Introduction
Esophageal carcinoma (EC), that occurs in the
squamous or glandular epithelium of the esophagus, represents the
predominant type of gastrointestinal tumors (1,2). EC is
further divided into esophageal squamous cell carcinoma (ESCC) and
esophageal adenocarcinoma (3).
Approximately 300,000 esophageal carcinoma-related deaths occur
every year worldwide, with its incidence and mortality rates
varying widely across countries. In China, studies indicate that
~150,000 people succumb to EC every year, with >90% of them
diagnosed with ESCC (4). Although
some progress has been achieved with regard to diagnosis and
treatment of ESCC, its five-year survival rate remains low
(2). It is, therefore, imperative
to develop antitumor drugs that effectively improve the overall
survival rates, while conferring low toxicity to patients. During
the development of antitumor drugs, numerous focus has been
directed towards active compounds from natural products. For
example, paclitaxel a new anti-microvascular drug extracted from
the bark of Taxus brevifolia, has been used in the treatment
of ESCC and ovarian carcinoma (5,6).
Similarly, vincristine, an alkaloid extracted from Catharanthus
roseus, is widely applied in the treatment of germinoma
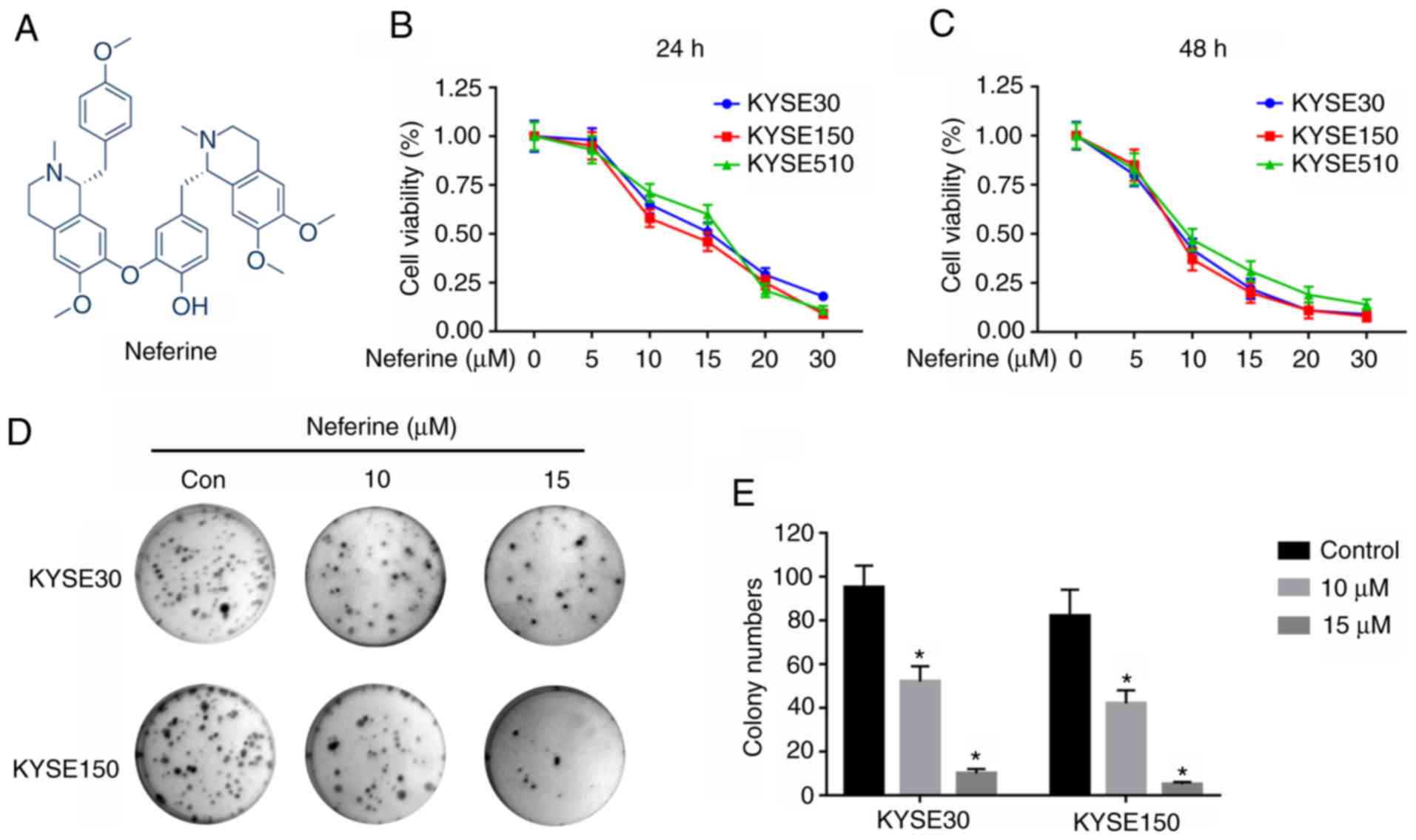
(7) and lymphoma (8). In addition, Neferine (Fig. 1A), an alkaloid extracted from
Nelumbo nucifera (lotus), has been revealed to actively
reduce blood lipid levels and anti-inflammation, and recently it
was reported to exert antitumor effects in multiple tumor cells
(9,10). Xu et al (11) revealed that neferine suppressed the
proliferation of ovarian carcinoma cells by provoking autophagy. In
osteosarcoma, neferine inhibited cell proliferation by triggering
cell cycle arrest (12). However,
its effects on ESCC remain unknown.
Abnormal alterations in the cell cycle is a hallmark
of cancer, and has been extensively exploited as a major target for
development of treatment therapies (13,14).
Previous studies have demonstrated that cyclins, along with
cyclin-dependent kinases (CDKs), are key regulators of cell cycle
progression. For instance, cyclin B1 is required to initiate
mitosis by modulating phosphorylation or dephosphorylation of
proteins (15). In addition, a
series of CDKs, such as p21, have been reported to function as
regulators during activation of cyclin B1 (16).
Apoptosis refers to the active and orderly death of
cells that maintains homeostasis of the internal environment under
physiological or pathological conditions (17,18).
Exposure of cells to internal pro-apoptotic factors, such as
activators of oncogenes, agents that cause DNA damage, cell
hypoxia, and deficiency of cell growth factors, has been revealed
to activate apoptosis of mitochondrial cells (19). Previous studies have demonstrated
that dysregulation of apoptosis can result in a variety of human
diseases, including development and regression of tumors (20,21).
In fact, dysregulation-related resistance to apoptosis is one of
the causes for tumorigenesis (22).
Reactive oxygen species (ROS) has been implicated in
tumorigenesis. Particularly, a moderate ROS level is required for
cell proliferation but excessive production of ROS induces
apoptosis causing cell death (23).
Furthermore, ROS has been revealed to modulate various pathways,
including the c-Jun N-terminal kinase (JNK), which is a key
regulator of apoptosis (24,25).
Recent studies demonstrated that the level of ROS could be tightly
controlled by the cellular antioxidant system. The transcription
factor nuclear factor erythroid 2-related factor 2 (Nrf2) is deemed
as the master regulator of the antioxidant system. Although
consistently exposed to a high level of ROS, cancer cells could
survive by upregulating the expression of Nrf2. In cerebral
ischaemia/reperfusion injury, the oxidative damage caused by
overproduced ROS could be removed by enhancing the expression of
Nrf2 (26–28). Hence, Nrf2 is usually regarded as an
upstream molecule of ROS.
The present study investigated the potential
anticancer activity of neferine in ESCC cells. It was revealed that
neferine induced cell cycle arrest and apoptosis in these cells.
