Introduction
Non-small cell lung cancer (NSCLC) is one of the
most common types of human lung malignancies and its incidence and
mortality have increased over the past decades worldwide (1,2).
Although surgical treatment is the mainstay for early cases, NSCLC
is frequently diagnosed at an advanced stage and metastasis and
relapse remain the major causes of therapy failure (3). Accumulating evidence has revealed that
activating mutations of oncogenes, including epidermal growth
factor receptor (EGFR), K-Ras and c-Met, are a driving force to
contribute to the tumorigenesis of certain NSCLCs (4). Targeting therapies towards such
driving mutations have emerged as first-line therapeutic strategies
for advanced NSCLC. However, most patients who initially respond to
targeted therapies eventually develop acquired resistance (5,6).
Further elucidation of the underlying mechanisms of resistance and
identification of novel anti-tumor agents for NSCLC treatment is
necessary.
Cell cycle progression and transitions are tightly
controlled by cyclins and cyclin-dependent kinases (CDKs). In
mammalian cells, three isoforms of cyclin D (D1, D2 and D3) have
been identified (7,8). The complex formed by cyclin D with
either CDK4 or CDK6 is required for retinoblastoma protein
phosphorylation and subsequent G1 to S phase progression (7,9).
Numerous studies revealed that cyclin D, particularly cyclin D1, is
overexpressed in multiple types of human cancer. Given the role of
cyclin D1 in mediating extracellular signaling with cell
proliferation, it is not surprising that overexpression of cyclin
D1 and hyperactivation of their cognate CDK kinases directly
contribute to unlimited neoplastic growth (10,11).
Thus, the development of novel inhibitors targeting cyclin
D1-CDK4/6 signaling is a promising antitumor strategy for clinical
treatment.
In the present study, cyclin D1 was determined to be
overexpressed in human NSCLC tissues and cell lines. Furthermore, a
natural product, Xanthohumol (Xanth), was identified as a potential
anti-tumor agent for NSCLC treatment. The inhibitory effect of
Xanth on NSCLC cells was determined in vitro and in
vivo and the underlying mechanism of the Xanth-mediated
anti-tumor activity was investigated.
Materials and methods
Cell culture and antibodies
The natural product Xanth, proteasome inhibitor
MG132 and cycloheximide were purchased from Selleck Chemicals. Cell
culture medium and supplements, including Dulbecco's modified
Eagle's medium (DMEM), RPMI-1640 and fetal bovine serum (FBS), were
obtained from Invitrogen (Thermo Fisher Scientific, Inc.). Human
NSCLC cell lines, including H520, H358, H1299, H23, HCC827 and
H1975, were purchased from the American Type Culture Collection
(ATCC). The H520 is an EGFR null cell, HCC827 (E746-A750 deletion)
and H1975 (L858R/T790M) are two NSCLC cells with EGFR activation
mutation, while H1299 and H23 are wild EGFR harbor cells. As EGFR
signaling plays a crucial role in NSCLC, cells with wild-type EGFR
or activation mutant were selected in the present study. The
immortalized human lung epithelial cell lines HBE and NL20 were
obtained from Sigma and ATCC, respectively. All the cells were
maintained in an incubator at 37°C in a humidified atmosphere with
5% CO2 according to the ATCC protocols and subjected to
a mycoplasma test every two months. The primary antibodies to
cyclin D1, c-Jun, Jun B, Jun D, Fos B, FOS-related antigen 1
(Fra1), phosphorylated (p)-Fra1, c-Fos, p-ERK1/2 and β-actin were
obtained from Cell Signaling Technology, Inc. The anti-Ki67
antibody for immunohistochemistry (IHC) was a product of Abcam.
Antibody conjugates were visualized by chemiluminescence (Thermo
Fisher Scientific, Inc.). The jetPEI (Qbiogene, Inc.) was used for
transient transfection following the standard protocol.
MTS assay
The MTS assay was performed as previously described
(12). In brief, NSCLC cells were
seeded in 96-well plates (3,000 cells/well) and maintained for 24
h. The cells were treated with various doses of Xanth for 72 h. The
cell viability was examined using the MTS Cell Proliferation Assay
kit (cat. no. G3580; Promega Corp.) following the standard
protocol.
Soft agar assay
The soft agar assay for colony formation was
performed as described previously (13). In brief, 3 ml Eagle's basal medium
containing 0.6% agar, 10% FBS and various doses of Xanth was used
for the agar base in a 6-well plate. NSCLC cells were counted and
suspended at a concentration of 8,000 cells/ml using Eagle's basal
medium containing 0.3% agar, 10% FBS and Xanth. The re-suspended
cells were overlaid into a 6-well plate with a 0.6% agar base and
maintained for 2 weeks. The number of colonies was counted under a
light microscope (Olympus).
Flow cytometry
Flow cytometric analysis was performed as described
previously (14). In brief, the
cells were treated with Xanth or DMSO (control) as indicated. Cells
were suspended at a concentration of 1×106 cells/ml with
PBS. The propidium iodide staining buffer containing RNase was
added to the cell suspension, followed by incubation at room
temperature for 15 min in the dark. The cells were washed with PBS
and analyzed with a FACSort flow cytometer (BD Biosciences).
Dual reporter assay
The dual reporter assay was performed as described
previously (15). In brief, the
Renilla luciferase reporter construct pRL-SV40 was
co-transfected with the pGL3-Basic control or the pGL3-AP-1 (cat.
no. 40342; Addgene) construct which contain three canonical AP-1
binding sites (TGACTCA) into human NSCLC cells using Lipofectamine
2000 (Thermo Fisher). The transfected cells were treated with Xanth
for another 24 h. Cell lysates were prepared using the
Dual-Luciferase Reporter Assay kit (cat. no. E1910; Promega Corp.)
and subjected to luciferase activity analysis. Renilla
luciferase activity was used as an internal control for
normalization.
RT-qPCR
NSCLC cells were treated with Xanth for 24 h, total
RNA was extracted using the Absolutely RNA® Purification
Kits (Agilent). SYBR®-Green Quantitative RT-qPCR Kit was
used in RT-qPCR. Amplification cycles were 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 55°C for 60 sec). The
RT-qPCR results were normalized to β-actin. Cyclin D1 primer
sequences used were: Forward, TCTACACCGACAACTCCATCCG; reverse,
TCTGGCATTTTGGAGAGGAAGTG.
Clinical tissue sample collection
All the surgical specimens were collected in
accordance with an Institutional Review Board-approved protocol.
NSCLC tissues and matched adjacent non-tumor tissues were collected
from 35 patients who provided written informed consent at the
Department of Pathology, Hunan Cancer Hospital of Central South
University (Changsha, China). The in vivo experiments were
approved by the Institutional Animal Care and Use Committee of
Central South University (Changsha, China).
