Introduction
Hepatocellular carcinoma (HCC) is the most common
type of liver cancer, and ranks fifth in terms of mortality rates
worldwide amongst all types of cancer, and its morbidity rates have
been increasing on a yearly basis (1). The outcomes of patients with HCC have
improved significantly over the past decades, which has been
ascribed to the development of improved local therapeutic
techniques and resection criteria; nonetheless, the prognosis of
patients with HCC remains poor, and the rates of distant metastasis
and local recurrence are also high (2–5). It is
well documented that dysregulated gene expression serves an
important role during the development and progression of cancer
(6–8). Certain cancer markers have been
identified for predicting the prognosis of surgical resection
outcomes; however, they may not always exhibit the best predictive
capacity (9,10). Therefore, identifying novel cancer
markers is required to improve prediction of clinical outcomes.
Additionally, understanding the mechanisms of action at the
molecular level underlying the involvement of predictive biomarkers
may highlight potential novel targets for the treatment of patients
with liver cancer.
Long non-coding RNAs (lncRNAs) are non-coding RNAs
that are 2.2 kb in length. Hox transcript antisense RNA
(HOTAIR) is a lncRNA encoded by the HOX gene cluster at the
HOXC site (11). HOTAIR is
upregulated in HCC compared with that in non-cancerous tissues, and
exerts its function through the activation of the Wnt/β catenin
signal transduction pathway and is associated with metastasis
(12). In vitro, it has been
demonstrated that HOTAIR promotes migration and invasion of
HCC cells (13,14). Furthermore, gene expression
profiling has shown that HOTAIR knockdown results in
upregulated astrotactin 1 (ASTN1) expression (15). ASTN1 is a neuronal adhesion
molecule, which is required for the physiological migration of
granule cells during brain development (16). However, its expression and functions
within tumor tissues remain largely unknown, and additional studies
are required to examine the role of ASTN1 in migration and invasion
in liver cancer.
The results of the present study showed that ASTN1
expression was decreased in liver cancer tissues compared with that
noted in matched adjacent normal liver tissues. ASTN1 expression
was upregulated or silenced to examine its functions in human liver
cancer cells. Overexpression of ASTN1 reduced liver cancer invasion
and migration through suppression of the Wnt signaling pathway.
Additionally, ASTN1 expression was associated with HCC immune
infiltration. Taken together, the results of the present study
suggest that ASTN1 may be used as a diagnostic and prognostic
marker in patients with liver cancer.
Materials and methods
Patients and tissue specimens
In the present study 145 consecutive HCC cases (mean
age, 47 years; age range, 22–75 years; 128 male and 17 female
patients) undergoing surgical resection at Sun Yat-sen University
Cancer Center during 2004 were immunohistochemically analyzed.
Their clinical data were acquired through reviewing medical
records. Informed consent was obtained from all patients, and the
study was approved by the Ethics Committee of Sun Yat-sen
University Cancer Center. Each case was followed once every month
for the first 6 months postoperatively, and once every 3 months
thereafter. This study was ended on December 31, 2017. Magnetic
resonance imaging (MRI) or computed tomography (CT) was used to
confirm tumor relapse or metastasis. Overall survival (OS) was
deemed as the duration from the date of surgical resection to the
date of death, whereas recurrence-free survival (RFS) was the
duration from the date of surgical resection to the date of
metastasis or relapse.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from cells or tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol, and subsequently
treated with DNase. The RNA was reverse transcribed into cDNA using
a RevertAid First-Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.). The thermocycling conditions was as follows:
Pre-degeneration at 95°C for 10 min, denaturation at 95°C for 5
sec; annealing at 60°C for 30 sec; followed by 40 cycles, and
extension at 60°C for 30 sec. qPCR was performed using SYBR-Green
qPCR Master mix (Thermo Fisher Scientific, Inc.) on an ABI 7300
system (Applied Biosystem; Thermo Fisher Scientific, Inc.). The
sequences of the primers used were: ASTN1 forward,
5′-CAATCTCTTCAATGGCTACAC-3′ and reverse,
5′-TCCTTTCCTCCAATCATCTAC-3′; and GAPDH forward,
5′-AATCCCATCACCATCTTC-3′ and reverse 5′-AGGCTGTTGTCATACTTC-3′.
GAPDH was used as the loading control. Experiments were repeated
three times. The 2−ΔΔCq method was used to normalize
target gene mRNA expression to GAPDH mRNA levels (17).
Bioinformatics analysis
An HCC dataset including 374 tumor and 50
non-carcinoma tissues was acquired from The Cancer Genome Atlas
project (TCGA; tcga-data.nci.nih.gov/tcga/). ASTN1 levels
between tumor and adjacent non-carcinoma tissues were compared
using a Student's t-test. The ASTN1 levels between tumor and
non-tumor tissues were compared in the GEO datasets, GSE22058
(18) and GSE14520 (19), using Integrative Molecular Database
of Hepatocellular Carcinoma (HCCDB) (20). ASTN1 expression in various
types of cancer was determined using the Oncomine database
(https://www.oncomine.org/) with the
selection criteria set as a significance threshold of P≤0.0001 and
a fold change of 2. UALCAN (ualcan.path.uab.edu/index.html) was used to show
ASTN1 expression and patient clinical stage information
based on gene expression. Survival analysis based on ASTN1
expression was performed using Kaplan-Meier-plotter (21). In addition, the pathways associated
with ASTN1 were identified based on TCGA HCC dataset using
gene set enrichment analysis (GSEA) using broad.mit.edu/gsea as described previously (22). Using Tumor Immune Estimation
Resource (TIMER), ASTN1 expression was analyzed across a
range of different types of cancer and immune infiltration in HCC
was estimated (23). The abundance
of six immune infiltrates (B cells, CD4+ T cells,
CD8+ T cells, neutrophils, macrophages and dendritic
cells) were estimated, and their association with ASTN1
expression were analyzed using Spearman's rank correlation
coefficient analysis. Scatter diagrams were drawn to show the
correlation between immune-related gene expression and ASTN1
expression.
Cell culture
HepG2, Hep3B, HCCLM3, Huh7, SKHEP1 and MIHA cells
were provided by The Cell Bank of the Type Culture Collection of
the Chinese Academy of Sciences, and cultured in DMEM supplemented
with 10% FBS (Invitrogen, Thermo Fisher Scientific, Inc.), in the
absence of antibiotics with 5% CO2 at 37°C and 99%
relative humidity.
Preparation and transfection of
lentivirus
The lentiviral vector pLKO.1-puro (Addgene, Inc.)
containing one of the three anti-ASTN1 short hairpin RNAs
(sh)RNAs or the negative control (shNC) was transfected into cells.
The sequences of the shRNAs were: shASTN1−1,
5′-GCCAGAGAAAGCGGATCAA-3′; shASTN1−2,
5′-GCAACTGCCAGATGGTCTT-3′; and shASTN1−3,
5′-CCTGGAACCTGACACCATT-3′. Human ASTN-1 cDNA was inserted
into a pLVX-puro lentiviral vector (Addgene, Inc.) using the
BamHI and EcoRI sites. Subsequently,
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect 293T cells with the
lentiviral vector and packaging plasmids according to the
manufacturer's protocol, to produce the lentiviruses. After 48 h of
transfection, the medium from transfected cells were collected,
mixed and filtered. The liver cancer cells were subjected to
specific lentiviral infection using the filtered medium.
Western blotting
Antibodies against ASTN1 (dilution 1:1,000; product
code ab154522), T-cell factor (TCF)1 (dilution 1:1,000; product
code ab183862), TCF4 (dilution 1:1,000; product code ab130014),
C-jun (Jun proto-oncogene) (dilution 1:5,000; product code
ab40766), C-myc (Myc proto-oncogene) (dilution 1:1,000; product
code ab32072), cyclooxygenase-2 (COX2) (dilution 1:500; product
code ab23672), metalloproteinase (MMP)2 (dilution 1:1,000; cat. no.
ab97779), MMP9 (dilution 1:500; product code ab119906), vascular
endothelial growth factor (VEGF) (dilution 1:300; product code
ab1316) (all from Abcam), β-catenin (dilution 1:1,000; cat. no.
8480) and GAPDH (dilution 1:2,000; cat. no. 5174) (both from Cell
Signaling Technology, Inc.) were used for western blotting. RIPA
lysis buffer (JRDUN) supplemented with a protease inhibitor
cocktail (Sigma Aldrich; Merck KGaA), was used to lyse tissues or
cells. A bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.) was used to measure protein concentration. A
total of ~25 µg protein lysate loaded on a 10 or 12% SDS gel,
resolved using SDS-PAGE and transferred to nitrocellulose membranes
(EMD Millipore). Membranes were incubated with the primary
antibodies overnight at 4°C, and subsequently incubated with a
horseradish peroxidase (HRP)-labeled secondary antibody (dilution
1:1,000; cat. no. A0208 from Beyotime Institute of Biotechnology)
for 1 h at room temperature. Enhanced chemiluminescence reagent
(EMD Millipore) was used to visualize the signals.
Transwell and wound healing
assays
Transwell assays were performed to determine the
migratory and invasive capacity of the cells. In the migration
assay, the transduced cells were plated in 24-well plates. A total
of 24 h later, cells were serum starved overnight, followed by
trypsin digestion; 200 µl serum-free medium containing
5×104 cells was added to the upper chamber of a
Transwell insert and 700 µl culture medium supplemented with 10%
FBS was added to the lower chamber. The cells were incubated for 24
h at 37°C. Subsequently, the cells which had migrated were fixed
using 4% paraformaldehyde and stained using 0.5% crystal violet,
and the number of cells stained was counted under a microscope. All
tests were performed three times. Similarly, invasion assays were
performed using a similar method to that of migration assay, with
the addition of 80 µl Matrigel® (Corning, Inc.) to the
upper chamber of the Transwell insert prior to addition of
cells.
For the wound healing assays, liver cancer cells
were cultured in wells, and once confluent, the monolayer was
scratched using a 10-µl pipette tip. Subsequently, the cells were
cultured using FBS-free DMEM. An inverted microscope (Olympus IX73)
was used to observe the migration of cells at 0, 12 and 24 h
post-scratching.
Tumor xenograft experiments in
vivo
Animal studies were performed in accordance with
guidelines released by the Experimental Animal Care Commission of
Sun Yat-sen University Cancer Center. Brief, 12 BALB/c nude mice
(4–5 weeks) (Shanghai SLAC Laboratory Animal Co., Ltd.) were raised
under specific pathogen-free conditions with a 12 h light/dark
cycle, and ad libitum access to water and food.