Mechanistically, neferine induced ROS-mediated activation of the
JNK signaling pathway by inhibiting Nrf2. Collectively, these
findings demonstrated that neferine holds great promise as a
treatment for ESCC.
Materials and methods
Reagents
Neferine (purity >98%) was acquired from
Selleckchem, whereas tert-Butylhydroquinone (tBHQ; B105351),
N-acetyl-L-cysteine (NAC; A105421) and SP600125 (SP; S125267) were
purchased from Shanghai Aladdin Biological Technology Co., Ltd.
Neferine was dissolved in DMSO (Sigma-Aldrich; Merck KGaA) at a
concentration of 100 mM as a primary stock solution and the desired
concentration of neferine for each experiment was obtained via
thinned with RPMI-1640 medium with 10% FBS, immediately before use.
The concentration of DMSO was lower than 1:3,000 in all
experiments.
Cell lines and cultures
Cancer cell lines, KYSE30, KYSE150 and KYSE510, were
obtained from Fengh Biotech and maintained in RPMI-1640 medium,
supplemented with 10% FBS (HyClone; GE Healthcare Life Sciences).
The cultures were incubated at 37°C, and 5% CO2.
Cell viability assay
Approximately 4,000 cells/well, were seeded
overnight in 96-well plates, then treated with varying
concentrations (0, 5, 10, 15, 20 and 30 µm) of neferine for 24 and
48 h at room temperature. Cell viability was determined by
detecting the absorbance at OD 450 nm using 10 µl Cell Counting
Kit-8 (NCM Biotech) according to the manufacturer's
instructions.
Clone formation assay
Approximately 1,000 cells/well, earlier incubated
with the aforementioned neferine concentrations, were seeded in
6-well plates and cultured for 14 days. The colonies were then
washed with PBS, fixed with 75% ethanol at room temperature for 15
min and stained using 0.1% crystal violet for another 15 min.
Finally, images were obtained using a digital camera (Olympus) and
the number of forming colonies (>10 cells per colony) was
measured by Gel Imaging Analysis System (Syngene).
Apoptosis assays
Approximately 5×104 cells were collected,
following overnight incubation with various neferine concentrations
(0, 10, 15 and 20 µm), then incubated with 5 µl Annexin V-FITC and
5 µl propidium iodide (Neobioscience) in darkness for 15 min at
room temperature. Thereafter, apoptosis was assessed using a flow
cytometer (BD Biosciences) and the results were analyzed with
FlowJo analysis software (version 10.0; FlowJo LLC).
Analysis of the cell cycle
Approximately 2×105 cells were plated in
a well of 6-well plates for 24 h, followed by another 24 h
incubation with neferine at 37°C. The cultures were then collected
and fixed with ice-cold 70% ethanol at 4°C overnight, followed by
determination of cell cycle distribution using a Cell Cycle
Detection Kit (Nanjing KeyGen Biotech Co., Ltd.) according to the
manufacturer's instructions.
Western blot analysis
Profiles of protein expression were analyzed using
western blot as previously described (21). The primary antibodies used in the
present study included E-cadherin (product code ab194982),
N-cadherin (product code ab18203), cyclin B1 (product code
ab32053), p21 (product code ab109520), Bax (product code ab32503),
Nrf2 (product code ab137550), and phosphorylated (p)-JNK (product
code ab4821) (all from Abcam), caspase-3 (cat. no. 19677-1-AP),
Bcl-2 (cat. no. 12789-1-Ap), Beclin-1 (cat. no. 11306-1-AP), JNK
(cat. no. 24164-1-AP) and caspase-9 (cat. no. 10380-1-AP) (all from
ProteinTech Group, Inc.). β-actin (cat. no. A19788)
(Sigma-Aldrich; Merck KGaA) was included as an internal control.
The blots were then incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies including anti-mouse IgG
(dilution 1:2,000; cat no. 7076) and anti-rabbit (dilution 1:2,000;
cat. no. 7074; both from Cell Signaling Technology, Inc.). The
protein bands were analyzed using ImageQuant LAS 4000/4010 (GE
Healthcare).
Determination of ROS production
Levels of ROS produced were measured using a ROS
Assay kit (Nanjing KeyGen Biotech Co., Ltd.), according to the
manufacturer's instructions, then the results analyzed using
fluorescent microscopy (magnification, ×100) and a flow cytometer
(FACScan; BD Biosciences) with FlowJo analysis software (version
10.0; FlowJo LLC).
Statistical analyses
All experiments were conducted using at least three
replicates, and the results were presented as the means ± standard
deviations (SD) of the means. A one-way analysis of variance
(ANOVA) was used to compare differences among multiple groups with
Tukey's post hoc test. An unpaired Student's t-test was employed
for comparisons between two groups. All data analyses were
performed in GraphPad Prism version 7 (GraphPad Software, Inc.) or
SPSS 22.0 (IBM Corp).
Results
Neferine suppresses cell
proliferation
To explore the antiproliferative activity of
neferine on ESCC cells, three cell lines (KYSE30, KYSE150 and
KYSE510) were incubated with varying (0, 5, 10, 15, 20 or 30 µM)
concentrations for 24 h (Fig. 1B)
and 48 h (Fig. 1C) and the
viability of the cells was determined using a CCK-8 assay. The
results revealed that neferine hindered the proliferation of the
three ESCC cell lines. Notably, the IC50 values, after
24 h, were 14.16±0.911, 13.03±1.162, and 14.67±1.353 µM in KYSE30,
KYSE150, and KYSE510 cells, respectively. A colony formation assay,
performed to evaluate the long-term effects of neferine on
proliferation of ESCC cells (Fig.
1D) revealed suppression of clonogenicity in KYSE30 and KYSE150
relative to the control group (Fig.
1E). Collectively, these results indicated that neferine
hindered proliferation of ESCC cells.
