Introduction
Lung cancer is the leading cause of cancer-related
death worldwide, accounting for more than 1.5 million deaths
annually (1). Adenocarcinoma, which
surpasses squamous cell carcinoma as the most frequent type of lung
cancer, is prone to malignant metastasis even at the early stages
(2). Despite improvements in
treatment for lung cancer, distant metastasis and cancer recurrence
are still the main causes of death in patients with lung
adenocarcinoma (3). Therefore, it
is essential to develop novel treatment strategies for preventing
the metastasis of lung adenocarcinoma.
Tumor metastasis is a multi-step process that
involves local invasion, intravasation, extravasation and
colonization, during which the formation of secondary tumors result
from the spreading of primary tumor cells to a distant site
(4,5). The first critical step in tumor
metastasis is invasion, which often requires the loss of
intercellular connections and acquisition of cell motility in tumor
epithelial cells (6).
Epithelial-mesenchymal transition (EMT), as characterized by a loss
of cell-cell adhesion, polarity and epithelial markers, is well
recognized to play a crucial role in tumor invasion (5). EMT occurs during embryonic development
and tissue organization under normal physiological conditions;
however, its dysregulation can interrupt epithelial homeostasis and
lead to various pathological conditions, including tumor metastasis
(7).
A number of clinical and experimental studies have
demonstrated that an inflammatory microenvironment may contribute
to tumor development and metastasis (8). Inflammatory cytokines, such as
interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interferon-γ
and IL-1β, are important components in an inflammatory
microenvironment (9,10). Among them, IL-6 is a key
inflammatory factor involved in the regulation of tumorigenesis,
progression and metastasis (11–13).
IL-6 can activate Janus kinase (JAK)/signal transducer and
activator of transcription 3 (STAT3) signaling pathway by binding
to the IL-6 receptor (IL-6R) complex, which consists of IL-6R and
gp130 (14). Phosphorylated STAT3
(p-STAT3; Y705) monomers can form dimers and subsequently
translocate into the nucleus, in order to regulate the
transcription of their target genes and eventually change the
fundamental cellular processes (15,16).
It has been confirmed that the STAT3 signaling pathway can
facilitate the EMT process by regulating the expression levels
EMT-associated genes, including E-cadherin, Snail and Twist
(6,17).
The naphthoquinone shikonin is the main active
ingredient of Zicao, a traditional Chinese herbal medicine
made from the root of Lithospermum erythrorhizon (18). Shikonin has a wide spectrum of
pharmacological effects as well as favorable pharmacokinetic
properties in vivo (19). A
number of studies have demonstrated that shikonin possesses strong
antitumor activity though the inhibition of tumor growth,
metastasis and angiogenesis in various cancer types (19,20).
Shikonin can also modulate cancer cell metabolism by inhibiting
tumor-specific pyruvate kinase-M2 (21). In addition, shikonin can promote
cell cycle arrest, induce necrosis and exacerbate necroptosis in
certain types of tumors (22,23).
Furthermore, shikonin exerts different pharmacological effects in
an inflammatory microenvironment (19). Considering the close association
between the inflammatory microenvironment and tumor metastasis, one
of its effects may be related to tumor inhibition (24). However, the potential antitumor
effects of shikonin in the inflammatory microenvironment and its
underlying molecular mechanisms remain largely unknown. The present
study investigated the effects of shikonin on tumor metastasis and
its underlying molecular mechanisms in an inflammatory
microenvironment. The findings of this study could provide new
insights into the mechanisms underlying the therapeutic effects of
shikonin in treating inflammation-related tumor metastasis.
Materials and methods
Reagents
Shikonin was obtained from MedchemExpress.
Lipopolysaccharide (LPS) and diamidino-phenyl-indole (DAPI) were
supplied by Sigma-Aldrich (a brand of Merck KGaA). Recombinant
human IL-6 and TNF-α were obtained from PeproTech. Human IL-6
neutralizing antibody (MAB206) and human TNF-α neutralizing
antibody (MAB610) were obtained from R&D Systems. Bovine serum
albumin (BSA) was purchased from Roche. All other reagents used in
this study were of analytical grade.
Cell culture
Two human lung adenocarcinoma cell lines (A549 and
H1299) and a human acute monocytic leukemia cell line (THP-1) were
purchased from the Cell Bank of the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). Cells were cultured
with F-12 medium (A549 cells; Gibco; Thermo Fisher Scientific,
Inc.) and RPMI-1640 medium (H1299 and THP-1 cells; Gibco; Thermo
Fisher Scientific, Inc.) containing 10% fetal bovine serum (FBS)
and maintained in a humidified atmosphere of 95% air and 5%
CO2 at 37°C.
THP-1 cell conditioned medium
THP-1 cell conditioned medium (THP-1-CM) was
prepared as previously described (6). Briefly, after adding 10 µg/ml LPS into
THP-1 cells (1×106 cells/ml) for 24 h, the supernatant
was collected by centrifuging at 1,520 × g for 15 min. Then, A549
and H1299 cells were treated with THP-1-CM and different
concentrations (0.25, 0.75 or 1.25 µM) of shikonin for 24 h.
Cell viability assay
Cell viability was determined using a Cell Counting
Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology). Briefly,
the cells (A549 and H1299) were seeded into 96-well plates at
5×103 cells/well and incubated under normal culture
conditions for 24 h, and then the cells were treated with the
indicated concentrations of shikonin and/or THP-1-CM for another 24
h. After treatment, 10 µl of CCK-8 solution was added into each
well of the 96-well plate (total medium 100 µl/well) and incubated
for 1 h at 37°C. Optical density values were detected using a
microplate reader (Model 550, Bio-Rad Laboratories) at 450 nm. The
experiment was independently repeated three times with five
replicates.
Wound healing assay
The migration ability of THP-1-CM-treated lung
adenocarcinoma cells (A549 and H1299) was evaluated using a wound
healing assay. Briefly, the cells were seeded in a 6-well plate at
5×105 cells/well and allowed to grow up to 80%
confluence. Subsequently, the cell monolayer was scratched with a
pipette tip to create a narrow wound-like gap. Shortly after
wounding, the cells were washed with phosphate-buffered saline
(PBS) and further treated with various concentrations of shikonin
and/or THP-1-CM for 24 h. The plates were photographed at 0 and 24
h with an inverted light microscope (IX53 Olympus; magnification
×40). The relative migrated distance was then analyzed. The
experiment was independently repeated three times with three
replicates.
Transwell chamber migration and
invasion assays
Chamber migration and invasion assays were performed
using a Transwell assay system (Corning Costar) as reported
previously (25,26). After treatment under the indicated
experimental conditions, the cells (A549 and H1299,
1×105 cells/chamber) suspended in 100 µl serum-free
medium were added to the upper chamber, while the lower chamber was
filled with complete medium containing 10% serum. The cells were
allowed to migrate at 37°C for 24 h. After removing non-migrated
cells, the membranes were fixed with 4% formaldehyde for 20 min. At
the end of fixation, the chambers were rinsed with PBS, and the
cells in the lower chamber were stained with 0.5% crystal violet
(Beyotime Institute of Biotechnology) for 10 min. Migrated cells
were quantified in five random fields per membrane, and each group
was assayed in triplicate. The invasion assay was performed as per
the migration assay, except that the filter membrane was coated
with Matrigel (BD Bioscience). The experiment was repeated three
times independently.
Western blotting
The cells (A549 and H1299) were seeded in 6-well
plates at 5×105 cells/well and allowed to grow up to
~70% confluence under normal culture conditions, then the cells
were treated with indicated administration conditions for 24 h.
After treatment, the total protein was isolated from cells by RIPA
buffer, and the concentrations of proteins were determined using a
bicinchoninic acid protein assay kit according to the
manufacturer's protocol (Beyotime Institute of Biotechnology).
Equal amounts of protein (30 µg protein per lane) from each sample
were separated by 10% SDS-PAGE and transferred to nitrocellulose
filter membrane at 100 mV for 75 min. After being blocked with 5%
non-fat skim milk [diluted with Tris-buffered saline containing
0.1% Tween 20 (TBST)] for 1 h at room temperature, the membrane was
incubated overnight with primary antibody (diluted with 2% bovine
serum albumin in TBST) at 4°C. The following primary antibodies
were used: anti-E-cadherin antibody (dilution 1:1,000; cat. no.
ab40772; Abcam), anti-N-cadherin antibody (dilution 1:1,000; cat.
no. ab18203; Abcam), anti-p-STAT3 antibody (dilution 1:2,000; cat.
no. ab76315; Abcam), anti-STAT3 antibody (dilution 1:2,500; cat.
no. ab119352; Abcam), anti-vimentin antibody (dilution 1:300;
bs-8533R; Biosynthesis Biotechnology), anti-Flag antibody (dilution
1:1,000; 20543-1-AP; Proteintech), anti-Snail antibody (dilution
1:500; 13099-1-AP; Proteintech), anti-p-JAK2 antibody (dilution
1:1,000; 3771; Cell Signaling Technology), anti-JAK2 antibody
(dilution 1:1,000; 3230; Cell Signaling Technology), and β-actin
(dilution 1:1,000; sc-47778; Santa Cruz Biotechnology, Inc.). On
the second day, blots were washed and incubated with anti-rabbit
(dilution 1:5,000; SA00001-2; Proteintech) or anti-mouse (dilution
1:5,000; SA00001-1; Proteintech) horseradish-peroxidase-conjugated
secondary antibody for 1 h. The protein bands were visualized with
an enhanced chemiluminescence western blot detection kit (Pierce
Biotechnology; Thermo Fisher Scientific, Inc.), and the protein
expression levels were semi-quantified by densitometry using ImageJ
v1.46r (National Institutes of Health) (27,28).
The experiment was repeated three times independently.