Establishment of tumor-bearing nude mice was routinely accomplished
through tail-vein injections of HCCLM3 cells infected with the
ASTN1-expressing or control vector virus (1×107
cells in 100 µl PBS). A total of 4 weeks after cell
transplantation, the mice were sacrificed and the number of lung
metastatic foci were counted. The tumors were extracted and
subjected to hematoxylin and eosin (H&E) and immunofluorescence
staining. The 5-µm paraffin sections were stained with H&E for
histological evaluation based on standard pathological methods. In
the immunofluorescence assay, the 5-µm sections were prepared from
the paraffin-embedded tissue blocks, followed by deparaffinization
and rehydration according to a standard protocol. After antigen
retrieval using citrate buffer solution (pH 6.0), tissues were
incubated with anti-ASTN1 (Santa-Cruz Biotechnology, Inc.; cat. no.
sc-514299) and anti-β-catenin (Abcam; cat. no. ab32572) overnight
at 4°C. Subsequently, anti-mouse and anti-rabbit horseradish
peroxidase (HRP)-conjugated fluorescent dye-labeled secondary
antibodies (cat. nos. A0428 and A0423; Beyotime Institute of
Biotechnology) were used to label and visualize target protein
expression in tissues.
Immunohistochemistry (IHC)
analysis
For IHC, 5-µm sections prepared from formalin-fixed
and paraffin-embedded tissue blocks were used for analysis.
Briefly, the paraffin-embedded sections were deparaffinized using
xylene and rehydrated using a series of solutions of decreasing
alcohol concentrations. Subsequently, tissues were placed in
boiling citrate buffer for antigen retrieval, and 3%
H2O2 was used to block endogenous peroxidase
activity. Tissues were incubated overnight with anti-ASTN1 antibody
(dilution, 1:150; cat. no. ab140533; Abcam) at 4°C. The following
day, the sections were washed and incubated using a HRP-polymer
anti-Rabbit IHC kit (Maixin) at room temperature, and developed
using DAB HRP Color Development kit (Maixin), followed by
hematoxylin counter-staining. Quantification of ASTN1 expression
was performed as described in our previous study (9).
Statistical methods
GraphPad Prism (GraphPad Software, Inc.) was used
for all statistical analyses. Differences between two groups were
compared using a Student's t-test. Difference between multiple
groups were compared using a one-way ANOVA with post hoc
contrasts by Tukey test. The area under the receiver operating
characteristic curve (AUC) was calculated using the pROC package in
R. A χ2 test was used to analyze the association between
ASTN1 expression levels and the clinicopathological
characteristics. The OS was compared between patients with high and
low ASTN1 expression levels using a log-rank test and Kaplan-Meier
analysis. Furthermore, univariate and multivariate Cox proportional
hazards regression models were used to determine survival-related
factors. P<0.05 was considered to indicate a statistically
significant difference.
Results
ASTN1 expression is downregulated in
HCC tissues relative to the matching adjacent normal tissue
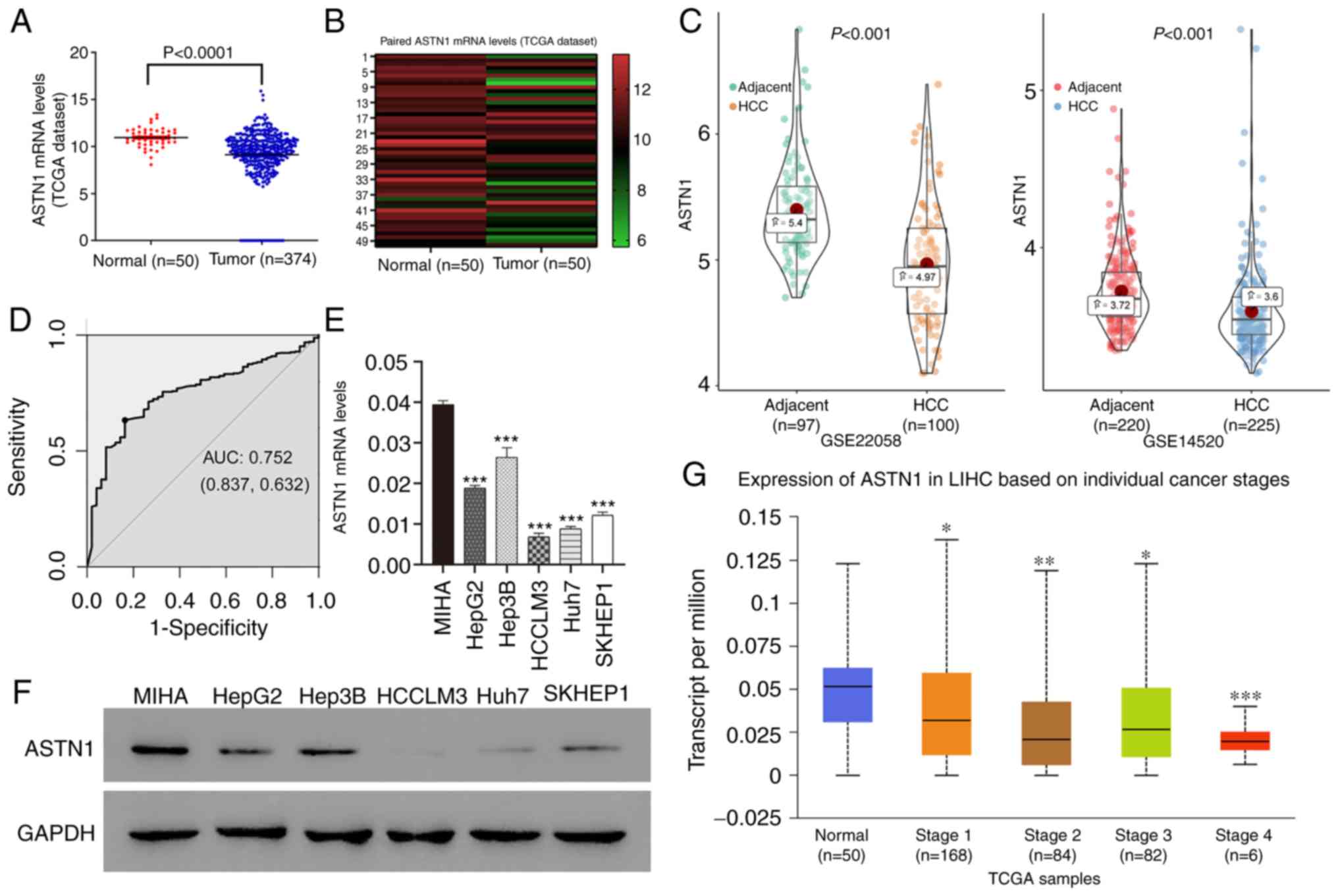
ASTN1 levels within human HCC tissues were
measured based on the TCGA dataset. ASTN1 expression levels
were downregulated in HCC tissues (n=374) compared with the normal
liver tissue (n=50) (9.14±0.14 vs. 10.95±0.14; P<0.0001;
Fig. 1A). Furthermore, ASTN1
expression in the 50 paired HCC and adjacent non-carcinoma tissue
samples showed that ASTN1 mRNA levels were lower in tumor
tissues compared with that in the paired non-carcinoma HCC tissues
(9.74±1.64 vs. 10.95±1.02; P<0.0001; Fig. 1B). Downregulated expression of
ASTN1 in HCC was further validated in the GSE22058
(P=8.600×10−11) and GSE14520 (P=2.480×10−7)
datasets (Fig. 1C). ASTN1
differential expression between HCC and adjacent samples in HCCDB
is shown in Fig. S1. ASTN1 protein
and mRNA expression levels were analyzed in 5 liver cancer cell
lines as well as in the MIHA normal liver cell line through western
blotting and RT-qPCR. ASTN1 levels were significantly lower in the
liver cancer cells compared with the normal liver cell line
(Fig. 1E and F). In addition,
receiver operating characteristic curve analysis suggested that
ASTN1 was a potential diagnostic marker for HCC, with an AUC
of 0.752 (Fig. 1D), and lower
ASTN1 levels were associated with advanced patient clinical
stage (Fig. 1G).
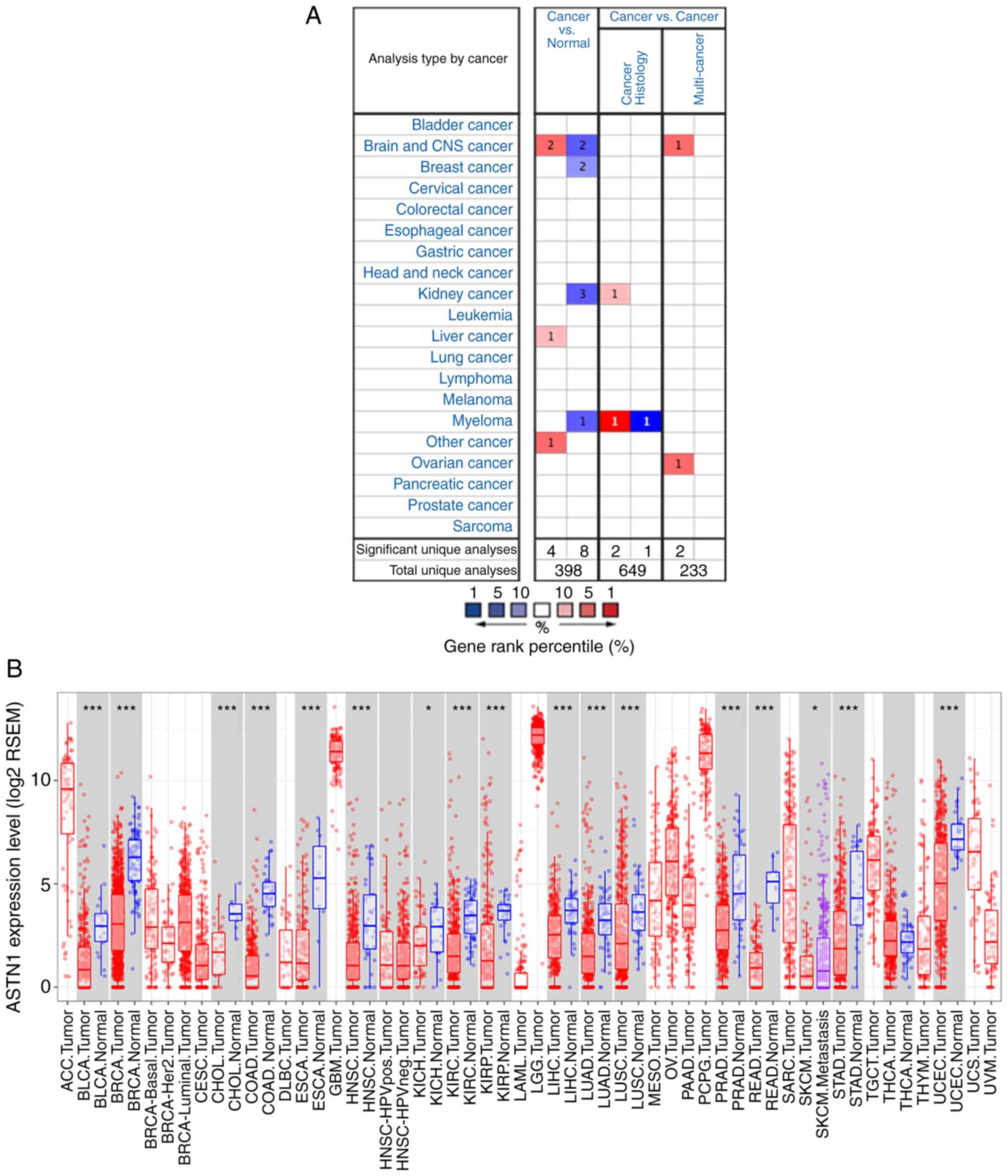
The Oncomine database showed that the ASTN1
expression was higher in two brain and CNS cancer studies, one
liver cancer study [relatively small population using a micro-array
with lack of validation (GSE6764)], and one other cancer study; low
in two brain and CNS cancer studies, two breast cancer studies,
three kidney cancer studies and one myeloma cancer study (Fig. 2A). The differential expression of
ASTN1 across all TCGA cancer cases in TIMER is shown in
Fig. 2B.