Western blot analysis
The whole-cell extract (WCE) was prepared using the
commercial radioimmunoprecipitation assay buffer (cat. no. PI89901;
Thermo Fisher Scientific, Inc.) and the protein concentration was
determined by the bicinchoninic acid protein assay (cat. no. 23228;
Thermo Fisher Scientific, Inc.). For EGF stimulation, NSCLC cells
were starved with 0.1% fetal bovine serum in RPMI-1640 medium
overnight and pretreated with Xanth for 2 h. EGF (50 ng/ml) was
added to the cell culture medium and maintained for 1 h, whole cell
lysates were collected for immunoblotting (IB) as described
previously (16). In brief, WCE (20
µg) was boiled with loading buffer for 5 min at 95°C and subjected
to SDS-PAGE, followed by electrotransfer to the polyvinylidene
difluoride membrane. The membrane was blocked with 5% non-fat milk,
followed by incubation with primary and secondary antibodies,
respectively. The antibodies against cyclin D1 (cat. no. 55506,
1:1,000), p21 (cat. no. 2947, 1:1000), p27 (cat. no. 3686,
1:1,000), c-Jun (cat. no. 9165, 1:1,000), JunB (cat. no. 3753,
1:1,000), JunD (cat. no. 5000, 1:1,000), FosB (cat. no. 2251,
1:1,000), Fra1 (cat. no. 5281, 1:1,000), c-Fos (cat. no. 2250,
1:1,000), p-ERK1/2 (cat. no. 4370, 1:1,000), pP90RSK (cat. no.
8753,1:1,000), p-Fra1 (cat. no. 5841, 1:1,000), Ubiquitin (cat. no.
3936, 1:1,000), Ki67 (cat. no. 9027, 1:1,000), anti-mouse IgG
HRP-linked antibody (cat. no. 7076, 1:10,000), and anti-rabbit IgG,
HRP-linked antibody (cat. no. 70764, 1:10,000), were purchased from
Cell Signaling Technology (Danvers, MA). The target protein was
visualized by chemiluminescence.
Generation of stable cyclin D1
knock-out cell lines
CRISPR-Cas9-mediated gene knockout was performed as
previously described (17). In
brief, single-guide (sg)RNAs (#1, GTTCGTGGCCTCTAAGATGA; #2,
GAAGCGTGTGAGGCGGTAGT) targeting cyclin D1 were used for the
construction of stable cell lines. In brief, NSCLC cells were
transfected with cyclin D1 sgRNA and selected by 1 µg/ml puromycin
for three weeks. Single colonies were chosen for further study.
In vivo tumor growth
The in vivo experiments were approved by the
Institutional Animal Care and Use Committee of Central South
University (Changsha, China). Mice were kept in colony cages with
free access to food and tap water and in standardized housing
conditions (natural 12 h light-dark cycle, temperature of 23±1°C,
relative humidity of 55±5%). The proper care and use of
experimental animals, including efforts to minimize suffering and
distress, use of analgesics or anaesthetics, was based on the Guide
for the Care and Use of Laboratory Animals (National Academies
Press, Washington, DC). The xenograft model was constructed by
subcutaneous injection of HCC827 cells (4×106) into the
right flank of 6-week-old athymic nude mice (n=5). Compound
treatment was initiated when the tumor reached an average volume of
100 mm3. The compound-treated group was administered
Xanth (10 mg/kg) every two days by i.p. injection. The health and
behaviour of mice were monitored every two days. The tumor volume
was determined according to the following formula:
Lengthxwidth2/2. The tumor-bearing mouse was euthanized
by CO2 when the tumor volume reached 700 mm3
(32 days). The fill rate of carbon dioxide is 30% of the chamber
volume per minute (3 liter/min), and the duration time is 5 min.
Death was further confirmed by cervical dislocation.
IHC
Tumor tissues from clinical samples and mouse
xenograft tumors were fixed and subjected to IHC analysis as
previously described (18). In
brief, the tissue slides were deparaffinized and rehydrated by
consecutive incubation with xylene and ethanol, followed by
submerging in sodium citrate buffer (10 mM, pH 6.0) and boiling for
10 min for antigen retrieval. The activity of endogenous peroxidase
was quenched by incubation with 3% H2O2 in
methanol for 10 min. Tissue slides were washed with PBS and blocked
with 50% goat serum albumin. After incubation with the primary and
secondary antibodies, the target protein was visualized using
3,3′-diaminobenzidine substrate and samples were counterstained
with hematoxylin.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 (GraphPad, Inc.). Quantitative data are expressed as the
mean ± standard deviation. The significance of differences between
groups was evaluated using the Student's t-test or One-way ANOVA.
Dunnett's method was used to compare treatment groups to a control
group, and Tukey's method was used to compare each group with every
other group. P<0.05 was considered to indicate a statistically
significant difference.
Results
High expression of cyclin D1 is
required for the maintenance of tumorigenic properties of NSCLC
cells
To determine the oncogenic function of cyclin D1 in
NSCLC, the protein levels of cyclin D1 were first examined in tumor
tissues. As shown in Fig. 1A,
cyclin D1 was significantly upregulated in human NSCLC tissues
compared with that in the paired non-tumor adjacent tissues. The IB
results suggested that cyclin D1 was overexpressed in all human
NSCLC cancer cell lines assessed: H520, H358, H1299, H23, HCC827
and H1975 (Fig. 1B). Furthermore,
stable cyclin D1-knockout cell lines were constructed from HCC827
and H1975 cells. The MTS data revealed that the viability of cells
with stable expression of sgCyclin D1 was significantly reduced
(Fig. 1C). To determine the role of
cyclin D1 in anchorage-independent growth of NSCLC cells, colony
formation was examined using the soft agar assay. The results
suggested that knockout of cyclin D1 inhibited colony formation of
H1975 and HCC827 cells, as the colony number was decreased >60%
when compared to that of cyclin D1-proficient cells (Fig. 1D). The results of the in vivo
tumor growth experiment indicated that depletion of cyclin D1
inhibited in vivo tumor development in the xenograft mouse
model. The sgCyclin D1-expressing HCC827 ×enograft tumors exhibited
a reduced tumor volume (Fig. 1E)
and a smaller tumor size (Fig. 1F and
G). These results suggested that cyclin D1 is highly expressed
in NSCLC tissues and cell lines, while knockout of cyclin D1
decreased the tumorigenic properties of NSCLC cells.