Quantitative real-time PCR
RNA isolation and RT-qPCR assay were performed as
previously described (29). The
cells (A549 and H1299) were seeded in 6-well plates at the number
of 5×105 cells/well and allowed to grow up to about 70%
confluence under normal culture conditions, then the cells were
treated with indicated administration conditions for 24 h. After
treatment, TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to isolate total RNA from the cell lines. RNA was
reverse-transcribed into cDNA by AMV reverse transcriptase
(Promega). Quantitative real-time PCR was carried out with SYBR
Premix Ex Taq (Takara Bio Inc.). All reactions were performed in
triplicate using an ABI Prism 7500 real-time PCR instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The relative
expression levels of target genes were determined after
normalization to β-actin level via the 2−ΔΔCq method
(30). The experiment was
independently repeated three times with three replicates. The
primers used in the reactions were as follows: E-cadherin (forward,
5′-CCACCAAAGTCACGCTGAAT-3′ and reverse, 5′-GGAGTTGGGAAATGTGAGC-3′),
N-cadherin (forward, 5′-AGCCTGGAACATATGTGATGA-3′ and reverse,
5′-CCATAAAACGTCATGGCAGTAA-3′), vimentin (forward,
5′-GGTGGACCAGCTAACCAACG-3′ and reverse,
5′-GTCAAGACGTGCCAGAGACG-3′), Snail (forward,
5′-GCCTTCAACTGCAAATACTGC-3′; reverse,
5′-CTTCTTGACATCTGAGTGGGTC-3′), β-actin (forward,
5′-TGGATCAGCAAGCAGGAGTA-3′ and reverse,
5′-TCGGCCACATTGTGAACTTT-3′).
Immunofluorescence
The immunofluorescence assay was performed as
previously described (31).
Briefly, the cells (A549 and H1299) were seeded on glass coverslips
in the special dishes and allowed to grow up to about 30%
confluence under normal culture conditions, then the cells were
treated with indicated administration conditions for 24 h. After
treatment, the cells werefixed with 4% paraformaldehyde for 20 min,
permeabilized with 0.5% Triton X-100, and blocked with 3% BSA for 1
h. The cells were then incubated with rabbit-anti-human antibodies
for E-cadherin (1:200 dilution; cat. no. 20874-1-AP; Proteintech),
N-cadherin (1:200 dilution; cat. no. 13116; Cell Signaling
Technology), vimentin (1:200 dilution; cat. no. 10366-1-AP;
Proteintech), and p-STAT3 (1:100 dilution; cat. no. 9145S; Cell
Signaling Technology) at 4°C overnight. After washing, the cells
were incubated with FITC-conjugated goat-anti-rabbit secondary
antibodies (1:200 dilution; cat. no. 31583; Invitrogen; Thermo
Fisher Scientific, Inc.) for 1 h, and the nuclei were
counterstained with DAPI. Finally, the stained cells were observed
using a confocal fluorescence microscope (FV1000-D, Olympus;
magnification ×800). The mean fluorescence intensities of
E-cadherin, N-cadherin and vimentin were quantified in five random
fields per sample using ImageJ v1.46r (National Institutes of
Health, Bethesda) according to the specified instructions. The
number of cells with p-STAT3 nuclear translocation and the
corresponding total number of cells were counted in at least five
random fields per sample by two independent observers in a blinded
(code-marked) approach, and the percentage of cells with nuclear
p-STAT3 translocation was also calculated. Each experiment was
performed in triplicate.
Enzyme-linked immunosorbent assay
Both IL-6 and TNF-α levels in the supernatant of
THP-1 cells were measured using a Quantikine enzyme-linked
immunosorbent assay (ELISA) kit (R&D Systems) according to the
manufacturer's instructions. The experiment was carried out in
triplicate and repeated three times independently.
Transient transfection
PcDNA3.1-Flag-STAT3 (Bioworld Technology, Inc.) was
transfected into cells (A549 and H1299) using Lipofectamine 2000™
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. After 24 h of the transfection,
the cells were treated with shikonin (1.25 µM) and IL-6 (50 ng/ml)
for another 24 h, then the subsequent experiments were
conducted.
Luciferase assay
Lung adenocarcinoma cells (A549 and H1299) were
seeded in 48-well plates at the number of 3×104
cells/well and cultured under normal conditions for 24 h, then
transfected with 0.2 µg pSTAT3-TA-luc (Beyotime Institute of
Biotechnology) and 0.01 µg pRL-TK Renilla (Beyotime Institute of
Biotechnology) using Lipofectamine 2000. Twenty-four hours after
transfection, the cells were treated with shikonin and IL-6 (50
ng/ml) for 24 h. After treatment, the cells were lysed using
passive lysis buffer, and the luciferase activity was detected
using a Dual Luciferase Reporter kit (Beyotime Institute of
Biotechnology). The experiment was carried out in triplicate and
repeated three times independently.
Tumor metastasis assay in vivo
All animal studies were approved by the Animal
Ethics Committee of The Fourth Military Medical University (Xi'an,
Shaanxi) (nos. 20150803 and 20170201). Nude mice (28–32 days old,
weighing 18–24 g, n=39) were obtained from Shanghai Laboratory
Animal Co. and maintained in air-conditioned rooms under controlled
lighting (12 h light/12 h dark). Standard laboratory water and food
were available ad libitum. The animal experimental
procedures were performed in strict accordance with the guidelines
approved by the Institutional Review Board of Xi Jing Hospital of
The Fourth Military Medical University, and conformed to the
principles for the ethical use of animals.
The tumor metastasis model was established based on
the experiences of our own and other studies (1,6).
Briefly, A549 cells were trypsinized and resuspended in PBS to
achieve an appropriate concentration (1×106 cells/ml),
and 0.2 ml of the cell suspension was injected into the tail veins
of athymic BALB/c nude mice (5).
The mice were weighed and randomly divided into the following three
groups (n=5 per group): Control group, 2.5 mg/kg shikonin group,
and 5 mg/kg shikonin group (32).
After 24 h of inoculation, shikonin was injected into the mice
every other day for 24 days via intraperitoneal injection.
Twenty-four hours after the last administration, the mice were
euthanized with sodium pentobarbital (50 mg/kg b.w., i.p.;
Sigma-Aldrich; Merck KGaA), followed by cervical dislocation. No
mouse progressed to cachexia or death during the experimental
period. Their lungs were rapidly excised, washed, and fixed in 4%
polyoxymethylene solution. To confirm the presence of metastatic
nodules as well as determine the location and extent of
micrometastatic foci, the number of metastatic nodules on the
entire lung surface was counted under a dissecting microscope
(magnification ×20) and the histological sections of lung tissue
were stained routinely with hematoxylin and eosin (H&E).
Antitumor effect in nude mice
A549 cells (1×107) were injected
subcutaneously into the flank area of male nude mice (5). When the tumors reached an average of
75 mm3 in size, all mice were randomly divided into
three groups (n=8 per group). The control group was administered
vehicle and the treatment groups were administered different doses
of shikonin (2.5 or 5 mg/kg) injected intraperitoneally every other
day. After 24 days, the mice were euthanized with sodium
pentobarbital (50 mg/kg b.w., i.p.; Sigma-Aldrich; Merck KGaA),
followed by cervical dislocation, and their tumor xenografts were
rapidly excised, weighed, and fixed in 4% paraformaldehyde for
immunohistochemical analysis. The tumor volumes were measured with
an electronic caliper by using the formula: Tumor volume
(mm3) = length × width2/2.
Immunohistochemistry
Immunohistochemical staining was performed according
to a previous description (5).
Paraformaldehyde-fixed tumor sections (4-µm in thickness) were
incubated with rabbit-anti-human monoclonal antibodies p-STAT3
(1:100 dilution; product code ab76315; Abcam), E-cadherin (1:500
dilution; product code ab227639, Abcam), N-cadherin (1:150
dilution; cat. no. 13116; Cell Signaling Technology, Inc.) and
vimentin (1:200 dilution; product code ab92547; Abcam), followed by
incubation with horseradish peroxidase-conjugated goat-anti-rabbit
secondary antibody (1:500 dilution; cat. no. WLA023; Wanleibio Co.,
Ltd.). The sections were then incubated with developing solution
and counterstained with hematoxylin. The color was fixed with acid
alcohol and dehydration steps. Finally, the sections were observed
with a light microscope (magnification ×100), and the
representative images were photographed. The experiment was
performed in triplicate.
Tissue microarray
A human lung adenocarcinoma tissue microarray (TMA)
containing 128 cases of lung adenocarcinoma specimens was
constructed by Xi'an Alenabio Biotechnology. The dot array was 1.5
mm in diameter, and each dot represented a tissue spot from one
individual specimen that was specifically selected and
pathologically confirmed. To compare the levels of IL-6 and CD68 in
lung adenocarcinoma tissues and those in normal lung tissues and
analyze the correlation between them, TMAs that composed 1.5-mm
diameter dots of 30 human lung adenocarcinoma tissues and 30
matched normal lung tissues were purchased from Outdo Biotech Co.,
Ltd. The study protocol was approved by the Institutional Review
Board of Xi Jing Hospital of The Fourth Military Medical
University. All experiments involving human subjects were performed
after obtaining informed consent, and in accordance with the
relevant guidelines and regulations.
Immunohistochemistry and TMA
score
Immunohisto-chemistry of TMA was performed as
described previously (33). The
anti-IL-6 antibody (1:100 dilution; bs-0781R; Bioss Biological
Technology) and anti-CD68 antibody (1:100 dilution; bs-0649R; Bioss
Biological Technology) were used for primary antibody incubation at
4°C overnight, while omission of the primary antibody served as a
negative control.
The immunohistochemical images of TMAs were scored
by applying a semi-quantitative immunoreactivity score (IRS) as
reported previously (1). Briefly,
category A documented the intensity of immunostaining as 0
(negative staining), 1 (weak staining) and 2 (strong staining).