Associations between ASTN1 expression
with clinicopathological parameters and prognosis in patients with
HCC
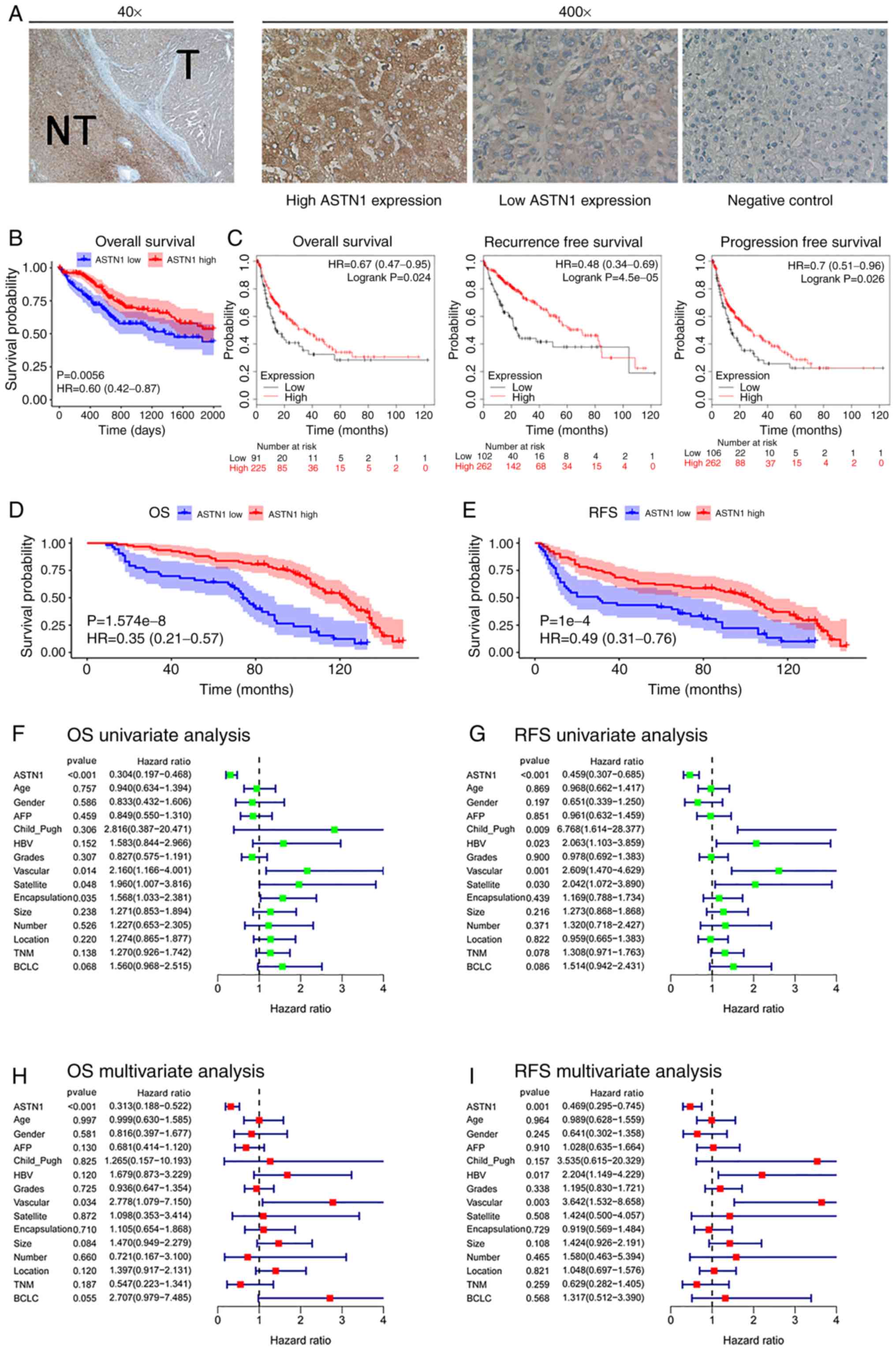
The associations between ASTN1 expression levels
with the clinicopathological parameters were analyzed. Further, the
ASTN1 levels in 145 HCC samples were also detected using IHC
(Fig. 3A). The 145 HCC patients
were split into two groups, based on ASTN1 expression; 92 patients
had levels of expression (score ≥6), and 53 patients had a low
(score <6) ASTN1 levels. ASTN1 levels were significantly
associated with the presence of microscopic satellite nodules
(P=0.018), microscopic vascular invasion (P=0.010), tumor grade
(P=0.003), encapsulation (P<0.001), Barcelona Clinic Liver
Cancer (BCLC) stage (P=0.024) and Tumor-Node-Metastasis stage
(P=0.007) as shown in Table I.
 | Table I.Association of ASTN1 with the
clinicopathological variables of the HCC patients. |
Table I.
Association of ASTN1 with the
clinicopathological variables of the HCC patients.
|
|
| ASTN1 level |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Case (n=145) | Low (n=53) | High (n=92) | P-value |
|---|
| Age (years) |
|
|
| 0.424 |
|
<45 | 73 | 29 | 44 |
|
|
≥45 | 72 | 24 | 48 |
|
| Sex |
|
|
| 0.338 |
|
Male | 128 | 45 | 83 |
|
|
Female | 17 | 8 | 9 |
|
| AFP (µg/l) |
|
|
| 0.805 |
|
<400 | 103 | 37 | 66 |
|
|
≥400 | 42 | 16 | 26 |
|
| Child_Pugh
score |
|
|
| 0.132 |
| 5 | 143 | 51 | 92 |
|
| 6 | 2 | 2 | 0 |
|
| HBV history |
|
|
| 0.248 |
| No | 20 | 5 | 15 |
|
|
Yes | 125 | 48 | 77 |
|
| Grades of
differentiation |
|
|
| 0.003 |
|
Low | 26 | 17 | 9 |
|
|
Medium | 99 | 30 | 69 |
|
|
High | 20 | 6 | 14 |
|
| Microscopic
vascular invasion |
|
| 0.010 |
|
|
Absent | 128 | 42 | 86 |
|
|
Present | 17 | 11 | 6 |
|
| Microscopic
satellite nodules |
|
| 0.018 |
|
|
Absent | 134 | 45 | 89 |
|
|
Present | 11 | 8 | 3 |
|
| Encapsulation |
|
|
| <0.001 |
|
Intact | 77 | 15 | 62 |
|
|
Destructed | 68 | 38 | 30 |
|
| Tumor size
(cm) |
|
|
| 0.264 |
|
<5 | 88 | 29 | 59 |
|
| ≥5 | 57 | 24 | 33 |
|
| Tumor number |
|
|
| >0.999 |
|
Single | 132 | 48 | 84 |
|
|
Multiple | 13 | 5 | 8 |
|
| Tumor location |
|
|
| 0.526 |
| Left
lobe | 43 | 15 | 28 |
|
| Right
lobe | 73 | 35 | 62 |
|
| Both
lobes | 5 | 3 | 2 |
|
| TNM stage |
|
|
| 0.007 |
| I | 113 | 35 | 78 |
|
| II | 23 | 12 | 11 |
|
|
III | 9 | 6 | 3 |
|
| BCLC stage |
|
|
| 0.024 |
| A | 127 | 43 | 84 |
|
| B | 15 | 7 | 8 |
|
| C | 3 | 3 | 0 |
|
Prognosis and survival analysis was performed using
the 365 cases from TCGA. Increased ASTN1 mRNA expression
levels was associated with improved OS rates (P=0.0056; Fig. 3B). KM-plotter was used to validate
the differences in OS based on ASTN1 expression, which
showed that OS, RFS and progression-free survival were
significantly different in patients with HCC cases between low and
high ASTN1 expression level groups (Fig. 3C). Low ASTN1 expression was
associated with shorter survival times. To further verify the above
findings, the ASTN1 levels among the recruited 145 HCC patients
were detected. The results suggested that patients with increased
ASTN1 expression levels had higher OS (P<0.0001; Fig. 3D) as well as RFS rates (P<0.0001;
Fig. 3E). According to results of
univariate Cox regression analysis, ASTN1, microscopic vascular
invasion, microscopic satellite nodules, and encapsulation were all
associated with OS rates (Fig. 3F).
Additionally, ASTN1, Child_Pugh, microscopic satellite nodule,
microscopic vascular invasion and HBV history were all associated
with RFS (Fig. 3G). To determine
whether ASTN1 was an independent factor for predicting patient
prognosis, multivariate analysis was performed on ASTN1 expression
levels and clinicopathological characteristics. According to the
Cox proportional hazards regression analysis, ASTN1 and microscopic
vascular invasion were independent risk factors of OS (Fig. 3H). Additionally, ASTN1, HBV history
and microscopic vascular invasion were independent risk factors
affecting patients RFS (Fig. 3I).
