/). Thanks to the work of
Professor Zefang Tang from Peking University (18), RNA sequencing expression data of
almost ten thousand cancers from TCGA and the GTEx datasets can be
analyzed using this web tool. In this pancreatic adenocarcinoma
dataset, the group cut-off level for CENPM expression was set as
50%. Samples with expression levels higher than this threshold were
considered as the high-expression cohort. The hazards ratio (HR)
was calculated based on the Cox PH Model. The dotted line shows the
95% confidence interval (CI).
Cell culture
hTERT-HPNE, BxPC-3, CFPAC-1, MIA PaCa-2, PANC-1 and
PATU-8988 cell lines were used to perform the experiments in the
present study. All cell lines were purchased from the Chinese
Academy of Sciences Cell Bank (China). DMEM with 4.5 g/l glucose
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.) was
used to culture HTERT-HPNE, MIA PaCa-2 and PANC-1 cell lines.
BxPC-3, CFPAC-1 and PATU-8988 cell lines were cultured in Roswell
Park Memorial Institute (RPMI)-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS, 100 U/ml penicillin
and 100 µg/ml streptomycin. Cells were incubated in a standard
atmosphere with 5% CO2 and at a temperature of 37°C.
RNA isolation and real-time
quantitative polymerase chain reaction (RT-qPCR)
Total RNA from cells was isolated according to the
RNAprep cell kit instructions (Tiangen Biotech Co, Ltd.). The
purity and concentration of RNA were examined by a nucleic acid
protein detector (DeNovix, Inc.). RNA samples were reverse
transcribed by a RevertAid First Strand kit (Thermo Fisher
Scientific, Inc.). qRT-PCR was performed using a 7500 Fast
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with SYBR Green Master (ROX) reagent (Roche Diagnostics). The
thermocycling conditions were as follows: Initial denaturation at
95°C for 10 min; 40 of cycles of denaturation for 15 sec at 95°C,
annealing for 1 min at 60°C and elongation for 1 min at 72°C; and
final extension for 10 min at 72°C. GAPDH was used for the
normalization of CENPM expression levels. The sequences of the
GAPDH primers used in the present study were forward
5′-GGACCTGACCTGCCGTCTAG-3′ and reverse 5′-GTAGCCCAGGATGCCCTTGA-3′.
The sequences of the CENPM primers used in the present study were
forward 5′-CTGGCGGACTCGATGCTCAAAG-3′ and reverse
5′-CGATTCACACTGGAGGGCAAAGG-3′. The results are presented with CT
values and were analyzed by the ΔΔCq method (19). Each sample was performed in
triplicate.
Cells transfection
Small interfering RNA (siRNA) for CENPM was designed
and generated by Shanghai GenePharma Co., Ltd. The sequences of the
negative controls (si-NC) were as follows: Sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. The sequences of the CENPM siRNAs were
as follows: CENPM-homo-172 (si-RNA1): Sense,
5′-GGACUCGAUGCUCAAAGAGTT-3′ and antisense,
5′-CUCUUUGAGCAUCGAGUCCTT-3′; and CENPM-homo-206 (si-RNA2): Sense,
5′-CUGAAGGUCCACUUGGCAATT-3′ and antisense,
5′-UUGCCAAGUGGACCUUCAGTT-3′. Cell interference was performed
according to the Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) protocol. Approximatelly 1×105 cells
of the PANC-1 and CFPAC-1 cell lines were seeded into 6-well plates
and cultured until they reached to 40–50% confluence. Lipofectamine
3000 reagent and siRNA were diluted in DMEM or RPMI-1640 medium and
then mixed at a ratio of 1:1, and subsequently they were incubated
at 37°C together for 15 min. The siRNA concentration of CENPM at
100 nmol/l was added to each well. Forty-eight hours later, cells
were harvested for subsequent experiments. Each transfection was
performed in triplicate.
Cell proliferation assay
Forty-eight hours after the siRNA transfection,
approximately 5,000 cells/well of PANC-1 and CFPAC-1 cells were
seeded into 96-well plates according to the Cell Counting Kit-8
(CCK-8; Dojindo Molecular Technologies, Inc.) protocol. Cell
proliferation assays were detected at 0, 24, 48, 72 and 96 h by a
Varioskan Flash multimode reader (Thermo Fisher Scientific, Inc.).
Approximately 500 cells/well of siRNA-transfected PANC-1 and
CFPAC-1 cells were seeded into 6-well plates and cultured with 10%
FBS medium. Cells were fixed with 4% paraformaldehyde at 4°C
(Solarbio Life Sciences) for 20 min when the colonies were evident
enough to be observed macroscopically. Colonies (consisting of at
least 50 cells) were observed by a light microscope at an ×100
magnification. The cells were then stained with 0.4% crystal violet
(Beyotime Institute of Biotechnology) at 37°C for 20 min. Images of
each well were captured using a camera (Canon). Each experiment was
performed in triplicate.
Cell cycle assay
si-NC and si-CENPM cells obtained were washed twice
using ice-cold PBS and were fixed in 70% ethanol at −20°C
overnight. Then, the cells were washed again and resuspended in 0.1
ml of staining buffer (FBS) (BD Pharmingen; BD Biosciences). After
washing the cells, 5 µl of 7-AAD (50 µg/ml) (BD Biosciences) was
added into the cell suspension and incubated in a dark room for 10
min at 37°C. Finally, a cell cycle assay was performed using a flow
cytometer (BD Accuri C6; BD Biosciences). The analysis software
used was ModFit version 5 (Verity Software House, Inc.).
Cell migration and invasion
assays
Approximately 5×104 cells/well of si-NC
or si-CENPM cells were seeded into Transwell chambers (with pore
size inserts of 8 µm) (Corning, Inc.). Due to the different
migration capacities of cells, PANC-1 cells migrated for 1 day,
while CFPAC-1 cells migrated for 3 days. In the invasion
experiments, Matrigel (Corning, Inc.) was diluted with DMEM or
RPMI-1640 medium at a ratio of 1:8 followed by incubation in the
chambers overnight. The cells were cultured in the upper chambers
and were then allowed to invade for 4 days for PANC-1 cells and 8
days for CFPAC-1 cells. All of the lower chambers were filled with
600 ml of DMEM or RPMI-1640 medium supplemented with 20% FBS. Upon
completion of migration or invasion, the chambers were fixed with
4% paraformaldehyde at 4°C for 20 min and stained with 0.4% crystal
violet at 37°C for 20 min carefully. Images were observed by light
microscope at an ×200 magnification, and five fields were randomly
selected for analysis. The analysis software uesd was VisionWorks
version 8 (UVP, Inc.).