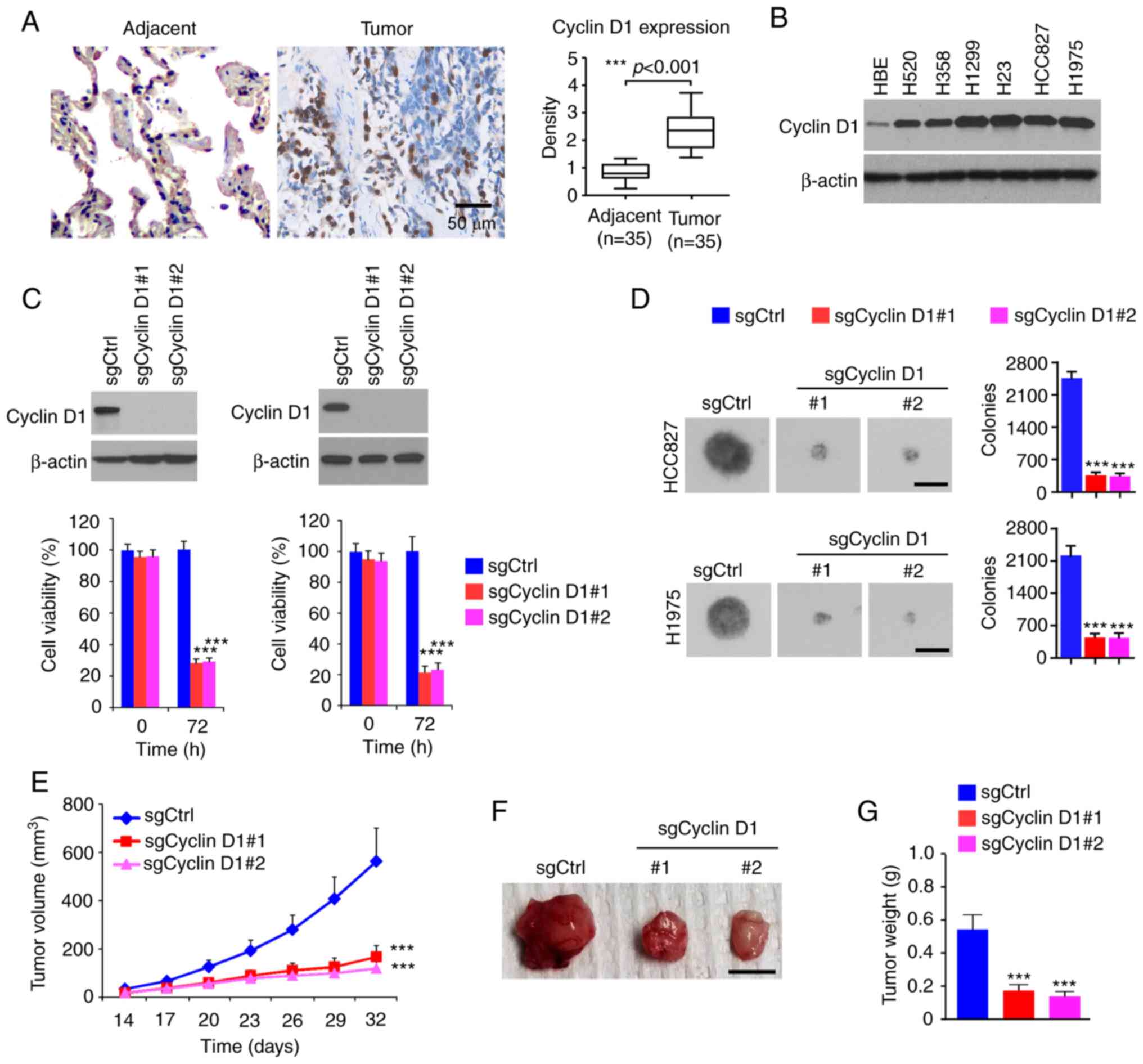 | Figure 1.Cyclin D1 is highly expressed in
NSCLC cells and required for the malignant phenotype. (A) IHC
staining analysis of cyclin D1 expression in 30 cases of matched
NSCLC patient tissues and adjacent tissues. Left panel,
representative IHC staining results of cyclin D1 (scale bar, 50
µm). Right panel, IHC score. (B) IB analysis of cyclin D1
expression in immortalized lung epithelial cells and NSCLC cells.
(C) Cell viability of HCC827 (left) and H1975 (right) cells
expressing sgCtrl or sgCyclin D1. Top, IB analysis of cyclin D1
expression. Bottom, cell viability assessed by an MTS assay. (D)
Colony formation of HCC827 and H1975 cells expressing sgCtrl or
sgCyclin D1 (scale bar, 200 µm). (E-G) In vivo tumor growth
of HCC827 cells expressing sgCtrl or sgCyclin D1: (E) Tumor volume,
(F) images of the tumors (scale bar, 1 cm), (G) tumor weight.
***P<0.001. NSCLC, non-small cell lung cancer; IHC,
immunohistochemistry; IB, immunoblot; Ctrl, control; sgCyclin D1,
single-guide RNA targeting cyclin D1. |
Xanth inhibits NSCLC cells in
vitro
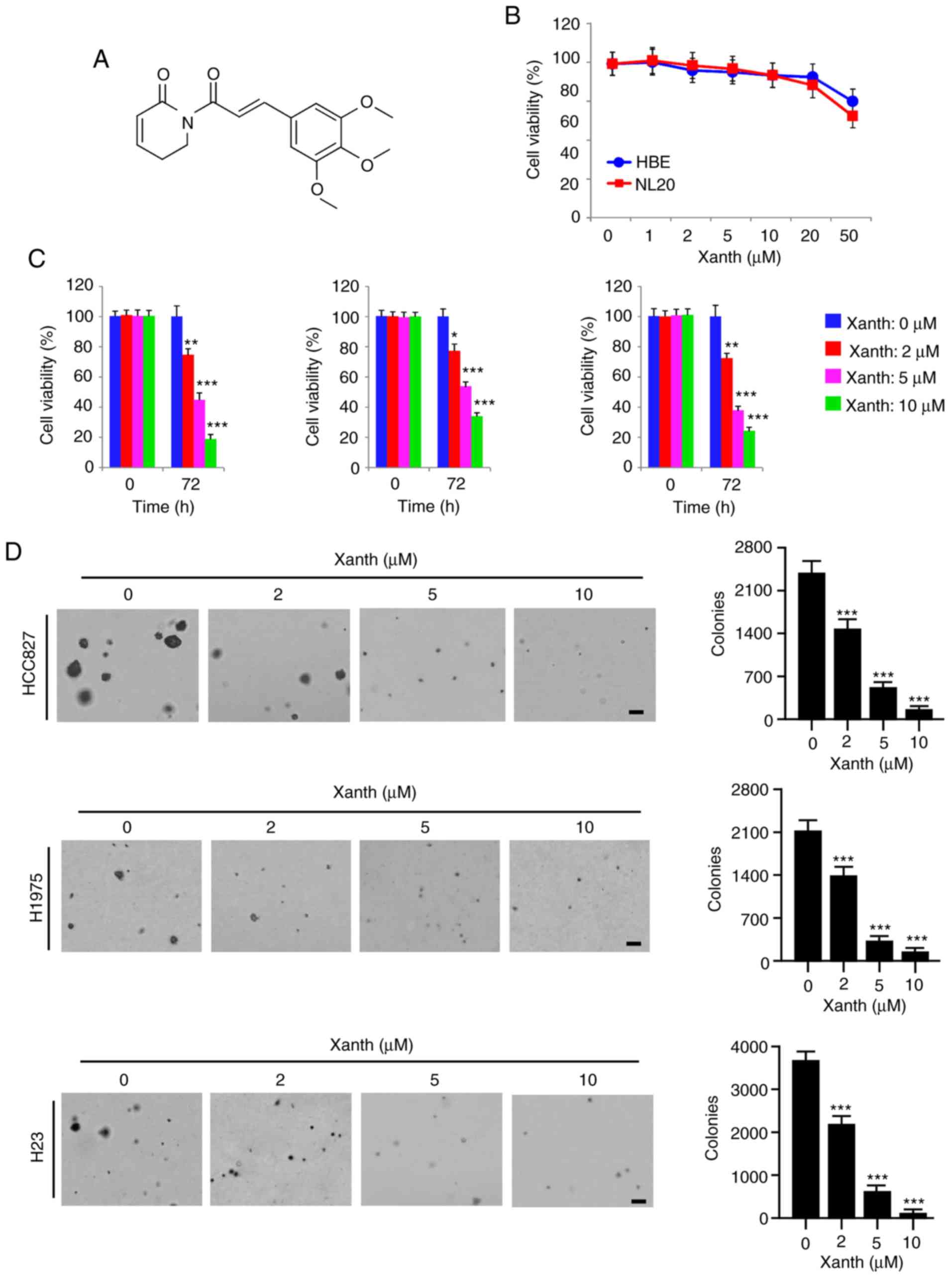
Accumulating evidence indicates that the natural
product Xanth (Fig. 2A) has potent
anti-tumor activity against multiple human cancer cell types
(19). However, the anti-tumor
effect of Xanth on NSCLC cells and the underlying mechanisms have
remained largely elusive. The present results indicated that Xanth
exhibited only slight cytotoxic effects on the immortalized lung
epithelial cell lines HBE and NL20, as the cell viability was not
significantly reduced after treatment with Xanth (Fig. 2B). However, the viability of
Xanth-treated NSCLC cell lines, including HCC827, H1975 and H23,
was decreased by Xanth in a dose-dependent manner (Fig. 2C). Treatment with Xanth for 72 h
reduced the cell viability >60% in all treated NSCLC cell lines.