Taken together, the above results suggest that, ASTN1 may serve as
an independent biomarker for prediction of OS and RFS in patients
with HCC [hazard ratio (HR), 0.313; 95% confidence interval (CI),
0.188–0.522; HR, 0.469; 95% CI, 0.295–0.745, respectively]. These
results suggest that ASTN1 may exhibit clinical value for the
diagnosis and prognosis of patients with HCC.
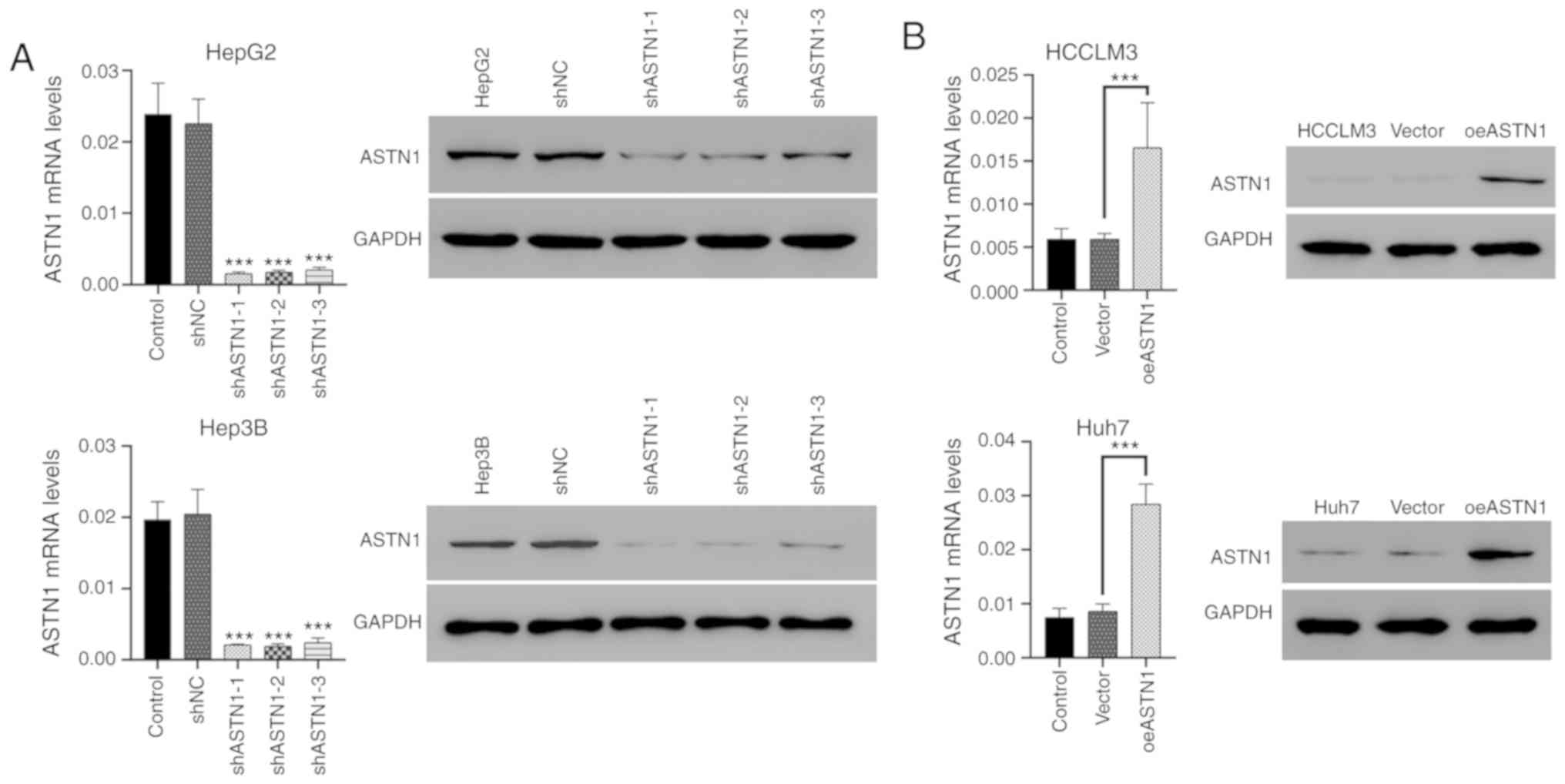
Effects of knockdown and
overexpression of ASTN1 levels in liver cancer cells
ASTN1 levels were significantly decreased in liver
cancer cells, particularly in the HCCLM3 and Huh7 cells. To further
investigate the role of ASTN1 in liver cancer, lentiviral
transfection was performed to overexpress ASTN1 (oeASTN1) in HCCLM3
and Huh7 cells, and to knock down expression (shASTN1 1–3) in HepG2
and Hep3B cells which exhibited higher levels of ASTN1 expression.
Following transfection for 48 h, RT-qPCR and western blotting were
performed. Transfection with control shNC or the empty vector did
not affect ASTN1 expression levels (Fig. 4A). To knockdown ASTN1 expression,
shASTN1 1–3 were transfected into cells, and this resulted in a
significant knockdown in ASTN1 expression in HepG2 and Hep3B cells;
shASTN1-3 exhibited the lowest knockdown efficiency and was thus
not used in subsequent experiments. ASTN1 mRNA and protein
expression levels were significantly upregulated in the
ASTN1 viral transfected cells compared with the
untransfected and empty vector virus-transfected cells (Fig. 4B).
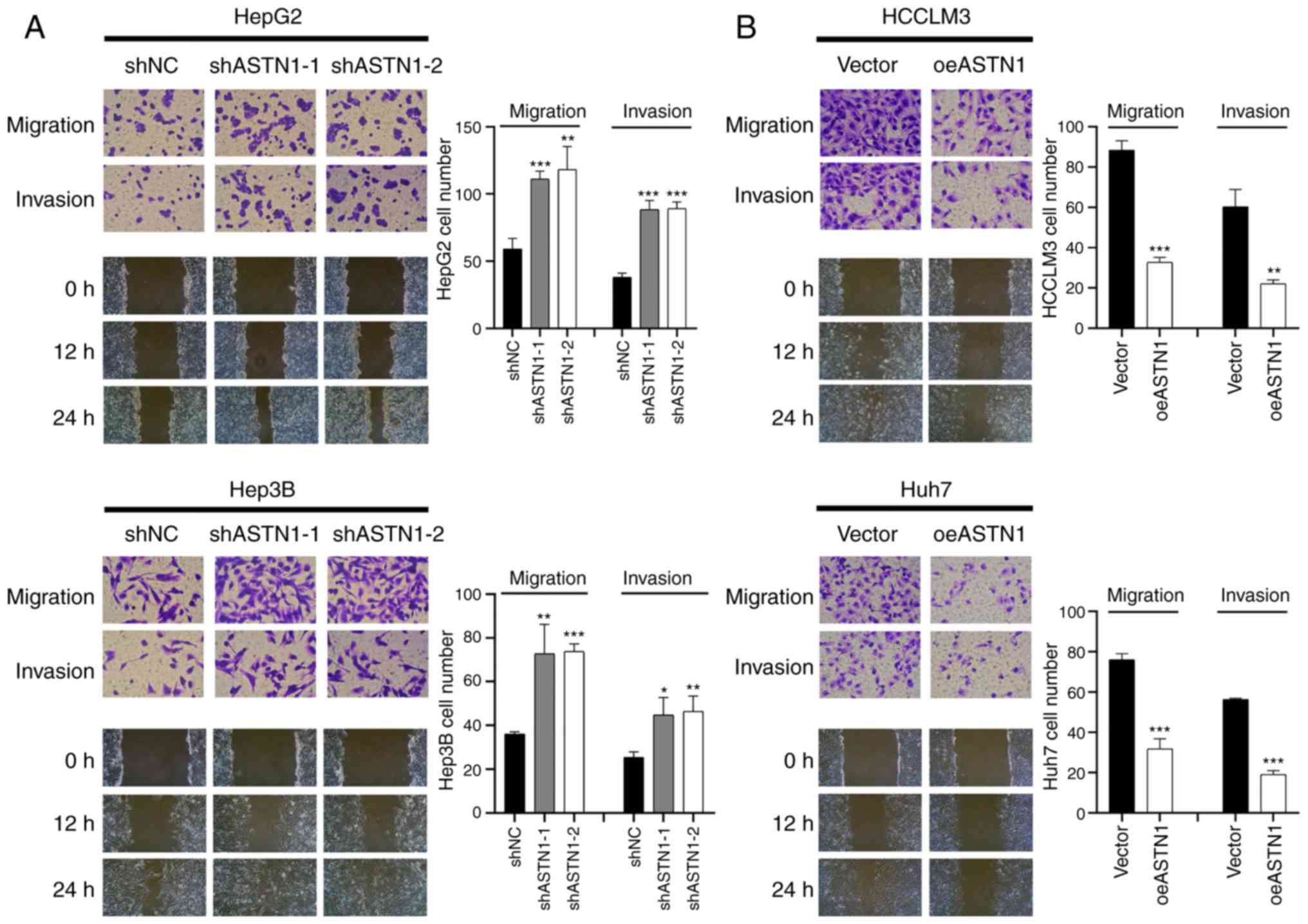
ASTN1 suppresses the migratory and
invasive capacities of liver cancer cells
Transwell invasion and migration assays were
performed to determine the role of ASTN1 in the invasive and
migratory capacities of liver cancer cells. Knockdown of ASTN1
expression in HepG2 and Hep3B cells significantly increased the
migratory and invasive capacity of the cells (Fig. 5A). Compared with the controls,
overexpression of ASTN1 in HCCLM3 and Huh7 cell lines resulted in a
significant downregulation in migration and invasion (Fig. 5B). These results suggest that ASTN1
suppressed the migratory and invasive capacity of liver cancer
cells.
ASTN1 suppresses the Wnt signal
transduction pathway in liver cancer
Pathways associated with ASTN1 were evaluated
among TCGA HCC samples using GSEA. ASTN1 levels were
negatively associated with REACTOME_SIGNALING_BY_WNT and
FEVR_CTNNB1_TARGETS_DN (Fig. 6A),
suggesting ASTN1 potentially affected HCC migration and invasion
through the Wnt/β-catenin signaling pathway.