Protein extraction and western blot
analysis
Seventy-two hours after siRNA transfection, cells
were collected and lysed in RIPA buffer (Beyotime Institute of
Biotechnology) supplemented with phenylmethanesulfonyl fluoride
(Sigma-Aldrich; Merck KGaA), PhosSTOP (Roche Diagnostics) and
DL-dithiothreitol (Beyotime Institute of Biotechnology). Cell
protein concentrations were measured using a BCA protein assay kit
(Beyotime Institute of Biotechnology) and equilibrated with PBS and
loading buffer. A total of 50 µg boiled protein per lane was
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred to a 0.45-µm
polyvinylidene difluoride (PVDF) membrane (EMD Millipore). The
membranes were incubated with 5% nonfat milk in Tris-buffered
saline (TBS), which contained 0.05% Tween-20, for 1 h at room
temperature. Each membrane was probed with various antibodies
overnight at 4°C. The following primary antibodies were used:
Anti-cyclin-dependent kinase (CDK)2 [product no. 18048S; Cell
Signaling Technology (CST), Inc.], anti-CDK6 (product no. 3136S;
CST), anti-cyclin D1 (product code ab134175; Abcam), anti-p21
(product no. 2947S; CST), anti-tubulin (product no. 2148S; CST),
anti-CENPM (cat. no. DF2314; Affinity), anti-mammalian target of
rapamycin (mTOR) (product code ab2732; Abcam), anti-phosphorylated
(p)-mTOR (Ser2448) (product no. 5536S; CST), anti-p70S6K (cat. no.
14485; ProteinTech Group, Inc.), anti-p-p70S6K (Thr421/Ser424)
(product no. 9204S; CST), and anti-GAPDH (product no. 2118S; CST).
All the antibodies were used at a dilution of 1:1,000. Then, the
membranes were incubated with HRP AffiniPure goat anti-rabbit IgG
(product no. BL003A; Biosharp) (1:5,000) for 1 h at room
temperature the following day. Protein bands were visualized using
Amersham Imager 600 (General Electric Company) and enhanced with
chemiluminescence (Thermo Fisher Scientific, Inc.). The analysis
software used was VisionWorks version 8.
Statistical analysis
All data were obtained from at least three
independent experiments. Data are presented as the mean values ±
SEM and were performed using t-tests or one-way ANOVA. Multiple
comparisons among the groups were performed using Sidak correction
method. P-values <0.05 were considered to indicate a
statistically significant difference. Statistical analyses were
achieved using SPSS 23.0 software (IBM, Corp.) and GraphPad Prism
version 7.01 (GraphPad Software, Inc.).
Results
CENPM is upregulated in pancreatic
cancer and associated with the survival rate
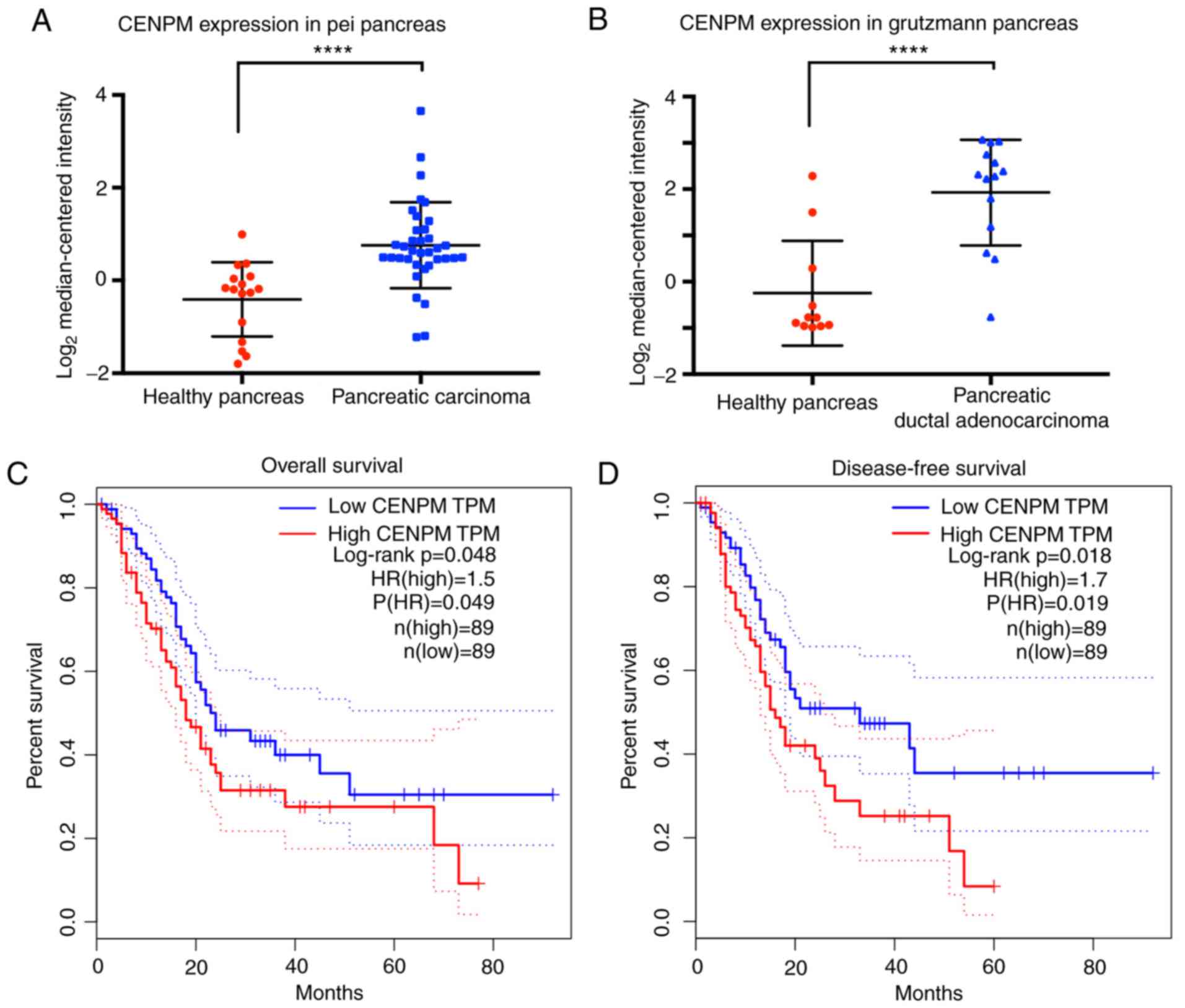
The data extracted from the Pei Pancreas database
and the Grutzmann Pancreas database (15,16)
revealed that the gene CENPM was significantly upregulated
(P<0.0001) in 50 cases of cancer patients with pancreatic
carcinoma or pancreatic ductal adenocarcinoma compared with 27
healthy cases (Fig. 1A and B).