Category B documented the percentage of immunoreactive cells as 1
(0-25%), 2 (26-50%), 3 (51-75%) and 4 (76-100%). The IRS
(range=0-8) was calculated by multiplying scores from categories A
and B. Samples with IRS scores of 0–3 were classified as low
expression, while scores of 4–8 were classified as high expression.
The results were evaluated by two independent pathologists who were
blinded to the clinical data.
Statistical analysis
Statistical analyses were performed with SPSS
version 16.0 software (SPSS Inc.). Data shown were obtained from at
least three independent experiments. Differences between groups
were compared using the Student's t-test or one-way analysis of
variance (ANOVA) followed by Bonferroni's test. For TMA, the
association between IL-6 levels and clinicopathological parameters
was evaluated using the Chi-square test. The correlation between
IL-6 and CD68 expression was assessed using the nonparametric
Spearman test. All data are expressed as mean ± standard deviation
(SD). A significant difference was defined as *P<0.05 or
**P<0.01 as indicated in the figures and defined in the figure
legends.
Results
Shikonin blocks THP-1-CM-induced
migration and invasion of human lung adenocarcinoma cells
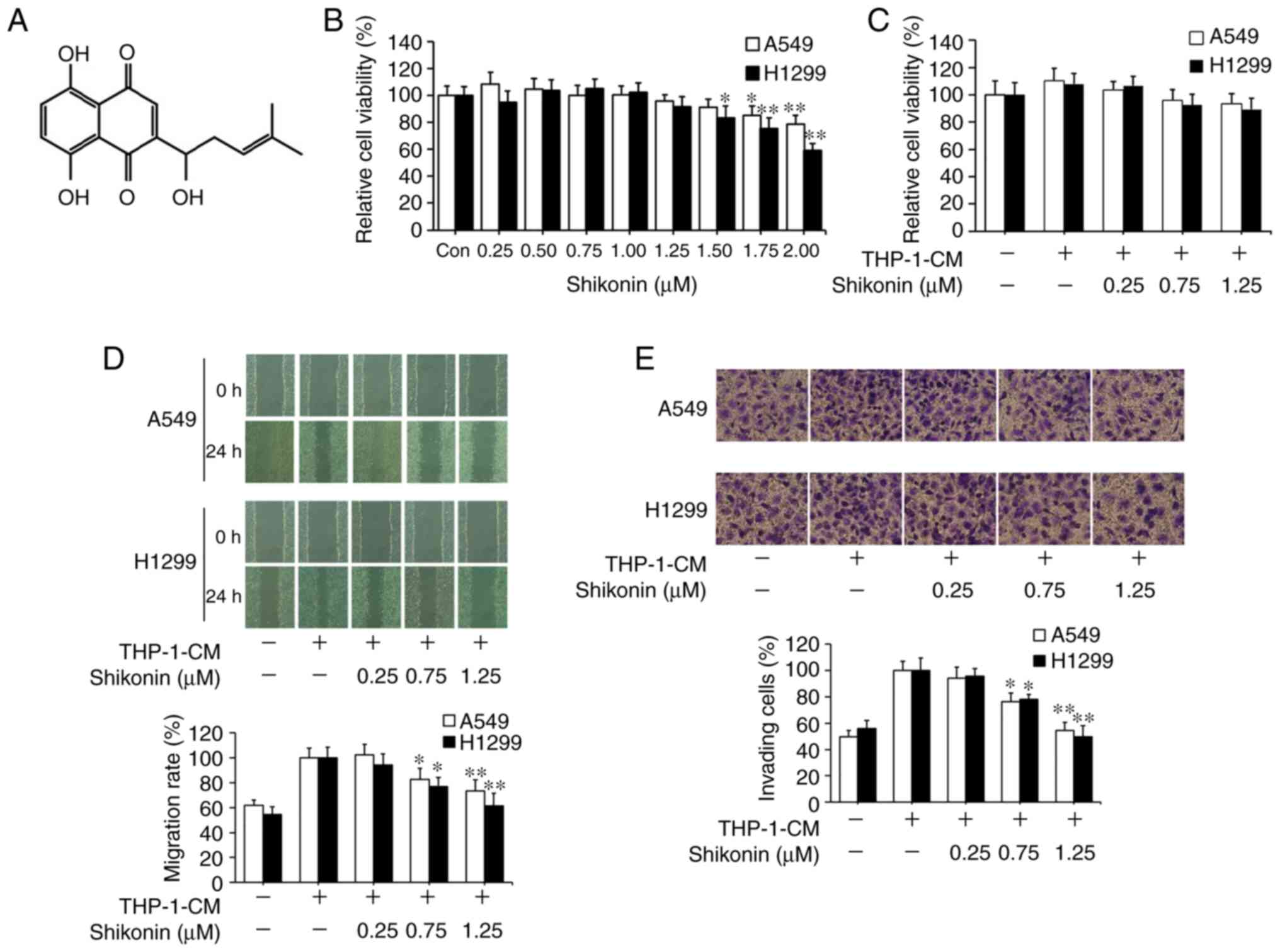
Shikonin has been reported as a potential antitumor
agent. Its chemical structure is shown in Fig. 1A. CCK-8 assay was first used to
assess the effects of shikonin on lung adenocarcinoma A549 and
H1299 cell viability under normal culture conditions. As shown in
Fig. 1B, the cytotoxic effects of
shikonin on both cell lines were in a dose-dependent manner, where
no obvious cytotoxicity was observed when its concentration was
equal to or less than 1.25 µM. Following that, THP-1-CM was used to
mimic the actual inflammatory microenvironment as described
previously (5,6). THP-1-CM alone or in combination with
0.25, 0.75 or 1.25 µM shikonin exerted no significant effect on the
viability of A549 and H1299 cells (Fig.
1C). Wound healing and chamber invasion assays were used to
assess the effects of shikonin on cell migration and invasion
induced by THP-1-CM. As shown in Fig.
1D, upon exposure to THP-1-CM alone, elevated wound closure
activity was observed in both cells lines with the scratch gap
almost filled, whereas the gap still existed in both cell lines
after co-treatment with THP-1-CM and shikonin (0.75 and 1.25 µM).
Similarly, chamber invasion assays revealed that shikonin inhibited
the invasion of A549 and H1299 cells after stimulation with
THP-1-CM (Fig. 1E). These results
indicate that shikonin can inhibit the migration and invasion
activities of lung adenocarcinoma cells induced by THP-1-CM.
Shikonin reverses the changes in the
expression levels of EMT markers induced by THP-1-CM in human lung
adenocarcinoma cells
Considering that EMT can be induced by various
cytokines within an inflammatory microenvironment and its
deregulation may lead to tumor metastasis, the markers associated
with EMT were examined in order to explore the molecular mechanism
underlying the inhibitory effects of shikonin on lung
adenocarcinoma cell migration and invasion activities. As shown in
Fig. 2A and B, the protein
expression levels of mesenchymal markers (N-cadherin and vimentin)
were found to be increased, while the level of the epithelial
marker (E-cadherin) was markedly decreased in cells cultured with
THP-1-CM alone; however, such changes in expression levels were
reversed by shikonin treatment. The alterations in the mRNA
expression levels of E-cadherin, N-cadherin and vimentin were
consistent with their protein expression levels (Fig. 2C). These results suggest that
shikonin can inhibit the migration and invasion activities of lung
adenocarcinoma cells by reversing THP-1-CM-induced EMT.
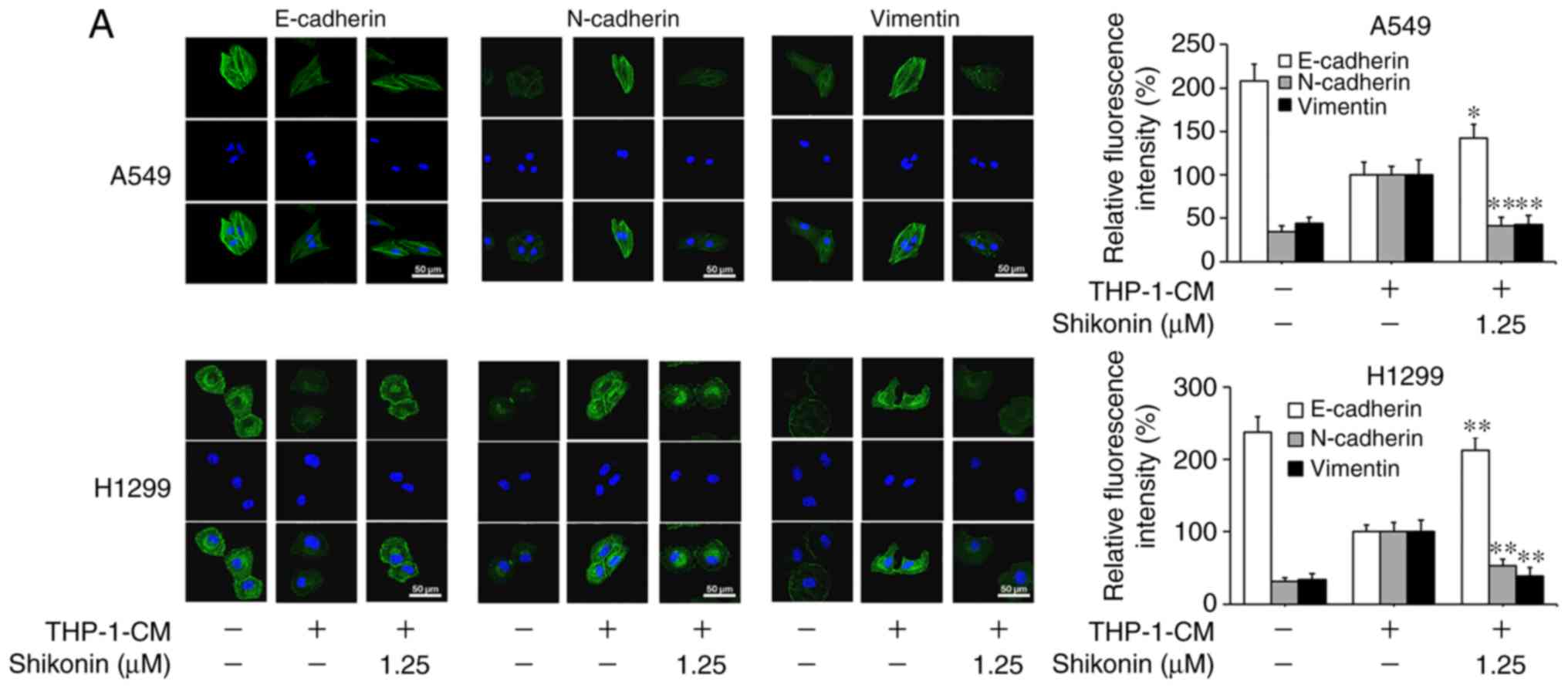 | Figure 2.Shikonin suppresses THP-1-CM-induced
epithelial-mesenchymal transition (EMT) in A549 and H1299 cells.