Furthermore, the results of the soft agar colony formation assay
suggested that the anchorage-independent cell growth of NSCLC cells
was significantly reduced with Xanth treatment (Fig. 2D). The Xanth exerted its inhibitory
effect on colony formation in a dose-dependent manner. The results
revealed that treatment with 10 µM Xanth decreased the colony
number >95% in HCC827, H1975 and H23 cells (Fig. 2D). These in vitro results
indicated that Xanth inhibits NSCLC cells, but not the immortalized
lung epithelial cells, in a dose-dependent manner.
Xanth reduces the protein levels of
cyclin D1 and suppresses Fra1 in NSCLC cells
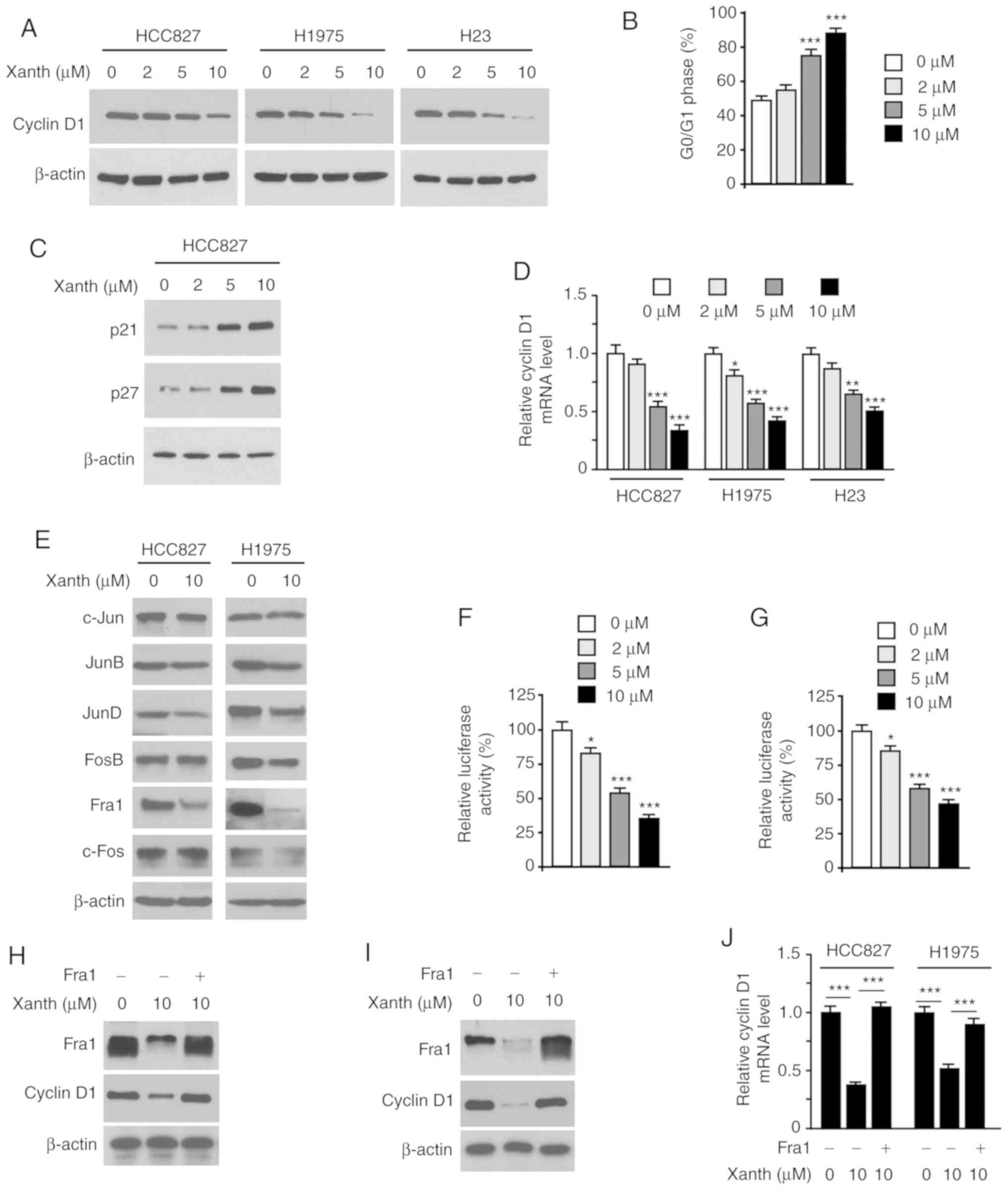
As Xanth exhibited a significant inhibitory effect
on NSCLC cell growth, it was then determined whether Xanth affected
cell cycle-associated proteins. The IB data revealed that Xanth
reduced the protein levels of cyclin D1 in HCC827, H1975 and H23
cells (Fig. 3A). Furthermore, flow
cytometric analysis revealed that Xanth induced cell cycle arrest
at the G0/G1 phase in HCC827 cells (Fig. 3B). Consistently with this, the IB
results revealed that Xanth increased the protein levels of p21 and
p27 (Fig. 3C). To investigate the
underlying mechanisms of how Xanth reduced cyclin D1 at the protein
level, Xanth-treated NSCLC cells were subjected to RT-qPCR analysis
of cyclin D1 expression. As shown in Fig. 3D, Xanth decreased the mRNA levels of
cyclin D1 in a dose-dependent manner, indicating that Xanth
suppressed cyclin D1 transcription. The AP-1 protein is one of the
most important transcription factors required for cyclin D1
transcription (20). The present
results suggested that Xanth slightly decreased the protein levels
of JunD but robustly reduced the expression of Fra1 in HCC827 and
H1975 (Fig. 3E) cells. Furthermore,
the reporter assay indicated that Xanth significantly reduced the
luciferase activity of AP-1 (Fig. 3F
and G). These results suggested that Fra1 is a critical
transcription factor for cyclin D1 expression in NSCLC cells.
Indeed, ectopic overexpression of Fra1 in HCC827 (Fig. 3H) and H1975 (Fig. 3I) cells compromised Xanth-induced
cyclin D1 downregulation. Consistently, the RT-qPCR results
revealed that overexpression of Fra1 restored cyclin D1 mRNA levels
in Xanth-treated NSCLC cells (Fig.