Overall survival and disease-free survival analyses
(Fig. 1C and D) extracted from
GEPIA revealed that patients with high CENPM expression presented
decreased survival percentages (P<0.05). An HR value over 1.0
indicated that CENPM was positively associated to pancreatic cancer
and suggested that the CENPM gene may act as a potential cancer
therapeutic target in pancreatic cancer treatment.
CENPM expression levels in pancreatic
cancer cell lines
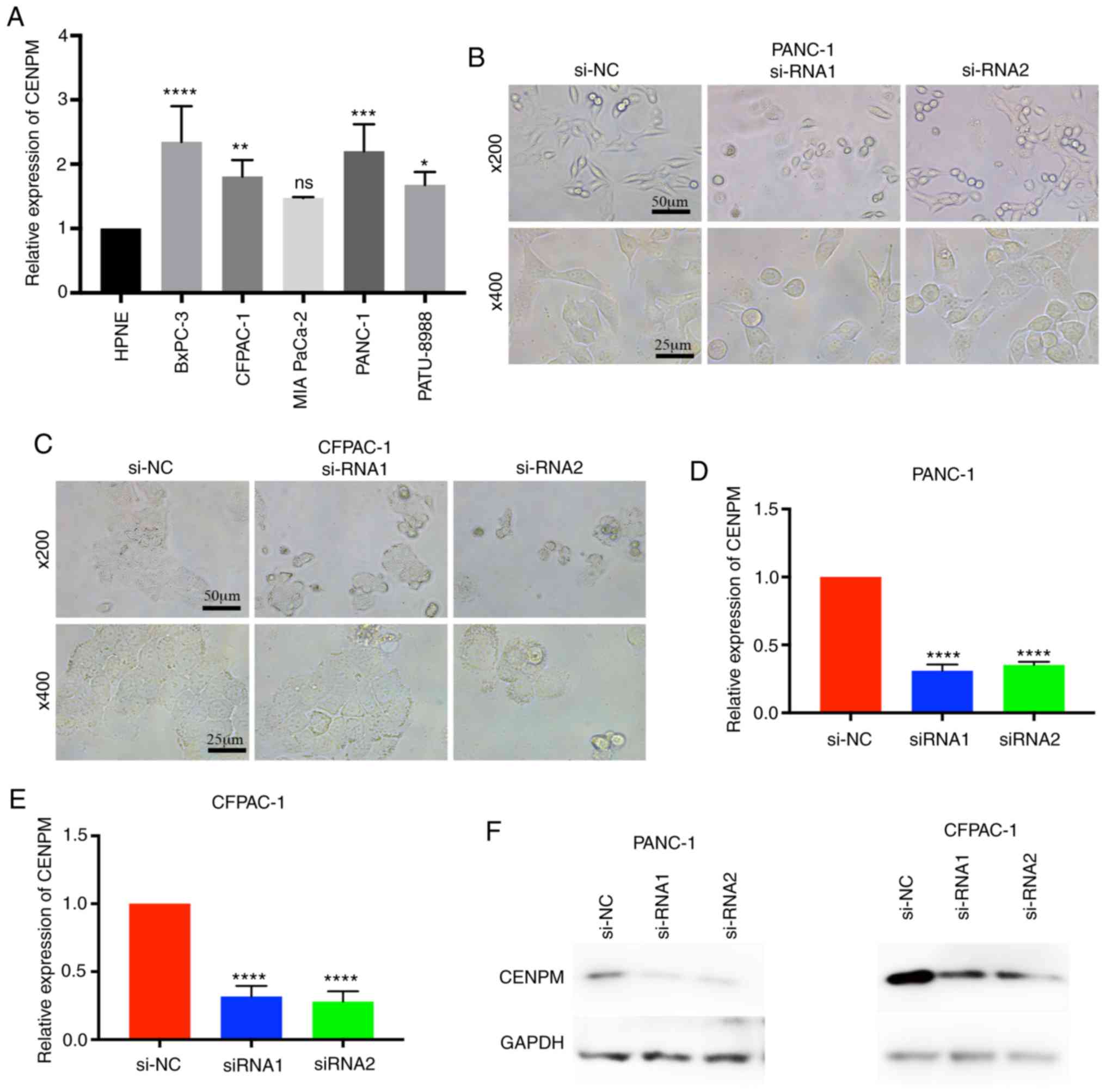
To determine whether CENPM was overexpressed in
pancreatic cancer cell lines, the relative expression level of
CENPM among various pancreatic cancer cell lines vs. the human
pancreatic nestin-expressing (hTERT-HPNE) cell line were
quantified. The data revealed that CENPM was overexpressed in
BxPC-3 (P<0.0001), CFPAC-1 (P<0.001), PANC-1 (P<0.0005)
and PATU-8988 (P<0.05) compared with hTERT-HPNE (Fig. 2A). In the present study, PANC-1 and
CFPAC-1 were selected for further research due to their relatively
higher expression levels and easy culture. Two effective siRNAs
were transfected to interfere in the expression of CENPM in these
two cell lines for subsequent experiments. With regard to the cell
morphologies of PANC-1 and CFPAC-1 cells it was observed that the
cells became round and underwent cell shrinkage when CENPM was
knocked down by siRNA (Fig. 2B and
C). The knockdown effect of the siRNA is presented in Fig. 2D-F. The inhibition levels of si-RNA1
and si-RNA2 in PANC-1 cells were 0.6908±0.01985 and 0.6486±0.02292
relative to si-NC (P<0.0001). The inhibition levels of si-RNA1
and si-RNA2 in CFPAC-1 were 0.6834±0.02452 and 0.7205±0.03211
relative to si-NC (P<0.0001).