Neferine induces cell cycle
arrest
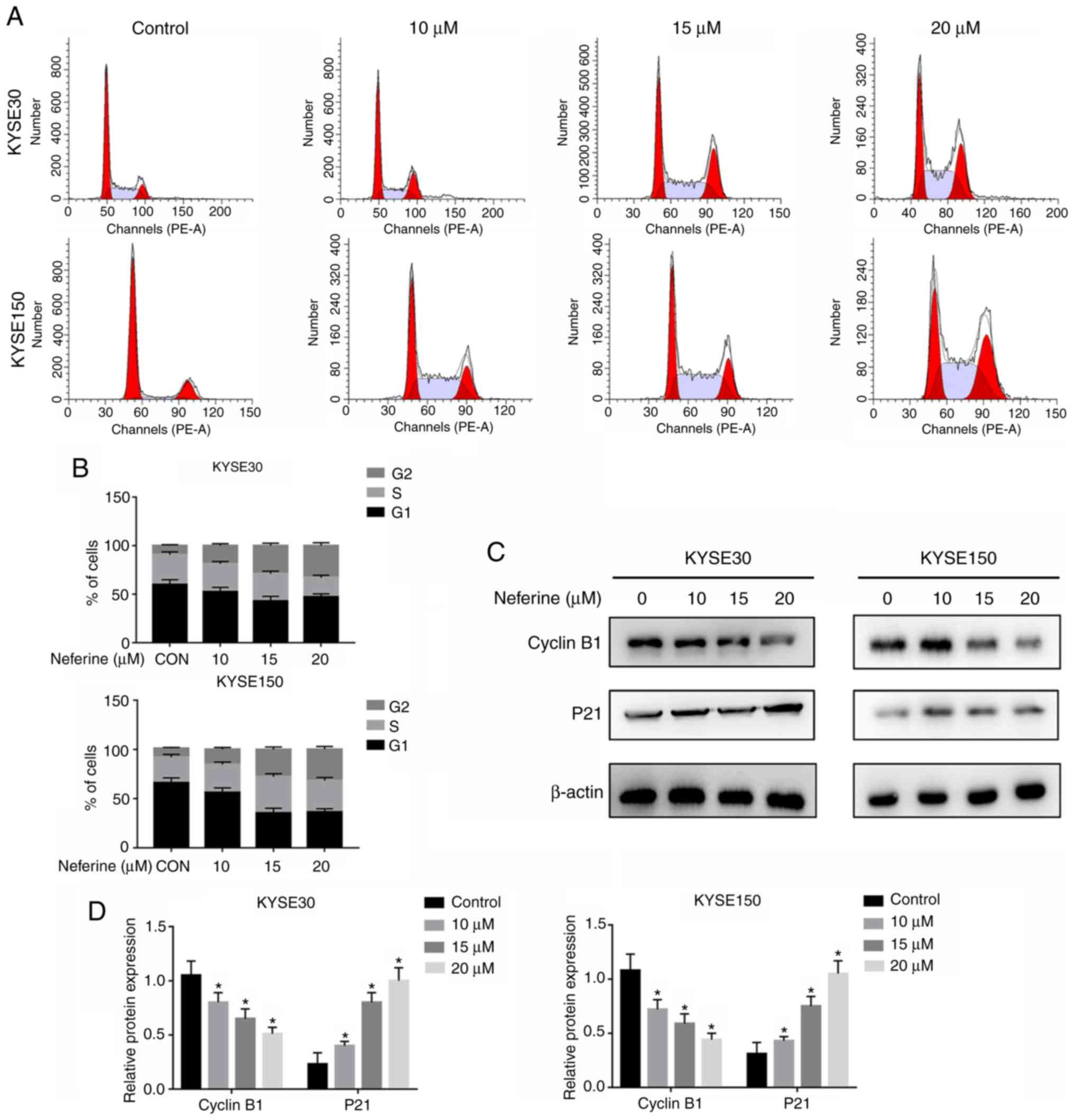
To ascertain whether neferine hinders ESCC
proliferation by regulating cell cycle distribution, KYSE30 and
KYSE150 cells were exposed to 4 different neferine concentrations
(0, 10, 15 and 20 µM) for 24 h, and then cell cycle distribution
was analyzed using flow cytometry (Fig.
2A). A higher G2/M-phase distribution in
neferine-treated KYSE30 and KYSE150 cells relative to the control
group (Fig. 2B) was revealed. The
proportion of G2/M phase cells in KYSE30 cells was
9.7±0.91% in the untreated group, while the percentages from
neferine treatment were 15.1±1.37 (10 µM), 22.8±1.84% (15 µM) and
33.0±3.25% (20 µM). In KYSE150 cells, the proportions of
G2/M phase cells were of 10.2±0.92% (untreated),
16.3±2.32% (10 µM), 25.3±2.45% (15 µM) and 33.2±3.41% (20 µM).
Next, western blot analysis was used to detect the expression of
cyclin B1 and p21, and the results revealed that neferine mediated
a downregulation of cyclin B1, but upregulation of p21 expression
(Fig. 2C and D). These results
indicated that neferine inhibited growth of ESCC cells by
modulating cell cycle-related proteins.
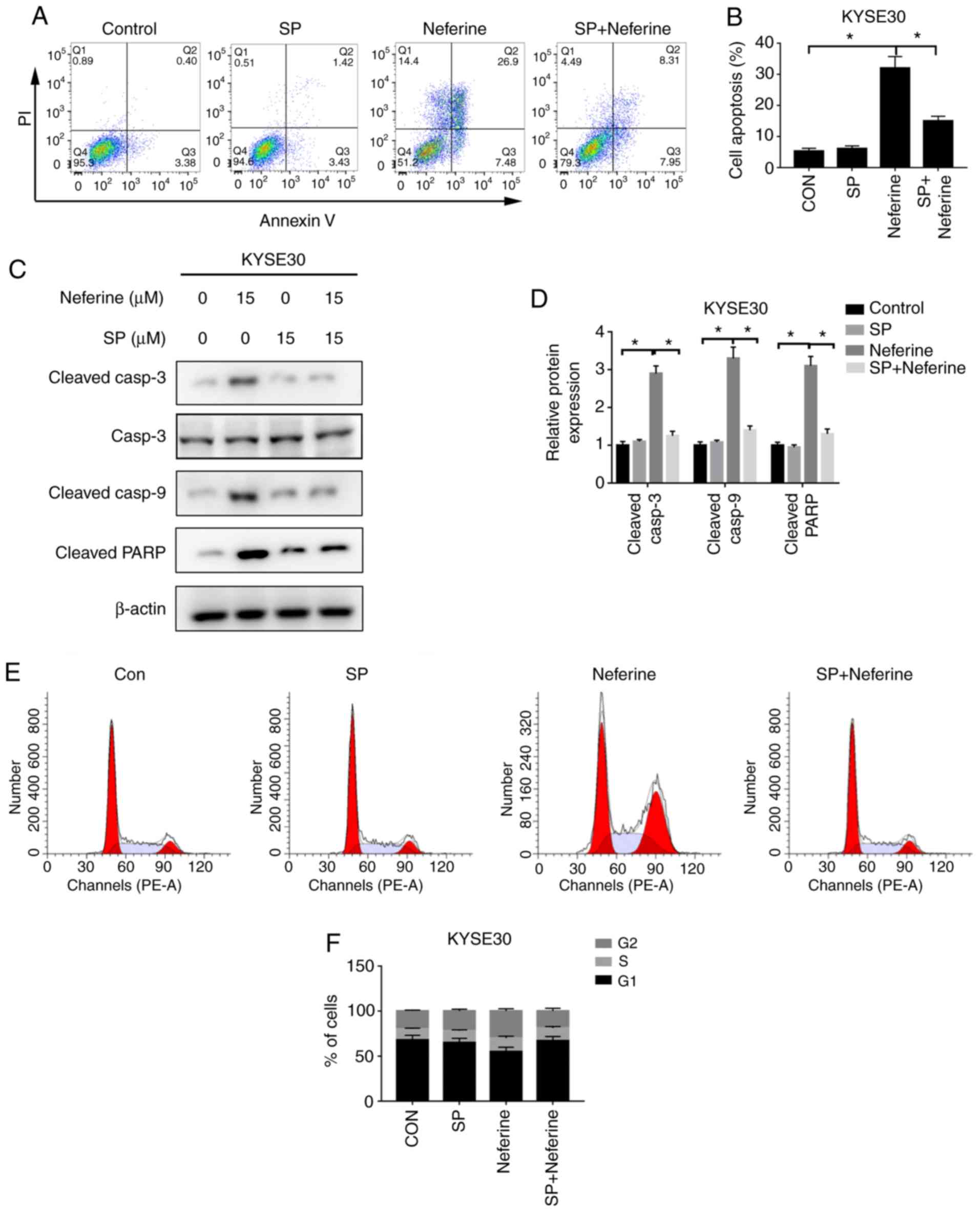
Neferine induces cell apoptosis
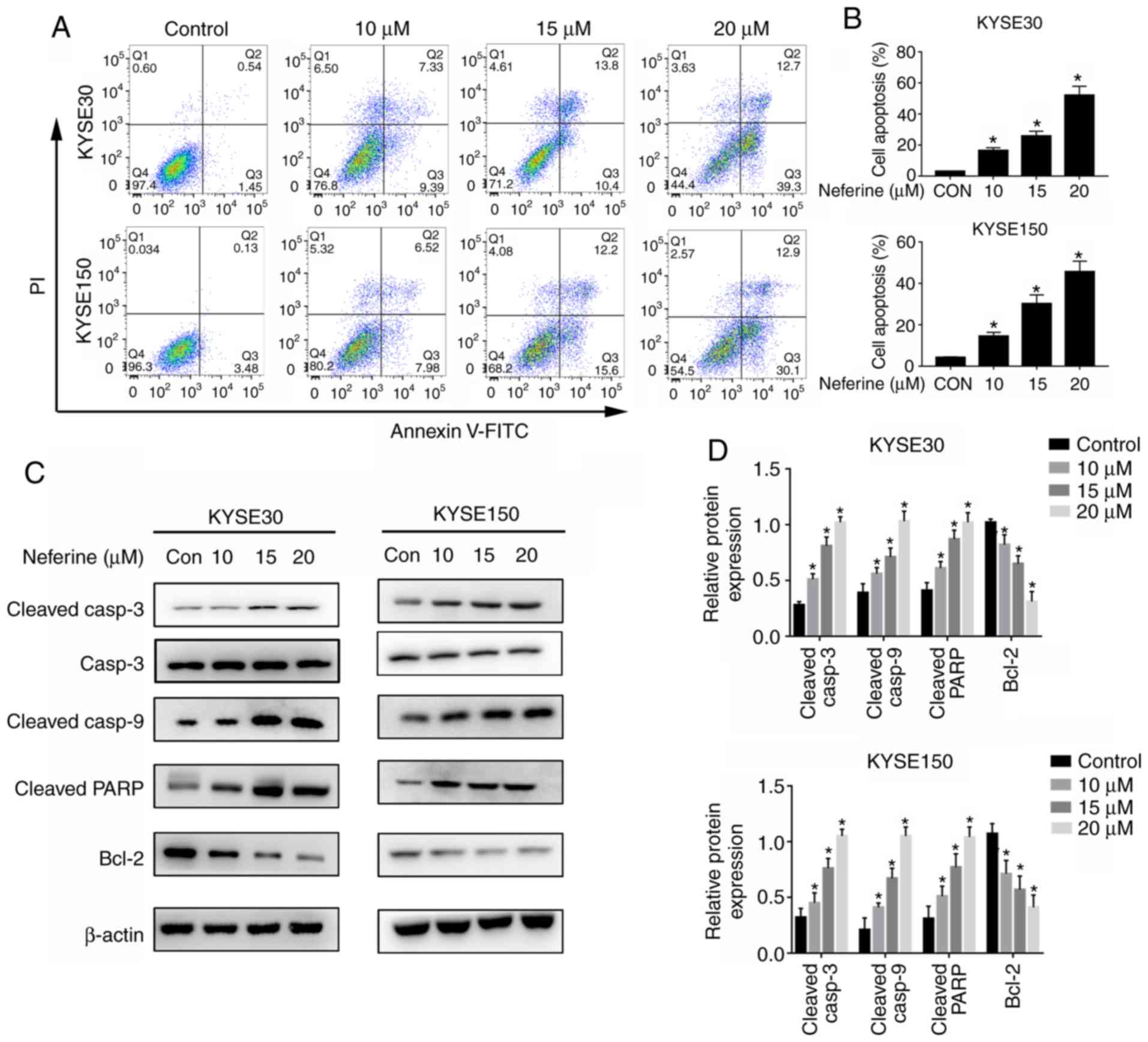
Apoptosis assays were performed to investigate the
ability of neferine to inhibit proliferation of ESCC. The results
revealed significantly higher apoptosis in neferine-treated KYSE30
and KYSE150 cells, relative to the control group (Fig. 3A and B). Specifically, apoptosis
rates in KYSE30 cells were 2.13±0.67% for the untreated group,
while those in neferine-treated cells were 16.55±2.45% (10 µM),
24.2±3.67% (15 µM) and 53.2±5.31% (20 µM). In KYSE150 cells, the