3J). Overall, these results suggest that Xanth reduces the
protein levels of cyclin D1 in a Fra1-dependent manner in NSCLC
cells.
Inhibition of ERK1/2 signaling is
required for Xanth-induced Fra1 reduction in NSCLC cells
To determine the underlying mechanisms of how Xanth
decreases the protein levels of Fra1, the signaling transduction in
Xanth-treated NSCLC cells was examined. Of note, the IB data
suggested that Xanth inhibited the activation of ERK1/2 signaling
in a dose-dependent manner. The phosphorylation of ERK1/2 and the
downstream target kinase P90RSK was robustly decreased after Xanth
treatment (Fig. 4A). Furthermore,
Xanth attenuated EGF-induced ERK1/2 and P90RSK phosphorylation in
NSCLC cells (Fig. 4B). Treatment
with the ERK1/2 signaling inhibitor PD98059 suppressed the protein
levels of Fra1 and cyclin D1 in HCC827, H1975 and H23 cells. In
addition, the phosphorylation of Fra1 on S265, a phosphorylation
residue that is catalyzed by ERK1/2, was inhibited in response to
PD98059 treatment (Fig. 4C). A
previous study suggested that Fra1 S265 phosphorylation promotes
Fra1 stability and inhibits protein degradation (21). Indeed, the IB results suggested that
Xanth reduced the half-life of Fra1 from nearly 2 h to 40 min
(Fig. 4D). Of note, the proteasome
inhibitor MG132 restored Fra1 protein levels in Xanth-treated NSCLC
cells (Fig. 4E), indicating that
Xanth promoted the ubiquitination-dependent protein degradation of
Fra1 in NSCLC cells. The ubiquitination analysis revealed that
Xanth promoted Fra1 ubiquitination in HCC827 cells (Fig. 4F). The S265D mutant was then
constructed, in which Ser265 was mutated to Asp to mimic the
constitutive phosphorylation of Fra1. The results indicated that
Xanth promoted the ubiquitination of wild-type Fra1 but not that of
the Fra1 S265D mutant (Fig. 4G).
Overall, these results suggested that inhibition of ERK1/2-mediated
Fra1 S265 phosphorylation is required for Xanth-induced Fra1
destruction in NSCLC cells.
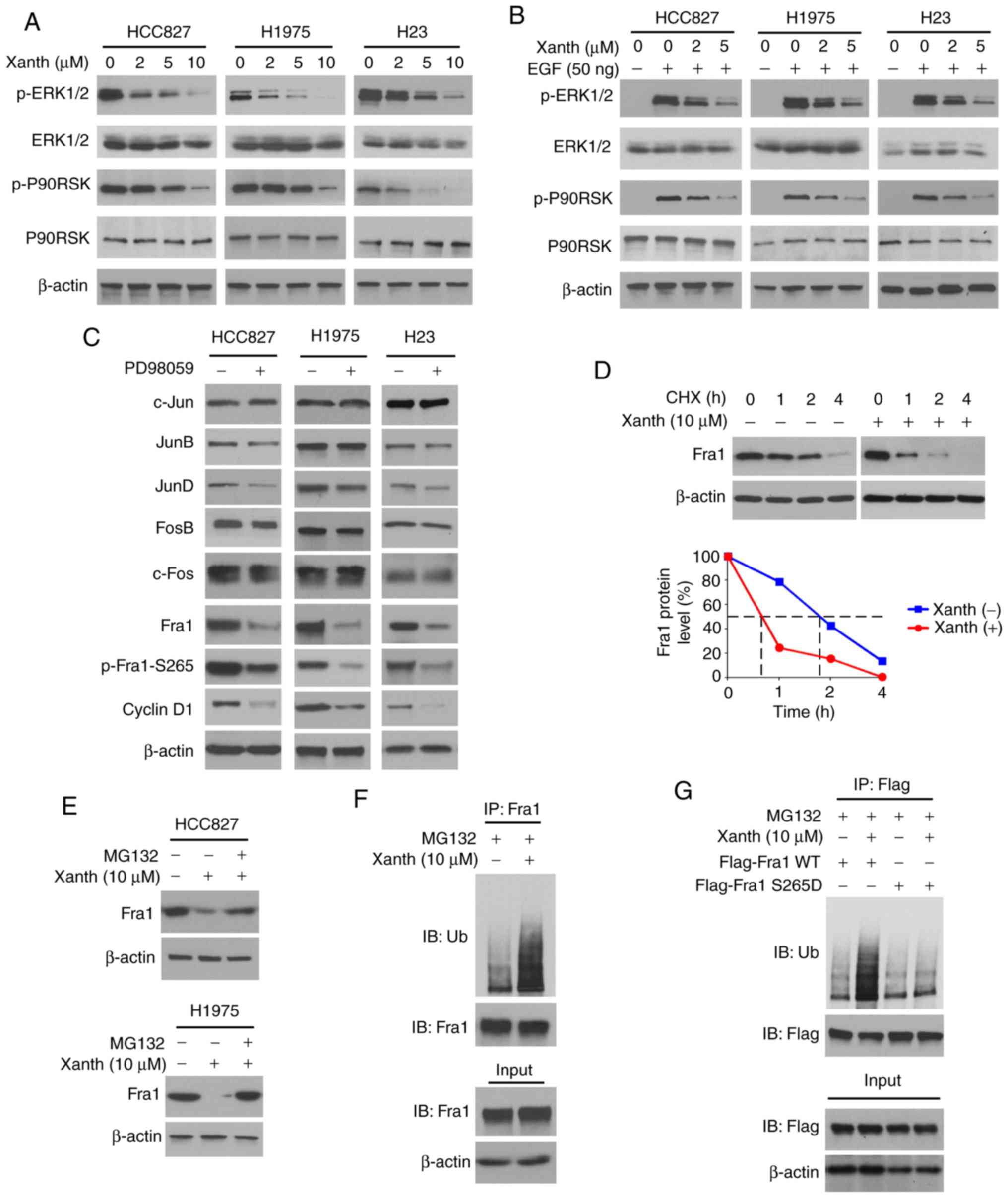 | Figure 4.Inhibition of ERK1/2 signaling is
required for Xanth-induced reduction of Fra1 in NSCLC cells. (A)
NSCLC cells were treated with Xanth for 24 h and subjected to IB
analysis. (B) NSCLC cells were starved with 0.1% fetal bovine serum
in RPMI-1640 medium overnight and pretreated with Xanth for 2 h.