CENPM gene knockdown inhibits
pancreatic cancer cell proliferation
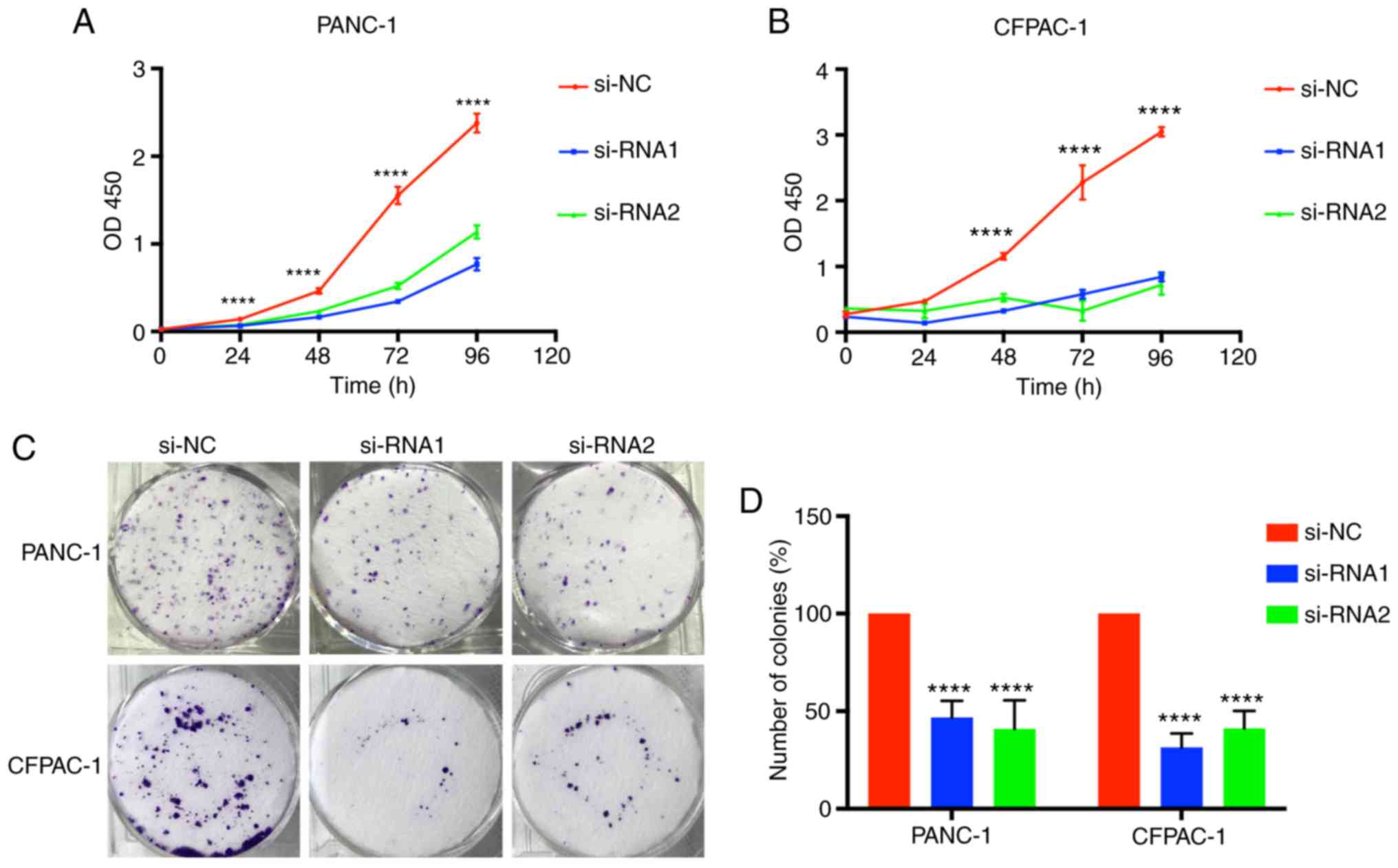
Tumor growth was associated with limitless
proliferation of tumor cells; therefore, considering the function
of CENPM in tumor cell proliferation was valuable. Time-dependent
proliferation of cells was calculated by CCK-8 assay, and the
values were analyzed at OD450 nm (Fig.
3A and B). Colony formation assays were also performed to
detect cell proliferation (Fig. 3C and
D). These results indicated that cell lines transfected with
si-RNA1 and si-RNA2 had significantly lower values (P<0.0001),
demonstrating that downregulation of CENPM could effectively
inhibit PANC-1 and CFPAC-1 proliferation compared with the si-NC
group.
CENPM gene knockdown affects the cell
cycle in pancreatic cancer cells
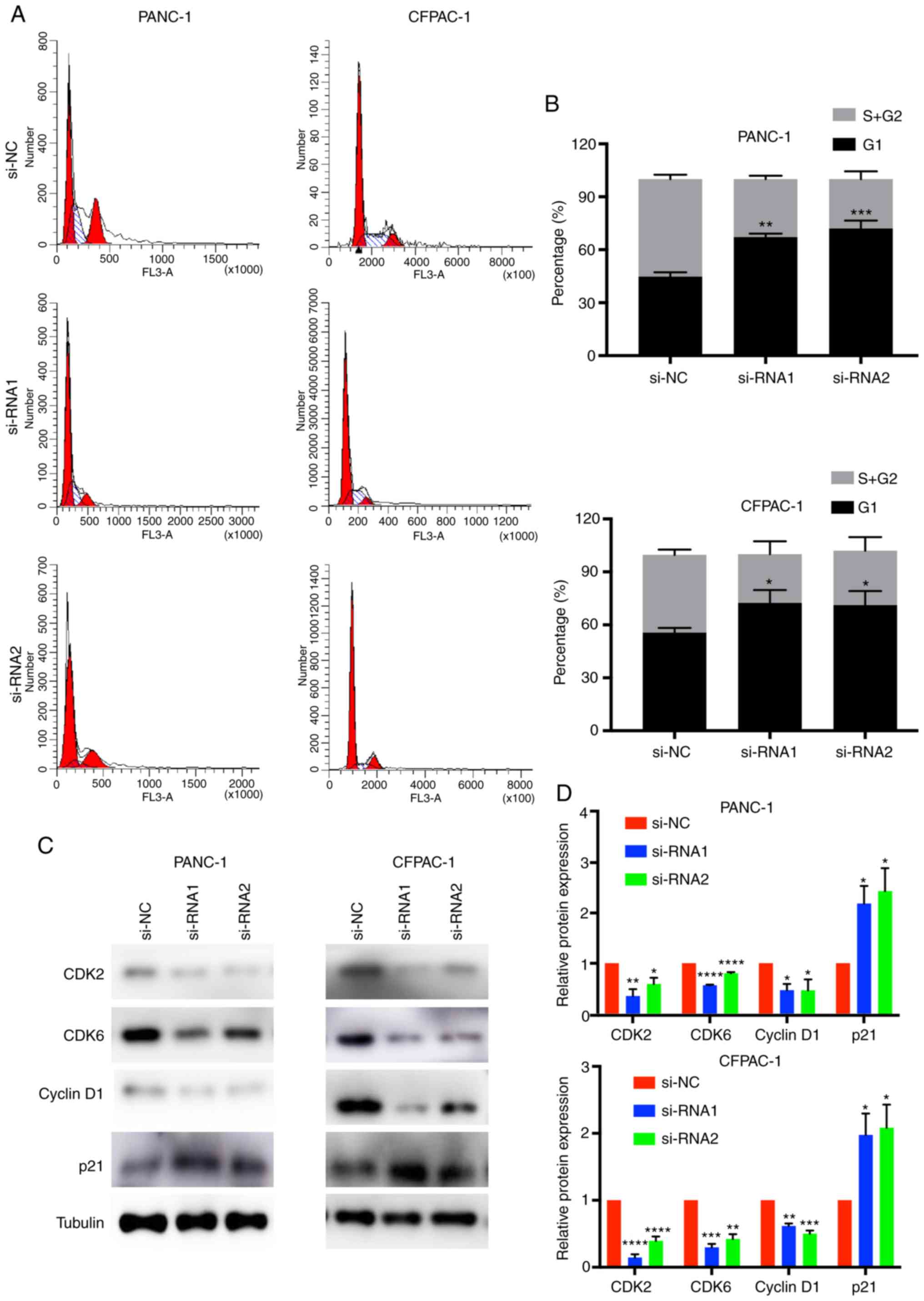
In order to better understand the effect of CENPM in
pancreatic cancer cells, cell cycle analysis was performed
(Fig. 4A and B). The results of
flow cytometric analysis revealed that the percentages of cells in
the G0/G1 phase of the CENPM-knockdown PANC-1 and CFPAC-1 cells
were higher than in the si-NC groups (P<0.05). In addition, the
percentages of the S+G2/M phase were decreased in the siRNA groups
(P<0.05).