(A) Immunofluorescence was used to visualize the protein expression
levels of E-cadherin, N-cadherin and vimentin after treatment with
THP-1-CM and/or shikonin for 24 h. Left, representative
immunofluorescence images of E-cadherin, N-cadherin and vimentin
(magnification ×800). Right, quantitative image analysis of the
signal intensity of E-cadherin, N-cadherin and vimentin. (B)
Western blot assays were used to detect the protein expression
levels of E-cadherin, N-cadherin and vimentin after treatment with
THP-1-CM and/or shikonin for 24 h. (C) Real-time PCR was used to
detect the mRNA levels of E-cadherin, N-cadherin and vimentin after
treatment with THP-1-CM and/or shikonin for 24 h. β-actin was used
as an internal control. Data are shown as the mean ± SD of three
independent experiments. *P<0.05, **P<0.01, compared with the
THP-1-CM-treated group. THP-1-CM, THP-1 cell conditioned
medium. |
IL-6 and TNF-α in THP-1-CM trigger EMT
in human lung adenocarcinoma cells
Based on previous findings that IL-6 and TNF-α can
promote EMT and migration in tumor cells (5,6,34), we
investigated the roles of IL-6 and TNF-α in THP-1-CM-induced EMT
and migration. As shown in Fig. 3A,
the levels of IL-6 and TNF-α markedly increased in the supernatants
from the LPS-challenged THP-1 cells, with their detectable levels
significantly reduced upon the addition of the respective
neutralizing antibodies. Moreover, the migration of A549 and H1299
cells induced by THP-1-CM was significantly attenuated by the
neutralizing anti-IL-6 antibody (Fig.
3B). The changes in the protein and mRNA expression levels of
EMT markers induced by THP-1-CM were also reversed upon the
addition of the anti-IL-6 antibody (Fig. 3C and D). Similar phenomena were also
observed after the addition of the anti-TNF-α antibody. These
results indicate that IL-6 and TNF-α play important roles in the
EMT of lung adenocarcinoma cells.
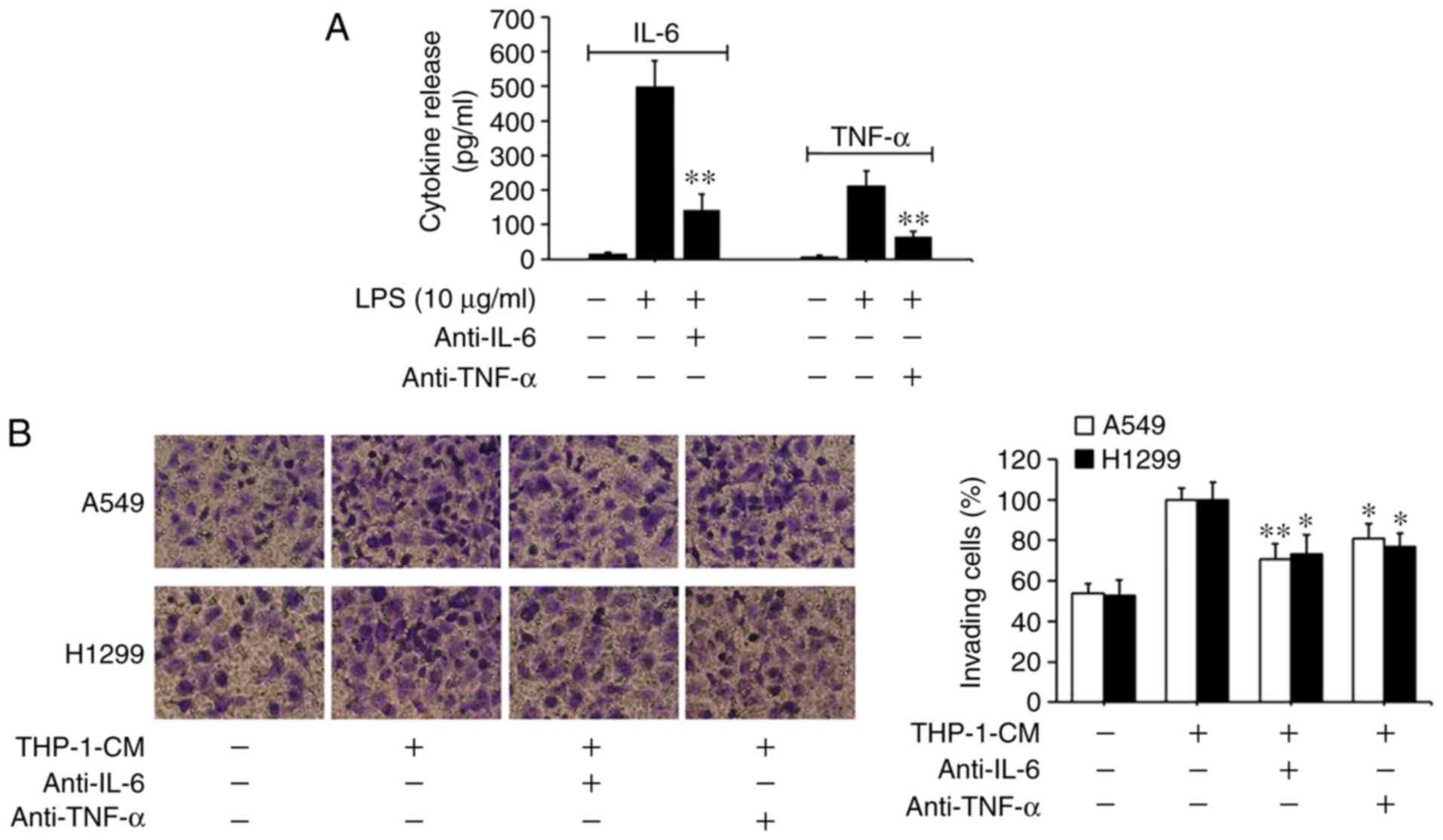 | Figure 3.Effect of IL-6 and TNF-α on the
migration and epithelial-mesenchymal transition (EMT) of A549 and
H1299 cells. (A) IL-6 and TNF-α secreted into the culture
supernatants were measured using ELISA kits after treatment with
LPS and anti-IL-6 or anti-TNF-α antibodies for 24 h in THP-1-CM.
**P<0.01, compared with the corresponding LPS-treated alone
group. (B) Effects of 24-h neutralization with anti-IL-6 or
anti-TNF-α antibody on the migration of A549 and H1299 cells, as
determined by the Transwell chamber migration assay. (C) The
protein levels of E-cadherin, N-cadherin and vimentin were detected
by western blotting with specific antibodies. Protein expression
levels were semi-quantified by densitometry analysis. (D) The
relative mRNA levels of E-cadherin, N-cadherin and vimentin were
detected by real-time PCR. β-actin was used as an internal control.
Data are shown as the mean ± SD of three independent experiments.
*P<0.05, **P<0.01, compared with the THP-1-CM-treated group.