EGF (50 ng/ml) was added to the cell culture medium and maintained
for 1 h. The cell lysate was subjected to IB analysis. (C) HCC827
(left), H1975 (middle) and H23 (right) cells were treated with
PD98059 or DMSO control for 24 h. The whole-cell lysate was
collected and subjected to IB analysis. (D) HCC827 cells were
treated with/without Xanth for 24 h, and cycloheximide was added to
the cell culture medium and maintained for various durations. The
cell lysate was subjected to IB analysis. (E) HCC827 and H1975
cells were treated with Xanth for 24 h, and the proteasome
inhibitor MG132 was added to the cell culture medium and cells were
maintained for another 6 h. The cell lysate was subjected to IB
analysis. (F) HCC827 cells were treated with Xanth for 24 h, the
proteasome inhibitor MG132 was added to the cell culture medium and
cells were maintained for another 6 h. The cell lysate was
subjected to an IP assay with the Fra1 antibody, followed by IB
analysis for examination of ubiquitination. (G) HCC827 cells were
transfected with various constructs as indicated and treated with
Xanth for 24 h. The proteasome inhibitor MG132 was added to the
cell culture medium and cells were maintained for another 6 h. The
cell lysate was subjected to IP assay with Flag antibody, followed
by IB analysis for examination of ubiquitination. Fra1, FOS-related
antigen 1; Xanth, xanthohumol; IB, immunoblot; IP,
immunoprecipitation. |
Xanth inhibits the in vivo tumor
development of NSCLC cells
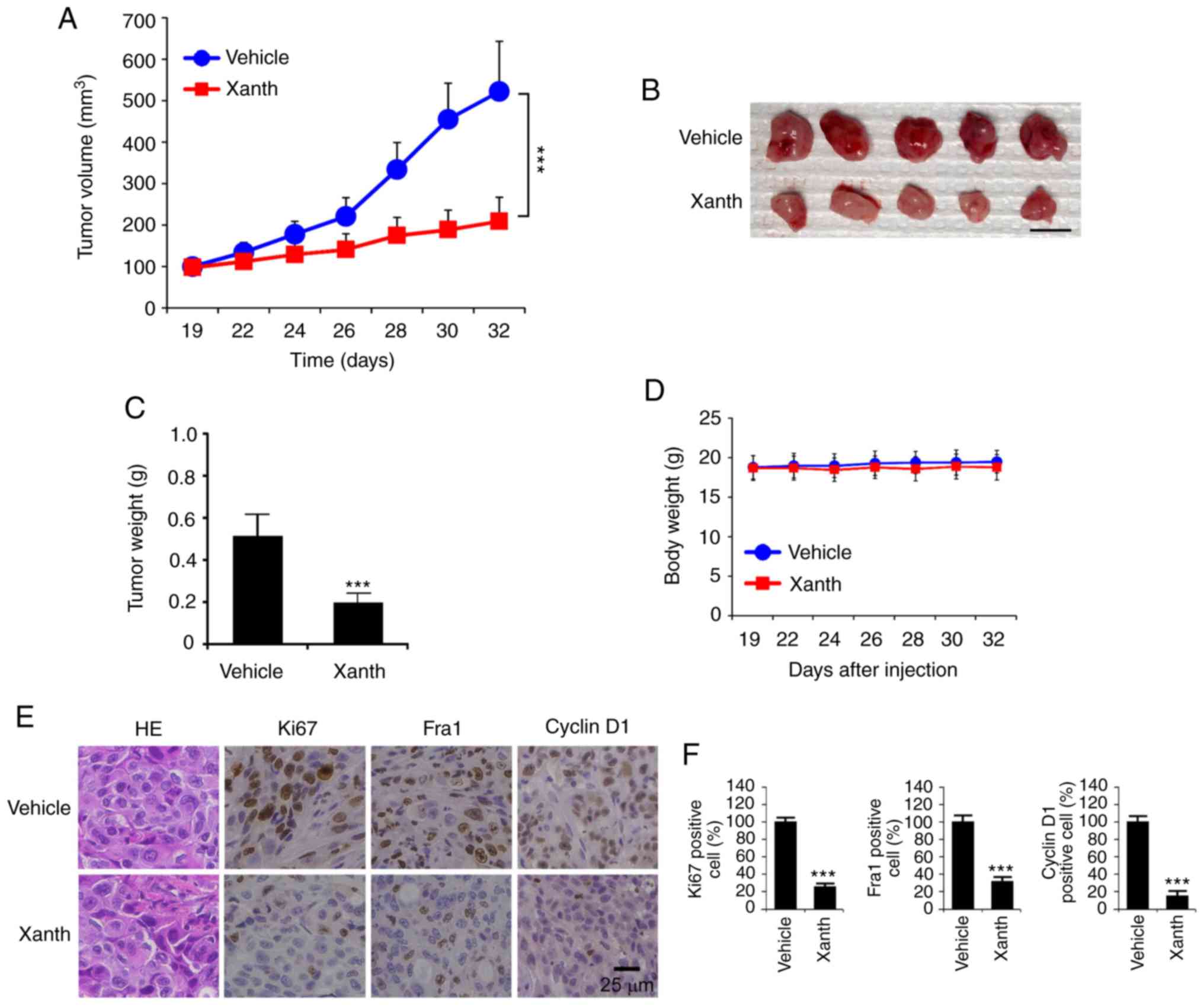
To determine the in vivo anti-tumor effect of
Xanth, it was tested in a xenograft mouse model established using
HCC827 cells. The results revealed that the average tumor volume of
the vehicle-treated group of HCC827-derived tumors was 522±121
mm3. By contrast, treatment with Xanth significantly
suppressed xenograft tumor growth, as the tumor volume was only
209±58 mm3 (Fig. 5A).
Furthermore, Xanth reduced the weight of the tumors >50% when
compared with that of the vehicle-treated group (Fig. 5B and C). Xanth did not cause a
significant body weight loss in the animals (Fig. 5D), suggesting that Xanth was
well-tolerated in them. The IHC staining result indicates that
Xanth reduced the population of Ki67-positive cells. Similarly, the
protein levels of Fra1 and cyclin D1 were significantly
downregulated in Xanth-treated xenograft tumors (Fig. 5E and F), which was consistent with
the in vitro results suggesting that Xanth reduced the
protein levels of Fra1 and cyclin D1. In conclusion, it was
indicated that Xanth inhibited the in vivo tumor growth of
NSCLC cells in a xenograft mouse model.
Discussion
The natural product Xanth was previously reported to
exhibit a potential anti-cancer effect against multiple human tumor
models (19). In vitro and
in vivo evidence indicated that Xanth inhibits the growth of
colorectal cancer (22), lung
cancer (23), prostate cancer
(24) and gastric cancer (25). At present, a panel of protein
kinases has been identified as potential protein targets of Xanth,
including PI3K/Akt (26), mTOR
(27) and hexokinase 2 (22). Xanth reduced the malignant
phenotypes of human cancer cells to suppress processes including
glycolysis (22), angiogenesis and
metastasis (28), and to promote
apoptosis (29). Although treatment
with Xanth induced cell cycle arrest, the underlying mechanism
remains elusive. In the present study, Xanth was indicated to
inhibit NSCLC cell growth in vitro and in vivo. A
further mechanistic study suggested that Xanth downregulated the
protein levels of cyclin D1 and induced cell cycle arrest in G0/G1
phase. Of note, it was demonstrated that Xanth reduces the
transcription of cyclin D1 in a Fra1-dependent manner. The present
data expanded the known anti-tumor mechanisms of Xanth and indicate
that targeting cyclin D1 and cell cycle progression offers an
alternative strategy for NSCLC prevention and treatment.
Cyclin D1 levels may be regulated transcriptionally,
translationally and post-translationally (8). Multiple transcription factors have
been identified to be required for cyclin D1 transcription,
including AP-1, EGFR, NF-κB, early growth response 1 and STAT5. A
panel of binding consensus for these transcription factors has been
identified within the human cyclin D1 promoter region (20). During the past decades, mitogenic
growth factors were the most intensively studied stimulators for
cyclin D1 transcription and are thought to be among the most
important ones. The mitogen-activated protein kinases (MAPKs), such
as the Ras-Raf-MAPK kinase-ERK signaling, have a crucial role in
mitogenic growth factor-induced cyclin D1 expression (30). Activation of ERK1/2 signaling
promotes the expression of AP-1 transcription factors and
eventually enhances cyclin D1 transcription (30,31).