CDKs are proteins involved in the cell cycle of
tumor cells (20). Cyclin D1 is a
cell cycle-related protein. p21 is a type of cyclin-dependent
kinase inhibitor (21). CDK2, CDK6,
cyclin D1 and p21 were detected by western blotting (Fig. 4C and D). The results revealed that
knockdown of CENPM decreased the expression levels of CDK2
(P<0.05), CDK6 (P<0.001), cyclin D1 (P<0.05) and increase
the expression levels of p21 (P<0.05). Collectively, low
expression of CENPM induced cell cycle arrest at the G1 phase in
pancreatic tumor cells.
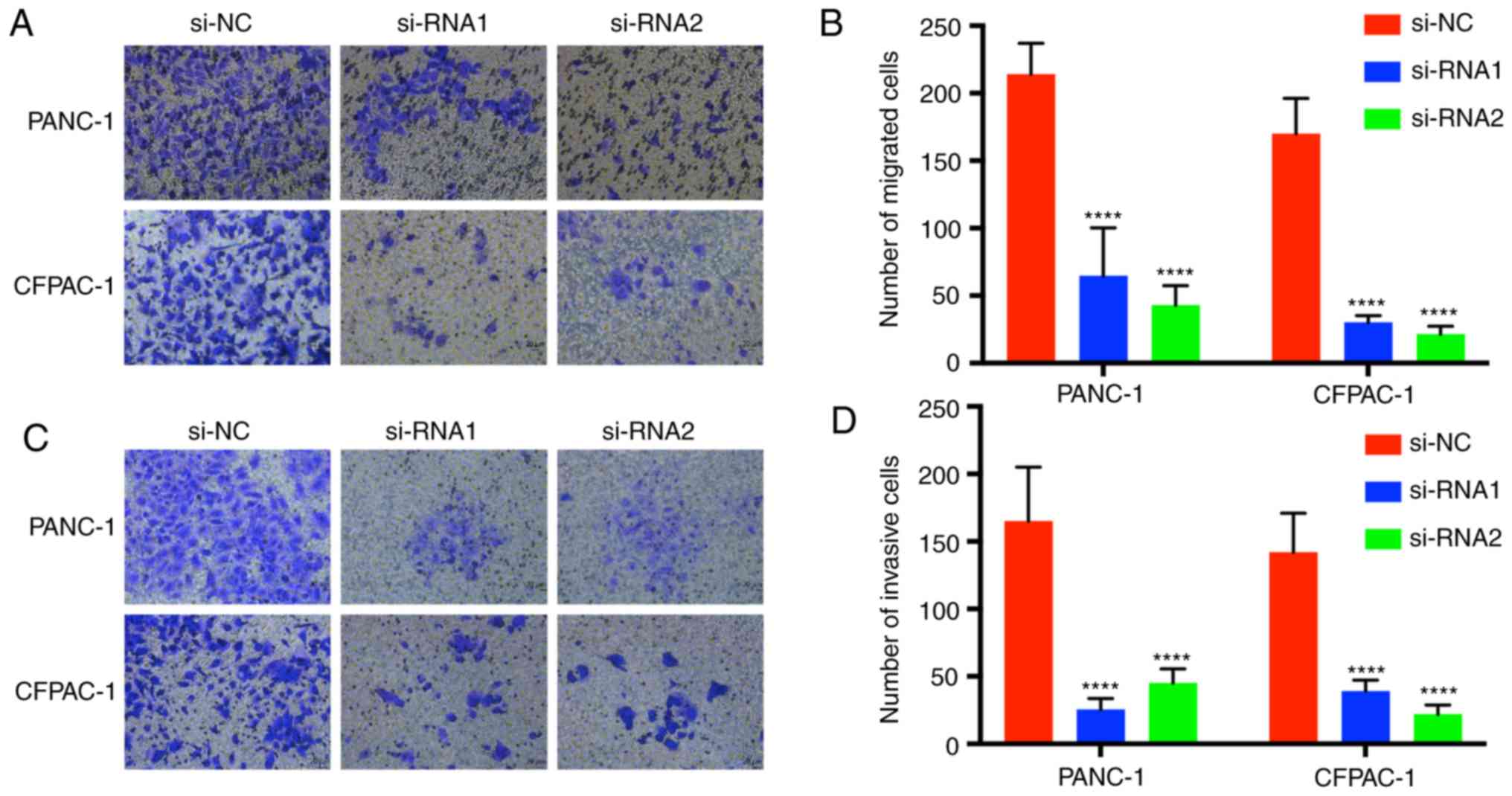
Downregulation of CENPM inhibits
pancreatic cancer cell migration and invasion
Transwell assays were performed to demonstrate the
migration and invasion function of CENPM in pancreatic tumor cells
(Fig. 5). Cells with migration
capacity can move from the upper chambers without FBS to the lower
chambers with a high concentration of FBS. The present results
revealed that knockdown of CENPM could efficiently hinder the
migration capacity of PANC-1 and CFPAC-1 cells compared with the
si-NC group (P<0.0001). The invasion assays also revealed a
similar trend.