apoptotic rates were 3.21±0.72% (untreated), 15.31±3.22% (10 µM),
27.3±3.45% (15 µM) and 43.2±4.21% (20 µM). Furthermore, neferine
treatment significantly increased the expression levels of cleaved
PARP as well as cleaved caspase-3 and 9, but downregulated the
expression of Bcl-2 (Fig. 3C and
D). These findings indicated that neferine successfully induced
apoptosis in ESCC cells.
Neferine increases ROS production and
activates the JNK pathway
Neferine has been demonstrated to cause excessive
ROS production leading to apoptosis (12). Based on this, its ability to induce
ROS production in ESCC cells was explored by treating KYSE30 and
KYSE150 cells with 10, 15 and 20 µM of neferine for 12 h (Fig. 4A), and then sequentially exposing
them to 15 µM for 6, 12 and 24 h (Fig.
4B). The results revealed significantly higher intracellular
ROS levels at 6, 12 and 24 h, in neferine-treated cells relative to
the control group. Similarly, higher ROS levels were detected in
KYSE30 and KYSE150 cells exposed to higher, compared to lower
neferine concentrations (Fig. 4A).
Then, the antioxidant, NAC was analyzed, using fluorescence
microscopy (Fig. 4C) and flow
cytometry (Fig. 4D) and it was
revealed that this ROS inhibitor significantly reduced the
production of ROS in ESCC cells following neferine treatment.
Numerous studies have reported a close relationship between ROS
production and activation of the JNK pathway (29,30).
Therefore, the expression of JNK pathway-related proteins was
assessed using western blot analysis and the results revealed
significantly higher levels of JNK phosphorylation in
neferine-treated ESCC cells (Fig. 4D
and E).
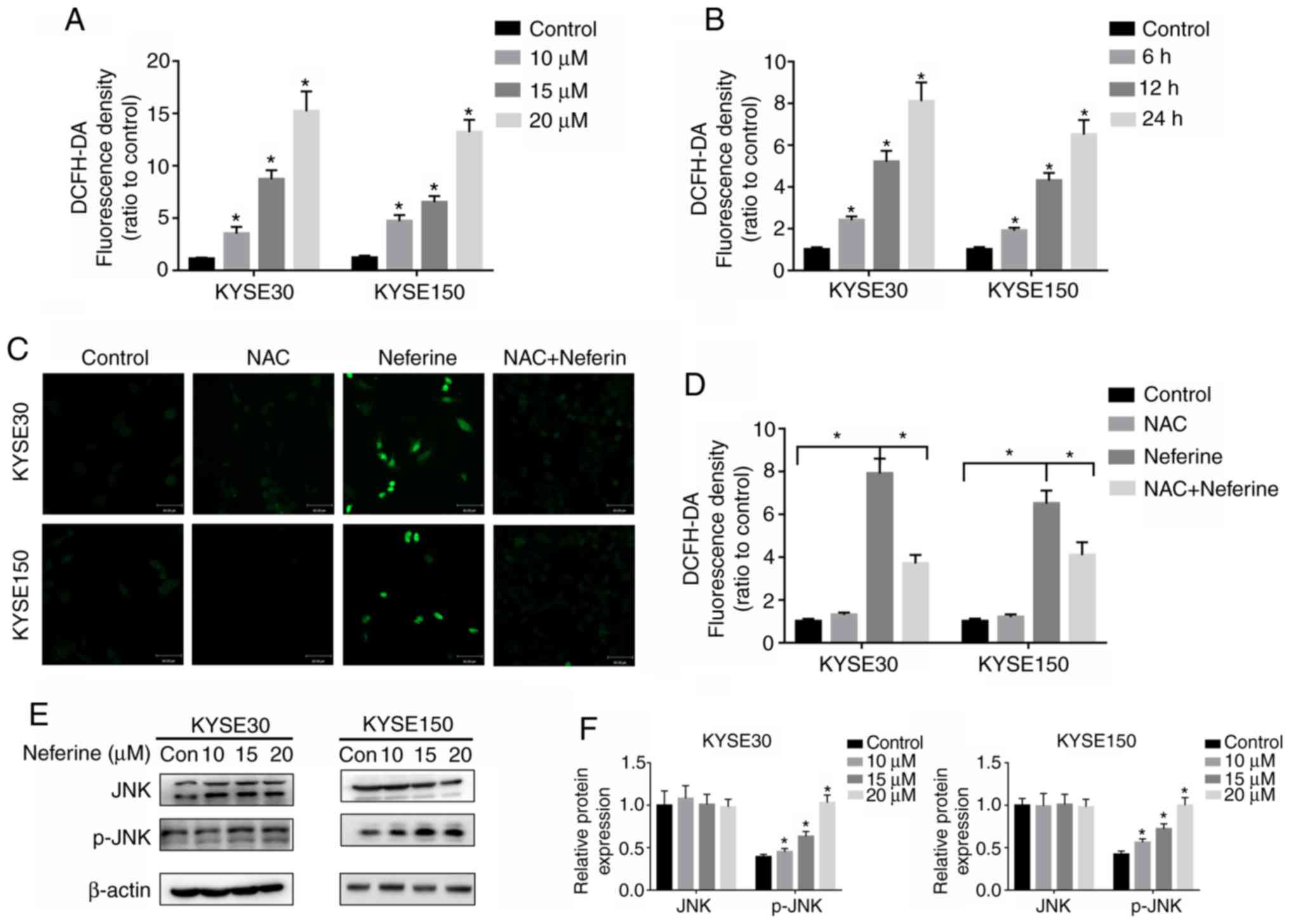 | Figure 4.Neferine induces the production of
ROS and activates the JNK pathway in ESCC cells. KYSE30 and KYSE150