Cyclin D1 is a nuclear protein. In the present study, IHC data in
patient-derived tissues showed that cyclin D1 was highly expressed
in tumor tissues. However, in adjacent tissues, there was no
significant nuclear staining of cyclin D1. The present results
suggested that treatment with Xanth inhibited the activation of
ERK1/2 signaling. Consistently, the protein levels of Fra1 were
robustly reduced. The dual reporter assay indicated that Xanth
inhibited the transcriptional activity of AP-1, as well as the mRNA
expression of cyclin D1. Furthermore, overexpression of Fra1
compromised this inhibitory effect of Xanth on NSCLC cells. This
evidence indicates that targeting the ERK1/2 signaling-mediated
AP-1 transcriptional activation contributed to the anti-tumor
effect of Xanth.
As an immediate early gene, Fra-1 is frequently
overexpressed in human cancers (32). Fra-1 is not able to transform the
cells on its own. However, a high expression of Fra1 promotes cell
proliferation and survival, as well as angiogenesis of human
cancers (32–34). Furthermore, overexpression of Fra1
confers chemo/radioresistance in multiple human cancer models
(35,36). The transcription and expression of
Fra1 were reported to be increased after stimulation with mitogenic
growth factors, including EGF, hepatocyte growth factor and
insulin-like growth factor 1. Activation of ERK1/2 signaling is
considered one of the most critical types of upstream signaling of
Fra1 after treatment with mitogenic growth factors (37). Of note, Fra1 is highly expressed in
K-Ras-driven cancer cells (38–40).
Similar to numerous key cell regulators, the expression of Fra1 is
tightly controlled by protein stability (41). A previous study indicated that
ERK1/2 kinase induced the phosphorylation of Fra1 on S265 and
compromised ubiquitination-mediated Fra1 destruction (21). Thus, the hyperactivation of ERK1/2
signaling not only promotes the transcription of Fra1 but also
increases protein stability. The present results suggested that
Xanth inhibited the activation of ERK1/2 signaling and attenuated
Fra1 phosphorylation at S265. The IB data revealed that treatment
with Xanth reduced the half-life of Fra1. Treatment with proteasome
inhibitor restored the protein levels of Fra1 in Xanth-treated
NSLCL cells. Xanth increased the ubiquitination of wild-type Fra1
but not that of the S265D mutant. The present results demonstrated
a novel anti-tumor effect of Xanth and suggested that the
inhibitory effect of Xanth on NSCLC cells was at least partially
dependent on Xanth-mediated Fra1 destruction.
In conclusion, the present study suggested that high
protein levels of cyclin D1 are required in NSCLC cells for
maintaining their malignant phenotype. The natural product Xanth
exerted an inhibitory effect on NSCLC cells by decreasing cyclin D1
in a Fra1-mediated, AP-1 transcription activity-dependent manner.
Furthermore, it was demonstrated that Xanth inhibited ERK1/2
signaling and Fra1 phosphorylation, which eventually caused
ubiquitination-dependent degradation of Fra1. The present study
enhanced the understanding of the anti-tumor mechanisms of Xanth
and indicated that Xanth is a promising chemotherapeutic agent for
NSCLC management.
Acknowledgements
The authors would like to thank Shiming Tan at the
Third Xingya Hospital, for technical support and providing critical
comments.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81904262, and 81972837) and
the Natural Science Foundation of Hunan Province (grant nos.
2018JJ3787, 2018JJ2604, and 2019JJ50682).
Availability of data and materials
All data and materials supporting the conclusion of
this study have been included within the article.
Authors' contributions
FG, ML, LZ, and WL designed the study. HZ, WL, FG,
ML, LZ, and WL performed experiments and/or contributed to data
analyses. FG, ML, LZ, and WL wrote the manuscript. All authors
provided critical review and revisions and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The in vivo experiments were approved by the
Institutional Animal Care and Use Committee of Central South
University (Changsha, China). Written informed consent was provided
by patients. There is no human subject participation.
Patient consent for publication
This study does not include any individual person's
data in any form.
Conflicts of interest
The authors have declared no conflicts of
interest.
References
|
1
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiang H, Chang Q, Xu J, Qian J, Zhang Y,
Lei Y, Han B and Chu T: New advances in antiangiogenic combination
therapeutic strategies for advanced non-small cell lung cancer. J
Cancer Res Clin Oncol. 146:631–645. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arbour KC and Riely GJ: Systemic therapy
for locally advanced and metastatic non-small cell lung cancer: A
review. JAMA. 322:764–774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skoulidis F and Heymach JV: Co-occurring
genomic alterations in non-small-cell lung cancer biology and
therapy. Nat Rev Cancer. 19:495–509. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santoni-Rugiu E, Melchior LC, Urbanska EM,
Jakobsen JN, Stricker K, Grauslund M and Sørensen JB: Intrinsic
resistance to EGFR-tyrosine kinase inhibitors in EGFR-mutant
non-small cell lung cancer: Differences and similarities with
acquired resistance. Cancers (Basel). 11:9232019. View Article : Google Scholar
|
|
6
|
Shah R and Lester JF: Tyrosine kinase
inhibitors for the treatment of EGFR mutation-positive
non-small-cell lung cancer: A clash of the generations. Clin Lung
Cancer. 21:e216–e228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qie S and Diehl JA: Cyclin D degradation
by E3 ligases in cancer progression and treatment. Semin Cancer
Biol. Jan 30–2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chou J, Quigley DA, Robinson TM, Feng FY
and Ashworth A: Transcription-associated cyclin-dependent kinases
as targets and biomarkers for cancer therapy. Cancer Discov.
10:351–370. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qie S and Diehl JA: Cyclin D1, cancer
progression, and opportunities in cancer treatment. J Mol Med
(Berl). 94:1313–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramos-García P, González-Moles MÁ, Ayén Á,
González-Ruiz L, Gil-Montoya JA and Ruiz-Ávila I: Predictive value
of CCND1/cyclin D1 alterations in the malignant transformation of
potentially malignant head and neck disorders: Systematic review
and meta-analysis. Head Neck. 41:3395–3407. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y, Li M, Yu X, Liu T, Li T, Zhou L,
Liu W, Li W and Gao F: Butein suppresses hepatocellular carcinoma
growth via modulating Aurora B kinase activity. Int J Biol Sci.
14:1521–1534. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao F, Yu X, Li M, Zhou L, Liu W, Li W and
Liu H: Deguelin suppresses non-small cell lung cancer by inhibiting
EGFR signaling and promoting GSK3β/FBW7-mediated Mcl-1
destabilization. Cell Death Dis. 11:1432020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu X, Liang Q, Liu W, Zhou L, Li W and Liu
H: Deguelin, an Aurora B kinase inhibitor, exhibits potent
anti-tumor effect in human esophageal squamous cell carcinoma.