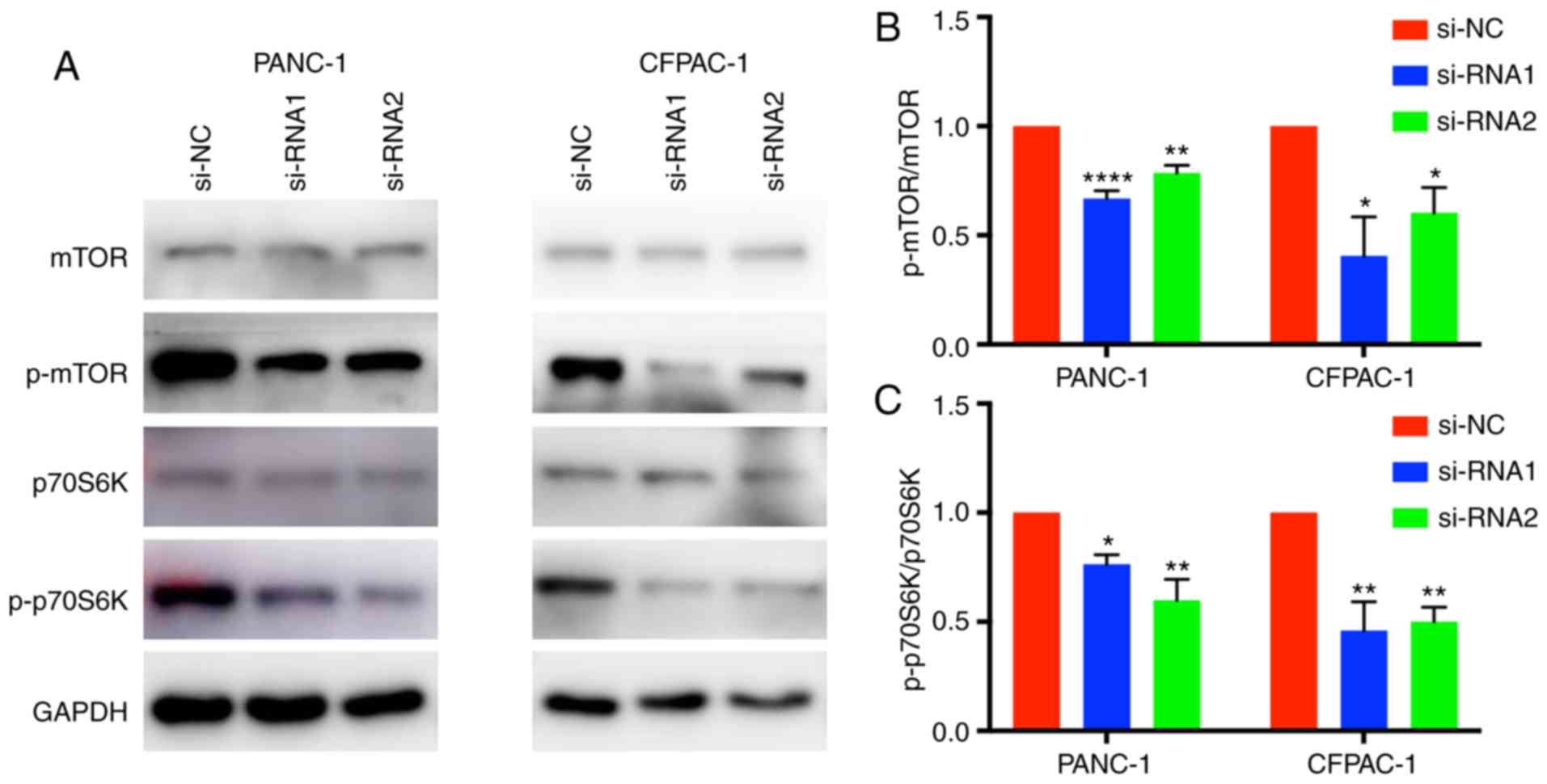
CENPM function may be regulated via
the mTOR/p70S6K signaling pathway
Western blotting was applied to elucidate the
mechanism by which CENPM regulates the migration and invasion
capacities of tumor cells. The phosphorylation levels of p70S6K and
mTOR were significantly reduced when CENPM was knocked down by
si-RNA-1 and si-RNA-2 (Fig. 6). The
mTOR/p70S6K pathway had an important role in cancer cell
metastasis, which may influence cell migration and invasion. These
results indicated that downregulation of CENPM inhibited
phosphorylation of the mTOR/p70S6K pathway thus reducing the
malignant degree of pancreatic tumors.
Discussion
Pancreatic carcinoma, a fatal disease with high
morbidity, has long been a tribulation for patients and doctors
(1). Pancreatic cancer is the
seventh cause of tumor-induced deaths and has a high
mortality/incidence ratio of up to 98% worldwide (3). In addition, its 5% five-year survival
rate makes this cancer even more serious (2,3).
The pathogenesis of pancreatic cancer has been
studied for several years, identifying contributing factors such as
smoking, heavy alcohol intake, high BMI, type II diabetes and
chronic pancreatitis (22).
Unfortunately, once pancreatic cancer is diagnosed, the only
treatment to cure the cancer is surgery. Further compounded, a
locally resectable tumor can result in post-surgery recurrence
(7).
Due to the limited options for therapy with
pancreatic cancer, it is imperative to explore new targeted
therapies and to provide different directions for clinical
treatment. Currently, gene editing technology is considered as a
curable treatment for pancreatic cancer (23,24).
The kinetochore is a surface structure on the
centromeric region of DNA and can be observed during every cell
cycle mitosis. The function of the kinetochore is to ensure the
correct segregation of chromosomes. CENPA is a leading marker in
the assembly of CENPA NAC, which is the beginning of kinetochore
construction. CENPM, along with CENPH, CENPC, CENPN, CENPU/50 and
CENPT, is physically closer to the CENPA nucleosome compared to
CENPA NAC (11). CENPM is highly
expressed in proliferating cells, such as activated lymphoid cells
and tumor cells (25). It is also
known as proliferation-associated nuclear element 1 (PANE1)
(25,26). Moreover, mitotic aberrations and
aneuploidy have been reported in CENPM-deficient cells (13,14,27).
Recently, Yu et al defined CENPM as a
complement to AFP in the diagnosis of hepatocellular carcinoma
(8); Xiao et al further
confirmed that CENPM was highly associated with hepatocellular
carcinoma progression and could be a candidate novel biomarker for
hepatocellular carcinoma (28).
Investigations of the underlying mechanisms of melanoma highlighted
that CENPM may play an important role in the metastases of melanoma
(9). Chen et al suggested
that CENPM may contribute to bladder cancer development and cancer
recurrence (12). In fact, bladder
cancer patients with high CENPM expression had markedly shorter
progression-free survival than those with low expression (10).
However, the mechanism between CENPM and pancreatic
carcinoma has not been explored. The present study aimed to
determine the function and mechanism of CENPM in the proliferation
and metastasis of pancreatic carcinoma.
CENPM, identified as ‘pseudo G-protein’, is
structurally related to Rab-family GTPases (29). The Rab GTPase family-associated
factors have been recognized as major regulators of the activity of
signaling pathways regulating cell growth and survival (30). A study identified Rab as promoting
oncogenesis by direct interaction with mTOR, resulting in
activation of the pathway (31).
mTOR (32) is a
protein that can influence cell growth via the stimulation of amino
acids, insulin, IGF-1, and ATP among other factors (33). Once mTOR is activated, mTORC1 can
regulate proteins, including ribosomal protein p70S6 kinases (S6Ks)
(34). p70S6K1 and p70S6K2 share a
large proportion of their kinase domains. p70S6K1, with 502 amino
acids, has been investigated more than p70S6K2. p70S6K has been
revealed to be highly expressed and activated in several breast
cancer cells (35). It has also
been revealed to play crucial roles in tumor angiogenesis, cellular
proliferation, migration and motility metastasis (36–38).