cells were pretreated with (A) 10, 15 and 20 µM neferine for 12 h
and incubated with (B) 15 µM for 6, 12 and 24 h. The ROS levels
were assessed using a flow cytometer. (C and D) KYSE30 and KYSE150
cells were treated with 15 µM neferine with or without 10 mM NAC
for 12 h, and the ROS levels were determined using fluorescence
microscopy. (E and F) KYSE30 and KYSE150 cells were treated with
neferine (0, 10, 15 and 20 µM) for 24 h, and the expression of JNK
and p-JNK was detected by western blotting. *P<0.05 vs. the
control or otherwise indicated in the image. ROS, reactive oxygen
species; JNK, c-Jun N-terminal kinase; ESCC, esophageal squamous
cell carcinoma; NAC, N-acetyl cysteine; p-JNK, phosphorylated
JNK. |
Neferine triggers cell cycle arrest
and apoptosis by increasing ROS production
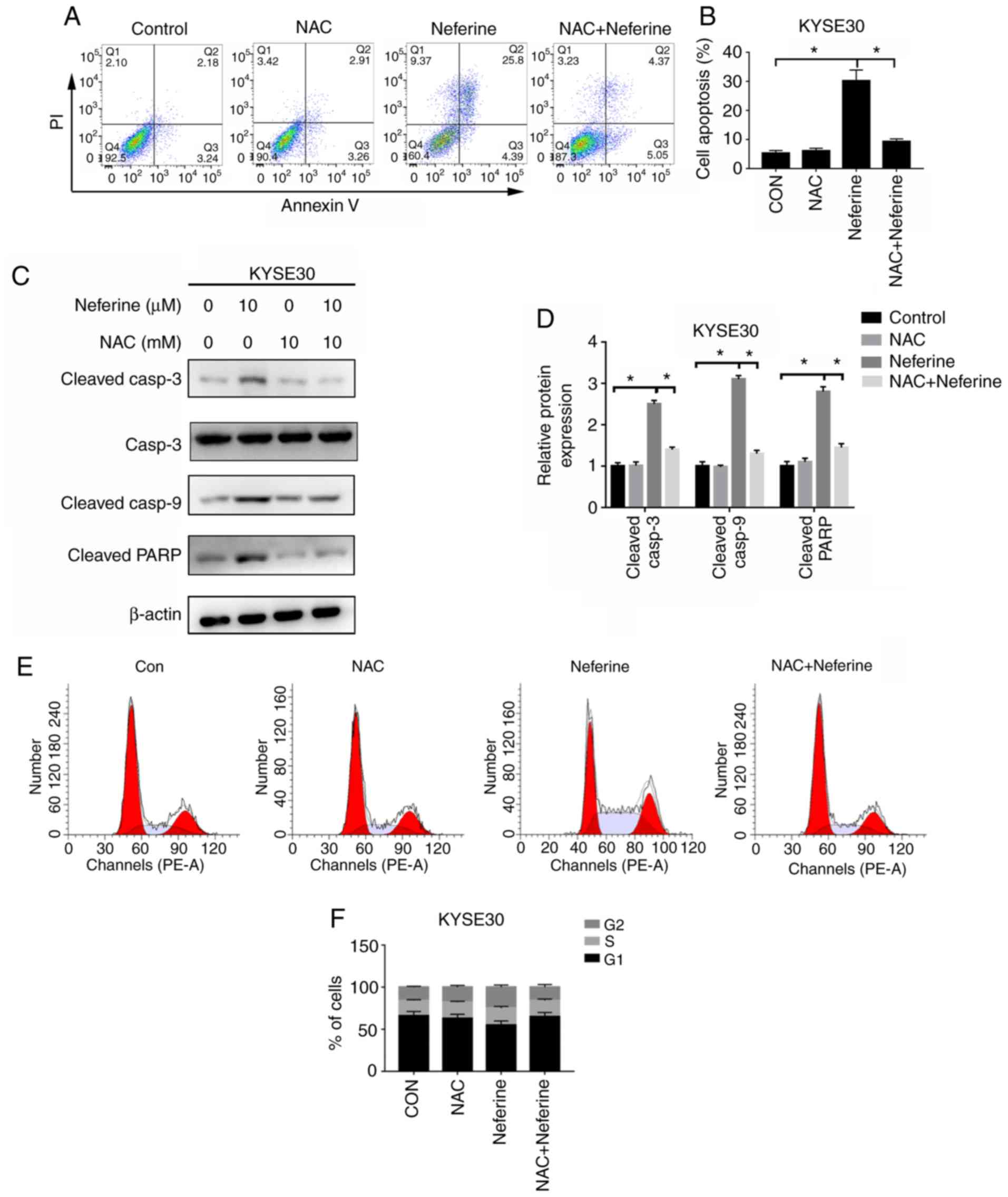
Since the antioxidant NAC could significantly reduce
the generation of ROS, subsequently, the effects of
neferine-mediated ROS accumulation on apoptosis and cycle arrest in
ESCC cells were investigated using NAC. Flow cytometric results
revealed that 2 h pretreatment with 10 mM NAC could reverse
neferine-induced apoptosis (Fig. 5A and
B), downregulate the expression of apoptotic-related proteins,
cleaved caspase-3, cleaved caspase-9 and cleaved PARP (Fig. 5C and D) and decrease the
neferine-induced G2/M cell cycle arrest (Fig. 5E and F). These results indicated
that neferine may affect apoptosis and the cell cycle of ESCC cells
by inducing ROS accumulation.
Neferine triggers cell cycle arrest
and apoptosis by activating the JNK pathway
The aforementioned findings suggested that neferine
induced activation of the JNK pathway, which is closely associated
with cell proliferation, the cell cycle and apoptosis.