THP-1-CM, THP-1 cell conditioned medium; LPS, lipopolysaccharide;
IL-6, interleukin-6; TNF-α, tumor necrosis factor-α. |
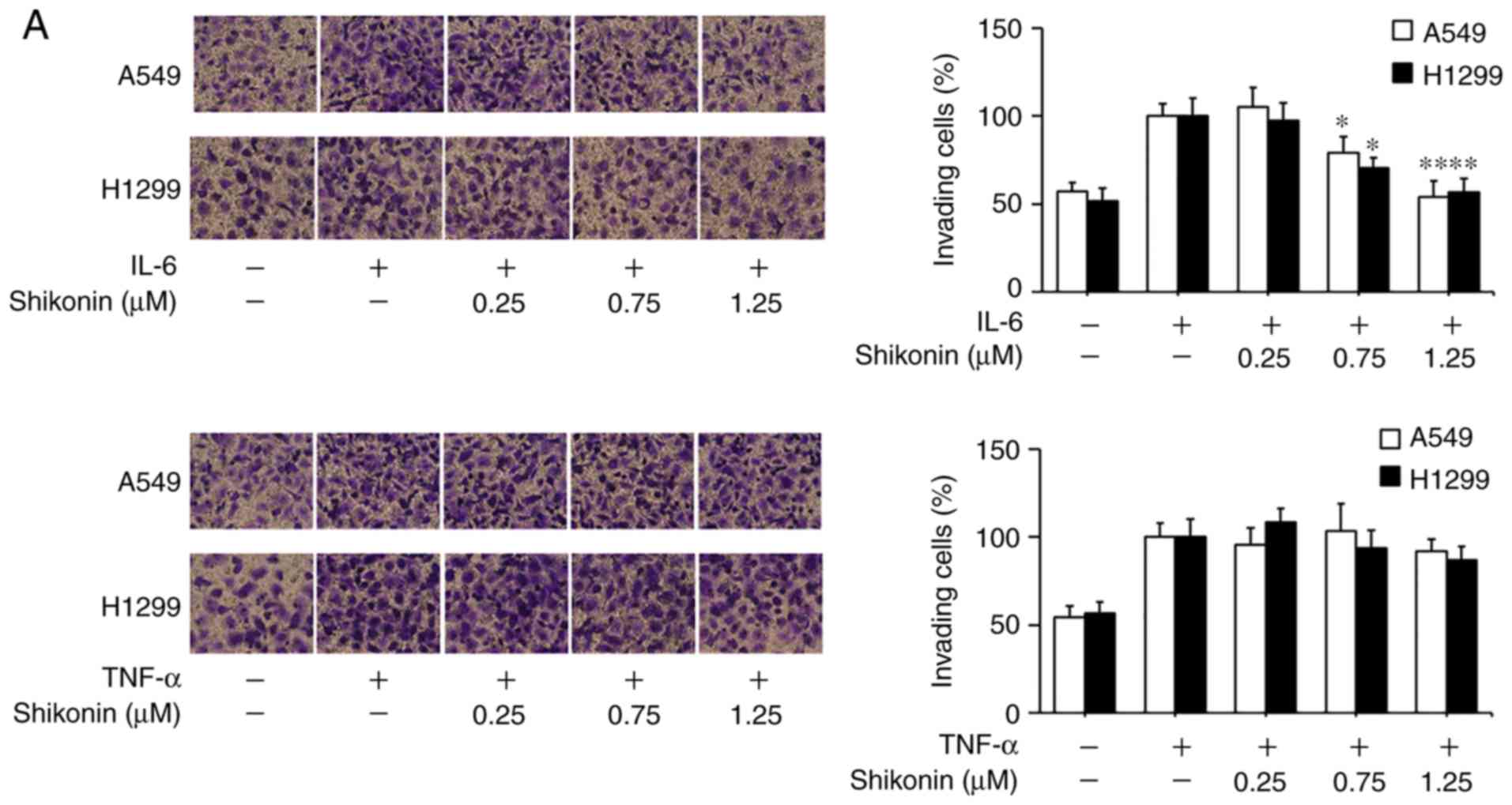
Shikonin suppresses IL-6-induced
migration and EMT in human lung adenocarcinoma cells
To gain further insights of the mechanisms
underlying the inhibitory effects of shikonin on EMT induced by
THP-1-CM, both cell lines were treated with IL-6 and TNF-α as
described previously (5). After
cultured in IL-6-supplemented media (50 ng/ml), both cell lines
displayed enhanced migration ability (Fig. 4A), which could be blocked by
shikonin administration. Furthermore, the results of western blot
analysis and real-time PCR assay revealed that shikonin restored
the downregulation of E-cadherin, while attenuated the upregulation
of N-cadherin, vimentin and Snail induced by IL-6 (Fig. 4B and C). However, shikonin did not
significantly inhibit the migration and EMT of A549 and H1299 cells
induced by 20 ng/ml TNF-α (Fig. 4D and
E), suggesting that only IL-6 and its downstream effectors are
responsible for the anti-metastatic effects of shikonin.
Shikonin inhibits IL-6-induced STAT3
activation in human lung adenocarcinoma cells
STAT3 is a downstream factor of IL-6, which
hyperactivates in the majority of human cancers and is closely
associated with poor prognosis (35). IL-6 can promote the EMT, migration
and invasion activities of tumor cells in an inflammatory
microenvironment via STAT3 activation and phosphorylation (3,6). As a
consequence, the effects of shikonin on IL-6-mediated STAT3
phosphorylation in A549 and H1299 cells were analyzed by western
blotting. As shown in Fig. 5A, the
addition of IL-6 markedly increased the level of p-STAT3 (Y705),
while shikonin treatment significantly suppressed the
overexpression of p-STAT3 induced by IL-6 in a dose-dependent
manner without affecting the total STAT3 level. In addition, the
results of immunofluorescence and luciferase reporter gene assays
indicated that shikonin could prevent the nuclear translocation of
p-STAT3 (Fig. 5B) and inhibit its
transactivation activity in both cell lines (Fig. 5C). However, there were no
significant inhibitory effects of shikonin on both p-JAK2 or JAK2
levels (Fig. 5D). To further verify
whether shikonin can exert its inhibitory effects through the
IL-6/STAT3 pathway, pcDNA3.1-Flag-STAT3 was used to overexpress
STAT3 in A549 and H1299 cells. As shown in Fig. 5E and F, after STAT3 overexpression,
the inhibitory effects of shikonin on STAT3 phosphorylation and EMT
induced by IL-6 were alleviated. Therefore, these results indicate
that shikonin can reverse IL-6-induced EMT by inhibiting the
activation of STAT3 in human lung adenocarcinoma cells.
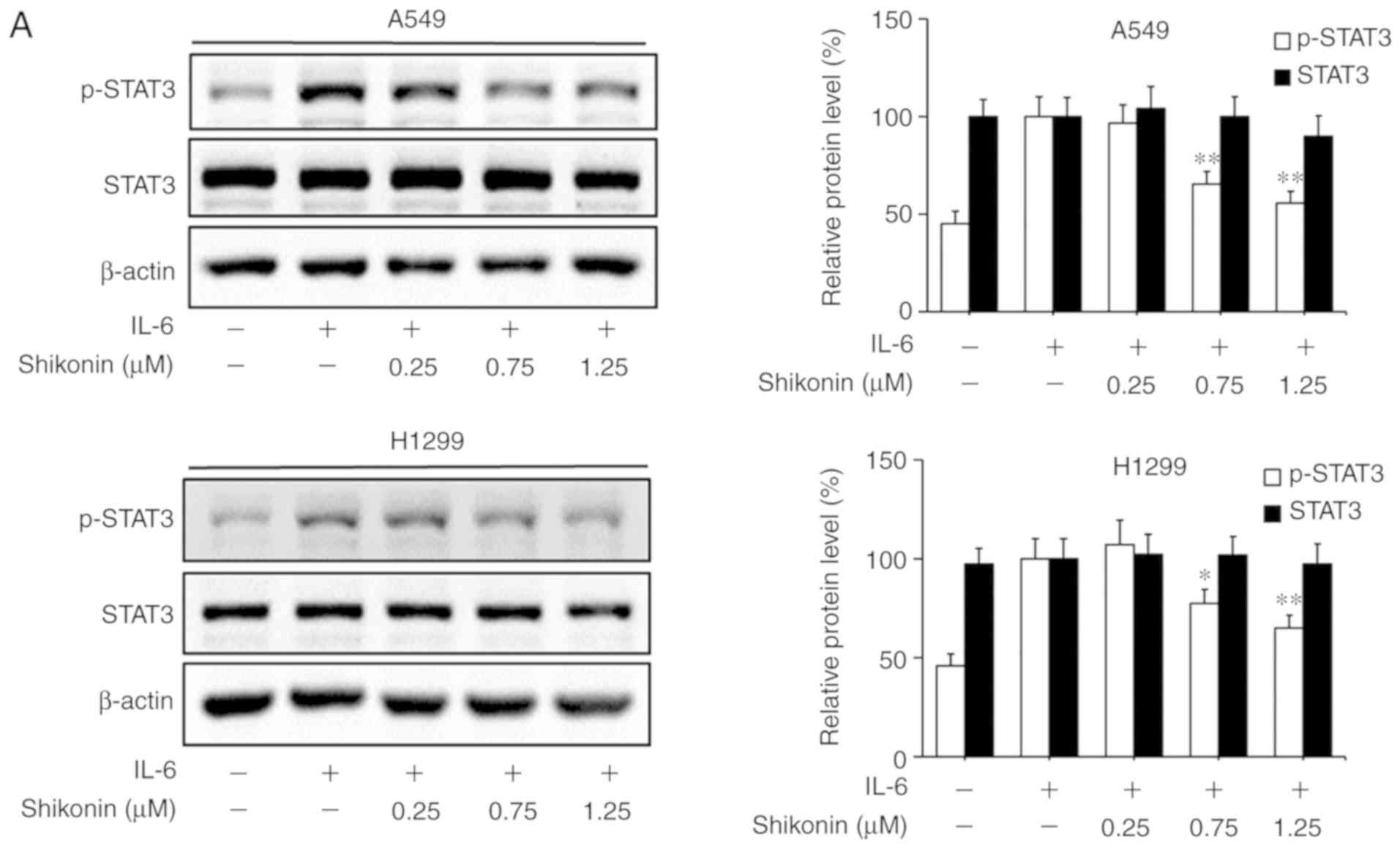 | Figure 5.Effects of shikonin on IL-6-induced
STAT3 activation in A549 and H1299 cells. (A) Expression levels of
phosphorylated (p)-STAT3 and STAT3 were detected by western
blotting after treatment with shikonin and IL-6 (50 ng/ml) for 24
h. Protein expression levels were semi-quantified by densitometric
analysis. *P<0.05, **P<0.01, compared with the IL-6-treated
group. (B) Immunofluorescence assays were performed to determine
the effects of shikonin on the nuclear translocation of p-STAT3
after treatment with shikonin (1.25 µM) and IL-6 (50 ng/ml) for 24
h. Upper panels, representative immunofluorescence images of
p-STAT3 staining (magnification ×800). Lower panel, the percentage
of cells with p-STAT3 nuclear translocation. **P<0.01, compared
with the IL-6-treated group. (C) Transactivation activities of
p-STAT3 in A549 and H1299 cells co-transfected with pSTAT3-TA-luc
and pRL-TK Renilla, and treated with shikonin and IL-6 (50 ng/ml)
for 24 h. Luciferase activity was detected by the Dual Luciferase
Reporter kit and normalized against the values for the
corresponding pRL-TK Renilla activity. *P<0.05, **P<0.01,
compared with the IL-6-treated group. (D) The protein expression
levels of p-JAK2 and JAK2 were detected by western blotting after
treatment with shikonin and IL-6 (50 ng/ml) for 24 h. β-actin was
used as an internal control. Protein expression levels were
semi-quantified by densitometry analysis. (E and F) After
transfection with STAT3 plasmids for 24 h, the cells were treated
with shikonin (1.25 µM) and IL-6 (50 ng/ml) for another 24 h. (E)
Expression levels of p-STAT3, Flag-STAT3, and STAT3 proteins were
detected by western blotting. (F) mRNA expression levels of
epithelial-mesenchymal transition (EMT)-related genes (E-cadherin,
N-cadherin, vimentin and Snail) were detected by real-time PCR.