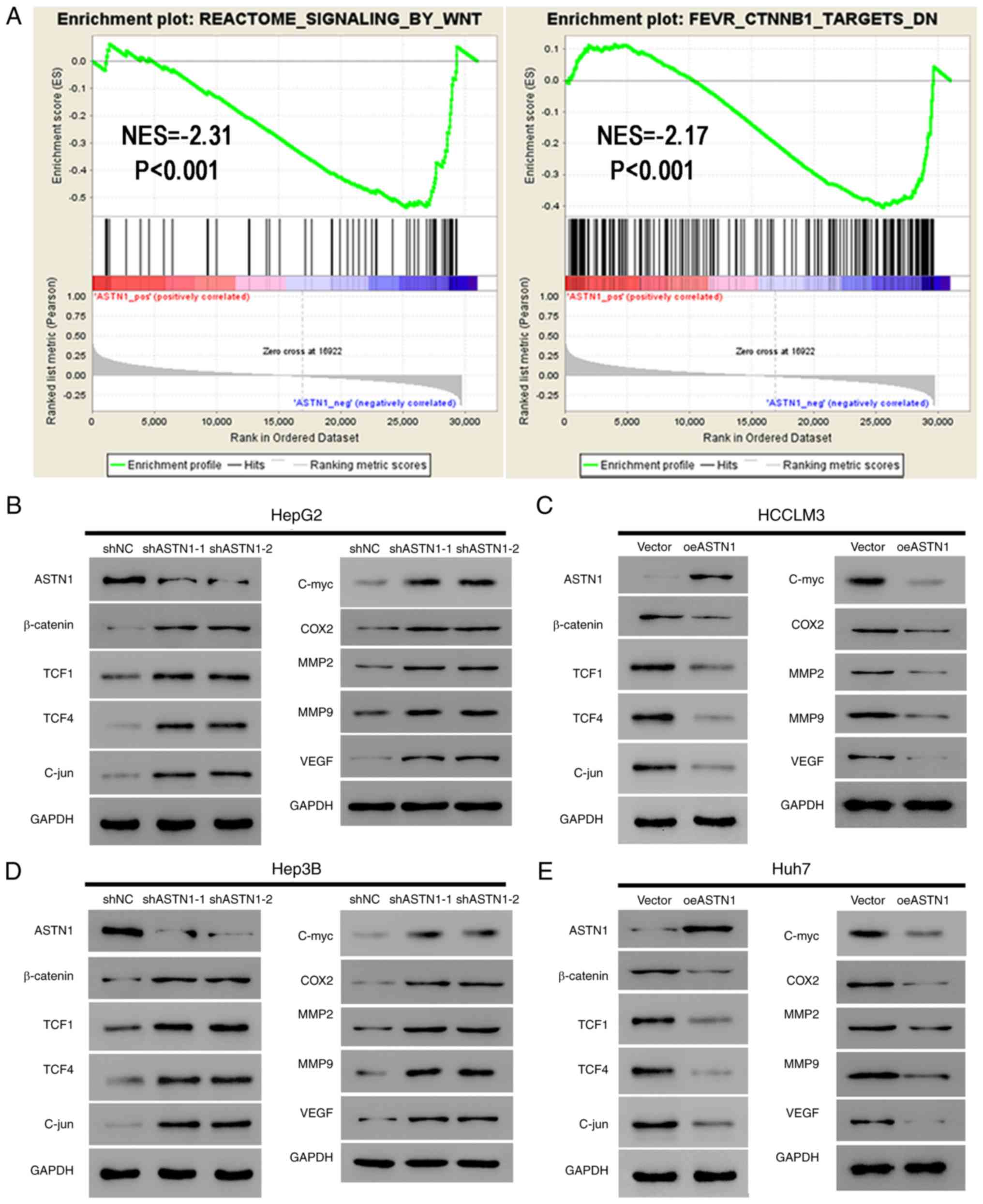 | Figure 6.ASTN1 suppresses the Wnt signaling
pathway in liver cancer cells. (A) As suggested by gene set
enrichment analysis, ASTN1 was negatively associated with the Wnt
signal transduction pathway and CTNNB1 in TCGA HCC samples.
Expression of the primary components of the Wnt signaling pathway
(β-catenin, TCF1, TCF4 and C-jun) and the downstream effectors
(C-myc, COX2, MM2, MMP9, and VEGF) of the Wnt signaling pathway
were assessed by western blotting in (B) HepG2 cells and (D) Hep3B
cells following ASTN1 silencing, and in (C) HCCLM3 cells and (E)
Huh7 cells following ASTN1 overexpression. HCC, hepatocellular
carcinoma; ASTN1, astrotactin 1; shNC, short hairpin negative
control; Vector, empty vector transfected cells; oeASTN1,
ASTN1-overexpressing vector; TCGA, The Cancer Genome Atlas; TCF,
T-cell factor; COX2, cyclooxygenase-2; MMP, metalloproteinase;
VEGF, vascular endothelial growth factor. |
Activation of the Wnt signaling pathway induces
invasion and proliferation of cells, which may serve a vital role
in enhancing carcinogenesis. In the present study, ASTN1
levels were negatively associated with the Wnt signal transduction
pathway; therefore, ASTN1 was hypothesized to inhibit the Wnt
signaling activity. Western blotting was performed to measure the
expression levels of the primary components (β-catenin, TCF1, TCF4
and C-jun) and the downstream effectors (C-myc, COX2, MMP2, MMP9
and VEGF) of the Wnt signaling pathway in liver cancer cells
overexpressing ASTN1 or with ASTN1 expression knocked down.
Knockdown of ASTN1 expression in HepG2 and Hep3B cells resulted in
a significant upregulation in β-catenin, TCF1, TCF4, C-jun, C-myc,
COX2, MM2, MMP9 and VEGF protein expression levels (Fig. 6B and D). Overexpression of ASTN1 in
HCCLM3 and Huh7 cells resulted in the opposite effect (Fig. 6C and E).
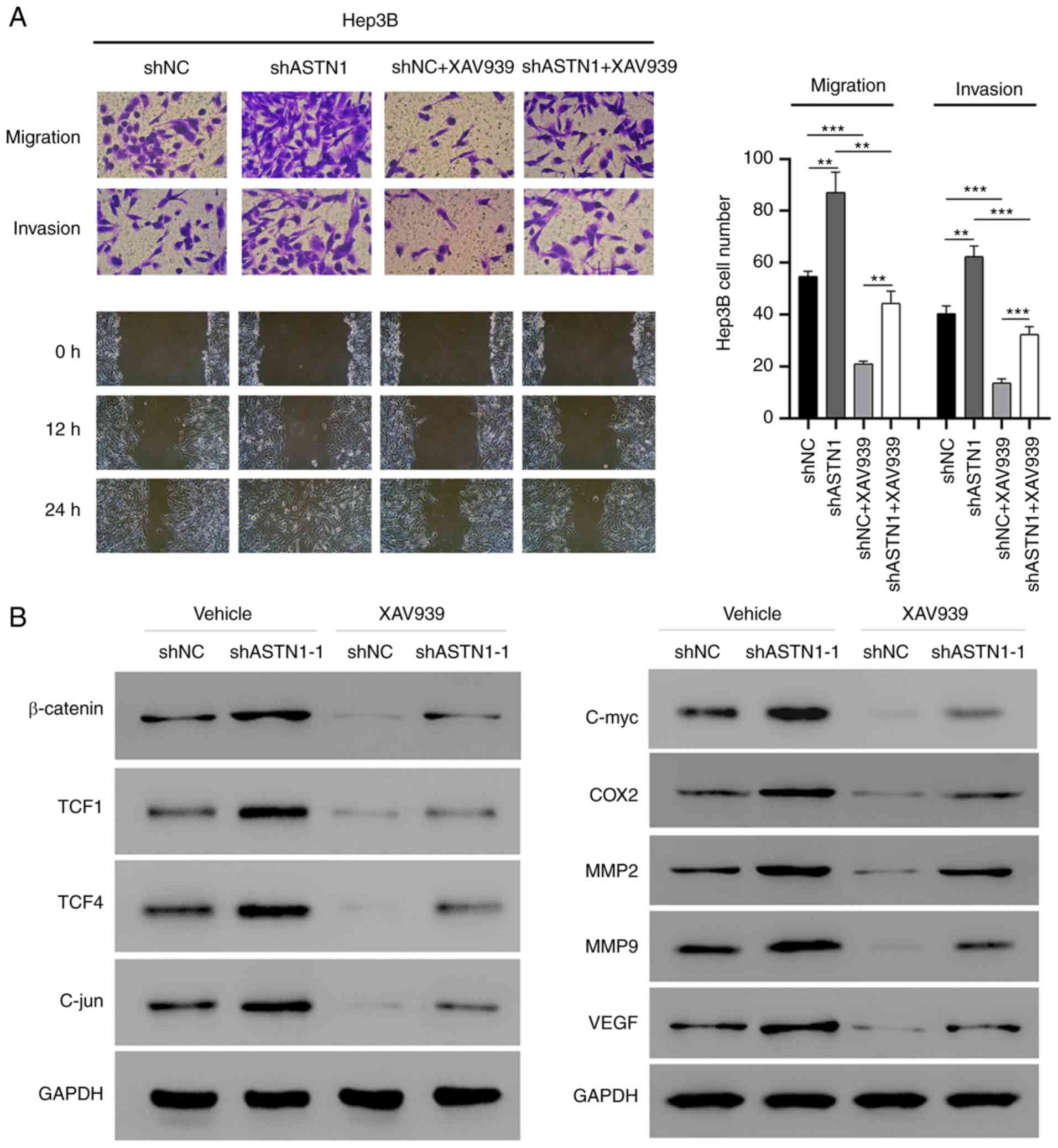
Wnt signaling mediates the effect of
ASTN1 on migration and invasion
To determine the role of Wnt signal transduction in
mediating the effect of ASTN1 on migration and invasion of cells,
ASTN1-silenced Hep3B cells were treated with XAV939 to inhibit the
Wnt signal transduction pathway (Fig.
7). ASTN1 silencing significantly increased migration and
invasion of cells, whereas XAV939 treatment suppressed these
effects. Furthermore, western blot analysis showed that β-catenin,
TCF1, TCF4, C-jun, C-myc, COX2, MM2, MMP9 and VEGF protein
expression levels were notably decreased in the Hep3B cells with
ASTN1 expression knocked down when treated with XAV939. These
results further verify the role of the Wnt signaling pathway in the
effects of ASTN1 on migration and invasion.