Consequently, the effects of neferine-mediated activation of the
JNK pathway in apoptosis and cycle arrest in ESCC cells were
explored, using SP, an inhibitor of the JNK pathway. Flow
cytometric results revealed that 2 h pretreatment with 15 µM SP
could reverse neferine-induced apoptosis (Fig. 6A and B) and downregulate the
expression of apoptotic-related proteins; cleaved caspase-3,
cleaved caspase-9 and cleaved PARP (Fig. 6C and D). In addition, SP-mediated
JNK inhibition resulted in a decrease of G2/M cell cycle
arrest (Fig. 6E and F). These
results indicated that neferine may affect apoptosis and the cell
cycle of ESCC cells by regulating activation of the JNK signaling
pathway.
Neferine triggers the ROS-mediated JNK
pathway with Nrf2 inhibition
It was further explored whether ROS participates in
the activation of the JNK signaling pathway. Results of the western
blot assay revealed that NAC successfully reversed the
phosphorylation levels of JNK induced by neferine (Fig. 7A and B). However, SP failed to
reduce neferine-induced ROS production (Fig. 7C). These results indicated that
neferine induced activation of the JNK signaling pathway by
stimulating production of ROS. In addition, it was observed that
neferine inhibited the expression of antioxidant, Nrf2 (Fig. 7D and E). Specifically, ESCC cells
pretreated with Nrf2 activator, tBHQ, partially offset the
proliferation inhibition effect of neferine on ESCC, compared with
the control group (Fig. 7F). These
data indicated that neferine could inhibit Nrf2 expression,
stimulate production of ROS and activate the JNK signaling pathway.
Moreover, the compound could also induce apoptosis and cell cycle
arrest in ESCC, which indicates a potential role in in management
of esophageal carcinoma.
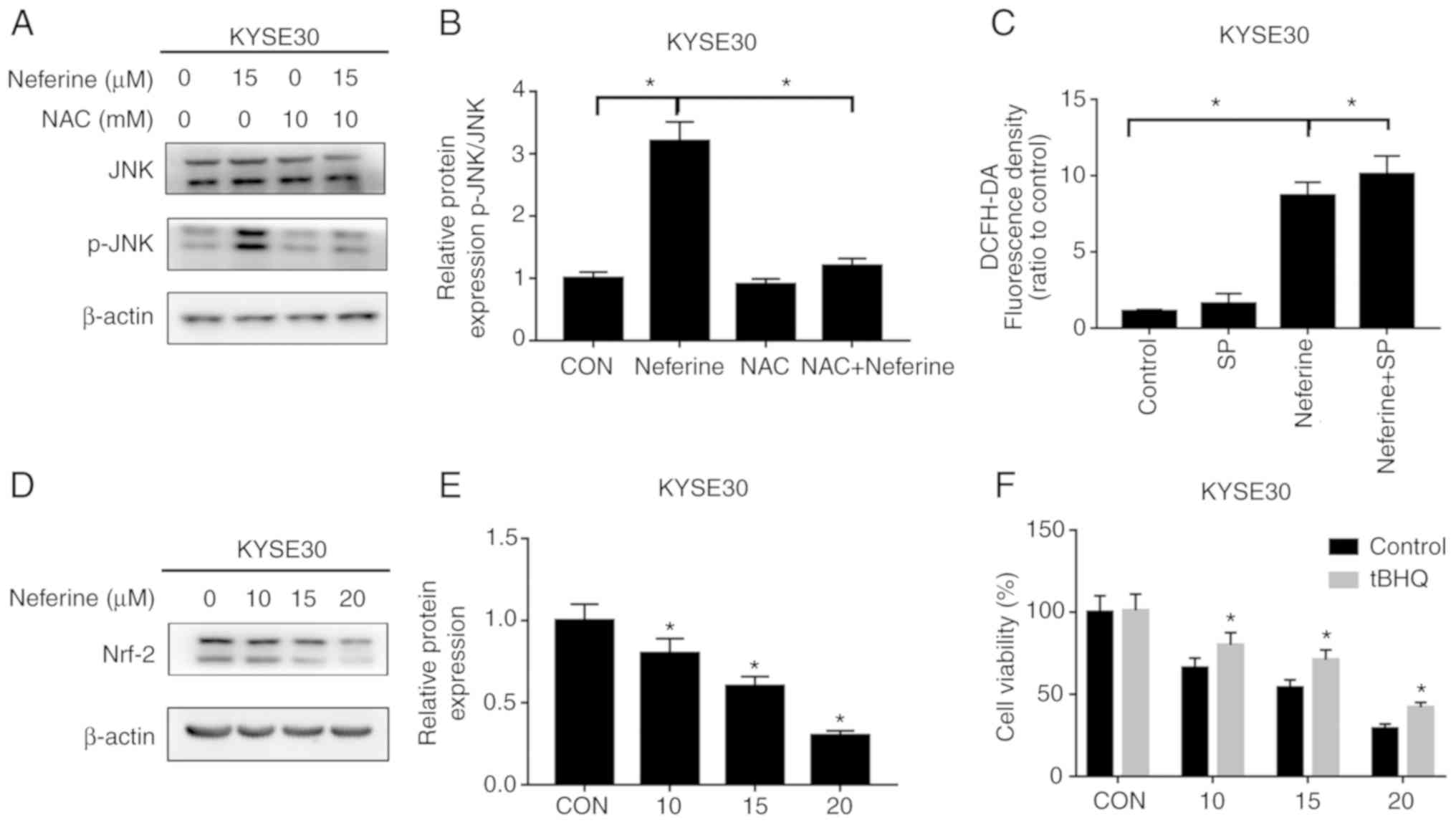 | Figure 7.Neferine induces ROS production and
activates JNK signaling by inhibiting Nrf2 expression. (A and B)
Cells were treated with 15 µM neferine with or without 10 mM NAC
for 12 h, and the expression of p-JNK and JNK was determined by
western blotting. (C) KYSE30 cells were treated with 15 µM neferine
with or without 15 mM SP for 12 h, and the ROS levels were assessed
using flow cytometry. (D and E) Cells were treated with 10, 15 and
20 µM neferine for 12 h, and the expression of Nrf2 was determined
by western blot analysis. (F) Cells were treated with 0,10, 15 and
20 µM of neferine, with or without 20 µM tBHQ for 12 h, and then
cell viability was determined with a CCK-8 assay. *P<0.05 vs.
the control or otherwise indicated in the image. ROS, reactive
oxygen species; JNK, c-Jun N-terminal kinase; NAC, N-acetyl
cysteine; SP, SP600125; tBHQ, tert-butylhydroquinone. |
Discussion
Esophageal squamous cell carcinoma is an aggressive
malignancy (1). Currently, ESCC
patients are mainly treated using surgical approaches, as well as
by radiotherapy or chemotherapy, depending on the pathological
results. Although chemotherapeutic drugs improve outcomes of
esophageal carcinoma patients to a certain extent, the prognosis of
ESCC remains dismal (2). Although
several antitumor drugs such as paclitaxel (6), vincristine (8) and colchicine (31), are available for clinical
application, they have limited efficacy in killing tumor cells.
This indicates that more effective anticancer drugs should be
designed to satisfy this unmet clinical need. In the present study,
the antitumor effect of neferine, an alkaloid extracted from lotus
leaves on ESCC was investigated. The results revealed that it
inhibited proliferation of esophageal carcinoma cells, thereby
causing G2/M phase arrest and inducing apoptosis. In
addition, neferine activated the JNK signaling pathway by
stimulating production of a high level of ROS and inhibiting Nrf2.
Overall, these findings indicated that neferine possesses a
significant anti-esophageal carcinoma effect.