Data are shown as the mean ± SD of three independent experiments.
*P<0.05, **P<0.01, compared with the IL-6-treated group;
#P<0.05, ##P<0.01, compared with the
combined shikonin (1.25 µM) and IL-6 (50 ng/ml) treatment group.
IL-6, interleukin-6; STAT3, signal transducer and activator of
transcription 3; JAK, Janus kinase. |
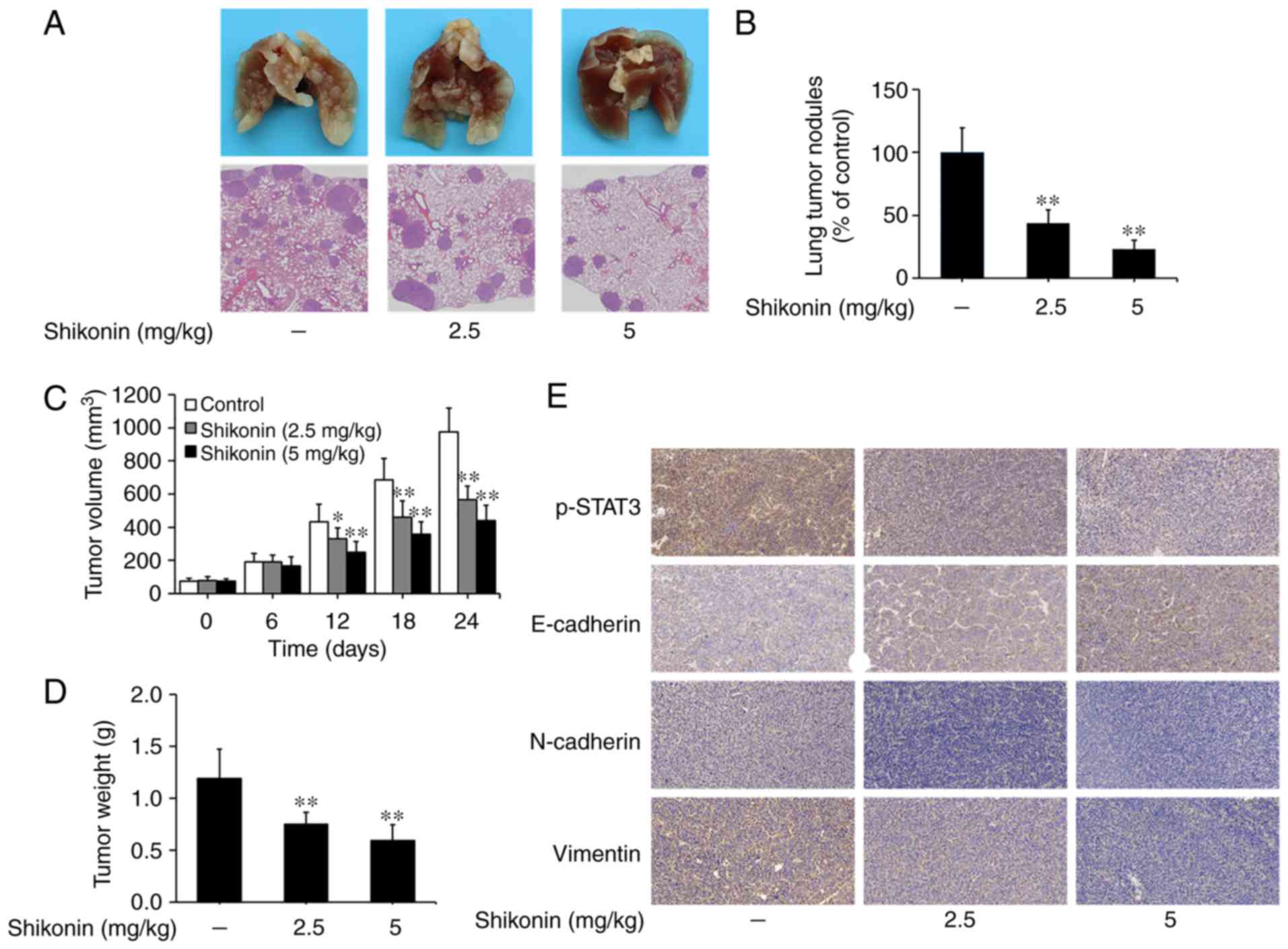
Shikonin inhibits the metastasis of
lung tumors in vivo
An artificial lung metastasis model was used to
study the anti-metastatic effects of shikonin in vivo
(5,6). In the present study, shikonin
treatment at 2.5 and 5 mg/kg significantly inhibited lung
metastasis, as shown in the image of lung tissue and H&E
staining assays (Fig. 6A). The
number of lung metastatic nodules was 102±20 in the vehicle control
group, while only 45±11 and 24±7 nodules were found in the groups
treated with 2.5 and 5 mg/kg shikonin, respectively (Fig. 6B). These results suggest that
shikonin can effectively suppress the metastasis of lung
adenocarcinoma in vivo.
The effects of shikonin on the growth of lung tumors
were studied using a nude mice xenograft model. As shown in
Fig. 6C and D, the volumes and
weights of tumor xenografts were significantly reduced in the
shikonin treatment groups compared to the vehicle control group.
Furthermore, through immunohistochemical staining, it was found
that the expression levels of p-STAT3, N-cadherin and vimentin were
decreased, while E-cadherin expression was increased in tumor
xenografts treated with shikonin (Fig.
6E). Taken together, these data suggest that shikonin can
prevent tumor metastasis and EMT by inhibiting STAT3 activation
in vivo.
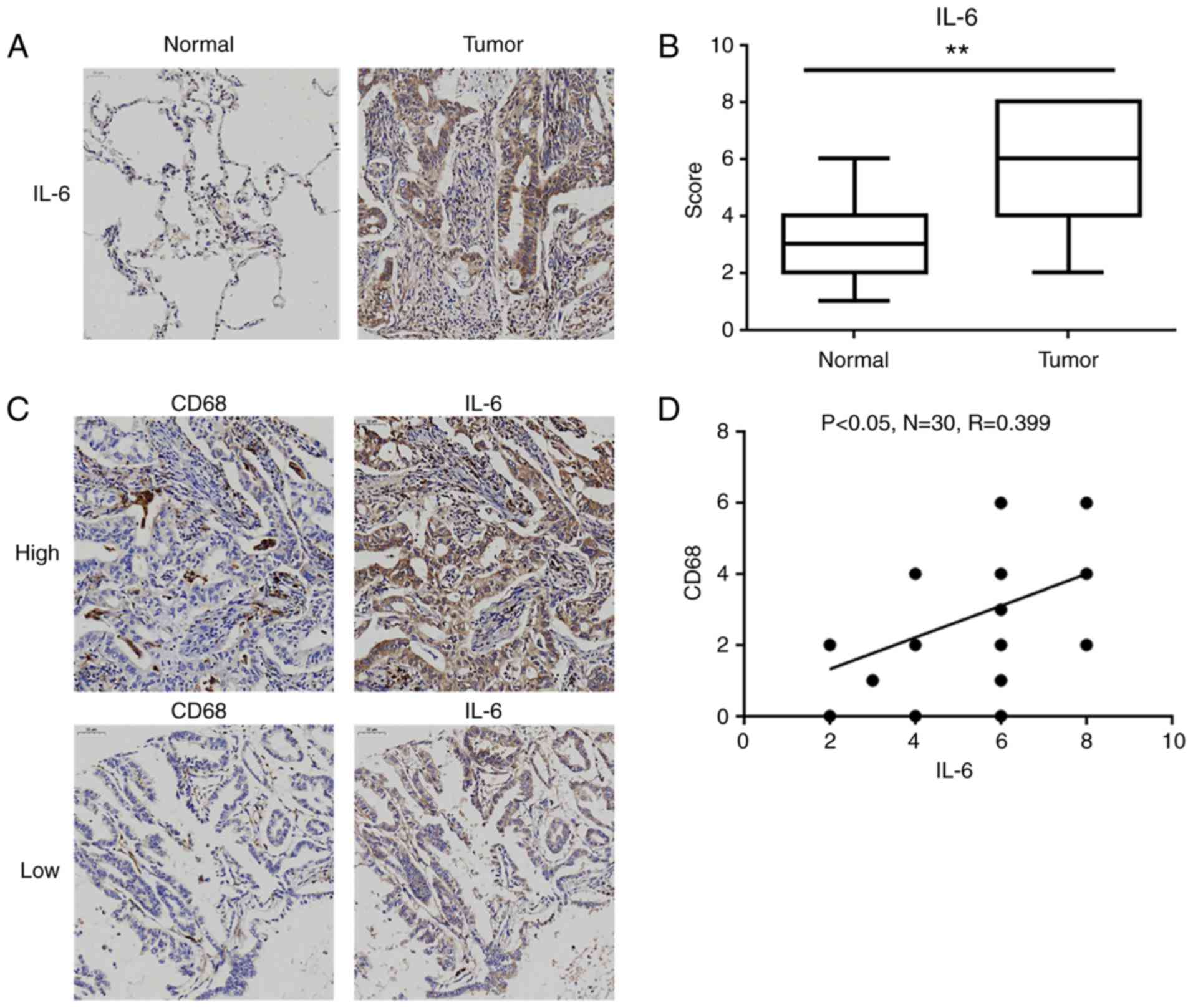
IL-6 expression is upregulated in
human lung adenocarcinoma tissues
As presented in Table
I, the expression level of IL-6 in tumor tissues was
significantly associated with tumor-node-metastasis (TNM) stage and
lymph node metastasis, but not with sex and age. Then, we further
compared the expression levels of IL-6 levels in lung
adenocarcinoma tissues and matched normal lung tissues. As shown in
Fig. 7A, the positive staining of
IL-6 was mainly observed in the cytoplasm of tumor cells and
inflammatory cells (e.g. macrophages), and the levels of IL-6 were
significantly elevated in lung adenocarcinoma tissues compared to
these level in the normal lung tissues (Fig. 7B). Furthermore, the distribution of
tumor-associated macrophages (TAMs) was assessed by the
well-characterized marker CD68. Although there was no significant
difference in CD68 levels between lung adenocarcinoma tissues and
normal lung tissues (data not shown), the level of CD68 was
significantly positively correlated with IL-6 expression in lung
adenocarcinoma tissues (R=0.399, P<0.05; Fig. 7C and D).