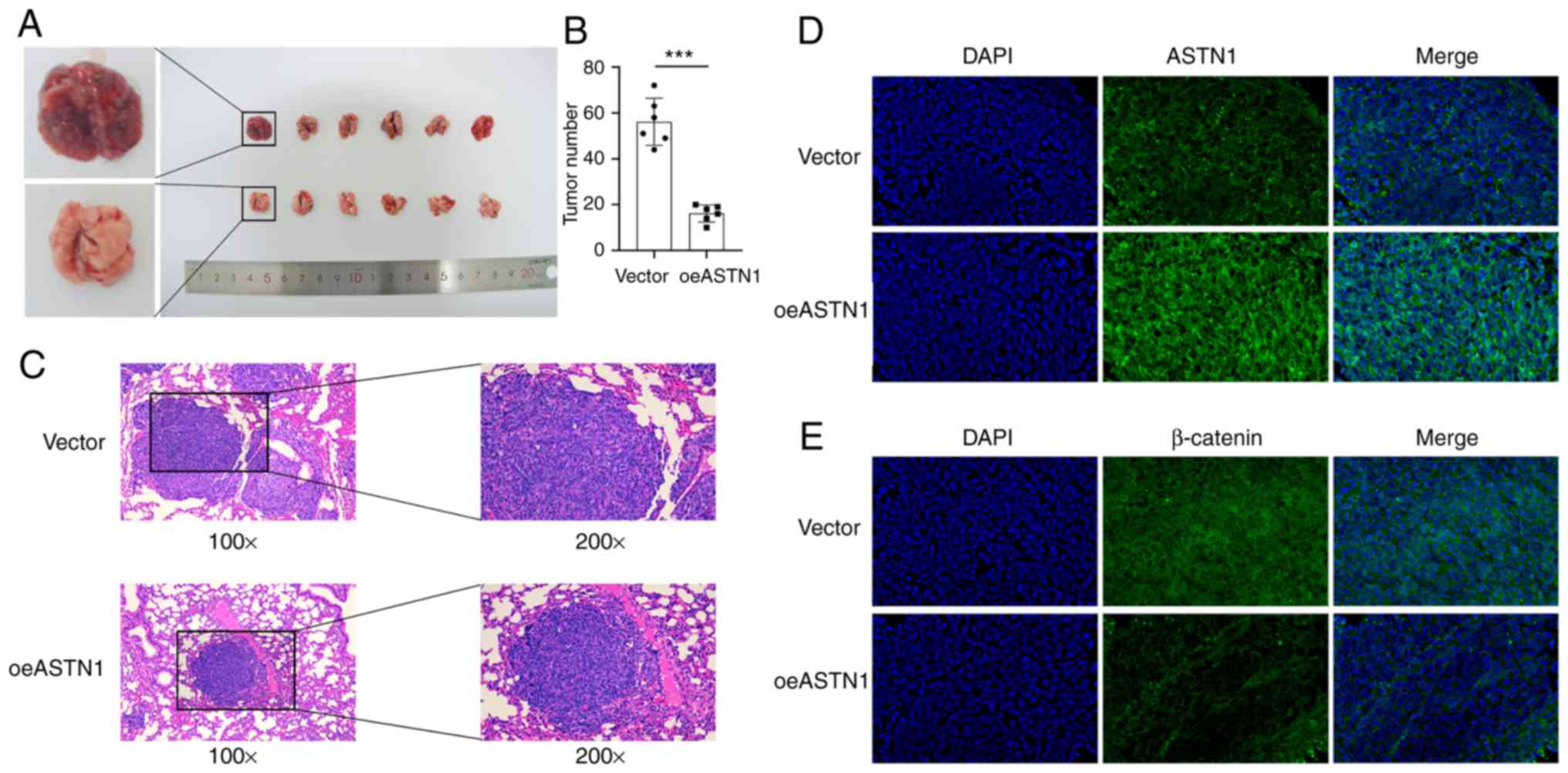
ASTN1 inhibits tumorigenesis of HCC
cells in vivo
To determine the role of ASTN1 levels in
tumorigenesis, HCCLM3 cells overexpressing ASTN1 (test group;
oeASTN1) or the cells transfected with an empty vector virus
(control group; vector), were injected into nude mice via the tail
vein. The results suggested that, ASTN1 overexpression exhibited
significantly slower tumor growth compared with the vector group
(Fig. 8A). After 4 weeks, the
number (Fig. 8B) and size (Fig. 8C) of tumors were significantly
reduced in mice injected with ASTN1 overexpressing cells compared
with the vector group. Furthermore, counterstaining of ASTN1 and
β-catenin was evaluated using immunofluorescence staining in
tissues (Fig. 8D and E), which
indicated that ASTN1 expression was upregulated, whereas β-catenin
expression was downregulated in the test group compared with the
control group. These results suggest that overexpression of ASTN1
notably reduced HCC tumor development and metastasis in
vivo, and this may have been mediated though inhibition of the
Wnt signaling pathway.
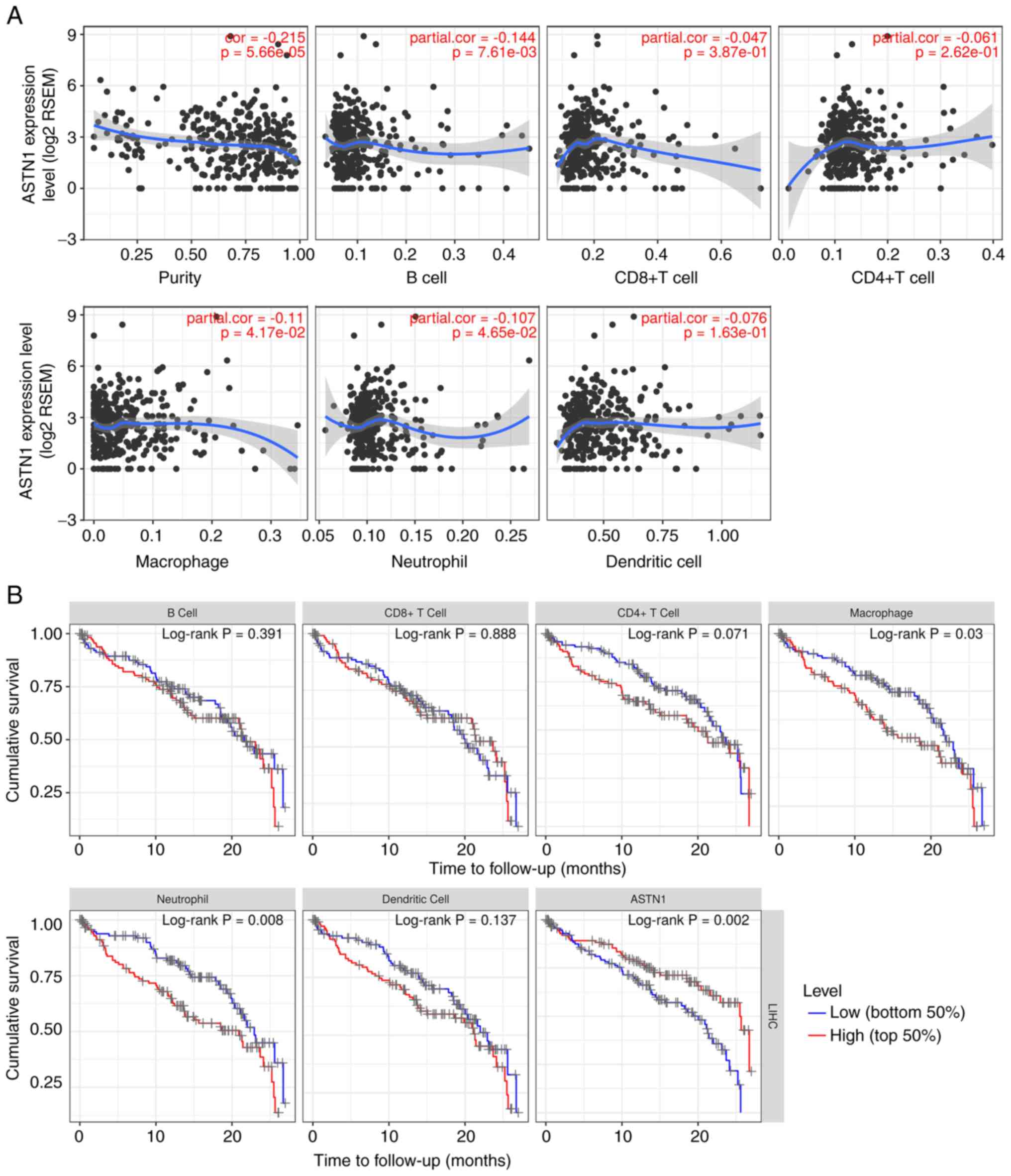
ASTN1 is correlated with immune
infiltration
The correlation between ASTN1 expression and
immune infiltration levels were investigated using TIMER. The
results showed that ASTN1 expression was significantly
negatively correlated with tumor purity, B cell, macrophage and
neutrophil infiltration (P<0.05; Fig. 9A). Patients with low macrophage
(P=0.030) or neutrophil counts (P=0.008), or increased ASTN1
levels (P=0.002) exhibited improved survival rates (Fig. 9B). As shown in Table II, the correlations between
ASTN1 expression and immune marker genes were also analyzed.
The detailed correlation diagram results are presented in Figs. S2 and S3.
 | Table II.Correlation analysis between ASTN1
and immune cell markers in TIMER. |
Table II.