Targeting the cell cycle has been a major approach
in the development of cancer treatments. Consequently, numerous
cell cycle-specific agents, including methotrexate, vinblastine and
cytarabine (32,33), have been identified and are widely
applied in the clinical management of malignant tumors. Previous
studies have revealed that neferine inhibits progression of
multiple malignancies, through cell cycle arrest, although this
effect varies (12). For instance,
Pham et al revealed that neferine hindered proliferation of
human neuroblastoma by blocking G2/M cell cycle
progression (9), while Xu et
al revealed that it could cause G1-phase arrest in ovarian
carcinoma cells by upregulating p21 and p27 (11). In the present study, it was observed
that neferine treatment increased the G2/M phase in ESCC
cells by downregulating the expression of cyclin B1 while
upregulating that of p21. The Cdk1/cyclin B1 complex is a crucial
initiator of mitosis, and has been reported to coordinate
G2/M progression (34).
Studies have revealed that activation of this complex can be
blocked by upregulating p21 (34,35).
In the present study, neferine suppressed proliferation of tumor
cells, by blocking cell cycle progression, although the mechanisms
underlying this phenomenon are unclear and require further
investigation.
Apoptosis, also known as programmed cell death, was
proposed by Kerr et al and refers to an active and inherent
programmed phenomenon (36). During
this process, cells maintain a balance of quantity through
proliferation and apoptosis, but once this balance is broken, it
results in certain diseases, such as cancer. Understanding the
relationship between apoptosis and cancer development presents new
insights into development of novel strategies for treatment of the
disease. Consequently, a variety of anticancer drugs have recently
been revealed to inhibit progression of tumor cells by inducing
apoptosis. For instance, schisantherin (37) hindered proliferation of human
gastric cancer cells by triggering apoptotic cell death. In the
present study, activation of caspase-3/9 and PARP was closely
associated with the apoptosis pathway. In addition, neferine
treatment induced apoptosis, mediated upregulation of the
expression of cleaved caspase-3/9 and PARP, and also resulted in
the downregulation of Bcl-2. Collectively, these results indicated
that neferine induced the apoptosis pathway in ESCC.
Recent studies have shown that ROS accelerates the
death of tumor cells, thereby effectively treating cancer (38,39).
High production of ROS decreases the mitochondrial transmembrane
potential and the release of cytochrome c, which then
activates a series of caspases, thereby inducing cell apoptosis. In
the present study, it was observed that neferine triggered ROS
production in a time- and dose-dependent manner, whereas
pretreatment with NAC, an inhibitor of ROS, reversed
neferine-induced cell cycle arrest and apoptosis. In addition to
apoptosis, neferine-triggered ROS production resulted in cell cycle
arrest.
Stress-activated kinases, JNK, are activated by
natural compounds such as longikaurin A (40), isoliensinine (41) and ampelopsin (42). In addition, studies have revealed
that ROS activates the JNK signaling pathway and promotes tumor
cell apoptosis (17,38,43).
Similarly, in the present study it was revealed that neferine
treatment increased the levels of JNK phosphorylation in esophageal
carcinoma cells. Conversely, pretreatment with SP, an inhibitor of
the JNK pathway, partially offset neferine-induced cell cycle
arrest and apoptosis. Furthermore, pretreatment with NAC attenuated
activation of the JNK pathway, however the addition of the JNK
inhibitor failed to reduce increase of nerefine-induced ROS. These
results indicated that neferine induced cell cycle arrest and
apoptosis through the ROS-mediated JNK pathway.
The present results revealed that neferine-treated
esophageal carcinoma cells exhibited downregulation of Nrf2
expression, and this was partially offset by Nrf2 activator tBHQ.
It was therefore hypothesized, that neferine may inhibit the
expression of Nrf2, induce production of ROS and activate the JNK
signaling pathway, thus causing cell cycle arrest and inducing
apoptosis. Nrf2 is an antioxidant factor that regulates cellular
redox state and is highly expressed in a variety of tumor cells
(44). In addition, this factor
induces and regulates the expression of a series of antioxidant
protein groups, and has been found to reduce cell damage caused by
ROS and electrophiles, thereby maintaining the cell in a stable
state and the dynamic balance of redox reaction (27,44,45).
However, there are certain limitations to the present study. All
the experiments were performed at the cellular level and the effect
of neferine on esophageal carcinoma requires in vivo
validation in a future study.
In summary, the present study revealed that neferine
can successfully suppress the growth of ESCC cells by blocking cell
cycle progression and triggering apoptosis. Furthermore, ROS
production and JNK phosphorylation are required in arresting cell
cycle progression and triggering apoptosis. Overall, neferine may
be a suitable candidate for developing novel therapies for
treatment of ESCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KA and YZ designed the study, performed the
laboratory experiments and wrote the manuscript. YL was responsible
for the statistical analysis. SY, ZH, MC, GuL and CD assisted with
the experiments and data analysis. JG and GaL reviewed and edited
the manuscript. All authors have read and approved the final
manuscript and agree to be accountable and agree to be accountable
for all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu X, Li Z, Li T, Long F, Lv Y, Liu L,
Liu X and Zhan Q: Osthole inhibits the PI3K/AKT signaling pathway
via activation of PTEN and induces cell cycle arrest and apoptosis
in esophageal squamous cell carcinoma. Biomed Pharmacother.
102:502–509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan X, Liu Y, Li G, Lan Z, Ma M, Li H,
Kong J, Sun J, Hou G, Hou X, et al: Blockade of immune-checkpoint
B7-H4 and lysine demethylase 5B in esophageal squamous cell
carcinoma confers protective immunity against P. gingivalis
infection. Cancer Immunol Res. 7:1440–1456. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan H, Zhou W, Yang Y, Xue L, Liu L and
Song Y: ISG15 promotes esophageal squamous cell carcinoma
tumorigenesis via c-MET/Fyn/β-catenin signaling pathway. Exp Cell
Res. 367:47–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu L and Chen L: Progress in research on
paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 24:402019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kundranda MN and Niu J: Albumin-bound
paclitaxel in solid tumors: Clinical development and future
directions. Drug Des Devel Ther. 9:3767–3777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scherz A, Feller K, Berezowska S, Genitsch
V and Zweifel M: Successful treatment of pituitary germinoma with
etoposide, cisplatin, vincristine, methotrexate and bleomycin
chemotherapy without radiotherapy. Anticancer Res. 37:3111–3115.
2017.PubMed/NCBI
|
|
8
|
Zhu B, Yu L and Yue Q: Co-delivery of
vincristine and quercetin by nanocarriers for lymphoma combination
chemotherapy. Biomed Pharmacother. 91:287–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pham DC, Chang YC, Lin SR, Fuh YM, Tsai MJ
and Weng CF: FAK and S6K1 inhibitor, neferine, dually induces
autophagy and apoptosis in human neuroblastoma cells. Molecules.
23:31102018. View Article : Google Scholar
|
|
10
|
Deng G, Zeng S, Ma J, Zhang Y, Qu Y, Han
Y, Yin L, Cai C, Guo C and Shen H: The anti-tumor activities of
Neferine on cell invasion and oxaliplatin sensitivity regulated by
EMT via Snail signaling in hepatocellular carcinoma. Sci Rep.
7:416162017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu L, Zhang X, Li Y, Lu S, Lu S, Li J,
Wang Y, Tian X, Wei JJ, Shao C and Liu Z: Neferine induces
autophagy of human ovarian cancer cells via p38 MAPK/JNK
activation. Tumour Biol. 37:8721–8729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Liu Z, Xu B, Sun Z, Gong Y and
Shao C: Neferine, an alkaloid ingredient in lotus seed embryo,
inhibits proliferation of human osteosarcoma cells by promoting p38
MAPK-mediated p21 stabilization. Eur J Pharmacol. 677:47–54. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cannell IG, Merrick KA, Morandell S, Zhu
CQ, Braun CJ, Grant RA, Cameron ER, Tsao MS, Hemann MT and Yaffe
MB: A pleiotropic RNA-binding protein controls distinct cell cycle
checkpoints to drive resistance of p53-defective tumors to
chemotherapy. Cancer Cell. 28:8312015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan X, Liu W, Chai Y, Hu L, Wang J and
Zhang Y: Identification of Hub genes in atypical teratoid/rhabdoid
tumor by bioinformatics analyses. J Mol Neurosci. 2020:(Online
ahead of print).
|
|
15
|
Saldivar JC, Hamperl S, Bocek MJ, Chung M,
Bass TE, Cisneros-Soberanis F, Samejima K, Xie L, Paulson JR,
Earnshaw WC, et al: An intrinsic S/G2 checkpoint enforced by ATR.