EBioMedicine. 26:100–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Yu X, Xia Z, Yu X, Xie L, Ma X, Zhou
H, Liu L, Wang J, Yang Y and Liu H: Repression of Noxa by Bmi1
contributes to deguelin-induced apoptosis in non-small cell lung
cancer cells. J Cell Mol Med. 22:6213–6227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu H, Li W, Yu X, Gao F, Duan Z, Ma X,
Tan S, Yuan Y, Liu L, Wang J, et al: EZH2-mediated Puma gene
repression regulates non-small cell lung cancer cell proliferation
and cisplatin-induced apoptosis. Oncotarget. 7:56338–56354. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou L, Yu X, Li M, Gong G, Liu W, Li T,
Zuo H, Li W, Gao F and Liu H: Cdh1-mediated Skp2 degradation by
dioscin reprogrammes aerobic glycolysis and inhibits colorectal
cancer cells growth. EBioMedicine. 51:1025702020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu X, Wang R, Zhang Y, Zhou L, Wang W, Liu
H and Li W: Skp2-mediated ubiquitination and mitochondrial
localization of Akt drive tumor growth and chemoresistance to
cisplatin. Oncogene. 38:7457–7472. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang CH, Sun TL, Xiang DX, Wei SS and Li
WQ: Anticancer activity and mechanism of xanthohumol: A prenylated
flavonoid from Hops (Humulus lupulus L.). Front Pharmacol.
9:5302018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klein EA and Assoian RK: Transcriptional
regulation of the cyclin D1 gene at a glance. J Cell Sci.
121:3853–3857. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Basbous J, Chalbos D, Hipskind R,
Jariel-Encontre I and Piechaczyk M: Ubiquitin-independent
proteasomal degradation of Fra-1 is antagonized by Erk1/2
pathway-mediated phosphorylation of a unique C-terminal
destabilizer. Mol Cell Biol. 27:3936–3950. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu W, Li W, Liu H and Yu X: Xanthohumol
inhibits colorectal cancer cells via downregulation of Hexokinases
II-mediated glycolysis. Int J Biol Sci. 15:2497–2508. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sławińska-Brych A, Zdzisińska B,
Dmoszyńska-Graniczka M, Jeleniewicz W, Kurzepa J, Gagoś M and
Stepulak A: Xanthohumol inhibits the extracellular signal regulated
kinase (ERK) signalling pathway and suppresses cell growth of lung
adenocarcinoma cells. Toxicology. 357-358:65–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kłósek M, Mertas A, Król W, Jaworska D,
Szymszal J and Szliszka E: Tumor necrosis factor-related
apoptosis-inducing ligand-induced apoptosis in prostate cancer
cells after treatment with xanthohumol-A natural compound present
in Humulus lupulus L. Int J Mol Sci. 17:8372016. View Article : Google Scholar
|
|
25
|
Wei S, Sun T, Du J, Zhang B, Xiang D and
Li W: Xanthohumol, a prenylated flavonoid from Hops, exerts
anticancer effects against gastric cancer in vitro. Oncol
Rep. 40:3213–3222. 2018.PubMed/NCBI
|
|
26
|
Silva AF, Faria-Costa G, Sousa-Nunes F,
Santos MF, Ferreira-Pinto MJ, Duarte D, Rodrigues I, Tiago
Guimarães J, Leite-Moreira A, Moreira-Gonçalves D, et al:
Anti-remodeling effects of xanthohumol-fortified beer in pulmonary
arterial hypertension mediated by ERK and AKT inhibition.
Nutrients. 11:5832019. View Article : Google Scholar
|
|
27
|
Guo D, Zhang B, Liu S and Jin M:
Xanthohumol induces apoptosis via caspase activation, regulation of
Bcl-2, and inhibition of PI3K/Akt/mTOR-kinase in human gastric
cancer cells. Biomed Pharmacother. 106:1300–1306. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito K, Matsuo Y, Imafuji H, Okubo T,
Maeda Y, Sato T, Shamoto T, Tsuboi K, Morimoto M, Takahashi H, et
al: Xanthohumol inhibits angiogenesis by suppressing nuclear
factor-κB activation in pancreatic cancer. Cancer Sci. 109:132–140.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Engelsgjerd S, Kunnimalaiyaan S, Kandil E,
Gamblin TC and Kunnimalaiyaan M: Xanthohumol increases death
receptor 5 expression and enhances apoptosis with the TNF-related
apoptosis-inducing ligand in neuroblastoma cell lines. PLoS One.
14:e02137762019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chambard JC, Lefloch R, Pouysségur J and
Lenormand P: ERK implication in cell cycle regulation. Biochim
Biophys Acta. 1773:1299–1310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tchakarska G and Sola B: The double
dealing of cyclin D1. Cell Cycle. 19:163–178. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dhillon AS and Tulchinsky E: FRA-1 as a
driver of tumour heterogeneity: A nexus between oncogenes and
embryonic signalling pathways in cancer. Oncogene. 34:4421–4428.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yun SI, Hong HK, Yeo SY, Kim SH, Cho YB
and Kim KK: Ubiquitin-specific protease 21 promotes colorectal
cancer metastasis by acting as a Fra-1 deubiquitinase. Cancers
(Basel). 12:2072020. View Article : Google Scholar
|
|
34
|
Zhang Z, Zhang Y, Zhang L, Pei Y, Wu Y,
Liang H, Zhang W and Zhang B: Incomplete radiofrequency ablation
provokes colorectal cancer liver metastases through heat shock
response by PKCα/Fra-1 pathway. Cancer Biol Med. 16:542–555.
2019.PubMed/NCBI
|
|
35
|
Tyagi A, Vishnoi K, Kaur H, Srivastava Y,
Roy BG, Das BC and Bharti AC: Cervical cancer stem cells manifest
radioresistance: Association with upregulated AP-1 activity. Sci
Rep. 7:47812017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu D, Chen S, Tan X, Li N, Liu C, Li Z,
Liu Z, Stupack DG, Reisfeld RA and Xiang R: Fra-1 promotes breast
cancer chemosensitivity by driving cancer stem cells from dormancy.
Cancer Res. 72:3451–3456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang X, Xie H, Dou Y, Yuan J, Zeng D and
Xiao S: Expression and function of FRA1 protein in tumors. Mol Biol
Rep. 47:737–752. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Román M, López I, Guruceaga E, Baraibar I,
Ecay M, Collantes M, Nadal E, Vallejo A, Cadenas S, Miguel ME, et
al: Inhibitor of differentiation-1 sustains mutant KRAS-driven
progression, maintenance, and metastasis of lung adenocarcinoma via
regulation of a FOSL1 network. Cancer Res. 79:625–638. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Keshamouni VG: Excavation of FOSL1 in the
ruins of KRAS-driven lung cancer. Am J Respir Cell Mol Biol.
58:551–552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Elangovan IM, Vaz M, Tamatam CR, Potteti
HR, Reddy NM and Reddy SP: FOSL1 promotes Kras-induced lung cancer
through amphiregulin and cell survival gene regulation. Am J Respir
Cell Mol Biol. 58:625–635. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gomard T, Jariel-Encontre I, Basbous J,
Bossis G, Moquet-Torcy G and Piechaczyk M: Fos family protein
degradation by the proteasome. Biochem Soc Trans. 36:858–863. 2008.
View Article : Google Scholar : PubMed/NCBI
|