Correlation analysis between ASTN1
and immune cell markers in TIMER.
|
|
| Correlation without
adjustment | Correlation
adjusted by purity |
|---|
|
|
|
|
|
|---|
| Cell types | Gene markers | Correlation | P-value | Correlation | P-value |
|---|
| CD8+ T
cells | CD8A | 0.034182 | 0.511591 | 0.081896 | 0.128411 |
|
| CD8B | 0.015941 | 0.759587 | 0.063422 | 0.239343 |
| T cells
(general) | CD3D | −0.12757 | 0.013933 | −0.10127 | 0.059859 |
|
| CD3E | 0.007557 | 0.884658 | 0.034257 | 0.52537 |
|
| CD2 | −0.03618 | 0.487164 | −0.00988 | 0.854771 |
| B cells | CD19 | 0.014771 | 0.776747 | 0.039349 | 0.465652 |
|
| CD79A | 0.11551 | 0.026093 | 0.13686 | 0.010818 |
| Monocytes | CD86 | −0.04087 | 0.432495 | −0.01351 | 0.802259 |
|
| CD115
(CSF1R) | 0.043919 | 0.398952 | 0.065139 | 0.226833 |
| TAMs | CCL2 | 0.180094 | 0.000491 | 0.191626 | 0.000337 |
|
| CD68 | −0.07161 | 0.168719 | −0.05022 | 0.351714 |
|
| IL10 | 0.002457 | 0.962381 | 0.023704 | 0.660375 |
| M1 macrophages | INOS
(NOS2) | 0.126491 | 0.01477 | 0.131225 | 0.01458 |
|
| IRF5 | −0.16654 | 0.001285 | −0.16246 | 0.002436 |
|
| COX2
(PTGS2) | 0.26749 | 1.69E-07 | 0.27122 | 3.01E-07 |
| M2 macrophages | CD163 | 0.136422 | 0.008511 | 0.161701 | 0.002554 |
|
| VSIG4 | 0.035601 | 0.494211 | 0.054388 | 0.313091 |
|
| MS4A4A | 0.050425 | 0.332751 | 0.066037 | 0.220477 |
| Neutrophils | CD66b
(CEACAM8) | 0.003447 | 0.94725 | −0.00637 | 0.906081 |
|
| CD11b
(ITGAM) | −0.17579 | 0.000671 | −0.16203 | 0.002503 |
|
| CCR7 | 0.1447 | 0.005231 | 0.147673 | 0.005923 |
| Natural killer
cells | KIR2DL1 | 0.081523 | 0.116985 | 0.102597 | 0.05658 |
|
| KIR2DL3 | 0.042478 | 0.41462 | 0.053668 | 0.319551 |
|
| KIR2DL4 | −0.06845 | 0.18832 | −0.02314 | 0.667981 |
|
| KIR3DL1 | 0.073519 | 0.157592 | 0.089658 | 0.095901 |
|
| KIR3DL2 | 0.056232 | 0.28001 | 0.097365 | 0.070477 |
|
| KIR3DL3 | −0.00972 | 0.851954 | 0.010157 | 0.850686 |
|
| KIR2DS4 | 0.01597 | 0.759156 | 0.024581 | 0.648643 |
| Dendritic
cells |
HLA-DPB1 | 0.01705 | 0.743422 | 0.047378 | 0.37963 |
|
|
HLA-DQB1 | −0.01022 | 0.844391 | 0.020789 | 0.699987 |
|
| HLA-DRA | 0.01886 | 0.717289 | 0.049302 | 0.360557 |
|
|
HLA-DPA1 | 0.032908 | 0.527457 | 0.056162 | 0.297547 |
|
| BDCA-1
(CD1C) | 0.188423 | 0.000262 | 0.181648 | 0.000687 |
|
| BDCA-4
(NRP1) | 0.193742 | 0.000173 | 0.219065 | 3.95E-05 |
|
| CD11c
(ITGAX) | −0.06498 | 0.211775 | −0.04713 | 0.382092 |
| Th1 | T-bet
(TBX21) | 0.111911 | 0.031157 | 0.127942 | 0.017264 |
|
| STAT4 | −0.03855 | 0.45914 | −0.03675 | 0.495677 |
|
| STAT1 | −0.06341 | 0.223052 | −0.05128 | 0.341569 |
|
| IFN-γ
(IFNG) | −0.11173 | 0.031431 | −0.0763 | 0.156743 |
|
| TNF-α
(TNF) | −0.00913 | 0.860897 | −0.00176 | 0.973948 |
| Th2 | GATA3 | 0.050592 | 0.331143 | 0.076333 | 0.156542 |
|
| STAT6 | 0.148376 | 0.004181 | 0.167407 | 0.00178 |
|
| STAT5A | 0.045432 | 0.382891 | 0.082603 | 0.125135 |
|
| IL13 | −0.01168 | 0.822557 | −0.00147 | 0.978293 |
| Tfh | BCL6 | 0.136255 | 0.008592 | 0.123545 | 0.02153 |
|
| IL21 | −0.00174 | 0.973357 | −0.01194 | 0.824809 |
| Th17 | STAT3 | 0.067729 | 0.19304 | 0.095733 | 0.075343 |
|
| IL17A | 0.076524 | 0.141253 | 0.069255 | 0.198757 |
| Treg | FOXP3 | 0.030033 | 0.564174 | 0.016873 | 0.754475 |
|
| CCR8 | −0.0099 | 0.849268 | −0.00339 | 0.949862 |
|
| STAT5B | 0.288155 | 1.59E-08 | 0.295426 | 2.13E-08 |
|
| TGFβ
(TGFB1) | 0.061255 | 0.239205 | 0.083081 | 0.122961 |
| T cell
exhaustion | PD-1
(PDCD1) | −0.0909 | 0.080357 | −0.05511 | 0.306696 |
|
| CTLA4 | −0.13813 | 0.007714 | −0.10896 | 0.042819 |
|
| LAG3 | −0.0897 | 0.08444 | −0.05067 | 0.347395 |
|
| TIM-3
(HAVCR2) | −0.11253 | 0.030226 | −0.08619 | 0.109528 |
|
| GZMB | 0.008927 | 0.863935 | 0.037332 | 0.488851 |
Discussion
HOTAIR is a lncRNA located on chromosome
12q13.13 at the HOXC site which is associated with cancer
progression (24). Numerous studies
examining the biological functions of HOTAIR during the
progression of HCC have suggested that expression of HOTAIR
is upregulated in hepatocellular carcinoma (HCC) tissues relative
to the normal tissues, and that HOTAIR may serve as a
potential marker of HCC (25).
Inspired by the bioinformatics analysis on genes associated with
invasiveness targeted by HOTAIR silencing, astrotactin 1
(ASTN1) has been identified as one of the top most
upregulated genes (15). Therefore,
ASTN1 is speculated to be associated with liver cancer progression.
To assess this hypothesis, ASTN1 expression was examined
using the TCGA HCC dataset as well as in patients who visited the
Sun Yat-sen University Cancer Center. According to the results of
the present study, ASTN1 expression was significantly
decreased in HCC tissues compared with non-carcinoma tissues. Using
Oncomine gene expression data we found that ASTN1 was
slightly increased in HCC when compared with the normal liver.
However, the Oncomine result should be interpreted with caution
since GSE6764: i) included a relatively small population (n=45),
ii) was based on microarray instead of RNA sequencing, and iii) had
lack of validation. Additionally, downregulated ASTN1 expression
levels in HCC were associated with a lower tumor grade, microscopic
vascular invasion, microscopic satellite nodule, destructed
encapsulation, advanced TNM stage, advanced BCLC stage and a less
favorable OS. These results highlight the potential of ASTN1 as a
candidate marker for diagnosis and prognostic prediction in
patients with HCC.
There are only a few existing studies that have
examined the effects of ASTN1 on tumor development, and only
one study has examined its role in small lung cancer patients
(26). Using exome sequencing,
Iwakawa et al (27) analyzed
44 cancer tissues from 38 cases of small cell lung cancer in a
Japanese cohort, as well as 38 normal controls, and there were
notable ASTN1 gene mutations identified, highlighting the
potential of ASTN1 during cancer development. The
involvement of ASTN1 in suppressing the migration of
neuronal cells has garnered increasing attention over the last
decades (28–30). Yi et al (31) reported that miR-sc3 enhanced the
proliferation and migration of Schwann cells through targeting of
ASTN1. Horn et al (28) demonstrated that neuron migration and
neuron-glia attachment were determined by neural cadherin (CDH2)
expression in glial cells, and ASTN1 enhanced cell migration
through directly interacting with CDH2 in neurons and glial cells.
CDH2 and ASTN1 formed a complex at the migration junction, which
served a critical role in the glial-mediated neuronal migration. In
the present study, cases with ASTN1 downregulation were associated
with increased risks of microscopic vascular invasion and advanced
clinical stage; therefore, the effects of ASTN1 on liver cancer
migration and invasion were examined. The role of ASTN1 in the
migratory and invasive capacity of liver cancer cells was assessed
in the present study through regulating the expression of ASTN1
using lentiviral transfection. To the best of our knowledge, the
present study is the first to show that ASTN1 may be considered a
tumor-suppressor gene in liver cancer.
At present, a number of signal transduction pathways
have been shown to participate in regulating the invasive and
metastatic capacity of liver cancer cells. Of these, the
Wnt/β-catenin signal transduction pathway is a classical pathway,
which serves a critical role in embryonic development as well as
self-renewal of adult tissues (32). When abnormally activated, this
pathway results in abnormal cell growth as well as malignant
transformation (33). Approximately
30% of HCC patients show abnormally high levels of Wnt/β-catenin
signal transduction pathway activity (34–36).
The abnormal activation of the Wnt/β-catenin signaling pathway
within HCC enhances cell proliferation and increases resistance to
sorafenib (37), and suppressing
the Wnt/β-catenin signaling pathway reduces the tumorigenic
potential (38). This suggests that
the excessive activation of the Wnt/β-catenin signal transduction
pathway facilitates HCC tumorigenesis. Therefore, it is essential
to explore the related mechanism of the hyperactivation of the
Wnt/β-catenin signal transduction pathway within liver cancer,
which may assist in the development of targeted therapeutics for
preventing tumor relapse. Nonetheless, the role of ASTN1 in
mediating the Wnt signal transduction pathway remains unclear. In
the present study, the GSEA results based on TCGA dataset suggested
that the ASTN1 levels were negatively associated with the
Wnt signal transduction pathway. Consistent with this, the
Wnt/β-catenin signal transduction pathway was also found to be
excessively activated in human liver cancer tissues. Therefore,
these results suggest that ASTN1 functioned via the Wnt/β-catenin
signal transduction pathway and downregulated several of the
downstream genes of this pathway. Overexpression of ASTN1 resulted
in a significant downregulation in β-catenin, TCF1, TCF4, C-jun,
C-myc, COX2, MM2, MMP9 and VEGF protein expression levels, which
are the primary signaling components or effectors downstream of the
Wnt signaling pathway, and are involved in the development of liver
cancer. Treatment with XAV939, an inhibitor of the Wnt/β-catenin
signaling pathway, significantly increased the migratory and
invasive capacity of ASTN1-overexpressing cells. Taken together,
these results suggest that the Wnt signaling transduction pathway
regulated the functions of ASTN1 with regards to the invasive and
migratory capacities of cells.
Recent advances in checkpoint inhibition therapy
have renewed investigative interest into tumor immunotherapy. The
present study also predicted the association between ASTN1
expression and different immune infiltration levels in HCC. The
results showed that the expression of ASTN1 in HCC was
significantly associated with increased infiltration of B cells,
macrophages and neutrophils. ASTN1 and marker genes of M1
macrophages were also significantly correlated. It has been shown
that SPON2 may inhibit the metastasis of HCC through the
recruitment of M1 macrophages (39). The Il-6/STAT3 pathway mediates the
polarization of M1 macrophages in HCC (40). These results suggest the potential
functional mechanism underlying ASTN1-mediated regulation of M1
macrophages in HCC, although further validation is required to
confirm this hypothesis. Additionally, the correlation between
ASTN1 and cancer immune infiltrates requires further
investigation.