 | Table I.Association between IL-6 expression
and clinicopathological features of the patients with lung
adenocarcinoma (N=128). |
Table I.
Association between IL-6 expression
and clinicopathological features of the patients with lung
adenocarcinoma (N=128).
|
| IL-6
expression |
|
|---|
|
|
|
|
|---|
| Clinical
parameters | Lowa, n | Highb, n | P-value |
|---|
| Sex |
|
| 0.214 |
|
Male | 24 | 52 |
|
|
Female | 22 | 30 |
|
| Age, years |
|
| 0.691 |
|
<60 | 23 | 44 |
|
|
≥60 | 23 | 38 |
|
| TNM stage |
|
| 0.007d |
| I | 19 | 17 |
|
| II | 14 | 19 |
|
|
III | 13 | 46 |
|
| Lymph node
metastasis |
|
| 0.013c |
|
N0 | 23 | 23 |
|
|
N1/N2/N3 | 23 | 59 |
|
Discussion
As a physiological process mediated by the innate
immune system, inflammation can protect the body against pathogenic
infections, environmental insults and injuries. However, recent
studies have demonstrated that inflammation and tumors are closely
associated (36). It has been shown
that the inflammatory microenvironment, which is an essential
component of the tumor microenvironment, plays a key role during
tumor promotion and metastasis (37). Inflammatory cells, such as
tumor-associated macrophages (TAMs), can induce tumor angiogenesis,
extracellular matrix breakdown and tissue remodeling, thus
aggravating tumor metastasis (38).
In addition, epithelial-mesenchymal transition (EMT) induction in
the inflammatory microenvironment can promote the metastatic
ability of tumor cells (39). EMT
in tumor cells is a process of the loss of cell-cell adhesion
ability as well as acquiring migratory and invasive properties.
During this process, the levels of epithelial markers, such as
E-cadherin, are decreased, while those of mesenchymal markers, such
as N-cadherin and vimentin, are increased (40). In the present study, we used
LPS-stimulated THP-1-CM to mimic the inflammatory microenvironment
in vitro according to previously reported methods (6,41,42).
It was found that shikonin could reverse EMT and inhibit the
migration and invasion of human lung adenocarcinoma cells within
the in vitro inflammatory microenvironment. Although our
experiments could not completely mimic the event of tumors cells
being persistently exposed to the inflammatory microenvironment
in vivo, our results at least indicated that shikonin could
inhibit EMT in lung adenocarcinoma cells exposed to soluble
inflammatory factors secreted by LPS-stimulated THP-1 cells.
Altogether, these findings suggest that shikonin possesses a potent
anti-metastatic effect within an inflammatory microenvironment.
Inflammatory cell infiltrates can secrete various
inflammatory cytokines into tumor cells to activate the major
inflammatory signaling pathways and facilitate tumor progression
and metastasis (43,44). IL-6, an important inflammatory
cytokine that regulates systemic and regional inflammation, can
promote the pathogenesis of tumors by enhancing tumor metastasis,
recruiting leukocytes and promoting angiogenesis (35). In the present study, the addition of
the anti-IL-6 antibody into THP-1-CM was able to inhibit the
migration and EMT of lung adenocarcinoma cells, suggesting that
IL-6 directly promotes EMT in an inflammatory microenvironment. In
addition, shikonin markedly reversed EMT and inhibited IL-6-induced
migration of lung adenocarcinoma cells. These findings suggest that
shikonin can exert anti-metastatic effects in an inflammatory
microenvironment via the IL-6-mediated signaling pathway.
As the downstream factor of IL-6, STAT3 is
hyperactivated in various human cancers, displays tumor-promoting
properties, and is associated with poor clinical prognosis
(35). Experimental studies have
found that activated STAT3 can regulate the expression levels of
EMT markers through Snail and Slug transcription factors, thus
aggravating EMT and tumor metastasis (17,45).
In the IL-6/STAT3 signaling pathway, the binding of cytokine IL-6
to the IL-6R complex, which consists of IL-6R and gp130, can
activate the JAK2 pathway. The activation of JAK2 can phosphorylate
STAT3 at Y705. p-STAT3 monomers can form dimers and subsequently
translocate into the nucleus to initiate the transcription of its
target genes (6). In this study, we
found that shikonin could inhibit IL-6-induced phosphorylation of
STAT3, prevent p-STAT3 from translocating into the nucleus, and
suppress the transactivation activity of p-STAT3. Furthermore, the
overexpression of STAT3 attenuated shikonin-inhibited STAT3
phosphorylation and EMT in lung adenocarcinoma cells upon exposure
to IL-6. These findings revealed that shikonin can inhibit
IL-6-induced EMT by inhibiting the activation of STAT3 in lung
adenocarcinoma cells. However, shikonin did not inhibit
IL-6-induced phosphorylation of JAK2 under our experimental
conditions. Based on previous studies, we speculate that shikonin
may inhibit JAK2-mediated activation of STAT3 by binding directly
to the STAT3 SH2 domain (46,47).
However, more studies are needed to identify the exact mechanism of
action.
Moreover, artificial lung metastasis and xenograft
models were used to determine the effects of shikonin on human lung
adenocarcinoma metastasis and growth in vivo. It was found
that shikonin treatment markedly suppressed the metastasis and
growth of lung adenocarcinoma. In addition, it was also noted that
shikonin inhibited STAT3 activation and EMT in a lung
adenocarcinoma xenograft. Furthermore, the results of tissue
microarrays indicated that the expression level of IL-6 was higher
in human lung adenocarcinoma tissues than that noted in normal lung
tissues. More importantly, the expression levels of IL-6 in human
lung adenocarcinoma tissues were significantly associated with TNM
stage and lymph node metastasis. Taken together, our results
demonstrate that shikonin has great potential for the suppression
of lung adenocarcinoma metastasis by inhibiting STAT3 activation
in vivo.
Notably, we speculate that other cytokines such as
IL-1β or IL-8 may also be expressed in THP-1-CM (48,49).
Actually, according to the general principle, most cytokines that
can be secreted by THP-1 cells after LPS stimulation should be
expressed in THP-1-CM. Whether shikonin has effects on the other
cytokines and their downstream pathways still requires further
investigations.
In conclusion, this study reports, for the first
time, that shikonin suppresses the EMT, migration and invasion
activities of human lung adenocarcinoma cells within the
inflammatory microenvironments involving the IL-6/STAT3 signaling
pathway. These findings provide additional insights into the
antitumor activities of shikonin, which may have valuable
implications for treating inflammation-related tumor
metastasis.
Acknowledgements
The authors would like to thank the staff of Rui
Zhang's laboratory for offering their assistance.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos.
8187102538, 81872421, 81172222, 81572504 and 81502405); the Natural
Science Foundation of Shaanxi Province (grant nos. 2019JQ889,
2017JM8086 and 2016JZ028); the State Key Laboratory of Cancer
Biology of China (grant nos. CBSKL201710 and CBSKL2017Z09); and the
Foundation of Xi'an Medical University (grant no. 2018DOC02).
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
TP and FZ contributed equally to this study. TP, XR
and ZL conceived and designed the experiments. TP and FZ carried
out the experiments in the study. FL and XG assisted in performing
the experiments. FZ analyzed the data and constructed the figures.
TP and FZ wrote the manuscript. XL revised the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The experiments involving human subjects were
approved by the Institutional Review Board of Xi Jing Hospital of
The Fourth Military Medical University (Xi'an, Shaanxi, China), and
in accordance with the relevant guidelines and regulations. All
animal studies were approved by the Animal Ethics Committee of The
Fourth Military Medical University (Xi'an, Shaanxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang D, Cao X, Wang F, Jiang, Feng D, Guo
H, Du L, Jin Y, Chen Y, Yin X and Li C: LFG-500, a novel synthetic
flavonoid, suppresses epithelial-mesenchymal transition in human
lung adenocarcinoma cells by inhibiting NLRP3 in inflammatory
microenvironment. Cancer Lett. 400:137–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Collins LG, Haines C, Perkel R and Enck
RE: Lung cancer: Diagnosis and management. Am Fam Physician.