Moreover, the mTOR/p70S6K signaling pathway was revealed to
suppress autophagy through phosphorylation of transcription factor
EB and restrain the expression of autophagy genes (39,40).
Holz et al revealed that p70S6K can directly regulate the
phosphorylation of mTOR at threonine 2446/serine 2448, which is a
portion of the regulatory repressor domain (41). In addition, the mTOR/S6K signaling
pathway has been demonstrated to be essential in several diseases,
such as cancer, diabetes and obesity (32).
In the present study, the CENPM gene was revealed to
be regulated in both pancreatic carcinoma and pancreatic ductal
adenocarcinoma compared with healthy tissues. The results revealed
that cells with low expression of CENPM could significantly inhibit
pancreatic cancer cell proliferation, cause cell cycle arrest at
the G1 phase and hinder pancreatic cancer cell migration and
invasion. Among total cellular protein, the expression of p-p70S6K
and p-mTOR was reduced due to low CENPM levels. This indicated that
the mTOR/p70S6K signaling pathway may be the underlying mechanism
of CENPM. CENPM may regulate the activity of the kinase by
interacting with the kinase domain and play an important role in
the phosphorylation of mTOR and p70S6K. However, the specific
molecular mechanisms remain unclear. Various cancer-related
signaling pathways, such as JAK/STAT (42), PI3K/Akt (43), JNK (44), Wnt (45), and MAPK/ERK (46), have been revealed to play pivotal
roles in tumor cell invasion and migration. The effect of CENPM in
the aforementioned pathways will be explored in future studies.
Although the biological function of CENPM in
pancreatic cancer was elucidated, the present study has some
limitations. First, in vivo experiments should be performed
to confirm the present conclusions. Next, clinical data, such as
serum or tissue from pancreatic cancer resections, need to be
collected for further research. In conclusion, it suggested that
CENPM may positively affect the tumorigenesis of pancreatic tumors
and could become a potential marker and a target for gene therapy
in pancreatic cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ performed most of the experiments of this study.
TZ and DL designed the work as well as acquired, analyzed and
interpreted the data. CH and HT performed some of the experiments.
XN conceived the study and performed some of the experiments. BC
contributed to the writing of the manuscript and revised it
critically for intellectual content. All authors read and approved
the final version of the manuscript and agree to be accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ducreux M, Seufferlein T, Van Laethem JL,
Laurent-Puig P, Smolenschi C, Malka D, Boige V, Hollebecque A and
Conroy T: Systemic treatment of pancreatic cancer revisited. Semin
Oncol. 46:28–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGuigan A, Kelly P, Turkington RC, Jones
C, Coleman HG and McCain RS: Pancreatic cancer: A review of
clinical diagnosis, epidemiology, treatment and outcomes. World J
Gastroenterol. 24:4846–4861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Wang B, Ren H and Chen W: miR-9-5p
inhibits pancreatic cancer cell proliferation, invasion and
glutamine metabolism by targeting GOT1. Biochem Biophys Res Commun.
509:241–248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang J, Liu Y, Zhang L, Tan J, Li E and
Li F: Overexpression of microRNA-519d-3p suppressed the growth of
pancreatic cancer cells by inhibiting ribosomal protein
S15A-mediated Wnt/beta-catenin signaling. Chem Biol Interact.
304:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goess R and Friess H: A look at the
progress of treating pancreatic cancer over the past 20 years.
Expert Rev Anticancer Ther. 18:295–304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu Z, Wang R, Chen F, Wang J and Huang X:
Five novel oncogenic signatures could be utilized as AFP-related
diagnostic biomarkers for hepatocellular carcinoma based on
next-generation sequencing. Dig Dis Sci. 63:945–957. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Wu F, Shi Y, Yang D, Xu M, Lai Y
and Liu Y: Identification of key candidate genes involved in
melanoma metastasis. Mol Med Rep. 20:903–914. 2019.PubMed/NCBI
|
|
10
|
Kim WT, Seo SP, Byun YJ, Kang HW, Kim YJ,
Lee SC, Jeong P, Song HJ, Choe SY, Kim DJ, et al: The anticancer
effects of garlic extracts on bladder cancer compared to cisplatin:
A common mechanism of action via centromere protein M. Am J Chin
Med. 46:689–705. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perpelescu M and Fukagawa T: The ABCs of
CENPs. Chromosoma. 120:425–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Q, Hu J, Deng J, Fu B and Guo J:
Bioinformatics analysis identified key molecular changes in bladder
cancer development and recurrence. Biomed Res Int.
2019:39179822019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foltz DR, Jansen LE, Black BE, Bailey AO,
Yates JR III and Cleveland DW: The human CENP-A centromeric
nucleosome-sassociated complex. Nat Cell Biol. 8:458–469. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Izuta H, Ikeno M, Suzuki N, Tomonaga T,
Nozaki N, Obuse C, Kisu Y, Goshima N, Nomura F, Nomura N and Yoda
K: Comprehensive analysis of the ICEN (Interphase Centromere
Complex) components enriched in the CENP-A chromatin of human
cells. Genes Cells. 11:673–684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grützmann R, Pilarsky C, Ammerpohl O,
Lüttges J, Böhme A, Sipos B, Foerder M, Alldinger I, Jahnke B,
Schackert HK, et al: Gene expression profiling of microdissected
pancreatic ductal carcinomas using high-density DNA microarrays.
Neoplasia. 6:611–622. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pei H, Li L, Fridley BL, Jenkins GD,
Kalari KR, Lingle W, Petersen G, Lou Z and Wang L: FKBP51 affects
cancer cell response to chemotherapy by negatively regulating Akt.
Cancer Cell. 16:259–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Daniel R, Rhodes JY and Arul M:
Chinnaiyan, ONCOMINE-A cancer microarray database and integrated
data-mining platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malumbres M: Cyclin-dependent kinases.
Genome Biol. 15(122)2014.PubMed/NCBI
|
|
21
|
Park EY, Woo Y, Kim SJ, Kim DH, Lee EK, De
U, Kim KS, Lee J, Jung JH, Ha KT, et al: Anticancer effects of a
new SIRT inhibitor, MHY2256, against Human Breast Cancer MCF-7
cells via regulation of MDM2-p53 binding. Int J Biolo Sci.