In conclusion, downregulated ASTN1 expression was
associated with a less favorable prognosis in patients with HCC. In
addition, overexpression of ASTN1 in liver cancer cells reduced the
migratory and invasive capacity of the cells, and reduced the
activity of the Wnt signal transduction pathway. ASTN1 was
shown to be correlated with immune infiltrates in HCC. Therefore,
it is of crucial significance to examine the underlying mechanism
of action of ASTN1 to develop novel preventative and therapeutic
strategies for treatment of liver cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81801804) and
Guangdong Provincial People's Hospital Project 2017 (2017bq05).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QFC, FS, PW, and WL conceived and designed the
experiments. QFC, FS, TH, and CH performed the experiments and
collected the data. QFC, FS, TH, CH and LS analyzed the data. QFC,
PW, and WL wrote the paper. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study of clinical samples was approved by the
Ethics Committee of Sun Yat-sen University Cancer Center and was
performed in accordance with the principles embodied in the
Declaration of Helsinki. All patients provided written informed
consent to participate in the study. All animal procedures were
approved by the Ethics Committee of Sun Yat-sen University Cancer
Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ASTN1
|
astrotactin 1
|
|
HCC
|
hepatocellular carcinoma
|
|
TCGA
|
The Cancer Genome Atlas
|
|
GEO
|
Gene Expression Omnibus
|
|
TIMER
|
Tumor Immune Estimation Resource
|
|
HOTAIR
|
Hox Transcript Antisense RNA
|
|
GSEA
|
gene set enrichment analysis
|
|
OS
|
overall survival
|
|
RFS
|
recurrence-free survival
|
|
shRNA
|
short hairpin RNA
|
|
mRNA
|
messenger RNA
|
|
IHC
|
immunohistochemistry
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
AUC
|
area under the roc curve
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Dasgupta P, Henshaw C, Youlden DR, Clark
PJ, Aitken JF and Baade PD: Global trends in incidence rates of
primary adult liver cancers: A systematic review and meta-analysis.
Front Oncol. 10:1712020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen QF, Jia ZY, Yang ZQ, Fan WL and Shi
HB: Transarterial chemoembolization monotherapy versus combined
transarterial chemoembolization-microwave ablation therapy for
hepatocellular carcinoma tumors ≤5 cm: A propensity analysis at a
single center. Cardiovasc Intervent Radiol. 40:1748–1755. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen L, Zeng Q, Guo P, Huang J, Li C, Pan
T, Chang B, Wu N, Yang L, Chen Q, et al: Dynamically
prognosticating patients with hepatocellular carcinoma through
survival paths mapping based on time-series data. Nat Commun.
9:22302018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen QF, Xia JG, Li W, Shen LJ, Huang T
and Wu P: Examining the key genes and pathways in hepatocellular
carcinoma development from hepatitis B virus-positive cirrhosis.
Mol Med Rep. 18:4940–4950. 2018.PubMed/NCBI
|
|
5
|
Chen QF, Wu PH, Huang T, Shen LJ, Huang ZL
and Li W: Efficacy of treatment regimens for advanced
hepatocellular carcinoma: A network meta-analysis of randomized
controlled trials. Medicine (Baltimore). 98:e174602019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang ZL, Li W, Chen QF, Wu PH and Shen
LJ: Eight key long non-coding RNAs predict hepatitis virus positive
hepatocellular carcinoma as prognostic targets. World J
Gastrointest Oncol. 11:983–997. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen QF, Li W, Wu P, Shen L and Huang ZL:
Alternative splicing events are prognostic in hepatocellular
carcinoma. Aging (Albany NY). 11:4720–4735. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen QF, Huang T, Si-Tu QJ, Wu P, Shen L,
Li W and Huang Z: Analysis of competing endogenous RNA network
identifies a poorly differentiated cancer-specific RNA signature
for hepatocellular carcinoma. J Cell Biochem. 121:2303–2317. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang T, Chen QF, Chang BY, Shen LJ, Li W,
Wu PH and Fan WJ: TFAP4 promotes hepatocellular carcinoma invasion
and metastasis via activating the PI3K/AKT signaling pathway. Dis
Markers. 2019:71292142019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeon Y, Kim H, Jang ES, Hong S, Kim JW,
Yoon YS, Cho JY, Han HS and Jeong SH: Expression profile and
prognostic value of glypican-3 in post-operative South Korean
hepatocellular carcinoma patients. APMIS. 124:208–215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao JZ, Li J, Du JL and Li XL: Long
non-coding RNA HOTAIR is a marker for hepatocellular carcinoma
progression and tumor recurrence. Oncol Lett. 11:1791–1798. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geng YJ, Xie SL, Li Q, Ma J and Wang GY:
Large intervening non-coding RNA HOTAIR is associated with
hepatocellular carcinoma progression. J Int Med Res. 39:2119–2128.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang T, He X, Chen A, Tan K and Du X:
LncRNA HOTAIR contributes to the malignancy of hepatocellular
carcinoma by enhancing epithelial-mesenchymal transition via
sponging miR-23b-3p from ZEB1. Gene. 670:114–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ono H, Motoi N, Nagano H, Miyauchi E,
Ushijima M, Matsuura M, Okumura S, Nishio M, Hirose T, Inase N and
Ishikawa Y: Long noncoding RNA HOTAIR is relevant to cellular
proliferation, invasiveness, and clinical relapse in small-cell
lung cancer. Cancer Med. 3:632–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng C, Heintz N and Hatten ME: CNS gene
encoding astrotactin, which supports neuronal migration along glial
fibers. Science. 272:417–419. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burchard J, Zhang C, Liu AM, Poon RT, Lee
NP, Wong KF, Sham PC, Lam BY, Ferguson MD, Tokiwa G, et al:
microRNA-122 as a regulator of mitochondrial metabolic gene network
in hepatocellular carcinoma. Mol Syst Biol. 6:4022010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roessler S, Jia HL, Budhu A, Forgues M, Ye
QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX and Wang XW: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lian Q, Wang S, Zhang G, Wang D, Luo G,
Tang J, Chen L and Gu J: HCCDB: A database of hepatocellular
carcinoma expression atlas. Genomics Proteomics Bioinformatics.
16:269–275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Menyhárt O, Nagy Á and Győrffy B:
Determining consistent prognostic biomarkers of overall survival
and vascular invasion in hepatocellular carcinoma. R Soc Open Sci.
5:1810062018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ezponda T and Licht JD: Molecular
pathways: Deregulation of histone h3 lysine 27 methylation in
cancer-different paths, same destination. Clin Cancer Res.
20:5001–5008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan SX, Tao QF, Wang J, Yang F, Liu L,
Wang LL, Zhang J, Yang Y, Liu H, Wang F, et al: Antisense long
non-coding RNA PCNA-AS1 promotes tumor growth by regulating
proliferating cell nuclear antigen in hepatocellular carcinoma.
Cancer Lett. 349:87–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang H: Cleave but not leave: Astrotactin
proteins in development and disease. IUBMB Life. 69:572–577. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwakawa R, Kohno T, Totoki Y, Shibata T,
Tsuchihara K, Mimaki S, Tsuta K, Narita Y, Nishikawa R, Noguchi M,
et al: Expression and clinical significance of genes frequently
mutated in small cell lung cancers defined by whole Exome/RNA
sequencing. Carcinogenesis. 36:616–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Horn Z, Behesti H and Hatten ME:
N-cadherin provides a cis and trans ligand for astrotactin that
functions in glial-guided neuronal migration. Proc Natl Acad Sci
USA. 115:10556–10563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Behesti H, Fore TR, Wu P, Horn Z, Leppert
M, Hull C and Hatten ME: ASTN2 modulates synaptic strength by
trafficking and degradation of surface proteins. Proc Natl Acad Sci
USA. 115:E9717–E9726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilson PM, Fryer RH, Fang Y and Hatten ME:
Astn2, a novel member of the astrotactin gene family, regulates the
trafficking of ASTN1 during glial-guided neuronal migration. J
Neurosci. 30:8529–8540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yi S, Wang S, Zhao Q, Yao C, Gu Y, Liu J,
Gu X and Li S: miR-sc3, a novel microRNA, promotes Schwann cell
proliferation and migration by targeting Astn1. Cell Transplant.
25:973–982. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lachenmayer A, Alsinet C, Savic R,
Cabellos L, Toffanin S, Hoshida Y, Villanueva A, Minguez B, Newell
P, Tsai HW, et al: Wnt-pathway activation in two molecular classes
of hepatocellular carcinoma and experimental modulation by
sorafenib. Clin Cancer Res. 18:4997–5007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guichard C, Amaddeo G, Imbeaud S, Ladeiro
Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M,
Degos F, et al: Integrated analysis of somatic mutations and focal
copy-number changes identifies key genes and pathways in
hepatocellular carcinoma. Nat Genet. 44:694–698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kan Z, Zheng H, Liu X, Li S, Barber TD,
Gong Z, Gao H, Hao K, Willard MD, Xu J, et al: Whole-genome
sequencing identifies recurrent mutations in hepatocellular
carcinoma. Genome Res. 23:1422–1433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Ye X, Zhang JB, Ouyang H, Shen Z,
Wu Y, Wang W, Wu J, Tao S, Yang X, et al: PROX1 promotes
hepatocellular carcinoma proliferation and sorafenib resistance by
enhancing β-catenin expression and nuclear translocation. Oncogene.
34:5524–5535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamashita T, Ji J, Budhu A, Forgues M,
Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al:
EpCAM-positive hepatocellular carcinoma cells are tumor-initiating
cells with stem/progenitor cell features. Gastroenterology.
136:1012–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang YL, Li Q, Yang XM, Fang F, Li J,
Wang YH, Yang Q, Zhu L, Nie HZ, Zhang XL, et al: SPON2 promotes
M1-like macrophage recruitment and inhibits hepatocellular
carcinoma metastasis by distinct integrin-Rho GTPase-Hippo
pathways. Cancer Res. 78:2305–2317. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yin Z, Ma T, Lin Y, Lu X, Zhang C, Chen S
and Jian Z: IL-6/STAT3 pathway intermediates M1/M2 macrophage
polarization during the development of hepatocellular carcinoma. J
Cell Biochem. 119:9419–9432. 2018. View Article : Google Scholar : PubMed/NCBI
|