75:56–63. 2007.PubMed/NCBI
|
|
3
|
Zhao Z, Cheng X, Wang Y, Han R, Li L,
Xiang T, He L, Long H, Zhu B and He Y: Metformin inhibits the
IL-6-induced epithelial-mesenchymal transition and lung
adenocarcinoma growth and metastasis. PLoS One. 9:e958842014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Deng Z, Wang Z, Wang D, Zhang L, Su
Q, Lai Y, Li B, Luo Z, Chen X, et al: Zipper-Interacting protein
kinase promotes epithelial-mesenchymal transition, invasion and
metastasis through AKT and NF-kB signaling and is associated with
metastasis and poor prognosis in gastric cancer patients.
Oncotarget. 6:8323–8338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song J, Feng L, Zhong R, Xia Z, Zhang L,
Cui L, Yan H, Jia X and Zhang Z: Icariside II inhibits the EMT of
NSCLC cells in inflammatory microenvironment via down-regulation of
Akt/NF-kappaB signaling pathway. Mol Carcinog. 56:36–48. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Yao J, Wu XP, Zhao L, Zhou YX,
Zhang Y, You QD, Guo QL and Lu N: Wogonin suppresses human alveolar
adenocarcinoma cell A549 migration in inflammatory microenvironment
by modulating the IL-6/STAT3 signaling pathway. Mol Carcinog.
54:E81–E93. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-Related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DeNardo DG, Andreu P and Coussens LM:
Interactions between lymphocytes and myeloid cells regulate pro-
versus anti-tumor immunity. Cancer Metastasis Rev. 29:309–316.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ostrand-Rosenberg S: Immune surveillance:
A balance between pro- and anti-tumor immunity. Curr Opin Genet
Dev. 18:11–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park EJ, Lee JH, Yu GY, He G, Ali SR,
Holzer RG, Osterreicher CH, Takahashi H and Karin M: Dietary and
genetic obesity promote liver inflammation and tumorigenesis by
enhancing IL-6 and TNF expression. Cell. 140:197–208. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeh HH, Lai WW, Chen HH, Liu HS and Su WC:
Autocrine IL-6-induced Stat3 activation contributes to the
pathogenesis of lung adenocarcinoma and malignant pleural effusion.
Oncogene. 25:4300–4309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lederle W, Depner S, Schnur S, Obermueller
E, Catone N, Just A, Fusenig NE and Mueller MM: IL-6 promotes
malignant growth of skin SCCs by regulating a network of autocrine
and paracrine cytokines. Int J Cancer. 128:2803–2814. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bromberg J and Wang TC: Inflammation and
cancer: IL-6 and STAT3 complete the link. Cancer Cell. 15:79–80.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang C, Yang G, Jiang T, Zhu G, Li H and
Qiu Z: The effects and mechanisms of blockage of STAT3 signaling
pathway on IL-6 inducing EMT in human pancreatic cancer cells in
vitro. Neoplasma. 58:396–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou G, Yang Z, Wang X, Tao R and Zhou Y:
TRAIL Enhances shikonin induced apoptosis through ros/jnk signaling
in cholangiocarcinoma cells. Cell Physiol Biochem. 42:1073–1086.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andujar I, Ríos JL, Giner RM and Recio MC:
Pharmacological properties of shikonin - A review of literature
since 2002. Planta Med. 79:1685–1697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Yang L, Oppenheim JJ and Howard
MZ: Cellular pharmacology studies of shikonin derivatives.
Phytother Res. 16:199–209. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Xie J, Jiang Z, Wang B, Wang Y and
Hu X: Shikonin and its analogs inhibit cancer cell glycolysis by
targeting tumor pyruvate kinase-M2. Oncogene. 30:4297–4306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yeh YC, Liu TJ and Lai HC: Shikonin
induces apoptosis, necrosis, and premature senescence of human a549
lung cancer cells through upregulation of p53 expression. Evid
Based Complement Alternat Med. 2015:6203832015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andújar I, Recio MC, Giner RM and Ríos JL:
Traditional Chinese medicine remedy to jury: The pharmacological
basis for the use of shikonin as an anticancer therapy. Curr Med
Chem. 20:2892–2898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heinrich EL, Walser TC, Krysan K, Liclican
EL, Grant JL, Rodriguez NL and Dubinett SM: The inflammatory tumor
microenvironment, epithelial mesenchymal transition and lung
carcinogenes. Cancer microenvironment: official journal of the
International Cancer Microenv. 5:5–18. 2012. View Article : Google Scholar
|
|
25
|
Zheng Y, Guo J, Zhou J, Lu J, Chen Q,
Zhang C, Qing C, Koeffler HP and Tong Y: FoxM1 transactivates PTTG1
and promotes colorectal cancer cell migration and invasion. BMC Med
Genomics. 8:492015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuo CL, Lai KC, Ma YS, Weng SW, Lin JP and
Chung JG: Gallic acid inhibits migration and invasion of SCC-4
human oral cancer cells through actions of NF-kappaB, ras and
matrix metalloproteinase-2 and −9. Oncol Rep. 32:355–361. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wallmeyer L, Dietert K, Sochorová M,
Gruber AD, Kleuser B, Vávrová K and Hedtrich S: TSLP is a direct
trigger for T cell migration in filaggrin-deficient skin
equivalents. Sci Rep. 7:7742017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to imagej: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan T, Zhang M, Zhang F, Yan G, Ru Y, Wang
Q, Zhang Y, Wei X, Xu X, Shen L, et al: NDRG2 overexpression
suppresses hepatoma cells survival during metabolic stress through
disturbing the activation of fatty acid oxidation. Biochem Biophys
Res Commun. 5:860–866. 2017. View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao M, Liu Y, Liu R, Qi J, Hou Y, Chang J
and Ren L: Upregulation of IL-11, an IL-6 family cytokine, promotes
tumor progression and correlates with poor prognosis in non-small
cell lung cancer. Cell Physiol Biochem. 45:2213–2224. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thakur R, Trivedi R, Rastogi N, Singh M
and Mishra DP: Inhibition of STAT3, FAK and Src mediated signaling
reduces cancer stem cell load, tumorigenic potential and metastasis
in breast cancer. Sci Rep. 5:101942015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang S, Wu X, Chen Y, Zhang J, Ding J,
Zhou Y, He S, Tan Y, Qiang F, Bai J, et al: Prognostic and
predictive role of JWA and XRCC1 expressions in gastric cancer.
Clin Cancer Res. 18:2987–2996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Wang HS, Zhou BH, Li CL, Zhang F,
Wang XF, Zhang G, Bu XZ, Cai SH and Du J: Epithelial-mesenchymal
transition (EMT) induced by TNF-alpha requires
AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer.
PLoS One. 8:e566642013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Diakos CI, Charles KA, McMillan DC and
Clarke SJ: Cancer-Related inflammation and treatment effectiveness.
Lancet Oncol. 15:e493–e503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pietila M, Ivaska J and Mani SA: Whom to
blame for metastasis, the epithelial-mesenchymal transition or the
tumor microenvironment? Cancer Lett. 380:359–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bingle L, Brown NJ and Lewis CE: The role
of tumour-associated macrophages in tumour progression:
Implications for new anticancer therapies. J Pathol. 196:254–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dehai C, Bo P, Qiang T, Lihua S, Fang L,
Shi J, Jingyan C, Yan Y, Guangbin W and Zhenjun Y: Enhanced
invasion of lung adenocarcinoma cells after co-culture with
THP-1-derived macrophages via the induction of EMT by IL-6. Immunol
Lett. 160:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie YG, Yu Y, Hou LK, Wang X, Zhang B and
Cao XC: FYN promotes breast cancer progression through
epithelial-mesenchymal transition. Oncol Rep. 36:1000–1006. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Budai MM, Varga A, Milesz S, Tőzsér J and
Benkő S: Aloe vera downregulates LPS-induced inflammatory cytokine
production and expression of NLRP3 inflammasome in human
macrophages. Mol Immunol. 56:471–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang HW, Wu T, Qi JY, Wang YQ, Luo XP and
Ning Q: Salidroside attenuates LPS-stimulated activation of THP-1
cell-derived macrophages through down-regulation of MAPK/NF-kB
signaling pathways. J Huazhong Univ Sci Technol Med Sci.
33:463–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33:S79–S84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lesina M, Wörmann SM, Neuhöfer P, Song L
and Algül H: Interleukin-6 in inflammatory and malignant diseases
of the pancreas. Semin Immunol. 26:80–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu YS, Chung I, Wong WF, Masamune A, Sim
MS and Looi CY: Paracrine IL-6 signaling mediates the effects of
pancreatic stellate cells on epithelial-mesenchymal transition via
Stat3/Nrf2 pathway in pancreatic cancer cells. Biochim Biophys Acta
Gen Subj. 1861:296–306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Qiu HY, Zhu X, Luo YL, Lin HY, Tang CY, Qi
JL, Pang YJ, Yang RW, Lu GH, Wang XM and Yang Yh: Identification of
new shikonin derivatives as antitumor agents targeting stat3 sh2
domain. Sci Rep. 7:28632017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qiu HY, Fu JY, Yang MK, Han HW, Wang PF,
Zhang YH, Lin HY, Tang CY, Qi JL, Yang RW, et al: Identification of
new shikonin derivatives as STAT3 inhibitors. Biochem Pharmacol.
146:74–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim YI, Choi KH, Kim SR, Goo TW and Park
SW: Bombyx mori hemocyte extract has anti-inflammatory effects on
human phorbol myristate acetate-differentiated THP1 cells via
TLR4-mediated suppression of the NF-kappaB signaling pathway. Mol
Med Rep. 16:4001–4007. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu X, Yin S, Chen Y, Wu Y, Zheng W, Dong
H, Bai Y, Qin Y, Li J, Feng S and Zhao P: LPSinduced
proinflammatory cytokine expression in human airway epithelial
cells and macrophages via NFkappaB, STAT3 or AP1 activation. Mol
Med Rep. 17:5484–5491. 2018.PubMed/NCBI
|