Science. 361:806–810. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Foijer F, Wolthuis RM, Doodeman V, Medema
RH and te Riele H: Mitogen requirement for cell cycle progression
in the absence of pocket protein activity. Cancer Cell. 8:455–466.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu W, Chai Y, Hu L, Wang J, Pan X, Yuan
H, Zhao Z, Song Y and Zhang Y: Polyphyllin VI induces apoptosis and
autophagy via reactive oxygen species mediated JNK and P38
activation in Glioma. Onco Targets Ther. 13:2275–2288. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020.(Online ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al Shahrani M, Balasubramaniam M,
Alshahrani MY, Saif A, Dera AA, Alasmari S, Abohassan M, Makkawi M,
Radhakrishnan S and Rajagopalan P: Computational and in vitro
characterization of ICY-5: A potential candidate promoting
mitochondrial apoptosis via the c-MET and STAT3 pathways. J Cell
Physiol. 2020.(Online ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buckley AM, Lynam-Lennon N, O'Neill H and
O'Sullivan J: Targeting hallmarks of cancer to enhance
radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol
Hepatol. 17:298–313. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Del Re DP, Amgalan D, Linkermann A, Liu Q
and Kitsis RN: Fundamental mechanisms of regulated cell death and
implications for heart disease. Physiol Rev. 99:1765–1817. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Soteriou D and Fuchs Y: A matter of life
and death: stem cell survival in tissue regeneration and tumour
formation. Nat Rev Cancer. 18:187–201. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fendt SM and Lunt SY: Dynamic ROS
regulation by TIGAR: Balancing anti-cancer and pro-metastasis
effects. Cancer Cell. 37:141–142. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Z, Song J, Nie L and Chen X: Reactive
oxygen species generating systems meeting challenges of
photodynamic cancer therapy. Chem Soc Rev. 45:6597–6626. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kahroba H, Shirmohamadi M, Hejazi MS and
Samadi N: The Role of Nrf2 signaling in cancer stem cells: From
stemness and self-renewal to tumorigenesis and chemoresistance.
Life Sci. 239:1169862019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferino A, Rapozzi V and Xodo LE: The
ROS-KRAS-Nrf2 axis in the control of the redox homeostasis and the
intersection with survival-apoptosis pathways: Implications for
photodynamic therapy. J Photochem Photobiol B. 202:1116722020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DeNicola GM, Karreth FA, Humpton TJ,
Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES,
et al: Oncogene-induced Nrf2 transcription promotes ROS
detoxification and tumorigenesis. Nature. 475:106–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan D, Huang S, Berger E, Liu L, Gross N,
Heinzmann F, Ringelhan M, Connor TO, Stadler M, Meister M, et al:
Kupffer cell-derived tnf triggers cholangiocellular tumorigenesis
through JNK due to chronic mitochondrial dysfunction and ROS.
Cancer Cell. 31:771–789.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Owusu-Ansah E, Yavari A, Mandal S and
Banerjee U: Distinct mitochondrial retrograde signals control the
G1-S cell cycle checkpoint. Nat Genet. 40:356–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui YJ, Ma CC, Zhang CM, Tang LQ and Liu
ZP: The discovery of novel indazole derivatives as tubulin
colchicine site binding agents that displayed potent antitumor
activity both in vitro and in vivo. Eur J Med Chem. 187:1119682020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mondal A, Gandhi A, Fimognari C, Atanasov
AG and Bishayee A: Alkaloids for cancer prevention and therapy:
Current progress and future perspectives. Eur J Pharmacol.
858:1724722019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salehi B, Selamoglu ZS, Mileski K, Pezzani
R, Redaelli M, Cho WC, Kobarfard F, Rajabi S, Martorell M, Kumar P,
et al: Liposomal Cytarabine as Cancer Therapy: From chemistry to
medicine. Biomolecules. 9:7732019. View Article : Google Scholar
|
|
34
|
Lin JH, Ting PC, Lee WS, Chiu HW, Chien
CA, Liu CH, Sun LY and Yang KT: Palmitic acid methyl ester induces
G2/M arrest in human bone marrow-derived mesenchymal stem cells via
the p53/p21 pathway. Stem Cells Int. 2019:76062382019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kreis NN, Louwen F and Yuan J: Less
understood issues: p21(Cip1) in mitosis and its therapeutic
potential. Oncogene. 34:1758–1767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kerr N, de Rivero Vaccari JP, Dietrich WD
and Keane RW: Neural-respiratory inflammasome axis in traumatic
brain injury. Exp Neurol. 323:1130802020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Z, Yu K, Hu Y, Su F, Gao Z, Hu T,
Yang Y, Cao X and Qian F: Schisantherin A induces cell apoptosis
through ROS/JNK signaling pathway in human gastric cancer cells.
Biochem Pharmacol. 173:1136732020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yuan YL, Jiang N, Li ZY, Song ZZ, Yang ZH,
Xue WH, Zhang XJ and Du Y: Polyphyllin VI induces apoptosis and
autophagy in human osteosarcoma cells by modulation of ROS/JNK
activation. Drug Des Devel Ther. 13:3091–3103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zou P, Zhang J, Xia Y, Kanchana K, Guo G,
Chen W, Huang Y, Wang Z, Yang S and Liang G: ROS generation
mediates the anti-cancer effects of WZ35 via activating JNK and ER
stress apoptotic pathways in gastric cancer. Oncotarget.
6:5860–5876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Che Y, Wang J, Yuan Z, Li Y, Lu Z, Zhang
Z, Zhang J, Wan J, Sun H, Chen Z, et al: The therapeutic effects of
Longikaurin A, a natural ent-kauranoid, in esophageal squamous cell
carcinoma depend on ROS accumulation and JNK/p38 MAPK activation.
Toxicol Lett. 280:106–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Wang X, Wu T, Li B, Liu T, Wang
R, Liu Q, Liu Z, Gong Y and Shao C: Isoliensinine induces apoptosis
in triple-negative human breast cancer cells through ROS generation
and p38 MAPK/JNK activation. Sci Rep. 5:125792015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo Z, Guozhang H, Wang H, Li Z and Liu N:
Ampelopsin inhibits human glioma through inducing apoptosis and
autophagy dependent on ROS generation and JNK pathway. Biomed
Pharmacother. 116:1085242019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang H, Zhang T, Sun W, Wang Z, Zuo D,
Zhou Z, Li S, Xu J, Yin F, Hua Y and Cai Z: Erianin induces
G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK
signaling pathway in human osteosarcoma cells in vitro and in vivo.
Cell Death Dis. 7:e22472016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Banerjee N, Wang H, Wang G and Khan MF:
Enhancing the Nrf2 antioxidant signaling provides protection
against trichloroethene-mediated inflammation and autoimmune
response. Toxicol Sci. 175:64–74. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou XL, Wu X, Zhu RR, Xu H, Li YY, Xu QR,
Liu S, Lai SQ, Xu X, Wan L, et al: Notch1-Nrf2 signaling crosstalk
provides myocardial protection by reducing ROS formation. Biochem
Cell Biol. 98:106–111. 2020. View Article : Google Scholar : PubMed/NCBI
|