12:1555–1567. 2016. View Article : Google Scholar
|
|
22
|
Bosetti C, Bertuccio P, Negri E, La
Vecchia C, Zeegers MP and Boffetta P: Pancreatic cancer: Overview
of descriptive epidemiology. Mol Carcinog. 51:3–13. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chu QD, Sun G, Pope M, Luraguiz N, Curiel
DT, Kim R, Li BD and Mathis JM: Virotherapy using a novel chimeric
oncolytic adenovirus prolongs survival in a human pancreatic cancer
xenograft model. Surgery. 152:441–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamamoto Y, Hiraoka N, Goto N, Rin Y,
Miura K, Narumi K, Uchida H, Tagawa M and Aoki K: A targeting
ligand enhances infectivity and cytotoxicity of an oncolytic
adenovirus in human pancreatic cancer tissues. J Control Release.
192:284–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bierie B, Edwin M, Melenhorst JJ and
Hennighausen L: The proliferation associated nuclear element
(PANE1) is conserved between mammals and fish and preferentially
expressed in activated lymphoid cells. Gene Expr Patterns.
4:389–395. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Renou JP, Bierie B, Miyoshi K, Cui Y,
Djiane J, Reichenstein M, Shani M and Hennighausen L:
Identification of genes differentially expressed in mouse mammary
epithelium transformed by an activated beta-catenin. Oncogene.
22:4594–4610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okada M, Cheeseman IM, Hori T, Okawa K,
McLeod IX, Yates JR III, Desai A and Fukagawa T: The CENP-H-I
complex is required for the efficient incorporation of newly
synthesized CENP-A into centromeres. Nat Cell Biol. 8:446–457.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao Y, Najeeb RM, Ma D, Yang K, Zhong Q
and Liu Q: Upregulation of CENPM promotes hepatocarcinogenesis
through mutiple mechanisms. J Exp Clin Cancer Res. 38:4582019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Basilico F, Maffini S, Weir JR, Prumbaum
D, Rojas AM, Zimniak T, De Antoni A, Jeganathan S, Voss B, van
Gerwen S, et al: The pseudo GTPase CENP-M drives human kinetochore
assembly. Elife. 3:e029782014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gopal Krishnan PD, Golden E, Woodward EA,
Pavlos NJ and Blancafort P: Rab GTPases: Emerging oncogenes and
tumor suppressive regulators for the editing of survival pathways
in cancer. Cancers (Basel). 12:2592020. View Article : Google Scholar
|
|
31
|
Thomas JD, Zhang YJ, Wei YH, Cho JH,
Morris LE, Wang HY and Zheng XF: Rab1A is an MTORC1 activator and a
colorectal oncogene. Cancer Cell. 26:754–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Magnuson B, Ekim B and Fingar DC:
Regulation and function of ribosomal protein S6 kinase (S6K) within
mTOR signalling networks. Biochem J. 441:1–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tavares MR, Pavan IC, Amaral CL,
Meneguello L, Luchessi AD and Simabuco FM: The S6K protein family
in health and disease. Life Sci. 131:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hall MN: mTOR-what does it do? Transplant
Proc. 40 (Suppl 10):S5–S8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holz MK and Blenis J: Identification of S6
kinase 1 as a novel mammalian target of rapamycin
(mTOR)-phosphorylating kinase. J Biol Chem. 280:26089–26093. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pende M, Um SH, Mieulet V, Sticker M, Goss
VL, Mestan J, Mueller M, Fumagalli S, Kozma SC and Thomas G:
S6K1(−/-)/S6K2(−/-) mice exhibit perinatal lethality and
rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation
and reveal a mitogen-activated protein kinase-dependent S6 kinase
pathway. Mol Cell Biol. 24:3112–3124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Skinner HD, Zhong XS, Gao N, Shi X and
Jiang BH: Arsenite induces p70S6K1 activation and HIF-1alpha
expression in prostate cancer cells. Mol Cell Biochem. 255:19–23.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Athamneh K, Alneyadi A, Alsamri H,
Alrashedi A, Palakott A, El-Tarabily KA, Eid AH, Al Dhaheri Y and
Iratni R: Origanum majorana essential Oil triggers p38
MAPK-mediated protective autophagy, apoptosis, and
caspase-dependent cleavage of P70S6K in colorectal cancer cells.
Biomolecules. 10:4122020. View Article : Google Scholar
|
|
39
|
Liu J, Ren Y, Hou Y, Zhang C, Wang B, Li
X, Sun R and Liu J: Dihydroartemisinin induces endothelial cell
autophagy through suppression of the Akt/mTOR pathway. J Cancer.
10:6057–6064. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martina JA, Chen Y, Gucek M and
Puertollano R: MTORC1 functions as a transcriptional regulator of
autophagy by preventing nuclear transport of TFEB. Autophagy.
8:913–914. 2012. View Article : Google Scholar
|
|
41
|
Holz MK, Ballif BA, Gygi SP and Blenis J:
mTOR and S6K1 mediate assembly of the translation preinitiation
complex through dynamic protein interchange and ordered
phosphorylation events. Cell. 123:569–580. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ashrafizadeh M, Rafiei H, Mohammadinejad
R, Afshar EG, Farkhondeh T and Samarghandian S: Potential
therapeutic effects of curcumin mediated by JAK/STAT signaling
pathway: A review. Phytother Res. Mar 10–2020.(Epub ahead of
print). View Article : Google Scholar
|
|
43
|
Xing J, Bhuria V, Bui KC, Nguyen MLT, Hu
Z, Hsieh CJ, Wittstein K, Stadler M, Wilkens L, Li J, et al:
Haprolid inhibits tumor growth of hepatocellular carcinoma through
Rb/E2F and Akt/mTOR inhibition. Cancers (Basel). 12:6152020.
View Article : Google Scholar
|
|
44
|
Ichimaru Y, Sano M, Kajiwara I, Tobe T,
Yoshioka H, Hayashi K, Ijichi H and Miyairi S: Indirubin 3′-oxime
inhibits migration, invasion, and metastasis InVivo in mice bearing
spontaneously occurring pancreatic cancer via blocking the RAF/ERK,
AKT, and SAPK/JNK Pathways. Transl Oncol. 12:1574–1582. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han F, Xu Q, Zhao J, Xiong P and Liu J:
ERO1L promotes pancreatic cancer cell progression through
activating the Wnt/catenin pathway. J Cell Biochem. 119:8996–9005.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang K, Li Y, Lian G, Lin H, Shang C, Zeng
L, Chen S, Li J, Huang C, Huang K and Chen Y: KRAS promotes tumor
metastasis and chemoresistance by repressing RKIP via the MAPK-ERK
pathway in pancreatic cancer. Int J Cancer. 142:2323–2334. 2018.
View Article : Google Scholar : PubMed/NCBI
|